Avian Diversity on a University Campus in the Mexican Chihuahuan Desert
Abstract
1. Introduction
2. Materials and Methods
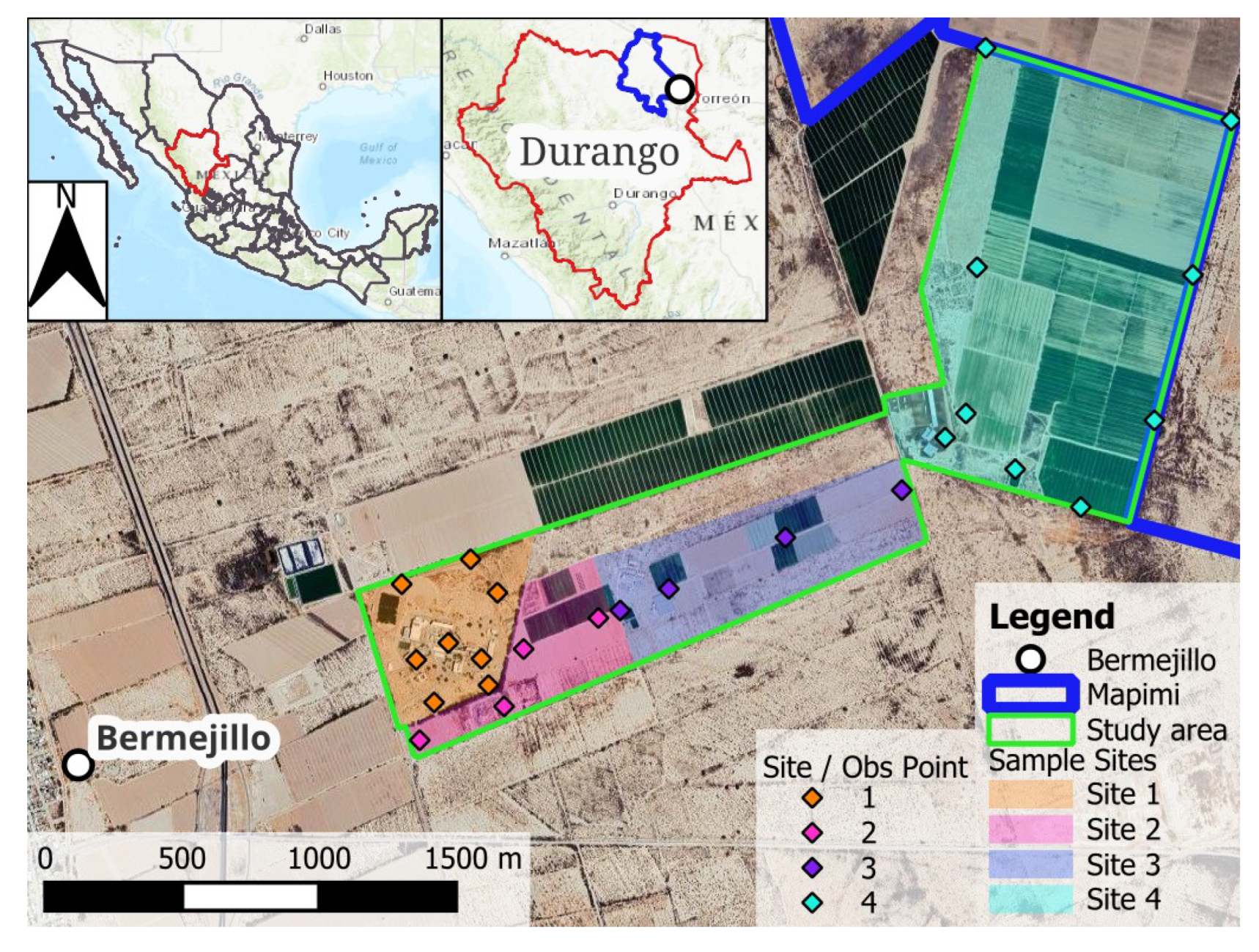
2.1. Study Area
2.2. Field Methods
2.3. Diversity Analysis
2.4. Statistical Analysis
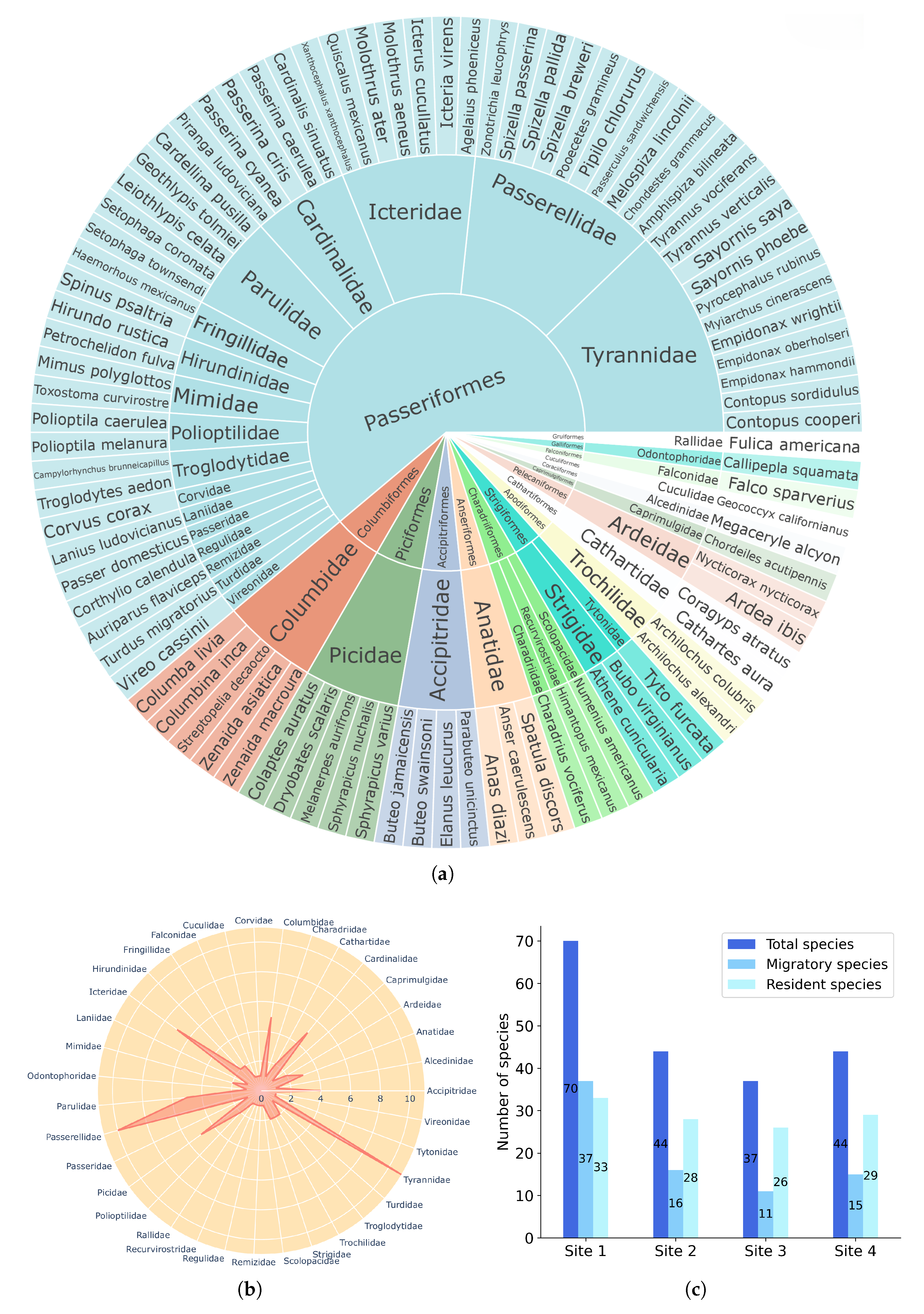
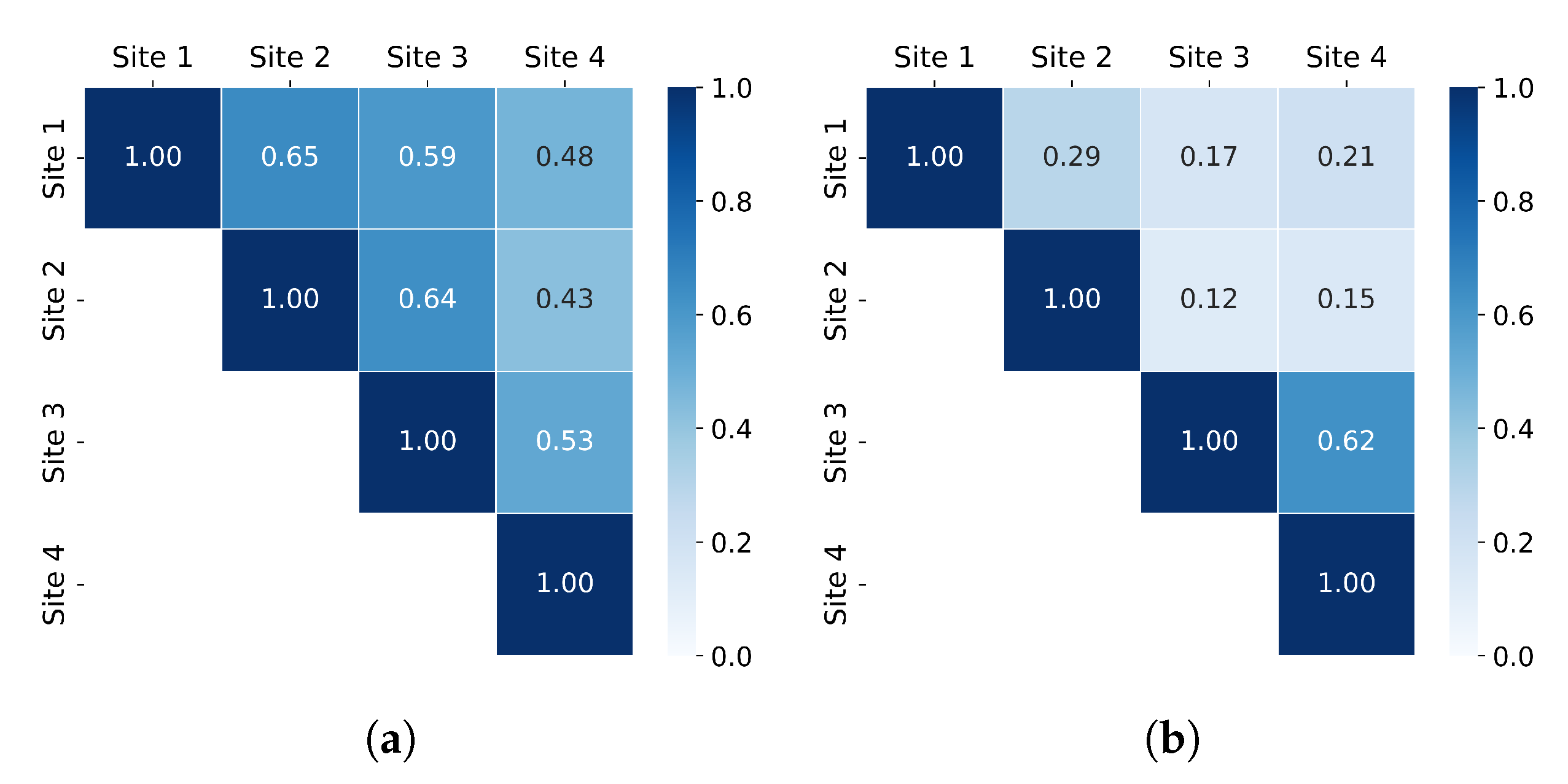
3. Results
4. Discussion
4.1. Richness and Abundance
4.2. Seasonality and Trophic Guilds
4.3. The Campus as a Refuge for Protected Birds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Specie | Comun Name | Seasonality | IUCN Red List | NOM-059 | Feeding Guild |
|---|---|---|---|---|---|
| Agelaius phoeniceus | Red-winged Blackbird | R | In | ||
| Amphispiza bilineata | Black-throated Sparrow | R | Om | ||
| Anas diazi | Mexican Duck | R | A | Om | |
| Anser caerulescens | Snow Goose | M | Om | ||
| Archilochus alexandri | Black-shinned Hummingbird | M | Ne | ||
| Archilochus colubris * | Ruby-throated Hummingbird | M | Ne | ||
| Ardea ibis | Western Cattle Egret | R | In, Pi | ||
| Athene cunicularia | Burrowing Owl | R | Ca | ||
| Auriparus flaviceps | Verdin | R | In, Fr | ||
| Bubo virginianus | Great Horned Owl | R | Ca | ||
| Buteo jamaicensis | Red-tailed Hawk | R | Ca | ||
| Buteo swainsoni | Swainson’s Hawk | M | Pr | Ca | |
| Callipepla squamata | Scaled Quail | R | Gr | ||
| Campylorhynchus brunneicapillus | Cactus Wren | R | In | ||
| Cardellina pusilla | Wilson’s Warbler | M | In | ||
| Cardinalis sinuatus | Pyrrhuloxia | R | Gr | ||
| Cathartes aura | Turkey Vulture | R | Sc | ||
| Charadrius vociferus | Killdeer | R | NT | In | |
| Chondestes grammacus | Lark Sparrow | R | Gr | ||
| Chordeiles acutipennis | Lesser Nighthawk | R | In | ||
| Colaptes auratus | Northern Flicker | M | In | ||
| Columba livia | Rock Pigeon | R | Gr | ||
| Columbina inca | Inca Dove | R | Gr | ||
| Contopus cooperi | Olive-sided Flycatcher | M | NT | In | |
| Contopus sordidulus | Western Wood-Pewee | M | In | ||
| Coragyps atratus | Black Vulture | R | Sc | ||
| Corthylio calendula | Ruby-crowned Kinglet | M | In | ||
| Corvus corax | Common Raven | R | Om | ||
| Dryobates scalaris | Ladder-backed Woodpecker | R | In | ||
| Elanus leucurus | White-tailed Kite | R | Ca | ||
| Empidonax hammondii | Hammond’s Flycatcher | M | In | ||
| Empidonax oberholseri | Dusky Flycatcher | M | In | ||
| Empidonax wrightii | Gray Flycatcher | M | In | ||
| Falco sparverius | American Kestrel | R | In, Ca | ||
| Fulica americana | American Coot | R | Om | ||
| Geococcyx californianus | Greater Roadrunner | R | Om | ||
| Geothlypis tolmiei | MacGillivray´s Warbler | M | A | In | |
| Haemorhous mexicanus | House Finch | R | Gr | ||
| Himantopus mexicanus | Black- necked Stilt | M | In, Pi | ||
| Hirundo rustica | Barn Swallow | M | In | ||
| Icteria virens | Yellow-breasted Chat | M | In, Fr | ||
| Icterus cucullatus | Hooded Oriole | M | In | ||
| Lanius ludovicianus | Loggerhead Shrike | R | NT | In, Ca | |
| Leiothlypis celata | Orange-crowned Warbler | M | In, Fr | ||
| Megaceryle alcyon | Belted Kingfisher | M | Pi | ||
| Megaceryle alcyon | Melanerpes aurifrons | R | In, Fr | ||
| Melospiza lincolnii | Lincoln’s Sparrow | M | Gr | ||
| Mimus polyglottos | Northern Mockingbird | R | Om | ||
| Molothrus aeneus | Bronzed Cowbird | R | In, Gr | ||
| Molothrus ater | Brown-headed Cowbird | R | In | ||
| Myiarchus cinerascens | Ash-throated Flycatcher | M | In | ||
| Numenius americanus | Long-billed Curlew | M | In, Ca | ||
| Nycticorax nycticorax | Black-crowned Night Heron | R | Pi, In, Ca | ||
| Parabuteo unicinctus | Harris’s Hawk | R | Pr | Ca | |
| Passer domesticus | House Sparrow | R | Gr | ||
| Passerculus sandwichensis | Savannah Sparrow | M | Gr, In | ||
| Passerina caerulea | Blue Grosbeak | M | Gr | ||
| Passerina ciris | Painted Bunting | M | Pr | Gr | |
| Passerina cyanea | Indigo Bunting | M | In, Gr | ||
| Petrochelidon fulva | Cave Swallow | M | In | ||
| Pipilo chlorurus | Green-tailed Towhee | M | Gr, In | ||
| Piranga ludoviciana | Western Tanager | M | In, Fr | ||
| Polioptila caerulea | Blue–gray Gnatcatcher | M | In | ||
| Polioptila melanura | Black-tailed Gnatcatcher | R | In | ||
| Pooecetes gramineus | Vesper Sparrow | M | Gr, In | ||
| Pyrocephalus rubinus | Vermilion Flycatcher | R | In | ||
| Quiscalus mexicanus | Great-tailed Grackle | R | Om | ||
| Sayornis phoebe | Eastern Phoebe | M | In | ||
| Sayornis saya | Say’s Phoebe | R | In | ||
| Setophaga coronata | Yellow-rumped Warbler | M | In, Fr | ||
| Setophaga townsendi | Townsend’s Warbler | M | In | ||
| Spatula discors | Blue-winged Teal | M | Om | ||
| Sphyrapicus nuchalis | Red-naped Sapsucker | M | In, Fr | ||
| Sphyrapicus varius | Yellow-bellied Sapsucker | M | In, Fr | ||
| Spinus psaltria | Lesser Golgfinch | R | Gr | ||
| Spizella breweri | Brewer’s Sparrow | M | In, Gr | ||
| Spizella pallida | Clay-colored Sparrow | M | Gr | ||
| Spizella passerina | Chipping Sparrow | M | Gr | ||
| Streptopelia decaocto | Turkish collar Dove | R | Gr | ||
| Toxostoma curvirostre | Curve-billed Thrasher | R | In | ||
| Troglodytes aedon | Northern House Wren | M | In | ||
| Turdus migratorius | American Robin | M | In | ||
| Tyrannus verticalis | Western Kingbird | M | In | ||
| Tyrannus vociferans | Cassin’s Kingbird | M | In | ||
| Tyto furcata | Barn Owl | R | Ca | ||
| Vireo cassinii * | Cassin’s Vireo | M | In | ||
| Xanthocephalus xanthocephalus | Yellow-headed Blackbird | M | Gr | ||
| Zenaida asiatica | White Dove | R | Gr | ||
| Zenaida macroura | Mourning Dove | R | Gr | ||
| Zonotrichia leucophrys | White-crowned Sparrow | M | Gr, In |


References
- Cisneros-Pineda, A.; Chaudhary, A.; Baldos, U.L.C.; Sung, Y.; Hertel, T. Demographic changes will shape planetary biodiversity. Sci. Total. Environ. 2025, 974, 179148. [Google Scholar] [CrossRef]
- Wu, S.; Liu, M.; Wang, D.; Zhang, Q. Vulnerability of biodiversity to social and ecological stressors in the Yarlung Tsangpo River Basin. J. Environ. Manag. 2025, 386, 125822. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, F.; Yan, Y.; Zhang, Q. Biodiversity in Urban Green Space: A Bibliometric Review on the Current Research Field and Its Prospects. Int. J. Environ. Res. Public Health 2022, 19, 12544. [Google Scholar] [CrossRef]
- Ramírez-Albores, J.E.; Sánchez-González, L.A.; Pérez-Suárez, M.; Navarro-Siguenza, A.G.; Franco-Maass, S. Greenspaces as shelters for the conservation of bird diversity in a big city. Urban Ecosyst. 2024, 27, 2047–2059. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Si, X.; Feng, G.; Slik, F.; Zhang, J. University campuses as valuable resources for urban biodiversity research and conservation. Urban For. Urban Greening 2021, 64, 127255. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, C.; Liu, J.; Si, X.; Feng, G. Species richness, phylogenetic and functional structure of bird communities in Chinese university campuses are associated with divergent variables. Urban Ecosyst. 2018, 21, 1213–1225. [Google Scholar] [CrossRef]
- Guthula, V.B.; Shrotriya, S.; Nigam, P.; Goyal, S.P.; Mohan, D.; Habib, B. Biodiversity significance of small habitat patches: More than half of Indian bird species are in academic campuses. Landscape Urban Plann. 2022, 222, 104552. [Google Scholar] [CrossRef]
- Sanllorente, O.; Ríos-Guisado, R.; Izquierdo, L.; Molina, J.L.; Mourocq, E.; Ibáñez-Álamo, J.D. The importance of university campuses for the avian diversity of cities. Urban For. Urban Greening 2023, 86, 128038. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, C.; Chen, S.; Zhang, Y.; Shi, H.; Chen, B.; Mao, L. Effects of landscape attributes on campuses bird species richness and diversity, Implications for Eco-Friendly Urban Planning. Sustainability 2021, 13, 5558. [Google Scholar] [CrossRef]
- Singh, R.; Tiwari, A.K.; Kumar, S.; Singh, G.S. Avifaunal diversity in managed urban ecosystem: A case study of Banaras Hindu University, Varanasi. Ecol. Quest. 2024, 35, 1–25. [Google Scholar] [CrossRef]
- Zhao, Z.; Borzée, A.; Li, J.; Chen, S.; Shi, H.; Zhang, Y. Urban bird community assembly mechanisms and driving factors in university campuses in Nanjing, China. Animals 2023, 13, 673. [Google Scholar] [CrossRef]
- Corcuera, P.; Navarro-Sigüenza, A.; Suárez-García, O. The Birds of the Cuatro Ciénegas basin, a wetland within the Chihuahuan desert. In Animal Diversity and Biogeography of the Cuatro Ciénegas Basin, 1st ed.; Springer: Cham, Switzerland, 2019; pp. 189–201. [Google Scholar] [CrossRef]
- Comer, P.J.; Hak, J.C.; Kindscher, K.; Muldavin, E.; Singhurst, J. Continent-scale landscape conservation design for temperate grasslands of the Great Plains and Chihuahuan Desert. Nat. Areas J. 2018, 38, 196–211. [Google Scholar] [CrossRef]
- González-Zamora, A.; Pérez-Morales, R. Chapter 4—Loss of biodiversity and effect of climate change on the vascular flora of the Chihuahuan desert of Mexico. In Terrestrial Biomes; Demolin-Leite, G.L., Ed.; Academic Press: New York, NY, USA, 2025; pp. 45–61. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen (Para Adaptarlo a Las Condiciones de la República Mexicana), 2nd ed.; Instituto de Geografía-UNAM: Mexico City, Mexico, 1973; p. 246. [Google Scholar]
- CONANP (Comisión Nacional de Areas Naturales Protegidas). Programa de Conservación y Manejo Reserva de la Biosfera Mapimí; Semarnat; Conanp: Mexico City, Mexico, 2006. [Google Scholar]
- García-Arévalo, A. Vascular plants of the Mapimí Biosphere Reserve, Mexico: A checklist. Sida Contrib. Bot. 2002, 20, 797–807. [Google Scholar]
- Gregory, R.D.; Gibbons, D.W.; Donald, P.F. Chapter 2: Bird census and survey techniques. In Bird Ecology and Conservation: A Handbook of Techniques; Oxford University Press: Oxford, UK, 2004; pp. 17–56. [Google Scholar] [CrossRef]
- Dunn, J.L.; Alderfer, J. Field Guide to the Birds of North America, 7th ed.; National Geographic Society: Washington, DC, USA, 2017. [Google Scholar]
- Sibley, D.A. The Sibley Guide to Birds, 2nd ed.; Knopf Publishing Group: New York, NY, USA, 2024. [Google Scholar]
- Madero, A.; Luz-Sada, M. Guía de la Observación de Aves de Nuevo León, 2nd ed.; Fondo Editorial Nuevo León: Nuevo León, Mexico, 2022. [Google Scholar]
- Chesser, R.T.; Billerman, S.M.; Burns, K.J.; Cicero, C.; Dunn, J.L.; Hernández-Baños, B.E.; Jiménez, R.A.; Johnson, O.; Mason, N.A.; Rasmussen, P.C. Check-List of North American Birds. American Ornithological Society. 2025. Available online: https://checklist.americanornithology.org/taxa/ (accessed on 7 April 2025).
- The IUCN Red List of Threatened Species. 2025. Available online: https://www.iucnredlist.org/ (accessed on 9 April 2025).
- Semarnat. Norma Oficial Mexicana NOM-059- Semarnat-2010. Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones Para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo. 2025. Available online: https://www.dof.gob.mx/normasOficiales/4254/semarnat/semarnat.htm (accessed on 14 April 2025).
- Berlanga, H.; Gómez da Silva, V.M.; Vargas-Canales, V.; Rodríguez-Contreras, L.A.; Sánchez-González, R.; Ortega-Álvarez, R.; Calderón-Parra, R. Aves de México: Lista Acualizada de Especies y Nombres Científicos. 2015. Available online: https://avesmx.conabio.gob.mx/Inicio.html (accessed on 16 April 2025).
- González-Salazar, C.; Martínez-Meyer, E.; López-Santiago, G. A hierarchical classification of trophic guilds for North American birds and mammals. Rev. Mex. Biodivers. 2014, 85, 931–941. [Google Scholar] [CrossRef]
- Hunter, J.D. MatPlotLib: A 2D Graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M. Seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Hernández Martínez, J.E.; Villarreal Wislar, C.; García Morales, R.; Guzmán, S.M.; Ibarra Vázquez, É.N.; Ramos Peña, B.; Maldonado Amaya, M.C. Monitoreo de aves en la Reserva de la Biosfera Mapimí. Huitzil 2019, 20, e-507. [Google Scholar] [CrossRef]
- Hernández-Ibarra, G.; Rodríguez-González, M.; Yáñez-Chávez, L.G. Aves del Matorral Desértico Micrófilo en el Sureste de Mapimí, Durango, 1st ed.; Yáñez Chávez, Luis Gerardo, Ciudad de México, CDMX: Mexico City, Mexico, 2025. [Google Scholar]
- Lepage, D. Durango Lista de Aves—Avibase—Listas de Aves del Mundo. 2025. Available online: https://avibase.bsc-eoc.org/checklist.jsp?lang=ES&p2=1&list=avibase&synlang=ES½oion=MXdu&version=text&lifelist=&highlight=0 (accessed on 16 June 2025).
- Latumahina, F.S.; Mardiatmoko, G.; Sahusilawane, J. Richness, diversity and evenness of birds in small island. J. Phys. Conf. Ser. 2020, 1463, 012023. [Google Scholar] [CrossRef]
- Patel, H.; Gajjar, G.; Bhatt, D.; Patel, K. An Annotated Checklist of Avifauna from Hemchandracharya North Gujarat University Campus, Patan, Gujarat, India. J. Biol. Stud. 2021, 3, 121–131. [Google Scholar] [CrossRef]
- Mariyappan, M.; Rajendran, M.; Velu, S.; Johnson, A.D.; Dinesh, G.K.; Solaimuthu, K.; Kaliyappan, M.; Sankar, M. Ecological Role and Ecosystem Services of Birds: A review. Int. J. Environ. Clim. Chang. 2023, 13, 76–87. [Google Scholar] [CrossRef]
- Mikles, C.S.; Aguillon, S.M.; Chan, Y.L.; Arcese, P.; Benham, P.M.; Lovette, I.J.; Walsh, J. Genomic differentiation and local adaptation on a microgeographic scale in a resident songbird. Mol. Ecol. 2020, 29, 4295–4307. [Google Scholar] [CrossRef]
- Liu, Y. Adaptations of Bird Community under the Influence of Human Activities: A Case Study on Nanjing Foreign Language School North Campus. Acad. J. Environ. Earth Sci. 2024, 6, 76–81. [Google Scholar] [CrossRef]
- Xing, X.; Qian, F.; Ma, K. From sky to ground: Decoding migratory bird’s habitat selection along the multi-scale sequence. Landsc. Ecol. 2025, 40, 33. [Google Scholar] [CrossRef]
- Norfolk, O.; Power, A.; Eichhorn, M.P.; Gilbert, F. Migratory bird species benefit from traditional agricultural gardens in arid South Sinai. J. Arid. Environ. 2015, 114, 110–115. [Google Scholar] [CrossRef]
- Schneider, N.A.; Griesser, M. Influence and value of different water regimes on avian species richness in arid inland Australia. Biodivers. Conserv. 2009, 18, 457–471. [Google Scholar] [CrossRef]
- Abdu, S.; Lee, A.T.; Cunningham, S.J. The presence of artificial water points structures an arid-zone avian community over small spatial scales. Ostrich 2018, 89, 339–346. [Google Scholar] [CrossRef]
- Albright, T.P.; Mutiibwa, D.; Gerson, A.R.; Smith, E.K.; Talbot, W.A.; O’Neill, J.J.; Wolf, B.O. Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proc. Natl. Acad. Sci. USA 2017, 114, 2283–2288. [Google Scholar] [CrossRef]
- Czenze, Z.J.; Kemp, R.; van Jaarsveld, B.; Freeman, M.T.; Smit, B.; Wolf, B.O.; McKechnie, A.E. Regularly drinking desert birds have greater evaporative cooling capacity and higher heat tolerance limits than non-drinking species. Funct. Ecol. 2020, 34, 1589–1600. [Google Scholar] [CrossRef]
- Møller, A.P.; Czeszczewik, D.; Flensted-Jensen, E.; Erritzøe, J.; Krams, I.; Laursen, K.; Liang, W.; Walankiewicz, W. Abundance of insects and aerial insectivorous birds in relation to pesticide and fertilizer use. Avian Res. 2021, 12, 43. [Google Scholar] [CrossRef]
- Kovinka, T.; Sharikov, A.; Massalskaya, T.; Volkov, S. Structure and heterogeneity of habitat determine diet of predators despite prey abundance: Similar response in Long-eared, Short-eared Owls and Common Kestrels. Avian Res. 2022, 14, 100072. [Google Scholar] [CrossRef]
- Nebel, S.; Mills, A.; Mccracken, J.D.; Taylor, P.D. Declines of aerial insectivores in North America follow a geographic gradient. Avian Conserv. Ecol. 2010, 5, 1. [Google Scholar] [CrossRef]
- Rosenberg, K.V.; Dokter, A.M.; Blancher, P.J.; Sauer, J.R.; Smith, A.C.; Smith, P.A.; Stanton, J.C.; Panjabi, A.; Helft, L.; Parr, M.; et al. Decline of the North American avifauna. Science 2019, 366, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Norris, A.R.; Frid, L.; Debyser, C.; De Groot, K.L.; Thomas, J.; Lee, A.; Cockle, K.L. Forecasting the Cumulative Effects of Multiple Stressors on Breeding Habitat for a Steeply Declining Aerial Insectivorous Songbird, the Olive-sided Flycatcher (Contopus cooperi). Front. Ecol. Evol. 2021, 9, 635872. [Google Scholar] [CrossRef]
- Shuford, W.D.; Gardali, T. California Bird Species of Special Concern: A Ranked Assessment of Species, Subspecies, and Distinct Populations of Birds of Immediate Conservation Concern in California, 7th ed.; Western Field Ornithologists: Camarillo, CA, USA, 2008. [Google Scholar]
- Smallwood, K.S.; Smallwood, N.L. Breeding Density and Collision Mortality of Loggerhead Shrike (Lanius ludovicianus) in the Altamont Pass Wind Resource Area. Diversity 2021, 13, 540. [Google Scholar] [CrossRef]
- Jackson, B.J.; Jackson, J.A. Killdeer (Charadrius vociferus), version 1.0. In Birds of the World; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Gupta, S.; Agrawal, A.; Saxena, K. Defense Strategies in Birds of Charadriidae Family. Proc. Zool. Soc. 2022, 75, 395–412. [Google Scholar] [CrossRef]
- Airola, D.A.; Estep, J.A.; Krolick, D.E.; Anderson, R.L.; Peters, J.R. Wintering areas and migration characteristics of Swainson’s Hawks that breed in the Central Valley of California. J. Raptor Res. 2019, 53, 237–252. [Google Scholar] [CrossRef]
- Furnas, B.J.; Wright, D.H.; Tennant, E.N.; O’Leary, R.M.; Kuehn, M.J.; Bloom, P.H.; Battistone, C.L. Rapid growth of the Swainson’s Hawk population in California since 2005. Ornitol. Appl. 2022, 124. [Google Scholar] [CrossRef]
- Dwyer, J.F.; Bednarz, J.C. Harris’s Hawk (Parabuteo unicinctus), version 1.0. In Birds of the World; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Battey, C.J.; Linck, E.B.; Epperly, K.L.; French, C.; Slager, D.L.; Sykes, P.W., Jr.; Klicka, J. A migratory divide in the Painted Bunting (Passerina ciris). Am. Nat. 2018, 191, 259–268. [Google Scholar] [CrossRef]
- González-Herrera, L.R.; Chablé-Santos, J.; Aguilar-Cordero, W.; Manríque-Saide, P. El comercio de aves silvestres en la ciudad de Mérida, Yucatán, México. Eco. y RR.AA. 2018, 5, 271–281. [Google Scholar] [CrossRef]
- Pitocchelli, J. MacGillivray’s Warbler (Geothlypis tolmiei), version 1.0. In Birds of the World; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Chesser, R.T.; Billerman, S.M.; Burns, K.J.; Cicero, C.; Dunn, J.L.; Kratter, A.W.; Winker, K. Sixty-first supplement to the American Ornithological Society’s check-list of North American birds. The Auk. 2020, 137, ukaa030. [Google Scholar] [CrossRef]
- Camacho-García, N.A.; Corcuera, P.; Lara, C.; Ugarte, I.H.S.; Soto, M.D.L.A. Habitat use and behavioral patterns of Cassin’s Kingbird (Tyrannus vociferans) in an urban park of Mexico City. Wilson J. Ornith. 2021, 132, 830–839. [Google Scholar] [CrossRef]
- Shaffer, J.A.; Igl, L.D.; Johnson, D.H.; Sondreal, M.L.; Goldade, C.M.; Nenneman, M.P.; Euliss, B. The effects of management practices on grassland birds—Clay-colored Sparrow (Spizella pallida). In The Effects of Management Practices on Grassland Birds: U.S. Geological Survey Professional Paper, 1st ed.; Geological Survey: Reston, VA, USA, 2023; p. 27. [Google Scholar] [CrossRef]
- Schlossberg, S.; Sterling, J.C. Gray Flycatcher (Empidonax wrightii), version 1.0. In Birds of the World; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Rodríguez González, M. Vireo de Cassin (Vireo cassinii). 2018. iNaturalist México. Available online: https://mexico.inaturalist.org/observations/9685451 (accessed on 16 June 2025).
- Maresh Nelson, S.B.; Ribic, C.A.; Niemuth, N.D.; Bernath-Plaisted, J.; Zuckerberg, B. Sensitivity of North American grassland birds to weather and climate variability. Conserv. Biol. 2024, 38, e14143. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.; Mata, V.A.; Lopes, P.B.; Pinho, C.J.; Chaves, C.; Correia, E.; Beja, P. Dietary metabarcoding reveals the simplification of bird–pest interaction networks across a gradient of agricultural cover. Mol. Ecol. 2024, 33, e17324. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Schuster, R.; Wilson, S.; Cooke, S.J.; Rodewald, A.D.; Bennett, J.R. Integrating season-specific needs of migratory and resident birds in conservation planning. Biol. Conserv. 2020, 252, 108826. [Google Scholar] [CrossRef]



| Shannon | Margalef | |||||
|---|---|---|---|---|---|---|
| Sites | Total | Resident | Migratory | Total | Resident | Migratory |
| Site 1 | 4.74 | 3.98 | 4.10 | 16.24 | 9.15 | 9.97 |
| Site 2 | 4.27 | 3.81 | 3.24 | 11.36 | 8.10 | 5.41 |
| Site 3 | 4.10 | 3.74 | 2.85 | 9.97 | 7.65 | 4.17 |
| Site 4 | 4.27 | 3.85 | 3.18 | 11.36 | 8.32 | 5.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Estrada, M.; Hernández-Ibarra, G.; Trucíos-Caciano, R.; Sandy-Pacheco, C.A. Avian Diversity on a University Campus in the Mexican Chihuahuan Desert. Sustainability 2025, 17, 8294. https://doi.org/10.3390/su17188294
Martínez-Estrada M, Hernández-Ibarra G, Trucíos-Caciano R, Sandy-Pacheco CA. Avian Diversity on a University Campus in the Mexican Chihuahuan Desert. Sustainability. 2025; 17(18):8294. https://doi.org/10.3390/su17188294
Chicago/Turabian StyleMartínez-Estrada, Moisés, Gonzalo Hernández-Ibarra, Ramón Trucíos-Caciano, and Clementina Araceli Sandy-Pacheco. 2025. "Avian Diversity on a University Campus in the Mexican Chihuahuan Desert" Sustainability 17, no. 18: 8294. https://doi.org/10.3390/su17188294
APA StyleMartínez-Estrada, M., Hernández-Ibarra, G., Trucíos-Caciano, R., & Sandy-Pacheco, C. A. (2025). Avian Diversity on a University Campus in the Mexican Chihuahuan Desert. Sustainability, 17(18), 8294. https://doi.org/10.3390/su17188294








