Suillus flavidus, a Peatland-Associated Mycorrhizal Fungus in Poland: Ecology, Distribution, Conservation Threats, and Sustainability Considerations
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Origin and Taxonomic Verification
2.2. Dystribution of Suillus flavidus and Ecologicala Data
3. Results
3.1. Verification of Herbarium Vouchers
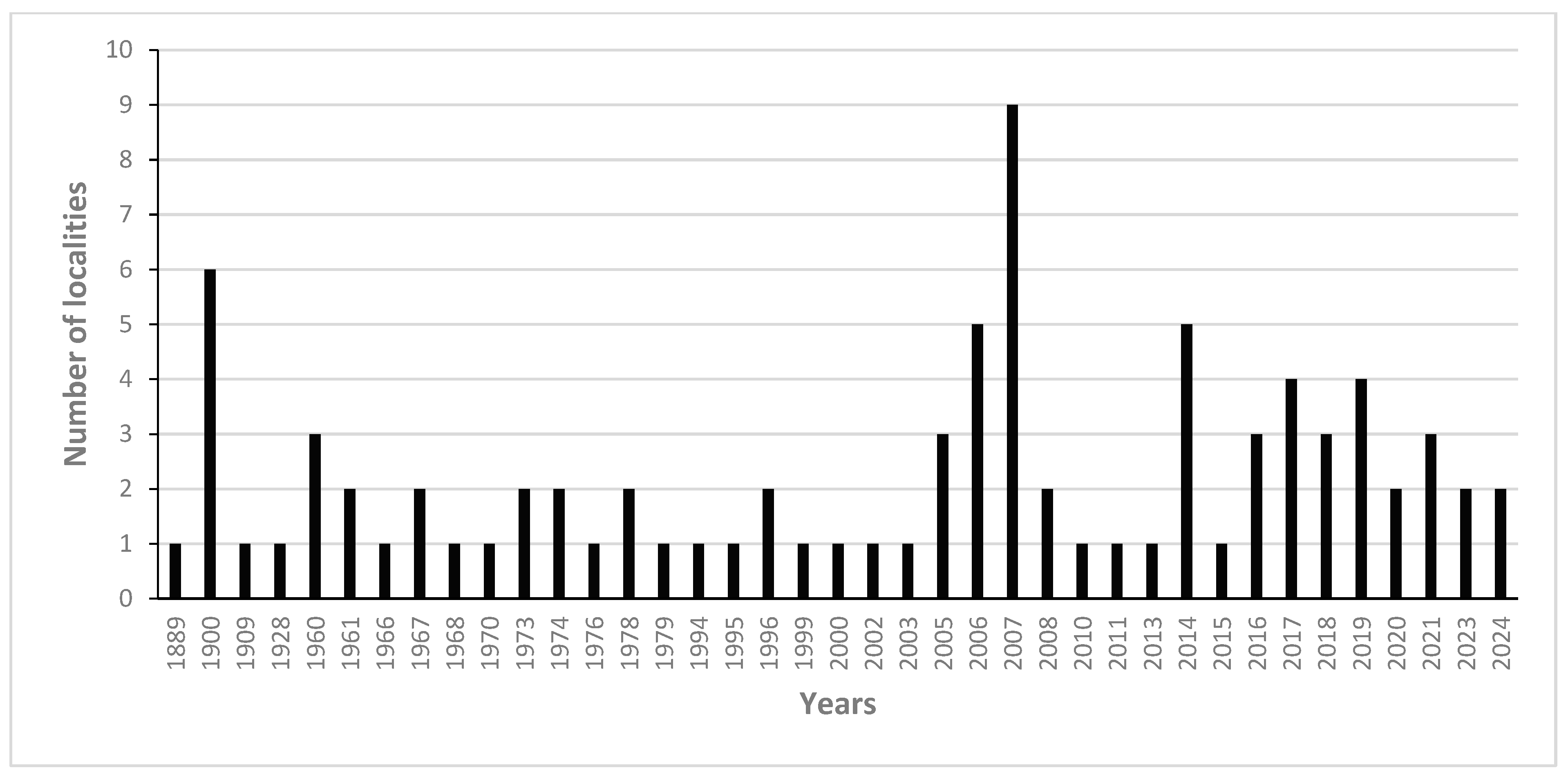
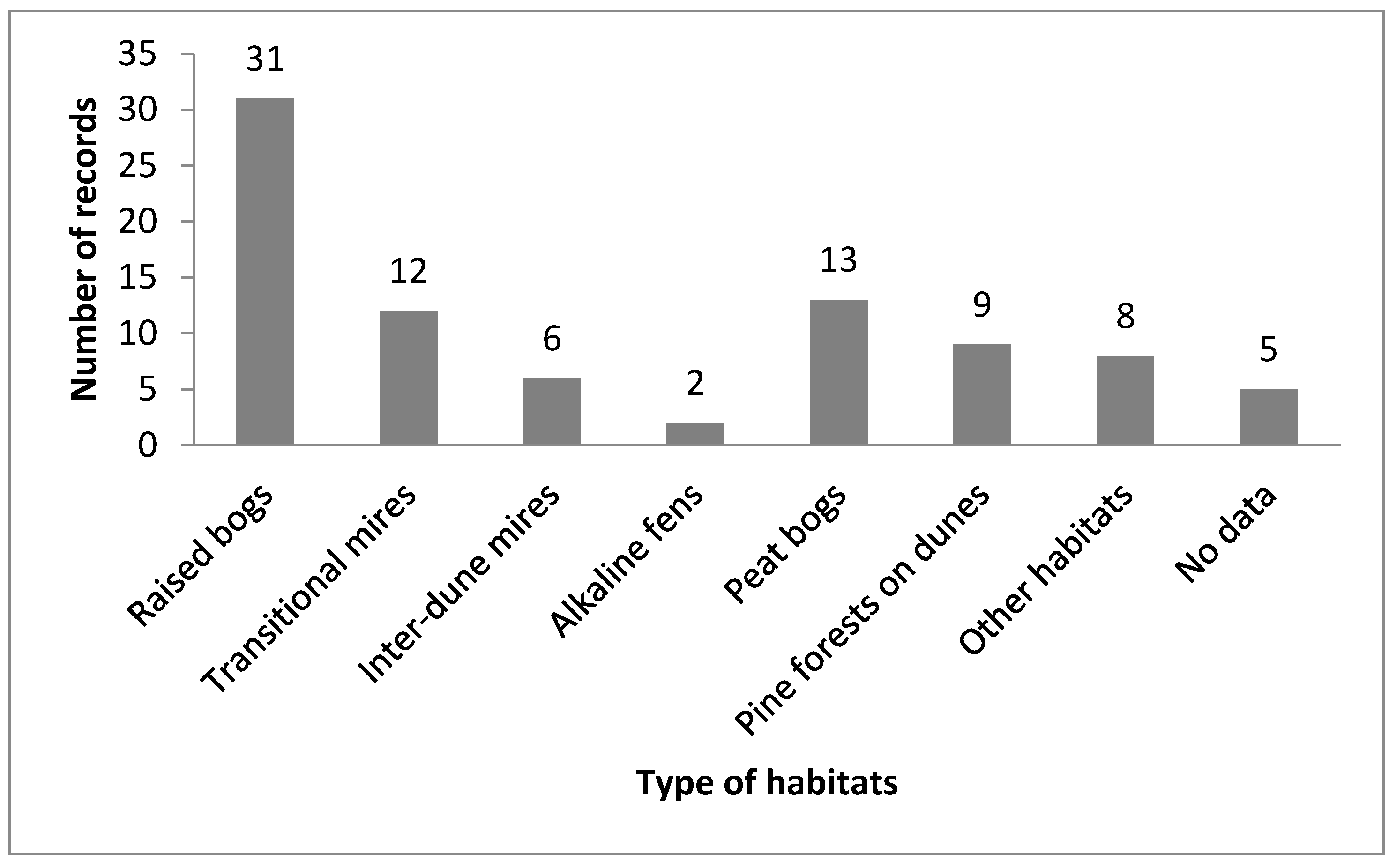
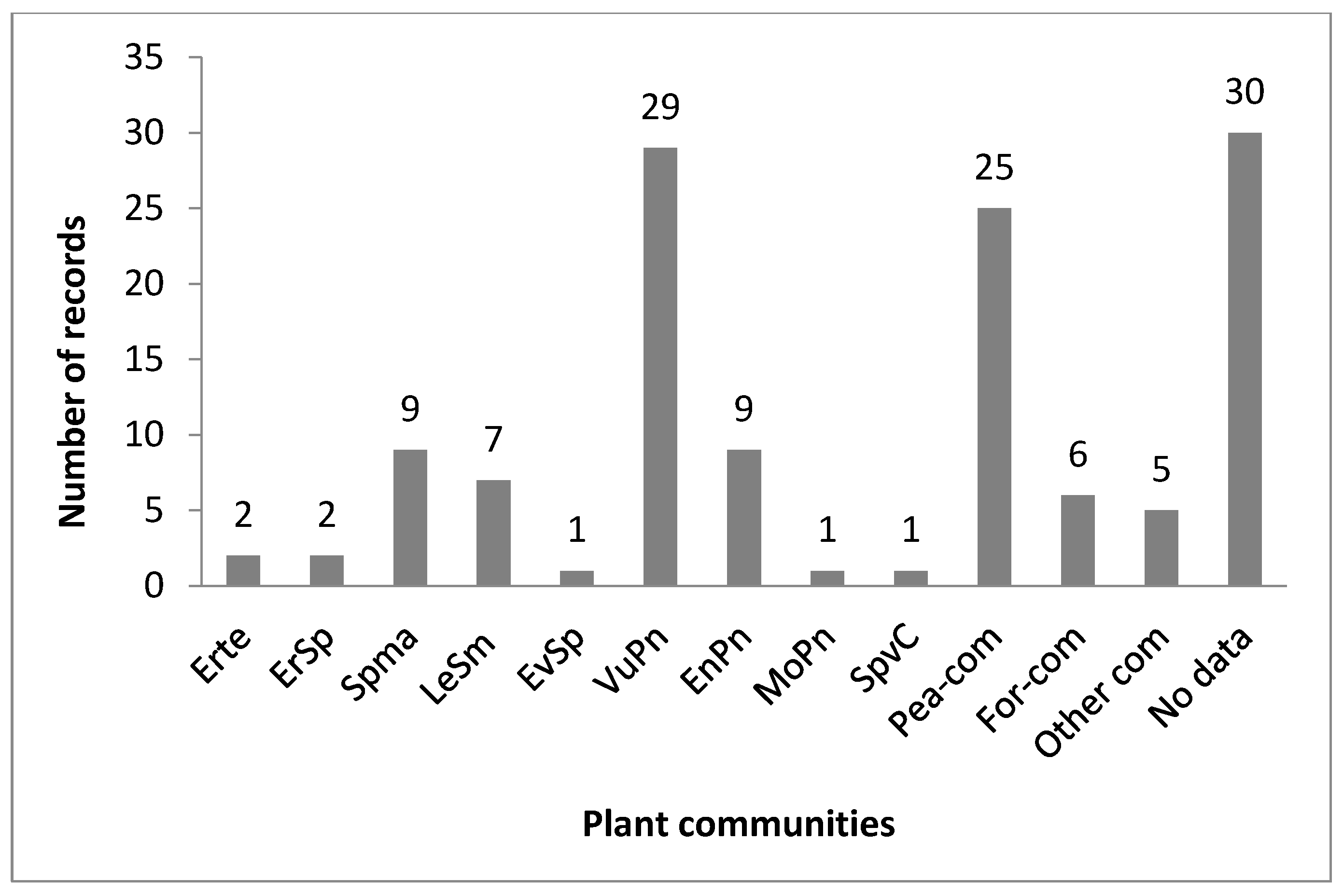
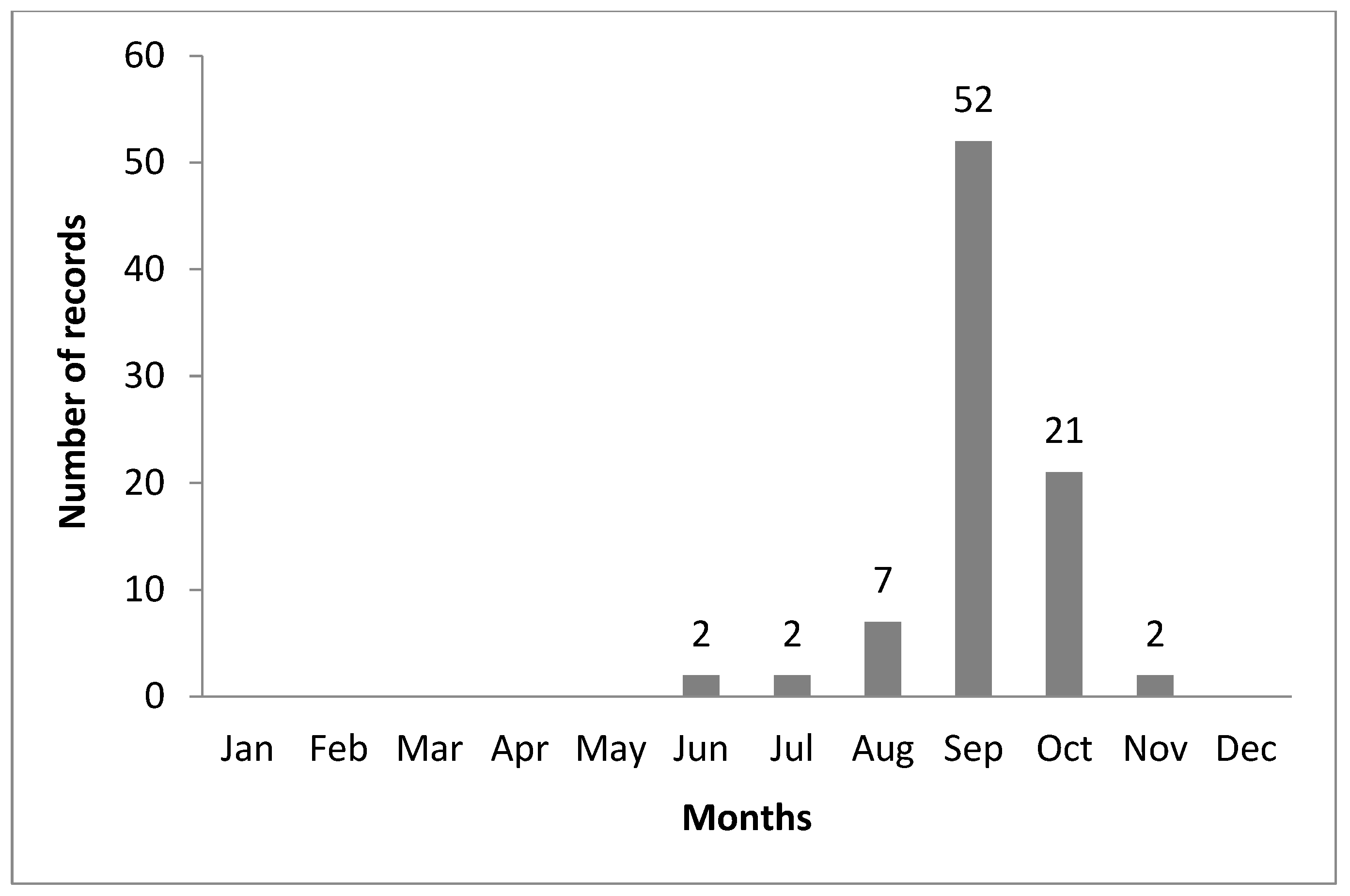
3.2. Current Distribution, Habitat, and Phenology
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Q.X.; Mueller, G.M.; Lutzoni, F.M.; Huang, Y.Q.; Guo, S.Y. Phylogenetic and biogeographic relationships of eastern Asian and eastern North American disjunct Suillus species (Fungi) as inferred from nuclear ribosomal RNA ITS sequences. Mol. Phylogenet. Evol. 2000, 17, 37–47. [Google Scholar] [CrossRef]
- Šutara, J. Central European genera of the Boletaceae and Suillaceae, with notes on their anatomical characters. Czech Mycol. 2005, 57, 1–50. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Vellinga, E.C.; Bruns, T.D.; Kennedy, P.G. Phylogenetic assessment of global Suillus ITS sequences supports morphologically defined species and reveals synonymous and undescribed taxa. Mycologia 2016, 108, 1216–1228. [Google Scholar]
- Zhang, R.; Shi, X.-F.; Liu, P.-G.; Wilson, A.W.; Mueller, G.M. Host Shift Speciation of the Ectomycorrhizal Genus Suillus (Suillineae, Boletales) and Biogeographic Comparison with Its Host Pinaceae. Front. Microbiol. 2022, 13, 831450. [Google Scholar] [CrossRef]
- Lofgren, L.; Nguyen, N.H.; Kennedy, P.G.; Pérez-Pazos, E.; Fletcher, J.; Liao, H.L.; Wang, H.; Zhang, K.; Ruytinx, J.; Smith, A.H.; et al. Suillus: An emerging model for the study of ectomycorrhizal ecology and evolution. New Phytol. 2024, 242, 1448–1475. [Google Scholar] [CrossRef]
- Wojewoda, W. Checklist of Polish larger Basidiomycetes. In Biodiversity of Poland; Mirek, Z., Ed.; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2003. [Google Scholar]
- Kujawa, A.; Gierczyk, B.; Snowarski, M. Bibliografia grzybów wielkoowocnikowych Polski. In Atlas Grzybów Polski; Snowarski, M., Ed.; 2025; Available online: http://www.grzyby.pl/grzyby-makroskopijne-Polski-w-literaturze-mikologicznej.htm (accessed on 27 May 2025).
- Klofac, W. A world-wide key to the genus Suillus. Österr. Z. Pilzk. 2013, 22, 211–278. [Google Scholar]
- Ronikier, M.; Miśkiewicz, A.; Mleczko, P. Presence and distribution of Suillus plorans in the Polisch Tatra Mts (Western Carpatians). Acta Soc. Bot. Pol. 2002, 71, 235–242. [Google Scholar] [CrossRef]
- Pietras, M.; Litkowiec, M.; Gołębiewska, J. Current and potential distribution of the ectomycorrhizal fungus Suillus lakei ((Murrill) A.H. Sm. & Thiers) in its invasion range. Mycorrhiza 2018, 28, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Bujakiewicz, A.; Lisiewska, M. Mikoflora zbiorowisk roślinnych Słowińskiego Parku Narodowego. Bad. Fizjogr. Pol. Zach. 1983, 34, 49–77. [Google Scholar]
- Dörfelt, H.; Bresinsky, A. Die Verbreitung und Ökologie ausgewählter Makromyceten Deutschlands. Distrib. Ecol. Sel. Macromycetes Germany. Z. Mykol. 2003, 69, 177–286. [Google Scholar]
- Fraiture, A.; Otto, P. (Eds.) Distribution, Ecology and Status of 51 Macromycetes in Europe: Results of the ECCF Mapping Programme; Botanic Garden Meise: Maise, Belgium, 2015. [Google Scholar]
- Kalamees, K.; Saar, I. Mycobiota of the Naissaar Nature Park (Estonia). Folia Cryptog. Est. 2006, 42, 25–41. [Google Scholar]
- Legon, N.W.; Henrici, A.; Roberts, P.J.; Spooner, B.M.; Watling, R. Checklist of the British and Irish Basidiomycota, 4th Update of the Printed Version Published 2005. 2009. Available online: http://www.basidiochecklist.info/ (accessed on 27 May 2025).
- Knudsen, H.; Taylor, A. Suillus Adans. In Funga Nordica. Agaricoid, Boletoid, Clavarioid, Cyphelloid and Gastroid Genera; Knudsen, H., Vesterholt, J., Eds.; Nordsvamp: Copenhagen, Denmark, 2012; pp. 203–207. [Google Scholar]
- GBIF Occurrence Download. Available online: https://doi.org/10.15468/39omei (accessed on 27 May 2025).
- Breitenbach, J.; Kränzlin, F. Fungi of Switzerland. 3. Boletales and Agarics 1st Part; Verlag Mycologia: Luzern, Switzerland, 1991. [Google Scholar]
- Škubla, P. Mycoflora Slovaca; Mycelium Edition: Bratislava, Slovakia, 2003. [Google Scholar]
- Dämon, W.; Krisai-Greilhuber, I. Die Pilze Österreichs. Verzeichnis und Rote Liste 2016. Teil: Makromyzeten; Österreichische Mykologische Gesellschaft: Wien, Austria, 2016. [Google Scholar]
- Dämmrich, F.; Gminder, A.; Hardtke, H.-J.; Karasch, P.; Schmidt, M.; Wehr, K. Datenbank der Pilze Deutschlands, Deutsche Gesellschaft für Mykologie e. V. (DGfM). 2025. Available online: http://www.pilze-deutschland.de/ (accessed on 22 May 2025).
- Gyosheva, M.; Natcheva, R.; Lambevska-Hristova, A. New data about macrofungal species diversity in Bulgaria. Phytol. Balc. 2018, 24, 305–313. [Google Scholar]
- Chinan, V. Macrofungi from “Gradinita” peat bog (Eastern Carpathians, Romania). Analele Stiintifice Ale Univ. Al. I. Cuza Iaşi 2011, 57, 35–40. [Google Scholar]
- Senn-Irlet, B.; Bieri, G.; Egli, S. Rote Liste der Gefahrdeten Arten der Schweiz (Rote Liste Grosspilze); Umwelt-Vollzug; Bundesamt für Umwelt, und WSL: Bern, Switzerland, 2007. [Google Scholar]
- Lizoň, P. Red list of Slovak fungi. Catathelasma 2001, 2, 25–33. [Google Scholar]
- Zíbarová, L.; Kolényova, M.; Tejklová, T.; Zehnálek, P. Červený seznam hub (makromycetů) České republiky (Red list of fungi (macromycetes) of the Czech Republic). Příroda 2024, 46, 1–192. [Google Scholar]
- Dämmrich, F.; Lotz-Winter, H.; Schmidt, M.; Pätzold, W.; Otto, P.; Schmitt, J.; Scholler, M.; Schurig, B.; Winterhoff, W.; Gminder, A.; et al. Rote Liste der Großpilze und vorläufige Gesamtartenliste der Ständer- und Schlauchpilze (Basidiomycota und Ascomycota) Deutschlands mit Ausnahme der Flechten und der phytoparasitischen Kleinpilze. In Rote Liste Gefährdeten Tiere, Pflanzen und Pilze Deutschlands. Bd. 8: Pilze (Teil 1). Großpilze [Red List of Endangered Animals, Plants and Fungi in Germany. Volume 8: Fungi (Part 1). Macrofungi]; Matzke-Hajek, G., Hofbauer, N., Ludwig, G., Eds.; Landwirtschaftsverlag–Naturschutz und Biologische Vielfalt: Münster, Germany, 2016; pp. 31–433. [Google Scholar]
- Wojewoda, W.; Ławrynowicz, M. Red List of the Macrofungi in Poland. In Red List of Plants and Fungi in Poland; Mirek, Z., Zarzycki, K., Wojewoda, W., Szeląg, Z., Eds.; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2006; pp. 53–70. [Google Scholar]
- Minister Środowiska. Rozporządzenie Ministra Środowiska z dn. 9 Października 2014 r. w Sprawie Ochrony Gatunkowej Grzybów; Dz. U. 2014 r., poz. 1408; Minister Środowiska: Wawelska, Poland, 2014. [Google Scholar]
- Stasińska, M. Maślak błotny (żółtawy) Suillus flavidus (Fr.) J. Presl. In Grzyby Chronione Polski. Rozmieszczenie, Zagrożenia, Rekomendacje Ochronne; Kujawa, A., Ruszkiewicz-Michalska, M., Kałucka, I.L., Eds.; Instytut Środowiska Rolniczego i Leśnego PAN: Poznań, Poland, 2020; pp. 324–325. [Google Scholar]
- The Global Fungal Red List Initiative. Available online: https://redlist.info/iucn/species_view/291273/ (accessed on 22 May 2025).
- Fluet-Chouinard, E.; Stocker, B.D.; Zhang, Z.; Malhotra, A.; Melton, J.R.; Poulter, B.; Kaplan, J.O.; Goldewijk, K.K.; Siebert, S.; Minayeva, T.; et al. Extensive global wetland loss over the past three centuries. Nature 2023, 614, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Schwalm, C.R.; Glendon, S.; Duffy, P.B. RCP8. 5 tracks cumulative CO2 emissions. Proc. Natl. Acad. Sci. USA 2020, 117, 19656–19657. [Google Scholar] [CrossRef]
- Trisos, C.H.; Merow, C.; Pigot, A.L. The projected timing of abrupt ecological disruption from climate change. Nature 2020, 580, 496–501. [Google Scholar] [CrossRef]
- Scheffers, B.R.; De Meester, L.; Bridge, T.C.; Hoffmann, A.A.; Pandolfi, J.M.; Corlett, R.T.; Butchart, S.H.; Pearce-Kelly, P.; Kovacs, K.M.; Dudgeon, D.; et al. The broad footprint of climate change from genes to biomes to people. Science 2016, 354, aaf7671. [Google Scholar] [CrossRef]
- Pigot, A.L.; Merow, C.; Wilson, A.; Trisos, C.H. Abrupt expansion of climate change risks for species globally. Nat. Ecol. Evol. 2023, 7, 1060–1071. [Google Scholar] [CrossRef]
- Svenning, J.-C.; Skov, F. Potential impact of climate change on the northern nemoral forest herb flora of Europe. Biodivers. Conserv. 2006, 15, 3341–3356. [Google Scholar] [CrossRef]
- Baldrian, P.; López-Mondéjar, R.; Kohout, P. Forest microbiome and global change. Nat. Rev. Microbiol. 2023, 21, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, H.; Zhao, X.; Lu, Z.; Sun, X.; Ding, G. Role of Suillus placidus in improving the drought tolerance of masson pine (Pinus massoniana Lamb.) seedlings. Forests 2021, 12, 332. [Google Scholar] [CrossRef]
- Qi, J.; Yin, D. Effects of Suillus luteus on the Growth, Photosynthesis, Stomata, and Root System of Pinus tabulaeformis Under Drought Stress. J. Plant Growth Regul. 2022, 42, 3486–3497. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Paź, S.; Frelich, L.E.; Jagodziński, A.M. How much does climate change threaten European forest tree species distributions? Glob. Change Biol. 2018, 23, 1150–1163. [Google Scholar] [CrossRef]
- Taccoen, A.; Piedallu, C.; Seynave, I.; Perez, V.; Gégout-Petit, A.; Nageleisen, L.M.; Bontemps, J.D.; Gégout, J.C. Background mortality drivers of European tree species: Climate change matters. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190386. [Google Scholar] [CrossRef]
- Pietras, M. First record of North American fungus Rhizopogon pseudoroseolus in Australia and prediction of its occurrence based on climatic niche and symbiotic partner preferences. Mycorrhiza 2019, 29, 397–401. [Google Scholar] [CrossRef]
- Pietras, M.; Kolanowska, M. Predicted potential occurrence of the North American false truffle Rhizopogon salebrosus in Europe. Fungal Ecol. 2019, 39, 225–230. [Google Scholar] [CrossRef]
- Kujawska, M.B.; Rudawska, M.; Stasińska, M.; Pietras, M.; Leski, T. Distribution and ecological traits of a rare and threatened fungus Hericium flagellum in Poland with the prediction of its potential occurrence in Europe. Fungal Ecol. 2021, 50, 101035. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Toots, M.; Diedhiou, A.G.; Henkel, T.W.; Kjøller, R.; Morris, M.H.; Nara, K.; Nouhra, E.; Peay, K.G.; et al. Towards global patterns in diversity and community structure of ectomycorrhizal fungi. Mol. Ecol. 2012, 21, 4160–4170. [Google Scholar] [CrossRef]
- Turetsky, M.R.; Benscoter, B.W.; Page, S.; Rein, G.; van der Werf, G.R.; Watts, A.C. Global vulnerability of peatlands to fire and carbon loss. Nat. Geosci. 2015, 8, 11–14. [Google Scholar] [CrossRef]
- Díaz, S.; Settele, J.; Brondízio, E.S.; Ngo, H.T.; Agard, J.; Arneth, J.; Balvaner, P.; Brauman, K.A.; Butchart, S.H.M.; Chan, K.M.A.; et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 2019, 366, eaax3100. [Google Scholar] [CrossRef]
- Stasińska, M. Macrofungi of raised and transitional bogs of Pomerania. Monogr. Bot. 2011, 101, 1–142. [Google Scholar] [CrossRef][Green Version]
- Skirgiełło, A. Materiały do poznania rozmieszczenia geograficznego grzybów wyższych w Europie. IV. Acta Mycol. 1972, 8, 191–218. [Google Scholar] [CrossRef]
- Łuszczyński, J. Grzyby macromycetes rezerwatu torfowiskowego Białe Ługi. In Rezerwat Torfowiskowy Białe Ługi; Żurek, S., Ed.; Wydawnictwo Homini: Bydgoszcz, Poland, 2001; pp. 185–204. [Google Scholar]
- Flisińska, Z. Grzyby Lubelszczyzny. Wielkoowocnikowe Podstawczaki (Basidiomycetes); Lubelskie Towarzystwo Naukowe: Lublin, Poland, 2004. [Google Scholar]
- Eichler, B. Boletus flavidus Fr. (grzyb żółtawy). Wszechświat 1901, 20, 638–639. [Google Scholar]
- Eichler, B. Drugi przyczynek do flory grzybów okolic Międzyrzecza. Pamiętn. Fizjogr. 1904, 18, 1–34. [Google Scholar]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. 2024. Available online: http://sweetgum.nybg.org/ih/ (accessed on 22 January 2025).
- Kujawa, A.; Gierczyk, B.; Ślusarczyk, T. Rejestr gatunków grzybów chronionych i zagrożonych. In Atlas Grzybów Polski; Snowarski, M., Ed.; 2025; Available online: https://www.grzyby.pl/rejestr-grzybow-chronionych-i-zagrozonych.htm (accessed on 10 April 2025).
- Kibby, G. British Boletales with Keys to Species, 7th ed.; Geoffrey Kibby: London, UK, 2016. [Google Scholar]
- Wojewoda, W. (Ed.) Atlas of the Geographical Distribution of Fungi in Poland; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2000. [Google Scholar]
- Matuszkiewicz, W. Przewodnik do oznaczania zbiorowisk roślinnych Polski. In Vademecum Geobotanicum. 3; Faliński, J.B., Ed.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2013. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. (Eds.) Vascular plants of Poland. An annotated checklist. In Biodiversity of Poland; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2020. [Google Scholar]
- Dahlberg, A.; Mueller, G.M. Applying IUCN red-listing criteria for assessing and reporting on the conservation status of fungal species. Fungal Ecol. 2011, 4, 147–162. [Google Scholar] [CrossRef]
- IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria; Version 16. 2024. Prepared by the Standards and Petitions Committee. Available online: https://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 27 August 2025).
- Błoński, F. Wyniki poszukiwań florystycznych skrytokwiatowych dokonanych w ciągu lata 1889 r. w obrębie 5-ciu powiatów Królestwa Polskiego. Pamiętn. Fizjogr. 1890, 10, 129–190. [Google Scholar]
- Stecki, K. Przyczynki do mykologii Galicyi. I. Grzyby okolic Rymanowa-Zdroju. Spraw. Kom. Fizjogr. 1910, 44, 49–56. [Google Scholar]
- Artsdatabanken. Available online: https://artsdatabanken.no/Taxon/Suillus%20flavidus/56076 (accessed on 27 May 2025).
- Gross, A.; Blaser, S.; Senn-Irlet, B.J. Swiss Fungi: National Data and Information Center for the Fungi of Switzerland [Database]. Version 2. 2024. Birmensdorf, Swiss Federal Institute WSL. Available online: https://www.wsl.ch/map_fungi (accessed on 21 May 2024).
- von Bonsdorff, T. Suillus flavidus. The FinBIF Checklist of Finnish Species 2024; Finnish Biodiversity Information Facility, Finnish Museum of Natural History, University of Helsinki: Helsinki, Finland, 2025; Available online: https://laji.fi/en/taxon/MX.71856 (accessed on 11 June 2025).
- Danmarks Svampeatlas. Available online: https://svampe.databasen.org/taxon/20730 (accessed on 27 May 2025).
- Ślusarczyk, T. Rzadkie i zagrożone grzyby wielkoowocnikowe w wybranych rezerwatach Polski Północnej i Zachodniej. Przegl. Przyr. 2020, 31, 90–108. [Google Scholar]
- Agentura Ochrany Přírody a Krajiny ČR. Available online: https://portal.nature.cz/w/druh-20629#/ (accessed on 11 June 2025).
- Tanneberger, F.; Tegetmeyer, C.; Busse, S.; Barthelmes, A.; Shumka, S.; Moles Marine, A.; Jenderedjian, K.; Steiner, G.M.; Essl, F.; Etzold, J.; et al. The peatland map of Europe. Mires Peat 2017, 19, 1–17. [Google Scholar]
- Tanneberger, F.; Moen, A.; Barthelmes, A.; Lewis, E.; Miles, L.; Sirin, A.; Tegetmeyer, C.; Joosten, H. Mires in Europe—Regional diversity, condition and protection. Diversity 2021, 13, 381. [Google Scholar] [CrossRef]
- Minasny, B.; Adetsu, D.V.; Aitkenhead, M.; Artz, R.R.E.; Baggaley, N.; Barthelmes, A.; Beucher, A.; Caron, J.; Conchedda, G.; Connolly, J.; et al. Mapping and monitoring peatland conditions from global to field scale. Biogeochemistry 2024, 167, 383–425. [Google Scholar] [CrossRef]
- Jasnowski, M.; Markowski, S.; Wołejko, T. Torfowiska. Mapa w skali 1:2,000,000. In Atlas Zasobów, Walorów i Zagrożeń Środowiska Geograficznego Polski; Kozłowski, S., Ed.; Instytut Geografii i Zagospodarowania Przestrzennego Kraju PAN: Warszawa, Poland, 1994. [Google Scholar]
- Ilnicki, P. Torfowiska i torf. In Wydawnictwo Akademii Rolniczej im; Augusta Cieszkowskiego: Poznań, Poland, 2002. [Google Scholar]
- Herbichowa, M.; Pawlaczyk, P.; Stańko, R. Conservation of Baltic Raised Bogs in Pomerania, Poland; Experience and Results of the LIFE04NAT/PL/000208 PLBALTBOGS Project; Wydawnictwo Klubu Przyrodników: Świebodzin, Poland, 2007. [Google Scholar]
- Szumińska, D.; Czapiewski, S.; Sewerniak, P. Natural and anthropogenic factors influencing changes in peatland management in Poland. Reg. Environ. Change 2023, 23, 5. [Google Scholar] [CrossRef]
- Wilga, M.S. Stanowisko maślaka błotnego Suillus flavidus (Fr.: Fr.) J.S. Presl w rezerwacie przyrody “Mierzeja Sarbska” koło Łeby. Przegl. Przyr. 2005, 16, 165–170. [Google Scholar]
- Kochanowski, J. Suillus flavidus. 2022. Available online: https://www.bio-forum.pl/messages/33/1303910.html (accessed on 5 October 2024).
- Jalas, J.; Suominen, J. (Eds.) Gymnospermae (Pinaceae to Ephedraceae). Atlas Florae Europaeae, 2; The Committee for Mapping the flora of Europe and Societas Biologica Fennica Vanamo: Helsinki, Finland, 1973. [Google Scholar]
- Burns, R.M.; Honkala, B.H. Silvics of North America: 1. Conifers; Agriculture Handbook 654; US Department of Agriculture Forest Service: Washington, DC, USA, 1990. [Google Scholar]
- Szczepkowski, A.; Olenderek, T. Suillus lakei (Murrill) A. H. Sm. & Thiers (Boletales, Basidiomycota) in Poland: New data. Acta Mycol. 2017, 52, 1098. [Google Scholar] [CrossRef][Green Version]
- Vellinga, E.C.; Wolfe, B.E.; Pringle, A. Global patterns of ectomycorrhizal introductions. New Phytol. 2009, 181, 960–973. [Google Scholar] [CrossRef]
- Policelli, N.; Bruns, T.D.; Vilgalys, R.; Nuñez, M.A. Suilloid fungi as global drivers of pine invasions. New Phytol. 2019, 222, 714–725. [Google Scholar] [CrossRef]
- Policelli, N.; Horton, T.R.; García, R.A.; Naour, M.; Pauchard, A.; Nuñez, M.A. Native and non-native trees can find compatible mycorrhizal partners in each other’s dominated areas. Plant Soil. 2020, 454, 285–297. [Google Scholar] [CrossRef]
- Brichta, J.; Šimůnek, V.; Bílek, L.; Vacek, Z.; Gallo, J.; Drozdowski, S.; Bravo-Fernández, J.A.; Mason, B.; Roig Gomez, S.; Hájek, V.; et al. Effects of Climate Change on Scots Pine (Pinus sylvestris L.) Growth across Europe: Decrease of Tree-Ring Fluctuation and Amplification of Climate Stress. Forests 2024, 15, 91. [Google Scholar] [CrossRef]
- Vašutová, M.; Vítovcová, K.; Manukjanová, A.; Prach, K. Fungal troublemakers–using indicator species with ephemeral fruitbodies to evaluate recovery of formerly extracted raised bogs. Ecol. Indic. 2023, 154, 110574. [Google Scholar] [CrossRef]
- Büntgen, U.; Kauserud, H.; Egli, S. Linking climate variability to mushroom productivity and phenology. Front. Ecol. Environ. 2012, 10, 14–19. [Google Scholar] [CrossRef]
- Gignac, L.D.; Halsey, L.A.; Vitt, D.H. A bioclimatic model for the distribution of Sphagnum-dominated peatlands in North America under present climatic conditions. J. Biogeogr. 2000, 27, 1139–1151. [Google Scholar] [CrossRef]
- Martínez-Peña, F.; De-Miguel, S.; Pukkala, T.; Bonet, J.A.; Ortega-Martínez, P.; Aldea, J.; de Aragón, J.M. Yield models for ectomycorrhizal mushrooms in Pinus sylvestris forests with special focus on Boletus edulis and Lactarius group deliciosus. For. Ecol. Manag. 2012, 282, 63–69. [Google Scholar] [CrossRef]
- Ágreda, T.; Águeda, B.; Olano, J.M.; Vicente-Serrano, S.M.; Fernández-Toirán, L.M. Increased evapotranspiration demand in a Mediterranean climate might cause a decline in fungal yields under global warming. Glob. Chang. Biol. 2015, 21, 3499–3510. [Google Scholar] [CrossRef] [PubMed]
- Andrew, C.; Heegaard, E.; Høiland, K.; Senn-Irlet, B.; Kuyper, T.W.; Krisai-Greilhuber, I.; Kirk, P.M.; Heilmann-Clausen, J.; Gange, A.C.; Egli, S.; et al. Explaining European fungal fruiting phenology with climate variability. Ecology 2018, 99, 1306–1315. [Google Scholar] [CrossRef]
- Boddy, L.; Büntgen, U.; Egli, S.; Gange, A.C.; Heegaard, E.; Kirk, P.M.; Mohammad, A.; Kauserud, H. Climate variation effects on fungal fruiting. Fungal Ecol. 2014, 10, 20–33. [Google Scholar] [CrossRef]
- Straatsma, G.; Ayer, F.; Egli, S. Species richness, abundance, and phenology of fungal fruit bodies over 21 years in a Swiss forest plot. Mycol. Res. 2001, 105, 515–523. [Google Scholar] [CrossRef]
- Compant, S.; van der Heijden, M.G.A.; Sessitsch, A. Climate change effects on beneficial plant–microorganism interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef]
- Stenström, E. The effects of flooding on the formation of ectomycorrhizae in Pinus sylvestris seedlings. Plant Soil 1991, 131, 247–250. [Google Scholar] [CrossRef]
- Rydin, H.; Jeglum, J.K. The Biology of Peatlands, 2nd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar] [CrossRef]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands: Background and Principles Including a Framework for Decision-Making; International Mire Conservation Group and International Peat Society: Devon, UK, 2002. [Google Scholar]
- Parker, T.C.; Clemmensen, K.E. Understanding the role of fungi in peatland degradation after drainage. New Phytol. 2023, 240, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.R.; Caporn, S.J.M.; Nwaishi, F.; Nilsson, R.H.; Sen, R. Bacterial and fungal communities in a degraded ombrotrophic peatland undergoing natural and managed re-vegetation. PLoS ONE 2015, 10, e0124726. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stasińska, M. Suillus flavidus, a Peatland-Associated Mycorrhizal Fungus in Poland: Ecology, Distribution, Conservation Threats, and Sustainability Considerations. Sustainability 2025, 17, 8244. https://doi.org/10.3390/su17188244
Stasińska M. Suillus flavidus, a Peatland-Associated Mycorrhizal Fungus in Poland: Ecology, Distribution, Conservation Threats, and Sustainability Considerations. Sustainability. 2025; 17(18):8244. https://doi.org/10.3390/su17188244
Chicago/Turabian StyleStasińska, Małgorzata. 2025. "Suillus flavidus, a Peatland-Associated Mycorrhizal Fungus in Poland: Ecology, Distribution, Conservation Threats, and Sustainability Considerations" Sustainability 17, no. 18: 8244. https://doi.org/10.3390/su17188244
APA StyleStasińska, M. (2025). Suillus flavidus, a Peatland-Associated Mycorrhizal Fungus in Poland: Ecology, Distribution, Conservation Threats, and Sustainability Considerations. Sustainability, 17(18), 8244. https://doi.org/10.3390/su17188244





