Influence of Location Type on the Regeneration and Growth of Pedunculate Oak (Quercus robur L.) in Central Europe: Implications for Sustainable Forest Land Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Measurement of Growth Characteristics and Light Conditions
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
- Light conditions are crucial for the early growth of pedunculate oak in artificial regeneration.
- Optimal growth occurred in gaps with lateral shelter and under-canopy area.
- Seedlings in gaps showed the greatest height and root collar diameter, supporting the improvement of plantation success.
- Clear-cut and inter-gap areas lead to poor seedling growth and low adaptability to cultivation.
- Regeneration conditions optimizing light align with sustainable and climate-adaptive forest management.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mearns, L.O.; Katz, R.W.; Schneider, S.H. Extreme High-Temperature Events: Changes in Their Probabilities with Changes in Mean Temperature. J. Appl. Meteorol. Climatol. 1984, 23, 1601–1613. [Google Scholar] [CrossRef]
- Goddess, C.M.; Palutikof, J.P.; Davies, T.D. A First Approach to Assessing Future Climate States in the UK over Very Long Timescales: Input to Studies of the Integrity of Radioactive Waste Repositories. Clim. Change 1990, 16, 115–139. [Google Scholar] [CrossRef]
- Segan, D.B.; Murray, K.A.; Watson, J.E.M. A Global Assessment of Current and Future Biodiversity Vulnerability to Habitat Loss–Climate Change Interactions. Glob. Ecol. Conserv. 2016, 5, 12–21. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis; Contribution to Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambrige University Press: Cambrige, UK, 2021. [Google Scholar]
- Savva, Y.; Oleksyn, J.; Reich, P.B.; Tjoelker, M.G.; Vaganov, E.A.; Modrzynski, J. Interannual Growth Response of Norway Spruce to Climate along an Altitudinal Gradient in the Tatra Mountains, Poland. Trees—Struct. Funct. 2006, 20, 735–746. [Google Scholar] [CrossRef]
- Ryan, M.G. Tree Responses to Drought. Tree Physiol. 2011, 31, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest Disturbances under Climate Change. Nat. Clim. Change 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent Insects, Pathogens and Drought Shape Changing Patterns in Oak Decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Knutzen, F.; Averbeck, P.; Barrasso, C.; Bouwer, L.M.; Gardiner, B.; Grünzweig, J.M.; Hänel, S.; Haustein, K.; Johannessen, M.R.; Kollet, S.; et al. Impacts on and Damage to European Forests from the 2018–2022 Heat and Drought Events. Nat. Hazards Earth Syst. Sci. 2025, 25, 77–117. [Google Scholar] [CrossRef]
- Petritan, A.M.; Biris, I.A.; Merce, O.; Turcu, D.O.; Petritan, I.C. Structure and Diversity of a Natural Temperate Sessile Oak (Quercus petraea L.)–European Beech (Fagus sylvatica L.) Forest. For. Ecol. Manag. 2012, 280, 140–149. [Google Scholar] [CrossRef]
- Perkins, D.; Uhl, E.; Biber, P.; Du Toit, B.; Carraro, V.; Rötzer, T.; Pretzsch, H. Impact of Climate Trends and Drought Events on the Growth of Oaks (Quercus robur L. and Quercus Petraea (Matt.) Liebl.) within and beyond Their Natural Range. Forests 2018, 9, 108. [Google Scholar] [CrossRef]
- Nölte, A.; Yousefpour, R.; Hanewinkel, M. Changes in Sessile Oak (Quercus petraea) Productivity under Climate Change by Improved Leaf Phenology in the 3-PG Model. Ecol. Model. 2020, 438, 109285. [Google Scholar] [CrossRef]
- Kašpar, J.; Tumajer, J.; Altman, J.; Altmanová, N.; Čada, V.; Čihák, T.; Doležal, J.; Fibich, P.; Janda, P.; Kaczka, R.; et al. Major Tree Species of Central European Forests Differ in Their Proportion of Positive, Negative, and Nonstationary Growth Trends. Glob. Change Biol. 2024, 30, e17146. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.F.; Buras, A.; Rammig, A.; Zang, C.S. Higher Susceptibility of Beech to Drought in Comparison to Oak. Dendrochronologia 2020, 64, 125780. [Google Scholar] [CrossRef]
- Basu, S.; Stojanović, M.; Jevšenak, J.; Buras, A.; Kulhavý, J.; Hornová, H.; Světlík, J. Pedunculate Oak Is More Resistant to Drought and Extreme Events than Narrow-Leaved Ash in Central European Floodplain Forests. For. Ecol. Manag. 2024, 561, 121907. [Google Scholar] [CrossRef]
- Stojanović, M.; Basu, S.; Mikhailov, S.; Hawryło, P.; Socha, J.; Mikac, S.; Trlin, D.; Seim, A.; Hotter, M.; Lapin, K.; et al. Distinct Climate Sensitivity and Resilience Mechanisms of Pedunculate Oak (Quercus robur L.) across a Temperature Gradient. For. Ecol. Manag. 2025, 596, 123069. [Google Scholar] [CrossRef]
- Annighöfer, P.; Beckschäfer, P.; Vor, T.; Ammer, C. Regeneration Patterns of European Oak Species (Quercus Petraea (Matt.) Liebl., Quercus robur L.) in Dependence of Environment and Neighborhood. PLoS ONE 2015, 10, e0134935. [Google Scholar] [CrossRef]
- Jaworski, A. Hodowla Lasu. T. 3: Charakterystyka Hodowlana Drzew i Krzewów Leśnych; Państwowe Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 2011. [Google Scholar]
- Eaton, E.; Caudullo, G.; Oliveira, S.; de Rigo, D. Quercus Robur and Quercus Petraea in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Central Statistical Office of Poland. Rocznik Statystyczny Leśnictwa; Główny Urząd Statystyczny: Warszawa, Poland, 2024. [Google Scholar]
- Dobrowolska, D. Effect of Stand Density on Oak Regeneration in Flood Plain Forests in Lower Silesia, Poland. For. Int. J. For. Res. 2008, 81, 511–523. [Google Scholar] [CrossRef]
- Chauvat, M.; Titsch, D.; Zaytsev, A.S.; Wolters, V. Changes in Soil Faunal Assemblages during Conversion from Pure to Mixed Forest Stands. For. Ecol. Manag. 2011, 262, 317–324. [Google Scholar] [CrossRef]
- Mölder, A.; Meyer, P.; Nagel, R. Integrative Management to Sustain Biodiversity and Ecological Continuity in Central European Temperate Oak (Quercus robur, Q. petraea) Forests: An Overview. For. Ecol. Manag. 2019, 437, 324–339. [Google Scholar] [CrossRef]
- Pardos, M.; Del Río, M.; Pretzsch, H.; Jactel, H.; Bielak, K.; Bravo, F.; Brazaitis, G.; Defossez, E.; Engel, M.; Godvod, K.; et al. The Greater Resilience of Mixed Forests to Drought Mainly Depends on Their Composition: Analysis along a Climate Gradient across Europe. For. Ecol. Manag. 2021, 481, 118687. [Google Scholar] [CrossRef]
- Larsen, D.R.; Johnson, P.S. Linking the Ecology of Natural Oak Regeneration to Silviculture. For. Ecol. Manag. 1998, 106, 1–7. [Google Scholar] [CrossRef]
- Sinha, A.; Rämö, J.; Malo, P.; Kallio, M.; Tahvonen, O. Optimal Management of Naturally Regenerating Uneven-Aged Forests. Eur. J. Oper. Res. 2017, 256, 886–900. [Google Scholar] [CrossRef]
- von Lüpke, B. Silvicultural Methods of Oak Regeneration with Special Respect to Shade Tolerant Mixed Species. For. Ecol. Manag. 1998, 106, 19–26. [Google Scholar] [CrossRef]
- Dey, D.C.; Jacobs, D.; McNabb, K.; Miller, G.; Baldwin, V.; Foster, G. Artificial Regeneration of Major Oak (Quercus) Species in the Eastern United States—A Review of the Literature. For. Sci. 2008, 54, 77–106. [Google Scholar] [CrossRef]
- Diaci, J.; Gyoerek, N.; Gliha, J.; Nagel, T.A. Response of Quercus robur L. Seedlings to North-South Asymmetry of Light within Gaps in Floodplain Forests of Slovenia. Ann. For. Sci. 2008, 65, 105. [Google Scholar] [CrossRef]
- Bolibok, L.; Andrzejczyk, T.; Drozdowski, S.; Szeligowski, H. Wysokość siedmioletnich odnowień dębowych na gniazdach w różnych warunkach siedliskowych. Leśne Pr. Badaw. 2011, 72, 163–170. [Google Scholar] [CrossRef][Green Version]
- Saha, S.; Kohnle, U.; Brang, P.; Ehring, A.; Geisel, J.; Leder, B.; Muth, M.; Petersen, R.; Peter, J.; Ruhm, W.; et al. Growth and Quality of Young Oaks (Quercus Robur and Quercus Petraea) Grown in Cluster Plantings in Central Europe: A Weighted Meta-Analysis. For. Ecol. Manag. 2012, 283, 106–118. [Google Scholar] [CrossRef]
- Dorota, D.; Przemysław, K.; Grażyna, O.; Bolibok, L. Effects of Stand Features and Soil Enzyme Activity on Spontaneous Pedunculate Oak Regeneration in Scots Pine Dominated Stands—Implication for Forest Management. For. Ecosyst. 2021, 8, 43. [Google Scholar] [CrossRef]
- Hochbichler, E. Methods of Oak Silviculture in Austria. Ann. Des. Sci. For. 1993, 50, 583–591. [Google Scholar] [CrossRef]
- Gross, K.; Homlicher, A.; Weinreich, A.; Wagner, E. Effect of Shade on Stomatal Conductance, Net Photosynthesis, Photochemical Efficiency and Growth of Oak Saplings. Ann. For. Sci. 1996, 53, 279–290. [Google Scholar] [CrossRef]
- Wagner, P.A.; Dreyer, E. Interactive Effects of Waterlogging and Irradiance on the Photosynthetic Performance of Seedlings from Three Oak Species Displaying Different Sensitivities (Quercus Robur, Q Petraea and Q Rubra). Ann. For. Sci. 1997, 54, 409–429. [Google Scholar] [CrossRef]
- Andrzejczyk, T.; Głodowski, Z. Wplyw Gatunkow Domieszkowych Na Wzrost i Pokroj Debu Szypulkowego (Quercus robur L.) w Uprawie Zalozonej Metoda Szymanskiego. Leśne Pr. Badaw. 2010, 71, 321–330. [Google Scholar] [CrossRef]
- Aleksandrowicz-Trzcińska, M.; Pewniak, B.; Żybura, H. Cechy jakościowe podrostów dębowych rosnących pod osłoną drzewostanów sosnowych. Sylwan 2018, 162, 267–276. [Google Scholar]
- Carlson, D.W.; Groot, A. Microclimate of Clear-Cut, Forest Interior, and Small Openings in Trembling Aspen Forest. Agric. For. Meteorol. 1997, 87, 313–329. [Google Scholar] [CrossRef]
- Prévost, M.; Raymond, P. Effect of Gap Size, Aspect and Slope on Available Light and Soil Temperature after Patch-Selection Cutting in Yellow Birch–Conifer Stands, Quebec, Canada. For. Ecol. Manag. 2012, 274, 210–221. [Google Scholar] [CrossRef]
- Gray, A.N.; Spies, T.A.; Easter, M.J. Microclimatic and Soil Moisture Responses to Gap Formation in Coastal Douglas-Fir Forests. Can. J. For. Research 2002, 32, 332–343. [Google Scholar] [CrossRef]
- Ritter, E.; Starr, M.; Vesterdal, L. Losses of Nitrate from Gaps of Different Sizes in a Managed Beech (Fagus sylvatica) Forest. Can. J. For. Res. 2005, 35, 308–319. [Google Scholar] [CrossRef]
- Sanders, T.; Pitman, R.; Broadmeadow, M. Species-Specific Climate Response of Oaks (Quercus Spp.) under Identical Environmental Conditions. iForest 2014, 7, 61–69. [Google Scholar] [CrossRef]
- Kuehne, C.; Pyttel, P.; Modrow, T.; Kohnle, U.; Bauhus, J. Seedling Development and Regeneration Success after 10 Years Following Group Selection Harvesting in a Sessile Oak (Quercus petraea [Mattuschka] Liebl.) Stand. Ann. For. Sci. 2020, 77, 71. [Google Scholar] [CrossRef]
- Sevillano, I.; Short, I.; Grant, J.; O’Reilly, C. Effects of Light Availability on Morphology, Growth and Biomass Allocation of Fagus Sylvatica and Quercus Robur Seedlings. For. Ecol. Manag. 2016, 374, 11–19. [Google Scholar] [CrossRef]
- Kohler, M.; Pyttel, P.; Kuehne, C.; Modrow, T.; Bauhus, J. On the Knowns and Unknowns of Natural Regeneration of Silviculturally Managed Sessile Oak (Quercus petraea (Matt.) Liebl.) Forests-a Literature Review. Ann. For. Sci. 2020, 77, 101. [Google Scholar] [CrossRef]
- Bobiec, A.; Reif, A.; Öllerer, K. Seeing the Oakscape beyond the Forest: A Landscape Approach to the Oak Regeneration in Europe. Landsc. Ecol. 2018, 33, 513–528. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated World Map of the Köppen-Geiger Climate Classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Institute of Meteorology and Water Management—National Research Institute. Climate of Poland 2020; Institute of Meteorology and Water Management—National Research Institute: Warszawa, Poland, 2021; Available online: https://klimat.imgw.pl/ (accessed on 29 July 2025).
- Institute of Meteorology and Water Management—National Research Institute. Climate of Poland 2024; Institute of Meteorology and Water Management—National Research Institute: Warszawa, Poland, 2025; Available online: https://imgw.pl/wp-content/uploads/2025/07/RAPORT-IMGW-PIB-Klimat-Polski-2024.pdf (accessed on 29 July 2025).
- Sikorska, E. Forest Sites. Part I. Lowland Habitat Types; Wyd. Akademii Rolniczej w Krakowie: Kraków, Poland, 1999. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2022: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences: Vienna, Austria, 2022. [Google Scholar]
- Haase, D.L. Morphological and Physiological Evaluations of Seedling Quality. In National Proceedings: Forest and Conservation Nursery Associations; Riley, L.E., Dumroese, R.K., Landis, T.D., Eds.; Proceedings RMRS-P-50; Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2006; pp. 3–8. [Google Scholar]
- Jaźwiński, J.; Banach, J.; Skrzyszewska, K.; Strejczek-Jaźwińska, P. Stabilność Adaptacji Potomstwa Buka Zwyczajnego Fagus Sylvatica L. Po Pięciu Latach wzrostuAdaptation Stability of European Beech Fagus sylvatica L. after Five Years of Growth. For. Res. Pap./Leśne Pr. Badaw. 2019, 80, 145–157. [Google Scholar] [CrossRef]
- Banach, J.; Małek, S.; Kormanek, M.; Durło, G. Growth of Fagus sylvatica L. and Picea abies (L.) Karst. Seedlings Grown in Hiko Containers in the First Year after Planting. Sustainability 2020, 12, 7155. [Google Scholar] [CrossRef]
- Frazer, G.; Canham, C.; Lertzman, K. Gap Light Analyzer (GLA): Imaging Software to Extract Canopy Structure and Gap Light Transmission Indices from True-Colour Sheye Photographs, Users Manual and Program Documentation; Simon Fraser University: Burnaby, BC, Canada, 1999. [Google Scholar]
- Bolibok, L. Equipment and Photo Exposure in Hemispherical Photography in Forest Research. For. Res. Pap. 2010, 71, 105–115. [Google Scholar] [CrossRef]
- Statistica (Data Analysis Software System), 2017. Available online: www.statsoft.com (accessed on 29 July 2025).
- Baguinon, N.; Merioles, M.; Alvarez, E.; Castillo, R. Principal Component Analysis in Detecting Site Quality, Habitats and Bio-Invasiveness. J. Nat. Stud. 2008, 7, 97–105. [Google Scholar]
- Bolibok, L.; Auchimik, J. Kształtowanie się wysokości upraw dębowych w centrum i na obrzeżu gniazd na siedlisku lasu mieszanego świeżego. Sylwan 2010, 154, 371–380. [Google Scholar]
- Bolibok, L. Regulacja warunków wzrostu odnowień na gniazdach–wpływ parametrów gniazd na oddziaływanie czynników biotycznych. Sylwan 2009, 153, 733–744. [Google Scholar]
- Szymkiewicz, B. Ębnia Gniazdowa Zupełna w Lasach Doświadczalnych SGGW Pod Rogowem. Sylwan 1972, 166, 23–37. [Google Scholar]
- Zabielski, B. Lasy Doświadczalne Wyższej Szkoły Rolniczej w Poznaniu; Wyd. WSP: Poznań, Poland, 1967. [Google Scholar]
- Zabielski, B.; Magnuski, K.; Ważyński, B.; Żółciak, E. Analiza Rozwoju Odnowień Dębowych w Drzewostanie Sosnowym Zagospodarowanym Rębnią Gniazdową; Rocz. Wyż. Szk. Rol. Pozn.: Poznań, Poland, 1963. [Google Scholar]
- Bernadzki, E. Cięcia Odnowieniowe. Poradnik Leśniczego; Państwowe Wydawnictwo Rolnicze i Leśne: Warszawa, Poland, 2000. [Google Scholar]
- Ceitel, J.; Perz, B. Sposób Morzfelda Przebudowy Składu Gatunkowego Drzewostanów. Sylwan 2006, 150, 23–34. [Google Scholar]
- Masternak, K.; Głębocka, K. Wpływ wielkości gniazd na wzrost dębu szypułkowego w drzewostanach zagospodarowanych rębnią gniazdową częściową. Agron. Sci. 2022, 77, 37. [Google Scholar] [CrossRef]
- Weiss, S.B. Vertical and Temporal Distribution of Insolation in Gaps in an Old-Growth Coniferous Forest. Can. J. For. Res. 2000, 30, 1953–1964. [Google Scholar] [CrossRef]
- Mariscal, M.J.; Martens, S.N.; Ustin, S.L.; Chen, J.; Weiss, S.B.; Roberts, D.A. Light-Transmission Profiles in an Old-Growth Forest Canopy: Simulations of Photosynthetically Active Radiation by Using Spatially Explicit Radiative Transfer Models. Ecosystems 2004, 7, 454–467. [Google Scholar] [CrossRef]
- Andrzejczyk, T.; Bolibok, L.; Buraczyk, W.; Drozdowski, S.; Szeligowski, H. Wpływ warunków siedliskowych na zróżnicowanie wysokości dębu na gniazdach. Sylwan 2014, 158, 404–413. [Google Scholar]
- Schlesinger, R.C.; Sander, I.L.; Davidson, K.R. Oak Regeneration Potential Increased by Shelterwood Treatments. North. J. Appl. For. 1993, 10, 149–153. [Google Scholar] [CrossRef]
- Gniot, M. Sukcesja debu w drzewostanach sosnowych na siedliskach borowych. Sylwan 2007, 151, 60–72. [Google Scholar]
- Magnuski, K.; Małys, L.; Gałecki, I. Charakterystyka Niektórych Cech Wzrostowych Dębu Szypułkowego (Quercus robur L.) Rosnącego w Kępach Po Rębniach Zupełnej, Częśąciowej i Zupełnej Gniazdowej; Rocz. Ak. Roln. w Poznaniu: Poznań, Poland, 1999; Volume 311. [Google Scholar]
- Paluch, R. Tempo Wzrostu Wysokości Dębu Szypułkowego (Quercus robur L.) w Dolnej Warstwie Drzewostanu Sosnowego. Sylwan 2013, 157, 909–916. [Google Scholar]
- Lüpke, B. Einfluss Der Konkurenz von Weichlaubholz Auf Das Wachstum Junger Traubeneichen. Forst Und Holz 1991, 46, 166–171. [Google Scholar]
- McShea, W.J.; Healy, W.M.; Devers, P.; Fearer, T.; Koch, F.H.; Stauffer, D.; Waldon, J. Forestry Matters: Decline of Oaks Will Impact Wildlife in Hardwood Forests. J. Wildl. Manag. 2007, 71, 1717–1728. [Google Scholar] [CrossRef]
- Rubio-Cuadrado, Á.; Gomez, C.; Rodríguez-Calcerrada, J.; Perea, R.; Gordaliza, G.J.; Camarero, J.; Montes, F.; Gil, L. Differential Response of Oak and Beech to Late Frost Damage: An Integrated Analysis from Organ to Forest. Agric. For. Meteorol. 2021, 297, 108243. [Google Scholar] [CrossRef]
- Čater, M.; Franc, B. Groundwater and Light Conditions as Factors in the Survival of Pedunculate Oak (Quercus robur L.) Seedlings. Eur. J. For. Res. 2006, 125, 419–426. [Google Scholar] [CrossRef]
- Ziegenhagen, B.; Kausch, W. Productivity of Young Shaded Oaks (Quercus robur L.) as Corresponding to Shoot Morphology and Leaf Anatomy. For. Ecol. Manag. 1995, 72, 97–108. [Google Scholar] [CrossRef]
- Van Hees, A.F.M. Growth and Morphology of Pedunculate Oak (Quercus robur L.) and Beech (Fagus sylvatica L.) Seedlings in Relation to Shading and Drought. Ann. Des. Sci. For. 1997, 54, 9–18. [Google Scholar] [CrossRef]
- Dickson, R.E.; Tomlinson, P.T. Oak Growth, Development and Carbon Metabolism in Response to Water Stress. Ann. For. Sci. 1996, 53, 181–196. [Google Scholar] [CrossRef]
- Bartsch, N.; Lüpke, B.; Röhrig, E. Waldbau Auf Ökologischer Grundlage; Ulmer: Stuttgart, Germany, 2020. [Google Scholar] [CrossRef]
- Reif, A.; Gärtner, S. Die natürliche Verjüngung der laubabwerfenden Eichenarten Stieleiche (Quercus robur L.) und Traubeneiche (Quercus petraea Liebl.)–eine Literaturstudie mit besonderer Berücksichtigung der Waldweide. Wald. Online AFSV-Berichte Der Arbeitsgemeinschaft Forstl. Standorts- Und Veg. 2007, 5, 79–116. [Google Scholar]
- Drozdowski, S.; Andrzejczyk, T.; Buraczyk, W.; Turkot, S. Wysokość dwunastoletnich odnowień dębu szypułkowego na różnej wielkości gniazdach o wydłużonym kształcie w kierunku wschód−zachód. Sylwan 2013, 157, 434–441. [Google Scholar]
- McNab, W.H. Factors Affecting Temporal and Spatial Soil Moisture Variation in and Adjacent to Group Selection Openings; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experimental Station: Radnor, PA, USA, 1991; pp. 475–488. [Google Scholar]
- York, R.A.; Battles, J.J.; Heald, R.C. Edge Effects in Mixed Conifer Group Selection Openings: Tree Height Response to Resource Gradients. For. Ecol. Manag. 2003, 179, 107–121. [Google Scholar] [CrossRef]
- Valkonen, S.; Ruuska, J.; Siipilehto, J. Effect of Retained Trees on the Development of Young Scots Pine Stands in Southern Finland. For. Ecol. Manag. 2002, 166, 227–243. [Google Scholar] [CrossRef]
- Gray, A.N.; Spies, T.A. Gap Size, Within-Gap Position and Canopy Structure Effects on Conifer Seedling Establishment. J. Ecol. 1996, 84, 635–645. [Google Scholar] [CrossRef]
- Coates, K.D. Tree Recruitment in Gaps of Various Size, Clearcuts and Undisturbed Mixed Forest of Interior British Columbia, Canada. For. Ecol. Manag. 2002, 155, 387–398. [Google Scholar] [CrossRef]
- Schwaiger, H.P.; Bird, D.N. Integration of Albedo Effects Caused by Land Use Change into the Climate Balance: Should We Still Account in Greenhouse Gas Units? For. Ecol. Manag. 2010, 260, 278–286. [Google Scholar] [CrossRef]
- Bristow, M.; Nichols, J.D.; Vanclay, J.K. Improving Productivity in Mixed-Species Plantations. For. Ecol. Manag. 2006, 233, 193–194. [Google Scholar] [CrossRef]
- Lobo, A.; Kjær, E.D.; Hansen, J.K. Adaptive Potential of Northern Pedunculate Oak: Genetic Variation in Growth and Phenology for a Warmer and Drier Future. Eur. J. For. Res. 2025, 144, 577–590. [Google Scholar] [CrossRef]
- Ducousso, A.; Bordacs, S. Technical Guidelines for Genetic Conservation and Use for Pedunculate and Sessile Oaks (Quercus Robur and Q. Petraea); International Plant Genetic Resources Institute: Rome, Italy, 2004; p. 6. [Google Scholar]
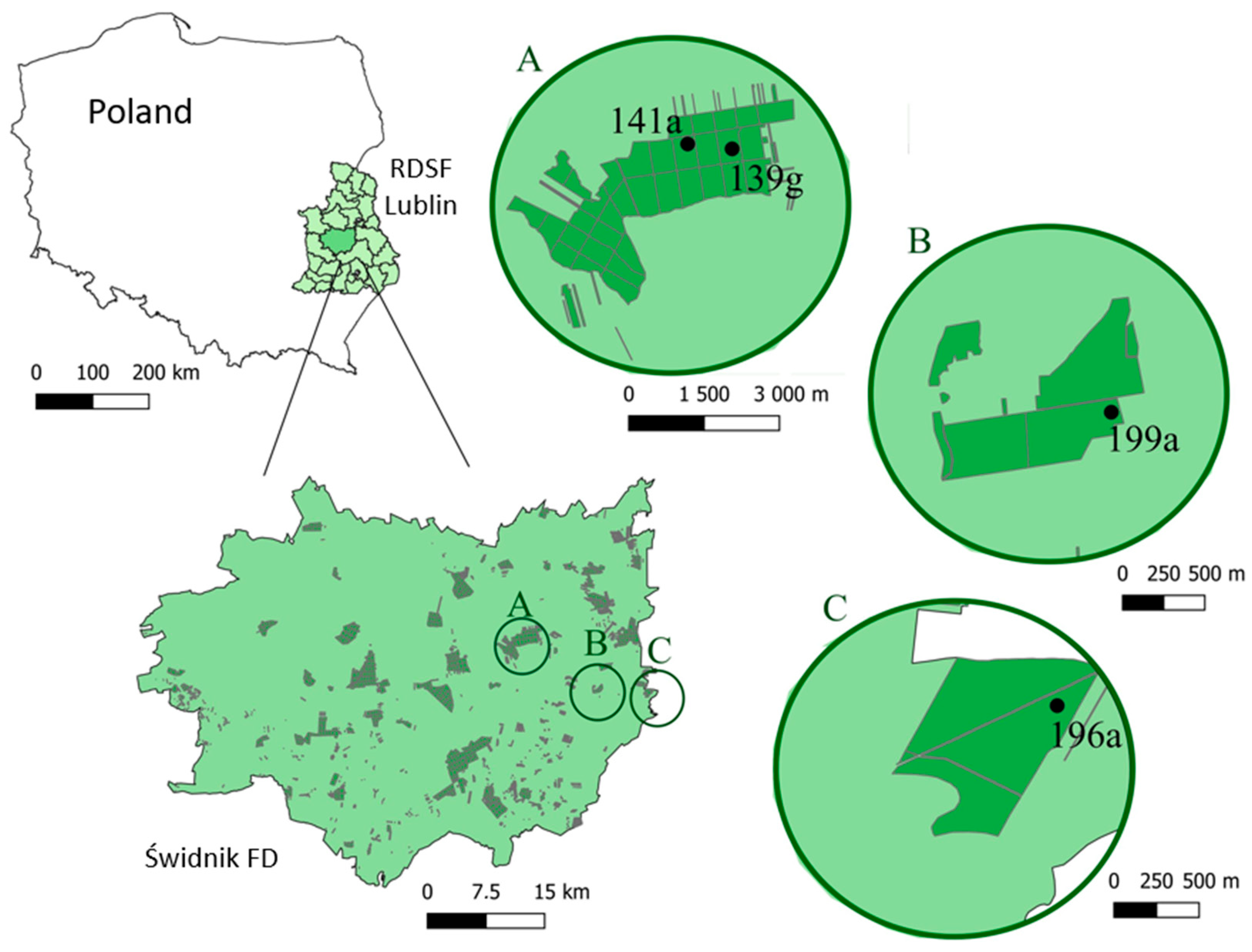


| Compartment | Type of Regeneration Site | Area [ha] | Species Composition of the Parent Stand [%] | Age of the Parent Stand [Years] | Plantation Area (% of Compartment) |
|---|---|---|---|---|---|
| 141a | Clear-cut area | 1.44 | 80 P * | 66 | 50 |
| 20 H, O | 66 | ||||
| 196a | Under-canopy area | 5.27 | 80 P | 104 | 60 |
| 20 O, B | 67 | ||||
| 199a | Gap | 2.35 | 90 P | 89 | 30 |
| 10 C | 69 | ||||
| 138d | Inter-gap area | 8.32 | 90 P | 109 | 30 |
| 10 O | 70 |
| Parameter | Clear-Cut | Under-Canopy | Gap | Inter-Gap | p Value | |
|---|---|---|---|---|---|---|
| Growth characteristics | H [cm] | 127.2 c ± 26.29 | 143.4 b ± 28.19 | 148.7 a ± 25.42 | 137.9 b ± 14.56 | <0.000 |
| D [cm] | 1.1 d ± 0.33 | 1.6 b ± 0.63 | 1.8 a ± 0.71 | 1.4 c ± 0.21 | ||
| SQ | 128.3 a ± 44.36 | 97.8 b ± 28.78 | 92.6 c ± 33.83 | 100.2 b ± 12.81 | ||
| Total light intensity | [MJ/m2/d] | 38.62 ± 1.45 | 17.21 ± 3.43 | 22.11 ± 2.25 | 34.70 ± 3.11 | 0.010 |
| % | 100 | 44.56 | 57.51 | 89.85 | ||
| Feature | Area | Cardinal Direction | p Value | ||||
|---|---|---|---|---|---|---|---|
| North | East | Central | South | West | |||
| H [cm] | Clear-cut | 130.1 ab ± 25.59 | 136.0 a ± 24.77 | 120.9 c ± 28.38 | 122.4 bc ± 24.46 | 125.1 bc ± 25.88 | <0.000 |
| Under-canopy | 140.3 b ± 26.31 | 141.1 b ± 25.53 | 154.3 a ± 32.86 | 142.5 b ± 26.82 | 139.9 b ± 27.34 | <0.000 | |
| Gap | 147.2 ± 28.0 | 150.4 ± 28.18 | 150.4 ± 22.08 | 148.7 ± 24.14 | 146.7 ± 24.70 | 0.400 | |
| Inter-gap | 140.6 ab ± 14.40 | 129.0 c ± 11.82 | 141.7 ab ± 14.82 | 143.3 a ± 11.52 | 135.2 bc ± 15.29 | <0.000 | |
| D [cm] | Clear-cut | 1.1 ± 0.30 | 1.1 ± 0.29 | 1.0 ± 0.33 | 1.1 ± 0.30 | 1.1 ± 0.42 | 0.271 |
| Under-canopy | 1.7 a ± 0.64 | 1.6 ab ± 0.78 | 1.7 a ± 0.63 | 1.5 b ± 0.52 | 1.5 b ± 0.53 | 0.022 | |
| Gap | 1.8 ± 0.70 | 1.8 ± 0.86 | 1.8 ± 0.62 | 1.8 ± 0.69 | 1.8 ± 0.66 | 0.812 | |
| Inter-gap | 1.4 b ± 0.18 | 1.3 c ± 0.19 | 1.5 a ± 0.19 | 1.5 ab ± 0.21 | 1.4 b ± 0.21 | <0.000 | |
| SQ | Clear-cut | 125.8 ab ± 44.70 | 137.3 a ± 43.75 | 125.8 ab ± 41.31 | 124.4 b ± 43.52 | 127.3 ab ± 46.90 | 0.042 |
| Under-canopy | 92.2 ± 31.08 | 100.1 ± 30.97 | 98.3 ± 26.47 | 98.5 ± 25.66 | 100.4 ± 28.98 | 0.051 | |
| Gap | 91.9 ± 32.36 | 96.1 ± 37.95 | 93.9 ± 31.84 | 90.7 ± 32.77 | 90.3 ± 34.23 | 0.371 | |
| Inter-gap | 103.2 a ± 12.44 | 103.3 a ± 12.53 | 94.1 b ± 11.28 | 100.2 ab ± 13.08 | 99.7 ab ± 12.89 | 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masternak, K.; Łukasik, M.; Czyżowski, P.; Gmitrowicz-Iwan, J.; Kowalczyk, K. Influence of Location Type on the Regeneration and Growth of Pedunculate Oak (Quercus robur L.) in Central Europe: Implications for Sustainable Forest Land Use. Sustainability 2025, 17, 8011. https://doi.org/10.3390/su17178011
Masternak K, Łukasik M, Czyżowski P, Gmitrowicz-Iwan J, Kowalczyk K. Influence of Location Type on the Regeneration and Growth of Pedunculate Oak (Quercus robur L.) in Central Europe: Implications for Sustainable Forest Land Use. Sustainability. 2025; 17(17):8011. https://doi.org/10.3390/su17178011
Chicago/Turabian StyleMasternak, Katarzyna, Michał Łukasik, Piotr Czyżowski, Joanna Gmitrowicz-Iwan, and Krzysztof Kowalczyk. 2025. "Influence of Location Type on the Regeneration and Growth of Pedunculate Oak (Quercus robur L.) in Central Europe: Implications for Sustainable Forest Land Use" Sustainability 17, no. 17: 8011. https://doi.org/10.3390/su17178011
APA StyleMasternak, K., Łukasik, M., Czyżowski, P., Gmitrowicz-Iwan, J., & Kowalczyk, K. (2025). Influence of Location Type on the Regeneration and Growth of Pedunculate Oak (Quercus robur L.) in Central Europe: Implications for Sustainable Forest Land Use. Sustainability, 17(17), 8011. https://doi.org/10.3390/su17178011







