Research on the Suitability of Building Integrated Agriculture—Taking Indoor Living Walls as an Example
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of Indoor Living Walls
2.2. Materials
2.3. Measurement Methods
2.3.1. Measurement of Indoor Microenvironment Indicators
2.3.2. Measurement of Vegetable Indicators
- (1)
- Morphological indicators of lettuce
- (2)
- Physiological indicators of lettuce
- (3)
- Yield indicators of lettuce
- (4)
- Quality indicators of lettuce
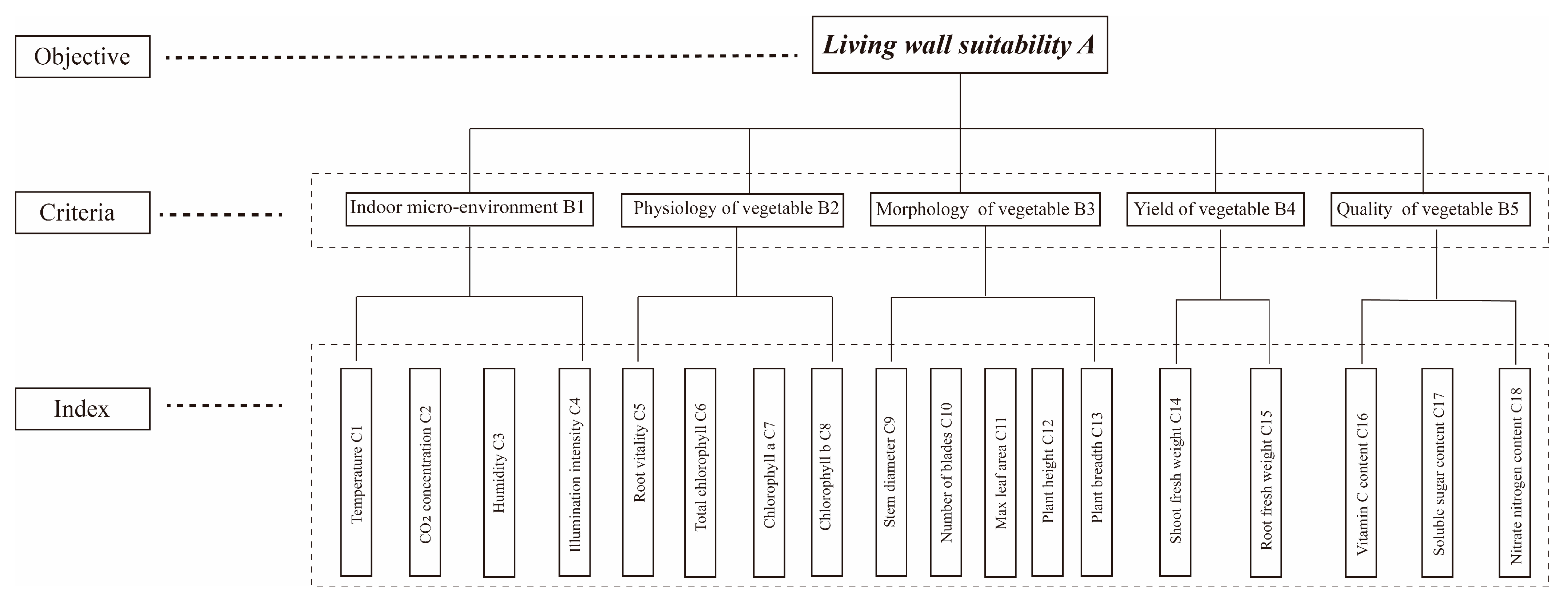
2.4. Selection of Evaluation Methods
2.5. Establishment of Evaluation Model
2.6. Determination of Evaluation Indicator Weights
2.6.1. Determination of AHP Weights
- (1)
- Construct comparison matrix
- (2)
- Weight calculation
- (3)
- Consistency check
2.6.2. Determination of Entropy Weights
- (1)
- Standardization of indicators
- (2)
- Calculate entropy weights to determine weights
2.6.3. Determination of AHP–Entropy Weight Combination Weights
2.7. Calculation of Suitability Scores
3. Results
3.1. Standard of Suitability Evaluation
3.2. Calculation of Evaluation Indicator Weights
3.2.1. AHP Weights
- (1)
- Objective layer A
- (2)
- Criteria layer B1
- (3)
- Criteria layer B2
- (4)
- Criteria layer B3
- (5)
- Criteria layer B4
- (6)
- Criteria layer B4
3.2.2. Entropy Weights
3.2.3. Combination Weights
3.3. Suitability Scores
4. Discussion
4.1. Environmental Suitability
4.2. Vegetable Suitability
4.3. Implications for Sustainability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BIA | Building integrated agriculture |
| ILWs | Indoor living walls |
| AHP | Analytic hierarchy process |
References
- Shi, K.; Chen, Y.; Yu, B.; Xu, T.; Li, L.; Huang, C.; Liu, R.; Chen, Z.; Wu, J. Urban Expansion and Agricultural Land Loss in China: A Multiscale Perspective. Sustainability 2016, 8, 790. [Google Scholar] [CrossRef]
- Jiang, L.; Deng, X.; Seto, K.C. The Impact of Urban Expansion on Agricultural Land Use Intensity in China. Land Use Policy 2013, 35, 33–39. [Google Scholar] [CrossRef]
- Gould, D.; Caplow, T. Building-Integrated Agriculture: A New Approach to Food Production. In Metropolitan Sustainability; Zeman, F., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing: Cambridge, UK, 2012; pp. 147–170. ISBN 978-0-85709-046-1. [Google Scholar]
- Wong, C.E.; Teo, Z.W.N.; Shen, L.; Yu, H. Seeing the Lights for Leafy Greens in Indoor Vertical Farming. Trends Food Sci. Technol. 2020, 106, 48–63. [Google Scholar] [CrossRef]
- Deng, L.; Deng, Q. The Basic Roles of Indoor Plants in Human Health and Comfort. Environ. Sci. Pollut. Res. 2018, 25, 36087–36101. [Google Scholar] [CrossRef]
- Berardi, U.; GhaffarianHoseini, A.; GhaffarianHoseini, A. State-of-the-Art Analysis of the Environmental Benefits of Green Roofs. Appl. Energy 2014, 115, 411–428. [Google Scholar] [CrossRef]
- Alhashimi, L.; Aljawi, L.; Gashgari, R.; Alamoudi, A. The Effect of Rooftop Garden on Reducing the Internal Temperature of the Rooms in Buildings. In Proceedings of the 4th World Congress on Mechanical, Chemical, and Material Engineering (MCM’18), Madrid, Spain, 16–18 August 2018. [Google Scholar]
- Chang, Y.-S.; Ho, M.-Y.; Wu, C.-W.; Chang, Y.-J. Indoor Plant Removal of Atmospheric CO2—Effects on Indoor Air Quality Improvement and Carbon Sequestration. Process Saf. Environ. Prot. 2025, 200, 107419. [Google Scholar] [CrossRef]
- Specht, K.; Siebert, R.; Hartmann, I.; Freisinger, U.B.; Sawicka, M.; Werner, A.; Thomaier, S.; Henckel, D.; Walk, H.; Dierich, A. Urban Agriculture of the Future: An Overview of Sustainability Aspects of Food Production in and on Buildings. Agric. Hum. Values 2014, 31, 33–51. [Google Scholar] [CrossRef]
- Ampim, P.A.Y.; Obeng, E.; Olvera-Gonzalez, E. Indoor Vegetable Production: An Alternative Approach to Increasing Cultivation. Plants 2022, 11, 2843. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, F.; Tahir, O.M.; Joni, R.A.; Fatemi, E. Opportunities and Challenges in Sustainability of Vertical Farming: A Review. J. Landsc. Ecol. 2018, 11, 35–60. [Google Scholar] [CrossRef]
- Al-Kodmany, K. The Vertical Farm: A Review of Developments and Implications for the Vertical City. Buildings 2018, 8, 24. [Google Scholar] [CrossRef]
- SANANBIO Official-Vertical Farming|Horticultural Lighting. Available online: https://www.sananbio.com/ (accessed on 7 August 2025).
- Pasona Urban Farm—KONODESIGNS. Available online: https://konodesigns.com/urban-farm/ (accessed on 7 August 2025).
- Pérez-Urrestarazu, L.; Fernández-Cañero, R.; Franco-Salas, A.; Egea, G. Vertical Greening Systems and Sustainable Cities. J. Urban Technol. 2015, 22, 65–85. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Zhang, Y. Investigation of the Indoor CO2 Removal Efficiency and Fresh Air Energy Savings of Living Walls in Office Spaces. J. Build. Eng. 2024, 90, 109422. [Google Scholar] [CrossRef]
- Thorpert, P.; Englund, J.-E.; Sang, Å.O. Shades of Green for Living Walls—Experiences of Color Contrast and Its Implication for Aesthetic and Psychological Benefits. Nat.-Based Solut. 2023, 3, 100067. [Google Scholar] [CrossRef]
- Wang, M.; Cao, J.; Jia, C.; Du, C.; Han, S.; Fukuda, H.; Gao, W.; Inoue, T. Effectiveness of a Dynamic Living Wall System of Plants on Indoor Thermal Environment in Summer—An Experimental Study. J. Build. Eng. 2024, 98, 111266. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Liu, H.; Liu, H. Physiological and Psychological Effects of Exposure to Different Types and Numbers of Biophilic Vegetable Walls in Small Spaces. Build. Environ. 2022, 225, 109645. [Google Scholar] [CrossRef]
- Shao, Y.; Li, J.; Zhou, Z.; Hu, Z.; Zhang, F.; Cui, Y.; Chen, H. The Effects of Vertical Farming on Indoor Carbon Dioxide Concentration and Fresh Air Energy Consumption in Office Buildings. Build. Environ. 2021, 195, 107766. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, T.; Yang, W.; Hu, X.; Li, C. A Review on the Impact of Outdoor Environment on Indoor Thermal Environment. Buildings 2023, 13, 2600. [Google Scholar] [CrossRef]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated Digital Image Analysis for Rapid and Accurate Measurement of Leaf Area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, H.; Yue, C.; Li, W.; Tong, Z.; Yang, P. Selection of Core Evaluation Indices and Construction of a Comprehensive Evaluation Method for Machine-Harvested Tea Plant Cultivars. Euphytica 2022, 218, 162. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Huang, J. Evaluation of Tea Frost Risk in Zhejiang Province Based on GIS. In Proceedings of the 2018 7th International Conference on Agro-Geoinformatics (Agro-Geoinformatics), Hangzhou, China, 6–9 August 2018; IEEE: New York, NY, USA, 2018; pp. 177–180. [Google Scholar]
- Wang, Z.; Yang, P.; Peng, H.; Li, C.; Yue, C.; Li, W.; Jiang, X. Comprehensive Evaluation of 47 Tea Germplasm Based on Entropy Weight Method and Grey Relational Degree. Genet. Resour. Crop Evol. 2021, 68, 3257–3270. [Google Scholar] [CrossRef]
- Abbasoğlu, M.S.; Kahramanoğlu, İ. Effects of Indoor Plants on Perceptions about Indoor Air Quality and Subjective Well-Being. J. Build. Eng. 2025, 106, 112563. [Google Scholar] [CrossRef]
- Wang, J.; Han, Z.; He, J.; Kang, H.; Li, Q.; Chen, H.; Zhang, X.; Miao, W.; Shang, X.; Chen, W.; et al. Exploring the Effects of Light–Water Interaction in Plant Factory to Improve the Yield and Quality of Panax Notoginseng (Burkill) F. H. Chen. Agronomy 2025, 15, 368. [Google Scholar] [CrossRef]
- van Heezik, Y.; Barratt, B.I.P.; Burns, B.R.; Clarkson, B.D.; Cutting, B.T.; Ewans, R.; Freeman, C.; Meurk, C.; Shanahan, D.F.; Simcock, R.; et al. A Rapid Assessment Technique for Evaluating Biodiversity to Support Accreditation of Residential Properties. Landsc. Urban Plan. 2023, 232, 104682. [Google Scholar] [CrossRef]
- Saaty, T.L. How to Make a Decision: The Analytic Hierarchy Process. Eur. J. Oper. Res. 1990, 48, 9–26. [Google Scholar] [CrossRef]
- Vaidya, O.S.; Kumar, S. Analytic Hierarchy Process: An Overview of Applications. Eur. J. Oper. Res. 2006, 169, 1–29. [Google Scholar] [CrossRef]
- Ishizaka, A.; Labib, A. Review of the Main Developments in the Analytic Hierarchy Process. Expert Syst. Appl. 2011, 38, 14336–14345. [Google Scholar] [CrossRef]
- Kordi, M.; Brandt, S.A. Effects of Increasing Fuzziness on Analytic Hierarchy Process for Spatial Multicriteria Decision Analysis. Comput. Environ. Urban Syst. 2012, 36, 43–53. [Google Scholar] [CrossRef]
- Dos Santos, P.H.; Neves, S.M.; Sant’Anna, D.O.; de Oliveira, C.H.; Carvalho, H.D. The Analytic Hierarchy Process Supporting Decision Making for Sustainable Development: An Overview of Applications. J. Clean. Prod. 2019, 212, 119–138. [Google Scholar] [CrossRef]
- Caha, J.; Burian, J. Comparison of Fuzzy AHP Algorithms for Land Suitability Assessment. In Dynamics in GIscience; Ivan, I., Horák, J., Inspektor, T., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 31–46. [Google Scholar]
- Quinn, B.; Schiel, K.; Caruso, G. Mapping Uncertainty from Multi-Criteria Analysis of Land Development Suitability, the Case of Howth, Dublin. J. Maps 2015, 11, 487–495. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Z.; Shen, S. Urban Green Space Suitability Evaluation Based on the AHP-CV Combined Weight Method: A Case Study of Fuping County, China. Sustainability 2018, 10, 2656. [Google Scholar] [CrossRef]
- Chen, H.; Liu, G.; Yang, Y.; Ye, X.; Shi, Z. Comprehensive Evaluation of Tobacco Ecological Suitability of Henan Province Based on GIS. Agric. Sci. China 2010, 9, 583–592. [Google Scholar] [CrossRef]
- Yue, J.; Li, Z.; Zuo, Z.; Wang, Y. Evaluation of Ecological Suitability and Quality Suitability of Panax Notoginseng Under Multi-Regionalization Modeling Theory. Front. Plant Sci. 2022, 13, 818376. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, J.; Han, S.; Ding, L.; Zhao, X.; Liu, X.; Deng, H. A Study on Plant Selection for Low-Carbon Rain Gardens Based on an AHP-TOPSIS Model. Sustainability 2024, 16, 2097. [Google Scholar] [CrossRef]
- Gao, Y.-H.; Han, B.; Miao, J.-J.; Jin, S.; Liu, H.-W. Research on Suitability Evaluation of Urban Engineering Construction Based on Entropy Weight Hierarchy-Cloud Model: A Case Study in Xiongan New Area, China. Appl. Sci. 2023, 13, 10655. [Google Scholar] [CrossRef]
- Ma, S.; Liu, C.; Zhang, R.; Wang, J.; Lu, D. Evaluation for Suitability of Underground Space Using Entropy Weight-analytic Hi-erarchy Process. Sci. Technol. Eng. 2021, 21, 10013–10020. [Google Scholar]
- Ma, C.-X.; Peng, F.-L. Evaluation of Spatial Performance and Supply-Demand Ratios of Urban Underground Space Based on POI Data: A Case Study of Shanghai. Tunn. Undergr. Space Technol. 2023, 131, 104775. [Google Scholar] [CrossRef]
- Choi, K.Y.; Paek, K.Y.; Lee, Y.B. Effect of Air Temperature on Tipburn Incidence of Butterhead and Leaf Lettuce in a Plant Factory. In Transplant Production in the 21st Century: Proceedings of the International Symposium on Transplant Production in Closed System for Solving the Global Issues on Environmental Conservation, Food, Resources and Energy; Kubota, C., Chun, C., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 166–171. ISBN 978-94-015-9371-7. [Google Scholar]
- Ahmed, H.A.; Yu-Xin, T.; Qi-Chang, Y. Optimal Control of Environmental Conditions Affecting Lettuce Plant Growth in a Controlled Environment with Artificial Lighting: A Review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Shibata, T.; Iwao, K.; Takano, T. Effect of Vertical Air Flowing on Lettuce Growing in a Plant Factory. Acta Hortic. 1995, 399, 175–182. [Google Scholar] [CrossRef]
- Ryu, D.K.; Kang, S.W.; Ngo, V.D.; Chung, S.O.; Choi, J.M.; Park, S.U.; Kim, S.J. Control of Temperature, Humidity, and CO2 Concentration in Small-Sized Experimental Plant Factory. Acta Hortic. 2014, 1037, 477–484. [Google Scholar] [CrossRef]
- Carvalho, D.R.A.; Torre, S.; Kraniotis, D.; Almeida, D.P.F.; Heuvelink, E.; Carvalho, S.M.P. Elevated Air Movement Enhances Stomatal Sensitivity to Abscisic Acid in Leaves Developed at High Relative Air Humidity. Front. Plant Sci. 2015, 6, 383. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of Different Light Intensities on Chlorophyll Fluorescence Characteristics and Yield in Lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of Environment Lighting on the Growth, Photosynthesis, and Quality of Hydroponic Lettuce in a Plant Factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Cheng, Z. Vegetable Cultivation Science-Individual Introduction, 2nd ed.; Science Press: Beijing, China, 2021; p. 195. [Google Scholar]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional Value, Bioactive Compounds and Health Benefits of Lettuce. J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- US Department of Agriculture; Agricultural Research Service; Nutrient Data Laboratory. National Nutrient Database for Standard Reference Release 28; US Department of Agriculture: Washington, DC, USA, 2015. [Google Scholar]
- López, A.; Javier, G.-A.; Fenoll, J.; Hellín, P.; Flores, P. Chemical Composition and Antioxidant Capacity of Lettuce: Comparative Study of Regular-Sized (Romaine) and Baby-Sized (Little Gem and Mini Romaine) Types. J. Food Compos. Anal. 2014, 33, 39–48. [Google Scholar] [CrossRef]
- Tomasi, N.; Pinton, R.; Costa, L.D.; Cortella, G.; Terzano, R.; Mimmo, T.; Scampicchio, M.; Cesco, S. New ‘Solutions’ for Floating Cultivation System of Ready-to-Eat Salad: A Review. Trends Food Sci. Technol. 2015, 46, 267–276. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) No 1258/2011 of 2 December 2011. Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels for Nitrates in Foodstuffs; European Commission: Luxembourg, 2011; Volume 32011R1258. [Google Scholar]
- Ahmed, H.A.; Tong, Y.; Li, L.; Sahari, S.Q.; Almogahed, A.M.; Cheng, R. Integrative Effects of CO2 Concentration, Illumination Intensity and Air Speed on the Growth, Gas Exchange and Light Use Efficiency of Lettuce Plants Grown under Artificial Lighting. Horticulturae 2022, 8, 270. [Google Scholar] [CrossRef]
- Zhou, J.; Li, P.; Wang, J. Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce. Horticulturae 2022, 8, 178. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root Traits Contributing to Plant Productivity under Drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Chen, L.; Xu, M.; Liu, C.; Hao, J.; Fan, S.; Han, Y. LsMYB15 Regulates Bolting in Leaf Lettuce (Lactuca sativa L.) Under High-Temperature Stress. Front. Plant Sci. 2022, 13, 921021. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fang, H.; Li, Z.; Yang, Q.; Wei, L.; Cheng, R. Effects of increased stereo multi-layer artificial light in natural light plant factory on yield and quality of lettuce. J. China Agric. Univ. 2019, 24, 92–99. [Google Scholar]
- Xie, P.; Wu, Z.; Wang, B.; Liu, N.; Liang, H.; Wang, L.; Tong, J. Effects of different nutrient solution temperatures on quality and tipburn of lettuce in plant factory. J. China Agric. Univ. 2025, 30, 65–75. [Google Scholar]
- Iqbal, Z.; Munir, M.; Sattar, M.N. Morphological, Biochemical, and Physiological Response of Butterhead Lettuce to Photo-Thermal Environments. Horticulturae 2022, 8, 515. [Google Scholar] [CrossRef]
- Sananbio Unmanned Plant Factory 3.0—Agricultural Technology for the Future. Available online: https://sananbio.com.cn/storyDetail/84 (accessed on 9 August 2025).
- Schonhof, I.; Kläring, H.-P.; Krumbein, A.; Claußen, W.; Schreiner, M. Effect of Temperature Increase under Low Radiation Conditions on Phytochemicals and Ascorbic Acid in Greenhouse Grown Broccoli. Agric. Ecosyst. Environ. 2007, 119, 103–111. [Google Scholar] [CrossRef]
- Miao, C.; Yang, S.; Xu, J.; Wang, H.; Zhang, Y.; Cui, J.; Zhang, H.; Jin, H.; Lu, P.; He, L.; et al. Effects of Light Intensity on Growth and Quality of Lettuce and Spinach Cultivars in a Plant Factory. Plants 2023, 12, 3337. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhang, W.; Li, J.; Huang, Z.; Tao, Y.; Hong, J.; Zhang, L.; Zhou, Y. Pre-Harvest Short-Term Continuous LED Lighting Improves the Nutritional Quality and Flavor of Hydroponic Purple-Leaf Lettuce. Sci. Hortic. 2024, 334, 113304. [Google Scholar] [CrossRef]

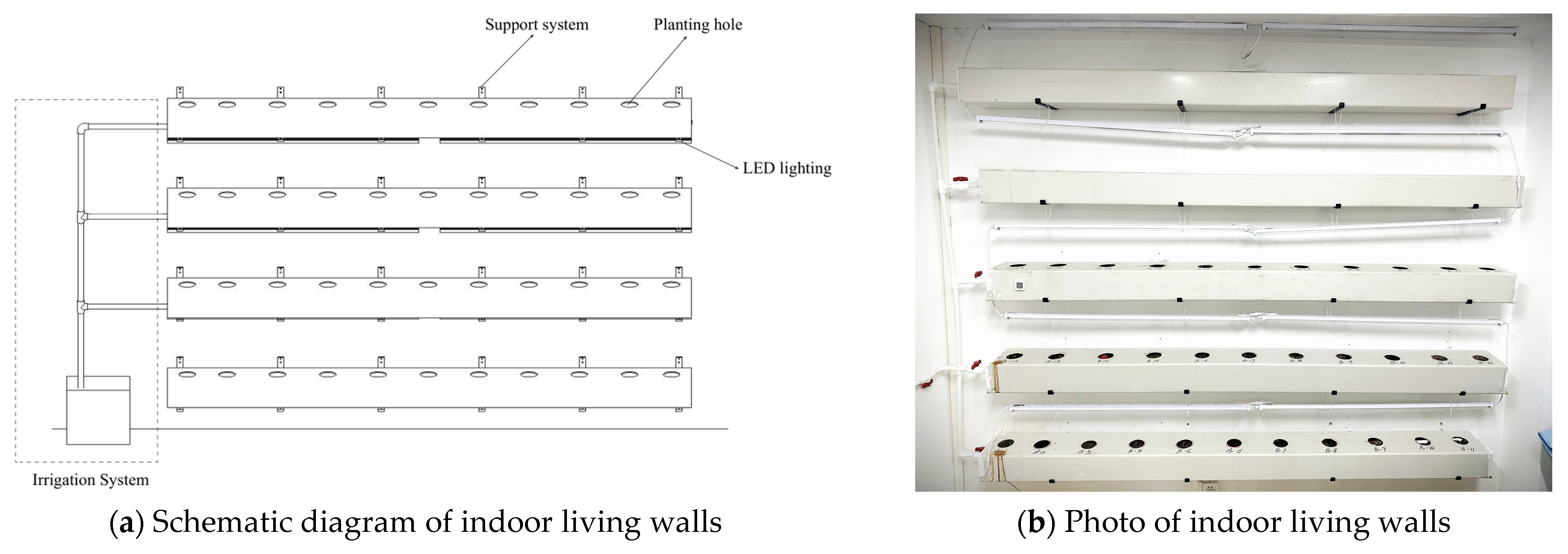
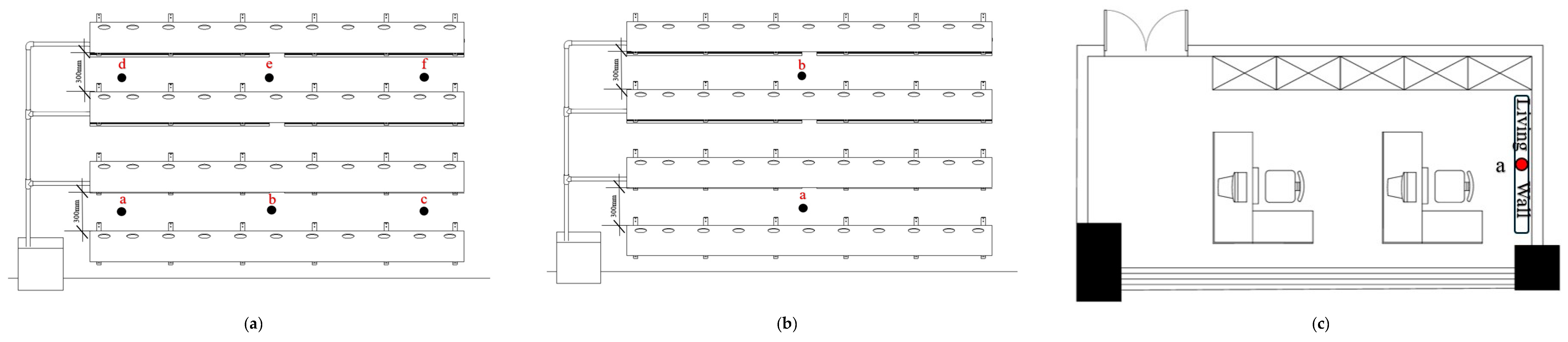
| Sample Group | Room Orientation | Set of ILWs | Height |
|---|---|---|---|
| R1-A | North | West | 0.4~0.85 m |
| R1-B | North | West | 1.3~1.75 m |
| R2-A | North | East | 0.4~0.85 m |
| R2-B | North | East | 1.3~1.75 m |
| R3-A | South | East | 0.4~0.85 m |
| R3-B | South | East | 1.3~1.75 m |
| R4-A | South | West | 0.4~0.85 m |
| R4-B | South | West | 1.3~1.75 m |
| Value | Meaning |
|---|---|
| 1 | The two elements are equally important when compared |
| 3 | The former is slightly more important than the latter |
| 5 | The former is significantly more important than the latter. |
| 7 | The former is extremely important compared to the latter |
| 9 | Indicates that the former element is more important than the latter |
| 2, 4, 6, 8 | The median value of the above adjacent judgments |
| The reciprocal of 1 to 9 | The importance of comparing the order of the corresponding two factors |
| n | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| RI | 0 | 0 | 0.58 | 0.9 | 1.12 | 1.24 | 1.32 | 1.41 | 1.45 | 1.51 | 1.49 | 1.54 | 1.56 |
| Indicator | Assignment Range | ||
|---|---|---|---|
| Suitable I | Moderately Suitable II | Unsuitable III | |
| Temperature C1 [43] | 22~25 °C | 18~22 °C | <18 or >25 °C |
| CO2 concentration content C2 [44] | ≤1500 ppm | ≤1000 ppm | ≤350 ppm |
| Humidity C3 [44,45,46,47] | 70~80% | 40~70 or 80~85% | <40 or >85% |
| Illumination intensity C4 [48,49,50] | 16~20 klx | <16 or >20 klx | <1.5 or >25 klx |
| Root vitality C5 | >82.508 μg/h/g FW | 58.015~82.508 μg/h/g FW | ≤58.015 μg/h/g FW |
| Total chlorophyll C6 | >1.061 mg/g FW | 0.842~1.061 mg/g FW | ≤0.842 mg/g FW |
| Chlorophyll a C7 | >0.601 mg/g FW | 0.482~0.601 mg/g FW | ≤0.482 mg/g FW |
| Chlorophyll b C8 | >0.492 mg/g FW | 0.396~0.492 mg/g FW | ≤0.396 mg/g FW |
| Stem diameter C9 | >8.7 mm | 5.3~8.7 mm | ≤5.3 mm |
| Number of blades C10 | >9 | >8 | ≤7 |
| Max leaf area C11 | >153.77 cm2 | 84.84~153.77 cm2 | ≤84.84 cm2 |
| Plant height C12 | >20.4 cm | 15.8~20.4 cm | ≤15.8 cm |
| Plant breadth C113 | >30.1 cm | 19.7~30.1 cm | ≤19.7 cm |
| Shoot fresh weight C14 | >33.59 g | 17.86~33.59 g | ≤17.86 g |
| Root fresh weight C15 | >2.4 g | 1.31–2.4 g | <1.31 g |
| Vitamin C content C16 [51,52,53] | >0.67 mg/g | 0.61~0.67 mg/g | <0.61 mg/g |
| Soluble sugar content C17 [53] | >11.8 mg/g | 8.1~11.8 mg/g | <8.1 mg/g |
| Nitrate nitrogen content C18 [54,55] | <3000 mg/kg | 3000~3500 mg/kg | >3500 mg/kg |
| Assignment 1 | 3 | 2 | 1 |
| A | B1 | B2 | B3 | B4 | B5 | |
|---|---|---|---|---|---|---|
| B1 | 1 | 1 | 1 | 1 | 1 | 0.2 |
| B2 | 1 | 1 | 1 | 1 | 1 | 0.2 |
| B3 | 1 | 1 | 1 | 1 | 1 | 0.2 |
| B4 | 1 | 1 | 1 | 1 | 1 | 0.2 |
| B5 | 1 | 1 | 1 | 1 | 1 | 0.2 |
| 1 = 5, CI 2 = 0, CR 3 = 0 | ||||||
| B1 | C1 | C2 | C3 | C4 | |
|---|---|---|---|---|---|
| C1 | 5 | 1 | 3 | 5 | 0.58 |
| C2 | 3 | 1 | 1 | 0.17 | |
| C3 | 1 | 1 | 1 | 0.15 | |
| C4 | 1 | 1 | 0.1 | ||
| 1 = 4.1871, CI 2 = 0.0624, CR 3 = 0.0693 | |||||
| B2 | C5 | C6 | C7 | C8 | |
|---|---|---|---|---|---|
| C5 | 5 | 5 | 3 | 1 | 0.55 |
| C6 | 3 | 3 | 1 | 0.25 | |
| C7 | 1 | 1 | 0.1 | ||
| C8 | 1 | 1 | 0.1 | ||
| 1 = 4.0455, CI 2 = 0.0152, CR 3 = 0.0168 | |||||
| B3 | C9 | C10 | C11 | C12 | C13 | |
|---|---|---|---|---|---|---|
| C9 | 3 | 4 | 1 | 2 | 3 | 0.4 |
| C10 | 2 | 3 | 1 | 1 | 0.21 | |
| C11 | 2 | 3 | 1 | 1 | 0.2 | |
| C12 | 1 | 2 | 0.12 | |||
| C13 | 1 | 0.07 | ||||
| 1 = 5.0778, CI 2 = 0.0194, CR 3 = 0.0173 | ||||||
| B4 | C14 | C15 | |
|---|---|---|---|
| C14 | 1 | 5 | 0.83 |
| C15 | 1 | 0.17 | |
| 1 = 2, CI 2 = 0, CR 3 = 0 | |||
| B5 | C16 | C17 | C18 | |
|---|---|---|---|---|
| C16 | 1 | 1 | 5 | 0.47 |
| C17 | 1 | 1 | 4 | 0.43 |
| C18 | 1/5 | 1/4 | 1 | 0.1 |
| 1 = 3.0057, CI 2 = 0.0028, CR 3 = 0.0049 | ||||
| Objective Layer | Criteria Layer | Indicator Layer | |||
|---|---|---|---|---|---|
| Suitability evaluation of indoor living walls | Indoor micro-environment B1 | 0.20 | Temperature C1 | 0.19 | 0.04 |
| CO2 concentration C2 | 0.20 | 0.04 | |||
| Humidity C3 | 0.23 | 0.05 | |||
| Illumination intensity C4 | 0.38 | 0.08 | |||
| Physiology of vegetable B2 | 0.20 | Root vitality C5 | 0.25 | 0.05 | |
| Total chlorophyll C6 | 0.23 | 0.05 | |||
| Chlorophyll a C7 | 0.33 | 0.07 | |||
| Chlorophyll b C8 | 0.19 | 0.04 | |||
| Morphology of vegetable B3 | 0.20 | Stem diameter C9 | 0.14 | 0.03 | |
| Number of blades C10 | 0.11 | 0.02 | |||
| Max leaf area C11 | 0.25 | 0.05 | |||
| Seeding height C12 | 0.24 | 0.05 | |||
| Plant breadth C13 | 0.26 | 0.05 | |||
| Yield of vegetable B4 | 0.20 | Shoot fresh weight C14 | 0.57 | 0.11 | |
| Root fresh weight C15 | 0.43 | 0.09 | |||
| Quality of vegetable B5 | 0.20 | Vitamin C content C16 | 0.47 | 0.09 | |
| Soluble sugar content C17 | 0.27 | 0.05 | |||
| Nitrate nitrogen content C18 | 0.26 | 0.05 |
| Indicator | W | Indicator | W | ||||
|---|---|---|---|---|---|---|---|
| C1 | 0.580 | 0.038 | 0.04 | C10 | 0.042 | 0.022 | 0.01 |
| C2 | 0.170 | 0.04 | 0.01 | C11 | 0.040 | 0.05 | 0.03 |
| C3 | 0.150 | 0.046 | 0.02 | C12 | 0.024 | 0.048 | 0.02 |
| C4 | 0.10 | 0.076 | 0.1 | C13 | 0.014 | 0.052 | 0.01 |
| C5 | 0.110 | 0.05 | 0.09 | C14 | 0.166 | 0.114 | 0.29 |
| C6 | 0.050 | 0.046 | 0.04 | C15 | 0.034 | 0.086 | 0.05 |
| C7 | 0.020 | 0.066 | 0.02 | C16 | 0.094 | 0.094 | 0.14 |
| C8 | 0.020 | 0.038 | 0.01 | C17 | 0.086 | 0.054 | 0.07 |
| C9 | 0.080 | 0.028 | 0.03 | C18 | 0.020 | 0.052 | 0.02 |
| Indicators | R1-A | R1-B | R2-A | R2-B | R3-A | R3-B | R4-A | R4-B | F | p |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature C1 (°C) | 19.5 ± 0.79 | 20.4 ± 0.91 | 22.17 ± 1.19 | 23.3 ± 1.12 | 24.75 ± 2.5 | 25.05 ± 2.62 | 25.5 ± 1.68 | 26.59 ± 1.8 | 6.613 | <0.001 |
| CO2 concentration C2 (ppm) | 586.15 ± 1.64 | 579.36 ± 1.18 | 704.73 ± 3.34 | 706.3 ± 1.51 | 677.85 ± 1.98 | 679.97 ± 1.45 | 695.06 ± 1.7 | 699.73 ± 26.82 | 36.46 | <0.001 |
| Humidity C3 (%) | 66.64 ± 2.71 | 61.05 ± 2.98 | 72.94 ± 7.9 | 71.44 ± 8.31 | 55.8 ± 13.31 | 54.76 ± 13.92 | 48.97 ± 3.1 | 46.24 ± 2.78 | 4.473 | <0.05 |
| Illumination intensity C4 (lx) | 1100.02 ± 2.39 | 1119.22 ± 1.35 | 1141.41 ± 2.92 | 1155.08 ± 1.78 | 1363.35 ± 2.18 | 1393.68 ± 1.08 | 1550.42 ± 4.21 | 1585.36 ± 3.41 | 18.125 | <0.001 |
| Root vitality C5 (μg/h/g FW) | 32.38 ± 0.97 | 39.58 ± 1.53 | 55.91 ± 2.05 | 63.34 ± 2.62 | 74.42 ± 1.47 | 80.22 ± 2 | 86.3 ± 0.84 | 91.14 ± 3.54 | 33.179 | <0.001 |
| Total chlorophyll C6 (mg/g FW) | 0.61 ± 0.04 | 0.72 ± 0.04 | 0.84 ± 0.08 | 0.9 ± 0.08 | 1.05 ± 0.06 | 1.02 ± 0.04 | 1.09 ± 0.05 | 1.24 ± 0.08 | 34.609 | <0.001 |
| Chlorophyll a C7 (mg/g FW) | 0.39 ± 0.03 | 0.4 ± 0.01 | 0.45 ± 0.05 | 0.47 ± 0.05 | 0.58 ± 0.06 | 0.58 ± 0.01 | 0.6 ± 0.02 | 0.68 ± 0.03 | 22.088 | <0.001 |
| Chlorophyll b C8 (mg/g FW) | 0.22 ± 0.01 | 0.32 ± 0.03 | 0.39 ± 0.01 | 0.43 ± 0.03 | 0.48 ± 0.03 | 0.42 ± 0.05 | 0.49 ± 0.03 | 0.56 ± 0.06 | 28.354 | <0.001 |
| Stem diameter C9 (mm) | 10.98 ± 0.09 | 11.85 ± 0.88 | 29.48 ± 0.66 | 31.6 ± 1.22 | 17.33 ± 0.47 | 18.15 ± 0.96 | 13.28 ± 0.48 | 14.63 ± 0.89 | 38.704 | <0.001 |
| Number of blades C10 | 6 ± 0.41 | 7 ± 0.58 | 9 ± 0.58 | 10 ± 0.58 | 8 ± 0.58 | 8 ± 0.58 | 7 ± 1.15 | 8 ± 0 | 11.032 | <0.001 |
| Max leaf area C11 (cm2) | 33.78 ± 1.43 | 36.25 ± 1.52 | 158.97 ± 4.15 | 165.41 ± 3.78 | 93.73 ± 5.74 | 97.05 ± 2.78 | 50.14 ± 0.95 | 57.96 ± 4.48 | 67.773 | <0.001 |
| Seeding height C12 (cm) | 9.9 ± 0.53 | 11.95 ± 0.84 | 27.58 ± 0.52 | 29.53 ± 1.08 | 16.66 ± 1.05 | 17.38 ± 0.49 | 11.63 ± 0.8 | 13.78 ± 1.05 | 28.262 | <0.001 |
| Plant breadth C13 (cm) | 10.98 ± 1.08 | 11.85 ± 1.6 | 29.48 ± 1.51 | 31.64 ± 1.41 | 17.33 ± 0.95 | 18.15 ± 1.72 | 13.28 ± 0.91 | 14.63 ± 0.77 | 13.161 | <0.001 |
| Shoot fresh weight C14 (g) | 33.78 ± 1.27 | 36.25 ± 1.09 | 158.98 ± 1.41 | 165.41 ± 1.63 | 93.73 ± 1.24 | 97.05 ± 1.72 | 50.14 ± 1.55 | 57.96 ± 1.64 | 47.947 | <0.001 |
| Root fresh weight C15 (g) | 8.72 ± 0.39 | 9.48 ± 0.4 | 35.83 ± 0.87 | 39.07 ± 2.39 | 16.42 ± 0.8 | 19.47 ± 1.42 | 10.13 ± 0.73 | 12.1 ± 1.7 | 20.906 | <0.001 |
| Vitamin C content C16 (mg/g) | 0.08 ± 0.02 | 0.08 ± 0.01 | 0.11 ± 0.02 | 0.12 ± 0.04 | 0.07 ± 0.02 | 0.07 ± 0.01 | 0.07 ± 0.02 | 0.08 ± 0.02 | 27.155 | <0.001 |
| Total soluble sugar content C17 (mg/g) | 5.08 ± 0.16 | 5.85 ± 0.12 | 8.48 ± 0.15 | 8.75 ± 0.05 | 7.19 ± 0.11 | 7.59 ± 0.02 | 6.58 ± 0.09 | 6.13 ± 0.08 | 42.08 | <0.001 |
| Nirate nitrogen C18 (mg/kg) | 223.77 ± 1.85 | 212.25 ± 2.54 | 148.69 ± 1.43 | 143.75 ± 0.92 | 133.72 ± 0.61 | 145.03 ± 1.58 | 110.49 ± 2.05 | 104.86 ± 0.66 | 69.095 | <0.001 |
| Group | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | C12 | C13 | C14 | C15 | C16 | C17 | C18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1-A | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 |
| R1-B | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 |
| R2-A | 3 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | 2 | 3 | 3 | 3 | 2 | 3 | 2 | 1 | 2 | 3 |
| R2-B | 3 | 2 | 3 | 1 | 2 | 2 | 1 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | 2 | 3 |
| R3-A | 3 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 3 |
| R3-B | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 3 |
| R4-A | 1 | 2 | 2 | 2 | 3 | 3 | 2 | 3 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 |
| R4-B | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 |
| Group | R1-A | R1-B | R2-A | R2-B | R3-A | R3-B | R4-A | R4-B |
| Score | 1.15 | 1.17 | 2.17 | 2.41 | 1.55 | 1.72 | 1.42 | 1.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, D.; Luo, X. Research on the Suitability of Building Integrated Agriculture—Taking Indoor Living Walls as an Example. Sustainability 2025, 17, 7984. https://doi.org/10.3390/su17177984
Mu D, Luo X. Research on the Suitability of Building Integrated Agriculture—Taking Indoor Living Walls as an Example. Sustainability. 2025; 17(17):7984. https://doi.org/10.3390/su17177984
Chicago/Turabian StyleMu, Dawei, and Xueke Luo. 2025. "Research on the Suitability of Building Integrated Agriculture—Taking Indoor Living Walls as an Example" Sustainability 17, no. 17: 7984. https://doi.org/10.3390/su17177984
APA StyleMu, D., & Luo, X. (2025). Research on the Suitability of Building Integrated Agriculture—Taking Indoor Living Walls as an Example. Sustainability, 17(17), 7984. https://doi.org/10.3390/su17177984




