Abstract
In this paper, the interaction between caking bituminous coal (HC) and two types of biomass, namely sunflower husks (SFHs) and walnut shells (WSs), was studied via lab-scale fixed-bed reactor experiments and thermogravimetric analysis (TGA). The dynamics of volatile matter composition and weight loss changes were analyzed for the initial biomass types and their 1:1 blends with HC during co-pyrolysis. Derivative thermogravimetry (DTG) revealed that during the co-pyrolysis of HC with biomass, the number of reaction stages increased to four, compared to three during individual pyrolysis, indicating synergistic thermal behavior. The apparent activation energy (Ea) of the blends was higher (62.8 kJ/mol for SFH/HC and 61.8 kJ/mol for WS/HC) than that of the individual HC (55.1 kJ/mol), SFHs (43.8 kJ/mol), and WSs (52.4 kJ/mol), confirming intensified reaction complexity. Co-pyrolysis resulted in higher methane (CH4) production, with the CH4:HAc (acetic acid) ratio increasing from 1.2 (WSs) and 1.7 (SFHs) to 1.9 (WS/HC) and 3.3 (SFH/HC). The non-additive behavior of blends is established, indicating the interactions between biomass and HC during co-pyrolysis. These findings support a more resilient and sustainable approach to producing fuels and reducing agents, particularly through the utilization of agricultural residues and waste biomass.
1. Introduction
Biomass is the only renewable energy in nature that contains a carbon resource in quantities large enough to be used as a substitute for fossil fuels (FFs) [1,2]. As an alternative to FFs, renewable energy sources from organic raw carbon bio-materials are increasingly used [3,4,5]. The most important of them is plant material, which is formed during photosynthesis [6], converting carbon dioxide (CO2) and water into energy-rich molecules. Plant biomass is a valuable renewable chemical raw material from which unique compounds can be obtained, as well as substitutes for FFs [7]. However, for the effective use of biomass as a fuel or reducing agent [8,9,10,11,12,13], it is necessary to consider that biomass from different sources has different properties [14]. Additionally, the efficiency of biomass processing depends on the use of thermochemical conversion technologies, such as direct combustion [15], gasification [16], thermal liquefaction [17], hydrogenation [18], and pyrolysis [19,20].
Pyrolysis is a thermochemical conversion [21] employed to convert carbonaceous materials into value-added products at temperatures ranging from 350 to 800 °C. Based on the process conditions (residence time, temperature, and heating rate) and target products, pyrolysis can be categorized into eight main types: carbonization, conventional pyrolysis, fast pyrolysis, flash-liquid pyrolysis, flash-gas pyrolysis, ultra-pyrolysis, vacuum pyrolysis, hydropyrolysis, and methanopyrolysis [22].
Biomass is comprised of three main pseudo-components: cellulose, hemicellulose, and lignin [23]. It also contains smaller amounts of extractives, such as terpenes, tannins, fatty acids, oils, and resins, along with moisture [24,25]. Biomass pyrolysis is a complex process that involves a series of parallel and sequential reactions, such as dehydration, depolymerization, isomerization, aromatization, and decarboxylation [26] under an anoxic environment. Generally, three main mechanisms can be considered: char formation, depolymerization, and fragmentation [27]. Additionally, secondary reactions like cracking or recombination can also occur [28]. The primary steps of char formation include dehydration (the removal of water molecules), carbonization (the breakdown of organic compounds into a solid, carbon-rich residue and volatile gases), and polymerization (reactions among the remaining materials that lead to the formation of complex carbon structures). Since biomass is composed of polymers such as cellulose, hemicellulose, and lignin, depolymerization involves the cleavage of the chemical bonds within these polymers. Fragmentation refers to the breakdown of biomass into smaller pieces or fragments during the pyrolysis process. This mechanism is characterized by physical breakdown, the release of small molecules, and the production of radicals. Likewise, secondary reactions, including cracking and recombination, may occur during biomass pyrolysis.
Caking coal is coal that under heat treatment without oxygen access begins to soften and form a coherent plastic layer, which can swell to fill the gaps of the coal particles or inert particles [29]. This ability is a prerequisite for a hard, coherent cellular coke or biocoke matrix formation [30,31]. Caking coal pyrolysis can be divided into three main zones. Below 350–400 °C, various processes occur before primary pyrolysis; first of all, processes of dehydration and desorption of occluded gases occur [32], and the transport of noncovalently bonded molecular phases. These processes depend on the coal rank, composition, temperature, and heating conditions, and are complicated by the heterogeneity of the coal [33]. When heated to 120 °C, physically bound moisture and coal-adsorbed gases (CO2, methane (CH4), air gases) are released. At temperatures above 200 °C, the release of water begins, which is formed during the thermal decomposition of the organic mass of coal (pyrogenetic water), as well as CO2. In the range of 250–325 °C, the decomposition process of carbonaceous matter intensifies. Water vapor (H2O), CO2, a certain amount of hydrogen sulfide, and organic sulfur compounds are intensively released. At this stage, the oxygen content in the coal is noticeably reduced. However, even in this temperature range, chemical bonds are split only at the end regions of coal macromolecules. Deep changes in the internal structure of the organic mass of coal have not yet occurred. At higher temperatures, ranging from 375 to 700 °C, the coal structure undergoes thermal decomposition, resulting in the formation of various hydrocarbons, including CH4, other alkanes, polycyclic aromatics, phenols, and nitrogen-containing compounds. In the temperature range of approximately 350–550 °C, caking coal often softens and becomes plastic [34]. Above 600 °C, this plastic layer undergoes repolymerization, forming a semicoke. Afterward, the semicoke hardens into coke, releasing CH4, hydrogen (H2), and traces of carbon oxides.
Considering the co-pyrolysis of coal and biomass or biomass-derived carbon materials, it is then important to study the interaction between different types of carbon material during pyrolysis [35,36]. During co-pyrolysis, various interactions can occur between coal and biomass; for instance, it can influence the breakdown of coal structure, thereby altering the overall pyrolysis kinetics. According to [37], during co-pyrolysis, biomass produces some quantity of H2 and hydroxyl radicals. These can promote the cracking of aromatic structures in coal into light volatile matter (VM). Additionally, interactions can impede the pyrolysis process, resulting in lower char yields and inferior biofuel properties.
Tian et al. [38] investigated the co-pyrolysis of miscanthus, three types of coal (lignite, bituminous coal, and anthracite), and their blends using the thermogravimetric analysis coupled to Fourier transform infrared spectroscopy (TGA-FTIR) method. The activation energies (Ea) for co-pyrolysis differed from those for the individual pyrolysis of miscanthus and coal. Kinetic analysis revealed that coal predominantly influenced the Ea. Increasing the biomass blend ratio decreased the Ea for miscanthus–lignite and miscanthus–bituminous coal blends but increased it for miscanthus–anthracite blends. FTIR analysis revealed emission profiles of CH4, carbon monoxide (CO), CO2, water, formic acid, phenol, and xylene. Products analyzed within the 250–400 °C temperature range corresponded to the decomposition of miscanthus. Higher biomass blend ratios resulted in the increased release of all analyzed products from miscanthus–lignite and miscanthus–bituminous coal blends, indicating a synergistic effect in co-pyrolysis.
Thermogravimetric and thermobalance reactor analyses were conducted under both non-isothermal and isothermal conditions by Jeong et al. [35] to investigate the kinetic characteristics of coking coals (weak and hard coking coal) mixed with biomass (yellow poplar) during pyrolysis. The study found that the Ea of weak coking coal (252–579 kJ/mol) was higher than that of hard coking coal (164–273 kJ/mol). Most hard coking coal/biomass blends had lower Ea (89–401 kJ/mol) compared to weak coking coal/biomass blends (128–456 kJ/mol) due to the inherently higher Ea of weak coking coal. Additionally, the Ea decreased with increasing biomass blend ratios for both types of coal/biomass blends at each conversion value.
In addition, Yang et al. [39] used thermogravimetry coupled with mass spectrometry to evaluate the pyrolysis of individual coal, individual walnut shells, and their blends. Synergistic reactions, in terms of gaseous product emissions, were observed only within the temperature range of approximately 573–773 K (300–500 °C) for blends with coal/walnut shell ratios of 1:5, 1:4, 1:3, and 1:2. This synergy was attributed to hydrogen transfer from H2 and hydroxyl radicals formed during walnut shell pyrolysis to the coal, leading to increased emissions of acetic acid and reduced residue formation.
Gohar et al. [40] studied the co-pyrolysis of bituminous coal–biomass blends containing hemp and sawdust, prepared in various ratios: 80:20, 60:40, 40:60, and 20:80. The kinetic parameters were calculated using the Coats–Redfern method with thirteen integral functions. The Ea for coal alone was 39 kJ/mol, while it was 60 kJ/mol for sawdust and 44 kJ/mol for hemp. Initially, as the biomass ratio in the blends increased, the Ea also increased. However, in the second pyrolysis stage (200–410 °C for sawdust-containing blend and 147–395 °C for the hemp-containing blend), the Ea decreased as the biomass ratio increased.
Bhattacharyya et al. [41] studied low-rank coal pyrolysis and co-pyrolysis of the coal and sawdust blend in a fixed-bed reactor under the temperatures of 773, 823, and 873 K (500, 550, and 600 °C) and a 30 K/min heating rate. It was concluded that co-pyrolysis led to a decrease in solid residue yield and an increase in gas and liquid products. Additionally, it was concluded that biomass can be blended with low-rank coal in higher amounts to increase the degradation rate.
Tauseef et al. [42] identified positive synergies in 80:20 and 60:40 coal–rice husk blends. Co-pyrolysis effectively transferred catalytically active species from rice husk to coal. Increasing the proportion of rice husk in the coal blend from 200 to 400 °C resulted in higher apparent Ea values, particularly for one-dimensional diffusional (64–100 kJ/mol) and phase interfacial reaction (30–52 kJ/mol) models.
On the other hand, some studies have reported no or weak interactions between coal and biomass during co-pyrolysis. The choice of coal for blends was focused on using sub-bituminous coal or high-rank coal. For instance, Lu et al. [43] used TGA to study the co-pyrolysis characteristics of fuel blends with five different ratios of biomass (both raw and torrefied wood) to anthracite: 100 wt.%, 75 wt.%, 50 wt.%, 25 wt.%, and 0 wt.%. The analysis showed that the co-pyrolysis characteristics of the biomass–anthracite blends were very similar to those of the individual components. Therefore, it was concluded that the interactions or synergistic effects between the raw/torrefied biomass and anthracite were minimal.
Vuthaluru [44] studied the thermal behavior of sub-bituminous coal, biomass materials (wood waste and wheat straw), and their blends in different ratios (10:90, 20:80, 30:70, and 50:50) during co-pyrolysis. The study found no interactions between coal and biomass during co-pyrolysis. The pyrolytic characteristics of the blends were additive, reflecting those of the individual fuels. Among the tested blends, the 20:80 ratio showed the lowest Ea: 91 kJ/mol for coal/wood waste and 79 kJ/mol for coal/wheat straw. Additionally, the optimum blend ratio for the co-pyrolysis of coal/wheat straw was found to be 50:50.
In addition to the above-analyzed results, complex interactions can be observed. Cao et al. [45] reported the observation of a strong positive interaction, a mild positive interaction, and a negative interaction when three different types of biomasses (wheat, rice, and bamboo straw) were co-pyrolyzed with middle volatile coal (VM is 26.24 wt.%). The co-pyrolysis interaction was strongest between coal and wheat straw, weaker between coal and rice straw, and showed a negative interaction between coal and bamboo straw. Generally, it was highlighted that co-pyrolysis can effectively reduce the apparent Ea. Additionally, alkali and alkaline earth metals on the surface of biochar can catalyze coal pyrolysis [46,47]. However, the hydrogen donors and free radicals generated during biomass pyrolysis inhibited the cross-linking and polymerization of macromolecular volatiles produced by coal pyrolysis.
To the best of our knowledge, the co-pyrolysis of well-caking bituminous coal (HC) and biomass using TGA, as well as the interactions during co-pyrolysis, have not been sufficiently studied. The study of co-pyrolysis of well-caking HC and biomass is of interest because it allows the use of biomass residues, reducing the reliance on fossil coal by replacing it with renewable carbon sources. Moreover, studying possible interactions in the co-pyrolysis of well-caking HC and biomass is important for the subsequent production of biofuel and bioreducing agents. Sunflower husks (SFHs) are chosen for their wide availability in agricultural regions, and walnut shells (WSs) for their dense structure and typically very low ash. Compositionally, SFHs typically contain 30 wt.% cellulose, 39 wt.% hemicellulose-rich neutral sugars, and 26 wt.% lignin (dry basis), whereas WSs are lignin-rich (35 wt.%) with 30 wt.% cellulose and 25 wt.% hemicellulose (dry basis) [48,49]. At the same time, HC is selected for its strong caking behavior relevant to fuel and reducing agent production. This approach supports the resilient and more sustainable production of metallurgical and energy carbon sources by utilizing locally available biomass. Therefore, this paper aims to investigate the co-pyrolysis characteristics of well-caking HC and biomass via a fixed-bed reactor, along with a focus on the chemical changes, thermal decomposition, and kinetic behavior under non-isothermal conditions.
2. Materials and Methods
2.1. Petrographic Analysis of HC
The preparation of HC samples and measurement of vitrinite reflectance were conducted according to ISO 7404-2:2009 and ISO 7404-5:2009, respectively [50,51,52]. Mean reflectance was measured on vitrinite or altered vitrinite using the Lucia petrographic complex. Sample preparation involved using the LECO PR-32 automatic press and the LECO GPX-300 grinding and polishing machine (LECO Instruments, St Joseph, MI, USA). Reflectance was measured on a polished sample surface under oil immersion with a refractive index of 1.515, using an Olympus microscope (Olympus Corporation, Tokyo, Japan) at 50× magnification and analyzed by Lucia Vitrinite 7.13 software. Reflectance measurements were performed at an average of 200 points.
2.2. Maximum Thickness of the Plastic Layer of HC
The maximum thickness of the HC plastic layer was measured using a special plastometric needle according to ISO/DTS 4699 [53]. In this method, 100 ± 1 g of HC with a particle size of less than 1.6 mm was heated at a rate of 3 °C/min from 250 °C to 730 °C. The maximum thickness of the plastic layer (y, mm) was then determined. The HC was subjected to a load and heated from below in a metal glass placed above a silicon carbide heater. The weight and position of the load were calculated to apply a pressure of 9.1 MPa on the HC. As the HC was heated, it underwent different stages of thermal decomposition depending on the distance from the heating surface. This results in the formation of a layer of coke and semi-coke, a layer of plastic mass, and above the plastic layer, untransformed coal that has not reached the plastic state. Plastometric shrinkage (x, mm) was determined in millimeters by the final decrease in the plastometric curve relative to the initial level of the curve.
2.3. Dilatometric Parameters of HC
The viscosity of the plastic layer and the dynamics of VM release determine the swelling of HC. Typically, the most significant swelling volumes occur when the plastic layer’s viscosity is minimal and the amount of released VM is maximal. In this state, the plastic layer offers less resistance to gas movement, making it more gas permeable. The dilatometric parameters of HC were determined according to [54]. In this study, 2 ± 0.01 g of compressed HC was heated in a metal tube under a pressure of 218 MPa. The heating period until the beginning of swelling, the swelling period, and the swelling index were identified under conditions where HC can swell freely in the tube. The measurement was conducted using a modified apparatus that allowed for the determination of HC dilatometric parameters at a uniform heating temperature of 500 °C, with fixed temperature intervals. Each trial was repeated twice.
2.4. Swelling Pressure of HC
The swelling pressure is a unique individual property of coal that occurs when coal is heated in a closed volume. The method used to determine the dynamics of swelling pressure is characterized by high sensitivity to changes in HC properties during heating [54]. The method involved rapidly heating an HC sample weighing 2 ± 0.01 g, which was pressed at a pressure of 218 MPa and a constant temperature of 500 °C, with an initial external load of 2 MPa. Each trial was repeated at least twice. Temperature intervals of time indicators were recorded during this study. It should be noted that the dilatometric parameters and the HC swelling pressure were determined in a single heating block.
2.5. Lab-Scale Fixed-Bed Reactor for Pyrolysis
For the fixed-bed lab-scale reactor studies, the diameter of the SFH pellets was 8 mm, and the maximum length was up to 12 mm. The size of hard coal and WSs was 10–7 mm, achieved by crushing in a laboratory jaw crusher. The weighed mixture was thoroughly mixed manually and then loaded into the retort.
For pyrolysis, the lab-scale fixed-bed reactor consists of a cylindrical retort (height 35 cm, inner diameter 12 cm), heated electrically, and controlled by two separated Proportional–Integral–Derivative (PID)–controllers. The sample was put in a cylindrical holder of 100 mm in height and 95 mm in inner diameter. Both parts were made of fiber-reinforced silica carbide (SiC)-ceramics to avoid reactions of CO, nitric oxide (NO), and pyrolysis chars with the wall and to avoid oxygen (O2) entering the reaction zone. The mounting and vessel for the sample bed were placed on a scale plate. The scale was mechanically separated from the retort by liquid sealing (synthetic thermal oil: Therminol 66). The scale was used to determine the weight loss of the sample during the pyrolysis process.
With this setup, it was possible to continuously measure the mass decrease in the sample during the pyrolysis process. The sample was introduced into the preheated reactor, allowing for rapid heating, which is comparable to real thermal conversion processes. A scheme of the setup can be found in Figure 1.
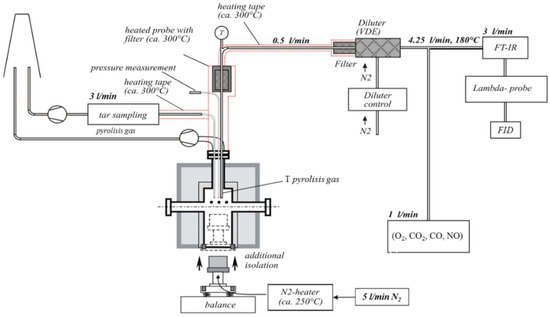
Figure 1.
Scheme of the lab-scale fixed-bed reactor including measurement set-up.
The following measurements/analyses were performed during each of the pyrolysis test runs:
- (1)
- Mass decrease in the sample over time (balance).
- (2)
- Concentrations of flue gas species over time:
- (a)
- Determination of H2O, CH4, ammonia (NH3), hydrogen cyanide (HCN), nitrous oxide (N2O), and basic hydrocarbons was performed with a multi-component FTIR spectroscopy device (Ansyco DX 4000, Vantaa, Finland).
- (b)
- CO2 and CO were measured with a multi-component non-dispersive infrared (NDIR) gas analyzer, which is equipped with an electrochemical sensor for O2 detection.
- (c)
- The amounts of total hydrocarbons (CxHy) were determined with a flame ionization detector (FID) (Messer Griesheim Modell VE7, Karlsfeld, Germany), which detects organic compounds by ionization in a burning H2 flame.
- (d)
- Wide band lambda sensor (O2).
- (3)
- Temperature measurements over time:
- (a)
- Five thermo-couples in the sample bed (3 different heights NiCr-Ni).
- (b)
- Thermo-couples in the gas phase (NiCr-Ni).
The initial sample and residues were analyzed, and detailed information about the release of inorganic species from the sample to the gas phase was obtained by calculating the mass balance for every relevant element.
A small, preheated purge gas stream (5 L/min N2) was injected through the sample bed. Sampling lines for product gases were also heated.
A pyrolysis was carried out to achieve 450 °C in 50 min with a heating rate of 8 K/min. The heating strategy was optimized to be as short as possible, and an attempt was made to minimize the temperature gradient over the sample bed. Moreover, blends with caking coal were tested using a segmental ramp. This ramp slowed in the 420 °C to 500 °C plastic layer range. The aim was to reduce measurement distortions and clarify the interactions between biomass and coal. Three test runs were carried out for each sample.
Inductively coupled plasma optical emission spectrometry (ICP-OES) was used to determine the inorganic concentration of HC, biomass samples, and blends. The procedure of measurement was performed according to [55,56].
2.6. Thermogravimetric Analysis
The thermal decomposition characteristics of the biomasses, HC, and their blends under non-isothermal conditions were determined via TGA (Netzsch STA 449 F3 Jupiter, Selb, Germany). All experiments were carried out under a nitrogen (N2) atmosphere with a flow rate of 100 mL/min at atmospheric pressure. The coal and biomass samples were ground, and fractions with a size of <150 μm were selected to avoid the influence of mass and heat transfer during the process of thermal decomposition. The mass of the sample was 5.0 ± 1.0 mg. Samples were weighed into the crucible first: 50% biomass, and then coal, and stirred for 3 min in the crucible itself, manually, with a wooden stick.
The reaction temperature range was up to 900 °C, and the heating rate was 5 K/min. All experimental runs were conducted twice.
TG (weight loss with temperature) and DTG (derivative thermogravimetry) curves were plotted and evaluated using OriginPro 2025 software.
The theoretical DTG curves of the blends were calculated by summing the weight loss rates of each component. These theoretical curves were then compared with experimental DTG curves to investigate the presence of chemical interactions between the blend components. The theoretical chemical interactions between HC and biomass during co-pyrolysis were determined according to the method reported in [41] and described as follows in Equation (1):
where , are the weight loss rates of the individual fuels and , are the mass fractions of HC and biomass (SFHs or WSs) in blends.
The Ea was calculated for all samples according to [57] and can be explained as follows. The thermal conversion for a single-step kinetic process can be defined using TGA data under various operating conditions, as shown in Equation (2):
where is an apparent conversion rate that depends on the temperature and partial pressure () of the reactive (1/min); is the fractional conversion degree of the material (0 < < 1.0); and represents the reaction model.
The fractional conversion degree () can be expressed using Equation (3):
where , , are the initial, instantaneous, and final masses (mg).
The apparent conversion reaction rate can be described as a function of temperature using the Arrhenius equation, as shown in Equation (4):
where A is the pre-exponential factor (1/min); Ea is the apparent activation energy (kJ/mol); and R is the universal gas constant (8.314 kJ/molK).
Furthermore, combining these equations results in Equation (5):
In the non-isothermal conversion process, TGA is conducted at a constant heating rate, as depicted by Equation (6):
Therefore, for non-isothermal conversion, Equation (7) is presented as follows:
The integral function , which is the most common integral form of a reaction model, can be derived using Equation (8):
where To is the initial absolute temperature (°C) and and is the temperature integral (or Arrhenius integral).
According to Coats and Redfern’s method [58], to obtain the kinetic parameters based on an integral model-fitting procedure, Equation (9) can be shown as follows:
Afterward, with this simplification, Equation (10) can be described as follows:
From the plot versus , the slope of the linear plot gives .
3. Results and Discussions
3.1. Characterization of the Properties of Caking HC and Two Biomass Samples
Caking HC and two biomass samples, namely SFHs and WSs, were taken to study the co-pyrolysis of HC and biomass. The biomass choice has the following practical reasons: the use of a material that is widely available and is present in large amounts in agricultural regions (SFHs), and a naturally compacted material (WSs) with a dense structure and a combination of very low ash and almost complete absence of sulfur compared to other types of biomass [59]. Proximate and ultimate sample analyses were determined according to [60,61] and are given in Table 1. For this study, HC with well-caking properties, widely used in blends for producing coke at coke-making plants, was taken. Samples of SFHs and WSs have lower fixed carbon (fCdb) and ash compared to HC, with values of 20.2 wt.% and 18.1 wt.% for fCdb and 3.0 wt.% and 0.9 wt.% for ash (Adb), respectively. Moreover, SFHs and WSs have higher VMdb than HC, with values of 76.84 wt.% and 81.02 wt.%, respectively.

Table 1.
Proximate and ultimate analyses, as well as selected characteristics for the samples.
The characteristics of HC are presented in Table 2. Caking properties and petrographic analysis reveal that well-quality coke can be obtained from this HC [52]. The mean reflectance of vitrinite of HC of 1.23% and a high fraction of caking macerals (Σcaking components of 89.6%) indicate a medium-rank, highly caking coal. The maximum plastic layer thickness of 13.0 mm and swelling index of 60.0 mm reflect a sustained plastic layer stage. Therefore, it is feasible to use this HC to improve the properties of the blends for coke production.

Table 2.
Petrographic analysis and caking properties of HC.
The characteristics of HC during swelling pressure measurements are summarized in Table 3. The period (a), lasting 342 s, represents the time (ta) before HC begins to soften, occurring between 0 and 376 °C. Following this, the plastic layer begins to form, with the onset of swelling pressure occurring over a relatively long duration (b) of 111 s, within the temperature range (tb) of 376–434 °C. The period (d) during which swelling pressure is active is shorter, lasting 80 s, with a temperature range (td) of 437–464 °C. This is due to the high viscosity of the plastic layer, as indicated by the period (f) of 250 s and the end of the plastic layer’s existence (tf) at 492 °C.

Table 3.
Swelling pressure of caking HC.
3.2. Lab-Scale Fixed-Bed Reactor Experiments
Table 4 shows the proximate and ultimate analyses, as well as selected characteristics of samples after individual pyrolysis of biomasses and co-pyrolysis of biomasses and HC (1:1). Samples are characterized by a decrease in VM, as well as an increase in the value of fCdb. After pyrolysis, the char yield was determined at 33% for WSs and 34% for SFHs. For the blends after co-pyrolysis, the char yields were 66% for WS/HC and 63% for SFH/HC. Assuming a good mixing based on masses, the char yield for SFH/HC was lower than expected, and the interaction of fuels may have occurred.

Table 4.
Proximate and ultimate analyses, as well as selected characteristics of the samples after pyrolysis and co-pyrolysis.
In general, typical HC is rich in aromatic C=C bonds, whereas biomass is rich in hydroxyl (O-H), ether, ester, (C-O) groups, etc., with highly variable content [38]. Figure 2 presents the van Krevelen diagram for HC, biomass, and their blends before and after pyrolysis. Biomass has higher concentrations of hydrogen and oxygen and a lower carbon content than HC, resulting in higher H/C and O/C atomic ratios. After pyrolysis, the carbon concentration in the biochar samples and blends is higher than in individual biomass samples. The carbon concentration in biochar from individual biomass samples increases compared to the initial biomass, while the hydrogen and oxygen contents decrease. This decrease in hydrogen and oxygen is due to deoxygenation and dehydration processes forming VM, primarily caused by the breaking of chemical bonds during pyrolysis [62].
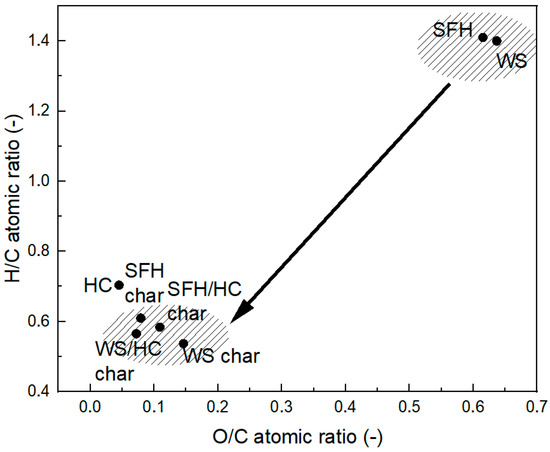
Figure 2.
van Krevelen diagram for HC, biomasses, and chars after pyrolysis.
The characteristics of volatile compounds (Figure 3a–h) include H2O, CO, CO2, CH4, acetic acid HAc (C2H4O2), propene (C3H6), methanol (CH3OH), acetaldehyde (C2H4O), and furfural (C5H4O2). Ethene (C2H4) was detected for SFH/HC, WS, and WS/HC samples. Sulfur dioxide (SO2) was also detected, but only in negligible amounts. The release of VM occurs in two main stages. The first stage is attributed to the evaporation of moisture. The second stage, with peaks between approximately 170 °C and 450 °C, results from the decomposition of various oxygen-containing functional groups. Figure 3a–h show that the main organic products in the gas phase were CH4 and HAc, with CH4 being the major component in all cases. However, the ratios were different. For WSs, the ratio CH4:HAc was roughly 1.2, whereas for SFHs, it was 1.7. For the blends with HC, the share of CH4 increased for WS and HC to 1.9 and for SFH and HC to 3.3.
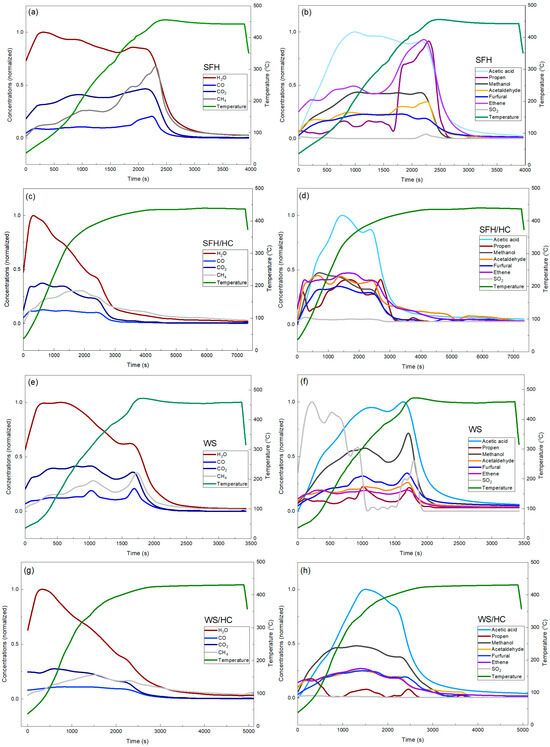
Figure 3.
Concentrations of the main products in the gas phase: (a,b) main products in the gas phase in SFH; (c,d) main products in the gas phase in SFH and HC blend; (e,f) main products in the gas phase in WSs; (g,h) main products in the gas phase in WS and HC blend.
Moreover, HAc, primarily produced from hemicellulose due to its abundant acetyl groups, is generated in higher amounts during the co-pyrolysis of SFH/HC and WS/HC compared to the individual pyrolysis of WSs and SFHs. It can be assumed that carbonyl and carboxyl-containing groups in the coal/WS blends can transform into HAc through radical stabilization, deacetylation, and isomerization [39].
ICP-OES results for the inorganic concentrations in biomass, HC, and their blends are presented in Table 5. The primary elements determined in all samples are Si, Al, Ca, Fe, K, Cl, S, Mg, and other elements in significantly smaller amounts. Phosphorus is found in relatively small amounts in HC but is noticeable in two biomass samples. Consequently, the phosphorus content is higher in the HC–biomass blends compared to individual HC samples. Notably, there is a decrease in most mineral species (Si, Al, Ca, K, S, and Cl) in the HC–biomass blends compared to the individual samples. However, for Fe and Na, the values in the blends increase compared to the individual samples. Moreover, the SFH and SFH/HC samples are characterized by a higher K value than the HC, WS, and WS/HC samples.

Table 5.
ICP-OES results of inorganic concentration of HC, biomass samples, and blends.
Since Table 5 shows the elemental concentrations measured on digested aliquots (mg/kg, total solids), the SFH/HC and WS/HC entries represent aliquots taken from nominal 1:1 (dry mass) bulk blends. Al and Si mainly occur in aluminosilicate minerals in the coal. On the other hand, SFHs and WSs contribute very little Al and Si and are low in ash overall. This leads to a varied distribution of analytes at the particle level. As a result, a small digestion subsample can yield Al–Si concentrations closer to the coal rather than the 1:1 mean value. Meanwhile, elements associated with biomass, like K and Ca, tend to be associated with biomass.
3.3. Thermogravimetric Analysis of Individual Caking Hard Coal, Two Different Types of Biomasses, and Blends of Hard Coal and Biomasses
Figure 4 shows the TG (Figure 4a,c,e,g,i) and DTG (Figure 4b,d,f,h,j) results of individual caking HC, two different types of biomasses, and blends of HC and biomasses at a constant heating rate from ambient temperature to 900 °C. Generally, the overall weight loss rate curve during individual pyrolysis of HC or two different types of biomasses (SFHs or WSs) can be characterized by three stages, as shown in Figure 4b,d,f. Individual pyrolysis is mainly associated with the second stage, in which the thermochemical decomposition of HC and biomass (SFHs or WSs) occurs. In the first stage, dehydration and evaporation of highly volatile compounds occur, while in the third stage, the formation of solid carbon residue predominates [43]. The total weight loss of HC did not change significantly before 340 °C, and at the end of stage 1, the weight loss was 3.0%, as shown in Figure 4a. According to the DTG profile, the temperature range for the thermochemical decomposition of HC is between 326 °C and 616 °C, with a weight loss of 19.9% at the end of stage 2. The onset temperature of the plastic layer stage was 423 °C, and the plastic layer stage was completed at 492 °C. At 462 °C, the weight loss was 10.9%, reaching a maximum weight loss rate of 0.7%/°C. Additionally, at this temperature, HC showed a plastic layer stage peak, which coincided with the td range of 437–464 °C (Table 3). As the temperature rose to 900 °C, the weight loss continued to increase steadily, reaching a maximum weight loss of 25.2%, with a gradually decreasing weight loss rate.
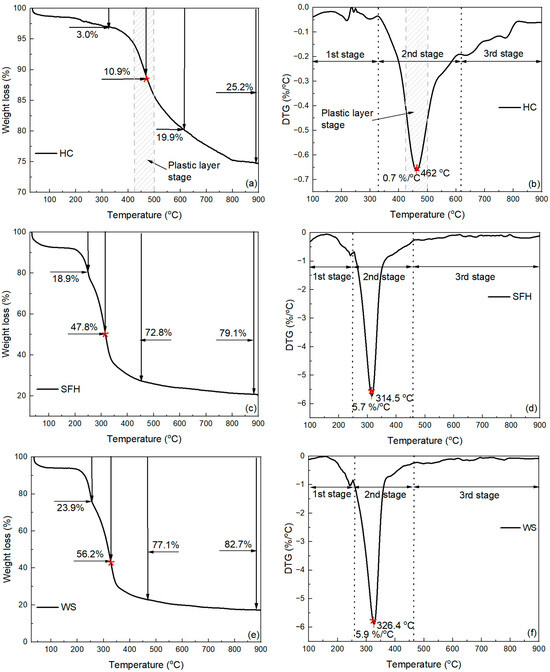
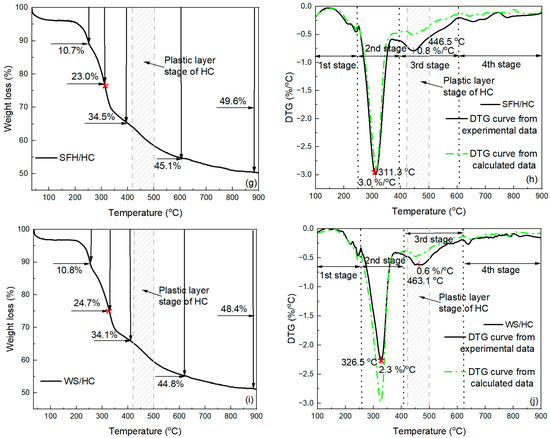
Figure 4.
Weight loss and DTG curves of caking HC, biomass samples, and a blend of caking HC with biomass: (a) weight loss of caking HC; (b) weight loss rate of caking HC; (c) weight loss of SFHs; (d) weight loss rate of SFHs; (e) weight loss of WSs; (f) weight loss rate of WSs; (g) weight loss of blend of 1:1 caking HC and SFHs; (h) weight loss rate of blend of 1:1 caking HC and SFHs (i) weight loss of blend of 1:1 caking HC and WSs (j) weight loss rate of blend of 1:1 caking HC and WS. The red asterisk on the TG curve refers to the maximum rate of mass loss according to the DTG curve.
According to the curves presented in Figure 4c, the weight losses at the end of the first stage were 18.9% for SFHs and 23.9% for WSs. Within the second stage, the decomposition reaction occurred earlier for the two biomass samples compared to HC. SFHs decomposed in the temperature range of 250–464 °C, and WSs in the temperature range of 252–465 °C, as indicated by the DTG curves (Figure 4d,f). For SFH and WS samples, the weight loss curves also show three main events during heat treatment, which are related to the decomposition of pseudo-components of biomass. Commonly, the decomposition of hemicellulose and cellulose occurs rapidly, predominantly for hemicellulose at 210–315 °C and cellulose at 315–400 °C. However, lignin is decomposed in a complex manner, as its weight loss can occur over a wide range of temperatures throughout the main heat treatment, up to 900 °C [63]. The first event corresponds to the breakdown peak, which can be attributed to the predominantly hemicellulose, as it has been found to break down over the temperature ranges of 250–334 °C (SFHs) and 252–340 °C (WSs). The second event can be attributed to the predominance of the peak breakdown of cellulose within the biomass, which occurs over the temperature ranges of 334–361 °C (SFHs) and 340–360 °C (WSs). Finally, the third event corresponds to the breakdown of lignin, which degrades in a wide temperature range throughout the entire heat treatment. The weight loss at the end of heat treatment was significantly higher than that of HC, with 79.1% for SFHs and 82.7% for WSs. The final weight loss values for the two biomass samples are consistent with the values presented in Table 1 for the VM. The DTG profile shows that the highest VM release for biomass (SFHs or WSs) and HC occurs in the second stage. The maximum decomposition rate of two samples of biomass (SFHs or WSs) is higher than that of HC and was 5.7%/°C and 5.9%/°C for SFHs and WSs, respectively. The DTG peaks of (SFHs and WSs) were observed at 314.5 °C and 326.4 °C, respectively. Likewise, at these temperatures, the weight loss is significantly higher than that of HC, being 47.8% for SFHs and 56.2% for WSs. Notably, the SFH sample is characterized by a lower total weight loss at the end of pyrolysis compared to WSs because SFHs have a higher ash content and lower VM values.
Figure 4g,i show the weight loss curves of the biomass blends with caking HC. The weight loss at the end of the first stage was 10.7% for the SFH/HC blend and 10.8% for the WS/HC blend, which was approximately half that of the individual biomass samples. For the SFH/HC blend, the second stage occurred within the temperature range of 242–393 °C, and the third stage occurred within the temperature range of 393–607 °C. During the co-pyrolysis of the WS/HC blend, the second stage was in the temperature range of 254–410 °C, and the third stage was in the temperature range of 410–609 °C. In this case, the weight loss curves for HC and SFH or WS blends are characterized by a lower total weight loss at the end of the heat treatment compared to the original biomass samples, and amount to 49.6% and 48.4% for the SFH/HC blend and WS/HC blend, respectively. In the case of co-pyrolysis of HC and biomass, using both SFH and WS components in the blend (Figure 4h,j), the number of reaction stages increases to four, compared to individual heating. This is due to the different thermal decomposition zones of HC and the two biomass samples.
For the blended samples, the DTG curves contain two main peaks, which distinguish these samples from the original HC sample and the two original biomass samples. Compared with individual samples, in addition to the main pronounced peak, which is often attributed primarily to cellulose decomposition [64,65,66], a mild decomposition process also develops at 446.5 °C for the SFH/HC blend and 463.1 °C for the WS/HC blend. The second mild peak of the blended samples results from the overlap of caking HC and lignin decomposition. Therefore, the second peak of the blended samples is closer to the peak that can be observed for the DTG curve of individual caking HC. The first DTG peak of the blended samples, corresponding to the maximum weight loss, is observed at approximately 311.3 °C (SFH/HC blend) and 326.5 °C (WS/HC blend), respectively. The maximum weight loss rate at these temperatures for the first DTG peak is 3.0%/°C (SFH/HC blend) and 2.3%/°C (WS/HC blend). As shown in Table 5, the SFH and SFH/HC samples have higher K values, which can catalyze the pyrolysis process [46,67,68]. This catalytic effect can cause a slight leftward shift in the DTG curve for SFHs, and SFH/HC compared to WSs and WS/HC, respectively. The second DTG peak, which can be considered, is at 446.5 °C for the SFH/HC blend and 463.1 °C for the WS/HC blend. The weight loss rate at these temperatures was 0.8%/°C (SFH/HC blend) and 0.6%/°C (WS/HC blend), respectively. It could be seen that the weight loss of HC is not significant in the temperature range, unlike the biomass of the samples, and is mainly caused by the low yield of HC volatiles at low temperatures. The weight loss of the blended samples is primarily attributed to the decomposition of biomass at lower temperatures. As was noted earlier, the process of individual heat treatment of the samples is mainly associated with the second stage, at which the main thermochemical decomposition occurs; however, for blends, the stages of thermochemical decomposition are extended by the addition of mild DTG peak and lie in the general temperature ranges of 242–607 °C for SFH/HC, 254–609 °C for WS/HC.
Figure 4h,j also show the theoretical DTG curves and the experimental ones. Notably, if there are no interactions during the thermochemical decomposition of biomass and HC, the pyrolysis characteristics of the blends will follow the behavior of the original samples in an additive manner. In both blends, the experimental DTG exceeds the theoretical additive curve in this region (Figure 4h,j), indicating a positive interaction between HC devolatilization and lignin-rich biomass fragments (for instance, -H radical transfer that promotes HC decomposition). By contrast, the first peak of WS/HC is slightly below its theoretical value, suggesting a small inhibition at lower temperatures. In the case of the SFH/HC blend, the theoretical DTG curve coincides with the experimental DTG curve, where the weight loss rate is assigned to SFH pseudo-components. However, for the second peak, which is associated with the decomposition of caking HC and lignin, the experimental DTG curve is characterized by a higher weight loss rate than the theoretical DTG curve. This difference can be explained by the fact that in cases of HC–biomass blend samples, some studies have found that a higher H/C atomic ratio of biomass compared with HC could facilitate HC decomposition [69]. Moreover, during the co-pyrolysis process of biomass and HC, some amounts of free radicals can be generated during biomass devolatilization, which act as hydrogen sources and may participate in the decomposition of HC [70]. Furthermore, it was well understood that the -H and -OH radicals significantly influenced HC pyrolysis by preventing secondary condensation, recombination, and crosslinking reactions, thereby decreasing secondary char yields and promoting volatile production [71]. Moreover, the increase in CH4 share and CH4:HAc ratio in blends (from 1.2 (WSs) and 1.7 (SFHs) to 1.9 (WS/HC) and 3.3 (SFH/HC)) may evidence enhanced deoxygenation and cracking under the blend conditions. Finally, inorganic catalysis (notably higher K in SFHs and SFH/HC) and the HC plastic layer range jointly increase vapor residence time and provide reactive surfaces.
In the case of the WS/HC blend, the experimental DTG curve for the peak, which also refers to the decomposition of pseudo-components of WSs, is characterized by a lower value of the weight loss rate compared to the theoretically calculated one. At the same time, the theoretical and experimental DTG peaks for the decomposition of HC and lignin part repeat the trend for the SFH/HC blend. Based on the obtained results, it can be concluded that the thermochemical decomposition associated with the decomposition of caking HC and lignin in the temperature range of 393–607 °C for SFH/HC and 410–609 °C for WS/HC occurred in a non-additive manner.
3.4. Kinetic Analysis
Table 6 presents the results for the apparent Ea of HC, two initial biomass samples, and two blend samples (SFH/HC and WS/HC) under slow heating conditions (5 K/min). The variation in values for the apparent Ea of HC was calculated in the temperature range of 360–500 °C when HC is in the plastic layer stage; for the two biomass samples, the temperature ranges were 250–350 °C, and for their blends with HC, the apparent Ea was estimated in the temperature range of 250–350 °C, as well as a small change that is observed in the high-temperature region of 445–600 °C for SFH/HC, and 440–600 °C for WS/HC.

Table 6.
Kinetic parameters for HC pyrolysis, biomass pyrolysis, and co-pyrolysis of blends of 1:1 caking HC and SFHs; caking HC and WSs.
The apparent Ea for the initial HC is the highest compared to individual heat treatments and corresponds to 55.1 kJ/mol. The apparent Ea of biomass samples was lower for SFHs at 43.8 kJ/mol and for WSs at 52.4 kJ/mol for the temperature range of 250–350 °C, after which there was a decrease in the apparent Ea at higher temperatures. The Ea values can be higher at lower temperatures, as biomass degassing occurs intensively in the temperature range of 250–350 °C, during which active thermal decomposition takes place.
The apparent Ea for blended samples is higher than that for the HC and biomass samples, which are 62.8 kJ/mol and 61.8 kJ/mol for SFH/HC and WS/HC blends, respectively. In general, an increase in the apparent Ea for blends was reported in [72], when using a blend of medium coalification degree coal and biomass (rice husk, corn stalk, or poplar wood) as blend components.
Moreover, the weight loss curves for the blends are characterized by lower values than the weight loss curves for individual pyrolysis of the two biomass samples, and the apparent Ea for both blends are characterized by higher values than the apparent Ea for individual biomass samples. In blends at lower temperature regions, the apparent Ea is higher, which indicates the predominance of decomposition of pseudo-components of biomass. However, the value of the apparent Ea then decreases and is 11.2 kJ/mol for SFH/HC and 9.9 kJ/mol for WS/HC in the temperature range of 445–600 °C (SFH/HC) and 440–600 °C (WS/HC). At higher temperatures, considering the previously shown weight loss rates for blends obtained from DTG curves, this part can be attributed to the decomposition of HC and lignin. Likewise, Ea values can be larger at lower temperatures, as thermochemical decomposition intensifies at lower temperatures.
The Arrhenius pre-exponential factor (A) represents the frequency factor and is related to the number of collisions that result in a reaction. Higher values of A indicate a higher probability of successful collisions and more complex reactions [73]. The values of the A increase for the samples are in the following order: SFH > WS > HC > SFH/HC WS/HC.
The above results imply that co-pyrolysis of HC with biomass requires more energy and a higher Ea than individual biomass pyrolysis. Moreover, in a blend where half of the HC is caking, and the non-caking part is introduced, i.e., the addition of biomass, this can affect the reduction in the plasticity of HC, the accumulation time of the caking layer of HC, and its transition to a plastic layer stage.
4. Conclusions
To investigate the co-pyrolysis characteristics of well-caking HC and biomass, with a focus on their thermal decomposition and the kinetics of HC–biomass blends under non-isothermal conditions, the selection of biomass samples was based on the following practical considerations: SFHs were chosen due to their wide availability and abundant presence in agricultural regions, and WSs were selected as a naturally compacted material with a dense structure, characterized by very low ash and an almost complete absence of sulfur compared to other types of biomass.
A lab-scale fixed-bed reactor was used to co-pyrolyze SFHs and WSs with caking HC at a 1:1 ratio. Post-pyrolysis, the char yield (mass char/mass feedstock, dry substance) was 33% for WSs and 34% for SFHs. For the blends, the char yield was 66% for the WS/HC blend and 63% for the SFH/HC blend. The char yield for the SFH/HC blend was lower than expected, suggesting interactions between the fuels. Additionally, the primary organic products in the gas phase were CH4 and HAc, with CH4 being the dominant component. The CH4 ratio was approximately 1.2 for WSs and 1.7 for SFHs. For the blends, the CH4 ratio increased to 1.9 for WS/HC and 3.3 for SFH/HC. The amount of HAc released was higher during the co-pyrolysis of WS/HC and SFH/HC blends than during the individual pyrolysis of WS and SFH samples.
TGA under non-isothermal conditions showed decreased weight loss in the blends compared to individual biomass samples, but more weight loss than individual HC. DTG results revealed that during the co-pyrolysis of HC and biomass, both in the case of using SFHs and WSs as components in the blend, the number of reaction stages increases to four compared to three stages during individual pyrolysis, which is associated with different zones of thermal degradation of HC and the two biomass samples. For the SFH/HC blend, the experimental DTG curve matched the theoretical curve during the SFH decomposition stage. Further, the HC and lignin decomposition stage showed a higher experimental weight loss rate than the theoretical one. In the case of a WS/HC blend, the experimental DTG curve for the peak, which primarily relates to the decomposition of the pseudo-components of the biomass, exhibited a lower rate of weight loss compared to the theoretically calculated one. At the same time, the theoretical and experimental DTG peaks for the predominantly HC and lignin decomposition stage show a similar manner to that observed for the SFH/HC blend.
The Ea of the blends was higher (62.8 kJ/mol and 61.8 kJ/mol for SFH/HC and WS/HC blends, respectively) than that of the individual biomasses (43.8 kJ/mol and 52.4 kJ/mol for SFHs and WSs, respectively) and HC (55.1 kJ/mol). In this case, the weight loss curves for the blends exhibited lower values compared to those for individual pyrolysis, and the DTG curves for the blends showed lower weight loss rates than those for the individual pyrolysis of biomass. The given values indicate that co-pyrolysis of HC with biomass requires more energy and a higher Ea than individual biomass pyrolysis. The values of the Arrhenius pre-exponential factor (A) increase in the order: SFH > WS > HC > SFH/HC WS/HC. This suggests that blends of HC with biomass have a higher likelihood of successful collisions and exhibit more complex reactions.
Practically, co-pyrolyzing biomass with well-caking bituminous coal offers the following: a wider devolatilization range with evidence of interactions near 390–610 °C; changed gas composition (CH4 share and CH4:HAc increase from 1.2 (WSs) and 1.7 (SFHs) to 1.9 (WS/HC) and 3.3 (SFH/HC)); and chars with higher fC and fuel ratio than the parent biomasses. These gains come with a somewhat higher apparent Ea for blends, reflecting more complex reactions but with possibilities to support partial fossil coal substitution in metallurgical applications. At an industrial scale, the identified dependencies are likely to persist. However, practical deployment will need specific unit operations for preparing mixtures and increased capacity for higher gas flow rates from co-pyrolysis.
Author Contributions
Conceptualization, L.K. and A.K.; methodology, L.K., A.K., P.S. and N.K.; investigation, L.K., A.K. and P.S.; writing—original draft preparation, L.K. and A.K.; writing—review and editing, P.S., S.R. and N.K.; project administration, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
The funding received from the European Union’s Horizon 2020 Research and Innovation Program under grant agreement number 731101 (BRISK2) is gratefully acknowledged.
Conflicts of Interest
Author Lina Kieush was employed by the company K1-MET GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships.
References
- Bridgwater, A.V. Renewable Fuels and Chemicals by Thermal Processing of Biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Kieush, L. Coal Pyrolysis Products Utilisation for Synthesis of Carbon Nanotubes. Pet. Coal 2019, 61, 461–463. [Google Scholar]
- DiGiovanni, C.; Echterhof, T. Progress Toward Biocarbon Utilization in Electric Arc Furnace Steelmaking: Current Status and Future Prospects. J. Sustain. Metall. 2024, 10, 2047–2067. [Google Scholar] [CrossRef]
- Safarian, S. To What Extent Could Biochar Replace Coal and Coke in Steel Industries? Fuel 2023, 339, 127401. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Loha, C.; Mahamood, R.M.; Jen, T.-C.; Alam, M.; Sarkar, I.; Das, P.; Akinlabi, E.T. An Overview of Biochar Production Techniques and Application in Iron and Steel Industries. Bioresour. Bioprocess. 2024, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Beadle, C.L.; Long, S.P. Photosynthesis—Is It Limiting to Biomass Production? Biomass 1985, 8, 119–168. [Google Scholar] [CrossRef]
- Ye, L.; Peng, Z.; Wang, L.; Anzulevich, A.; Bychkov, I.; Kalganov, D.; Tang, H.; Rao, M.; Li, G.; Jiang, T. Use of Biochar for Sustainable Ferrous Metallurgy. JOM 2019, 71, 3931–3940. [Google Scholar] [CrossRef]
- Ng, K.W.; Liu, K.; Huang, X. Biocarbon Utilization in Cokemaking by Partial Briquetting. Fuel 2024, 365, 131283. [Google Scholar] [CrossRef]
- Castro-Díaz, M.; Vega, M.F.; Díaz-Faes, E.; Barriocanal, C.; Musa, U.; Snape, C. Evaluation of Demineralized Lignin and Lignin-Phenolic Resin Blends to Produce Biocoke Suitable for Blast Furnace Operation. Fuel 2019, 258, 116125. [Google Scholar] [CrossRef]
- Kieush, L.; Schenk, J.; Pfeiffer, A.; Koveria, A.; Rantitsch, G.; Hopfinger, H. Investigation on the Influence of Wood Pellets on the Reactivity of Coke with CO2 and Its Microstructure Properties. Fuel 2022, 309, 122151. [Google Scholar] [CrossRef]
- Wei, R.; Meng, K.; Long, H.; Xu, C. Biomass Metallurgy: A Sustainable and Green Path to a Carbon-Neutral Metallurgical Industry. Renew. Sustain. Energy Rev. 2024, 199, 114475. [Google Scholar] [CrossRef]
- Kieush, L.; Schenk, J.; Koveria, A.; Hrubiak, A.; Hopfinger, H.; Zheng, H. Evaluation of Slag Foaming Behavior Using Renewable Carbon Sources in Electric Arc Furnace-Based Steel Production. Energies 2023, 16, 4673. [Google Scholar] [CrossRef]
- Kieush, L.; Koveria, A.; Zhu, Z.Q.; Boyko, M.; Sova, A.; Yefimenko, V. Application of Biomass Pellets for Iron Ore Sintering. MSF 2021, 1045, 17–31. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-Fuels from Thermochemical Conversion of Renewable Resources: A Review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Ram, M.; Mondal, M.K. Biomass Gasification. In Biofuels and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 253–276. ISBN 9780323852692. [Google Scholar]
- Demirbaş, A. Mechanisms of Liquefaction and Pyrolysis Reactions of Biomass. Energy Convers. Manag. 2000, 41, 633–646. [Google Scholar] [CrossRef]
- Zhang, L.; Rao, T.U.; Wang, J.; Ren, D.; Sirisommboonchai, S.; Choi, C.; Machida, H.; Huo, Z.; Norinaga, K. A Review of Thermal Catalytic and Electrochemical Hydrogenation Approaches for Converting Biomass-Derived Compounds to High-Value Chemicals and Fuels. Fuel Process. Technol. 2022, 226, 107097. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction Mechanisms and Multi-Scale Modelling of Lignocellulosic Biomass Pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Kieush, L.; Schenk, J.; Koveria, A.; Rantitsch, G.; Hrubiak, A.; Hopfinger, H. Utilization of Renewable Carbon in Electric Arc Furnace-Based Steel Production: Comparative Evaluation of Properties of Conventional and Non-Conventional Carbon-Bearing Sources. Metals 2023, 13, 722. [Google Scholar] [CrossRef]
- Kumar, K.P.; Remya, N. Technoeconomic Analysis of Biofuel Production from Agricultural Residues through Pyrolysis. In Green Approach to Alternative Fuel for a Sustainable Future; Elsevier: Amsterdam, The Netherlands, 2023; pp. 463–469. ISBN 9780128243183. [Google Scholar]
- Bhaskar, T.; Balagurumurthy, B.; Singh, R.; Poddar, M.K. Thermochemical Route for Biohydrogen Production. In Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2013; pp. 285–316. ISBN 9780444595553. [Google Scholar]
- Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T. Biocarbon Production Using Three-Stage Pyrolysis and Its Preliminary Suitability to the Iron and Steel Industry. Energies 2024, 17, 3131. [Google Scholar] [CrossRef]
- Orfão, J.J.M.; Antunes, F.J.A.; Figueiredo, J.L. Pyrolysis Kinetics of Lignocellulosic Materials—Three Independent Reactions Model. Fuel 1999, 78, 349–358. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass Pyrolysis Kinetics: A Comparative Critical Review with Relevant Agricultural Residue Case Studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Slezak, R.; Unyay, H.; Szufa, S.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Reactors Torrefaction vs. Biomass Pyrolizers—Part 2. Energies 2023, 16, 2212. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic Biomass Pyrolysis: A Review of Product Properties and Effects of Pyrolysis Parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass Pyrolysis: A Review of the Process Development and Challenges from Initial Researches up to the Commercialisation Stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- ISO 15585:2006(E); Hard Coal—Determination of Caking Index. International Organization for Standardization: Geneva, Switzerland, 2006.
- Hu, W.; Wang, Q.; Zhao, X.; Yang, S.; Wu, H.; Zhang, S.; Sun, J. Relevance between Various Phenomena during Coking Coal Carbonization. Part 3: Understanding the Properties of the Plastic Layer during Coal Carbonization. Fuel 2021, 292, 120371. [Google Scholar] [CrossRef]
- Sciazko, M.; Mertas, B.; Stepien, L. Kinetic Modelling of Coking Coal Fluidity Development. J Therm Anal Calorim 2020, 142, 977–990. [Google Scholar] [CrossRef]
- Arenillas, A.; Rubiera, F.; Pevida, C.; Pis, J.J. A Comparison of Different Methods for Predicting Coal Devolatilisation Kinetics. J. Anal. Appl. Pyrolysis 2001, 58–59, 685–701. [Google Scholar] [CrossRef]
- Koveria, A.; Kieush, L.; Saik, P.; Lozynskyi, V. Metallurgical Coke Production with Biomass Additives. Part 2. Production and Characterization of Laboratory Biocokes. In Modern Technologies in Energy and Transport; Boichenko, S., Zaporozhets, A., Yakovlieva, A., Shkilniuk, I., Eds.; Studies in Systems, Decision and Control; Springer: Cham, Switzerland, 2024; Volume 510, pp. 287–306. ISBN 9783031443503. [Google Scholar]
- Nowak, M.A.; Paul, A.D.; Srivastava, R.D.; Radziwon, A. Coal Conversion. In Encyclopedia of Energy; Elsevier: Amsterdam, The Netherlands, 2004; pp. 425–434. ISBN 9780121764807. [Google Scholar]
- Jeong, H.M.; Seo, M.W.; Jeong, S.M.; Na, B.K.; Yoon, S.J.; Lee, J.G.; Lee, W.J. Pyrolysis Kinetics of Coking Coal Mixed with Biomass under Non-Isothermal and Isothermal Conditions. Bioresour. Technol. 2014, 155, 442–445. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, S.; Zhao, J.; Chen, L.; Meng, H. Synergistic Effect on Thermal Behavior during Co-Pyrolysis of Lignocellulosic Biomass Model Components Blend with Bituminous Coal. Bioresour. Technol. 2014, 169, 220–228. [Google Scholar] [CrossRef]
- Yuan, S.; Dai, Z.; Zhou, Z.; Chen, X.; Yu, G.; Wang, F. Rapid Co-Pyrolysis of Rice Straw and a Bituminous Coal in a High-Frequency Furnace and Gasification of the Residual Char. Bioresour. Technol. 2012, 109, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Jiao, H.; Cai, J.; Wang, J.; Yang, Y.; Bridgwater, A.V. Co-Pyrolysis of Miscanthus Sacchariflorus and Coals: A Systematic Study on the Synergies in Thermal Decomposition, Kinetics and Vapour Phase Products. Fuel 2020, 262, 116603. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, A.; Zhao, W.; Yang, Z.; Li, H. Thermochemical Behaviors, Kinetics and Gas Emission Analyses during Co-Pyrolysis of Walnut Shell and Coal. Thermochim. Acta 2019, 673, 26–33. [Google Scholar] [CrossRef]
- Gohar, H.; Khoja, A.H.; Ansari, A.A.; Naqvi, S.R.; Liaquat, R.; Hassan, M.; Hasni, K.; Qazi, U.Y.; Ali, I. Investigating the Characterisation, Kinetic Mechanism, and Thermodynamic Behaviour of Coal-Biomass Blends in Co-Pyrolysis Process. Process Saf. Environ. Prot. 2022, 163, 645–658. [Google Scholar] [CrossRef]
- Bhattacharyya, M.; Shadangi, K.P.; Purkayastha, R.; Mahanta, P.; Mohanty, K. Co-Pyrolysis of Coal and Biomass Blends: Impact of Pyrolysis Temperature and Biomass Blending on Thermal Stability of Coal, and Composition of Pyrolysis Products. Process Saf. Environ. Prot. 2024, 187, 1010–1021. [Google Scholar] [CrossRef]
- Tauseef, M.; Ansari, A.A.; Khoja, A.H.; Naqvi, S.R.; Liaquat, R.; Nimmo, W.; Daood, S.S. Thermokinetics Synergistic Effects on Co-Pyrolysis of Coal and Rice Husk Blends for Bioenergy Production. Fuel 2022, 318, 123685. [Google Scholar] [CrossRef]
- Lu, K.-M.; Lee, W.-J.; Chen, W.-H.; Lin, T.-C. Thermogravimetric Analysis and Kinetics of Co-Pyrolysis of Raw/Torrefied Wood and Coal Blends. Appl. Energy 2013, 105, 57–65. [Google Scholar] [CrossRef]
- Vuthaluru, H.B. Thermal Behaviour of Coal/Biomass Blends during Co-Pyrolysis. Fuel Process. Technol. 2004, 85, 141–155. [Google Scholar] [CrossRef]
- Cao, Z.; Xu, Q.; Kang, H.; Shi, J.; Lu, X.; Chen, B.; Guo, L. Synergistic Interactions between Lignite and Biomass during Co-Pyrolysis from Volatile Release, Kinetics, and Char Structure. J. Energy Inst. 2024, 114, 101662. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the Catalytic Effects of Alkali and Alkaline Earth Metals (AAEMs) Including Sodium, Potassium, Calcium and Magnesium on the Pyrolysis of Lignocellulosic Biomass and on the Co-Pyrolysis of Coal with Biomass. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Luo, Z.; Zhu, X. Investigation on the Catalytic Behavior of Alkali Metals and Alkaline Earth Metals on the Biomass Pyrolysis Assisted with Real-Time Monitoring. Energy Fuels 2020, 34, 12654–12664. [Google Scholar] [CrossRef]
- De’Nobili, M.D.; Bernhardt, D.C.; Basanta, M.F.; Rojas, A.M. Sunflower (Helianthus Annuus L.) Seed Hull Waste: Composition, Antioxidant Activity, and Filler Performance in Pectin-Based Film Composites. Front. Nutr. 2021, 8, 777214. [Google Scholar] [CrossRef]
- Domingos, I.; Ferreira, J.; Cruz-Lopes, L.P.; Esteves, B. Liquefaction and Chemical Composition of Walnut Shells. Open Agric. 2022, 7, 249–256. [Google Scholar] [CrossRef]
- ISO 7404-2:2009; Methods for the Petrographic Analysis of Coals—Part 2: Methods of Preparing Coal Samples. ISO: Geneva, Switzerland, 2009.
- ISO 7404-5:2009; Methods for the Petrographic Analysis of Coals—Part 5: Method of Determining Microscopically the Reflectance of Vitrinite. ISO: Geneva, Switzerland, 2009.
- Kieush, L.; Koveria, A.; Schenk, J.; Rysbekov, K.; Lozynskyi, V.; Zheng, H.; Matayev, A. Investigation into the Effect of Multi-Component Coal Blends on Properties of Metallurgical Coke via Petrographic Analysis under Industrial Conditions. Sustainability 2022, 14, 9947. [Google Scholar] [CrossRef]
- ISO/DTS 4699; Hard Coal—Determination of Plastometric Indices—Manual Sapozhnikov Penetration Plastometer Method. International Organization for Standardization: Geneva, Switzerland, 2020.
- Koveria, A.; Kieush, L.; Usenko, A.; Sova, A. Study of Cellulose Additive Effect on the Caking Properties of Coal. Min. miner. depos. 2023, 17, 1–8. [Google Scholar] [CrossRef]
- Sommersacher, P.; Brunner, T.; Obernberger, I.; Kienzl, N.; Kanzian, W. Application of Novel and Advanced Fuel Characterization Tools for the Combustion Related Characterization of Different Wood/Kaolin and Straw/Kaolin Mixtures. Energy Fuels 2013, 27, 5192–5206. [Google Scholar] [CrossRef]
- DIN 51729-11:1998-11; Testing of Solid Fuels—Determination of Chemical Composition of Fuel Ash—PART 11: Determination by Inductively Coupled Plasma Emission Spectrometry (ICP-OES). German Institute for Standardisation: Berlin, Germany, 1998.
- El-Sayed, S.A.; Khass, T.M.; Mostafa, M.E. Thermal Degradation Behaviour and Chemical Kinetic Characteristics of Biomass Pyrolysis Using TG/DTG/DTA Techniques. Biomass Conv. Bioref. 2024, 14, 17779–17803. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Wei, R.; Zhang, L.; Cang, D.; Li, J.; Li, X.; Xu, C.C. Current Status and Potential of Biomass Utilization in Ferrous Metallurgical Industry. Renew. Sustain. Energy Rev. 2017, 68, 511–524. [Google Scholar] [CrossRef]
- ASTM D3172-13; Standard Practice for Proximate Analysis of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM E870-82(2019); Standard Test Methods for Analysis of Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2019.
- Manmeen, A.; Kongjan, P.; Palamanit, A.; Jariyaboon, R. Biochar and Pyrolysis Liquid Production from Durian Peel by Using Slow Pyrolysis Process: Regression Analysis, Characterization, and Economic Assessment. Ind. Crops Prod. 2023, 203, 117162. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Yeng, L.C.; Wahit, M.U.; Othman, N. Thermal and flexural properties of regenerated cellulose(rc)/poly(3-hydroxybutyrate)(phb)biocomposites. J. Teknol. 2015, 75, 107–112. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Santana, R.M.C.; Zattera, A.J. Materials Produced from Plant Biomass: Part II: Evaluation of Crystallinity and Degradation Kinetics of Cellulose. Mat. Res. 2012, 15, 421–427. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.-P. The Effect of the Biomass Components Lignin, Cellulose and Hemicellulose on TGA and Fixed Bed Pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Ye, Z.; Li, S.; Pan, Z.; Wang, J.; Hu, Z.-T.; Xu, C.C.; Hu, M. Towards Enhanced Understanding of the Synergistic Effects between Potassium and Calcium in Biomass Catalyzed Pyrolysis. J. Anal. Appl. Pyrolysis 2024, 184, 106848. [Google Scholar] [CrossRef]
- Liu, N.; Dou, C.; Yang, X.; Bai, B.; Zhu, S.; Tian, J.; Wang, Z.; Xu, L.; Shen, B. Effects of Pretreatment Procedure, Compositional Feature and Reaction Condition on the Devolatilization Characteristics of Biomass during Pyrolysis Process: A Review. J. Energy Inst. 2025, 118, 101943. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, L.; Xu, S. Effects of Feedstock on Co-Pyrolysis of Biomass and Coal in a Free-Fall Reactor. J. Fuel Chem. Technol. 2011, 39, 728–734. [Google Scholar] [CrossRef]
- Morgan, T.J.; Kandiyoti, R. Pyrolysis of Coals and Biomass: Analysis of Thermal Breakdown and Its Products. Chem. Rev. 2014, 114, 1547–1607. [Google Scholar] [CrossRef]
- Park, D.K.; Kim, S.D.; Lee, S.H.; Lee, J.G. Co-Pyrolysis Characteristics of Sawdust and Coal Blend in TGA and a Fixed Bed Reactor. Bioresour. Technol. 2010, 101, 6151–6156. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wang, J.; Qiao, Y.; Huang, H.; Xu, L.; Tian, Y. Understanding the Pyrolysis Synergy of Biomass and Coal Blends Based on Volatile Release, Kinetics and Char Structure. Biomass Bioenergy 2023, 168, 106687. [Google Scholar] [CrossRef]
- Merdun, H.; Laougé, Z.B. Kinetic and Thermodynamic Analyses during Co-Pyrolysis of Greenhouse Wastes and Coal by TGA. Renew. Energy 2021, 163, 453–464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).