Carbon-Based Nanomaterials in Water and Wastewater Treatment Processes
Abstract
1. Introduction
2. Carbon-Based Nanomaterials
2.1. Quality and Durability of Carbon-Based Nanomaterials—GO
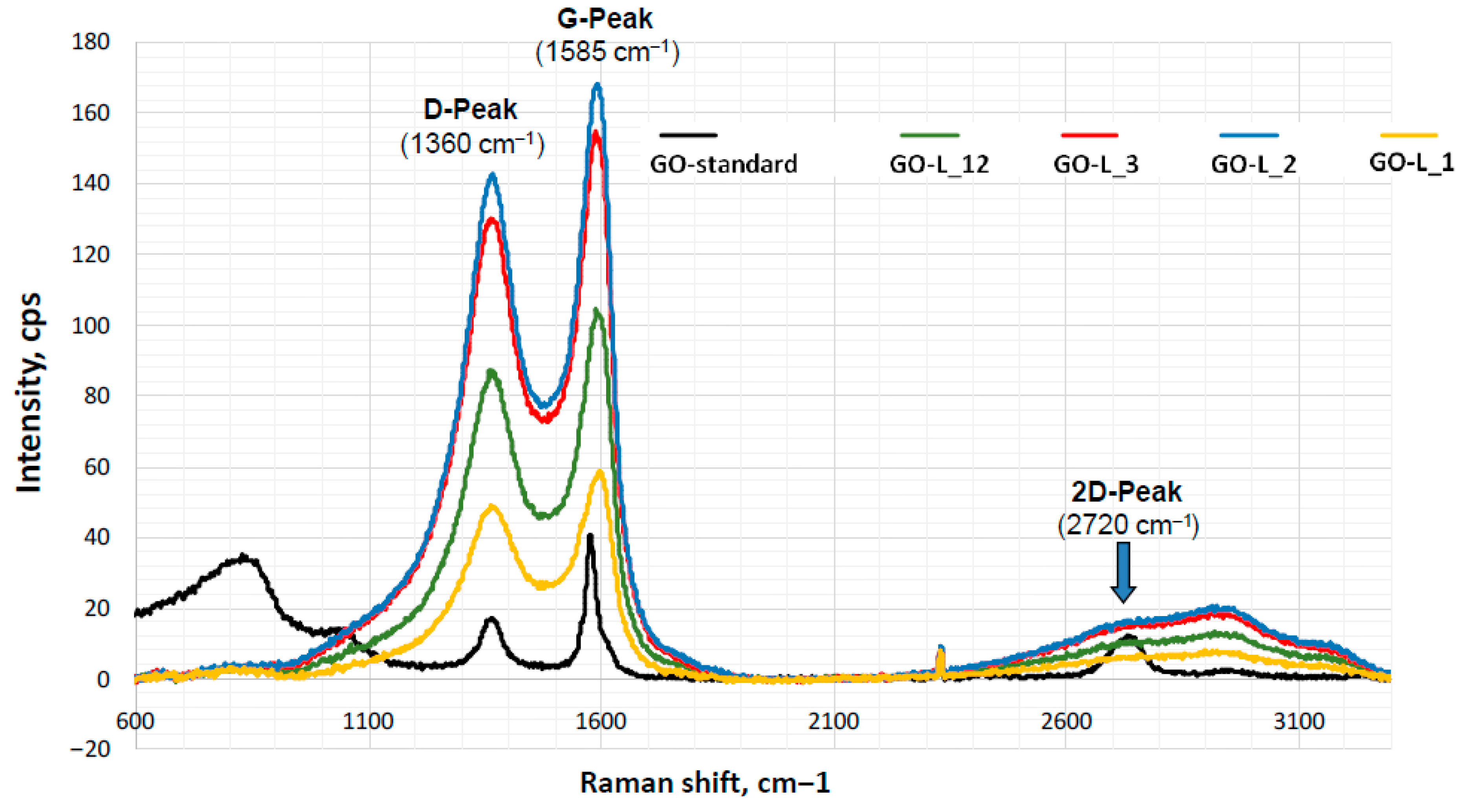
2.2. Preliminary Research on Changes in the Properties of Graphene Oxide During Its Storage (Aging) in the Form of an Aqueous Suspension or Solid Form After Freeze-Drying
3. Selected Applications of Carbon-Based Nanomaterials
- adsorption;
- disinfection;
- membrane processes;
- photocatalytic processes.
3.1. Adsorption
3.2. Disinfection
- direct impact on bacterial cell structures);
- penetration through the micro-organism’s cell membrane;
- oxidation of selected cellular components;
- hydroxyl radicals (as part of the reaction of NPs as photocatalysts;
- production of dissolved metal ions that may contribute to the destruction of some cellular components.
3.3. Membrane Processes
- Membranes based on nonporous polymer and aligned CNTs, which make composite membranes permeable.
- Polymer-based membranes where blended CNTs modify the physicochemical characteristics of the composite membrane.
3.4. Catalytic Processes
4. Impact of CNMs on the Aquatic Environment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A Review of Removal of Pollutants from Water/Wastewater Using Different Types of Nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 825910. [Google Scholar] [CrossRef]
- Smith, S.C.; Rodrigues, D.F. Carbon-Based Nanomaterials for Removal of Chemical and Biological Contaminants from Water: A Review of Mechanisms and Applications. Carbon 2015, 91, 122–143. [Google Scholar] [CrossRef]
- Jain, K.; Patel, A.S.; Pardhi, V.P.; Flora, S.J.S. Nanotechnology in Wastewater Management: A New Paradigm Towards Wastewater Treatment. Molecules 2021, 26, 1797. [Google Scholar] [CrossRef]
- Barrejón, M.; Prato, M. Carbon Nanotube Membranes in Water Treatment Applications. Adv. Mater. Interfaces 2022, 9, 2101260. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered Nanomaterials for Water Treatment and Remediation: Costs, Benefits, and Applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Sadegh, H.; Ali, G.A.M.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-ghoshekandi, R.; Nadagouda, M.N.; Sillanpää, M.; Megiel, E. The Role of Nanomaterials as Effective Adsorbents and Their Applications in Wastewater Treatment. J. Nanostructure Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Kumar, S.; Ahlawat, W.; Bhanjana, G.; Heydarifard, S.; Nazhad, M.M.; Dilbaghi, N. Nanotechnology-Based Water Treatment Strategies. J. Nanosci. Nanotechnol. 2014, 14, 1838–1858. [Google Scholar] [CrossRef]
- Soni, R.; Pal, A.K.; Tripathi, P.; Lal, J.A.; Kesari, K.; Tripathi, V. An Overview of Nanoscale Materials on the Removal of Wastewater Contaminants. Appl. Water Sci. 2020, 10, 189. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon Nanomaterials: Production, Impact on Plant Development, Agricultural and Environmental Applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef]
- Freixa, A.; Acuña, V.; Sanchís, J.; Farré, M.; Barceló, D.; Sabater, S. Ecotoxicological Effects of Carbon Based Nanomaterials in Aquatic Organisms. Sci. Total Environ. 2018, 619–620, 328–337. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Singh, A. Adsorptive Removal of Heavy Metals, Dyes, and Pharmaceuticals: Carbon-Based Nanomaterials in Focus. Carbon 2024, 217, 118621. [Google Scholar] [CrossRef]
- Li, Z.; Gao, B.; Chen, G.Z.; Mokaya, R.; Sotiropoulos, S.; Li Puma, G. Carbon Nanotube/Titanium Dioxide (CNT/TiO2) Core–Shell Nanocomposites with Tailored Shell Thickness, CNT Content and Photocatalytic/Photoelectrocatalytic Properties. Appl. Catal. B Environ. 2011, 110, 50–57. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Removal of Methylene Blue from Aqueous Solution with Self-Assembled Cylindrical Graphene-Carbon Nanotube Hybrid. Chem. Eng. J. 2012, 192, 156–163. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhao, L.; Wu, W.; Lu, G.; Xu, F.; Tong, Y.; Liu, W.; Du, J. Enhanced Adsorption of Malachite Green onto Carbon Nanotube/Polyaniline Composites. J. Appl. Polym. Sci. 2013, 127, 2475–2482. [Google Scholar] [CrossRef]
- Ma, L.; Chen, A.; Lu, J.; Zhang, Z.; He, H.; Li, C. In Situ Synthesis of CNTs/Fe-Ni/TiO2 Nanocomposite by Fluidized Bed Chemical Vapor Deposition and the Synergistic Effect in Photocatalysis. Particuology 2014, 14, 24–32. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, C.; Dichiara, A.B.; Jiang, W.; Gu, J. Cellulose Nanofibril/Carbon Nanomaterial Hybrid Aerogels for Adsorption Removal of Cationic and Anionic Organic Dyes. Nanomaterials 2020, 10, 169. [Google Scholar] [CrossRef]
- Li, Z.; Kang, W.; Han, Z.; Yan, J.; Cheng, B.; Liu, Y. Hierarchical MnOx@PVDF/MWCNTs Tree-like Nanofiber Membrane with High Catalytic Oxidation Activity. J. Alloys Compd. 2019, 780, 805–815. [Google Scholar] [CrossRef]
- Hintsho, N.; Petrik, L.; Nechaev, A.; Titinchi, S.; Ndungu, P. Photo-Catalytic Activity of Titanium Dioxide Carbon Nanotube Nano-Composites Modified with Silver and Palladium Nanoparticles. Appl. Catal. B Environ. 2014, 156–157, 273–283. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Joneidi-Yekta, H.; Mohseni, M. Adsorption of Anionic and Cationic Dyes from Aqueous Solution Using Gelatin-Based Magnetic Nanocomposite Beads Comprising Carboxylic Acid Functionalized Carbon Nanotube. Chem. Eng. J. 2017, 308, 1133–1144. [Google Scholar] [CrossRef]
- Anku, W.W.; Oppong, S.O.-B.; Shukla, S.K.; Agorku, E.S.; Govender, P.P. Palladium-Doped-ZrO2-Multiwalled Carbon Nanotubes Nanocomposite: An Advanced Photocatalyst for Water Treatment. Appl. Phys. A 2016, 122, 579. [Google Scholar] [CrossRef]
- Fugetsu, B.; Satoh, S.; Shiba, T.; Mizutani, T.; Lin, Y.-B.; Terui, N.; Nodasaka, Y.; Sasa, K.; Shimizu, K.; Akasaka, T.; et al. Caged Multiwalled Carbon Nanotubes as the Adsorbents for Affinity-Based Elimination of Ionic Dyes. Environ. Sci. Technol. 2004, 38, 6890–6896. [Google Scholar] [CrossRef]
- Jung, G.; Kim, H. Synthesis and Photocatalytic Performance of PVA/TiO2/graphene-MWCNT Nanocomposites for Dye Removal. J. Appl. Polym. Sci. 2014, 131, app.40715. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Jiang, R.; Xiao, L.; Zeng, G.M. Preparation, Characterization, Adsorption Kinetics and Thermodynamics of Novel Magnetic Chitosan Enwrapping Nanosized γ-Fe2O3 and Multi-Walled Carbon Nanotubes with Enhanced Adsorption Properties for Methyl Orange. Bioresour. Technol. 2010, 101, 5063–5069. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of Methylene Blue from Aqueous Solution by Graphene. Colloids Surf. B Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, X.; Yang, J.; Shan, X.; Yang, L.; Zhang, Y.; Li, X.; Gao, M. ZnO-Graphene Composite for Photocatalytic Degradation of Methylene Blue Dye. Catal. Commun. 2012, 29, 29–34. [Google Scholar] [CrossRef]
- Lei, P.; Zhou, Y.; Dong, C.; Liu, Y.; Shuang, S. CoNi-MOF-Graphene Magnetic Nanocomposites for the Electrocatalytic Detection of Glucose and the Efficient Removal of Organic Dyes. ACS Appl. Nano Mater. 2023, 6, 9369–9375. [Google Scholar] [CrossRef]
- Nguyen, D.C.T.; Oh, W.-C. Ternary Self-Assembly Method of Mesoporous Silica and Cu2O Combined Graphene Composite by Nonionic Surfactant and Photocatalytic Degradation of Cationic-Anionic Dye Pollutants. Sep. Purif. Technol. 2018, 190, 77–89. [Google Scholar] [CrossRef]
- Ma, J.; Sun, Y.; Zhang, M.; Yang, M.; Gong, X.; Yu, F.; Zheng, J. Comparative Study of Graphene Hydrogels and Aerogels Reveals the Important Role of Buried Water in Pollutant Adsorption. Environ. Sci. Technol. 2017, 51, 12283–12292. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wei, J.; Lv, S.; Guo, Z.; Jiang, F. Removal of Organic Dyes in Environmental Water onto Magnetic-Sulfonic Graphene Nanocomposite. CLEAN Soil Air Water 2013, 41, 992–1001. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, B. Sulfonated Graphene Nanosheets as a Superb Adsorbent for Various Environmental Pollutants in Water. Environ. Sci. Technol. 2015, 49, 7364–7372. [Google Scholar] [CrossRef]
- Khan Rind, I.; Sarı, A.; Tuzen, M.; Lanjwani, M.F.; Saleh, T.A. Synthesis of Graphene/Silica Composites and Its Removal Efficiency of Methylene Blue Dye from Water. Inorg. Chem. Commun. 2023, 158, 111507. [Google Scholar] [CrossRef]
- Bu, J.; Yuan, L.; Zhang, N.; Liu, D.; Meng, Y.; Peng, X. High-Efficiency Adsorption of Methylene Blue Dye from Wastewater by a Thiosemicarbazide Functionalized Graphene Oxide Composite. Diam. Relat. Mater. 2020, 101, 107604. [Google Scholar] [CrossRef]
- Vo, T.S.; Vo, T.T.B.C.; Suk, J.W.; Kim, K. Recycling Performance of Graphene Oxide-Chitosan Hybrid Hydrogels for Removal of Cationic and Anionic Dyes. Nano Converg. 2020, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Liu, Y.; Feng, J. Low-Density, Mechanical Compressible, Water-Induced Self-Recoverable Graphene Aerogels for Water Treatment. ACS Appl. Mater. Interfaces 2017, 9, 22456–22464. [Google Scholar] [CrossRef]
- Chen, H.; Liu, T.; Meng, Y.; Cheng, Y.; Lu, J.; Wang, H. Novel Graphene Oxide/Aminated Lignin Aerogels for Enhanced Adsorption of Malachite Green in Wastewater. Colloids Surf. Physicochem. Eng. Asp. 2020, 603, 125281. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Mengting, Z.; Fu, D.; Yeap, S.K.; Othman, M.H.D.; Avtar, R.; Ouyang, T. Functionalizing TiO2 with Graphene Oxide for Enhancing Photocatalytic Degradation of Methylene Blue (MB) in Contaminated Wastewater. J. Environ. Manag. 2020, 270, 110871. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, H.; She, X.; Wang, T.; She, F.; Kong, L. Selective Removal of Anionic Dyes Using Poly(N,N-Dimethyl Amino Ethylmethacrylate) Functionalized Graphene Oxide. RSC Adv. 2016, 6, 67242–67251. [Google Scholar] [CrossRef]
- Kharismadewi, D.; Haldorai, Y.; Nguyen, V.H.; Tuma, D.; Shim, J.-J. Synthesis of Graphene Oxide-Poly(2-Hydroxyethyl Methacrylate) Composite by Dispersion Polymerization in Supercritical CO2: Adsorption Behavior for the Removal of Organic Dye. Compos. Interfaces 2016, 23, 719–739. [Google Scholar] [CrossRef]
- Sheng, G.; Zhu, S.; Wang, S.; Wang, Z. Removal of Dyes by a Novel Fly Ash-Chitosan-Graphene Oxide Composite Adsorbent. RSC Adv. 2016, 6, 17987–17994. [Google Scholar] [CrossRef]
- Katubi, K.M.M.; Alsaiari, N.S.; Alzahrani, F.M.; Siddeeg, S.M.; Tahoon, M.A. Synthesis of Manganese Ferrite/Graphene Oxide Magnetic Nanocomposite for Pollutants Removal from Water. Processes 2021, 9, 589. [Google Scholar] [CrossRef]
- Moon, Y.-E.; Jung, G.; Yun, J.; Kim, H.-I. Poly(Vinyl Alcohol)/Poly(Acrylic Acid)/TiO2/Graphene Oxide Nanocomposite Hydrogels for pH-Sensitive Photocatalytic Degradation of Organic Pollutants. Mater. Sci. Eng. B 2013, 178, 1097–1103. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, P.; Wang, C.; Sun, H. Highly Efficient Removal of Ionic Dyes in Aqueous Solutions Using Magnetic 3D Reduced Graphene Oxide Aerogel Supported Nano Zero-Valent Iron. Environ. Eng. Res. 2023, 29, 230149. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lu, J.-S.; Yang, S.-W. Highly Visible-Light-Responsive Cu2O/rGO Decorated with Fe3O4@SiO2 Nanoparticles as a Magnetically Recyclable Photocatalyst. Nanotechnology 2018, 29, 305606. [Google Scholar] [CrossRef]
- Ye, J.; Dai, J.; Yang, D.; Li, C.; Yan, Y.; Wang, Y. 2D/2D Confinement Graphene-Supported Bimetallic Sulfides/g-C3N4 Composites with Abundant Sulfur Vacancies as Highly Active Catalytic Self-Cleaning Membranes for Organic Contaminants Degradation. Chem. Eng. J. 2021, 418, 129383. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, W.; Chen, M.; Ma, L.; Yang, B.; Liang, Q.; Chen, Y. A Mussel-Induced Method to Fabricate Reduced Graphene Oxide/Halloysite Nanotubes Membranes for Multifunctional Applications in Water Purification and Oil/Water Separation. Chem. Eng. J. 2018, 336, 263–277. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Yang, K.; Li, M.; Luan, X.; Sun, Y.; Wang, H.; Sun, Q.; Tang, K.; Zheng, H.; et al. Adsorption of Methylene Blue by Nicandra physaloides (L.) Gaertn Seed Gum/Graphene Oxide Aerogel. Environ. Technol. 2022, 43, 2342–2351. [Google Scholar] [CrossRef]
- Stafiej, A.; Pyrzynska, K. Adsorption of Heavy Metal Ions with Carbon Nanotubes. Sep. Purif. Technol. 2007, 58, 49–52. [Google Scholar] [CrossRef]
- Rosenzweig, S.; Sorial, G.A.; Sahle-Demessie, E.; McAvoy, D.C. Optimizing the Physical-Chemical Properties of Carbon Nanotubes (CNT) and Graphene Nanoplatelets (GNP) on Cu(II) Adsorption. J. Hazard. Mater. 2014, 279, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Anitha, K.; Namsani, S.; Singh, J.K. Removal of Heavy Metal Ions Using a Functionalized Single-Walled Carbon Nanotube: A Molecular Dynamics Study. J. Phys. Chem. A 2015, 119, 8349–8358. [Google Scholar] [CrossRef]
- Alagappan, P.N.; Heimann, J.; Morrow, L.; Andreoli, E.; Barron, A.R. Easily Regenerated Readily Deployable Absorbent for Heavy Metal Removal from Contaminated Water. Sci. Rep. 2017, 7, 6682. [Google Scholar] [CrossRef]
- Moghaddam, H.K.; Pakizeh, M. Experimental Study on Mercury Ions Removal from Aqueous Solution by MnO2/CNTs Nanocomposite Adsorbent. J. Ind. Eng. Chem. 2015, 21, 221–229. [Google Scholar] [CrossRef]
- Wang, S.; Gong, W.; Liu, X.; Yao, Y.; Gao, B.; Yue, Q. Removal of Lead(II) from Aqueous Solution by Adsorption onto Manganese Oxide-Coated Carbon Nanotubes. Sep. Purif. Technol. 2007, 58, 17–23. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; McKay, G. Super High Removal Capacities of Heavy Metals (Pb2+ and Cu2+ ) Using CNT Dendrimer. J. Hazard. Mater. 2017, 336, 146–157. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, X.; Wu, Q.; Han, J.; Jiang, J. Assembly of Polyacrylamide-Sodium Alginate-Based Organic-Inorganic Hydrogel with Mechanical and Adsorption Properties. Polymers 2019, 11, 1239. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Adsorption of Divalent Heavy Metal Ions from Water Using Carbon Nanotube Sheets. J. Hazard. Mater. 2011, 185, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, N.M.; Reta, N.; Dalal, H.; Ellis, A.V.; Shapter, J.; Voelcker, N.H. Enhanced Adsorption of Mercury Ions on Thiol Derivatized Single Wall Carbon Nanotubes. J. Hazard. Mater. 2013, 261, 534–541. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Shao, D.; Hu, J.; Wang, X. Adsorption of Ni(II) on Oxidized Multi-Walled Carbon Nanotubes: Effect of Contact Time, pH, Foreign Ions and PAA. J. Hazard. Mater. 2009, 166, 109–116. [Google Scholar] [CrossRef]
- Tang, W.-W.; Zeng, G.-M.; Gong, J.-L.; Liu, Y.; Wang, X.-Y.; Liu, Y.-Y.; Liu, Z.-F.; Chen, L.; Zhang, X.-R.; Tu, D.-Z. Simultaneous Adsorption of Atrazine and Cu(II) from Wastewater by Magnetic Multi-Walled Carbon Nanotube. Chem. Eng. J. 2012, 211–212, 470–478. [Google Scholar] [CrossRef]
- Ji, L.; Zhou, L.; Bai, X.; Shao, Y.; Zhao, G.; Qu, Y.; Wang, C.; Li, Y. Facile Synthesis of Multiwall Carbon Nanotubes/Iron Oxides for Removal of Tetrabromobisphenol A and Pb(Ii). J. Mater. Chem. 2012, 22, 15853. [Google Scholar] [CrossRef]
- Xu, D.; Tan, X.; Chen, C.; Wang, X. Removal of Pb(II) from Aqueous Solution by Oxidized Multiwalled Carbon Nanotubes. J. Hazard. Mater. 2008, 154, 407–416. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Liu, M.; Chen, X.; Li, Y.; Yang, Y. Mechanistic Insight into the Simultaneous Removal of Cr(VI) and Phosphate by a Novel Versatile Bimetallic Material. J. Environ. Chem. Eng. 2024, 12, 114446. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, C.; Yu, J.; Jiang, X. Adsorption Properties of a Novel 3D Graphene/MgO Composite for Heavy Metal Ions. J. Cent. South Univ. 2019, 26, 813–823. [Google Scholar] [CrossRef]
- Hao, L.; Song, H.; Zhang, L.; Wan, X.; Tang, Y.; Lv, Y. SiO2/Graphene Composite for Highly Selective Adsorption of Pb(II) Ion. J. Colloid Interface Sci. 2012, 369, 381–387. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Zheng, X.; Lv, W.; Wang, M.; Yang, Q.-H.; Kang, F. Adsorption of Lead(II) Ions from Aqueous Solution on Low-Temperature Exfoliated Graphene Nanosheets. Langmuir 2011, 27, 7558–7562. [Google Scholar] [CrossRef]
- Babaei, M.; Azar, P.A.; Tehrani, M.S.; Farjaminezhad, M.; Hussain, S.W. Green and Simple Synthesized Graphene/MnO2 Quantum Dot Nanocomposite: Characterization and Application as an Efficient Adsorbent for Solid-Phase Extraction of Heavy Metals. J. Nanostructure Chem. 2022, 12, 249–261. [Google Scholar] [CrossRef]
- Fang, F.; Kong, L.; Huang, J.; Wu, S.; Zhang, K.; Wang, X.; Sun, B.; Jin, Z.; Wang, J.; Huang, X.-J.; et al. Removal of Cobalt Ions from Aqueous Solution by an Amination Graphene Oxide Nanocomposite. J. Hazard. Mater. 2014, 270, 1–10. [Google Scholar] [CrossRef]
- Zare-Dorabei, R.; Ferdowsi, S.M.; Barzin, A.; Tadjarodi, A. Highly Efficient Simultaneous Ultrasonic-Assisted Adsorption of Pb(II), Cd(II), Ni(II) and Cu (II) Ions from Aqueous Solutions by Graphene Oxide Modified with 2,2′-Dipyridylamine: Central Composite Design Optimization. Ultrason. Sonochem. 2016, 32, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, L.; Ma, J.; Tian, Y. Preparation of Graphene Oxide/Chitosan Complex and Its Adsorption Properties for Heavy Metal Ions. Green Process. Synth. 2020, 9, 294–303. [Google Scholar] [CrossRef]
- Zhao, G.; Ren, X.; Gao, X.; Tan, X.; Li, J.; Chen, C.; Huang, Y.; Wang, X. Removal of Pb(Ii) Ions from Aqueous Solutions on Few-Layered Graphene Oxide Nanosheets. Dalton Trans. 2011, 40, 10945. [Google Scholar] [CrossRef]
- Mi, X.; Huang, G.; Xie, W.; Wang, W.; Liu, Y.; Gao, J. Preparation of Graphene Oxide Aerogel and Its Adsorption for Cu2+ Ions. Carbon 2012, 50, 4856–4864. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Moazen Ferdowsi, S.; Zare-Dorabei, R.; Barzin, A. Highly Efficient Ultrasonic-Assisted Removal of Hg(II) Ions on Graphene Oxide Modified with 2-Pyridinecarboxaldehyde Thiosemicarbazone: Adsorption Isotherms and Kinetics Studies. Ultrason. Sonochem. 2016, 33, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.; Basu, T.; Debnath, S.; Ghosh, A.K.; De, A.; Ghosh, U.C. Mechanistic Insight for the Sorption of Cd(II) and Cu(II) from Aqueous Solution on Magnetic Mn-Doped Fe(III) Oxide Nanoparticle Implanted Graphene. J. Chem. Eng. Data 2013, 58, 2809–2818. [Google Scholar] [CrossRef]
- Fang, Q.; Zhou, X.; Deng, W.; Liu, Z. Hydroxyl-Containing Organic Molecule Induced Self-Assembly of Porous Graphene Monoliths with High Structural Stability and Recycle Performance for Heavy Metal Removal. Chem. Eng. J. 2017, 308, 1001–1009. [Google Scholar] [CrossRef]
- Li, L.; Fan, L.; Sun, M.; Qiu, H.; Li, X.; Duan, H.; Luo, C. Adsorbent for Chromium Removal Based on Graphene Oxide Functionalized with Magnetic Cyclodextrin–Chitosan. Colloids Surf. B Biointerfaces 2013, 107, 76–83. [Google Scholar] [CrossRef]
- Li, C.; Yan, Y.; Zhang, Q.; Zhang, Z.; Huang, L.; Zhang, J.; Xiong, Y.; Tan, S. Adsorption of Cd2+ and Ni2+ from Aqueous Single-Metal Solutions on Graphene Oxide-Chitosan-Poly(Vinyl Alcohol) Hydrogels. Langmuir 2019, 35, 4481–4490. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Chai, L.; Li, M. Effects of Single- and Multi-Organic Acid Ligands on Adsorption of Copper by Fe3O4/Graphene Oxide-Supported DCTA. J. Colloid Interface Sci. 2016, 478, 288–295. [Google Scholar] [CrossRef]
- Vilela, D.; Parmar, J.; Zeng, Y.; Zhao, Y.; Sánchez, S. Graphene-Based Microbots for Toxic Heavy Metal Removal and Recovery from Water. Nano Lett. 2016, 16, 2860–2866. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Luo, C.; Wang, X.; Duan, H. Removal of Pb2+ from Water Environment Using a Novel Magnetic Chitosan/Graphene Oxide Imprinted Pb2+. Int. J. Biol. Macromol. 2016, 86, 505–511. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Y.; Zeng, G.; Wang, H.; You, S.; Hu, X.; Tan, X.; Chen, A.; Guo, F. Effects of Inorganic Electrolyte Anions on Enrichment of Cu(II) Ions with Aminated Fe3O4/Graphene Oxide: Cu(II) Speciation Prediction and Surface Charge Measurement. Chemosphere 2015, 127, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.K.; Lim, B.S.; Yoon, Y.; Kwag, S.H.; Park, W.K.; Song, Y.H.; Yang, W.S.; Ahn, Y.-T.; Kang, J.-W.; Yoon, D.H. Efficient Removal of Arsenic by Strategically Designed and Layer-by-Layer Assembled PS@+rGO@GO@Fe3O4 Composites. J. Environ. Manag. 2017, 201, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Al-Yaari, M.; Saleh, T.A. Removal of Lead from Wastewater Using Synthesized Polyethyleneimine-Grafted Graphene Oxide. Nanomaterials 2023, 13, 1078. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ding, L.; Wu, X.; Deng, F.; Kang, R.; Luo, X. Enhancing the Hg(II) Removal Efficiency from Real Wastewater by Novel Thymine-Grafted Reduced Graphene Oxide Complexes. Ind. Eng. Chem. Res. 2016, 55, 6845–6853. [Google Scholar] [CrossRef]
- Chen, J.H.; Xing, H.T.; Sun, X.; Su, Z.B.; Huang, Y.H.; Weng, W.; Hu, S.R.; Guo, H.X.; Wu, W.B.; He, Y.S. Highly Effective Removal of Cu(II) by Triethylenetetramine-Magnetic Reduced Graphene Oxide Composite. Appl. Surf. Sci. 2015, 356, 355–363. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Dąbek, L.; Świątkowski, A. The Use of Modified Multi-Walled Carbon Nanotubes for the Removal of Selected Pharmaceuticals from the Aqueous Environment. Desalination Water Treat. 2023, 288, 60–71. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xu, W.; Cui, M.; Wang, M.; Chen, B.; Sun, Y.; Chen, K.; Li, L.; Du, Q.; et al. Filtration and Adsorption of Tetracycline in Aqueous Solution by Copper Alginate-Carbon Nanotubes Membrane Which Has the Muscle-Skeleton Structure. Chem. Eng. Res. Des. 2022, 183, 424–438. [Google Scholar] [CrossRef]
- Ma, Q.; Chu, Y.; Ni, X.; Zhang, J.; Chen, H.; Xu, F.; Wang, Y. CeO2 Modified Carbon Nanotube Electrified Membrane for the Removal of Antibiotics. Chemosphere 2023, 310, 136771. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Deng, S.; Huang, Q.; Nie, Y.; Wang, B.; Huang, J.; Yu, G. Regenerable Granular Carbon Nanotubes/Alumina Hybrid Adsorbents for Diclofenac Sodium and Carbamazepine Removal from Aqueous Solution. Water Res. 2013, 47, 4139–4147. [Google Scholar] [CrossRef]
- Yanyan, L.; Kurniawan, T.A.; Albadarin, A.B.; Walker, G. Enhanced Removal of Acetaminophen from Synthetic Wastewater Using Multi-Walled Carbon Nanotubes (MWCNTs) Chemically Modified with NaOH, HNO3/H2SO4, Ozone, and/or Chitosan. J. Mol. Liq. 2018, 251, 369–377. [Google Scholar] [CrossRef]
- Cai, N.; Larese-Casanova, P. Sorption of Carbamazepine by Commercial Graphene Oxides: A Comparative Study with Granular Activated Carbon and Multiwalled Carbon Nanotubes. J. Colloid Interface Sci. 2014, 426, 152–161. [Google Scholar] [CrossRef]
- Barrios-Bermúdez, N.; González-Avendaño, M.; Lado-Touriño, I.; Cerpa-Naranjo, A.; Rojas-Cervantes, M. Fe-Cu Doped Multiwalled Carbon Nanotubes for Fenton-like Degradation of Paracetamol Under Mild Conditions. Nanomaterials 2020, 10, 749. [Google Scholar] [CrossRef]
- Rosli, F.A.; Ahmad, H.; Jumbri, K.; Abdullah, A.H.; Kamaruzaman, S.; Fathihah Abdullah, N.A. Efficient Removal of Pharmaceuticals from Water Using Graphene Nanoplatelets as Adsorbent. R. Soc. Open Sci. 2021, 8, 201076. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and Removal of Tetracycline Antibiotics from Aqueous Solution by Graphene Oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Li, W.; Yao, B.; Zheng, Y.; Zhang, G.; Zhi, D.; Zhou, Y. Efficient Degradation of Chlortetracycline by Graphene Supported Cobalt Oxide Activated Peroxydisulfate: Performances and Mechanisms. Processes 2023, 11, 1381. [Google Scholar] [CrossRef]
- Needham, E.M.; Sidney, S.M.; Chimka, J.R.; Fairey, J.L. Trihalomethane, Dihaloacetonitrile, and Total N-Nitrosamine Precursor Adsorption by Carbon Nanotubes: The Importance of Surface Oxides and Pore Volume. Environ. Sci. Water Res. Technol. 2016, 2, 1004–1013. [Google Scholar] [CrossRef]
- Bai, H.; Zan, X.; Zhang, L.; Sun, D.D. Multi-Functional CNT/ZnO/TiO2 Nanocomposite Membrane for Concurrent Filtration and Photocatalytic Degradation. Sep. Purif. Technol. 2015, 156, 922–930. [Google Scholar] [CrossRef]
- Fagan, S.B.; Girão, E.C.; Filho, J.M.; Filho, A.G.S. First Principles Study of 1,2-dichlorobenzene Adsorption on Metallic Carbon Nanotubes. Int. J. Quantum Chem. 2006, 106, 2558–2563. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J. The Preparation of Novel Polyvinyl Alcohol (PVA)-Based Nanoparticle/Carbon Nanotubes (PNP/CNTs) Aerogel for Solvents Adsorption Application. J. Colloid Interface Sci. 2020, 569, 254–266. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Mahvi, A.H.; Rastkari, N.; Saeedi, R.; Nazmara, S.; Iravani, E. Adsorption of Bisphenol A (BPA) from Aqueous Solutions by Carbon Nanotubes: Kinetic and Equilibrium Studies. Desalination Water Treat. 2015, 54, 84–92. [Google Scholar] [CrossRef]
- Gotovac, S.; Honda, H.; Hattori, Y.; Takahashi, K.; Kanoh, H.; Kaneko, K. Effect of Nanoscale Curvature of Single-Walled Carbon Nanotubes on Adsorption of Polycyclic Aromatic Hydrocarbons. Nano Lett. 2007, 7, 583–587. [Google Scholar] [CrossRef]
- Al-Khateeb, I.; Mohammed, A.M.; Haider, A.J.; Al-Douri, Y. Removal of Benzene from Aqueous Solution Using Carbon Nanotube Synthesized from Fuel Oil Waste. Adv. Mater. Res. 2014, 925, 105–109. [Google Scholar] [CrossRef]
- Saleh, T.A.; Gondal, M.A.; Drmosh, Q.A.; Yamani, Z.H.; AL-yamani, A. Enhancement in Photocatalytic Activity for Acetaldehyde Removal by Embedding ZnO Nano Particles on Multiwall Carbon Nanotubes. Chem. Eng. J. 2011, 166, 407–412. [Google Scholar] [CrossRef]
- Guo, X.; Huang, Y.; Yu, W.; Yu, X.; Han, X.; Zhai, H. Multi-Walled Carbon Nanotubes Modified with Iron Oxide and Manganese Dioxide (MWCNTs-Fe3O4-MnO2) as a Novel Adsorbent for the Determination of BPA. Microchem. J. 2020, 157, 104867. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Chi, W.; Wang, Y.; Yue, X.; Huang, Q.; Yu, C. Catalytic Degradation of Phenol and P-Nitrophenol Using Fe3O4/MWCNT Nanocomposites as Heterogeneous Fenton-Like Catalyst. Water. Air. Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Li, S.; Guo, S.; Yang, H.; Gou, G.; Ren, R.; Li, J.; Dong, Z.; Jin, J.; Ma, J. Enhancing Catalytic Performance of Au Catalysts by Noncovalent Functionalized Graphene Using Functional Ionic Liquids. J. Hazard. Mater. 2014, 270, 11–17. [Google Scholar] [CrossRef]
- Maliyekkal, S.M.; Sreeprasad, T.S.; Krishnan, D.; Kouser, S.; Mishra, A.K.; Waghmare, U.V.; Pradeep, T. Graphene: A Reusable Substrate for Unprecedented Adsorption of Pesticides. Small 2013, 9, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Vellaichamy, B.; Periakaruppan, P. Silver Nanoparticle-Embedded RGO-Nanosponge for Superior Catalytic Activity towards 4-Nitrophenol Reduction. RSC Adv. 2016, 6, 88837–88845. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; Rhadfi, T.; Mckay, G.; Al-marri, M.; Abdala, A.; Hilal, N.; Hussien, M.A. Enhancing Oil Removal from Water Using Ferric Oxide Nanoparticles Doped Carbon Nanotubes Adsorbents. Chem. Eng. J. 2016, 293, 90–101. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, P.; Zhang, L.; Lu, W.; Zhang, G.; Huang, Y.; Zhang, J.; Chen, T. Construction of Superhydrophilic and Under-Water Superoleophobic Carbon-Based Membranes for Water Purification. RSC Adv. 2016, 6, 73399–73403. [Google Scholar] [CrossRef]
- Won, G.; Kim, S.; Chang, Y.J.; Go, Y.; Kim, S.W. Magnetic Carbon Nanotube Sorbents with Macropores Formed by Salts for Oily Wastewater Treatment. J. Ind. Eng. Chem. 2025, 142, 463–475. [Google Scholar] [CrossRef]
- Huang, J.; Yan, Z. Adsorption Mechanism of Oil by Resilient Graphene Aerogels from Oil-Water Emulsion. Langmuir 2018, 34, 1890–1898. [Google Scholar] [CrossRef]
- Hegab, H.M.; Elmekawy, A.; Aubry, C.; Kallem, P.; Wadi, V.S.; Banat, F.; Hasan, S.W. 3D Hierarchical Aquaporin-like Nanoporous Graphene Membrane with Engineered Tripartite Nanochannels for Efficient Oil/Water Separation. Npj Clean Water 2024, 7, 10. [Google Scholar] [CrossRef]
- Junaidi, N.F.D.; Othman, N.H.; Shahruddin, M.Z.; Alias, N.H.; Marpani, F.; Lau, W.J.; Ismail, A.F. Fabrication and Characterization of Graphene Oxide-Polyethersulfone (GO-PES) Composite Flat Sheet and Hollow Fiber Membranes for Oil-Water Separation. J. Chem. Technol. Biotechnol. 2020, 95, 1308–1320. [Google Scholar] [CrossRef]
- Zhan, Y.; He, S.; Wan, X.; Zhao, S.; Bai, Y. Thermally and Chemically Stable Poly(Arylene Ether Nitrile)/Halloysite Nanotubes Intercalated Graphene Oxide Nanofibrous Composite Membranes for Highly Efficient Oil/Water Emulsion Separation in Harsh Environment. J. Membr. Sci. 2018, 567, 76–88. [Google Scholar] [CrossRef]
- Rahman, M.M.; Balkhoyor, H.B.; Asiri, A.M. Phenolic Sensor Development Based on Chromium Oxide-Decorated Carbon Nanotubes for Environmental Safety. J. Environ. Manag. 2017, 188, 228–237. [Google Scholar] [CrossRef]
- Duong-Viet, C.; Liu, Y.; Ba, H.; Truong-Phuoc, L.; Baaziz, W.; Nguyen-Dinh, L.; Nhut, J.-M.; Pham-Huu, C. Carbon Nanotubes Containing Oxygenated Decorating Defects as Metal-Free Catalyst for Selective Oxidation of H2S. Appl. Catal. B Environ. 2016, 191, 29–41. [Google Scholar] [CrossRef]
- Jafari, A.; Mahvi, A.H.; Nasseri, S.; Rashidi, A.; Nabizadeh, R.; Rezaee, R. Ultrafiltration of Natural Organic Matter from Water by Vertically Aligned Carbon Nanotube Membrane. J. Environ. Health Sci. Eng. 2015, 13, 51. [Google Scholar] [CrossRef]
- Baek, Y.; Kim, C.; Seo, D.K.; Kim, T.; Lee, J.S.; Kim, Y.H.; Ahn, K.H.; Bae, S.S.; Lee, S.C.; Lim, J.; et al. High Performance and Antifouling Vertically Aligned Carbon Nanotube Membrane for Water Purification. J. Membr. Sci. 2014, 460, 171–177. [Google Scholar] [CrossRef]
- Shao, D.; Jiang, Z.; Wang, X.; Li, J.; Meng, Y. Plasma Induced Grafting Carboxymethyl Cellulose on Multiwalled Carbon Nanotubes for the Removal of UO2 2+ from Aqueous Solution. J. Phys. Chem. B 2009, 113, 860–864. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Astinchap, B.; Moradian, R. Enhancing Antifouling Capability of PES Membrane via Mixing with Various Types of Polymer Modified Multi-Walled Carbon Nanotube. J. Membr. Sci. 2013, 444, 184–191. [Google Scholar] [CrossRef]
- Lee, S.-H.; Pumprueg, S.; Moudgil, B.; Sigmund, W. Inactivation of Bacterial Endospores by Photocatalytic Nanocomposites. Colloids Surf. B Biointerfaces 2005, 40, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, D.; Chen, Y.; He, J.; Li, Q. Mechanistic Study of Highly Effective Phosphate Removal from Aqueous Solutions over a New Lanthanum Carbonate Fabricated Carbon Nanotube Film. J. Environ. Manag. 2024, 359, 120938. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Yin, H.; Liu, Z.; Luan, E.; Zhao, F.; Tang, Z.; Liu, S. Three-Dimensional Graphene/Pt Nanoparticle Composites as Freestanding Anode for Enhancing Performance of Microbial Fuel Cells. Sci. Adv. 2015, 1, e1500372. [Google Scholar] [CrossRef]
- Prince, J.A.; Bhuvana, S.; Anbharasi, V.; Ayyanar, N.; Boodhoo, K.V.K.; Singh, G. Ultra-Wetting Graphene-Based Membrane. J. Membr. Sci. 2016, 500, 76–85. [Google Scholar] [CrossRef]
- Musico, Y.L.F.; Santos, C.M.; Dalida, M.L.P.; Rodrigues, D.F. Surface Modification of Membrane Filters Using Graphene and Graphene Oxide-Based Nanomaterials for Bacterial Inactivation and Removal. ACS Sustain. Chem. Eng. 2014, 2, 1559–1565. [Google Scholar] [CrossRef]
- Pavagadhi, S.; Tang, A.L.L.; Sathishkumar, M.; Loh, K.P.; Balasubramanian, R. Removal of Microcystin-LR and Microcystin-RR by Graphene Oxide: Adsorption and Kinetic Experiments. Water Res. 2013, 47, 4621–4629. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Lu, J.; Wang, Y.; Li, G.; Zhao, F. Anti-Bacterial Properties of Ultrafiltration Membrane Modified by Graphene Oxide with Nano-Silver Particles. J. Colloid Interface Sci. 2016, 484, 107–115. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, D.; Qi, P. Synthesis and Characterization of Silver Nanoparticle and Graphene Oxide Nanosheet Composites as a Bactericidal Agent for Water Disinfection. J. Colloid Interface Sci. 2011, 360, 463–470. [Google Scholar] [CrossRef]
- Anand, A.; Unnikrishnan, B.; Mao, J.-Y.; Lin, H.-J.; Huang, C.-C. Graphene-Based Nanofiltration Membranes for Improving Salt Rejection, Water Flux and Antifouling—A Review. Desalination 2018, 429, 119–133. [Google Scholar] [CrossRef]
- Markovic, M.; Kumar, A.; Andjelkovic, I.; Lath, S.; Kirby, J.K.; Losic, D.; Batley, G.E.; McLaughlin, M.J. Ecotoxicology of Manufactured Graphene Oxide Nanomaterials and Derivation of Preliminary Guideline Values for Freshwater Environments. Environ. Toxicol. Chem. 2018, 37, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, B.; Xu, H.; Liu, H.; Wang, M.; He, Y.; Pan, B. Nanomaterials-Enabled Water and Wastewater Treatment. NanoImpact 2016, 3–4, 22–39. [Google Scholar] [CrossRef]
- Sarkar, B.; Mandal, S.; Tsang, Y.F.; Kumar, P.; Kim, K.-H.; Ok, Y.S. Designer Carbon Nanotubes for Contaminant Removal in Water and Wastewater: A Critical Review. Sci. Total Environ. 2018, 612, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Dwivedi, A.D.; Lee, W.-N.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.-H.; Fu, J. Application of Nanotechnologies for Removing Pharmaceutically Active Compounds from Water: Development and Future Trends. Environ. Sci. Nano 2018, 5, 27–47. [Google Scholar] [CrossRef]
- Cheriyamundath, S.; Vavilala, S.L. Nanotechnology-Based Wastewater Treatment. Water Environ. J. 2021, 35, 123–132. [Google Scholar] [CrossRef]
- Kumar, V.; Kim, K.-H.; Park, J.-W.; Hong, J.; Kumar, S. Graphene and Its Nanocomposites as a Platform for Environmental Applications. Chem. Eng. J. 2017, 315, 210–232. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive Removal of Antibiotics from Aqueous Solution Using Carbon Materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of Nanomaterials in Water Treatment Applications: A Review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Joshi, R.; Chughtai, A.A.; Macintyre, C.R. Graphene Modified Multifunctional Personal Protective Clothing. Adv. Mater. Interfaces 2019, 6, 1900622. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of Graphite Oxide and Reduced Graphene Oxide Obtained from Different Graphite Precursors and Oxidized by Different Methods Using Raman Spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef]
- Perera, D.; Abeywickrama, A.; Zen, F.; Colavita, P.E.; Jayasundara, D.R. Evolution of Oxygen Functionalities in Graphene Oxide and Its Impact on Structure and Exfoliation: An Oxidation Time Based Study. Mater. Chem. Phys. 2018, 220, 417–425. [Google Scholar] [CrossRef]
- Luo, L.; Peng, T.; Yuan, M.; Sun, H.; Dai, S.; Wang, L. Preparation of Graphite Oxide Containing Different Oxygen-Containing Functional Groups and the Study of Ammonia Gas Sensitivity. Sensors 2018, 18, 3745. [Google Scholar] [CrossRef]
- Nuncira, J.; Seara, L.M.; Sinisterra, R.D.; Caliman, V.; Silva, G.G. Long-Term Colloidal Stability of Graphene Oxide Aqueous Nanofluids. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 407–417. [Google Scholar] [CrossRef]
- Li, C.; Lu, Y.; Yan, J.; Yu, W.; Zhao, R.; Du, S.; Niu, K. Effect of Long-Term Ageing on Graphene Oxide: Structure and Thermal Decomposition. R. Soc. Open Sci. 2021, 8, 202309. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Zou, Y.; Yang, Y. A UV-Light Induced Photochemical Method for Graphene Oxide Reduction. J. Mater. Sci. 2017, 52, 12742–12750. [Google Scholar] [CrossRef]
- Chlanda, A.; Kowiorski, K.; Małek, M.; Kijeńska-Gawrońska, E.; Bil, M.; Djas, M.; Strachowski, T.; Swieszkowski, W.; Lipińska, L. Morphology and Chemical Purity of Water Suspension of Graphene Oxide FLAKES Aged for 14 Months in Ambient Conditions. A Preliminary Study. Materials 2021, 14, 4108. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Świderska-Dąbrowska, R.; Piaskowski, K.; Lewandowska, L.; Fenert, B.; Mitura, K.A.; Baran, M.J. Carbon-Based and Related Nanomaterials as Active Media for Analytical, Biomedical, and Wastewater Processing Applications. In Pure and Functionalized Carbon Based Nanomaterials; Zarzycki, P.K., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 326–363. ISBN 9781351032308. [Google Scholar]
- Piaskowski, K.; Zarzycki, P.K. Carbon-Based Nanomaterials as Promising Material for Wastewater Treatment Processes. Int. J. Environ. Res. Public. Health 2020, 17, 5862. [Google Scholar] [CrossRef] [PubMed]
- Ham, H.; Van Khai, T.; Park, N.-H.; So, D.S.; Lee, J.-W.; Gil Na, H.; Jung Kwon, Y.; Yeon Cho, H.; Woo Kim, H. Freeze-Drying-Induced Changes in the Properties of Graphene Oxides. Nanotechnology 2014, 25, 235601. [Google Scholar] [CrossRef]
- Albert, E.L.; Che Abdullah, C.A.; Shiroshaki, Y. Synthesis and Characterization of Graphene Oxide Functionalized with Magnetic Nanoparticle via Simple Emulsion Method. Results Phys. 2018, 11, 944–950. [Google Scholar] [CrossRef]
- Rana, K.; Kaur, H.; Singh, N.; Sithole, T.; Siwal, S.S. Graphene-Based Materials: Unravelling Its Impact in Wastewater Treatment for Sustainable Environments. Mater. 2024, 3, 100107. [Google Scholar] [CrossRef]
- Carbon Nanotube Price: A Comparison Between Asia and Europe. 2025. Available online: https://graphenerich.com/carbon-nanotube-price-a-comparison-between-asia-and-europe/ (accessed on 15 August 2025).
- Navigating the Financial Landscape of Carbon Nanotubes Production Costs. 2024. Available online: https://theprocurementexpert.com/carbon-nanotubes-production-costs/ (accessed on 15 August 2025).
- Tripathy, J.; Mishra, A.; Pandey, M.; Thakur, R.R.; Chand, S.; Rout, P.R.; Shahid, M.K. Advances in Nanoparticles and Nanocomposites for Water and Wastewater Treatment: A Review. Water 2024, 16, 1481. [Google Scholar] [CrossRef]
- Nyairo, W.N.; Shikuku, V.O.; Sanou, Y. Carbon Nanotubes in Water Treatment: Progress and Challenges. In Innovative Nanocomposites for the Remediation and Decontamination of Wastewater; IGI Global: Hershey, PA, USA, 2022; pp. 171–184. ISBN 9781668445532. [Google Scholar]
- Sophia A., C.; Lima, E.C. Removal of Emerging Contaminants from the Environment by Adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef]
- Aslam, M.M.-A.; Kuo, H.-W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized Carbon Nanotubes (CNTs) for Water and Wastewater Treatment: Preparation to Application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Kwiecińska-Mydlak, A. The Application of Nanomaterial Adsorbents for the Removal of Impurities from Water and Wastewaters: A Review. Desalination Water Treat. 2020, 185, 1–26. [Google Scholar] [CrossRef]
- Epelle, E.I.; Okoye, P.U.; Roddy, S.; Gunes, B.; Okolie, J.A. Advances in the Applications of Nanomaterials for Wastewater Treatment. Environments 2022, 9, 141. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Saleh, T.A. Synthesis and Characterization of Alumina-Coated Carbon Nanotubes and Their Application for Lead Removal. J. Hazard. Mater. 2011, 185, 17–23. [Google Scholar] [CrossRef]
- Joseph, L.; Flora, J.R.V.; Park, Y.-G.; Badawy, M.; Saleh, H.; Yoon, Y. Removal of Natural Organic Matter from Potential Drinking Water Sources by Combined Coagulation and Adsorption Using Carbon Nanomaterials. Sep. Purif. Technol. 2012, 95, 64–72. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Duttagupta, S.P.; Chatterjee, A.K.; Mukherji, S. Potential of Carbon Nanomaterials for Removal of Heavy Metals from Water. Desalination 2008, 232, 145–156. [Google Scholar] [CrossRef]
- Ceroni, L.; Benazzato, S.; Pressi, S.; Calvillo, L.; Marotta, E.; Menna, E. Enhanced Adsorption of Methylene Blue Dye on Functionalized Multi-Walled Carbon Nanotubes. Nanomaterials 2024, 14, 522. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Astruc, D. Nanomaterials for Removal of Toxic Elements from Water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- Wei, Y.; Duan, G.; Huang, Y.; Han, X.; Zhang, C.; He, S.; Zhao, H.; Ma, C.; Jiang, S. Application of Magnetic Graphene in the Field of Wastewater Treatment: A Review. J. Water Process Eng. 2025, 74, 107766. [Google Scholar] [CrossRef]
- Hao, X.; Yang, S.; E, T.; Liu, L.; Ma, D.; Li, Y. Graphene Oxide/Montmorillonite Composite Aerogel with Slit-Shaped Pores: Selective Removal of Cu2+ from Wastewater. J. Alloys Compd. 2022, 923, 166335. [Google Scholar] [CrossRef]
- Sharma, S.K.; Ranjani, P.; Mamane, H.; Kumar, R. Preparation of Graphene Oxide-Doped Silica Aerogel Using Supercritical Method for Efficient Removal of Emerging Pollutants from Wastewater. Sci. Rep. 2023, 13, 16448. [Google Scholar] [CrossRef]
- Choi, W.S.; Lee, H.-J. Nanostructured Materials for Water Purification: Adsorption of Heavy Metal Ions and Organic Dyes. Polymers 2022, 14, 2183. [Google Scholar] [CrossRef]
- Thines, R.K.; Mubarak, N.M.; Nizamuddin, S.; Sahu, J.N.; Abdullah, E.C.; Ganesan, P. Application Potential of Carbon Nanomaterials in Water and Wastewater Treatment: A Review. J. Taiwan Inst. Chem. Eng. 2017, 72, 116–133. [Google Scholar] [CrossRef]
- Deng, C.-H.; Gong, J.-L.; Zeng, G.-M.; Niu, C.-G.; Niu, Q.-Y.; Zhang, W.; Liu, H.-Y. Inactivation Performance and Mechanism of Escherichia Coli in Aqueous System Exposed to Iron Oxide Loaded Graphene Nanocomposites. J. Hazard. Mater. 2014, 276, 66–76. [Google Scholar] [CrossRef]
- Zekić, E.; Vuković, Ž.; Halkijević, I. Application of Nanotechnology in Wastewater Treatment. Građevinar 2018, 70, 315–323. [Google Scholar] [CrossRef]
- Saccucci, M.; Bruni, E.; Uccelletti, D.; Bregnocchi, A.; Sarto, M.S.; Bossù, M.; Di Carlo, G.; Polimeni, A. Surface Disinfections: Present and Future. J. Nanomater. 2018, 2018, 8950143. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Zhang, Y.; Tian, M.; He, T.; Liu, J.; Chen, V. Polymeric Antimicrobial Membranes Enabled by Nanomaterials for Water Treatment. J. Membr. Sci. 2018, 550, 173–197. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.-S.; Chae, S.-R. Fouling in Membrane Bioreactors: An Updated Review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, A.O.E.; Galal, A.; Hussein, M.Z.; El Sayed, I.E.-T. Graphene Functionalization by 1,6-Diaminohexane and Silver Nanoparticles for Water Disinfection. J. Nanomater. 2016, 2016, 1485280. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial Nanomaterials for Water Disinfection and Microbial Control: Potential Applications and Implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Mao, Y.; Ding, L. Carbon Nanotubes as Antimicrobial Agents for Water Disinfection and Pathogen Control. J. Water Health 2018, 16, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, M.; Zhang, S.; Pan, B. Application Potential of Carbon Nanotubes in Water Treatment: A Review. J. Environ. Sci. 2013, 25, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Lilly, M.; Dong, X.; McCoy, E.; Yang, L. Inactivation of Bacillus anthracis Spores by Single-Walled Carbon Nanotubes Coupled with Oxidizing Antimicrobial Chemicals. Environ. Sci. Technol. 2012, 46, 13417–13424. [Google Scholar] [CrossRef]
- Salam, M.A.; Obaid, A.Y.; El-Shishtawy, R.M.; Mohamed, S.A. Synthesis of Nanocomposites of Polypyrrole/Carbon Nanotubes/Silver Nano Particles and Their Application in Water Disinfection. RSC Adv. 2017, 7, 16878–16884. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Gong, J.-L.; Zeng, G.-M.; Ou, X.-M.; Song, B.; Guo, M.; Zhang, J.; Liu, H.-Y. Antimicrobial Behavior Comparison and Antimicrobial Mechanism of Silver Coated Carbon Nanocomposites. Process Saf. Environ. Prot. 2016, 102, 596–605. [Google Scholar] [CrossRef]
- Dinh, N.X.; Chi, D.T.; Lan, N.T.; Lan, H.; Van Tuan, H.; Van Quy, N.; Phan, V.N.; Huy, T.Q.; Le, A.-T. Water-Dispersible Silver Nanoparticles-Decorated Carbon Nanomaterials: Synthesis and Enhanced Antibacterial Activity. Appl. Phys. A 2015, 119, 85–95. [Google Scholar] [CrossRef]
- Klerk, C.D.; Fosso-Kankeu, E.; Waanders, F.B. Evaluation of the Antibacterial Activity of Metal Impregnated Multi-Walled Carbon Nanotubes: Impact of Domestic Wastewater as Supporting Medium. Desalination Water Treat. 2017, 99, 272–281. [Google Scholar] [CrossRef]
- El-Newehy, M.; Thamer, B.M.; El-Hamshary, H.; Moydeen AbdulHameed, M. Engineered Multi-Walled Carbon Nanotubes for Disinfecting Wastewater. Mater. Chem. Phys. 2023, 308, 128262. [Google Scholar] [CrossRef]
- Ferré-Pujol, P.; Obata, S.; Raya, J.; Bianco, A.; Katayama, H.; Kato, T.; Nishina, Y. Reversible Chemical Modifications of Graphene Oxide for Enhanced Viral Capture and Release in Water. Carbon 2025, 234, 120015. [Google Scholar] [CrossRef]
- Zhang, Y.; Chung, T.-S. Graphene Oxide Membranes for Nanofiltration. Curr. Opin. Chem. Eng. 2017, 16, 9–15. [Google Scholar] [CrossRef]
- Lazarenko, N.S.; Golovakhin, V.V.; Shestakov, A.A.; Lapekin, N.I.; Bannov, A.G. Recent Advances on Membranes for Water Purification Based on Carbon Nanomaterials. Membranes 2022, 12, 915. [Google Scholar] [CrossRef]
- Ahn, C.H.; Baek, Y.; Lee, C.; Kim, S.O.; Kim, S.; Lee, S.; Kim, S.-H.; Bae, S.S.; Park, J.; Yoon, J. Carbon Nanotube-Based Membranes: Fabrication and Application to Desalination. J. Ind. Eng. Chem. 2012, 18, 1551–1559. [Google Scholar] [CrossRef]
- Manawi, Y.; Kochkodan, V.; Hussein, M.A.; Khaleel, M.A.; Khraisheh, M.; Hilal, N. Can Carbon-Based Nanomaterials Revolutionize Membrane Fabrication for Water Treatment and Desalination? Desalination 2016, 391, 69–88. [Google Scholar] [CrossRef]
- Sun, H.; Wu, P. Tuning the Functional Groups of Carbon Quantum Dots in Thin Film Nanocomposite Membranes for Nanofiltration. J. Membr. Sci. 2018, 564, 394–403. [Google Scholar] [CrossRef]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M.A. Remediation of Wastewater Using Various Nano-Materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon Nanotube Membranes for Water Purification: A Bright Future in Water Desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Al-anzi, B.S.; Siang, O.C. Recent Developments of Carbon Based Nanomaterials and Membranes for Oily Wastewater Treatment. RSC Adv. 2017, 7, 20981–20994. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Mansoor, B.; Mansour, A.; Khraisheh, M. Functional Graphene Nanosheets: The next Generation Membranes for Water Desalination. Desalination 2015, 356, 208–225. [Google Scholar] [CrossRef]
- Hegab, H.M.; Zou, L. Graphene Oxide-Assisted Membranes: Fabrication and Potential Applications in Desalination and Water Purification. J. Membr. Sci. 2015, 484, 95–106. [Google Scholar] [CrossRef]
- Koo, C.H.; Lau, W.J.; Lai, G.S.; Lai, S.O.; Thiam, H.S.; Ismail, A.F. Thin-Film Nanocomposite Nanofiltration Membranes Incorporated with Graphene Oxide for Phosphorus Removal. Chem. Eng. Technol. 2018, 41, 319–326. [Google Scholar] [CrossRef]
- Song, N.; Gao, X.; Ma, Z.; Wang, X.; Wei, Y.; Gao, C. A Review of Graphene-Based Separation Membrane: Materials, Characteristics, Preparation and Applications. Desalination 2018, 437, 59–72. [Google Scholar] [CrossRef]
- Yang, G.C.C.; Chen, Y.-C.; Yang, H.-X.; Yen, C.-H. Performance and Mechanisms for the Removal of Phthalates and Pharmaceuticals from Aqueous Solution by Graphene-Containing Ceramic Composite Tubular Membrane Coupled with the Simultaneous Electrocoagulation and Electrofiltration Process. Chemosphere 2016, 155, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Yusof, N.; Tan, Y.H. Graphene Oxide Incorporated Thin Film Nanocomposite Nanofiltration Membrane for Enhanced Salt Removal Performance. Desalination 2016, 387, 14–24. [Google Scholar] [CrossRef]
- Elkholy, R.A.; Khalil, E.M.; Farag, A.B.; Abo El-Fadl, M.M.; El-Aassar, A.M. Photocatalytic Degradation of Organic Pollutants in Wastewater Using Different Nanomaterials Immobilized on Polymeric Beads. Desalination Water Treat. 2020, 193, 117–128. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous Photocatalysis and Its Potential Applications in Water and Wastewater Treatment: A Review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Gandhi, M.R.; Vasudevan, S.; Shibayama, A.; Yamada, M. Graphene and Graphene-Based Composites: A Rising Star in Water Purification—A Comprehensive Overview. ChemistrySelect 2016, 1, 4358–4385. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, H.; Wu, C.; Feng, S.; Alsaedi, A.; Hayat, T.; Chen, C. Facile Synthesis of Magnetic Fe3O4/Graphene Composites for Enhanced U(VI) Sorption. Appl. Surf. Sci. 2018, 444, 691–698. [Google Scholar] [CrossRef]
- Lai, C.; Wang, M.-M.; Zeng, G.-M.; Liu, Y.-G.; Huang, D.-L.; Zhang, C.; Wang, R.-Z.; Xu, P.; Cheng, M.; Huang, C.; et al. Synthesis of Surface Molecular Imprinted TiO2/Graphene Photocatalyst and Its Highly Efficient Photocatalytic Degradation of Target Pollutant under Visible Light Irradiation. Appl. Surf. Sci. 2016, 390, 368–376. [Google Scholar] [CrossRef]
- Park, J.; Jin, T.; Liu, C.; Li, G.; Yan, M. Three-Dimensional Graphene-TiO2 Nanocomposite Photocatalyst Synthesized by Covalent Attachment. ACS Omega 2016, 1, 351–356. [Google Scholar] [CrossRef]
- Solomon, R.V.; Lydia, I.S.; Merlin, J.P.; Venuvanalingam, P. Enhanced Photocatalytic Degradation of Azo Dyes Using Nano Fe3O4. J. Iran. Chem. Soc. 2012, 9, 101–109. [Google Scholar] [CrossRef]
- Tju, H.; Taufik, A.; Saleh, R. Enhanced UV Photocatalytic Performance of Magnetic Fe3O4/CuO/ZnO/NGP Nanocomposites. J. Phys. Conf. Ser. 2016, 710, 012005. [Google Scholar] [CrossRef]
- Zhu, Y.; Xue, J.; Xu, T.; He, G.; Chen, H. Enhanced Photocatalytic Activity of Magnetic Core-Shell Fe3O4@Bi2O3-RGO Heterojunctions for Quinolone Antibiotics Degradation under Visible Light. J. Mater. Sci. Mater. Electron. 2017, 28, 8519–8528. [Google Scholar] [CrossRef]
- Thangavel, S.; Thangavel, S.; Raghavan, N.; Krishnamoorthy, K.; Venugopal, G. Visible-Light Driven Photocatalytic Degradation of Methylene-Violet by rGO/Fe3O4/ZnO Ternary Nanohybrid Structures. J. Alloys Compd. 2016, 665, 107–112. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Ingrosso, C.; Placido, T.; Striccoli, M.; Curri, M.L.; Agostiano, A.; Comparelli, R. Nanocomposite Materials for Photocatalytic Degradation of Pollutants. Catal. Today 2017, 281, 85–100. [Google Scholar] [CrossRef]
- Xin, L.; Hu, J.; Xiang, Y.; Li, C.; Fu, L.; Li, Q.; Wei, X. Carbon-Based Nanocomposites as Fenton-Like Catalysts in Wastewater Treatment Applications: A Review. Materials 2021, 14, 2643. [Google Scholar] [CrossRef]
- Nair, K.M.; Kumaravel, V.; Pillai, S.C. Carbonaceous Cathode Materials for Electro-Fenton Technology: Mechanism, Kinetics, Recent Advances, Opportunities and Challenges. Chemosphere 2021, 269, 129325. [Google Scholar] [CrossRef]
- Jame, S.A.; Zhou, Z. Electrochemical Carbon Nanotube Filters for Water and Wastewater Treatment. Nanotechnol. Rev. 2016, 5. [Google Scholar] [CrossRef]
- Rokhsat, E.; Akhavan, O. Improving the Photocatalytic Activity of Graphene Oxide/ZnO Nanorod Films by UV Irradiation. Appl. Surf. Sci. 2016, 371, 590–595. [Google Scholar] [CrossRef]
- She, P.; Yin, S.; He, Q.; Zhang, X.; Xu, K.; Shang, Y.; Men, X.; Zeng, S.; Sun, H.; Liu, Z. A Self-Standing Macroporous Au/ZnO/Reduced Graphene Oxide Foam for Recyclable Photocatalysis and Photocurrent Generation. Electrochim. Acta 2017, 246, 35–42. [Google Scholar] [CrossRef]
- Bai, X.; Sun, C.; Liu, D.; Luo, X.; Li, D.; Wang, J.; Wang, N.; Chang, X.; Zong, R.; Zhu, Y. Photocatalytic Degradation of Deoxynivalenol Using Graphene/ZnO Hybrids in Aqueous Suspension. Appl. Catal. B Environ. 2017, 204, 11–20. [Google Scholar] [CrossRef]
- Xiao, W.; Li, B.; Yan, J.; Wang, L.; Huang, X.; Gao, J. Three Dimensional Graphene Composites: Preparation, Morphology and Their Multi-Functional Applications. Compos. Part Appl. Sci. Manuf. 2023, 165, 107335. [Google Scholar] [CrossRef]
- Luan, V.H.; Tien, H.N.; Hur, S.H. Fabrication of 3D Structured ZnO Nanorod/Reduced Graphene Oxide Hydrogels and Their Use for Photo-Enhanced Organic Dye Removal. J. Colloid Interface Sci. 2015, 437, 181–186. [Google Scholar] [CrossRef]
- Mariafrancesca, B.; Aleksey, V.N.; Aleksey, V.E.; Donatella, A.; Anna, N.; Leonardo, D.D.; Alexandr, I.M.; Fiore, P.N.; Giovanni, D.F. Improving the Catalytic Performance of TiO2 by Its Surface Deposition on CNT Buckypapers for Use in the Removal of Wastewater Pollutants. New Carbon Mater. 2025, 40, 438–455. [Google Scholar] [CrossRef]
- Du, T.; Adeleye, A.S.; Keller, A.A.; Wu, Z.; Han, W.; Wang, Y.; Zhang, C.; Li, Y. Photochlorination-Induced Transformation of Graphene Oxide: Mechanism and Environmental Fate. Water Res. 2017, 124, 372–380. [Google Scholar] [CrossRef]
- Suji, S.; Harikrishnan, M.; Vickram, A.S.; Nibedita, D.; Saranya, V.; Thiruvengadam, S.; Kamaraj, C.; Gnanasekaran, L.; Goyal, K.; Ali, H.; et al. Ecotoxicological Evaluation of Nanosized Particles with Emerging Contaminants and Their Impact Assessment in the Aquatic Environment: A Review. J. Nanoparticle Res. 2025, 27, 112. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the Environment: Where Do We Come from, Where Do We Go To? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef]
- Ahmad, J.; Naeem, S.; Ahmad, M.; Usman, A.R.A.; Al-Wabel, M.I. A Critical Review on Organic Micropollutants Contamination in Wastewater and Removal through Carbon Nanotubes. J. Environ. Manag. 2019, 246, 214–228. [Google Scholar] [CrossRef]
- Hu, X.; Ren, C.; Kang, W.; Mu, L.; Liu, X.; Li, X.; Wang, T.; Zhou, Q. Characterization and Toxicity of Nanoscale Fragments in Wastewater Treatment Plant Effluent. Sci. Total Environ. 2018, 626, 1332–1341. [Google Scholar] [CrossRef]
- Lalwani, G.; D’Agati, M.; Khan, A.M.; Sitharaman, B. Toxicology of Graphene-Based Nanomaterials. Adv. Drug Deliv. Rev. 2016, 105, 109–144. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Iglesias, O.; Collado, S.; Oulego, P.; Díaz, M. Graphene-Family Nanomaterials in Wastewater Treatment Plants. Chem. Eng. J. 2017, 313, 121–135. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, W.; Xu, Z.; Peng, W.; Luo, S. Comparative Effects of Graphene and Graphene Oxide on Copper Toxicity to Daphnia Magna: Role of Surface Oxygenic Functional Groups. Environ. Pollut. 2018, 236, 962–970. [Google Scholar] [CrossRef]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and Environmental Risks of Nanomaterials: Challenges and Future Needs. J. Environ. Sci. Health Part C 2009, 27, 1–35. [Google Scholar] [CrossRef]
- Vasyukova, I.; Gusev, A.; Tkachev, A. Reproductive Toxicity of Carbon Nanomaterials: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2015, 98, 012001. [Google Scholar] [CrossRef]
- Fan, W.; Liu, Y.; Xu, Z.; Wang, X.; Li, X.; Luo, S. The Mechanism of Chronic Toxicity to Daphnia Magna Induced by Graphene Suspended in a Water Column. Environ. Sci. Nano 2016, 3, 1405–1415. [Google Scholar] [CrossRef]
- Chen, M.; Yin, J.; Liang, Y.; Yuan, S.; Wang, F.; Song, M.; Wang, H. Oxidative Stress and Immunotoxicity Induced by Graphene Oxide in Zebrafish. Aquat. Toxicol. 2016, 174, 54–60. [Google Scholar] [CrossRef]
- Laux, P.; Riebeling, C.; Booth, A.M.; Brain, J.D.; Brunner, J.; Cerrillo, C.; Creutzenberg, O.; Estrela-Lopis, I.; Gebel, T.; Johanson, G.; et al. Challenges in Characterizing the Environmental Fate and Effects of Carbon Nanotubes and Inorganic Nanomaterials in Aquatic Systems. Environ. Sci. Nano 2018, 5, 48–63. [Google Scholar] [CrossRef]
- Gautam, P.K.; Singh, A.; Misra, K.; Sahoo, A.K.; Samanta, S.K. Synthesis and Applications of Biogenic Nanomaterials in Drinking and Wastewater Treatment. J. Environ. Manag. 2019, 231, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Liu, Q.; Zhu, Y.; Yang, H.; Chen, H. Preparation of High-Value Carbon Nanotubes from Real Waste Plastic towards the Negative Carbon Technology. Carbon Capture Sci. Technol. 2024, 13, 100258. [Google Scholar] [CrossRef]
- Pandey, G.; Jain, P. Assessing the Nanotechnology on the Grounds of Costs, Benefits, and Risks. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9. [Google Scholar] [CrossRef]
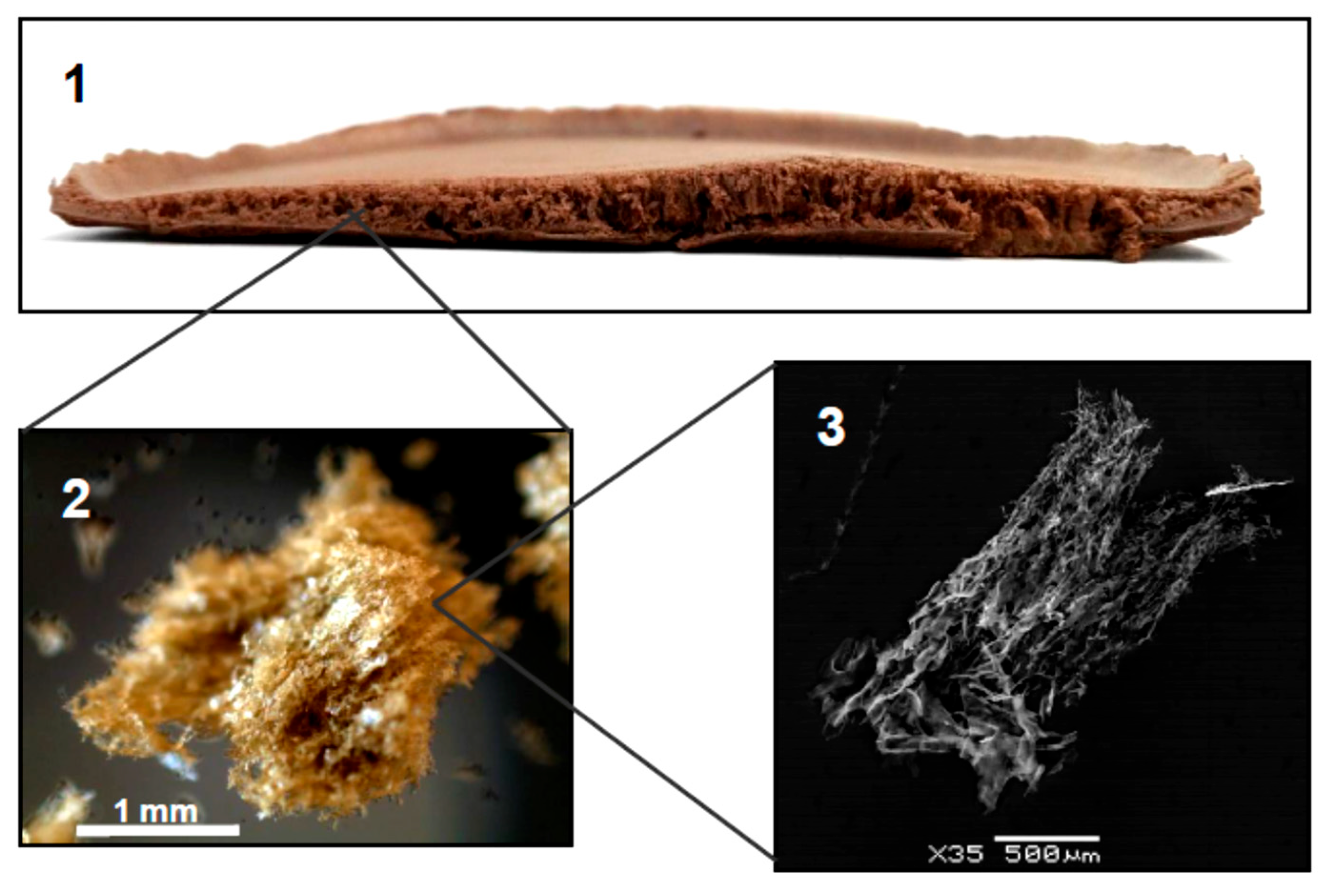
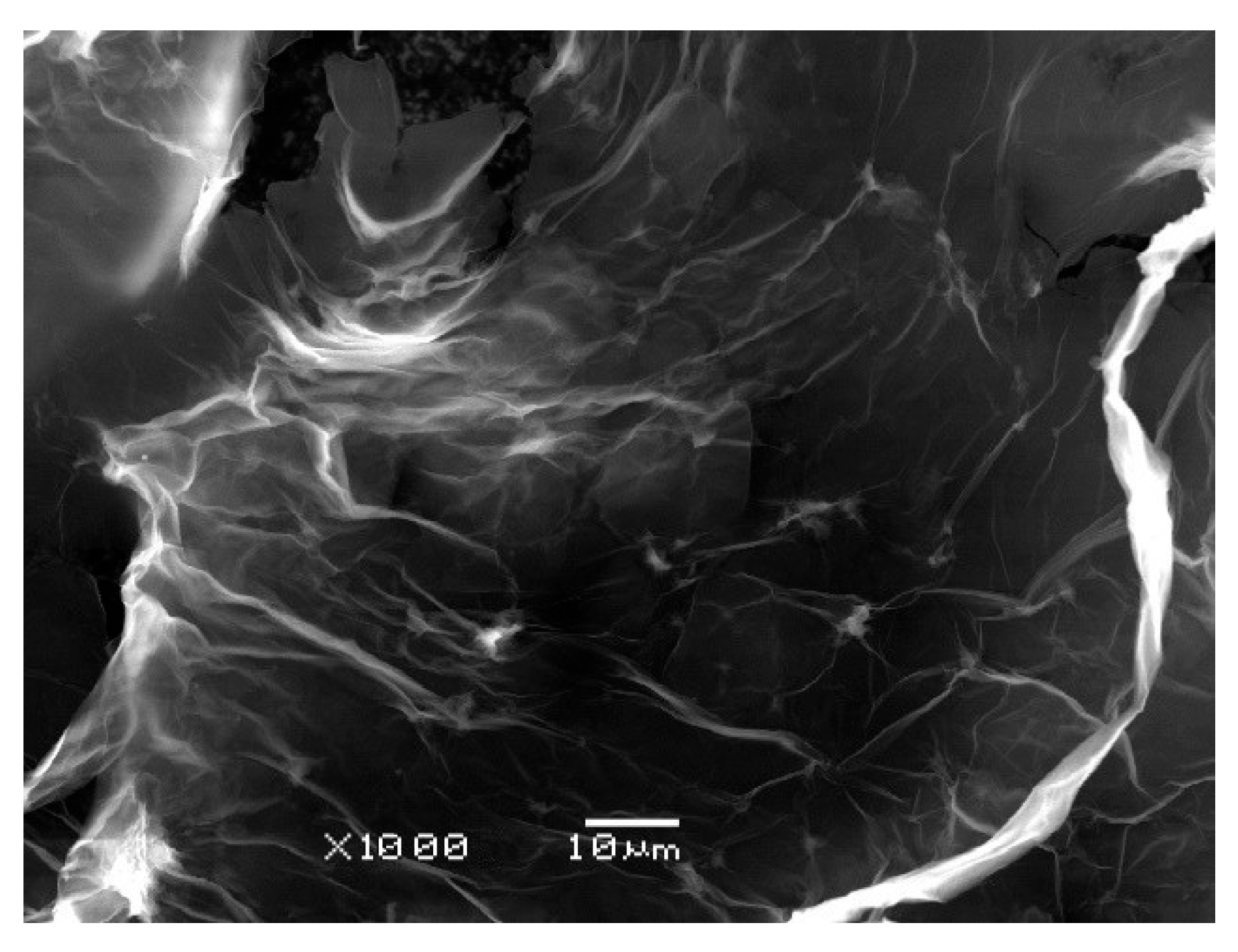



| Carbon-Based Nanomaterials (CNMs) | ||
|---|---|---|
| Carbon nanotubes (CNTs): Single-Walled (SWCNTs) Multi-Walled (MWCNTs) | Graphene (Gr) | Graphene-family nanomaterials (GFNs): Graphene oxide (GO), Reduced graphene oxide (rGO) |
| Removal/degradation of dyes: | ||
| CNT/TiO2 [12] Graphene G-CNT [13] CNT/PANI polyaniline [14] CNTs/Fe-Ni/TiO2 [15] Cellulose Nanofibrils CNF-CNT [16] MnOx@polyvinylidene fluoride MnOx@PVDF/MWCNTs [17] Titanium Dioxide with Silver Ag-TiO2/MWCNT [18] Titanium Dioxide with Palladium Pd-TiO2/MWCNT [18] MWCTs entrapped in gelatin with embedded magnetic nanoparticles Gel-CNT-MNPs [19] Palladium-doped–ZrO2 Pd-ZrO2-MWCNTs [20] MWCNTs encapsulated in alginate microvesicles with Ba2+ Ba2+-ALG/MWCNT [21] TiO2/graphene-MWCNT nanocomposite immobilized in poly(vinyl alcohol) PVA/TiO2/Gr-MWCNT [22] Magnetic Chitosan Enwrapping Nanosized γ-Fe2O3 m-CS/γ-Fe2O3/MWCNTs [23] | Gr [24] ZnO and graphene composites ZnO/Grs [25] cobalt (Co) and nickel (Ni) metal–organic frameworks CNMs–Gr [26] SiO2/Cu2O-graphene [27] Graphene hydrogel GH [28] Graphene aerogel GA [28] cellulose nanofibrils graphene nanoplates CNF-GnP [16] magnetic-sulfonic graphene nanocomposite Gr-SO3H/Fe3O4 [29] Sulfonated Graphene Nanosheets GNSs [30] Graphene nanoplatelets GNP-SiO2 [31] | Thiosemicarbazide functionalized GO-TSC-GO [32] Chitosan GO-CTS [33] Poly(vinyl alcohol) GO/PVA [34] GO/aminated lignin aerogel GALA [35] GO-TiO2 [36] Poly(N,N-dimethyl amino ethylmethacrylate) GO-PDMAEMA [37] Poly(2-hydroxyethyl methacrylate) GO-PHEMA [38] Fly ash with chitosan FCGO [39] MnFe2O4/GO [40] Poly(vinyl alcohol)/poly(acrylic acid) PVA/PAA/TiO2/GO [41] Aerogel with zero-valent iron rGOA-nZVI [42] Cu2O-rGO/Fe3O4@SiO2 [43] 2D bimetallic sulfides/N-doped FeCoS/N-rGO [44] Reduced graphene oxide/halloysite nanotubes polydopamine PDA/rGO/HNTs [45] Nicandra physaloides (L.) Gaertn seed, gum NPG/GO [46] |
| Removal of metal ions: | ||
| CNTs [47,48] Hydroxyl Functionalized CNT-OH [49] Carboxyl Functionalized CNT-COO− [49] Amide Functionalized CNT-CONH2 [49] Supported-epoxidized carbon nanotube SENT [50] MnO2/CNTs [51,52] Poly-amidoamine Dendrimer PAMAM/CNT [53] Polyacrylamide-Sodium Alginate PAAM-SA-CNT [54] Oxidized CNTs [55] Thiol-derivatized SWCNT-SH [56] MWCNTs [57] Magnetic MWCNT MMWCNT [58] Fe3O4 nanoparticles with 3-aminopropyltriethoxysilane MWCNTs/Fe3O4-NH2 [59] Oxidized Oxi-MWCNTs [60] Carboxyl Functionalized MWCNT-COOH [48] Hydroxyl Functionalized MWCNT-OH [48] La(OH)3 and CaO2 fabricated CNT La-Ca-CNT [61] | Graphene/MgO [62] SiO2/Graphene [63] Graphene hydrogel GH [28] Graphene aerogel GA [28] Graphene nanosheets GNSs [64] Sulfonated Graphene Nanosheets GNSs [30] Gr/MnO2-QD quantum dot [65] Graphene nanoplatelets GNP [48] | Amination GO-NH2 [66] 2,2′-dipyridylamine GO-DPA [67] Chitosan GO-CTS [68] Few-layered FGO [69] GO aerogels [70] 2-pyridinecarboxaldehyde thiosemicarbazone GO/2-PTSC [71] Mn-doped Fe(III) oxide nanoparticle implanted graphene GMIO [72] Glycol-GO [73] Cyclodextrin–chitosan CCGO [74] Manganese Ferrite MnFe2O4/GO [40] Chitosan-Poly(vinyl alcohol) GO-CS-PVA hydrogels [75] Fe3O4 1,2-diaminocyclohexanetetraacetic acid Fe3O4/GO/DCTA [76] GOx-microbots [77] magnetic chitosan Pb2+ Pb-MCGO [78] Aminated Fe3O4 AMGO [79] Polystyrene PS@ + rGO@GO@Fe3O4 PG-Fe3O4 [80] Polyethyleneimine-Grafted PEI/GO [81] Thymine-Grafted rGO-Thy [82] Halloysite nanotubes polydopamine PDA/rGO/HNTs [45] Triethylenetetramine-magnetic TET-MrGO [83] |
| Removal/degradation of pharmaceuticals: | ||
| CNTs [84] Copper alginate CA-CNTs [85] CeO2@CNT membrane [86] Alumina Hybrid CNTs/Al2O3 [87] Modified MWCNTs [88] Carboxyl Functionalized MWCNT-COOH [84,89] Hydroxyl Functionalized MWCNT-OH [84] Alumina Hybrid MWCNTs/Al2O3 [87] Fe-Cu Doped MWCNTs Fe-Cu/CNT[90] | Graphene hydrogel GH [28] Graphene aerogel GA [28] Graphene nanoplatelets GNP [91] | GO [92] Co3O4/rGO [93] 2D bimetallic sulfides/N-doped FeCoS/N-rGO [44] |
| Removal/degradation of organic substances: | ||
| CNTs [94] CNT/ZnO/TiO2 [95] Metallic SWNTs [96] PVA-based Polymer Nanoparticles PNP/CNTs [97] SWCNTs [98,99] MWCNTs [98,100] Magnetic MWCNT MMWCNT [58] ZnO nano particles on Multiwall Carbon Nanotubes MWNT/ZnO [101] iron oxide and manganese dioxide MWCNTs/Fe3O4-MnO2 [102] Fe3O4 nanoparticles with 3-aminopropyltriethoxysilane MWCNTs/Fe3O4-NH2 [59] Fe3O4 decorated MWCNT Fe3O4/MWCNT [103] | Sulfonated Graphene Nanosheets GNSs [30] Au nanoparticles anchored on the Ionic Liquid of 3,4,9,10-perylene tetracarboxylic acid-noncovalent functionalized graphene Au/PDIL-GS [104] | GO [105] rGO [105] 2D bimetallic sulfides/N-doped FeCoS/N-rGO [44] Silver nanospheres (Ag-NSs) rGO nanosponge RGONS/Ag-NSs [106] |
| Separation of oil/water: | ||
| Ferric Oxide Nanoparticles Doped CNT/Fe2O3 [107] Silver Nanoparticles Polyacrylic Acid Ag/PAA-CNTs [108] Magnetic CNT with macropores formed by salts [109] | Graphene aerogel GA [110] nanoporous graphene NPG [111] | Polyethersulfone GO-PES [112] Poly(arylene ether nitrile) halloysite nanotubes polydopamine (PEN)/HNTs@GO-PDA [113] Halloysite nanotubes polydopamine PDA/rGO/HNTs [45] |
| Other: | ||
| Chromium Oxide-decorated Cr2O3-CNT [114] Oxidized CNTs [115] Vertically Aligned Carbon Nanotubes on Anodized Aluminum Oxide AAO-CNT [116] vertically aligned VA CNT [117] Plasma Induced Grafting Carboxymethyl Cellulose MWCNT-g-CMC [118] Polymerized Citric Acid (CA), Acrylic Acid (AA) and Acrylamide (AAm) modified MWCNT/PCA, PAA, PAAm [119] TiO2-MWNTs [120] Lanthanum Carbonate CNT LC-CNT [121] La(OH)3- and CaO2-fabricated CNT La-Ca-CNT [61] | graphene aerogel (GA) decorated with platinum nanoparticles GA/Pt [122] graphene-poly(acrylonitrile-co-maleimide) G-PANCMI [123] poly(N-vinylcarbazole)-graphene (PVK-G) [124] | GO [125] GO-Ag [126,127] Poly(N-vinylcarbazole) PVK-GO [124] |
| Element | Weight% | Atomic% |
|---|---|---|
| C | 55.10 | 62.53 |
| O | 43.06 | 36.69 |
| S | 1.85 | 0.78 |
| Totals | 100.00 |
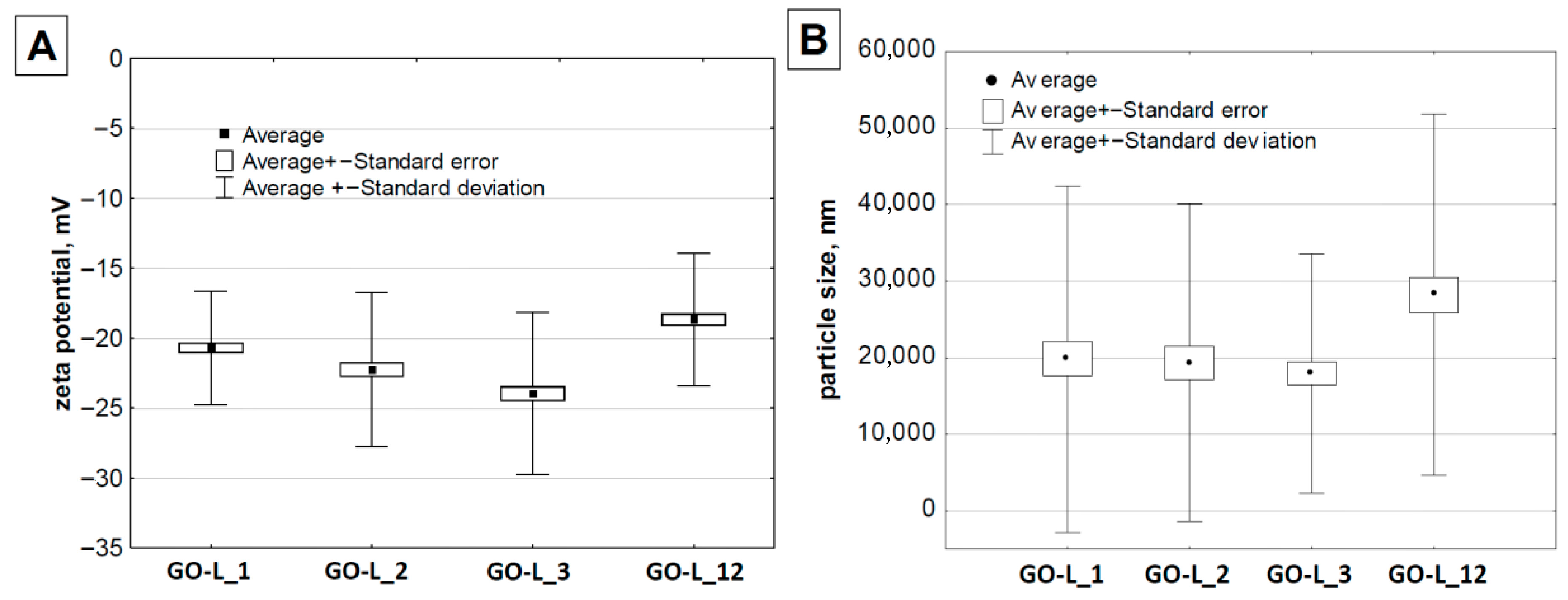
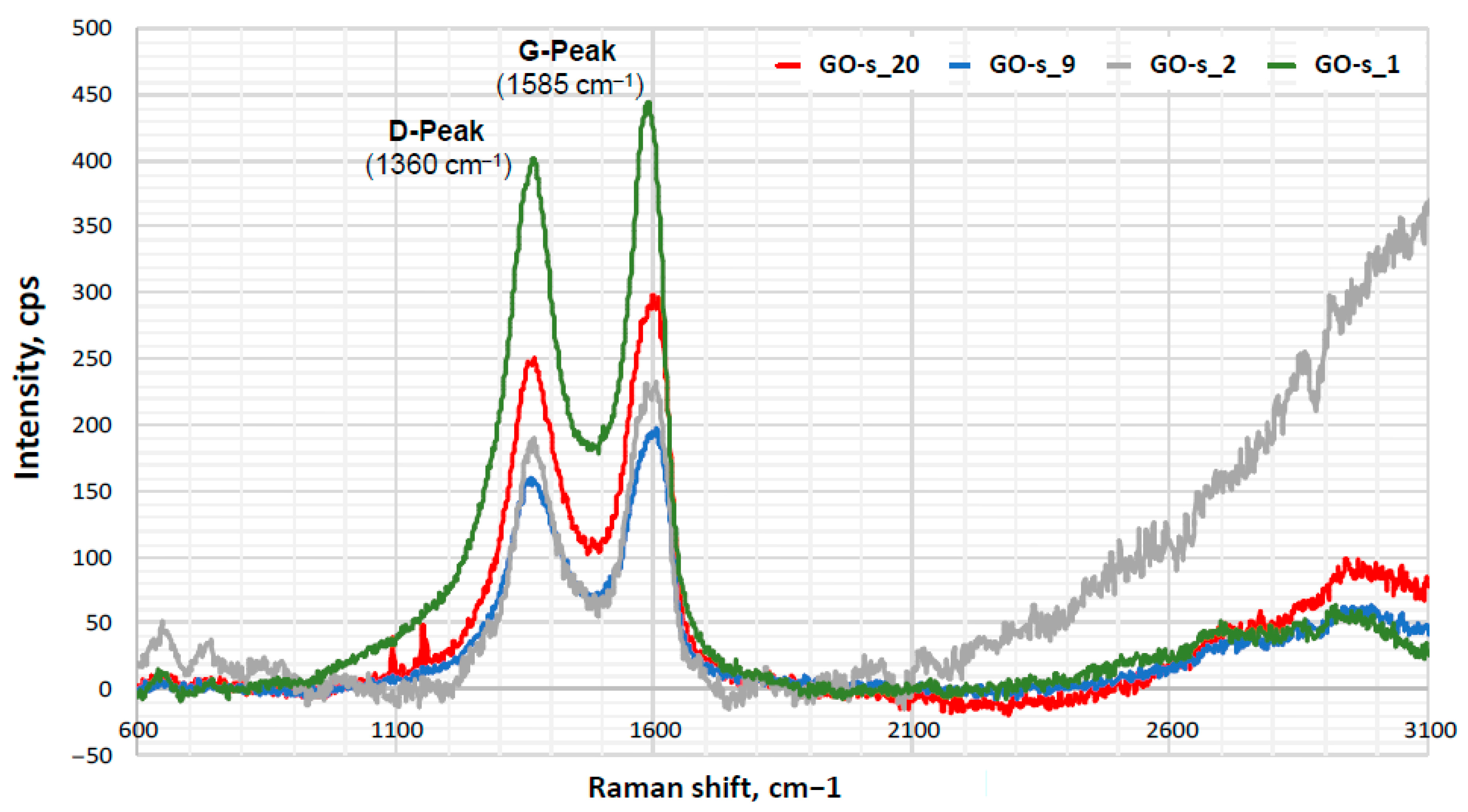
| Sample | ID/IG | I2D/IG |
|---|---|---|
| GO-s_1 | (0.91) | 0.09 |
| GO-s_2 | 0.81 | (0.74) |
| GO-s_9 | 0.80 | 0.17 |
| GO-s_20 | 0.83 | 0.15 |
| GO-L_1 | 0.83 | 0.10 |
| GO-L_2 | 0.85 | 0.095 |
| GO-L_3 | 0.84 | 0.090 |
| GO-L_12 | 0.83 | 0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piaskowski, K.; Świderska-Dąbrowska, R.; Dąbrowski, T. Carbon-Based Nanomaterials in Water and Wastewater Treatment Processes. Sustainability 2025, 17, 7414. https://doi.org/10.3390/su17167414
Piaskowski K, Świderska-Dąbrowska R, Dąbrowski T. Carbon-Based Nanomaterials in Water and Wastewater Treatment Processes. Sustainability. 2025; 17(16):7414. https://doi.org/10.3390/su17167414
Chicago/Turabian StylePiaskowski, Krzysztof, Renata Świderska-Dąbrowska, and Tomasz Dąbrowski. 2025. "Carbon-Based Nanomaterials in Water and Wastewater Treatment Processes" Sustainability 17, no. 16: 7414. https://doi.org/10.3390/su17167414
APA StylePiaskowski, K., Świderska-Dąbrowska, R., & Dąbrowski, T. (2025). Carbon-Based Nanomaterials in Water and Wastewater Treatment Processes. Sustainability, 17(16), 7414. https://doi.org/10.3390/su17167414






