Abstract
This study addresses the use of porcelain polishing waste as a pyro-expansive agent in clay-based formulations for the production of lightweight aggregates, aiming to reduce the consumption of natural resources and mitigate environmental impacts. In line with circular economy principles and sustainable construction goals, this study investigates the potential use of porcelain polishing waste as a pyro-expansive agent in clay-based formulations for producing sustainable lightweight aggregates. Using the Taguchi method and ANOVA, the effects of key processing parameters were evaluated. The results demonstrated a broad range of volumetric changes, from shrinkage of 40.84% to expansion of 91.69%, depending on the formulation and processing conditions. The aggregates exhibited specific mass values ranging from 0.99 g/cm3 to 2.36 g/cm3, water absorption up to 3.29%, and mechanical strength from 4.57 MPa to 39.87 MPa. Notably, nine of the sixteen experimental conditions met the technical standards for classification as LWA, indicating suitability for applications in high-strength, structural, and non-structural lightweight concretes, as well as lightweight mortars. The performance of these materials was directly linked to the chemical and mineralogical characteristics of the precursors and the proportion of pyro-expansive waste used. Overall, the findings suggest that 50% of the produced aggregates are viable for high-performance concrete applications, offering an environmentally responsible alternative to virgin raw materials and contributing to sustainable waste valorization in the ceramic and construction industries.
1. Introduction
Sustainability has gained increasing prominence as urbanization and the rapid growth of the global population have led to the overexploitation of natural resources [1]. This includes the clays used to produce artificial lightweight aggregates, which are being continuously mined, resulting in the degradation of ecosystems. This issue is not only related to reducing material waste but also to actions that promote the intelligent use of natural resources, which can have a positive impact on social, economic, and regional development [2]. The United Nations Environment Programme (UNEP) and the International Solid Waste Association (ISWA) published a study in 2024, projecting that by 2050, global waste production will reach approximately 3.8 billion tons, affecting society, the environment, and the global economy. This underscores the urgent need to recover and reuse waste, which will become valuable resources [3].
In Brazil, the production of commercial lightweight aggregates is limited to the Southeast region, where the only manufacturer of this product is located. This restricts the use of such material in other areas of the country, as its application becomes costly due to transportation expenses. In addition to the high cost, the monopolization of manufacturing in a single location results in environmental damage, since longer distances are required for product distribution, thus increasing CO2 emissions [4].
Over the years, several studies have emerged addressing the production of lightweight aggregates with the incorporation of waste materials such as sludge [5,6,7], coal ash [8,9], and agricultural residues [10,11], as well as various other waste types reused in lightweight aggregate production [12,13,14,15,16,17,18,19,20,21]. There is a need for solid waste management worldwide, in addition to other environmental concerns, such as the uncontrolled extraction of finite natural resources.
Ceramic tiles, widely used materials around the world, most of them being porcelain tiles, are responsible for generating large amounts of waste, approximately 15 million and 200 thousand tons per year in Brazil [22]. The growth of this sector leads to a significant increase in waste production, one of which is porcelain polishing residue (PPR), which is improperly disposed of and accumulates over the years, resulting in environmental damage that can become irreversible, such as soil, air, and groundwater contamination [23,24].
Several authors have studied the chemical composition of porcelain polishing residue [25,26,27], with common findings indicating high levels of silica (SiO2) and alumina (Al2O3), respectively. Among the oxides, the one with the most significant content is magnesium oxide (MgO). These characteristics make the residue attractive to produce lightweight aggregates, such as silica and alumina, when sintered, which can form a glassy phase essential for trapping gases released from other chemical components [28], such as magnesium oxide, which gives the aggregate lightness.
Established methodologies for producing lightweight aggregates include the Riley diagram, based on the chemical compatibility of materials that, when subjected to high temperatures, expand and become lighter due to the formation of internal porosity. Another widely used approach is the fluxing oxide sum (SiO2/ΣOF), which considers the proportion of oxides that promote liquid phase formation during sintering. However, as highlighted by several authors [28,29,30], these methods have limitations: for instance, the Riley diagram does not account for mineralogical composition or the presence of wastes with unconventional behavior, while the ΣOF method may overlook factors such as particle size distribution, heating rate, or interactions between oxides within the firing parameters. There are studies across various fields of knowledge that use the Taguchi method as a tool for process improvement, showing satisfactory results within each area researched [31,32,33].
Given the above, the development of sustainable and regionally sourced lightweight aggregates becomes necessary, as the use of waste contributes to reducing environmental impacts while also encouraging the local economy and enabling the production of aggregates in other regions of the country. Thus, the objective of this study was to investigate the development of artificial lightweight aggregate using porcelain polishing residue as a pyro-expansive agent in formulations with clays.
2. Methodology
2.1. Collection and Processing of Raw Materials
The raw materials used were obtained in the municipality of João Pessoa, in the state of Paraíba. The porcelain polishing residue was sourced from the porcelain tile industry, while the clays (ferruginous and kaolinitic) came from the coastal area.
The processing of these raw materials initially involved drying them in a drying oven, (Solab SL-102, Piracicaba, SP, Brazil) with air circulation and renewal at 110 °C for 24 h, to remove the residual moisture from the clays and the water present in the residue derived from the industry. After drying, the porcelain polishing residue was ground in an alumina disc mill to reduce particle size, and the ferruginous clay was disaggregated using a porcelain mortar and pestle. Subsequently, both the clays and the residue were sieved through a 170-mesh screen to obtain fine powders that would result in more uniform and consistent expansion during sintering [34].
2.2. Characterization of Raw Materials
For the characterization of the raw materials, particle size distribution, chemical composition, and mineralogical composition analyses were performed in order to assess potential expansion behavior and material characteristics after sintering [16]. To determine the particle size distribution of the raw materials, a dry-method particle size analyzer (CILAS 1090, Orléans, France) was used, covering a range from 0.10 µm to 500 µm across 100 size classes.
The ferruginous clay had an average diameter of 63.22 µm, suggesting a similarity to fine sand (60 µm to 200 µm). However, 50% of the material had a diameter smaller than 26.50 µm, indicating characteristics more consistent with silt (2 µm to 60 µm). The kaolinitic clay and the porcelain polishing residue contained finer materials, with average diameters of 3.80 µm and 6.69 µm, respectively, both suggesting a similarity to silt (2 µm to 60 µm).
Although the particle size distributions, when compared to the Brazilian standard, suggest that the raw materials are similar to silt, there are still portions of extremely fine particles, comparable to clay (<2 µm) [35].
The chemical composition of the raw materials was determined through energy-dispersive X-ray fluorescence (XRF) analysis using the EDX-720 equipment (Waltham, MA, USA), and the results are presented in Table 1.

Table 1.
Chemical composition of the ferruginous and kaolinitic clays and the PPR.
It is observed that all three raw materials contain high levels of inorganic oxides (SiO2 and Al2O3), which is a positive factor to produce lightweight aggregates, as these oxides facilitate the trapping of released gases. The higher content of magnesium oxide (MgO) in the ferruginous clay and the porcelain polishing residue suggests that gas release may occur more easily. In contrast, potassium oxide (K2O), a natural fluxing agent, indicates the potential for the formation of a glassy phase and densification of the material. The iron oxide (Fe2O3), found in greater quantity in the ferruginous clay and responsible for its reddish color, also suggests that samples containing this clay may exhibit enhanced gas release.
The higher SiO2/ΣOF ratio observed in the kaolinitic clay indicates that materials produced from it may have higher strengths. It is also noted that the ferruginous clay contains higher levels of fluxing oxides (OF)—Fe2O3, MgO, K2O, and CaO—than the kaolinitic clay. The sum of fluxing oxides (ΣOF) influences the material’s expansion, as a higher total implies more gas release. The inorganic oxides (SiO2 and Al2O3) play a crucial role in controlling the material’s viscosity, helping to trap the gases that are released [29]. In this context, aggregates produced with ferruginous clay (ΣOF 14.34) are more likely to expand than those made with kaolinitic clay (ΣOF 2.86).
The mineralogical composition of the precursor materials was determined by X-ray diffraction (XRD) using the XRD 7000 equipment (Shimadzu, Kyoto, Japan). For radiation, copper Kα was used (40 kV/30 mA), with a scanning speed of 2°/min, a step size of 0.02°, and a scan range from 5° to 55°, yielding the results presented in Figure 1, Figure 2 and Figure 3.
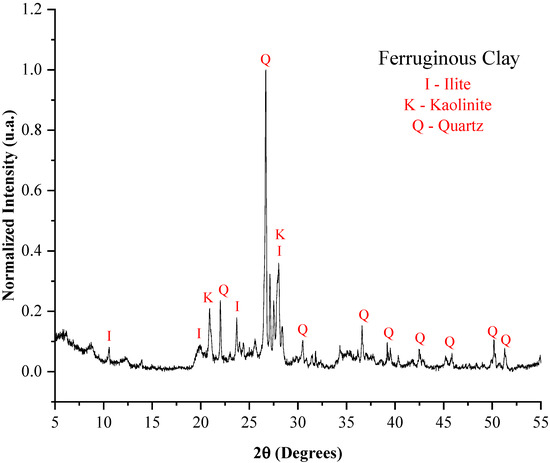
Figure 1.
X-ray diffraction pattern of the ferruginous clay.
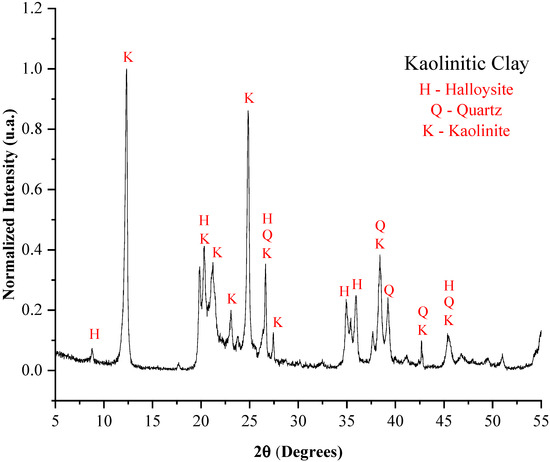
Figure 2.
X-ray diffraction pattern of the kaolinitic clay.
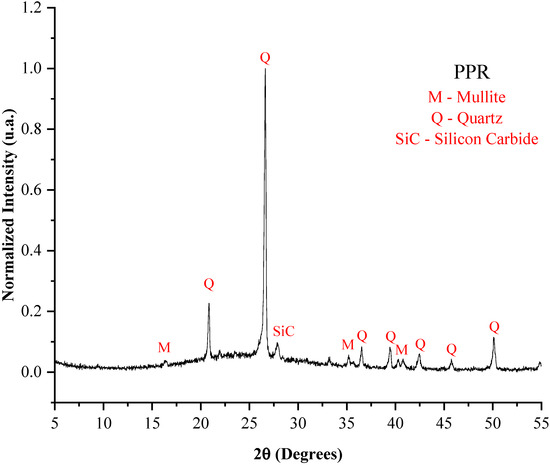
Figure 3.
X-ray diffraction pattern of the PPR.
The kaolinite found in the clays, the illite present in the ferruginous clay, and the halloysite identified in the kaolinitic clay are described as hydrated aluminum silicates, and their presence reflects the high concentrations of inorganic oxides (SiO2 and Al2O3). These minerals are significant in the production of lightweight aggregates due to the dehydroxylation process, which releases water in the form of vapor [36,37], contributing to pore formation.
The high silica content in the samples justifies the presence of quartz in all three precursor materials. Its presence is essential for stabilizing the aggregate matrix and increasing its strength [38], and also because it has a strong influence on the viscosity of the liquid phase, playing a crucial role in the expansion process. Similarly, mullite was identified in the porcelain polishing residue [39].
The presence of silicon carbide (SiC) in the PPR is explained by the abrasive material used during the polishing stage of porcelain tiles, which can contribute to expandability when subjected to high temperatures, due to the porosity generated from gas release [40].
2.3. Preparation and Characterization of Samples
Following a critical analysis of the study by [22], which identified the compositions that showed the best overall performance in terms of bloating index, density, water absorption, and mechanical strength, a composition consisting of 40% clay and 60% porcelain polishing residue was selected.
The input parameters for the Taguchi method were defined as follows: heating rate, sintering temperature, sintering time, and type of clay used [31]. Each parameter had four levels, with the heating rate ranging from 10 °C/min to 40 °C/min, sintering temperature from 1180 °C to 1240 °C, sintering time from 10 min to 25 min, and the type of clay being either kaolinitic (white) or ferruginous (red). Based on these factors, an orthogonal L16 matrix (43 × 21) was determined, requiring 16 experimental mixtures.
Table 2 presents the experimental program, as determined by Minitab 21 software, for the Taguchi method.

Table 2.
Experimental program determined using the Taguchi method.
The desired outputs, or evaluated responses, were bloating index, mass loss, specific gravity, water absorption [31], and crushing strength. The experimental procedures followed some of the methods established by [16]. For each test, a total of 25 samples were analyzed.
Using an analytical precision balance (Gehaka, AG200, São Paulo, SP, Brazil), the masses of the samples were measured for pellet preparation. After mixing the powders, moisture (distilled water) was added to provide the necessary plasticity for the extrusion forming process. For the ferruginous clay composition, 35% moisture was used, and for the kaolinitic clay composition, 40% moisture was added, resulting in two plastic masses.
The masses were left exposed to the air for 24 h. Next, pellet extrusion was performed using an extruder with a 10 mm diameter nozzle. Immediately after extrusion, the pellets were manually shaped, reaching a diameter of 17 mm. Once formed, the pellets were again left to air-dry for 24 h to begin gradually removing moisture, thereby preventing potential disintegration during the sintering stage due to rapid water loss.
Subsequently, the pellets were placed in a circulating air oven (Solab, SL-102, Piracicaba, SP, Brazil) at 110 °C for 24 h. Finally, the sintering process was carried out using the experimental program described in Table 2, using a Fortlab MC 1200/450 electric furnace (Guangdong Province, China).
To determine the bloating index, three diameter measurements were taken from the pellets using a digital caliper (precision of 0.01 mm), and their average volume was calculated (Equation (1)). This procedure was performed for both the oven-dried samples (initial volume) and the sintered samples (final volume), in order to capture the dimensional change.
where
- —average bloating index, in percentage (%);
- —initial volume (before sintering), in cm3;
- —final volume (after sintering), in cm3.
To determine mass loss (Equation (2)), the masses of the dried and sintered samples were measured using a precision balance.
where
- —average mass loss, in percentage (%);
- —dry mass, in grams;
- —sintered mass, in grams.
The specific gravity test (Equation (3)) was performed according to NBR 6458 (ABNT, 2025) [41], in which the saturated (surface-dry) and immersed masses are measured.
where
- —average specific gravity, in g/cm3;
- —sintered mass, in grams;
- —saturated mass, in grams;
- —immersed mass, in grams;
- —density of water (g/cm3) at the test temperature (°C).
The water absorption test (Equation (4)) was conducted based on an adaptation of NBR 6458 (ABNT, 2025) [41], in which the dry and saturated masses were measured. Measurements were taken on both whole and broken samples to assess the formation of a superficial impermeable layer and the connectivity of internal pores.
where
- —average water absorption, in percentage (%);
- —saturated mass, in grams;
- —sintered mass, in grams.
The crushing strength test was carried out using a universal testing machine (Shimadzu AG-X, Tokyo, Japan) with a load capacity of 10 kN. The loading rate applied was 1 mm/min.
For a better understanding of the mineralogical and microstructural behavior of the produced aggregates, four samples were selected for X-ray diffraction (XRD) and scanning electron microscopy (SEM) analyses. The selection was based on differences in performance observed in the physical tests.
2.4. Application Catalog and Statistical Modeling
For the development of the application catalog, the main properties of lightweight aggregates (specific gravity, water absorption, and crushing strength) were considered, along with the primary applications of the product: high-strength concrete, structural and non-structural lightweight concrete, lightweight mortars, geotechnics, gardening and landscaping, and thermal–acoustic insulation. The results obtained for each group of samples were compared with standards [42], commercial lightweight aggregate catalogs [43,44], and established literature [45,46], resulting in Table 3.

Table 3.
Reference values for the classification of lightweight aggregates.
For the statistical modeling using the Taguchi method and analysis of variance ANOVA, Minitab software was employed, while the surface plots were generated with the assistance of Matlab R2024a software.
Initially, the Taguchi analysis was performed for each response defined in the experimental program (Table 2), where the control factors influencing each property were analyzed, obtaining the signal-to-noise ratios necessary to rank the parameters that impacted each specific property, along with their respective graphs, enabling the determination of the optimal levels of each factor to achieve the desired results. The signal-to-noise (S/N) ratio was used to analyze the effects that the factors proposed in Table 1 have on the final properties of interest in this study. The S/N ratios are divided into three categories: smaller is better, larger is better, and nominal is best. For the properties analyzed in this study, the following definitions were applied: bloating index and crushing strength were set as larger is better, while mass loss, specific gravity, and water absorption were set as smaller is better.
For the analysis of variance, a generalized linear model was applied, which, similarly to the Taguchi ranking, yielded the contribution of each factor to each property analyzed. A confidence level of 95% and a significance level of 5% were adopted. Additionally, ANOVA used the F-test to identify the most relevant factors.
Finally, surface plots were produced for the main properties of the lightweight aggregates, namely specific gravity, water absorption, and crushing strength.
3. Results and Discussion
3.1. Technological Tests
Figure 4, Figure 5 and Figure 6 refer, respectively, to the comparison between the bloating index and mass loss, specific gravity and crushing strength, and water absorption. The results are presented as mean values, and error bars were included in the figures to represent the measurement error limits.
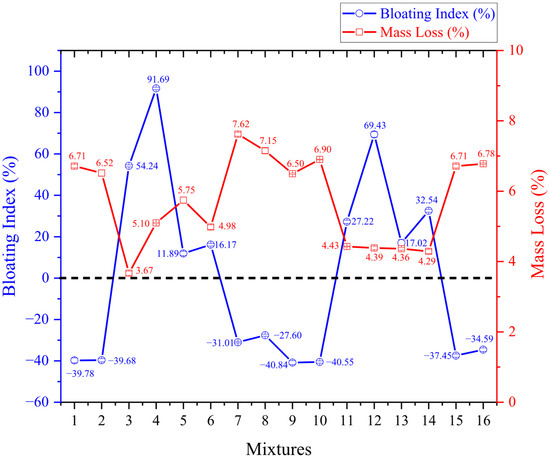
Figure 4.
Bloating index versus mass loss.
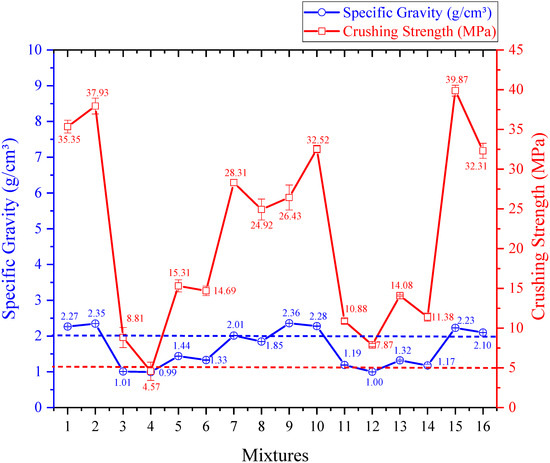
Figure 5.
Specific gravity versus crushing strength.
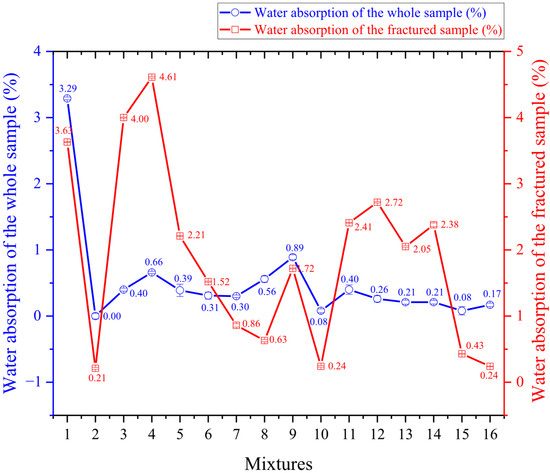
Figure 6.
Water absorption.
As shown in Figure 4, the experiments using kaolinitic clay exhibited shrinkage. It can be said that the lack of expansion in the kaolinitic clay samples was expected, given that this clay contains low levels of fluxing oxides (its total reaching only 2.86), which prevents the release and trapping of gases when heated. The combination of the chemical components of the kaolinitic clay and the porcelain polishing residue was not suitable to induce expansion in the material. A similar case is reported in the study by [27], which also found it challenging to generate expansion in aggregates developed with kaolinitic clay, as well as Moreno-Maroto et al. [47], who suggest that this type of clay should not be used in lightweight aggregate production due to the shrinkage it undergoes during sintering.
The shrinkage of the samples can be attributed to the dehydroxylation of kaolinite, a component of their mineralogy. It is evident that the most significant mass losses (6.5% in experiment 9 to 7.62% in experiment 7) occurred in the kaolinitic clay experiments and were associated with pellet shrinkage (40.84% in experiment 9 to 27.6% in experiment 8). The highest shrinkage occurred in the samples subjected to the lowest sintering temperatures (1180 °C and 1200 °C). That is, in addition to the kaolinitic clay lacking expansion potential, the incorporation of porcelain polishing residue into the mixture and heating it at low temperatures may further exacerbate sample shrinkage.
Unlike the samples made with kaolinitic clay, the ones produced with ferruginous clay exhibited high expandability (11.89% in experiment 5 to 91.69% in experiment 4). This behavior is attributed to the higher content of fluxing oxides in the chemical composition of ferruginous clay (its total reaches 14.34), particularly iron oxide, which is also responsible for the clay’s reddish color. Another factor contributing to the expansion is the decomposition of silicon carbide (SiC) found in the porcelain polishing residue. When subjected to high temperatures, the gases within the sample are released and trapped by the formation of the viscous liquid phase, causing the material to expand. Other studies have also reported similar results with ferruginous clay, as seen in the work of Moreno-Maroto et al. [14], which investigated several types of clays for lightweight aggregate production, found that ferruginous clay showed the most significant expansion. Likewise, Pontes et al. [22] also used porcelain polishing residue to produce lightweight aggregates and observed significant expansion values when ferruginous clay was used, reaching up to 187%.
Increasing the sintering temperature (up to 1240 °C) resulted in greater expansion in the ferruginous clay samples, with the expansion decreasing as the sintering temperature was reduced (down to 1180 °C). Despite the wide variation in expansion, little difference was observed in mass loss. Souza et al. [10] indicate that most mass loss occurs at temperatures below 1000 °C, suggesting that increasing the temperature beyond this point does not significantly contribute to further mass loss.
In general, lower heating rates at the same sintering temperature resulted in greater expansion and less shrinkage. This is because slower heating allows more time for gas entrapment and the formation of the viscous phase, which retains the gases—an essential factor for material expansion. Regarding sintering time, it was observed that longer durations (at a constant temperature) led to less shrinkage and greater expansion. This likely occurs because shorter times may not be sufficient for the material to expand fully.
As shown in Figure 5, seven experiments (1, 2, 7, 9, 10, 15, and 16) resulted in apparent densities above 2 g/cm3, which exceeds the normative threshold for classifying lightweight aggregates.
Since the samples made with kaolinitic clay exhibited shrinkage, the pellets became denser, resulting in higher specific gravity values (from 2.01 g/cm3 in experiment 7 to 2.36 g/cm3 in experiment 9). Among the kaolinitic clay samples, only experiment 8 met the criteria to be classified as a lightweight aggregate in terms of specific gravity (1.85 g/cm3).
When ferruginous clay was used (experiments 3, 4, 5, 6, 11, 12, 13, and 14), the samples exhibited different behavior. All these samples exhibited specific gravity values below 2.00 g/cm3, thereby qualifying as lightweight aggregates (ranging from 0.99 g/cm3 in experiment 4 to 1.44 g/cm3 in experiment 5). This result is due to the mass loss and expansion that occurred in the samples, which led to pore formation and imparted lightness to the material, consequently reducing its specific gravity. Similar results were reported by Pontes et al. [22], who found that higher sintering temperatures led to lower densities. In contrast, samples sintered at lower temperatures exhibited higher densities, in some cases exceeding the threshold for lightweight aggregates.
It is also observed that the temperature of 1200 °C yielded the lowest apparent densities for ferruginous clay samples (0.99 g/cm3 in experiment 4 and 1.00 g/cm3 in experiment 12), which is consistent with the high expansion previously noted.
Regarding sintering time, it was observed that shorter durations resulted in higher densities, likely because insufficient time limits the release of gases that contribute to the material’s lightness. Conversely, slower heating rates led to lighter materials, whereas higher rates caused disordered pore formation, which is prone to collapse and results in increased density.
The samples made with kaolinitic clay had higher apparent densities, low water absorption, and high crushing strength (from 24.92 MPa in experiment 8 to 39.87 MPa in experiment 15), due to the shrinkage that made them denser and more compact.
On the other hand, the ferruginous clay samples did not show high crushing strength compared to those made with kaolinitic clay, as their internal porosity made them more fragile. Nevertheless, they still presented satisfactory values for lightweight aggregates (from 4.57 MPa in experiment 4 to 15.31 MPa in experiment 5), except for experiment 4, which resulted in a value below the threshold recommended for high-strength concrete (5.00 MPa).
Overall, as shown in Figure 6, the samples presented water absorption values within the recommended range (0% to 20%) for the main applications of lightweight aggregates (concrete), varying from 0% in experiment 2 to 3.29% in experiment 1.
It was observed that water absorption increased in all samples when the test was conducted on fractured pellets. However, the most significant increases occurred in the samples with ferruginous clay, whereas the samples with kaolinitic clay showed minimal differences. This is because the ferruginous clay samples tend to form a glassy phase, like a film encasing the sample, which, during whole-sample testing, prevents water from reaching the internal pores, resulting in low absorption values, as can be observed in [15]; the sample in which a vitreous layer was formed exhibited greater resistance to water absorption. When the test is performed on fractured samples, the internal pores come into greater contact with the water, leading to higher absorption.
Although the ferruginous clay samples demonstrated high expandability, low specific gravity, and significant pore formation, their water absorption remained low due to the reduced surface porosity caused by the gradual formation of the liquid phase during sintering. However, when water absorption tests were performed on fractured samples, a higher absorption was confirmed as the internal pores were directly exposed to water. Pontes et al. [22] reported minimum absorption values of 0.83% in samples made with ferruginous clay, which are consistent with the results found in this study.
The samples produced with kaolinitic clay also showed low water absorption, as their structure became denser and more compact.
To explore the relationship between physical performance and mineralogical–microstructural features, four samples were selected based on the most pronounced differences observed in the physical tests. In the kaolinitic clay group, sample 1 showed unusually high water absorption, and sample 8 was the only one classified as lightweight. In the ferruginous clay group, sample 4 exhibited the highest expansion, while sample 5 achieved the highest mechanical strength. These samples were analyzed by XRD and SEM to identify the crystalline phases and microstructural features associated with these distinct behaviors. The X-ray diffraction, copper Kα was used (40 kV/30 mA), with a scanning speed of 2°/min, a step size of 0.02°, and a scan range from 10° to 80°analyses for the four selected samples are shown in Figure 7.
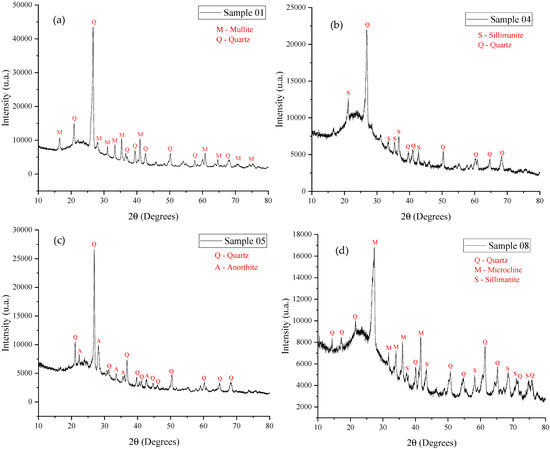
Figure 7.
X-ray diffraction: (a) sample 1 (1180 °C); (b) sample 4 (1240 °C); (c) sample 5 (1180 °C); and (d) sample 8 (1240 °C).
The presence of silica in all three raw materials (kaolinitic clay, ferruginous clay, and porcelain polishing residue), when sintered at high temperatures (above 1000 °C), can form quartz, which explains its presence in all the analyzed samples (all of which were sintered above 1000 °C).
Alumina, also present in raw materials, when combined with silica and sintered at lower temperatures, can form mullite, as observed in sample 1 (1180 °C). Additionally, sillimanite was identified in samples 4 and 8 (1240 °C), which were sintered at slightly higher temperatures.
Calcium oxide, found in the ferruginous clay and the porcelain polishing residue, in the presence of silica and alumina, can promote the formation of anorthite, which appeared in sample 5 (1180 °C) under low-temperature sintering.
Potassium, present in the kaolinitic clay (and also in the porcelain polishing residue), when combined with the main elements (silica and alumina) and sintered at higher temperatures, may lead to the formation of microcline, identified in sample 8 (1240 °C).
From a mineralogical perspective, kaolinite, halloysite, and illite present in the raw materials (Figure 1, Figure 2 and Figure 3), all of which are lamellar phases, are known to undergo thermal decomposition above 1000 °C, leading to the formation of mullite and sillimanite. Quartz is stable at high temperatures and remains present in all samples analyzed after sintering. The formation of anorthite and microcline is consistent with the presence of calcium-rich phases in the raw materials, such as illite. Thus, the development of mineralogical phases after sintering is well correlated with the phases identified in the raw materials.
Each mineral present in the samples plays an important role in modifying the expansive microstructure of the materials. Quartz, being a hard and stable mineral, contributes to mechanical strength, but its low reactivity limits expansion. Anorthite, a calcium- and aluminum-rich feldspathic mineral, acts as a flux during sintering, facilitating the formation of the glassy phase, which promotes expansion and the development of a porous microstructure. Microcline, another potassium feldspar, also contributes to the generation of the glassy phase, but its influence may vary depending on the sintering temperature and the chemical composition of the mixture. Sillimanite, a resistant aluminosilicate mineral, contributes to structural stability, assisting in the formation of a solid matrix. Finally, mullite, formed from the reaction between alumina and silica at high temperatures, imparts high mechanical strength and thermal stability but may reduce porosity.
The SEM images are shown in Figure 8. In all figures, the presence of well-defined pores and grains is readily observable. The kaolinitic clay samples (Figure 8a,d) showed a relatively low number of pores. In contrast, the ferruginous clay samples displayed a greater quantity of well-distributed pores (Figure 8b,c), confirming the visual analysis.
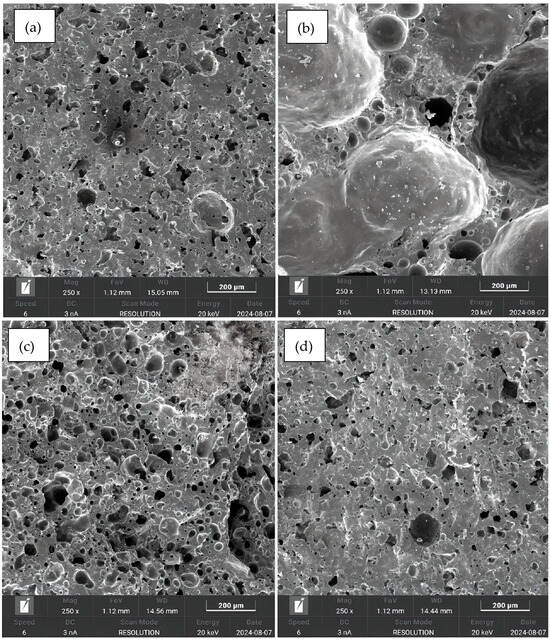
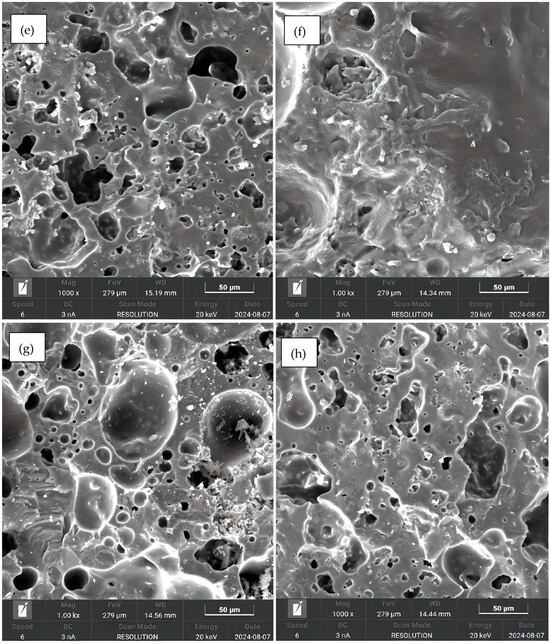
Figure 8.
SEM at 500 μm: (a) sample 1; (b) sample 4; (c) sample 1; (d) sample 4; (e) sample 5; (f) sample 8; (g) sample 5; and (h) sample 8.
The SEM observations support the results of the crushing strength tests, since the kaolinitic clay samples, with lower porosity, showed higher strength, while the ferruginous clay samples, with higher porosity, had lower crushing strength. The presence or absence of pores also supports the findings on specific gravity of the aggregates: the more pores, the greater the expansion and the lower the density.
The morphology observed via scanning electron microscopy converges with the water absorption results from the fractured samples, indicating that both techniques complement each other in characterizing the porous structures of the aggregates. While the ferruginous clay samples exhibited more pores in the SEM images, they also showed higher water absorption compared to the kaolinitic clay samples, which contained fewer pores and lower water absorption values. This correlation suggests that denser microstructures correspond to lower porosity, while more porous and interconnected microstructures are associated with higher absorption. When comparing SEM data with the absorption results from whole samples, a divergence is observed that can be explained by the presence of isolated pores not connected to the surface, which do not allow water penetration [48]. Therefore, the water absorption results corroborate the findings obtained from the SEM images, supporting the interpretation of the porous structure on the aggregates. Furthermore, as addressed in [49], surface porosity directly influences both the impermeability and the water absorption capacity of lightweight aggregates, and the combined use of SEM and the water absorption test contributes to a broader understanding of their porosity characteristics.
3.2. Applications
Based on the limits of specific gravity, water absorption, and strength required for a material to be classified as a lightweight aggregate (Table 3), an analysis was carried out using the results obtained from the 16 experiments. This analysis generated Table 4, which indicates whether each experiment meets the criteria for any of the main applications of lightweight aggregates.

Table 4.
Applications of the produced aggregates.
It was observed that seven experiments (1, 2, 7, 9, 10, 15, and 16) do not meet the criteria for any industrial applications. These correspond to the samples made with kaolinitic clay that exhibited apparent densities greater than 2.00 g/cm3. Only one experiment using kaolinitic clay fulfilled the necessary criteria and was classified as a lightweight aggregate (experiment 8), that is, it exhibited a density below 2.00 g/cm3, which is the threshold for an aggregate to be classified as lightweight. On the other hand, almost all ferruginous clay samples met the requirements for the three main applications (high-strength concrete, structural lightweight concrete, and non-structural lightweight concrete and lightweight mortars), as they exhibited densities below 2.00 g/cm3, water absorption between 0% and 20% for high-strength concrete and structural lightweight concrete, as well as absorption between 0% and 34% for non-structural lightweight concrete and lightweight mortars. These samples also met the strength requirements, with all exceeding 5.00 MPa (for high-strength concrete), 2.30 MPa (for structural lightweight concrete), and 1.80 MPa (for non-structural lightweight concrete and lightweight mortars), except experiment 4, which did not qualify for high-strength concrete because it did not reach the minimum required strength of 5 MPa. However, none of the 16 experiments met the criteria for geotechnical applications, gardening and landscaping, or thermal–acoustic insulation, as none of the samples reached the required water absorption range (10% to 38%).
It can be concluded that it was possible to produce lightweight aggregates with commercial potential for the three main engineering applications (concretes) using kaolinitic and ferruginous clays from the coast of Paraiba, combined with porcelain polishing residue in the formulations and variations in sintering parameters.
3.3. Statistical Analyses
Each result is influenced by a unique set of variables, making direct comparisons difficult. Therefore, the Taguchi method and analysis of variance ANOVA were applied to statistically determine how each variable affects the properties of the analyzed aggregates.
3.3.1. Taguchi Method
Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13 present the signal-to-noise ratio results for the analysis of bloating index, mass loss, specific gravity, water absorption, and crushing strength versus controllable variables.
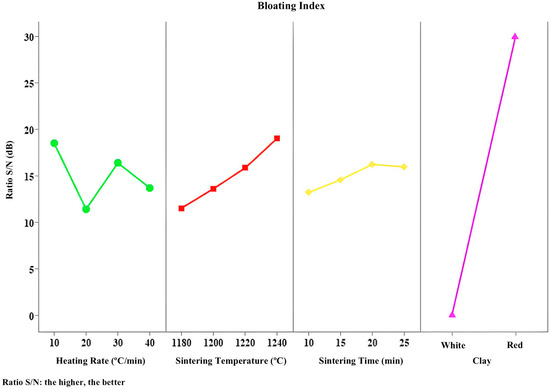
Figure 9.
S/R responses to bloating index.
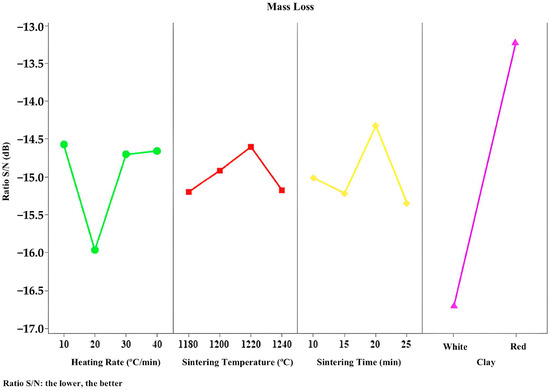
Figure 10.
S/N responses to mass loss.
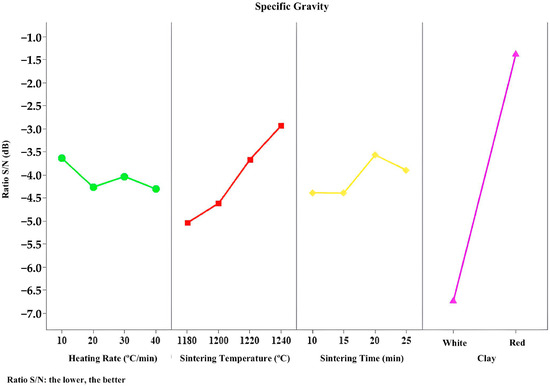
Figure 11.
S/N responses to specific gravity.
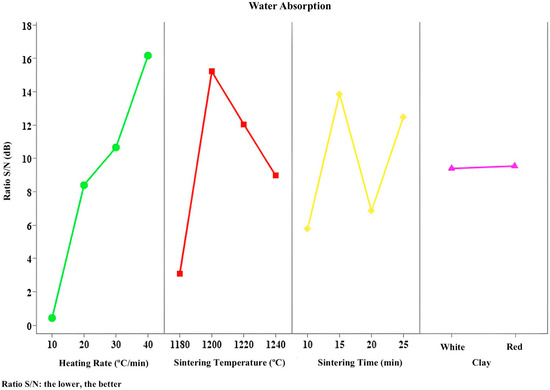
Figure 12.
S/R responses to water absorption.
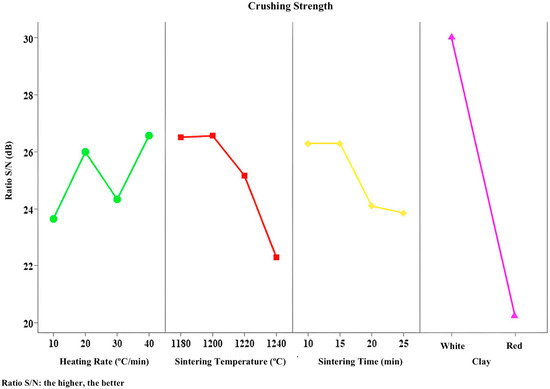
Figure 13.
S/R responses to crushing strength.
In Figure 9, it can be observed that the type of clay used is the most influential factor in achieving greater material expansion. Indeed, as discussed earlier, regardless of the heating parameter levels adopted, ferruginous clay expands, while kaolinitic clay does not. This is due to the chemical and mineralogical characteristics of the raw materials, particularly the specific properties of each clay type. The curve corresponding to the clay type is the steepest, and thus the most significant. The greater the variation in the curve, the more relevant that parameter is to the process.
Figure 10 shows that the type of clay is also the most influential factor in mass loss, followed by heating rate, sintering time, and sintering temperature. For this property analyzed, the higher the signal-to-noise ratio, the lower the variance in mass loss around the desired value (the lower, the better). It is worth noting the importance of having more than two levels for each factor, because where a straight line would typically connect two points, there may be a third between them. This third point could culminate in the loss of a more suitable area for the desired result.
It is possible to notice in Figure 11 that the type of clay used is the factor with the most significant influence on achieving a lower specific gravity. As previously mentioned, specific gravity is associated with material expansion, which in turn is related to the type of clay used. The curves for clay type and sintering temperature are the steepest, making them the most significant and therefore the most relevant parameters in this process.
The curves shown in Figure 12 indicate that the heating rate is the most impactful factor on water absorption (for whole samples), followed by sintering temperature, sintering time, and clay type. The type of clay has minimal relevance when it comes to water absorption.
Figure 13, which displays the signal-to-noise ratio for crushing strength versus controllable variables, shows that the type of clay is once again the most influential factor for this property. Indeed, crushing strength is related to the type of clay used, since, as seen earlier, the use of kaolinitic clay, regardless of other heating parameters, resulted in the highest strength values, while replacing it with ferruginous clay led to a decrease in strength. These findings are directly linked to the chemical and mineralogical characteristics of the clays used.
3.3.2. Analysis of Variance
From Table 5, it can be observed that the parameter with the greatest contribution to material expansion was the type of clay, followed by sintering temperature.

Table 5.
ANOVA analysis for bloating index.
The Taguchi and ANOVA analyses converge in identifying the parameters with the most significant influence on this property. Thus, the ideal combination for achieving greater expansion is the use of ferruginous clay, which has a higher fluxing oxide content and directly contributes to material expansion; a sintering temperature of 1240 °C, as higher temperatures promote pore formation and consequent expansion; a heating rate of 10 °C/min, as slower rates facilitate the trapping of gases and formation of pores that lead to higher expansion; and a sintering time of 20 min, which allows the pores to form and stabilize, resulting in material expansion.
In the ANOVA test for mass loss (Table 6), it is noted that the ranking of the most influential factors to mass loss converges with the Taguchi method, resulting in the definition of the use of ferruginous clay, a heating rate of 10 °C/min, a sintering time of 20 min and a sintering temperature of 1220 °C.

Table 6.
ANOVA analysis of mass loss.
For specific gravity (Table 7), it is again observed that the parameter with the most significant influence was the type of clay, followed by sintering temperature.

Table 7.
ANOVA analysis for specific gravity.
The Taguchi and ANOVA analyses once more converge in identifying the most influential parameters for this property. Therefore, the optimal combination for achieving lower specific gravity includes the use of ferruginous clay, since its chemical and mineralogical composition favors expansion and thus results in lower density; a sintering temperature of 1240 °C, which, like the clay type, is associated with greater expansion; a sintering time of 20 min, also favorable for expansion and reduced specific gravity; and a heating rate of 10 °C/min, which promotes greater expansion and lower density.
In the ANOVA test for water absorption (Table 8), it can be noted that the ranking of the factors with the greatest contribution to absorption closely resembles the order established by the Taguchi method, resulting in the optimal conditions of a heating rate of 40 °C/min, sintering temperature of 1220 °C, sintering time of 15 min, and the use of ferruginous clay.

Table 8.
ANOVA analysis for water absorption.
Finally, Table 9 shows the parameters that contributed most to the crushing strength, namely type of clay, followed by sintering time, sintering temperature, and heating rate, with the latter being the least influential factor.

Table 9.
ANOVA analysis for crushing strength.
The analyses closely align in identifying the most influential parameters for this property. Thus, the ideal combination to achieve higher crushing strength involves the use of kaolinitic clay, because due to its low content of fluxing oxides, the material undergoes shrinkage and becomes denser, which enhances strength; a sintering temperature of 1200 °C, since lower temperatures inhibit pore formation, increasing density and strength; a heating rate of 40 °C/min, because a faster rate can cause the collapse of any developing porous structure, resulting in a denser material; and a sintering time of 10 min, as shorter times may limit full pore development, thereby increasing the density and strength of the material.
3.3.3. Surface Charts
Figure 14, Figure 15 and Figure 16 present results for specific gravity, water absorption, and crushing strength as a function of the main variables identified through the Taguchi method.
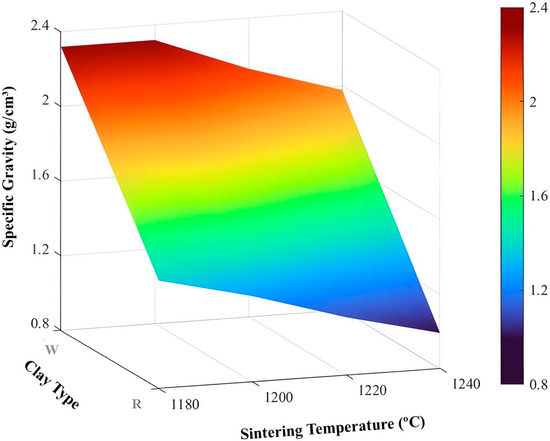
Figure 14.
Surface for specific gravity.
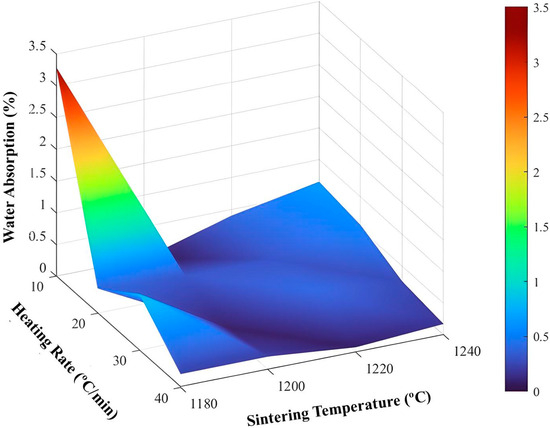
Figure 15.
Surface for water absorption.
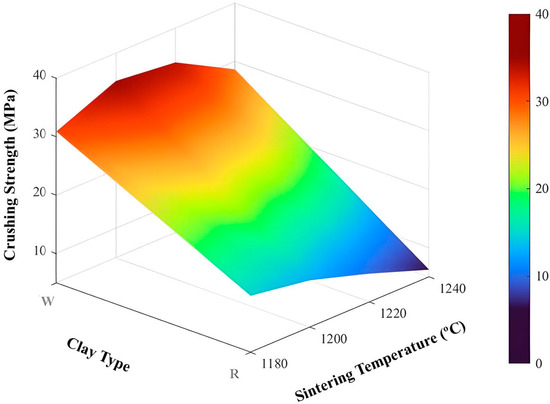
Figure 16.
Surface for crushing strength.
As shown in Figure 14, the samples made with kaolinitic clay exhibit higher apparent densities compared to those made with ferruginous clay. Furthermore, the higher the sintering temperature, the lower the specific gravity.
Figure 15 shows that, in general, regardless of the heating rate and sintering temperature used, water absorption remains at low levels.
The results in Figure 16 indicate that the use of kaolinitic clay yields aggregates with high crushing strength, whereas ferruginous clay results in aggregates with lower strength. The graph also shows that for kaolinitic clay, intermediate sintering temperatures of 1200 °C and 1220 °C led to higher strength values. In contrast, for ferruginous clay, lower sintering temperatures result in the highest strength values.
4. Conclusions
This study demonstrated that, although fixed parameters were established for the aggregates, such as pellet size and residue proportions, their expandability was high when ferruginous clay was used in the composition, and nonexistent when kaolinitic clay was used, as shrinkage occurred. Regarding mass loss, it was observed that the highest percentages were associated with the samples made with kaolinitic clay.
Some samples presented specific gravity values above the established limits for lightweight aggregates, which is influenced by the bloating index, since the samples that did not meet the criteria for lightweight aggregates in terms of specific gravity were those that underwent shrinkage. Water absorption and crushing strength showed satisfactory results, with the highest strengths observed in the kaolinitic clay samples.
Although the literature suggests [22] that porcelain polishing residue promotes expansion due to its favorable mineralogical composition for gas formation and liquid phase generation, as well as high contents of fluxing oxides, this was not confirmed for the samples containing kaolinitic clay, within the studied proportions and conditions.
Future studies should consider employing mercury intrusion porosimetry (MIP) to obtain greater precision in determining the ratio between open and closed pores in lightweight aggregates. Although the present study provides relevant information on the porous structure through the techniques applied, these methods are not the most suitable for accurately differentiating pore types. Incorporating MIP in subsequent research will allow for a more detailed assessment of pore connectivity and morphology, improving the understanding of how the porous structure influences water absorption and mechanical performance.
This study presents some limitations in the testing methods that should be addressed in future research to provide a more comprehensive understanding of the materials. The pore analysis would benefit from the use of mercury intrusion porosimetry (MIP), which offers a more precise quantification of open and closed pores. Although the grain size distribution, chemical and mineralogical composition, and applied physical property tests provided interesting information, the inclusion of additional techniques could strengthen the assessment of the performance and applicability of the aggregates. Recognizing these limitations, future studies are encouraged to expand the experimental scope to deepen the knowledge about the potential of clay-based formulations with porcelain polishing waste for the production of sustainable lightweight aggregates.
The statistical methods applied in the field of materials engineering, such as the Taguchi method and analysis of variance (ANOVA) used in this study, proved to be valuable tools for identifying which production factors should receive greater attention from professionals, with the aim of reducing economic losses, improving and standardizing performance, and enhancing the quality of products delivered to society. The combined application of these techniques enabled the identification and quantification of the influence of the main process factors on the properties of lightweight aggregates, optimizing parameters based on a reduced number of experiments and providing statistical robustness to the results obtained, particularly in studies focused on sustainability and the reuse of industrial waste.
It is possible to produce lightweight aggregates using clays from the coastal region of Paraíba and porcelain polishing residue as a pyro-expansive agent, by adjusting the sintering parameters (heating rate, temperature, and time) according to the desired property, making it a technically viable alternative.
Author Contributions
Conceptualization, V.S.M.d.O., J.A.d.S.N., G.L.d.N., M.A.S.d.A. and R.P.S.D.; Methodology, V.S.M.d.O., J.A.d.S.N. and G.L.d.N.; Validation, R.P.S.D.; Formal analysis, V.S.M.d.O., J.A.d.S.N., G.L.d.N., M.A.S.d.A., R.P.S.D. and C.M.P.; Investigation, V.S.M.d.O., J.A.d.S.N. and G.L.d.N.; Writing—original draft, V.S.M.d.O., J.A.d.S.N. and G.L.d.N.; Writing—review & editing, M.A.S.d.A., R.P.S.D. and C.M.P.; Visualization, M.A.S.d.A., R.P.S.D. and C.M.P.; Supervision, M.A.S.d.A. and R.P.S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, Y.; Zhao, F. Impact of Energy Depletion, Human Development, and Income Distribution on Natural Resource Sustainability. Resour. Policy 2023, 83, 103531. [Google Scholar] [CrossRef] [PubMed]
- Kępniak, M.; Łukowski, P. Multicriteria Analysis of Cement Mortar with Recycled Sand. Sustainability 2024, 16, 1773. [Google Scholar] [CrossRef]
- United Nations Environment Programme. 2021 Global Status Report for Buildings and Construction. 2021. Available online: https://globalabc.org/sites/default/files/2021-10/GABC_Buildings-GSR-2021_BOOK.pdf (accessed on 17 August 2024).
- López-Acevedo, F.J.; Herrero, M.J.; Escavy Fernández, J.I.; González Bravo, J. Potential Reduction in Carbon Emissions in the Transport of Aggregates by Switching from Road-Only Transport to an Intermodal Rail/Road System. Sustainability 2024, 16, 9871. [Google Scholar] [CrossRef]
- Mañosa, J.; Formosa, J.; Giro-Paloma, J.; Maldonado-Alameda, A.; Quina, M.J.; Chimenos, J.M. Valorisation of Water Treatment Sludge for Lightweight Aggregate Production. Constr. Build. Mater. 2021, 269, 121335. [Google Scholar] [CrossRef]
- Tang, C.W.; Cheng, C.K. Sustainable Use of Sludge from Industrial Park Wastewater Treatment Plants in Manufacturing Lightweight Aggregates. Materials 2022, 15, 1785. [Google Scholar] [CrossRef] [PubMed]
- Thukkaram, S.; Arun Kumar, A. Behaviour of Sewage Sludge Based Lightweight Aggregate in Geopolymer Concrete. Mater. Res. Express 2024, 11, 055501. [Google Scholar] [CrossRef]
- Lu, N.; Chen, H.; Chen, J.; Cao, Y.F. Ceramic Aggregate Material Formulated with MSWI Fly Ash and Fuel Ash for Use as Filter Media. Minerals 2023, 13, 845. [Google Scholar] [CrossRef]
- Alqenai, Y.; Balapour, M.; Zooyousefin, M.; Shresthal, N.; Hsuan, Y.G.; Farnam, Y. Investigating Effects of Sintering Mean Residence Time on Engineering Properties of Coal Ash-Based Lightweight Aggregate. Int. J. Appl. Ceram. Technol. 2025, 22, e14854. [Google Scholar] [CrossRef]
- de Souza, N.S.L.; dos Anjos, M.A.S.; Sá, M.d.V.V.A.d.; de Farias, E.C.; de Souza, M.M.; Branco, F.G.; Pereira, A. Evaluation of Sugarcane Bagasse Ash for Lightweight Aggregates Production. Constr. Build. Mater. 2021, 271, 121604. [Google Scholar] [CrossRef]
- Din, N.A.M.; Muthusamy, K.; Embong, R.; Krishnaraj, L. Recycling of Oil Palm Shell as Aggregate in Concrete: A Review. Construction 2024, 4, 28–36. [Google Scholar] [CrossRef]
- Dondi, M.; Cappelletti, P.; D’Amore, M.; de Gennaro, R.; Graziano, S.F.; Langella, A.; Raimondo, M.; Zanelli, C. Lightweight Aggregates from Waste Materials: Reappraisal of Expansion Behavior and Prediction Schemes for Bloating. Constr. Build. Mater. 2016, 127, 394–409. [Google Scholar] [CrossRef]
- Soltan, A.M.M.; Kahl, W.A.; El-Raoof, F.A.; El-Kaliouby, B.A.H.; Serry, M.A.K.; Abdel-Kader, N.A. Lightweight Aggregates from Mixtures of Granite Wastes with Clay. J. Clean. Prod. 2016, 117, 139–149. [Google Scholar] [CrossRef]
- Moreno-Maroto, J.M.; González-Corrochano, B.; Alonso-Azcárate, J.; Rodríguez, L.; Acosta, A. Manufacturing of Lightweight Aggregates with Carbon Fiber and Mineral Wastes. Cem. Concr. Compos. 2017, 83, 335–348. [Google Scholar] [CrossRef]
- Mendonça de Souza, M.; Barbosa, N.P.; dos Anjos, M.A.S.; Aguiar, J.G.C.; da Silva Neto, J.A.; Pederneiras, C.M. Recycling Red Ceramic Waste as a Raw Material for Lightweight Aggregates. Appl. Sci. 2025, 15, 5729. [Google Scholar] [CrossRef]
- da Silva Neto, J.A.; dos Anjos, M.A.S.; Dutra, R.P.S.; Mendonça de Souza, M.; Pederneiras, C.M. Development of Sustainable Artificial Lightweight Aggregates with Binary Mixtures of Waste Rich in Aluminosilicate and Carbonate in Kaolinitic Clay. Sustainability 2025, 17, 2017. [Google Scholar] [CrossRef]
- da Silva Neto, J.A.; dos Anjos, M.A.S.; Dutra, R.P.S.; Mendonça de Souza, M.; Pederneiras, C.M. Optimization via Taguchi of Artificial Lightweight Aggregates Obtained from Kaolinite Clay and Ceramic Waste: Development and Industrial Applications. Buildings 2025, 15, 2003. [Google Scholar] [CrossRef]
- de Sousa, J.T.F.; dos Anjos, M.A.S.; Neto, J.A.d.S.; de Farias, E.C.; Branco, F.G.; Pederneiras, C.M. Self-Compacting Concrete with Artificial Lightweight Aggregates from Sugarcane Ash and Calcined Scheelite Mining Waste. Appl. Sci. 2025, 15, 452. [Google Scholar] [CrossRef]
- Wichmann, I.; Firdous, R.; Stephan, D. Upcycling of Demolition Material from Concrete and Brick for the Production of Cold-Bound, Alkali-Activated Lightweight Aggregates. Mater. Struct. 2023, 56, 135. [Google Scholar] [CrossRef]
- Resende, D.M.; de Carvalho, J.M.F.; Paiva, B.O.; Gonçalves, G.d.R.; Costa, L.C.B.; Peixoto, R.A.F. Sustainable Structural Lightweight Concrete with Recycled Polyethylene Terephthalate Waste Aggregate. Buildings 2024, 14, 609. [Google Scholar] [CrossRef]
- Han, S.; Ju, T.; Meng, F.; Lin, L.; Li, J.; Chen, K.; Jiang, J. Comprehensive Study of Recycling Municipal Solid Waste Incineration Fly Ash in Lightweight Aggregate with Bloating Agent: Effects of Water Washing and Bloating Mechanism. Sci. Total Environ. 2023, 881, 163267. [Google Scholar] [CrossRef] [PubMed]
- Pontes, I.C.; Neto, J.A.S.; Campos, L.F.A.; Anjos, M.A.S.; Dutra, R.P.S. Avaliação Mecânica de Agregados Leves Obtidos Com Diferentes Argilas e Percentuais de Resíduo Do Polimento Do Porcelanato. In Proceedings of the 5th Luso-Brazilian Congress of Sustainable Construction Materials, Lisbon, Portugal, 6–8 November 2024. [Google Scholar]
- Almeida, M.I.; Dias, A.C.; Demertzi, M.; Arroja, L. Environmental Profile of Ceramic Tiles and Their Potential for Improvement. J. Clean. Prod. 2016, 131, 583–593. [Google Scholar] [CrossRef]
- Soares Filho, J.E.; Aurich, J.C.; Sousa, F.J.P.; Nascimento, R.M.; Paskocimas, C.A.; Silva, A.H.A. Polishing Performance of Eco-Friendly Porcelain Stoneware Tiles Reusing Bricks and Roof Tiles Wastes. J. Clean. Prod. 2020, 256, 120362. [Google Scholar] [CrossRef]
- Ramos, G.A.; de Matos, P.R.; Pelisser, F.; Gleize, P.J.P. Effect of Porcelain Tile Polishing Residue on Eco-Efficient Geopolymer: Rheological Performance of Pastes and Mortars. J. Build. Eng. 2020, 32, 101699. [Google Scholar] [CrossRef]
- Santos, H.M.M.; Jochem, L.F.; de Matos, P.R.; Casagrande, C.A.; Marinho, É.P.; Szeląg, M.; de Nóbrega, A.C.V. Porcelain Tile Polishing Residue in Concrete as an Additive or Replacement for Portland Cement. Appl. Sci. 2023, 13, 2824. [Google Scholar] [CrossRef]
- De Souza, N.S.L.; Dos Anjos, M.A.S.; De Sá, M.d.V.V.A.; De Farias, E.C.; Mello, L.C.D.A. Development of Lightweight Aggregates from Crushing of Ornamental Stones (Granites and Marbles) and Clay. Rev. Mater. 2020, 25, e-12559. [Google Scholar] [CrossRef]
- Riley, C.M. Relation of Chemical Properties to the Bloating of Clays. J. Am. Ceram. Soc. 1951, 34, 121–128. [Google Scholar] [CrossRef]
- Ayati, B.; Ferrándiz-Mas, V.; Newport, D.; Cheeseman, C. Use of Clay in the Manufacture of Lightweight Aggregate. Constr. Build. Mater. 2018, 162, 124–131. [Google Scholar] [CrossRef]
- Fenner, J.; Zeller, A.; Liebezeit, S.; Knorr, M.; Schnell, A.; Mettke, L.; Goldmann, D. Investigation of Industrial Residues and Waste Materials to Expand the Raw Material Base for the Production of Lightweight Aggregates. Mater. Proc. 2023, 15, 71. [Google Scholar]
- Chen, H.J.; Chang, S.N.; Tang, C.W. Application of the Taguchi Method for Optimizing the Process Parameters of Producing Lightweight Aggregates by Incorporating Tile Grinding Sludge with Reservoir Sediments. Materials 2017, 10, 1294. [Google Scholar] [CrossRef]
- Bamshad, O.; Mahdikhani, M.; Ramezanianpour, A.M.; Maleki, Z.; Majlesi, A.; Habibi, A.; Delavar, M.A. Prediction and Multi-Objective Optimization of Workability and Compressive Strength of Recycled Self-Consolidating Mortar Using Taguchi Design Method. Heliyon 2023, 9, e16381. [Google Scholar] [CrossRef]
- Vignesh, R.; Abdul-Rahim, A. New Insights into the Production of Sustainable Synthetic Aggregates and Their Microstructural Evaluation. Mater. Constr. 2023, 73, e324. [Google Scholar] [CrossRef]
- Nunes, J.J.B.d.C.; Teixeira, A.M.A.J.; Saraiva, R.M.D.d.C. Caracterização Morfológica Do Agregado Leve de Argila Expandida Brasileira Com Utilização Do AIMS. Ambiente Construído 2021, 21, 213–227. [Google Scholar] [CrossRef]
- NBR 6502:1995; Rocks and Soils–Terminology. Brazilian Association of Technical Standards (ABNT): Rio de Janeiro, Brazil, 1995.
- Izadifar, M.; Thissen, P.; Steudel, A.; Kleeberg, R.; Kaufhold, S.; Kaltenbach, J.; Schuhmann, R.; Dehn, F.; Emmerich, K. Comprehensive Examination of Dehydroxylation of Kaolinite, Disordered Kaolinite, and Dickite: Experimental Studies and Density Functional Theory. Clays Clay Min. 2020, 68, 319–333. [Google Scholar] [CrossRef]
- Daou, I.; Lecomte-Nana, G.L.; Tessier-Doyen, N.; Peyratout, C.; Gonon, M.F.; Guinebretiere, R. Probing the Dehydroxylation of Kaolinite and Halloysite by in Situ High Temperature X-ray Diffraction. Minerals 2020, 10, 480. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Atmaca, N. Cold Bonded and Low Temperature Sintered Artificial Aggregate Production by Using Waste Materials. Period. Polytech. Civ. Eng. 2023, 67, 112–122. [Google Scholar] [CrossRef]
- Balapour, M.; Thway, T.; Rao, R.; Moser, N.; Garboczi, E.J.; Hsuan, Y.G.; Farnam, Y. A Thermodynamics-Guided Framework to Design Lightweight Aggregate from Waste Coal Combustion Fly Ash. Resour. Conserv. Recycl. 2022, 178, 106050. [Google Scholar] [CrossRef]
- Cuevas, K.; Lopez, M. The Effect of Expansive Agent and Cooling Rate in the Performance of Expanded Glass Lightweight Aggregate as an Internal Curing Agent. Constr. Build. Mater. 2021, 271, 121505. [Google Scholar] [CrossRef]
- NBR 6458; Soils—Determination of the Specific Mass of Solids, the Apparent Specific Mass and the Water Absorption of the Fraction Retained in the Sieve with an Opening of 2.0 mm. Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 2025.
- EN 13055:2016; Lightweight Aggregates–Part 1: Lightweight Aggregates for Concrete, Mortar and Grout. European Committee for Standardization (CEN): Brussels, Belgium, 2016.
- CINEXPAN, Expanded Clay. Technical Data Sheet Type1506.Pdf. Available online: https://www.Cinexpan.Com.Br/Pdf/Ficha-Tecnica-Tipo-1506.Pdf (accessed on 15 July 2024).
- CINEXPAN, Expanded Clay. Technical Data Sheet Type 2215.Pdf. Available online: https://www.Cinexpan.Com.Br/Pdf/Ficha-Tecnica-Tipo-2215.Pdf (accessed on 15 July 2024).
- De Santis, B.C.; Rossignolo, J.A. Influence of Calcined Clay Lightweight Aggregates on the Mechanical Properties of Structural Concretes. Rev. Mater. 2015, 20, 399–406. [Google Scholar] [CrossRef]
- Lyra, G.P.; dos Santos, V.; De Santis, B.C.; Rivaben, R.R.; Fischer, C.; Pallone, E.M.d.J.A.; Rossignolo, J.A. Reuse of Sugarcane Bagasse Ash to Produce a Lightweight Aggregate Using Microwave Oven Sintering. Constr. Build. Mater. 2019, 222, 222–228. [Google Scholar] [CrossRef]
- Moreno-Maroto, J.M.; Uceda-Rodríguez, M.; Cobo-Ceacero, C.J.; Cotes-Palomino, T.; Martínez-García, C.; Alonso-Azcárate, J. Studying the Feasibility of a Selection of Southern European Ceramic Clays for the Production of Lightweight Aggregates. Constr. Build. Mater. 2020, 237, 117583. [Google Scholar] [CrossRef]
- La Russa, M.F.; Ruffolo, S.A.; Belfiore, C.M.; Aloise, P.; Randazzo, L.; Rovella, N.; Pezzino, A.; Montana, G. Study of the Effects of Salt Crystallization on Degradation of Limestone Rocks. Period. Mineral. 2013, 82, 113–127. [Google Scholar] [CrossRef]
- Souza, M.M.; Barbosa, N.P.; Anjos, M.A.S.; Farias, E.C.; Aguiar, J.G.C.; Silva Neto, J.A. Sustainable Lightweight Aggregates from Diatomite Residue. Sustaibanility 2025, 17, 6508. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).