An Overview of Heavy Metal Contamination in Water from Agriculture: Origins, Monitoring, Risks, and Control Measures
Abstract
1. Introduction
2. Prospects of HMs Resulting from Agriculture
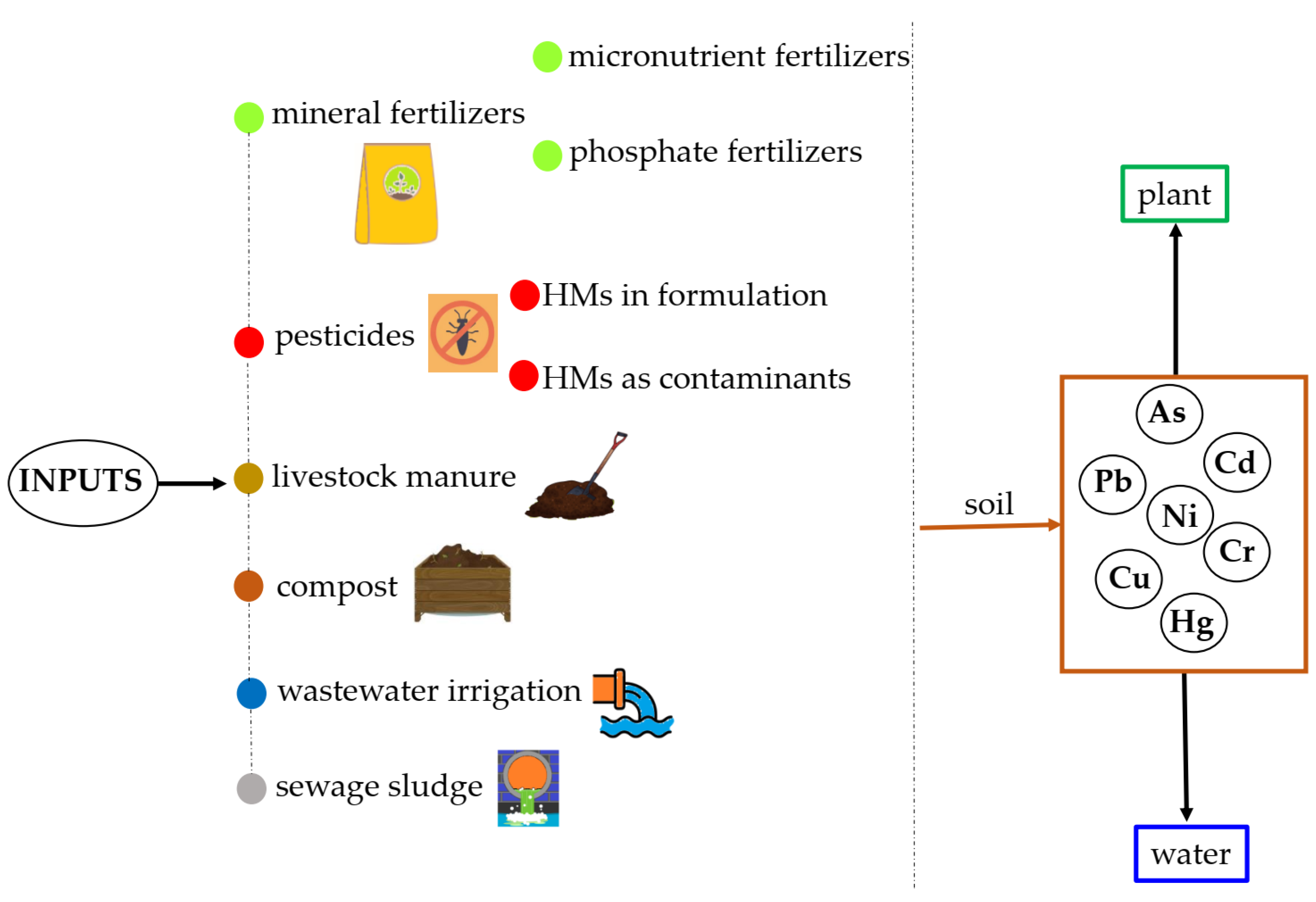
2.1. Agricultural Inputs as Sources of HMs
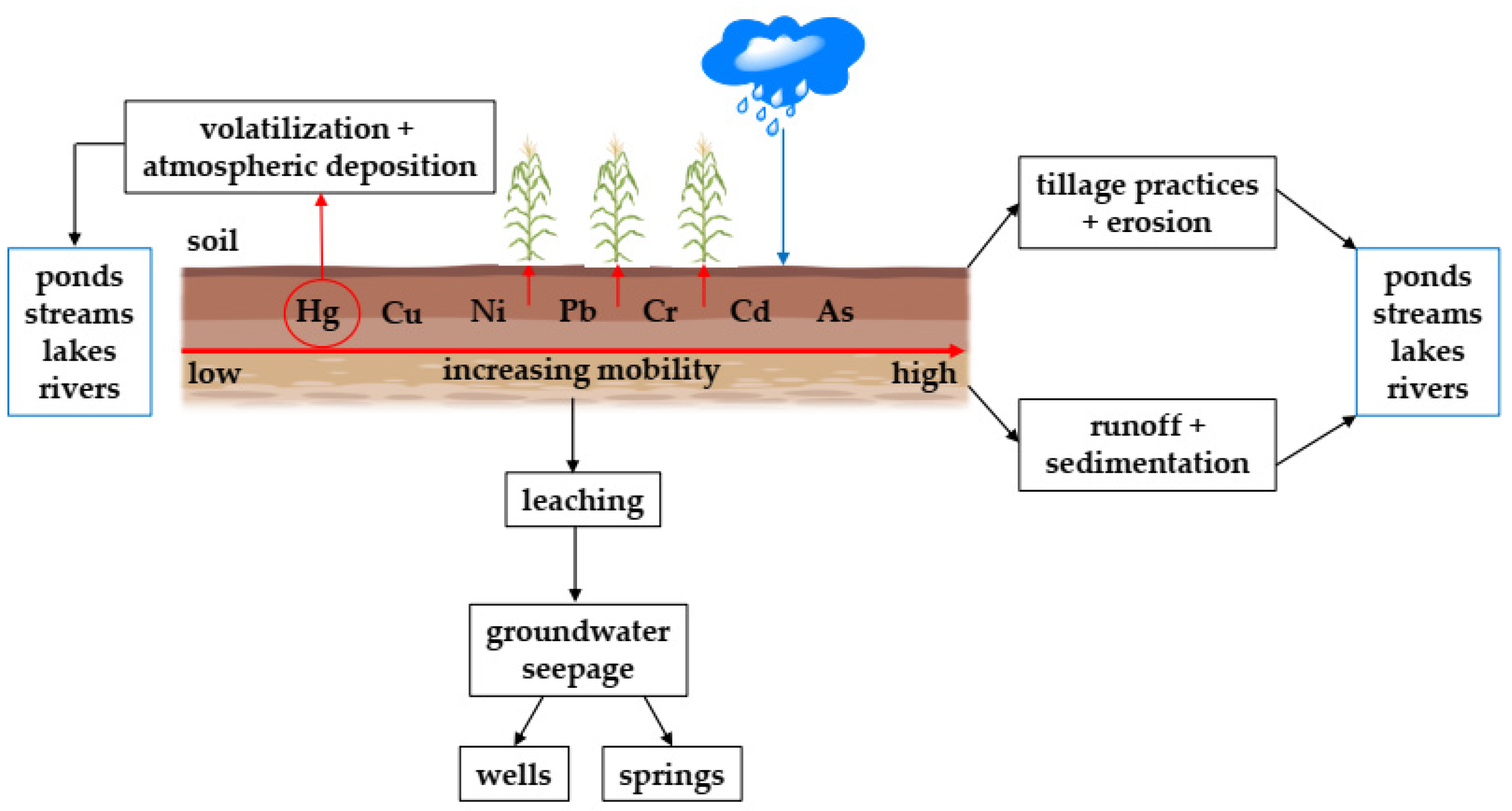
2.2. HMs Transport Pathways from Agricultural Fields to Water Bodies
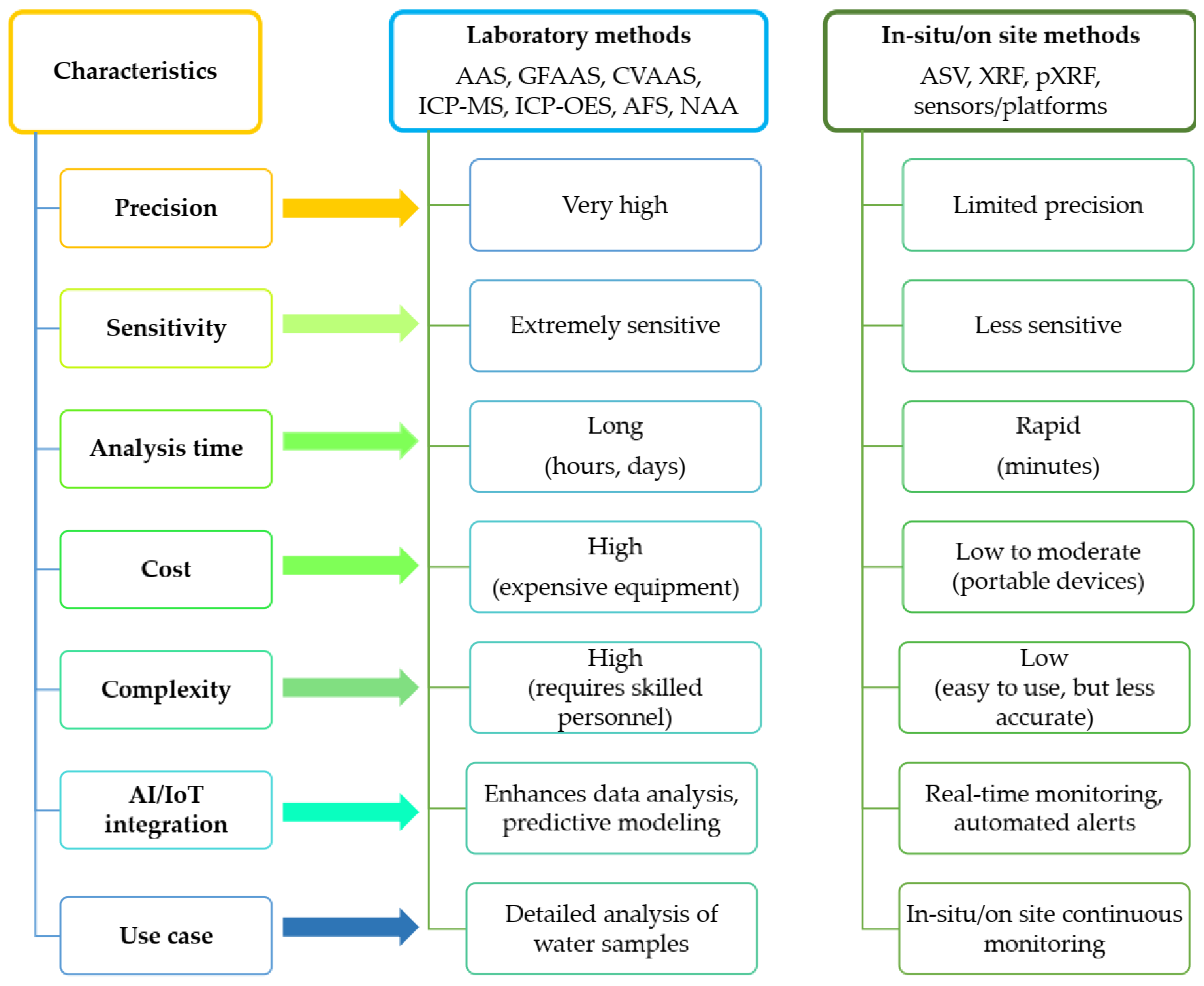
3. Methods Used for Detection of HMs in Water Samples
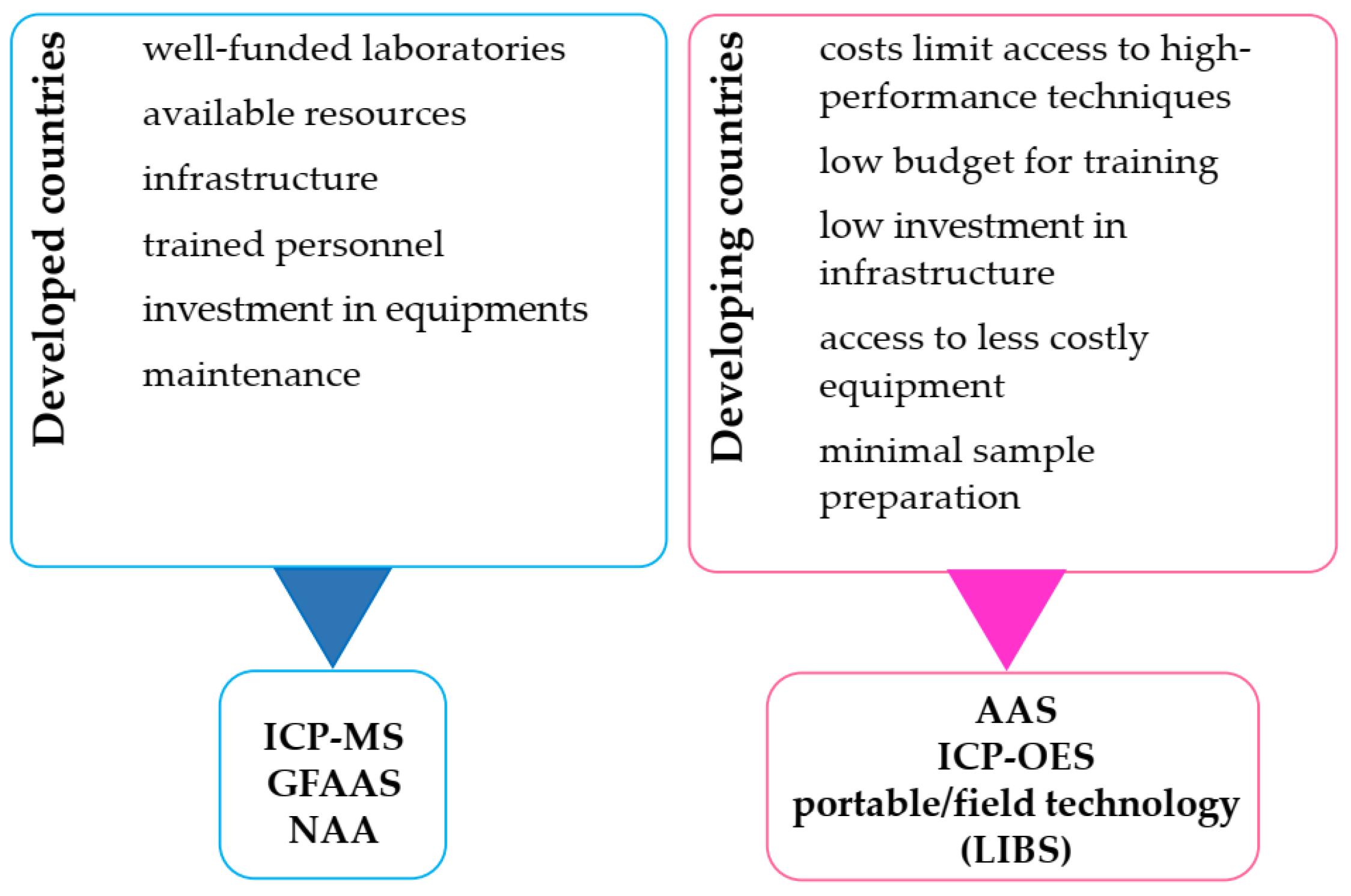
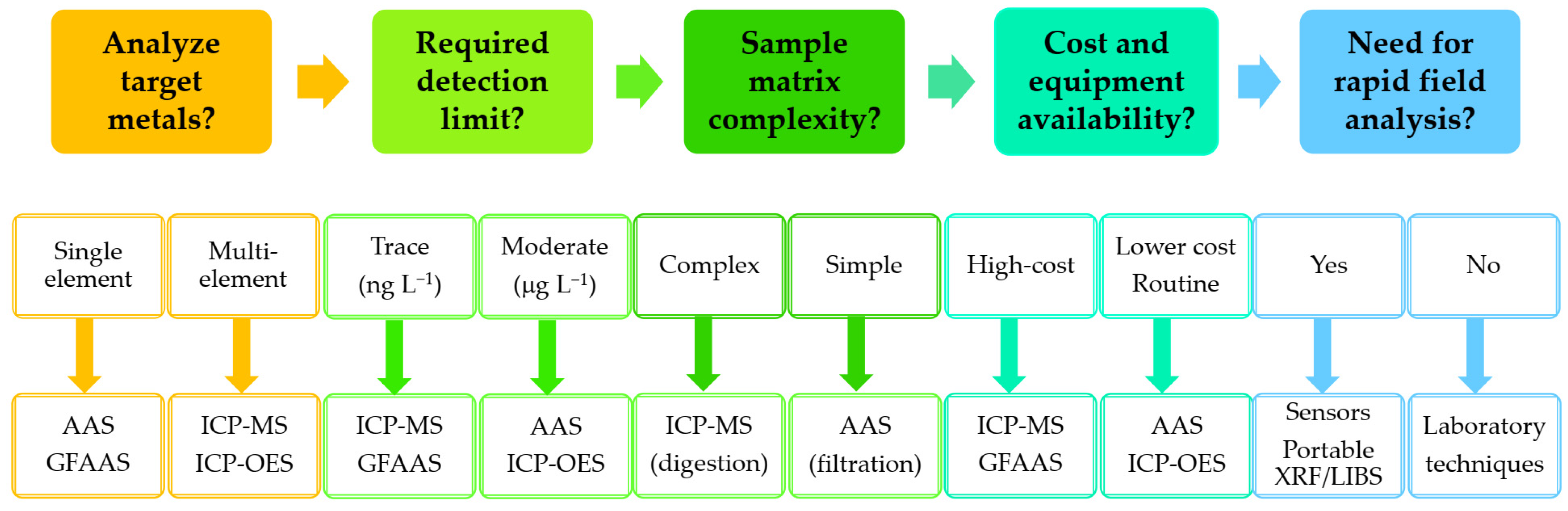
3.1. Laboratory Techniques for the Detection of HMs in Water Samples
| Detection Method | Target Metals | Sample Preparation/Concentration | Detection Range | Advantages (A) and Limitations (L) | Ref. |
|---|---|---|---|---|---|
| AAS | Cd, Co, Cr, Cu, Ni, Pb, Zn | - preservation of water samples was ensured with ultrapure HNO3 - prior to analysis, water samples were filtered | mg L−1-μg L−1 | A: high precision, very low detection limits L: expensive equipment, relatively longer analysis times | [82,83] |
| Cd, Cr, Co, Cu, Mn, Ni, Pb, Zn | - water samples were concentrated before quantification (excepting Fe, Zn) | [84] | |||
| Cr, Fe, Mn, Ni, Pb | - microwave digestion was performed of water samples with an acid mixture (67% HNO3: 98%H2SO4: 37%HCl: 40%HF = 2:1:1:1) | [62] | |||
| Cd, Co, Cr, Ni, Pb | - 10 mL water sample was treated with 2 mL 8-hydroxyquinoline and 0.2 mL Cu(II) solution (coprecipitation procedure) - then, centrifugation was performed, and the resulting precipitate was dissolved in 1 mL HNO3 | [60] | |||
| Cd, Cr, Mn, Ni, Pb | - water samples were filtered (0.45 μm pore size) and the pH adjusted to 9 - we coprecipitated targeted ions with the aid of Cu(II)-dibenzyldithiocarbamate precipitate, which was then dissolved in 0.5 mL concentrated HNO3 | [61] | |||
| GFAAS | Hg | - a 2% solution of KMnO4 was added to the water sample to convert mercury from organo-mercuric compounds into its ionic form and help prevent evaporation and loss of metals - measurements were conducted 24 h later, after adding a 20% solution of SnCl2 to the sample | μg L−1 | A: high sensitivity for trace metals; small sample volumes required; L: expensive, use of matrix modifiers; useful for detection of Pb and Hg, in particular | [84] |
| Cd | - microwave digestion was performed of water samples with an acid mixture (67% HNO3: 98%H2SO4: 37%HCl: 40%HF = 2:1:1:1) | [63] | |||
| Pb | - water samples were filtered (filter membrane with 0.45 μm pore size) - continuous-flow microextraction was conducted with the addition of chelation reagent 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone | [67] | |||
| Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn | - 3 L of water sample was concentrated at 80 °C till reaching a final volume of 50 mL - 4 mL 98% H2SO4 was added and digested by a Digesdahl apparatus (3 min) - 10 mL 30% H2O2 was added and heated until oxidation was finished - the resulting mixture was filtered and diluted to 50 mL with deionized water | [63] | |||
| As, Cd, Cr, Pb | - water samples were filtered (0.45 μm pore size) and we adjusted the pH to lower than 2 - 50 mL acidified sample was treated with 5 mL HNO3 and boiled at 130 °C till the volume was 25–30 mL | [64] | |||
| CVAAS | Hg | - water samples spiked with Hg+2 ions were mineralized in a photoreactor thermostatted at 25 °C with a luminous intensity of 3.81 mW cm−2 in the presence of 100 mgTiO2 and 0.01 mol L−1 potassium persulfate - we used a preconcentration system composed of a mini-column packed with 100 mg of 2-aminothiazol-modified silica gel - we carried out elution with 100 μL 2 mol L−1 HCl | ng L−1 | A: highly sensitive for mercury detection L: limited to mercury; possible interferences | [68] |
| Hg | - 500 mL water was treated in a separatory funnel with 2.5 mL 20 N H2SO4 and 1.5 mL 0.5% KMnO4 - the mixture was neutralized with 5 mL 10N NaOH and 1.5 mL 10% NH2OH∙HCl and then allowed to rest for 20 min - chelating agent was added (1.5 mL 10% EDTA) - mercury extraction was performed with 10 mL 0.01% dithizone-toluene - toluene was evaporated from the dithizone-toluene phase, leaving behind mercury for further analysis - samples resulting after evaporation were digested (2 mL of HNO3:HClO4 = 1:1 and 5 mL of H2SO4) - finally, 1 mL SnCl2 solution was added as a reductant | [69] | |||
| ICP-MS | Cd, Cr, Cu, Pb, Zn | - after sampling, a drop of 50% HNO3 was added to maintain a pH < 2 - 5 mL aqua regia (1:1) was added to 20 mL water sample | μg L−1-ng L−1 | A: extremely sensitive; multielement analysis; speciation analysis (able to distinguish between different oxidation states); low detection limits L: expensive technique; trained personnel; expensive maintenance for apparatus | [78] |
| As, Cd, Co, Mn, Ni, Pb, Zn | - water samples were stabilized with ultrapure 0.5% HNO3 | [71] | |||
| As, Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn | - water samples were filtered (filter membrane with 0.45 μm pore size) - HNO3 was added to acidify the samples for preservation at pH < 2 to prevent oxidation and bacterial growth | [85] | |||
| Cd, Co, Cr, Cu, Mn, Ni, Pb, Zn | - 90 mL water samples were digested with 10 mL concentrated HNO3 at 100 °C - the resulting digestate was filtered and diluted with 0.01 N HNO3 | [72] | |||
| ICP-OES | As, Cd, Cr, Cu, Fe, Hg, Mn, Ni, Pb, Zn | - water samples were pre-acidified with HNO3 (5 mL L−1) - a 10 mL aliquot was subjected to digestion (0.2 mL concentrated HNO3 and 0.5 mL concentrated HCl) - the mixture was heated at 90–95 °C until the final volume was reduced to 3–5 mL - the digested sample was then diluted to a final volume of 10 mL with deionized water | μg L−1 | A: multielement analysis; fast analysis L: less sensitive than ICP-MS | [74] |
| As, Cd, Cr, Cu, Mn, Ni, Pb, Zn | - water samples were acidified with 65% HNO3 until reaching pH 1–2 in order to prevent precipitation and retention on the walls of the vessels | [75] | |||
| Cd, Co, Cr, Cu, Mn, Ni, Pb, Zn | - 100 mL water samples were acidified with HNO3:HCl = 3:1 - the acidified sample was heated until the final volume was 25 mL - the samples were filtered and diluted to 100 mL final volume | [76] | |||
| As, Cd, Hg, Pb | - prior to analysis, water samples were filtered using a 100 mm Whatman filter | [86] | |||
| AFS | As, Hg | - after sampling, a drop of 50% HNO3 was added to maintain a pH < 2 - 5 mL aqua regia (1:1) was added to a 20 mL water sample | μg L−1-ng L−1 | A: high sensitivity for Hg, As; selective detection L: limited element range; need to train personnel | [78] |
| NAA | Cr, Cu, Fe, Mn, Zn | - 1 L of water sample was evaporated to 20 mL - 0.5 mL was stored in a polyethylene vial - measurement of γ-ray was carried out with a high-purity germanium detector | μg L−1-ng L−1 | A: non-destructive; highly sensitive; used for a wide range of samples; not affected by the errors typically associated with sample preparation L: expensive; requires access to a neutron source or nuclear reactor | [79] |
3.2. Techniques for In Situ and On-Site Measurement of HMs
3.3. Integration of Artificial Intelligence and Internet of Things in HM Detection
4. Regulatory Standards and Legislative Measures for HMs in Water
4.1. Regulations and Legislation Related to HM Levels in Water
4.2. Challenges in Enforcement
5. Impact of HMs on Water Quality and Aquatic Organisms
5.1. Impact of HMs on Water Quality
- (a)
- Heavy metal contamination load (CL) can be calculated using the following equation [118]: CL = HC x Q x 86.4, where CL = heavy metal contamination load (kg day−1), HC = heavy metal content in contaminated water (mg L−1), and Q = flow rate (m3 s−1);
- (b)
- (c)
- Pollution index (PI) is calculated on the basis of individual metal concentration. The calculation for this parameter and water classification according to PI values are reported by Goher et al. [128];
- (d)
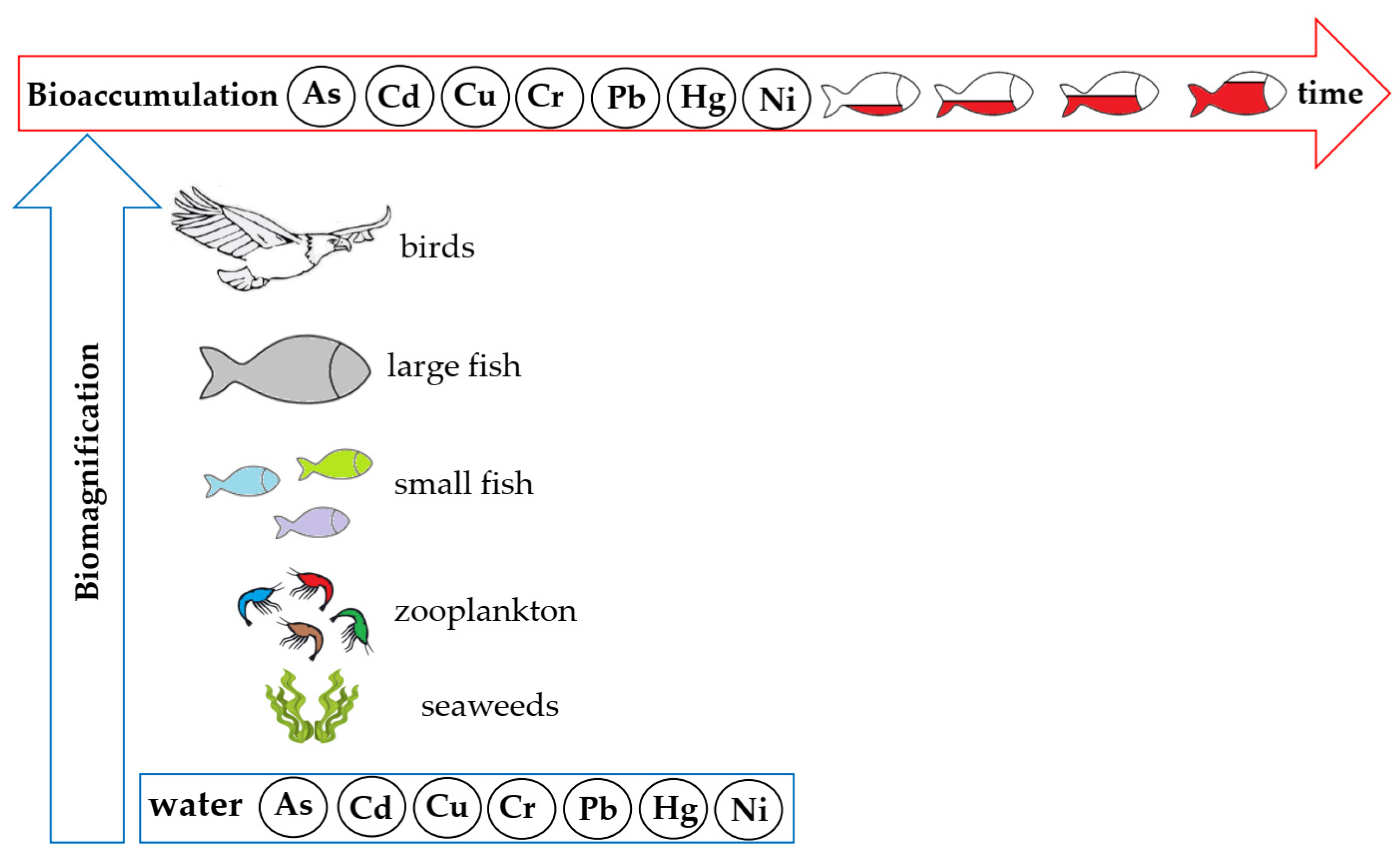
5.2. Impact of HMs on Aquatic Organisms
6. Approaches to Mitigate HM Contamination Resulting from Agriculture
6.1. Best Agricultural Practices
6.2. Supplementary Metal Pollution Control Strategies
6.3. Public Awareness and Community Involvement
7. Conclusions and Future Perspectives
- -
- Development of high-sensitivity and high-selectivity sensors, along with other smart monitoring tools, for accurate and real-time field measurements;
- -
- Integration of smart technologies (IoT-enabled systems, remote sensing) into environmental monitoring to enhance detection and early warning capabilities;
- -
- Development of precise, field-ready, and affordable methods for heavy metal monitoring to ensure widespread accessibility, real-time risk assessment, and effective pollution management;
- -
- Incorporation of digital tools and AI-powered systems into precision agriculture to enable smarter input management and improved environmental monitoring;
- -
- Advancement of bioremediation and phytoremediation strategies to enhance their efficiency, flexibility, and affordability in removing heavy metals from the environment;
- -
- Design of multifunctional soil amendments (e.g., biochar, organic amendments) that simultaneously improve soil fertility and immobilize heavy metal contaminants to prevent their leaching into water bodies.
- -
- Enforce stricter quality standards for agricultural inputs to limit heavy metal content;
- -
- Reinforce regulatory frameworks to ensure compliance with environmental protection measures;
- -
- Expand farmer education programs focused on input management and sustainable agricultural practices to prevent environmental pollution.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Appenroth, K.-J. What are “heavy metals” in Plant Sciences? Acta Physiol. Plant 2010, 32, 615–619. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. [Google Scholar] [CrossRef]
- Zaynab, M.; Al-Yahuai, R.; Ameen, Y.; Sharif, Y.; Ali, L.; Fatima, M.; Khan, K.A.; Li, S. Health and environmental effects of heavy metals. J. King Saud Univ. Sci. 2022, 34, 101653. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Trophic transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/webs—Concepts and implications for wildlife and human health. Hum. Ecol. Risk Assess. 2019, 25, 1353–1376. [Google Scholar] [CrossRef]
- Khurana, P.; Pulicharla, R.; Brar, S.K. Antibiotic-metal complexes in wastewaters: Fate and treatment trajectory. Environ. Intl. 2021, 157, 106863. [Google Scholar] [CrossRef]
- Barus, B.S.; Chen, K.; Cai, M.; Li, R.; Chen, H.; Li, C.; Wang, J.; Cheng, S.-Y. Heavy metal adsorption and release on polystyrene particles at various salinities. Front. Mar. Sci. 2021, 8, 671802. [Google Scholar] [CrossRef]
- Tumwesigye, E.; Nnadozie, C.F.; Akamagwuna, F.; Noundou, X.S.; Nyakairu, G.W.; Odume, O.N. Microplastics as vectors of chemical contaminants and biological agents in freshwater ecosystems: Current knowledge status and future perspectives. Environ. Pollut. 2023, 330, 121829. [Google Scholar] [CrossRef]
- He, Z.; Yang, X.; Stoffella, P. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Madjar, R. Agrochemistry, Plant and Soil; INVEL-Multimedia: Ilfov, Romania, 2008. (In Romanian) [Google Scholar]
- DalCorso, G.; Manara, A.; Piasetin, S.; Furini, A. Nutrient metal element in plants. Metallomics 2014, 6, 1770–1788. [Google Scholar] [CrossRef]
- Madjar, R.M.; Vasile Scăețeanu, G.; Sandu, M.A. Nutrient Water Pollution from Unsustainable Patterns of Agricultural Systems, Effects and Measures of Integrated Farming. Water 2024, 16, 3146. [Google Scholar] [CrossRef]
- Vasile Scăețeanu, G.; Madjar, R.M. The Control of Nitrogen in Farmlands for Sustainability in Agriculture. Sustainability 2025, 17, 5619. [Google Scholar] [CrossRef]
- Wajid, K.; Khan, Z.I.; Nadeem, M.; Bashir, H.; Chen, F.; Ugulu, I. Effect of organic manure and mineral fertilizers on bioaccumulation and translocation of trace metals in maize. Bull. Environ. Contam. Toxicol. 2020, 104, 649–657. [Google Scholar] [CrossRef]
- Khatun, J.; Intekhab, A.; Dhak, D. Effect of uncontrolled fertilization and heavy metal toxicity associated with arsenic (As), lead (Pb) and cadmium (Cd), and possible remediation. Toxicology 2022, 477, 153274. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Gimeno-Garcia, E.; Andreu, V.; Boluda, R. Heavy metals incidence in the application of inorganic fertilizers and pesticides to rice farming soils. Environ. Poll. 1996, 92, 19–25. [Google Scholar] [CrossRef]
- Modaihsh, A.S.; Al-Sewailem, M.S. Heavy metals content of commercial inorganic fertilizers marketed in the Kingdon of Saudi Arabia. In Proceedings of the Fourth International Conference on Precision Agriculture, Nis, Yugoslavia, 13–15 October 1999; pp. 1745–1754. [Google Scholar] [CrossRef]
- Jiao, W.; Chen, W.; Chang, A.; Page, A. Environmental risks of trace elements associated with long-term phosphate fertilizers applications: A review. Environ. Poll. 2012, 168, 44–53. [Google Scholar] [CrossRef]
- Suciu, A.; De Vivo, R.; Rizzati, N.; Capri, E. Cd content in phosphate fertilizer: Which potential risk for the environment and human health? Curr. Opin. Env. Sci. Health 2022, 30, 100392. [Google Scholar] [CrossRef]
- Nisti, M.B.; Saueia, C.R.; Malheiro, L.H.; Groppo, G.H.; Mazzilli, B.P. Lixiviation of natural radionuclides and heavy metals in tropical soils amended with phosphogypsum. J. Environ. Radioact. 2015, 144, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Weiksnar, K.; Clavier, K.; Robey, N.; Townsend, T. Changes in trace metal concentrations through the phosphogypsum lifecycle. Sci. Total Environ. 2022, 851, 158163. [Google Scholar] [CrossRef]
- Tayibi, H.; Choura, M.; Lopez, F.; Alguacil, F.; Lopez-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.E.; Duis, L.; Brown, R.; Kemp, T. Trace Element Content of Selected Fertilizers and Micronutrient Source Materials. Commun. Soil Sci. Plant Anal. 2005, 36, 1591–1609. [Google Scholar] [CrossRef]
- Oyeyemi, K.; Aizebeokhai, A.; Okagbue, H. Geostatistical exploration of dataset assessing the heavy metal contamination in Ewekoro limestone, Southwestern Nigeria. Data Briefs 2017, 14, 110–117. [Google Scholar] [CrossRef]
- Srivastava, V.; Sarkar, A.; Singh, S.; Singh, P.; De Araujo, A.S.F.; Singh, R.P. Agroecological Responses of Heavy Metal Pollution with Special Emphasis on Soil Health and Plant Performances. Front. Environ. Sci. 2017, 5, 64. [Google Scholar] [CrossRef]
- De Jesus, R.; Silva, L.; Castro, J.; De Azevedo Neto, A.; De Jesus, R.; Ferreira, S. Determination of mercury in phosphate fertilizers by cold vapor atomic absorption spectrometry. Talanta 2013, 106, 293–297. [Google Scholar] [CrossRef]
- Hashem, M.A.; Rahman, M.A.; Hasan, M.; Momen, M.A.; Lamia, Q.F.; Sahen, M.S.; Maoya, M. Effect of agricultural fertilizers on arsenic leaching from sediment under aerobic conditions. CSCEE 2024, 10, 100794. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.W.; Bordallo, S.U.; Meyer, E.; Duarte, Z.V.S.; Schmitt, J.K.; Garlet, L.P.; Kokkonen da Silva, A.A.; Moura-Bueno, J.M.; Bastos de Melo, G.W.; Brunetto, G.; et al. Heavy Metal-Based Fungicides Alter the Chemical Fractions of Cu, Zn, and Mn in Vineyards in Southern Brazil. Agronomy 2024, 14, 969. [Google Scholar] [CrossRef]
- Schneider, L. When toxic chemicals refuse to die—an examination of the prolonged mercury pesticide use in Australia. Elem. Sci. Anthr. 2021, 9, 053. [Google Scholar] [CrossRef]
- Bhat, A.; Ravi, K.; Tian, F.; Singh, B. Arsenic Contamination Needs Serious Attention: An Opinion and Global Scenario. Pollutants 2024, 4, 196–211. [Google Scholar] [CrossRef]
- Quinteros, E.; Ribo, A.; Mejia, R.; Lopez, A.; Belteton, W.; Comandari, A.; Orantes, C.; Pleites, E.; Hernandez, C.; Lopez, D. Heavy metals and pesticide exposure from agricultural activities and former agrochemical factory in a Salvadoran rural community. Environ. Sci. Pollut. Res. 2017, 24, 1662–1676. [Google Scholar] [CrossRef]
- Defarge, N.; De Vendomois, S.; Seralini, G.E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Toxicol. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Hejna, M.; Moscatelli, A.; Onelli, E.; Baldi, A.; Pilu, S.; Rossi, L. Evaluation of concentration of heavy metals in animal rearing system. Ital. J. Anim. Sci. 2019, 18, 1372–1384. [Google Scholar] [CrossRef]
- Liu, W.-R.; Zeng, D.; She, L.; Su, W.-X.; He, D.-C.; Wu, G.-Y.; Ma, X.-R.; Jiang, S.J.; Jiang, C.-H.; Ying, G.-G. Comparisons of pollution characteristics, emission situations, and mass loads for heavy metals in the manures of different livestock and poultry in China. Sci. Total Environ. 2020, 734, 139023. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Feng, Q. Comparison of the Potential Ecological and Human Health Risks of Heavy Metals from Sewage Sludge and Livestock Manure for Agricultural Use. Toxics 2021, 9, 145. [Google Scholar] [CrossRef]
- Hejna, M.; Onelli, E.; Moscatelli, A.; Bellotto, M.; Cristiani, C.; Stroppa, N.; Rossi, L. Heavy-Metal Phytoremediation from Livestock Wastewater and Exploitation of Exhausted Biomass. Int. J. Environ. Res. Public Health 2021, 18, 2239. [Google Scholar] [CrossRef]
- Nicholson, F.A.; Chambers, B.J.; Williams, J.R.; Unwin, R.J. Heavy metal contents of livestock feeds and animal manures in England and Wales. Bioresour. Technol. 1999, 70, 23–31. [Google Scholar] [CrossRef]
- Rivera, F.R.A.Z.; Prado Panp, B.; Guedron, S.; Palomino, L.M.; Ponce de Leon Hill, C.; Grabach, C.S. Impact of the change in irrigation practices from untreated to treated wastewater on the mobility of potentially toxic elements (PTEs) in soil irrigates for decades. J. Soils Sediments 2023, 23, 2726–2743. [Google Scholar] [CrossRef]
- Aftab, K.; Iqbal, S.; Khan, M.R.; Busquets, R.; Noreen, R.; Ahmad, N.; Kazimi, S.G.T.; Karami, A.M.; Al Suliman, N.M.S.; Ouladsmane, M. Wastewater-Irrigated Vegetables Are a Significant Source of Heavy Metal Contaminants: Toxicity and Health Risks. Molecules 2023, 28, 1371. [Google Scholar] [CrossRef]
- Aksouh, M.Y.; Boudieb, N.; Benosmane, N.; Moussaoui, Y.; Michalski, R.; Klyta, J.; Kończyk, J. Presence of Heavy Metals in Irrigation Water, Soils, Fruits, and Vegetables: Health Risk Assessment in Peri-Urban Boumerdes City, Algeria. Molecules 2024, 29, 4187. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.K.; Madheshiya, P.; Khare, A.K.; Tiwari, S. Heavy metal contamination in the wastewater irrigated soil and bioaccumulation in cultivated vegetables: Assessment of human health risk. J. Food Compos. Anal. 2024, 128, 106054. [Google Scholar] [CrossRef]
- Atta, M.I.; Zehra, S.S.; Dai, D.-Q.; Ali, H.; Naveed, K.; Ali, I.; Sarwar, M.; Ali, B.; Iqbal, R.; Bawazeer, S.; et al. Amassing of heavy metals in soils, vegetables and crop plants irrigated with wastewater: Health risk assessment of heavy metals in Dera Ghazi Khan, Punjab, Pakistan. Front. Plant Sci. 2023, 13, 1080635. [Google Scholar] [CrossRef]
- Abbas, M.T.; Wadaan, M.A.; Ullah, H.; Farooq, M.; Fozia, F.; Ahmad, I.; Khan, M.F.; Baabbad, A.; Ullah, Z. Bioaccumulation and Mobility of Heavy Metals in the Soil-Plant System and Health Risk Assessment of Vegetables Irrigated by Wastewater. Sustainability 2023, 15, 15321. [Google Scholar] [CrossRef]
- Olejnik, D. Evaluation of the Heavy Metals Content in Sewage Sludge from Selected Rural and Urban Wastewater Treatment Plants in Poland in Terms of Its Suitability for Agricultural Use. Sustainability 2024, 16, 5198. [Google Scholar] [CrossRef]
- Voca, N.; Leto, J.; Peter, A.; Brandic, I.; Suric, J.; Bilandzija, N. Environmental benefits: Investigating the effects of municipal sewage sludge on the productivity and energy quality of Miscanthus. Agrolife Sci. J. 2024, 13, 243–252. [Google Scholar]
- Marin, E.; Rusănescu, C.O. Agricultural Use of Urban Sewage Sludge from the Wastewater Station in the Municipality of Alexandria in Romania. Water 2023, 15, 458. [Google Scholar] [CrossRef]
- Tănase, V.; Vrînceanu, N.; Preda, M.; Motelică, D.M. Residual effects of fertilization with sewage sludge compost on cropland. Agrolife Sci. J. 2017, 6, 195–204. [Google Scholar]
- Smith, S. A critical review of the bioavailability and impacts of heavy metals in municipal solid waste composts compared to sewage sludge. Environ. Int. 2009, 35, 142–156. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Balgobind, A.; Pillay, B. Bioavailability of Heavy Metals in Soil: Impact on Microbial Biodegradation of Organic Compounds and Possible Improvement Strategies. Int. J. Mol. Sci. 2013, 14, 10197–10228. [Google Scholar] [CrossRef]
- Yin, Y.; Impellitteri, C.; You, S.-J.; Allen, H. The importance of organic matter distribution and extract soil: Solution ratio on the desorption of heavy metals from soils. Sci. Total Environ. 2002, 287, 107–119. [Google Scholar] [CrossRef]
- Priya, A.K.; Muruganandam, M.; Ali, S.S.; Kornaros, M. Clean-Up of Heavy Metals from Contaminated Soil by Phytoremediation: A Multidisciplinary and Eco-Friendly Approach. Toxics 2023, 11, 422. [Google Scholar] [CrossRef]
- Enya, O.; Heaney, N.; Iniama, G.; Lin, C. Effects of heavy metals on organic matter decomposition in inundated soils: Microcosm experiment and field examination. Sci. Total Environ. 2020, 724, 138223. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Wang, P.; Shen, X.; Qiu, S.; Zhang, L.; Ma, Y.; Liang, J. Clay-Based Materials for Heavy Metals Adsorption: Mechanisms, Advancements, and Future Prospects in Environmental Remediation. Crystals 2024, 14, 1046. [Google Scholar] [CrossRef]
- Schlüter, K. Review: Evaporation of mercury from soils. An integration and synthesis of current knowledge. Environ.Geol. 2000, 39, 249–271. [Google Scholar] [CrossRef]
- Buszewski, B.; Kowalkowski, T. A new model of heavy metal transport in the soil using nonlinear artificial neural networks. Environ. Eng. Sci. 2006, 23, 589–595. [Google Scholar] [CrossRef]
- Qiao, P.; Wang, S.; Li, J.; Zhao, Q.; Wei, Y.; Lei, M.; Yang, J.; Zhang, Z. Process, influencing factors, and simulation of the lateral transport of heavy metals in surface runoff in a mining area driven by rainfall: A review. Sci. Total Environ. 2023, 857, 159119. [Google Scholar] [CrossRef] [PubMed]
- Ipeaiyeda, A.R.; Ayoade, A.R. Flame atomic absorption spectrometric determination of heavy metals in aqueous solution and surface water preceded by co-precipitation procedure with copper (II) 8-hydroxyquinoline. Appl. Water Sci. 2017, 7, 4449–4459. [Google Scholar] [CrossRef]
- Tuzen, M.; Soylak, M. Multi-element coprecipitation for separation and enrichment of heavy metal ions for their flame atomic absorption spectrometric determinations. J. Hazard. Mat. 2009, 162, 724–729. [Google Scholar] [CrossRef]
- Radulescu, C.; Gulama, I.D.; Stihi, C.; Ionita, I.; Chilian, A.; Necula, C.; Chelarescu, E.D. Determination of heavy metal levels in water and therapuetic mud by atomic absorption spectrometry. Rom. J. Phys. 2014, 59, 1057–1066. [Google Scholar]
- Shanbehzadeh, S.; Dastjerdi, M.V.; Hassanzadeh, A.; Kiyanizadeh, T. Heavy metals in water and sediment: A case study of Tembi river. J. Environ. Public Health 2014, 858720. [Google Scholar] [CrossRef]
- Ali, M.M.; Ali, M.L.; Islam, M.S.; Rahman, M.Z. Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environ. Nanotechnol. Monit. Manag. 2016, 5, 27–35. [Google Scholar] [CrossRef]
- Bakirdere, S.; Yaroglu, T.; Tirik, N.; Demiroz, M.; Fidan, A.K.; Maruldali, O.; Karaca, A. Determination of As, Cd and Pb in tap water and bottled water by using optimized GFAAS system with Pd-Mg and Ni modifiers. J. Spectrosc. 2013, 824817. [Google Scholar] [CrossRef]
- Assubaie, F. Assessment of the levels of some heavy metals in water in Alahsa Oasis farms, Saudi Arabia, with analysis by atomic absorption spectrometry. Arab. J. Chem. 2015, 8, 240–245. [Google Scholar] [CrossRef]
- Cao, J.; Liang, P.; Liu, R. Determination of trace lead in water samples by continuous flow microextraction combined with graphite furnace atomic absorption spectrometry. J. Hazard. Chem. 2008, 152, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Pa, F.A.; Alcantara, I.L.; Roldan, P.S.; Padilha, C.C.F.; Araujo, A.B.; Valente, J.P.S.; Florentino, A.O.; Padilha, P.M. Determination of Hg in water by CVAAS using 2-aminothiazole modified silica. Eclética Química 2005, 30, 47–55. [Google Scholar] [CrossRef]
- Maha, M.M.; Matsuyama, A.; Arima, T.; Sainoki, A. Assessment of Total Mercury Levels Emitted from ASGM into Soil and Groundwater in Chami Town, Mauritania. Sustainability 2024, 16, 7992. [Google Scholar] [CrossRef]
- Abdelmonen, B.H.; Kamal, L.; Elbaz, R.; Khalifa, M.; Abdelnaser, A. From contamination to detection: The growing threat of heavy metals. Heliyon 2025, 11, e41713. [Google Scholar] [CrossRef]
- Voica, C.; Kovacs, M.H.; Dehelean, A.; Ristoiu, D.; Iordache, A. ICP-MS determinations of heavy metals in surface waters from Transylvania. Rom. Journ. Phys. 2012, 57, 1184–1193. [Google Scholar]
- Lipy, E.P.; Rahman, M.T.; Islam, S.; Raknuzzaman, M.; Islam, M.M.; Akhter, S.; Islam, D.; Naim, M.R.; Moniruzzaman, M.; Biswas, A.; et al. Occurence of heavy metals in fish species, water and sediments of the Pasur River in Bangladesh along with assessments of ecological and human risks. Discov. Environ. 2025, 3, 49. [Google Scholar] [CrossRef]
- Alhabri, I.; Al-Hakimi, A.; Al-Hazmy, S.; Albadri, A.E.A.E. Determination of trace and heavy metals in bottled drinking water in Yemen By ICP-MS. Results Chem. 2024, 8, 101558. [Google Scholar] [CrossRef]
- González-Díaz, R.L.; de Anda, J.; Shear, H.; Padilla-Tovar, L.E.; Lugo-Melchor, O.Y.; Olvera-Vargas, L.A. Assessment of Heavy Metals in Surface Waters of the Santiago–Guadalajara River Basin, Mexico. Hydrology 2025, 12, 37. [Google Scholar] [CrossRef]
- Biedunkova, O.; Kuznietsov, P.; Trach, Y. Temporal Dynamics and Sources of Heavy Metals in an Aquatic Ecosystem: An Applied Study. Eng. Proc. 2025, 87, 30. [Google Scholar] [CrossRef]
- Dilebo, W.B.; Anchiso, M.D.; Kidane, T.T.; Ayalew, M.E. Assessment of selected heavy metals concentration level of drinking water in Gazer Town and selected Kebele, South Ari District, Southern Ethiopia. Intl. J. Anal. Chem. 2023, 1524850. [Google Scholar] [CrossRef]
- Didukh-Shadrina, S.L.; Losev, V.N.; Samoilo, A.; Trofimchuk, A.K.; Nesterenko, P.N. Determination of Metals in Natural Waters by Inductively Coupled Plasma Optical Emission Spectroscopy after Preconcentration on Silica Sequentially Coated with Layers of Polyhexamethylene Guanidinium and Sulphonated Nitrosonaphthols. Int. J. Anal. Chem. 2019, 467631. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhou, G.; Yang, R.; Cheng, Z.; Sun, B. Heavy Metals and As in Ground Water, Surface Water, and Sediments of Dexing Giant Cu-Polymetallic Ore Cluster, East China. Water 2022, 14, 352. [Google Scholar] [CrossRef]
- Rozana, K.; Sukirno; Pradasiwi, D.S.; Murniashih, S. Assessment of heavy metals concentration in the water around the rea of Adipala Cilacap steam power plant using neutron activation analysis. J. Phys. Conf. Ser. 2020, 1436, 012084. [Google Scholar] [CrossRef]
- Parmar, D.; Srivastava, R.; Baruah, P. Lased induced breakdown spectroscopy: A robust technique for the detection of trace metals in water. Mater. Today Proc. 2023, 77, 234–239. [Google Scholar] [CrossRef]
- Tian, H.; Jiao, L.; Dong, D. Rapid determination of trace cadmium in drinking water using laser-induced breakdown spectroscopy coupled with chelating resin enrichment. Sci. Rep. 2019, 9, 10443. [Google Scholar] [CrossRef]
- Ristea, E.; Pârvulescu, O.C.; Lavric, V.; Oros, A. Assessment of Heavy Metal Contamination of Seawater and Sediments Along the Romanian Black Sea Coast: Spatial Distribution and Environmental Implications. Sustainability 2025, 17, 2586. [Google Scholar] [CrossRef]
- Mussakulkyzy, A.; Opp, C.; Amirgaliev, N.; Madibekov, A.; Ismukhanova, L.; Zhadi, A. Transmission of Heavy Metals in River Water and Self-Purification Capacity of Ile River. Appl. Sci. 2025, 15, 6548. [Google Scholar] [CrossRef]
- Rajkovic, M.B.; Lacnjevac, C.M.; Ralevic, N.R.; Stojanović, M.D.; Tosković, D.V.; Pantelic, G.K.; Ristic, N.M.; Jovanic, S. Identification of Metals (Heavy and Radioactive) in Drinking Water by an Indirect Analysis Method Based on Scale Tests. Sensors 2008, 8, 2188–2207. [Google Scholar] [CrossRef]
- Bai, M.; Zhang, C.; Bai, Y.; Wang, T.; Qu, S.; Qi, H.; Zhang, M.; Tan, C.; Zhang, C. Occurrence and Health Risks of Heavy Metals in Drinking Water of Self-Supplied Wells in Northern China. Int. J. Environ. Res. Public Health 2022, 19, 12517. [Google Scholar] [CrossRef]
- Kamagate, M.; Lanciné, T.; Berthe, K.A.N.; Droh Lanciné, G.; Kriaa, K.; Assadi, A.A.; Zhang, J.; Tahraoui, H. Assessment of the Human Health Risks Associated with Heavy Metals in Surface Water Near Gold Mining Sites in Côte d’Ivoire. Water 2025, 17, 1891. [Google Scholar] [CrossRef]
- Borrill, A.; Reily, N.; Macpherson, J. Addressing the practicalities of anodic stripping voltametry for heavy metal detection: A tutorial review. Analyst 2019, 144, 6834. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Compton, R. Anodic stripping coltametry using underpotential deposition allows sub 10 ppb measurement of total As and As(III) in water. Talanta 2022, 247, 123578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, S.; Wu, Z.; Zhang, F.; Jin, B.; Yang, C. Review of Underwater In Situ Voltammetry Analyzers for Trace Metals. Chemosensors 2024, 12, 158. [Google Scholar] [CrossRef]
- Eksperiandova, L.P.; Makarovska, Y.N.; Blank, A.B. Determination of small quantities of heavy metals in water soluble salts and natural water by X-ray fluorescence. Anal.Chim. Acta 1998, 371, 105–108. [Google Scholar] [CrossRef]
- Venvik, G.; Boogaard, F.C. Portable XRF Quick-Scan Mapping for Potential Toxic Elements Pollutants in Sustainable Urban Drainage Systems: A Methodological Approach. Science 2020, 2, 64. [Google Scholar] [CrossRef]
- Zhou, S.; Yuan, Z.; Cheng, Q.; Zhang, Z.; Yang, J. Rapid in situ determination of heavy metal concentrations in polluted water via portable XRF: Using Cu and Pb as example. Environ. Pollut. 2018, 243, 1325–1333. [Google Scholar] [CrossRef]
- Dhillon, A.; Singh, N.; Nair, M.; Kumar, D. Analytical methods to determine and sense heavy metal pollutants using Mxene and Mxene-based composites: Mechanistic prophecy into sensing properties. Chemosphere 2022, 303, 135166. [Google Scholar] [CrossRef]
- Sapna, K.; Sharma, V.; Kumar, M.; Kulshrestha, V. A modified Mxene composite sensor with sulphur impurities for electrochemical detection of lead in the aqueous system. Nanoscale 2025, 17, 7229–7243. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ma, L.; Zhou, L.; Liu, G.; Jiang, Y.; Gao, J. Preparation and Application of Bismuth/MXene Nano-Composite as Electrochemical Sensor for Heavy Metal Ions Detection. Nanomaterials 2020, 10, 866. [Google Scholar] [CrossRef] [PubMed]
- Sallam, G.; Shaban, S.; Nassar, A.; El-Khouly, M. Water soluble porphyrin as optical sensor for the toxic heavy metal ions in an aqueous medium. Spectrochim. Acta A 2020, 241, 118609. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Lai, Q.; Fan, W.; Zhang, Y.; Liu, Z. Advances in Portable Heavy Metal Ion Sensors. Sensors 2023, 23, 4125. [Google Scholar] [CrossRef]
- Xiao, W.; Xiao, M.; Fu, Q.; Yu, S.; Shen, H.; Bian, H.; Tang, Y. A Portable Smart-Phone Readout Device for the Detection of Mercury Contamination Based on an Aptamer-Assay Nanosensor. Sensors 2016, 16, 1871. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Khan, A.; Ganguly, A.; Kaswan, K.; Suresh, S.; Cheng, Y.-Y.; Lee, K.-M.; Yu, J.-H.; Lin, Z.-H. Real-time wireless detection of heavy metal ions using a self-powered triboelectric nanosensor integrated with an autonomous thermoelectric generator-powered robotic system. Adv. Sci. 2025, 12, 2410424. [Google Scholar] [CrossRef]
- Lahari, S.A.; Kumawat, N.; Amreen, K.; Ponnalagu, R.N.; Goel, S. IoT integrated and deep learning assisted electrochemical sensor for multiplexed heavy metal sensing in water samples. Clean Water 2025, 8, 10. [Google Scholar] [CrossRef]
- Devnath, A.; Lee, G.; Hanjoo, J.; Alimkhanuly, B.; Patil, S.; Kadyrov, A.; Lee, S. IoT-enabled novel heterostructure FET-based hybrid sensor for real-time arsenic detection. Sens. Actuators B Chem. 2024, 417, 136146. [Google Scholar] [CrossRef]
- Mazhar, T.; Talpur, D.B.; Shloul, T.A.; Ghadi, Y.Y.; Haq, I.; Ullah, I.; Ouahada, K.; Hamam, H. Analysis of IoT Security Challenges and Its Solutions Using Artificial Intelligence. Brain Sci. 2023, 13, 683. [Google Scholar] [CrossRef]
- Sebestyen, H.; Popescu, D.E.; Zmaranda, R.D. A Literature Review on Security in the Internet of Things: Identifying and Analysing Critical Categories. Computers 2025, 14, 61. [Google Scholar] [CrossRef]
- United Nations. The 17 Goals. Available online: https://sdgs.un.org/goals (accessed on 10 June 2025).
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32000L0060 (accessed on 10 June 2025).
- Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the Protection of Groundwater Against Pollution and Deterioration. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006L0118-20140711 (accessed on 29 June 2025).
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast). Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj/eng (accessed on 29 June 2025).
- Directive 2006/7/EC of the European Parliament and of the Council of 15 February 2006 Concerning the Management of Bathing Water Quality and Repealing Directive 76/160/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006L0007-20140101 (accessed on 29 June 2025).
- Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 86/280/EEC and Amending Directive 2000/60/EEC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/eli/dir/2008/105/oj (accessed on 29 June 2025).
- Council Directive of 21 May 1991 Concerning Urban Waste Water Treatment (91/271/EEC). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A01991L0271-20140101 (accessed on 29 June 2025).
- Council Directive of 12 December 1991 Concerning the Protection of Waters Against Pollution Caused by Nitrates from Agricultural Sources (91/676/EEC). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A01991L0676-20081211 (accessed on 29 June 2025).
- Directive 2007/60/EC of the European Parliament and of the Council of 23 October 2007 on the Assessment and Management of Flood Risks (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32007L0060 (accessed on 29 June 2025).
- WHO. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 29 June 2025).
- USEPA. Issue Paper on the Environmental Chemistry of Metals. Available online: https://www.epa.gov/scientific-leadership/issue-paper-environmental-chemistry-metals (accessed on 29 June 2025).
- Boyd, D. The Water We Drink: An International Comparison of Drinking Water Quality Standards and Guidelines; A Report Prepared for the David Suzuki Foundation Healthy Environment, Healthy Canadian Series; David Suzuki Foundation: Vancouver, BC, Canada, 2006. [Google Scholar]
- Ghezzi, L.; D’Orazio, M.; Doveri, M.; Lelli, M.; Petrini, R.; Giannecchini, R. Groundwater and potentially toxic elements in a dismissed mining area: Thallium contamination of drinking spring water in the Apuan Alps (Tuscany, Italy). J. Geochem. Explor. 2019, 197, 84–92. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Jiang, X.; Zhang, Y.; Zhang, J.; Liu, Y.; Guo, H.; Li, Z.; Fu, Z. The interaction between nutrients and heavy metals in lakes and rivers entering lakes. Ecol. Indic. 2024, 161, 111963. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, M.; Lan, J.; Huang, Y.; Zhang, J.; Huang, S.; Yang, Y.; Ru, J. Water Quality Degradation Due to Heavy Metal Contamination: Health Impacts and Eco-Friendly Approaches for Heavy Metal Remediation. Toxics 2023, 11, 828. [Google Scholar] [CrossRef]
- Georgaki, M.-N.; Charalambous, M.; Kazakis, N.; Talias, M.A.; Georgakis, C.; Papamitsou, T.; Mytiglaki, C. Chromium in Water and Carcinogenic Human Health Risk. Environments 2023, 10, 33. [Google Scholar] [CrossRef]
- Dragan, F.; Lestyan, M.; Lupu, V.V.; Marcu, F.M.; Cozma, A.; Fodor, K.; Ciubara, A.; Moisa, C.F.; Teaha, D.; Lupu, A.; et al. The Threat of Mercury Poisoning by Fish Consumption. Appl. Sci. 2023, 13, 369. [Google Scholar] [CrossRef]
- Han, J.; Zhang, R.; Tang, J.; Chen, J.; Zheng, C.; Zhao, D.; Wang, J.; Zhang, H.; Qi, X.; Wu, X.; et al. Occurrence and Exposure Assessment of Nickel in Zhejiang Province, China. Toxics 2024, 12, 169. [Google Scholar] [CrossRef]
- Mohan, V.; Nithila, P.; Reddy, J. Estimation of heavy metals in drinking water and development of heavy metal pollution index. J. Environ. Sci. Health 1996, A31, 283–289. [Google Scholar] [CrossRef]
- Edet, A.E.; Offiong, O.E. Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 2002, 57, 295–304. [Google Scholar] [CrossRef]
- Eldaw, E.; Huang, T.; Elubid, B.; Khalifa Mahamed, A.; Mahama, Y. A Novel Approach for Indexing Heavy Metals Pollution to Assess Groundwater Quality for Drinking Purposes. Int. J. Environ. Res. Public Health 2020, 17, 1245. [Google Scholar] [CrossRef]
- Moldovan, A.; Török, A.I.; Kovacs, E.; Cadar, O.; Mirea, I.C.; Micle, V. Metal Contents and Pollution Indices Assessment of Surface Water, Soil, and Sediment from the Arieș River Basin Mining Area, Romania. Sustainability 2022, 14, 8024. [Google Scholar] [CrossRef]
- Dange, S.; Arumugam, K.; Vijayaraghavalu, S.S. Geochemical insights into heavy metal contamination and health hazards in Palar River Basin: A pathway to sustainable solutions. Ecol. Indic. 2024, 166, 112568. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Dai, Y.; Xu, M.; Wang, D.; Han, Y.; Liang, W.; Shi, Y.; Song, F.; Yao, L.; et al. Health Risk of Heavy Metals in Drinking Water Sources of Water-Carrying Lakes Affected by Retreating Polder: A Case Study of Luoma Lake. Water 2024, 16, 2699. [Google Scholar] [CrossRef]
- Goher, M.; Hassan, A.; Abdel-Moniem, I.; Fahmy, A.; El-sayed, S. Evaluation of surface water quality and heavy metal indices of Ismailia Canal, Nile River, Egypt. Egypt. J. Aquat. Res. 2014, 40, 225–233. [Google Scholar] [CrossRef]
- Phaenark, C.; Phankamolsil, Y.; Sawangproh, W. Ecological and health implication in Thai Fauna: A systematic review. Ecotoxicol. Environ. Saf. 2024, 285, 117086. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Jain, A.; Yadav, S.; Dubey, A.; Trivedi, S. A review on heavy metal-induced toxicity in fishes: Bioaccumulation, antioxidant defense system, histopathological manifestations, and transcriptional profiling of genes. J. Trace Elem. Med. Biol. 2024, 83, 127377. [Google Scholar] [CrossRef]
- Ray, S.; Vashishth, R. From water to plate: Reviewing the bioaccumulation of heavy metals in fish and unraveling human health risks in the food chain. Emerg. Contam. 2024, 10, 100358. [Google Scholar] [CrossRef]
- Jamil Emon, F.; Rohani, M.F.; Sumaiya, N.; Tuj Jannat, M.F.; Akter, Y.; Shahjahan, M.; Abdul Kari, Z.; Tahiluddin, A.B.; Goh, K.W. Bioaccumulation and Bioremediation of Heavy Metals in Fishes—A Review. Toxics 2023, 11, 510. [Google Scholar] [CrossRef]
- Akter Suchana, S.; Ahmed, M.S.; Islam, M.; Rahman, M.L.; Rohani, M.F.; Ferdusi, T.; Ahmmad, A.K.S.; Fatema, M.K.; Badruzzaman, M.; Shahjahan, M. Chromium exposure causes structural abberations of erythrocytes, gills, liver, kidney and genetic damage in striped catfish Pangasianodon hypophthalmus. Biol. Trace Elem. Res. 2021, 199, 3869–3885. [Google Scholar] [CrossRef]
- Wan, Y.; Huang, Q.; Wang, Q.; Yu, Y.; Su, D.; Qiao, Y.; Li, H. Accumulation and bioavailability of heavy metals in an acid soil and their uptake by paddy rice under continuous application of chicken and swine manure. J. Hazard. Mater. 2020, 384, 121293. [Google Scholar] [CrossRef]
- Singh, A.; Agrawal, M.; Marshall, F. The role of organic vs. inorganic fertilizers in reducing phytoavailability of heavy metals in a wastewater-irrigated area. Ecol. Eng. 2010, 36, 1733–1740. [Google Scholar] [CrossRef]
- Puschenreiter, M.; Horak, O.; Friesl, W.; Hartl, W. Low-cost agricultural measures to reduce heavy metal transfer into the food chain—A review. Plant Soil Environ. 2005, 51, 1–11. [Google Scholar] [CrossRef]
- Su, Y.; Kwong, R.; Tang, W.; Yang, Y.; Zhong, H. Straw return enhances the risks of metals in soil? Ecotoxicol. Environ. Saf. 2021, 207, 111201. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, L.; Zhai, L.; Zhang, H.; Wang, S.; Zou, J.; Shen, Z.; Lian, C.; Chen, Y. Analysis of the long-term effectiveness of biochar immobilization remediation on heavy metal contaminated soil and the potential environmental factors weakening the remediation effect: A review. Ecotoxicol. Environ. Saf. 2021, 207, 111261. [Google Scholar] [CrossRef] [PubMed]
- Phiri, Z.; Moja, N.; Nkambule, T.; De Kock, L.-A. Utilization of biochar for remediation of heavy metals in aqueous environments: A review and bibliometric analysis. Heliyon 2024, 10, e25785. [Google Scholar] [CrossRef] [PubMed]
- Getahun, S.; Kefale, H.; Gelaye, Y. Application of precision agriculture technologies for sustainable crop production and environmental sustainability: A systematic review. Sci. World J. 2024, 2126734. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, Z.; Ji, H.; Qiu, F.; Zhao, D.; Bredar, A.; Farnum, B. Simultaneous control of soil erosion and arsenic leaching at disturbed land using polyacrylamide modified magnetite nanoparticles. Sci. Total Environ. 2020, 702, 134997. [Google Scholar] [CrossRef]
- Correia, A.A.S.; Casaleiro, P.D.F.; Figueiredo, D.T.R.; Moura, M.S.M.R.; Rasteiro, M.G. Key-Parameters in Chemical Stabilization of Soils with Multiwall Carbon Nanotubes. Appl. Sci. 2021, 11, 8754. [Google Scholar] [CrossRef]
- Elango, D.; Devi, K.D.; Jeyabalakrishnan, H.K.; Rajendran, K.; Haridass, V.K.T.; Dharmaraj, D.; Charuchandran, C.V.; Wang, W.; Fakude, M.; Mishra, R.; et al. Agronomic, breeding, and biotechnological interventions to mitigate heavy metal toxicity problems in agriculture. J. Agric. Food Res. 2022, 10, 100374. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavaş, İ.; Ünay, A.; Abdel-DAIM, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of Floating Aquatic Plants in Phytoremediation of Heavy Metals Polluted Water: A Review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef]
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of Heavy Metals from Soil and Aquatic Environment: An Overview of Principles and Criteria of Fundamental Processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Karnwal, A.; Martolia, S.; Dohroo, A.; Al-Tawaha, A.R.M.S.; Malik, T. Exploring bioremediation strategies for heavy metals and POPs pollution: The role of microbes, plants, and nanotechnology. Front. Environ. Sci. 2024, 12, 1397850. [Google Scholar] [CrossRef]
- Swain, R.; Sahoo, B. Mapping of heavy metal pollution in river water at daily time-scale using spatio-temporal fusion of MODIS-aqua and Landsat satellite imageries. J. Environ. Manag. 2017, 192, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, J.; Zhang, H.; Lin, H. Progress in Remote Sensing of Heavy Metals in Water. Remote Sens. 2024, 16, 3888. [Google Scholar] [CrossRef]
- Rasheed, T.; Shafi, S.; Sher, F. Smart nano-architectures as potential sensing tools for detecting heavy metal ions in aqueous matrices. TrEAC 2022, 36, e00179. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, H.; Shin, W.H.; Oh, J.-M.; Koo, S.-M.; Kim, Y.; Lee, T.; Yu, B.J.; Park, C. Development of Colorimetric Whole-Cell Biosensor for Detection of Heavy Metals in Environment for Public Health. Int. J. Environ. Res. Public Health 2021, 18, 12721. [Google Scholar] [CrossRef]
- Velusamy, K.; Periyasamy, S.; Kumar, S.; Rangasamy, G.; Pauline, M.N.; Ramaraju, P.; Mohanasundaram, S.; Vo, D.-V.N. Biosensor for heavy metals detection in wastewater: A review. Food Chem. Toxicol. 2022, 168, 113307. [Google Scholar] [CrossRef]
- Syu, W.-J.; Chang, T.-K.; Pan, S.-Y. Establishment of an Automatic Real-Time Monitoring System for Irrigation Water Quality Management. Int. J. Environ. Res. Public Health 2020, 17, 737. [Google Scholar] [CrossRef]
- Essamlali, I.; Nhaila, H.; El Khaili, M. Advances in machine learning and IoT for water quality monitoring: A comprehensive review. Heliyon 2024, 10, e27920. [Google Scholar] [CrossRef]

| Sample | Cd | Cr | Cu | Ni | Pb | Zn |
|---|---|---|---|---|---|---|
| WW, mg L−1 | 0.8 | 0.87 | 0.4 | 0.38 | 0.36 | 0.28 |
| 0.64 | 0.82 | 0.37 | 0.31 | 0.33 | 0.23 | |
| 0.07 | 0.11 | 0.18 | 0.16 | 0.09 | 0.08 | |
| SS, mg kg−1 | 3.20 | 63 | 35.40 | 78 | 74.50 | 31.50 |
| 3.35 | 56 | 38 | 85.50 | 70.75 | 33 | |
| 1.25 | 34.25 | 16.75 | 36.50 | 46.25 | 15.75 | |
| VS, mg kg−1 | 4.53 | 21.15 | 19 | 28.4 | 20.5 | 14.9 |
| 5.69 | 22.24 | 17.35 | 31.87 | 22.70 | 12.27 | |
| 2.35 | 15.89 | 11.38 | 17.15 | 7.33 | 8.64 |
| Pollutant | EQS (Surface Water) [107] | Drinking Water | ||||
|---|---|---|---|---|---|---|
| AA-EQS Inland Surface Waters | AA-EQS Other Surface Waters | MAC-EQS Inland Surface Waters | EU Legislation [107] | WHO [113] | USEPA [114] | |
| Arsenic (As) | - | - | - | - | 10 | 10 |
| Cadmium (Cd) * | ≤0.08 (Class 1) 0.08 (Class 2) 0.09 (Class 3) 0.15 (Class 4) 0.25 (Class 5) | 0.2 | ≤0.45 (Class 1) 0.45 (Class 2) 0.6 (Class 3) 0.9 (Class 4) 1.5 (Class 5) | 5 | 3 | 5 |
| Copper (Cu) | - | - | - | 2000 | 2000 | 1300 |
| Chromium (Cr) | - | - | - | 25 | 50 | 100 |
| Lead (Pb) | 7.2 | 7.2 | NA | 5 | 10 | 15 |
| Mercury (Hg) | - | - | - | 1 | 6 | 2 |
| Nickel (Ni) | 20 | 20 | NA | 20 | 70 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madjar, R.M.; Vasile Scăețeanu, G. An Overview of Heavy Metal Contamination in Water from Agriculture: Origins, Monitoring, Risks, and Control Measures. Sustainability 2025, 17, 7368. https://doi.org/10.3390/su17167368
Madjar RM, Vasile Scăețeanu G. An Overview of Heavy Metal Contamination in Water from Agriculture: Origins, Monitoring, Risks, and Control Measures. Sustainability. 2025; 17(16):7368. https://doi.org/10.3390/su17167368
Chicago/Turabian StyleMadjar, Roxana Maria, and Gina Vasile Scăețeanu. 2025. "An Overview of Heavy Metal Contamination in Water from Agriculture: Origins, Monitoring, Risks, and Control Measures" Sustainability 17, no. 16: 7368. https://doi.org/10.3390/su17167368
APA StyleMadjar, R. M., & Vasile Scăețeanu, G. (2025). An Overview of Heavy Metal Contamination in Water from Agriculture: Origins, Monitoring, Risks, and Control Measures. Sustainability, 17(16), 7368. https://doi.org/10.3390/su17167368









