Remediation of Heavy Metal-Contaminated Soils Using Phosphate-Enriched Sewage Sludge Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Preparation
2.2. Characterization Methods
2.3. Soil Incubation Experiment for Heavy Metal Remediation
3. Results
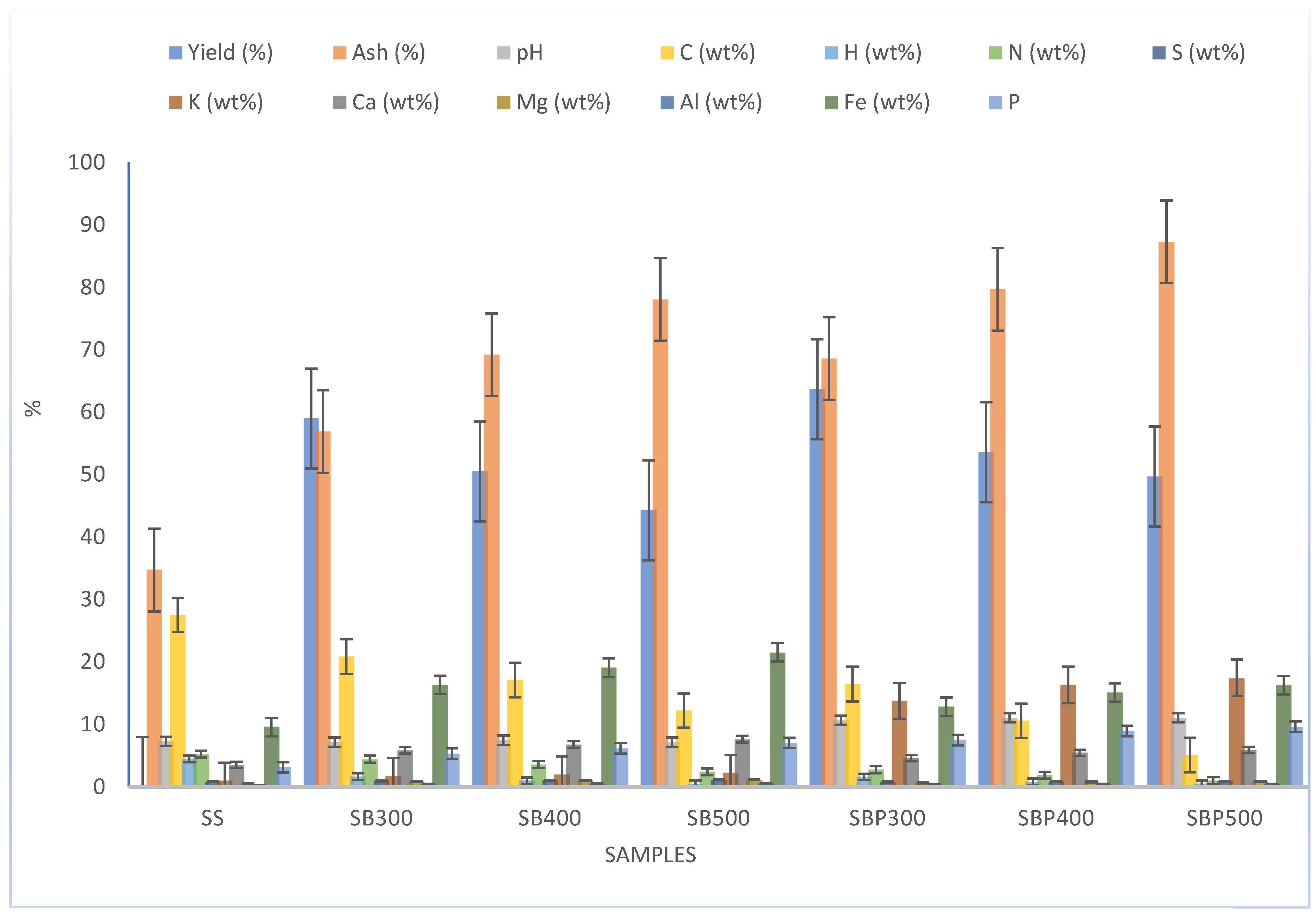
3.1. Material Characterization
3.2. Interaction Mechanisms Between Biochar and Heavy Metals in Soil
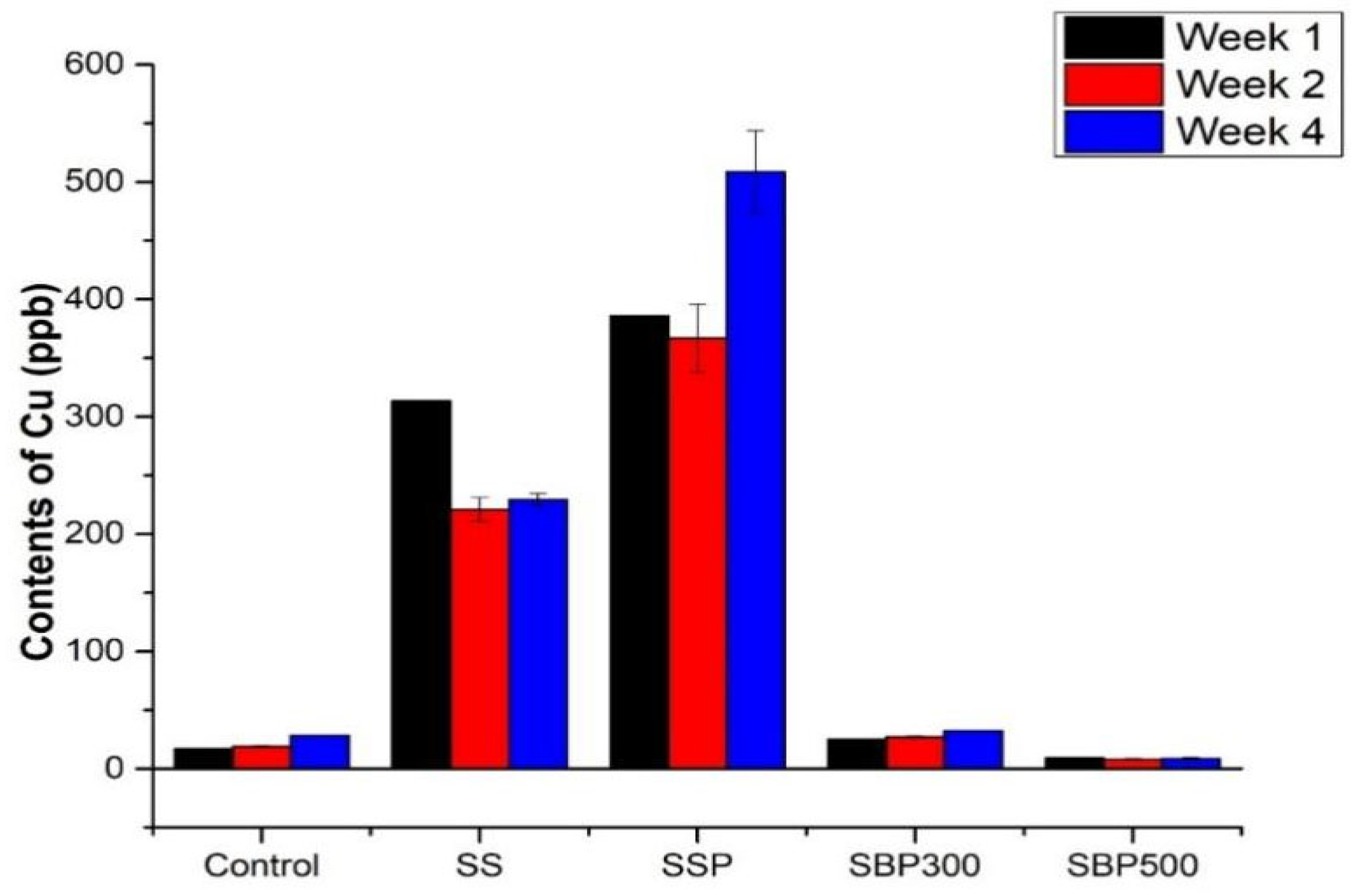
3.2.1. Copper (Cu)
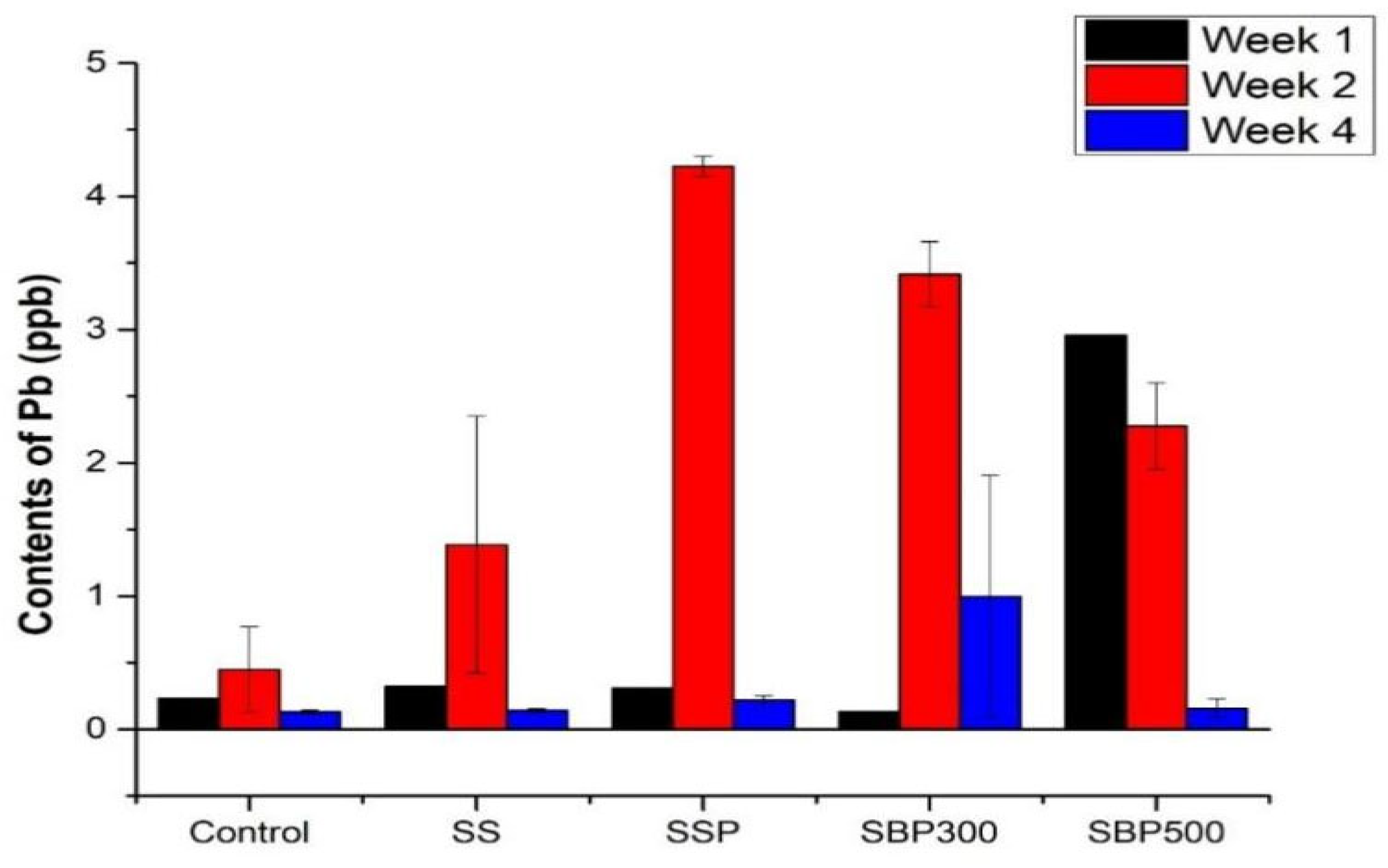
3.2.2. Lead (Pb)
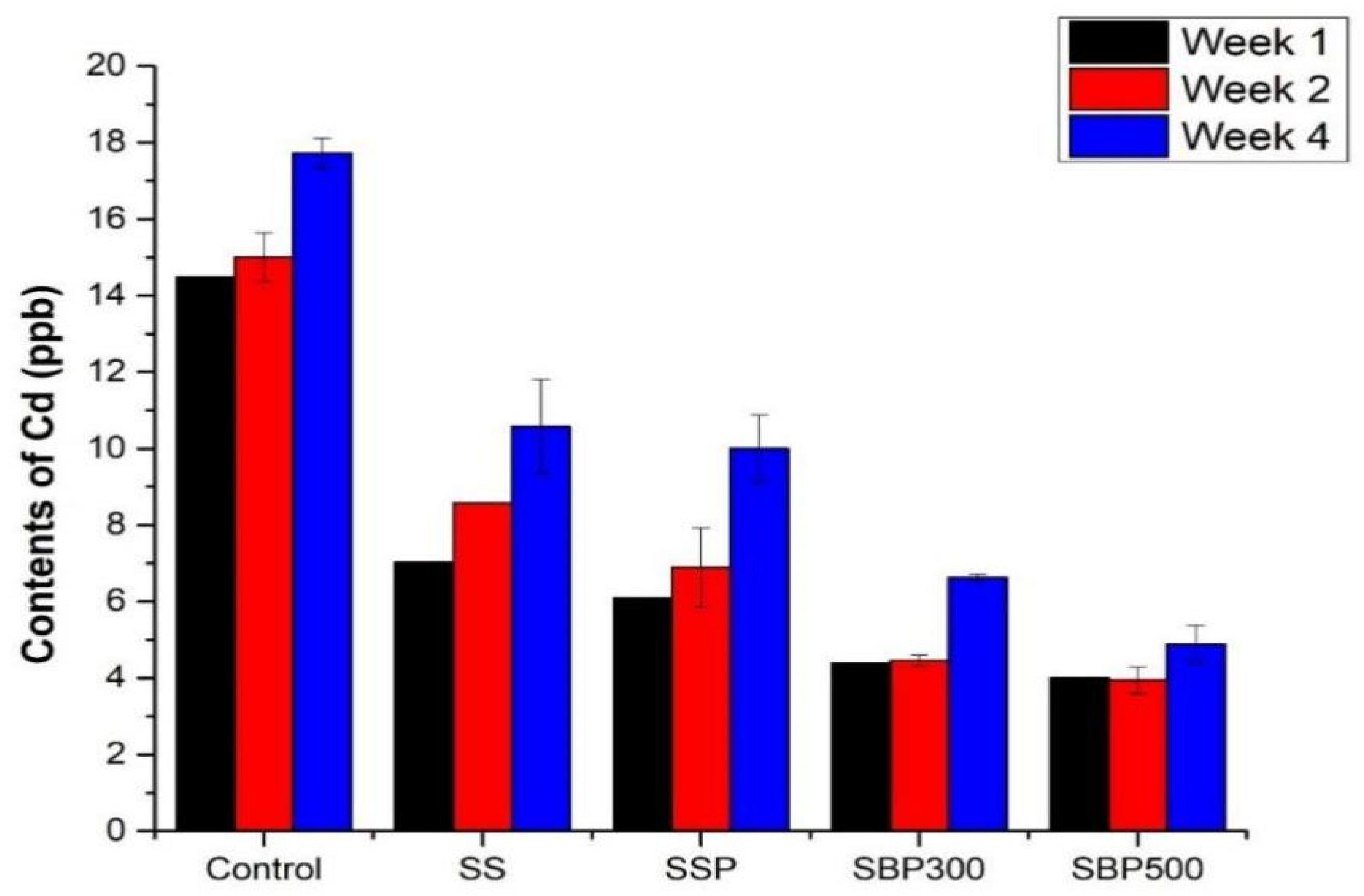
3.2.3. Cadmium (Cd)
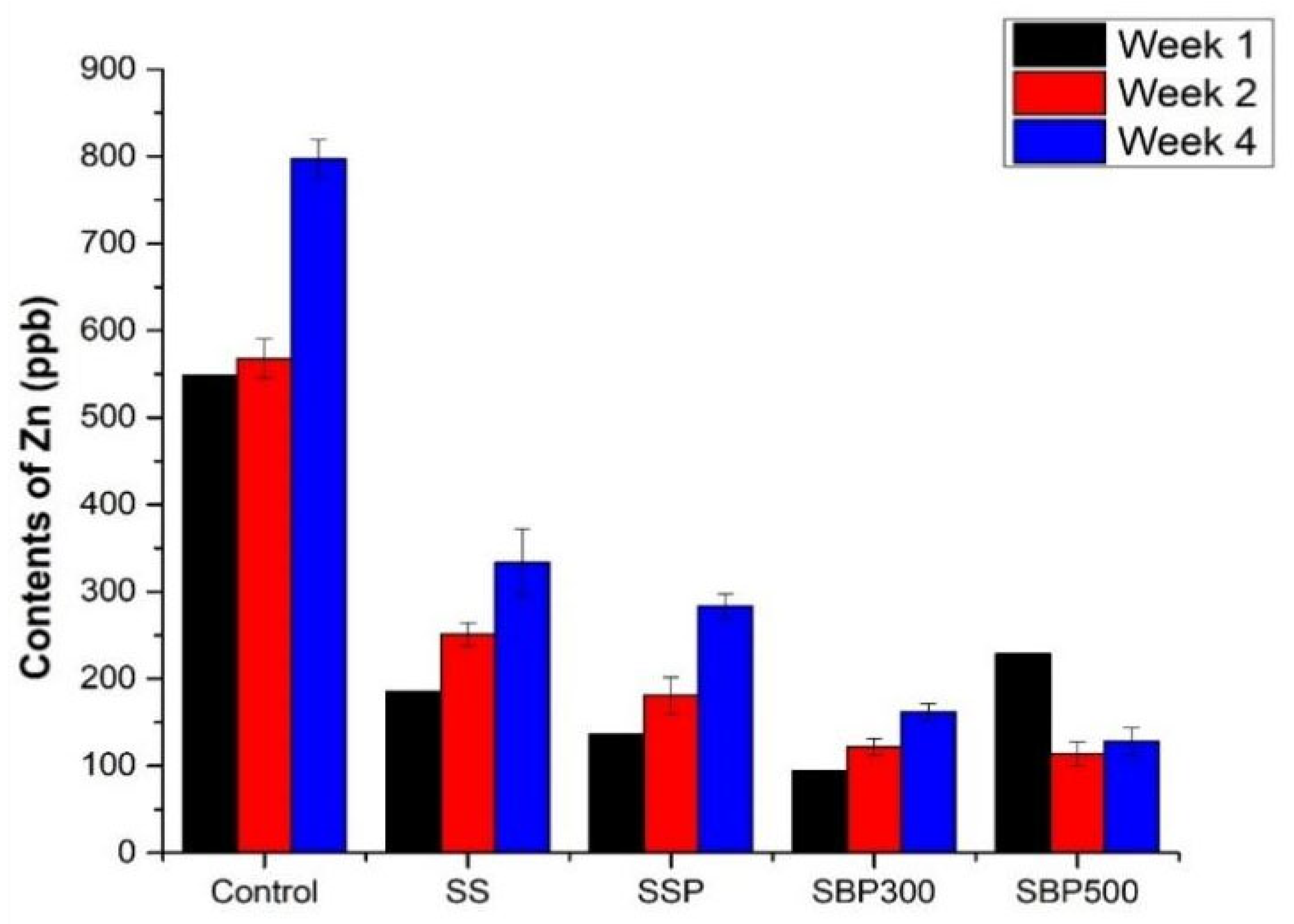
3.2.4. Zinc (Zn)
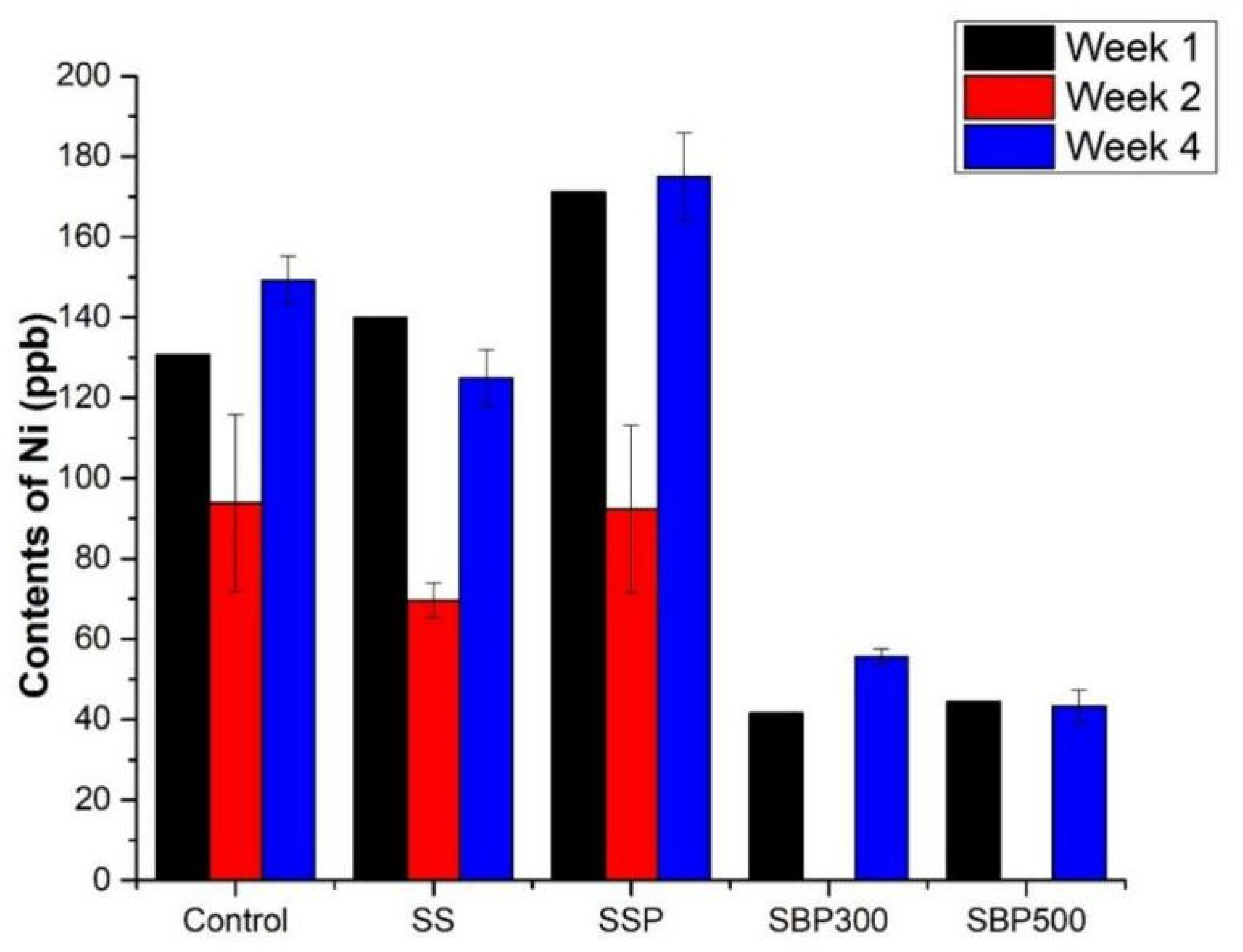
3.2.5. Nickel (Ni)
4. Discussion
4.1. Characteristics of Sewage Sludge-Derived Biochar
4.2. Sewage Sludge-Derived Biochar Interplay with Heavy Metals
4.2.1. Dual Mechanisms of Copper Retention: Surface Complexation and Hydroxide Precipitation
4.2.2. Phosphate-Facilitated Lead Immobilization Through Complexation and Precipitation
4.2.3. Cadmium Immobilization via Apatite Formation and pH-Induced Precipitation
4.2.4. Zinc Sequestration Through Adsorption and Ion Exchange on Biochar Surfaces
4.2.5. Limited Nickel Retention: Weak Phosphate Affinity and Surface Complexation Constraints
4.3. Mechanistic Insights: pH-Mediated and Ligand-Specific Pathways of Metal Immobilization
4.3.1. pH-Mediated Reactions: Precipitation and Charge Neutralization
4.3.2. Ligand-Specific Interactions: Complexation and Ion Exchange
4.4. Environmental Implications and Remediation Potential
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy Metal Pollution and Human Biotoxic Effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Bin Emran, T.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ.—Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total Concentrations and Sources of Heavy Metal Pollution in Global River and Lake Water Bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Petruzzelli, G. Soil Contamination and Remediation Strategies. Current Research and Future Challenge. EGU Gen. Assem. Conf. Abstr. 2012, 14, 7963. [Google Scholar]
- Singh, B.P.; Hatton, B.J. Influence of Biochars on Nitrous Oxide Emission and Nitrogen Leaching from Two Contrasting Soils. J. Environ. Qual. 2010, 39, 1224–1235. [Google Scholar] [CrossRef]
- Joseph, S.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; Zwieten, V.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An Investigation into the Reactions of Biochar in Soil. Aust. J. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Lim, J.E.; Lee, S.E.; Cho, J.S.; Moon, D.H.; Hashimoto, Y.; Ok, Y.S. Speciation and Phytoavailability of Lead and Antimony in a Small Arms Range Soil Amended with Mussel Shell, Cow Bone and Biochar: EXAFS Spectroscopy and Chemical Extractions. Chemosphere 2014, 95, 433–441. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-Char Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Wang, S.; Xu, Y.; Norbu, N.; Wang, Z. Remediation of Biochar on Heavy Metal Polluted Soils. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 042113. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of Metal Sorption by Biochars: Biochar Characteristics and Modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Rees, F.; Simonnot, M.O.; Morel, J.L. Short-Term Effects of Biochar on Soil Heavy Metal Mobility Are Controlled by Intra-Particle Diffusion and Soil pH Increase. Eur. J. Soil Sci. 2014, 65, 149–161. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar Reduces the Bioavailability and Phytotoxicity of Heavy Metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Kopecký, M.; Kolář, L. A Meta-analysis on the Impacts of Different Oxidation Methods on the Surface Area Properties of Biochar. Land Degrad. Dev. 2023, 34, 299–312. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Neugschwandtner, R.W.; Konvalina, P.; Kopecký, M.; Moudrý, J.; Perná, K.; Murindangabo, Y.T. The Impact of Pyrolysis Temperature on Biochar Properties and Its Effects on Soil Hydrological Properties. Sustainability 2022, 14, 14722. [Google Scholar] [CrossRef]
- Uchimiya, M.; Lima, I.M.; Klasson, K.T.; Wartelle, L.H. Contaminant Immobilization and Nutrient Release by Biochar Soil Amendment: Roles of Natural Organic Matter. Soil Sediment Contam. 2010, 26, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Lima, I.; Xing, B.; Gaskin, J.; Steiner, C.; Das, K.; Ahmedna, M.; Rehrah, D.; Watts, D.; Busscher, W. Characterization of Designer Biochar Produced at Different Temperatures and Their Effects on a Loamy Sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Sachdeva, S.; Kumar, R.; Sahoo, P.K.; Nadda, A.K. Recent Advances in Biochar Amendments for Immobilization of Heavy Metals in an Agricultural Ecosystem: A Systematic Review. Environ. Pollut. 2023, 319, 120937. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, H.; Lu, Y.-Y.; Ren, Z.-Q.; Gao, N.; Wang, J.-J.; Huang, B.-C.; Jin, R.-C. In-Situ Synthesis of Lanthanum-Coated Sludge Biochar for Advanced Phosphorus Adsorption. J. Environ. Manag. 2025, 373, 123607. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, G.I. Prediction of the Impact of Ecological Restoration Technology on the Restoration of Heavy Metal Pollution in Agricultural Soil. Geol. Ecol. Landsc. 2024, 1–17. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, Q.; Kumar, A.; Zhang, W.; Yu, Z.; Hui, D.; Zhang, C.; Shan, S. Effect of Degradable Microplastics, Biochar and Their Coexistence on Soil Organic Matter Decomposition: A Critical Review. TrAC Trends Anal. Chem. 2025, 183, 118082. [Google Scholar] [CrossRef]
- Mayer, B.K.; Baker, L.A.; Boyer, T.H.; Drechsel, P.; Mac, G.; Munir Hanjra, A.; Parameswaran, P.; Stoltzfus, J.; Paul Westerhoff, A.; Rittmann, B.E. Total Value of Phosphorus Recovery. Environ. Sci. Technol. 2016, 50, 6606–6620. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Duraisamy, P.; Mani, A.; Arulmozhiselvan, K. Immobilization and Phytoavailability of Cadmium in Variable Charge Soils. I. Effect of Phosphate Addition. Plant Soil 2003, 250, 83–94. [Google Scholar] [CrossRef]
- Cárdenas- Aguiar, E.; Gascó, G.; Paz-Ferreiro, J.; Méndez, A. The Effect of Biochar and Compost from Urban Organic Waste on Plant Biomass and Properties of an Artificially Copper Polluted Soil. Int. Biodeterior. Biodegrad. 2017, 124, 223–232. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Plasencia, P.; Gascó, G.; Méndez, A. Biochar from Pyrolysis of Deinking Paper Sludge and Its Use in the Remediation of Zn-polluted Soils. Land. Degrad. Dev. 2017, 28, 355–360. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil Amendments for Immobilization of Potentially Toxic Elements in Contaminated Soils: A Critical Review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Karthik, V.; Periyasamy, S.; Dharneesh, S.; Duvakeesh, G.K.; Gizaw, D.G.; Vijayashankar, T. Biochar as a Sustainable Adsorbent for Heavy Metal Removal From Polluted Waters: A Comprehensive Outlook. J. Chem. 2024, 2024, 8217730. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Ćwieląg-Piasecka, I. Effect of Biochar Application on Heavy MetalMobility in Soils Impacted by CopperSmelting Processes. Pol. J. Environ. Stud. 2020, 29, 1749–1757. [Google Scholar] [CrossRef]
- Lwin, C.S.; Seo, B.-H.; Kim, H.-U.; Owens, G.; Kim, K.-R. Application of Soil Amendments to Contaminated Soils for Heavy Metal Immobilization and Improved Soil Quality—A Critical Review. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Ghodszad, L.; Reyhanitabar, A.; Maghsoodi, M.R.; Asgari Lajayer, B.; Chang, S.X. Biochar Affects the Fate of Phosphorus in Soil and Water: A Critical Review. Chemosphere 2021, 283, 131176. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, S.; Cheng, P.; Zhang, S.; Sun, Y. Effects of Soil Amendments on Heavy Metal Immobilization and Accumulation by Maize Grown in a Multiple-Metal-Contaminated Soil and Their Potential for Safe Crop Production. Toxics 2020, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Kooij, J.; Yang, P.-T.; Bruun, S.; Magid, J.; Gro Nielsen, U.; Theil Kuhn, L.; Müller-Stöver, D. Phosphorus Speciation in Different Sewage Sludges and Their Biochars and Its Implications for Movement of Labile Phosphate in Two Soils. J. Environ. Manag. 2024, 370, 122565. [Google Scholar] [CrossRef]
- Peirce, J.J.; Vesilind, P.A.; Weiner, R. Environmental Pollution and Control, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1998; ISBN 978-0750698993. [Google Scholar]
- Protogene, M.; Murindangabo, Y.T.; Frouz, J.; Brom, J. Characterization, Fractionation and Untapped Potential of Phosphate-Amended Sewage Sludge Biochar in Soil-Plant Systems. Chemosphere 2024, 367, 143565. [Google Scholar] [CrossRef]
- Roberts, D.A.; Cole, A.J.; Whelan, A.; de Nys, R.; Paul, N.A. Slow Pyrolysis Enhances the Recovery and Reuse of Phosphorus and Reduces Metal Leaching from Biosolids. Waste Manag. 2017, 64, 133–139. [Google Scholar] [CrossRef]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar Production by Sewage Sludge Pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Junior, A.; Guo, M. Efficacy of Sewage Sludge Derived Biochar on Enhancing Soil Health and Crop Productivity in Strongly Acidic Soil. Front. Soil Sci. 2023, 3, 1066547. [Google Scholar] [CrossRef]
- Song, W.; Guo, M. Quality Variations of Poultry Litter Biochar Generated at Different Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2012, 94, 138–145. [Google Scholar] [CrossRef]
- Stella Mary, G.; Sugumaran, P.; Niveditha, S.; Ramalakshmi, B.; Ravichandran, P.; Seshadri, S. Production, Characterization and Evaluation of Biochar from Pod (Pisum sativum), Leaf (Brassica oleracea) and Peel (Citrus sinensis) Wastes. Int. J. Recycl. Org. Waste Agric. 2016, 5, 43–53. [Google Scholar] [CrossRef]
- Chatterjee, R.; Sajjadi, B.; Chen, W.-Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Effect of Pyrolysis Temperature on PhysicoChemical Properties and Acoustic-Based Amination of Biochar for Efficient CO2 Adsorption. Front. Energy Res. 2020, 8, 85. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Liu, N.; Sun, Z.; Fu, S.; Zhan, X.; Yang, J.; Zhou, R.; Zhang, H.; Zhang, J.; et al. Pyrolysis Temperature Had Effects on the Physicochemical Properties of Biochar. Plant Soil Environ. 2023, 69, 363–373. [Google Scholar] [CrossRef]
- Guo, M. The 3R Principles for Applying Biochar to Improve Soil Health. Soil Syst. 2020, 4, 9. [Google Scholar] [CrossRef]
- de Souza Souza, C.; Bomfim, M.R.; da Conceição de Almeida, M.; de Souza Alves, L.; de Santana, W.N.; da Silva Amorim, I.C.; Santos, J.A.G. Induced Changes of Pyrolysis Temperature on the Physicochemical Traits of Sewage Sludge and on the Potential Ecological Risks. Sci. Rep. 2021, 11, 974. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Bhatia, D.; Dhiman, J.; Samuel, J.; Prasad, R.; Singh, J. A Sustainable Paradigm of Sewage Sludge Biochar: Valorization, Opportunities, Challenges and Future Prospects. J. Clean. Prod. 2020, 269, 122259. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, K.; Jin, J.; Gao, B.; Yan, Y.; Han, L.; Wu, F.; Xing, B. Properties of the Plant- and Manure-Derived Biochars and Their Sorption of Dibutyl Phthalate and Phenanthrene. Sci. Rep. 2014, 4, 5295. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Sun, M.; Liu, H.; Liu, D.; Dai, J. Cadmium Immobilization in Soil Using Phosphate Modified Biochar Derived from Wheat Straw. Sci. Total Environ. 2024, 926, 171614. [Google Scholar] [CrossRef] [PubMed]
- Peiris, C.; Alahakoon, Y.A.; Malaweera Arachchi, U.; Mlsna, T.E.; Gunatilake, S.R.; Zhang, X. Phosphorus-Enriched Biochar for the Remediation of Heavy Metal Contaminated Soil. J. Agric. Food Res. 2023, 12, 100546. [Google Scholar] [CrossRef]
- Sha, H.; Li, J.; Wang, L.; Nong, H.; Wang, G.; Zeng, T. Preparation of Phosphorus-Modified Biochar for the Immobilization of Heavy Metals in Typical Lead-Zinc Contaminated Mining Soil: Performance, Mechanism and Microbial Community. Environ. Res. 2023, 218, 114769. [Google Scholar] [CrossRef]
- Zeng, G.; Wan, J.; Huang, D.; Hu, L.; Huang, C.; Cheng, M.; Xue, W.; Gong, X.; Wang, R.; Jiang, D. Precipitation, Adsorption and Rhizosphere Effect: The Mechanisms for Phosphate-Induced Pb Immobilization in Soils—A Review. J. Hazard. Mater. 2017, 339, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Moore, F.; González, M.-E.; Khan, N.; Curaqueo, G.; Sanchez-Monedero, M.; Rilling, J.; Morales, E.; Panichini, M.; Mutis, A.; Jorquera, M.; et al. Copper Immobilization by Biochar and Microbial Community Abundance in Metal-Contaminated Soils. Sci. Total Environ. 2018, 616–617, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kwak, J.-H.; Islam, M.S.; Naeth, M.A.; Gamal El-Din, M.; Chang, S.X. Biochar Surface Complexation and Ni(II), Cu(II), and Cd(II) Adsorption in Aqueous Solutions Depend on Feedstock Type. Sci. Total Environ. 2020, 712, 136538. [Google Scholar] [CrossRef] [PubMed]
- Hummel, W.; Curti, E. Nickel Aqueous Speciation and Solubility at Ambient Conditions: A Thermodynamic Elegy. Monatsh Chem. 2003, 134, 941–973. [Google Scholar] [CrossRef]
- El-Naggar, A.; Ahmed, N.; Mosa, A.; Niazi, N.K.; Yousaf, B.; Sharma, A.; Sarkar, B.; Cai, Y.; Chang, S.X. Nickel in Soil and Water: Sources, Biogeochemistry, and Remediation Using Biochar. J. Hazard. Mater. 2021, 419, 126421. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Wang, Y.; Wang, N.; Ma, F.; Yuan, Y. Effects of Ageing on Surface Properties of Biochar and Bioavailability of Heavy Metals in Soil. Agriculture 2024, 14, 1631. [Google Scholar] [CrossRef]
- Wang, Z.; Geng, C.; Bian, Y.; Zhang, G.; Zheng, C.; An, C. Effect of Oxidative Aging of Biochar on Relative Distribution of Competitive Adsorption Mechanism of Cd2+ and Pb2+. Sci. Rep. 2022, 12, 11308. [Google Scholar] [CrossRef] [PubMed]
- Illera, V.; Garrido, F.; Serrano, S.; García-González, M.T. Immobilization of the Heavy Metals Cd, Cu and Pb in an Acid Soil Amended with Gypsum- and Lime-rich Industrial By-products. Eur. J. Soil Sci. 2004, 55, 135–145. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical Characterization of Rice Straw-Derived Biochar for Soil Amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Rechberger, M.V.; Kloss, S.; Wang, S.L.; Lehmann, J.; Rennhofer, H.; Ottner, F.; Wriessnig, K.; Daudin, G.; Lichtenegger, H.; Soja, G.; et al. Enhanced Cu and Cd Sorption after Soil Aging of Woodchip-Derived Biochar: What Were the Driving Factors? Chemosphere 2019, 216, 463–471. [Google Scholar] [CrossRef]
- Zhang, C.; Shan, B.; Zhu, Y.; Tang, W. Remediation Effectiveness of Phyllostachys Pubescens Biochar in Reducing the Bioavailability and Bioaccumulation of Metals in Sediments. Environ. Pollut. 2018, 242, 1768–1776. [Google Scholar] [CrossRef]
- Wagner, A.; Kaupenjohann, M. Biochar Addition Enhanced Growth of Dactylis glomerata L. and Immobilized Zn and Cd but Mobilized Cu and Pb on a Former Sewage Field Soil. Eur. J. Soil Sci. 2015, 66, 505–515. [Google Scholar] [CrossRef]
- Ahmad, M.; Manzoor, K.; Venkatachalam, P.; Ikram, S. Kinetic and Thermodynamic Evaluation of Adsorption of Cu(II) by Thiosemicarbazide Chitosan. Int. J. Biol. Macromol. 2016, 92, 910–919. [Google Scholar] [CrossRef]
- Lu, D.; Hetrick, S.; Moran, E.; Li, G. Application of Time Series Landsat Images to Examining Land-Use/Land-Cover Dynamic Change. Photogramm. Eng. Remote Sens. 2012, 78, 747–755. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Kim, B.-Y.; Ahn, J.-H.; Lee, Y.H.; Zhang, M.; Moon, D.H.; Al-Wabel, M.I.; Lee, S.S. Impact of Soybean Stover- and Pine Needle-Derived Biochars on Pb and As Mobility, Microbial Community, and Carbon Stability in a Contaminated Agricultural Soil. J. Environ. Manag. 2016, 166, 131–139. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Gao, B.; Harris, W. Dairy-Manure Derived Biochar Effectively Sorbs Lead and Atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef]
- Ho, S.H.; Chen, Y.D.; Yang, Z.K.; Nagarajan, D.; Chang, J.S.; Ren, N.Q. High-Efficiency Removal of Lead from Wastewater by Biochar Derived from Anaerobic Digestion Sludge. Bioresour. Technol. 2017, 246, 142–149. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative Distribution of Pb2+ Sorption Mechanisms by Sludge-Derived Biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, J.; Niazi, N.K.; Müller, K.; Chu, Y.; Zhang, L.; Yuan, G.; Lu, K.; Song, Z.; Wang, H. Influence of Pyrolysis Temperature on Lead Immobilization by Chemically Modified Coconut Fiber-Derived Biochars in Aqueous Environments. Environ. Sci. Pollut. Res. 2016, 23, 22890–22896. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Feng, C.; Huang, W.; Lei, Z.; Zhang, Z. Study on Interaction between Phosphorus and Cadmium in Sewage Sludge during Hydrothermal Treatment by Adding Hydroxyapatite. Bioresour. Technol. 2014, 159, 176–181. [Google Scholar] [CrossRef]
- Gao, X.; Peng, Y.; Zhou, Y.; Adeel, M.; Chen, Q. Effects of Magnesium Ferrite Biochar on the Cadmium Passivation in Acidic Soil and Bioavailability for Packoi (Brassica chinensis L.). J. Environ. Manag. 2019, 251, 109610. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xia, W.; Lu, P. Study on Adsorption Characteristics of Biochar on Heavy Metals in Soil. Korean J. Chem. Eng. 2017, 34, 1867–1873. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, S.; Chen, Y.; Wang, F.; Yang, D.; Ren, J.; Lu, H.; Ping, L.; Chai, Y. Biochar Reduces Cadmium Accumulation in Rice Grains in a Tungsten Mining Area-Field Experiment: Effects of Biochar Type and Dosage, Rice Variety, and Pollution Level. Environ. Geochem. Health 2019, 41, 43–52. [Google Scholar] [CrossRef]
- Yin, D.; Wang, X.; Peng, B.; Tan, C.; Ma, L.Q. Effect of Biochar and Fe-Biochar on Cd and As Mobility and Transfer in Soil-Rice System. Chemosphere 2017, 186, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.-T.; Wu, P.; Qin, Q.-Y.; Huang, Y.-N.; Wang, Y.-J.; Zhou, D.-M. Screening of Wheat Straw Biochars for the Remediation of Soils Polluted with Zn (II) and Cd (II). J. Hazard. Mater. 2019, 362, 311–317. [Google Scholar] [CrossRef]
- Li, H.; Ye, X.; Geng, Z.; Zhou, H.; Guo, X.; Zhang, Y.; Zhao, H.; Wang, G. The Influence of Biochar Type on Long-Term Stabilization for Cd and Cu in Contaminated Paddy Soils. J. Hazard. Mater. 2016, 304, 40–48. [Google Scholar] [CrossRef]
- LAHORI, A.H.; GUO, Z.; ZHANG, Z.; LI, R.; MAHAR, A.; AWASTHI, M.K.; SHEN, F.; SIAL, T.A.; KUMBHAR, F.; WANG, P.; et al. Use of Biochar as an Amendment for Remediation of Heavy Metal-Contaminated Soils: Prospects and Challenges. Pedosphere 2017, 27, 991–1014. [Google Scholar] [CrossRef]
- Puga, A.P.; Abreu, C.A.; Melo, L.C.A.; Beesley, L. Biochar Application to a Contaminated Soil Reduces the Availability and Plant Uptake of Zinc, Lead and Cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef]
- Liang, J.; Yang, Z.; Tang, L.; Zeng, G.; Yu, M.; Li, X.; Wu, H.; Qian, Y.; Li, X.; Luo, Y. Changes in Heavy Metal Mobility and Availability from Contaminated Wetland Soil Remediated with Combined Biochar-Compost. Chemosphere 2017, 181, 281–288. [Google Scholar] [CrossRef]
- Rajakaruna, N.; Boyd, R.S. The Edaphic Factor- Its Role in Shaping the Biotic World Evolution under Extreme Edaphic Conditions.Pdf. 2008. Available online: https://nrajakaruna.wordpress.com/wp-content/uploads/2013/10/edaphic-factor.pdf (accessed on 1 July 2025).
- Yang, X.; Lu, K.; McGrouther, K.; Che, L.; Hu, G.; Wang, Q.; Liu, X.; Shen, L.; Huang, H.; Ye, Z.; et al. Bioavailability of Cd and Zn in Soils Treated with Biochars Derived from Tobacco Stalk and Dead Pigs. J. Soils Sediments 2017, 17, 751–762. [Google Scholar] [CrossRef]
- Liu, L.; Huang, L.; Huang, R.; Lin, H.; Wang, D. Immobilization of Heavy Metals in Biochar Derived from Co-Pyrolysis of Sewage Sludge and Calcium Sulfate. J. Hazard. Mater. 2021, 403, 123648. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, J.; Wang, L.; Yang, G.; Zhang, X. Effect of Biochar on Heavy Metal Speciation of Paddy Soil. Water Air Soil Pollut. 2015, 226, 429. [Google Scholar] [CrossRef]
- Muhammad, N.; Ge, L.; Chan, W.P.; Khan, A.; Nafees, M.; Lisak, G. Impacts of Pyrolysis Temperatures on Physicochemical and Structural Properties of Green Waste Derived Biochars for Adsorption of Potentially Toxic Elements. J. Environ. Manag. 2022, 317, 115385. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Sun, Y.; Huang, Q.; Xu, Y.; Jia, H. Sustainable Exploitation and Safe Utilization of Biochar: Multiphase Characterization and Potential Hazard Analysis. Bioresour. Technol. 2023, 383, 129241. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A Review of Biochar as a Low-Cost Adsorbent for Aqueous Heavy Metal Removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Baloch, S.B.; Ali, S.; Bernas, J.; Moudrý, J.; Konvalina, P.; Mushtaq, Z.; Murindangabo, Y.T.; Onyebuchi, E.F.; Baloch, F.B.; Ahmad, M.; et al. Wood Ash Application for Crop Production, Amelioration of Soil Acidity and Contaminated Environments. Chemosphere 2024, 357, 141865. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd Allah, E.F.; et al. Biochar Production and Characteristics, Its Impacts on Soil Health, Crop Production, and Yield Enhancement: A Review. Plants 2024, 13, 166. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, J.; Zhang, S.; Zhang, X.; Chen, H. Effect of Phosphorus-Modified Biochars on Immobilization of Cu (II), Cd (II), and As (V) in Paddy Soil. J. Hazard. Mater. 2020, 390, 121349. [Google Scholar] [CrossRef]
- Uchimiya, M.; Lima, I.M.; Thomas Klasson, K.; Chang, S.; Wartelle, L.H.; Rodgers, J.E. Immobilization of Heavy Metal Ions (CuII, CdII, NiII, and PbII) by Broiler Litter-Derived Biochars in Water and Soil. J. Agric. Food Chem. 2010, 58, 5538–5544. [Google Scholar] [CrossRef]
- Harikishore Kumar Reddy, D.; Lee, S.-M. Magnetic Biochar Composite: Facile Synthesis, Characterization, and Application for Heavy Metal Removal. Colloids Surf. A Physicochem. Eng. Asp. 2014, 454, 96–103. [Google Scholar] [CrossRef]
- Adhikari, S.; Gascó, G.; Méndez, A.; Surapaneni, A.; Jegatheesan, V.; Shah, K.; Paz-Ferreiro, J. Influence of Pyrolysis Parameters on Phosphorus Fractions of Biosolids Derived Biochar. Sci. Total Environ. 2019, 695, 133846. [Google Scholar] [CrossRef]
- Kargar, M.; Clark, O.G.; Hendershot, W.H.; Jutras, P.; Prasher, S.O. Immobilization of Trace Metals in Contaminated Urban Soil Amended with Compost and Biochar. Water Air Soil Pollut. 2015, 226, 191. [Google Scholar] [CrossRef]
- Rinklebe, J.; Shaheen, S.M.; Frohne, T. Amendment of Biochar Reduces the Release of Toxic Elements under Dynamic Redox Conditions in a Contaminated Floodplain Soil. Chemosphere 2016, 142, 41–47. [Google Scholar] [CrossRef]
- Kumari, S.; Mishra, A. Heavy Metal Contamination. In Soil Contamination—Threats and Sustainable Solutions; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Nyiramigisha, P.; Komariah; Sajidan. Harmful Impacts of Heavy Metal Contamination in the Soil and Crops Grown Around Dumpsites. Rev. Agric. Sci. 2021, 9, 271–282. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K. Heavy Metals Toxicity in Plants: An Overview on the Role of Glutathione and Phytochelatins in Heavy Metal Stress Tolerance of Plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Pujari, M.; Kapoor, D. Heavy Metals in the Ecosystem: Sources and Their Effects. In Heavy Metals in the Environment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–7. [Google Scholar]
- Singh, A.; Sharma, A.; Verma, R.K.; Chopade, R.L.; Pandit, P.P.; Nagar, V.; Aseri, V.K.; Choudhary, S.; Awasthi, G.; K. Awasthi, K.; et al. Heavy Metal Contamination of Water and Their Toxic Effect on Living Organisms. In The Toxicity of Environmental Pollutants; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Authman, M.M. Use of Fish as Bio-Indicator of the Effects of Heavy Metals Pollution. J. Aquac. Res. Dev. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Danovaro, R.; Cocozza di Montanara, A.; Corinaldesi, C.; Dell’Anno, A.; Illuminati, S.; Willis, T.J.; Gambi, C. Bioaccumulation and Biomagnification of Heavy Metals in Marine Micro-Predators. Commun. Biol. 2023, 6, 1206. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E. Trophic Transfer, Bioaccumulation, and Biomagnification of Non-Essential Hazardous Heavy Metals and Metalloids in Food Chains/Webs—Concepts and Implications for Wildlife and Human Health. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 1353–1376. [Google Scholar] [CrossRef]
- Azeh Engwa, G.; Udoka Ferdinand, P.; Nweke Nwalo, F.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World—New Tricks for an Old Dog? IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Ali, S.; Wang, D.; Kaleri, A.R.; Baloch, S.B.; Brtnicky, M.; Kucerik, J.; Mustafa, A. Physiological Responses and Phytoremediation Abilities of Cucumber (Cucumis sativus L.) under Cesium and Strontium Contaminated Soils. Agronomy 2022, 12, 1311. [Google Scholar] [CrossRef]
- Rehan, M.; Alsohim, A.S. Bioremediation of Heavy Metals. In Environmental Chemistry and Recent Pollution Control Approaches; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation Technologies and Their Mechanism for Removal of Heavy Metal from Contaminated Soil: An Approach for a Sustainable Environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef]
- Akhtar, F.Z.; Archana, K.M.; Krishnaswamy, V.G.; Rajagopal, R. Remediation of Heavy Metals (Cr, Zn) Using Physical, Chemical and Biological Methods: A Novel Approach. SN Appl. Sci. 2020, 2, 267. [Google Scholar] [CrossRef]
- Chen, H.M.; Zheng, C.R.; Tu, C.; Shen, Z.G. Chemical Methods and Phytoremediation of Soil Contaminated with Heavy Metals. Chemosphere 2000, 41, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Dada, E.; Njoku, K.; Osuntoki, A.; Akinola, M. A Review of Current Techniques of <in Situ> Physico-Chemical and Biological Remediation of Heavy Metals Polluted Soil. Ethiop. J. Environ. Stud. Manag. 2015, 8, 606. [Google Scholar] [CrossRef]
- Liao, X.; Li, Y.; Miranda-Avilés, R.; Zha, X.; Anguiano, J.H.H.; Moncada Sánchez, C.D.; Puy-Alquiza, M.J.; González, V.P.; Garzon, L.F.R. In Situ Remediation and Ex Situ Treatment Practices of Arsenic-Contaminated Soil: An Overview on Recent Advances. J. Hazard. Mater. Adv. 2022, 8, 100157. [Google Scholar] [CrossRef]
- Arora, V.; Khosla, B. Conventional and Contemporary Techniques for Removal of Heavy Metals from Soil. In Biodegradation Technology of Organic and Inorganic Pollutants; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K.S.M. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery From Various Environments-A Review. Front. Environ. Sci. 2019, 7, 66. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Li, J.; Dissanayake, P.D.; Suvarna, M.; Li, L.; Yuan, X.; Sarkar, B.; Tsang, D.C.W.; Rinklebe, J.; Wang, X.; et al. Prediction of Soil Heavy Metal Immobilization by Biochar Using Machine Learning. Environ. Sci. Technol. 2022, 56, 4187–4198. [Google Scholar] [CrossRef]
- Gogoi, L.; Narzari, R.; Chutia, R.S.; Borkotoki, B.; Gogoi, N.; Kataki, R. Remediation of Heavy Metal Contaminated Soil: Role of Biochar. In Advances in Chemical Pollution, Environmental Management and Protection; Elsevier: Amsterdam, The Netherlands, 2021; pp. 39–63. [Google Scholar]
- Phiri, Z.; Moja, N.T.; Nkambule, T.T.I.; de Kock, L.-A. Utilization of Biochar for Remediation of Heavy Metals in Aqueous Environments: A Review and Bibliometric Analysis. Heliyon 2024, 10, e25785. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Zhu, Y.; Fang, W.; Tan, Y.; He, Z.; Liao, H. Research Status, Trends, and Mechanisms of Biochar Adsorption for Wastewater Treatment: A Scientometric Review. Environ. Sci. Eur. 2024, 36, 25. [Google Scholar] [CrossRef]
- Aftab, B.; Ok, Y.S.; Cho, J.; Hur, J. Targeted Removal of Organic Foulants in Landfill Leachate in Forward Osmosis System Integrated with Biochar/Activated Carbon Treatment. Water Res. 2019, 160, 217–227. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the Total and Bioavailable Polycyclic Aromatic Hydrocarbons and Dioxins in Biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Sun, Z.-F.; Pan, X.-W.; Tan, J.-Y.; Yang, S.-S.; Wu, J.-T.; Chen, C.; Yuan, Y.; Ren, N.-Q. Sewage Sludge Derived Biochar for Environmental Improvement: Advances, Challenges, and Solutions. Water Res. X 2023, 18, 100167. [Google Scholar] [CrossRef] [PubMed]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The Presence of Contaminations in Sewage Sludge—The Current Situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbasabire, P.; Murindangabo, Y.T.; Brom, J.; Byukusenge, P.; Ufitikirezi, J.d.D.M.; Uwihanganye, J.; Umurungi, S.N.; Ntezimana, M.G.; Karimunda, K.; Bwimba, R. Remediation of Heavy Metal-Contaminated Soils Using Phosphate-Enriched Sewage Sludge Biochar. Sustainability 2025, 17, 7345. https://doi.org/10.3390/su17167345
Mbasabire P, Murindangabo YT, Brom J, Byukusenge P, Ufitikirezi JdDM, Uwihanganye J, Umurungi SN, Ntezimana MG, Karimunda K, Bwimba R. Remediation of Heavy Metal-Contaminated Soils Using Phosphate-Enriched Sewage Sludge Biochar. Sustainability. 2025; 17(16):7345. https://doi.org/10.3390/su17167345
Chicago/Turabian StyleMbasabire, Protogene, Yves Theoneste Murindangabo, Jakub Brom, Protegene Byukusenge, Jean de Dieu Marcel Ufitikirezi, Josine Uwihanganye, Sandra Nicole Umurungi, Marie Grace Ntezimana, Karim Karimunda, and Roger Bwimba. 2025. "Remediation of Heavy Metal-Contaminated Soils Using Phosphate-Enriched Sewage Sludge Biochar" Sustainability 17, no. 16: 7345. https://doi.org/10.3390/su17167345
APA StyleMbasabire, P., Murindangabo, Y. T., Brom, J., Byukusenge, P., Ufitikirezi, J. d. D. M., Uwihanganye, J., Umurungi, S. N., Ntezimana, M. G., Karimunda, K., & Bwimba, R. (2025). Remediation of Heavy Metal-Contaminated Soils Using Phosphate-Enriched Sewage Sludge Biochar. Sustainability, 17(16), 7345. https://doi.org/10.3390/su17167345







