1. Introduction
The importance of water as a vital resource is undeniable. Population growth and anthropogenic activities contribute to water pollution. This increases the need to find economical and safe methods for its treatment to give it some other use or for its return to the environment without generating negative impacts on local inhabitants [
1]. It is also essential to reduce energy consumption and enhance sustainability in pollutant removal technologies, as this contributes to the achievement of several Sustainable Development Goals (SDGs). Particularly, Goal 6: Clean Water and Sanitation, specifically Target 6.3, which aims to improve water quality by reducing pollution, eliminating dumping, and minimizing the release of hazardous chemicals and materials, as well as halving the proportion of untreated wastewater and substantially increasing recycling and safe reuse globally. It also supports Goal 7: Affordable and Clean Energy, particularly Target 7.3, which seeks to double the global rate of improvement in energy efficiency. Additionally, it aligns with Goal 12: Responsible Consumption and Production, especially Target 12.4, which focuses on the environmentally sound management of chemicals and all wastes throughout their life cycle, according to agreed international frameworks, to significantly reduce their release into the air, water, and soil, thereby minimizing their adverse effects on human health and the environment [
2].
Among the most prevalent contaminants in water are coliform bacteria, organic matter, suspended solids, fertilizers, pesticides, and, significantly, heavy metals [
3]. Mining can significantly impact water quality and aquatic ecosystems through several mechanisms, with acid mine drainage (AMD) being one of the most well-known and harmful. This acid leaches heavy metals from surrounding rocks, creating a toxic, acidic runoff that can enter nearby rivers, lakes, and groundwater. AMD severely lowers pH levels in water, harming aquatic life and contaminating drinking water sources [
4]. Lead in water has led to worrying situations worldwide, affecting public health. In 2014, the city of Flint, Michigan, experienced a crisis when changing the source of its water supply, and the Flint River water began to corrode lead pipes, releasing high levels of the mineral in water and affecting the health of thousands of residents, especially children [
5]. Lead poisoning is a major global health concern, particularly affecting children. Lead exposure has resulted in the poisoning of one-third of children globally, with the primary sources being the use of lead pipes and activities related to mining [
6]. According to a 2020 study published by the United Nations Children’s Fund (UNICEF), an estimated 800 million children worldwide have blood lead levels at or above five micrograms per deciliter (µg/dL). This report was the first to comprehensively assess the global scale of childhood lead exposure [
7]. In
Table 1, data on lead global production and usage are shown [
8].
Lead represents one of the main minerals produced in Bolivia, giving rise to considerable lead emissions into the environmental matrices in areas close to mining operations, especially near roads, fruit orchards, and industrial zones [
9,
10]. This phenomenon affects the flora, fauna, and inhabitants who reside near production areas or lead deposits [
11]. Lead can be absorbed into soil or contaminate water, affecting vegetative species and other living beings [
12]. Lead bioaccumulation in the soil, water, and air produces an increase in environmental pollution rates [
13]. There is an alarming situation regarding lead in different bodies of water, reaching a wide presence like other heavy metals [
14]. The production volumes of lead extracted in Bolivia since 2010 are shown in
Table 2 [
15].
The exploitation of lead in San Cristóbal mine (one of the largest mine facilities in Bolivia) could last up to 147 years [
16]. According to the International Lead and Zinc Study Group (ILZSG), global lead production continues to remain elevated, highlighting the ongoing demand for and extraction of lead worldwide [
8]. In Bolivia, this trend is likely to continue, with lead mining operations potentially expanding, which in turn could lead to further contamination of local water sources. This persistent production and extraction of lead contribute to both global pollution and localized environmental challenges, particularly in water quality, exacerbating the risk of lead contamination for surrounding communities.
Given the problems caused by heavy metals, various technologies have been developed for their elimination or inactivation. Some removal methods are membrane filtration, ion exchange, adsorption by activated carbon, and coagulation–flocculation, among others [
17].
Research on membrane filtration technology is constantly growing and has yielded positive results [
18,
19,
20]. The study by Vera et al. involved using osmosis membranes to remove Pb and Cd from mining wastewater. The study achieved an osmosis rejection of 98.77% for Pb and 98.30% for Cd [
19]. Another successful experience was recorded in the removal of metal ions such as lead, achieving removal percentages of 98.9% and 98.7% using RO and nanofiltration (NF), respectively [
19]. Reverse osmosis membranes have demonstrated high efficiency in removing not only lead but also other toxic metals, reinforcing their potential as a versatile and reliable water treatment technology. In the case of arsenic, removal rates ranging from 90% to 99% have been achieved, while tests involving hexavalent chromium have shown removal efficiencies as high as 99.9% [
21]. In addition, Villena Martines et al. reached a rejection rate greater than 99% using heavy metals such as arsenic and lead [
22]. On the other hand, low-pressure reverse osmosis studies were addressed, showing effective results on municipal wastewater treatment [
23]. The use of low pressures in reverse osmosis obtained percentages of rejection greater than 90% in anionic pollutants from wastewater [
24], showing that low pressures confer a reduction in environmental impact while minimizing energy consumption [
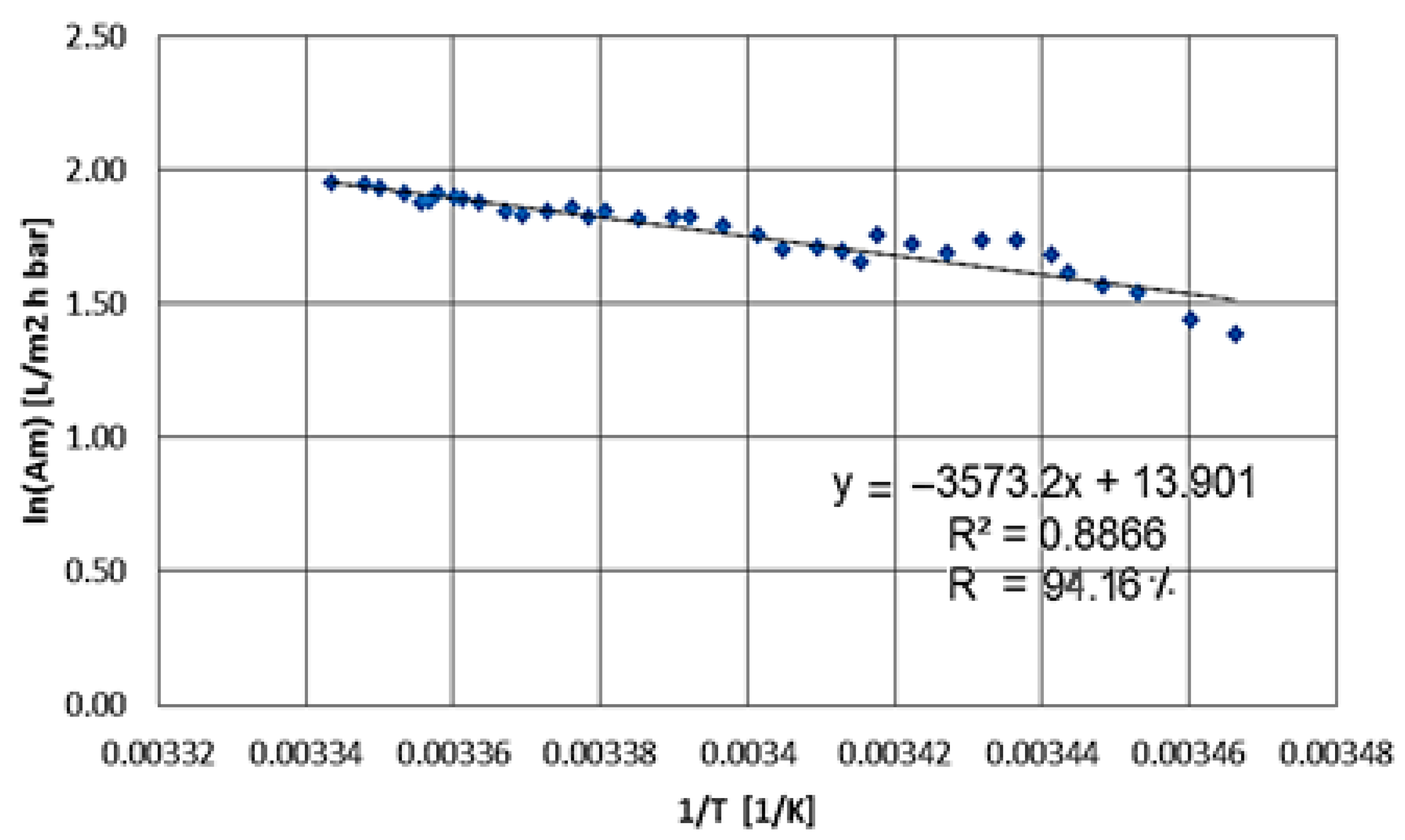
25]. The objective of this research was to optimize the working pressure range for RO, using a pilot plant to remove lead and seeking to maintain high metal removal with more sustainable energy costs. With the demand for the removal of heavy metals, such as lead from water, the need arises to obtain high efficiencies with existing technologies and at the same time a low energy cost, aiming at sustainability.
5. Conclusions
The negative impact of lead on the environment and public health is of great concern. Like other heavy metals, lead is mined in large quantities and released into the environment, often without undergoing any treatment process. Illegal mining emerges as a significant source of lead contamination of water resources. There are active technologies that achieve high removal rates, close to 100%, but high operating costs limit their application. This research offers a vision of the Bolivian context and the danger that the world faces due to the presence of lead in water.
Experimentation through RO membrane filtration has yielded promising results. The conductivity rejection percentages in membrane tests ranged between 92.98% and 96.13%. Regarding the lead rejection rate concerning the lead concentration, significant values were obtained, all around 100%, with a maximum rejection rate of lead of 99.75%.
The use of technology such as membrane filtration contemplates various potential applications in different industrial sectors. Significant advances and improvements in this technology allow for the regulation of more environmentally friendly working conditions without compromising removal efficiency. During experimentation, it was highlighted that controlling conditions on a pilot scale contributes to reductions in operational failures and continuous monitoring of removal performance. The process reduced energy consumption by 30% compared to conventional operation conditions. At the same time, the average flux produced almost tripled with respect to that when normal conditions for RO are used.
RO emerges as a quick solution to address the contamination of water bodies, which demands immediate action. RO is a great precedent to begin decontaminating mining liabilities in different regions of the world. Although this article focuses on lead removal, the use of membranes can cover a large number of contaminants present in bodies of water. The results obtained are a contribution to the SGDs, specifically Goal 6 Target 6.3, because the present study address future research to minimize and remove hazardous chemicals such as lead in untreated wastewater from mining, Goal 7 Target 7.3, because reducing energy costs involves increasing energy efficiency, and Goal 12 Target 12.4, on the management of chemicals—in this case, lead—to significantly reduce their release into water and minimize their negative impacts on human health and the environment.
Although our study with a single heavy metal, lead, had good results, the proposal for optimization and reduction in energy costs must continue to be evaluated using in situ contaminated water. Future research should focus on the application of conditions found for the removal of other metals separately, as well as on sets of heavy metals present in reality. It must be evaluated whether, under similar conditions or other conditions, there are high removals with low pressures. This will allow a set of sustainable parameters to be evaluated using reverse osmosis membranes in conditions closer to reality.