Why Does the Water Color in a Natural Pool Turn into Reddish-Brown “Pumpkin Soup”?
Abstract
1. Introduction
2. Study Area and Methods
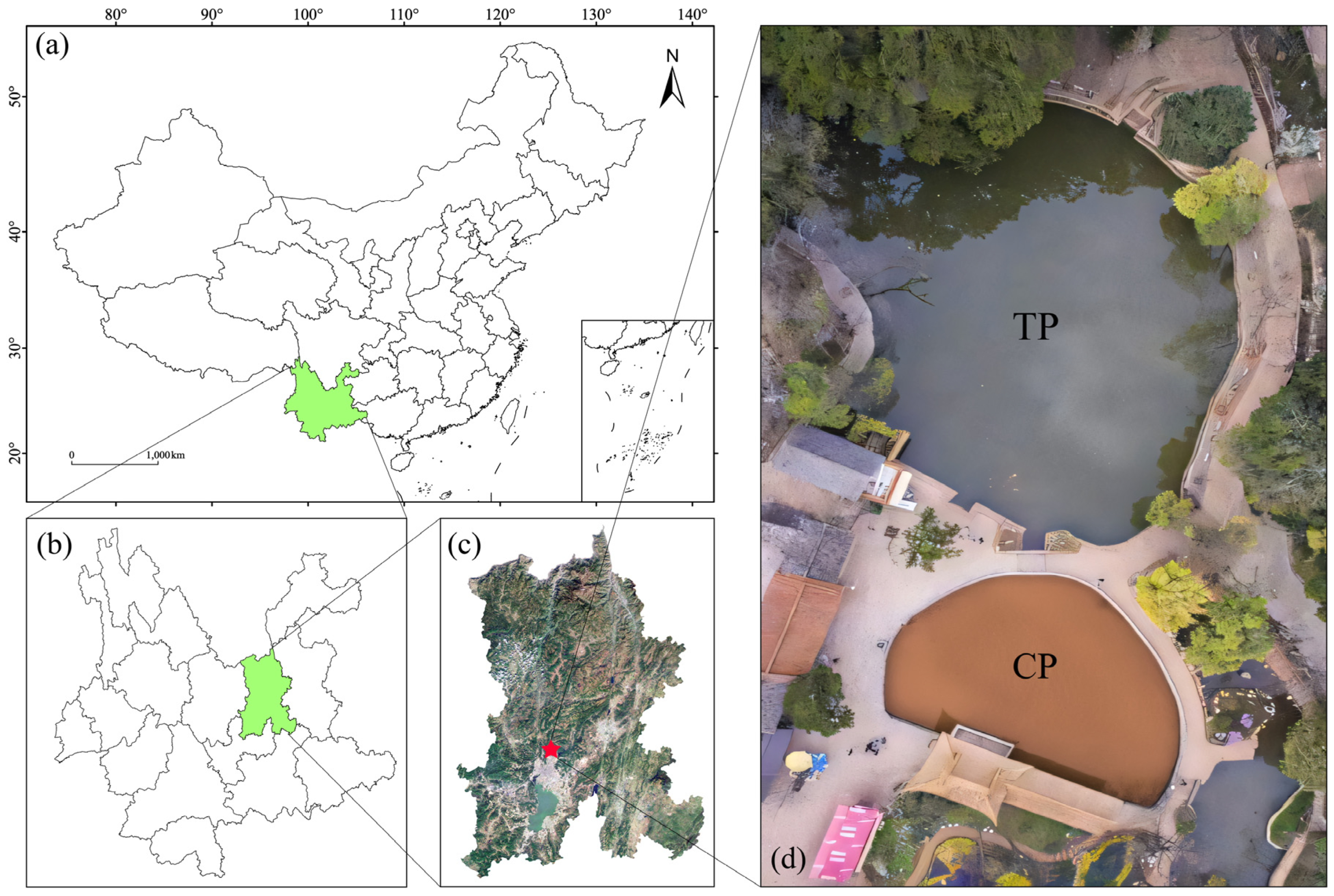
2.1. Overview of the Study Area
2.2. Sample Collection and Identification
2.3. Species Diversity Analysis
3. Results and Discussion
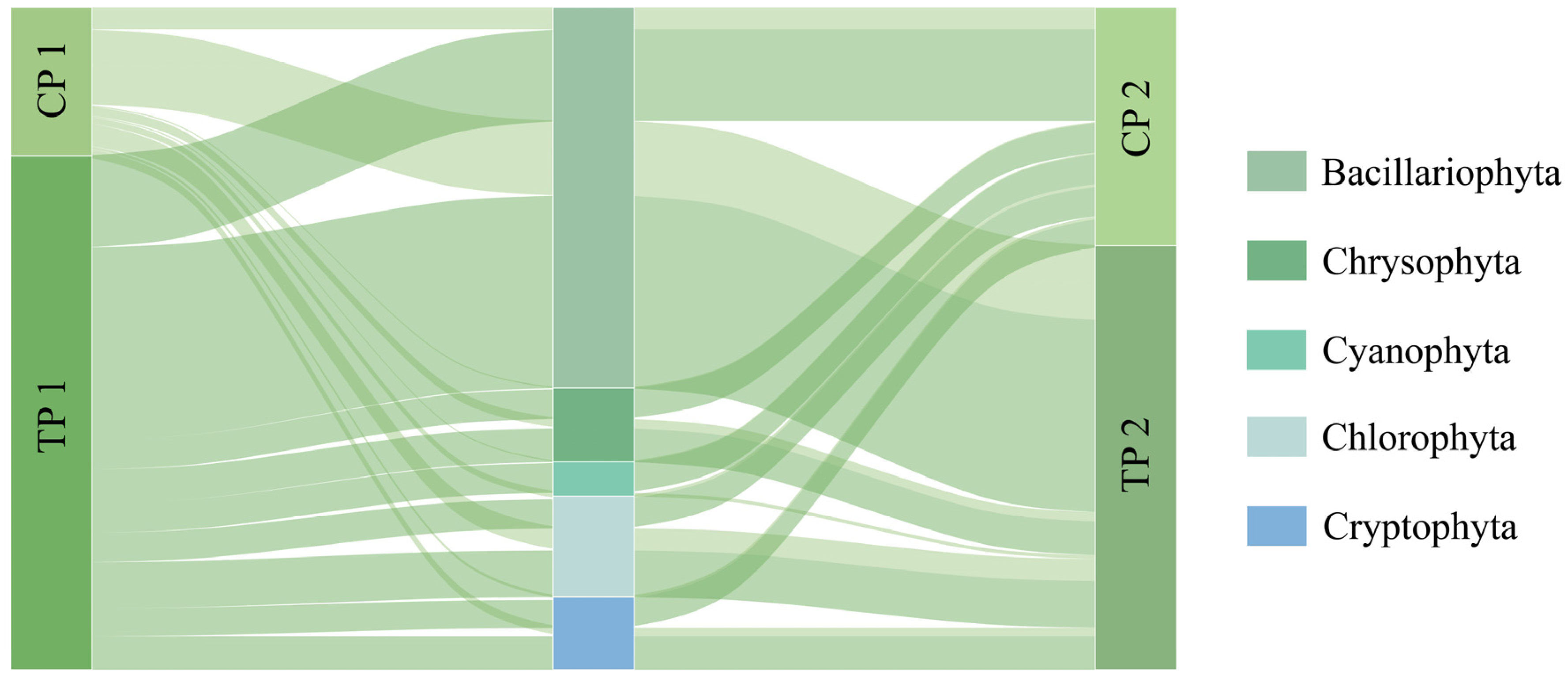
3.1. Phytoplankton Community Structure and Dominant Species
3.2. Comparison of Water Quality Physicochemical Parameters
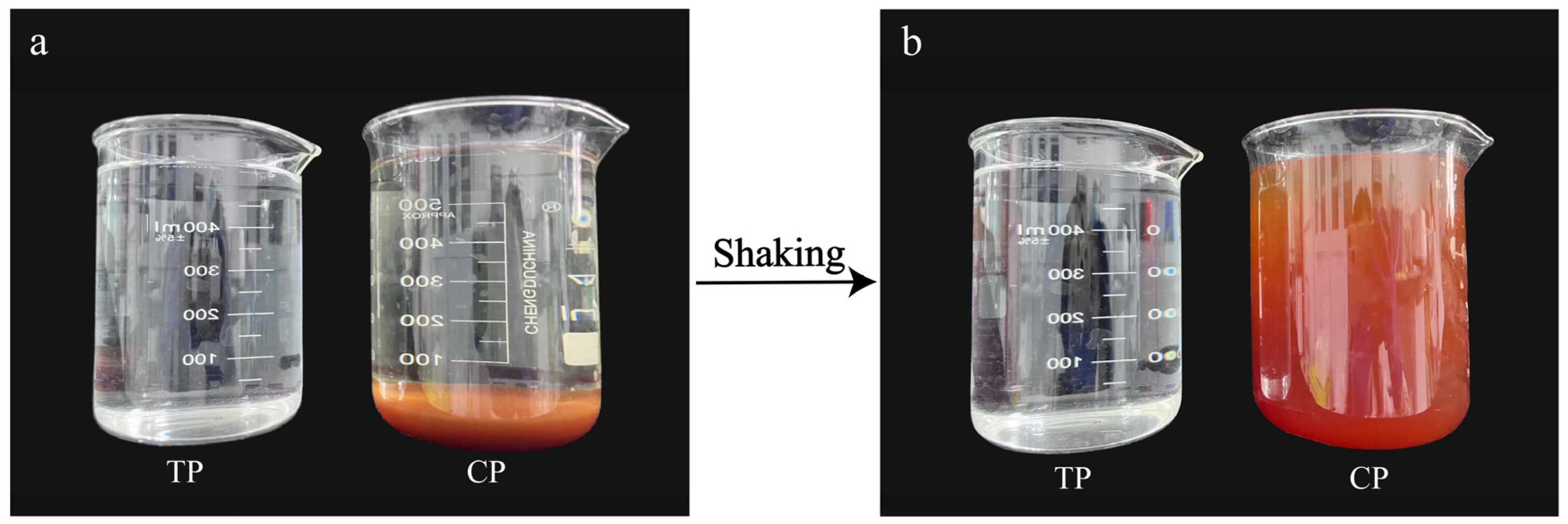
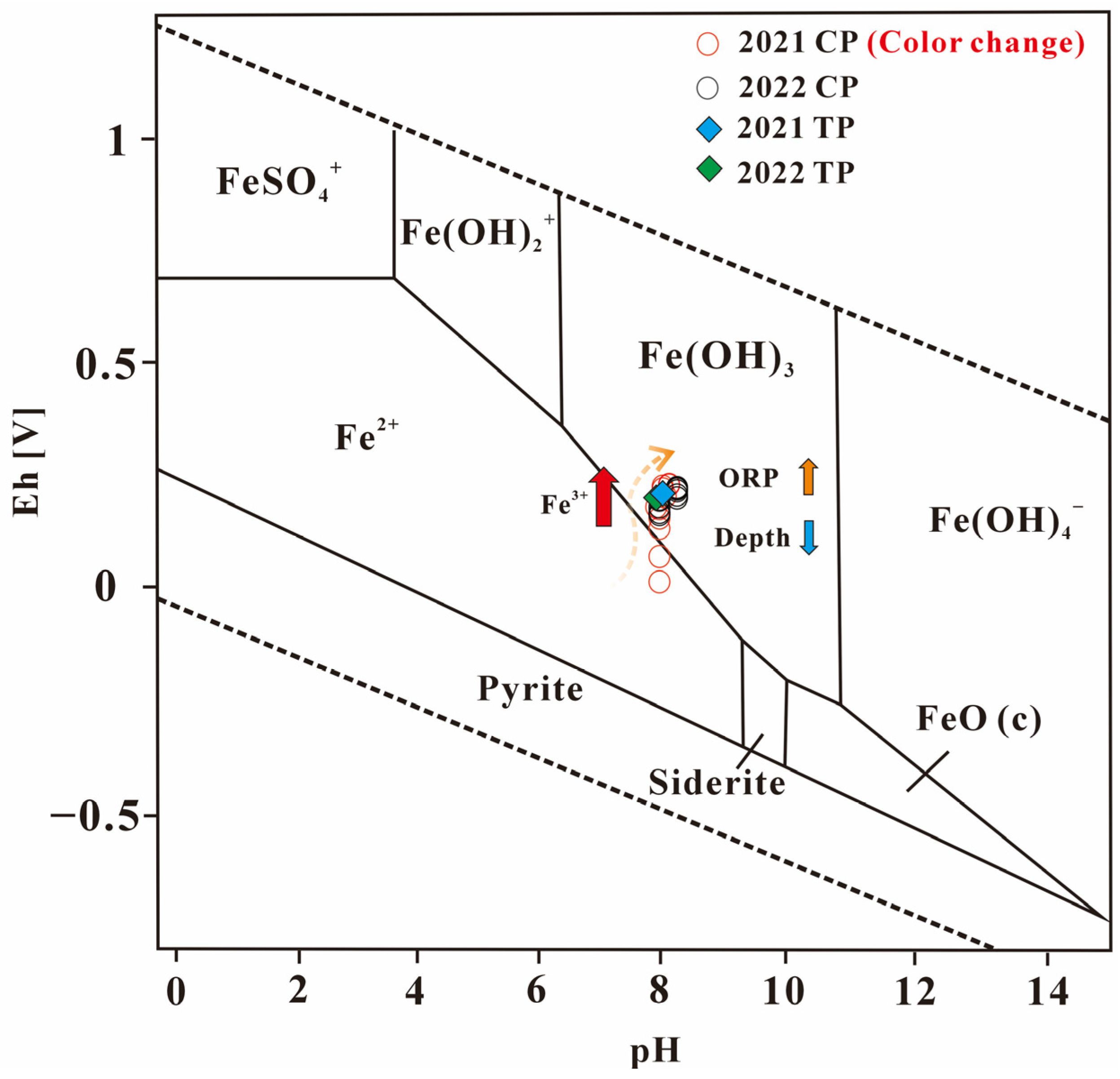
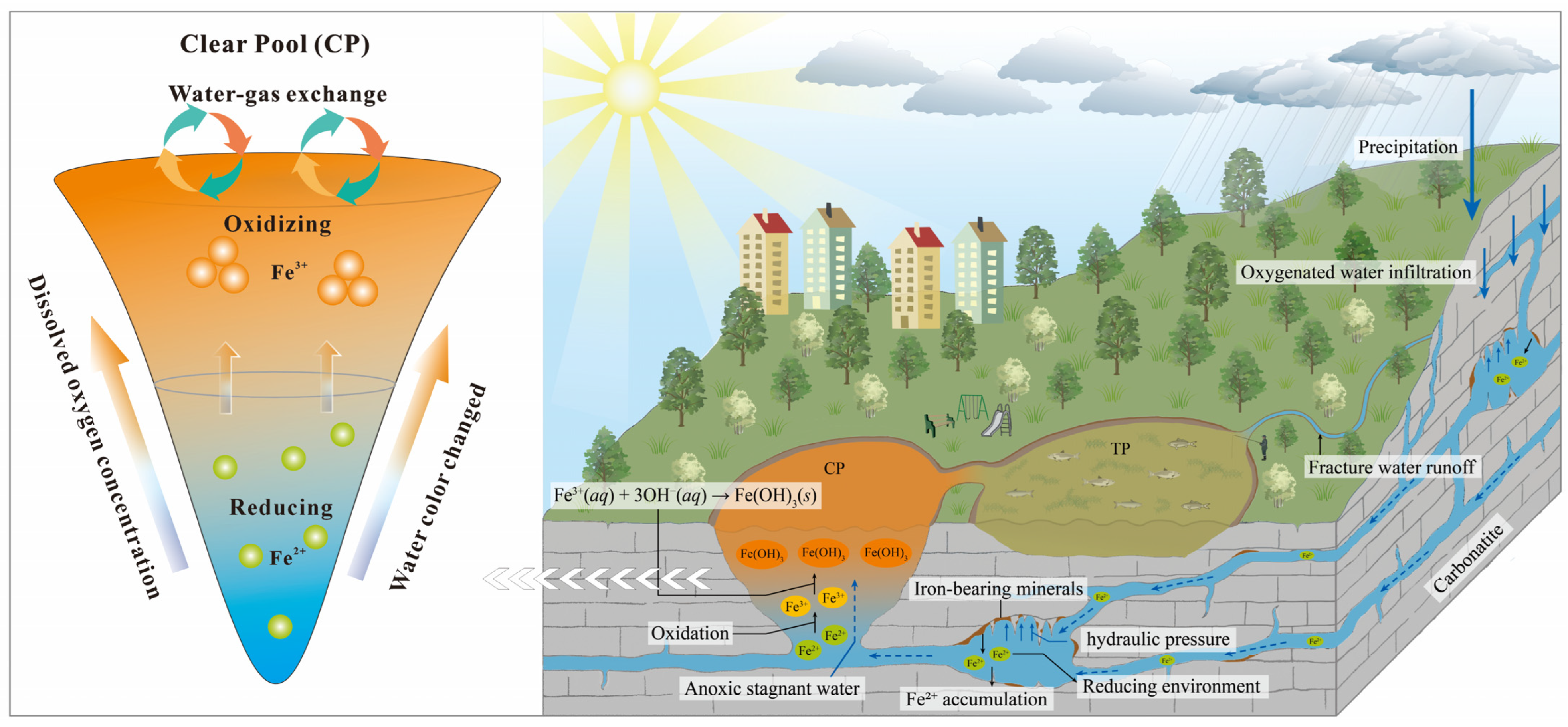
3.3. Role of Fe(OH)3 Colloids in Water Coloration
3.4. Mechanistic Exploration of Water Color Variations in HP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, I.; Gessner, U.; Dietz, A.J.; Kuenzer, C. Global WaterPack—A 250m resolution dataset revealing the daily dynamics of global inland water bodies. Remote Sens. Environ. 2017, 198, 345–362. [Google Scholar] [CrossRef]
- Adrian, R.; O’Reilly, C.M.; Zagarese, H.; Baines, S.B.; Hessen, D.O.; Keller, W.; Livingstone, D.M.; Sommaruga, R.; Straile, D.; Van Donk, E.; et al. Lakes as sentinels of climate change. Limnol. Oceanogr. 2009, 54, 2283–2297. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.K.; Gurlin, D.; Pahlevan, N.; Alikas, K.; Conroy, T.; Anstee, J.; Balasubramanian, S.V.; Barbosa, C.C.F.; Binding, C.; Bracher, A.; et al. GLORIA—A globally representative hyperspectral in situ dataset for optical sensing of water quality. Sci. Data 2023, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, J.; Qin, B.; Zhu, G.; Zhang, Y.; Jeppesen, E.; Tong, Y. Importance and vulnerability of lakes and reservoirs supporting drinking water in China. Fundam. Res. 2023, 3, 265–273. [Google Scholar] [CrossRef]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhang, W.; Cao, C.; Zhang, F.; Shen, Q.; Zhang, X.; Zhang, B. A dataset of remote-sensed Forel-Ule Index for global inland waters during 2000–2018. Sci. Data 2021, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Wu, T.; Li, X.; Wang, S.; Shen, Q. A new method for accurate inversion of Forel-Ule index using MODIS images—Revealing the water color evolution in China’s large lakes and reservoirs over the past two decades. Water Res. 2024, 255, 121560. [Google Scholar] [CrossRef]
- Zhao, Z.; Shi, K.; Peng, Y.; Wang, W.; Lai, L.; Zhang, Y.; Zhou, Y.; Zhang, Y.; Qin, B. Widespread decrease in chromophoric dissolved organic matter in Chinese lakes derived from satellite observations. Remote Sens. Environ. 2023, 298, 113848. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, Y.; Liu, X.; Wang, M.; Qin, B. Remote sensing of diffuse attenuation coefficient of photosynthetically active radiation in Lake Taihu using MERIS data. Remote Sens. Environ. 2014, 140, 365–377. [Google Scholar] [CrossRef]
- Shen, X.; Ke, C.-Q.; Duan, Z.; Cai, Y.; Li, H.; Xiao, Y. Satellite Observations Reveal Widespread Color Variations in Global Lakes Since the 1980s. Water Resour. Res. 2025, 61, e2023WR036926. [Google Scholar] [CrossRef]
- Kessouri, F.; McWilliams, J.C.; Bianchi, D.; Sutula, M.; Renault, L.; Deutsch, C.; Feely, R.A.; McLaughlin, K.; Ho, M.; Howard, E.M.; et al. Coastal eutrophication drives acidification, oxygen loss, and ecosystem change in a major oceanic upwelling system. Proc. Natl. Acad. Sci. USA 2021, 118, e2018856118. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Wang, S.; Zhang, B.; Mao, Z.; Zhang, F.; Li, J. Earth observation reveals the shifting patterns of China’s lake colour driven by climate change and land cover. J. Environ. Manag. 2024, 370, 122809. [Google Scholar] [CrossRef]
- Topp, S.N.; Pavelsky, T.M.; Dugan, H.A.; Yang, X.; Gardner, J.; Ross, M.R.V. Shifting Patterns of Summer Lake Color Phenology in Over 26,000 US Lakes. Water Resour. Res. 2021, 57, e2020WR029123. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y.; Tao, J.; Ye, L.; Wang, H.; Shan, K.; Jeppesen, E.; Song, L. Water depth and land-use intensity indirectly determine phytoplankton functional diversity and further regulate resource use efficiency at a multi-lake scale. Sci. Total Environ. 2022, 834, 155303. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Tao, J.; Zhou, Y.; Yang, H.; Yang, X.; Li, Y.; Zhou, Q.; Jeppesen, E. Concentrations of dissolved organic matter and methane in lakes in Southwest China: Different roles of external factors and in-lake biota. Water Res. 2022, 225, 119190. [Google Scholar] [CrossRef]
- Hu, H.; Wei, Y. The Freshwater Algae of China—Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. [Google Scholar]
- Pavelková, A.; Cencerová, V.; Zeman, J.; Antos, V.; Nosek, J. Reduction of chlorinated hydrocarbons using nano zero-valent iron supported with an electric field. Characterization of electrochemical processes and thermodynamic stability. Chemosphere 2021, 265, 128764. [Google Scholar] [CrossRef]
- Li, D.; Chang, F.; Zhang, Y.; Duan, L.; Liu, Q.; Li, H.; Hu, G.; Zhang, X.; Gao, Y.; Zhang, H. Arsenic migration at the sediment-water interface of anthropogenically polluted Lake Yangzong, Southwest China. Sci. Total Environ. 2023, 879, 163205. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lei, S.; Zhang, M.; Hughes, C.; Crawford, J.; Liu, Z.; Qu, D. Spatial and seasonal isotope variability in precipitation across China: Monthly isoscapes based on regionalized fuzzy clustering. J. Clim. 2022, 35, 3411–3425. [Google Scholar] [CrossRef]
- Margalef, R. Information Theory in Ecology; Royal Academy of Sciences and Arts of Barcelona: Barcelona, Spain, 1973. [Google Scholar]
- Spellerberg, I.F.; Fedor, P.J. A tribute to Claude Shannon (1916–2001) and a plea for more rigorous use of species richness, species diversity and the ‘Shannon–Wiener’ Index. Glob. Ecol. Biogeogr. 2003, 12, 177–179. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Kociolek, J. A worldwide listing and biogeography of freshwater diatom genera: A phylogenetic perspective. Diatom Res. 2018, 33, 509–534. [Google Scholar] [CrossRef]
- Duong, T.T.; Nguyen, H.Y.; Le, T.P.Q.; Nguyen, T.K.; Tran, T.T.H.; Le, N.D.; Dang, D.K.; Vu, T.N.; Panizzo, V.; McGowan, S. Transitions in diatom assemblages and pigments through dry and wet season conditions in the Red River, Hanoi (Vietnam). Plant Ecol. Evol. 2019, 152, 163–177. [Google Scholar] [CrossRef]
- Manoylov, K.M.; Ognjanova-Rumenova, N.; Stevenson, R.J. Morphotype variations in subfossil diatom species of Aulacoseira in 24 Michigan Lakes, USA. Acta Bot. Croat. 2009, 68, 401–419. [Google Scholar]
- Yang, C.; Nan, J.; Li, J. Driving factors and dynamics of phytoplankton community and functional groups in an estuary reservoir in the Yangtze River, China. Water 2019, 11, 1184. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, J.; Liao, J.; Shi, R.; Li, T.; Guo, Y.; Long, A. Characteristics of phytoplankton biomass, primary production and community structure in the modaomen channel, pearl river estuary, with special reference to the influence of saltwater intrusion during neap and spring tides. PLoS ONE 2016, 11, e0167630. [Google Scholar] [CrossRef]
- Wang, C.; Wu, N.; Li, W.; Liu, Q.; Lai, Z.; Fohrer, N. Curved filaments of Aulacoseira complex as ecological indicators in the Pearl River, China. Ecol. Indic. 2020, 118, 106722. [Google Scholar] [CrossRef]
- Thomas, K.E.; Hall, R.I.; Scrimgeour, G.J. Evaluating the use of algal pigments to assess the biological condition of streams. Environ. Monit. Assess. 2013, 185, 7895–7913. [Google Scholar] [CrossRef]
- El Semary, N.A. Iron-Marine Algal Interactions and Impacts: Decreasing Global Warming by Increasing Algal Biomass. Sustainability 2022, 14, 10372. [Google Scholar] [CrossRef]
- Duppeti, H.; Chakraborty, S.; Das, B.S.; Mallick, N.; Kotamreddy, J. Rapid assessment of algal biomass and pigment contents using diffuse reflectance spectroscopy and chemometrics. Algal Res. 2017, 27, 274–285. [Google Scholar] [CrossRef]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Gaoa, G. Nitrogen and phosphorus inputs control phytoplankton growth in eutrophic Lake Taihu, China. Limnol. Oceanogr. 2010, 55, 420–432. [Google Scholar] [CrossRef]
- Fowler, R.A.; Warner, K.A.; Gawley, W.G.; Saros, J.E. Paleolimnological comparison of algal changes in a clear-versus a brown-water lake over the last two centuries in the northeastern USA. J. Paleolimnol. 2022, 67, 289–305. [Google Scholar] [CrossRef]
- Pitblado, J.R. Landsat views of Sudbury (Canada) area acidic and nonacidic lakes. Can. J. Fish. Aquat. Sci. 1992, 49, 33–39. [Google Scholar] [CrossRef]
- Ma, R.; Tang, J.; Dai, J.; Zhang, Y.; Song, Q. Absorption and scattering properties of water body in Taihu Lake, China: Absorption. Int. J. Remote Sens. 2006, 27, 4277–4304. [Google Scholar] [CrossRef]
- Su, C.; Zhang, X.; Sun, Y.; Meng, S.; Cui, X.; Fei, Y. Hydrochemical characteristics and evolution processes of karst groundwater in Pingyin karst groundwater system, North China. Environ. Earth Sci. 2023, 82, 67. [Google Scholar] [CrossRef]
- Sun, H.; Bian, K.; Wang, T.; Jin, Z.; Niu, Z. Hydrogeochemical Characteristics and Genetic Analysis of Karst Groundwater in the Fengfeng Mining Area. Water 2023, 15, 4049. [Google Scholar] [CrossRef]
- Arvola, L.; Rask, M.; Huotari, J.; Tulonen, T.; Kahilainen, K.K.; Ruuhijärvi, J.; Lindberg, H.; Viitala, R.; Blanchet, C.; Arzel, C. Chemical responses of small boreal lakes to atmospheric and catchment drivers over four decades. Sci. Total Environ. 2025, 968, 178696. [Google Scholar] [CrossRef]
- Škerlep, M.; Steiner, E.; Axelsson, A.L.; Kritzberg, E.S. Afforestation driving long-term surface water browning. Glob. Change Biol. 2020, 26, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, C.J.; Mladenov, N.; Wall, C.B.; Hollman, K.; Tran, C.H.; Symons, C.C.; Shurin, J.B. Life after a fiery death: Fire and plant biomass loading affect dissolved organic matter in experimental ponds. Glob. Change Biol. 2024, 30, e17061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, C.; Adeyeye, O.; Yang, W.; Liang, X. Source and Mobilization Mechanism of Iron, Manganese and Arsenic in Groundwater of Shuangliao City, Northeast China. Water 2020, 12, 534. [Google Scholar] [CrossRef]
- Liu, K.; Qiao, X.; Li, B.; Sun, Y.; Li, Z.; Pu, C. Characteristics of deuterium excess parameters for geothermal water in Beijing. Environ. Earth Sci. 2016, 75, 1485. [Google Scholar] [CrossRef]
- Williams, M.D.; Oostrom, M. Oxygenation of anoxic water in a fluctuating water table system: An experimental and numerical study. J. Hydrol. 2000, 230, 70–85. [Google Scholar] [CrossRef]
- Kohfahl, C.; Massmann, G.; Pekdeger, A. Sources of oxygen flux in groundwater during induced bank filtration at a site in Berlin, Germany. Hydrogeol. J. 2009, 17, 571–578. [Google Scholar] [CrossRef]
- Grenthe, I.; Stumm, W.; Laaksuharju, M.; Nilsson, A.; Wikberg, P. Redox potentials and redox reactions in deep groundwater systems. Chem. Geol. 1992, 98, 131–150. [Google Scholar] [CrossRef]
- Bao, J.; Wu, X.; Zhang, Q.; Yuan, D.; Guo, F.; Liu, F. Unveiling the nitrogen transport and transformation in different karst aquifers media. J. Hydrol. 2023, 620, 129335. [Google Scholar] [CrossRef]
- Kazak, E.S.; Pozdniakov, S.P. Field study and reactive simulation of iron migration in groundwater during the riverbank filtration. Appl. Geochem. 2021, 124, 104817. [Google Scholar] [CrossRef]
- Xia, X.; Teng, Y.; Zhai, Y. Biogeochemistry of iron enrichment in groundwater: An indicator of environmental pollution and its management. Sustainability 2022, 14, 7059. [Google Scholar] [CrossRef]
- Smith, R.L.; Kent, D.B.; Repert, D.A.; Böhlke, J. Anoxic nitrate reduction coupled with iron oxidation and attenuation of dissolved arsenic and phosphate in a sand and gravel aquifer. Geochim. Cosmochim. Acta 2017, 196, 102–120. [Google Scholar] [CrossRef]
- Mahler, B.; Bourgeais, R. Dissolved oxygen fluctuations in karst spring flow and implications for endemic species: Barton Springs, Edwards aquifer, Texas, USA. J. Hydrol. 2013, 505, 291–298. [Google Scholar] [CrossRef]
- Linnik, P.; Osadchyi, V.; Osadcha, N.; Linnik, R. Redox potential as an important characteristic of the chemical and biological state of surface waters. Chem. Ecol. 2023, 39, 640–672. [Google Scholar] [CrossRef]
- Ford, D.; Williams, P.D. Karst Hydrogeology and Geomorphology; John Wiley & Sons: London, UK, 2007. [Google Scholar]
- Smith, L.; Watzin, M.C.; Druschel, G. Relating sediment phosphorus mobility to seasonal and diel redox fluctuations at the sediment–water interface in a eutrophic freshwater lake. Limnol. Oceanogr. 2011, 56, 2251–2264. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Iron uptake and growth limitation in oceanic and coastal phytoplankton. Mar. Chem. 1995, 50, 189–206. [Google Scholar] [CrossRef]
- Raven, J.A. The iron and molybdenum use efficiencies of plant growth with different energy, carbon and nitrogen sources. New Phytol. 1988, 109, 279–287. [Google Scholar] [CrossRef]
- Barbeau, K.; Rue, E.; Bruland, K.W.; Butler, A. Photochemical cycling of iron in the surface ocean mediated by microbial iron (III)-binding ligands. Nature 2001, 413, 409–413. [Google Scholar] [CrossRef]
- Rose, A.L.; Waite, T.D. Kinetics of iron complexation by dissolved natural organic matter in coastal waters. Mar. Chem. 2003, 84, 85–103. [Google Scholar] [CrossRef]
| Dominant Species | Margalef | Shannon | Simpson | Pielou | ||
|---|---|---|---|---|---|---|
| 2021 | CP 1 | Aulacoseira granulate var. angustissima | 1.01 | 1.77 | 0.74 | 0.63 |
| Aulacoseira granulata | ||||||
| TP 1 | Aulacoseira granulate var. angustissima, | 1.52 | 2.07 | 0.78 | 0.62 | |
| Aulacoseira sp. | ||||||
| 2022 | CP 2 | Aulacoseira ambigua | 1.33 | 2.05 | 0.78 | 0.71 |
| Aulacoseira sp. | ||||||
| TP 2 | Aulacoseira ambigua | 1.33 | 1.88 | 0.71 | 0.62 | |
| Aulacoseira sp. |
| Parameters | 2021 | 2022 | ||
|---|---|---|---|---|
| CP 1 | TP 1 | CP 2 | TP 2 | |
| TN (mg L−1) | 1.99 | 4.27 | 2.61 | 1.85 |
| TP (mg L−1) | 0.10 | 0.05 | 0.10 | 0.15 |
| DTN (mg L−1) | 1.53 | 3.71 | 2.60 | 1.71 |
| DTP (mg L−1) | 0.01 | 0.02 | 0.02 | 0.01 |
| PO43− (mg L−1) | 0.00 | 0.01 | 0.02 | 0.01 |
| NO3-N (mg L−1) | 0.83 | 2.79 | 2.27 | 1.16 |
| Chl-a (μg L−1) | 5.76 | 57.61 | 8.86 | 65.76 |
| DOC (mg L−1) | 3.52 | 5.36 | 4.75 | 8.45 |
| DO (mg L−1) | 6.28 | 7.21 | 6.45 | 7.15 |
| WT (°C) | 18.6 | 21.7 | 18.7 | 21.4 |
| pH | 8.3 | 8.2 | 8.2 | 8.1 |
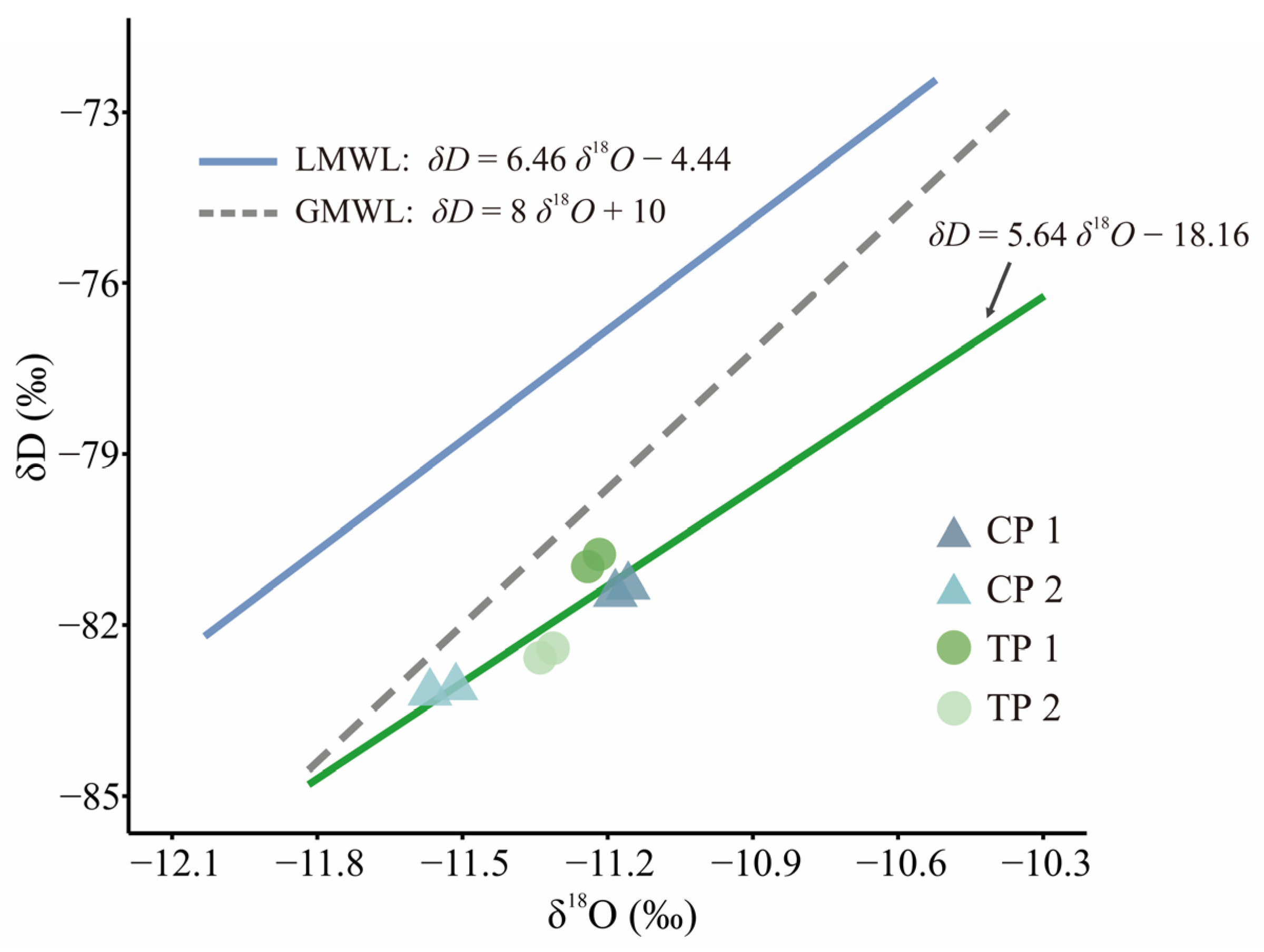
| δ18O (‰) | −11.16 | −11.24 | −11.51 | −11.31 |
| δD (‰) | −81.32 | −80.97 | −83.09 | −82.41 |
| Elements | CP 1 | TP 1 |
|---|---|---|
| As | 5.9 × 10−1 | 4.11 × 10−1 |
| Be | 5.56 × 10−4 | 2.50 × 10−3 |
| Cd | 5.37 × 10−3 | 7.48 × 10−3 |
| Co | 1.96 × 10−1 | 2.29 × 10−1 |
| Cr | 1.15 | 1.81 |
| Cu | 6.26 × 10−1 | 5.07 × 10−1 |
| Fe | 4.85 × 102 | 6.01 × 102 |
| Mn | −6.38 × 10−5 | 1.77 × 10−5 |
| Mo | 7.31 × 10−1 | 2.95 × 10−1 |
| Ni | 5.16 | 6.20 |
| Pb | 4.53 × 10−2 | 5.50 × 10−2 |
| Sb | 1.26 × 10−1 | 7.98 × 10−2 |
| Se | 2.72 × 10−1 | 1.99 × 10−1 |
| Ti | 3.96 × 10 | 4.98 × 10 |
| Tl | 7.02 × 10−3 | 6.53 × 10−3 |
| V | 1.24 | 1.46 |
| Zn | 3.27 | 5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Zhao, M.; Liu, Q.; Duan, L.; Li, H.; Zhang, Y.; Gao, Q.; Zhang, H.; Qiu, B. Why Does the Water Color in a Natural Pool Turn into Reddish-Brown “Pumpkin Soup”? Sustainability 2025, 17, 7255. https://doi.org/10.3390/su17167255
Li D, Zhao M, Liu Q, Duan L, Li H, Zhang Y, Gao Q, Zhang H, Qiu B. Why Does the Water Color in a Natural Pool Turn into Reddish-Brown “Pumpkin Soup”? Sustainability. 2025; 17(16):7255. https://doi.org/10.3390/su17167255
Chicago/Turabian StyleLi, Donglin, Mingyang Zhao, Qi Liu, Lizeng Duan, Huayu Li, Yun Zhang, Qingyan Gao, Haonan Zhang, and Bofeng Qiu. 2025. "Why Does the Water Color in a Natural Pool Turn into Reddish-Brown “Pumpkin Soup”?" Sustainability 17, no. 16: 7255. https://doi.org/10.3390/su17167255
APA StyleLi, D., Zhao, M., Liu, Q., Duan, L., Li, H., Zhang, Y., Gao, Q., Zhang, H., & Qiu, B. (2025). Why Does the Water Color in a Natural Pool Turn into Reddish-Brown “Pumpkin Soup”? Sustainability, 17(16), 7255. https://doi.org/10.3390/su17167255






