Valorization of Sugarcane Bagasse in Thailand: An Economic Analysis of Ethanol and Co-Product Recovery via Organosolv Fractionation
Abstract
1. Introduction
2. Materials and Methods
2.1. Process Synthesis and Design
- is the interaction parameter between components i and j;
- is a weighting factor;
- xj is the mole fraction of component j;
- αij is a non-randomness parameter (typically between 0.2 and 0.47).
- k typically indexes all components in the denominators to normalize the interactions with respect to component i or j;
- m is used similarly to k but specifically for summing interactions between component j and all other components m (inside the inner bracket) in the second term of the equation.
- Roles:
- : Denominator of the first term (normalizing interaction contributions to component i);
- : Denominator inside the brackets in the second term (normalizing interactions related to component j).
- : Numerator inside the bracket (weighted average interaction toward j from all m).
2.2. Process Setup for Organosolv Fractionation
2.3. Economic Analysis
- i = interest rate or desired rate of return (per year);
- n = project lifetime or payback period (years).
2.4. Sensitivity Analysis
3. Results
3.1. Scenario
3.2. Economic Evaluation
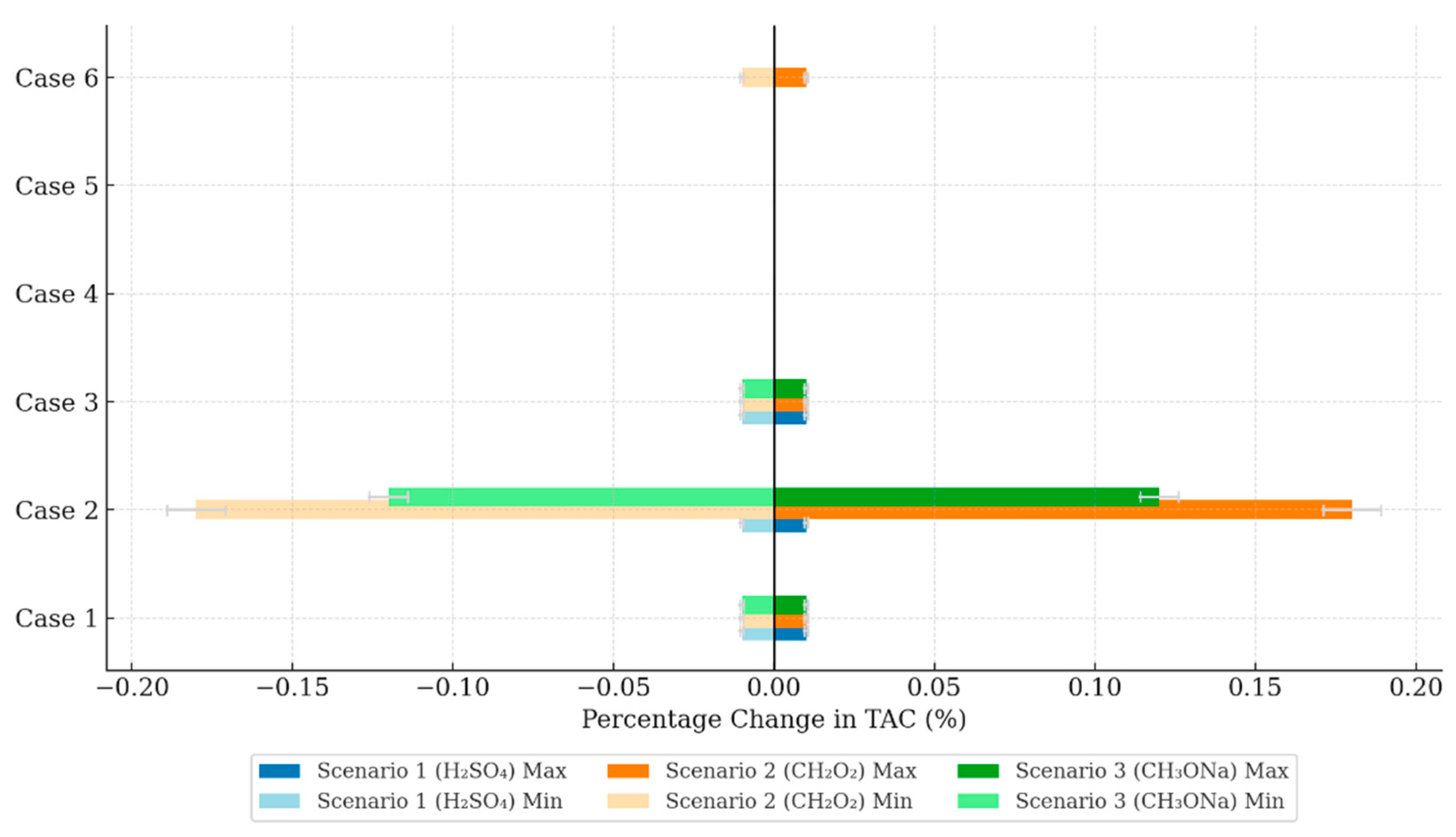
3.3. Assessment of Sensitivity Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Energy Statistics of Thailand 2023, Energy Policy and Planning Office (EPPO), Ministry of Energy, Thailand. Available online: https://www.eppo.go.th/index.php/th/component/k2/item/19566-energy-statistics-2566 (accessed on 1 June 2025).
- Preethi, M.G.; Kumar, G.; Karthikeyan, O.P.; Varjani, S.; Rajesh, B.J. Lignocellulosic biomass as an optimistic feedstock for the production of biofuels as valuable energy source: Techno-economic analysis, Environmental Impact Analysis, Breakthrough and Perspectives. Environ. Technol. Innov. 2021, 24, 102080. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Qiu, X.; Xu, C. Hydrothermal treatment of lignocellulosic biomass towards low-carbon development: Production of high-value-added bioproducts. EnergyChem 2024, 6, 100133. [Google Scholar] [CrossRef]
- Jayakumar, M.; Gindaba, G.T.; Gebeyehu, K.B.; Periyasamy, S.; Jabesa, A.; Baskar, G.; John, B.I.; Pugazhendhi, A. Bioethanol production from agricultural residues as lignocellulosic biomass feedstock’s waste valorization approach: A comprehensive review. Sci. Total Environ. 2023, 879, 163158. [Google Scholar] [CrossRef]
- Alternative Energy Development Plan: AEDP2015, Department of Renewable Energy Development and Energy Efficiency, Ministry of Energy, Thailand. 2015. Available online: https://www.eppo.go.th/images/POLICY/ENG/AEDP2015ENG.pdf (accessed on 11 June 2025).
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A.A.-O. Lignocellulosic Biomass Valorization for Bioethanol Production: A Circular Bioeconomy Approach. BioEnergy Res. 2022, 15, 1820–1841. [Google Scholar] [CrossRef] [PubMed]
- Raina, N.; Boonmee, R.; Kirdponpattara, S.; Narasingha, M.; Sriariyanun, M.; Phitsuwan, P.; Chuetor, S. Process performance evaluation of different chemical pretreatments of lignocellulosic biomass for bioethanol production. Ind. Crops Prod. 2024, 211, 118207. [Google Scholar] [CrossRef]
- Joseph, A.M.; Tulasi, Y.; Shrivastava, D.; Kiran, B. Techno-economic feasibility and exergy analysis of bioethanol production from waste. Energy Convers. Manag. X 2023, 18, 100358. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Sganzerla, W.G.; Larnaudie, V.; Veersma, R.J.; van Erven, G.; Shiva; Ríos-González, L.J.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; Ferrari, M.D.; et al. Advances in process design, techno-economic assessment and environmental aspects for hydrothermal pretreatment in the fractionation of biomass under biorefinery concept. Bioresour. Technol. 2023, 369, 128469. [Google Scholar] [CrossRef]
- Varriale, L.; Geib, D.; Ulber, R. Short-term adaptation as a tool to improve bioethanol production using grass press-juice as fermentation medium. Appl. Microbiol. Biotechnol. 2024, 108, 393. [Google Scholar] [CrossRef]
- Weerasai, K.; Laosiripojana, N.; Imman, S.; Kreetachat, T.; Suriyachai, N. Reusable alkaline catalyzed organosolv pretreatment and delignification of bagasse for sugar platform biorefinery. Biomass Convers. Biorefin. 2023, 13, 1751–1761. [Google Scholar] [CrossRef]
- Suriyachai, N.; Champreda, V.; Kraikul, N.; Techanan, W.; Laosiripojana, N. Fractionation of lignocellulosic biopolymers from sugarcane bagasse using formic acid-catalyzed organosolv process. 3 Biotech 2018, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Panakkal, E.J.; Sriariyanun, M.; Ratanapoompinyo, J.; Yasurin, P.; Cheenkachorn, K.; Rodiahwati, W.; Tantayotai, P. Influence of Sulfuric Acid Pretreatment and Inhibitor of Sugarcane Bagasse on the Production of Fermentable Sugar and Ethanol. Appl. Sci. Eng. Prog. 2021, 15. [Google Scholar] [CrossRef]
- Kreetachat, T.; Suriyachai, N.; Khongchamnan, P.; Suwannahong, K.; Wongcharee, S.; Sakulthaew, C.; Chokejaroenrat, C.; Imman, S. Optimization of Acid-Catalyzed Hydrolysis and Simultaneous Saccharification and Fermentation for Enhanced Ethanol Production from Sweet Stalk Sorghum. Catalysts 2025, 15, 379. [Google Scholar] [CrossRef]
- Gutierrez, S.; Mangone, F.; Vergara, P.; Gonzalez, V.; Ferreira, J.P.; Villar, J.C.; Garcia-Ochoa, F. Lignocellulosic biomass pre-treatments by diluted sulfuric acid and ethanol-water mixture: A comparative techno-economic analysis. Bioresour. Technol. Rep. 2023, 23, 101514. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Deng, B.; Huang, C.; Zhu, J.; Liang, L.; He, X.; Wei, Y.; Qin, C.; Liang, C.; et al. Application and prospect of organic acid pretreatment in lignocellulosic biomass separation: A review. Int. J. Biol. Macromol. 2022, 222, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Baral, N.R.; Shah, A. Comparative techno-economic analysis of steam explosion, dilute sulfuric acid, ammonia fiber explosion and biological pretreatments of corn stover. Bioresour. Technol. 2017, 232, 331–343. [Google Scholar] [CrossRef]
- Rodrigues Gurgel da Silva, A.; Giuliano, A.; Errico, M.; Rong, B.-G.; Barletta, D. Economic value and environmental impact analysis of lignocellulosic ethanol production: Assessment of different pretreatment processes. Clean Technol. Environ. Policy 2019, 21, 637–654. [Google Scholar] [CrossRef]
- Areeya, S.; Jayex Panakkal, E.; Sriariyanun, M.; Kangsadan, T.; Tawai, A.; Amornraksa, S.; Hartley, U.; Yasurin, P. A Review on Chemical Pretreatment of Lignocellulosic Biomass for the Production of Bioproducts: Mechanisms, Challenges and Applications. Appl. Sci. Eng. Prog. 2023, 16, 6767. [Google Scholar] [CrossRef]
- Fu, R.; Kang, L.; Zhang, C.; Fei, Q. Application and progress of techno-economic analysis and life cycle assessment in biomanufacturing of fuels and chemicals. Green Chem. Eng. 2023, 4, 189–198. [Google Scholar] [CrossRef]
- Abdou Alio, M.; Marcati, A.; Pons, A.; Vial, C. Modeling and simulation of a sawdust mixture-based integrated biorefinery plant producing bioethanol. Bioresour. Technol. 2021, 325, 124650. [Google Scholar] [CrossRef]
- Bisotti, F.; Gilardi, M.; Berglihn, O.T.; Tschentscher, R.; Hansen, L.D.; Horn, S.J.; Várnai, A.; Wittgens, B. From laboratory scale to innovative spruce-based biorefinery. Note I: Conceptual process design and simulation. In Computer Aided Chemical Engineering; Manenti, F., Reklaitis, G.V., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 53, pp. 2449–2454. [Google Scholar]
- Solarte-Toro, J.C.; Rueda-Duran, C.A.; Ortiz-Sanchez, M.; Cardona Alzate, C.A. A comprehensive review on the economic assessment of biorefineries: The first step towards sustainable biomass conversion. Bioresour. Technol. Rep. 2021, 15, 100776. [Google Scholar] [CrossRef]
- Chong, T.Y.; Cheah, S.A.; Ong, C.T.; Wong, L.Y.; Goh, C.R.; Tan, I.S.; Foo, H.C.Y.; Lam, M.K.; Lim, S. Techno-economic evaluation of third-generation bioethanol production utilizing the macroalgae waste: A case study in Malaysia. Energy 2020, 210, 118491. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Bioresour. Technol. 2021, 320, 124533. [Google Scholar] [CrossRef]
- Ansaloni, L.; Fabbri, D.; Spigno, G. Valorization of macroalgal biomass for the production of renewable chemicals: A review. Ind. Crops Prod. 2021, 159, 113051. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol. Natl. Renew. Energy Lab. 2011, 275–3000. [Google Scholar]
- Rocha, G.J.d.M.; Silva, F.T.; Curvelo, A.A.d.S.; Araujo, G.T. A fast and accurate method for determination of cellulose and polyoses by HPLC. Proceedings 1997. Available online: https://repositorio.usp.br/item/000929622 (accessed on 11 June 2025).
- Tiwari, S.; Yadav, J.; Gaur, R.; Singh, R.; Verma, T.; Yadav, J.S.; Pandey, P.K.; Rath, S.K. Multistep Structural and Chemical Evaluation of Sugarcane Baggase, Pretreated With Alkali for Enhancing the Enzymatic Saccharification by Cellulase and Xylanase of the Pseudomonas sp. CVB-10 (MK443365) and Bacillus paramycoides T4 (MN370035) Mix-Culture System. Front. Energy Res. 2022, 9, 726010. [Google Scholar] [CrossRef]
- Oh, H.W.; Lee, S.C.; Woo, H.C.; Kim, Y.H. Energy-efficient recovery of fermented butyric acid using octyl acetate extraction. Biotechnol. Biofuels Bioprod. 2022, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Viell, J.; Harwardt, A.; Seiler, J.; Marquardt, W. Is biomass fractionation by Organosolv-like processes economically viable? A conceptual design study. Bioresour. Technol. 2013, 150, 89–97. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.R.G.; Errico, M.; Rong, B.-G. Solvent Recycle and Impurity Purge Evaluation for Organosolv Pretreatment Method for Bioethanol Production from Lignocellulosic Biomass. In Computer Aided Chemical Engineering; Espuña, A., Graells, M., Puigjaner, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 40, pp. 1141–1146. [Google Scholar]
- Saadan, R.; Hachimi Alaoui, C.; Ihammi, A.; Chigr, M.; Fatimi, A. A Brief Overview of Lignin Extraction and Isolation Processes: From Lignocellulosic Biomass to Added-Value Biomaterials. In Proceedings of the International Electronic Conference on Forests 2024, Virtual, 23–25 September 2024. [Google Scholar]
- Gavin, T.; Ray, S. Chemical Engineering Design: Principles, Practice and Economics of Plant and Process Design, 3rd ed.; Elsevier: London, UK, 2021. [Google Scholar]
- Prachuab, P. Renewable Energy Strategies for Sustainable Development in Thailand; Thammasat University: Bangkok, Thailand, 2019. [Google Scholar]
- Echemi. Weekly Chemical Prices. Available online: https://www.echemi.com/weekly-price.html (accessed on 20 June 2024).
- Authority, P.W. Provincial Waterworks Authority website. Available online: https://www.pwa.co.th (accessed on 20 June 2024).
- Electricity Generating Authority of Thailand. Available online: https://www.egat.co.th/home/egat-price (accessed on 20 June 2024).
- Lim, K.L.; Wong, W.Y.; James Rubinsin, N.; Loh, S.K.; Lim, M.T. Techno-Economic Analysis of an Integrated Bio-Refinery for the Production of Biofuels and Value-Added Chemicals from Oil Palm Empty Fruit Bunches. Processes 2022, 10, 1965. [Google Scholar] [CrossRef]
- Hafyan, R.H.; Mohanarajan, J.; Uppal, M.; Kumar, V.; Narisetty, V.; Maity, S.K.; Sadhukhan, J.; Gadkari, S. Integrated biorefinery for bioethanol and succinic acid co-production from bread waste: Techno-economic feasibility and life cycle assessment. Energy Convers. Manag. 2024, 301, 118033. [Google Scholar] [CrossRef]
- Wirawan, S.S.; Solikhah, M.D.; Widiyanti, P.T.; Nitamiwati, N.P.D.; Romelan, R.; Heryana, Y.; Nurhasanah, A.; Sugiyono, A. Unlocking Indonesia’s sweet sorghum potential: A techno-economic analysis of small-scale integrated sorghum-based fuel grade bioethanol industry. Bioresour. Technol. Rep. 2024, 25, 101706. [Google Scholar] [CrossRef]
- Nair, L.G.; Agrawal, K.; Verma, P. Organosolv pretreatment: An in-depth purview of mechanics of the system. Bioresour Bioprocess 2023, 10, 50. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Wang, Z.; Dien, B.S.; Slininger, P.J.W.; Singh, V. Economic Analysis of Cellulosic Ethanol Production from Sugarcane Bagasse Using a Sequential Deacetylation, Hot Water and Disk-Refining Pretreatment. Processes 2019, 7, 642. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Lachos-Perez, D.; Buller, L.S.; Zabot, G.L.; Forster-Carneiro, T. Cost analysis of subcritical water pretreatment of sugarcane straw and bagasse for second-generation bioethanol production: A case study in a sugarcane mill. Biofuels Bioprod. Biorefin. 2022, 16, 435–450. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Solarte-Toro, J.C.; Galán-Martín, Á.; Ruiz, E.; Castro, E.; Ortiz-Sánchez, M.; Cardona Alzate, C.A. Olive leaves upgrading applying a novel two-stage organosolv pretreatment: Techno-economic and environmental assessment. Biochem. Eng. J. 2024, 207, 109317. [Google Scholar] [CrossRef]
- Peng, J.; Xu, H.; Wang, W.; Kong, Y.; Su, Z.; Li, B. Techno-economic analysis of bioethanol preparation process via deep eutectic solvent pretreatment. Ind. Crops Prod. 2021, 172, 114036. [Google Scholar] [CrossRef]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A. Analysis, Synthesis, and Design of Chemical Processes, 5th ed.; Pearson: London, UK, 2018. [Google Scholar]
- Parascanu, M.M.; Sanchez, N.; Sandoval-Salas, F.; Carreto, C.M.; Soreanu, G.; Sanchez-Silva, L. Environmental and economic analysis of bioethanol production from sugarcane molasses and agave juice. Environ. Sci. Pollut. Res. Int. 2021, 28, 64374–64393. [Google Scholar] [CrossRef]
- Gadkari, S.; Narisetty, V.; Maity, S.K.; Manyar, H.; Mohanty, K.; Jeyakumar, R.B.; Pant, K.K.; Kumar, V. Techno-Economic Analysis of 2,3-Butanediol Production from Sugarcane Bagasse. ACS Sustain. Chem. Eng. 2023, 11, 8337–8349. [Google Scholar] [CrossRef]
- Martinez-Hernandez, E.; Cui, X.; Scown, C.D.; Amezcua-Allieri, M.A.; Aburto, J.; Simmons, B.A. Techno-economic and greenhouse gas analyses of lignin valorization to eugenol and phenolic products in integrated ethanol biorefineries. Biofuels Bioprod. Biorefin. 2019, 13, 978–993. [Google Scholar] [CrossRef]
- Liu, F.; Dong, X.; Zhao, X.; Wang, L. Life cycle assessment of organosolv biorefinery designs with the complete use of biomass. Energy Convers. Manag. 2021, 246, 114653. [Google Scholar] [CrossRef]
- Wenger, J.; Pichler, S.; Näyhä, A.; Stern, T. Practitioners’ Perceptions of Co-Product Allocation Methods in Biorefinery Development—A Case Study of the Austrian Pulp and Paper Industry. Sustainability 2022, 14, 2619. [Google Scholar] [CrossRef]
- Priadi, H.; Awad, S.; Villot, A.; Andres, Y.; Purwanto, W.W. Techno-enviro-economic analysis of second-generation bioethanol at plant-scale by different pre-treatments of biomass from palm oil waste. Energy Convers. Manag. X 2024, 21, 100522. [Google Scholar] [CrossRef]
- Gubicza, K.; Nieves, I.U.; Sagues, W.J.; Barta, Z.; Shanmugam, K.T.; Ingram, L.O. Techno-economic analysis of ethanol production from sugarcane bagasse using a Liquefaction plus Simultaneous Saccharification and co-Fermentation process. Bioresour. Technol. 2016, 208, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kautto, J.; Realff, M.; Ragauskas, A.; Kässi, T. Economic Analysis of an Organosolv Process for Bioethanol Production. Bioresources 2014, 9, 6041–6072. [Google Scholar] [CrossRef]
- Correia, B.; Matos, H.A.; Lopes, T.F.; Marques, S.; Gírio, F. Sustainability Assessment of 2G Bioethanol Production from Residual Lignocellulosic Biomass. Processes 2024, 12, 987. [Google Scholar] [CrossRef]
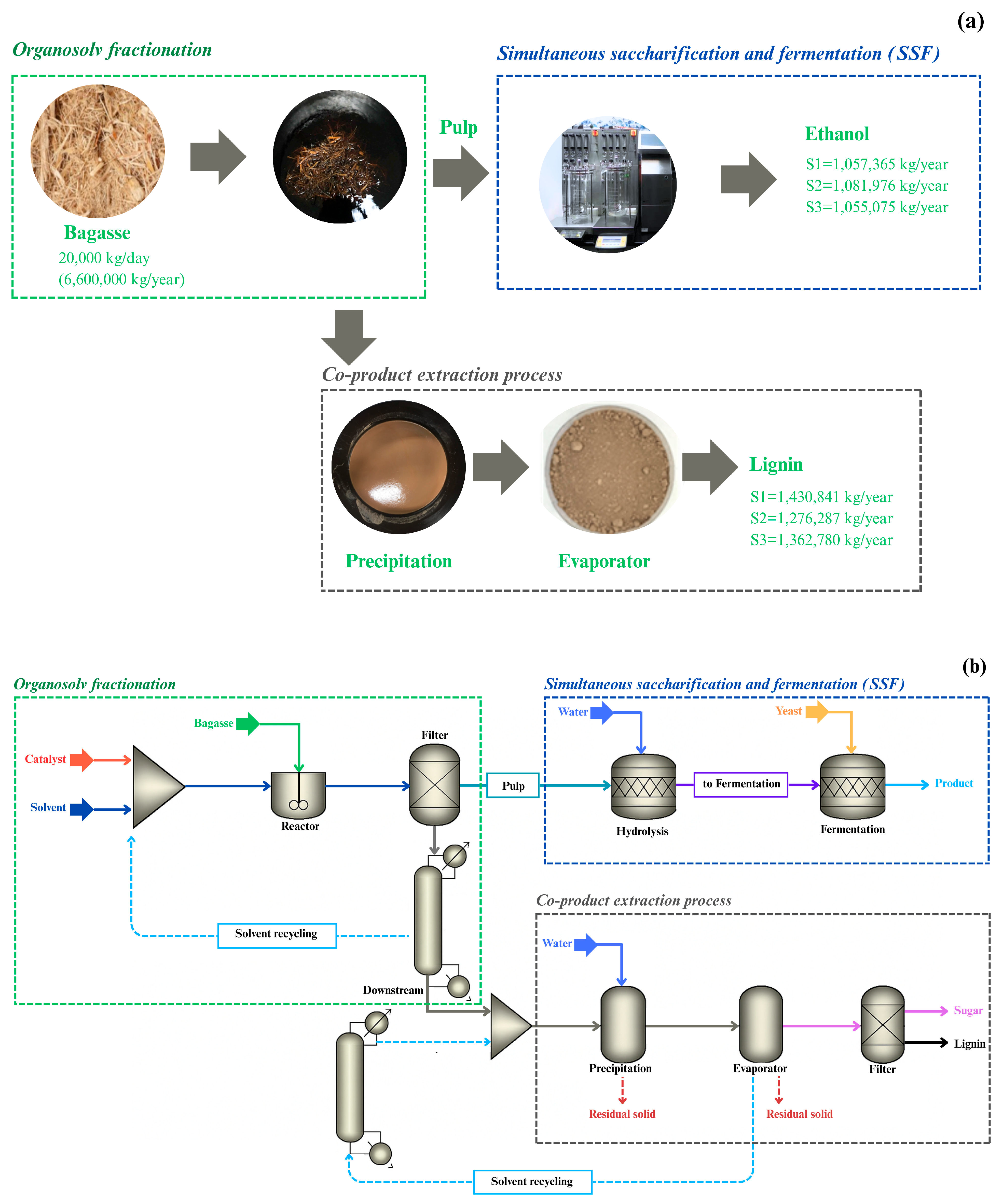
| Composition | Brazil [28] | China [29] | Thailand [11] * |
|---|---|---|---|
| Cellulose | 42.19 | 39.52 | 38.30 |
| Xylan | 27.60 | 25.63 | 20.70 |
| Lignin | 21.56 | 30.36 | 23.70 |
| Ash | 2.84 | 1.45 | 4.20 |
| Other | 5.63 | 1.72 | 13.00 |
| Input | Unit | Price | Reference |
|---|---|---|---|
| Bagasse | USD/ton | 14.00 | [35] |
| C2H6O | USD/L | ~0.80 | [36] |
| H2O | USD/L | ~0.00028 | [37] |
| H2SO4 | USD/L | ~0.16 | [36] |
| CH2O2 | USD/L | ~0.49 | [36] |
| C4H8O2 | USD/L | ~1.30 | [36] |
| CH3ONa | USD/kg | ~0.60 | [36] |
| CH3OH | USD/L | ~0.28 | [36] |
| Electricity | USD/KV | 2.1 | [38] |
| Units | Bagasse | Downstream | Lignin | Sugar | Pulp | to Fermentation | Product | |
|---|---|---|---|---|---|---|---|---|
| Scenario 1; Organosolv Fractionation by H2SO4 | ||||||||
| Cellulose | kg/year | 2,797,815.00 | 213,473.28 | 196,395.42 | 17,077.86 | 2,584,341.72 | 516,868.34 | 516,868.34 |
| Xylan | kg/year | 1,504,830.00 | 1,449,452.26 | 246,406.88 | 1,203,045.37 | 55,377.74 | 55,377.74 | 55,377.74 |
| Lignin | kg/year | 1,731,285.00 | 1,572,353.04 | 1,430,841.26 | 141,511.77 | 158,931.96 | 158,931.96 | 158,931.96 |
| Ash | kg/year | 306,810.00 | 253,670.51 | - | 253,670.51 | 53,139.49 | 53,139.49 | 53,139.49 |
| Extractives | kg/year | 964,260.00 | 904,668.73 | - | 904,668.73 | 59,591.27 | 59,591.27 | 59,591.27 |
| Enzyme | kg/year | - | - | - | - | - | - | - |
| Ethanol | kg/year | - | 2,041,590.44 | - | 3583.91 | - | - | 1,057,365.16 |
| Co2 | kg/year | - | - | - | - | - | - | 1,010,101.99 |
| Glucose | kg/year | - | - | - | - | - | 2,297,087.79 | 229,708.78 |
| Water | kg/year | - | 1,089,128.38 | - | 155,788.26 | - | 8,536,287.65 | 10,603,698.80 |
| Xylose | kg/year | - | - | - | - | - | - | - |
| Yeast | kg/year | - | - | - | - | - | - | 5,823,742.37 |
| H2SO4 | kg/year | - | 73,050.00 | - | 73,048.73 | - | - | - |
| CH2O2 | kg/year | - | - | - | - | - | - | - |
| CH3OH | kg/year | - | - | - | - | - | - | - |
| Scenario 2; Organosolv fractionation by CH2O2 | ||||||||
| Cellulose | kg/year | 2,797,815.00 | 153,320.26 | 141,054.64 | 12,265.62 | 2,644,494.74 | 528,898.95 | 528,898.95 |
| Xylan | kg/year | 1,504,830.00 | 1,351,487.82 | 229,752.93 | 1,121,734.89 | 153,342.18 | 153,342.18 | 153,342.18 |
| Lignin | kg/year | 1,731,285.00 | 1,402,513.98 | 1,276,287.72 | 126,226.26 | 328,771.02 | 328,771.02 | 328,771.02 |
| Ash | kg/year | 306,810.00 | 175,311.23 | - | 175,311.23 | 131,498.77 | 131,498.77 | 131,498.77 |
| Extractives | kg/year | 964,260.00 | 869,280.39 | - | 869,280.39 | 94,979.61 | 94,979.61 | 94,979.61 |
| Enzyme | kg/year | - | - | - | - | - | - | - |
| Ethanol | kg/year | - | 583,311.56 | - | - | - | - | 1,081,976.34 |
| Co2 | kg/year | - | - | - | - | - | - | 1,033,613.08 |
| Glucose | kg/year | - | - | - | - | - | 2,350,554.71 | 235,055.47 |
| Water | kg/year | - | 1,561,084.02 | - | - | - | 8,530,940.88 | 10,646,472.99 |
| Xylose | kg/year | - | - | - | - | - | - | - |
| Yeast | kg/year | - | - | - | - | - | - | 5,775,662.21 |
| H2SO | kg/year | - | - | - | - | - | - | - |
| CH2O2 | kg/year | - | 709,162.09 | - | - | - | - | - |
| CH3OH | kg/year | - | 687,926.46 | - | - | - | - | - |
| Scenario 3; Organosolv fractionation by CH3ONa | ||||||||
| Cellulose | kg/year | 2,797,815.00 | 219,068.91 | 201,543.40 | 17,525.51 | 2,578,746.09 | 515,749.22 | 515,749.22 |
| Xylan | kg/year | 1,504,830.00 | 423,760.13 | 72,039.22 | 351,720.91 | 1,081,069.87 | 1,081,069.87 | 1,081,069.87 |
| Lignin | kg/year | 1,731,285.00 | 1,497,561.53 | 1,362,780.99 | 134,780.54 | 233,723.48 | 233,723.48 | 233,723.48 |
| Ash | kg/year | 306,810.00 | 87,655.62 | - | 87,655.62 | 219,154.38 | 219,154.38 | 219,154.38 |
| Extractives | kg/year | 964,260.00 | 547,892.53 | - | 547,892.53 | - | 416,367.47 | 416,367.47 |
| Enzyme | kg/year | - | - | - | - | - | - | - |
| Ethanol | kg/year | - | - | - | - | - | - | 1,055,075.74 |
| Co2 | kg/year | - | - | - | - | - | - | 1,007,914.91 |
| Glucose | kg/year | - | - | - | - | - | 2,292,114.12 | 229,211.41 |
| Water | kg/year | - | - | - | - | - | 8,536,785.02 | 10,599,719.80 |
| Xylose | kg/year | - | - | - | - | - | - | - |
| Yeast | kg/year | - | - | - | - | - | - | 5,828,214.94 |
| H2SO4 | kg/year | - | - | - | - | - | - | - |
| CH2O2 | kg/year | - | - | - | - | - | - | - |
| CH3OH | kg/year | - | 2,896,143.95 | - | - | - | - | - |
| CH3ONa | kg/year | - | 365,250.00 | - | - | 365,250.00 | - | - |
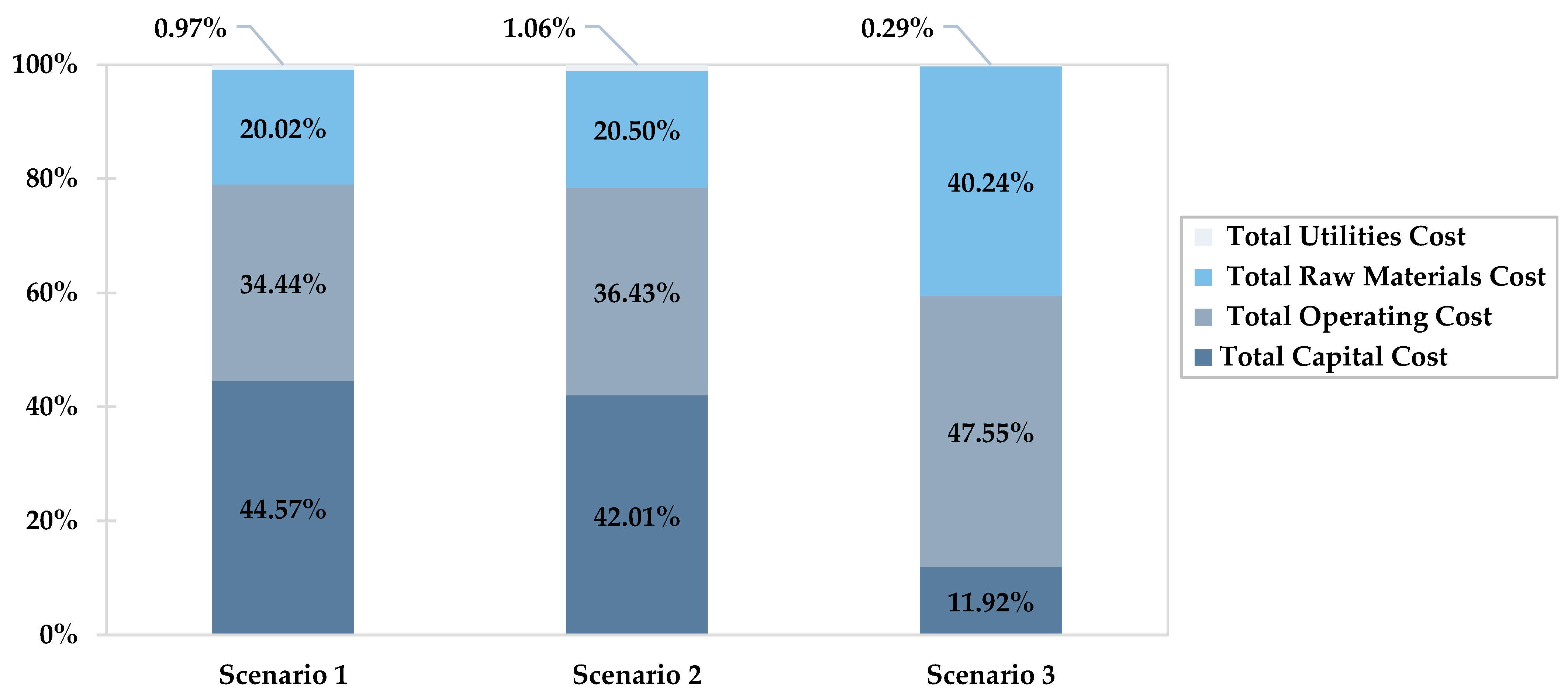
| Cost Analysis | Unit | Scenario 1 | Scenario 2 | Scenario 3 |
|---|---|---|---|---|
| Total Capital Cost | USD | 4,358,930 | 3,671,550 | 3,640,210 |
| Total Operating Cost | USD/Year | 3,368,230 | 3,183,650 | 14,526,300 |
| Total Raw Materials Cost | USD/Year | 1,957,890 | 1,791,750 | 12,292,500 |
| Total Product Sales | USD/Year | 43,710,900 | 43,291,000 | 43,950,800 |
| Total Utilities Cost | USD/Year | 94,615 | 92,066 | 92,288 |
| Desired Rate of Return | Year | 20 | 20 | 20 |
| Equipment Cost | USD | 295,500 | 242,900 | 262,300 |
| Total Installed Cost | USD | 1,391,500 | 1,066,700 | 1,079,100 |
| Electricity rate | kW | 79.64 | 77.49 | 77.68 |
| Electricity cost | USD/H | 11.94 | 11.62 | 11.65 |
| TAC | USD | 4,263,365 | 3,927,396 | 15,273,841 |
| TAC | million USD | 4.26 | 3.92 | 15.27 |
| Parameter Changed | Min | Baseline | Max | Unit | TAC (Million US$/Year) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min | Change (%) | Baseline | Max | Change (%) | ||||||
| Scenario 1; Organosolv fractionation by H2SO4 catalyst | ||||||||||
| Raw material cost | Case 1 | 12.84 | 14.00 | 15.70 | US$/ton | 4.25 | −0.01 | 4.26 | 4.27 | 0.01 |
| Chemicals cost | Case 2 | different for water, ethanol and H2SO4 | 4.25 | −0.01 | 4.26 | 4.27 | 0.01 | |||
| Utilities Cost | Case 3 | 0.14 | 0.15 | 0.17 | US$/kWhr | 4.25 | −0.01 | 4.26 | 4.27 | 0.01 |
| Utilities consumption | Case 4 | 75.71 | 84.12 | 92.53 | KW | 4.26 | 0.00 | 4.26 | 4.26 | 0.00 |
| Temperature change | Case 5 | 153.00 | 170.00 | 187.00 | °C | 4.26 | 0.00 | 4.26 | 4.26 | 0.00 |
| Pressure change | Case 6 | 18.00 | 20.00 | 22.00 | bar | 4.26 | 0.00 | 4.26 | 4.26 | 0.00 |
| Scenario 2; Organosolv fractionation by CH2O2 catalyst | ||||||||||
| Raw material cost | Case 1 | 12.84 | 14.00 | 15.70 | US$/ton | 3.91 | −0.01 | 3.92 | 4.02 | 0.01 |
| Chemicals cost | Case 2 | different for water, ethanol and CH2O2 | 3.74 | −0.18 | 3.92 | 4.10 | 0.18 | |||
| Utilities Cost | Case 3 | 0.14 | 0.15 | 0.17 | US$/kWhr | 3.91 | −0.01 | 3.92 | 3.93 | 0.01 |
| Utilities consumption | Case 4 | 69.74 | 77.49 | 85.24 | KW | 3.92 | 0.00 | 3.92 | 3.92 | 0.00 |
| Temperature change | Case 5 | 143.10 | 159.00 | 174.90 | °C | 3.92 | 0.00 | 3.92 | 3.92 | 0.00 |
| Pressure change | Case 6 | 18.00 | 20.00 | 22.00 | bar | 3.91 | −0.01 | 3.92 | 3.93 | 0.01 |
| Scenario 3; Organosolv fractionation by CH3ONa catalyst | ||||||||||
| Raw material cost | Case 1 | 12.84 | 14.00 | 15.70 | US$/ton | 15.26 | −0.01 | 15.27 | 15.28 | 0.01 |
| Chemicals cost | Case 2 | different for water, ethanol and CH3ONa | 15.15 | −0.12 | 15.27 | 15.15 | 0.12 | |||
| Utilities Cost | Case 3 | 0.14 | 0.15 | 0.17 | US$/kWhr | 15.26 | −0.01 | 15.27 | 15.28 | 0.01 |
| Utilities consumption | Case 4 | 69.91 | 77.68 | 85.45 | KW | 15.27 | 0.00 | 15.27 | 15.27 | 0.00 |
| Temperature change | Case 5 | 135.00 | 150.00 | 165.00 | °C | 15.27 | 0.00 | 15.27 | 15.27 | 0.00 |
| Pressure change | Case 6 | 18.00 | 20.00 | 22.00 | bar | 15.27 | 0.00 | 15.27 | 15.27 | 0.00 |
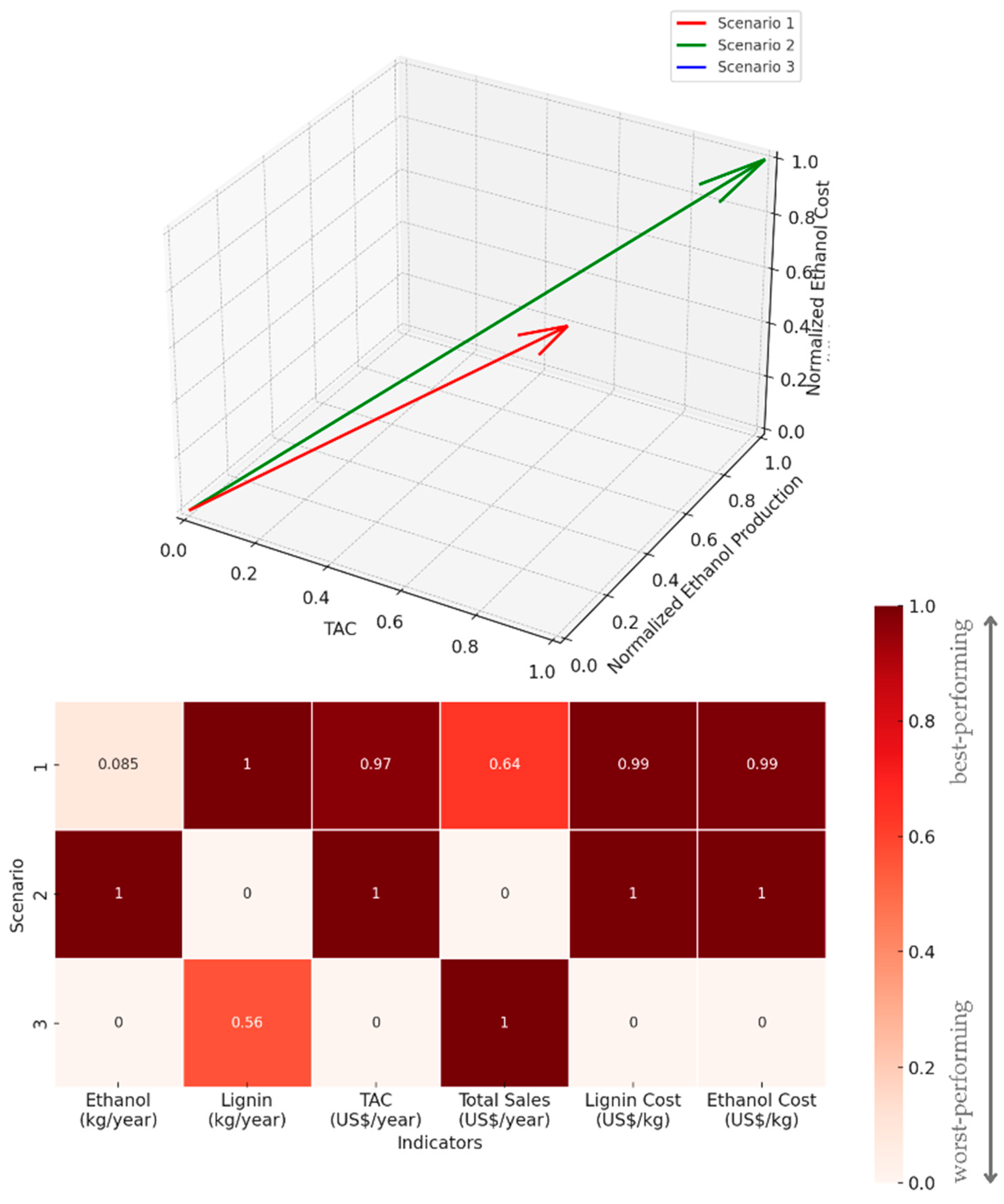
| Scenario | Ethanol Production | Lignin Production | TAC | Total Product Sales | Cost of Lignin Production | Cost of Ethanol Production | |
|---|---|---|---|---|---|---|---|
| (kg/Year) | (kg/Year) | USD/Year) | (USD/Year) | (USD/kg) | (USD/kg) | (USD/L) * | |
| 1 | 1,057,365 | 1,430,841 | 4,263,365 | 43,710,900 | 1.88 | 1.49 | 1.20 |
| 2 | 1,081,976 | 1,276,287 | 3,927,396 | 43,291,000 | 1.84 | 1.45 | 1.14 |
| 3 | 1,055,075 | 1,362,780 | 15,273,841 | 43,950,800 | 6.96 | 5.49 | 4.33 |
| Biomass | Main Process | Technical Notes | MESP (USD/L) | Reference |
|---|---|---|---|---|
| Sugarcane bagasse | Liquefaction + SSF + Co-Ferm | Recombinant E. coli (LY01); pH 6.0; no detoxification required | 0.50–0.63 | [54] |
| Hardwood (generic) | Organosolv + enzymatic hydrolysis | Ethanol-water 50:50; no catalyst or organic solvent recovery reported | 0.81 (base), 0.53 (lignin @1000 USD/t) | [55] |
| Wheat straw | Organosolv + lignin valorization (eugenol) | H2SO4 used as catalyst in organosolv; lignin valorized into eugenol | 0.53 | [50] |
| Eucalyptus residues | Steam explosion + enzymatic hydrolysis + fermentation | Steam explosion at 200 °C for 10 min; enzyme: Cellic CTec2; fed-batch fermentation | 2.37 | [56] |
| Corn stover | Steam explosion + enzymatic hydrolysis + fermentation | Similar to eucalyptus; low yield from corn stover contributes to high MESP | 2.65 | [56] |
| Sugarcane bagasse | Organosolv + value-based allocation | Organosolv fractionation by H2SO4 | 1.20 | The current research (Scenario 1) |
| Sugarcane bagasse | Organosolv + value-based allocation | Organosolv fractionation by CH2O2 | 1.14 | The current research (Scenario 2) |
| Sugarcane bagasse | Organosolv + value-based allocation | Organosolv fractionation by CH3ONa | 4.33 | The current research (Scenario 3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaowdang, S.; Suriyachai, N.; Imman, S.; Kreetachat, N.; Chuetor, S.; Wongcharee, S.; Suwannahong, K.; Nukunudompanich, M.; Kreetachat, T. Valorization of Sugarcane Bagasse in Thailand: An Economic Analysis of Ethanol and Co-Product Recovery via Organosolv Fractionation. Sustainability 2025, 17, 7145. https://doi.org/10.3390/su17157145
Khaowdang S, Suriyachai N, Imman S, Kreetachat N, Chuetor S, Wongcharee S, Suwannahong K, Nukunudompanich M, Kreetachat T. Valorization of Sugarcane Bagasse in Thailand: An Economic Analysis of Ethanol and Co-Product Recovery via Organosolv Fractionation. Sustainability. 2025; 17(15):7145. https://doi.org/10.3390/su17157145
Chicago/Turabian StyleKhaowdang, Suphalerk, Nopparat Suriyachai, Saksit Imman, Nathiya Kreetachat, Santi Chuetor, Surachai Wongcharee, Kowit Suwannahong, Methawee Nukunudompanich, and Torpong Kreetachat. 2025. "Valorization of Sugarcane Bagasse in Thailand: An Economic Analysis of Ethanol and Co-Product Recovery via Organosolv Fractionation" Sustainability 17, no. 15: 7145. https://doi.org/10.3390/su17157145
APA StyleKhaowdang, S., Suriyachai, N., Imman, S., Kreetachat, N., Chuetor, S., Wongcharee, S., Suwannahong, K., Nukunudompanich, M., & Kreetachat, T. (2025). Valorization of Sugarcane Bagasse in Thailand: An Economic Analysis of Ethanol and Co-Product Recovery via Organosolv Fractionation. Sustainability, 17(15), 7145. https://doi.org/10.3390/su17157145











