Use of Amino Acids and Organic Waste Extracts to Improve the Quality of Liquid Nitrogen–Calcium–Magnesium Fertilizers
Abstract
1. Introduction
2. Materials and Methods
2.1. The Production of Liquid Nitrogen–Calcium–Magnesium Fertilizers
2.2. Bioactive Substances Used in the Liquid Fertilizers
2.3. Analytical Methods for Determining Plant Nutrient Concentrations
2.4. Physical Methods for Determining Properties of Liquid Fertilizers
2.5. Instrumental Analysis Methods for Determining Properties of Liquid Fertilizers
2.6. Statistical Analysis
3. Results and Discussion
3.1. Decomposition of Dolomite by Nitric Acid
3.2. Neutralization and Nitrogen Enrichment of Dolomite Decomposition Solutions
3.3. Influence of Amino Acid Concentrate Naturamin-WSP on the Crystallization Temperature of Liquid Fertilizers
3.4. Influence of Organic Digestate Extracts on the Crystallization Temperature of Liquid Fertilizers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Easwaran, C.; Christopher, S.R.; Moorthy, G.; Mohan, P.; Marimuthu, R.; Koothan, V.; Nallusamy, S. Nano hybrid fertilizers: A review on the state of the art in sustainable agriculture. Sci. Total Environ. 2024, 929, 172533. [Google Scholar] [CrossRef] [PubMed]
- McArthur, J.W.; McCord, G.C. Fertilizing growth: Agricultural inputs and their effects in economic development. J. Dev. Econ. 2017, 127, 133–152. [Google Scholar] [CrossRef]
- Basavegowda, N.; Baek, K. Current and future perspectives on the use of nanofertilizers for sustainable agriculture: The case of phosphorus nanofertilizer. 3 Biotech 2021, 11, 357. [Google Scholar] [CrossRef]
- Majaron, V.F.; da Silva, M.G.; Bortoletto-Santos, R.; Klaic, R.; Ribeiro, S.J.L.; Polito, W.L.; Bevilaqua, D.; Farinas, C.S.; Ribeiro, C. Bioactive Materials with Microorganisms can Enhance the Micronutrients Solubilization and Sulfate availability from Low Reactive Sources: Insight for application as Coating Fertilizer Granules. J. Polym. Environ. 2022, 30, 2602–2613. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Al-Amri, S.M.; El-Enany, A.E. Enhancing Rhizobium–Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change. Agriculture 2023, 13, 2092. [Google Scholar] [CrossRef]
- Rosa, L.; Gabrielli, P. Energy and food security implications of transitioning synthetic nitrogen fertilizers to net-zero emissions. Environ. Res. Lett. 2023, 18, 014008. [Google Scholar] [CrossRef]
- Karami, S.; Soltani, S.; Jasemi, K. Positive and Negative Impact of Nitrogen Fertilizer on Soil Properties and Nutrient Dynamic. Asian J. Res. Agric. For. 2023, 9, 233–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, C.; Su, Y.; Peng, W.; Lu, R.; Liu, Y.; Huang, H.; He, X.; Yang, M.; Zhu, S. Soil Acidification caused by excessive application of nitrogen fertilizer aggravates soil-borne diseases: Evidence from literature review and field trials. Agric. Ecosyst. Environ. 2022, 340, 108176. [Google Scholar] [CrossRef]
- Ahmed, M.; Rauf, M.; Mukhtar, Z.; Saeed, N.A. Excessive use of nitrogenous fertilizers: An unawareness causing serious threats to environment and human health. Environ. Sci. Pollut. Res. 2017, 24, 26983–26987. [Google Scholar] [CrossRef]
- Bijay-Singh; Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Guo, C.; Liu, X.; He, X. A global meta-analysis of crop yield and agricultural greenhouse gas emissions under nitrogen fertilizer application. Sci. Total Environ. 2022, 831, 154982. [Google Scholar] [CrossRef] [PubMed]
- Pajura, R.; Maslon, A.; Czarnota, J. The Use of Waste to Produce Liquid Fertilizers in Terms of Sustainable Development and Energy Consumption in the Fertilizer Industry–A Case Study from Poland. Energies 2023, 16, 1747. [Google Scholar] [CrossRef]
- Smol, M. Transition to Circular Economy in the Fertilizer Sector–Analysis of Recommended Directions and End-Users Perception of Waste-Based Products in Poland. Energies 2021, 14, 4312. [Google Scholar] [CrossRef]
- Fernández-Delgado, M.; del Amo-Mateos, E.; Lucas, S.; García-Cubero, M.T.; Coca, M. Liquid fertilizer production from organic waste by conventional and microwave-assisted extraction technologies: Techno-economic and environmental assessment. Sci. Total Environ. 2022, 806, 150904. [Google Scholar] [CrossRef]
- Lateef, A.; Nazir, R.; Jamil, N.; Alam, S.; Shah, R.; Khan, M.N.; Saleem, M. Synthesis and characterization of zeolite based nano–composite: An environment friendly slow release fertilizer. Microporous Mesoporous Mater. 2016, 232, 174–183. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Y.; Degryse, F.; Huang, C.; Hou, C.; Wu, L.; Jiang, R.; Mclaughlin, M.J.; Zhang, F. Magnesium-fortified phosphate fertilizers improve nutrient uptake and plant growth without reducing phosphorus availability. Pedosphere 2022, 32, 744–751. [Google Scholar] [CrossRef]
- Yadav, V.K.; Yadav, K.K.; Cabral-Pinto, M.M.S.; Choudhary, N.; Gnanamoorthy, G.; Tirth, V.; Prasad, S.; Khan, A.H.; Islam, S.; Khan, N.A. The Processing of Calcium Rich Agricultural and Industrial Waste for Recovery of Calcium Carbonate and Calcium Oxide and Their Application for Environmental Cleanup: A Review. Appl. Sci. 2021, 11, 4212. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Kuang, Y.; Wang, N. Amino Acids Biostimulants and Protein Hydrolysates in Agricultural Sciences. Plants 2024, 13, 210. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. The Application of Arbuscular Mycorrhizal Fungi as Microbial Biostimulant, Sustainable Approaches in Modern Agriculture. Plants 2023, 12, 3101. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W. Various Techniques for Molecular and Rapid Detection of Infectious and Epidemic Diseases. Lett. Org. Chem. 2023, 20, 779–801. [Google Scholar] [CrossRef]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valorization 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Martínez-Cuenca, M.-R.; Bermejo, A.; Legaz, F.; Quiñones, A. Liquid Organic Fertilizers for Sustainable Agriculture: Nutrient Uptake of Organic versus Mineral Fertilizers in Citrus Trees. PLoS ONE 2016, 11, e0161619. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Delgado, M.; del Amo-Mateos, E.; Lucas, S.; García-Cubero, M.T.; Coca, M. Recovery of organic carbon from municipal mixed waste compost for the production of fertilizers. J. Clean. Prod. 2020, 265, 121805. [Google Scholar] [CrossRef]
- Bloem, E.; Albihn, A.; Elving, J.; Hermann, L.; Lehmann, L.; Sarvi, M.; Schaaf, T.; Schick, J.; Turtola, E.; Ylivainio, K. Contamination of organic nutrient sources with potentially toxic elements, antibiotics and pathogen microorganisms in relation to P fertilizer potential and treatment options for the production of sustainable fertilizers: A review. Sci. Total Environ. 2017, 607–608, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.K.; Yaseen, T.; Traversa, A.; Ben Kheder, M.; Brunetti, G.; Cocozza, C. Effects of the main extraction parameters on chemical and microbial characteristics of compost tea. Waste Manag. 2016, 52, 62–68. [Google Scholar] [CrossRef]
- Tortosa, G.; Alburquerque, J.A.; Bedmar, E.J.; Ait-Baddi, G.; Cegarra, J. Strategies to produce commercial liquid organic fertilisers from “alperujo” composts. J. Clean. Prod. 2014, 82, 37–44. [Google Scholar] [CrossRef]
- Spaccini, R.; Cozzolino, V.; Di Meo, V.; Savy, D.; Drosos, M.; Piccolo, A. Bioactivity of humic substances and water extracts from compost made by ligno-cellulose wastes from biorefinery. Sci. Total Environ. 2019, 646, 792–800. [Google Scholar] [CrossRef]
- Saito, B.; Seckler, M.M. Alkaline extraction of humic substances from peat applied to organic-mineral fertilizer production. Braz. J. Chem. Eng. 2014, 31, 675–682. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Anielak, A.M.; Kleczek, A.; Luszczek, B. Innovative Method of Extraction of Humic Substances from Digestate Sludge and Assessment of the Impact of Their on the Growth of Selected Plants. Energies 2023, 16, 1283. [Google Scholar] [CrossRef]
- Cristina, G.; Camelin, E.; Ottone, C.; Garofalo, S.F.; Jorquera, L.; Castro, M.; Fino, D.; Schiappacasse, M.C.; Tommasi, T. Recovery of humic acids from anaerobic sewage sludge: Extraction characterization and encapsulation in alginate beads. Int. J. Biol. Macromol. 2020, 164, 277–285. [Google Scholar] [CrossRef]
- Datta, A.; Sanyal, S.K.; Saha, S. A study on natural and synthetic humic acids and their complexing ability towards cadmium. Plant Soil 2001, 235, 115–125. [Google Scholar] [CrossRef]
- Retsch. Woven Wire Mesh Sieves—Ø 200 mm/203 mm. Available online: https://www.retsch.com/products/sieving/test-sieves/mesh-sieves-200-203-mm?utm_source=chatgpt.com (accessed on 13 January 2025).
- Paleckiene, R.; Navikaite, R.; Slinksiene, R. Peat as a Raw Material for Plant Nutrients and Humic Substances. Sustainability 2021, 13, 6354. [Google Scholar] [CrossRef]
- Gerhardt. Vapodesk Distillation System. Available online: https://www.dijkstra.net/media/catalog/category/Dijkstra_Vereenigde_Gerhardt_Vapodest.pdf?utm_source=chatgpt.com (accessed on 13 January 2025).
- Zhang, J.Z.; Fan, X.H.; Xue, L.H. Continuous determination of calcium and magnesium in iron ore by EDTA complexometric titration. Miner. Metall. Process. 2012, 28, 269–275. [Google Scholar]
- LST EN 13039:2012; Soil Improvers and Growing Media—Determination of Organic Matter Content and Ash. Lithuanian Standardisation Department: Vilnius, Lithuania, 2012.
- LST EN 1484:1997; Water Analysis—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). Available online: https://standards.iteh.ai/catalog/standards/cen/7d0a16de-63ee-4536-a6f4-9ec990809a08/en-1484-1997?srsltid=AfmBOoo3tbFBj3nxJgMNf9hqn33fJft20C-7zxlOrFpNoOHFazpUaziP (accessed on 27 July 2025).
- ISO 23381:2020; Determination of Salt Out (Crystallization) Temperature of Liquid Fertilizers. ISO: Geneva, Switzerland, 2020.
- Malvern Panalytical. X–ray Diffraction (XRD). Available online: https://www.malvernpanalytical.com/en/products/technology/xray-analysis/x-ray-diffraction (accessed on 27 July 2025).
- Hitachi. SEM (Scanning Electron Microscopes). Available online: https://www.hitachi-hightech.com/eu/en/products/microscopes/sem-tem-stem/sem/ (accessed on 20 January 2015).
- Didžiulytė, E.; Šlinkšienė, R. Liquid Nitrogen-Calcium-Magnesium Fertilizer with Extract from Biodegradable Materials; Poster Session at Open Readings 2025. 16 May 2025. Conference Poster. Available online: https://openreadings.eu/wp-content/latex/perm/15521d900d04838a93909d9ac988d3856eb4ff0e39597b6daf4c243a7c289481/abstract.pdf (accessed on 17 May 2025).
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, L 170, 1–114. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj (accessed on 5 February 2024).
- European Commission. Harmonised Standards for Fertilising Products. Single Market and Economy—European Commission. Available online: https://single-market-economy.ec.europa.eu/single-market/goods/european-standards/harmonised-standards/fertilising-products-standards_en (accessed on 16 June 2025).
- Wen, Y.; Sánchez-Román, M.; Li, Y.; Wang, C.; Han, Z.; Zhang, L.; Gao, Y. Nucleation and stabilization of Eocene dolomite in evaporative lacustrine deposits from central Tibetan plateau. Sedimentology 2020, 67, 3333–3354. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, H.; Liu, B.; Liu, H.; Shi, K.; Guo, R.; Zhang, X. Petrology and geochemical characteristics of dolomite in the Middle Permian Maoukou Formation, central Sichuan. Pet. Res. 2017, 2, 366–377. [Google Scholar] [CrossRef]
- Wilson, J.F.; Fodor, L.M.; Kenton, J.R. Fertilizer Solution Containing Sulfur and Having Low Crystallization Temperature. CA1129667A, 17 August 1982. Available online: https://patents.google.com/patent/CA1129667A/en (accessed on 1 February 2025).
- Yin, X.; Li, D.; Tan, Y.; Wu, X.; Yu, X.; Zeng, D. Solubility phase diagram of the Ca(NO3)2–Mg(NO3)2−H2O system. J. Chem. Eng. Data 2014, 59, 4026–4030. [Google Scholar] [CrossRef]
- Ergashev, D.; Khamdamova, S.; Khakimov, O.; Akramjonov, A. Study of the Physical and Chemical Processes of New Type Fertilizer Based on Local Raw Materials. Available online: https://www.e3s-conferences.org/articles/e3sconf/pdf/2024/93/e3sconf_iceste2024_03082.pdf?utm_source=chatgpt.com (accessed on 15 June 2025).
- Didžiulytė, E.; Šlinkšienė, R. Analysis and Usage Perspective of Solid Digestate. Proceedings 2023, 92, 2034. [Google Scholar] [CrossRef]
- Jinjiangmelamine. Solubility of Urea Overview. Available online: https://jinjiangmelamine.com/solubility-of-urea/?utm_source=chatgpt.com (accessed on 15 June 2025).
- Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, X.; Zhang, Y. The Impact of Humic Acid Fertilizers on Crop Yield and Nitrogen Use Efficiency: A Meta-Analysis. Agronomy 2024, 14, 2763. [Google Scholar] [CrossRef]
- Nargesi, M.M.; Sedaghathoor, S.; Hashemabadi, D. Effect of foliar application of amino acid, humic acid and fulvic acid on the oil content and quality of olive. Saudi J. Biol. Sci. 2022, 29, 3473–3481. [Google Scholar] [CrossRef] [PubMed]
- Jančaitienė, K.; Sidaraitė, R. Liquid complex Fertilizers with Bio-Additives. Proceedings 2023, 92, 2030. [Google Scholar] [CrossRef]
- Valieri, G. Acid Composition Based on Leonardite and Amino Acids. Application No. AU 2016221585 B2, 9 August 2018. Available online: https://patents.google.com/patent/AU2016221585B2/en (accessed on 5 June 2025).
- Phibunwatthanawong, T.; Riddech, N. Liquid organic fertilizer production for growing vegetables under hydroponic condition. Int. J. Recycl. Org. Waste Agric. 2019, 8, 369–380. [Google Scholar] [CrossRef]
- Greenlive. pH Regulation Properties of Liquid Fertilizers. Greenlive—Agro & Environment News, Greenlive.com.tr. Available online: https://www.greenlive.com.tr/en/ph-regulation-properties-of-liquid-fertilizers/ (accessed on 15 June 2025).
- Sviklas, A.; Shlinkshene, R. Liquid fertilizers based on dolomite, nitric acid, and ammonia. Russ. J. Appl. Chem. 2003, 76, 1885–1890. [Google Scholar] [CrossRef]
- Saidullaeva, G.A.; Tadjiyeva, X.S.; Abdullaeva, M.T. Obtaining liquid fertilizers with physiologically active substances. J. Med. Pract. Nurs. 2024, 2, 2938–3765. [Google Scholar]
- Henderson, B.C.R.; Sanderson, J.M.; Fowles, A. A review of the foliar application of individual amino acids as biostimulants in plants. Discov. Agric. 2025, 3, 69. [Google Scholar] [CrossRef]
- VanIperen. CalMag Liquid. Available online: https://www.vaniperen.com/products/calmag-liquid-fertilizer/?utm_source.com (accessed on 15 July 2025).
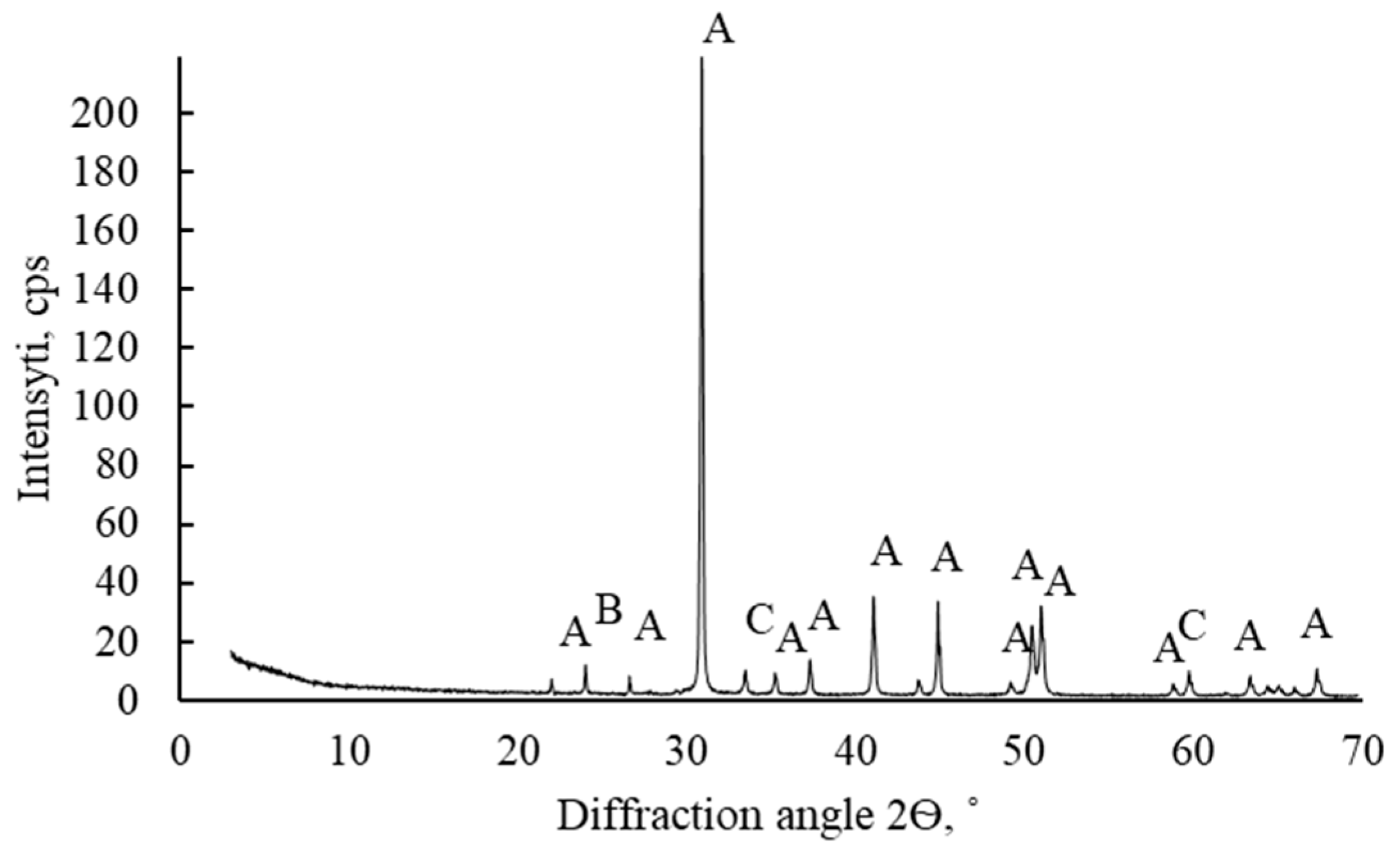

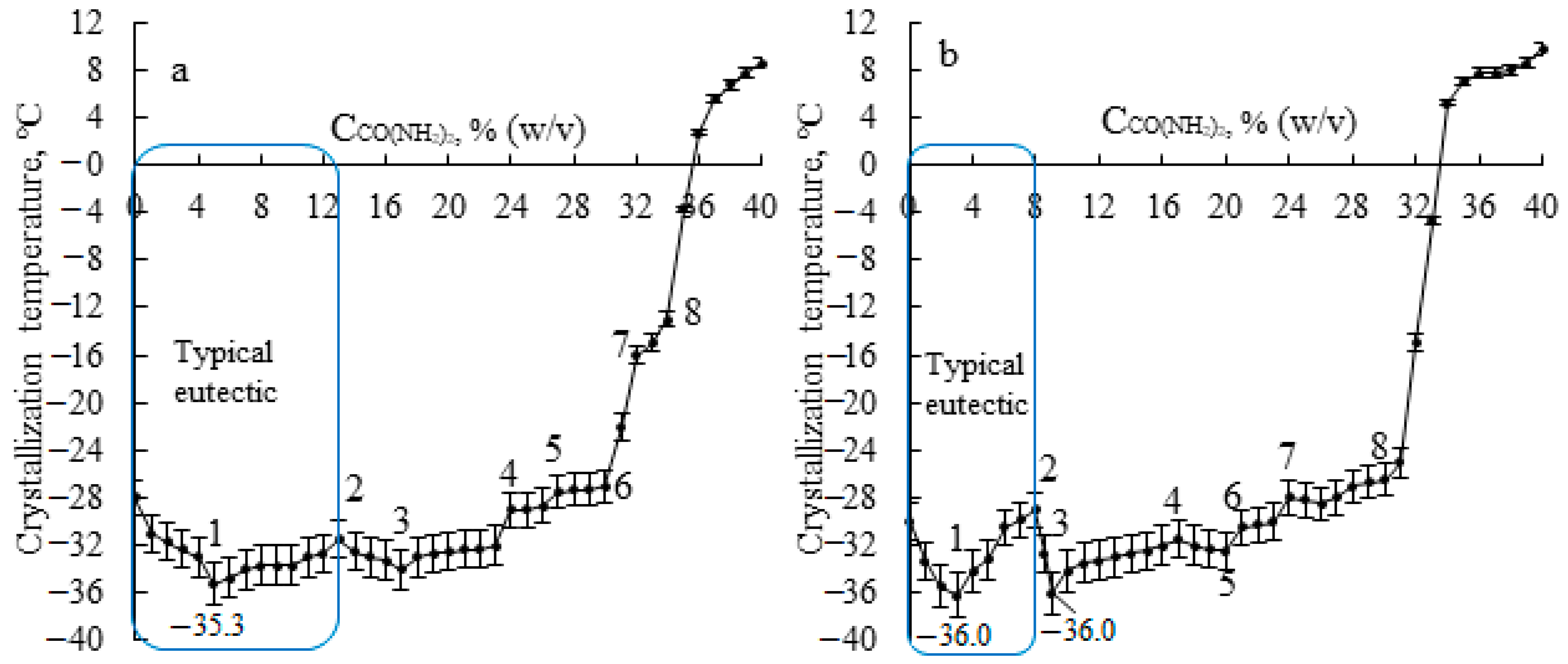



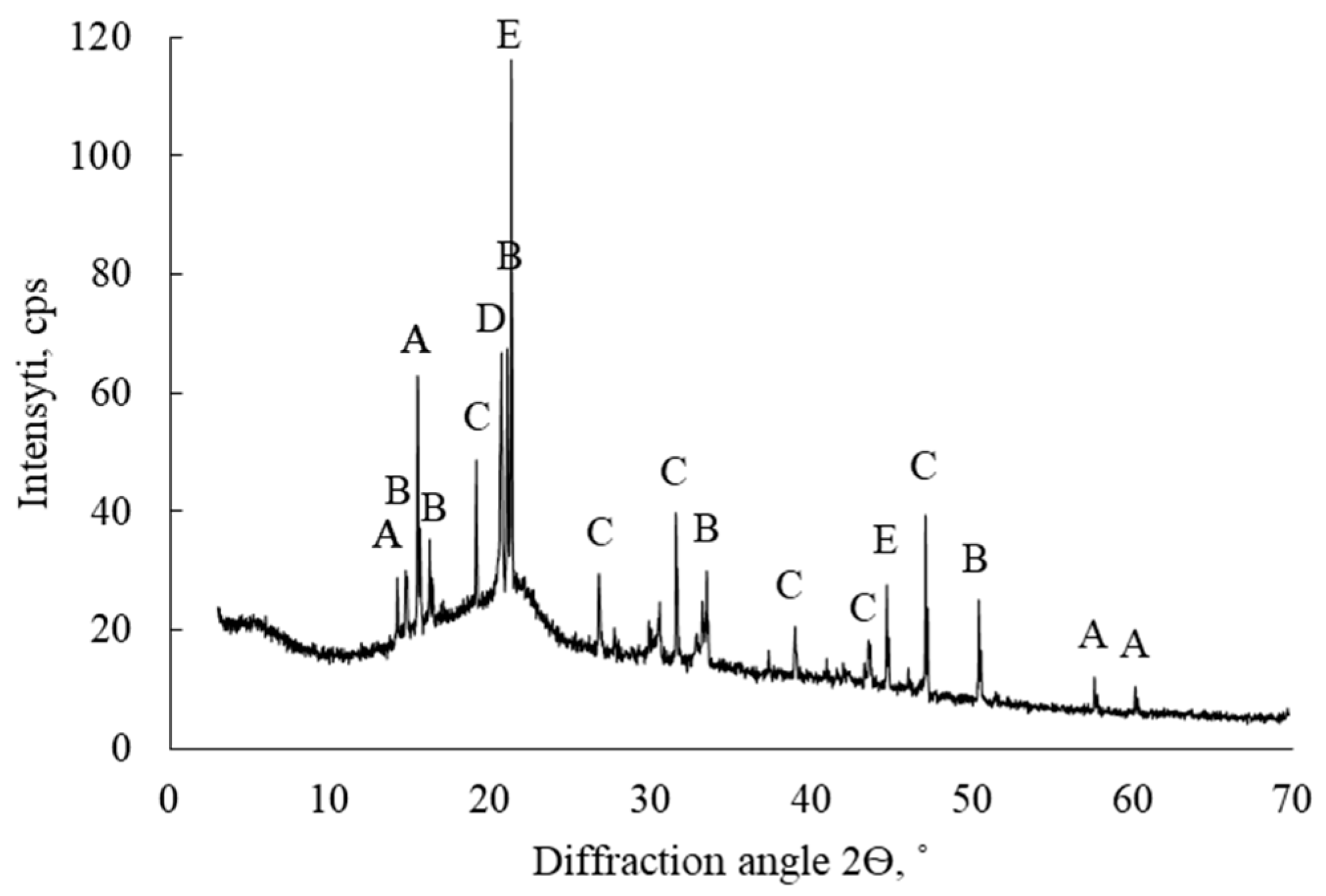



| Chemical Element Concentration, % | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cu | Fe | Co | Mn | Cr | Ni | Pb | Cd | Zn |
| 2.4 · 10−5 | 1.5 · 10−3 | 5.0 · 10−5 | 5.5 · 10−4 | 1.6 · 10−4 | – | 2.5 · 10−5 | – | 1.1 · 10−4 |
| Extraction Solvent | |||||||
|---|---|---|---|---|---|---|---|
| H2O | 0.5 M KOH | ||||||
| Temperature, °C | Time, Hours | TOC Concentration, mg/L | Temperature, °C | Time, Hours | TOC Concentration, mg/L | ||
| WD | CD | WD | CD | ||||
| Magnetic Stirring | |||||||
| 23 | 3 | 266 ± 0.01 | 417 ± 0.56 | 23 | 3 | 1170 ± 0.59 | 1517 ± 0.08 |
| 6 | 290 ± 0.07 | 442 ± 0.25 | 6 | 2351 ± 0.41 | 2419 ± 0.21 | ||
| 9 | 293 ± 0.17 | 417 ± 0.46 | 9 | 1930 ± 0.52 | 2080 ± 0.54 | ||
| 50 | 3 | 166 ± 0.02 | 235 ± 0.55 | 50 | 3 | 2672 ± 0.03 | 2926 ± 0.56 |
| 6 | 271 ± 0.02 | 347 ± 0.41 | 6 | 2084 ± 0.21 | 3834 ± 0.22 | ||
| 9 | 363 ± 0.01 | 161 ± 0.01 | 9 | 3315 ± 0.20 | 3407 ± 0.91 | ||
| 70 | 3 | 368 ± 0.01 | 508 ± 0.26 | 70 | 3 | 1884 ± 0.36 | 3050 ± 0.25 |
| 6 | 483 ± 0.02 | 541 ± 0.09 | 6 | 3954 ± 0.68 | 3649 ± 0.41 | ||
| 9 | 571 ± 0.15 | 503 ± 0.05 | 9 | 3466 ± 0.54 | 3615 ± 0.43 | ||
| 90 | 3 | 534 ± 0.26 | 574 ± 0.01 | 90 | 3 | 4008 ± 0.50 | 3552 ± 0.58 |
| 6 | 534 ± 0.05 | 424 ± 0.01 | 6 | 4495 ± 0.52 | 4072 ± 0.33 | ||
| 9 | 513 ± 0.02 | 444 ± 0.54 | 9 | 4032 ± 0.05 | 4232 ± 0.48 | ||
| Ultrasonic Bath | |||||||
| 30 | 3 | 247 ± 0.01 | 648 ± 0.02 | 30 | 3 | 1704 ± 0.25 | 1526 ± 0.02 |
| 6 | 407 ± 0.01 | 523 ± 0.03 | 6 | 1781 ± 0.05 | 2193 ± 0.02 | ||
| 9 | 366 ± 0.01 | 689 ± 0.51 | 9 | 2865 ± 0.54 | 2555 ± 0.39 | ||
| 50 | 3 | 322 ± 0.16 | 576 ± 0.02 | 50 | 3 | 1900 ± 0.95 | 1808 ± 0.42 |
| 6 | 401 ± 0.06 | 568 ± 0.09 | 6 | 2484 ± 0.52 | 2508 ± 0.85 | ||
| 9 | 438 ± 0.08 | 715 ± 0.08 | 9 | 3060 ± 0.15 | 2685 ± 0.45 | ||
| 60 | 3 | 310 ± 0.01 | 609 ± 0.45 | 60 | 3 | 2732 ± 0.02 | 2560 ± 0.66 |
| 6 | 378 ± 0.02 | 599 ± 0.63 | 6 | 2682 ± 0.50 | 2483 ± 0.64 | ||
| 9 | 465 ± 0.02 | 634 ± 0.22 | 9 | 3023 ± 0.51 | 2752 ± 0.44 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Didžiulytė, E.; Šlinkšienė, R. Use of Amino Acids and Organic Waste Extracts to Improve the Quality of Liquid Nitrogen–Calcium–Magnesium Fertilizers. Sustainability 2025, 17, 7081. https://doi.org/10.3390/su17157081
Didžiulytė E, Šlinkšienė R. Use of Amino Acids and Organic Waste Extracts to Improve the Quality of Liquid Nitrogen–Calcium–Magnesium Fertilizers. Sustainability. 2025; 17(15):7081. https://doi.org/10.3390/su17157081
Chicago/Turabian StyleDidžiulytė, Eglė, and Rasa Šlinkšienė. 2025. "Use of Amino Acids and Organic Waste Extracts to Improve the Quality of Liquid Nitrogen–Calcium–Magnesium Fertilizers" Sustainability 17, no. 15: 7081. https://doi.org/10.3390/su17157081
APA StyleDidžiulytė, E., & Šlinkšienė, R. (2025). Use of Amino Acids and Organic Waste Extracts to Improve the Quality of Liquid Nitrogen–Calcium–Magnesium Fertilizers. Sustainability, 17(15), 7081. https://doi.org/10.3390/su17157081






