Abstract
The high pH and heavy metal leaching of argon oxygen decarburization (AOD) slag limit its application in agriculture. Slag carbonation can aid in decreasing slag alkalinity and inhibit heavy metal release; the environmental safety of utilizing carbonated AOD slag (CAS) as a fertilizer remains a topic of significant debate, however. In this work, pakchoi (Brassica chinensis L.) was planted in CAS-fertilized soil to investigate the accumulation and migration behavior of heavy metals in the soil–plant system and perform an associated risk assessment. Our results demonstrated that CAS addition increases Ca, Si, and Cr concentrations but decreases Mg and Fe concentrations in soil leachates. Low rates (0.25–1%) of CAS fertilization facilitate the growth of pakchoi, resulting in the absence of soil contamination and posing no threat to human health. At the optimal slag addition rate of 0.25%, the pakchoi leaf biomass, stem biomass, leaf area, and seedling height increased by 34.2%, 17.2%, 26.3%, and 8.7%, respectively. The accumulation of heavy metals results in diverging characteristics in pakchoi. Cr primarily accumulates in the roots; in comparison, Pb, Cd, Ni, and Hg preferentially accumulate in the leaves. The migration rate of the investigated heavy metals from the soil to pakchoi follows the order of Cr > Cd > Hg > Ni > Pb; in comparison, that from the roots to the leaves follows the order Cd > Ni > Hg > Cr > Pb. Appropriate utilization of CAS as a mineral fertilizer can aid in improving pakchoi yield, achieving sustainable economic benefits, and preventing environmental pollution.
1. Introduction
Sustainable development has become a focal point for both governments and society as a whole. The rapid consumption of steel products has resulted in the generation of substantial amounts of solid waste in the steel industry. Stainless steel slag (SSS) is one of the main forms of solid waste produced during the stainless steel production process. On a statistical basis, the annual discharge of SSS has exceeded 10 million tons in China [1]. SSS comprises CaO, SiO2, and MgO, and similar to the utilization of other forms of steel slag, it is suitable for use in the production of roadbed materials and cement [2,3,4]. Despite its advantages, corresponding recycling rates remain low. Large quantities of SSS are deposited in slag repositories, wasting a considerable amount of land resources and resulting in significant issues related to ecological pollution [5]. As part of efforts to prevent environmental pollution, China levied a tax of 25 RMB/ton on metallurgical slag in 2018. In 2020, the country amended the law to increase penalties for the discharge of metallurgical slag [6]. There is clear evidence that SSS treatment is becoming an economic burden for the stainless steel industry.
The utilization of industrial solid waste as a mineral fertilizer is a common practice in agriculture [7,8]. In one study, steel mill slag was reported to improve the fruit quality of melon (Cucumis melo L.) [9]. Si-Fe slag has been proven to improve rice productivity [10]. In parallel with these results, Mg slag could be utilized as a mineral fertilizer to facilitate maize growth [11]. Metallurgical slag is characterized by the presence of heavy metals, such as Cr, V, Ni, Mo, etc. [12,13]. The inappropriate application of metallurgical slag as a mineral fertilizer can result in heavy metal accumulation in soil. The accumulated heavy metals can enter water bodies, making these elements bioavailable to aquatic organisms. The toxicity of heavy metals is amplified from lower trophic levels to higher orders through the food chain [14]. In addition, heavy metals can also migrate into plants through their roots, ultimately posing health risks to humans [15,16,17,18]. Han et al. [19] demonstrated in their study that vegetables planted close to electronic waste processing sites exhibit high heavy metal pollution index values. In another study, Hossain et al. [20] demonstrated that the consumption of plants contaminated with heavy metals increases the likelihood of cancer development over the long term.
Based on the two-step process of stainless steel smelting, SSS comprises argon oxygen decarburization (AOD) slag and electronic arc furnace (EAF) slag. As one of the common forms of SSS, AOD slag contains nutrient elements of Ca, Si, Fe, etc., and it has been proven to be effective in improving pakchoi (Brassica chinensis L.) yield [21]. AOD slag is a form of alkaline solid waste. AOD slag contains less than 2% CR [12]. Its large-scale use in agriculture is limited due to its high pH and toxic Cr content. The process of slag carbonation has been reported to decrease steel slag leachate pH from 12.6 to 9.4 and also inhibit the release of Cr from AOD slag [22,23]. Using carbonated AOD slag (CAS) as a mineral fertilizer may result in greater fertilizer efficiency and pose a reduced risk to the environment compared to the original form of AOD slag (OAS). Nevertheless, the potential risk of heavy metal accumulation in the soil–plant system due to the use of CAS as a mineral fertilizer should be investigated in order to prevent water and soil pollution and improve soil fertility.
In this study, peat soil and pakchoi were selected to explore the influence of CAS fertilization on heavy metal accumulation in soil and pakchoi and the associated environmental risk. Through batch leaching tests, the leaching behavior of nutrient elements (e.g., Ca, Si, Mg, and Fe) and heavy metals (e.g., Cr, Ni, Pb, Hg, and Cd) from CAS-fertilized soil was investigated. Pot experiments were performed to assess the fertilizer efficiency of CAS. The accumulation and migration behavior of heavy metals in the soil–plant system were explored by examining the heavy metal content of pakchoi and the soil after planting and calculating the biological transfer coefficient (BTC) and biological accumulation coefficient (BAC). The health risk posed by CAS fertilization was assessed using target hazard quotient (THQ) and estimated daily intake (EDI).
2. Materials and Methods
2.1. OAS and CAS
OAS, a by-product produced in the stainless steel refining process, was obtained from the Qingshan steel industry, China. The main chemical components of the obtained OAS were CaO (62.012 wt.%), SiO2 (26.315 wt.%), MgO (4.94 wt.%), Al2O3 (1.642 wt.%), and Fe2O3 (0.272 wt.%). The primary heavy metal present in the OAS was Cr2O3 with a weight content of 0.358 wt.%. Following drying at 105 °C for 6 h and passing through a 200-mesh sieve (74 μm), the remaining OAS was used for the carbonation test.
The carbonation test was performed at L/S = 0.2 using a 500 mL stainless steel autoclave reactor. First, 200 g of OAS with a particle size < 74 μm and 40 mL of deionized water were added to the reactor to form the slurry. The obtained slurry was fully stirred until it became porous and fluffy. Next, high-purity CO2 was injected into the sealed chamber, and carbonation treatment was performed at room temperature (25 °C), with a 100% CO2 atmosphere and 5 bar pressure for 5, 10, 15, and 20 min. Following the carbonation test, the sampled CAS was subjected to thermogravimetric–derivative thermogravimetric analysis (TG-DTA). TG-DTA measurements were conducted using a TGA-HTG3 TG-DTA analyzer (produced by Beijing Hengjiu Experimental Equipment Co., Ltd, Beijing, China) operated with a N2 atmosphere, at a controlled heating rate of 20 °C min−1, over the temperature range of 20 to 1000 °C. The carbonation degree of the OAS was evaluated using the weight gain method [24,25]. Based on the low pH and Cr leaching concentration [24], the CAS with a carbonation degree of 25.64% (carbonated for 20 min) was employed for batch leaching tests, pakchoi planting, and mineralogical analysis.
2.2. Soil
Klasmann peat soil 876# was purchased from a soil company in Hebei, China. The detailed methods employed for soil analysis can be found in our previous study [21]. The air-dried soil with particle sizes < 2 mm was mixed with the CAS (<74 μm) at different rates (including 0.25%, 0.5%, 1%, 2%, 4%, and 8%) in w/w to prepare the slag–soil mixtures.
2.3. Batch Leaching Tests
Batch leaching tests were performed in triplicate to investigate the water-soluble behavior of the slag–soil mixtures. First, a 90 g solid sample was added to a 1 L glass bottle, and 900 mL deionized water was added to produce the L/S = 10. Subsequently, the bottle was horizontally shaken at 200 rpm and 25 °C for 24 h using a THZ-82A gas bath thermostatic vibrator (produced by Changzhou Guoyu Instrument Manufacturing Co., Ltd, Changzhou, China) [26]. After being shaken, the supernatant was passed through a 0.45 μm membrane filter (Changde BKMAN Biotechnology Co., Ltd., Changde, China).
2.4. Pot Experiments
Pakchoi seeds were soaked for 30 min with deionized water before sowing. Plastic pots (with a diameter of 19.0 cm and a height of 17.3 cm) filled with the slag–soil mixture (1.0 kg pot−1) were used for pakchoi seed sowing. Each pot contained 50 seeds and was placed in a growth chamber under a light period of 16 h at 25 °C and a dark period of 8 h at 20 °C. The water holding capacity of the pakchoi seedlings was maintained through regular deionized water irrigation. The pakchoi seedlings were selected, leaving 20 seedlings in the respective pot at the two-leaf stage. The soil and pakchoi seedlings were sampled after their growth for a period of 28 days. The pot experiments were conducted based on a completely randomized design. The pot experiment for each slag addition was performed in triplicate to ensure reliability.
2.5. Characterization
2.5.1. Mineralogical Characterization
The mineralogical component of the CAS was obtained through powder X-ray diffraction (XRD) with the use of a Brucker D8 Advance X-ray diffractometer (produced by Bruker Corporation, Bilerika, MA, United States of America). Using an EQ UINOX55 spectrometer (produced by Bruker Corporation, Bilerika, MA, United States of America) in the range of 400 cm−1 to 4000 cm−1, Fourier-transform infrared (FTIR) spectroscopy measurement was performed to further detect the mineralogical component.
2.5.2. Leachate Characterization
The water-soluble element concentrations of the original soil and soil–slag mixtures in the filtered leachate were examined using Agilent ICPMS7800 inductively coupled plasma mass spectrometry (ICP-MS, produced by Agilent Technologies, Inc, Santa Clara, United States of America). The pH values of the original soil and soil–slag mixture leachate were determined using a PHS-3C pH meter (produced by Shanghai Yidian Scientific Instrument Co., Ltd, Shanghai, China). All of the solution measurements were repeated in triplicate.
2.5.3. Characterization of Pakchoi and Soil Following Growth for 28 Days
The leaf area, biomass, and seedling height were measured using the fresh sample, expressed as fresh weight (FW). The chlorophyll content of the pakchoi leaves was determined using a UV-1800 ultraviolet–visible (UV-VIS, produced by Shimadzu Corporation, Lzumo, Japan) spectrophotometer. Approximately 0.2 g of the fresh mass leaf was crushed in a mortar with 2 mL of 80% acetone to extract the chlorophyll. The obtained extract was placed in a 10 mL test tube. Next, the utilized mortar was rinsed using 2 mL of 80% acetone and the content thereof was placed in a test tube. Subsequently, 80% acetone was added to the test tube to produce a final volume of 10 mL. After centrifugation at 10,000 rpm for 5 min, the solution was detected using the UV-VIS spectrophotometer at absorbances of 663 nm and 645 nm, respectively. Chlorophyll content measurement was repeated in triplicate. The chlorophyll a, chlorophyll b, and total chlorophyll contents were determined using the following equations:
For malondialdehyde (MDA) measurements, 1 g of the fresh mass leaf was ground using trichloroacetic acid (TCA) and quartz sand to form a homogenate. This homogenate was centrifuged at 4000 rpm for 10 min. The supernatant was reacted with 0.6% thiobarbituric acid (TBA) in a water bath at 90 °C for 15 min and then cooled at room temperature. This solution was centrifuged at 4000 rpm for 15 min. Thereafter, the absorbance of the supernatant was measured at 532 nm and 600 nm on the UV-VIS spectrophotometer. The MDA content was calculated by subtracting the A600 value from the A532 value and calibrated using the molar extinction coefficient of 155 mM−1 cm−1.
After the evaluation of the above parameters, the harvested seedlings were oven-dried at 60 °C until a constant weight was achieved. The water content of the pakchoi amounted to roughly 91.7%. The dried root, stem, and leaf were digested with HNO3 using microwave digestion equipment (MWD-630, produced by Shanghai Metash Instruments Co., Ltd., Shanghai, China). The obtained solutions of the root, stem, and leaf were filtrated and diluted. Thereafter, the heavy metal (e.g., Pb, Hg, Cd, Ni, and Cr) contents of the pakchoi root, stem, and leaf following growth for 28 days were examined with ICP-MS equipment using the diluted solution, expressed as dry weight (DW). The soil following growth for 28 days was sampled, mixed, and air-dried. The total Pb, Hg, Cd, Ni, and Cr contents in the dried soil were measured using the same methods of USEPA 3050B as for the original soil.
2.6. Data Processing
To study the migration mechanism of heavy metals (e.g., Pb, Hg, Cd, Ni, and Cr) from the soil to pakchoi, the biological transfer coefficient (BTC) and the biological accumulation coefficient (BAC) were determined using Equations (4) and (5) [27,28], respectively:
where Croot, Cleaf, and Cstem denote the mean concentrations of Pb, Hg, Cd, Ni, and Cr in the root, leaf, and stem of the pakchoi seedlings, respectively, and Csoil denotes the mean concentration of Pb, Hg, Cd, Ni, and Cr in the soil.
The target hazard quotient (THQ) and the estimated daily intake (EDI, in mg kg−1 d−1) were determined to evaluate the non-carcinogenic risk relative to the respective individual risk from the consumption of CAS-fertilized pakchoi seedlings using Equations (6) and (7) [11,29,30], respectively:
where AT is the averaging time in terms of non-carcinogens (365 d yr−1 × exposure yrs, assumed as 70 yrs); Wfood is the ingestion rate of pakchoi seedlings (0.2762 kg d−1); ED is the exposure duration (70 yrs); EFr represents the exposure frequency (365 d yr−1); BW denotes the average body weight of adults in China (60 kg); and Cmetal is the mean concentration of Pb, Hg, Cd, Ni, and Cr in pakchoi seedlings’ above-ground parts (stem and leaf), in mg kg−1. The oral reference dose (RfD) was 0.0035, 0.0005, 0.0005, 0.02, and 1.5 mg kg−1 d−1 in terms of Pb, Hg, Cd, Ni, and Cr, respectively [11,27,31].
2.7. Statistical Analysis
The obtained experimental data was analyzed with the use of SPSS version 25.0. The normality of the obtained data was evaluated using the Shapiro–Wilk test. Homogeneity of variance was assessed using Levene’s test. One-way analysis of variance (ANOVA) was performed; subsequently, Duncan’s test was carried out at a p < 0.05 significance level to test for differences in the means of the different treatments in achieving statistical significance.
3. Results and Discussion
3.1. Mineralogical Components of the CAS
Figure 1a presents the XRD patterns of the CAS. The major minerals in CAS include dicalcium silicate (Ca2SiO4, ICDD PDF-#49-1672), merwinite (Ca3Mg(SiO4)2, ICDD PDF-#89-2432), augite (Ca(Mg0.85Al0.15) (SiO1.70Al0.30)O6, ICDD PDF-#78-1391), calcite (CaCO3, ICDD PDF-#05-0586), fluorite (CaF2, ICDD PDF-#75-0363), and periclase (MgO, ICDD PDF-#71-1176). Due to the low heavy metal content of AOD slag, the heavy metal-bearing phase was not determined using XRD.
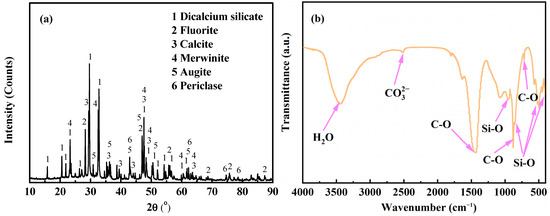
Figure 1.
XRD patterns and FTIR spectra of the CAS: (a) XRD patterns and (b) FTIR spectra.
Our FTIR results (Figure 1b) demonstrated that the broad peak identified at 3450 cm−1 belongs to the O-H bond in absorbed H2O molecules [32]. The peaks ascribed to the Si-O bond in silicates were examined in the region of 500–1200 cm−1 [12,33], which are provided by dicalcium silicate, merwinite, and augite phases, corresponding to the results of the XRD analysis. The small peak identified at 2510 cm−1 represents carbonate in calcite [34]. The calcite also displays a C-O asymmetric stretching vibration (v3) band peak at 1390 cm−1, a C-O out-of-plane bending vibration (v2) band peak at 875 cm−1, and a C-O in-plane bending vibration (v4) band peak at 713 cm−1 [12,34]. Some other peaks at around 1080, 1640, and 1800 cm−1 belong to the Si-O-Si bands of the C-S-H phases [12,33]. Due to the poor crystallinity of the C-S-H phases, this phase was not identified using XRD analysis.
3.2. Effects of CAS Fertilization on the Heavy Metal Content of the Original Soil
The original soil exhibited mean values of organic matter (OM) of 601.17 g kg−1, total N of 7.06 g kg−1, total P of 0.72 g kg−1, total K of 1.01 g kg−1, and a pH of 6.13. The effects of CAS addition on the total Cr, Ni, Pb, Cd, and Hg contents in the original soil are illustrated in Table 1. Increasing the CAS addition from 0% to 8% significantly enhanced the total Cr content from 3.518 mg kg−1 to 231.721 mg kg−1 and the total Ni content from 5.212 mg kg−1 to 12.060 mg kg−1 in the original soil. These increases are attributed to the high Cr and Ni contents in the CAS. As almost no Pb, Cd, and Hg were present in the CAS, the addition of CAS to the soil had little effect on the total content of Pb, Cd, and Hg in the original soil.

Table 1.
Effects of CAS addition on total Cr, Ni, Pb, Cd, and Hg contents in the original soil.
3.3. Effects of CAS Fertilization on Elemental Release from the Soil
The leachate pH values and water-soluble element concentrations of the original soil and the soil–slag mixture were measured and are illustrated in Figure 2. As presented in Figure 2a, the addition of CAS to the soil increased the soil leachate pH. The pH increased from 5.81 to 8.57 as the slag addition rate was increased from 0% to 8%. This increase is attributed to the dissolution of the alkaline phases, such as dicalcium silicate, merwinite, and periclase, in the CAS, releasing OH− ions into the soil [35]. In addition, it is recognized that the hydrolysis of carbonates also contributes to the enhancement of pH [23].
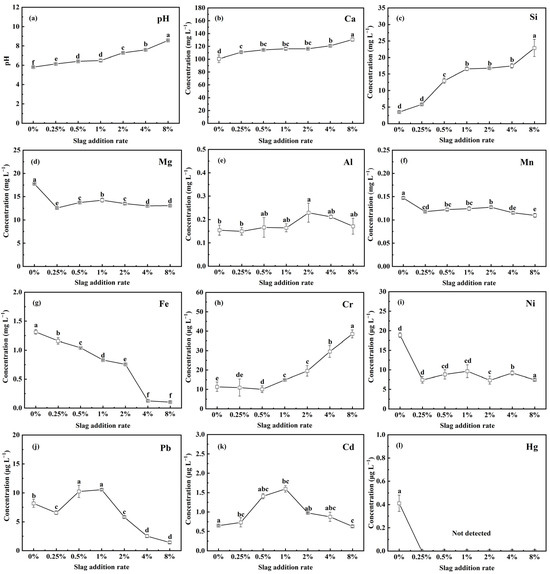
Figure 2.
Evolution of pH and elemental release concentration of CAS-fertilized soil as a function of slag addition rate: (a) pH, (b) Ca, (c) Si, (d) Mg, (e) Al, (f) Mn, (g) Fe, (h) Cr, (i) Ni, (j) Pb, (k) Cd, and (l) Hg. Lowercase letters indicate statistical significance at p < 0.05 in accordance with Duncan’s test, and the error bars represent the standard deviation (SD).
Regarding nutrient elements, as illustrated in Figure 2b–g, introducing CAS into the soil leads to a continuous increase in Ca leached concentration from 100.55 mg L−1 to 130.69 mg L−1 and Si leached concentration from 3.51 mg L−1 to 22.86 mg L−1, respectively. The Si leached concentration of the 8% CAS-fertilized soil was 6.5 times higher than that of the peat soil. When the CAS addition rate was increased from 0% to 8%, the Mg leached concentration declined from 17.75 mg L−1 to 13.08 mg L−1; in comparison, that of Fe decreased from 1.31 mg L−1 to 0.10 mg L−1. The decline in the Fe leached concentration was minor at slag addition rates of 0.25–2%. A significant decrease in Fe concentration was observed at a slag addition rate > 2%. Mg and Fe leached concentrations are correlated with pH. In alkaline solution, Mg and Fe ions will be precipitated as hydroxides [35], thus leading to a decrease in their concentrations.
Regarding heavy metals, as illustrated in Figure 2h–l, introducing CAS into the soil leads to an increase in Cr and Cd leached concentrations but a decrease in Pb, Ni, and Hg leached concentrations in the original soil. The Pb, Hg, Cd, Cr, and Ni leached concentrations of the CAS-fertilized soil were relatively low at slag rates of 0–8%. The maximum leached concentrations of Cr, Ni, Pb, Cd, and Hg were determined to be 38.54 μg L−1, 18.85 μg L−1, 10.55 μg L−1, 1.59 μg L−1, and 0.41 μg L−1, respectively. Excluding Cr, the Ni, Pb, Cd, and Hg contents of the CAS leachate were minimal. The pH of the leachate has a significant influence on major changes in Ni, Pb, Cd, and Hg leached concentration. Although the CAS contains a certain amount of Cr, the low slag addition rates (0.25–0.5%) do not lead to the increased Cr leached concentration of the original soil.
3.4. Effects of CAS Fertilization on Pakchoi Seedling Growth
The effects of CAS fertilization on pakchoi seedling growth are illustrated in Figure 3. Compared with the control, the addition of 0.25%, 0.5%, and 1% CAS to the soil increased the leaf biomass, stem biomass, and leaf area of the pakchoi seedlings (Figure 3a–c). The seedling height increased at slag addition rates of 0.25% to 0.5%; in comparison, it declined when the slag addition rate was ≥1% (Figure 3d). At the optimal slag addition rate of 0.25%, the leaf biomass, stem biomass, leaf area, and seedling height increased by 34.2%, 17.2%, 26.3%, and 8.7%, respectively. With respect to slag addition rates of 0.25–8%, the above four parameters tended to increase first and then decrease with the rising slag rate, suggesting that low rates (≤1%) of CAS fertilization facilitate pakchoi growth; in comparison, high rates, particularly ≥8%, have negative effects. Figure 3e presents images of the pakchoi seedlings following germination for 28 days.
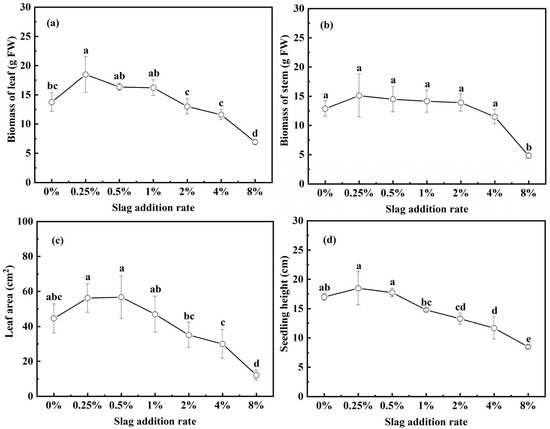
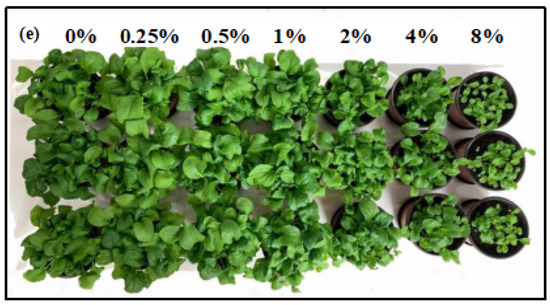
Figure 3.
Evolution of the biomass, leaf area, and seedling height of pakchoi seedlings as a function slag addition rate: (a) biomass of the leaf, (b) biomass of the stem, (c) leaf area, (d) seedling height, and (e) pakchoi seedling images after germinating for 28 days. Lowercase letters indicate statistical significance at p < 0.05 in accordance with Duncan’s test, and the error bars represent the SD.
Chlorophyll content is a vital factor in determining the effects of treatment on photosynthetic ability and net primary production, correlating with plant growth [36]. As depicted in Figure 4a–c, the CAS treatment reduced the chlorophyll contents (both chlorophyll a and chlorophyll b) of the pakchoi seedlings, primarily due to the decreased Fe and Mg leached concentrations, with the decrease in Fe being particularly pronounced [37]. As depicted in Figure 4d, the reduction in chlorophyll content was accompanied by an improved chl a–chl b ratio. This finding indicates that the degradation rate of chlorophyll a is lower than that of chlorophyll b.
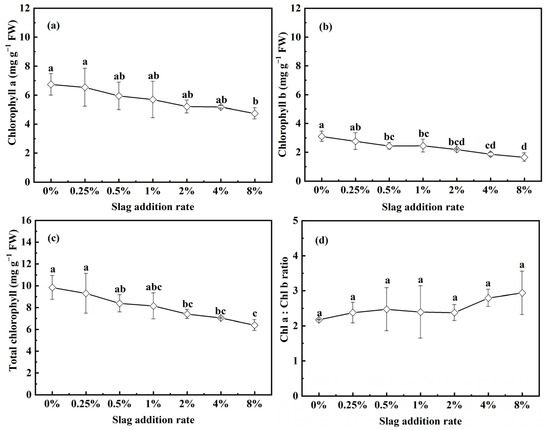
Figure 4.
Effects of CAS addition rate on the chlorophyll content of pakchoi seedlings: (a) chlorophyll a content, (b) chlorophyll b content, (c) total chlorophyll content (chlorophyll a + chlorophyll b), and (d) chlorophyll a–chlorophyll b ratio. Lowercase letters indicate statistical significance at p < 0.05 in accordance with Duncan’s test, and the error bars represent the SD.
Introducing slag into the soil will induce alterations in soil properties, in turn impacting pakchoi growth. At low slag addition rates (0.25–1%), the soil pH ranged from 6.14 to 6.50, which is favorable to the growth of pakchoi. Moreover, the Mg and Fe leached concentrations did not decline to values low enough to have an effect on the synthesis of chlorophyll in the pakchoi seedlings. The addition of CAS significantly enhances the Si content of the soil, even at addition rates of 0.25–1%. Si has been reported to increase the photosynthesis rate, biomass, and nutrient accumulation of plants via enhanced verticality, improving disease and pest resistance [7,38]. The increased Si leached concentration of soil contributes to the improvement of pakchoi seedling growth. At high slag addition rates (8%), the soil pH and the Ca leached concentration increase to high levels. P in the soil will therefore be precipitated as calcium phosphate, resulting in extremely low P availability [39]. The authors of a number of studies have suggested that P deficiency leads to a reduction in plant growth [40,41]. In addition, introducing significant amounts of slag into the soil decreases the Fe leached concentration to a very low level while simultaneously increasing the Cr leached concentration to a relatively high level. Deficiency in Fe and the accumulation of Cr in pakchoi seedlings hinder the synthesis of chlorophyll, inhibiting pakchoi growth [40,42].
3.5. Heavy Metal Accumulation and Migration in the Soil–Plant System
The potential heavy metal accumulation risks induced by the CAS fertilization of soil and pakchoi were evaluated by measuring the Cr, Ni, Pb, Cd, and Hg contents of the soil after planting and harvesting of the plant. As illustrated in Table 2, increasing the slag addition rate significantly enhanced the Cr and Ni contents of the soil. The Cr and Ni contents of the utilized soil were 155.9 times and 6.5 times higher than those of the original soil, respectively, at a slag addition rate of 8%. The Pb, Cd, and Hg contents of the soil did not significantly increase following the application of CAS to the soil. It is noteworthy that the Pb and Cd contents of the soil after the growth of pakchoi were slightly higher than those of the original soil. This finding may be attributed to the inhomogeneity of the soil. The Cr content of the soil with a slag addition rate of 8% was higher than the limit specified by the Chinese standard [43]. High rates (≥8%) of CAS fertilization will result in soil contamination.

Table 2.
Total Cr, Ni, Pb, Cd, and Hg contents in soil after the growth of pakchoi.
The results presented in Figure 5a–e demonstrate that increasing the CAS fertilization rate enhances the Cr and Cd accumulation rates of the pakchoi seedlings; in comparison, this treatment has little effect on the accumulation rate of Ni, Pb, and Hg. The accumulation rate of Cr follows the order of root > stem > leaf; that of Ni, Cd, and Hg follows the order of leaf > stem > root; and that of Pb follows the order of leaf > root > stem in the pakchoi seedlings. These findings indicate that the upward migration rate of Cr from the root to the leaf is slow in the pakchoi seedlings. Following the absorption of heavy metals from the soil into the pakchoi, the absorbed Cr preferentially accumulated in the roots; in comparison, Ni, Cd, Pb, and Hg preferentially accumulated in the above-ground parts. This pattern may be attributed to the fact that the upward migration rate of Ni, Pb, Cd, and Hg in the pakchoi seedlings is faster than that of Cr. It is noteworthy that Cd accumulation in the roots was higher than in the stems at slag addition rates of 4% and 8%. This finding may be attributed to the increased pH, impacting the migration rate of Cd.
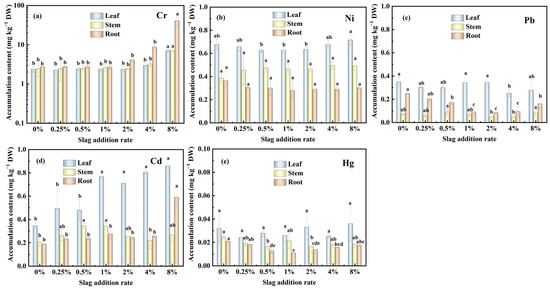
Figure 5.
Effects of CAS addition rate on heavy metal accumulation in the leaf, stem, and root of pakchoi seedlings: (a) Cr, (b) Ni, (c) Pb, (d) Cd, and (e) Hg. Lowercase letters indicate statistical significance at p < 0.05 in accordance with Duncan’s test, and the error bars represent the SD.
Within the slag addition rate range of 0–8%, the maximum accumulation rate of Cr, Ni, Pb, Cd, and Hg in the above-ground parts of the fresh pakchoi are 1.17 mg kg−1, 0.10 mg kg−1, 0.03 mg kg−1, 0.09 mg kg−1, and 0.004 mg kg−1, respectively. Based on the Chinese standard [44], the heavy metal limits in food are as follows: Cr ≤ 0.5 mg kg−1, Ni ≤ 1.0 mg kg−1, Pb ≤ 0.3 mg kg−1, Cd ≤ 0.2 mg kg−1, and Hg ≤ 0.01 mg kg−1. The Ni, Pb, Cd, and Hg accumulation rate of the harvested pakchoi meets the limit specified by the Chinese standard [44]. However, the Cr accumulation rate of the pakchoi grown in 4% and 8% CAS-fertilized soil exceeded the limit. This finding indicates that low rates (0.25–2%) of CAS fertilization for pakchoi growth are safe; in comparison, high rates (4% and 8%) may pose health risks.
To investigate the migration mechanism of heavy metals in the soil–plant system, the BAC and BTC values were determined. As Cr in CAS is mainly present in the insoluble spinel phase [45], using the heavy metal content of the CAS-fertilized soil to calculate the above two parameters may result in low values. Accordingly, the BAC and BTC values were determined using the original soil (0%). As shown in Figure 6, the BAC values followed the order of Cr (3.38) > Cd (2.89) > Hg (2.59) > Ni (0.71) > Pb (0.09). This finding indicates that the migration rate of the above five heavy metals from the soil to the pakchoi follows the order of Cr > Cd > Hg > Ni > Pb. Islam et al. [27] investigated the migration behavior of heavy metals in the soil–plant system using four different vegetables, namely, brinjal (Solanum melongena), bottle gourd (Lagenaria siceraria), pumpkin (Cucurbita maxima), and tomato (Solanum lycopersicum). Their study results indicated that the migration rate of the investigated heavy metals from the soil to the plant followed the order of Cd > Ni > Pb > Cr. This discrepancy may be attributed to the fact that the Cr content of the CAS-fertilized soil was high, which improved the migration rate of Cr from the soil to the pakchoi. The BTC value follows the order of Cd (2.94) > Ni (2.92) > Hg (2.71) > Cr (1.75) > Pb (1.69). The upward migration rate of Cd from the pakchoi root to the leaf was the fastest; in comparison, that of Pb was the slowest.
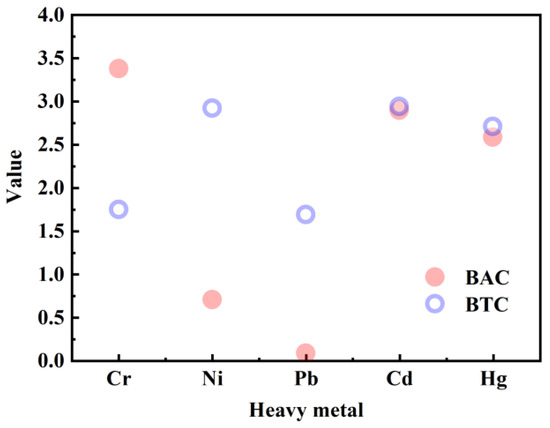
Figure 6.
BAC and BTC values of heavy metals in the pakchoi seedlings.
Heavy metal accumulation in plants results in multiple toxic effects by inducing reactive oxygen species (ROS) production, including H2O2, O2−, O3, and 1O2 [42]. MDA, a decomposition product, content was examined to investigate the effect of CAS fertilization on the extent of ROS damage to the pakchoi seedlings. As illustrated in Figure 7, the MDA content of the pakchoi seedlings did not increase compared with the control with low rates (0.25–1%) of CAS fertilization. At slag addition rates of 2%, 4%, and 8%, however, the MDA content increased by 6.1%, 10.7%, and 21.4%, respectively. As demonstrated in the results, the low rates (0.25–1%) of CAS fertilization did not increase the extent of ROS damage to the pakchoi seedlings. Notably, high rates (≥2%) of CAS fertilization induced the upregulation of ROS damage level. Moreover, the change trend of the MDA content was similar to the Cr accumulation rate. Thus, Cr accumulation in pakchoi seedlings may primarily lead to enhanced ROS damage through CAS fertilization. To avoid potential heavy metal damage to the soil–plant system through the use of CAS as a fertilizer, specific mitigation strategies can be implemented, such as the application of biochar, appropriate irrigation and water management, and planting crop cultivars with low heavy metal accumulation [46].
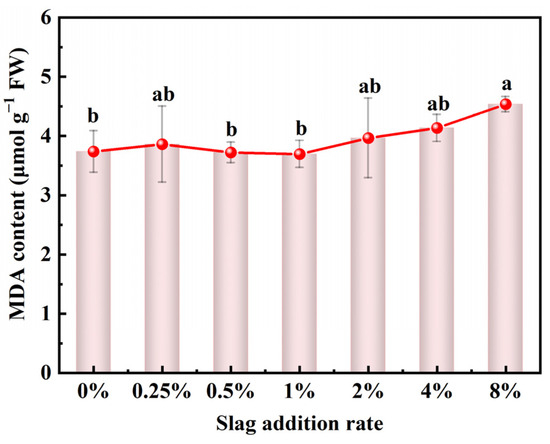
Figure 7.
Effects of CAS addition rate on the MDA content of pakchoi seedlings. Lowercase letters indicate statistical significance at p < 0.05 in accordance with Duncan’s test, and the error bars represent the SD.
3.6. Health Risk Assessment
The results presented in Table 3 illustrate the calculated EDI and THQ values. The EDI value of Cr is the highest among the five heavy metals. With the addition of CAS to the soil, no significant increase in the EDI values of Ni, Pb, Cd, and Hg was observed. Compared with the control, low rates (0.25–2%) of CAS application do not impact the EDI value of Cr. The increase in the EDI value of Cr was observed at slag addition rates of 4% and 8%. This finding can be attributed to the fact that the application of CAS in the soil increases the Cr accumulation rate while having little effect on the Ni, Pb, Cd, and Hg accumulation rate of pakchoi. All of the EDI values of the measured heavy metals are lower than 1, suggesting that the consumption of this pakchoi is safe. The THQ values of the five heavy metals were calculated to evaluate the health risks they pose. The respective THQ values of Cr, Ni, Pb, Cd, and Hg were lower than 1, also indicating that consumption of this pakchoi presents a low risk. Hossain et al. [20] also assessed the health risk of heavy metal contents in Piper betel using the THQ value. The THQ values of the individual metals As, Pb, Cu, Zn, Cr, and Cd were all less than 1, suggesting that consuming only one heavy metal from betel poses a low risk.

Table 3.
EDI and THQ values of heavy metals in the pakchoi seedlings.
It is noteworthy that the minerals in CAS can be more easily dissolved under acid rain conditions, resulting in an increase in heavy metal leaching concentration. In addition, heavy metals with a low valence state may be oxidized to a high valance state in the soil–plant system, enhancing their toxicity. The long-term stability and heavy metal oxidation states should therefore be further investigated when using CAS as a fertilizer.
4. Conclusions
Introducing CAS into peat soil increases Ca and Si leached concentrations while simultaneously decreasing Mg and Fe leached concentrations in the soil. No significant increase in Ni, Pb, Cd, or Hg leached concentration was recorded in our experiment; in comparison, a marked increase in the Cr leached concentration of the soil was noted. Low rates (0.25–1%) of CAS fertilization improved the growth of pakchoi seedlings; in contrast, high rates (≥2%) resulted in the opposite effect. No Ni, Pb, Cd, and Hg pollution in the soil and pakchoi seedlings was observed in this study. The application of CAS during pakchoi planting could induce Cr contamination of the soil at a slag addition rate ≥ 8%. The accumulation rate of Cr follows the order of root > stem > leaf, that of Ni, Cd, and Hg follows the order of leaf > stem > root, and that of Pb follows the order of leaf > root > stem in the pakchoi seedlings. The migration rate of the investigated heavy metals from the soil to the pakchoi follows the order of Cr > Cd > Hg > Ni > Pb; in comparison, that from the root to the leaf follows the order of Cd > Ni > Hg > Cr > Pb. The EDI and THQ values of heavy metals indicate that the consumption of pakchoi grown on peat soil with a CAS content of 0~8% poses no risk. From the above results, we can conclude that applying 0.25–1% CAS to soil as a mineral fertilizer is beneficial in increasing pakchoi seedling yield and does not pose a risk to health or induce contamination of the soil.
Author Contributions
Methodology, Y.Z.; investigation, L.Z., Z.Y., B.L. and S.C.; resources, L.Z.; writing—original draft preparation, S.C.; writing—review and editing, B.L.; funding acquisition, B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Hebei Natural Science Foundation (No. E2024209158, No. E2022209093), the Central Guidance Local Science and Technology Development Fund Project of Hebei Province (236Z1102G), the Tangshan Municipal Project of Science and Technology (No. 22130228H), and the Key Program of North China University of Science and Technology (No. ZD-ST-202312).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author upon request. The data are not publicly available due to the data also forming part of an ongoing study.
Acknowledgments
The author would like to thank North China University of Science and Technology for providing the materials and testing equipment for the experiments.
Conflicts of Interest
Author Liangjin Zhang was employed by the North China University of Science and Technology, Central Research Institute of Building and Construction Co. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| SSS | Stainless steel slag |
| AOD | Argon oxygen decarburization |
| EAF | Electronic arc furnace |
| CAS | Carbonated AOD slag |
| OAS | Original AOD slag |
| BTC | Biological transfer coefficient |
| BAC | Biological accumulation coefficient |
| THQ | Target hazard quotient |
| EDI | Estimated daily intake |
| ROS | Reactive oxygen species |
References
- Wang, Z.; Sohn, I. Selective elemental concentration during the solidification of stainless steel slags for increased Cr recovery with MnO addition. J. Am. Ceram. Soc. 2020, 103, 6012–6024. [Google Scholar] [CrossRef]
- Rosales, J.; Cabrera, M.; Agrela, F. Effect of stainless steel slag waste as a replacement for cement in mortars. Mechanical and statistical study. Constr. Build. Mater. 2017, 142, 444–458. [Google Scholar] [CrossRef]
- Sas, Z.; Sha, W.; Soutsos, M.; Doherty, R.; Bondar, D.; Gijbels, K.; Schroeyers, W. Radiological characterisation of alkali-activated construction materials containing red mud, fly ash and ground granulated blast-furnace slag. Sci. Total Environ. 2019, 659, 1496–4504. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ling, T.C.; Pan, S.Y. Environmental benefit assessment of steel slag utilization and carbonation: A systematic review. Sci. Total Environ. 2022, 806, 150280. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Zhang, X.; Ding, Z.; Zhang, M.; Guo, M. A clean approach for detoxification of industrial chromium-bearing stainless steel slag: Selective crystallization control and binary basicity effect. J. Hazard. Mater. 2023, 446, 130746. [Google Scholar] [CrossRef]
- Deng, L.; Yao, B.; Lu, W.; Zhang, M.; Li, H.; Chen, H.; Zhao, M.; Du, Y.; Zhang, M.; Ma, Y.; et al. Effect of SiO2/Al2O3 ratio on the crystallization and heavy metal immobilization of glass ceramics derived from stainless steel slag. J. Non-Cryst. Solids 2022, 593, 121770. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kang, Y.G.; Sohn, K.M.; Kim, P.J.; Galgo, S.J.C. Creating new value of blast furnace slag as soil amendment to mitigate methane emission and improve rice cropping environments. Sci. Total Environ. 2022, 806, 150961. [Google Scholar] [CrossRef]
- Lin, S.; Wang, W.; Sardans, J.; Lan, X.; Fang, Y.; Singh, B.P.; Xu, X.; Wiesmeier, M.; Tariq, A.; Zeng, F.; et al. Effects of slag and biochar amendments on microorganisms and fractions of soil organic carbon during flooding in a paddy field after two years in southeastern China. Sci. Total Environ. 2022, 824, 153783. [Google Scholar] [CrossRef]
- Preston, H.A.F.; Nunes, G.H.D.S.; Preston, W.; Souza, E.B.D.; Mariano, R.D.L.R.; Datnoff, L.E.; Nascimento, C.W.A.D. Slag-based silicon fertilizer improves the resistance to bacterial fruit blotch and fruit quality of melon grown under field conditions. Crop Prot. 2021, 147, 105460. [Google Scholar] [CrossRef]
- Ali, M.A.; Oh, J.H.; Kim, P.J. Evaluation of silicate iron slag amendment on reducing methane emission from flood water rice farming. Agr. Ecosyst. Environ. 2008, 128, 21–26. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Y.L.; Li, H.; Cheng, F. Evaluating heavy metal accumulation and potential risks in soil-plant systems applied with magnesium slag-based fertilizer. Chemosphere 2018, 197, 382–412. [Google Scholar] [CrossRef] [PubMed]
- Baciocchi, R.; Costa, G.; Di Gianfilippo, M.; Polettini, A.; Pomi, R.; Stramazzo, A. Thin-film versus slurry-phase carbonation of steel slag: CO2 uptake and effects on mineralogy. J. Hazard. Mater. 2015, 283, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Hobson, A.J.; Stewart, D.I.; Bray, A.W.; Mortimer, R.J.G.; Mayes, W.M.; Rogerson, M.; Burke, I.T. Mechanism of vanadium leaching during surface weathering of basic oxygen furnace steel slag blocks: A microfocus X-ray absorption spectroscopy and electron microscopy study. Environ. Sci. Technol. 2017, 51, 7823–7830. [Google Scholar] [CrossRef] [PubMed]
- Anandkumar, A.; Li, J.; Prabakaran, K.; Jia, Z.; Leng, Z.; Nagarajan, R.; Du, D. Accumulation of toxic elements in an invasive crayfish species (Procambarus clarkii) and its health risk assessment to humans. J. Food Compos. Anal. 2020, 88, 103449. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, M.; Wang, J.; Zhang, Z.; Duan, C.; Wang, X.; Zhao, S.; Bai, X.; Li, Z.; Li, Z.; et al. A global meta-analysis of heavy metal(loid)s pollution in soils near copper mines: Evaluation of pollution level and probabilistic health risks. Sci. Total Environ. 2022, 835, 155441. [Google Scholar] [CrossRef]
- Su, C.; Wang, J.; Chen, Z.; Meng, J.; Yin, G.; Zhou, Y.; Wang, T. Sources and health risks of heavy metals in soils and vegetables from intensive human intervention areas in South China. Sci. Total Environ. 2023, 857, 159389. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhang, Q.C.; Kang, S.G.; Li, M.Y.; Zhang, M.Y.; Xu, W.M.; Xiang, P.; Ma, L.Q. Heavy metal(loid)s in agricultural soil from main grain production regions of China: Bioaccessibility and health risks to humans. Sci. Total Environ. 2023, 858, 159819. [Google Scholar] [CrossRef]
- Kama, R.; Liu, Y.; Aidara, M.; Kpalari, D.F.; Song, J.; Diatta, S.; Sulemana, H.; Li, H.; Li, Z. Plant-soil feedback combined with straw incorporation under Maize/Soybean intercropping increases heavy metals migration in soil-plant system and soil HMRG abundance under livestock wastewater irrigation. J. Soil Sci. Plant Nut. 2024, 24, 7090–7104. [Google Scholar] [CrossRef]
- Han, Z.; Wang, N.; Zhang, H.; Yang, X. Heavy metal contamination and risk assessment of human exposure near an e-waste processing site. Acta Agr. Scand. B-S.P. 2017, 67, 119–125. [Google Scholar] [CrossRef]
- Hossain, M.M.; Tripty, S.J.; Shishir, M.Z.A.; Wang, S.; Hossain, I.; Geng, A.; Han, S.; Zhu, D. Malondialdehyde and heavy metal contents in Piper betel: Possible risks of heavy metals in human health. J. Food Compos. Anal. 2024, 134, 106540. [Google Scholar] [CrossRef]
- Cai, S.; Ren, Q.; Zeng, Y.; Wang, L.; Zhang, Y.; Liu, B.; Li, J. Toxicity assessment of the utilization of AOD slag as a mineral fertilizer for pakchoi (Brassica chinensis L.) planting. J. Clean. Prod. 2022, 256, 120377. [Google Scholar] [CrossRef]
- Isteri, V.; Ohenoja, K.; Hanein, T.; Kinoshita, H.; Tanskanen, P.; Illikainen, M.; Fabritius, T. Production and properties of ferrite-rich CSAB cement from metallurgical industry residues. Sci. Total Environ. 2020, 712, 136208. [Google Scholar] [CrossRef]
- Huijgen, W.; Witkamp, G.J.; Comans, R. Mineral CO2 sequestration by steel slag carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Zeng, Y.N.; Li, J.G.; Zhang, Y.Z.; Zhao, Q.Z. Carbonation of argon oxygen decarburization stainless steel slag and its effect on chromium leachability. J. Clean. Prod. 2020, 328, 129617. [Google Scholar] [CrossRef]
- Fang, Y.; Chang, J. Rapid hardening beta-C2S mineral and microstructure changes activated by accelerated carbonation curing. J. Therm. Anal. Calorim. 2017, 129, 681–689. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, X.; Hong, W.; Khalid, Z.; Lv, G.; Jiang, X. Leaching behavior and comprehensive toxicity evaluation of heavy metals in MSWI fly ash from grate and fluidized bed incinerators using various leaching methods: A comparative study. Sci. Total Environ. 2024, 914, 169595. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Mamun, M.H.A.; Masunaga, S. Trace metals in soil and vegetables and associated health risk assessment. Environ. Monit. Assess. 2014, 186, 8727–8739. [Google Scholar] [CrossRef]
- Qi, C.; Wu, F.; Deng, Q.; Liu, G.; Mo, C.; Liu, B.; Zhu, J. Distribution and accumulation of antimony in plants in the super-large Sb deposit areas, China. Microchem. J. 2011, 97, 44–51. [Google Scholar] [CrossRef]
- Ji, K.; Kim, J.; Lee, M.J.; Park, S.; Kwon, H.J.; Cheong, H.K.; Jang, J.Y.; Kim, D.S.; Yu, S.; Kim, Y.W.; et al. Assessment of exposure to HMs and health risks among residents near abandoned metal mines in Goseong, Korea. Environ. Pollut. 2013, 178, 322–328. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Y.; Liu, S.; He, J.; Long, F. Concentration and potential health risk of HMs in market vegetable in Chongqing, China. Ecotoxicol. Environ. Saf. 2011, 74, 1664–1669. [Google Scholar] [CrossRef]
- Storelli, M.M. Potential human health risks from metals (Hg, Cd, and Pb) and polychlorinated biphenyls (PCBs) via seafood consumption: Estimation of target hazard quotients (THQs) and toxic equivalents. Food Chem. Toxicol. 2008, 46, 2782–2788. [Google Scholar] [CrossRef]
- Salman, M.; Cizer, O.; Pontikes, Y.; Santos, R.M.; Snellings, R.; Vandewalle, L.; Blanpain, B.; Balen, K.V. Effect of accelerated carbonation on AOD stainless steel slag for its valorisation as a CO2-sequestering construction material. Chem. Eng. J. 2014, 246, 39–52. [Google Scholar] [CrossRef]
- Kriskova, L.; Pontikes, Y.; Cizer, O.; Mertens, G.; Veulemans, W.; Geysen, D.; Jones, P.T.; Vandewalle, L.; Van Balen, K.; Blanpain, B. Effect of mechanical activation on the hydraulic properties of stainless steel slags. Cement Concrete Res. 2012, 42, 778–788. [Google Scholar] [CrossRef]
- Chen, Z.M.; Li, R.; Liu, J. Preparation and properties of carbonated steel slag used in cement cementitious materials. Constr. Build. Mater. 2021, 283, 122667. [Google Scholar] [CrossRef]
- De Windt, L.; Chaurand, P.; Rose, J. Kinetic of steel slag leaching: Batch tests and modeling. Waste Manage. 2011, 31, 225–235. [Google Scholar] [CrossRef]
- Pietrini, F.; Iori, V.; Beone, T.; Mirabile, D.; Zacchini, M. Effects of a ladle furnace slag added to soil on morpho-physiological and biochemical parameters of Amaranthus paniculatus L. plants. J. Hazard. Mater. 2017, 329, 339–347. [Google Scholar] [CrossRef]
- Ghafariyan, M.H.; Malakouti, M.J.; Dadpour, M.R.; Stroeve, P.; Mahmoudi, M. Effects of magnetite nanoparticles on soybean chlorophyll. Environ. Sci. Technol. 2013, 47, 10645–10652. [Google Scholar] [CrossRef]
- Basu, S.; Kumar, G. Exploring the significant contribution of silicon in regulation of cellular redox homeostasis for conferring stress tolerance in plants. Plant Physiol. Bioch. 2021, 166, 393–404. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Zwiazek, J.J. Responses of reclamation plants to high root zone pH: Effects of phosphorus and calcium availability. J. Environ. Qual. 2016, 45, 1652–1662. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants; Elsevier Ltd.: Waltham, MA, USA, 2012. [Google Scholar]
- Lynch, J.; Lauchli, A.; Emanuel, E. Vegetative growth of the common bean in response to phosphorus nutrition. Crop. Sci. 1991, 31, 380–387. [Google Scholar] [CrossRef]
- Ao, M.; Chen, X.; Deng, T.; Sun, S.; Tang, Y.; Morel, J.L.; Qiu, R.; Wang, S. Chromium biogeochemical behaviour in soil-plant systems and remediation strategies: A critical review. J. Hazard. Mater. 2022, 424, 127233. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil environment quality-Risk control standard for soil contamination of agriculture land. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- GB2762-2022; National standard for food safety-Limits of pollutants in food. Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- Kim, E.; Spooren, J.; Broos, K.; Horckmans, L.; Quaghebeur, M.; Vrancken, K.C. Selective recovery of Cr from stainless steel slag by alkaline roasting followed by water leaching. Hydrometallurgy 2015, 158, 139–148. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, Y.; Zhu, Y.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).