Towards a Circular Economy: Unlocking the Potentials of Cigarette Butt Recycling as a Resource for Seashore Paspalum Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Products of CB Recycling Process
2.2. Preparation of Plant Growing Substrates
2.3. Plant Material and Growth Conditions
2.4. Chlorophyll Fluorescence
2.5. Photosynthetic Pigments and Antioxidants
2.6. Biometric Measurements
2.7. Statistical Analysis
3. Results
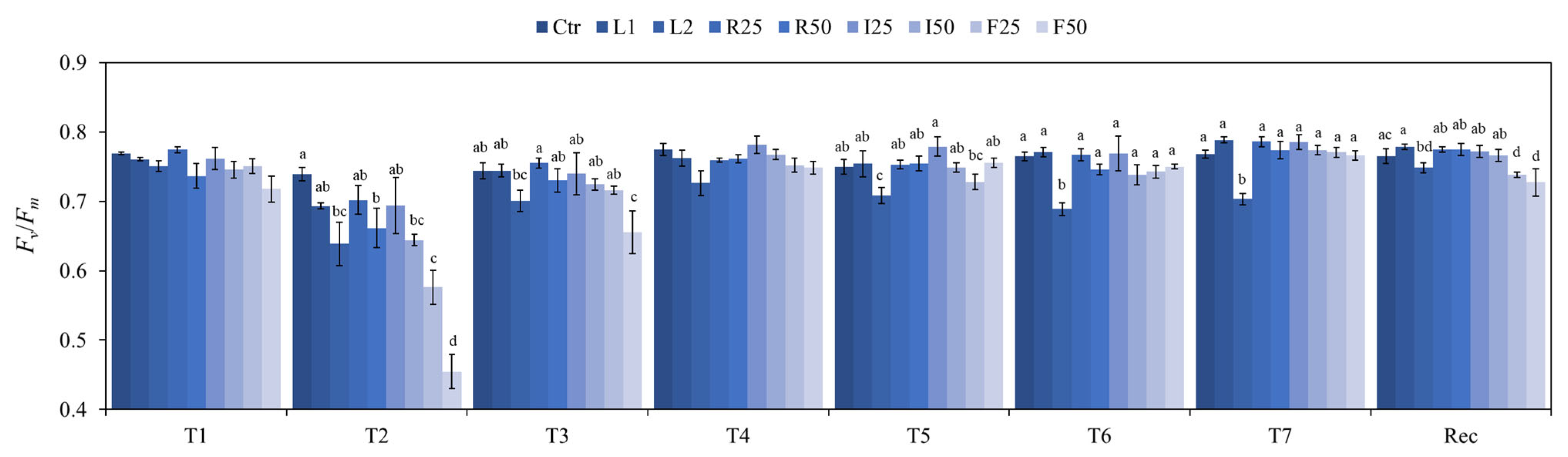
3.1. Chlorophyll Fluorescence
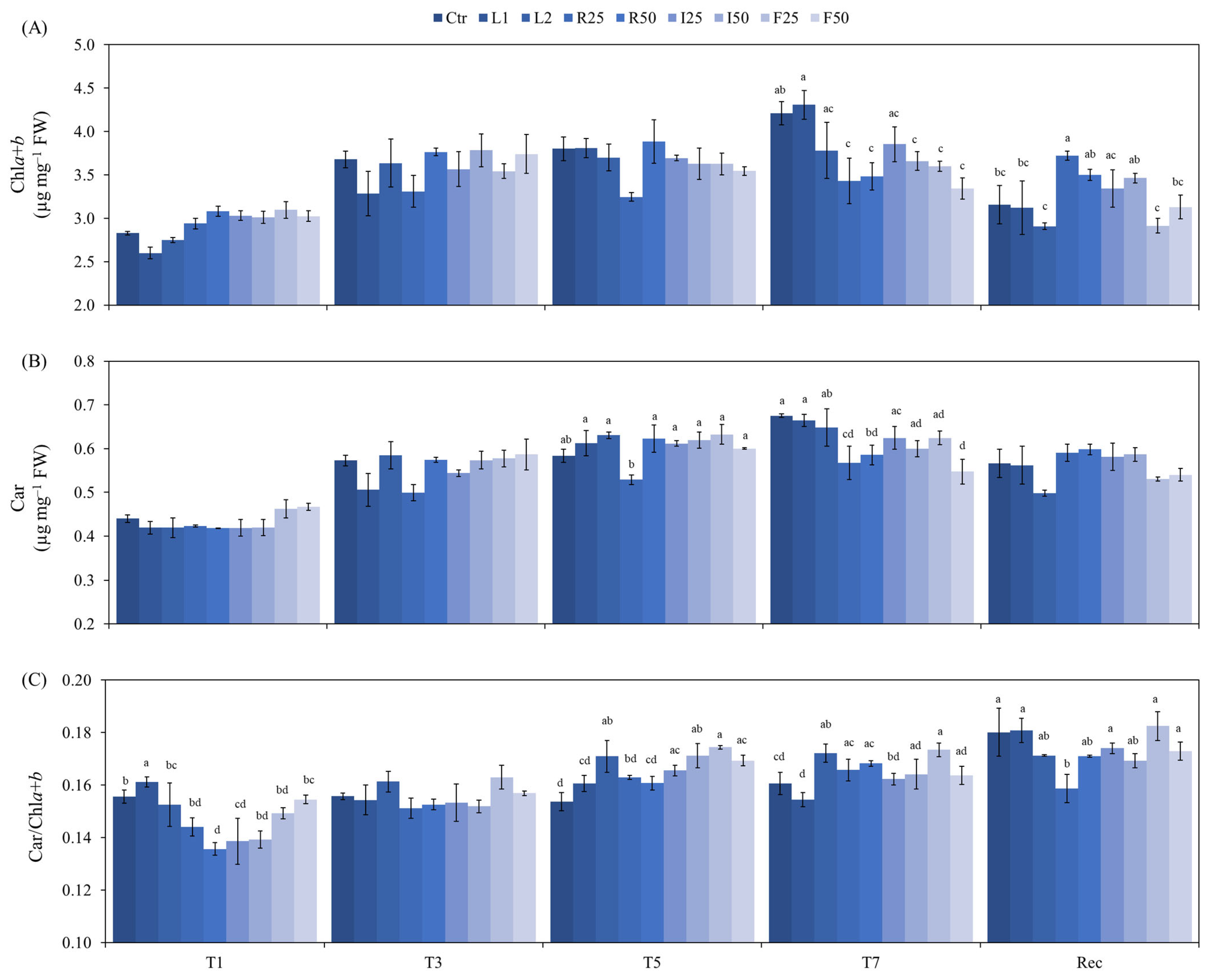
3.2. Biochemical Analysis
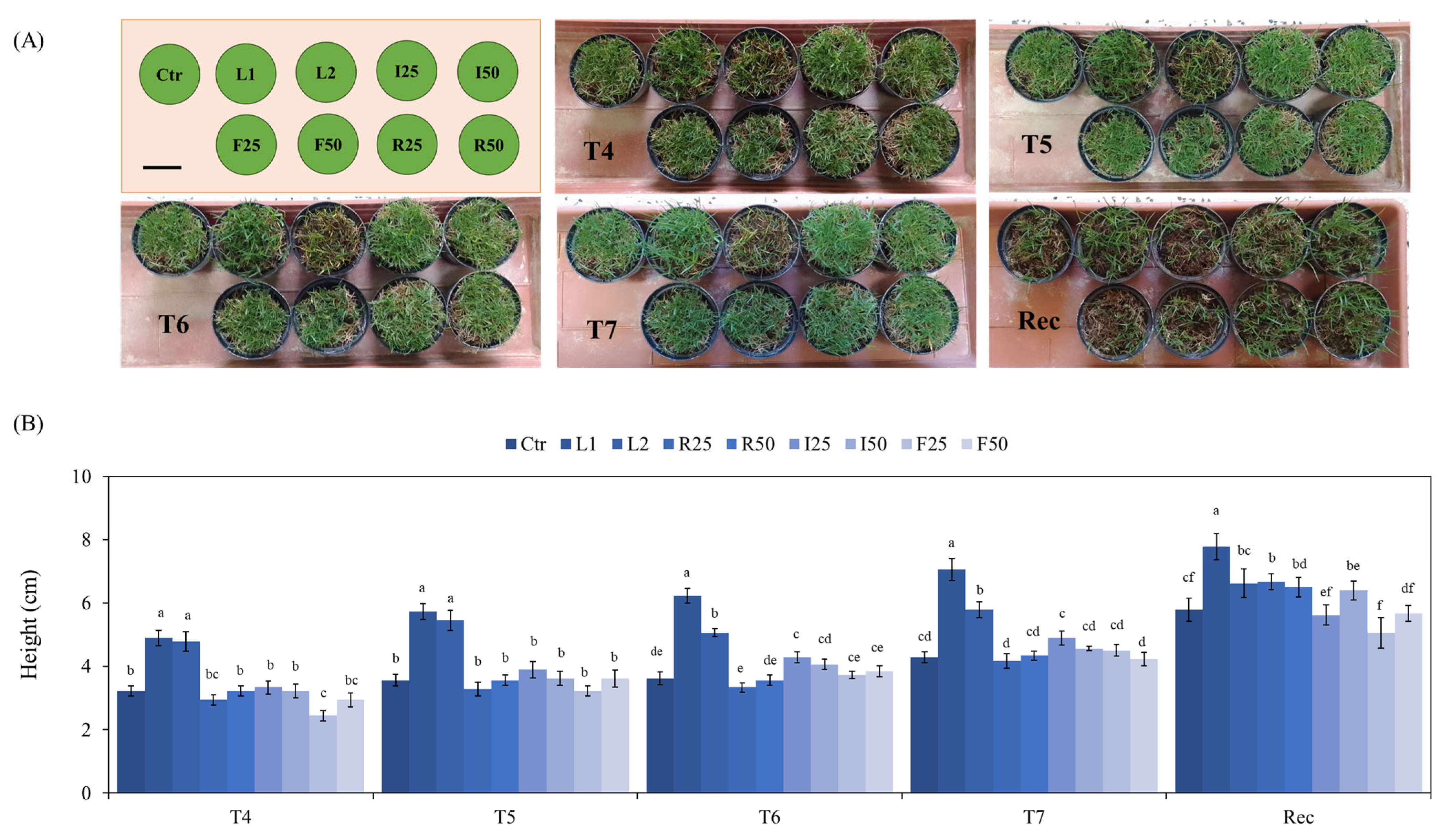
3.3. Turf Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghasemi, A.; Golbini Mofrad, M.M.; Parseh, I.; Hassani, G.; Mohammadi, H.; Hayati, R.; Alinejad, N. Cigarette butts as a super challenge in solid waste management: A review of current knowledge. Environ. Sci. Pollut. Res. 2022, 29, 51269–51280. [Google Scholar] [CrossRef]
- World Health Organization. Global Report on Trends in Prevalence of Tobacco Use 2000–2025, 4th ed.; World Health Organization: Geneva, Switzerland, 2021; ISBN 9789240000032. [Google Scholar]
- World Health Organization. Tobacco and Its Environmental Impact: An Overview; World Health Organization: Geneva, Switzerland, 2017; ISBN 9789241512497. [Google Scholar]
- Italian National Institute of Statistics Smoking Habits in Italy. Available online: https://esploradati.istat.it/databrowser/#/it/dw/categories/IT1,Z0810HEA,1.0/HEA_STYLE/HEA_STYLE_SMOK/IT1,83_63_DF_DCCV_AVQ_PERSONE_204,1.0 (accessed on 5 May 2025).
- Marinello, S.; Lolli, F.; Gamberini, R.; Rimini, B. A second life for cigarette butts? A review of recycling solutions. J. Hazard. Mater. 2020, 384, 121245. [Google Scholar] [CrossRef]
- Bonanomi, G.; Maisto, G.; De Marco, A.; Cesarano, G.; Zotti, M.; Mazzei, P.; Libralato, G.; Staropoli, A.; Siciliano, A.; De Filippis, F.; et al. The fate of cigarette butts in different environments: Decay rate, chemical changes and ecotoxicity revealed by a 5-years decomposition experiment. Environ. Pollut. 2020, 261, 114108. [Google Scholar] [CrossRef] [PubMed]
- Korobushkin, D.I.; Garibian, P.G.; Pelgunova, L.A.; Zaitsev, A.S. The earthworm species Eisenia fetida accelerates the decomposition rate of cigarette butts on the soil surface. Soil Biol. Biochem. 2020, 151, 108022. [Google Scholar] [CrossRef]
- Green, D.S.; Tongue, A.D.W.; Boots, B. The ecological impacts of discarded cigarette butts. Trends Ecol. Evol. 2022, 37, 183–192. [Google Scholar] [CrossRef]
- Moroz, I.; Scapolio, L.G.B.; Cesarino, I.; Leão, A.L.; Bonanomi, G. Toxicity of cigarette butts and possible recycling solutions—A literature review. Environ. Sci. Pollut. Res. 2021, 28, 10450–10473. [Google Scholar] [CrossRef]
- Yousefi, M.; Kermani, M.; Farzadkia, M.; Godini, K.; Torkashvand, J. Challenges on the recycling of cigarette butts. Environ. Sci. Pollut. Res. 2021, 28, 30452–30458. [Google Scholar] [CrossRef]
- Mariotti, L.; Huarancca Reyes, T.; Curadi, M.; Guglielminetti, L. Recycling cigarette filters as plant growing substrate in soilless system. Horticulturae 2022, 8, 135. [Google Scholar] [CrossRef]
- FOCUS Progetto Focus—Raccolta Differenziata Mozziconi di Sigaretta. Available online: https://www.comune.capannori.lu.it/progetti/progetto-focus-raccolta-differenziata-mozziconi-di-sigaretta/# (accessed on 7 May 2025).
- Chiellini, C.; Mariotti, L.; Huarancca Reyes, T.; de Arruda, E.J.; Fonseca, G.G.; Guglielminetti, L. Remediation capacity of different microalgae in effluents derived from the cigarette butt cleaning process. Plants 2022, 11, 1770. [Google Scholar] [CrossRef] [PubMed]
- Huarancca Reyes, T.; Mariotti, L.; Chiellini, C.; Guglielminetti, L.; Fonseca, G.G. UV-B irradiation effect on microalgae performance in the remediation of effluent derived from the cigarette butt cleaning process. Plants 2022, 11, 2356. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.; Bhatt, U.; Singh, H.; Kumar, D.; Sharma, J.; Strasser, R.J.; Soni, V. Ecotoxicological assessment of cigarette butts on morphology and photosynthetic potential of Azolla pinnata. BMC Plant Biol. 2024, 24, 300. [Google Scholar] [CrossRef] [PubMed]
- Sadıç, E.; Çelik, T.A. Environmental hazards of light cigarette butts and filters: Physiological, cytotoxic, and genotoxic assessment using the Allium cepa L. assay. Environ. Sci. Pollut. Res. 2025, 32, 8415–8425. [Google Scholar] [CrossRef] [PubMed]
- Montalvão, M.F.; Sampaio, L.L.G.; Gomes, H.H.F.; Malafaia, G. An insight into the cytotoxicity, genotoxicity, and mutagenicity of smoked cigarette butt leachate by using Allium cepa as test system. Environ. Sci. Pollut. Res. 2019, 26, 2013–2021. [Google Scholar] [CrossRef]
- Mansouri, N.; Etebari, M.; Ebrahimi, A.; Ebrahimpour, K.; Rahimi, B.; Hassanzadeh, A. Genotoxicity and phytotoxicity comparison of cigarette butt with cigarette ash. Environ. Sci. Pollut. Res. 2020, 27, 40383–40391. [Google Scholar] [CrossRef]
- Jakimiuk, A.; Bulak, A.; Barroso, P.M.; Podlasek, A.; Vaverková, M.D. Impact of Cigarette Butts on Plant Germination Based on Sinapis alba L. and Hordeum vulgare L. Seeds. J. Ecol. Eng. 2022, 23, 226–237. [Google Scholar] [CrossRef]
- Green, D.S.; Boots, B.; Olah-Kovacs, B.; Palma-Diogo, D. Disposable e-cigarettes and cigarette butts alter the physiology of an aquatic plant Lemna minor (Lemnaceae). Sci. Total Environ. 2023, 892, 164457. [Google Scholar] [CrossRef]
- Green, D.S.; Boots, B.; Da Silva Carvalho, J.; Starkey, T. Cigarette butts have adverse effects on initial growth of perennial ryegrass (gramineae: Lolium perenne L.) and white clover (leguminosae: Trifolium repens L.). Ecotoxicol. Environ. Saf. 2019, 182, 109418. [Google Scholar] [CrossRef]
- Selmar, D.; Radwan, A.; Abdalla, N.; Taha, H.; Wittke, C.; El-Henawy, A.; Alshaal, T.; Amer, M.; Kleinwächter, M.; Nowak, M.; et al. Uptake of nicotine from discarded cigarette butts—A so far unconsidered path of contamination of plant-derived commodities. Environ. Pollut. 2018, 238, 972–976. [Google Scholar] [CrossRef]
- Ajibade, S.; Simon, B.; Takács, A.; Gulyás, M. Effects of Cigarette Butt Leachate on the Growth of White Mustard (Sinapis alba L.) and Soil Properties: A Preliminary Study. Pollutants 2024, 4, 515–536. [Google Scholar] [CrossRef]
- Núñez, M.d.P.; López Loveira, E.G.; Domínguez, S.E.; Calfayan, L.M.; Itria, R.F.; Butler, M. Assessment of nicotine and degradation products in cigarette butts leachates after detoxification by white rot fungi. J. Hazard. Mater. 2025, 492, 138059. [Google Scholar] [CrossRef]
- Zittel, R.; da Silva, C.P.; Domingues, C.E.; Seremeta, D.C.H.; da Cunha, K.M.; de Campos, S.X. Availability of nutrients, removal of nicotine, heavy metals and pathogens in compounds obtained from smuggled cigarette tobacco compost associated with industrial sewage sludge. Sci. Total Environ. 2020, 699, 134377. [Google Scholar] [CrossRef]
- Momeni, L.; Mohsenzadeh, S. The effect of toxic substances of cigarette filter on the growth and some physiological characteristics of tall fescue (Festuca arundinacea) in soil and hydroponic media. Glob. Res. Environ. Sustain. 2023, 1, 27–35. [Google Scholar]
- Beard, J.B. Origin, biogeographical migrations and diversifications of turfgrasses. In Turfgrass History and Literature; Michigan State University Press: East Lansing, MI, USA, 2012; pp. 1–26. [Google Scholar]
- Pompeiano, A.; Huarancca Reyes, T.; Moles, T.M.; Villani, M.; Volterrani, M.; Guglielminetti, L.; Scartazza, A. Inter- and intraspecific variability in physiological traits and post-anoxia recovery of photosynthetic efficiency in grasses under oxygen deprivation. Physiol. Plant. 2017, 161, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, M.; Volterrani, M.; Magni, S.; Caturegli, L.; Minelli, A.; Leto, C.; La Bella, S.; Tuttolomondo, T.; Virga, G.; Grossi, N. Seashore paspalum in the mediterranean transition zone: Phenotypic traits of twelve accessions during and after establishment. Ital. J. Agron. 2017, 12, 808. [Google Scholar] [CrossRef]
- Mariotti, L.; Huarancca Reyes, T.; Ramos-Diaz, J.M.; Jouppila, K.; Guglielminetti, L. Hormonal regulation in different varieties of Chenopodium quinoa Willd. exposed to short acute UV-B irradiation. Plants 2021, 10, 858. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Pompeiano, A.; Ranieri, A.; Volterrani, M.; Guglielminetti, L.; Scartazza, A. Photosynthetic performance of five cool-season turfgrasses under UV-B exposure. Plant Physiol. Biochem. 2020, 151, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Packer, L., Douce, R., Eds.; Academic Press Inc.: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. ISBN 9780121820480. [Google Scholar]
- Jalal, A.; Oliveira Junior, J.C.d.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; Reis, A.R. dos Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 111225. [Google Scholar] [CrossRef]
- Kessler, D.; Bhattacharya, S.; Diezel, C.; Rothe, E.; Gase, K.; Schöttner, M.; Baldwin, I.T. Unpredictability of nectar nicotine promotes outcrossing by hummingbirds in Nicotiana attenuata. Plant J. 2012, 71, 529–538. [Google Scholar] [CrossRef]
- Ali, A.H.; Abdelrahman, M.; El-Sayed, M.A. Alkaloid role in plant defense response to growth and stress. In Bioactive Molecules in Plant Defense; Ogaiah, S., Abdelrahman, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 145–158. ISBN 978-3-030-27164-0. [Google Scholar]
- Lewerenz, L.; Abouzeid, S.; Yahyazadeh, M.; Hijazin, T.; Selmar, D. Novel cognitions in allelopathy: Implications from the “horizontal natural product transfer”. Plants 2022, 11, 3264. [Google Scholar] [CrossRef]
- Noble, R.E. Effect of cigarette smoke on seed germination. Sci. Total Environ. 2001, 267, 177–179. [Google Scholar] [CrossRef]
- Selmar, D.; Engelhardt, U.H.; Hänsel, S.; Thräne, C.; Nowak, M.; Kleinwächter, M. Nicotine uptake by peppermint plants as a possible source of nicotine in plant-derived products. Agron. Sustain. Dev. 2015, 35, 1185–1190. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, X.; Chu, P.; Shi, H.; Wang, R.; Li, J.; Zheng, S. Cultivation and application of nicotine-degrading bacteria and environmental functioning in tobacco planting soil. Bioresour. Bioprocess. 2023, 10, 10. [Google Scholar] [CrossRef]
- Alkhatib, R.; Alkhatib, B.; Abdo, N. Impact of exogenous nicotine on the morphological, physio-biochemical, and anatomical characteristics in Capsicum annuum. Int. J. Phytoremediat. 2022, 24, 666–674. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, T.; Wakeel, A.; Cheema, Z.A. Differential response of maize and mungbean to tobacco allelopathy. Exp. Agric. 2014, 50, 611–624. [Google Scholar] [CrossRef]
- Cheng, Y.-D.; Bai, Y.-X.; Jia, M.; Chen, Y.; Wang, D.; Wu, T.; Wang, G.; Yang, H.-W. Potential risks of nicotine on the germination, growth, and nutritional properties of broad bean. Ecotoxicol. Environ. Saf. 2021, 209, 111797. [Google Scholar] [CrossRef]
- Guertal, E.A.; Datnoff, L.E. Silicon in turfgrass: A review. Crop Sci. 2021, 61, 3861–3876. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef]
- Taher, D.; Nofal, E.; Hegazi, M.; El-Gaied, M.A.; El-Ramady, H.; Solberg, S.Ø. Response of warm season turf grasses to combined cold and salinity stress under foliar applying organic and inorganic amendments. Horticulturae 2023, 9, 49. [Google Scholar] [CrossRef]
- He, Y.; Xiao, H.; Wang, H.; Chen, Y.; Yu, M. Effect of silicon on chilling-induced changes of solutes, antioxidants, and membrane stability in seashore paspalum turfgrass. Acta Physiol. Plant. 2010, 32, 487–494. [Google Scholar] [CrossRef]
- Jan, S.; Bhardwaj, S.; Singh, B.; Kapoor, D. Silicon efficacy for the remediation of metal contaminated soil. 3 Biotech 2024, 14, 212. [Google Scholar] [CrossRef] [PubMed]
- Thiravetyan, P.; Treesubsuntorn, C.; Sriprapat, W. Phytoremediation of BTEX by Plants. In Phytoremediation; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 1, pp. 283–293. ISBN 978-3-319-10394-5. [Google Scholar]
- Tarigholizadeh, S.; Sushkova, S.; Rajput, V.D.; Ranjan, A.; Arora, J.; Dudnikova, T.; Barbashev, A.; Mandzhieva, S.; Minkina, T.; Wong, M.H. Transfer and degradation of PAHs in the soil–plant system: A review. J. Agric. Food Chem. 2024, 72, 46–64. [Google Scholar] [CrossRef]
- Fan, Q.; Raymer, P.L.; Bahri, B.A.; Jespersen, D. Dose-dependent physiological effects of UV-C radiation on seashore paspalum. Plant Physiol. Biochem. 2024, 208, 108514. [Google Scholar] [CrossRef] [PubMed]
- Zainul Armir, N.A.; Zulkifli, A.; Gunaseelan, S.; Palanivelu, S.D.; Salleh, K.M.; Che Othman, M.H.; Zakaria, S. Regenerated cellulose products for agricultural and their potential: A review. Polymers 2021, 13, 3586. [Google Scholar] [CrossRef]
- Lima, R.G.; Maranni, M.; Araujo, L.O.; Maciel, B.M.; Canassa, T.; Caires, A.R.L.; Cena, C. Preparation and phytotoxicity evaluation of cellulose acetate nanoparticles. Polymers 2022, 14, 5022. [Google Scholar] [CrossRef]
- Pompeiano, A.; Huarancca Reyes, T.; Moles, T.M.; Guglielminetti, L.; Scartazza, A. Photosynthetic and growth responses of Arundo donax L. plantlets under different oxygen deficiency stresses and reoxygenation. Front. Plant Sci. 2019, 10, 408. [Google Scholar] [CrossRef]
- Basit, F.; Khalid, M.; El-Keblawy, A.; Sheteiwy, M.S.; Sulieman, S.; Josko, I.; Zulfiqar, F. Hypoxia stress: Plant’s sensing, responses, and tolerance mechanisms. Environ. Sci. Pollut. Res. 2024, 31, 63458–63472. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Mancilla-Álvarez, E.; Bello-Bello, J.J. In vitro response of sugarcane (Saccharum spp. Hybrid) plantlets to flooding stress. J. Biotechnol. 2024, 393, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Huarancca Reyes, T.; Scartazza, A.; Castagna, A.; Cosio, E.G.; Ranieri, A.; Guglielminetti, L. Physiological effects of short acute UVB treatments in Chenopodium quinoa Willd. Sci. Rep. 2018, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Erdal, N.B.; Hakkarainen, M. Degradation of cellulose derivatives in laboratory, man-made, and natural environments. Biomacromolecules 2022, 23, 2713–2729. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huarancca Reyes, T.; Volterrani, M.; Guglielminetti, L.; Scartazza, A. Towards a Circular Economy: Unlocking the Potentials of Cigarette Butt Recycling as a Resource for Seashore Paspalum Growth. Sustainability 2025, 17, 6976. https://doi.org/10.3390/su17156976
Huarancca Reyes T, Volterrani M, Guglielminetti L, Scartazza A. Towards a Circular Economy: Unlocking the Potentials of Cigarette Butt Recycling as a Resource for Seashore Paspalum Growth. Sustainability. 2025; 17(15):6976. https://doi.org/10.3390/su17156976
Chicago/Turabian StyleHuarancca Reyes, Thais, Marco Volterrani, Lorenzo Guglielminetti, and Andrea Scartazza. 2025. "Towards a Circular Economy: Unlocking the Potentials of Cigarette Butt Recycling as a Resource for Seashore Paspalum Growth" Sustainability 17, no. 15: 6976. https://doi.org/10.3390/su17156976
APA StyleHuarancca Reyes, T., Volterrani, M., Guglielminetti, L., & Scartazza, A. (2025). Towards a Circular Economy: Unlocking the Potentials of Cigarette Butt Recycling as a Resource for Seashore Paspalum Growth. Sustainability, 17(15), 6976. https://doi.org/10.3390/su17156976






