Improvement of Unconfined Compressive Strength in Granite Residual Soil by Indigenous Microorganisms
Abstract
1. Introduction
2. Materials
2.1. Physical Properties of Granite Residual Soil
2.2. Culture Medium and Cementing Solution
2.2.1. Culture Medium
2.2.2. Cementing Solution
3. Procedures
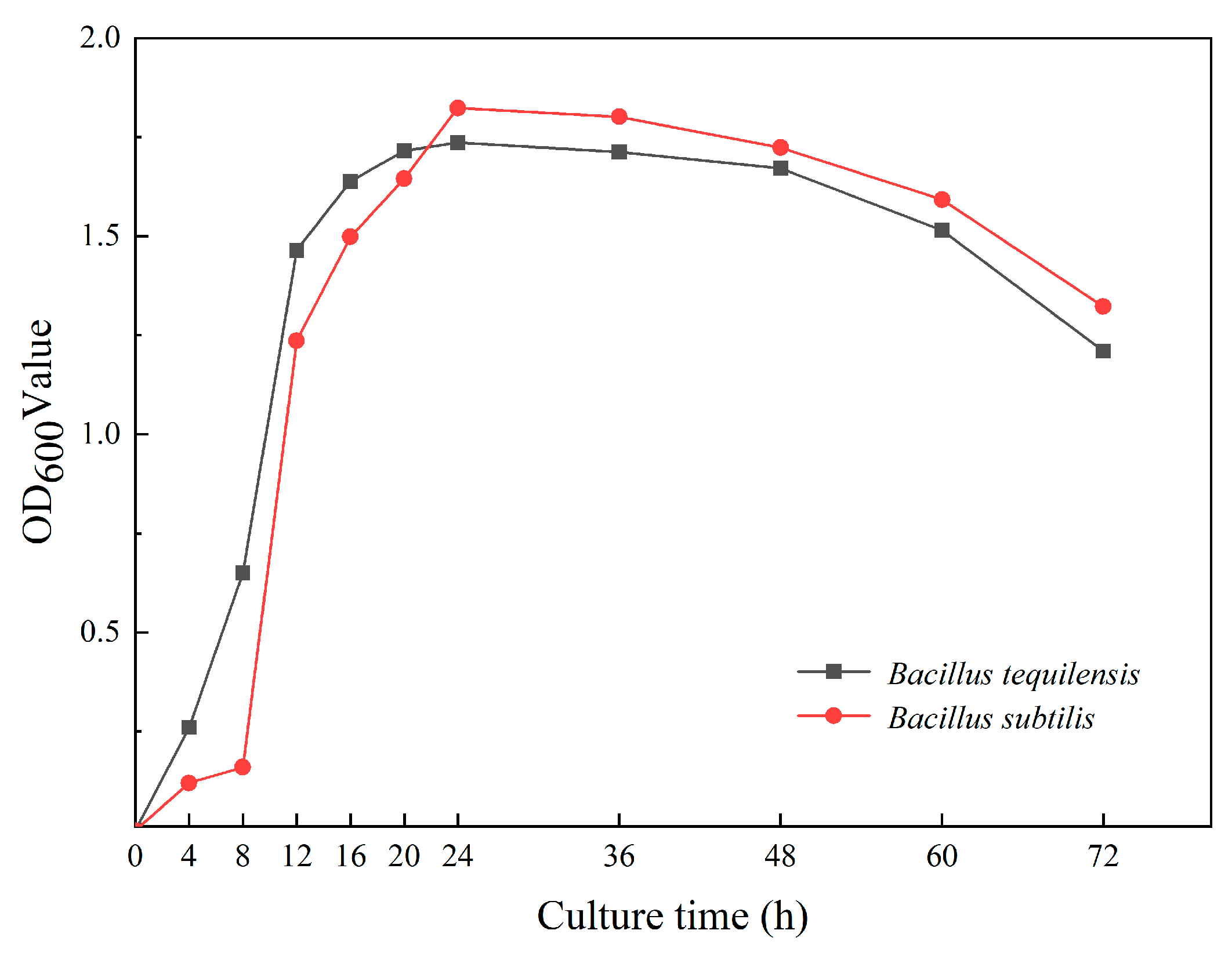
3.1. Screening and Purification of Urease-Producing Strains
3.2. Bacterial Concentration and Urease Activity Test
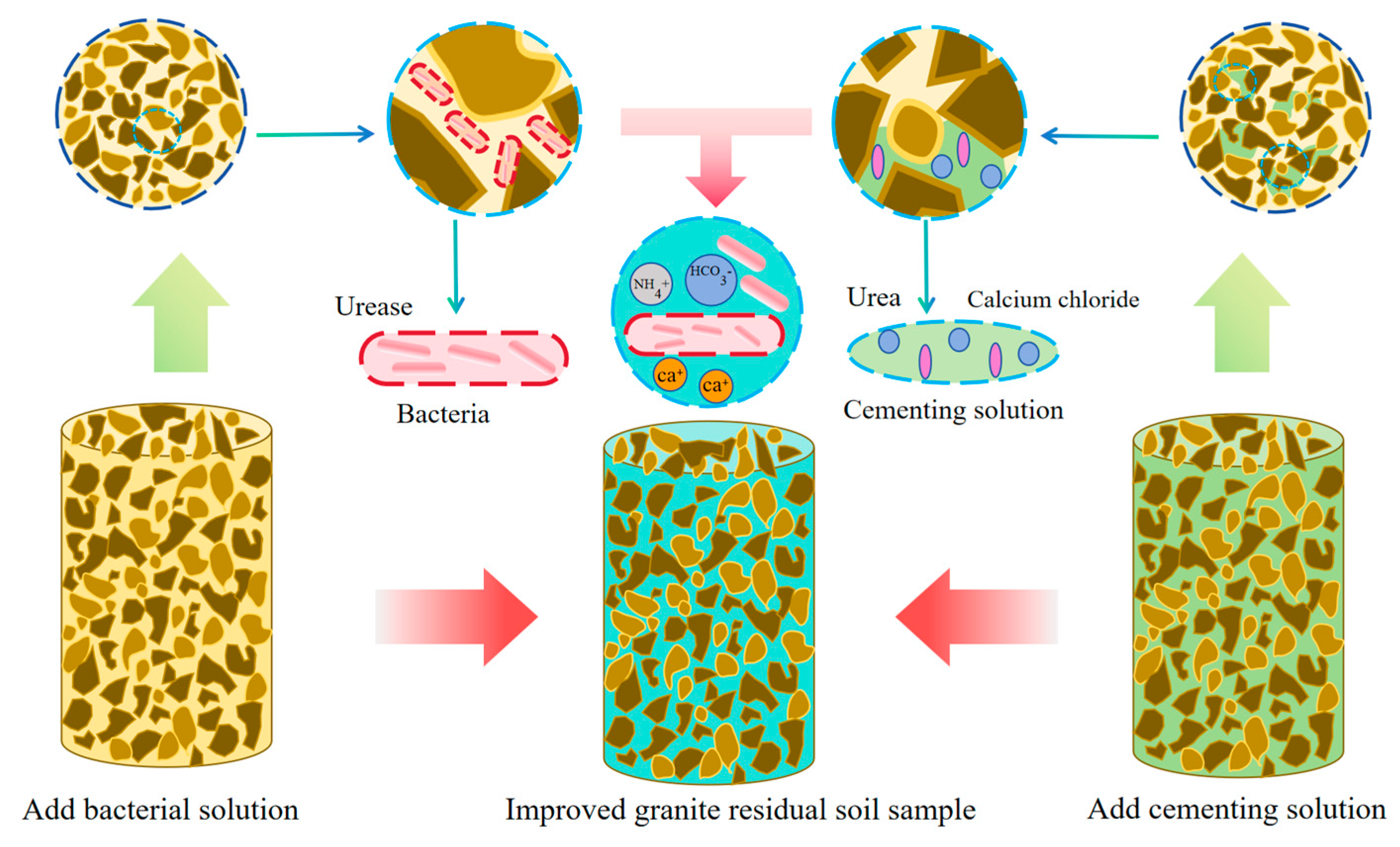
3.3. Microbial Improvement of the Granite Residual Soil Test
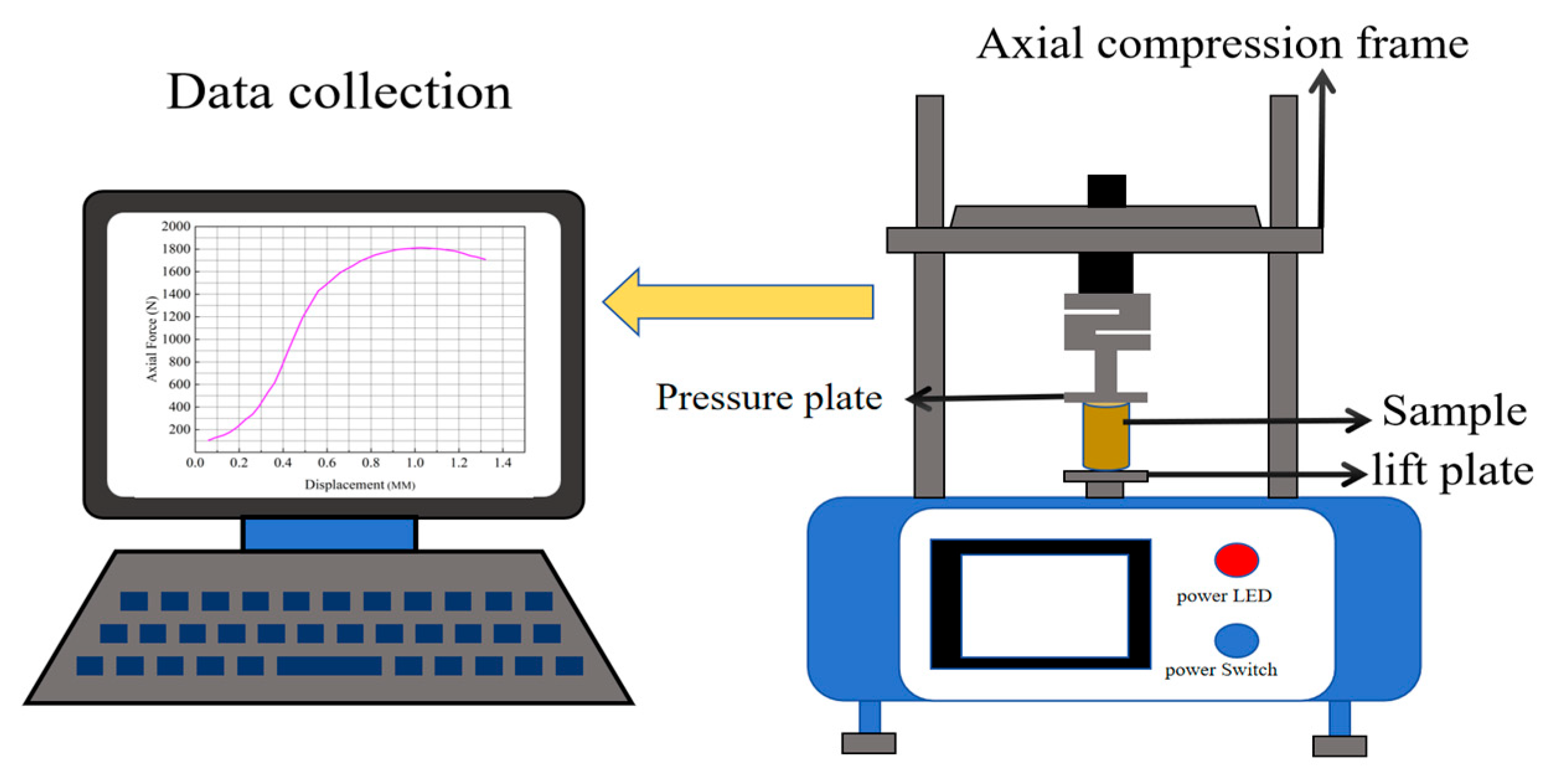
3.4. Unconfined Compressive Strength Analyses
3.5. SEM and XRD Analyses
4. Analysis of the Test Results of Unconfined Compressive Strength
4.1. Analysis of Stress–Strain Curves of Granite Residual Soil Improved by Microorganisms
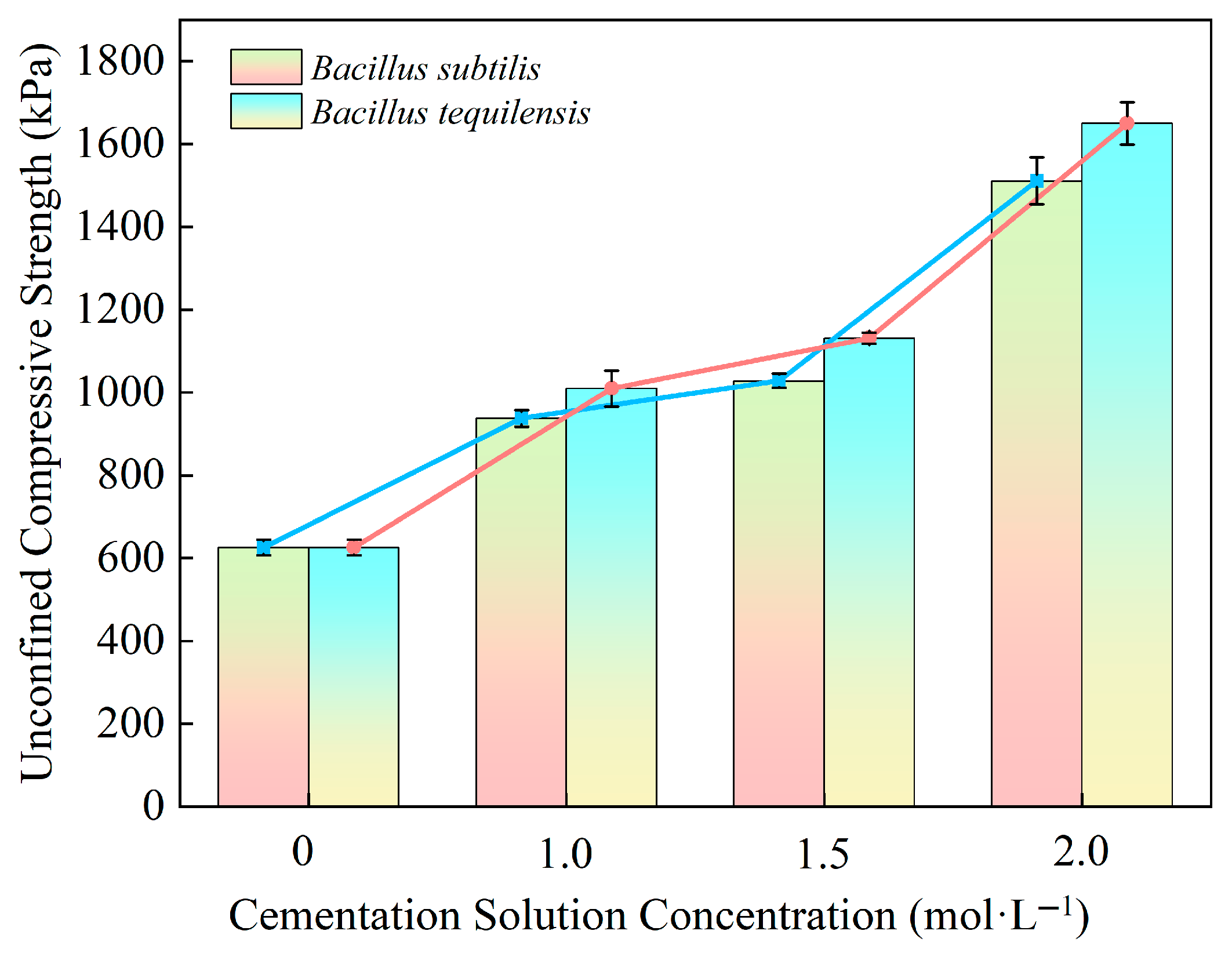
4.2. Analysis of Compressive Strength of Granite Residual Soil Improved by Microorganisms
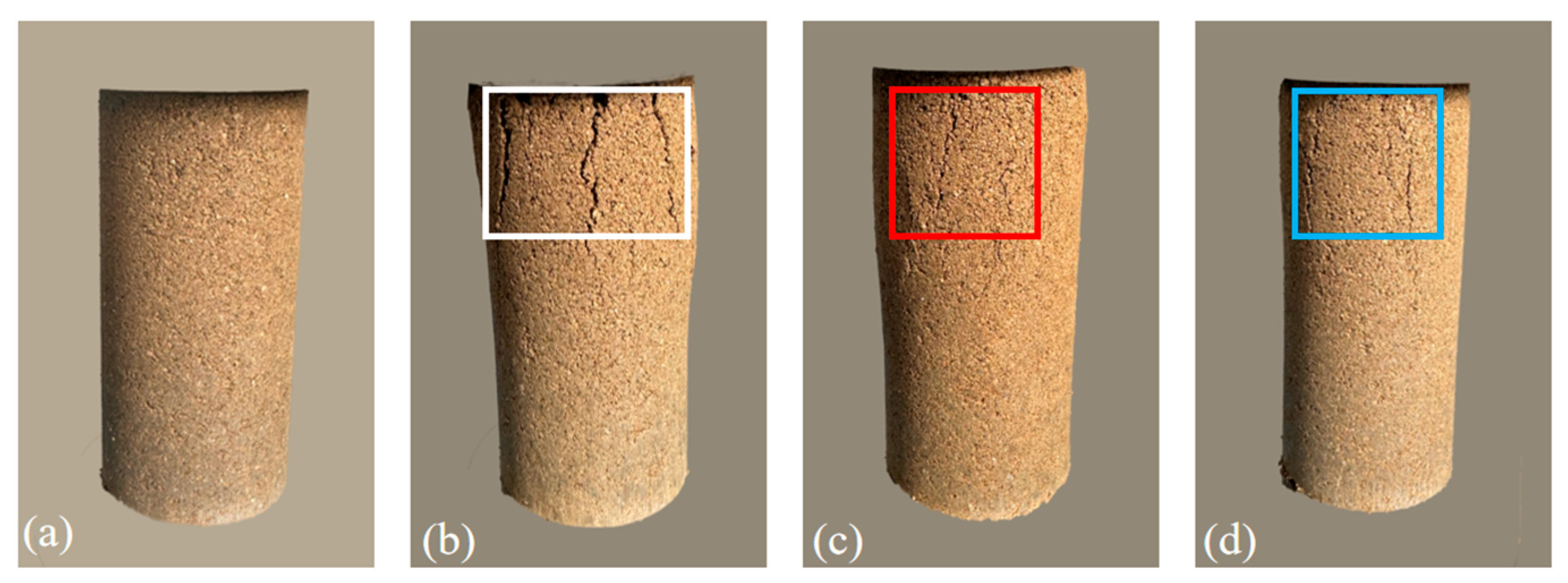
4.3. Analysis of the Failure Strain in Granite Residual Soil Improved by Microorganisms
5. Analysis of Microscopic Test Results
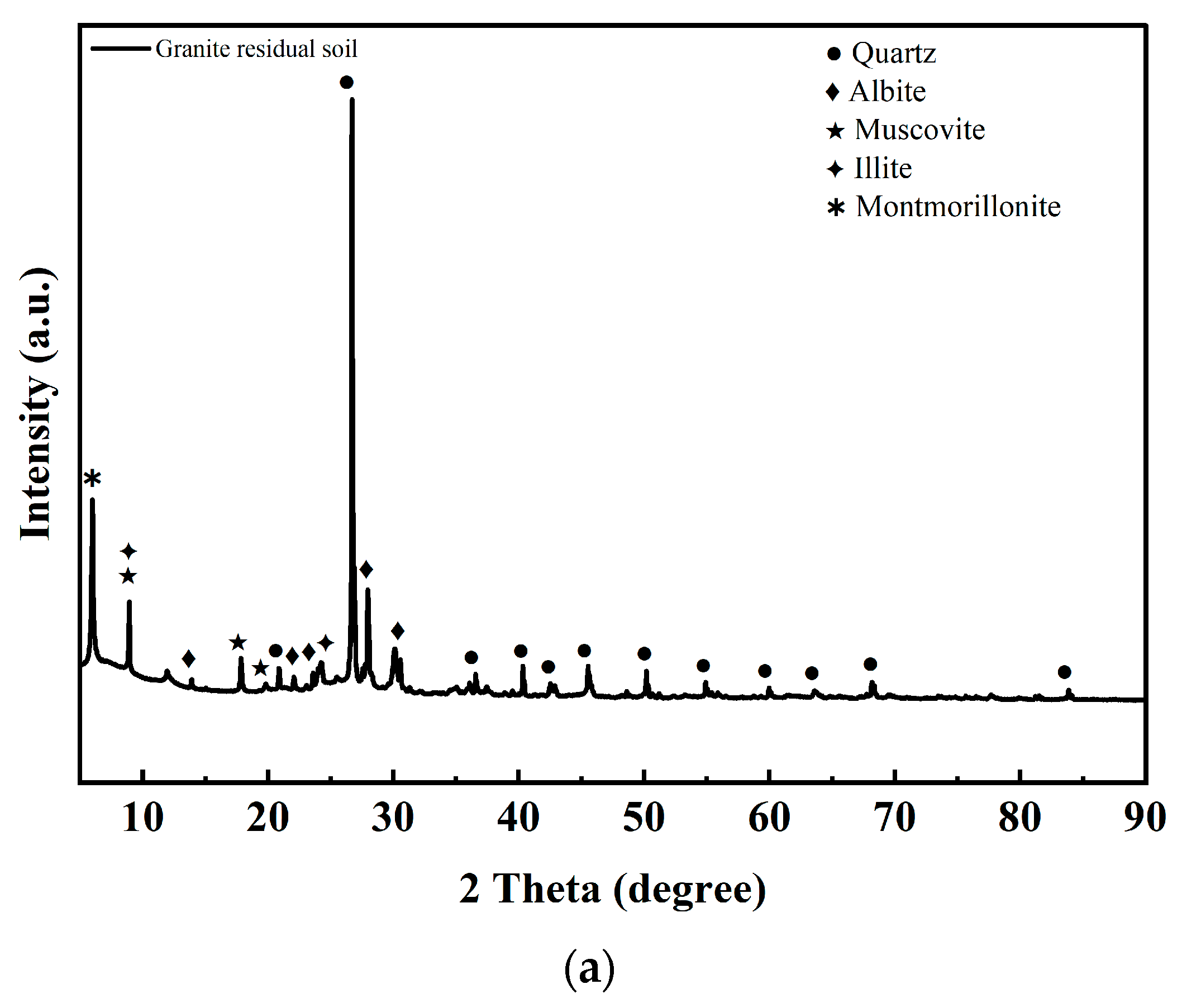
5.1. XRD Results
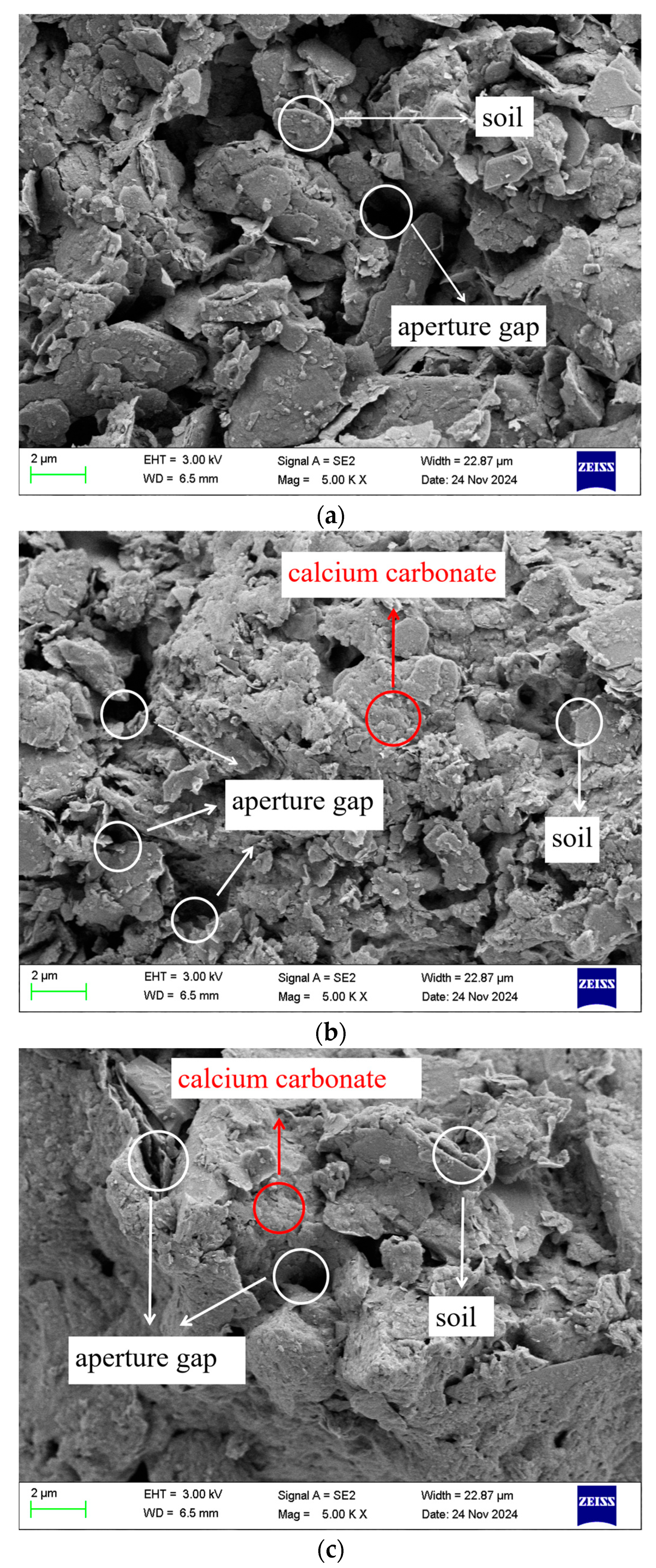
5.2. SEM Results
6. Discussion
- Although the effects of varying cementing solution concentrations were examined, scenarios involving concentrations exceeding 2.0 mol/L were not investigated. This is because high concentrations of calcium ions may inhibit urease activity, thereby negatively impacting the soil improvement process. In future research, higher concentrations will be tested to validate this hypothesis. Additionally, the curing process in the present study was conducted under constant temperature conditions; therefore, temperature was not considered as a variable factor. Furthermore, parameters such as the pH value of the cementing solution, the organic content in the bacterial solution, and the cation exchange capacity (CEC) of the soil were not included in the current analysis. These factors will be further examined in subsequent studies.
- The current study exhibits limitations in the quantitative characterization of the underlying mechanism. While XRD analysis was primarily employed for qualitative confirmation of calcite formation, the absence of peak area statistics in the experimental design phase hinders the establishment of a quantitative correlation between calcium carbonate content and macroscopic strength at this stage. Although SEM observations provided insights into the distribution of cementing substances, the variation patterns of porosity and calcium carbonate precipitation were not systematically analyzed. Future research will focus on conducting quantitative assessments at these microscopic scales.
7. Conclusions
- The stress–strain curves of both the improved and unimproved soil samples exhibit strain-softening behavior; however, the residual stress of the improved soil sample is markedly higher than that of the unimproved sample.
- Both Bacillus subtilis and Bacillus tequilensis demonstrate significant efficacy in enhancing the unconfined compressive strength of granite residual soil from the Hanzhong area. The improvement effect increases with the concentration of the cementing solution, reaching a maximum at a concentration of 2.0 mol/L. Among the two bacterial strains, Bacillus tequilensis exhibits superior performance, achieving a peak strength of 1649.7 kPa, representing an increase of 163.7%.
- The failure strain of the granite residual soil is significantly improved through the application of Bacillus subtilis and Bacillus tequilensis, effectively mitigating the brittle failure characteristics of the soil. At low concentrations of cementing solution, Bacillus subtilis-treated soil exhibits a higher failure strain than that treated with Bacillus tequilensis. However, the latter performs better at higher concentrations. Both bacterial strains significantly enhance the failure morphology of the soil, markedly reducing the width and number of cracks.
- SEM and XRD analyses indicate that the improved granite residual soil exhibits evident calcium carbonate cementation, which reduces interparticle porosity. The bonding force and density between soil particles are significantly enhanced, thereby contributing to the increased unconfined compressive strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Zhang, R.; Han, L.; Goh, A. Engineering properties of the Bukit Timah Granitic residual soil in Singapore. Undergr. Space 2019, 4, 98–108. [Google Scholar] [CrossRef]
- Coutinho, R.Q.; Silva, M.M.; dos Santos, A.N.; Lacerda, W.A. Geotechnical characterization and failure mechanism of landslide in granite residual soil. J. Geotech. Geoenviron. Eng. 2019, 145, 05019004. [Google Scholar] [CrossRef]
- Dang, F.; Kang, J.; Luo, C.; Li, W.; Guo, H.; Luo, Y. Feasibility Study on Stabilizing Granite Residual Soil Using Montmorillonite. Results Eng. 2025, 26, 105679. [Google Scholar] [CrossRef]
- Ji, X.; Lu, H.; Dai, C.; Ye, Y.; Cui, Z.; Xiong, Y. Characterization of properties of soil–rock mixture prepared by the laboratory vibration compaction method. Sustainability 2021, 13, 11239. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Q.; Xu, H.; Wang, Y.; Tang, L. Influences of different modifiers on the disintegration of improved granite residual soil under wet and dry cycles. Int. J. Min. Sci. Technol. 2022, 32, 831–845. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Q.; Zhao, L.; Zhou, X. Research on the strength and disintegration characteristics of the granite residual soil improved with cement. J. Hunan Univ. Sci. Technol. (Nat. Sci. Ed.) 2016, 31, 54–59. [Google Scholar] [CrossRef]
- Liu, F.; Chen, S.; Sun, H.; Liu, H. Shear Characteristics of Granite Residual Geogrid-Soil Interface with Different Water Contents. J. Tongji Univ. (Nat. Sci.) 2023, 51, 222–228. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Gu, X. Mechanical test and mechanism study of granite residual soil modified by fiber and nanomaterials. J. Water Resour. Water Eng. 2022, 33, 185–191. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Miranda, T.; Oliveira, D.; Silva, R. Soil stabilisation using alkaline activation of fly ash for self compacting rammed earth construction. Constr. Build. Mater. 2012, 36, 727–735. [Google Scholar] [CrossRef]
- Chew, S.; Kamruzzaman, A.; Lee, F. Physicochemical and engineering behavior of cement treated clays. J. Geotech. Geoenviron. Eng. 2004, 130, 696–706. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.; Wang, Y.; Jiang, N. A critical review of biomineralization in environmental geotechnics: Applications, trends, and perspectives. Biogeotechnics 2023, 1, 100003. [Google Scholar] [CrossRef]
- Dubey, A.A.; Dhami, N.K.; Ravi, K.; Mukherjee, A. Erosion mitigation with biocementation: A review on applications, challenges, & future perspectives. Rev. Environ. Sci. Bio/Technol. 2023, 22, 1059–1091. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Mukherjee, A.; Huang, M.; Achal, V. Biomineralization in metakaolin modified cement mortar to improve its strength with lowered cement content. J. Hazard. Mater. 2017, 329, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; He, X.; Zaman, M.; Ma, G.; Zhao, C. Review of strength improvements of biocemented soils. Int. J. Geomech. 2022, 22, 03122001. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, C.; Xiao, Y. Reaction principles, deposition and failure mechanisms and theories of biomineralization: Progress and challenges. Chin. J. Geotech. Eng. 2023, 51, 222–228. [Google Scholar] [CrossRef]
- Gowthaman, S.; Nakashima, K.; Kawasaki, S. Freeze-thaw durability and shear responses of cemented slope soil treated by microbial induced carbonate precipitation. Soils Found. 2020, 60, 840–855. [Google Scholar] [CrossRef]
- Cardoso, R.; Pires, I.; Duarte, S.O.; Monteiro, G.A. Effects of clay’s chemical interactions on biocementation. Appl. Clay Sci. 2018, 156, 96–103. [Google Scholar] [CrossRef]
- Tiwari, N.; Satyam, N.; Sharma, M. Micro-mechanical performance evaluation of expansive soil biotreated with indigenous bacteria using MICP method. Sci. Rep. 2021, 11, 10324. [Google Scholar] [CrossRef]
- Liang, S.; Xiao, X.; Fang, C.; Feng, D.; Wang, Y. Experimental study on the mechanical properties and disintegration resistance of microbially solidified granite residual soil. Crystals 2022, 12, 132. [Google Scholar] [CrossRef]
- Peng, J.; Wen, Z.; Liu, Z.; Sun, Y.; Feng, Q.; He, J. Experimental research on MICP-treated organic clay. Chin. J. Geotech. Eng. 2019, 41, 733–740. [Google Scholar] [CrossRef]
- Cui, M.; Zheng, J.; Lai, H. Experimental study of effect of particle size on strength of bio-cemented sand. Rock Soil Mech. 2016, 37, 397–402. [Google Scholar] [CrossRef]
- Tobler, D.J.; Maclachlan, E.; Phoenix, V.R. Microbially Mediated Plugging of Porous Media and the Impact of Differing Injection Strategies. Ecol. Eng. 2012, 42, 270–278. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, W. Influence of curing test conditions on the strength of microorganism reinforced soil mass. J. For. Eng. 2023, 8, 170–175. [Google Scholar] [CrossRef]
- Huang, W. Experimental Study on Solidifying Characteristics of Granite Residual Soil Byconcentration of Cementing Fluid and Times of Infusion. Master’s Thesis, Changsha University of Science and Technology, Changsha, China, 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.X.; Cheng, X.H. Role of calcium sources in the strength and microstructure of microbial mortar. Constr. Build. Mater. 2015, 77, 160–167. [Google Scholar] [CrossRef]
- Hu, Q.; Song, W.; Hu, J. Study of the Mechanical Properties and Water Stability of Microbially Cured, Coir-Fiber-Reinforced Clay Soil. Sustainability 2023, 15, 13261. [Google Scholar] [CrossRef]
- GB/T 50123-2019; Geotechnical Test Method Standard. China Planning Press: Beijing, China, 2019.
- Ma, X. Experimental study on soil-water characteristic curve and strength test of granite residual soil solidified by Bacillus Pasteur. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2023. [Google Scholar] [CrossRef]
- Whiffin V, S. Microbial CaCO3 Precipitation for the Production of Biocement. Doctoral Dissertation, Murdoch University, Murdoch, Australia, 2004. [Google Scholar]
- Huang, C.; Du, Q.; Qu, L. Influences of Calcium Source and Curing Method on the Uniformity and Strength of Sand Loess Treated by MICP. J. Civ. Environ. Eng. 2025, pp. 1–10. Available online: http://kns.cnki.net/kcms/detail/50.1218.TU.20241209.0953.002.html (accessed on 9 December 2024).
- Liu, H.; Ma, G.; Zhao, C.; Zhang, J.; He, X.; Xiao, Y. Macro-and miero-mechanical regime of biotreated calcareous sand. J. Civ. Environ. Eng. 2020, 42, 205–206. [Google Scholar]
- Chu, J.; Ivanov, V.; Naeimi, M.; Stabnikov, V.; Liu, H.-L. Optimization of calcium-based bioclogging and biocementation of sand. Acta Geotech. 2014, 9, 277–285. [Google Scholar] [CrossRef]
- Cui, M.-J.; Zheng, J.-J.; Zhang, R.-J.; Lai, H.-J.; Zhang, J. Influence of cementation level on the strength behaviour of bio-cemented sand. Acta Geotech. 2017, 12, 971–986. [Google Scholar] [CrossRef]






| Materials | Maximum Dry Density (g·cm−3) | Dry Density (g·cm−3) | Natural Moisture Content (%) | Optimal Moisture Content (%) | Liquid Limit (%) | Plastic Limit (%) | Plasticity Index | Void Ratio | Specific Gravity |
|---|---|---|---|---|---|---|---|---|---|
| Granite residual soil | 2 | 1.8 | 8.2 | 9.8 | 16.8 | 10.5 | 6.3 | 0.5 | 2.7 |
| Bacterial Strain | Urease Activity [(mmol/(L·min)] |
|---|---|
| Bacillus subtilis | 6.657 |
| Bacillus tequilensis | 8.275 |
| Bacterial Strain | Concentration of Cementing Solution/(mol/L) | Number |
|---|---|---|
| Blank control group | 0 | GRS-0 |
| Bacillus subtilis | 0.5 | BS-0.5 |
| 1 | BS-1.0 | |
| 2 | BS-2.0 | |
| Bacillus tequilensis | 0.5 | BT-0.5 |
| 1 | BT-1.0 | |
| 2 | BT-2.0 |
| Sum of Squares | Degrees of Freedom | Mean Square | F-Statistic | p-Value | ||
|---|---|---|---|---|---|---|
| Bacillus subtilis | Between groups | 3 | ||||
| Within groups | 8 | |||||
| Total | 11 | |||||
| Bacillus tequilensis | Between groups | 3 | ||||
| Within groups | 8 | |||||
| Total | 11 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, M.; Peng, H.; Kang, J.; Guo, H.; Luo, Y.; Tao, M. Improvement of Unconfined Compressive Strength in Granite Residual Soil by Indigenous Microorganisms. Sustainability 2025, 17, 6895. https://doi.org/10.3390/su17156895
Wang Y, Li M, Peng H, Kang J, Guo H, Luo Y, Tao M. Improvement of Unconfined Compressive Strength in Granite Residual Soil by Indigenous Microorganisms. Sustainability. 2025; 17(15):6895. https://doi.org/10.3390/su17156895
Chicago/Turabian StyleWang, Ya, Meiqi Li, Hao Peng, Jiaxin Kang, Hong Guo, Yasheng Luo, and Mingjiang Tao. 2025. "Improvement of Unconfined Compressive Strength in Granite Residual Soil by Indigenous Microorganisms" Sustainability 17, no. 15: 6895. https://doi.org/10.3390/su17156895
APA StyleWang, Y., Li, M., Peng, H., Kang, J., Guo, H., Luo, Y., & Tao, M. (2025). Improvement of Unconfined Compressive Strength in Granite Residual Soil by Indigenous Microorganisms. Sustainability, 17(15), 6895. https://doi.org/10.3390/su17156895






