Functional Connectivity in Future Land-Use Change Scenarios as a Tool for Assessing Priority Conservation Areas for Key Bird Species: A Case Study from the Chaco Serrano
Abstract
1. Introduction
2. Materials and Methods
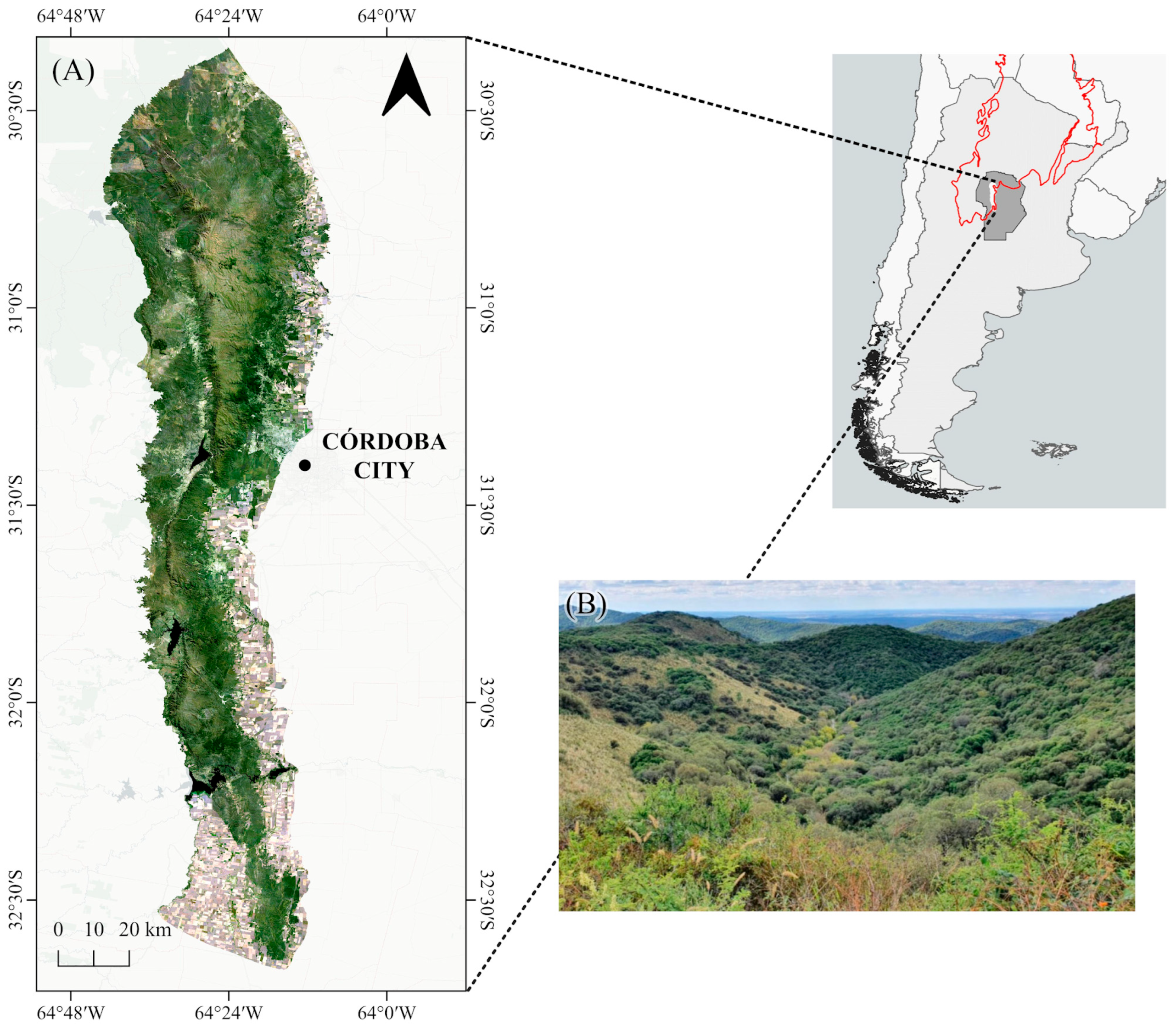
2.1. Study Area
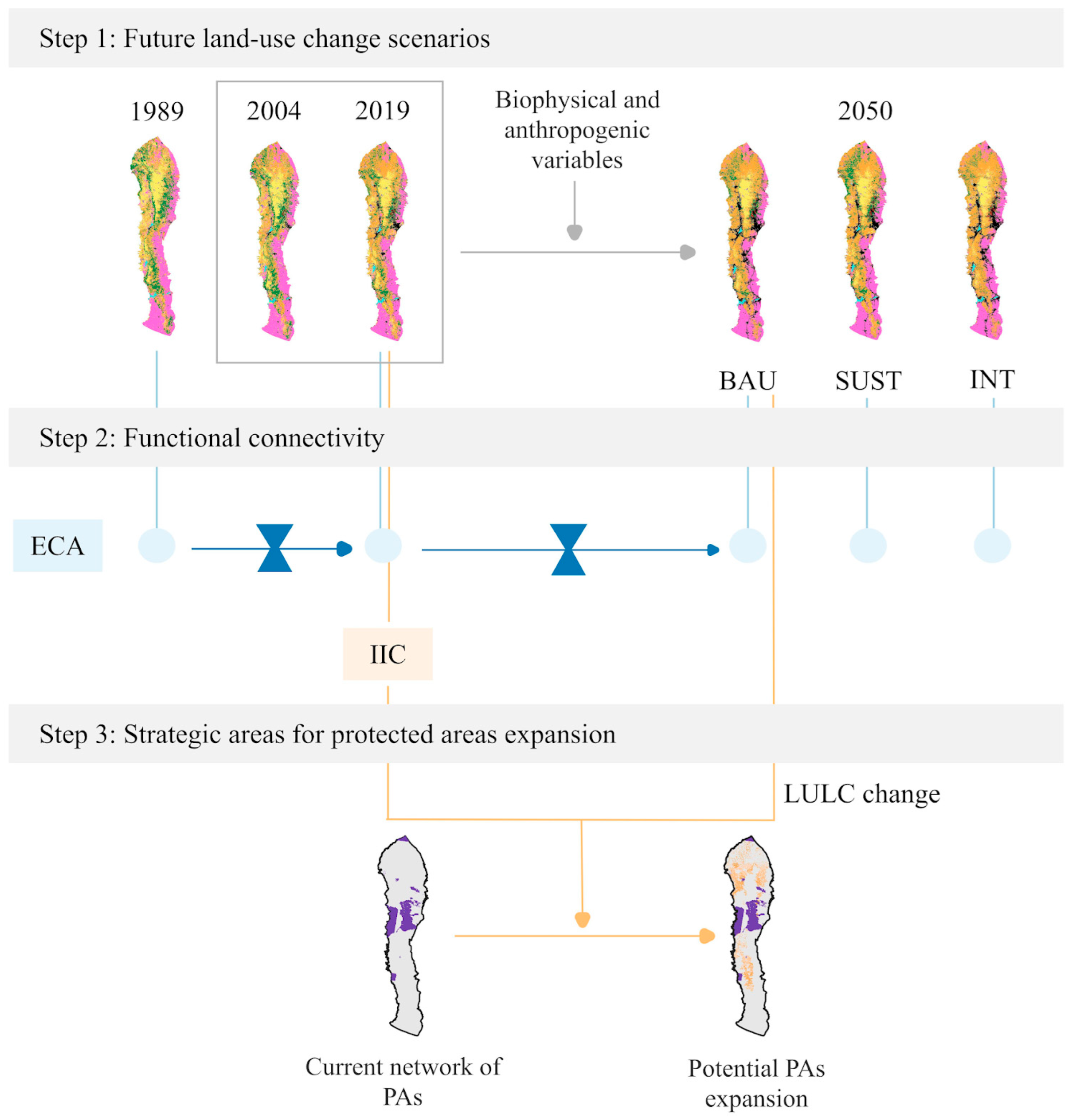
2.2. Future Land-Use Change Scenarios
2.3. Bird Species
2.4. Functional Connectivity
2.5. Strategic Areas for Protected Area Expansion
3. Results
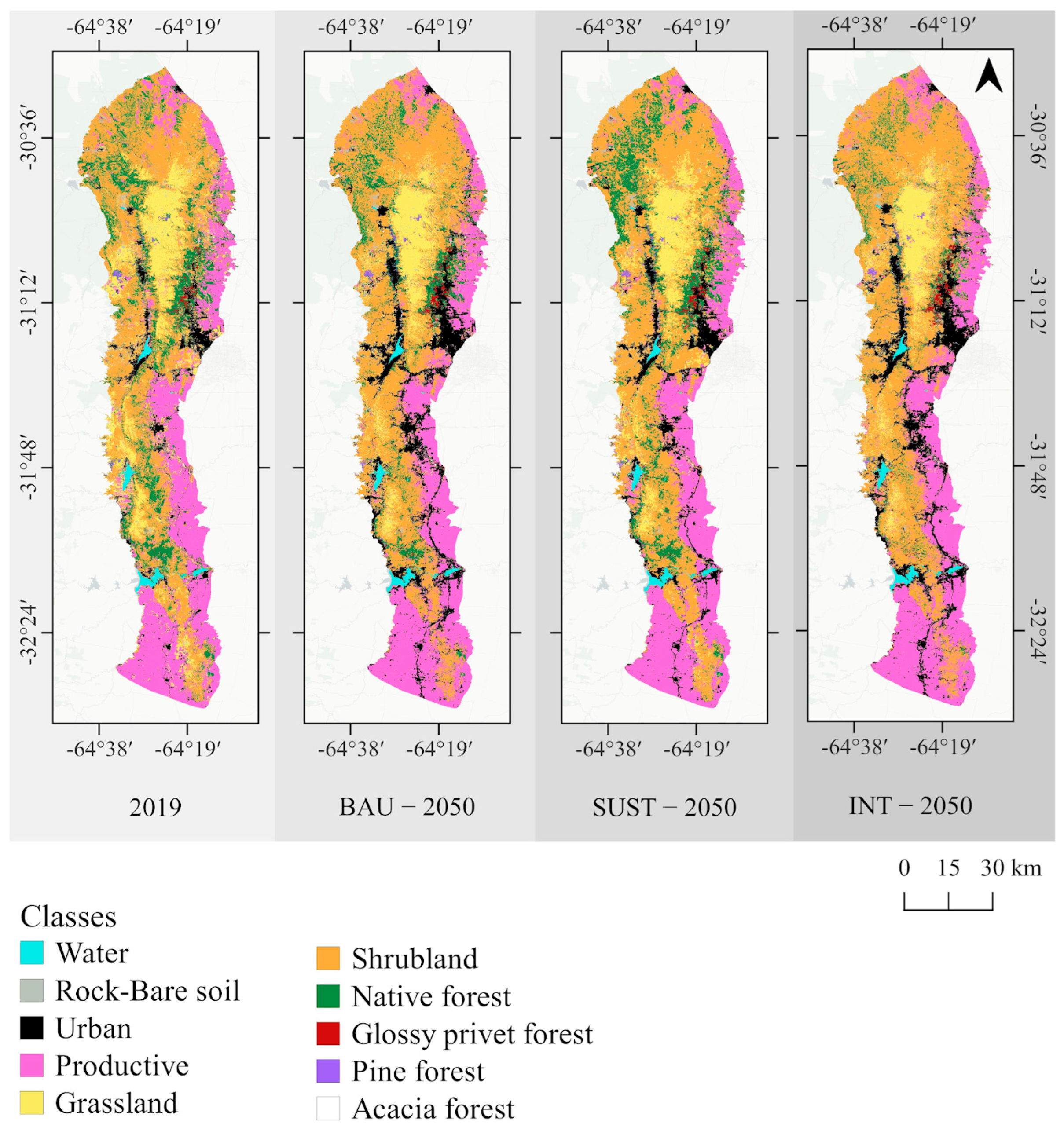
3.1. Future Land-Use Change Scenarios
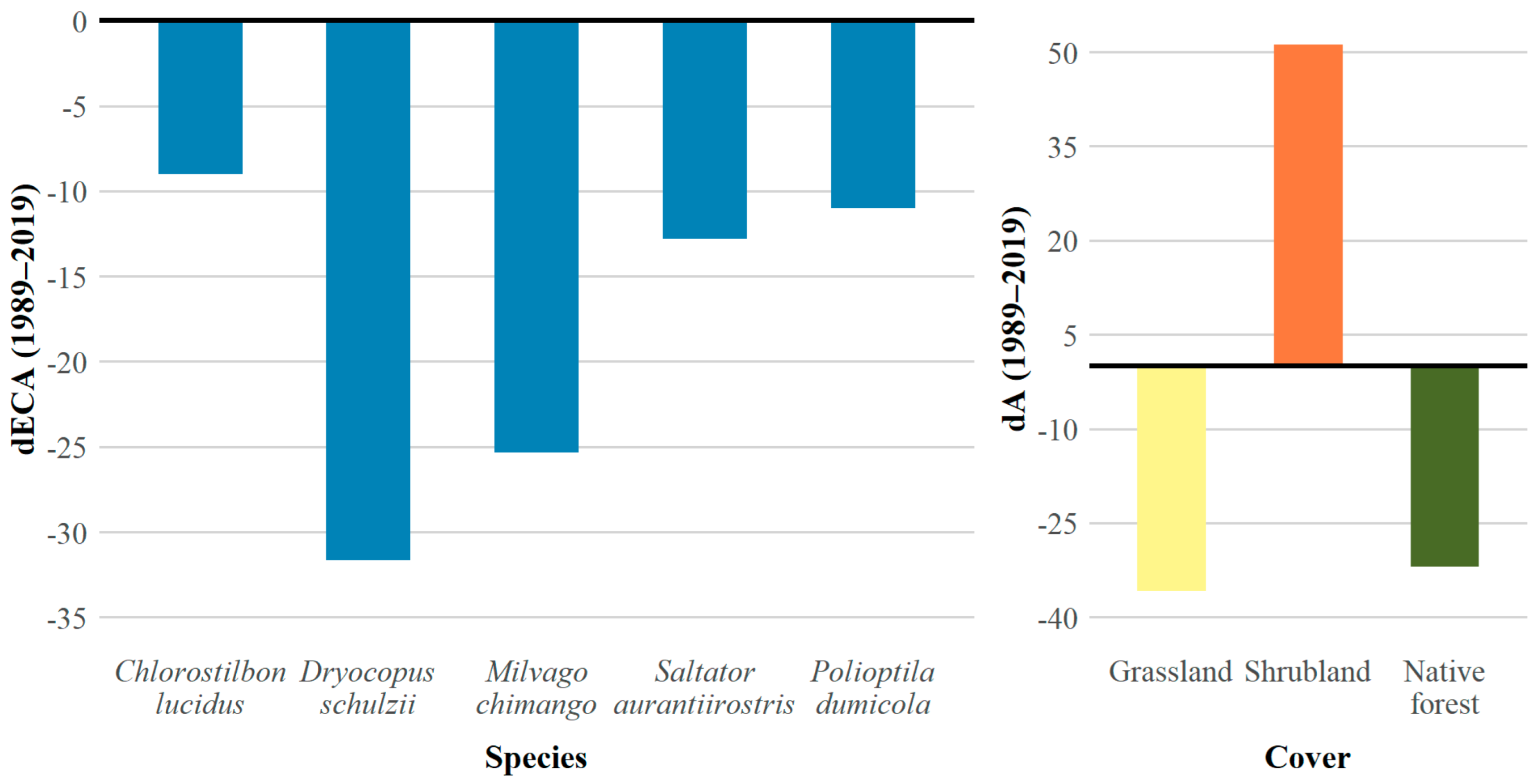
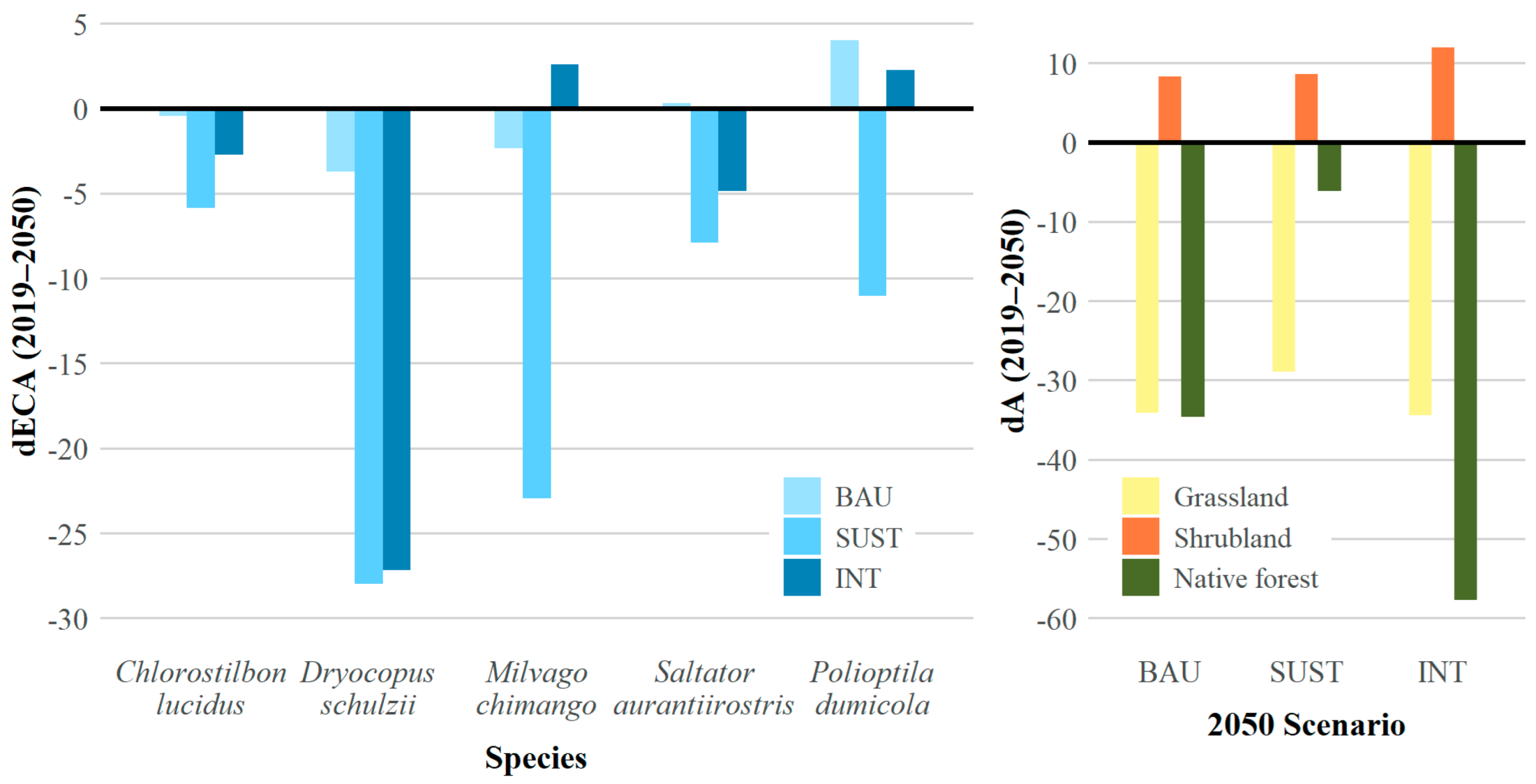
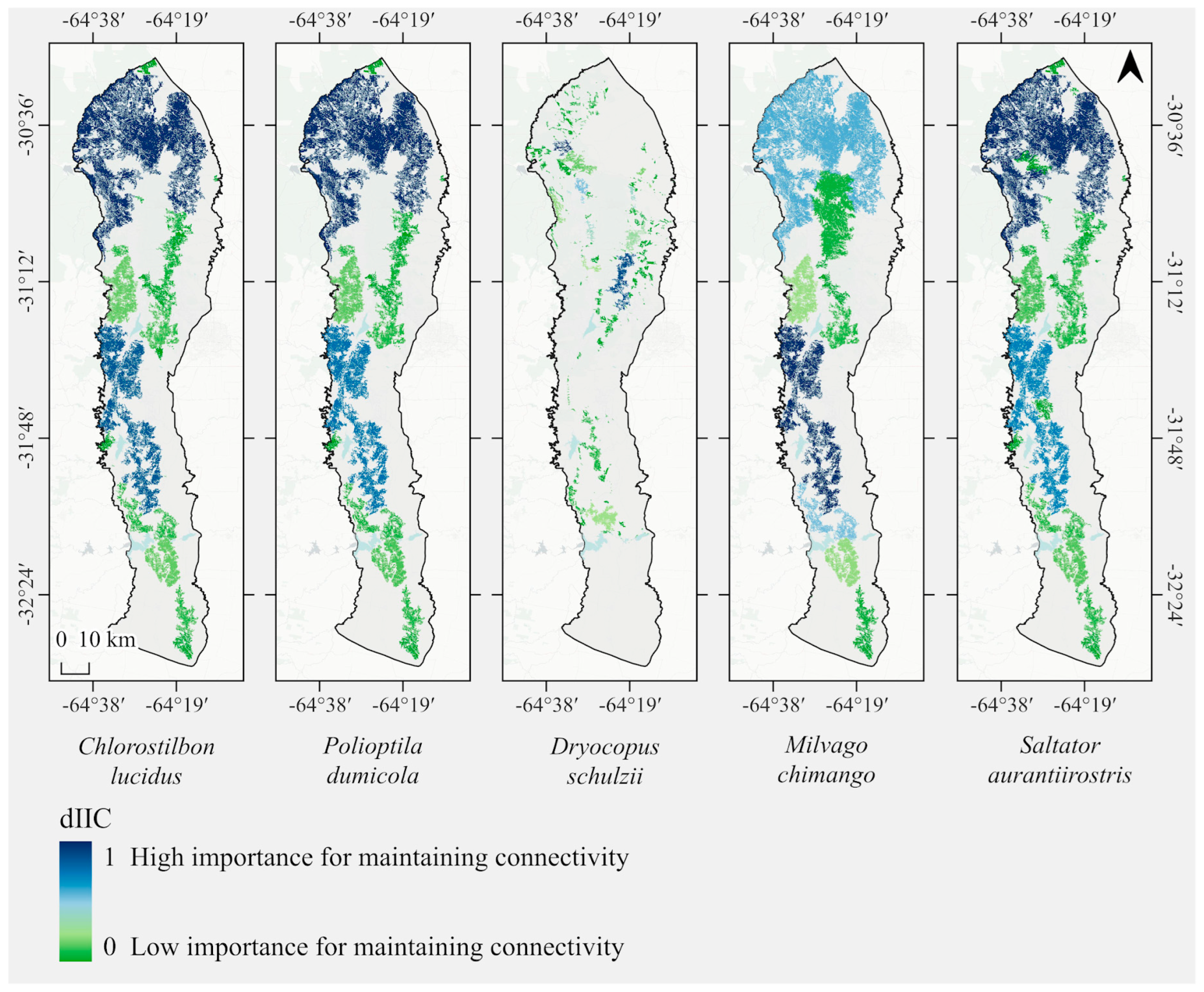
3.2. Functional Connectivity
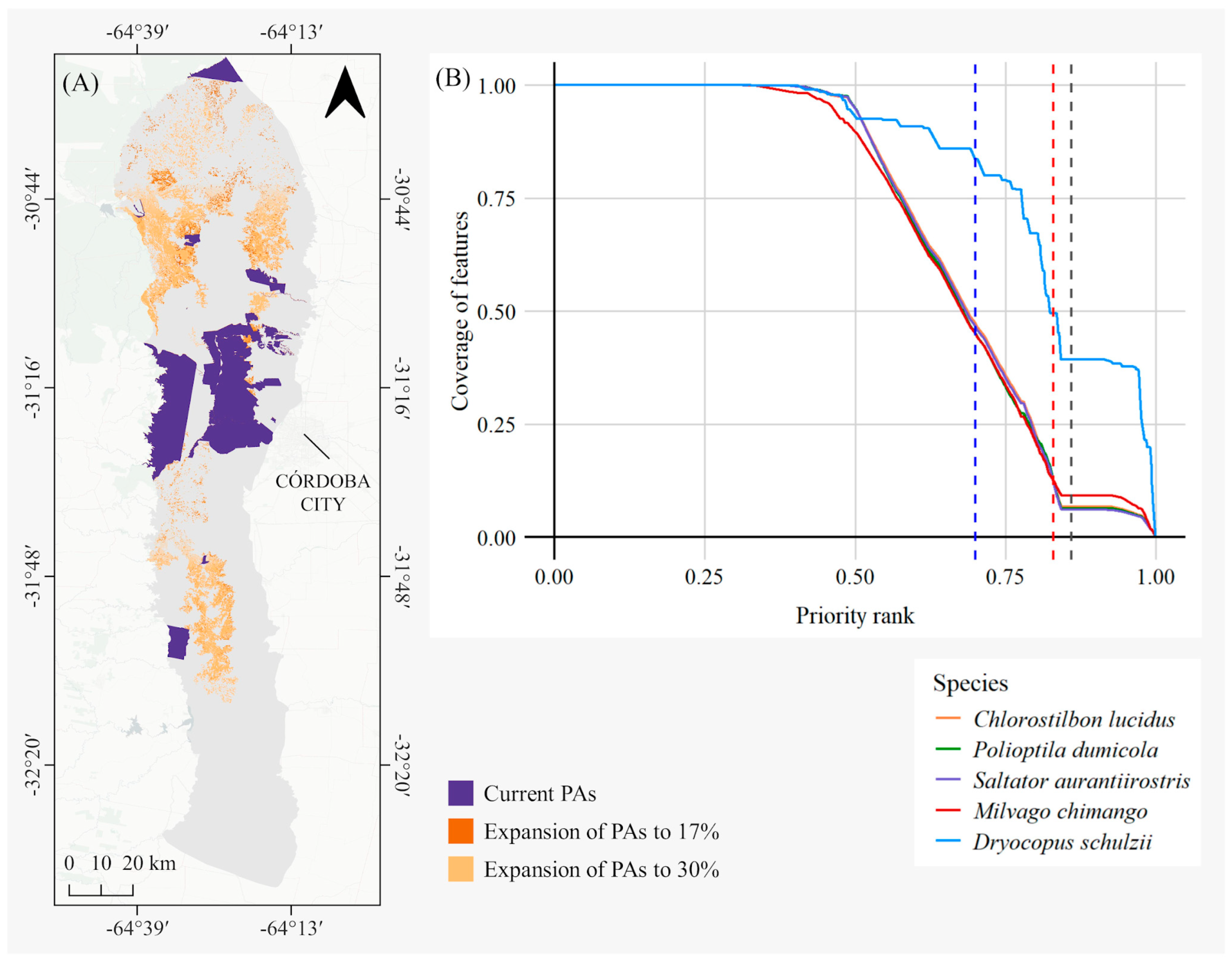
3.3. Strategic Areas for Protected Area Expansion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LULC | Land-Use/Land-Cover |
| PAs | Protected areas |
| BAU | Business-as-usual scenario |
| SUST | Sustainability scenario |
| INT | Intensification scenario |
| IUCN | International Union for Conservation of Nature |
| ECA | Equivalent Connected Area index |
| IIC | Integral Index of Connectivity |
| dECA | Relative change in equivalent connected area |
| dA | Relative change in habitat area |
Appendix A. Future Land-Use Change Scenario Modeling
- Urban Zone: a 300 m buffer surrounding the main urban areas. This distance was chosen because it approximates the extent of urban expansion between 2004 and 2019.
- Non-Urban Zone: the area outside the main urban settlements and their buffer zones.
- Northwest Zone: a native forest area located in the northern part of the study area, away from extensive urbanization and invasions of exotic tree species.
| Variable | Description | Source |
|---|---|---|
| Physical-environmental | ||
| Altitude (m.a.s.l.) | DEM NASA SRTM version 3.0 [98] | |
| Slope (degrees) | Derived from the digital elevation model | |
| Orientation (degrees) | ||
| Temperature (°C) | Mean value for the period 1940–2000 | WorldClim BIO12 [99] |
| Annual precipitation (mm) | ||
| Aridity | Martonne aridity index | Atlas Climático digital de la República Argentina [100] |
| Distance to water (m) | Distance to watercourse or waterbody | Administración Provincial de Recursos Hídricos |
| Native forest patch area (ha) | The pixel value represents the area of the native forest patch it is part of | [52] |
| Distance to grassland (m) | Distance from each raster pixel to the corresponding class, cover type, or feature of interest | [52] |
| Distance to shrubland (m) | ||
| Distance to native forest (m) | ||
| Distance to glossy privet forest (m) | ||
| Distance to pine forest (m) | ||
| Antropic | ||
| Fire frecuency | Fire frecuency between 2004 and 2018 | [101] |
| Population density (Hab/km2) | Population density derived from the 2001 and 2010 national census data | [102] |
| Distance to roads (m) | Distance to primary, secondary, and tertiary roads | Mapas Córdoba–IDECOR |
| Distance to urban areas (m) | Distance from each raster pixel to the corresponding class, cover type, or feature of interest | [52] |
| Distance to productive areas (m) | ||
Appendix B
| Name | |
|---|---|
| National PAs | La Calera Ascochinga |
| Provincial PAs | Reserva forestal natural Uritorco Reserva forestal natural Sierras de Punilla Reserva hídrica natural los Gigantes Reserva hídrica natural la Quebrada Corredor biogeográfico Chaco Árido |
| Municipal PAs | Área protegidas AP1 Villa Carlos Paz Reserva urbana San Martín Reserva natural Quisquisacate Reserva forestal natural Sierra de Cuniputo Reserva hídrica natural Salsipuedes Reserva de uso múltiple Villa General Belgrano Reserva hídrica natural Villa Cerro Azul Reserva natural de uso múltiple de la Rancherita Reserva natural municipal el Portecelo Reserva ecológica recreativa Cuesta Blanca Reserva natural cultural recreativa municipal Tanti Reserva parque recreativo natural Río Yuspe Reserva natural comunal Camin Cosquín Reserva hídrica recreativa natural Saldán Inchin Reserva hídrica recreativa natural Bamba Área natural protegidas Villa Cielo Reserva los Manantiales Reserva hídrica recreativa Villa Allende Reserva hídrica recreativa los Quebrachitos Reserva Tiu Mayu Reserva hídrica recreativa natural Mendiolaza Área de protección Alta Gracia |
Appendix C
| 2019 | 2050 | |||
|---|---|---|---|---|
| BAU | SUST | INT | ||
| Urban | 50 822.72 | 109 688.39 | 73 307.73 | 111 754.18 |
| Productive | 215 900.50 | 206 441.30 | 205 228.32 | 216 827.65 |
| Grassland | 114 156.38 | 75 161.11 | 81 138.69 | 74 826.44 |
| Shrubland | 323 393.63 | 350 368.13 | 351 369.91 | 362 057.75 |
| Native forest | 108 200.53 | 70 764.31 | 101 543.96 | 45 774.14 |
| Glossy privet forest | 4 147.11 | 4 981.21 | 4 916.26 | 6 318.85 |
| Pine forest | 2 393.91 | 3 124.80 | 2 980.50 | 2 997.23 |
Appendix D. dECA and dA Values
| 1989–2019 | 2019–2050 | |||
|---|---|---|---|---|
| BAU | SUST | INT | ||
| Chlorostilbon lucidus | −15.19 | −0.43 | −5.85 | −2.71 |
| Dryocopus schulzii | −33.83 | −3.71 | −27.97 | −27.18 |
| Milvago chimango | −26.93 | −2.36 | −22.97 | 2.58 |
| Saltator aurantiirostris | −14.54 | 0.31 | −7.92 | −4.87 |
| Polioptila dumicola | −7.36 | 4.00 | −11.01 | 2.25 |
| 1989–2019 | 2019–2050 | |||
|---|---|---|---|---|
| BAU | SUST | INT | ||
| Grassland | −35.82 | −34.16 | −28.92 | −34.45 |
| Shrubland | 51.15 | 8.34 | 8.65 | 11.96 |
| Native forest | −31.93 | −34.60 | −6.15 | −57.70 |
References
- Smith, P.; House, J.I.; Bustamante, M.; Sobocká, J.; Harper, R.; Pan, G.; West, P.C.; Clark, J.M.; Adhya, T.; Rumpel, C.; et al. Global Change Pressures on Soils from Land Use and Management. Glob. Change Biol. 2019, 22, 1008–1028. [Google Scholar] [CrossRef]
- Song, X.P.; Hansen, M.C.; Stehman, S.V.; Potapov, P.V.; Tyukavina, A.; Vermote, E.F.; Townshend, J.R. Global land change from 1982 to 2016. Nature 2018, 560, 639–643. [Google Scholar] [CrossRef]
- Winkler, K.; Fuchs, R.; Rounsevell, M.; Herold, M. Global land use changes are four times greater than previously estimated. Nat. Commun. 2021, 12, 2501. [Google Scholar] [CrossRef] [PubMed]
- Pimm, S.L.; Raven, P. Extinction by numbers. Nature 2000, 403, 843–845. [Google Scholar] [CrossRef]
- Davison, C.W.; Rahbek, C.; Morueta-Holme, N. Land-use change and biodiversity: Challenges for assembling evidence on the greatest threat to nature. Glob. Change Biol. 2021, 27, 5414–5429. [Google Scholar] [CrossRef] [PubMed]
- Jaureguiberry, P.; Titeux, N.; Wiemers, M.; Bowler, D.E.; Coscieme, L.; Golden, A.S.; Jacob, U. The direct drivers of recent global anthropogenic biodiversity loss. Sci. Adv. 2022, 8, eabm9982. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- Faria, D.; Morante-Filho, J.C.; Baumgarten, J.; Bovendorp, R.S.; Cazetta, E.; Gaiotto, F.A.; Mariano-Neto, E.; Mielke, M.S.; Pessoa, M.S.; Rocha-Santos, L.; et al. The breakdown of ecosystem functionality driven by deforestation in a global biodiversity hotspot. Biol. Conserv. 2023, 283, 110126. [Google Scholar] [CrossRef]
- Taylor, B.M.; McIlwain, J.L.; Kerr, A.M. Marine reserves and reproductive biomass: A case study of a heavily targeted reef fish. PLoS ONE 2012, 7, e39599. [Google Scholar] [CrossRef]
- Ghosh-Harihar, M.; An, R.; Athreya, R.; Borthakur, U.; Chanchani, P.; Chetry, D.; Datta, A.; Harihar, A.; Karanth, K.K.; Mariyam, D.; et al. Protected areas and biodiversity conservation in India. Biol. Conserv. 2019, 237, 114–124. [Google Scholar] [CrossRef]
- Geldmann, J.; Manica, A.; Burgess, N.D.; Coad, L.; Balmford, A. A global-level assessment of the effectiveness of protected areas at resisting anthropogenic pressures. Proc. Natl. Acad. Sci. USA 2019, 116, 23209–23215. [Google Scholar] [CrossRef] [PubMed]
- Marcos, C.; Díaz, D.; Fietz, K.; Forcada, A.; Ford, A.; García-Charton, J.A.; Goñi, R.; Lenfant, P.; Mallo, S.; Mouillot, D.; et al. Reviewing the ecosystem services, societal goods, and benefits of marine protected areas. Front. Mar. Sci. 2021, 8, 613819. [Google Scholar] [CrossRef]
- Convention on Biological Diversity (CBD). Strategic Plan for Biodiversity 2011–2020 and the Aichi Targets. In Decisions Adopted by the Conference of the Parties to the Convention on Biological Diversity at Its Tenth Meeting; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2010. [Google Scholar]
- Convention on Biological Diversity (CBD). Report of the Open-ended Working Group on the Post-2020 Global Biodiversity Framework on its third meeting. In Proceedings of the Open-Ended Working Group on the Post-2020 Global Biodiversity Framework. Third Meeting, Online, 23 August–3 September 2021. [Google Scholar]
- Rudnick, D.; Ryan, S.; Beier, P.; Cushman, S.A.; Dieffenbach, F.; Epps, C.W.; Gerber, L.; Hartter, J.; Jenness, J.S.; Kintsch, J.; et al. The role of landscape connectivity in planning and implementing conservation and restoration priorities. Issues Ecol. 2012, 16, 20. [Google Scholar]
- Keeley, A.T.; Beier, P.; Jenness, J.S. Connectivity metrics for conservation planning and monitoring. Biol. Conserv. 2021, 255, 109008. [Google Scholar] [CrossRef]
- Correa Ayram, C.A.; Mendoza, M.E.; Etter, A.; Salicrup, D.R.P. Habitat connectivity in biodiversity conservation: A review of recent studies and applications. Prog. Phys. Geogr. 2016, 40, 7–37. [Google Scholar] [CrossRef]
- Pascual-Hortal, L.; Saura, S. Comparison and development of new graph-based landscape connectivity indices: Towards the prioritization of habitat patches and corridors for conservation. Landsc. Ecol. 2006, 21, 959–967. [Google Scholar] [CrossRef]
- Brussaard, L.; Caron, P.; Campbell, B.; Lipper, L.; Mainka, S.; Rabbinge, R.; Babin, D.; Pulleman, M. Reconciling biodiversity conservation and food security: Scientific challenges for a new agriculture. Curr. Opin. Environ. Sustain. 2010, 2, 34–42. [Google Scholar] [CrossRef]
- Mosciaro, M.J.; Calamari, N.C.; Peri, P.L.; Montes, N.F.; Seghezzo, L.; Ortiz, E.; Rejalaga, L.; Barral, P.; Villarino, S.; Mastrangelo, M.; et al. Future scenarios of land use change in the Gran Chaco: How far is zero deforestation? Reg. Environ. Change 2022, 22, 115. [Google Scholar] [CrossRef]
- Malek, Z.; Boerboom, L.; Glade, T. Future forest cover change scenarios with implications for landslide risk: An example from Buzau Subcarpathians. Environ. Manag. 2015, 56, 1228–1243. [Google Scholar] [CrossRef]
- Tejada, G.; Dalla-Nora, E.; Cordoba, D.; Lafortezza, R.; Ovando, A.; Assis, T.; Aguiar, A.P. Deforestation scenarios for the Bolivian lowlands. Environ. Res. 2016, 144, 49–63. [Google Scholar] [CrossRef]
- IPBES. Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Zenodo: Geneva, Switzerland, 2019. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. In Climate Change 2023: Synthesis Report; Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar]
- Dockerty, T.; Lovett, A.; Appleton, K.; Sunnenberg, B.A. Developing scenarios and visualizations to illustrate potential policy and climatic influences on future agricultural landscapes. Agric. Ecosyst. Environ. 2006, 114, 103–120. [Google Scholar] [CrossRef]
- Alcamo, J.; Henrichs, T. Chapter two towards guidelines for environmental scenario analysis. Dev. Integr. Environ. Assess. 2008, 2, 13–35. [Google Scholar]
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050; FAO: Rome, Italy, 2018. [Google Scholar]
- Gavier Pizarro, G.; Calamari, N.; Piquer-Rodríguez, M.; Kuemmerle, T. El método de construcción de escenarios aplicado al Ordenamiento Territorial. In El Ordenamiento Territorial Rural en Argentina. Bases Conceptuales, Herramientas y Experiencias; FAO: Rome, Italy, 2014. [Google Scholar]
- Mori, A.S.; Isbell, F.; Seidl, R. β-diversity, community assembly, and ecosystem functioning. Trends Ecol. Evol. 2018, 33, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Torres, D.A.; Díaz, S.; Cordier, J.M.; Torres, R.; Caron, M.; Nori, J. Analyzing individual drivers of global changes promotes inaccurate long-term policies in deforestation hotspots: The case of Gran Chaco. Biol. Conserv. 2022, 269, 109536. [Google Scholar] [CrossRef]
- Moilanen, A.; Montesino Pouzols, F.; Meller, L.; Veach, V.; Arponen, A.; Leppanen, J.; Kujala, H. Spatial Conservation Planning Methods and Software Zonation; University of Helsinki, Department of Biosciences: Helsinki, Finland, 2014. [Google Scholar]
- Mumby, P.J.; Elliott, I.A.; Eakin, C.M.; Skirving, W.; Paris, C.B.; Edwards, H.J.; Enriquez, S.; Iglesias-Prieto, R.; Cherubin, L.M.; Stevens, J.R. Reserve design for uncertain responses of coral reefs to climate change. Ecol. Lett. 2011, 14, 132–140. [Google Scholar] [CrossRef]
- Jacoboski, L.I.; Hartz, S.M. Using functional diversity and taxonomic diversity to assess effects of afforestation of grassland on bird communities. Perspect. Ecol. Conserv. 2020, 18, 103–108. [Google Scholar] [CrossRef]
- Boscolo, D.; Metzger, J.P. Is bird incidence in Atlantic forest fragments influenced by landscape patterns at multiple scales? Landsc. Ecol. 2009, 24, 907–918. [Google Scholar] [CrossRef]
- Albanesi, S.; Dardanelli, S.; Bellis, L.M. Effects of fire disturbance on bird communities and species of mountain Serrano forest in central Argentina. J. For. Res. 2014, 19, 105–114. [Google Scholar] [CrossRef]
- Thorn, S.; Bässler, C.; Brandl, R.; Burton, P.J.; Cahall, R.; Campbell, J.L.; Castro, J.; Choi, C.Y.; Cobb, T.; Donato, D.C.; et al. Impacts of salvage logging on biodiversity: A meta-analysis. J. Appl. Ecol. 2018, 55, 279–289. [Google Scholar] [CrossRef]
- Dardanelli, S. Dinámica de Comunidades de Aves en Fragmentos de Bosque de la Provincia de Córdoba. Ph.D. Thesis, Facultad de Ciencias Exactas, Físicas y Naturales, Universidad Nacional de Córdoba, Córdoba, Argentina, 2006. [Google Scholar]
- Pidgeon, A.M.; Radeloff, V.C.; Flather, C.H.; Lepczyk, C.A.; Clayton, M.K.; Hawbaker, T.J.; Hammer, R.B. Associations of forest bird species richness with housing and landscape patterns across the USA. Ecol. Appl. 2007, 17, 1989–2010. [Google Scholar] [CrossRef]
- Paritsis, J.; Aizen, M. Effects of exotic conifer plantations on the biodiversity of understory plants, epigeal beetles, and birds in Nothofagus dombeyi forests. For. Ecol. Manag. 2008, 255, 1575–1583. [Google Scholar] [CrossRef]
- Szumik, C.; Aagesen, L.; Casagranda, D.; Arzamendia, V.; Baldo, D.; Claps, L.E.; Zuloaga, F.O. Detecting areas of endemism with a taxonomically diverse data set: Plants, mammals, reptiles, amphibians, birds, and insects from Argentina. Cladistics 2012, 28, 317–329. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Townshend, J. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerle, T.; Altrichter, M.; Baldi, G.; Cabido, M.; Camino, M.; Cuellar, E.; Zak, M. Forest conservation: Remember Gran Chaco. Science 2017, 355, 465. [Google Scholar] [CrossRef] [PubMed]
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Gordon, J.E. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar] [CrossRef]
- Laurance, W.F.; Sayer, J.; Cassman, K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014, 29, 107–116. [Google Scholar] [CrossRef]
- Baumann, M.; Israel, C.; Piquer-Rodríguez, M.; Gavier Pizarro, G.; Volante, J.N.; Kuemmerle, T. Deforestation and cattle expansion in the Paraguayan Chaco 1987–2012. Reg. Environ. Change 2017, 17, 1179–1191. [Google Scholar] [CrossRef]
- Buchadas, A.; Baumann, M.; Meyfroidt, P.; Kuemmerle, T. Uncovering major types of deforestation frontiers across the world’s tropical dry woodlands. Nat. Sustain. 2022, 5, 619–627. [Google Scholar] [CrossRef]
- Romero-Muñoz, A.; Fandos, G.; Benítez-López, A.; Kuemmerle, T. Habitat destruction and overexploitation drive widespread declines in all facets of mammalian diversity in the Gran Chaco. Glob. Change Biol. 2021, 27, 755–767. [Google Scholar] [CrossRef]
- González, E.; Rossetti, M.R.; Moreno, M.L.; Bernaschini, M.L.; Cagnolo, L.; Musicante, M.L.; Valladares, G. Habitat loss and fragmentation in Chaco forests: A review of the responses of insect communities and consequences for ecosystem processes. In Insect Decline and Conservation in the Neotropics; Santos, J.C., Fernandes, G.W., Eds.; Springer: Cham, Switzerland, 2024; pp. 129–162. [Google Scholar]
- Piquer-Rodríguez, M.; Torella, S.; Gavier Pizarro, G.; Volante, J.N.; Somma, D.; Ginzburg, R.; Kuemmerle, T. Effects of past and future land conversions on forest connectivity in the Argentine Chaco. Landsc. Ecol. 2015, 30, 817–833. [Google Scholar] [CrossRef]
- Piquer-Rodríguez, M.; Butsic, V.; Gärtner, P.; Macchi, L.; Baumann, M.; Pizarro, G.G.; Volante, J.N.; Gasparri, I.N.; Kuemmerle, T. Drivers of agricultural land-use change in the Argentine Pampas and Chaco regions. Appl. Geogr. 2018, 91, 111–122. [Google Scholar] [CrossRef]
- TNC. Gran Chaco Americano Ecoregional Assessment. In Fundación Vida Silvestre Argentina, Fundación Para el Desarrollo Sustentable del Chaco (Del Chaco) and Wildlife Conservation Society Bolivia (WCS), 1st ed.; Nature Conservancy: Arlington, VA, USA, 2005. [Google Scholar]
- Arcamone, J.R.; Bellis, L.M.; Silvetti, L.E.; Gavier Pizarro, G.I. 30 years of land cover changes within a global deforestation front: Insights from the Chaco Serrano Mountains. Land Degrad. Dev. 2025, 36, 2854–2867. [Google Scholar] [CrossRef]
- Gavier Pizarro, G.I.; Bucher, E.H. Deforestación de las Sierras Chicas de Córdoba (Argentina) en el período 1970–1997. Acad. Nac. Cienc. Córdoba 2004, 101, 1–27. [Google Scholar]
- Giorgis, M.A.; Cingolani, A.M.; Chiarini, F.; Chiapella, J.; Barboza, G.; Ariza Espinar, L.; Cabido, M. Composición florística del Bosque Chaqueño Serrano de la provincia de Córdoba, Argentina. Kurtziana 2011, 36, 9–43. [Google Scholar]
- Giorgis, M.A.; Cingolani, A.M.; Gurvich, D.E.; Tecco, P.A.; Chiapella, J.; Chiarini, F.; Cabido, M. Changes in floristic composition and physiognomy are decoupled along elevation gradients in central Argentina. Appl. Veg. Sci. 2017, 20, 558–571. [Google Scholar] [CrossRef]
- Cingolani, A.M.; Giorgis, M.A.; Hoyos, L.E.; Cabido, M. La vegetación de las montañas de Córdoba (Argentina) a comienzos del siglo XXI: Un mapa base para el ordenamiento territorial. Bol. Soc. Argent. Bot. 2022, 57, 51–60. [Google Scholar] [CrossRef]
- Cabido, M.; Zeballos, S.R.; Zak, M.; Carranza, M.L.; Giorgis, M.A.; Cantero, J.J.; Acosta, A.T. Native woody vegetation in central Argentina: Classification of Chaco and Espinal forests. Appl. Veg. Sci. 2018, 21, 298–311. [Google Scholar] [CrossRef]
- Giorgis, M.A.; Palchetii, M.V.; Morera, R.; Cabido, M.; Chiapella, J.O.; Cingolani, A.M. Flora vascular de las montañas de Córdoba (Argentina): Características y distribución de las especies a través del gradiente altitudinal. Bol. Soc. Argent. Bot. 2021, 56, 327–345. [Google Scholar] [CrossRef]
- Naval Fernández, M.C.; Albornoz, J.; Bellis, L.M.; Baldini, C.; Arcamone, J.; Silvetti, L.; Argañaraz, J.P. Megaincendios 2020 en Córdoba: Incidencia del fuego en áreas de valor ecológico y socioeconómico. Ecol. Austral 2023, 33, 136–151. [Google Scholar] [CrossRef]
- Bellis, L.M.; Astudillo, A.; Gavier Pizarro, G.; Dardanelli, S.; Landi, M.; Hoyos, L. Glossy privet (Ligustrum lucidum) invasion decreases Chaco Serrano forest bird diversity but favors its seed dispersers. Biol. Invasions 2021, 23, 723–739. [Google Scholar] [CrossRef]
- Silvetti, L.E.; Gavier Pizarro, G.; Solari, L.M.; Arcamone, J.R.; Bellis, L.M. Land use changes and bird diversity in subtropical forests: Urban development as the underlying factor. Biodivers. Conserv. 2023, 32, 385–403. [Google Scholar] [CrossRef]
- Agudelo-Henríquez, W.J. Escenarios Futuros de Deforestación Como Herramienta Para Evaluar Políticas de Manejo en un Sector de las Sierras Chicas de Córdoba, Argentina. Master’s Thesis, Centro de Zoología Aplicada, Córdoba, Argentina, 2015. [Google Scholar]
- IPLAM Plan Vial y Usos del Suelo. Ley 9841. Regulación de Usos del Suelo. 2012. Available online: https://www.idecor.gob.ar/accede-al-mapa-del-plan-metropolitano-de-usos-del-suelo-de-cordoba/ (accessed on 1 February 2023).
- Soares-Filho, B.S.; Rodrigues, H.; Costa, W.; Schlesinger, P. Modeling Environmental Dynamics with Dinamica EGO. Belo Horizonte, Minas Gerais. 2009. Available online: https://dinamicaego.com/dokuwiki/doku.php?id=guidebook_start (accessed on 1 February 2023).
- Silvetti, L.; Arcamone, J.; Gavier Pizarro, G.; Landi, M.; Bellis, L.M. Land use change scenarios and their implications for bird conservation in subtropical forests. Forests 2025, 16, 1001. [Google Scholar] [CrossRef]
- Stotz, D.F.; Fitzpatrick, J.W.; Parker, T.A., III; Moskovits, D.K. Neotropical Birds: Ecology and Conservation; University of Chicago Press: Chicago, IL, USA, 1996. [Google Scholar]
- De La Ossa, V.; De La Ossa-Lacayo, A. Aspects of population density and natural history of Milvago chimachima (Aves: Falconidae) in the urban area of Sincelejo (Sucre, Colombia). Univ. Sci. 2011, 16, 63–69. [Google Scholar] [CrossRef]
- Díaz Vélez, M.C.; Silva, W.R.; Pizo, M.A.; Galetto, L. Movement patterns of frugivorous birds promote functional connectivity among Chaco Serrano woodland fragments in Argentina. Biotropica 2015, 47, 475–483. [Google Scholar] [CrossRef]
- Wynia, A.L.; Jiménez, J.E. Assessment of larvae availability on Magellanic woodpecker foraging behavior. Bosque 2019, 40, 81–86. [Google Scholar] [CrossRef]
- Saura, S.; Estreguil, C.; Mouton, C.; Rodríguez-Freire, M. Network analysis to assess landscape connectivity trends: Application to European forests (1990–2000). Ecol. Indic. 2011, 11, 407–416. [Google Scholar] [CrossRef]
- Hernández, A.; Miranda, M.; Arellano, E.C.; Saura, S.; Ovalle, C. Landscape dynamics and their effect on the functional connectivity of a Mediterranean landscape in Chile. Ecol. Indic. 2015, 48, 198–206. [Google Scholar] [CrossRef]
- Saura, S.; Pascual-Hortal, L. A new habitat availability index to integrate connectivity in landscape conservation planning: Comparison with existing indices and application to a case study. Landsc. Urban Plan. 2007, 83, 91–103. [Google Scholar] [CrossRef]
- Godínez-Gómez, O.; Correa Ayram, C.A. Makurhini: Analyzing Landscape Connectivity. R Package Version. 2020. Available online: https://connectscape.github.io/Makurhini/ (accessed on 1 April 2025).
- Moilanen, A.; Lehtinen, P.; Kohonen, I.; Jalkanen, J.; Virtanen, E.A.; Kujala, H. Novel methods for spatial prioritization with applications in conservation, land use planning and ecological impact avoidance. Methods Ecol. Evol. 2022, 13, 1062–1072. [Google Scholar] [CrossRef]
- Di Minin, E.; Veach, V.; Lehtomäki, J.; Montesino Pouzols, F.; Moilanen, A. A Quick Introduction to Zonation; University of Helsinki: Helsinki, Finland, 2014. [Google Scholar]
- Williams, S.H.; Scriven, S.A.; Burslem, D.F.; Hill, J.K.; Reynolds, G.; Agama, A.L.; Brodie, J.F. Incorporating connectivity into conservation planning for the optimal representation of multiple species and ecosystem services. Conserv. Biol. 2020, 34, 934–942. [Google Scholar] [CrossRef]
- Xu, Y.; Si, Y.; Wang, Y.; Zhang, Y.; Prins, H.H.; Cao, L.; de Boer, W.F. Loss of functional connectivity in migration networks induces population decline in migratory birds. Ecol. Appl. 2019, 29, e01960. [Google Scholar] [CrossRef]
- Albert, C.H.; Rayfield, B.; Dumitru, M.; Gonzalez, A. Applying network theory to prioritize multispecies habitat networks that are robust to climate and land-use change. Conserv. Biol. 2017, 31, 1383–1396. [Google Scholar] [CrossRef]
- Gonzalez, A.; Thompson, P.; Loreau, M. Spatial ecological networks: Planning for sustainability in the long-term. Curr. Opin. Environ. Sustain. 2017, 29, 187–197. [Google Scholar] [CrossRef]
- Petersen, W.J.; Savini, T.; Chutipong, W.; Kamjing, A.; Phosri, K.; Tantipisanuh, N.; Ngoprasert, D. Predicted Pleistocene–Holocene range and connectivity declines of the vulnerable fishing cat and insights for current conservation. J. Biogeogr. 2022, 49, 1494–1507. [Google Scholar] [CrossRef]
- Martinez Pardo, J.; Saura, S.; Insaurralde, A.; Di Bitetti, M.S.; Paviolo, A.; De Angelo, C. Much more than forest loss: Four decades of habitat connectivity decline for Atlantic Forest jaguars. Landsc. Ecol. 2023, 38, 41–57. [Google Scholar] [CrossRef]
- Kremen, C.; Merenlender, A.M. Landscapes that work for biodiversity and people. Science 2018, 362, eaau6020. [Google Scholar] [CrossRef]
- Pedroza-Arceo, N.M.; Weber, N.; Ortega-Argueta, A. A knowledge review on integrated landscape approaches. Forests 2022, 13, 312. [Google Scholar] [CrossRef]
- Williams, D.R.; Clark, M.; Buchanan, G.M.; Ficetola, G.F.; Rondinini, C.; Tilman, D. Proactive conservation to prevent habitat losses to agricultural expansion. Nat. Sustain. 2021, 4, 314–322. [Google Scholar] [CrossRef]
- Diengdoh, V.L.; Ondei, S.; Amin, R.J.; Hunt, M.; Brook, B.W. Landscape functional connectivity for butterflies under different scenarios of land-use, land-cover, and climate change in Australia. Biol. Conserv. 2023, 277, 109825. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, H.; Gu, X. Integrating species distribution and piecewise linear regression model to identify functional connectivity thresholds to delimit urban ecological corridors. Comput. Environ. Urban Syst. 2024, 113, 102177. [Google Scholar] [CrossRef]
- Jellesmark, S.; Blackburn, T.M.; Dove, S.; Geldmann, J.; Visconti, P.; Gregory, R.D.; Hoffmann, M. Assessing the global impact of targeted conservation actions on species abundance. bioRxiv 2022. [Google Scholar] [CrossRef]
- Pereira, H.M.; Ferrier, S.; Walters, M.; Geller, G.N.; Jongman, R.H.G.; Scholes, R.J.; Bruford, M.W.; Brummitt, N.; Butchart, S.H.M.; Cardoso, A.C.; et al. Essential biodiversity variables. Science 2013, 339, 277–278. [Google Scholar] [CrossRef]
- Schneider, C. Situación de las Áreas Protegidas de la Provincia de Córdoba. Asoc. Conserv. Estud. Nat. (ACEN) Áreas Protegidas Prov. Córdoba 2020, 2, 57. [Google Scholar]
- Lehtomäki, J.; Kusumoto, B.; Shiono, T.; Tanaka, T.; Kubota, Y.; Moilanen, A. Spatial conservation prioritization for the East Asian islands: A balanced representation of multitaxon biogeography in a protected area network. Divers. Distrib. 2019, 25, 414–429. [Google Scholar] [CrossRef]
- Cox, R.L.; Underwood, E.C. The importance of conserving biodiversity outside of protected areas in Mediterranean ecosystems. PLoS ONE 2011, 6, e14508. [Google Scholar] [CrossRef]
- Laurance, W.F.; Useche, D.C.; Rendeiro, J.; Kalka, M.; Bradshaw, C.J.; Sloan, S.P.; McGraw, W.S. Averting biodiversity collapse in tropical forest protected areas. Nature 2012, 489, 290–294. [Google Scholar] [CrossRef]
- Häkkilä, M.; Le Tortorec, E.; Brotons, L.; Rajasärkkä, A.; Tornberg, R.; Mönkkönen, M. Degradation in landscape matrix has diverse impacts on diversity in protected areas. PLoS ONE 2017, 12, e0184792. [Google Scholar] [CrossRef] [PubMed]
- Beaudry, F.; Ferris, M.C.; Pidgeon, A.M.; Radeloff, V.C. Identifying areas of optimal multispecies conservation value by accounting for incompatibilities between species. Ecol. Model. 2016, 332, 74–82. [Google Scholar] [CrossRef]
- Meyer, C.; Weigelt, P.; Kreft, H. Multidimensional biases, gaps and uncertainties in global plant occurrence information. Ecol. Lett. 2016, 19, 992–1006. [Google Scholar] [CrossRef]
- Leménager, T.; King, D.; Elliott, J.; Gibbons, H.; King, A. Greater than the sum of their parts: Exploring the environmental complementarity of state, private and community protected areas. Glob. Ecol. Conserv. 2014, 2, 238–247. [Google Scholar] [CrossRef]
- McGowan, J.; Beaumont, L.J.; Smith, R.J.; Chauvenet, A.L.; Harcourt, R.; Atkinson, S.C.; Possingham, H.P. Conservation prioritization can resolve the flagship species conundrum. Nat. Commun. 2020, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Farr, T.G.; Rosen, P.A.; Caro, E.; Crippen, R.; Duren, R.; Hensley, S.; Alsdorf, D. The shuttle radar topography mission. Rev. Geophys. 2007, 45, RG2004. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Bianchi, A.R.; Cravero, S.A.C. Atlas Climático Digital de la República Argentina; Ediciones INTA: Salta, Argentina, 2010. [Google Scholar]
- Marinelli, M.; Bustos, S.; Viotto, S.; Clemente, J.; Benítez, J.; Mari, N.; Argañaraz, J. Elaboración de la base de datos de incendios 1987–2018 para las Sierras de Córdoba mediante imágenes Landsat. In Proceedings of the IV Congreso Nacional de Ciencia y Tecnología Ambiental, Florencio Varela, Argentina, 2–5 December 2019. [Google Scholar]
- Rodríguez, G.M. Cartografía Censal Digitalizada del INDEC. Revisada y Corregida, Años 1991, 2001 y 2010. 2018. Available online: https://ri.conicet.gov.ar/handle/11336/149711 (accessed on 21 December 2021).
- Cheng, L.L.; Liu, M.; Zhan, J.Q. Land use scenario simulation of mountainous districts based on Dinamica EGO model. J. Moun. Sci. 2020, 7, 289–303. [Google Scholar] [CrossRef]
| Species | Functional Role/Habitat Requirement | Reproductive Strategy | Territoriality | Abundance Status | Conservation Status (IUCN) | Sensibility |
|---|---|---|---|---|---|---|
| Chlorostilbon lucidus Glittering-bellied Emerald | Pollinator species found in shrubland habitats and forest understories | Solitary breeder; nests in shrubs or low branches | Low territoriality | Common | Least Concern | Favorable |
| Polioptila dumicola Masked Gnatcatcher | Insectivore in forest, forest understory, and shrubland | Builds cup-shaped nests; socially monogamous | Territorial during breeding | Common | Least Concern | Mean |
| Dryocopus schulzii Black-bodied Woodpecker | Ecosystem engineer in mature forests (creates cavities) | Cavity nester; low reproductive rate | Highly territorial | Rare | Vulnerable | High |
| Milvago chimango Chimango Caracara | Opportunistic scavenger in open habitats (grassland/shrubland) | Opportunistic breeder; nests in trees or structures | Low territoriality | Abundant | Least Concern | Low |
| Saltator aurantiirostris Golden-billed Saltator | Seed disperser in shrubland and forest understory | Builds open-cup nests; monogamous pairs | Territorial | Common | Least Concern | Low |
| Specie | Habitat Suitability | Distance Threshold (m.) | ||
|---|---|---|---|---|
| Grassland | Shrubland | Native Forest | ||
| Chlorostilbon lucidus | 0 | 1 | 0.5 | 500 |
| Polioptila dumicola | 0 | 1 | 1 | 500 |
| Dryocopus schulzii | 0 | 0 | 1 | 1000 |
| Milvago chimango | 1 | 0.5 | 0 | 1800 |
| Saltator aurantiirostris | 0 | 1 | 0.5 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcamone, J.R.; Silvetti, L.E.; Bellis, L.M.; Baldini, C.; Alvarez, M.P.; Naval-Fernández, M.C.; Albornoz, J.V.; Gavier Pizarro, G. Functional Connectivity in Future Land-Use Change Scenarios as a Tool for Assessing Priority Conservation Areas for Key Bird Species: A Case Study from the Chaco Serrano. Sustainability 2025, 17, 6874. https://doi.org/10.3390/su17156874
Arcamone JR, Silvetti LE, Bellis LM, Baldini C, Alvarez MP, Naval-Fernández MC, Albornoz JV, Gavier Pizarro G. Functional Connectivity in Future Land-Use Change Scenarios as a Tool for Assessing Priority Conservation Areas for Key Bird Species: A Case Study from the Chaco Serrano. Sustainability. 2025; 17(15):6874. https://doi.org/10.3390/su17156874
Chicago/Turabian StyleArcamone, Julieta Rocío, Luna Emilce Silvetti, Laura Marisa Bellis, Carolina Baldini, María Paula Alvarez, María Cecilia Naval-Fernández, Jimena Victoria Albornoz, and Gregorio Gavier Pizarro. 2025. "Functional Connectivity in Future Land-Use Change Scenarios as a Tool for Assessing Priority Conservation Areas for Key Bird Species: A Case Study from the Chaco Serrano" Sustainability 17, no. 15: 6874. https://doi.org/10.3390/su17156874
APA StyleArcamone, J. R., Silvetti, L. E., Bellis, L. M., Baldini, C., Alvarez, M. P., Naval-Fernández, M. C., Albornoz, J. V., & Gavier Pizarro, G. (2025). Functional Connectivity in Future Land-Use Change Scenarios as a Tool for Assessing Priority Conservation Areas for Key Bird Species: A Case Study from the Chaco Serrano. Sustainability, 17(15), 6874. https://doi.org/10.3390/su17156874






