Assessing Two Decades of Organic Farming: Effects on Soil Heavy Metal Concentrations and Biodiversity for Sustainable Management
Abstract
1. Introduction
2. Materials and Methods
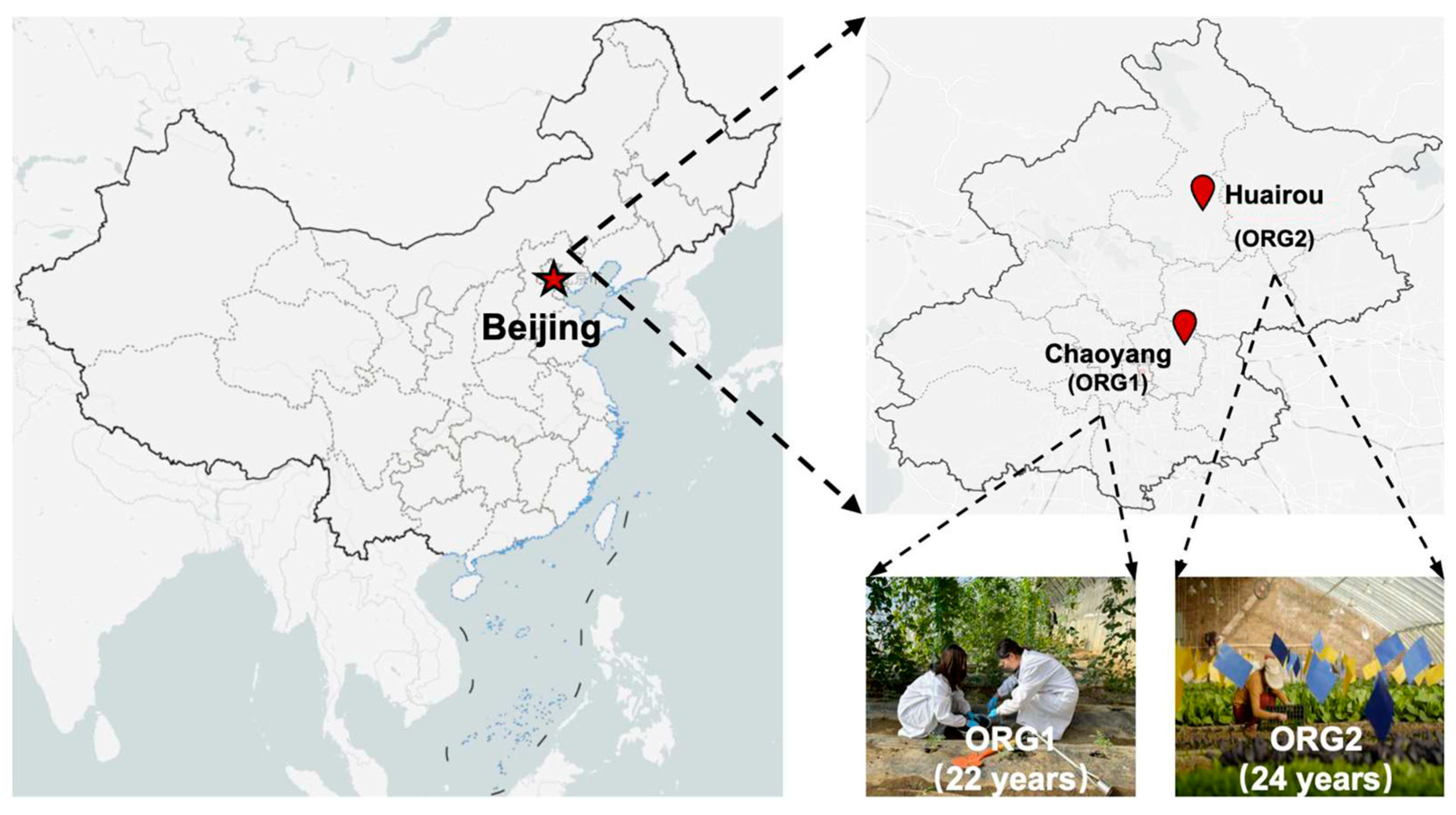
2.1. Studying and Sampling Farms
2.2. Soil Sampling
2.3. Soil Properties and Heavy Metal Analysis
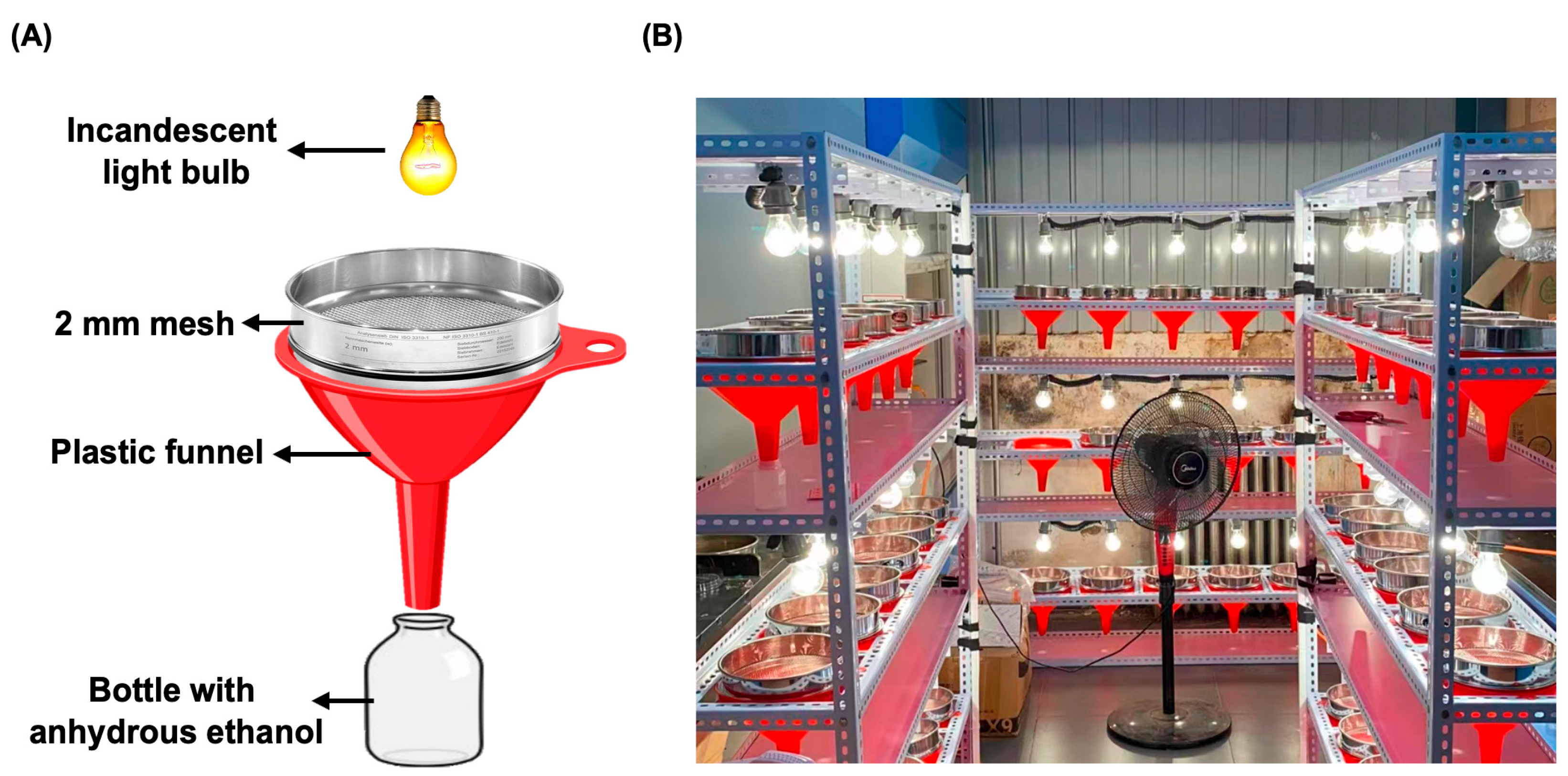
2.4. Collembola Extraction and Identification
2.5. Soil Microbial Analysis
2.6. Statistical Analysis
2.6.1. Heavy Metal Risk Assessment
2.6.2. Collembola Biodiversity Index
2.6.3. Data Processing
3. Results
3.1. Soil Properties of Organic and Conventional Farms
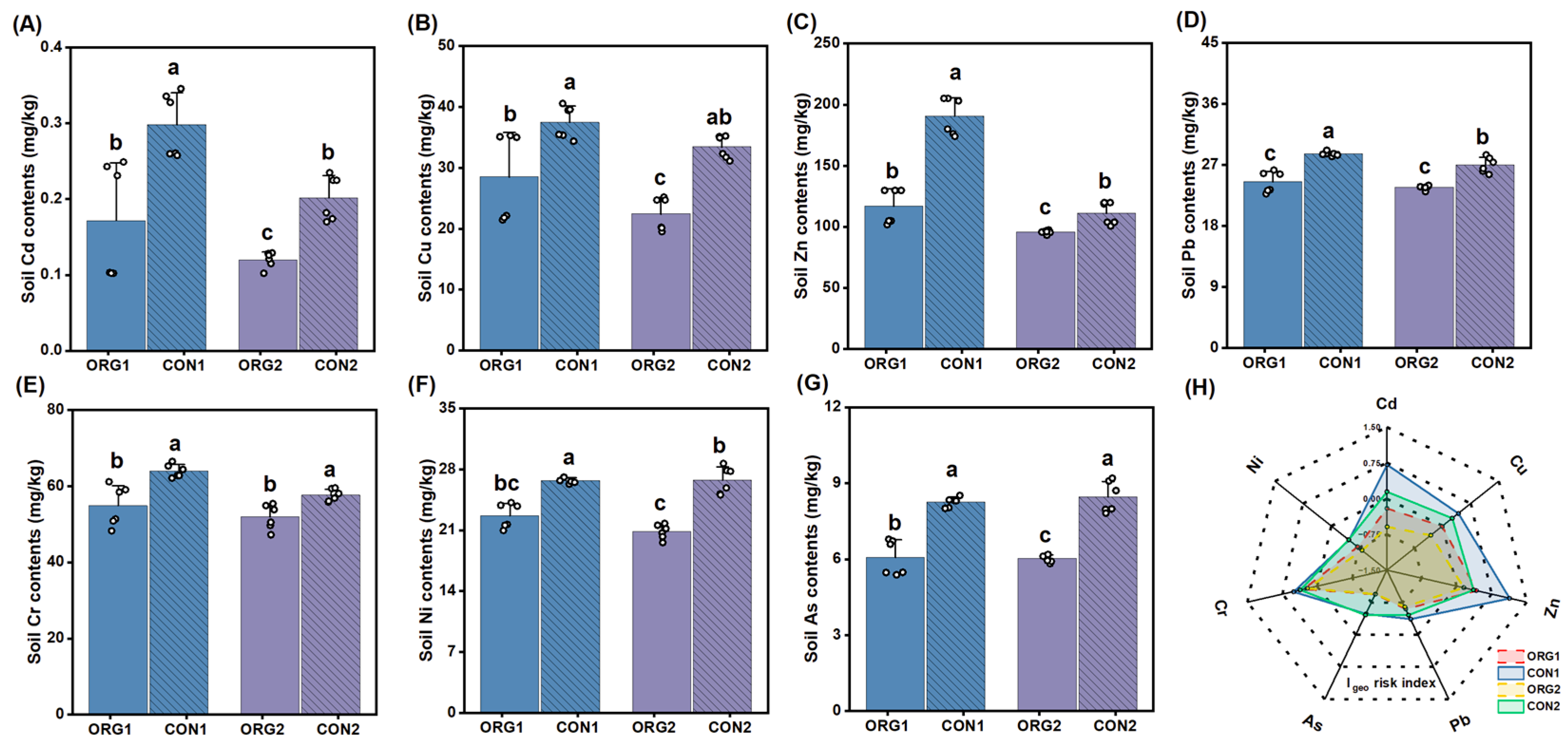
3.2. Heavy Metal Accumulation and Potential Risks
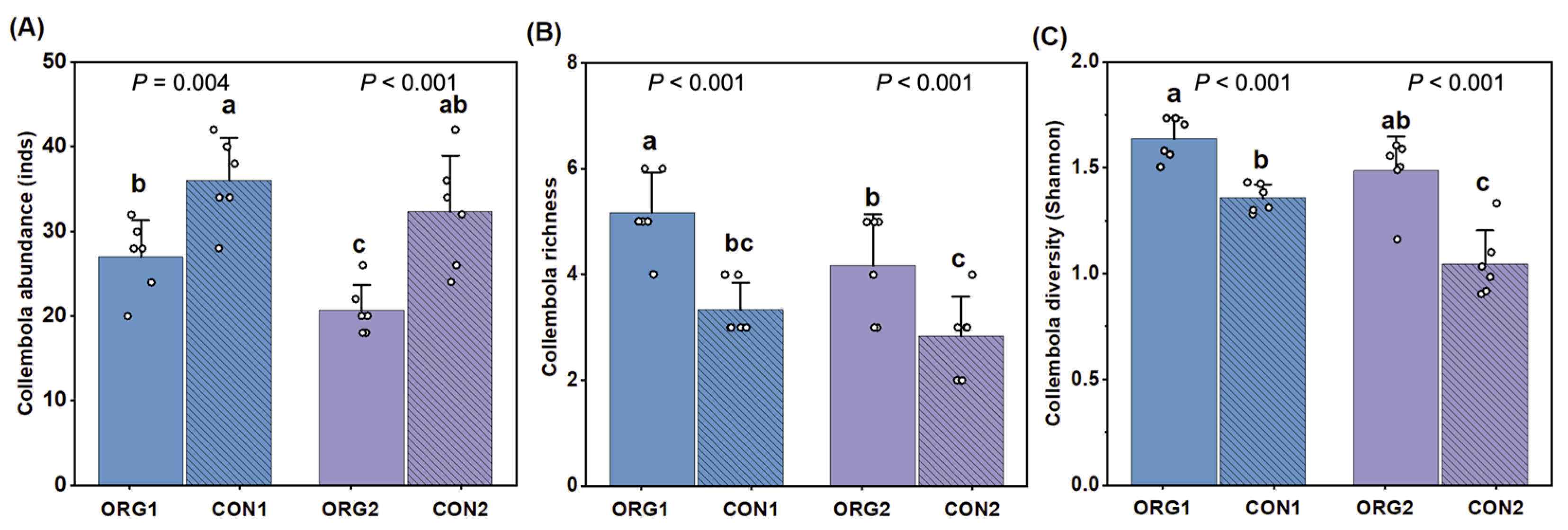
3.3. Soil Collembola Diversity of Organic and Conventional Farms
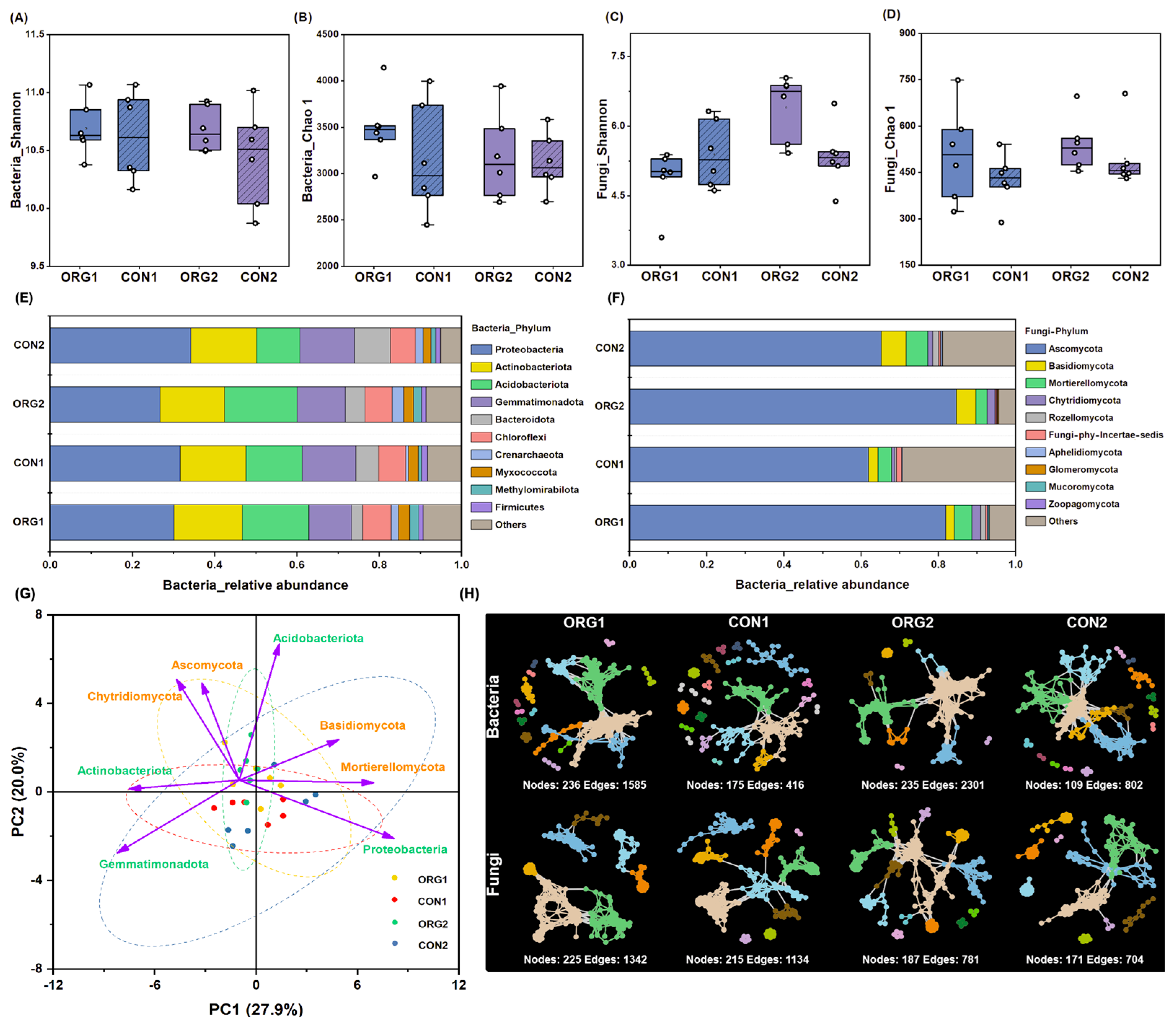
3.4. Soil Microbial Diversity and Community of Organic and Conventional Farms
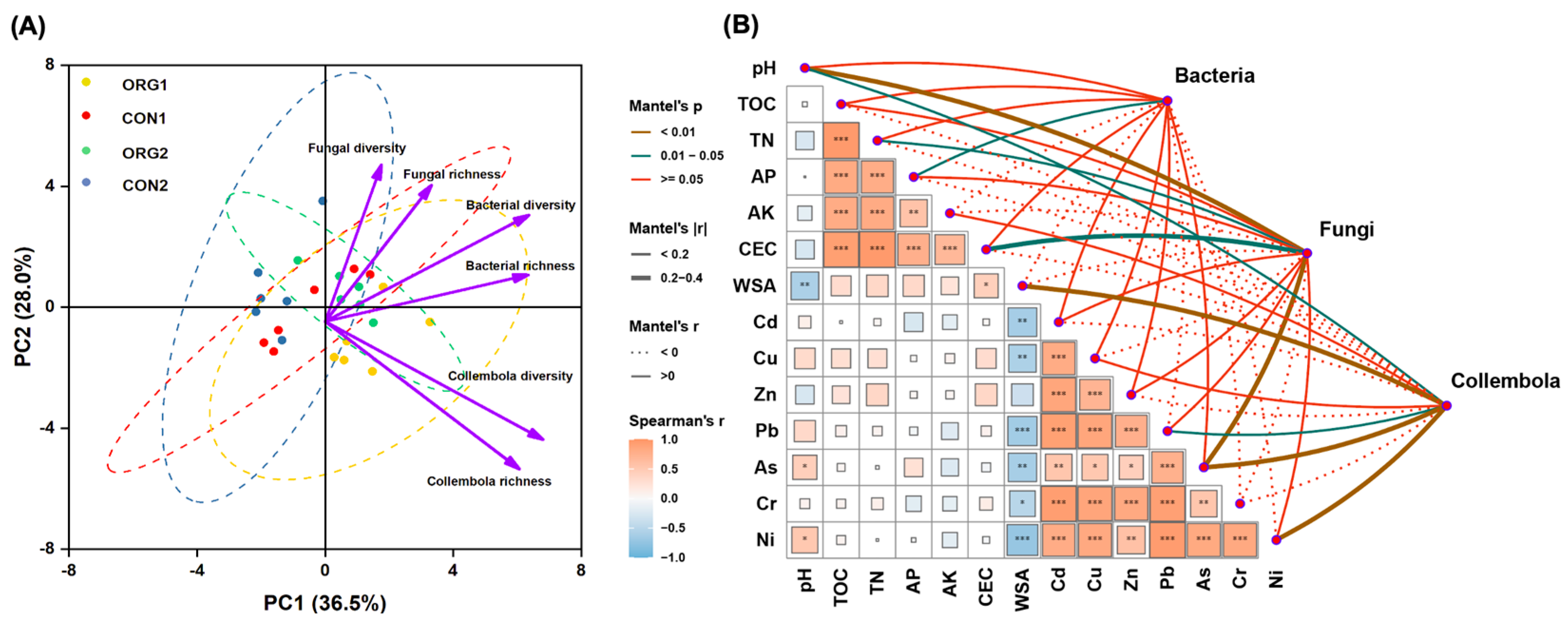
3.5. Soil Factors Impacting the Soil Biodiversity
4. Discussion
4.1. Influence of Organic Practice on Reducing Heavy Metal Accumulation
4.2. Influence of Organic Practice on Harnessing Soil Biodiversity
4.3. Implications for Sustainable Agricultural Management
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; McKenna, B.A.; Lombi, E. Soil and the intensification of agriculture for global food security. Environ. Int. 2019, 132, 105078. [Google Scholar] [CrossRef]
- Abudulai, M.; Nboyine, J.A.; Quandahor, P.; Seidu, A.; Traore, F.; Abudulai, M. Agricultural intensification causes decline in insect biodiversity. Glob. Decline Insects 2022, 1, 43–64. [Google Scholar]
- Sonderegger, T.; Pfister, S. Global assessment of agricultural productivity losses from soil compaction and water erosion. Environ. Sci. Technol. 2021, 55, 12162–12171. [Google Scholar] [CrossRef]
- Gomiero, T. Soil degradation, land scarcity and food security: Reviewing a complex challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Wen, W.; Zhuang, Y.; Jiang, T.; Li, W.; Li, H.; Cai, W.; Xu, D.; Zhang, L. “Period-area-source” hierarchical management for agricultural non-point source pollution in typical watershed with integrated planting and breeding. J. Hydrol. 2024, 635, 131198. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Z.; Lu, Y.; Shan, S.; Zhuang, H.; Gong, C.; Cui, X.; Zhang, F.; Li, P. Facilitating mitigation of agricultural non-point source pollution and improving soil nutrient conditions: The role of low temperature co-pyrolysis biochar in nitrogen and phosphorus distribution. Bioresour. Technol. 2024, 394, 130179. [Google Scholar] [CrossRef]
- Pérez-Lucas, G.; Navarro, G.; Navarro, S. Adapting agriculture and pesticide use in Mediterranean regions under climate change scenarios: A comprehensive review. Eur. J. Agron. 2024, 161, 127337. [Google Scholar] [CrossRef]
- Hou, D.; Jia, X.; Wang, L.; McGrath, S.P.; Zhu, Y.-G.; Hu, Q.; Zhao, F.-J.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar] [CrossRef]
- Sarker, A.; Kim, J.-E.; Islam, A.R.M.T.; Bilal, M.; Rakib, M.R.J.; Nandi, R.; Rahman, M.M.; Islam, T. Heavy metals contamination and associated health risks in food webs—A review focuses on food safety and environmental sustainability in Bangladesh. Environ. Sci. Pollut. Res. 2022, 29, 3230–3245. [Google Scholar] [CrossRef]
- Gamage, A.; Gangahagedara, R.; Gamage, J.; Jayasinghe, N.; Kodikara, N.; Suraweera, P.; Merah, O. Role of organic farming for achieving sustainability in agriculture. Farming Syst. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Muller, A.; Schader, C.; El-Hage Scialabba, N.; Brüggemann, J.; Isensee, A.; Erb, K.-H.; Smith, P.; Klocke, P.; Leiber, F.; Stolze, M.; et al. Strategies for feeding the world more sustainably with organic agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Ruis, S.J.; Francis, C.A. Do organic farming practices improve soil physical properties? Soil Use Manag. 2024, 40, e12999. [Google Scholar] [CrossRef]
- Gao, J.; Han, H.; Gao, C.; Wang, Y.; Dong, B.; Xu, Z. Organic amendments for in situ immobilization of heavy metals in soil: A review. Chemosphere 2023, 335, 139088. [Google Scholar] [CrossRef]
- Wu, P.; Guo, Z.; Hua, K.; Wang, D. Long-term application of organic amendments changes heavy metals accumulation in wheat grains by affecting soil chemical properties and wheat yields. J. Soils Sediments 2023, 23, 2136–2147. [Google Scholar] [CrossRef]
- Bai, Z.; Caspari, T.; Gonzalez, M.R.; Batjes, N.H.; Mäder, P.; Bünemann, E.K.; de Goede, R.; Brussaard, L.; Xu, M.; Ferreira, C.S.S. Effects of agricultural management practices on soil quality: A review of long-term experiments for Europe and China. Agric. Ecosyst. Environ. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; Van Der Heijden, M.G. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Ponge, J.-F.; Pérès, G.; Guernion, M.; Ruiz-Camacho, N.; Cortet, J.; Pernin, C.; Villenave, C.; Chaussod, R.; Martin-Laurent, F.; Bispo, A. The impact of agricultural practices on soil biota: A regional study. Soil Biol. Biochem. 2013, 67, 271–284. [Google Scholar] [CrossRef]
- Filser, J.; Mebes, K.-H.; Winter, K.; Lang, A.; Kampichler, C. Long-term dynamics and interrelationships of soil Collembola and microorganisms in an arable landscape following land use change. Geoderma 2002, 105, 201–221. [Google Scholar] [CrossRef]
- Coulibaly, S.F.M.; Coudrain, V.; Hedde, M.; Brunet, N.; Mary, B.; Recous, S.; Chauvat, M. Effect of different crop management practices on soil Collembola assemblages: A 4-year follow-up. Appl. Soil Ecol. 2017, 119, 354–366. [Google Scholar] [CrossRef]
- Sauma-Sánchez, T.; Alcorta, J.; Tamayo-Leiva, J.; Díez, B.; Bezuidenhout, H.; Cowan, D.A.; Ramond, J.-B. Functional redundancy buffers the effect of poly-extreme environmental conditions on southern African dryland soil microbial communities. FEMS Microbiol. Ecol. 2024, 100, fiae157. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; Volume 9, pp. 539–579. [Google Scholar]
- Bremner, J.M.; Mulvaney, C. Nitrogen—Total. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; Volume 9, pp. 595–624. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA Circular No. 939; U.S. Government Printing Office: Washington, DC, USA, 1954.
- Elliott, E. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci. Soc. Am. J. 1986, 50, 627–633. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Tan, X.; Hou, D.; Fang, L.; Lin, A.; Li, F.; Duan, G. Long-term effectiveness of heavy metal (loid) stabilization: Development of an assessing method. Environ. Pollut. 2025, 368, 125798. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Huang, C.; Hou, R.; Hou, D. Long-term soil remediation using layered double hydroxides: Field evidence for simultaneous immobilization of both cations and oxyanions. Environ. Pollut. 2025, 366, 125417. [Google Scholar] [CrossRef]
- Yin, W. Pictorical Keys to Soil Animals of China; Science Press: Beijing, China, 2000. [Google Scholar]
- Qiao, Z.; Liu, D.; Gong, X.; Schädler, M.; Zhang, S.; Yan, Q.; Liu, X.; Xie, Z.; Chang, L.; Wu, D.; et al. Land-use change reshapes communities and guild structure of Collembola across a wide geographic range of the temperate zone. Appl. Soil Ecol. 2025, 209, 106036. [Google Scholar] [CrossRef]
- Alamgir, M.; Islam, M.; Hossain, N.; Kibria, M.; Rahman, M. Assessment of heavy metal contamination in urban soils of Chittagong City, Bangladesh. Int. J. Plant Soil Sci. 2015, 7, 362–372. [Google Scholar] [CrossRef]
- Yuan, D.; Li, P.; Yan, C.; Wang, J.; Bai, X.; Wei, Y.; Wang, C.; Kou, Y. Risk Assessment of Heavy Metals in Road-Deposited Sediments and Correlation Distribution of DOM and Heavy Metals in Beijing, China. Toxics 2025, 13, 308. [Google Scholar] [CrossRef]
- Dickman, M. Some indices of diversity. Ecology 1968, 49, 1191–1193. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental impact of different agricultural management practices: Conventional vs. organic agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Weissengruber, L.; Möller, K.; Puschenreiter, M.; Friedel, J.K. Long-term soil accumulation of potentially toxic elements and selected organic pollutants through application of recycled phosphorus fertilizers for organic farming conditions. Nutr. Cycl. Agroecosystems 2018, 110, 427–449. [Google Scholar] [CrossRef]
- Ghani, J.; Nawab, J.; Khan, S.; Khan, M.A.; Ahmad, I.; Ali, H.M.; Siddiqui, M.H.; Funari, V.; Dinelli, E. Organic amendments minimize the migration of potentially toxic elements in soil–plant system in degraded agricultural lands. Biomass Convers. Biorefinery 2024, 14, 6547–6565. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Baloch, M.Y.J.; Zhang, W.; Sultana, T.; Akram, M.; Al Shoumik, B.A.; Khan, M.Z.; Farooq, M.A. Utilization of sewage sludge to manage saline–alkali soil and increase crop production: Is it safe or not? Environ. Technol. Innov. 2023, 32, 103266. [Google Scholar] [CrossRef]
- Fang, X.; Zhong, X.; Cui, Z.; Zhang, Y.; Du, L.; Yang, Y.; Lv, J. Distribution and remediation techniques of heavy metals in soil aggregates perspective: A review. Water Air Soil Pollut. 2023, 234, 631. [Google Scholar] [CrossRef]
- Jiao, W.; Chen, W.; Chang, A.C.; Page, A.L. Environmental risks of trace elements associated with long-term phosphate fertilizers applications: A review. Environ. Pollut. 2012, 168, 44–53. [Google Scholar] [CrossRef]
- JIANG, R.; LYU, Y.; SHEN, S. Assessment of heavy metal content and pollution in organic and conventional farming soils in North China. Chin. J. Eco-Agric. 2015, 23, 877–885. [Google Scholar]
- Zhou, X.-Y.; Wang, X.-R. Impact of industrial activities on heavy metal contamination in soils in three major urban agglomerations of China. J. Clean. Prod. 2019, 230, 1–10. [Google Scholar] [CrossRef]
- Bengtsson, J.; Ahnström, J.; Weibull, A.C. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2005, 42, 261–269. [Google Scholar] [CrossRef]
- Liu, H.; Meng, J.; Bo, W.; Cheng, D.; Li, Y.; Guo, L.; Li, C.; Zheng, Y.; Liu, M.; Ning, T. Biodiversity management of organic farming enhances agricultural sustainability. Sci. Rep. 2016, 6, 23816. [Google Scholar] [CrossRef]
- Menta, C.; Remelli, S. Soil health and arthropods: From complex system to worthwhile investigation. Insects 2020, 11, 54. [Google Scholar] [CrossRef]
- Liu, S.; Hao, C.; Xie, Z.; Wu, Y.; Liang, A.; Chang, L.; Wu, D.; Chen, T.-W. Conservation tillage impacts on soil biodiversity: Additional insights from the Collembola-associated bacteria. Agric. Ecosyst. Environ. 2024, 362, 108827. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, S.; Qian, R.; Ochoa-Hueso, R.; Wang, X.; Wang, J.; Liao, L.; Liu, G.; Li, Q.; Zhang, C. Plant and microbial β diversities are better predictors of ecosystem functioning than their α diversities, but aridity weakens these associations. Plant Soil 2024, 1–20. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Olesen, J.E.; van Groenigen, K.J.; Hui, D.; He, X.; Chen, G.; Deng, Q. Mycorrhizal association controls soil carbon-degrading enzyme activities and soil carbon dynamics under nitrogen addition: A systematic review. Sci. Total Environ. 2024, 948, 175008. [Google Scholar] [CrossRef]
- Awasthi, A.; Singh, M.; Soni, S.K.; Singh, R.; Kalra, A. Biodiversity acts as insurance of productivity of bacterial communities under abiotic perturbations. ISME J. 2014, 8, 2445–2452. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; Han, X.; Wang, X.; Guo, Z.; Lu, X.; Zou, W.; Yan, J. Microbial responses of soil fertility to depth of tillage and incorporation of straw in a haplic chernozem in northeast China. Chin. Geogr. Sci. 2023, 33, 693–707. [Google Scholar] [CrossRef]
- Runnel, K.; Tedersoo, L.; Krah, F.-S.; Piepenbring, M.; Scheepens, J.; Hollert, H.; Johann, S.; Meyer, N.; Bässler, C. Toward harnessing biodiversity–ecosystem function relationships in fungi. Trends Ecol. Evol. 2025, 40, 180–190. [Google Scholar] [CrossRef]
- Hagmann, D.F.; Goodey, N.M.; Mathieu, C.; Evans, J.; Aronson, M.F.; Gallagher, F.; Krumins, J.A. Effect of metal contamination on microbial enzymatic activity in soil. Soil Biol. Biochem. 2015, 91, 291–297. [Google Scholar] [CrossRef]
| Farm | pH | SOC (g/kg) | TN (g/kg) | AP (g/kg) | AK (g/kg) | CEC (cmol/kg) | WSA (%) |
|---|---|---|---|---|---|---|---|
| ORG1 | 8.00 ± 0.16 b | 21.3 ± 4.00 a | 2.33 ± 0.50 a | 0.28 ± 0.17 a | 0.51 ± 0.04 a | 16.8 ± 1.10 ab | 17.5 ± 2.84 ab |
| CON1 | 7.94 ± 0.21 b | 20.5 ± 12.2 a | 2.42 ± 1.10 a | 0.33 ± 0.19 a | 0.41 ± 0.06 b | 18.1 ± 4.80 a | 14.7 ± 3.23 b |
| ORG2 | 7.92 ± 0.17 b | 10.5 ± 0.44 b | 1.22 ± 0.10 b | 0.24 ± 0.01 a | 0.34 ± 0.00 c | 14.3 ± 0.72 bc | 20.2 ± 2.90 a |
| CON2 | 8.45 ± 0.16 a | 12.2 ± 1.36 b | 1.16 ± 0.42 b | 0.27 ± 0.05 a | 0.37 ± 0.05 bc | 12.9 ± 1.75 c | 11.0 ± 0.59 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Guo, J.; Zhao, H.; Qu, G.; Han, S.; Huang, C. Assessing Two Decades of Organic Farming: Effects on Soil Heavy Metal Concentrations and Biodiversity for Sustainable Management. Sustainability 2025, 17, 6817. https://doi.org/10.3390/su17156817
Chen Y, Guo J, Zhao H, Qu G, Han S, Huang C. Assessing Two Decades of Organic Farming: Effects on Soil Heavy Metal Concentrations and Biodiversity for Sustainable Management. Sustainability. 2025; 17(15):6817. https://doi.org/10.3390/su17156817
Chicago/Turabian StyleChen, Yizhi, Jianning Guo, Hanyue Zhao, Guangyu Qu, Siqi Han, and Caide Huang. 2025. "Assessing Two Decades of Organic Farming: Effects on Soil Heavy Metal Concentrations and Biodiversity for Sustainable Management" Sustainability 17, no. 15: 6817. https://doi.org/10.3390/su17156817
APA StyleChen, Y., Guo, J., Zhao, H., Qu, G., Han, S., & Huang, C. (2025). Assessing Two Decades of Organic Farming: Effects on Soil Heavy Metal Concentrations and Biodiversity for Sustainable Management. Sustainability, 17(15), 6817. https://doi.org/10.3390/su17156817






