Bioprocess Integration of Candida ethanolica and Chlorella vulgaris for Sustainable Treatment of Organic Effluents in the Honey Industry
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Samples and Microorganisms
2.1.1. Industrial Effluent
2.1.2. Sewage Effluent
2.1.3. Microbial Strains and Their Maintenance
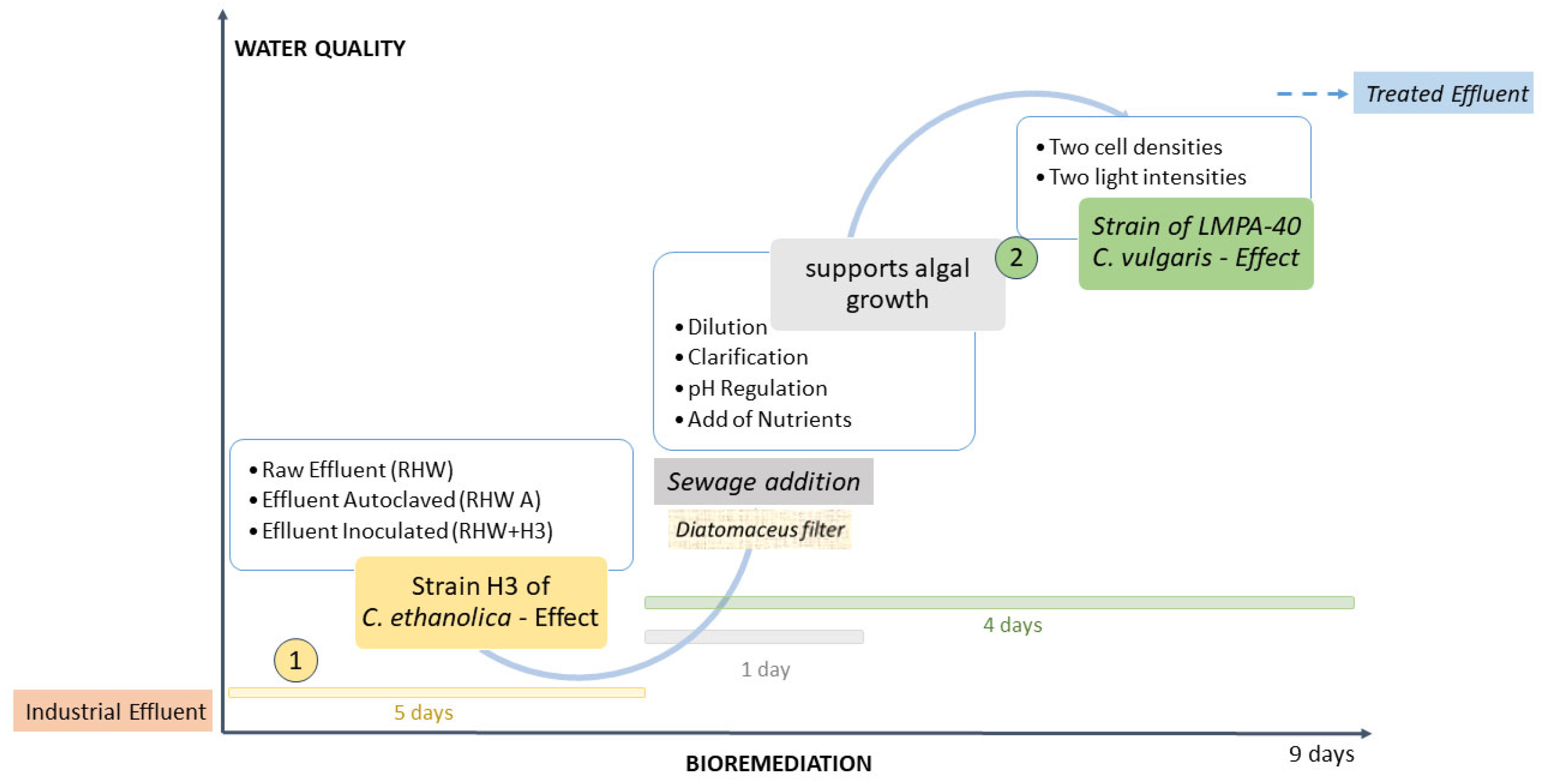
2.2. Experimental Design and Measurements
- 1.
- Cell density: RW was inoculated with C. vulgaris to achieve the following initial concentrations:
- 2.
- Light intensity:
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
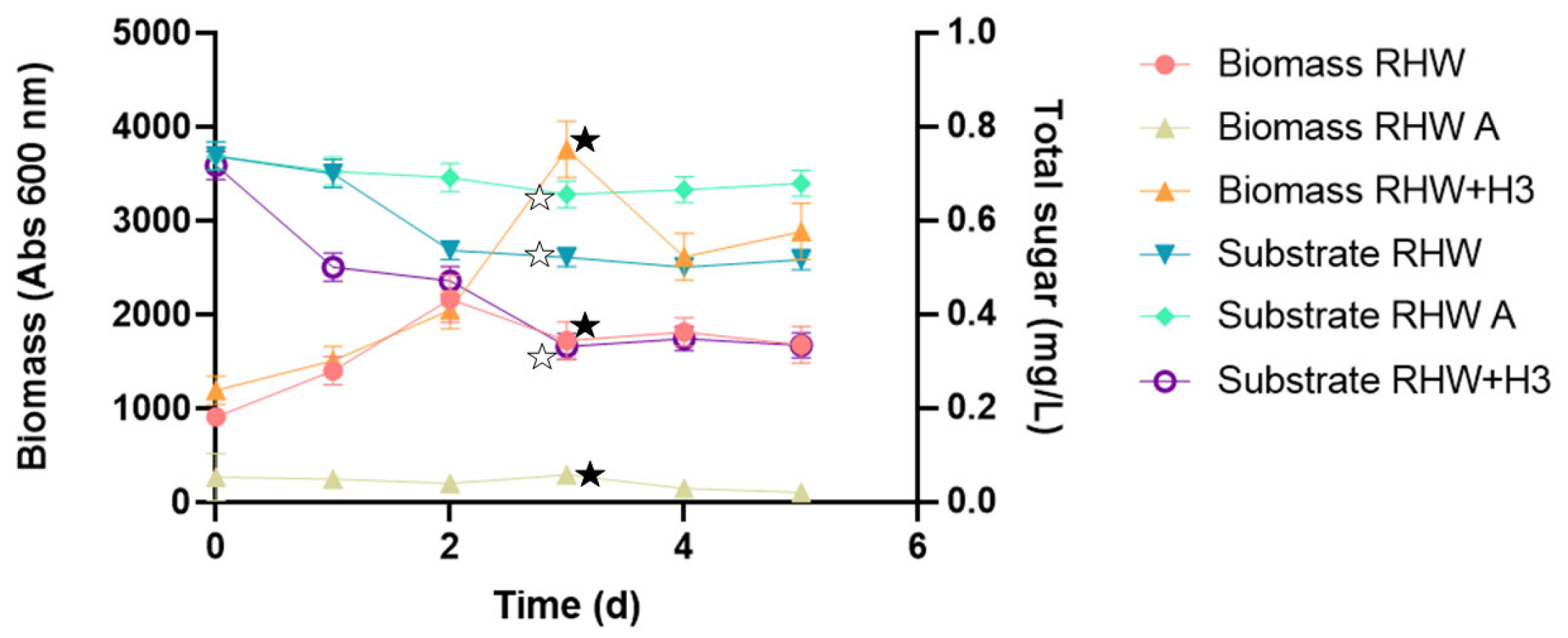
3.1. Yeast Treatment
3.2. C. vulgaris Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Secretaría de Agricultura, Secretaría de Agricultura, Ganadería y Pesca. Available online: https://magyp.gob.ar/apicultura/material_descarga.php (accessed on 12 September 2023).
- OEC-WORLD. The Observatory of Economic Complexity. Available online: https://oec.world/es/profile/hs/honey (accessed on 20 August 2023).
- Sathya, K.; Nagarajan, K.; Carlin Geor Malar, G.; Rajalakshm, S.; Raja Lakshm, P. A comprehensive review on comparison among effluent treatment methods and modern methods of treatment of industrial wastewater effluent from different sources. Appl. Water Sci. 2022, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, A.; Chapagain, A.; Aldaya, M.; Mekonnen, M. The Water Footprint Assessment: Manual Setting the Global Standard; EarthScan: Washington, DC, USA, 2011; Volume 77, ISBN 978-1-84981-279-8. Available online: https://digitalcommons.unl.edu/wffdocs/77 (accessed on 20 May 2024).
- Hossain, M.L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. A review of commonly used methodologies for assessing the antibacterial activity of honey and honey products. Antibiotics 2022, 11, 975. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef] [PubMed]
- Kwakman, P.H.S.; Velde, A.A.T.; de Boer, L.; Speijer, D.; Christina Vandenbroucke-Grauls, M.J.; Zaat, S.A.J. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Novoa, J.G.; Domínguez, F.G.; Pajot, H.; de Cabo, L.I.; Navarro Llorens, J.M.; Marconi, P.L. Isolation and assessment of highly sucrose-tolerant yeast strains for honey processing factory’s effluent treatment. AMB Express. 2024, 14, 125. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Halder, G.N.; Gunapati, O.; Indrama, T.; Tiwari, O.N. Chapter 17-Bioremediation of organic and inorganic pollutants using microalgae. New Future Dev. Microb. Biotechnol. Bioeng. 2019, 1, 223–235. [Google Scholar] [CrossRef]
- de Cabo Laura, L.; Marconi, P.L. Estrategias de Remediación Para las Cuencas de dos ríos Urbanos de Llanura: Matanza-Riachuelo y Reconquista; de Cabo Laura, L., Marconi, P.L., Eds.; Fundación Azara: Ciudad Autónoma de Buenos Aires, Argentina, 2021; ISBN 978-987-3781-74-2. [Google Scholar]
- Rajamanickam, R.; Selvasembian, R. Insights into the potential of Chlorella species in the treatment of hazardous pollutants from industrial effluent. World J. Microbiol. Biotechnol. 2025, A41, 135. [Google Scholar] [CrossRef] [PubMed]
- Groppa, M.D.; Trentini, A.; Zawoznik, M.; Bigi, R.; Nadra, C.; Marconi, P. Optimization of a bioremediation strategy for an urban stream of Matanza-Riachuelo Basin. Int. J. Environ. Eng. 2019, 13, 418–424. [Google Scholar]
- Marconi, P.L.; Trentini, A.; Zawoznik, M.; Nadra, C.; Mercadé, J.M.; Sánchez Novoa, J.G.; Orozco, D.; Groppa, M.D. Development and testing of a 3D-printable polylactic acid device to optimize a water bioremediation process. AMB Express. 2020, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- González-López, F.; Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C. Evaluation of a Landfill Leachate Bioremediation System Using Spirulina sp. Sustainability 2025, 17, 2385. [Google Scholar] [CrossRef]
- Najar-Almanzor, C.E.; Velasco-Iglesias, K.D.; Solis-Bañuelos, M.; González-Díaz, R.L.; Guerrero-Higareda, S.; Fuentes-Carrasco, O.J.; García-Cayuela, T.; Carrillo-Nieves, D. Chlorella vulgaris-mediated bioremediation of food and beverage wastewater from industries in Mexico: Results and perspectives towards sustainability and circular economy. Sci. Total Environ. 2024, 940, 173753. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Nayak, M.; Ghosh, A. A review on co-culturing of microalgae: A greener strategy towards sustainable biofuels production. Sci. Total Environ. 2022, 802, 149765. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, E.; Borowski, S.; Kręgiel, D. Cultivation of yeasts on liquid digestate to remove organic pollutants and nutrients and for potential application as co-culture with microalgae. J. Environ. Manag. 2024, 362, 121351. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Patel, A.; Mehtani, J.; Pruthi, P.A.; Pruthi, V.; Poluri, K.M. Co-culturing of oleaginous microalgae and yeast: Paradigm shift towards enhanced lipid productivity. Environ. Sci. Pollut. Res. Int. 2019, 26, 16952–16973. [Google Scholar] [CrossRef] [PubMed]
- Ashtiani, V.; Jalili, H.; Rahaie, M.; Sedighi, M.; Amrane, A. Effect of mixed culture of yeast and microalgae on acetyl-CoA carboxylase and Glycerol-3-phosphate acyltransferase expression. J. Biosci. Bioeng. 2021, 131, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Suastes-Rivas, J.K.; Hernández-Altamirano, R.; Mena-Cervantes, V.Y.; Valdez-Ojeda, R.; Toledano-Thompson, T.; Tovar-Gálvez, L.R.; López-Adrián, S.; Chairez, I. Efficient production of fatty acid methyl esters by a wastewater-isolated microalgae-yeast co-culture. Env. Sci. Pollut. Res. Int. 2020, 27, 28490–28499. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Karitani, Y.; Yamada, R.; Matsumoto, T.; Ogino, H. Co-utilization of microalgae and heterotrophic microorganisms improves wastewater treatment efficiency. App. Microbiol. Biotechnol. 2024, 108, 468. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Guo, L.; Zhao, Y.; Jin, C.; She, Z.; Gao, M. Enhancing microalgae growth and product accumulation with carbon source regulation: New perspective for the coordination between photosynthesis and aerobic respiration. Chemosphere 2021, 278, 130435. [Google Scholar] [CrossRef] [PubMed]
- Castillo, T.; Ramos, D.; García-Beltrán, T.; Brito-Bazan, M.; Galindo, E. Mixotrophic cultivation of microalgae: An alternative to produce high-value metabolites. Biochem. Engin. J. 2021, 176, 108183. [Google Scholar] [CrossRef]
- Hyungseok, Y.; Kyu-Hong, A.; Hyung-Jib, L.; Kwang-Hwan, L.; Youn-Jung, K.; Kyung-Guen, S. Nitrogen removal from synthetic wastewater by simultaneous nitrification and denitrification (SND) via nitrite in an intermittently-aerated reactor. Water Res. 1999, 33, 145–154. [Google Scholar] [CrossRef]
- Marconi, P.L.; Alvarez, M.A.; Klykov, S.P.; Kurakov, V.V. Application of a mathematical model for production of recombinant antibody 14D9 by Nicotiana tabacum cell suspension batch culture. BioProcess Int. 2014, 12, 42–49. [Google Scholar]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington DC, USA, 2017. [Google Scholar]
- Di Rienzo, J.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina, 2013. Available online: http://www.infostat.com.ar (accessed on 13 November 2019).
- Tukey, J. Some selected quick and easy methods of statistical analysis. Trans. N. Y. Acad. Sci. 1953, 16, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Escuredo, O.; Dobre, I.; Fernández-González, M.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tafere, D.A. Chemical composition and uses of Honey: A Review. J. Food Sci. Nutr. Res. 2021, 4, 194–201. [Google Scholar] [CrossRef]
- Young, G.W.Z.; Blundell, R. A review on the phytochemical composition and health applications of honey. Heliyon 2023, 9, e12507. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission, 2019. Standard for Honey CXS 12-1981 (Amended 2019). C.A.S.F. Honey. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 20 May 2024).
- Fattori, S.B. “LA MIEL” Propiedades, Composición y Análisis Físico- Químico. s.l., 2004. Available online: www.apimondia.org (accessed on 23 May 2024).
- D’Amore, T.; Russell, I.; Stewart, G.G. Sugar utilization by yeast during fermentation. J. Ind. Microbiol. 1989, 4, 315–323. [Google Scholar] [CrossRef]
- Gómez, J.A.; Höffner, K.; Barton, P.I. From sugars to biodiesel using microalgae and yeast. Green Chem. 2016, 18, 461–475. [Google Scholar] [CrossRef]
- Parkinson, J.; Gordon, R. Beyond micromachining: The potential of diatoms. Trends Biotechnol. 1999, 17, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen-Jensen, M. Proposal for the Treatment of Effluents from the Production of Craft Beer. Degree Thesis in Civil Engineering, UNCPBA, Buenos Aires, Argentina, 2020. [Google Scholar]
- Leung, S.M.; Little, J.C.; Holst, T.; Love, N.G. Air/water oxygen transfer in a biological aerated filter. J. Environ. Engin. 2006, 132, 181–189. [Google Scholar] [CrossRef]
- Sánchez-Zurano, A.; Lafarga, T.; Morales-Amaral, M.D.M.; Gómez-Serrano, C.; Fernández-Sevilla, J.M.; Acién-Fernández, F.G.; Molina-Grima, E. Wastewater treatment using Scenedesmus almeriensis: Effect of operational conditions on the composition of the microalgae-bacteria consortia. J. Appl. Phycol. 2021, 33, 3885–3897. [Google Scholar] [CrossRef]
- Trentini, A.; Groppa, M.; Zawoznik, M.; Bigi, R.; Perelman, P.; Marconi, P. Biorremediación del lago Lugano de la Ciudad Autónoma de Buenos Aires por algas unicelulares—Estudios preliminares para su posterior utilización. Terra Mundus 2017, 4, 1–10. [Google Scholar]
- Van Do, T.C.; Nguyen, T.N.T.; Tran, D.T.; Le, T.G.; Nguyen, V.T. Semi-continuous removal of nutrients and biomass production from domestic wastewater in raceway reactors using Chlorella variabilis TH03-bacteria consortia. Environ. Technol. Innov. 2020, 20, 101172. [Google Scholar] [CrossRef]
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Kyndt, J.; Martinez, A. Heterotrophic growth of microalgae: Metabolic aspects. World, J. Microb. Biot. 2015, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Michelon, W.; Pirolli, M.; Mezzari, M.P.; Soares, H.M.; da Silva, M.L.B. Residual sugar from microalgae biomass harvested from phycoremediation of swine wastewater digestate. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2019, 79, 2203–2210. [Google Scholar] [CrossRef] [PubMed]
- Dubey, K.K.; Kumar, S.; Dixit, D.; Kumar, P.; Kumar, D.; Jawed, A.; Haque, S. Implication of industrial waste for biomass and lipid production in Chlorella minutissima under autotrophic, heterotrophic, and mixotrophic grown conditions. Appl. Biochem. Biotechnol. 2015, 176, 1581–1595. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, D.; Martinez-Rodriguez, O.A.; Martinez, A. Heterotrophic cultivation of microalgae: Production of metabolites of commercial interest. J. Chem. Technol. Biotechnol. 2017, 92, 925–936. [Google Scholar] [CrossRef]
- Pérez-García, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, K.H.; Ligaray, M.; Choi, M.J. Organic matter composition of manure and its potential impact on plant growth. Sustainability 2019, 11, 2346. [Google Scholar] [CrossRef]
- Fida, S.; Yasmeen, M.; Adnan, R.; Zeeshan, M. Treatment methods for sugar rich wastewater: A review. Clean. Water 2025, 3, 100067. [Google Scholar] [CrossRef]


| Untreated Samples | ||
|---|---|---|
| Parameter | RHW | RTW |
| pH | 4.33 ± 0.03 | 6.57 ± 0.16 |
| COD (mg O2/L) | 27,167 ± 192 | 731 ± 33 |
| Total sugar (mg/L) | 3690 ± 275 | 10 ± 0.02 |
| NH4-N (mg/L) | 0.02 ± 0.00 | 32.9 ± 2.8 |
| Soluble reactive phosphorous (mg/L) | 0.03 ± 0.00 | 2.50 ± 0.39 |
| Escherichia coli (CFU/100 mL) | 0 | 51,200 ± 2550 |
| Total coliforms (CFU/100 mL) | 0 | 74,800 ± 4580 |
| Fecal coliforms (CFU/100 mL) | 0 | 126,000 ± 5820 |
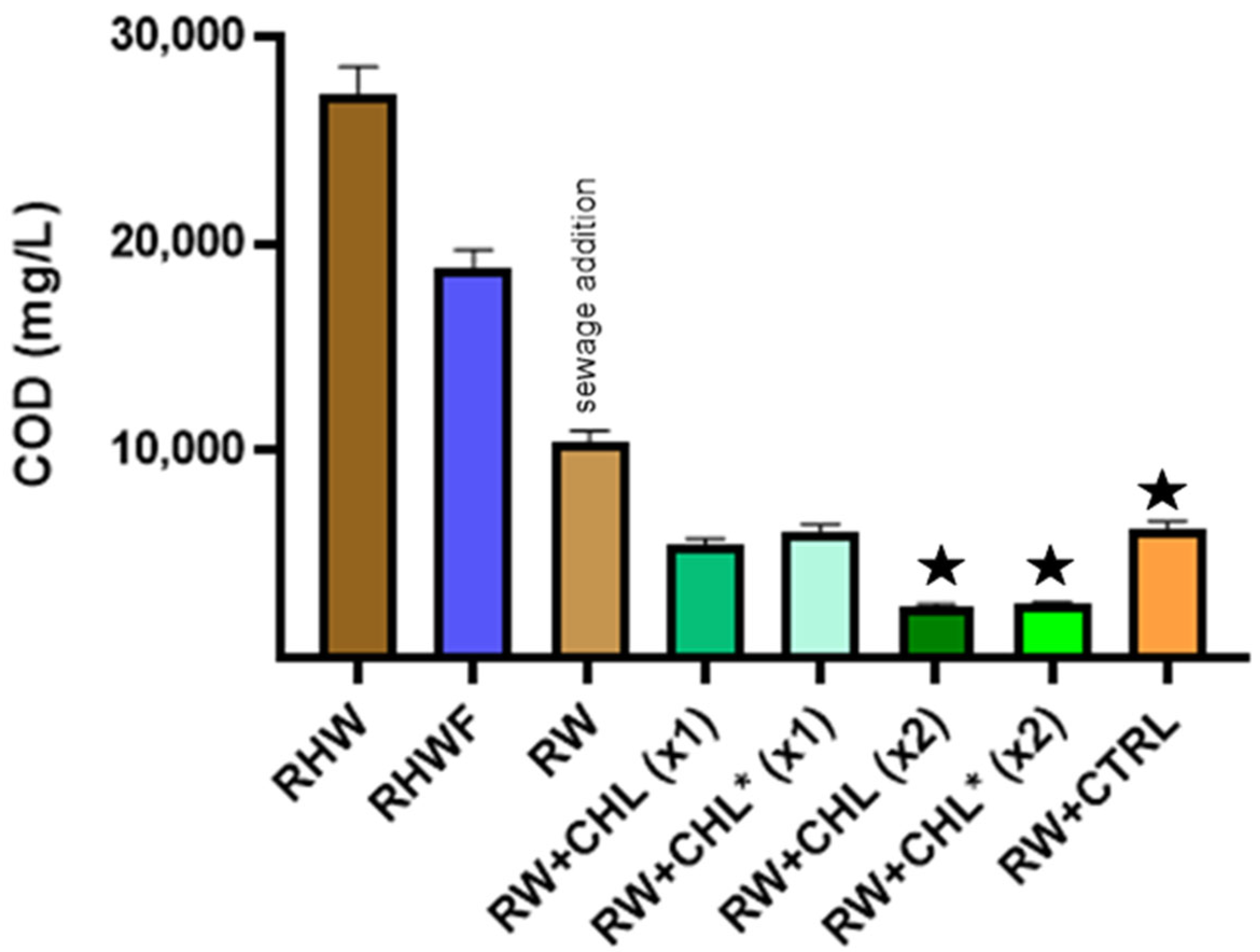
| Yeast Treatment | Filtration | Sewage Addition | Microalgae Treatment | ||||
|---|---|---|---|---|---|---|---|
| Parameter | RHW + H3 | RHWF | RW | RW + CHL (X1) | RW + CHL * (X1) | RW + CHL (X2) | RW + CHL * (X2) |
| pH | 3.96 ± 0.06 | 3.98 ± 0.06 | 7.23 ± 0.00 | 5.83 ± 0.29 | 8.00 ± 0.00 | 7.30 ± 0.29 | 7.50 ± 0.00 |
| COD (mg O2/L) | 29,533 ± 882 | 18,766 ± 189 | 10,433 ± 639 | 5486 ± 355 | 6120 ± 240 | 2486 ± 158 | 2553 ± 124 |
| Total sugar (mg/L) | 1672 ± 6.15 | 1539 ± 57.0 | 953 ± 17.0 | 893 ± 10.8 | 810 ± 30.8 | 602 ± 5.31 | 774 ± 15.4 |
| NH4-N (mg/L) | 0.03 ± 0.01 | 0.02 ± 0.00 | 19.1 ± 1.92 | 5.22 ± 0.16 | 3.79 ± 0.15 | 1.99 ± 0.18 | 1.39 ± 0.05 |
| Soluble reactive phosphorous (mg/L) | 0.03 ± 0.01 | 0.03 ± 0.00 | 1.31 ± 0.12 | 0.84 ± 0.08 | 0.64 ± 0.19 | 0.51 ± 0.19 | 0.27 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez Novoa, J.G.; Rodriguez, N.; Debandi, T.; Navarro Llorens, J.M.; de Cabo, L.I.; Marconi, P.L. Bioprocess Integration of Candida ethanolica and Chlorella vulgaris for Sustainable Treatment of Organic Effluents in the Honey Industry. Sustainability 2025, 17, 6809. https://doi.org/10.3390/su17156809
Sánchez Novoa JG, Rodriguez N, Debandi T, Navarro Llorens JM, de Cabo LI, Marconi PL. Bioprocess Integration of Candida ethanolica and Chlorella vulgaris for Sustainable Treatment of Organic Effluents in the Honey Industry. Sustainability. 2025; 17(15):6809. https://doi.org/10.3390/su17156809
Chicago/Turabian StyleSánchez Novoa, Juan Gabriel, Natalia Rodriguez, Tomás Debandi, Juana María Navarro Llorens, Laura Isabel de Cabo, and Patricia Laura Marconi. 2025. "Bioprocess Integration of Candida ethanolica and Chlorella vulgaris for Sustainable Treatment of Organic Effluents in the Honey Industry" Sustainability 17, no. 15: 6809. https://doi.org/10.3390/su17156809
APA StyleSánchez Novoa, J. G., Rodriguez, N., Debandi, T., Navarro Llorens, J. M., de Cabo, L. I., & Marconi, P. L. (2025). Bioprocess Integration of Candida ethanolica and Chlorella vulgaris for Sustainable Treatment of Organic Effluents in the Honey Industry. Sustainability, 17(15), 6809. https://doi.org/10.3390/su17156809







