Urbanization Compromises the Sustainability of Coastal Ecosystems: Insights from the Reproductive Traits of the Bioindicator Clam Donax trunculus
Abstract
1. Introduction
2. Materials and Methods
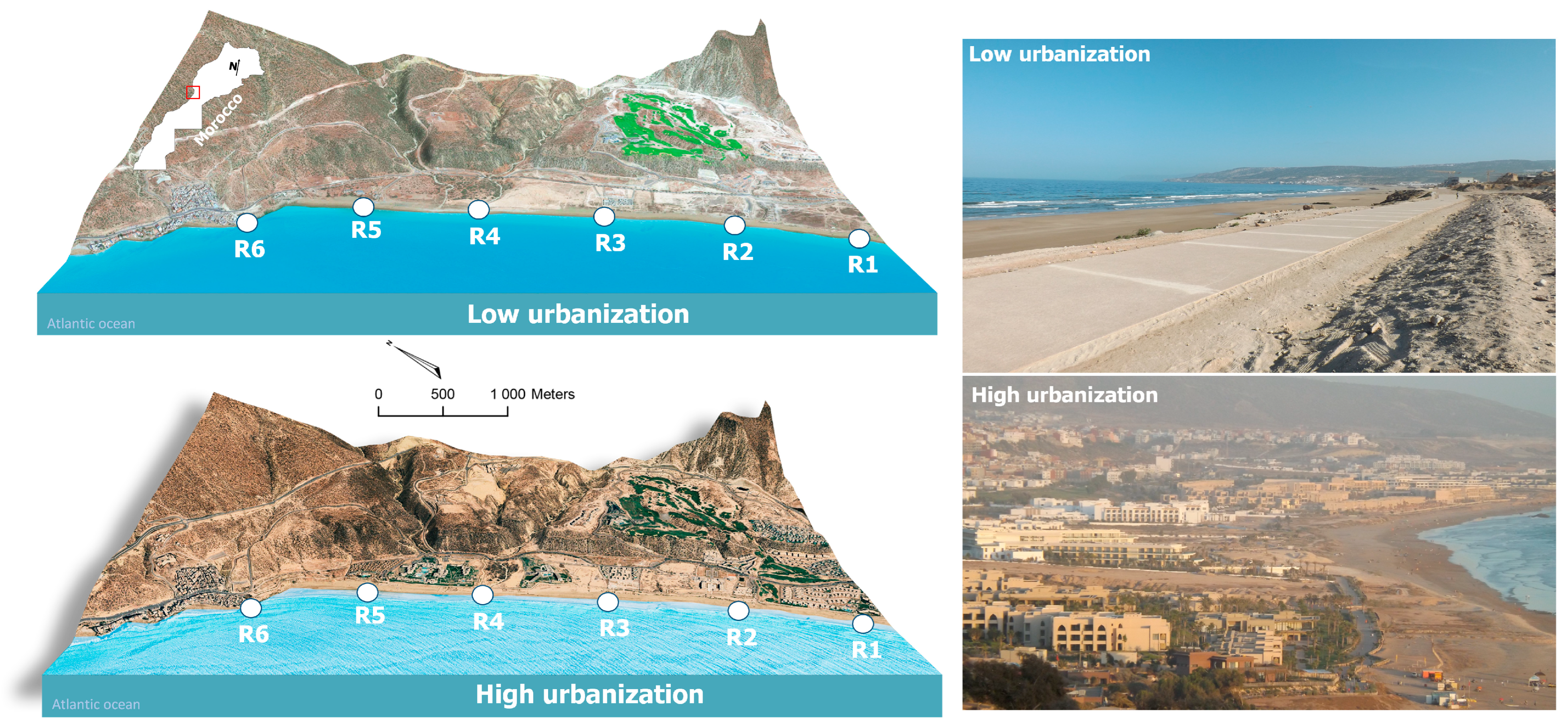
2.1. Study Area
2.2. Sampling
2.3. Gonadal Analysis of Donax trunculus
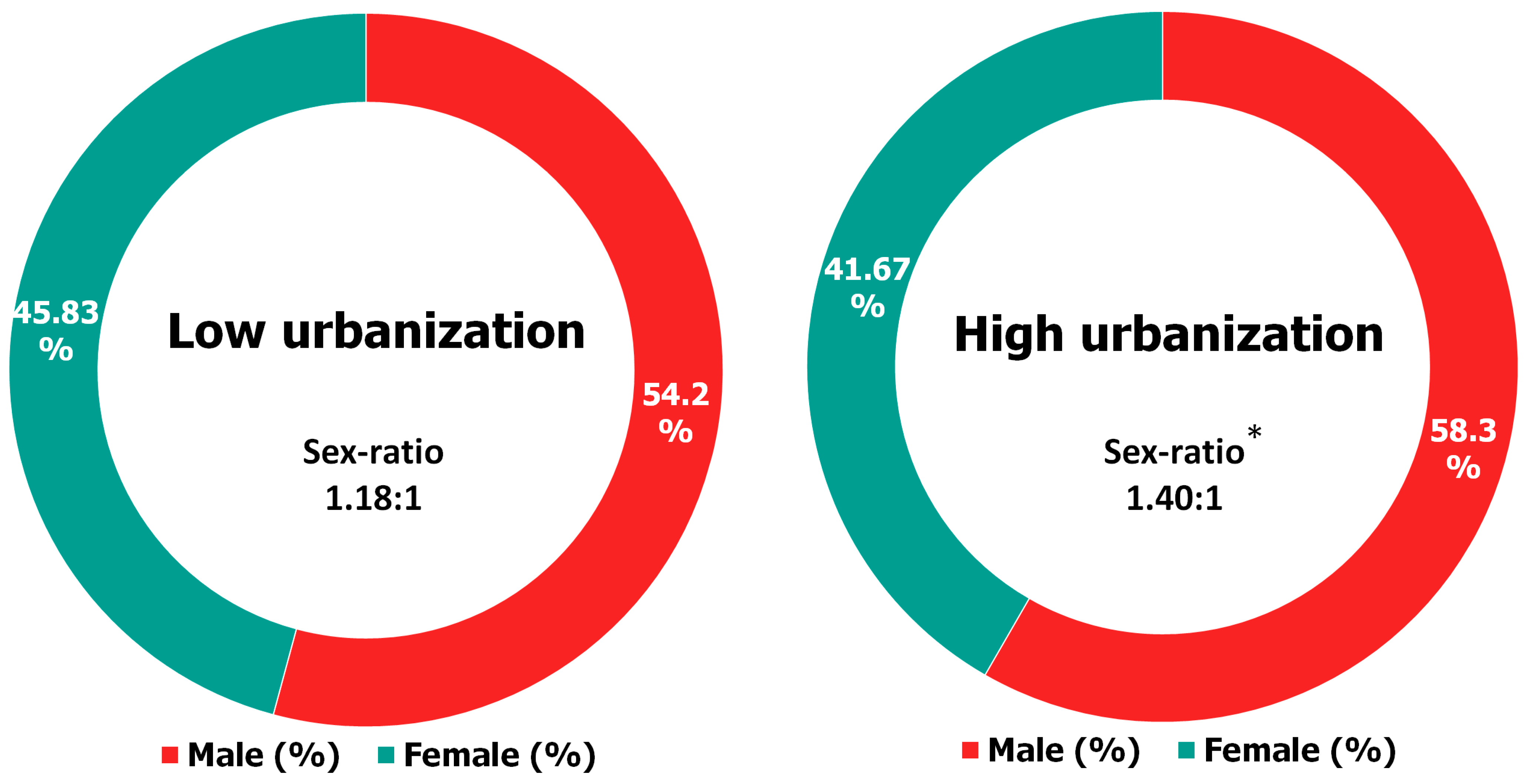
2.4. Sex Ratio Determination
2.5. Abundance and Biomass of Donax trunculus
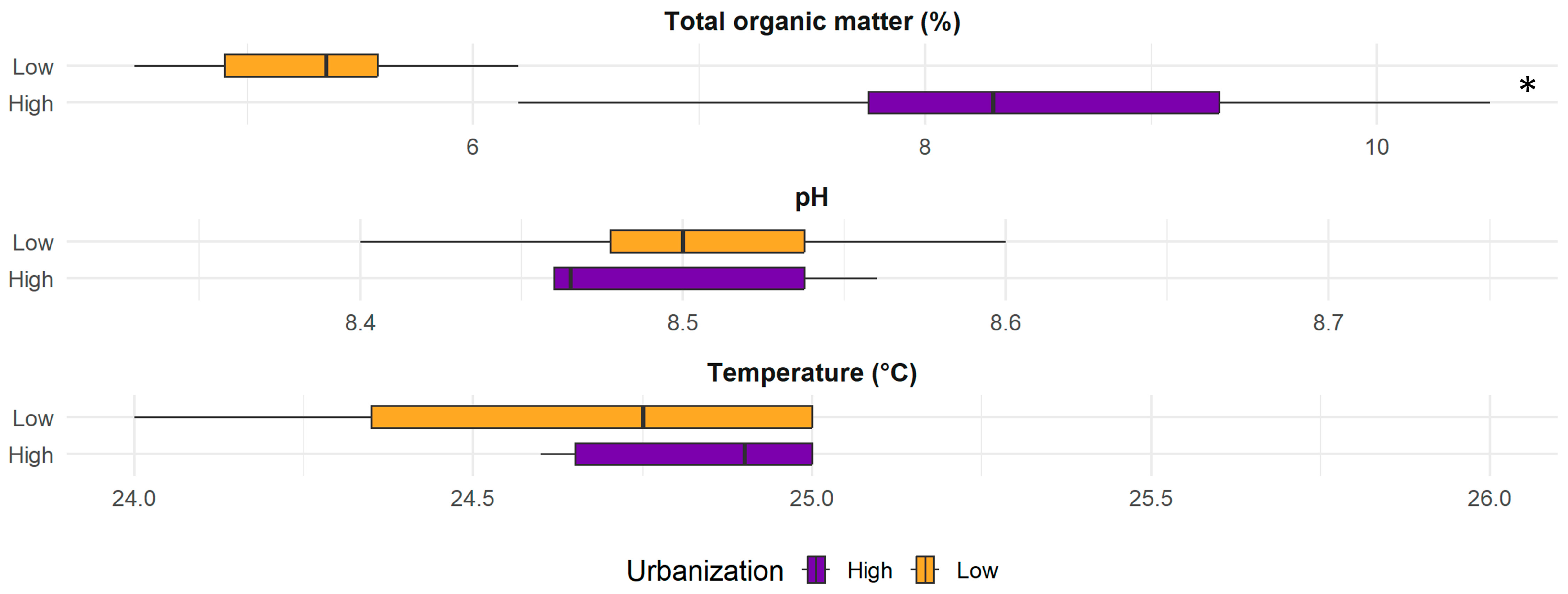
2.6. Physicochemical Analysis of Sediment
2.7. Data Analysis
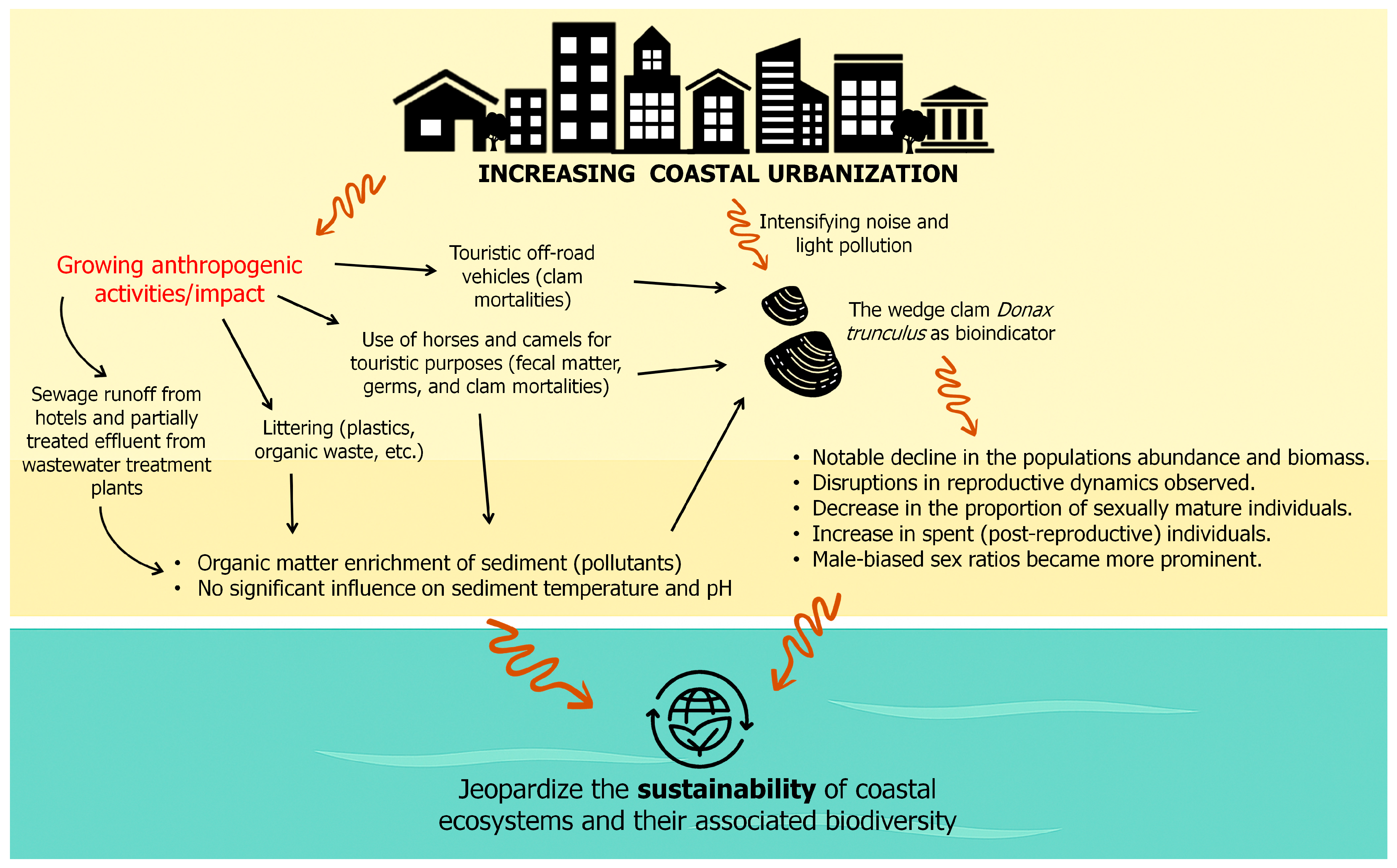
3. Results and Discussion
4. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Defeo, O.; McLachlan, A.; Armitage, D.; Elliott, M.; Pittman, J. Sandy Beach Social–Ecological Systems at Risk: Regime Shifts, Collapses, and Governance Challenges. Front. Ecol. Environ. 2021, 19, 564–573. [Google Scholar] [CrossRef]
- McLachlan, A.; Defeo, O. (Eds.) Fisheries. In The Ecology of Sandy Shores, 2nd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 331–374. ISBN 978-0-12-809467-9. [Google Scholar]
- Costa, L.L.; Zalmon, I.R.; Fanini, L.; Defeo, O. Macroinvertebrat es as Indicators of Human Disturbances on Sandy Beaches: A Global Review. Ecol. Indic. 2020, 118, 106764. [Google Scholar] [CrossRef]
- Tlili, S.; Minguez, L.; Giamberini, L.; Geffard, A.; Boussetta, H.; Mouneyrac, C. Assessment of the Health Status of Donax Trunculus from the Gulf of Tunis Using Integrative Biomarker Indices. Ecol. Indic. 2013, 32, 285–293. [Google Scholar] [CrossRef]
- Ben-Haddad, M.; Abelouah, M.R.; Hajji, S.; De-la-torre, G.E.; Abou Oualid, H.; Rangel-Buitrago, N.; Ait Alla, A. The Wedge Clam Donax trunculus L., 1758 as a Bioindicator of Microplastic Pollution. Mar. Pollut. Bull. 2022, 178, 113607. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.S.; Barboza, C.A.M.; Skinner, V.B.; Cabrini, T.M.B. Crustaceans as Ecological Indicators of Metropolitan Sandy Beaches Health. Ecol. Indic. 2016, 62, 154–162. [Google Scholar] [CrossRef]
- Burt, J.A.; Killilea, M.E.; Ciprut, S. Coastal Urbanization and Environmental Change: Opportunities for Collaborative Education across a Global Network University. Reg. Stud. Mar. Sci. 2019, 26, 100501. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of Heavy Metal Pollution from Anthropogenic Activities and Remediation Strategies: A Review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Gracia, A.; Rangel-Buitrago, N.; Oakley, J.A.; Williams, A.T. Use of Ecosystems in Coastal Erosion Management. Ocean. Coast. Manag. 2018, 156, 277–289. [Google Scholar] [CrossRef]
- Fanini, L.; Piscart, C.; Pranzini, E.; Kerbiriou, C.; Le Viol, I.; Pétillon, J. The Extended Concept of Littoral Active Zone Considering Soft Sediment Shores as Social-Ecological Systems, and an Application to Brittany (North-Western France). Estuar. Coast. Shelf Sci. 2021, 250, 107148. [Google Scholar] [CrossRef]
- Day, J.W.; Gunn, J.D.; Burger, J.R. Diminishing Opportunities for Sustainability of Coastal Cities in the Anthropocene: A Review. Front. Environ. Sci. 2021, 9, 663275. [Google Scholar] [CrossRef]
- Stojanovic, T.A.; Farmer, C.J.Q. The Development of World Oceans & Coasts and Concepts of Sustainability. Mar. Policy 2013, 42, 157–165. [Google Scholar] [CrossRef]
- Costa, L.L.; Tavares, D.C.; Suciu, M.C.; Rangel, D.F.; Zalmon, I.R. Human-Induced Changes in the Trophic Functioning of Sandy Beaches. Ecol. Indic. 2017, 82, 304–315. [Google Scholar] [CrossRef]
- La Valle, P.; Nicoletti, L.; Finoia, M.G.; Ardizzone, G.D. Donax Trunculus (Bivalvia: Donacidae) as a Potential Biological Indicator of Grain-Size Variations in Beach Sediment. Ecol. Indic. 2011, 11, 1426–1436. [Google Scholar] [CrossRef]
- Tlili, S.; Mouneyrac, C. The Wedge Clam Donax Trunculus as Sentinel Organism for Mediterranean Coastal Monitoring in a Global Change Context. Reg. Environ. Change 2019, 19, 995–1007. [Google Scholar] [CrossRef]
- Chahouri, A.; Ouchene, H.; Yacoubi, B.; Moukrim, A.; Banaoui, A. Reproductive Cycle of Two Marine Sentinel Bivalve Species (Donax Trunculus and Scrobicularia Plana) in the Agadir Bay, Southern Morocco. Reg. Stud. Mar. Sci. 2022, 56, 102611. [Google Scholar] [CrossRef]
- Chelyadina, N.; Pospelova, N.; Popov, M. Effects of Environmental Factors on Changing Sex Structure of Cultivated Mussels (Mytilus Galloprovincialis, Lamarck, 1819) in the Coastal Zone of the Black Sea. Int. Rev. Hydrobiol. 2021, 106, 183–190. [Google Scholar] [CrossRef]
- Rossi, F.; Forster, R.M.; Montserrat, F.; Ponti, M.; Terlizzi, A.; Ysebaert, T.; Middelburg, J.J. Human Trampling as Short-Term Disturbance on Intertidal Mudflats: Effects on Macrofauna Biodiversity and Population Dynamics of Bivalves. Mar. Biol. 2007, 151, 2077–2090. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Morales, S.; López-Landavery, E.A.; Giffard-Mena, I.; Ramírez-Álvarez, N.; Gómez-Reyes, R.J.E.; Díaz, F.; Galindo-Sánchez, C.E. Transcriptomic Response of the Crassostrea Virginica Gonad after Exposure to a Water-Accommodation Fraction of Hydrocarbons and the Potential Implications in Reproduction. Mar. Genom. 2019, 43, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Birch, G.F. A Review and Critical Assessment of Sedimentary Metal Indices Used in Determining the Magnitude of Anthropogenic Change in Coastal Environments. Sci. Total Environ. 2023, 854, 158129. [Google Scholar] [CrossRef] [PubMed]
- Tlili, S.; Jemai, D.; Brinis, S.; Regaya, I. Microplastics Mixture Exposure at Environmentally Relevant Conditions Induce Oxidative Stress and Neurotoxicity in the Wedge Clam Donax Trunculus. Chemosphere 2020, 258, 127344. [Google Scholar] [CrossRef] [PubMed]
- Hellou, J.; Yeats, P.; Steller, S.; Gagné, F. Chemical Contaminants and Biological Indicators of Mussel Health during Gametogenesis. Environ. Toxicol. Chem. 2003, 22, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Miglioli, A.; Balbi, T.; Fabbri, E. Physiological Roles of Serotonin in Bivalves: Possible Interference by Environmental Chemicals Resulting in Neuroendocrine Disruption. Front. Endocrinol. 2022, 13, 792589. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.; Sokolov, E.P.; Haider, F.; Oppermann, C.; Kragl, U.; Ruth, W.; Stock, M.; Glufke, S.; Winkel, E.J.; Sokolova, I.M. Effects of a Common Pharmaceutical, Atorvastatin, on Energy Metabolism and Detoxification Mechanisms of a Marine Bivalve Mytilus Edulis. Aquat. Toxicol. 2019, 208, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Xu, L.; Song, H.; Feng, J.; Hu, Z.; Yang, M.J.; Shi, P.; Li, Y.R.; Guo, Y.J.; Li, H.Z.; et al. Examination of the Regulation of Energy Metabolism, Antioxidant Response, and Ammonia Detoxification in Hard Clam, Mercenaria Mercenaria, under Hypersalinity Stress. Aquaculture 2023, 563, 738916. [Google Scholar] [CrossRef]
- Breitwieser, M.; Viricel, A.; Graber, M.; Murillo, L.; Becquet, V.; Churlaud, C.; Fruitier-Arnaudin, I.; Huet, V.; Lacroix, C.; Pante, E.; et al. Short-Term and Long-Term Biological Effects of Chronic Chemical Contamination on Natural Populations of a Marine Bivalve. PLoS ONE 2016, 11, 0150184. [Google Scholar] [CrossRef] [PubMed]
- Lamine, I.; Ait Alla, A.; Elazzaoui, A.; Meryam, N.; Moukrim, A. Assessment of Reproductive Cycle, Parasitism and Biological Indices of the Wedge Clam Donax Trunculus along the Taghazout Coastline (on the Moroccan Atlantic Coast). Reg. Stud. Mar. Sci. 2021, 47, 101971. [Google Scholar] [CrossRef]
- Hamdani, A.; Soltani, N.; Zaidi, N. Growth and Reproduction of Donax Trunculus from the Gulf of Annaba (Northeast Algeria) in Relation to Environmental Conditions. Environ. Sci. Pollut. Res. 2020, 27, 41656–41667. [Google Scholar] [CrossRef] [PubMed]
- De La Huz, R.; Lastra, M.; López, J. The Influence of Sediment Grain Size on Burrowing, Growth and Metabolism of Donax trunculus L. (Bivalvia: Donacidae). J. Sea Res. 2002, 47, 85–95. [Google Scholar] [CrossRef]
- Maria, T.F.; De Troch, M.; Vanaverbeke, J.; Esteves, A.M.; Vanreusel, A. Use of Benthic vs Planktonic Organic Matter by Sandy-Beach Organisms: A Food Tracing Experiment with 13 C Labelled Diatoms. J. Exp. Mar. Bio. Ecol. 2011, 407, 309–314. [Google Scholar] [CrossRef]
- Tlili, S.; Métais, I.; Ayache, N.; Boussetta, H.; Mouneyrac, C. Is the Reproduction of Donax Trunculus Affected by Their Sites of Origin Contrasted by Their Level of Contamination? Chemosphere 2011, 84, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.L.; Fanini, L.; Zalmon, I.R.; Defeo, O.; McLachlan, A. Cumulative Stressors Impact Macrofauna Differentially According to Sandy Beach Type: A Meta-Analysis. J. Environ. Manag. 2022, 307, 114594. [Google Scholar] [CrossRef] [PubMed]
- Orlando, L.; Ortega, L.; Defeo, O. Perspectives for Sandy Beach Management in the Anthropocene: Satellite Information, Tourism Seasonality, and Expert Recommendations. Estuar. Coast. Shelf Sci. 2021, 262, 107597. [Google Scholar] [CrossRef]
- Vázquez, E.; Woodin, S.A.; Wethey, D.S.; Peteiro, L.G.; Olabarria, C. Reproduction Under Stress: Acute Effect of Low Salinities and Heat Waves on Reproductive Cycle of Four Ecologically and Commercially Important Bivalves. Front. Mar. Sci. 2021, 8, 685282. [Google Scholar] [CrossRef]
- Ben-Haddad, M.; Abelouah, M.R.; Lamine, I.; Hajji, S.; Noureddine, S.; Rangel-Buitrago, N.; Ait Alla, A. Trace Metals in Urbanized Coasts: The Central Atlantic of Morocco as a Case Study. Mar. Pollut. Bull. 2023, 186, 114455. [Google Scholar] [CrossRef] [PubMed]
- Aouiche, I.; Daoudi, L.; Anthony, E.J.; Sedrati, M.; Harti, A.; Ziane, E. The Impact of Storms in the Morphodynamic Evolution of a Human-Impacted Semi-Sheltered Beach (Agadir Bay, Morocco). J. Afr. Earth Sci. 2016, 115, 32–47. [Google Scholar] [CrossRef]
- M’hamed, N.; Anthony, E.J.; Mhamed, A.; Abderrahmane, O. Multi-Decadal Shoreline Change, Inherited Coastal Morphology and Sediment Supply in the Souss-Massa Littoral Cell (Morocco), and a Prognosis with Sea-Level Rise. J. Afr. Earth Sci. 2022, 196, 104672. [Google Scholar] [CrossRef]
- Aouiche, I.; Daoudi, L.; Anthony, E.J.; Sedrati, M.; Ziane, E.; Harti, A.; Dussouillez, P. Anthropogenic Effects on Shoreface and Shoreline Changes: Input from a Multi-Method Analysis, Agadir Bay, Morocco. Geomorphology 2016, 254, 16–31. [Google Scholar] [CrossRef]
- Ben-Haddad, M.; Lamine, I.; Moukrim, A.; Bergayou, H.; Abou Oualid, J.; Ait Alla, A. State Diagnosis of Macrozoobenthic Biodiversity in the Intertidal Zone of the Sandy Coast of Taghazout (Southwestern of Morocco). J. Ecol. Eng. 2021, 22, 126–137. [Google Scholar] [CrossRef]
- Micallef, A.S.; Williams, A.T. Application of Function Analysis to Bathing Areas in the Maltese Islands. J. Coast. Conserv. 2003, 9, 147–158. [Google Scholar] [CrossRef]
- Ben-Haddad, M.; Hajji, S.; Abelouah, M.R.; Rangel-Buitrago, N.; Ait Alla, A. From Urbanization to Bioaccumulation: A Comparative Study of Metal Pollution and Biomarker Responses in Donax Trunculus along a Touristic Coastal Area in Morocco. Reg. Stud. Mar. Sci. 2023, 65, 103106. [Google Scholar] [CrossRef]
- González, S.A.; Yáñez-Navea, K.; Muñoz, M. Effect of Coastal Urbanization on Sandy Beach Coleoptera Phaleria Maculata (Kulzer, 1959) in Northern Chile. Mar. Pollut. Bull. 2014, 83, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ben-Haddad, M.; Hajji, S.; Abelouah, M.R.; Costa, L.L.; Rangel-Buitrago, N.; Ait Alla, A. Has the “Covid–19” Lockdown an Impact on Beach Faunal Communities? The Central Atlantic Coast of Morocco as a Case Study. Mar. Pollut. Bull. 2022, 185, 114259. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; de la Vega, C. Relationships between Fresh Weight, Dry Weight, Ash Free Dry Weight, Carbon and Nitrogen Content for Selected Vertebrates. J. Exp. Mar. Bio. Ecol. 2016, 481, 41–48. [Google Scholar] [CrossRef]
- Touch, N.; Hibino, T.; Takata, H.; Yamaji, S. Loss on Ignition-Based Indices for Evaluating Organic Matter Characteristics of Littoral Sediments. Pedosphere 2017, 27, 978–984. [Google Scholar] [CrossRef]
- Gosling, E. Reproduction, Settlement and Recruitment. In Bivalve Molluscs; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 131–168. ISBN 9780470995532. [Google Scholar]
- Cottrell, R.S.; Black, K.D.; Hutchison, Z.L.; Last, K.S. The Influence of Organic Material and Temperature on the Burial Tolerance of the Blue Mussel, Mytilus Edulis: Considerations for the Management of Marine Aggregate Dredging. PLoS ONE 2016, 11, 0147534. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, I.; Quagliariello, A.; Peruzza, L.; Martino, M.E.; Dalla Rovere, G.; Iori, S.; Asnicar, D.; Ciscato, M.; Fabrello, J.; Corami, F.; et al. Contaminants from Dredged Sediments Alter the Transcriptome of Manila Clam and Induce Shifts in Microbiota Composition. BMC Biol. 2023, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.X.; Shu, M.A.; Chai, X.L.; Shao, Y.Q.; Wu, H.X.; Sun, C.S.; Yang, S.B. Effect of Chronic Sublethal Exposure of Major Heavy Metals on Filtration Rate, Sex Ratio, and Gonad Development of a Bivalve Species. Bull. Environ. Contam. Toxicol. 2014, 92, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Shin, Y.K.; Hung, S.S.O.; Romano, N.; Cheon, Y.P.; Kim, J.W. Reproductive Impairment and Intersexuality in Gomphina Veneriformis (Bivalvia: Veneridae) by the Tributyltin Compound. Anim. Cells Syst. 2015, 19, 61–68. [Google Scholar] [CrossRef]
- Sibaja-Cordero, J.A.; Camacho-García, Y.E.; Azofeifa-Solano, J.C.; Alvado-Arranz, B. Ecological Patterns of Macrofauna in Sandy Beaches of Costa Rica: A Pacific-Caribbean Comparison. Estuar. Coast. Shelf Sci. 2019, 223, 94–104. [Google Scholar] [CrossRef]
- Ortega-Cisneros, K.; de Lecea, A.M.; Smit, A.J.; Schoeman, D.S. Resource Utilization and Trophic Niche Width in Sandy Beach Macrobenthos from an Oligotrophic Coast. Estuar. Coast. Shelf Sci. 2017, 184, 115–125. [Google Scholar] [CrossRef]
- Rong, Y.; Tang, Y.; Ren, L.; Taylor, W.D.; Razlutskij, V.; Naselli-Flores, L.; Liu, Z.; Zhang, X. Effects of the Filter-Feeding Benthic Bivalve Corbicula Fluminea on Plankton Community and Water Quality in Aquatic Ecosystems: A Mesocosm Study. Water 2021, 13, 1827. [Google Scholar] [CrossRef]
- Pegado, T.; Andrades, R.; Noleto-Filho, E.; Franceschini, S.; Soares, M.; Chelazzi, D.; Russo, T.; Martellini, T.; Barone, A.; Cincinelli, A.; et al. Meso- and Microplastic Composition, Distribution Patterns and Drivers: A Snapshot of Plastic Pollution on Brazilian Beaches. Sci. Total Environ. 2024, 907, 167769. [Google Scholar] [CrossRef] [PubMed]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics Removal in Wastewater Treatment Plants: A Critical Review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Augusto, M.; Abude, R.R.S.; Cardoso, R.S.; Cabrini, T.M.B. Local Urbanization Impacts Sandy Beach Macrofauna Communities over Time. Front. Mar. Sci. 2023, 10, 1158413. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Thompson, L.M.C.; Walker, S.J. Mortalities Caused by Off-Road Vehicles (ORVs) to a Key Member of Sandy Beach Assemblages, the Surf Clam Donax Deltoides. Hydrobiologia 2008, 610, 345–350. [Google Scholar] [CrossRef]
- Saleem, F.; Li, E.; Edge, T.A.; Tran, K.L.; Schellhorn, H.E. Identification of Potential Microbial Risk Factors Associated with Fecal Indicator Exceedances at Recreational Beaches. Environ. Microbiome 2024, 19, 4. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Haddad, M.; Hajji, S.; Abelouah, M.R.; Ait Alla, A. Urbanization Compromises the Sustainability of Coastal Ecosystems: Insights from the Reproductive Traits of the Bioindicator Clam Donax trunculus. Sustainability 2025, 17, 6622. https://doi.org/10.3390/su17146622
Ben-Haddad M, Hajji S, Abelouah MR, Ait Alla A. Urbanization Compromises the Sustainability of Coastal Ecosystems: Insights from the Reproductive Traits of the Bioindicator Clam Donax trunculus. Sustainability. 2025; 17(14):6622. https://doi.org/10.3390/su17146622
Chicago/Turabian StyleBen-Haddad, Mohamed, Sara Hajji, Mohamed Rida Abelouah, and Aicha Ait Alla. 2025. "Urbanization Compromises the Sustainability of Coastal Ecosystems: Insights from the Reproductive Traits of the Bioindicator Clam Donax trunculus" Sustainability 17, no. 14: 6622. https://doi.org/10.3390/su17146622
APA StyleBen-Haddad, M., Hajji, S., Abelouah, M. R., & Ait Alla, A. (2025). Urbanization Compromises the Sustainability of Coastal Ecosystems: Insights from the Reproductive Traits of the Bioindicator Clam Donax trunculus. Sustainability, 17(14), 6622. https://doi.org/10.3390/su17146622






