Extracts of Hechtia spp. as Novel Coagulants Reduce the Pollutant Load of Whey
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining the Extracts
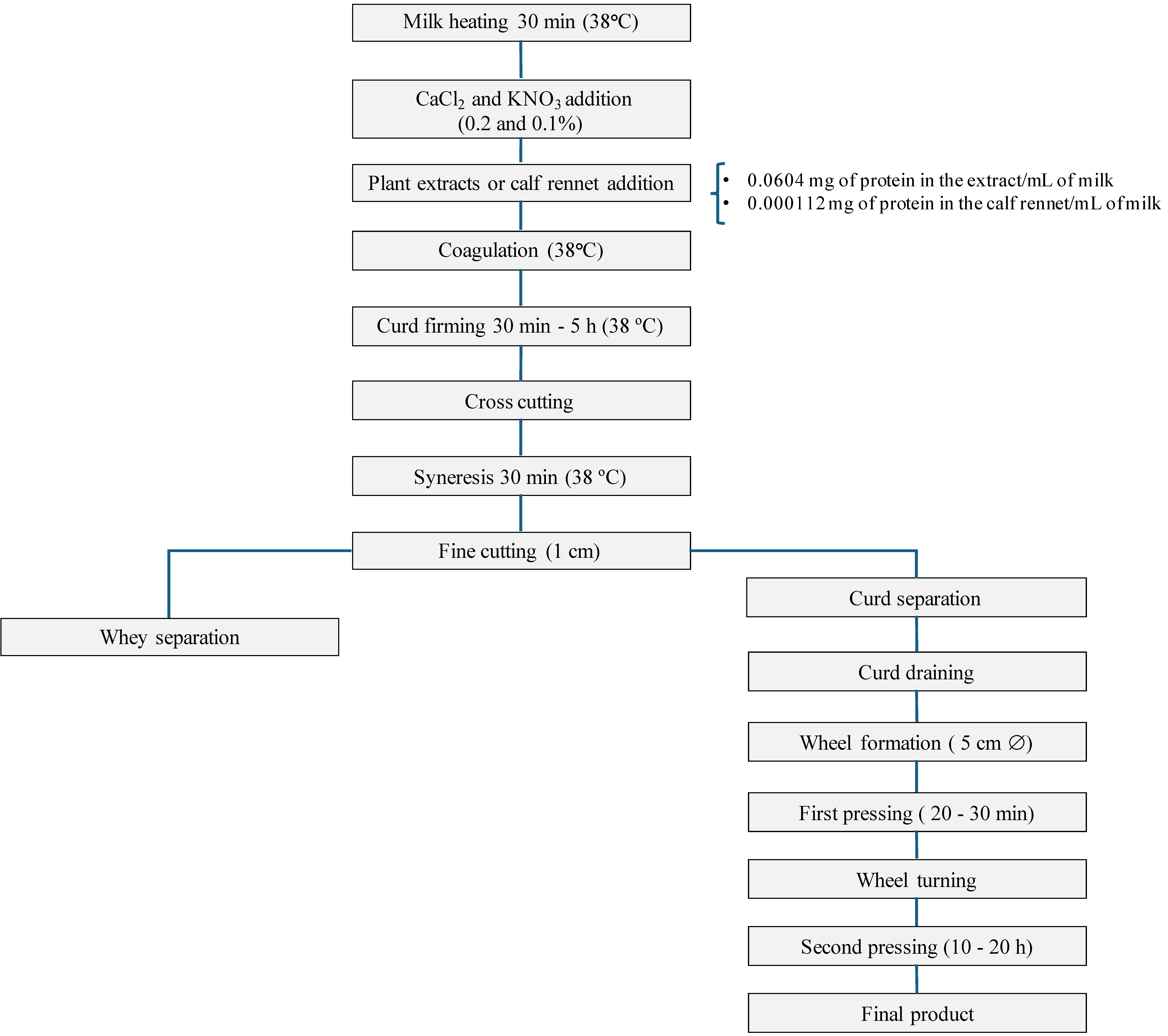
2.2. Cheese Production Process
2.3. Texture Analyses
2.4. Determination of Color in Cheeses
2.5. Determination of the Remaining Macromolecules in Whey
2.6. Determination of the BOD and COD of the Whey
2.7. Statistical Analysis
3. Results and Discussion
3.1. Yields of Extracts
3.2. Coagulation Time Test
3.3. Color of the Cheese Manufactured
3.4. Textural Profile Analysis (TPA)
3.5. Residual Macromolecules in the Whey
3.6. BOD and COD Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JVL | Juice Green Liquid (leaves blended and filtered) |
| JVP | Juice Green Press (leaves pressed and filtered) |
| EP | Parenchymatous Tissue (mixed and filtered) |
| PT | Parenchymatous Tissue (cut into cubes) |
| HT | Whole plant (cut into cubes) |
| ET | Chlorenchymatous tissue (cut into cubes) |
| RB | Rosette base (Rosette blended and filtered) |
References
- Gomes, S.; Belo, A.T.; Alvarenga, N.; Dias, J.; Lage, P.; Pinheiro, C.; Pinto-Cruz, C.; Brás, T.; Duarte, M.F.; Martins, A.P. Characterization of Cynara cardunculus L. flower from Alentejo as a coagulant agent for cheesemaking. Int. Dairy J. 2019, 91, 178–184. [Google Scholar] [CrossRef]
- Alvarenga, N.; Fernandes, J.; Gomes, S.; Baltazar, T.; Fiates, V.; Fidalgo, L.G.; Santos, T.; Conceicao, C.; Dias, J. Impact of different Cynara cardunculus L. extracts on the physicochemical, microbial, and sensory properties of Serpa cheese. Int. Dairy J. 2025, 162, 106159. [Google Scholar] [CrossRef]
- Vioque, M.; Gómez, R.; Sánchez, E.; Mata, C.; Tejada, L.; Fernández-Salguero, J. Chemical and microbiological characteristics of ewes’ milk cheese manufactured with extracts from flowers of Cynara cardunculus and Cynara humilis as coagulants. J. Agric. Food Chem. 2020, 48, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Voșgan, Z.; Dumuța, A.; Mihali, C.; Dippong, T.; Mihalescu, L.; Marian, M.; Mihalescu, B. The influence of different forms of black cumin (Nigella sativa L.) on the characteristics of sheep’s curd cheese. Front. Sustain. Food Syst. 2024, 8, 1413008. [Google Scholar] [CrossRef]
- Pacifico, S.; Caputo, E.; Piccolella, S.; Mandrich, L. Exploring New Fruit-and Vegetable-Derived Rennet for Cheese Making. Appl. Sci. 2024, 14, 2257. [Google Scholar] [CrossRef]
- Bathmanathan, R.; Yahya, Y.A.C.; Yusoff, M.M.; Vejayan, J. Utilizing coagulant plants in the development of functional dairy foods and beverages: A mini review. J. Biol. Sci. 2019, 19, 259–271. [Google Scholar] [CrossRef]
- Vieira, D.F.V.; Barbosa, M. Cheese-making with a vegetable rennet from Cardo (Cynara cardunculus). J. Dairy Res. 1972, 39, 335–343. [Google Scholar] [CrossRef]
- Borges, E.J.; de Castro, M.T.; de Freitas, A.R.F.; Borges, A.C.; dos Santos, P.A. Development and physicochemical characterization of Artisanal Minas Canastra cheese produced with Cynara cardunculus L. Rev. Inst. Laticínios Cândido Tostes 2018, 73, 136–148. [Google Scholar] [CrossRef]
- Ordiales, E.; Martín, A.; Benito, M.J.; Fernández, M.; Casquete, R.; de Guía Córdoba, M. Influence of the technological properties of vegetable rennet (Cynara cardunculus) on the physicochemical, sensory and rheological characteristics of ‘Torta del Casar’ cheese. Int. J. Dairy Technol. 2018, 67, 402–409. [Google Scholar] [CrossRef]
- Tejada, L.; Gomez, R.; Fernández-Salguero, J. Sensory characteristics of ewe milk cheese made with three types of coagulant: Calf rennet, powdered vegetable coagulant and crude aqueous extract from Cynara cardunculus. J. Food Qual. 2007, 30, 91–103. [Google Scholar] [CrossRef]
- Fernández-Salguero, J.; Sanjuán, E. Influence of vegetable and animal rennet on proteolysis during ripening in ewes’ milk cheese. Food Chem. 1999, 64, 177–183. [Google Scholar] [CrossRef]
- Barracosa, P.; Simões, I.; Martins, A.P.; Barros, M.; Pires, E. Biochemical diversity of cardoon flowers (Cynara cardunculus L.): Predicting PDO Mediterranean cheese textures. Food Biosci. 2021, 39, 100805. [Google Scholar] [CrossRef]
- Gutiérrez-Méndez, N.; Balderrama-Carmona, A.; García-Sandoval, S.E.; Ramírez-Vigil, P.; Leal-Ramos, M.Y.; García-Triana, A. Proteolysis and rheological properties of cream cheese made with a plant-derived coagulant from Solanum elaeagnifolium. Foods 2019, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- García, X.; López, M. Effect of vegetable coagulant, microbial coagulant and calf rennet on physicochemical, proteolysis, sensory and texture profiles of fresh goats’ cheese. Dairy Sci. Technol. 2012, 92, 691–707. [Google Scholar] [CrossRef]
- Colombo, M.L.; Cimino, C.V.; Bruno, M.A.; Hugo, A.; Liggieri, C.; Fernández, A.; Vairo-Cavalli, S. Artichoke cv. Francés flower extract as a rennet substitute: Effect on textural, microstructural, microbiological, antioxidant properties, and sensory acceptance of miniature cheeses. J. Sci. Food Agric. 2020, 101, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio, N.M.A.; Pérez, E.B.E. Plantas Útiles del Estado de Hidalgo; UAEH: Hidalgo, Mexico, 2006; Volume 3. [Google Scholar]
- Hornung-Leoni, C.T. Avances sobre usos etnobotánicos de las Bromeliaceae en Latinoamérica. Bol. Latinoam. Y Del Caribe De Plantas Med. Y Aromat. 2011, 10, 297–314. [Google Scholar]
- Poništ, J.; Dubšíková, V.; Schwarz, M.; Samešová, D. Methods of processing whey waste from dairies. A review. Environ. Prot. Eng. 2021, 47, 4. [Google Scholar] [CrossRef]
- Martínez-Ruiz, N.R.; López-Díaz, J.A. Optimización de la extracción y estandarización de un cuajo vegetal para la elaboración de queso asadero. Cienc. Front. 2008, 6, 173–176. [Google Scholar]
- Kim, S.; Park, J.B.; Hwang, I.K. Quality attributes of various varieties of Korean red pepper powders (Capsicum annuum L.) and color stability during sunlight exposure. J. Food Sci. 2002, 67, 2957–2961. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.E.; Trussell, R. Métodos Normalizados Para el Análisis de Aguas Potables y Residuales; Díaz de Santos Publishing House, SA: Madrid, Spain, 1992; pp. 5/12–5/19. [Google Scholar]
- Mokua, S.K.; Mbaria, J.M.; Maitho, T.E.; Moriasi, G.A. Ethnobotanical documentation, phytochemical screening, and cytotoxicity evaluation of medicinal plants used to manage snakebite envenomation in Mwingi West subcounty, Kenya. Evid.-Based Complement. Altern. Med. 2021, 1, 4167296. [Google Scholar] [CrossRef] [PubMed]
- Zreen, Z.; Hameed, A.; Kiran, S.; Farooq, T.; Zaroog, M.S. A comparative study of Diospyros malabarica (Gaub) extracts in various polarity-dependent solvents for evaluation of phytoconstituents and biological activities. BioMed Res. Int. 2022, 1, 4746223. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.A.; Lazza, C.M.; Errasti, M.E.; López, L.M.; Caffini, N.O.; Pardo, M.F. Milk clotting and proteolytic activity of an enzyme preparation from Bromelia hieronymi fruits. LWT-Food Sci. Technol. 2010, 43, 695–701. [Google Scholar] [CrossRef]
- Waheed, M.; Hussain, M.B.; Majeed, M.; Rehman, T.U.; Khan, M.U.; Shariati, M.A.; Glinushkin, A.P. Quality evaluation of cheese prepared by plant coagulants: Carica papaya and Moringa oleifera leaves. Russ. J. Agric. Socio-Econ. Sci. 2017, 64, 232–239. [Google Scholar] [CrossRef]
- Silva, S.V.; Barros, R.M.; Malcata, F.X. Hydrolysis of caseins by extracts of Cynara cardunculus precipitated by ammonium sulfate. J. Food Sci. 2002, 67, 1746–1751. [Google Scholar] [CrossRef]
- Hoxha, M.; Zhupa, I.; Mara, V.; Shumka, S. Assessment of coagulation and proteolytic activity of the flower extract from thistle plant species. J. Hyg. Eng. Des. 2022, 38, 180–186. [Google Scholar]
- Nazish, H.A.; Gulzar, N.; Nadeem, M.; Rafiq, S.; Sameen, A.; Ajmal, M.; Murtaza, S.; Saleem, I.M. Efficacy of Withania coagulans fruit extract as a coagulant for Mozzarella cheese at different coagulation temperatures from curd formation to pizza top. J. Food Process. Preserv. 2022, 46, e17167. [Google Scholar] [CrossRef]
- El-Kholy, A.M. Ras cheese making with vegetable coagulant-a comparison with calf rennet. World J. Dairy Food. Sci. 2015, 10, 82–89. [Google Scholar]
- Tesfaw, A.T.; Sewmehon, Y.M.; Tegegne, A.T.; Alemu, G.B.; Mersha, N.T.; Yohannes, T.G.; Negash, A.W.; Jiru, T.M. Exploring cheese production enzymes from various plants as an alternative to Calf rennet. Discov. Food 2024, 4, 141. [Google Scholar] [CrossRef]
- Sulmiyati, S.; Malelak, G.E.M. Coagulation power comparison between fresh and powdered biduri (Calotropis gigantea) leaf extract in making suspesi soft cheese. Int. Food Res. J. 2023, 30, 1341–1350. [Google Scholar] [CrossRef]
- Nasr, A.I.; Mohamed, A.I.A.; Hamid, O.I. Characterization of partially purified milk-clotting enzyme from sunflower (Helianthus annuus) seeds. Food Sci. Nutr. 2016, 4, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Mbye, M.; Mohamed, H.; Raziq, A.; Kamal-Eldin, A. The effects of camel chymosin and Withania coagulans extract on camel and bovine milk cheeses. Sci. Rep. 2021, 11, 13573. [Google Scholar] [CrossRef] [PubMed]
- Araghi, F.E.; Nodushan, R.M.; Jafarpour, A.; Moslehishad, M. Optimizing the effect of plant protease on different properties of analog cheese containing functional corn leachate. Food Sci. Nutr. 2023, 11, 2719–2732. [Google Scholar] [CrossRef] [PubMed]
- Correia, P.; Vítor, A.; Tenreiro, M.; Correia, A.C.; Madanelo, J.; Guiné, R. Effect of different thistle flower ecotypes as milk-clotting in Serra da Estrela cheese. Nutr. Food Sci. 2016, 46, 458–475. [Google Scholar] [CrossRef]
- Guiama, V.D.; Koube, J.; Ngah, E.; Beka, R.G.; Bindzi, J.M. Solanum aethiopicum Extract Used as Coagulant Affected Nutritional and Rheological Characteristics of Cheese. Am. J. Food Nutr. 2021, 9, 31–42. [Google Scholar]
- Mohsin, A.Z.; Norsah, E.; Marzlan, A.A.; Abd, R.M.H.; Hussin, A.S.M. Exploring the applications of plant-based coagulants in cheese production: A review. Int. Dairy J. 2024, 148, 105792. [Google Scholar] [CrossRef]
- Ben Amira, A.; Besbes, S.; Attia, H.; Blecker, C. Milk-clotting properties of plant rennets and their enzymatic, rheological, and sensory role in cheese making: A review. Int. J. Food Prop. 2017, 20 (Suppl. 1), S76–S93. [Google Scholar] [CrossRef]
- Tejada, L.; Abellán, A.; Cayuela, J.M.; Martínez-Cacha, A. Sensorial characteristics during ripening of the Murcia al vino goat’s milk cheese: The effect of the type of coagulant used and the size of the cheese. J. Sens. Stud. 2006, 21, 333–347. [Google Scholar] [CrossRef]
- Galán, E.; Prados, F.; Pino, A.; Tejada, L.; Fernández-Salguero, J. Influence of different amounts of vegetable coagulant from cardoon Cynara cardunculus and calf rennet on the proteolysis and sensory characteristics of cheeses made with sheep milk. Int. Dairy J. 2008, 18, 93–98. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Perea-Gutiérrez, T.C.; Lugo-Sánchez, M.E.; Ramirez-Suarez, J.C.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Cordoba, B. Comparison of the milk-clotting properties of three plant extracts. Food Chem. 2013, 141, 1902–1907. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Figueroa, R.H.; López-Malo, A.; Palou, E.; Mani-López, E. Reduced-Fat Cream Cheese with Lactobacillus acidophilus LA-5 as a Probiotic: Physicochemical, Sensory, and Fermentation Characteristics. ACS Food Sci. Technol. 2025, 5, 1699–1709. [Google Scholar] [CrossRef]
- Abd El-Gawad, M.A.; Ahmed, N.S. Cheese yield as affected by some parameters review. Acta Sci. Pol. Technol. Aliment. 2011, 10, 131–153. [Google Scholar]
- Buchanan, D.; Martindale, W.; Romeih, E.; Hebishy, E. Recent advances in whey processing and valorisation: Technological and environmental perspectives. Int. J. Dairy Technol. 2023, 76, 291–312. [Google Scholar] [CrossRef]
- Hoover, A.; Hu, H.; Wright, C. Procter & Gamble Chemical Analysis Summary of Biomass Waste for Anaerobic Digestion. Technical Report. United States: N. p. 2016. Available online: https://www.osti.gov/biblio/1871299 (accessed on 17 April 2025).
- Kazimierowicz, J.; Zieliński, M.; Bartkowska, I.; Dębowski, M. Effect of acid whey pretreatment using ultrasonic disintegration on the removal of organic compounds and anaerobic digestion efficiency. Int. J. Environ. Res. Public Health 2022, 19, 11362. [Google Scholar] [CrossRef] [PubMed]
- Abdelhakam, K.E.; Faraht, F.H.O.; Elkihir, N.M. Effect of dryer & drying methods on quality and nutritional value of dried sweet and sour whey produced from white Cheese. Omdurman Islam. Univ. J. 2022, 18, 358–373. [Google Scholar] [CrossRef]
- Thakur, M.; Singh, K.; Khedkar, R. Phytochemicals: Extraction process, safety assessment, toxicological evaluations, and regulatory issues. In Functional and Preservative Properties of Phytochemicals; Academic Press: New York, NY, USA, 2020; pp. 341–361. [Google Scholar]
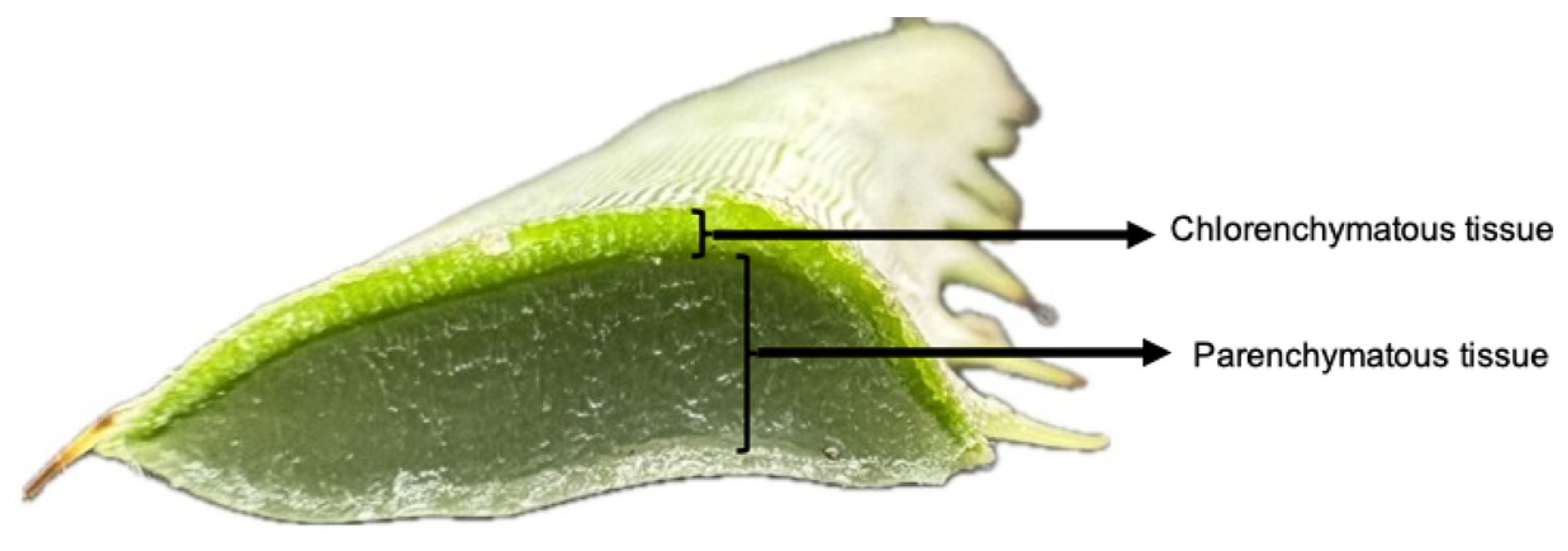
| Species | Sample | Yield (mL of Extract/g of Sample) |
|---|---|---|
| H. glomerata | JVL | 0.22 |
| JVP | 0.22 | |
| EP | 0.62 | |
| PT | WE | |
| HT | WE | |
| ET | WE | |
| BR | 0.18 | |
| H. podantha | JVL | 0.30 |
| JVP | 0.30 | |
| EP | 0.66 | |
| PT | WE | |
| HT | WE | |
| ET | WE | |
| BR | 0.35 |
| Species | Extract | Coagulation Time (h) |
|---|---|---|
| H. glomerata | JVL | WC |
| JVP | 9 | |
| EP | WC | |
| PT | WC | |
| HT | WC | |
| ET | WC | |
| RB | WC | |
| H. podantha | JVL | 3 |
| JVP | 8 | |
| EP | 5 | |
| PT | 9 | |
| HT | WC | |
| ET | WC | |
| RB | 2 |
| Species | Extract | L* | a* | b* | ΔE | C* | h* |
|---|---|---|---|---|---|---|---|
| H. glomerata | JVP | 21.80 ± 0.78 f | 0.36 ± 0.53 f | 18.82 ± 0.68 f | 42.11 ± 0.88 a | 18.83 ± 0.67 f | 1.55 ± 0.03 a |
| H. podantha | JVL | 26.97 ± 0.85 e | −2.68 ± 0.46 g | 25.22 ± 0.98 e | 35.07 ± 0.88 c | 25.37 ± 0.95 e | −1.46 ± 0.02 d |
| JVP | 26.05 ± 0.66 e | 2.87 ± 0.44 e | 19.68 ± 0.74 f | 38.40 ± 0.14 b | 19.89 ± 0.78 f | 1.43 ± 0.02 b | |
| EP | 41.62 ± 0.99 d | 6.70 ± 0.20 c | 41.05 ± 0.40 c | 11.76 ± 0.69 g | 41.60 ± 0.37 c | 1.41 ± 0.01 b | |
| PT | 71.59 ± 0.72 a | −0.70 ± 0.12 f | 31.30 ± 0.99 d | 32.22 ± 0.12 d | 31.30 ± 0.99 d | −1.55 ± 0.03 e | |
| RB | 21.76 ± 0.35 f | 14.84 ± 0.68 a | 18.90 ± 0.86 f | 26.79 ± 1.12 e | 24.05 ± 0.32 e | 0.90 ± 0.04 c | |
| Control (calf rennet) | 47.91 ± 0.26 c | 9.60 ± 0.47 b | 50.51 ± 0.96 b | --- | 51.42 ± 0.98 b | 1.38 ± 0.01 b | |
| Commercial cheese | 65.25 ± 0.79 b | 4.06 ± 0.80 d | 54.34 ± 0.58 a | 18.64 ± 0.58 f | 54.50 ± 0.58 a | 1.50 ± 0.01 a | |
| Species | Extract | Hardness (N) | Adhesiveness (mJ) | Cohesiveness | Springiness (mm) | Gumminess (N) | Chewiness (mJ) | |
|---|---|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | |||||||
| H. glomerata | JVP | 11.33 ± 0.34 c | 8.20 ± 0.96 b | 1.97 ± 0.78 b | 2.10 ± 0.50 b | −0.56 ± 0.02 a | 23.65 ± 5.11 b | −13.17 ± 2.40 ab |
| H. podantha | JVP | 11.91 ± 0.25 bc | 7.65 ± 1.08 b | 8.20 ± 3.24 a | 1.87 ± 0.25 b | −0.58 ± 0.52 a | 22.24 ± 2.67 b | −12.03 ± 10.80 ab |
| PT | 17.26 ± 0.38 a | 15.44 ± 1.91 a | 0.60 ± 0.20 b | 2.47 ± 0.96 b | −0.47 ± 0.20 a | 42.80 ± 17.44 ab | −17.97 ± 1.16 ab | |
| JVL | 11.58 ± 0.46 c | 7.73 ± 1.38 b | 4.47 ± 3.85 ab | 1.66 ± 0.36 b | −0.42 ± 0.36 a | 19.19 ± 4.05 b | −8.13 ± 7.38 a | |
| EP | 10.78 ± 0.47 c | 7.10 ± 0.06 b | 3.10 ± 2.34 ab | 2.43 ± 0.27 b | −0,75 ± 0.13 a | 22.46 ± 5.83 b | −16.90 ± 5.49 ab | |
| Control (calf rennet) | 12.82 ± 0.64 b | 9.22 ± 3.61 b | 1.83 ± 0.68 b | 2.08 ± 1.26 b | −0.59 ± 0.26 a | 17.16 ± 1.60 b | −13.43 ± 3.20 ab | |
| Commercial cheese | 9.24 ± 0.35 d | 8.25 ± 0.58 b | 0.90 ± 0.56 b | 6.26 ± 2.21 a | −0.13 ± 0.59 a | 57.38 ± 18.70 a | −23.87 ± 1.00 b | |
| Species | Extract | Residual Protein g of Protein/L of Whey | Residual Carbohydrates g Eq Lactose/L of Whey |
|---|---|---|---|
| H. glomerata | JVP | 0.60 ± 0.02 c | 39.39 ± 0.47 f |
| H. podantha | JVL | 0.74 ± 0.01 b | 75.19 ± 0.38 d |
| JVP | 0.73 ± 0.02 b | 98.61 ± 0.14 c | |
| EP | 0.76 ± 0.01 b | 56.49 ± 0.54 e | |
| PT | 0.79 ± 0.04 b | 56.32 ± 0.07 e | |
| RB | 0.92 ± 0.02 a | 149.49 ± 0.83 a | |
| Control (calf rennet) | 0.78 ± 0.02 b | 111.60 ± 0.69 b | |
| Species | Extract | COD mg of O2/L | BOD mg of O2/L |
|---|---|---|---|
| H. glomerata | JVP | 213,936.97 ± 37,676.15 ab | 165,801.15 ± 29,199.02 ab |
| H. podantha | JVL | 336,144.83 ± 28,916.07 ab | 260,512.25 ± 22,409.96 ab |
| JVP | 235,010.61 ± 180,070.23 ab | 182,133.22 ± 139,554.43 ab | |
| EP | 150,427.37 ± 14,240.24 b | 116,581.21 ± 11,036.19 b | |
| PT | 339,993.90 ± 84,015.30 ab | 263,495.27 ± 65,111.86 ab | |
| RB | 473,748.97 ± 179,175.50 a | 367,155.45 ± 138,861.01 a | |
| Control (calf rennet) | 418,899.77 ± 71,900.06 ab | 324,647.32 ± 55,722.55 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cruz, L.; Mosqueda-Avalos, M.A.; Negrete-Rodríguez, M.d.l.L.X.; Conde-Barajas, E.; Flores-Martínez, N.L.; Bernardino-Nicanor, A. Extracts of Hechtia spp. as Novel Coagulants Reduce the Pollutant Load of Whey. Sustainability 2025, 17, 6579. https://doi.org/10.3390/su17146579
González-Cruz L, Mosqueda-Avalos MA, Negrete-Rodríguez MdlLX, Conde-Barajas E, Flores-Martínez NL, Bernardino-Nicanor A. Extracts of Hechtia spp. as Novel Coagulants Reduce the Pollutant Load of Whey. Sustainability. 2025; 17(14):6579. https://doi.org/10.3390/su17146579
Chicago/Turabian StyleGonzález-Cruz, Leopoldo, Miguel Angel Mosqueda-Avalos, María de la Luz Xochilt Negrete-Rodríguez, Eloy Conde-Barajas, Norma Leticia Flores-Martínez, and Aurea Bernardino-Nicanor. 2025. "Extracts of Hechtia spp. as Novel Coagulants Reduce the Pollutant Load of Whey" Sustainability 17, no. 14: 6579. https://doi.org/10.3390/su17146579
APA StyleGonzález-Cruz, L., Mosqueda-Avalos, M. A., Negrete-Rodríguez, M. d. l. L. X., Conde-Barajas, E., Flores-Martínez, N. L., & Bernardino-Nicanor, A. (2025). Extracts of Hechtia spp. as Novel Coagulants Reduce the Pollutant Load of Whey. Sustainability, 17(14), 6579. https://doi.org/10.3390/su17146579








