Development of Sustainable Technology for Effective Reject Water Treatment
Abstract
1. Introduction
| Technology | Removal Efficiency | Advantages | Disadvantages | Treatment Cost/Energy Usage | Reference |
|---|---|---|---|---|---|
| ANNAMOX | Up to 90% N-NH4+ | High nitrogen compound removal rates Lower sludge production and hence a reduction in sewage sludge processing costs Resilience of Anammox bacteria to high nitrogen loads Significantly lower energy consumption as compared to conventional nitrification–denitrification bioreactors Reduced chemical usage due to elimination of external carbon use | Anammox bacteria need specific conditions to grow Sensitivity of Anammox bacteria to presence of inhibitors such as heavy metals Long period of bioreactor startup Minor effect phosphate removal | 0.2–0.3 kWh/kg-N removed | [27,28,29] |
| SHARON | 50–90% N-NH4+ | Energy efficient technology using 40% less added carbon than full nitrification Small size of the reactors No need to provide external carbon sources to bioreactor | Partial removal of ammonia nitrogen, necessity to combine with other technologies Specific operational conditions, e.g., high temperature (30–40 °C) and pH of 7–8 Need for strict control of operating parameters, e.g., dissolved oxygen Minor effect on phosphate removal | 0.8–1.3 kWh/kg-N removed | [30,31,32] |
| SHARON-ANAMMOX | Up to 71% N-NH4+ | Highly efficient in treating high-strength ammonia side streams, e.g., reject waters, leachate, and other industrial wastewaters Compact and modular reactors as compared to conventional nitrification–denitrification bioreactors. Low sludge production More energy efficient as compared to conventional biological reactors | Complex two-stage process Each stage needs specific process conditions such as temperature and pH Sensitivity to inhibitory compounds typically presented in industrial wastewaters Use is limited to specific types of wastewaters Requires advanced monitoring systems High investment cost Minor effect on phosphate removal | - | [30,33] |
| DAMO | 80–95% N-NH4+ | Operates under anaerobic conditions, thus a low-energy-demand technology Low sludge production Simultaneous methane removal Suitability for high nitrogen loads | Long startup period Sensitivity to temperature and pH The process requires methane as an electron donor As a standalone process: low efficiency of ammonia removal, higher removal rate in combination with Annamox No effect on phosphate content | - | [34,35,36] |
| CANDO | 80–95% N-NH4+ | Energy efficiency Converts nitrogen compounds into fuel (N2O) Lower greenhouse gas emissions | The need for strict process control Needs an organic carbon source to conduct denitrification | 2–3 MJ per kg of N removed | [37,38] |
| Air stripping | 50–98% N-NH4+ | Simple equipment construction Insensitive to toxic substances Efficient technique | High energy demand Requires certain conditions such as pH, temperature, and flow rate Time-consuming process No effect on phosphate content | 0.3–0.8 kWh/m3 | [39,40] |
| Hydrodynamic cavitation | Up to 45% N-NH4+ | Easy operation Simple device construction Energy efficiency Possibility to remove other pollutants | As a sole method: low removal efficacy Necessity to combine with other methods to achieve high removal rates Possibility to generate toxic intermediates | 0.02–0.1 USD/m3 | [25] |
| Reverse osmosis | 60–99% N-NH4+ | Low energy requirement Compact and easy design High efficiency Easily adaptable to a specific wastewater composition | High cost of purchasing membranes Possibility of fouling membranes by colloidal matter and formation of biofilms on their surface Necessity to often clean the membranes The metals present in wastewater, such as Fe and Mg, might decrease membrane potential | 0.50 kWh/m3 | [41,42] |
| Microwave radiation | 80% N-NH4+ 35% P-PO43− | Moderate cost of operation (as compared to other technologies) Suitable for high ammonium concentration Simultaneous removal of ammonia nitrogen and phosphates | Affected by pH and radiation time, initial ammonia concentration, and aeration Evaporation of NH3 Difficult to achieve full-scale application | 4.8 kW per reactor (capacity of 5 m3/d) | [43,44,45] |
| Ion exchange and adsorption | 80–95% N-NH4+ and P-PO43 | Low cost of technology Easy operation Possibility to modify adsorbents to adequate wastewater composition Availability and diversity of adsorbents Effectively removes ammonium and phosphates Efficient technology for low levels of ammonium nitrogen and phosphates | Necessity to provide particular process conditions, e.g., pH ranges Depending on the absorbent, the removal efficiency varies significantly Additional energy and costs relating to regeneration Necessity of waste brine treatment or disposal | 0.05–0.2 kWh/m3 | [10] |
| Enhanced biological phosphorus removal | 70–95% P-PO43 | Reduced chemical usage Possibility of removing nitrogen compounds | Significant sensitivity to environmental conditions | 0.3–0.6 kWh/m3 | [46] |
| Struvite precipitation | 70–90% P-PO43 20–30% for nitrogen compounds | Generation of product with a high fertilizer value that contains both phosphorus and nitrogen Low energy demand Efficient for high-strength wastewater | Requires specific pH values and magnesium-to-phosphate ratio Requires chemical dosing of magnesium salts | 0.1–4.6 kWh/kg struvite | [47,48,49] |
| Electrochemical methods | Up to 98% P-PO43 | Simple device construction Short hydraulic retention time Less sludge volume Adaptability to specific conditions Relatively low-cost method | High energy consumption as compared to other methods Electrode degradation: necessity to replace Necessity to maintain adequate process conditions Possibility of generating by-products | 0.18–11.29 kWh/m3 for aluminum electrode and 0.24–8.47 kWh/m3 for iron electrode | [50] |
2. Materials and Methods
2.1. Characterization of Materials
2.2. Experimental Methodology
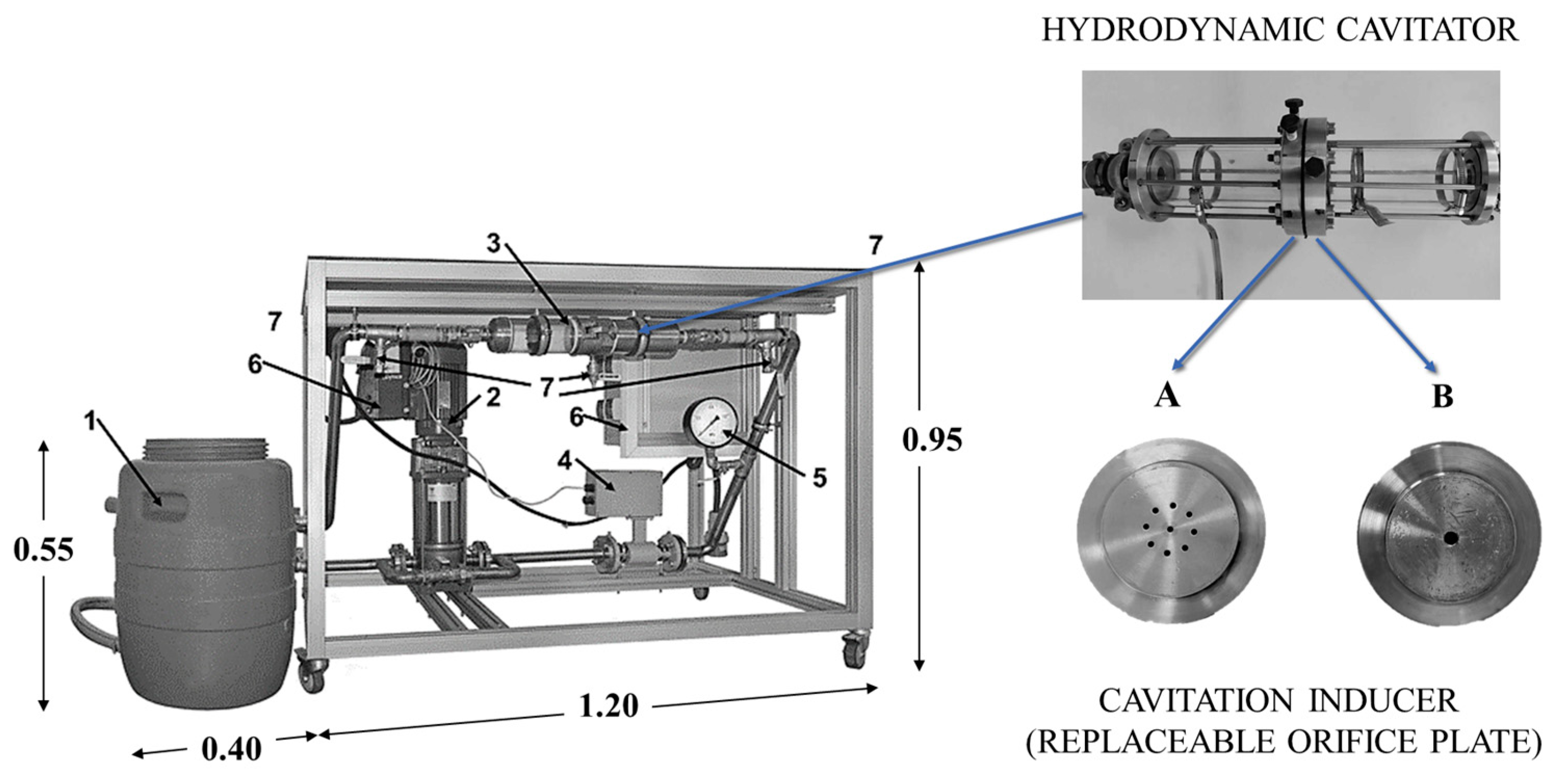
- A: orifice plate with 9 holes, each with a diameter of 1 mm;
- B: orifice plate with one concentric hole with a diameter of 3/10 mm.
2.3. Laboratory Installation
2.4. Evaluation of Process Performance
2.5. Kinetic Evaluation
2.6. Energy and Cost Evaluation
2.7. Statistical Analyses
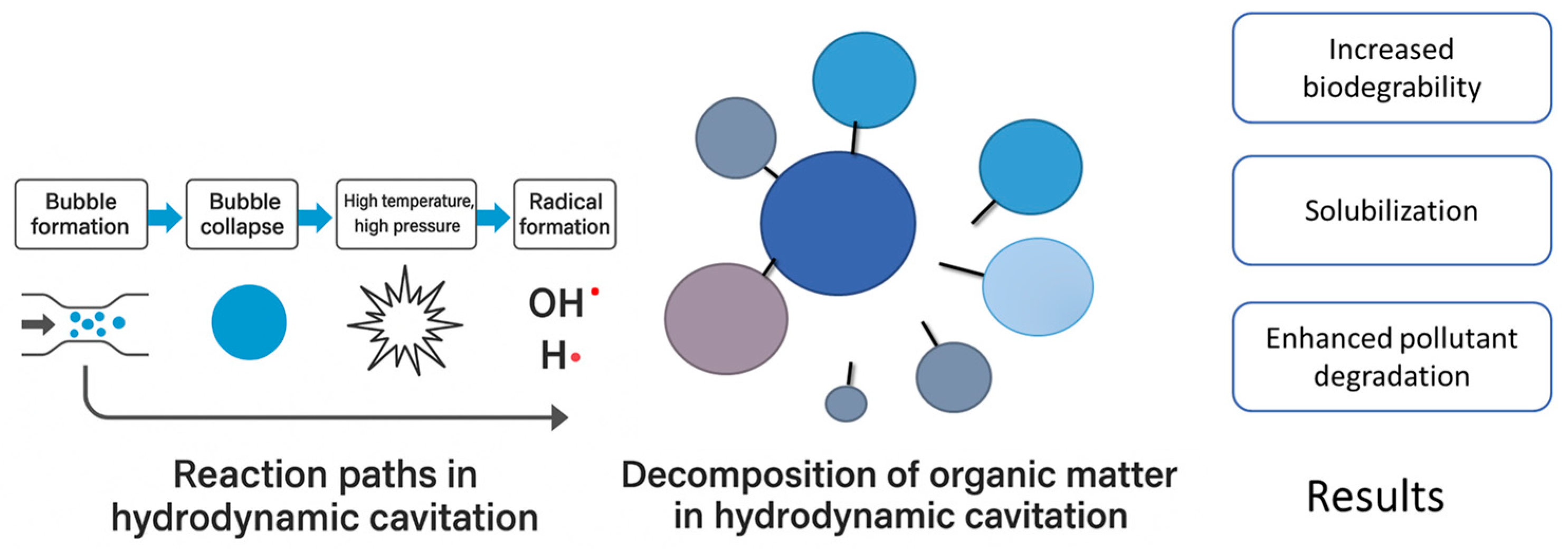
3. Results and Discussion
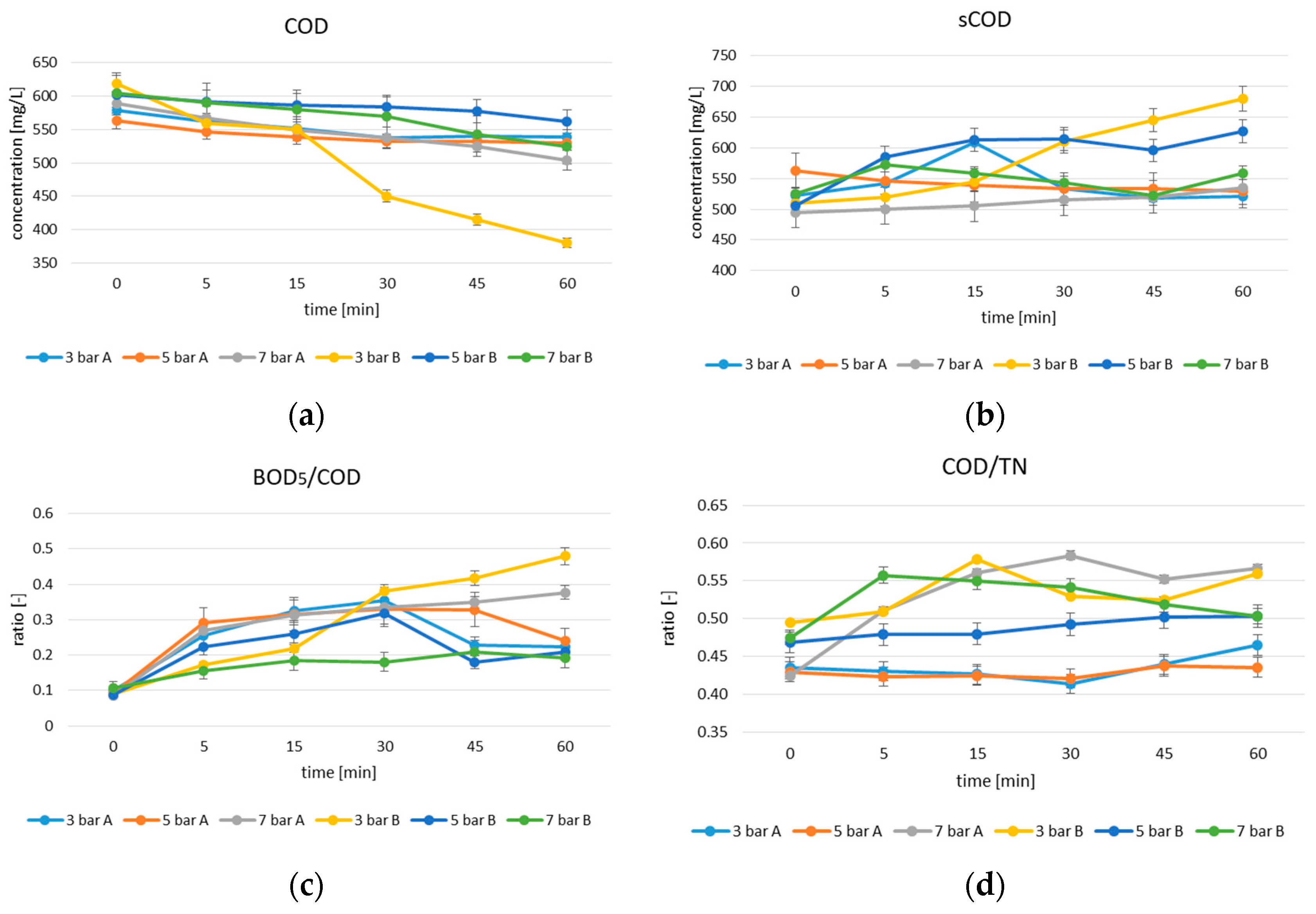
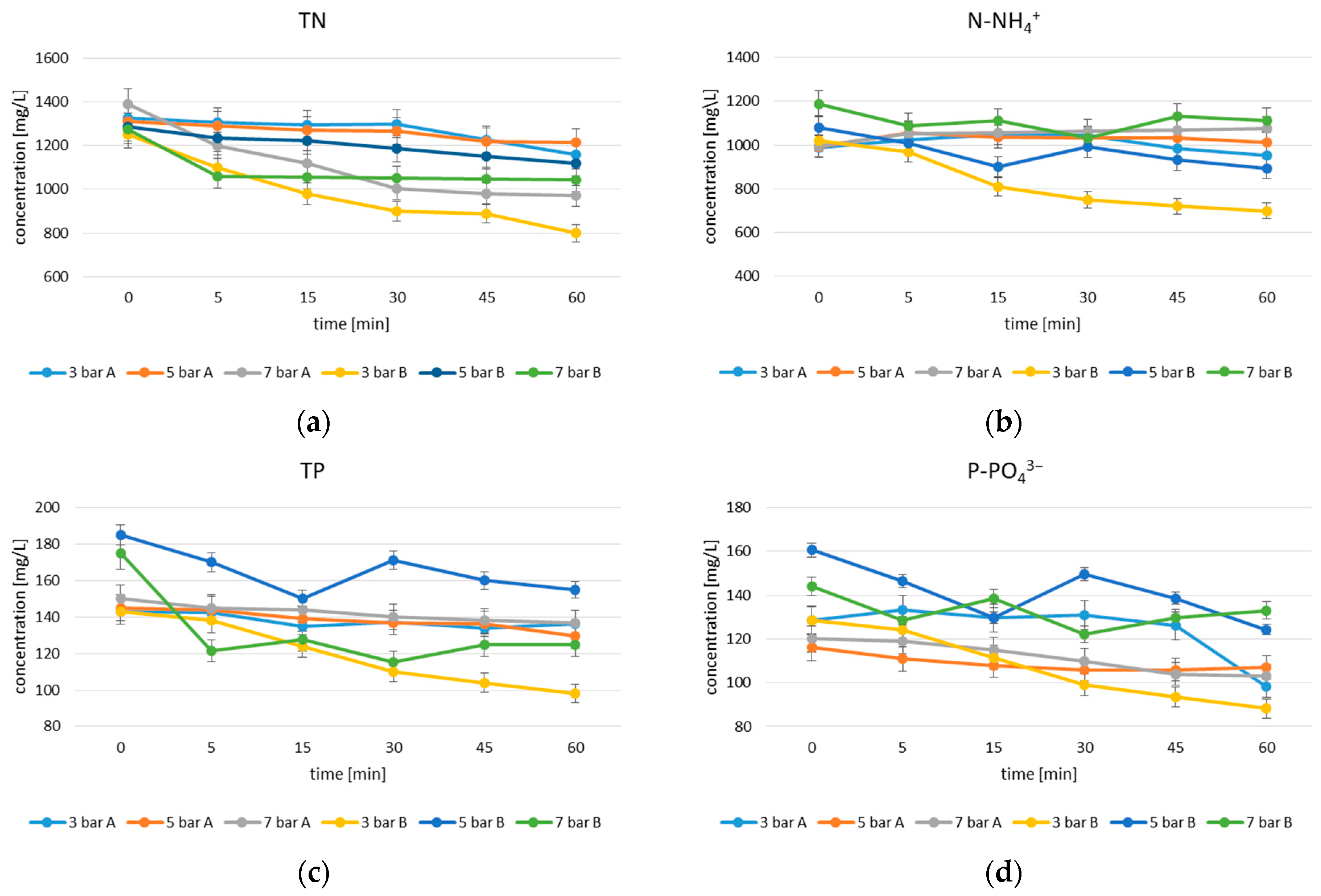
3.1. Experiment 1
3.2. Experiment 2
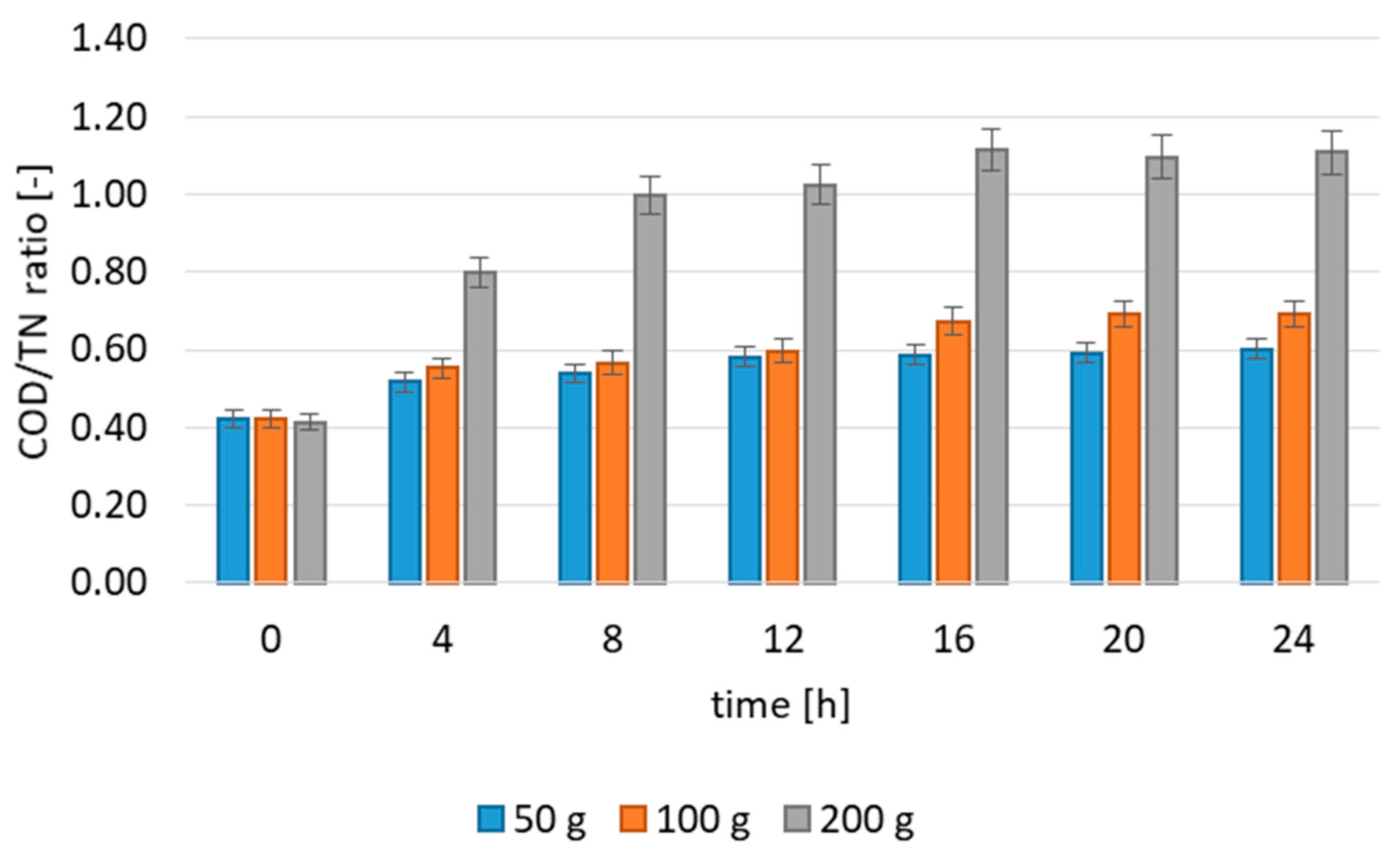
3.2.1. Effect of Contact Time and Dose
3.2.2. Adsorption Kinetics
3.3. Energy and Cost Evaluation and Future Prospects
- A solution to the problem of zeolite regeneration, thus reducing the cost of reagents and the formation of waste brine;
- An analysis of zeolite in the context of the adsorbed nutrients and heavy metals;
- An evaluation of the effectiveness of using synthetic zeolites generated from wastes;
- The influence of pH, T, and agitation speed on adsorption performance;
- Isotherm studies aiming to optimize adsorption experiments and describe the occurring mechanisms within adsorption;
- The effect of the proposed technology on conventional biological treatment.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANAMMOX | anaerobic ammonium oxidation |
| BI | biodegradability index |
| BOD5 | biological oxygen demand |
| C | cost of materials |
| COD | chemical oxygen demand |
| sCOD | soluble chemical oxygen demand |
| CANDO | coupled aerobic–anoxic nitrous decomposition operation |
| CANON | completely autotrophic nitrogen removal over nitrite |
| DAMO | denitrifying anaerobic methane oxidation |
| E | energy demand |
| EA | energy needed to perform adsorption |
| EHC | energy needed to perform HC |
| ER | energy needed to zeolite regeneration |
| HC | hydrodynamic cavitation |
| N-NH4+ | ammonia nitrogen |
| P | the power of low-speed stirrer |
| P-PO43− | phosphates |
| PR | power output of the pump |
| q | adsorption capacity |
| RE | removal efficiency |
| RW | reject water |
| SHARON-ANAMMOX | single-reactor system for high activity ammonia removal over nitrite- anaerobic ammonium oxidation |
| T | temperature |
| t | mixing time |
| TN | total nitrogen |
| TP | total phosphorus |
| tR | time to perform regeneration |
| TSSs | total suspended solids |
| VSSs | volatile suspended solids |
| WWTPs | wastewater treatment plants |
References
- Zhang, Y.; Gong, H.; Zhu, D.; Lu, D.; Zhou, S.; Wang, Y.; Dai, X. A two-stage partial nitritation-denitritation/anammox (PN-DN/A) process to treat high-solid anaerobic digestion (HSAD) reject water: Verification based on pilot-scale and full-scale projects. Water Res. X 2024, 22, 100213. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Zhou, S.; Lu, D.; Diao, S.; Shi, W.; Gong, H.; Dai, X. Evolution of dissolved organic nitrogen (DON) during sludge reject water treatment revealed by FTICR-MS. Sci. Total Environ. 2023, 893, 164944. [Google Scholar] [CrossRef] [PubMed]
- Flores-Alsina, X.; Vangsgaard, A.K.; Uri-Carreño, N.; Nielsen, P.H.; Gernaey, K.V. Quantifying, predicting, and mitigating nitrous oxide emissions in a full-scale partial nitritation/anammox reactor treating reject water. Water Res. 2025, 278, 123200. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Gong, H.; Xu, Y.; Xu, E.; Yang, D.; Gu, G.; Dai, X. One-stage partial nitritation—Anammox treatment of RW from high-solid-sludge anaerobic digestion with thermal hydrolysis pretreatment: Inhibition and system recovery. J. Environ. 2022, 10, 107958. [Google Scholar] [CrossRef]
- Karmann, C.; Mágrová, A.; Jeníček, P.; Bartáček, J.; Kouba, V. Advances in nitrogen removal and recovery technologies from RW: Economic and environmental perspectives. Bioresour. Technol. 2024, 391, 129888. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Gong, H.; Diao, S.; Shi, W.; Yin, R.; Dai, X. Enhanced sludge settlement of two stage PN/Anammox for reject water treatment with respective diatomite addition. Sci. Total Environ. 2023, 877, 162784. [Google Scholar] [CrossRef] [PubMed]
- Parde, D.; Behera, M.; Dash, R.R.; Bhunia, P. A review on anammox processes: Strategies for enhancing bacterial growth and performance in wastewater treatment. IBBS 2024, 191, 105812. [Google Scholar] [CrossRef]
- Liang, Z.; Han, H.; Yi, J.; Dai, X. Modified integrated fixed-film activated sludge process: Advanced nitrogen removal for low-C/N domestic wastewater. Chemosphere 2022, 307 Pt 2, 135827. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Sui, Q.; Yu, D.; Gui, S.; Zhang, K.; Wei, Y. Effective enrichment of anaerobic ammonia oxidation sludge with feast-starvation strategy: Activity, sedimentation, growth kinetics, and microbial community. Bioresour. Technol. 2023, 388, 129730. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Chen, Z.; Osman, A.I.; Rooney, D.W.; Yap, P.S. Strategies for ammonia recovery from wastewater: A review. Environ. Chem. Lett. 2024, 22, 2699–2751. [Google Scholar] [CrossRef]
- Farghali, M.; Osman, A.I.; Mohamed, I.M.A.; Chen, Z.; Chen, L.; Ihara, I.; Yap, P.S.; Rooney, D.W. Strategies to save energy in the context of the energy crisis: A review. Environ. Chem. Lett. 2023, 21, 2003–2039. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ni, J.; Zhu, Z.; Zuo, X. Simultaneous ammonium and phosphate removal with Mg-loaded chitosan carbonized microsphere: Influencing factors and removal mechanism. Environ. Res. 2023, 228, 115850. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Sun, H.; Jia, L.; Wu, W.; Wang, J. Simultaneous biological removal of nitrogen and phosphorus from secondary effluent of wastewater treatment plants by advanced treatment: A review. Chemosphere 2022, 296, 134054. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Chen, M.; Zhang, D. Effects of hydrodynamic cavitation combined with snail enzyme treatment on the structure and functional properties of water-soluble dietary fiber in rice husks. Ultrason. Sonochem. 2025, 113, 107236. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Sonawane, S.H.; Shyam, P.; Sun, X.; Manickam, S. Hybrid hydrodynamic cavitation (HC) technique for the treatment and disinfection of lake water. Ultrason. Sonochem. 2023, 97, 106454. [Google Scholar] [CrossRef] [PubMed]
- Karaçoban, D.; Topaç, E.; Dindar, F.O.; Keskinler, B. Effect of Orifice Induced Hydrodynamic Cavitation on the Properties of Waste Activated Sludge. KSCE J. Civ. Eng. 2024, 28, 1151–1161. [Google Scholar] [CrossRef]
- Warade, A.R.; Shinde, G.B.; Gaikwad, R.; Hakke, V.S.; Sonawane, S.H.; Lingayat, A.B. Intensification of pharmaceutical wastewater treatment using hydrodynamic cavitation process. Mater. Today Proc. 2022, 77, 692–697. [Google Scholar] [CrossRef]
- Kumari, P.; Ghosh, S.; Mondal, P. Hybrid process of hydrodynamic cavitation and photocatalytic oxidation for degradation of pesticides in water. Chem. Eng. Process. 2025, 209, 110147. [Google Scholar] [CrossRef]
- Jadhav, S.P.; Gogate, P.R. Hydrodynamic cavitation induced degradation of Brilliant Blue dye intensified using various additives. Journal of the Indian Chemical Society. J. Indian Chem. Soc. 2024, 102, 101540. [Google Scholar] [CrossRef]
- Darandale, G.R.; Jadhav, M.V.; Warade, A.R.; Vikas, S.H. Hydrodynamic cavitation a novel approach in wastewater treatment: A review. Mater. Today 2023, 77, 960–968. [Google Scholar] [CrossRef]
- Bis, M.; Montusiewicz, A.; Ozonek, J.; Pasieczna-Patkowska, S. Application of hydrodynamic cavitation to improve the biodegradability of mature landfill leachate. Ultrason. Sonochem. 2015, 26, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Lebiocka, M. Application of Hydrodynamic Cavitation to Improve the Biodegradability of Municipal Wastewater. J. Ecol. Eng. 2020, 21, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Gawande, G.D.; Chougule, S.; Bangar, S.; Dethe, A.; Rathod, A.; Kulkarni, A. Hydrodynamic cavitation and its hybridization with Fenton process as a promising AOP for dairy wastewater treatment. Mater. Today Proc. 2024. [Google Scholar] [CrossRef]
- Korpe, S.; Polisetty, V.R.; Sonawane, S.H. Performance Evaluation of Hydrodynamic Cavitation in Combination with Aops for Degradation of Tannery Wastewater. J. Environ. Chem. Eng. 2023, 11, 109731. [Google Scholar] [CrossRef]
- Patil, P.B.; Bhandari, V.M.; Ranade, V.V. Improving efficiency for removal of ammoniacal nitrogen from wastewaters using hydrodynamic cavitation. Ultrason. Sonochem. 2021, 70, 105306. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, L.; An, Y.; Wang, W.; Li, H.; Lian, Z. Simultaneous removal of manganese and ammonium nitrogen from mine water using sodium hexametaphosphate modified natural zeolite loaded by 3D MnOx. J. Environ. Chem. Eng. 2024, 12, 112509. [Google Scholar] [CrossRef]
- Cho, S.; Kambey, C.; Nguyen, V.K. Performance of Anammox Processes for Wastewater Treatment: A Critical Review on Effects of Operational Conditions and Environmental Stresses. Water 2020, 12, 20. [Google Scholar] [CrossRef]
- Ni, S.Q.; Ahmad, H.A.; Zhao, Y.; Li, Q.; Dong, Y.; Cui, Z. Energy-Efficient Anaerobic Ammonia Removal: From Laboratory to Full-Scale Application. In Microbial Bioremediation & Biodegradation, 1st ed.; Shah, M., Ed.; Springer: Singapore, 2020; pp. 505–526. [Google Scholar] [CrossRef]
- Shourjeh, M.S.; Kowal, P.; Lu, X.; Xie, L.; Drewnowski, J. Development of Strategies for AOB and NOB Competition Supported by Mathematical Modeling in Terms of Successful Deammonification Implementation for Energy-Efficient WWTPs. Processes 2021, 9, 562. [Google Scholar] [CrossRef]
- Shalini, S.S.; Joseph, K. Nitrogen management in landfill leachate: Application of SHARON, ANAMMOX and combined SHARON-ANAMMOX process. Waste Manag. 2012, 32, 2385–2400. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Bisen, M.; Kulshreshtha, S.; Kumar, L.; Choudhury, S.R.; Nath, M.J.; Mandal, M.; Kumar, A.; Patel, S.K.S. Advancement in Anaerobic Ammonia Oxidation Technologies for Industrial Wastewater Treatment and Resource Recovery: A Comprehensive Review and Perspectives. Bioengineering 2025, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Van Kempen, R.; Mulder, J.W.; Uijterlinde, C.A.; Loosdrecht, M.C.M. Overview: Full scale experience of the SHARON® process for treatment of rejection water of digested sludge dewatering. Water Sci. Technol. 2001, 44, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Shalini, S.S.; Joseph, K. Combined SHARON and ANAMMOX processes for ammoniacal nitrogen stabilisation in landfill bioreactors. Bioresour. Technol. 2018, 250, 723–732. [Google Scholar] [CrossRef]
- Peng, L.; Nie, W.B.; Ding, J.; Ni, B.J.; Liu, Y.; Han, H.J.; Xie, G.J. Denitrifying Anaerobic Methane Oxidation and Anammox Process in a Membrane Aerated Membrane Bioreactor: Kinetic Evaluation and Optimization. Environ. Sci. Technol. 2020, 54, 6968–6977. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, G.; Sivakumar, M.; Wu, J. Enhancing integrated denitrifying anaerobic methane oxidation and Anammox processes for nitrogen and methane removal: A review. Crit. Rev. Environ. Sci. Technol. 2022, 53, 390–415. [Google Scholar] [CrossRef]
- Molina-Macías, A.K.; Londono, Y.A.; Penuela, G.A. Denitrifying anaerobic methane oxidation and its applications for wastewater treatment. Int. J. Environ. Sci. Technol. 2023, 20, 2209–2228. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Z.; Pan, S.; Kalehbasti, P.R.; Zhang, W. Benchmarking sidestream shortcut nitrogen removal processes against nitrous oxide recovery from a life cycle perspective. J. Clean. Prod. 2023, 384, 135530. [Google Scholar] [CrossRef]
- Wang, Z.; Woo, S.G.; Yao, Y.; Cheng, H.H.; Wu, Y.J.; Criddle, C.S. Nitrogen removal as nitrous oxide for energy recovery: Increased process stability and high nitrous yields at short hydraulic residence times. Water Res. 2020, 173, 115575. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, A.; Sabzalipour, S.; Takdatsan, A.; Yengejeh, R.J.; Khafaie, M.A. Ammonia removal form municipal wastewater by air stripping process: An experimental study. S. Afr. J. Chem. Eng. 2021, 36, 134–141. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, H.; Lee, E. Influence of Ammonia Stripping Parameters on the Efficiency and Mass Transfer Rate of Ammonia Removal. Appl. Sci. 2021, 11, 441. [Google Scholar] [CrossRef]
- Shin, C.; Szczuka, A.; Jiang, R.; Mitch, W.A.; Criddlea, C.S. Optimization of reverse osmosis operational conditions to maximize ammonia removal from the effluent of an anaerobic membrane bioreactor Environ. Sci. Water Res. Technol. 2021, 7, 739–747. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, J.; Liu, Y. Necessity of direct energy and ammonium recovery for carbon neutral municipal wastewater reclamation in an innovative anaerobic MBR-biochar adsorption-reverse osmosis process. Water Res. 2022, 211, 118058. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.; Chen, L.; Kruger, K. Microwave irradiated ammonia nitrogen removal from anaerobically digested liquid dairy manure: A response surface methodology and artificial neural network-based optimization and modeling. J. Environ. Chem. Eng. 2022, 10, 108279. [Google Scholar] [CrossRef]
- Vialkova, E.; Obukhova, M.; Belova, L. Microwave Irradiation in Technologies of Wastewater and Wastewater Sludge Treatment: A Review. Water 2021, 13, 1784. [Google Scholar] [CrossRef]
- Lin, L.; Chen, J.; Xu, Z.; Yuan, S.; Cao, M.; Liu, H.; Lu, X. Removal of ammonia nitrogen in wastewater by microwave radiation: A pilot-scale study. J. Hazard. Mater. 2009, 168, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.; Mackey, B.; Chadalavada, S.; Kainthola, J.; Heck, P.; Goel, R. Enhanced Bio-P removal: Past, present, and future—A comprehensive review. Chemosphere 2022, 309 Pt 2, 136518. [Google Scholar] [CrossRef] [PubMed]
- Enyemadze, I.; Momade, F.; Oduro-Kwarteng, S.; Essandoh, H. Phosphorus recovery by struvite precipitation: A review of the impact of calcium on struvite quality. J. Water Sanit. Hyg. Dev. 2021, 11, 706–718. [Google Scholar] [CrossRef]
- Kékedy-Nagy, L.; Abolhassani, M.; Sultana, R.; Anari, Z.; Brye, K.R.; Pollet, B.G.; Greenlee, L.F. The effect of anode degradation on energy demand and production efficiency of electrochemically precipitated struvite. J. Appl. Electrochem. 2022, 52, 205–215. [Google Scholar] [CrossRef]
- Santos, A.F.; Mendes, L.S.; Alvarenga, P.; Gando-Ferreira, L.M.; Quina, M.J. Nutrient Recovery via Struvite Precipitation from Wastewater Treatment Plants: Influence of Operating Parameters, Coexisting Ions, and Seeding. Water 2024, 16, 1675. [Google Scholar] [CrossRef]
- Wang, Z.; Anand, D.; He, Z. Phosphorus Recovery from Whole Digestate through Electrochemical Leaching and Precipitation. Environ. Sci. Technol. 2023, 57, 10107–10116. [Google Scholar] [CrossRef] [PubMed]
- Szaja, A. Development of the novel strategy for effective reject water treatment. In 5th International Conference Strategies Toward Green Deal Implementation: Water, Raw Materials & Energy in Green Transition: Abstract Book; Smol, M., Ed.; Mineral and Energy Economy Research Institute Polish Academy of Sciences: Cracow, Poland, 2024; p. 105. [Google Scholar]
- Woszuk, A.; Franus, W. Properties of the Warm Mix Asphalt involving clinoptilolite and Na-P1 zeolite additives. Constr. Build. Mater. 2016, 114, 556–563. [Google Scholar] [CrossRef]
- Szaja, A.; Montusiewicz, A.; Lebiocka, M.; Skrzypiec, J. An Application of Orifice Hydrodynamic Cavitation Reactor for Tertiary Treatment of Wastewater Treatment Plant Effluents. Adv. Sci. Technol. Res. J. 2023, 17, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Lebiocka, M.; Montusiewicz, A.; Grządka, E.; Pasieczna-Patkowska, S.; Montusiewicz, J.; Szaja, A. Hydrodynamic Cavitation as a Method of Removing Surfactants from Real Carwash Wastewater. Molecules 2024, 29, 4791. [Google Scholar] [CrossRef] [PubMed]
- Yeneneh, A.M.; Al Balushi, K.; Jafary, T.; Al Marshudi, A.S. Hydrodynamic Cavitation and Advanced Oxidation for Enhanced Degradation of Persistent Organic Pollutants: A Review. Sustainability 2024, 16, 4601. [Google Scholar] [CrossRef]
- Ye, J.; Huang, L.; Zhang, Y.; Zhong, C. The impact of low COD/N ratio on denitrification performance and microbial community in an intermittent aeration moving bed membrane bioreactor. Process. Biochem. 2025, 150, 189–201. [Google Scholar] [CrossRef]
- Wang, B.; Su, H.; Zhang, B. Hydrodynamic cavitation as a promising route for wastewater treatment—A review. Chem. Eng. 2021, 412, 128685. [Google Scholar] [CrossRef]
- Zheng, H.; Zheng, Y.; Zhu, J. Recent Developments in Hydrodynamic Cavitation Reactors: Cavitation Mechanism, Reactor Design, and Applications. Engineering 2022, 19, 180–198. [Google Scholar] [CrossRef]
- Lei, E.; Yuan, X.; Xiang, K.; Shao, Z.; Hong, F.; Huang, Y. Research progress of hydrodynamic cavitation reactors in the field of water treatment: A review. J. Water Process. Eng. 2024, 66, 105997. [Google Scholar] [CrossRef]
- Taşdemir, A.; Cengiz, İ.; Yildiz, E.; Bayhan, Y.K. Investigation of ammonia stripping with a hydrodynamic cavitation reactor. Ultrason. Sonochem. 2020, 60, 104741. [Google Scholar] [CrossRef] [PubMed]
- Fleite, S.N.; Ayude, M.A.; Ranade, V.V.; Cassanello, M.C. Hydrodynamic cavitation effects on advanced oxidation processes and mass transfer: A conceptual model. Chem. Eng. J. Adv. 2024, 18, 100603. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, F.; Zhu, K.; Wang, Z.; Ning, J. Degradation of ammonia nitrogen by an economic combined hydrodynamic cavitation method. Environ. Sci. Pollut. Res. 2023, 30, 72782–72792. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Tang, C.D.; Wu, Z.L.; Wang, W.M.; Zhang, Y.F.; Zhao, Y.; Cravotto, G. Eutrophic water purification efficiency using a combination of hydrodynamic cavitation and ozonation on a pilot scale. Environ. Sci. Pollut. Res. 2015, 22, 6298–6307. [Google Scholar] [CrossRef] [PubMed]
- Dölle, K.D.; Bargen, M. Phosphor Removal from Waste Water Using Hydrodynamic Cavitation. J. Sci. Res. Rep. 2017, 14, 1–11. [Google Scholar] [CrossRef][Green Version]
- Huang, X.; Bai, J.; Li, K.; Zhao, Y.; Tian, W.; Hu, C. Preparation of Clay/Biochar Composite Adsorption Particle Performance for Ammonia Nitrogen Removal from Aqueous Solution. J. Ocean Univ. China 2020, 19, 729–739. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Li, Y.; Liu, T.; Zhang, N.; Wang, M.; Yang, S.; Wang, W.; Jin, P. Evaluation of the adsorption of ammonium-nitrogen and phosphate on a granular composite adsorbent derived from zeolite. Environ. Sci. Pollut. Res. 2019, 26, 17632–17643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, Z.; Qian, S.; Li, Z.; Wang, Z.; Ma, Y. Preparation of NaA zeolite with graphite tailings and its adsorption of ammonia nitrogen. Sci. Rep. 2024, 14, 28359. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Gu, S.; Cheng, H.; Xing, D.; Twagirayezu, G.; Wang, X.; Ning, W.; Mao, M. Removal of Phosphate from Aqueous Solution by Zeolite-Biochar Composite: Adsorption Performance and Regulation Mechanism. Appl. Sci. 2022, 12, 5334. [Google Scholar] [CrossRef]
- Luo, Q.; Wei, J.; Guo, Z.; Song, Y. Adsorption and immobilization of phosphorus from water and sediments using a lanthanum-modified natural zeolite: Performance, mechanism and effect. Sep. Purif. Technol. 2024, 329, 125187. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, X.; Wei, J.; Zhang, J.; Guo, Z.; Song, Y. High-efficiency lanthanum-modified zeolite adsorbents for phosphorus control and algal suppression: Preparation, characterization and mechanistic insights. Sep. Purif. Technol. 2025, 352, 128146. [Google Scholar] [CrossRef]
- Priya, E.; Kumar, S.; Verma, C.; Sarkar, S.; Maji, P.K. A comprehensive review on technological advances of adsorption for removing nitrate and phosphate from waste water. J. Water Process. Eng. 2022, 49, 103159. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Huo, S.; Li, F.; Li, K. Recent progress in metal-based composites toward adsorptive removal of phosphate: Mechanisms, behaviors, and prospects. J. Chem. Eng. 2022, 446, 137081. [Google Scholar] [CrossRef]
- Zakutevskyy, O.; Shaposhnikova, T.; Kotynska, L.; Biedrzycka, A.; Skwarek, E. Sorption of ammonium and phosphate ions from aqueous solutions by carbon and mineral sorbents. Physicochem. Probl. Miner. Process. 2022, 58, 150285. [Google Scholar] [CrossRef]
- Bahmanzadegan, F.; Ghaemi, A. A comprehensive review on novel zeolite-based adsorbents for environmental pollutant. J. Hazard. Mater. Adv. 2025, 17, 100617. [Google Scholar] [CrossRef]
- Adam, M.R.; Othman, M.H.; Samah, R.A.; Puteh, M.H.; Ismail, A.F.; Mustafa, A.I.; Rahman, M.A.; Jaafar, J. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Han, B.; Butterly, C.; Zhang, W.; He, J.; Chen, D. Adsorbent Materials for Ammonium and Ammonia Removal: A Review. J. Clean. Prod. 2020, 283, 124611. [Google Scholar] [CrossRef]
- Widiastuti, N.; Wu, H.; Ang, H.M.; Zhang, D. Removal of 1687 ammonium from greywater using natural zeolite. Desalination 2011, 277, 15–23. [Google Scholar] [CrossRef]
- Mazloomi, F.; Jalali, M. Ammonium removal from aqueous 1547 solutions by natural Iranian zeolite in the presence of organic 1548 acids, cations and anions. J. Environ. Chem. Eng. 2016, 4, 240–249. [Google Scholar] [CrossRef]
- Karadag, D.; Tok, S.; Akgul, E.; Turan, M.; Ozturk, M.; Demir, A. Ammonium removal from sanitary landfill leachate 1463 using natural Gördes clinoptilolite. J. Hazard. Mater. 2008, 153, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, G.; Chen, H.; Zhou, S.; Sun, X.; Wang, D.; Huang, L.; Li, Z. Simultaneous removal of nitrogen and phosphorus from urban sewage by synthetic zeolites adsorption: Performance, characterization, and mechanism. Desalination Water Treat. 2023, 303, 59–69. [Google Scholar] [CrossRef]
- Hermassi, M.; Valderrama, C.; Moreno, N.; Font, O.; Querol, X.; Batis, N.; Cortina, J.L. Powdered Ca-activated zeolite for phosphate removal from treated waste-water. J. Chem. Technol. Biotechnol. 2016, 91, 1962–1971. [Google Scholar] [CrossRef]
- de Magalhães, L.F.; da Silva, G.R.; Peres, A.E.C.; Kooh, M.R.R. Zeolite Application in Wastewater Treatment. Adsorpt. Sci. Technol. 2022, 2022, 4544104. [Google Scholar] [CrossRef]
- Jia, Z.G.; Han, C.; Wu, L.; Zhang, D.; Li, M. Biotemplated synthesis of hollow nickel silicate fiber for organic dye contaminants and its selective adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129219. [Google Scholar] [CrossRef]
- Cusioli, L.F.; Quesada, H.B.; Andrade, M.B.; Gomes, R.G.; Bergamasco, R. Application of a novel low-cost adsorbent functioned with iron oxide nanoparticles for the removal of triclosan present in contaminated water. Microporous Mesoporous Mater. 2021, 325, 111328. [Google Scholar] [CrossRef]
- Demiti, G.M.; Fachina, Y.J.; Januário, E.F.; Scaliante, M.H.; Rodríguez, M.T.; Bergamasco, R. Removing pharmaceuticals from water with natural and modified zeolites: Kinetics, thermodynamics, and competitive adsorption in a multi-drug system. J. Mol. Liq. 2025, 418, 126688. [Google Scholar] [CrossRef]

| Parameter | Unit | Reject Water |
|---|---|---|
| Chemical oxygen demand (COD) | mg/L | 592 ± 19 |
| Soluble chemical oxygen demand (sCOD) | mg/L | 519 ± 21 |
| Biological oxygen demand (BOD5) | 8.42 ± 0.7 | |
| Total nitrogen (TN) | mg/L | 1305 ± 45 |
| Total phosphorus (TP) | mg/L | 156.9 ± 16.9 |
| Ammonia nitrogen (N-NH4+) | mg/L | 1044.5 ± 71.3 |
| Phosphates (P-PO43−) | mg/L | 132.9 ± 15 |
| Total suspended solids (TSSs) | mg/L | 117.7 ± 2.1 |
| Volatile suspended solids (VSSs) | mg/L | 91.4 ± 2.4 |
| pH | - | 8.36 ± 0.17 |
| Biological oxygen demand-to-chemical oxygen demand ratio (BOD5/COD) | - | 0.094 ± 0.007 |
| Chemical oxygen demand-to-total nitrogen ratio (COD/TN) | - | 0.45 ± 0.02 |
| Parameter | A | B |
|---|---|---|
| Number of holes | 9 | 1 |
| Diameter of holes | 1 mm | 3/1 mm |
| α: constriction parameter of orifice | 4 mm−1 | 4 mm−1 |
| β: constriction parameter of orifice | 0.0023 | 0.0023 |
| Number of holes | 9 | 1 |
| Diameter of holes | 1 mm | 3/1 mm |
| Total area of holes | 7.0686 mm2 | 7.0686 mm2 |
| Time | TSSs | VSSs | pH | T | TSSs | VSSs | pH | T | Energy Usage |
|---|---|---|---|---|---|---|---|---|---|
| min | mg/L | mg/L | - | °C | mg/L | mg/L | - | °C | kWh |
| 3 bar | |||||||||
| CAVITATION INDUCER A | CAVITATION INDUCER B | ||||||||
| 0 | 94 ± 2.1 | 86 ± 2.7 | 8.27 ± 0.06 | 16.1 ± 0.2 | 91 ± 7.1 | 69 ± 4.1 | 8.57 ± 0.07 | 16.5 ± 0.2 | 0 |
| 5 | 142 ± 3.7 | 103 ± 2.9 | 8.41 ± 0.10 | 16.9 ± 0.4 | 134 ± 7.3 | 89 ± 3.3 | 8.61 ± 0.11 | 17.1 ± 0.4 | 0.041 |
| 15 | 202 ± 2.1 | 103 ± 4.1 | 8.48 ± 0.10 | 18.6 ± 1.2 | 156 ± 8.9 | 125 ± 9.0.7 | 8.65 ± 0.08 | 17 ± 0.8 | 0.079 |
| 30 | 212 ± 4.7 | 116 ± 5.1 | 8.62 ± 0.06 | 22.4 ± 0.6 | 190 ± 8.9 | 106 ± 8.7 | 8.68 ± 0.01 | 18.6 ± 0.7 | 0.241 |
| 45 | 203 ± 5.3 | 116 ± 6.1 | 8.65 ± 0.11 | 25.6 ± 0.7 | 179 ± 11.2 | 126 ± 9.8 | 8.71 ± 0.05 | 21 ± 1.3 | 0.350 |
| 60 | 214 ± 3.8 | 131 ± 6.0 | 8.74 ± 0.08 | 28.6 ± 0.5 | 258 ± 13.7 | 168 ± 10.4 | 8.78 ± 0.11 | 22.5 ± 1.7 | 0.486 |
| 5 bar | |||||||||
| 0 | 86 ± 2.2 | 81 ± 3.0 | 8.42 ± 0.11 | 18.7 ± 2.1 | 98 ± 8.1 | 73.18 ± 2.9 | 8.31 ± 0.06 | 17.9 ± 1.2 | 0 |
| 5 | 132 ± 3.1 | 99 ± 1.7 | 8.50 ± 0.12 | 20.5 ± 2.0 | 156 ± 10.1 | 140.4 ± 3.8 | 8.38 ± 0.05 | 18.7 ± 1.1 | 0.059 |
| 15 | 135 ± 4.2 | 99 ± 3.1 | 8.59 ± 0.04 | 22.8 ± 2.5 | 168 ± 14.0 | 144 ± 10 | 8.40 ± 0.03 | 20.1 ± 2.0 | 0.121 |
| 30 | 136 ± 3.6 | 140 ± 4.2 | 8.67 ± 0.09 | 26.9 ± 2.7 | 189 ± 14.2 | 156 ± 8.1 | 8.42 ± 0.12 | 24.1 ± 1.0 | 0.351 |
| 45 | 131 ± 4.1 | 136 ± 3.9 | 8.76 ± 0.11 | 29.3 ± 3.2 | 209.4 ± 15.9 | 174 ± 8.0 | 8.45 ± 0.06 | 25.7 ± 2.0 | 0.515 |
| 60 | 135 ± 4.1 | 133 ± 3.0 | 8.77 ± 0.08 | 31.9 ± 3.5 | 249 ± 15.1 | 189 ± 9.1 | 8.50 ± 0.11 | 29.7 ± 2.2 | 0.689 |
| 7 bar | |||||||||
| 0 | 86 ± 2.5 | 82 ± 4.3 | 8.36 ± 0.02 | 18.1 ± 3.5 | 96.8 ± 7.9 | 71 ± 3.3 | 8.23 ± 0.07 | 18 ± 1.9 | 0 |
| 5 | 134 ± 3.0 | 102 ± 5.1 | 8.45 ± 0.11 | 25.0 ± 1.7 | 126 ± 14.0 | 124 ± 9.57 | 8.22 ± 0.09 | 24.1 ± 2.1 | 0.069 |
| 15 | 137 ± 2.3 | 104 ± 2.5 | 8.49 ± 0.07 | 29.4 ± 2.5 | 210 ± 17.5 | 164 ± 10.7 | 8.27 ± 0.12 | 28.8 ± 2.3 | 0.161 |
| 30 | 139 ± 4.5 | 110 ± 4.5 | 8.53 ± 0.07 | 34.5 ± 3.7 | 222 ± 24.3 | 167 ± 143.9 | 8.35 ± 0.11 | 34.1 ± 3.5 | 0.483 |
| 45 | 140 ± 3.0 | 115 ± 6.0 | 8.61 ± 0.11 | 37.1 ± 1.9 | 232.2 ± 20.5 | 177 ± 15.3 | 8.44 ± 0.09 | 37.5 ± 3.7 | 0.703 |
| 60 | 143 ± 5.9 | 120 ± 6.0 | 8.78 ± 0.07 | 39.4 ± 2.0 | 246 ± 21.1 | 212 ± 18.1 | 8.52 ± 0.11 | 38.9 ± 3.9 | 0.923 |
| Model | Parameter | Unit | Ammonia Nitrogen | Phosphates | ||||
|---|---|---|---|---|---|---|---|---|
| Zeolite dose | 50 g | 100 g | 200 g | 50 g | 100 g | 200 g | ||
| Experimental data | qe | mg/g | 5.4 | 3.5 | 2.7 | 1.06 | 0.70 | 0.40 |
| PFO | qe | mg/g | 6.94 | 4.50 | 3.29 | 1.23 | 0.73 | 0.38 |
| k1 | h−1 | 0.17 | 0.14 | 0.33 | 0.09 | 0.119 | 0.19 | |
| R2 | - | 0.998 | 0.981 | 0.998 | 0.991 | 0.997 | 0.993 | |
| PSO | qe | mg/g | 8.52 | 5.64 | 3.65 | 1.78 | 0.98 | 0.466 |
| k2 | h−1 | 0.02 | 0.03 | 0.14 | 0.04 | 0.09 | 0.36 | |
| R2 | - | 0.9993 | 0.9871 | 0.9991 | 0.9887 | 0.9991 | 0.9945 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szaja, A.; Sawicka, M.; Smagała, R. Development of Sustainable Technology for Effective Reject Water Treatment. Sustainability 2025, 17, 6548. https://doi.org/10.3390/su17146548
Szaja A, Sawicka M, Smagała R. Development of Sustainable Technology for Effective Reject Water Treatment. Sustainability. 2025; 17(14):6548. https://doi.org/10.3390/su17146548
Chicago/Turabian StyleSzaja, Aleksandra, Maria Sawicka, and Rafał Smagała. 2025. "Development of Sustainable Technology for Effective Reject Water Treatment" Sustainability 17, no. 14: 6548. https://doi.org/10.3390/su17146548
APA StyleSzaja, A., Sawicka, M., & Smagała, R. (2025). Development of Sustainable Technology for Effective Reject Water Treatment. Sustainability, 17(14), 6548. https://doi.org/10.3390/su17146548







