Advanced Municipal Wastewater Treatment and Bioproduct Generation via Optimized Autotrophic and Mixotrophic Microalgal Cultivation
Abstract
1. Introduction
2. Materials and Methods
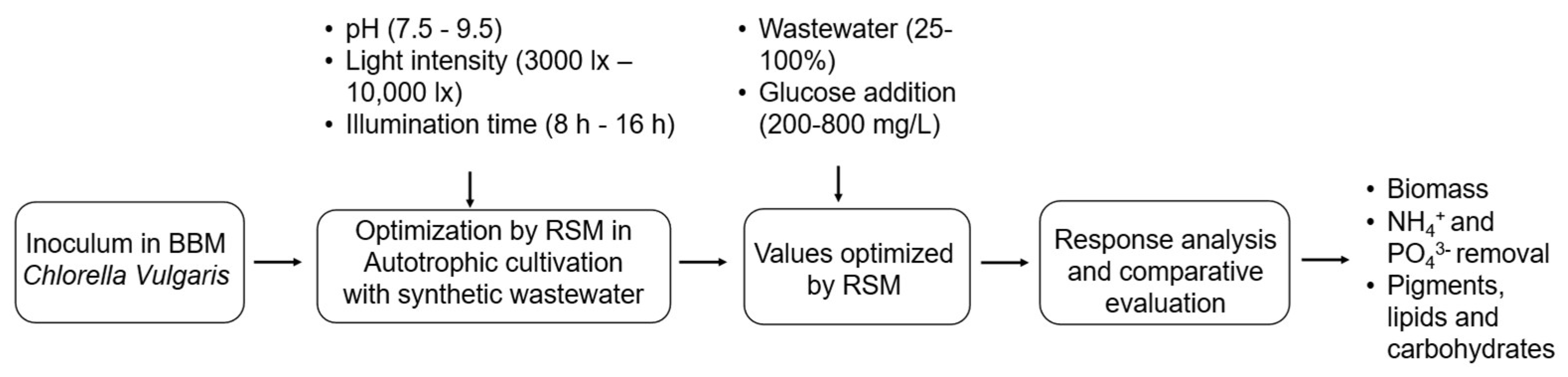
2.1. Microalgae Strain, Mediums and Cultivation
2.2. Experimental Analysis by RSM
2.3. Analytical Methods
2.3.1. Biomass Growth
2.3.2. Determination of Pigments, Carbohydrates and Lipids
2.3.3. Nutrient Analysis and COD
3. Results and Discussion
3.1. Optimization of Operational Parameters in Autotrophic Conditions
3.1.1. Statistical Analysis
3.1.2. Variable Interaction
3.1.3. Numerical Optimization
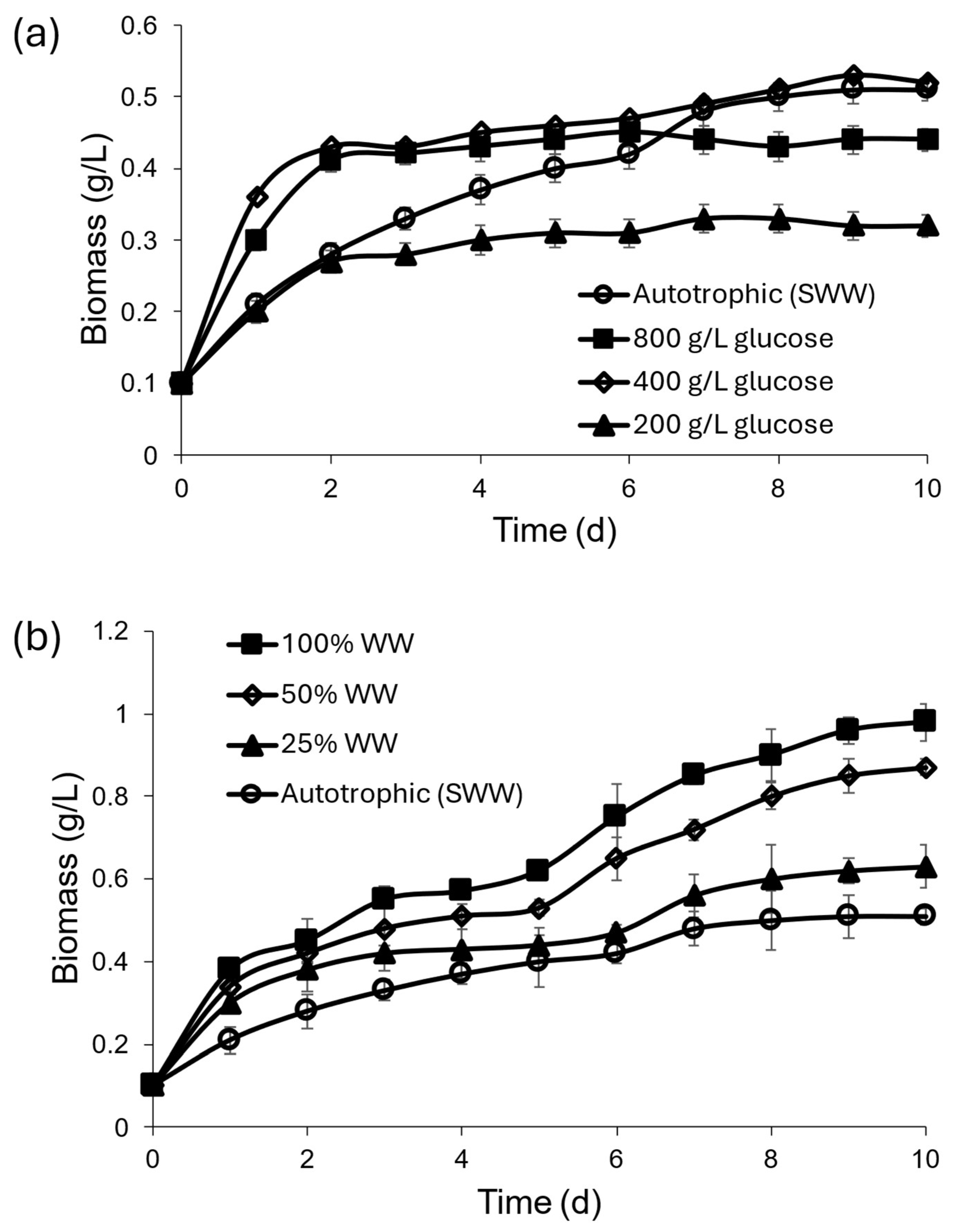
3.2. Mixotrophic Cultivation Under Optimal Conditions
3.3. Advancements in Microalgal Carbon Utilization and Municipal Wastewater Treatment
3.4. Study Limitations and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kratzer, R.; Murkovic, M. Food ingredients and nutraceuticals from microalgae: Main product classes and biotechnological production. Foods 2021, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Liu, J.; Shen, Z.; Li, A.; Ma, T.; Feng, Q.; Sun, Y. Treatment of high-nitrate wastewater mixtures from MnO2 industry by Chlorella vulgaris. Bioresour. Technol. 2019, 291, 121836. [Google Scholar] [CrossRef] [PubMed]
- Alazaiza, M.Y.D.; He, S.; Su, D.; Abu Amr, S.S.; Toh, P.Y.; Bashir, M.J.K. Bashir. Sewage Water Treatment Using Chlorella Vulgaris Microalgae for Simultaneous Nutrient Separation and Biomass Production. Separations 2023, 10, 229. [Google Scholar] [CrossRef]
- Sravan, J.S.; Matsakas, L.; Sarkar, O. Advances in Biological Wastewater Treatment Processes: Focus on Low-Carbon Energy and Resource Recovery in Biorefinery Context. Bioengineering 2024, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Salbitani, G.; Carfagna, S. Ammonium utilization in microalgae: A sustainable method for wastewater treatment. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Pérez-Mora, L.S.; Mejia-Da-Silva, L.d.C.; Cezare-Gomes, E.d.A.; Santo, É.D.E.; Gohara-Beirigo, A.K.; Matsudo, M.C.; Nardin, B.M.; Sant’anna, C.L.; de Carvalho, J.C.M. Phycoremediation Processes for Secondary Effluent from Sewage Treatment Plants Using Photosynthetic Microorganisms: A Review. Appl. Microbiol. 2023, 3, 400–416. [Google Scholar] [CrossRef]
- Mai, C.; Mojiri, A.; Palanisami, S.; Altaee, A.; Huang, Y.; Zhou, J.L. Wastewater Hydroponics for Pollutant Removal and Food Production: Principles, Progress and Future Outlook. Water 2023, 15, 2614. [Google Scholar] [CrossRef]
- Ramírez Mérida, L.G.; Rodríguez Padrón, R.A. Application of microalgae in wastewater: Opportunity for sustainable development. Front. Environ. Sci. 2023, 11, 1238640. [Google Scholar] [CrossRef]
- Goh, P.S.; Ahmad, N.A.; Lim, J.W.; Liang, Y.Y.; Kang, H.S.; Ismail, A.F.; Arthanareeswaran, G. Microalgae-Enabled Wastewater Remediation and Nutrient Recovery through Membrane Photobioreactors: Recent Achievements and Future Perspective. Membranes 2022, 12, 1094. [Google Scholar] [CrossRef] [PubMed]
- Satiro, J.; Gomes, A.; Florencio, L.; Simões, R.; Albuquerque, A. Effect of microalgae and bacteria inoculation on the startup of bioreactors for paper pulp wastewater and biofuel production. J. Environ. Manag. 2024, 362, 121305. [Google Scholar] [CrossRef] [PubMed]
- Bhuyar, P.; Trejo, M.; Dussadee, N.; Unpaprom, Y.; Ramaraj, R.; Whangchai, K. Microalgae cultivation in wastewater effluent from tilapia culture pond for enhanced bioethanol production. Water Sci. Technol. 2021, 84, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Varsani, V.; Vyas, S.J.; Dudhagara, D.R. Development of bio-based material from the Moringa oleifera and its bio-coagulation kinetic modeling–A sustainable approach to treat the wastewater. Heliyon 2022, 8, e10447. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, A.A. Clean Water Production from Urban Sewage by Algae-Based Treatment Techniques, a Reflection of Case Studies. Sustainability 2025, 17, 3107. [Google Scholar] [CrossRef]
- Popa, M.D.; Simionov, I.-A.; Petrea, S.M.; Georgescu, P.L.; Ifrim, G.A.; Iticescu, C. Efficiency of Microalgae Employment in Nutrient Removal (Nitrogen and Phosphorous) from Municipal Wastewater. Water 2025, 17, 260. [Google Scholar] [CrossRef]
- Maltsev, Y.; Kulikovskiy, M.; Maltseva, S. Nitrogen and phosphorus stress as a tool to induce lipid production in microalgae. Microb. Cell Factories 2023, 22, 239. [Google Scholar] [CrossRef] [PubMed]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, N.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142168. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, C.J. A note on the chlorophyll and phaeophytin content of the chlorophyll maximum. Limnol. Oceanog. 1965, 10, 482–483. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington DC, USA, 1998. [Google Scholar]
- Gao, Y.; Bernard, O.; Fanesi, A.; Perré, P.; Lopes, F. The impact of light/dark regimes on structure and physiology of Chlorella vulgaris biofilms. Front. Microbiol. 2023, 14, 1250866. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.S.; Kim, Y.S.; Yoon, H.S. Effect of Different Cultivation Modes (Photoautotrophic, Mixotrophic, and Heterotrophic) on the Growth of Chlorella sp. and Biocompositions. Front. Bioeng. Biotechnol. 2021, 9, 774143. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.K.; Mishra, I. Progress of sustainable development goal 7: Clean and green energy for all as the biggest challenge to combat climate crisis. Environ. Sustain. 2022, 5, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Mehariya, S.; Karthikeyan, O.P.; Verma, P. Influence of Carbon Sources on Biomass and Biomolecule Accumulation in Picochlorum sp. Cultured under the Mixotrophic Condition. Int. J. Environ. Res. Public Health 2022, 19, 3674. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Shan, S.; Li, X.; Xu, Q.; Yan, X.; Ruan, R.; Cheng, P. Carbon and energy metabolism for the mixotrophic culture of Chlorella vulgaris using sodium acetate as a carbon source. Front. Microbiol. 2024, 15, 1436264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, D.; Cheng, K.W.; Chen, F. Investigation of carbon and energy metabolic mechanism of mixotrophy in Chromochloris zofingiensis. Biotechnol. Biofuels 2021, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.; Chakraborty, S. Synergistic effects between autotrophy and heterotrophy in optimization of mixotrophic cultivation of Chlorella sorokiniana in bubble-column photobioreactors. Algal Res. 2020, 46, 101799. [Google Scholar] [CrossRef]
- Ward, V.C.A.; Rehmann, L. Fast media optimization for mixotrophic cultivation of Chlorella vulgaris. Sci. Rep. 2019, 9, 19262. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Zhang, C.; Yang, Q.; Zhang, X.; Chen, W.; Zhu, X.; Xu, Q.; Tan, Q. Photoheterotroph improved the growth and nutrient levels of Chlorella vulgaris and the related molecular mechanism. Appl. Microbiol. Biotechnol. 2024, 108, 269. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Huang, Y.; Xia, A.; Fu, Q.; Liao, Q.; Zeng, W.; Zheng, Y.; Zhu, X. Optimizing culture conditions for heterotrophic-assisted photoautotrophic biofilm growth of Chlorella vulgaris to simultaneously improve microalgae biomass and lipid productivity. Bioresour. Technol. 2018, 270, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Pei, H. The growth and lipid accumulation of Scenedesmus quadricauda during batch mixotrophic/heterotrophic cultivation using xylose as a carbon source. Bioresour. Technol. 2018, 263, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Gaur, S.; Kaur, M.; Kalra, R.; Rene, E.R.; Goel, M. Application of microbial resources in biorefineries: Current trend and future prospects. Heliyon 2024, 10, e28615. [Google Scholar] [CrossRef] [PubMed]
- Aditi; Bhardwaj, R.; Yadav, A.; Swapnil, P.; Meena, M. Characterization of microalgal β-carotene and astaxanthin: Exploring their health-promoting properties under the effect of salinity and light intensity. Biotechnol. Biofuels Bioprod. 2025, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Plöhn, M.; Scherer, K.; Stagge, S.; Jönsson, L.J.; Funk, C. Utilization of Different Carbon Sources by Nordic Microalgae Grown Under Mixotrophic Conditions. Front. Mar. Sci. 2022, 9, 830800. [Google Scholar] [CrossRef]
- Arora, N.; Philippidis, G.P. Insights into the physiology of Chlorella vulgaris cultivated in sweet sorghum bagasse hydrolysate for sustainable algal biomass and lipid production. Sci. Rep. 2021, 11, 6779. [Google Scholar] [CrossRef] [PubMed]
- Cordoba-Perez, M.; de Lasa, H. CO2-derived carbon capture using microalgae and sodium bicarbonate in a photobioCREC unit: Kinetic modeling. Processes 2021, 9, 1296. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M.; Koniuszy, A. Utilisation of co2 from sodium bicarbonate to produce chlorella vulgaris biomass in tubular photobioreactors for biofuel purposes. Sustainability 2021, 13, 9118. [Google Scholar] [CrossRef]
- Yu, H.C.; Lay, C.H.; Abdul, P.M.; Wu, J.Y. Enhancing Lipid Production of Chlorella sp. by Mixotrophic Cultivation Optimization. Processes 2023, 11, 1892. [Google Scholar] [CrossRef]
- Kong, W.; Yang, S.; Wang, H.; Huo, H.; Guo, B.; Liu, N.; Zhang, A.; Niu, S. Regulation of biomass, pigments, and lipid production by Chlorella vulgaris 31 through controlling trophic modes and carbon sources. J. Appl. Phycol. 2020, 32, 1569–1579. [Google Scholar] [CrossRef]
- Whangchai, K.; Mathimani, T.; Sekar, M.; Shanmugam, S.; Brindhadevi, K.; Van Hung, T.; Chinnathambi, A.; Alharbi, S.A.; Pugazhendhi, A. Synergistic supplementation of organic carbon substrates for upgrading neutral lipids and fatty acids contents in microalga. J. Environ. Chem. Eng. 2021, 9, 105482. [Google Scholar] [CrossRef]
- Mohammadi, F.S.; Arabian, D. Optimization of Chlorella vulgaris cultivation grown in waste molasses syrup using mixture design. J. Am. Oil Chem. Soc. 2022, 100, 45–56. [Google Scholar] [CrossRef]
- Trinh-Dang, M.; Kim, O.T.T. Effects of Some Nutritional Factors on the Growth of Chlorella vulgaris in a Mixotrophic Cultivation. J. Adv. Biol. Biotechnol. 2023, 26, 1–8. [Google Scholar] [CrossRef]
- Dewi, R.N.; Mahreni; Nur, M.M.A.; Siahaan, A.A.; Ardhi, A.C. Enhancing the biomass production of microalgae by mixotrophic cultivation using virgin coconut oil mill effluent. Environ. Eng. Res. 2022, 28, 220059. [Google Scholar] [CrossRef]
- Tan, Y.H.; Chai, M.K.; Ooi, Y.K.; Wong, L.S. Assessment of Domestic Wastewaters as Potential Growth Media for Chlorella vulgaris and Haematococcus pluvialis. Pertanika J. Sci. Technol. 2022, 30, 565–580. [Google Scholar] [CrossRef]
- Seyhaneyıldız Can, Ş.; Can, E.; Yılmaz, K. Lipid content and wastewater treatment potential of Chlorella vulgaris and Scenedesmus obliquus isolated from Uzuncayır Dam Lake. Oceanol. Hydrobiol. Stud. 2024, 53, 310–320. [Google Scholar] [CrossRef]
- Salgueiro, J.L.; Perez-Rial, L.; Maceiras, R.; Sanchez, A.; Cancela, A. Transforming Wastewater into Biofuel: Nutrient Removal and Biomass Generation with Chlorella vulgaris. Energies 2024, 17, 4911. [Google Scholar] [CrossRef]
- Mojiri, A.; Ozaki, N.; Kazeroon, R.A.; Rezania, S.; Baharlooeian, M.; Vakili, M.; Farraji, H.; Ohashi, A.; Kindaichi, T.; Zhou, J.L. Contaminant Removal from Wastewater by Microalgal Photobioreactors and Modeling by Artificial Neural Network. Water 2022, 14, 4046. [Google Scholar] [CrossRef]
- Huo, X.; Chai, R.; Gou, L.; Zhang, M.; Guo, M. Facile synthesis of composite polyferric magnesium–silicate–sulfate coagulant with enhanced performance in water and wastewater. Int. J. Miner. Met. Mater. 2024, 31, 574–584. [Google Scholar] [CrossRef]
- Zhou, X.C.; Chen, S.Q.; Zhou, M.J.; Li, M.; Lan, S.; Feng, T. Highly efficient cobalt-based amorphous catalyst for peroxymonosulfate activation toward wastewater remediation. Rare Met. 2023, 42, 1160–1174. [Google Scholar] [CrossRef]
- He, C.; Liu, Y.; Qi, M.; Liu, Z.; Wei, Y.; Fujita, T.; Wang, G.; Ma, S.; Yang, W. A functionalized activated carbon adsorbent prepared from waste amidoxime resin by modifying with H3PO4 and ZnCl2 and its excellent Cr(VI) adsorption. Int. J. Miner. Met. Mater. 2024, 31, 585–598. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W. Multifunctional materials with controllable superwettability for oil–water separation and removal of pollutants: Design, emerging applications, and challenges. Carbon Neutralization 2023, 2, 378–412. [Google Scholar] [CrossRef]
- Sátiro, J.; Cunha, A.; Gomes, A.P.; Simões, R.; Albuquerque, A. Optimization of Microalgae–Bacteria Consortium in the Treatment of Paper Pulp Wastewater. Appl. Sci. 2022, 12, 579. [Google Scholar] [CrossRef]
- Galang, M.G.K.; Chen, J.; Cobb, K.; Zarra, T.; Ruan, R. Reduction of biogenic CO2 emissions, COD and nutrients in municipal wastewater via mixotrophic co-cultivation of Chlorella vulgaris–aerobic-activated sludge consortium. Environ. Technol. 2025, 46, 3348–3362. [Google Scholar] [CrossRef] [PubMed]
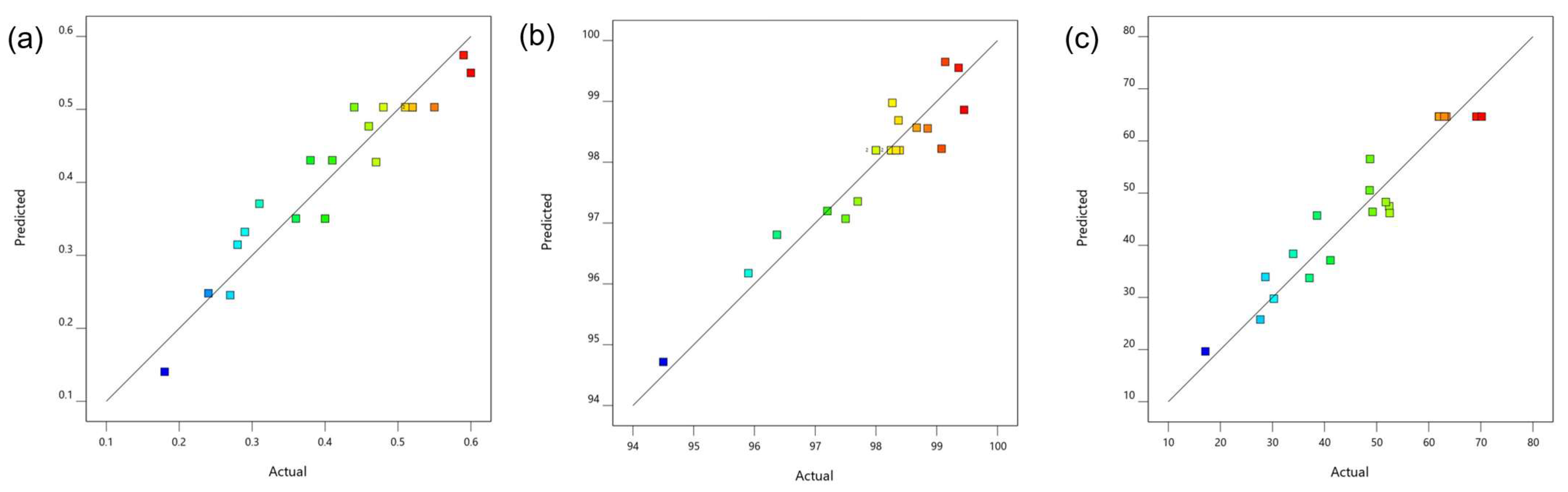

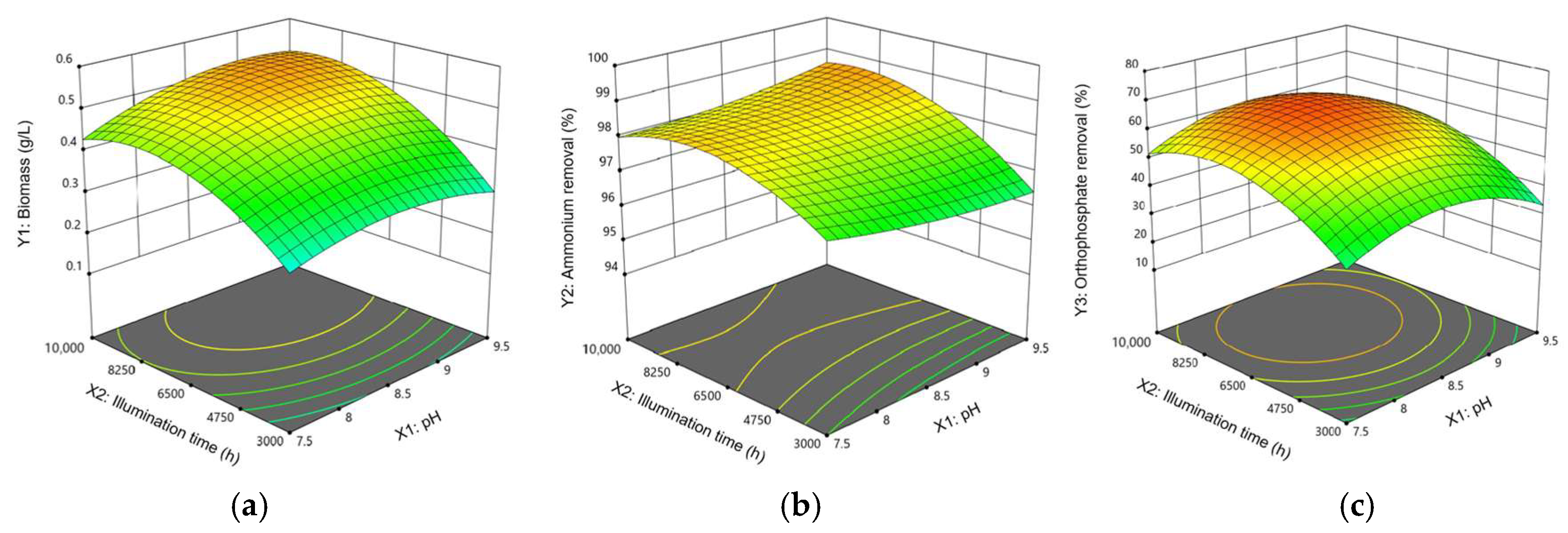
| Run. | X1: pH | X2: Light Intensity | X3: Illumination Time (h) | Y1: Biomass (g/L) | Y2: Ammonium Removal (%) | Y3: Orthophosphate Removal (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| (lux) | Exp. | Pred. | Exp. | Pred. | Exp. | Pred. | |||
| 1 | 8.50 | 6500 | 18.73 | 0.60 | 0.55 | 99.14 | 99.64 | 48.65 | 50.53 |
| 2 | 8.50 | 6500 | 12 | 0.48 | 0.50 | 98.33 | 98.20 | 61.95 | 64.67 |
| 3 | 8.50 | 6500 | 5.27 | 0.31 | 0.38 | 98.85 | 98.57 | 48.74 | 56.52 |
| 4 | 9.50 | 10,000 | 8 | 0.47 | 0.43 | 98.27 | 97.69 | 52.50 | 46.17 |
| 5 | 8.50 | 6500 | 12 | 0.52 | 0.50 | 98.25 | 98.20 | 69.17 | 64.67 |
| 6 | 8.50 | 6500 | 12 | 0.55 | 0.50 | 98.39 | 98.20 | 70.17 | 64.67 |
| 7 | 7.50 | 3000 | 8 | 0.24 | 0.25 | 96.37 | 96.82 | 41.13 | 37.09 |
| 8 | 7.50 | 3000 | 16 | 0.28 | 0.32 | 99.08 | 96.92 | 30.27 | 29.75 |
| 9 | 9.50 | 3000 | 8 | 0.27 | 0.25 | 97.70 | 97.37 | 37.10 | 33.71 |
| 10 | 8.50 | 6500 | 12 | 0.44 | 0.50 | 98.25 | 98.20 | 63.38 | 64.67 |
| 11 | 7.50 | 10,000 | 16 | 0.46 | 0.48 | 99.36 | 99.56 | 51.76 | 48.29 |
| 12 | 8.50 | 614 | 12 | 0.18 | 0.14 | 94.50 | 94.72 | 17.12 | 19.64 |
| 13 | 8.50 | 12,386 | 12 | 0.38 | 0.43 | 97.20 | 97.21 | 38.56 | 45.71 |
| 14 | 9.50 | 10,000 | 16 | 0.59 | 0.58 | 99.45 | 98.87 | 49.22 | 46.39 |
| 15 | 7.50 | 10,000 | 8 | 0.40 | 0.35 | 97.50 | 98.38 | 52.41 | 47.47 |
| 16 | 9.50 | 3000 | 16 | 0.29 | 0.33 | 95.90 | 97.47 | 27.67 | 25.77 |
| 17 | 8.50 | 6500 | 12 | 0.51 | 0.50 | 98.00 | 98.20 | 63.00 | 64.67 |
| 18 | 10.18 | 6500 | 12 | 0.41 | 0.43 | 98.67 | 98.57 | 28.64 | 33.90 |
| 19 | 6.82 | 6500 | 12 | 0.36 | 0.36 | 98.37 | 98.68 | 33.97 | 38.34 |
| 20 | 8.50 | 6500 | 12 | 0.52 | 0.50 | 98.00 | 98.20 | 62.00 | 64.67 |
| Source | Sum of Squares | df | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| Model | 0.25 | 9 | 0.028 | 10.63 | 0.00065 |
| 0.0077 | 1 | 0.0077 | 2.9 | 0.1195 | |
| 0.1013 | 1 | 0.1013 | 38.2 | 0.0001 | |
| 0.0388 | 1 | 0.0388 | 14.62 | 0.0034 | |
| 0.0032 | 1 | 0.0032 | 1.21 | 0.2978 | |
| 0.0002 | 1 | 0.0002 | 0.0754 | 0.7892 | |
| 0.0018 | 1 | 0.0018 | 0.6785 | 0.4293 | |
| 0.0229 | 1 | 0.0229 | 8.61 | 0.0149 | |
| 0.0852 | 1 | 0.0853 | 32.16 | 0.0002 | |
| 0.0033 | 1 | 0.0033 | 1.23 | 0.2926 | |
| Residual | 0.0265 | 10 | 0.0027 | ||
| Lack of Fit | 0.019 | 5 | 0.0038 | 2.62 | 0.1572 |
| Pure Error | 0.0073 | 5 | 0.0015 | ||
| Cor Total | 0.2802 | 19 |
| Source | Sum of Squares | df | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| Model | 25.64 | 9 | 2.85 | 10.07 | 0.0006 |
| 0.0173 | 1 | 0.0173 | 0.061 | 0.8099 | |
| 7.43 | 1 | 7.43 | 26.25 | 0.0004 | |
| 1.44 | 1 | 1.44 | 5.1 | 0.0476 | |
| 0.918 | 1 | 0.9180 | 3.24 | 0.1018 | |
| 3.37 | 1 | 3.37 | 11.9 | 0.0062 | |
| 0.5671 | 1 | 0.5671 | 2.00 | 0.1872 | |
| 0.3337 | 1 | 0.3337 | 1.18 | 0.3029 | |
| 9.04 | 1 | 9.04 | 31.93 | 0.0002 | |
| 1.48 | 1 | 1.48 | 5.22 | 0.0454 | |
| Residual | 2.83 | 10 | 0.2829 | ||
| Lack of Fit | 2.69 | 5 | 0.5383 | 19.51 | 0.0027 |
| Pure Error | 0.1379 | 5 | 0.0276 | ||
| Cor Total | 28.47 | 19 |
| Source | Sum of Squares | df | Mean Square | F-Value | Prob > F |
|---|---|---|---|---|---|
| Model | 3993.89 | 9 | 443.77 | 12.52 | 0.0002 |
| 23.84 | 1 | 23.84 | 0.6729 | 0.4312 | |
| 819.29 | 1 | 819.29 | 23.12 | 0.0007 | |
| 43.49 | 1 | 43.49 | 1.23 | 0.2938 | |
| 2.18 | 1 | 2.18 | 0.0616 | 0.8089 | |
| 0.18 | 1 | 0.18 | 0.0051 | 0.9446 | |
| 33.46 | 1 | 33.46 | 0.9443 | 0.3541 | |
| 1465.76 | 1 | 1465.76 | 41.37 | <0.0001 | |
| 1843.48 | 1 | 1843.48 | 52.03 | <0.0001 | |
| 223.35 | 1 | 223.35 | 6.3 | 0.0309 | |
| Residual | 354.31 | 10 | 35.43 | ||
| Lack of Fit | 285.28 | 5 | 57.06 | 4.13 | 0.0728 |
| Pure Error | 69.03 | 5 | 13.81 | ||
| Cor Total | 4348.2 | 19 |
| Parameter | Conditions | ||||||
|---|---|---|---|---|---|---|---|
| Optimal conditions pH | 8.44 | ||||||
| Light intensity (lux) | 8433 | ||||||
| Illumination time (h) | 16 | ||||||
| SWW | SWW + 200 g/L glucose | SWW + 400 g/L glucose | SWW + 800 g/L glucose | WW 25% | WW 50% | WW 100% | |
| Response variables | |||||||
| Biomass (g/L) pred. | 0.542 | ||||||
| Biomass (g/L) exp. | 0.534 ± 0.03 | 0.32 ± 0.01 | 0.51 ± 0.03 | 0.46 ± 0.04 | 0.66 ± 0.03 | 0.88 ± 0.02 | 0.98 ± 0.06 |
| Ammonium removal (%) pred. | 98.87 | ||||||
| Ammonium removal (%) exp. | 97.66 ± 1.69 | 97.83 ± 0.45 | 93.32 ± 2.32 | 93.32 ± 3.65 | 91.23 ± 4.06 | 97.25 ± 2.73 | 94.90 ± 0.57 |
| Orthophosphate removal (%) pred. | 60.01 | ||||||
| Orthophosphate removal (%) exp. | 58.78 ± 2.72 | 53.79 ± 0.50 | 75.46 ± 2.24 | 63.47 ± 0.88 | 54.80 ± 3.51 | 71.14 ± 2.22 | 64.92 ± 3.26 |
| Biochemical profile | |||||||
| Total carbohydrate (%) | 28.46 ± 1.52 | 25.81 ± 2.01 | 26.25 ± 0.89 | 24.21 ± 2.35 | 22.67 ± 0.97 | 29.57 ± 1.78 | 27.14 ± 3.04 |
| Total lipid (%) | 21.40 ± 0.94 | 21.90 ± 0.37 | 19.35 ± 1.03 | 22.27 ± 0.83 | 19.46 ± 0.86 | 22.31 ± 1.51 | 25.20 ± 1.39 |
| Chlorophyll-a (mg/mL) | 7.46 ± 0.89 | 3.80 ± 0.55 | 3.98 ± 0.28 | 3.99 ± 0.40 | 1.73 ± 0.49 | 4.27 ± 0.71 | 8.50 ± 0.88 |
| Chlorophyll-b (mg/mL) | 3.53 ± 0.18 | 1.65 ± 0.14 | 1.18 ± 0.15 | 1.44 ± 0.18 | 0.79 ± 0.20 | 1.48 ± 0.29 | 3.10 ± 0.51 |
| Carotenoids (mg/mL) | 2.01 ± 0.06 | 1.02 ± 0.13 | 1.38 ± 0.11 | 1.19 ± 0.12 | 0.84 ± 0.27 | 1.50 ± 0.26 | 2.81 ± 0.33 |
| Kinetic parameters | |||||||
| Biomass productivity (mg/L/day) | 41.45 ± 2.87 | 21.27 ± 0.26 | 41.20 ± 2.76 | 34.97 ± 4.08 | 57.40 ± 2.55 | 77.40 ± 0.50 | 86.41 ± 5.34 |
| Specific growth rate (1/day) | 0.149 ± 0.018 | 0.105 ± 0.030 | 0.162 ± 0.026 | 0.147 ± 0.028 | 0.197 ± 0.003 | 0.209 ± 0.013 | 0.211 ± 0.016 |
| Lipid productivity (mg/L/day) | 329.32 ± 45.30 | 337.01 ± 36.53 | 307.69 ± 1.53 | 342.56 ± 19.07 | 299.48 ± 13.98 | 343.07 ± 34.64 | 312.30 ± 23.78 |
| µuptake ammonium (1/day) | 0.602 ± 0.02 | 0.665 ± 0.01 | 0.581 ± 0.01 | 0.543 ± 0.01 | 0.463 ± 0.01 | 0.523 ± 0.02 | 0.565 ± 0.01 |
| µuptake orthophosphate(1/day) | 0.085 ± 0.01 | 0.077 ± 0.01 | 0.147 ± 0.01 | 0.129 ± 0.01 | 0.079 ± 0.01 | 0.217 ± 0.02 | 0.104 ± 0.01 |
| Strain | Reference | Carbon Source | Findings |
|---|---|---|---|
| Chlorella vulgaris, Coelastrella sp., Desmodesmus sp., Chlorococcum sp., and Scotiellopsis reticulata | [33] | Methanol, glycerol, and xylose | Xylose at 6 g/L and methanol at 3% for Chlorococcum sp. and Scotiellopsis reticulata, while Chlorococcum sp. with glycerol at 20 g/L |
| Chlorella vulgaris | [34] | Weet sorghum bagasse hydrolysate | Optimal biomass production (3.44 g/L) and lipid productivity (120 mg/L/d) when cultivated mixotrophically with 25% v/v sweet sorghum bagasse hydrolysate |
| Chlorella vulgaris | [35] | NaHCO3 | A selectivity of up to 33.0%, achieving a maximum organic carbon formation rate of 1.18 mmol/L/day at 28 mM NaHCO3 |
| Chlorella vulgaris | [36] | NaHCO3 | 2 g/L sodium bicarbonate increased biomass productivity (7.0 ± 1.0 mg/L/d and lipid content (26 ± 4% |
| Chlorella sp. | [37] | Peptone, urea, yeast extract, NH4Cl, (NH4)2SO4, NH4NO3, NaNO3, and KNO3 | 1 g/L glucose and 0.2 g/L (NH4)2SO4 at pH 10 for the highest total FAMEs content (59%) |
| Chlorella vulgaris | [38] | Glucose, maltose, sodium acetate, sucrose, glycerol and xylose | Enhanced growth with glucose, maltose, and sodium acetate at concentrations of 2 and 10 g/L, which also significantly increased lipid production while decreasing chlorophyll and carotenoid biosynthesis under mixotrophic and heterotrophic conditions |
| Chlorella vulgaris | [39] | Acetate, dextrose and bicarbonate | 2.7 g/L biomass yield and 20.8% lipid content with 0.4% acetate, further enhanced to 27% total lipid content and 69% neutral lipid content with the combined supplementation of 0.4% acetate and 0.6% dextrose |
| Chlorella vulgaris | [40] | Molasses, NaNO3, and K2HPO4 | Lipid productivity of 115 mg/L/d with 9.5 g/L molasses, 5 g/L NaNO3, and 0.15 g/L K2HPO4 |
| Chlorella vulgaris | [41] | Glucose, sodium acetate, and sucrose | glucose supplementation at a C:N ratio of 18:1 (52.92 mmol/L carbon and 2.94 mmol/L nitrogen), yielding a maximum growth rate of 0.58/day |
| Chlorella vulgaris and Botryococcus braunii | [42] | Virgin coconut oil mill effluent with glucose and glycerol | Chlorella vulgaris showed optimal biomass production at 5.34 g/L in 20% virgin coconut oil mill effluent, while Botryococcus braunii achieved its best biomass yield of 5.60 g/L under mixotrophic conditions with an 80:20 glucose to glycerol ratio |
| Strain | Reference | Strategy | Findings |
|---|---|---|---|
| Chlorella vulgaris and Haematococcus pluvialis | [43] | Real municipal wastewater at 10%, 20%, 50%, and 80% | Chlorella vulgaris exhibited a biomass concentration of 0.227 g/L and over 88% removal efficiency for total nitrogen, total phosphorus, and total ammonia nitrogen in 50% domestic wastewater, while Haematococcus pluvialis achieved over 80% total ammonia nitrogen removal in 50% and 80% wastewater |
| Chlorella vulgaris | [3] | Real municipal wastewater at 50%, 60%, 70%, 80%, and 90% | Maximum removals of 84% COD, 95% NH3-N, and 97% phosphorus, and peak biomass production typically occurring by day 12, extending to day 14 at an 80% wastewater mixing ratio |
| Chlorella vulgaris and Scenedesmus obliquus | [44] | Real municipal wastewater at 0.25%, 50%, and 75% | Both strains demonstrated optimal biomass and lipid content increases, along with effective nutrient removal, when cultured in wastewater diluted to 0.25%, 50%, and 75% concentrations over a 20-day period |
| Chlorella vulgaris | [45] | Synthetic wastewater varying nitrate, nitrite and COD | 97% nitrate, 90% nitrite, and 90.6% COD was removed, yielding biomass with a 20% fatty acid extraction rate |
| Chlorella vulgaris | [46] | Synthetic wastewater with high concentrations (>10 mg/L) of total ammonia nitrogen, COD, caffeine and N,N-diethyl-m-toluamide | Removal rates of 82.3% total ammonia nitrogen, 67.7% COD, 85.7% caffeine, and 73.3% N,N-diethyl-m-toluamide |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nápoles-Armenta, J.; Romero-Soto, I.C.; Samaniego-Moreno, L.; Díaz-Tenorio, L.M.; Soto, L.A.L.; Mora-Orozco, C.D.L.; Pérez, R.G.; Martínez-Orozco, E.; García-Gómez, C.; Pérez-Valencia, L.I. Advanced Municipal Wastewater Treatment and Bioproduct Generation via Optimized Autotrophic and Mixotrophic Microalgal Cultivation. Sustainability 2025, 17, 6539. https://doi.org/10.3390/su17146539
Nápoles-Armenta J, Romero-Soto IC, Samaniego-Moreno L, Díaz-Tenorio LM, Soto LAL, Mora-Orozco CDL, Pérez RG, Martínez-Orozco E, García-Gómez C, Pérez-Valencia LI. Advanced Municipal Wastewater Treatment and Bioproduct Generation via Optimized Autotrophic and Mixotrophic Microalgal Cultivation. Sustainability. 2025; 17(14):6539. https://doi.org/10.3390/su17146539
Chicago/Turabian StyleNápoles-Armenta, Juan, Itzel Celeste Romero-Soto, Luis Samaniego-Moreno, Lourdes Mariana Díaz-Tenorio, Luis Alonso Leyva Soto, Celia De La Mora-Orozco, Rafael González Pérez, Edgardo Martínez-Orozco, Celestino García-Gómez, and Laura Izascum Pérez-Valencia. 2025. "Advanced Municipal Wastewater Treatment and Bioproduct Generation via Optimized Autotrophic and Mixotrophic Microalgal Cultivation" Sustainability 17, no. 14: 6539. https://doi.org/10.3390/su17146539
APA StyleNápoles-Armenta, J., Romero-Soto, I. C., Samaniego-Moreno, L., Díaz-Tenorio, L. M., Soto, L. A. L., Mora-Orozco, C. D. L., Pérez, R. G., Martínez-Orozco, E., García-Gómez, C., & Pérez-Valencia, L. I. (2025). Advanced Municipal Wastewater Treatment and Bioproduct Generation via Optimized Autotrophic and Mixotrophic Microalgal Cultivation. Sustainability, 17(14), 6539. https://doi.org/10.3390/su17146539








