Fingerprinting Agro-Industrial Waste: Using Polysaccharides from Cell Walls to Biomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Agro-Wastes Evaluated
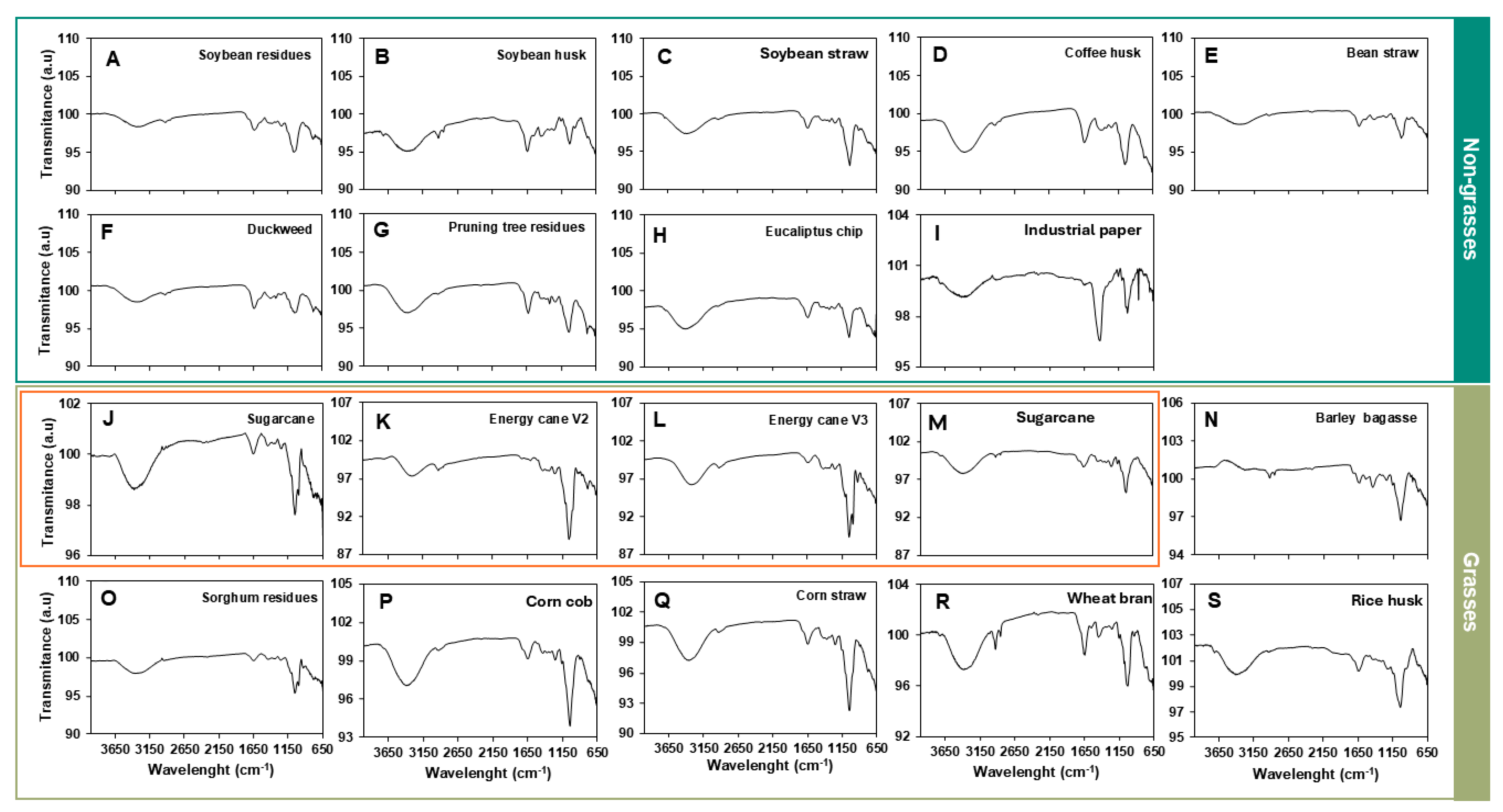
2.2. Compositional Characterization by Fourier-Transform Infrared Spectroscopy (FT-IR)
2.3. Elemental Composition and Calorific Power
2.4. Lignin Quantification
2.5. Cell Wall Obtention and Fractionation
2.6. Neutral Monosaccharides’ Hydrolysis and Quantification
2.7. Uronic Acid Determination
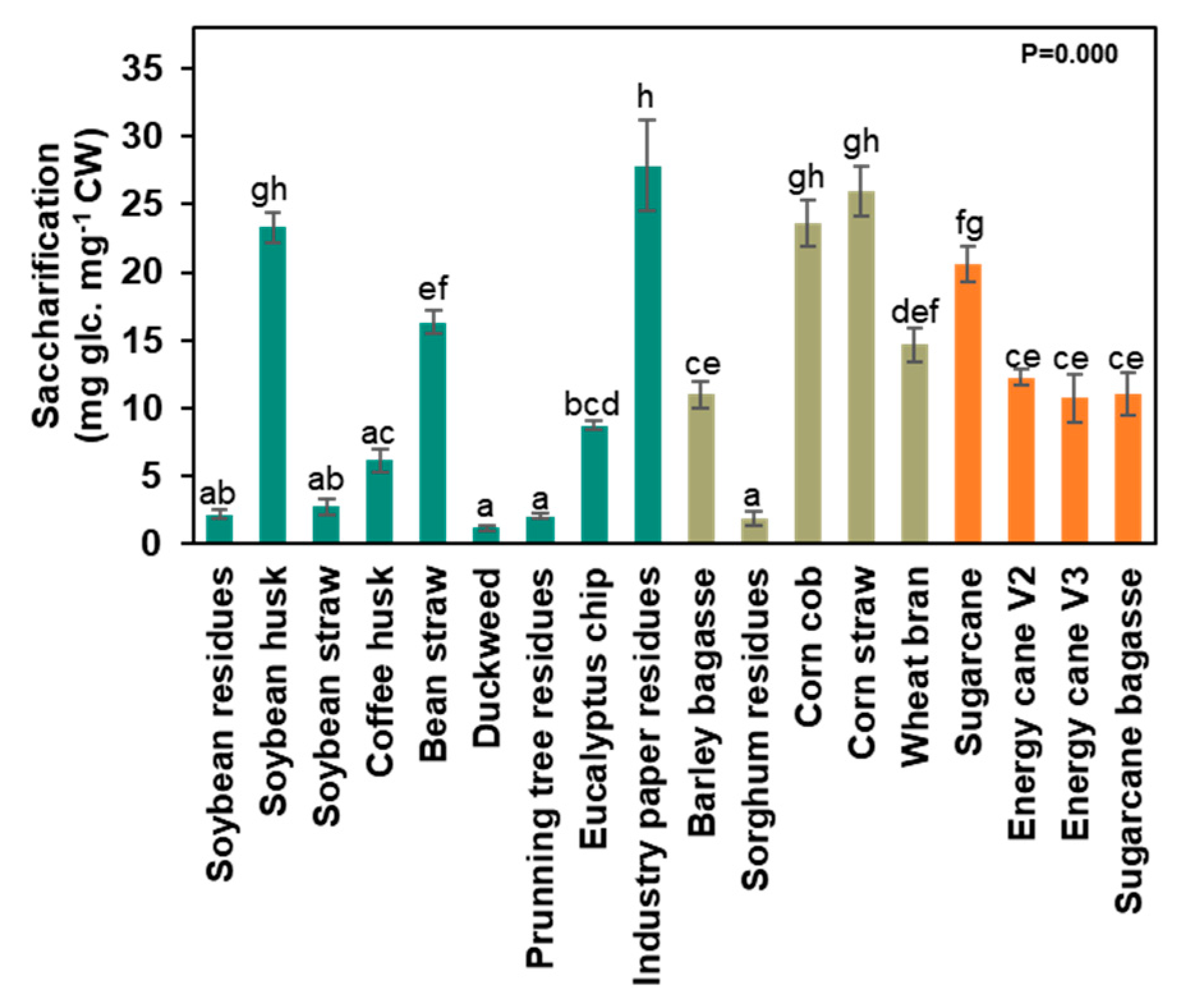
2.8. Cell Wall Saccharification
2.9. Data Analysis
3. Results
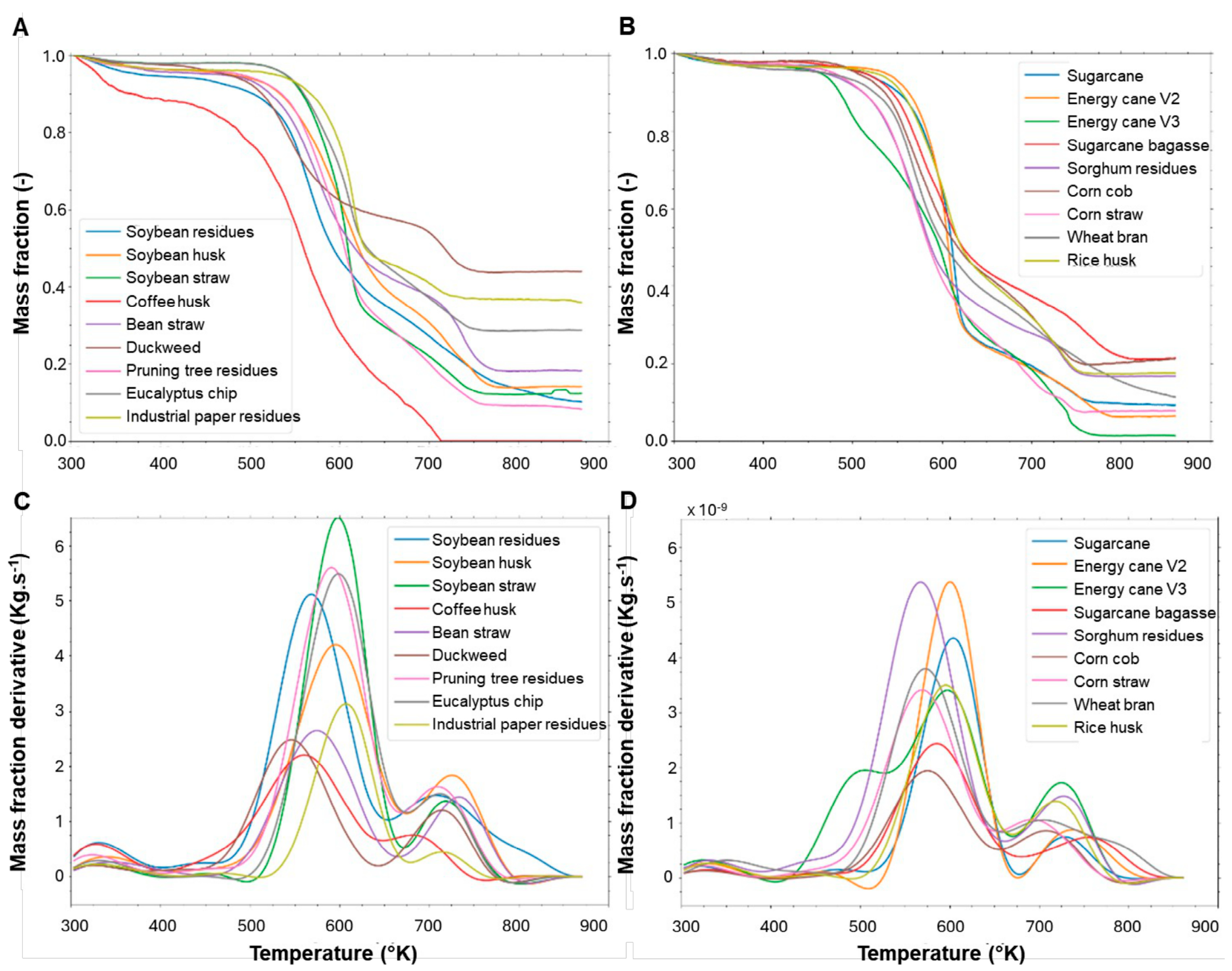
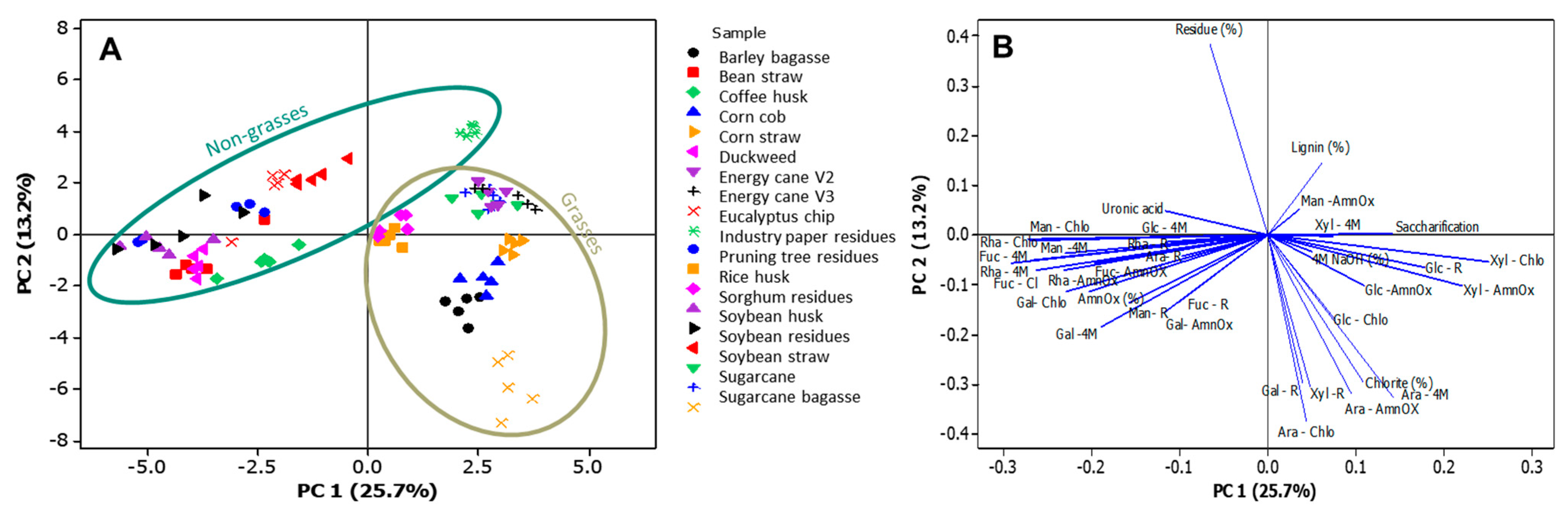
3.1. Agro-Wastes Have Similar Industrial Characteristics
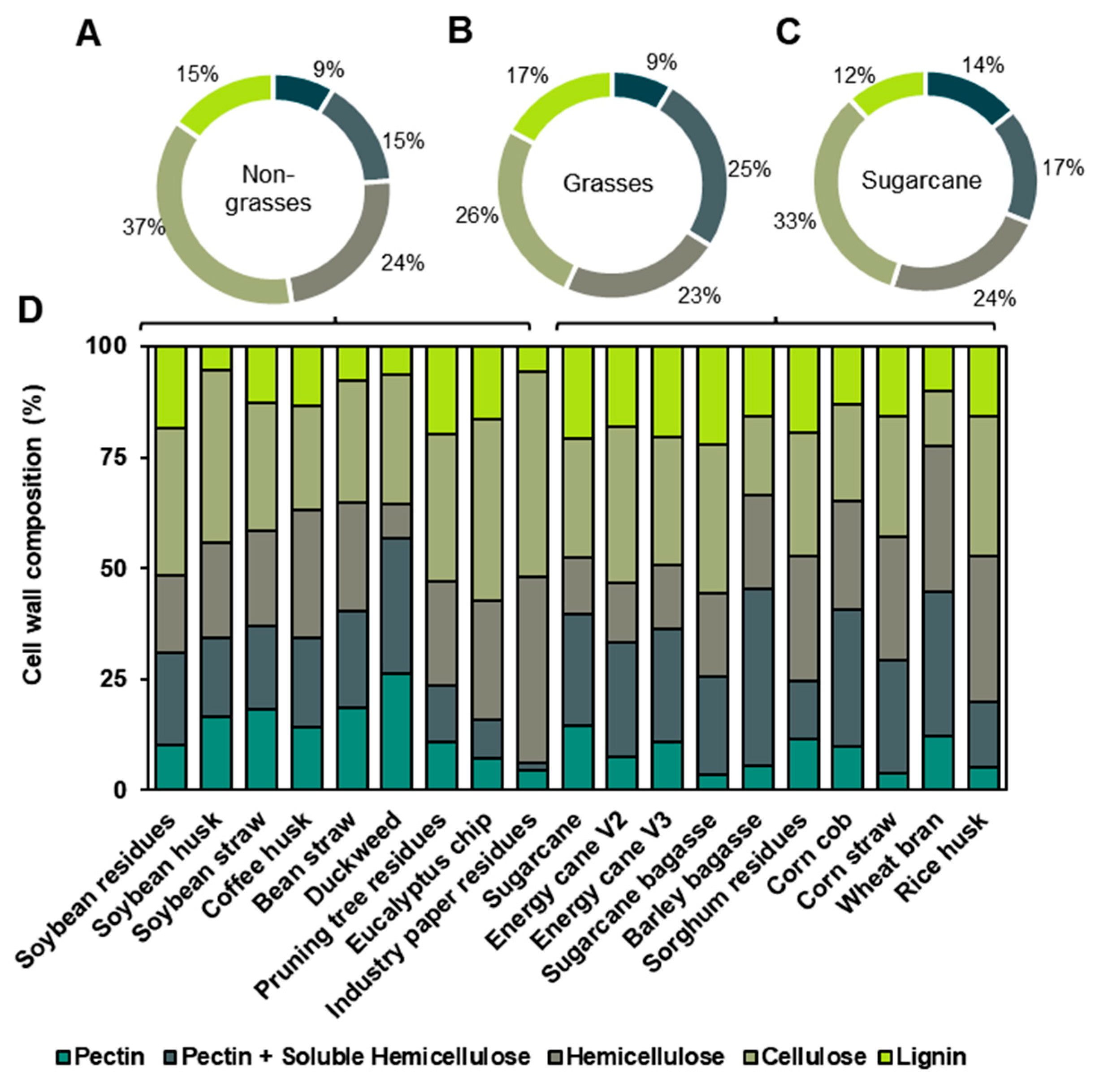
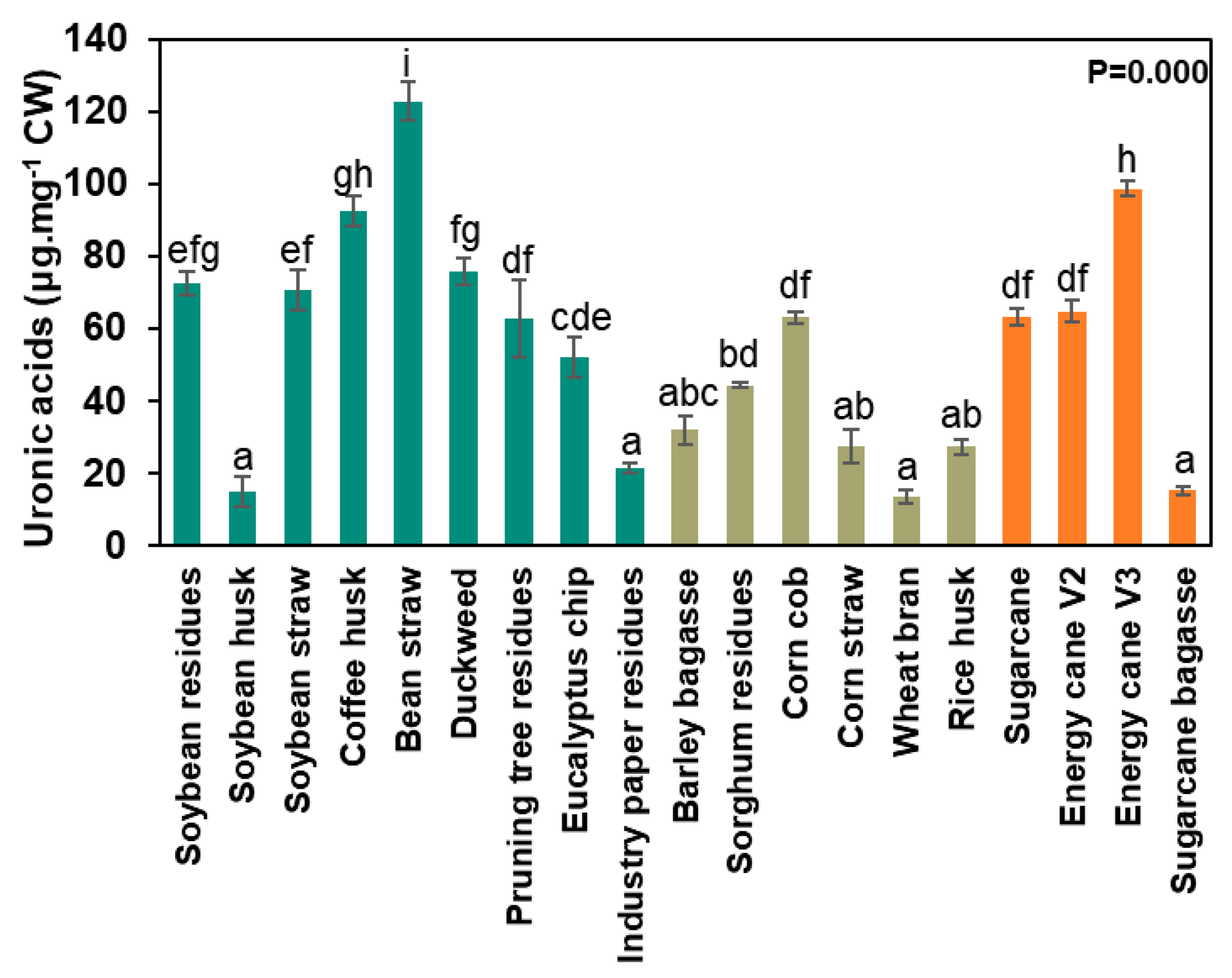
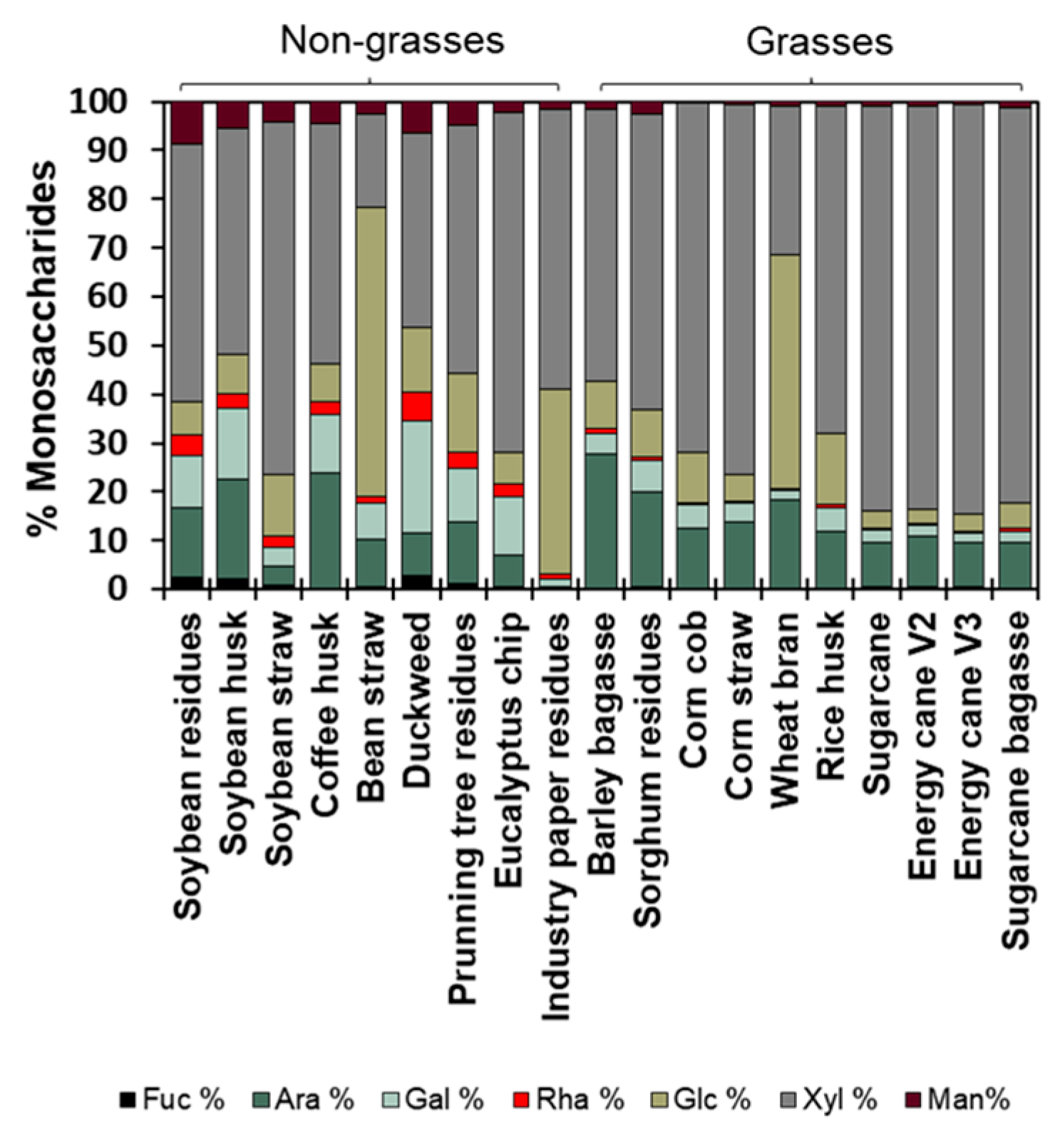
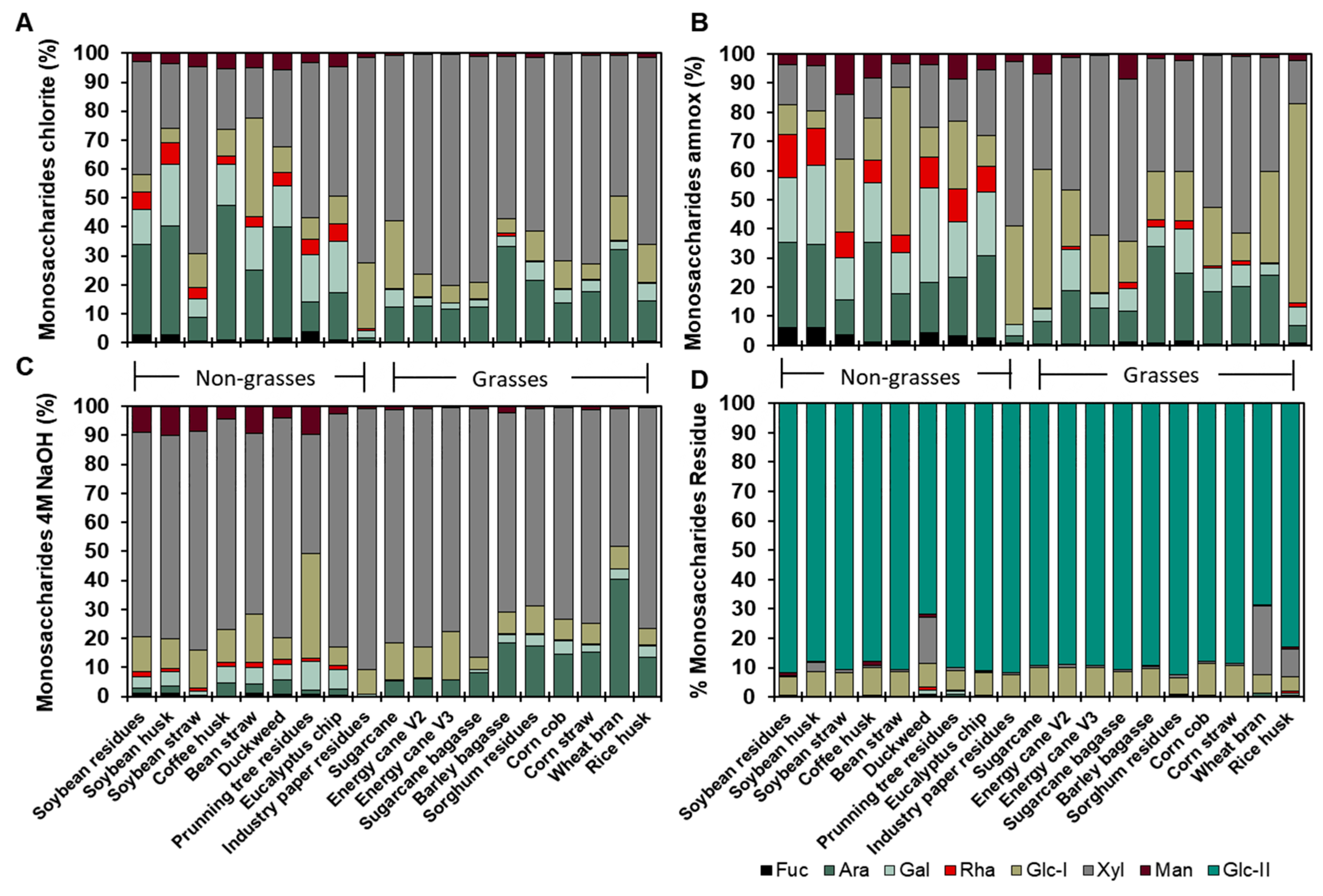
3.2. Agro-Wastes Have Different Proportions of Cell Wall Domains
3.3. Fine Composition of the Cell Wall Agro-Wastes
4. Discussion
4.1. Agro-Waste Characterization and Its Potential
4.2. Cell Wall Components and Their Polysaccharides’ Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- HLPE High Level Panel of Experts. Nutrition and Food Systems. Comm. World Food Secur. (CFS) 2017, 44, 150. [Google Scholar]
- de Coninck, H.; Revi, A.; Babiker, M.; Bertoldi, P.; Buckeridge, M.; Cartwright, A.; Dong, W.; Ford, J.; Fuss, S.; Hourcade, J.C.; et al. Strenthening and Implementing the Global Response. In Global Warming of 1.5 °C: Summary for Policy Makers; IPCC—The Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018; pp. 313–443. [Google Scholar]
- Mishra, A.; Kumar, M.; Medhi, K. Biomass Energy with Carbon Capture and Storage (BECCS). In Current Developments in Biotechnology and Bioengineering: Sustainable Bioresources for the Emerging Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2020; pp. 399–427. ISBN 9780444643094. [Google Scholar]
- Martinez-Burgos, W.J.; Bittencourt Sydney, E.; Bianchi Pedroni Medeiros, A.; Magalhães, A.I.; de Carvalho, J.C.; Karp, S.G.; Porto de Souza Vandenberghe, L.; Junior Letti, L.A.; Thomaz Soccol, V.; de Melo Pereira, G.V.; et al. Agro-Industrial Wastewater in a Circular Economy: Characteristics, Impacts and Applications for Bioenergy and Biochemicals. Bioresour. Technol. 2021, 341, 125795. [Google Scholar] [CrossRef]
- Mancini, S.D.; de Medeiros, G.A.; Paes, M.X.; de Oliveira, B.O.S.; Antunes, M.L.P.; de Souza, R.G.; Ferraz, J.L.; Bortoleto, A.P.; de Oliveira, J.A.P. Circular Economy and Solid Waste Management: Challenges and Opportunities in Brazil. Circ. Econ. Sustain. 2021, 1, 261–282. [Google Scholar] [CrossRef]
- Yang, N.-H.N.; Bertassini, A.C.; Mendes, J.A.J.; Gerolamo, M.C. The ‘3CE2CE’ Framework—Change Management Towards a Circular Economy: Opportunities for Agribusiness. Circ. Econ. Sustain. 2021, 1, 697–718. [Google Scholar] [CrossRef]
- Verbančič, J.; Lunn, J.E.; Stitt, M.; Persson, S. Carbon Supply and the Regulation of Cell Wall Synthesis. Mol. Plant 2017, 11, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, D. Pectin Structure and Biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Maleki, S.S.; Mohammadi, K.; Ji, K. Characterization of Cellulose Synthesis in Plant Cells. Sci. World J. 2016, 2016, 8641373. [Google Scholar] [CrossRef]
- Carpita, N.C.; Gibeaut, D.M. Structural Models of Primary Cell Walls in Flowering Plants: Consistency of Molecular Structure with the Physical Properties of the Walls during Growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef]
- Silva, G.B.; Ionashiro, M.; Carrara, T.B.; Crivellari, A.C.; Tiné, M.A.S.S.; Prado, J.; Carpita, N.C.; Buckeridge, M.S. Cell Wall Polysaccharides from Fern Leaves: Evidence for a Mannan-Rich Type III Cell Wall in Adiantum raddianum. Phytochemistry 2011, 72, 2352–2360. [Google Scholar] [CrossRef]
- McCann, M.C.; Roberts, K. Changes in Cell Wall Architecture during Cell Elongation. J. Exp. Bot. 1994, 45, 1683–1691. [Google Scholar] [CrossRef]
- de Souza, A.P.; Leite, D.C.C.; Pattathil, S.; Hahn, M.G.; Buckeridge, M.S. Composition and Structure of Sugarcane Cell Wall Polysaccharides: Implications for Second-Generation Bioethanol Production. Bioenergy Res. 2013, 6, 564–579. [Google Scholar] [CrossRef]
- McCann, M.C.; Carpita, N.C. Designing the Deconstruction of Plant Cell Walls. Curr. Opin. Plant Biol. 2008, 11, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Bosneaga, E.; Auer, M. Plant Cell Walls throughout Evolution: Towards a Molecular Understanding of Their Design Principles. J. Exp. Bot. 2009, 60, 3615–3635. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic Agriculture Wastes as Biomass Feedstocks for Second-Generation Bioethanol Production: Concepts and Recent Developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.H.; Ngamcharussrivichai, C. Recent Advances in Lignocellulosic Biomass for Biofuels and Value-Added Bioproducts—A Critical Review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef] [PubMed]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated Lignocellulosic Value Chains in a Growing Bioeconomy: Status Quo and Perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Singh, A.T.; Sharma, M.; Sharma, M.; Dutt Sharma, G.; Kumar Passari, A.; Bhasin, S. Valorization of Agro-Industrial Residues for Production of Commercial Biorefinery Products. Fuel 2022, 322, 124284. [Google Scholar] [CrossRef]
- Chapple, C.; Carpita, N. Plant Cell Walls as Targets for Biotechnology. Curr. Opin. Plant Biol. 1998, 1, 179–185. [Google Scholar] [CrossRef]
- Cesarino, I.; Araújo, P.; Domingues, A.P.; Mazzafera, P. An Overview of Lignin Metabolism and Its Effect on Biomass Recalcitrance. Braz. J. Bot. 2012, 35, 303–311. [Google Scholar] [CrossRef]
- ASTM D2974; Standard Test Methods for Determining the Water (Moisture) Content, Ash Content, and Organic Material of Peat and Other Organic Soils. ASTM: Philadelphia, PA, USA, 1987.
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2013; pp. 1–530. [Google Scholar] [CrossRef]
- Van Acker, R.; Vanholme, R.; Storme, V.; Mortimer, J.C.; Dupree, P.; Boerjan, W. Lignin Biosynthesis Perturbations Affect Secondary Cell Wall Composition and Saccharification Yield in Arabidopsis thaliana. Biotechnol. Biofuels 2013, 6, 46. [Google Scholar] [CrossRef]
- Fukushima, R.S.; Kerley, M.S. Use of Lignin Extracted from Different Plant Sources as Standards in the Spectrophotometric Acetyl Bromide Lignin Method. J. Agric. Food Chem. 2011, 59, 3505–3509. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, R.S.; Kerley, M.S.; Ramos, M.H.; Porter, J.H.; Kallenbach, R.L. Comparison of Acetyl Bromide Lignin with Acid Detergent Lignin and Klason Lignin and Correlation with in Vitro Forage Degradability. Anim. Feed. Sci. Technol. 2015, 201, 25–37. [Google Scholar] [CrossRef]
- Chang, X.F.; Chandra, R.; Berleth, T.; Beatson, R.P. Rapid, Microscale, Acetyl Bromide-Based Method for High-Throughput Determination of Lignin Content in Arabidopsis thaliana. J. Agric. Food Chem. 2008, 56, 6825–6834. [Google Scholar] [CrossRef]
- Carpita, N.C. Fractionation of Hemicelluloses from Maize Cell Walls with Increasing Concentrations of Alkali. Phytochemistry 1984, 23, 1089–1093. [Google Scholar] [CrossRef]
- Gorshkova, T.A.; Wyatt, S.E.; Salnikov, V.V.; Gibeaut, D.M.; Lozovaya, V.V.; Carpita, N.C.; Ibragimov, R. Cell-Wall Polysaccharides of Developing Flax Plants. Plant Physiol. 1996, 110, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Filisetti-Cozzi, T.M.C.C.; Carpita, N.C. Measurement of Uronic Acids without Interference from Neutral Sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Gomez, L.D.; Whitehead, C.; Barakate, A.; Halpin, C.; McQueen-Mason, S.J. Automated Saccharification Assay for Determination of Digestibility in Plant Materials. Biotechnol. Biofuels 2010, 3, 23. [Google Scholar] [CrossRef]
- Leong, H.Y.; Chang, C.K.; Khoo, K.S.; Chew, K.W.; Chia, S.R.; Lim, J.W.; Chang, J.S.; Show, P.L. Waste Biorefinery towards a Sustainable Circular Bioeconomy: A Solution to Global Issues. Biotechnol. Biofuels 2021, 14, 87. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Karpichev, Y.; Pandey, A.; Chandra Kuhad, R.; Bhat, R.; Punia, R.; Aghbashlo, M.; Tabatabaei, M.; Gupta, V.K. Advancement in Valorization Technologies to Improve Utilization of Bio-Based Waste in Bioeconomy Context. Renew. Sustain. Energy Rev. 2020, 131, 109965. [Google Scholar] [CrossRef]
- Kumar, A.; Singh Negi, Y.; Choudhary, V.; Kant Bhardwaj, N. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Fávaro, S.L.; Lopes, M.S.; Vieira de Carvalho Neto, A.G.; Rogério de Santana, R.; Radovanovic, E. Chemical, Morphological, and Mechanical Analysis of Rice Husk/Post-Consumer Polyethylene Composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 154–160. [Google Scholar] [CrossRef]
- Mondal, M.I.H.; Yeasmin, M.S.; Rahman, M.S. Preparation of Food Grade Carboxymethyl Cellulose from Corn Husk Agrowaste. Int. J. Biol. Macromol. 2015, 79, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, M.; Asghar, A.; Ramzan, N.; Aslam, U.; Bello, M.M. Impacts of Non-Oxidative Torrefaction Conditions on the Fuel Properties of Indigenous Biomass (Bagasse). Waste Manag. Res. 2020, 38, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.P.; Ferreira, A.N.; de Albuquerque, F.S.; de Almeida Barros, A.C.; da Luz, J.M.R.; Gomes, F.S.; Pereira, H.J.V. Box–Behnken Experimental Design for the Optimization of Enzymatic Saccharification of Wheat Bran. Biomass Convers. Biorefin. 2022, 12, 5597–5604. [Google Scholar] [CrossRef]
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A. Study on the Physical and Chemical Composition of Agro Wastes for the Production of 5-Hydroxymethylfurfural. Bioresour. Technol. 2018, 247, 821–828. [Google Scholar] [CrossRef]
- Antolini, D.; Piazzi, S.; Menin, L.; Baratieri, M.; Patuzzi, F. High Hydrogen Content Syngas for Biofuels Production from Biomass Air Gasification: Experimental Evaluation of a Char-Catalyzed Steam Reforming Unit. Int. J. Hydrogen Energy 2022, 47, 27421–27436. [Google Scholar] [CrossRef]
- Majumder, A.K.; Jain, R.; Banerjee, P.; Barnwal, J.P. Development of a New Proximate Analysis Based Correlation to Predict Calorific Value of Coal. Fuel 2008, 87, 3077–3081. [Google Scholar] [CrossRef]
- Krieger Filho, G.C.; Costa, F.; Torraga Maria, G.F.; Bufacchi, P.; Trubachev, S.; Shundrina, I.; Korobeinichev, O. Kinetic Parameters and Heat of Reaction for Forest Fuels Based on Genetic Algorithm Optimization. Thermochim. Acta 2022, 713, 179228. [Google Scholar] [CrossRef]
- Saccani, A.; Sisti, L.; Manzi, S.; Fiorini, M. PLA Composites Formulated Recycling Residuals of the Winery Industry. Polym. Compos. 2019, 40, 1378–1383. [Google Scholar] [CrossRef]
- Gulsunoglu-Konuskan, Z.; Kilic-Akyilmaz, M. Microbial Bioconversion of Phenolic Compounds in Agro-Industrial Wastes: A Review of Mechanisms and Effective Factors. J. Agric. Food Chem. 2022, 70, 6901–6910. [Google Scholar] [CrossRef]
- El-Ramady, H.; Brevik, E.C.; Bayoumi, Y.; Shalaby, T.A.; El-Mahrouk, M.E.; Taha, N.; Elbasiouny, H.; Elbehiry, F.; Amer, M.; Abdalla, N.; et al. An Overview of Agro-Waste Management in Light of the Water-Energy-Waste Nexus. Sustainability 2022, 14, 15717. [Google Scholar] [CrossRef]
- Grandis, A.; Leite, D.C.C.; Tavares, E.Q.P.; Arenque-Musa, B.C.; Gaiarsa, J.W.; Martins, M.C.M.; de Souza, A.P.; Gomez, L.D.; Fabbri, C.; Mattei, B.; et al. Cell Wall Hydrolases Act in Concert during Aerenchyma Development in Sugarcane Roots. Ann. Bot. 2019, 124, 1067–1089. [Google Scholar] [CrossRef] [PubMed]
- van Maris, A.J.A.A.; Abbott, D.A.; Bellissimi, E.; van den Brink, J.; Kuyper, M.; Luttik, M.A.H.H.; Wisselink, H.W.; Scheffers, W.A.; van Dijken, J.P.; Pronk, J.T. Alcoholic Fermentation of Carbon Sources in Biomass Hydrolysates by Saccharomyces Cerevisiae: Current Status. Antonie Leeuwenhoek 2006, 90, 391–418. [Google Scholar] [CrossRef]
- Moysés, D.N.; Reis, V.C.B.; de Almeida, J.R.M.; de Moraes, L.M.P.; Torres, F.A.G. Xylose Fermentation by Saccharomyces Cerevisiae: Challenges and Prospects. Int. J. Mol. Sci. 2016, 17, 207. [Google Scholar] [CrossRef]
- Cunha, J.T.; Soares, P.O.; Romaní, A.; Thevelein, J.M.; Domingues, L. Xylose Fermentation Efficiency of Industrial Saccharomyces Cerevisiae Yeast with Separate or Combined Xylose Reductase/Xylitol Dehydrogenase and Xylose Isomerase Pathways. Biotechnol. Biofuels 2019, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Saxena, I.M.; Brown, R.M. Cellulose Biosynthesis: Current Views and Evolving Concepts. Ann. Bot. 2005, 96, 9–21. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, N.; Phillips, G.C.; Xu, J. Growing Lemna Minor in Agricultural Wastewater and Converting the Duckweed Biomass to Ethanol. Bioresour. Technol. 2012, 124, 485–488. [Google Scholar] [CrossRef]
- Liu, J.; Willför, S.; Xu, C. A Review of Bioactive Plant Polysaccharides: Biological Activities, Functionalization, and Biomedical Applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Palantöken, S.; Bethke, K.; Zivanovic, V.; Kalinka, G.; Kneipp, J.; Rademann, K. Cellulose Hydrogels Physically Crosslinked by Glycine: Synthesis, Characterization, Thermal and Mechanical Properties. J. Appl. Polym. Sci. 2020, 137, 48380. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Kim, T.H.; Liu, K.; Ma, M.G.; Choi, S.E.; Si, C. Multifunctional Lignin-Based Composite Materials for Emerging Applications. Front. Bioeng. Biotechnol. 2021, 9, 708976. [Google Scholar] [CrossRef] [PubMed]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Fabbri, F.; Bischof, S.; Mayr, S.; Gritsch, S.; Jimenez Bartolome, M.; Schwaiger, N.; Guebitz, G.M.; Weiss, R. The Biomodified Lignin Platform: A Review. Polymers 2023, 15, 1694. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bossche, G.; Van Den Bosch, S.; Koelewijn, S.-F.; Ennaert, T.; Sels, B.F. Catalysis in Lignocellulosic Biorefineries: The Case of Lignin Conversion. In Nanotechnology Catalysis; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2017; pp. 537–584. [Google Scholar]
- Esquena-Moret, J. A Review of Xyloglucan: Self-Aggregation, Hydrogel Formation, Mucoadhesion and Uses in Medical Devices. Macromol 2022, 2, 562–590. [Google Scholar] [CrossRef]
- Mishra, A.; Malhotra, A.V. Tamarind Xyloglucan: A Polysaccharide with Versatile Application Potential. J. Mater. Chem. 2009, 19, 8528–8536. [Google Scholar] [CrossRef]
- Yan, J.; Jia, X.; Feng, L.; Yadav, M.; Li, X.; Yin, L. Rheological and Emulsifying Properties of Arabinoxylans from Various Cereal Brans. J. Cereal Sci. 2019, 90, 102844. [Google Scholar] [CrossRef]
- González-Estrada, R.; Calderón-Santoyo, M.; Carvajal-Millan, E.; De Jesús Ascencio Valle, F.; Ragazzo-Sánchez, J.A.; Brown-Bojorquez, F.; Rascón-Chu, A. Covalently Cross-Linked Arabinoxylans Films for Debaryomyces hansenii Entrapment. Molecules 2015, 20, 11373–11386. [Google Scholar] [CrossRef]
- He, H.J.; Qiao, J.; Liu, Y.; Guo, Q.; Ou, X.; Wang, X. Isolation, Structural, Functional, and Bioactive Properties of Cereal Arabinoxylan—A Critical Review. J. Agric. Food Chem. 2021, 69, 15437–15457. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Arya, S.K. Mannans: An Overview of Properties and Application in Food Products. Int. J. Biol. Macromol. 2018, 119, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.-D.; Kim, D.; Paek, S.-H.; Oh, S.-E. Plant Cell Wall Polysaccharides as Potential Resources for the Development of Novel Prebiotics. Biomol. Ther. 2012, 20, 371–379. [Google Scholar] [CrossRef]
- Singla, A.; Gupta, O.P.; Sagwal, V.; Kumar, A.; Patwa, N.; Mohan, N.; Ankush; Kumar, D.; Vir, O.; Singh, J.; et al. Beta-Glucan as a Soluble Dietary Fiber Source: Origins, Biosynthesis, Extraction, Purification, Structural Characteristics, Bioavailability, Biofunctional Attributes, Industrial Utilization, and Global Trade. Nutrients 2024, 16, 900. [Google Scholar] [CrossRef] [PubMed]
- Beck, E.; Hopf, H. Branched-Chain Sugars and Sugar Alcohols. Methods Plant Biochem. 1990, 2, 235–289. [Google Scholar] [CrossRef]
- Eckey-Kaltenbach, H.; Heller, W.; Sonnenbichler, J.; Zetl, I.; Schäfer, W.; Ernst, D.; Sandermann, H. Oxidative Stress and Plant Secondary Metabolism: 6″-O-Malonylapiin in Parsley. Phytochemistry 1993, 34, 687–691. [Google Scholar] [CrossRef]
- Mølhøj, M.; Verma, R.; Reiter, W.D. The Biostnthesis of the Branched-Chain Sugar D-Apiose in Plants: Functional Cloning and Characterization of a UDP-D-Apiose/UDP-D-Xylose Synthase from Arabidopsis. Plant J. 2003, 35, 1781–1791. [Google Scholar] [CrossRef]
- Pičmanová, M.; Møller, B.L. Apiose: One of Nature’s Witty Games. Glycobiology 2016, 26, 430–442. [Google Scholar] [CrossRef]
- Zanutto, F.V.; Boldrin, P.K.; Varanda, E.A.; De Souza, S.F.; Sano, P.T.; Vilegas, W.; Dos Santos, L.C. Characterization of Flavonoids and Naphthopyranones in Methanol Extracts of Paepalanthus chiquitensis Herzog by HPLC-ESI-IT-MSn and Their Mutagenic Activity. Molecules 2012, 18, 244–262. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Buckeridge, M.S.; de Souza, A.P. Breaking the “Glycomic Code” of Cell Wall Polysaccharides May Improve Second-Generation Bioenergy Production from Biomass. Bioenergy Res. 2014, 7, 1065–1073. [Google Scholar] [CrossRef]
| Agro-Waste | Species | Location | |
|---|---|---|---|
| Non-grasses | Soybean residues | Glycine max var IAC Foscarin 31 | Instituto Agrônomico de Campinas |
| Soybean husk | Glycine max | Ribeirão Preto-SP | |
| Soybean straw | Glycine max | Maringá, PR | |
| Coffee husk | Coffea | Ribeirão Preto-SP | |
| Bean straw | Phaseolus vulgaris | Ribeirão Preto-SP | |
| Duckweed | Lemna minor 8627 | Rutgers Duckweed Stock Cooperative | |
| Prunning tree residues | - | University of São Paulo | |
| Eucalyptus chip | Eucalyptus | Suzano papel e celulose | |
| Industry paper residues | - | Suzano papel e celulose | |
| Grasses | Barley bagasse | Hordeum vulgare | Brewery Colorado, Ribeirão Preto-SP |
| Sorghum residues | Sorghum bicolor | Embrapa | |
| Corn cob | Zea mays | Ribeirão Preto-SP | |
| Corn straw | Zea mays | Ribeirão Preto-SP | |
| Wheat bran | Triticum | Ribeirão Preto-SP | |
| Rice husk | Oryza sativa | Ribeirão Preto-SP | |
| Sugarcane | Saccharum sp 80-3280 | Piracicaba-SP | |
| Energy cane V2 | Saccharum | Piracicaba-SP | |
| Energy cane V3 | Saccharum | Piracicaba-SP | |
| Sugarcane bagasse | Saccharum | Guarani-São Paulo-SP |
| C% | H% | N% | O% | Ashes % | HHV [MJ·Kg−1] | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soybean residues | 39.87 | ± | 0.21 | cd | 5.84 | ± | 0.07 | be | 1.82 | ± | 0.16 | ef | 43.68 | ± | 0.26 | ac | 8.79 | ± | 0.06 | cd | 16.07 | ± | 0.04 | de |
| Soybean husk | 41.52 | ± | 0.28 | efg | 6.43 | ± | 0.08 | fgh | 2.11 | ± | 0.03 | fg | 46.90 | ± | 0.42 | bcd | 3.05 | ± | 0.24 | ab | 17.12 | ± | 0.22 | egi |
| Soybean straw | 43.22 | ± | 0.19 | ghi | 6.12 | ± | 0.08 | cdeg | 0.50 | ± | 0.04 | bd | 45.93 | ± | 0.22 | ad | 4.23 | ± | 0.09 | abc | 17.45 | ± | 0.15 | fgi |
| Coffee husk | 41.81 | ± | 0.36 | efg | 6.00 | ± | 0.07 | beg | 1.60 | ± | 0.03 | e | 47.14 | ± | 0.77 | bcd | 3.46 | ± | 0.37 | abc | 16.69 | ± | 0.28 | eg |
| Bean straw | 37.57 | ± | 0.15 | bc | 5.59 | ± | 0.06 | b | 1.87 | ± | 0.02 | ef | 47.54 | ± | 0.60 | cd | 7.44 | ± | 0.51 | bd | 14.60 | ± | 0.17 | bc |
| Duckweed | 36.33 | ± | 0.38 | b | 5.64 | ± | 0.07 | bc | 3.68 | ± | 0.07 | i | 44.41 | ± | 0.18 | ad | 9.94 | ± | 0.33 | de | 14.47 | ± | 0.16 | bc |
| Pruning tree residues | 42.95 | ± | 0.12 | gh | 5.76 | ± | 0.03 | bd | 0.83 | ± | 0.05 | d | 47.37 | ± | 1.07 | cd | 4.13 | ± | 0.10 | abc | 16.80 | ± | 0.11 | eg |
| Eucalyptus chip | 36.14 | ± | 0.51 | b | 4.71 | ± | 0.20 | a | 0.38 | ± | 0.02 | bc | 41.12 | ± | 3.96 | a | 17.65 | ± | 4.35 | fg | 13.54 | ± | 0.39 | b |
| Industrial paper residues | 32.33 | ± | 0.06 | a | 4.41 | ± | 0.02 | a | 0.00 | ± | 0.00 | a | 41.26 | ± | 0.16 | a | 21.99 | ± | 0.21 | g | 11.76 | ± | 0.05 | a |
| Barley bagasse | 45.74 | ± | 0.83 | j | 6.69 | ± | 0.10 | h | 3.01 | ± | 0.12 | h | 41.40 | ± | 0.36 | a | 3.17 | ± | 0.75 | ab | 19.46 | ± | 0.42 | j |
| Sorghum residues | 39.03 | ± | 0.38 | cd | 5.67 | ± | 0.11 | bd | 1.99 | ± | 0.13 | fg | 49.20 | ± | 0.25 | d | 4.12 | ± | 0.31 | abc | 15.10 | ± | 0.26 | cd |
| Corn cob | 43.01 | ± | 0.05 | gh | 5.96 | ± | 0.13 | bef | 0.39 | ± | 0.04 | bc | 48.53 | ± | 0.95 | cd | 2.11 | ± | 0.78 | ab | 16.97 | ± | 0.24 | egh |
| Corn straw | 41.95 | ± | 0.34 | fg | 6.04 | ± | 0.12 | beg | 0.72 | ± | 0.04 | cd | 49.32 | ± | 0.77 | d | 1.98 | ± | 0.81 | ab | 16.60 | ± | 0.27 | ef |
| Wheat bran | 40.54 | ± | 0.16 | df | 6.49 | ± | 0.02 | gh | 2.32 | ± | 0.03 | g | 47.48 | ± | 0.20 | cd | 3.18 | ± | 0.35 | ab | 16.79 | ± | 0.05 | eg |
| Rice husk | 37.61 | ± | 0.31 | bc | 5.58 | ± | 0.03 | b | 0.40 | ± | 0.05 | bc | 41.82 | ± | 0.29 | ab | 14.60 | ± | 0.37 | ef | 15.06 | ± | 0.14 | cd |
| Sugarcane | 44.98 | ± | 0.68 | hj | 6.29 | ± | 0.20 | eh | 0.38 | ± | 0.05 | bc | 47.49 | ± | 0.34 | cd | 1.35 | ± | 0.14 | a | 18.17 | ± | 0.09 | i |
| Energy cane V2 | 45.20 | ± | 0.17 | ij | 6.14 | ± | 0.04 | cdeg | 0.23 | ± | 0.04 | ab | 47.78 | ± | 0.19 | cd | 0.66 | ± | 0.26 | a | 18.05 | ± | 0.09 | hi |
| Energy cane V3 | 44.36 | ± | 0.78 | hj | 6.16 | ± | 0.09 | deg | 0.24 | ± | 0.05 | ab | 48.51 | ± | 0.49 | cd | 0.73 | ± | 0.18 | a | 17.71 | ± | 0.22 | gi |
| Sugarcane bagasse | 44.63 | ± | 0.11 | hj | 6.28 | ± | 0.05 | eh | 0.34 | ± | 0.01 | ab | 47.37 | ± | 0.14 | cd | 1.39 | ± | 0.06 | a | 18.04 | ± | 0.07 | hi |
| p-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | ||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagliuso, D.; Grandis, A.; de Castro Juraski, A.; Azzoni, A.R.; Teixeira de Morais Polizeli, M.d.L.; Villanueva, H.H.; Krieger Filho, G.C.; Silveira Buckeridge, M. Fingerprinting Agro-Industrial Waste: Using Polysaccharides from Cell Walls to Biomaterials. Sustainability 2025, 17, 6362. https://doi.org/10.3390/su17146362
Pagliuso D, Grandis A, de Castro Juraski A, Azzoni AR, Teixeira de Morais Polizeli MdL, Villanueva HH, Krieger Filho GC, Silveira Buckeridge M. Fingerprinting Agro-Industrial Waste: Using Polysaccharides from Cell Walls to Biomaterials. Sustainability. 2025; 17(14):6362. https://doi.org/10.3390/su17146362
Chicago/Turabian StylePagliuso, Débora, Adriana Grandis, Amanda de Castro Juraski, Adriano Rodrigues Azzoni, Maria de Lourdes Teixeira de Morais Polizeli, Helio Henrique Villanueva, Guenther Carlos Krieger Filho, and Marcos Silveira Buckeridge. 2025. "Fingerprinting Agro-Industrial Waste: Using Polysaccharides from Cell Walls to Biomaterials" Sustainability 17, no. 14: 6362. https://doi.org/10.3390/su17146362
APA StylePagliuso, D., Grandis, A., de Castro Juraski, A., Azzoni, A. R., Teixeira de Morais Polizeli, M. d. L., Villanueva, H. H., Krieger Filho, G. C., & Silveira Buckeridge, M. (2025). Fingerprinting Agro-Industrial Waste: Using Polysaccharides from Cell Walls to Biomaterials. Sustainability, 17(14), 6362. https://doi.org/10.3390/su17146362






