Abstract
Combining advanced extraction technologies with non-pollutant solvents represents a sustainable approach toward valorizing medicinal plants and aligns with the principles of green chemistry. This study aimed to evaluate the efficiency of microwave-assisted extraction (MAE) combined with natural deep eutectic solvents (NADES) to extract bioactive compounds from the underexplored leaves and bark of Salix amplexicaulis Bory & Chaub. Additionally, the potential of NADES as sustainable alternatives to conventional solvents was assessed through a comparative evaluation of MAE-NADES with MAE–water and traditional ethanol maceration. NADES based on lactic acid–glycerol, lactic acid–glucose, glycerol–glucose, and glycerol–urea were synthesized by heating and stirring. Willow extracts were characterized by HPLC-DAD, resulting in the identification and quantification of seven phenolic acids and four flavonoids. Lactic acid–glucose (5:1)-based NADES extracted the highest number of phenolics in the greatest amount from the bark and leaves of S. amplexicaulis. MAE-NADES offers a fast, cost-effective preparation, high extraction efficiency, and environmentally friendly properties, opening new perspectives on the valorization of S. amplexicaulis in the pharmaceutical field. Furthermore, NADES provide a promising alternative to water and toxic organic solvents for extracting bioactives.
1. Introduction
The bark of willow species (Salix spp., Salicaceae) is an ancient remedy significant in the treatment of pain, fever, and inflammatory processes [1]. The anti-inflammatory, analgesic, and antipyretic effects of the willow bark extracts result from the synergistic action of its bioactive components: salicin and its derivatives, flavonoids, and phenolic acids [2,3,4]. Even though the medicinal use of willow bark has significantly declined in the era of synthetic non-steroidal anti-inflammatory drugs (NSAIDs) [5], there has been a growing interest in using willow bark extracts as natural alternatives that exert fewer side effects [6]. The genus Salix comprises over 500 [7] predominantly woody species distributed across all continents except Antarctica [6]. Despite being an easily accessible and renewable source of active principles for numerous applications in the pharmaceutical industry, only the bark of a few species, including S. purpurea, S. daphnoides, and S. fragilis, is recognized as an officinal herbal drug. This plant material is commonly utilized in the preparation of various products, such as dry and liquid extracts, tinctures, infusions, decoctions, and powdered forms [8]. Although traditionally regarded as waste, with limited significance for the extraction of active principles, willow leaves are increasingly recognized as a valuable source of bioactives [9,10]. Salix amplexicaulis Bory & Chaub. is a sub-Mediterranean species distributed across the Balkan Peninsula, northern and central Anatolia, and locally in southern Italy [11]. It is a shrub ranging in height from 2 to 5 m [12]. There is very little data regarding this willow species in the scientific literature. In our previous research, S. amplexicaulis was identified as a species with the most favorable chemical profile among six analyzed willow species, being superior to the officinal S. purpurea and S. fragilis [9].
Due to variations in the physicochemical characteristics of bioactive components, it is crucial to determine the most efficient extraction method for the selected compounds [13,14]. Traditional extraction techniques, such as maceration, are progressively being replaced by novel assisted technologies. Microwave-assisted extraction (MAE) is an advanced, sustainable, and green extraction method that, compared to traditional techniques, minimizes solvent use while providing a shorter processing time and improved extraction efficiency. A more efficient and faster extraction of targeted components, with improved solubility and diffusion, is achieved by applying heat. MAE further enhances this process by causing cell disruption through rapid energy absorption, improving mass transfer and compound recovery [15].
In addition to the choice of extraction method, successful extraction also depends on the selection of the solvent, as its physical and chemical characteristics significantly affect the yield of the target compounds [16]. Although water is a widely accessible, economical, and safe solvent, its effectiveness in extracting many organic bioactives is constrained by its high polarity and potential to cause chemical degradation [17]. Furthermore, because of their various adverse effects, organic solvents are commonly avoided [18]. Scientists discovered that water and lipids alone are not sufficient solvents for important biological processes in living organisms, such as survival under extreme cold or heat conditions and the biosynthesis of poorly soluble macromolecules in water. As a result, they identified alternative solvent systems. Natural deep eutectic solvents (NADES) are created in the body by combining primary metabolites in specific proportions [19,20]. Cellular components such as sugars, alcohols, amino acids, organic acids, and choline derivatives serve as the structural elements of NADES. The formation of NADES involves the development of van der Waals and hydrogen bonds, resulting in a reduction in melting point [21]. NADES are novel, economical, and environmentally friendly solvents with favorable physicochemical properties, such as biodegradability, low volatility, good solubilization capacity, and adjustable viscosity and polarity, which make them suitable for the extraction of bioactive compounds from plant materials [22]. The literature states that NADES enable the sustainable extraction of polyphenols [23].
Our previous research has proven that MAE using water as a solvent is effective for isolating bioactive components from the bark and leaves of S. amplexicaulis [9]. To date, there are no studies in the scientific literature on applying NADES as solvents to extract bioactive compounds from species of the genus Salix alone or in combination with MAE. In an effort to preserve the environment, reduce the negative impact of humans in the field of chemistry, and enhance extraction performance, NADES have been synthesized and applied in this study. Applying green solvents, such as NADES, in combination with advanced extraction methods, such as MAE, presents new opportunities in environmentally sustainable technologies. As a continuation of our research on the extraction and phytochemical investigation of the underexplored S. amplexicaulis, this study aimed to assess the efficiency of extracting bioactive compounds from the leaves and bark of S. amplexicaulis using an environmentally friendly strategy that combines MAE with various NADES. Moreover, the potential of NADES to replace conventional solvents was assessed by comparing MAE-NADES with MAE–water and traditional ethanol maceration. Such a comparative analysis may contribute to the development of more sustainable extraction methods applicable in the fields of phytochemistry and pharmaceuticals. In addition, this study seeks to provide an evaluation of the impact of different NADES formulations on extraction efficiency, aiming to maximize the amount of active principles that can be obtained from S. amplexicaulis.
2. Materials and Methods
2.1. Plant Material
Bark and leaves of S. amplexicaulis were collected in the locality of the Pečenjevce settlement, north-east Serbia (43°06′01′′ N, 21°54′60′′ E). This region is characterized by a temperate continental climate [24], a developed hydrographic network, and fertile alluvial soils [25], which together provide favorable ecological conditions for the growth of Salix species, naturally inhabiting riparian zones along watercourses [26]. Moreover, the preservation of natural habitats in the Pečenjevce area, with minimal anthropogenic influence, promotes rich biodiversity among flora and fauna. The plant identification was conducted through morphological examination, involving comparison of observed traits with species descriptions and reference herbarium specimens. A voucher specimen was identified and deposited in the Herbarium BUNS, University of Novi Sad (no. 2-1482). After air-drying, the plant material was stored at room temperature in a dark and dry place inside double paper bags until analysis. Before extraction, the dried plant material was pulverized using an electric mill (Bosch, Gerlingen, Germany) and then sieved through a 0.35 mesh (d = 0.35 mm) produced by Retsch GmbH & Co. KG (Haan, Germany).
2.2. Chemicals
The chemicals used in the study were the following: methanol (HPLC grade), acetonitrile (HPLC grade), and acetic acid (HPLC grade), obtained from J. T. Baker (Deventer, The Netherlands); glycerol, lactic acid, and urea, obtained from Avena Lab (Vršac, Serbia); 70% ethanol, obtained from Zorka Pharma (Šabac, Serbia); D-glucose monohydrate p.a., obtained from Lach-Ner (Neratovice, Czech Republic); gallic acid (>97%), chlorogenic acid (>95%), caffeic acid (>98%), para-hydroxybenzoic acid (>99%), vanillic acid (>97%), sinapic acid (>98%), syringic acid (>95%), trans-cinnamic acid (>99%), rutin (>94%), naringenin (>98%), and epicatechin (>98%), obtained from Sigma—Aldrich (St. Louis, MO, USA); quercetin (>99%), obtained from Extrasynthese (Genay Cedex, France); p-coumaric acid (>95%), obtained from Fluka (Buchs, Switzerland); deionized water.
2.3. NADES Preparation
One of the most commonly used methods for the synthesis of NADES is the heating and stirring method [27], due to its cost-effectiveness and the ability to control the temperature during preparation, which helps to maintain the stability of heat-sensitive NADES components [17,20]. Water was included in the composition of NADES to reduce the temperature during synthesis, save time, decrease the viscosity of NADES (ahigher viscosity indicates a lower extraction efficiency), and adjust the solubilizing capacity [20]. It has been shown that the optimal water content is 20% (v/v) [28]. An analytical balance (ACJ 100-4; KERN & SOHN GmbH, Balingen, Germany) was used to weigh the NADES components. The synthesis was conducted using a magnetic stirrer (Velp Scientifica, Usmate Velate, Italy) with continuous stirring (1100 rpm) and heating (50–80 °C) until a homogeneous and transparent liquid was formed. Table 1 provides data on the synthesized NADES.

Table 1.
Various NADES combinations synthesized using the heating and stirring method.
2.4. Extract Preparation
An analytical balance was used to weigh 0.25 g of plant material (bark/leaves of S. amplexicaulis), which was poured with 5 mL of the appropriate solvent or solvent mixture. Table 2 presents a detailed overview of the sample preparation process, along with the selected extraction method for each sample.

Table 2.
Sample preparation for the selected extraction method.
For samples S1–S5 and S7–S11, the selected extraction method was MAE, performed using a microwave oven (Bosch BFL523MS0/06; Munich, Germany). The samples were exposed to radiation at a power of 180 W, for three cycles of 8 s (total extraction time: 24 s), to prevent overheating of the samples. Elevated temperatures can cause sample overheating, resulting in degradation of active compounds. Therefore, pauses were implemented between cycles, during which the samples were taken out of the oven and allowed to cool to room temperature [29]. After extraction, all extracts were centrifuged for 15 min at 3500 rpm in a Sigma 2–7 centrifuge, SIGMA Laborzentrifugen GmbH (Osterode am Harz, Germany). The supernatants were collected and filtered. The filtrates were preserved in a dark environment prior to High-Performance Liquid Chromatography (HPLC) analysis.
Samples S6 and S12 were macerated for 48 h at room temperature, using 70% (v/v) ethanol (EtOH).
2.5. HPLC Analysis
The chemical characterization of S. amplexicaulis extracts was conducted using a modified HPLC method on an Agilent HP 1100 HPLC-diode array detection (HPLC-DAD) system with an autosampler injector (Santa Clara, CA, USA) [30]. A Zorbax CB-C18 column (4.6 mm × 150 mm, i.d., 5 µm particle size) was used; a mobile phase of 0.1% aqueous acetic acid (A) and 0.1% acetic acid in acetonitrile (B) was delivered in gradient mode with a flow rate of 1 mL/min. UV detection was set at 280 nm, with an injection volume of 10 μL [9]. NADES extracts are compatible with separation techniques, such as HPLC [31]. For HPLC analysis, NADES extracts were diluted 10-fold with methanol and filtered through a membrane filter directly into a vial, and 10 µL of each sample was injected into the HPLC system. Aqueous and ethanolic extracts were filtered through a membrane filter directly into a vial, and 10 µL of sample was injected into the HPLC system. Before injecting the extracts, the method was validated using standards for the target compounds, and calibration curves were generated for each standard. The identification of active principles was carried out by comparing their retention times and UV spectra with those of the corresponding standards. Each identified bioactive compound in the bark and leaf extracts was quantified using the calibration curves of standard substances and expressed as mg/g of dry plant material (mg/g DM).
2.6. Principal Component Analysis (PCA)
PCA was performed using the Statistica v.12 software package (Stat Soft Inc., Tulsa, OK, USA). Different solvents used for extraction represented the rows (cases), while the quantity of bioactive compounds represented the columns (variables). Before calculations were carried out, all data sets were standardized.
2.7. Statistical Analysis
The results are presented as the means of triplicate measurements ± standard deviations. Statistical data analysis was performed using IBM SPSS software, version 22. The comparison of means of the measured parameters was performed using a one-way ANOVA with Tukey’s post hoc test, at a significance level of p < 0.05.
3. Results and Discussion
As environmental awareness continues to grow, the efficient and environmentally responsible use of natural resources has become increasingly important [32]. In this study, the potential of NADES in combination with MAE for isolating phenolic compounds from the bark and leaves of S. amplexicaulis was evaluated. Table 3 presents data obtained from the chemical characterization of the bark and leaf extracts of S. amplexicaulis.

Table 3.
Chemical characterization of the bark and leaf extracts of S. amplexicaulis (mg/g dry plant material).
HPLC examination revealed the presence of 11 bioactive compounds in the S. amplexicaulis bark extracts, including gallic acid, chlorogenic acid, vanillic acid, syringic acid, p-coumaric acid, sinapic acid, and trans-cinnamic acid, along with the flavonoids epicatechin, rutin, quercetin, and naringenin. Using the same identification technique, the presence of these active principles was also confirmed in the S. amplexicaulis leaf extracts, with the exception of vanillic and syringic acid. Figure 1 shows a representative chromatogram of the bark extract.
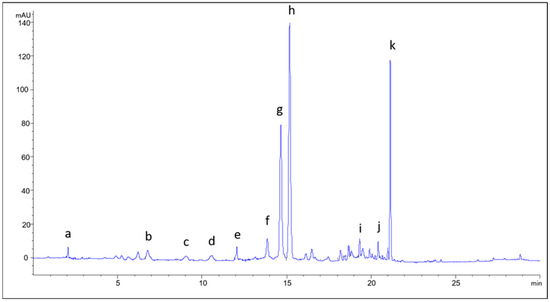
Figure 1.
Chromatogram of the bark extract of S. amplexicaulis obtained using NADES 2: a—gallic acid; b—chlorogenic acid; c—vanillic acid; d—syringic acid; e—epicatechin; f—p-coumaric acid; g—sinapic acid; h—rutin; i—quercetin; j—trans-cinnamic acid; k—naringenin.
The most abundant bioactive compounds in the observed extracts were rutin, sinapic acid, and naringenin. Previous research confirms that rutin and naringenin are among the most abundant flavonoids in various species of the genus Salix [33,34,35]. The therapeutic use of extracts from Salix species is primarily attributed to the presence of salicin. However, the identified phenolic acids and flavonoids in S. amplexicaulis extracts contribute to beneficial effects on human health [36]. These components exhibit anti-inflammatory, antipyretic, antibacterial, and antitumor activity [37]. They also show antioxidant activity through the scavenging of reactive oxygen species, protecting biological systems from oxidative damage, which is associated with the development of cancer, atherosclerosis, diabetes, accelerated aging, and Alzheimer’s disease [36].
The efficiency of phenolic compound extraction depended on the extraction method, the applied solvent, and the examined plant material (bark/leaves). The key differences in temperature, extraction time, and impact on thermolabile compounds caused differences in extraction efficiency between MAE and maceration [38]. When it comes to extraction solutions, the components of NADES affect their physical characteristics, leading to differences in extraction performance [39]. Additionally, differences in viscosity, solubilization capacity [20], and polarity toward targeted active compounds (selectivity), as well as the ability to stabilize active principles [39], result in varying separation capacities between NADES, water, and 70% ethanol. When discussing plant material, the degree of grinding, storage time, environmental conditions [40], and structure are factors that affect the yield.
The gallic acid content ranged from 0.26 to 2.39 mg/g DM and 0.24 to 2.38 mg/g DM in bark and leaf extracts, respectively. The highest concentration of gallic acid was found in bark samples S1 and S2, while leaf samples S7 and S8 contained slightly lower amounts of this compound. The results indicate that NADES systems composed of lactic acid (NADES 1 and 2) are effective solvents for extracting gallic acid from both plant organs, with comparable extraction yields. Also, these NADES were tenfold more effective than water for extracting gallic acid. The enhanced extraction of gallic acid in NADES 1 and 2 can be attributed to its improved solubility in these media. In contrast, NADES 3, NADES 4, and 70% EtOH were not suitable solvents for the extraction of this phenolic acid from either the bark or the leaves. The obtained results highlight the crucial role of the solvent’s polar nature in the efficient extraction of phenolics and are in agreement with other studies [41].
The concentration of chlorogenic acid in bark extracts ranged from 0.38 to 2.30 mg/g DM, while in leaf extracts the range was 0.08 to 1.9 mg/g DM. The bark extract obtained with MAE-NADES 4 (S4) represented the sample with the highest amount of extracted chlorogenic acid. MAE of leaves (S11) resulted in the highest concentration of this acid in the aqueous extract. In the case of bark, NADES showed superior performance compared with the conventional solvents, enabling the extraction of 2-fold and 2.5-to-6-fold higher amounts of chlorogenic acid than ethanol and water, respectively. In contrast, the chlorogenic acid concentration in NADES leaf extracts was lower than in the aqueous extract, but was approximately 15 times higher than in the extract obtained by maceration with 70% ethanol. The chlorogenic acid content in all NADES bark and leaf extracts, as well as the aqueous leaf extract of the tested species, was higher compared to ultrasonic bark extracts of the following species: S. alba, S. triandra, S. fragilis, S. myrsinifolia, and S. petandra [42].
Vanillic and syringic acids were detected in bark extracts only, with amounts ranging from 0.04 to 0.35 mg/g DM and 0.1 to 0.18 mg/g DM, respectively. NADES 2 was the most effective solvent for the extraction of both phenolic acids, enabling eight times higher amounts of vanillic acid compared to water.
The content of p-coumaric acid ranged from 0.05 to 0.51 mg/g DM and from 0.3 to 1.58 mg/g DM in the bark and leaves, respectively. MAE of the leaves using NADES 3 as the extraction solvent enabled the isolation of the greatest amount of this phenolic acid. A higher content of p-coumaric acid in the leaves compared to the bark is associated with the greater exposure of the leaves to UV radiation. The photoisomerization of p-coumaric acid plays a key role in providing a mechanism for protection against UV radiation [43]. The concentration of p-coumaric acid in the NADES (1, 2, and 4) extracts of S. amplexicaulis bark was higher compared to the amount isolated from the ethanol bark extracts of S. alba, S. babylonica, and S. triandra. All four NADES extracts of S. amplexicaulis leaves had a higher content of p-coumaric acid compared to the ethanol extracts of the bark of S. alba, S. babylonica, S. triandra, and S. aegyptiaca [37].
The phenolic acid isolated in the highest amount was sinapic acid. Its content ranged from 3.68 to 9.90 mg/g DM and from 2.50 to 5.91 mg/g DM in bark and leaf extracts, respectively. The combination of NADES 4 with MAE enabled the highest amount of sinapic acid in both the bark extract (S4) and the leaf extract (S10). In the extraction from the leaves, all four NADES showed higher efficiency compared to water as a solvent, as well as ethanol maceration. The efficiency of NADES 4 for the extraction of this phenolic acid is in accordance with a study in which a mixture of glycerol–urea (1:1) was highlighted as one of the most suitable solvents for the extraction of phenolic compounds from the plant Agrimonia eupatoria L. [44].
Trans-cinnamic acid was the least abundant phenolic acid, ranging from 0.006 to 0.08 mg/g DM and from 0.004 to 0.041 mg/g DM in bark and leaf extracts, respectively. NADES 2 was the most effective solvent, while water was the least effective. Using NADES as solvents, trans-cinnamic acid was extracted in higher amounts than by maceration with 70% ethanol.
The content of epicatechin ranged from 0.27 to 1.78 mg/g DM and from 0.12 to 0.33 mg/g DM in the bark and leaves, respectively. This is the only active principle in the bark for which it has been shown that maceration with 70% EtOH is the method that isolates the greatest amount. In contrast, epicatechin was not extracted from the S. amplexicaulis leaves by maceration with 70% EtOH. However, using NADES 1, the highest content of this flavonoid was obtained from the leaf extract (S7).
Rutin was the active principle isolated in the highest amount from both the bark and the leaves, ranging from 10.96 to 29.55 mg/g DM and from 5.61 to 16.82 mg/g DM, respectively. The use of NADES 3 with MAE from the bark enabled the highest content of rutin in S3. These findings are in agreement with a study by Jurić et al. (2021) [45], who showed that NADES systems containing an alcohol and sugar component enable efficient extraction of flavonoid compounds. The use of NADES 1 with MAE from the leaves enabled the highest content of rutin in S7. The rutin content in the NADES extracts of the bark and leaves was significantly higher compared to the water extracts. The extraction of rutin from leaves using all four types of NADES and from bark using NADES 2, 3, and 4, showed greater efficiency compared to maceration with ethanol. Significantly higher amounts of rutin were found in all the examined extracts of the bark and leaves of S. amplexicaulis compared to the ethanol extracts of the bark of S. alba, S. babylonica, S. triandra, S. purpurea, and S. aegyptiaca [37]. It was also determined that the bioavailability of rutin after oral administration was higher when applied with the NADES system compared to its application as an aqueous suspension [17].
The content of quercetin ranged from 0.31 to 1.56 mg/g DM and from 0.3 to 2.04 mg/g DM in the bark and leaf extracts, respectively. Compared to the bark, a higher amount of quercetin was isolated from the leaves. MAE-NADES 2 enabled the isolation of the highest amount of quercetin from the bark, while it was MAE-NADES 4 that enabled this in the case of the leaves. NADES were effective solvents, extracting 4 to 5 times and 2.5 to 3 times more quercetin from bark than water and ethanol, respectively. NADES proved even more effective for extracting quercetin from leaves, the amount being 4.5 to 7 times and 3.5 to 5 times higher than that in water and ethanol extract, respectively. All examined NADES extracts of the bark and leaves contained higher amounts of quercetin compared to the ethanol extracts of the bark of S. alba, S. babylonica, S. triandra, and S. purpurea [37].
The content of naringenin ranged from 0.35 to 4.63 mg/g DM and from 0.47 to 3.4 mg/g DM in bark and leaf extracts, respectively. The MAE-NADES 2 system proved to be the most effective for isolating the greatest amount of this compound from bark and leaves. The use of MAE with water as the solvent resulted in the extraction of the lowest amounts of naringenin from bark and leaves. Extraction of the bark using NADES as a solvent achieved 8.5 to 13 times higher naringenin contents compared to water and 7.5 to approximately 12 times higher contents compared to maceration with ethanol. When it comes to the leaves, the naringenin content in the NADES extracts was six to seven times higher than in the water extract and four to five times higher than in the ethanol extract. All NADES extracts of the bark and leaves of S. amplexicaulis had significantly higher naringenin contents compared to the ethanol extract of the bark of S. alba, S. babylonica, S. triandra, and S. purpurea [37]. The phenolic profile of many plants is significantly determined by the genotype, environment, growth stage, harvest time, and preservation conditions of the plant material [46]. The applied extraction method, solvent, particle size of the dried plant material, time, and storage conditions are factors that affect the efficiency of extraction [40]. These effects on the phenolic profile and extraction efficiency can explain the variations between S. amplexicaulis and the compared species of the Salix genus.
The efficiency of the applied solvents in extracting phenolic and flavonoid compounds from the bark and leaves of S. amplexicaulis was further analyzed by PCA. Within the initial data matrix, different solvents used for extraction represented the objects of PCA, while the concentration of phenolic acids and flavonoids in the analyzed samples represented the variables. Using PCA, the original data set was decomposed into loading and score vectors, resulting in new variables known as principal components (PCs). The first two PCs (PC1 and PC2) accounted for most of the variation in the data set: 70.62% and 81.19% of the total variance of the initial data matrix, for bark and leaves, respectively.
Figure 2 presents the PCA results for bark extracts.
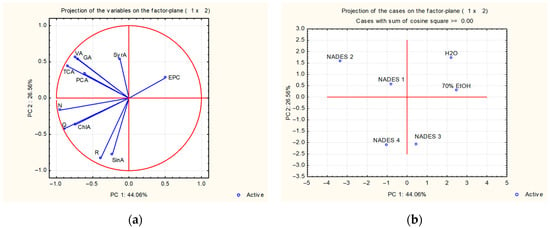
Figure 2.
Principal component analysis of bark extracts. Projection of the examined (a) variables—contents of phenolic acids and flavonoids—and (b) cases—solvents used for extraction in the space defined by PC1 and PC2. GA, gallic acid; ChlA, chlorogenic acid; VA, vanillic acid; PCA, p-coumaric acid; SyrA, syringic acid; SinA, sinapic acid; TCA, trans-cinnamic acid; EPC, epicatechin; R, rutin; Q, quercetin; N, naringenin.
PCA analysis of bark showed that the variability described by PC1 mostly correlated with the levels of naringenin, quercetin, trans-cinnamic, vanillic, chlorogenic, and gallic acid, while PC2 mostly correlated with rutin, sinapic, and syringic acid concentrations (Figure 2a).
PCA divided the solvents used for S. amplexicaulis bark extraction into four clusters. PC1 allowed the separation of solvents that efficiently extracted phenolic compounds (NADES 1, NADES 2, and NADES 4) from those that performed less efficiently (NADES 3, water, and 70% ethanol), while PC2 enabled further separation within each of the two groups. As shown in Figure 2b, NADES 1 and NADES 2 were clustered together in the upper left quadrant of the loading plot, indicating their similar efficiency in extracting certain phenolic acids, such as gallic acid. This can be explained by the fact that NADES consisting of organic acids, such as lactic acid in NADES 1 and 2, favor the extraction of phenolic acids. It is also evident that NADES 2 is well separated from NADES 1 and favors the accumulation of trans-cinnamic, vanillic, p-coumaric, and syringic acid. Extraction using NADES 4 as a separate cluster was associated with high levels of sinapic and chlorogenic acid, as well as the flavonoid compounds quercetin, naringenin, and rutin.
Traditional solvents, water, and 70% ethanol were clustered together in the upper right quadrant of the loading plot, indicating their similar efficiency in extracting phenolics from bark of S. amplexicaulis and are associated with epicatechin accumulation. PCA also indicated that NADES 3, a separate cluster in the lower right quadrant of the loading plot, was poor in terms of observed phenolic compounds.
PCA analysis of leaf extracts of S. amplexicaulis (Figure 3a) showed that the variability described by PC1 mostly correlated with the concentration of the flavonoid compounds naringenin, rutin, and quercetin, as well as sinapic acid, while PC2 mostly correlated with amounts of p-coumaric and chlorogenic acid and epicatechin.
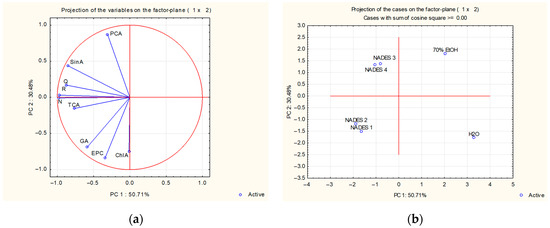
Figure 3.
Principal component analysis of leaf extracts. Projection of the examined (a) variables—content of phenolic acids and flavonoids—and (b) cases—solvents used for extraction in the space defined by PC1 and PC2. GA, gallic acid; ChlA, chlorogenic acid; PCA, p-coumaric acid; SinA, sinapic acid; TCA, trans-cinnamic acid; EPC, epicatechin; R, rutin; Q, quercetin; N, naringenin.
PCA showed that the solvents used for leaf extraction (Figure 3b) were also divided into four clusters, but with slight differences in comparison to bark (Figure 2b). PC1 facilitated the classification of solvents into two groups, according to their efficiency in extracting phenolics from S. amplexicaulis leaves. NADES extraction proved superior in efficiency in comparison to water and 70% ethanol extraction. As shown in Figure 3, the latter two extracts were poor in terms of extracting the determined compounds. PC2 allowed further separation of NADES 1 and NADES 2 from NADES 3 and NADES 4. NADES 1 and NADES 2 favored the accumulation of gallic, chlorogenic, and trans-cinnamic acid, as well as the flavonoid compounds epicatechin and naringenin. The extracts obtained by NADES 3 and NADES 4 were characterized by high levels of p-coumaric and sinapic acid, as well as quercetin and rutin.
The isolation of phenolic acids and flavonoids is determined by the choice of extraction solvent [37]. MAE-NADES enhanced the extraction efficiency of gallic, vanillic, syringic, p-coumaric, sinapic, and trans-cinnamic acid and flavonoids—rutin, quercetin, and naringenin—compared to the aqueous and ethanol extracts. An increasing amount of research highlights the benefits of NADES application in isolating polar and non-polar substances compared to water, ethanol, methanol, ethyl acetate, and acetone [21]. The different components that form NADES determine variations in polarity, viscosity, and the ability to dissolve bioactive compounds, which are directly related to the extraction efficiency. High solubility in NADES is achieved due to dipole–dipole interactions and hydrogen bonding between NADES and bioactive compounds [39]. Among the applied solvents, the greatest number of phenolic compounds in the highest quantity were extracted using NADES 2 from the bark and leaves of S. amplexicaulis, which can be explained by its ability to isolate both polar and non-polar compounds. The obtained results are consistent with a study in which, among eight different NADES composed of constituents such as choline chloride, alcohols, carboxylic acids, and sugars, the mixture of lactic acid–glucose (5:1) was the most effective for extracting phenolic compounds from mango bark [28]. The greater extraction efficiency of the lactic acid–glucose (5:1) mixture compared to ethanol has been confirmed in studies on the isolation of phenolics from grapefruit bark [47], mango bark [28], anthocyanins from Catharanthus roseus (L.) G. Don [48], and phenolic components from the processing residues of onion, olive, tomato, and pear [31].
By reducing the extraction time, achieving a higher extraction yield, minimizing energy consumption and cost, and ensuring purity, as well as easy control, MAE represents an adequate replacement for conventional extraction methods [49]. During MAE, NADES can absorb microwave radiation and convert it into heat, causing damage to the cellular structures of the plant material and facilitating the transfer of analytes into the solvent matrix [50], thereby highlighting the advantages of using this combination.
Besides a high extraction efficiency, a solvent should ensure stability during extraction and sample storage [18]. The application of NADES may increase the stability and extend the preservation time of bioactive compounds in extracts [39]. A study on the extraction of phenolic compounds from agro-food industrial by-products demonstrates that these compounds remained stable over 60 days in the eutectic solvent [31]. Due to specific interactions between phenolic compounds and NADES components, the diffusion of bioactive molecules is reduced, as is their exposure to oxygen, which leads to decreased oxidative degradation [48]. The interactions become stronger with the reduction in water content in NADES, which increases their ability to stabilize active compounds [51]. In addition, their antimicrobial effects on Gram-positive and Gram-negative bacteria, as well as on yeasts, together with their cryoprotective properties, contribute to the prolonged shelf life of the extracts [52].
Properties such as non-flammability, minimal toxicity, and low volatility emphasize the safety of NADES as solvents, making them promising for use in the food industry and in pharmaceutical, nutraceutical, and cosmetic formulations. Additionally, NADES enhance the biological activity of active compounds [39]. NADES extracts have potential for the development of novel products for human applications. These extracts, enriched with salicin, salicylic derivatives, flavonoids, and phenolic acids, exhibit a synergistic effect [2,3,4] that positions them as a potential natural replacement for NSAIDs [6]. However, further research is essential to evaluate their biological effects and toxicological safety [17].
The potential for solvent recycling and the efficient recovery of extracted bioactive compounds represent significant aspects in the design of green and sustainable extraction techniques. NADES are solvents that enable the recovery of bioactive compounds, after which they can be recycled, thereby reducing solvent waste [53]. For example, the study demonstrated that the counter-current separation method effectively enabled the isolation of analytes from the NADES matrix, achieving quantitative recovery values of 95.7% for rutin, 94.6% for quercetin, 97.0% for kaempferol, and 96.7% for daidzein [54]. Another study demonstrated that the use of macroporous resins can enable the recovery of phenolic compounds from an extract of mulberry (Morus alba L.) leaves obtained using a choline chloride–glycerol eutectic mixture and MAE. The observed recovery yields of the seven phenolic compounds ranged from 77.81% to 83.77%. Later, the recovered eutectic mixture was suitable for efficient reuse three times [55]. Additionally, dilution of the NADES extract containing anthocyanins enabled a 99.46% recovery of these compounds using macroporous resin, while also allowing highly efficient solvent recycling. The addition of water enables the breaking of bonds between NADES and the active compound [56]. Moreover, the biodegradability of NADES represents an important characteristic for its ecotoxicological impact. The presence of water facilitates the biodegradation of NADES [17].
Furthermore, the valorization of S. amplexicaulis leaves, which are typically regarded as industrial waste, supports circular economy principles by converting plant by-products into valuable raw materials.
4. Conclusions
A key step in the analytical process is extraction, usually in the presence of organic solvents, which represent a serious threat to the environment and human health. In this study, guided by the principles of green chemistry, NADES solvents were synthesized and applied, along with MAE, an innovative extraction method, for isolating active principles from the bark and leaves of the underexplored S. amplexicaulis. The MAE–NADES system demonstrated clear advantages over conventional maceration for isolating phenolics from S. amplexicaulis, including faster and more cost-effective preparation, higher efficiency, and greater environmental acceptability. PCA further supported the superior efficiency of NADES to extract phenolic acids and flavonoids from the bark and leaves of S. amplexicaulis in comparison to water and 70% ethanol. NADES consisting of lactic acid–glucose (5:1) showed improved extraction capacity for most phenolics characterized in the bark and leaves of S. amplexicaulis. Overall, NADES systems have shown significant potential to replace water and toxic organic solvents. Furthermore, the results suggest that S. amplexicaulis leaves may serve as a significant source for the extraction of active principles, thereby reducing waste in industry.
This combination presents a sustainable approach and represents a significant advancement in the field of natural product research. It offers potential for application across different plant species, enabling the selective extraction of specific phenolic compounds. In addition, it opens the possibility of the application of these extracts in the field of pharmacy. However, further investigations are required to gain a better understanding of the complex nature of NADES systems and their application in the extraction of bioactive components.
Author Contributions
Conceptualization, E.G.; methodology, N.G.-L. and E.G.; software, E.G.; validation, N.G.-L. and B.T.; formal analysis, M.V. and E.G.; investigation, M.V. and E.G.; resources, N.G.-L.; data curation, M.V. and E.G.; writing—original draft preparation, M.V. and E.G.; writing—review and editing, N.G.-L. and B.T.; visualization, E.G.; supervision, N.G.-L.; project administration, N.G.-L., B.T., and E.G.; funding acquisition, N.G.-L., B.T., and E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hungarian Tempus Public Foundation and the Ministry of Culture and Innovation (Grant No. HTP4KNVYZ2025/EG-00105-2025) and the Provincial Secretariat for Higher Education and Scientific Research, Province of Vojvodina (Grant No. 003077388 2024 09418 003 000 000 001/1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| MAE | Microwave-assisted extraction |
| NADES | Natural deep eutectic solvents |
| EtOH | Ethanol |
| DM | Dry plant material |
| PCA | Principal component analysis |
References
- Maistro, E.L.; Terrazzas, P.M.; Sawaya, A.C.H.F.; Rosa, P.C.P.; Perazzo, F.F.; de Mascarenhas Gaivão, I.O. In Vivo Toxicogenic Potential of Salix alba (Salicaceae) Bark Extract. J. Toxicol. Environ. Health A 2022, 85, 121–130. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Schmidt, M.; Jäggi, R.; Metz, J.; Khayyal, M.T. Willow Bark Extract: The Contribution of Polyphenols to the Overall Effect. Wien Med. Wochenschr. 2007, 157, 348–351. [Google Scholar] [CrossRef]
- Schmid, B.; Kötter, I.; Heide, L. Pharmacokinetics of Salicin after Oral Administration of a Standardised Willow Bark Extract. Eur. J. Clin. Pharmacol. 2001, 57, 387–391. [Google Scholar] [CrossRef]
- Tawfeek, N.; Mahmoud, M.F.; Hamdan, D.I.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, Pharmacology and Medicinal Uses of Plants of the Genus Salix: An Updated Review. Front. Pharmacol. 2021, 12, 593856. [Google Scholar] [CrossRef]
- Maroon, A.; Bost, J.; Maroon, J. Natural Anti-Inflammatory Agents for Pain Relief. Surg. Neurol. Int. 2010, 1, 80. [Google Scholar] [CrossRef]
- Baker, P.; Charlton, A.; Johnston, C.; Leahy, J.J.; Lindegaard, K.; Pisano, I.; Prendergast, J.; Preskett, D.; Skinner, C. A Review of Willow (Salix spp.) as an Integrated Biorefinery Feedstock. Ind. Crops Prod. 2022, 189, 115823. [Google Scholar] [CrossRef]
- Aleman, R.S.; Marcia, J.; Duque-Soto, C.; Lozano-Sánchez, J.; Montero-Fernández, I.; Ruano, J.A.; Hoskin, R.T.; Moncada, M. Effect of Microwave and Ultrasound-Assisted Extraction on the Phytochemical and In Vitro Biological Properties of Willow (Salix alba) Bark Aqueous and Ethanolic Extracts. Plants 2023, 12, 2533. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Salix [Various Species Including S. purpurea L., S. daphnoides Vill., S. fragilis L.], Cortex; EMA: London, UK, 2017. [Google Scholar]
- Gligorić, E.; Igić, R.; Srđenović Čonić, B.; Kladar, N.; Teofilović, B.; Grujić, N. Chemical Profiling and Biological Activities of “Green” Extracts of Willow Species (Salix L., Salicaceae): Experimental and Chemometric Approaches. Sustain. Chem. Pharm. 2023, 32, 100981. [Google Scholar] [CrossRef]
- Gligorić, E.; Igić, R.; Teofilović, B.; Grujić-Letić, N. Phytochemical Screening of Ultrasonic Extracts of Salix Species and Molecular Docking Study of Salix-Derived Bioactive Compounds Targeting Pro-Inflammatory Cytokines. Int. J. Mol. Sci. 2023, 24, 11848. [Google Scholar] [CrossRef]
- Kailis, N.; Eleftheriadou, E. Contribution to the description and distribution of Salix × velchevii (Salicaceae). Phytol. Balcan. 2011, 17, 279–282. [Google Scholar]
- Cronk, Q.; Ruzzier, E.; Belyaeva, I.; Percy, D. Salix Transect of Europe: Latitudinal Patterns in Willow Diversity from Greece to Arctic Norway. Biodivers. Data J. 2015, 3, e6258. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Mar. Drugs 2022, 20, 677. [Google Scholar] [CrossRef]
- Verdum, M.; Jové, P. Novel Sustainable Alternatives for the Study of the Chemical Composition of Cork. Sustainability 2024, 16, 575. [Google Scholar] [CrossRef]
- Li, J. Evaluation of Fatty Tissue Representative Solvents in Extraction of Medical Devices for Chromatographic Analysis of Devices’ Extractables and Leachables Based on Abraham General Solvation Model. J. Chromatogr. A 2022, 1676, 463240. [Google Scholar] [CrossRef]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The Perspectives of Natural Deep Eutectic Solvents in Agri-Food Sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Fu, R.; Zhang, L.; Wang, D.; Wang, S. Enhanced Extraction of Natural Pigments from Curcuma longa L. Using Natural Deep Eutectic Solvents. Ind. Crops Prod. 2019, 140, 111620. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- de los Ángeles Fernández, M.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural Deep Eutectic Solvents-Mediated Extractions: The Way Forward for Sustainable Analytical Developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Liu, Y.; Wu, K.; Zhu, Y.; Lu, H.; Liang, B. Insights into the Relationships between Physicochemical Properties, Solvent Performance, and Applications of Deep Eutectic Solvents. Environ. Sci. Pollut. Res. 2021, 28, 35537–35563. [Google Scholar] [CrossRef]
- Alsaidi, R.; Thiemann, T. Use of Natural Deep Eutectic Solvents (NADES) in Food Science and Food Processing. Sustainability 2025, 17, 2293. [Google Scholar] [CrossRef]
- Milovanovic, B.; Ducic, V.; Radovanovic, M.; Milivojevic, M. Climate Regionalization of Serbia According to Köppen Climate Classification. J. Geogr. Inst. Jovan Cvijic SASA 2017, 67, 103–114. [Google Scholar] [CrossRef]
- Horejs, B.; Bulatović, A.; Meyer, C.; Milić, B.; Schneider, S.; Schlöffel, M.; Stevanović, V. Prehistoric Landscapes of the Pusta Reka Region (Leskovac). New Investigations along the Southern Morava River. J. Serb. Archaeol. Soc. 2018, 34, 23–51. [Google Scholar]
- Kuzovkina, Y.A.; Quigley, M.F. Willows Beyond Wetlands: Uses of Salix L. Species for Environmental Projects. Water Air Soil Pollut. 2005, 162, 183–204. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Lanjekar, K.J.; Gokhale, S.; Rathod, V.K. Utilization of Waste Mango Peels for Extraction of Polyphenolic Antioxidants by Ultrasound-Assisted Natural Deep Eutectic Solvent. Bioresour. Technol. Rep. 2022, 18, 101074. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel Extraction of Polyphenols from Sour Cherry Pomace Using Natural Deep Eutectic Solvents–Ultrafast Microwave-Assisted NADES Preparation and Extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef]
- Miljić, U.; Puškaš, V.; Cvejić Hogervorst, J.; Torović, L. Phenolic Compounds, Chromatic Characteristics and Antiradical Activity of Plum Wines. Int. J. Food Prop. 2017, 20, 2022–2033. [Google Scholar] [CrossRef][Green Version]
- de los Ángeles Fernández, M.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel Approaches Mediated by Tailor-Made Green Solvents for the Extraction of Phenolic Compounds from Agro-Food Industrial by-Products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Enayat, S.; Banerjee, S. Comparative antioxidant activity of extracts from leaves, bark and catkins of Salix aegyptiaca sp. Food Chem. 2009, 116, 23–28. [Google Scholar] [CrossRef]
- Gligorić, E.; Igić, R.; Suvajdžić, L.; Grujić-Letić, N. Species of the Genus Salix L.: Biochemical Screening and Molecular Docking Approach to Potential Acetylcholinesterase Inhibitors. Appl. Sci. 2019, 9, 1842. [Google Scholar] [CrossRef]
- Gligorić, E.; Igić, R.; Suvajdžić, L.; Teofilović, B.; Turk-Sekulić, M.; Grujić-Letić, N. Methodological Aspects of Extraction, Phytochemical Characterization and Molecular Docking Studies of Salix caprea L. Bark and Leaves. Acta. Chim. Slov. 2019, 66, 821–830. [Google Scholar] [CrossRef]
- Piątczak, E.; Dybowska, M.; Płuciennik, E.; Kośla, K.; Kolniak-Ostek, J.; Kalinowska-Lis, U. Identification and Accumulation of Phenolic Compounds in the Leaves and Bark of Salix alba (L.) and Their Biological Potential. Biomolecules 2020, 10, 1391. [Google Scholar] [CrossRef]
- Saracila, M.; Panaite, T.D.; Papuc, C.P.; Criste, R.D. Heat Stress in Broiler Chickens and the Effect of Dietary Polyphenols, with Special Reference to Willow (Salix spp.) Bark Supplements—A Review. Antioxidants 2021, 10, 686. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- Boeing, J.S.; Barizão, É.O.; e Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of Solvent Effect on the Extraction of Phenolic Compounds and Antioxidant Capacities from the Berries: Application of Principal Component Analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef]
- Nam, Y.H.; Ahn, S.M.; Seo, G.J.; Kim, N.W.; Shin, S.W.; Nuankaew, W.; Murughanantham, N.; Pandian, S.; Hwang, J.S.; Hong, B.N.; et al. Optimization of NADES-Based Green Extraction of Ellagitannins from Rambutan Peel with Enhanced Antioxidant Activity. Food Chem. 2025, 475, 143308. [Google Scholar] [CrossRef]
- Köhler, A.; Förster, N.; Zander, M.; Ulrichs, C. Inter- and Intraspecific Diversity of Salix Bark Phenolic Profiles—A Resource for the Pharmaceutical Industry. Fitoterapia 2023, 170, 105660. [Google Scholar] [CrossRef]
- González Moreno, A.; de Cózar, A.; Prieto, P.; Domínguez, E.; Heredia, A. Radiationless Mechanism of UV Deactivation by Cuticle Phenolics in Plants. Nat. Commun. 2022, 13, 1786. [Google Scholar] [CrossRef]
- Lazović, M.; Cvijetić, I.; Jankov, M.; Milojković-Opsenica, D.; Trifković, J.; Ristivojević, P. Efficiency of Natural Deep Eutectic Solvents to Extract Phenolic Compounds from Agrimonia eupatoria: Experimental Study and In Silico Modelling. Plants 2022, 11, 2346. [Google Scholar] [CrossRef]
- Jurić, T.; Mićić, N.; Potkonjak, A.; Milanov, D.; Dodić, J.; Trivunović, Z.; Popović, B.M. The Evaluation of Phenolic Content, in Vitro Antioxidant and Antibacterial Activity of Mentha Piperita Extracts Obtained by Natural Deep Eutectic Solvents. Food Chem. 2021, 362, 130226. [Google Scholar] [CrossRef]
- Ben Ahmed, Z.; Yousfi, M.; Viaene, J.; Dejaegher, B.; Demeyer, K.; Mangelings, D.; Vander Heyden, Y. Seasonal, Gender and Regional Variations in Total Phenolic, Flavonoid, and Condensed Tannins Contents and in Antioxidant Properties from Pistacia atlantica ssp. Leaves. Pharm. Biol. 2017, 55, 1185–1194. [Google Scholar] [CrossRef]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green Extraction of Polyphenols from Grapefruit Peels Using High Voltage Electrical Discharges, Deep Eutectic Solvents and Aqueous Glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of Natural Deep Eutectic Solvents to the Extraction of Anthocyanins from Catharanthus roseus with High Extractability and Stability Replacing Conventional Organic Solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. Microwave-Assisted Extraction of Pectin from Grape Pomace. Sci. Rep. 2022, 12, 12722. [Google Scholar] [CrossRef]
- Ivanović, M.; Islamčević Razboršek, M.; Kolar, M. Innovative Extraction Techniques Using Deep Eutectic Solvents and Analytical Methods for the Isolation and Characterization of Natural Bioactive Compounds from Plant Material. Plants 2020, 9, 1428. [Google Scholar] [CrossRef]
- Cao, J.; Cao, J.; Wang, H.; Chen, L.; Cao, F.; Su, E. Solubility Improvement of Phytochemicals Using (Natural) Deep Eutectic Solvents and Their Bioactivity Evaluation. J. Mol. Liq. 2020, 318, 113997. [Google Scholar] [CrossRef]
- Mišan, A.; Pojić, M. Applications of NADES in Stabilizing Food and Protecting Food Compounds against Oxidation. Adv. Bot. Res. 2021, 97, 333–359. [Google Scholar] [CrossRef]
- González-Laredo, R.F.; Sayago-Monreal, V.I.; Moreno-Jiménez, M.R.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Landeros-Macías, L.F.; Rosales-Castro, M. Natural Deep Eutectic Solvents (NaDES) as an Emerging Technology for the Valorisation of Natural Products and Agro-food Residues: A Review. Int. J. Food Sci. Technol. 2023, 58, 6660–6673. [Google Scholar] [CrossRef]
- Liu, Y.; Garzon, J.; Friesen, J.B.; Zhang, Y.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Countercurrent Assisted Quantitative Recovery of Metabolites from Plant-Associated Natural Deep Eutectic Solvents. Fitoterapia 2016, 112, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.-Z.; Cui, Q.; Wang, L.-T.; Meng, Y.; Yu, L.; Li, Y.-Y.; Fu, Y.-J. A Green and Integrated Strategy for Enhanced Phenolic Compounds Extraction from Mulberry (Morus alba L.) Leaves by Deep Eutectic Solvent. Microchem. J. 2020, 154, 104598. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Radojčić Redovniković, I. Enabling Technologies for the Extraction of Grape-Pomace Anthocyanins Using Natural Deep Eutectic Solvents in up-to-Half-Litre Batches Extraction of Grape-Pomace Anthocyanins Using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).