Effects of Vegetation Heterogeneity on Butterfly Diversity in Urban Parks: Applying the Patch–Matrix Framework at Fine Scales
Abstract
1. Introduction
2. Materials and Methods
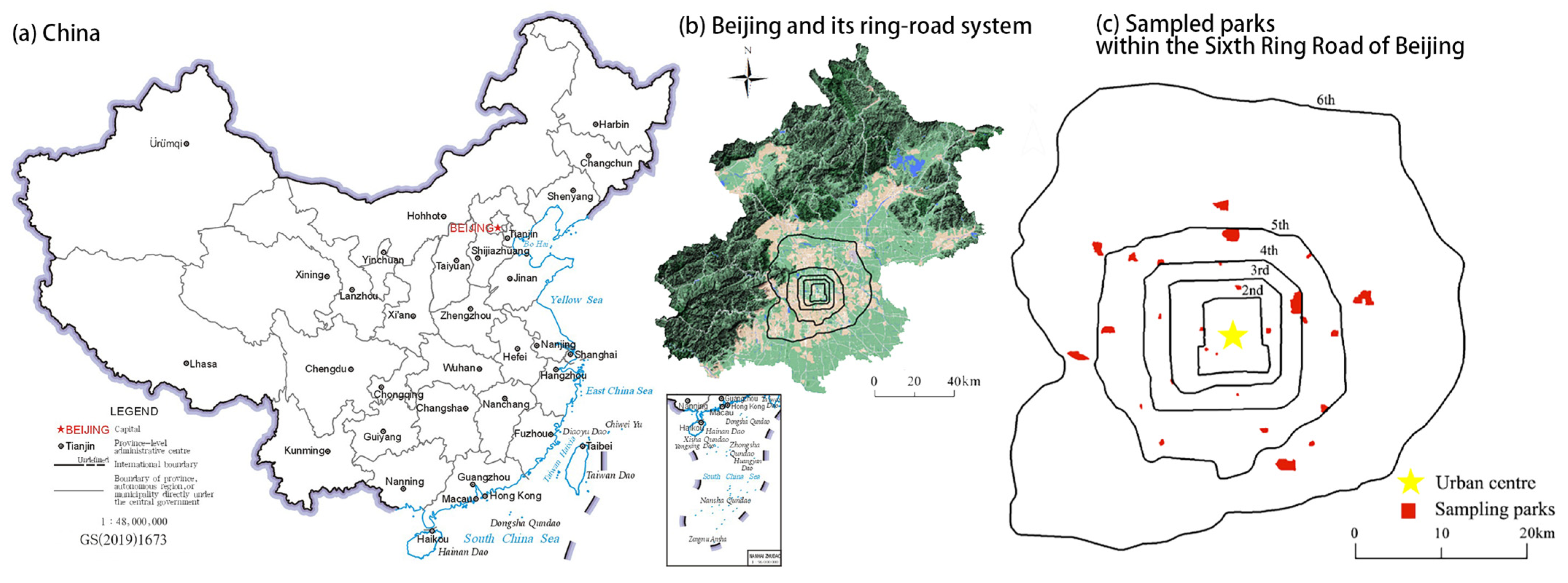
2.1. Study Area and Study Sites
2.2. Butterfly Surveys
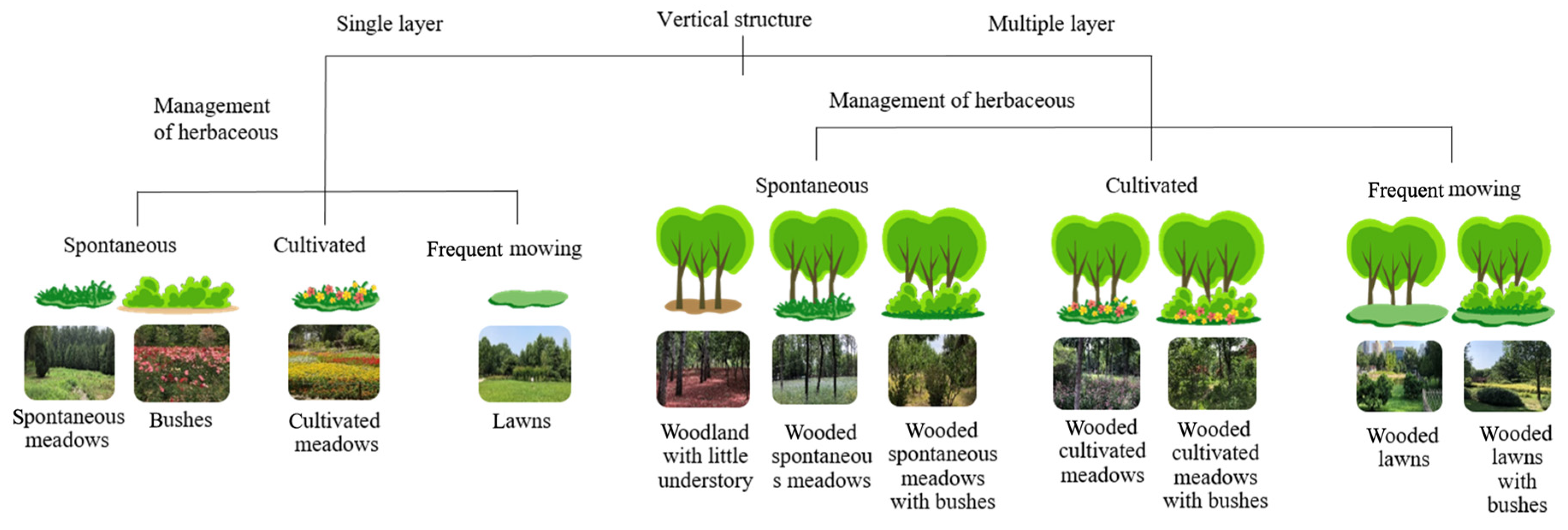
2.3. Mapping of Vegetation Units and Spatial Metrics of Green Spaces
2.4. Data Analysis
2.4.1. Diversity and Abundance Indices
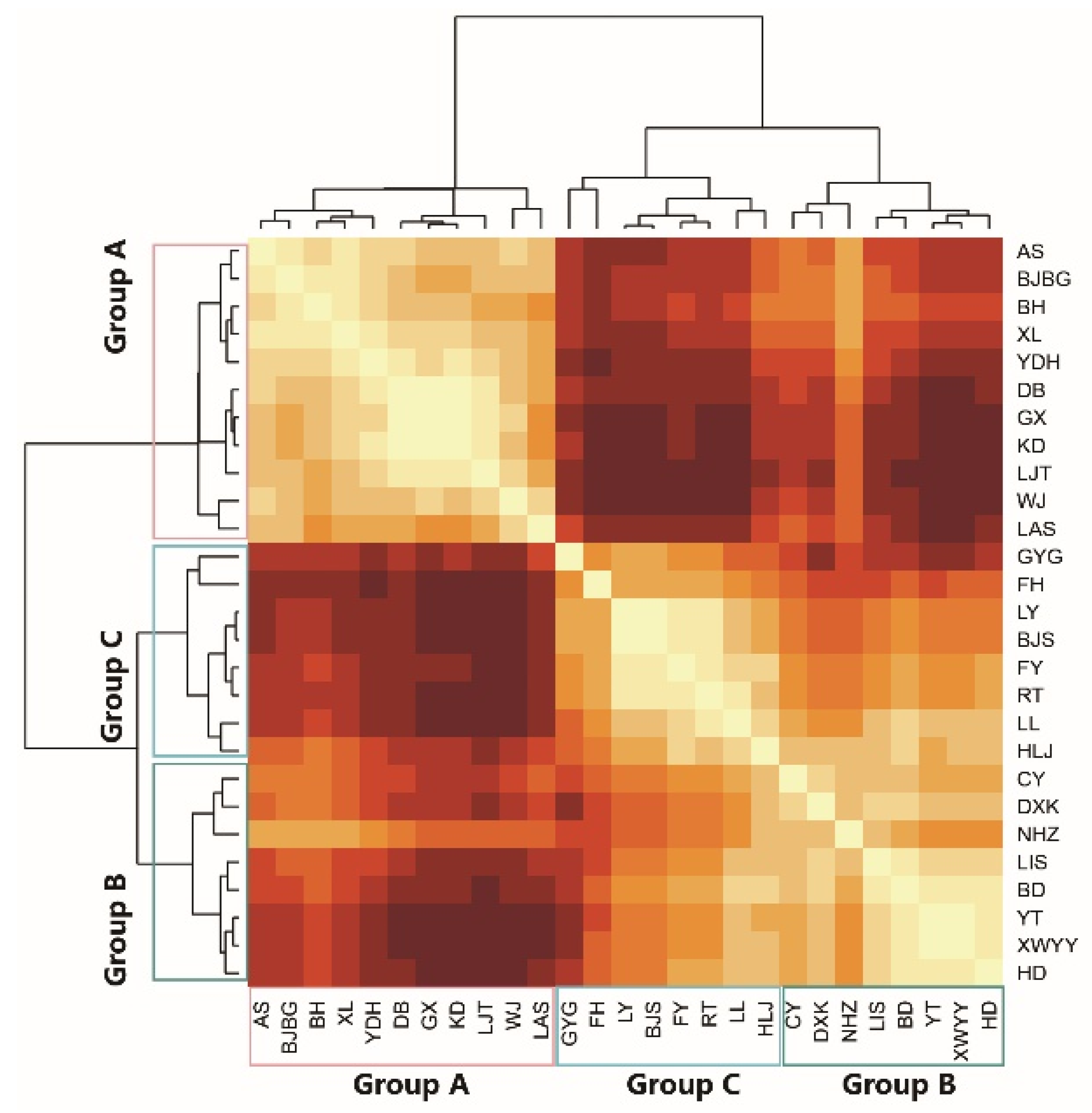
2.4.2. Clustering Analysis
2.4.3. Analysis of Variance (ANOVA)
2.4.4. Generalized Linear Models (GLMs)
2.4.5. Generalized Additive Models (GAMs)
2.4.6. Random Forest Models (RF)
3. Results
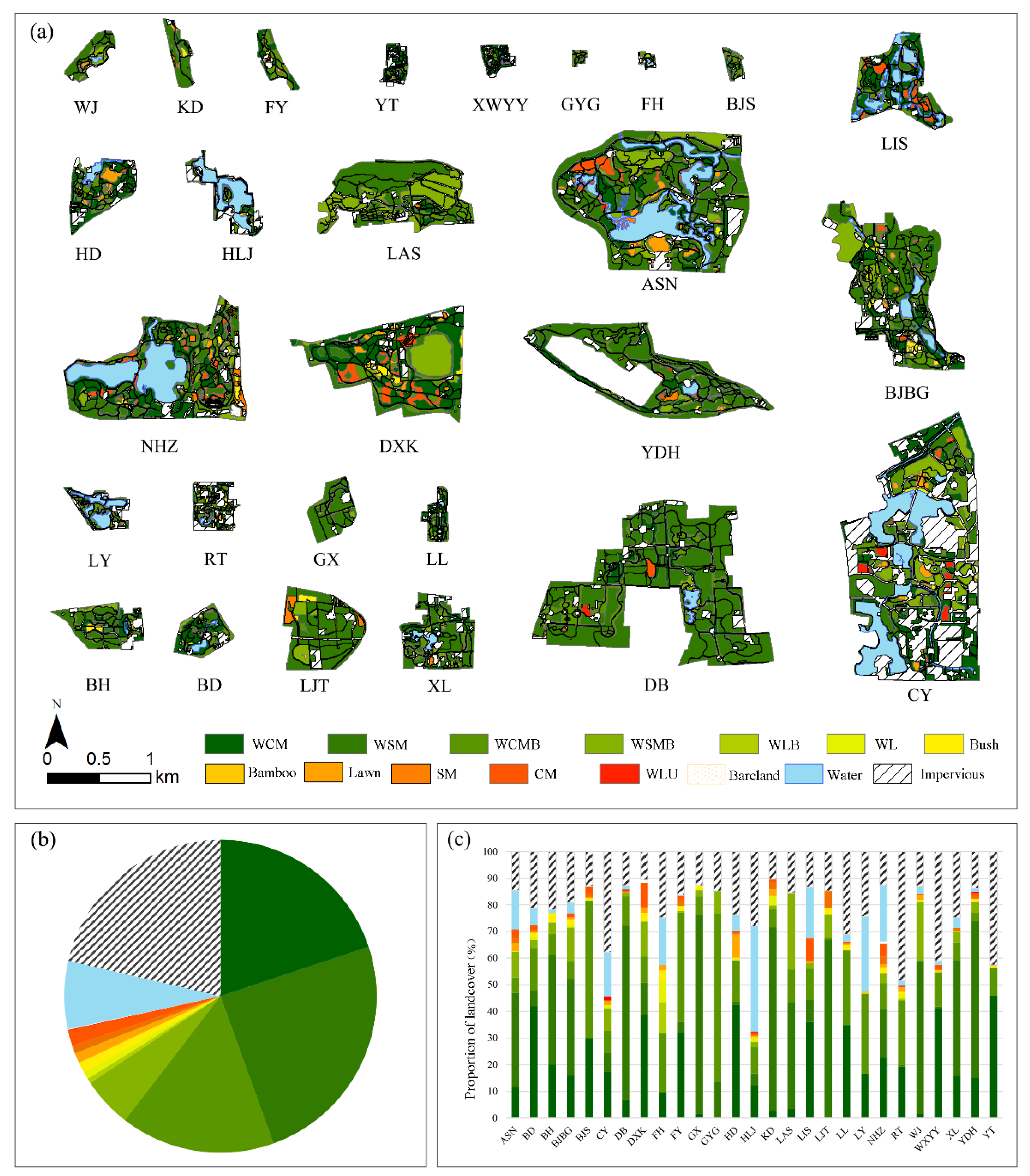
3.1. Overall Characteristics
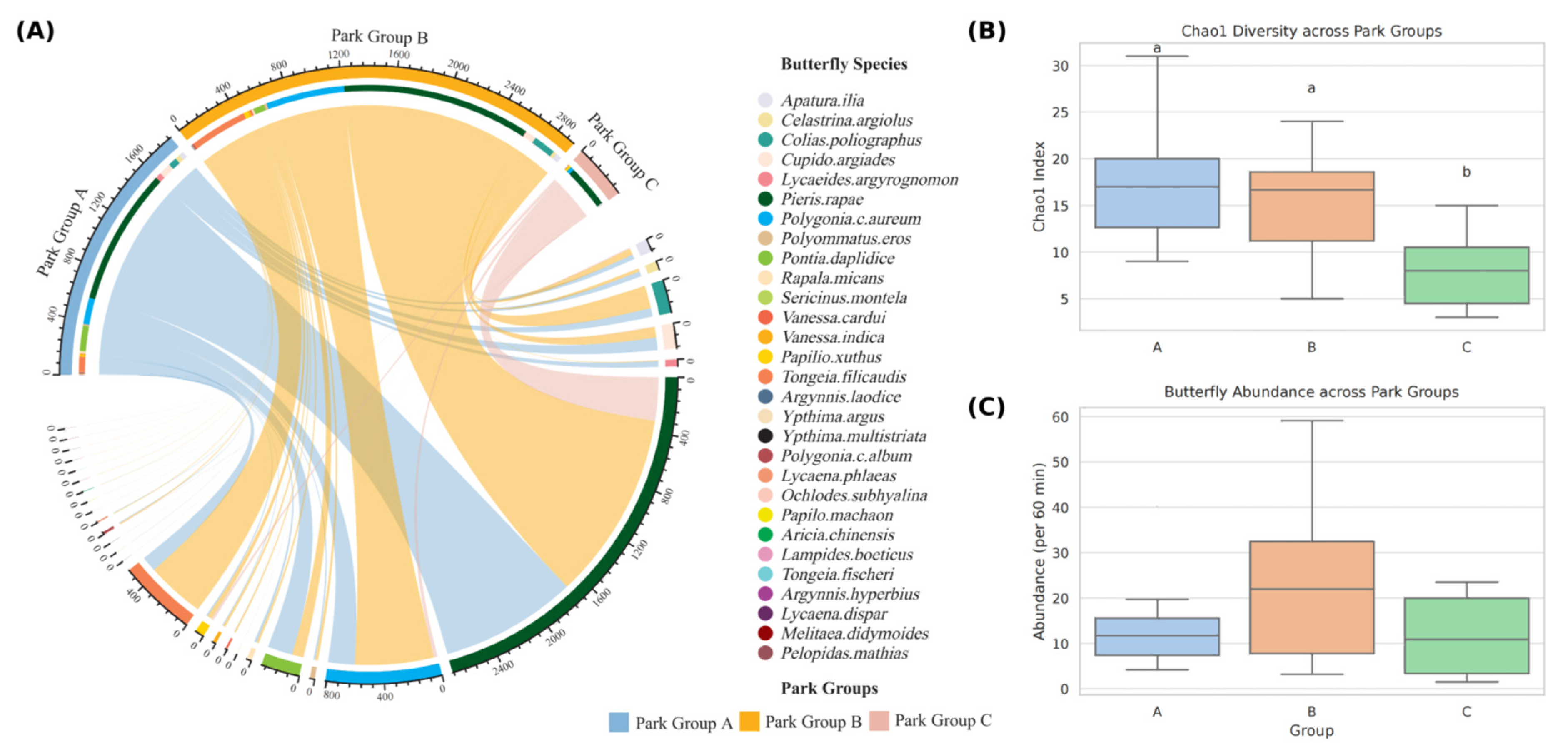
3.1.1. Overall Butterfly Community Composition
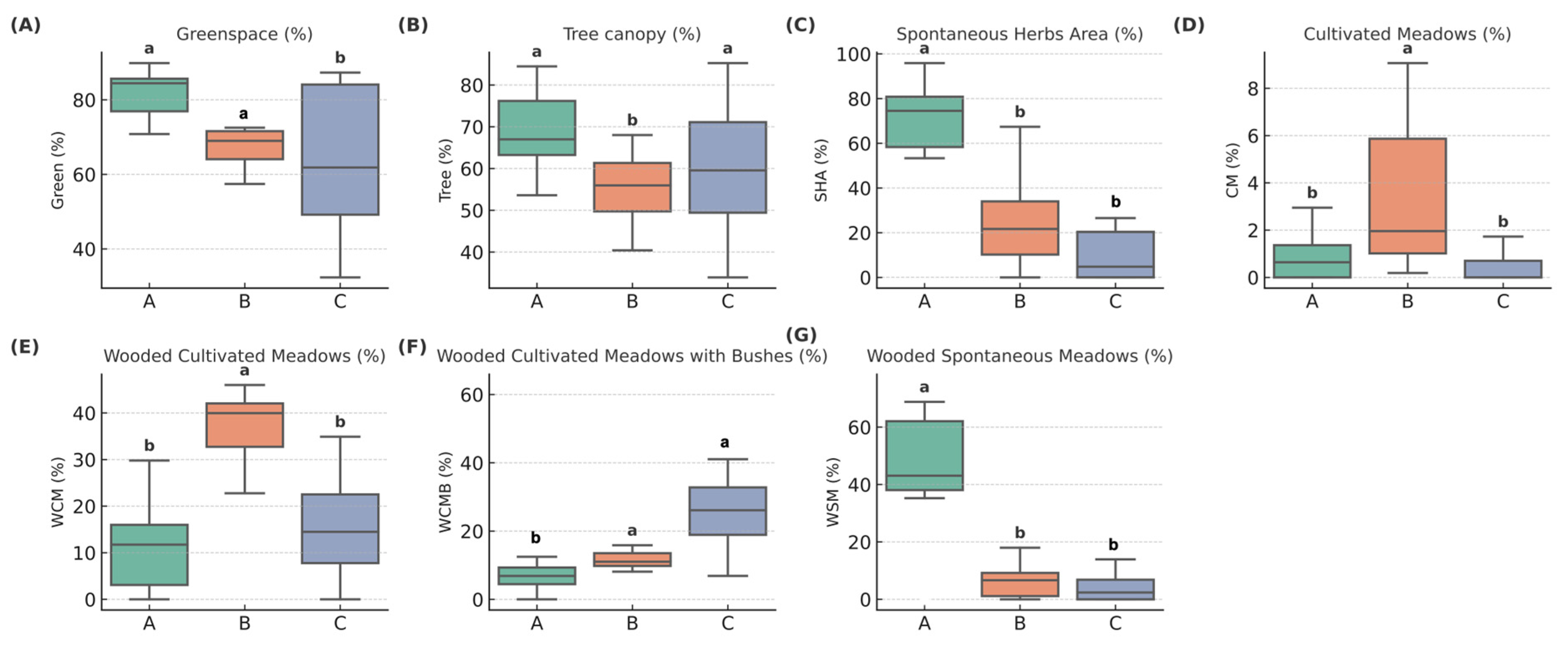
3.1.2. Intra-Park Land Cover and Vegetation Unit Composition
3.2. Comparison of Park Group Characteristics and Butterfly Diversity
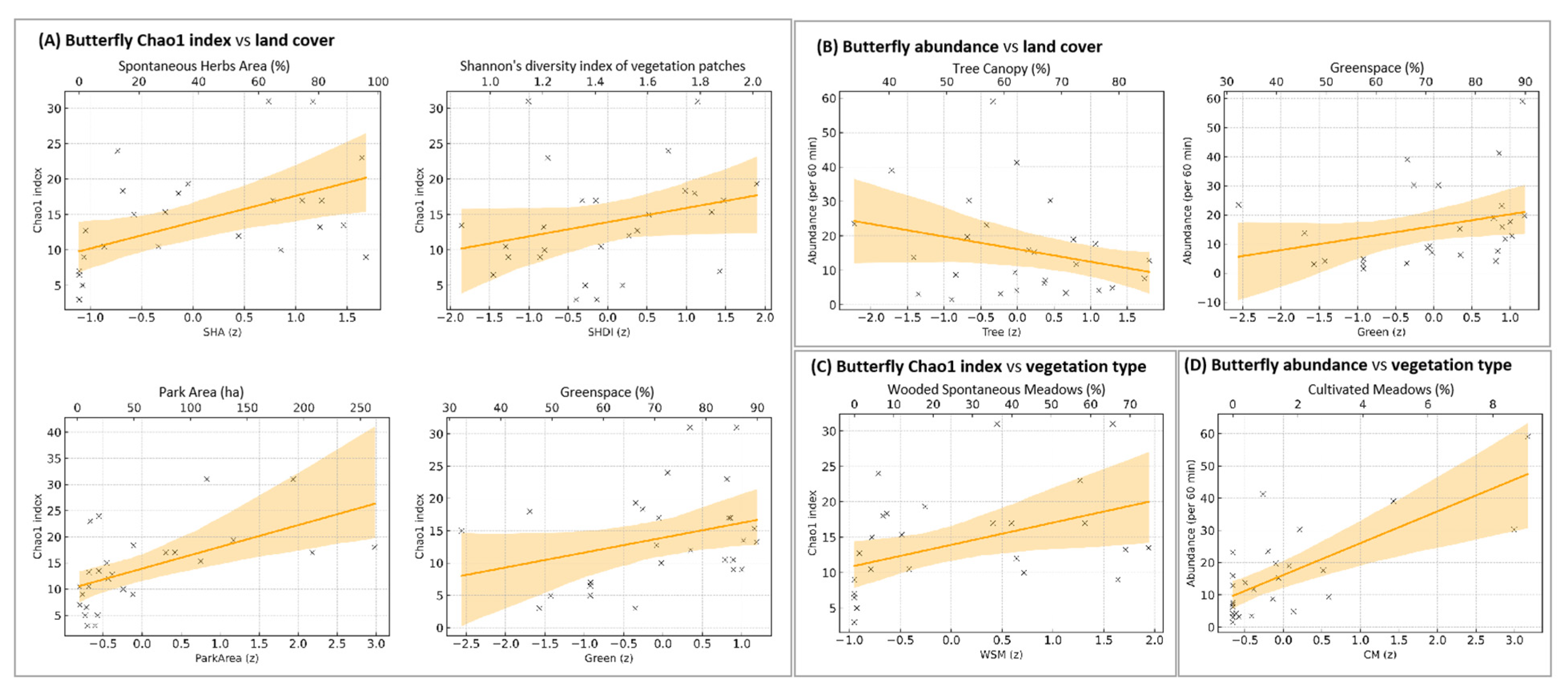
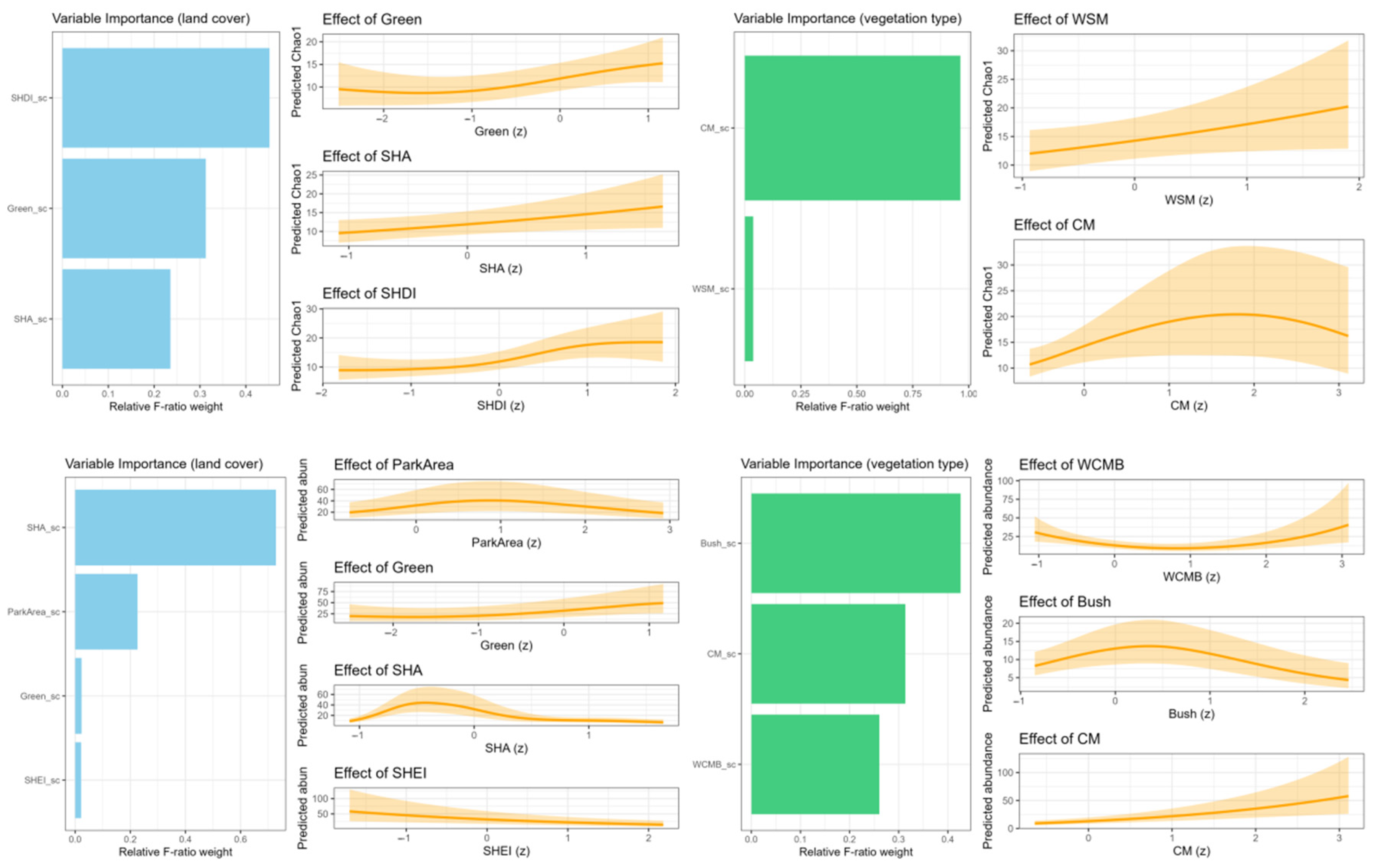
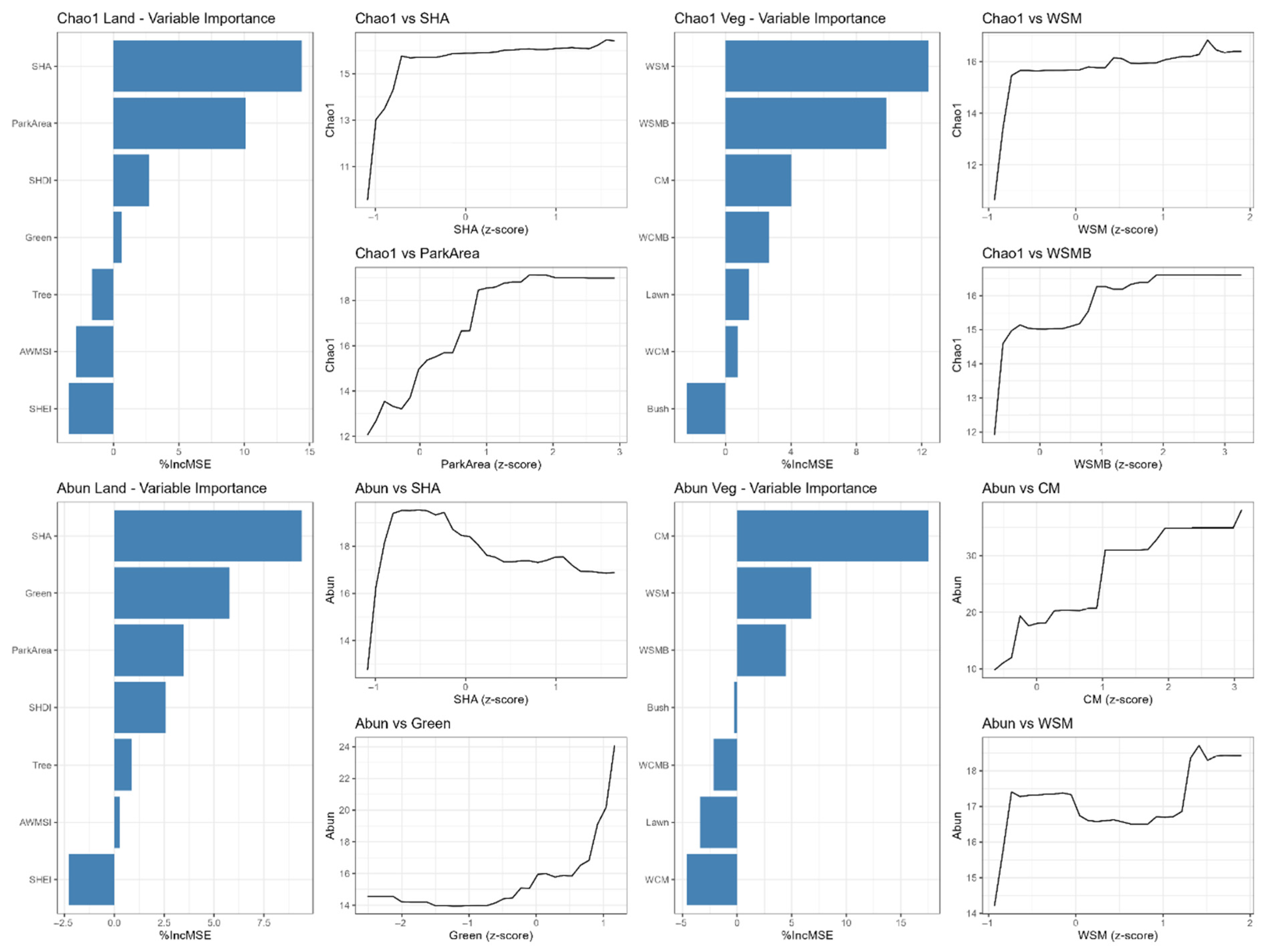
3.3. Drivers of Butterfly Diversity
3.3.1. Effects on Butterfly Species Richness
3.3.2. Effects on Butterfly Abundance
4. Discussion
4.1. Butterfly Community in Beijing Urban Parks
4.2. Fine-Scale Microhabitat Heterogeneity Drives Butterfly Richness
4.3. Divergent Drivers of Butterfly Richness and Abundance
4.4. Applying the Patch–Matrix Framework at the Intra-Park Scale
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Our World in Data Data Page: Share of the Population Living in Urban Areas 2025. Available online: https://ourworldindata.org/grapher/share-of-population-urban (accessed on 24 June 2025).
- World Urbanization Prospects: The 2018 Revision; United Nations: New York, NY, USA, 2018.
- Aronson, M.F.J.; Sorte, F.A.L.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A Global Analysis of the Impacts of Urbanization on Bird and Plant Diversity Reveals Key Anthropogenic Drivers. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133330. [Google Scholar] [CrossRef]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities Are Hotspots for Threatened Species. Glob. Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Forister, M.L.; McCall, A.C.; Sanders, N.J.; Fordyce, J.A.; Thorne, J.H.; O’Brien, J.; Waetjen, D.P.; Shapiro, A.M. Compounded Effects of Climate Change and Habitat Alteration Shift Patterns of Butterfly Diversity. Proc. Natl. Acad. Sci. USA 2010, 107, 2088–2092. [Google Scholar] [CrossRef] [PubMed]
- Warren, M.S.; Maes, D.; Van Swaay, C.A.M.; Goffart, P.; Van Dyck, H.; Bourn, N.A.D.; Wynhoff, I.; Hoare, D.; Ellis, S. The Decline of Butterflies in Europe: Problems, Significance, and Possible Solutions. Proc. Natl. Acad. Sci. USA 2021, 118, e2002551117. [Google Scholar] [CrossRef]
- Sing, K.-W.; Jusoh, W.F.A.; Hashim, N.R.; Wilson, J.-J. Urban Parks: Refuges for Tropical Butterflies in Southeast Asia? Urban. Ecosyst. 2016, 19, 1131–1147. [Google Scholar] [CrossRef]
- Zhang, S.; Han, D.; She, J.; Shen, Q.; Wang, C. The Value of Pocket Parks in Preserving Urban Butterfly Diversity. Urban. For. Urban. Green. 2024, 99, 128467. [Google Scholar] [CrossRef]
- Lizée, M.-H.; Manel, S.; Mauffrey, J.-F.; Tatoni, T.; Deschamps-Cottin, M. Matrix configuration and patch isolation influences override the species–area relationship for urban butterfly communities. Landscape Ecol. 2012, 27, 159–169. [Google Scholar] [CrossRef]
- Luppi, M.; Dondina, O.; Orioli, V.; Bani, L. Local and Landscape Drivers of Butterfly Richness and Abundance in a Human-Dominated Area. Agric. Ecosyst. Environ. 2018, 254, 138–148. [Google Scholar] [CrossRef]
- Öckinger, E.; Van Dyck, H. Landscape Structure Shapes Habitat Finding Ability in a Butterfly. PLoS ONE 2012, 7, e41517. [Google Scholar] [CrossRef]
- Adams, B.J.; Li, E.; Bahlai, C.A.; Meineke, E.K.; McGlynn, T.P.; Brown, B.V. Local- and Landscape-scale Variables Shape Insect Diversity in an Urban Biodiversity Hot Spot. Ecol. Appl. 2020, 30, e02089. [Google Scholar] [CrossRef]
- Han, D.; Zhang, C.; Wang, C.; She, J.; Sun, Z.; Zhao, D.; Bian, Q.; Han, W.; Yin, L.; Sun, R.; et al. Differences in Response of Butterfly Diversity and Species Composition in Urban Parks to Land Cover and Local Habitat Variables. Forests 2021, 12, 140. [Google Scholar] [CrossRef]
- Peng, M.-H.; Hung, Y.-C.; Liu, K.-L.; Neoh, K.-B. Landscape Configuration and Habitat Complexity Shape Arthropod Assemblage in Urban Parks. Sci. Rep. 2020, 10, 16043. [Google Scholar] [CrossRef]
- Zeng, H.; Wang, J.; Guan, M.; Lu, Y.; Liu, H.; Zhao, D. Effects of Vegetation Structure and Environmental Characteristics on Pollinator Diversity in Urban Green Spaces. Urban. For. Urban. Green. 2023, 84, 127928. [Google Scholar] [CrossRef]
- Norton, B.A.; Evans, K.L.; Warren, P.H. Urban Biodiversity and Landscape Ecology: Patterns, Processes and Planning. Curr. Landsc. Ecol. Rep. 2016, 1, 178–192. [Google Scholar] [CrossRef]
- Aronson, M.F.; Lepczyk, C.A.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S.; Nilon, C.H.; Vargo, T. Biodiversity in the City: Key Challenges for Urban Green Space Management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef]
- Hwang, Y.H.; See, S.C.; Patil, M.A. Short-Term Vegetation Changes in Tropical Urban Parks: Patterns and Design-Management Implications. Urban. For. Urban. Green. 2021, 64, 127240. [Google Scholar] [CrossRef]
- Forman, R.T.T. Urban Ecology: Science of Cities; Cambridge University Press: Cambridge, UK, 2014; ISBN 978-1-107-00700-0. [Google Scholar]
- Sing, K.-W.; Dong, H.; Wang, W.-Z.; Wilson, J.-J. Can Butterflies Cope with City Life? Butterfly Diversity in a Young Megacity in Southern China. Genome 2016, 59, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Tam, K.C.; Bonebrake, T.C. Butterfly Diversity, Habitat and Vegetation Usage in Hong Kong Urban Parks. Urban. Ecosyst. 2015, 19, 721–733. [Google Scholar] [CrossRef]
- Sebek, P.; Vodka, S.; Bogusch, P.; Pech, P.; Tropek, R.; Weiss, M.; Zimova, K.; Cizek, L. Open-Grown Trees as Key Habitats for Arthropods in Temperate Woodlands: The Diversity, Composition, and Conservation Value of Associated Communities. For. Ecol. Manag. 2016, 380, 172–181. [Google Scholar] [CrossRef]
- Silaeva, T.; Andreychev, A.; Kiyaykina, O.; Balčiauskas, L. Taxonomic and Ecological Composition of Forest Stands Inhabited by Forest Dormouse Dryomys Nitedula (Rodentia: Gliridae) in the Middle Volga. Biologia 2021, 76, 1475–1482. [Google Scholar] [CrossRef]
- Nielsen, A.B.; Van Den Bosch, M.; Maruthaveeran, S.; Van Den Bosch, C.K. Species Richness in Urban Parks and Its Drivers: A Review of Empirical Evidence. Urban. Ecosyst. 2014, 17, 305–327. [Google Scholar] [CrossRef]
- Dennis, R.L.H. Butterfly Biology Systems: Connections and Interactions in Life History and Behaviour; CABI: Wallingford, UK; Boston, MA, USA, 2020; ISBN 978-1-78924-357-4. [Google Scholar]
- Cornelis, J.; Hermy, M. Biodiversity Relationships in Urban and Suburban Parks in Flanders. Landsc. Urban. Plan. 2004, 69, 385–401. [Google Scholar] [CrossRef]
- Khera, N.; Mehta, V.; Sabata, B.C. Interrelationship of Birds and Habitat Features in Urban Greenspaces in Delhi, India. Urban. For. Urban. Green. 2009, 8, 187–196. [Google Scholar] [CrossRef]
- Lizée, M.-H.; Tatoni, T.; Deschamps-Cottin, M. Nested Patterns in Urban Butterfly Species Assemblages: Respective Roles of Plot Management, Park Layout and Landscape Features. Urban. Ecosyst. 2016, 19, 205–224. [Google Scholar] [CrossRef]
- Perović, D.; Gámez-Virués, S.; Börschig, C.; Klein, A.-M.; Krauss, J.; Steckel, J.; Rothenwöhrer, C.; Erasmi, S.; Tscharntke, T.; Westphal, C. Configurational Landscape Heterogeneity Shapes Functional Community Composition of Grassland Butterflies. J. Appl. Ecol. 2015, 52, 505–513. [Google Scholar] [CrossRef]
- Turner, M.G.; Gardner, R.H. Landscape Ecology in Theory and Practice: Pattern and Process; Springer: New York, NY, USA, 2015; ISBN 978-1-4939-2793-7. [Google Scholar]
- Wang, M.; Zhang, H.; Fan, S.; Hao, P.; Dong, L. A Zoning-Based Solution for Hierarchical Forest Patch Mosaic in Urban Parks. Urban. For. Urban. Green. 2021, 65, 127352. [Google Scholar] [CrossRef]
- McGarigal, K.; Ene, E.; Cushman, S. FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical Maps. 2023 Computer software program produced by the authors. Available online: https://www.fragstats.org (accessed on 9 June 2025).
- Kerr, J.T.; Southwood, T.R.E.; Cihlar, J. Remotely Sensed Habitat Diversity Predicts Butterfly Species Richness and Community Similarity in Canada. Proc. Natl. Acad. Sci. USA 2001, 98, 11365–11370. [Google Scholar] [CrossRef]
- Chang, C.-R.; Chien, H.-F.; Shiu, H.-J.; Ko, C.-J.; Lee, P.-F. Multiscale Heterogeneity within and beyond Taipei City Greenspaces and Their Relationship with Avian Biodiversity. Landsc. Urban. Plan. 2017, 157, 138–150. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Escobar, F.; Rueda-Hernández, R.; Avendaño-Reyes, S.; Baena, M.; Bandala, V.; Chacón-Zapata, S.; Guillén-Servent, A.; González-García, F.; Lorea-Hernández, F.; et al. City “Green” Contributions: The Role of Urban Greenspaces as Reservoirs for Biodiversity. Forests 2016, 7, 146. [Google Scholar] [CrossRef]
- Han, D.; Wang, C.; Sun, Z.; She, J.; Yin, L.; Bian, Q.; Han, W. Microhabitat Preferences of Butterflies in Urban Parks: Both Vegetation Structure and Resources Are Decisive. Urban. For. Urban. Green. 2022, 71, 127552. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, R.; Qiu, J. Decline in the Diversity of Willow Trunk-Dwelling Weevils (Coleoptera: Curculionoidea) as a Result of Urban Expansion in Beijing, China. J. Insect Conserv. 2011, 15, 367–377. [Google Scholar] [CrossRef]
- Pollard, E.; Yates, T.J. Monitoring Butterflies for Ecology and Conservation: The British Butterfly Monitoring Scheme; Conservation Biology Series; Chapman & Hall: London, UK, 1993; ISBN 978-0-412-40220-3. [Google Scholar]
- Wu, C.; Xu, Y. Butterflies of China; The Straits Publishing House: Fuzhou, China, 2017; ISBN 978-7-5567-0302-9. [Google Scholar]
- Yang, H.; Wang, C.; Yu, P. Beijing Butterfly; Scientific and Technical Documents Publishing House: Beijing, China, 1994; ISBN 978-7-5023-2185-7. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002; ISBN 978-0-387-95364-9. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, Second Edition, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2017; ISBN 978-1-315-37027-9. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards, T.C.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random Forests for Classification in Ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning; Springer Series in Statistics; Springer: New York, NY, USA, 2009; ISBN 978-0-387-84857-0. [Google Scholar]
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Wood, S. Mgcv: Mixed GAM Computation Vehicle with Automatic Smoothness Estimation. R package version 1.9-3, 2025. Available online: https://cran.r-project.org/web/packages/mgcv/mgcv.pdf (accessed on 4 April 2025).
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R. News 2002, 2, 18–22. [Google Scholar]
- Greenwell, B.M. Pdp: An R Package for Constructing Partial Dependence Plots. R J. 2017, 9, 421–436. [Google Scholar]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R package version 2.6-10, 2025. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 29 January 2025).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Scheipl, F.; Grothendieck, G.; Green, P.; et al. Lme4: Linear Mixed-Effects Models Using “Eigen” and S4. R package version 1.1-37, 2025. Available online: https://cran.r-project.org/web/packages/lme4/lme4.pdf (accessed on 26 March 2025).
- Bartoń, K. MuMIn: Multi-Model Inference. R package version 1.48-11, 2025. Available online: https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf (accessed on 1 April 2025).
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). Version 2.3-4, 2023. Available online: https://cran.r-project.org/web/packages/AICcmodavg/AICcmodavg.pdf (accessed on 2 June 2025).
- Breiman, L.; Cutler, A.; Liaw, A.; Wiene, M. randomForest: Breiman and Cutlers Random Forests for Classification and Regression. R package version 4.7-1.2, 2024. Available online: https://cran.r-project.org/web/packages/randomForest/randomForest.pdf (accessed on 22 September 2024).
- Gu, Z. Circlize: Circular Visualization. R package version 0.4.16, 2024. Available online: https://cran.r-project.org/web/packages/circlize/circlize.pdf (accessed on 20 February 2024).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T.; et al. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. R package version 3.5.2, 2025. Available online: https://ggplot2.tidyverse.org/reference/ggplot2-package.html (accessed on 9 April 2025).
- Wilke, C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for “ggplot2”. R package version 1.2.0, 2025. Available online: https://wilkelab.org/cowplot/ (accessed on 2 June 2025).
- Sing, K.-W.; Luo, J.; Wang, W.; Jaturas, N.; Soga, M.; Yang, X.; Dong, H.; Wilson, J.-J. Ring Roads and Urban Biodiversity: Distribution of Butterflies in Urban Parks in Beijing City and Correlations with Other Indicator Species. Sci. Rep. 2019, 9, 7653. [Google Scholar] [CrossRef]
- Blair, R.B.; Launer, A.E. Launer Butterfly Diversity and Human Land Use: Species Assemblages along an Urban Grandient. Biol. Conserv. 1997, 80, 113–125. [Google Scholar] [CrossRef]
- Lee, C.M.; Park, J.W.; Kwon, T.-S.; Kim, S.-S.; Ryu, J.W.; Jung, S.J.; Lee, S.K. Diversity and Density of Butterfly Communities in Urban Green Areas: An Analytical Approach Using GIS. Zool. Stud. 2015, 54, 4. [Google Scholar] [CrossRef]
- Kitahara, M.; Fujii, K. Biodiversity and Community Structure of Temperate Butterfly Species within a Gradient of Human Disturbance: An Analysis Based on the Concept of Generalist vs. Specialist Strategies. Res. Popul. Ecol. 1994, 36, 187–199. [Google Scholar] [CrossRef]
- Clark, P.J.; Reed, J.M.; Chew, F.S. Effects of Urbanization on Butterfly Species Richness, Guild Structure, and Rarity. Urban. Ecosyst. 2007, 10, 321–337. [Google Scholar] [CrossRef]
- Han, D.; Han, C.; Wang, C.; She, J.; Bian, Q.; Han, W.; Yin, L. Butterfly Diversity and Dominant Species Niche in Different Urbanization Zones of Beijing. J. Chin. Urban. For. 2023, 21, 74–81. [Google Scholar]
- Pietrzak, S.; Pabis, K. Life on Green Patches: Diversity and Seasonal Changes of Butterfly Communities Associated With Wastelands of the Post-Industrial Central European City. Ecol. Evol. 2024, 14, e70695. [Google Scholar] [CrossRef] [PubMed]
- Öckinger, E.; Dannestam, Å.; Smith, H.G. The Importance of Fragmentation and Habitat Quality of Urban Grasslands for Butterfly Diversity. Landsc. Urban. Plan. 2009, 93, 31–37. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Mata, L.; Mackie, J.A.; Hahs, A.K.; Stork, N.E.; Williams, N.S.G.; Livesley, S.J. Increasing Biodiversity in Urban Green Spaces through Simple Vegetation Interventions. J. Appl. Ecol. 2017, 54, 1874–1883. [Google Scholar] [CrossRef]
- Huang, S.; Lin, Y.; Dong, J.; Lin, Y.; Su, Z.; Li, J.; Zhang, Y.; Jin, J.; Fu, W. Relationship between Plant Habitat Types and Butterfly Diversity in Urban Mountain Parks. Forests 2024, 15, 1390. [Google Scholar] [CrossRef]
- Ranalli, R.; Borghesan, S.; Labra, M.; Biella, P. From Cities to Refuges for Pollinators: Urban Practices for Enhancing Pollinator Habitats in Changing Landscapes. J. Appl. Ecol. 2025, 1–8. [Google Scholar] [CrossRef]
- Braem, S.; Turlure, C.; Nieberding, C.; Van Dyck, H. Oviposition Site Selection and Learning in a Butterfly under Niche Expansion: An Experimental Test. Anim. Behav. 2021, 180, 101–110. [Google Scholar] [CrossRef]
- Hordley, L.; Fox, R. Wildlife-Friendly Garden Practices Increase Butterfly Abundance and Species Richness in Urban and Arable Landscapes. Sci. Total Environ. 2024, 929, 171503. [Google Scholar] [CrossRef]
- Aguilera, G.; Ekroos, J.; Persson, A.S.; Pettersson, L.B.; Öckinger, E. Intensive Management Reduces Butterfly Diversity over Time in Urban Green Spaces. Urban. Ecosyst. 2019, 22, 335–344. [Google Scholar] [CrossRef]
- Garbuzov, M.; Fensome, K.A.; Ratnieks, F.L.W. Public Approval plus More Wildlife: Twin Benefits of Reduced Mowing of Amenity Grass in a Suburban Public Park in Saltdean, UK. Insect Conserv. Divers. 2015, 8, 107–119. [Google Scholar] [CrossRef]
- Chan, M.; Tsang, T.P.N.; Dingle, C.; Early, R.; Sorte, C.J.B.; Bonebrake, T.C. Microhabitat Coverage Influences Avian Species Composition More than Habitat Heterogeneity in Hong Kong Urban Parks. Urban. For. Urban. Green. 2024, 101, 128519. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Shreeve, T.G.; Van Dyck, H. Habitats and Resources: The Need for a Resource-Based Definition to Conserve Butterflies. Biodivers. Conserv. 2006, 15, 1943–1966. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal Species Diversity Driven by Habitat Heterogeneity/Diversity: The Importance of Keystone Structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar]
- Hicks, D.M.; Ouvrard, P.; Baldock, K.C.R.; Baude, M.; Goddard, M.A.; Kunin, W.E.; Mitschunas, N.; Memmott, J.; Morse, H.; Nikolitsi, M.; et al. Food for Pollinators: Quantifying the Nectar and Pollen Resources of Urban Flower Meadows. PLoS ONE 2016, 11, e0158117. [Google Scholar] [CrossRef]
- Wenzel, A.; Grass, I.; Belavadi, V.V.; Tscharntke, T. How Urbanization Is Driving Pollinator Diversity and Pollination—A Systematic Review. Biol. Conserv. 2020, 241, 108321. [Google Scholar] [CrossRef]
- Stelzer, R.J.; Chittka, L.; Carlton, M.; Ings, T.C. Winter Active Bumblebees (Bombus terrestris) Achieve High Foraging Rates in Urban Britain. PLoS ONE 2010, 5, e9559. [Google Scholar] [CrossRef]
- Han, D.; Wang, C.; Yin, L. Characteristics of butterfly-nectar plant network in Beijing’s urban parks and identifying important nectariferous plant species. Acta Ecol. Sin. 2021, 41, 8892–8905. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Dapporto, L.; Sparks, T.H.; Williams, S.R.; Greatorex-Davies, J.N.; Asher, J.; Roy, D.B. Turnover and Trends in Butterfly Communities on Two British Tidal Islands: Stochastic Influences and Deterministic Factors. J. Biogeogr. 2010, 37, 2291–2304. [Google Scholar] [CrossRef]
- Marcantonio, M.; Voda, R.; Da Re, D.; Igot, Q.; Dennis, R.L.H.; Vielfaure, A.; Vanwambeke, S.O.; Nieberding, C.M. The Effect of Habitat on Insect Movements: Experimental Evidence from Wild-Caught Butterflies. Insects 2023, 14, 737. [Google Scholar] [CrossRef]
- Evans, K.L.; Warren, P.H.; Gaston, K.J. Species-Energy Relationships at the Macroecological Scale: A Review of the Mechanisms. Biol. Rev. 2005, 80, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.M.; Good, K.D.; Moretti, M.; Kremer, P.; Wadzuk, B.; Traver, R.; Smith, V. Towards the Intentional Multifunctionality of Urban Green Infrastructure: A Paradox of Choice? NPJ Urban. Sustain. 2024, 4, 1–13. [Google Scholar] [CrossRef]
- Derby Lewis, A.; Bouman, M.J.; Winter, A.M.; Hasle, A.F.; Stotz, D.F.; Johnston, M.K.; Klinger, K.R.; Rosenthal, A.; Czarnecki, C.A. Does Nature Need Cities? Pollinators Reveal a Role for Cities in Wildlife Conservation. Front. Ecol. Evol. 2019, 7, 220. [Google Scholar] [CrossRef]
- Lausch, A.; Blaschke, T.; Haase, D.; Herzog, F.; Syrbe, R.; Lutz, T.; Walz, U. Understanding and Quantifying Landscape Structure—A Review on Relevant Process Characteristics, Data Models and Landscape Metrics. Ecol. Model. 2015, 295, 31–41. [Google Scholar] [CrossRef]
| Categories | Metrics (Abbreviation) | Description | Equation (Range) |
|---|---|---|---|
| Composition of Park Land Cover | Percentage of green space (Green) | Represents the proportion of the total park area that is covered by green spaces. It serves as a measure of the landscape composition and the dominance of a particular patch type (%). | [0, 100] |
| Percentage of tree canopy (Tree) | Denotes the proportion of the total area occupied by tree canopy (%), reflecting the extent of arboreal cover within the park. | ||
| Percentage of spontaneous herbs area (SHA) | Measures the proportion of the park’s total area that is covered by spontaneously occurring herbs. This metric serves as an indicator of lower management intensity for the understory vegetation (%). | ||
| Percentage of water body (Water) | Specifies the percentage of the park area occupied by water bodies (e.g., ponds, lakes, streams), reflecting the hydrological component of the landscape (%). | ||
| Percentage of impervious surface (Grey) | Defines the proportion of the total area that is covered by impervious surfaces such as roads, buildings, and paved areas. This metric is used to assess the level of urban infrastructure within the park (%). | ||
| Vegetation Patch Configuration | Shannon’s diversity index of vegetation patches (SHDI) | A widely employed metric in ecology, SHDI accounts for both the richness (number of patch types) and the evenness (abundance distribution among types) of vegetation patches. An SHDI value of 0 indicates the presence of only a single patch type, while the index increases without an upper limit as the number of patch types increases and their proportions become more uniformly distributed. | [0, ∞) |
| Shannon’s evenness index of vegetation patches (SHEI) | Interpreted here as a measure of patch dominance, SHEI assumes a value of 0 when a single patch type predominates completely and attains a maximum of 1 when all patch types are represented in equal proportion (unitless). | [0, 1) | |
| Area-weighted mean patch shape index of vegetation patches (AWMSI) | Represents the average shape complexity of vegetation patches, weighted by their area. An AWMSI value of 1 corresponds to perfectly circular patches, while increasing values indicate more irregular and complex patch shapes (unitless). | [1, ∞) | |
| Composition of vegetation units | Percentage of wooded cultivated meadows (WCM) | Percentage of the total area occupied by wooded cultivated meadows (%). | [0, 100] |
| Percentage of wooded spontaneous meadows (WSM) | Percentage of the total area occupied by wooded spontaneous meadows (%). | ||
| Percentage of wooded cultivated meadows with bushes (WCMB) | Percentage of the total area occupied by wooded cultivated meadows with bushes (%). | ||
| Percentage of wooded spontaneous meadows with bushes (WSMB) | Percentage of the total area occupied by wooded spontaneous meadows with bushes (%). | ||
| Percentage of bushes (Bush) | Percentage of the total area occupied by bushes (%). | ||
| Percentage of lawns (Lawn) | Percentage of the total area occupied by lawns (%). | ||
| Percentage of cultivated meadows (CM) | Percentage of the total area occupied by cultivated meadows (%). | ||
| Percentage of spontaneous meadows (SM) | Percentage of the total area occupied by spontaneous meadows (%). | ||
| Percentage of woodland with little understory (WLU) | Percentage of the total area occupied by woodland with no understory (%). | ||
| Percentage of wooded lawn (WL) | Percentage of the total area occupied by a wooded lawn (%). | ||
| Percentage of wooded lawn with bushes (WLB) | Percentage of the total area occupied by wooded lawns with bushes (%). | ||
| Percentage of bamboos (Bamboos) | Percentage of the total area occupied by bamboos (%). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Wang, C.; She, J.; Sun, Z.; Yin, L. Effects of Vegetation Heterogeneity on Butterfly Diversity in Urban Parks: Applying the Patch–Matrix Framework at Fine Scales. Sustainability 2025, 17, 6289. https://doi.org/10.3390/su17146289
Han D, Wang C, She J, Sun Z, Yin L. Effects of Vegetation Heterogeneity on Butterfly Diversity in Urban Parks: Applying the Patch–Matrix Framework at Fine Scales. Sustainability. 2025; 17(14):6289. https://doi.org/10.3390/su17146289
Chicago/Turabian StyleHan, Dan, Cheng Wang, Junying She, Zhenkai Sun, and Luqin Yin. 2025. "Effects of Vegetation Heterogeneity on Butterfly Diversity in Urban Parks: Applying the Patch–Matrix Framework at Fine Scales" Sustainability 17, no. 14: 6289. https://doi.org/10.3390/su17146289
APA StyleHan, D., Wang, C., She, J., Sun, Z., & Yin, L. (2025). Effects of Vegetation Heterogeneity on Butterfly Diversity in Urban Parks: Applying the Patch–Matrix Framework at Fine Scales. Sustainability, 17(14), 6289. https://doi.org/10.3390/su17146289






