Abstract
In this study, we selected the production processes and main products of three typical chemical enterprises in Shanghai, namely SH Petrochemical (part of the oil-refining sector), SK Ethylene, and HS Chlor-Alkali, to quantitatively assess the synergistic effects across technology, policy, and emission mechanisms. The localized air pollutant levels and greenhouse gas emissions of the three enterprises were calculated. The synergistic effects between the end-of-pipe emission reductions for air pollutants and greenhouse gas emissions were analyzed using the pollutant reduction synergistic and cross-elasticity coefficients, including technology comparisons (e.g., acrylonitrile gas incineration (AOGI) technology vs. traditional flare). Based on these data, we used the SimaPro software and the CML-IA model to conduct a life cycle environmental impact assessment regarding the production and upstream processes of their unit products. By combining the life cycle method and the scenario simulation method, we predicted the trends in the environmental impacts of the three chemical enterprises after the implementation of low-carbon development policies in the chemical industry in 2030. We also quantified the synergistic effects of localized air pollutant and greenhouse gas (GHG) emission reductions within the low-carbon development scenario by using cross-elasticity coefficients based on life cycle environmental impacts. The research results show that, for every ton of air pollutant reduced through end-of-pipe treatment measures, the HS Chlor-Alkali enterprise would increase its maximum CO2 emissions, amounting to about 80 tons. For SK Ethylene, the synergistic coefficient for VOC reduction and CO2 emissions when using AOGI thermal incineration technology is superior to that for traditional flare thermal incineration. The activities of the three enterprises had an impact on several environmental indicators, particularly the fossil fuel resource depletion potential, accounting for 69.48%, 53.94%, and 34.23% of their total environmental impact loads, respectively. The scenario simulations indicate that, in a low-carbon development scenario, the overall environmental impact loads of SH Petrochemical (refining sector), SK Ethylene, and HS Chlor-Alkali would decrease by 3~5%. This result suggests that optimizing the upstream power structure, using “green hydrogen” instead of “grey hydrogen” in hydrogenation units within refining enterprises, and reducing the consumption of electricity and steam in the production processes of ethylene and chlor-alkali are effective measures in reducing carbon emissions in the chemical industry. The quantification of the synergies based on life cycle environmental impacts revealed that there are relatively strong synergies for air pollutant and GHG emission reductions in the oil-refining industry, while the chlor-alkali industry has the weakest synergies.
1. Introduction
Owing to the potential consequences of global climate change, reducing greenhouse gas (GHG) emissions has become a common goal for all countries in the world [1,2]. The chemical industry’s activities entail high energy consumption and high emissions. In China, this industry has become one of the main sources of carbon dioxide emissions [3]. At the same time, production processes in the chemical industry give rise to a variety of air pollutant emissions, posing a threat to the atmosphere and human health [4]. Studies have shown that air pollutant and GHG emissions are homologous [5], and the realization of synergistic reductions in the emissions of air pollutants and GHGs is vital for the sustainable development of the chemical industry.
Scholars around the world have extensively studied the synergistic effects of reducing air pollutant and GHG emissions to explore effective strategies [6]. At the regional scale, Bollen et al. applied the MERGE model and confirmed that localized air pollutant and GHG emission reductions had significant synergistic benefits [7]. At the national scale, Brendemoen and Vennemo found that GHG emission reduction is important in improving the local ambient air quality [8]. Mardones et al. used a computable general equilibrium (CGE) model to study the impacts of introducing an environmental tax in Chile, and the results showed that a significant reduction in net carbon dioxide (CO2) emissions provided co-benefits for local air pollutant reductions [9]. At the city scale, Mexico conducted a study on the co-benefits of air pollution control programs in urban agglomerations in 2009 with the support of the Integrated Environmental Strategies (IES) program organized by the U.S. Federal Environmental Protection Agency [10]. At the sectoral level, the Greenhouse Gas and Air pollution Interactions and Synergies (GAINS) model developed by the International Institute for Applied Systems Analysis (IIASA) was used to evaluate 3500 end-of-pipe technologies for air pollutants and 350 end-of-pipe technologies for structural CO2 reduction [11]. Wagner et al. applied the GAINS model to derive the marginal abatement cost curves of GHGs and air pollutants for three scenarios, confirming that GHG abatement policies can simultaneously contribute to local air quality improvement [12]. Dandan Liu et al. proposed a method for the evaluation of the synergistic effects of technologies in pollution reduction and carbon mitigation in the petroleum refining industry. This model evaluates the synergistic control efficacy of technologies from multiple perspectives, including pollution–carbon synergy, the synergistic reduction of pollutant–carbon emissions, cost–benefit analysis, and environmental benefits. The developed methodology was validated through its application to typical petroleum refining technology. The results confirm the synergistic benefits of pollution and carbon reduction through five petroleum refining technologies [13].
Chinese scholars have focused on the synergistic effects of policy measures such as energy use reduction, energy efficiency improvement, and clean energy substitution on air pollutants and GHG emission reduction, but the scale of research on the synergistic effects of end-of-pipe measures is still insufficient [14,15]. Studies have shown that many localized air pollutant abatement measures, especially structural abatement, can synergistically reduce GHG emissions, but the high energy consumption of end-of-pipe treatment facilities may also lead to an increase in GHG emissions [16,17,18]. Researchers combining life cycle assessment (LCA) and scenario modeling have demonstrated that LCA not only enhances our understanding of environmental impacts but also enables a deeper analysis of the synergies between energy efficiency and emission reduction policies through scenario simulations [19].
In general, research in this field remains active, especially at the meso–micro level, where quantitative studies on synergistic emission reduction in specific industries or enterprises, as well as the development of synergistic control programs, are still scarce [20]. Additionally, data on the synergistic coefficients are scarce, making it difficult for scholars to accurately assess the synergistic emission reduction potential and actual effects of various emission reduction measures and technologies. (The synergistic coefficient is the amount of greenhouse gases that may be emitted or synergistically abated by abatement of local air pollutants per unit mass. If the synergistic coefficient is positive, it indicates that the abatement technology can synergistically abate greenhouse gases at the same time as the abatement of pollutants, with a positive synergistic effect; if the synergistic coefficient is negative, it indicates that the abatement technology will increase the emission of greenhouse gases alongside the abatement of pollutants, with a negative synergistic effect.) Currently, domestic and foreign studies on the synergistic effects of reducing air pollutant and GHG emissions mainly focus on the synergistic analysis of emissions. Critically, the synergistic effects are not only reflected in the levels of emissions but also in the extent of ecological and environmental impacts. Furthermore, quantitative analyses of the synergistic effects of reducing of air pollutant and GHG emissions in industries and enterprises are still relatively rare.
Taking the Chinese chemical industry as an example, we selected three typical enterprises, namely oil refining, ethylene, and chlor-alkali manufacturing, to quantitatively study the synergistic effects of reducing conventional air pollutant and GHG emissions. By carefully accounting for air pollutant and GHG emissions, we analyzed the synergistic effects of different air pollutant end-of-pipe measures on GHG emissions to provide specific data support and guidance for the industry and enterprises in optimizing emission reduction technologies. Moreover, we adopted the life cycle assessment methodology to carry out an environmental impact assessment and a synergistic effect analysis based on the LCA of the main products of the three typical enterprises (producing 1 ton of refined diesel fuel, 1 ton of ethylene, and 1 ton of chlor-alkali). We also expanded the synergistic effect analysis to the level of ecological and environmental impacts, providing scientific evidence and data support to aid in the development of targeted emission reduction measures and policies.
2. Research Subjects and Methods
2.1. Subjects of Study and Data Sources
Three typical enterprises were selected for this research: SH Petrochemical (in the oil-refining sector), SK Ethylene, and HS Chlor-Alkali. SH Petrochemical produces gasoline, diesel, and other products derived from crude oil as feedstock. SK Ethylene produces petrochemicals, such as ethylene, propylene, polyethylene, polypropylene, and styrene, derived from naphtha and propane as cracked feedstocks. HS Chlor-Alkali project produces chlorine and caustic soda, and it also produces dichloromethane from the byproducts of hydrochloric acid and ethylene supplied by SK as feedstocks to produce ethylene dichloride. The scope of this study regarding emissions accounting for the three enterprises is shown in Figure 1, Figure 2 and Figure 3.
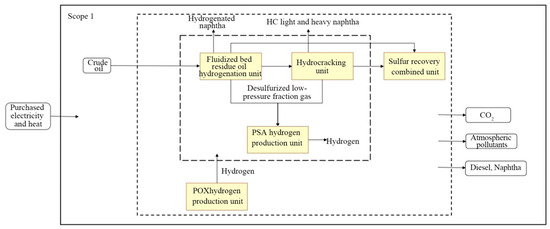
Figure 1.
Accounting scope for SH Petrochemical (refining sector).
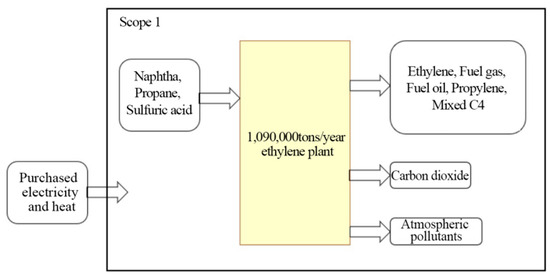
Figure 2.
SK Ethylene’s accounting scope.
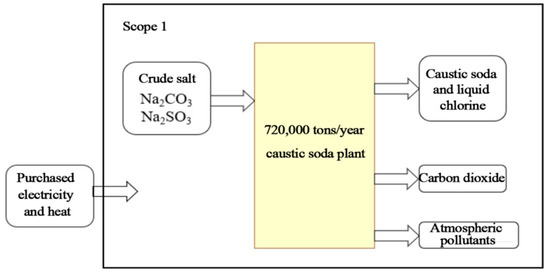
Figure 3.
HS Chlor-alkali’s accounting scope.
The basic data used for emissions accounting regarding SH Petrochemical (part of the refining sector) are derived from the EIA report of this enterprise’s technological transformation project, with 2021 serving as the base year; the air pollutant and GHG emissions of SK Ethylene are derived from this company’s retrospective EIA report, with the base year of 2017; and the data used for accounting for HS Chlor-Alkali’s emissions are derived from its retrospective EIA report, with the base year of 2017.
2.2. Methodology
2.2.1. Methods Used to Account for Emissions of Air Pollutants and GHGs
The air pollutants released by SH Petrochemical and SK Ethylene primarily consist of conventional pollutants (NOx, TSP, and SO2) and volatile organic compounds (VOCs). HS Chlor-Alkali utilizes cleaner energy sources such as natural gas and hydrogen, with its emissions mainly comprising process exhausts that include specific pollutants (Cl2 and HCl) and VOCs. The conventional air pollutants (NOx, TSP, and SO2) and specific pollutants (Cl2 and HCl) emitted by these enterprises are categorized as organized emissions, whereas the VOCs are classified into organized and unorganized emissions. According to the 2017 air pollutant accounting guidelines released by the Ministry of Ecology and Environment, the methods for the calculation of enterprises’ actual emissions of major pollutants include the direct measurement method, the material balance method, and the production and emission coefficient method [21]. Table 1 details the accounting methods used by SH Petrochemical (in the refining sector), SK Ethylene, and HS Chlor-Alkali, with specific accounting equations provided in Appendix A.

Table 1.
Accounting methods for organized and unorganized emissions used by the chemical enterprises studied.
In accordance with the “Shanghai Municipal Chemical Industry Greenhouse Gas Emission Accounting and Reporting Methods” promulgated by the Shanghai Development and Reform Commission, petrochemical enterprises primarily account for CO2 emissions, barring exceptional circumstances [22]. CO2 emissions from net purchased electricity and heat are calculated using the coefficient method, while other emission sources are assessed via the carbon balance method, with detailed equations provided in Appendix A.
2.2.2. Synergy Analysis Methodology
- (1)
- Synergistic coefficients
The end-of-pipe treatment process for air pollutants has synergistic effects on GHG emissions. These effects can be categorized into direct effects and indirect effects [23]. The direct effect refers to GHG emissions generated by applying the end-of-pipe treatment process to air pollutants or via chemical reactions in this process within the geographical boundaries of an enterprise [24]. According to its chemical reaction equation, the limestone–gypsum method of desulfurization generates direct carbon dioxide emissions; in other words, there is a negative synergistic emission effect, and every 1 kg of SO2 removed brings 0.68 kg of CO2 emissions.
The indirect effects are mainly reflected in the GHG emissions indirectly generated by the end-of-pipe treatment facilities via power consumption. In both theoretical and on-site research methods, the values of electricity consumption per unit of desulfurization, denitrification, and dedusting are comprehensively used as the key indicators to measure the electricity consumption of chemical enterprises’ end-of-pipe treatment facilities [24,25]. Using the known motor power and the annual amount of desulfurization, denitrification, and dust removal equipment used, the electricity consumption coefficient can be calculated via the following equation:
where the subscript a denotes the type of air pollutant; is the coefficient of electricity consumption (kWh/t); is the average power of the end-of-pipe treatment equipment (kw); t is the annual operation time of the end-of-pipe treatment facility (h); and is the amount of air pollutant removed (t).
The coefficient of synergy is defined as the amount of additional CO2 emitted per ton of air pollutant removed (t, both direct and indirect).
- (2)
- Cross-elasticity coefficients based on emissions
The pollutant reduction cross-elasticity coefficient can be used to quantitatively analyze the synergistic effect between end-of-pipe reductions in air pollutant and CO2 emissions. It is calculated using the following equation:
where Els denotes the cross-elasticity coefficient; denote the direct CO2 emissions (t) and indirect emissions (t) in the end-of-pipe treatment process, respectively; is the total amount of CO2 emissions within the accounting scope of the enterprise (t); is the quantity of air pollutants removed (t); and is the quantity air pollutants generated (t).
The cross-elasticity coefficient is mainly used to measure the degree of response of changes in the emission reduction of one pollutant to changes in the emission reduction of another pollutant. When ElsCO2/a < 0, it indicates that changing the end-of-pipe treatment facility or technology has a negative synergistic effect and the CO2 emissions increase while air pollutant emissions decrease [26]. For each end-of-pipe treatment technology employed, the larger the absolute value of ElsCO2/a, the greater its impact on CO2 emissions [26,27,28].
- (3)
- Life cycle environmental impact assessment methodology
Life cycle assessment (LCA) is a comprehensive assessment method that considers a product’s resources, energy consumption, and environmental emissions throughout its life cycle. The “Life Cycle Assessment—Principles and Framework” (ISO 14040:2006), issued by the International Organization for Standardization (ISO) in 2006, provides the principles of and a framework for LCA, allowing the extensive adoption of this method worldwide [29,30].
Life cycle impact assessment (LCIA) is the core component of LCA, and its goal is to transform the data collected in a life cycle inventory into comparable environmental impact indicators. In LCIA, two main evaluation models exist: midpoint and endpoint models [31]. The midpoint model is an environmental problem-oriented approach, widely used in China because of its universality and relative simplicity. The endpoint model, on the other hand, is more concerned with impacts on human health, but the evaluation results yielded by the endpoint model are usually characterized by higher uncertainty. Thus, there are some limitations in its application [32]. Owing to a lack of localized research on life cycle environmental impact assessment models, Chinese scholars have more often adopted the midpoint assessment model when conducting life cycle environmental impact assessments regarding chemical enterprises.
SimaPro 9.4.0.2, a software product with a robust life cycle database and rich environmental impact assessment models, is currently one of the mainstream assessment tools. This software contains several authoritative databases, such as Ecoinvent, Agri-footprint, Industry Data 2.0, etc., which provide sufficient upstream data for researchers and greatly facilitate life cycle assessment and environmental impact analysis. In this study, the CML-IA midpoint model in the SimaPro software was used to carry out a life cycle environmental impact assessment, taking the emission characteristics of chemical enterprises into account [33,34]. Midpoint environmental impact indicators were selected for calculation and analysis (for details of the types of indicators and standardization factors, please refer to Appendix B, Table A1). The indicators included the abiotic resource depletion potential (ADP element), fossil fuel depletion potential (ADP fossil), global warming potential (GWP), ozone depletion potential (ODP), human toxicity potential (HTP), freshwater aquatic ecotoxicity potential (FAETP), marine aquatic ecotoxicity potential (MAETP), terrestrial ecotoxicity potential (TETP), photochemical smog potential (POCP), acidification potential (AP), eutrophication potential (EDP), and eutrophication potential (EP).
- (4)
- Cross-elasticity coefficients based on ecological impacts
The use of standardized methods for indicators enables a comparison of the levels of environmental impact between different indicators [29]. Furthermore, selecting region-specific environmental impact standardization factors eliminates the differences in magnitude between midpoint indicators [26]. After applying these approaches, all standardized indicators were summed to derive the overall environmental impact load value of the product, which was calculated as follows:
where the subscript c denotes the environmental impact indicator; is the total environmental impact load value; is the environmental impact load value of an indicator; is the environmental impact indicator characterization value; and is the standardization factor.
The synergistic effect of emission reduction policies is reflected in the changes in various indicators. Based on the characterization method used for the CML-IA model, CO2 is characterized as a GWP factor, while SO2, NOx, and particulate matter are characterized as AP, HTP, and POCP, indicating that these pollutants have potential impacts on global climate change, human health, acidification, and photochemical smog formation [35]. Thus, four environmental impact indicators were selected: GWP, HTP, AP, and POCP. The cross-elasticity coefficients facilitate the development of a quantitative equation for the synergistic effect of atmospheric pollutant and GHG emission reductions within the future low-carbon development scenario based on the life cycle environmental impacts:
where the subscript BAU denotes the baseline scenario (the policy-as-usual scenario); subscript s denotes the low-carbon development scenario; SEA/C is the cross-elasticity coefficient based on ecological and environmental impacts; ΔEIAP and ΔEIGHG are the relative magnitudes of change in the environmental impact loads (in percent) associated with atmospheric pollutants and GHGs; EIAP is the value of the environmental impact load associated with the emission of air pollutants; and EIGHG represents the GWP load associated with GHG emissions.
A positive indicates that GHG emission reduction has a positive synergistic effect with respect to air pollutant emission reduction; on the contrary, if it is negative, it has a negative synergistic effect. If SE is equal to 0, it indicates that the change in GHG emissions will not have an impact on air pollutant emissions and their environmental impacts. When |SEA/C| = 1, it indicates that, under a certain policy and measure scenario, the two factors above both have the same role and effect within that scenario [14,26]. For the GHG emission reduction policy within the low-carbon development scenario, if |SEA/C| > 1, the policy has a more significant effect on air pollutant emissions while reducing GHG emissions. Conversely, |SEA/C| < 1 indicates that the policy has a more significant effect on GHG emissions and a relatively weak synergistic effect on air pollutant reduction [14].
2.2.3. Scenario Analysis Methodology
Scenario analysis is a forecasting methodology in which researchers systematically assess potential situations and their consequences by assuming that particular phenomena or trends will persist in the future [3]. Based on a detailed study of three enterprises, and in light of the goal of the Chinese chemical industry to achieve peak carbon emissions by 2030, we made reasonable assumptions about the changes that may occur in this industry’s production processes under the influence of relevant policies. The three chemical companies’ baseline and low-carbon development scenarios were designed by adjusting the energy structure, process, and air pollution emissions in the life cycle inventory. The base year was set to 2021, and the baseline scenario was developed accordingly. The study’s time frame was from 2021 to 2035.
In developing the baseline scenario, we relied on existing measures, excluding potential future policy adjustments or technological innovations. The enterprises’ levels of utilization of energy reduction and efficiency technologies were maintained at the current level in the base year, serving as a reference point in assessing the emission reduction potential of the low-carbon development scenario [36]. In the model, the source structure of enterprises’ purchased electricity was based on installed capacity data for various power sources in the base year published by the National Bureau of Statistics [26] and on the upstream power structure above. The purchased electricity was proportionally allocated to five types of power: thermal power, hydropower, nuclear power, wind power, and solar power. The life cycle inventory data for these five types of electricity production were obtained from the Ecoinvent database in the SimaPro software.
The low-carbon development scenarios were mainly based on China’s 2030 carbon peak policies. Carbon emissions from refining and chemical enterprises can be categorized into two main groups based on their sources: carbon emissions from fossil fuel combustion and carbon emissions from processes [3]. The relevant parameters of the life cycle evaluation under the different scenarios were established by adjusting the input and output data of the life cycle inventories of the three chemical enterprises. The upstream power structures of the enterprises changed significantly, and the proportion of power consumed by the enterprises changed when adjusting the upstream power structure according to the planning targets for the five types of installed power capacity for 2030 defined in China Energy Outlook 2030 [37]. These enterprises will realize technological improvements and upgrades under the low-carbon development scenario. We considered initiatives developed by oil refineries to adopt green hydrogen instead of grey hydrogen, ethylene, or chlor-alkali in order to reduce their energy consumption, such as that of electricity and steam, through technological advancement and management optimization. Based on these adjusted data, the life cycle inventory was updated to reflect the environmental impacts of the enterprises under different scenarios. Appendix B (Table A2) details the scenario settings and related parameters.
3. Results
3.1. Accounting for Air Pollutant and GHG Emissions
3.1.1. Accounting for Air Pollutant Emissions
- (1)
- Refinery Enterprises
Table 2 summarizes the total emissions of organized atmospheric pollutants from the processes and combustion activities of SH Petrochemical (in the refining sector). Detailed control measures and corresponding emission calculations are provided in Appendix C (Table A3 and Table A4). Table 2 clearly reveals that the annual organized emissions of SO2, NOx, and particulate matter from SH Petrochemical amount to 43.81 tons, 397.27 tons, and 45.96 tons, respectively. Notably, NOx emissions occur across multiple production units, with fuel combustion making the largest contribution, accounting for 89.47% of the total emissions (Figure 4). Particulate matter emissions also predominantly stem from fuel combustion, constituting 95.37% of the total particulate emissions in the refining sector. The generation and emission of SO2 are primarily concentrated in the sulfur recovery units and fuel combustion processes, with annual production amounts of 600.94 tons and 290.52 tons, respectively. After desulfurization using the regenerable absorption process for SOx cleanup (RASOC) and sodium hydroxide desulfurization, the removal efficiency exceeds 90%. NMHC, CO, and H2S emissions are mainly associated with processing operations, likely due to SH Petrochemical’s use of asphalt and petroleum coke gasification for hydrogen production, generating CO and H2S as major byproducts. Additionally, during the removal of acidic gases in the partial oxidation (POX) hydrogen production units, significant amounts of NMHC are generated in the exhaust. Organized VOC emissions are primarily concentrated in the combustion of fuel during the heating of crude oil, while process-related VOC emissions are mainly unorganized, originating from sealing leaks (Table 3).

Table 2.
Summary of SH Petrochemical’s organic air pollutant emission accounting results (t/a).
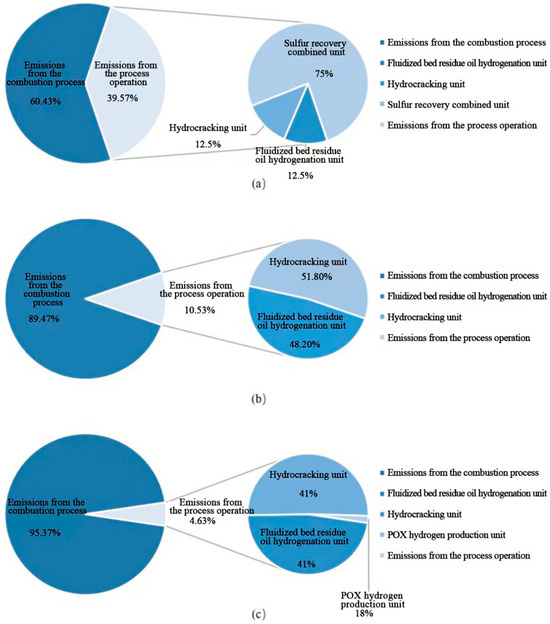
Figure 4.
SH Petrochemical’s SO2, NOx, and particulate matter emission source percentages. (a) Proportions of different emission sources in SO2 emissions; (b) proportions of different emission sources in NOx emissions; and (c) proportions of different emission sources in particulate matter emissions.

Table 3.
SH Petrochemical’s unorganized VOC emissions accounting.
Table 3 summarizes the calculated results regarding the unorganized VOC emissions generated by SH Petrochemical (refining sector), with an annual VOC generation capacity of 88.21 tons. The primary emission source is leakage from sealing points, with the hydrocracking unit for residual oil having the most sealing points and the most significant VOC emissions, reaching 50.73 tons per year. As there is no VOC removal process in the facility used, SH Petrochemical’s total VOC generation and emission degree from organized and unorganized sources for 2021 amounts to about 164.91 tons. Among these sources, unorganized emissions slightly exceed organized emissions, accounting for 53.49%.
- (2)
- Ethylene Producers
Table 4 and Table 5 present the results regarding the emissions of conventional air pollutants and VOCs by SK Ethylene. Table A5 and Table A6 in Appendix C provide a comprehensive overview of the pollutant emission accounting results for each compound. As illustrated in Table 4, the aggregate annual emissions of NOx, TSP, and SO2 are 2237.94 tons, 112.56 tons, and 235.44 tons, respectively. NOx is generated and emitted by multiple production units, with processing units generating and emitting the highest amounts. TSP is predominantly generated by processing units, with a generation rate of 2002.01 tons. However, these emissions decreased to 99.75 tons after efficient dust removal, primarily for units lacking dedusting equipment. SO2 generation and emission are also primarily concentrated in the processing units, primarily via sulfuric acid recovery. SO2 generated and emitted by processing units accounted for 85.43% and 77.50% of ethylene production, respectively.

Table 4.
SK Ethylene’s conventional air pollutant accounting results (t/a).

Table 5.
SK Ethylene’s VOC emission accounting results (t/a).
SK Ethylene emitted 1255.6 tons of VOCs in 2017. Among the various pollution sources, the generation and emission of VOCs from organized emissions were the largest, mainly concentrated in combustion flue gas emissions and flare emissions.
- (3)
- Chlor-Alkali Producers
The atmospheric pollutant results for HS Chlor-Alkali are presented in Table 6. The specific pollutants emitted by this company are Cl2 and HCl, with annual emissions of 0.05 tons and 0.11 tons, respectively. The caustic soda unit is the primary source of Cl2, accounting for 99.63% of the total Cl2 generated by this company. As a result of efficient end-of-pipe treatment, this company’s Cl2 removal efficiency is 99.91%, so the caustic soda unit only represents a 20.00% contribution to the company’s total Cl2 emissions. Of all plants, caustic soda plants produce the highest amounts of HCl, accounting for 98.58% of the entire company’s HCl emissions. Falling film absorption and water jet ejector end treatments significantly reduce the HCl emissions.

Table 6.
HS Chlor-Alkali’s air pollutant accounting (t/a).
Table 7 summarizes the organized and unorganized VOC emission accounting results for HS Chlor-Alkali (detailed in Appendix C, Table A7). The annual VOC emissions for HS Chlor-Alkali amount to 166.55 tons. The highest unorganized VOC emissions result from volatilization losses during the storage and blending of organic liquids and leaks from dynamic and static equipment seals, accounting for 99.95% of the company’s total VOC emissions. The organized VOC emissions are limited to process-related emissions, which are minimal after applying efficient high-temperature incineration end-of-pipe treatment technology.

Table 7.
HS Chlor-Alkali’s VOC emission accounting (t/a).
3.1.2. Accounting for Carbon Dioxide Emissions
Data on the electricity and heat, fueled by natural gas, produced by SH Petrochemical (refining sector), SK Ethylene, and HS Chlor-Alkali were supplied by the Shanghai Chemical Industry Zone (SCZ) Cogeneration Project, and these companies’ CO2 emission factors were calculated according to the emission factor method specified in the “2020 Average Carbon Dioxide Emission Factor of China’s Regional and Provincial Electricity Grids” document. The CO2 emissions of SH Petrochemical (refining sector), SK Ethylene, and HS Chlor-Alkali are presented in Table 8. The parameters and results are described in Appendix C (Table A8, Table A9, Table A10, Table A11, Table A12, Table A13, Table A14 and Table A15).

Table 8.
Summary of carbon dioxide emissions generated by SH Petrochemical (refining sector), SK Ethylene, and HS Chlor-Alkali (t/a).
According to our calculations, the total carbon dioxide emissions generated by SH Petrochemical (refining sector) amount to 4.41 million tons annually, with fuel combustion accounting for 97.1% of these emissions.
SK Ethylene emits 1.03 million tons of CO2 annually, with its emission sources including fuel combustion, flare combustion, process production, direct carbon emissions from end-of-pipe treatment, and CO2 emissions embedded in net purchased electricity and heat, accounting for 29.6%, 20.3%, 0.2%, 2.8%, and 47.1%, respectively. Indirect CO2 emissions from the end treatment of ethylene projects—that is, carbon emissions from end-treatment electricity consumption—are included in the CO2 emissions implied by the net purchased electricity and heat.
HS Chlor-Alkali emits 0.73 million tons of CO2 annually, with its emission sources comprising fuel combustion, process production, direct carbon emissions from end-of-pipe treatment, and CO2 emissions embedded in net purchased electricity and heat, totaling four categories. CO2 emissions embedded in net purchased electricity and heat account for 97.5%, while direct carbon emissions from end-of-pipe treatment, fuel combustion, and process production account for 0.019%, 0.43%, and 2.03%, respectively. Indirect CO2 emissions from end-of-pipe treatment due to electricity consumption are included in the CO2 emissions embedded in the net purchased electricity and heat.
Among the three enterprises, SH Petrochemical has the highest CO2 emissions by far, followed by SK Ethylene, with HS Chlor-Alkali having the lowest emissions. Summing the CO2 emissions from different sources across these enterprises reveals that fuel combustion is the most significant contributor, accounting for 74.4% of the total emissions. Indirect CO2 emissions from purchased electricity and heat are also substantial, comprising 21.4% and representing a major source of carbon emissions for these enterprises.
3.2. Air Pollution End-of-Pipe Management Synergies
End-of-pipe technologies have been widely employed to reduce the emission of air pollutants during chemical production. Although technologies can significantly reduce air pollutant emissions, the chemical reactions and power consumption accompanying the removal of these pollutants may increase CO2 emissions. Therefore, when utilizing end-of-pipe technologies, their synergistic effects with respect to air pollutant level reduction and GHG emission need to be comprehensively considered. The synergistic coefficients of the corresponding end-of-pipe treatments for different air pollutants for SH Petrochemical (refining sector), SK Ethylene, and HS Chlor-Alkali are shown in Table 9, Table 10, and Table 11, respectively, and are detailed in Appendix D (Table A16, Table A17, Table A18, Table A19, Table A20 and Table A21).

Table 9.
SH Petrochemical’s synergistic coefficients.

Table 10.
Synergistic coefficients for SK Ethylene.

Table 11.
Synergistic coefficients for HS Chlor-Alkali.
As shown in Table 9, the synergistic coefficients of NOx, TSP, and SO2 emission reduction are 1.89, 0.0041, and 7.40 tCO2/ton of pollutants, respectively; in other words, every ton of NOx, TSP, and SO2 emission reduction leads to 1.89, 0.0041, and 7.40 tons of carbon dioxide emissions. The synergistic coefficients of SO2 emission reduction are much higher than those of the other three pollutants, while the synergistic coefficients of TSP emission reduction are the lowest. Further analysis shows that the RASOC renewable wet flue gas desulfurization technology used in the combined sulfur recovery unit consumes more power and has a higher power consumption factor, resulting in greater indirect emissions of CO2.
As listed in Table 10, the synergistic coefficients for VOC abatement in SK Ethylene’s end-of-pipe treatment approach are considerably higher than those for NOx and particulate matter. Conversely, the synergistic coefficients for particulate matter abatement are the lowest. In the abatement of VOCs, the direct and indirect CO2 emissions generated by the flare thermal incineration method are higher, amounting to 2.4 times the level observed for the AOGI thermal incineration method. The technologies employed for end-of-pipe treatment in the power center and the sulfuric acid recovery sector include ammonia flue gas desulfurization (FGD) (the simultaneous removal of SO2 and particulate matter), selective catalytic reduction (SCR) (the removal of NOx), and organocatalytic flue gas desulfurization and denitrification (the simultaneous removal of SO2 and NOx), with synergistic effects. The synergistic coefficient of ammonia FGD is notably higher, reaching 53.1 tCO2/t, 1.2 tCO2/t, and 4.2 tCO2/t. Evidently, the synergistic coefficients of ammonia desulfurization and dust removal are the highest, followed by organic catalytic flue gas desulfurization and denitrification—a result that may be due to the great amounts of power that these two methods consume, resulting in high indirect CO2 emissions.
As indicated in Table 11, the pollutants associated with the chlor-alkali process are relatively unique, with a high electricity consumption coefficient for end-of-pipe treatment, resulting in increased electricity usage during the pollutant reduction process. The synergistic coefficients for the reductions in the emissions of Cl2, HCl, and VOCs are 26.90, 425.96, and 70.67 tCO2 per ton of pollutant, respectively, implying that, for every ton of Cl2, HCl, and VOCs removed, there is an increase of 26.9, 426.0, and 71.0 tons in carbon dioxide emissions, respectively.
Table 12 presents the cross-elasticity coefficients for atmospheric pollutants and the associated end-of-pipe control technologies for all three enterprises. All coefficients exhibit negative values, confirming that, while end-of-pipe treatments reduce the atmospheric pollutant levels, they concurrently increase the CO2 emissions. Critically, the lower absolute value of the cross-elasticity coefficient indicates a weaker synergistic effect, indicating that GHG emissions increase at a slower rate per unit reduction in atmospheric pollutants.

Table 12.
Cross-elasticity coefficients for pollutant reductions for end-of-pipe air pollutant management technologies at SH Petrochemical, SK Ethylene, and HS Chlor-Alkali.
Regarding SH Petrochemical, the RASOC wet flue gas desulfurization method led to a 97.81% reduction in SO2 emissions (587.79 tons), while also generating 4351.97 tons of CO2, with a growth rate (the proportion of CO2 emissions related to end-of-pipe treatment with respect to the total CO2 emissions of the company) of 0.1%. This method had the largest absolute value of the cross-elasticity coefficient (0.00101) among the three emission reduction approaches used by SH Petrochemical, indicating that this method results in a relatively high growth rate for carbon dioxide emissions. Regarding SK Ethylene, flare thermal incineration removed 22,900 tons of VOCs (with a reduction rate of 98%), while directly and indirectly emitting 211,000 tons of CO2, with an increase rate of 20.47%. Its cross-elasticity coefficient’s absolute value was 0.209, the largest among the emission reduction approaches employed by SK Ethylene. In contrast, the bag filter approach yielded the smallest absolute value of the cross-elasticity coefficient, amounting to only 7.51 × 10−6. For HS Chlor-Alkali, reducing 10.7 tons of Cl2 (with a reduction rate of 99.53%) resulted in only 287.83 tons of CO2 emissions, with an increase rate of 0.04%—the smallest absolute value of the cross-elasticity coefficient in this project—indicating that the Cl2 end-of-pipe reduction technology leads to a relatively small rate of growth in CO2 emissions. For this company, reducing 9.11 tons of VOCs (with a reduction rate of 5.19%) resulted in 643.82 tons of CO2 emissions, with an increase rate of 0.09% and an absolute value of the cross-elasticity coefficient of 0.0171, which is relatively large, indicating that the VOC end-of-pipe reduction technology has a relatively significant impact on CO2 emissions.
3.3. Life Cycle Environmental Impact Assessment for Typical Products
The CML-IA model in the SimaPro software was used to characterize and standardize the life cycle inventory data. The environmental impact loads of the 11 indicators were cumulated to derive the life cycle environmental impact assessment results corresponding to each ton of product produced by SH Petrochemical (refining sector), SK Ethylene, and HS Chlor-Alkali. The characterization and standardization results obtained are shown in Appendix E.
Normalization eliminated the dimensional disparities between the environmental impact indicators, allowing for a comparison between the different indicators [37]. The calculations indicated that the environmental impact loads per ton of product for SH Petrochemical (refining sector), SK Ethylene, and HS Chlor-Alkali were 5.44 × 10−9, 7.05 × 10−9, and 6.46 × 10−10, respectively. SK Ethylene exhibited the highest environmental impact per ton of ethylene produced, whereas HS Chlor-Alkali exhibited the lowest impact per ton of chlor-alkali produced. Figure 5 delineates the proportions of the environmental impact loads for each indicator per unit product produced by these three enterprises. The figure reveals that the predominant environmental impacts for the production of one ton of product for these chemical enterprises are ADP fossil, MAETP, GWP, and AP, exhibiting a convergent trend. Notably, the impact on ADP fossil is the most pronounced, constituting 69.48%, 53.94%, and 34.23% of each enterprise’s total environmental impact load, respectively. The extensive utilization of fossil raw materials and fossil energy consumption during production and upstream electricity generation significantly contributes to the elevated proportion of the environmental impact load attributed to ADP fossil. The environmental impacts of the global warming potential and acidification potential predominantly originate from the emissions of carbon dioxide, sulfur dioxide, and nitrogen oxides during production. The marine aquatic ecotoxicity impact primarily results from the discharge of wastewater containing toxic substances during production.
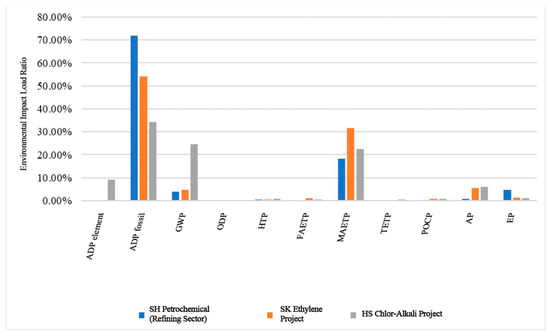
Figure 5.
Percentages of environmental impact loads of the products of the three petrochemical companies analyzed.
3.4. Synergistic Effects Based on Impacts on Ecological Environment Under Different Future Scenarios
Table 13 presents the incremental ratios of the life cycle environmental impacts of producing one ton of refined diesel in the oil-refining industry, one ton of ethylene in the ethylene industry, and one ton of chlor-alkali in the chlor-alkali industry under the low-carbon development scenario, compared with the environmental impacts in the baseline scenario. The results reveal 5.5% and 2.1% reductions in the environmental impact loads of air pollutants in the oil-refining and ethylene industries, respectively, suggesting that optimizing the upstream electric power structure and utilizing “green hydrogen” and analogous measures effectively reduce their environmental impact loads. In contrast, the upstream power structure adjustment and process-related energy-saving measures exhibit a comparatively diminished effect in terms of reducing air pollutants in the chlor-alkali industry, with a mere 0.29% decrease in the environmental impact load of air pollutants associated with producing one ton of product. This decline can be attributed to the nature of air pollutants in the chlor-alkali industry, primarily consisting of specific pollutants such as chlorine gas and hydrogen chloride, making them less directly associated with energy consumption.

Table 13.
Proportion of change in life cycle environmental impact load between low-carbon development scenario and baseline scenario.
Furthermore, the GHG-related environmental impact loads of the three industries are 17%, 8.8%, and 15% lower in the low-carbon development scenario, with relatively substantial emission reductions, suggesting that optimizing the upstream electric power structure and process improvement can reduce GHG emissions. This reduction in environmental impact is particularly pronounced in the oil-refining and chlor-alkali sectors, which substantially impact GHGs. The findings underscore the efficacy of strategies such as optimizing the upstream electric power structure and implementing process improvements to achieve substantial GHG emission reductions and significantly mitigate the environmental impacts of the production of these goods.
Table 14 presents the cross-elasticity coefficients (based on life cycle environmental impacts) for the low-carbon development scenario relative to the baseline scenario. In the low-carbon development scenario, the SEA/C of the oil-refining, ethylene, and chlor-alkali industries is greater than zero, with all values being less than 1. This result indicates that the environmental impacts of reducing GHG and air pollutant emissions have a positive synergistic effect and exhibit positive synergies. The SEA/C values for the oil-refining, ethylene, and chlor-alkali industries are 0.32, 0.24, and 0.019, respectively. It is evident that the rate of change in the environmental impact loads associated with GHG emission reductions is greater than that of atmospheric pollutant emission reductions, suggesting that, in the low-carbon development scenario, the synergistic effect of policy measures targeting carbon peaking on air pollutant emissions is relatively weak. The oil-refining industry has the most considerable SEA/C value, indicating that air pollutant and GHG abatement have relatively strong synergistic effects. By contrast, the chlor-alkali industry has the smallest SEA/C value and the weakest synergistic effect. Likely because of the distinct production processes and raw materials used in this sector, the chlor-alkali industry emits primary air pollutants such as chlorine and hydrogen chloride, which bear less relevance to energy consumption.

Table 14.
Cross-elasticity coefficients based on life cycle environmental impacts for low-carbon development scenario compared to baseline scenario.
4. Discussion
(1) Comparison of emission accounting methods and analysis of data discrepancies in chemical industry: Accounting for localized air pollutant and GHG emissions is foundational for quantitative research and synergistic emission reduction practices. In the chemical industry, the emission of major pollutants can be accounted for via field measurement, material balance, and analogical methods. Field measurement involves the on-site monitoring of pollutant emissions, offering high accuracy and enabling the analysis of the emission characteristics of the source. However, this method is limited by its expensiveness and instrument dependence [38]. The material balance method, based on the law of conservation of matter, is highly accurate and inexpensive. However, it requires precise knowledge of material usage and alterations in the production process—information that is often challenging to obtain, particularly in complex production processes [39]. The analogical method is a technique used to indirectly calculate the emissions of air pollutants generated by a target enterprise. This method has a wide range of applications but is less accurate. The quality of the raw materials used by different enterprises, differences in the production process, and the operational status will affect the accounting results [39]. The field measurement and material balance methods were adopted in this study. We compared the accounting results obtained in this study with the Second National Census on Pollution Sources (2017) (SNCPS) database and the Environmental Statistics Database. According to the Second Census, the NOx emissions of SH Petrochemical (in the refining sector) amounted to 414.6 tons, while the figure obtained in this study was slightly lower. This discrepancy can be attributed, at least in part, to our use of 2021 data, as China further strengthened its efforts to reduce emissions from the petrochemical and other heavily polluting industries between 2017 and 2021, resulting in a decrease in the annual emissions of pollutants from all refineries in the country. This phenomenon has resulted in a consistent decline in the annual emissions of pollutants from all refineries across the country. According to the 2017 annual environmental statistics, the NOx, particulate matter, and SO2 emissions generated by SK Ethylene boilers amounted to 391.0 tons, 7.8 tons, and 37.5 tons, respectively. The emissions of NOx in the power center unit found in this study amounted to 348.9 tons, which is slightly lower than in the environmental statistics, while the emissions of particulate matter and SO2 amounted to 12.8 tons and 53.0 tons, slightly higher than in the environmental statistics. This is possibly due to the fact that, with the increase in ethylene production, the power center’s increased load resulted in elevated particulate matter and SO2 emissions, concurrently augmenting nitrogen oxide generation. However, in 2017, this ethylene company appended a novel SCR denitrification apparatus to all three boilers within the power center, culminating in a substantial reduction in emissions.
The main reason behind the Chinese chemical industry’s high contribution to GHG emissions is its use of the carbon balance and emission factor methods. The carbon balance method requires comprehensive and precise data, including detailed material inputs and outputs, energy use data, and carbon content parameters that align with an enterprise’s actual circumstances [26]. The accuracy of the emission factor method is contingent on the emission factors employed. Therefore, the utilization of emission factors by enterprises in China should be prioritized when accounting for these entities [40]. A comparison of the environmental statistics from 2021 revealed that the CO2 emissions of SH Petrochemical’s refining segment amounted to 5.2 million tons per year. This figure exceeds the result reported in the present study. The potential causes of this discrepancy may include the process of CO2 emission, which is associated with the hydrocracking process, which, in turn, may have been underestimated due to the absence of relevant data. This potential oversight could have led to inaccurate calculations of the process emissions, leading to a lower result.
The synergistic coefficient of air pollutant emission reduction for chlor-alkali projects is much higher than that of oil-refining and ethylene production enterprises. Evidently, the synergistic coefficients of different manufacturers and the end-of-pipe technologies for different pollutants can be diverse. Thus, the synergistic coefficients of end-of-pipe technologies are affected by a variety of factors, such as the products, pollutant types, and treatment technologies. According to the “dual-carbon” goal, when selecting end-of-pipe technologies, in addition to considering their emission reduction effects on specific pollutants, it is also necessary to comprehensively consider their effects on GHG emissions, their technical feasibility, and the synergistic benefits, among other factors. The research in this paper can help the chemical industry and enterprises to select decarbonized pollution treatment technologies and realize simultaneous air pollution and carbon reduction. In the foreseeable future, the end-of-pipe treatment of localized air pollutants will remain an important means of reducing pollutant emissions and improving the air quality. Therefore, conducting a systematic study on the synergistic coefficients of end-of-pipe treatment technologies for various pollutants in different industries and enterprises, and establishing a corresponding database, will provide data support and a basis for the selection of decarbonized end-of-pipe treatment technologies in different industries and enterprises.
(2) Quantification and implications of synergistic effects of pollution and carbon reduction in end-of-pipe treatment technologies for chemical enterprises: The synergistic effects of localized air pollutant and GHG emission reduction are evident in the changes in emissions and their ecological impacts. Quantitative research on synergistic effects, based on ecological and environmental impacts, can assist industries and enterprises in comprehensively considering their production characteristics and environmental impacts, thereby helping them to formulate targeted pollution and carbon reduction strategies. In this study, we incorporated the life cycle approach into the quantitative analysis of synergies, combined it with simulations of low-carbon development scenarios, and quantitatively evaluated the synergistic effects of emission reduction in terms of environmental impacts. The study of synergistic effects can be expanded from the accounting of emissions to the assessment of environmental impacts, thus providing a technical reference for the development of relevant policies and the assessment of implementation effects. Our findings indicate that the oil-refining industry, through its use of crude oil as a primary raw material, is a significant consumer of fuel oil and electricity. China’s thermal-power-based energy structure is characterized by substantial fossil fuel consumption, contributing to the depletion of fossil resources and ensuring that it has the greatest environmental impact as a proportion of the total load. The oil-refining industry must prioritize optimizing its energy structures, enhancing its energy efficiency, and achieving energy conservation and emission reduction. Secondly, the three enterprises examined in this study also exhibited higher values of MAETP loads. Notably, SK Ethylene demonstrated the highest impact load value for this indicator, underscoring the need for this enterprise to address potential impacts on the marine ecosystem that may arise from its production processes. The ADP element and GWP of HS Chlor-Alkali accounted for the largest proportions of their total environmental impact loads, a fact that may be attributed to the substantial consumption of salts and abiotic resources in the chlor-alkali production process. Moreover, this finding underscores the need for HS Chlor-Alkali to implement continuous improvements in its utilization of abiotic resources and regulation of carbon dioxide emissions. An examination of the synergistic effects, grounded in the life cycle environmental impacts in future scenarios, reveals that the oil-refining industry has the highest cross-elasticity coefficient, indicating strong synergies between air pollutant and GHG emission reduction. In contrast, the chlor-alkali industry exhibits the lowest value and the weakest synergistic effect. In the future, this research is anticipated to be extended to various sectors, including diverse chemical manufacturing enterprises. The findings will serve as a reference with which government management departments can formulate more targeted and synergistic emission reduction policies across different industries.
(3) Absence of real-time monitoring data: The absence of real-time monitoring data constitutes a limitation of this study. Because of the availability of real-time CEMS data and its focus on major pollution sources and discharge outlets, it lacks comprehensive coverage. Furthermore, issues such as sensor aging and data loss can compromise data reproducibility. While real-time CEMS data remain limited in our study context (particularly for VOC fugitive sources), we maximized the data robustness through cross-validation methods (e.g., material balance calculations). We believe that, with broader deployment and technological refinements of real-time CEMS, such monitoring data will see wider application in the future.
(4) Use of static electricity emission factors: At present, real-time CEMS data mainly focus on the major pollution sources and major discharge outlets, and they do not cover all of them. Moreover, there are problems such as sensor aging and data loss, which will affect the reproducibility of the data as well. While real-time CEMS data remain limited in our study context (particularly for VOC fugitive sources), we maximized the data robustness through cross-validation (e.g., using material balance calculations). However, the embodied CO2 emissions from the net purchased electricity were calculated using the emission factor approach. As a fundamental tool in current greenhouse gas accounting systems, this methodology is designated as the standard method in the IPCC Guidelines and China’s Provincial Greenhouse Gas Inventory Compilation Guidelines. The emission factors, uniformly published annually by the state (Study on CO2 Emission Factors for China’s Regional Power Grids), prevent reporting deviations arising from enterprise-selected factors, thus enhancing the comparability of emission data across enterprises and industries. Furthermore, the emission factors applied in the calculations corresponded to activity-level data, thereby improving the reliability of the results. However, the annual average power emission factor adopted lacks the dynamic nature of spatiotemporal differentiation. Therefore, in the future, research should fully consider spatiotemporally refined accounting—for instance, a hierarchical factor system can be established.
(5) Promotion of research results: In the study of the co-benefits between atmospheric pollutant and greenhouse gas emission reductions, the accuracy of the results is highly dependent on the precision of the accounted emissions. Future research will include the accounting of emissions data from various enterprises in the chemical industry over multiple years. By comparatively analyzing the synergy coefficients and cross-elasticity coefficients, we aim to derive more generalizable patterns from these data.
5. Conclusions
The emission inventory analysis indicates that refining and ethylene production predominantly emit SO2, NOx, particulate matter, and VOCs. In contrast, chlor-alkali production primarily discharges specific pollutants such as Cl2, HCl, and VOCs. Regarding CO2 emissions, the principal contributors in refining processes are emissions generated by fuel combustion and those associated with net purchased electricity and heat. In ethylene production, the key emission sources include fuel combustion, flare combustion, and emissions linked to net purchased electricity and heat. For chlor-alkali production, the emission sources encompass fuel combustion, process emissions, direct carbon emissions from end-of-pipe treatments, and implied emissions from net purchased electricity and heat.
The results of our synergy analysis indicate significant variability in the synergistic coefficients for emission reduction in all three enterprises and across different end-of-pipe technologies. The synergistic coefficients for one air pollutant treatment vary significantly across chemical industries and production sectors. Overall, for each ton of air pollutant reduced, SH Petrochemical, SK Ethylene, and HS Chlor-Alkali increase their CO2 emissions by 3.8, 7.1, and 81.8 tons, respectively.
The life cycle environmental impact assessment results indicate that the environmental impact load values per ton product for SH Petrochemical (oil-refining sector), SK Ethylene Project, and HS Chlor-Alkali are 5.4 × 10−9, 7.0 × 10−9, and 6.5 × 10−10, respectively. SK Ethylene exhibited the highest environmental impact per unit product, while HS Chlor-Alkali showed the lowest. The primary environmental impacts associated with the production processes of these three companies include ADP fossil, GWP, MAETP, and AP, with some convergence in these areas. Additionally, ADP fossil presented the highest environmental impact loading ratio.
The scenario analysis results reveal that implementing low-carbon development policies can substantially mitigate the environmental impacts of the chemical industry, particularly concerning AP, GWP, and MAETP. Under a low-carbon development scenario, the total environmental impact load values for SH Petrochemical (in the refining sector), SK Ethylene, and HS Chlor-Alkali decrease by 3.2%, 3.9%, and 4.9%, respectively, compared to the baseline scenario. Consequently, optimizing the upstream power structure, substituting “green hydrogen” for “grey hydrogen” in refinery hydrogenation units, and reducing energy consumption, such as that of electricity and steam, in ethylene and chlor-alkali production processes are effective strategies for the achievement of low-carbon development in the chemical industry. Under the scenario of low-carbon development, the environmental impact loads resulting from atmospheric pollutant and greenhouse gas emissions can be synergistically reduced for all three enterprises mentioned above. In particular, the refinery enterprise demonstrates strong synergistic effects, whereas the chlor-alkali enterprise exhibits relatively weaker synergistic effects.
Author Contributions
Conceptualization, W.M.; data curation, Y.C.; investigation, Q.G., Y.C. and Y.X.; writing—original draft, Q.G.; writing—review and editing, W.T. and W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China Special Project—Research on the Optimization of Synergistic Paths and Realization Mechanisms of Pollution Reduction and Carbon Reduction in Key Regions (Grant No. 72243008).
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A
SH Petrochemical, SK Ethylene, and HS Chlor-Alkali’s organized waste gas is accounted for by field measurement, the material balance algorithm, and analogy, and the leading accounting equations are as follows.
Sulfur dioxide emissions from the heating furnace are calculated using the material balance algorithm with the following equation:
where denotes the SO2 production in the accounting period, t; denotes the fuel consumption in the accounting period, t; and denotes the sulfur content in fuel, %.
The amount of SO2 generated in the tail gas of the sulfur recovery unit is calculated by the field measurement method:
where E denotes the amount of SO2 produced during the accounting period, kg; denotes the flow rate of acid gas during the accounting period, m3; denotes the volume fraction of H2S in the acidic gas, %; and denotes the sulfur recovery rate, %.
NOx, particulate matter, and NMHC can be accounted for using the analogy method. By analogy with the NOx emission concentration of the heating furnace in similar existing projects, the NOx concentration in the exhaust gas of the heating furnace of SH Petrochemical is found to be no more than 40 mg/Nm3; the concentration of particulate matter in the exhaust gas of the process heating furnace is no more than 2.0 mg/Nm3; and the concentration of NMHC in the exhaust gas of the process heating furnace is no more than 5.0 mg/Nm3.
VOC emissions from flaring operations can be quantified through two equations. When actually measuring the flare,
where denotes the VOC generation volume of the flare, kg; denotes the flow rate of the flare gas, m3/h; denotes the working time of the flare, h; denotes the volume fraction of VOCs; denotes the molecular weight of VOCs, kg/kmol; and denotes the combustion efficiency of the flare, %.
When no actual measurements were taken of the flare, the flare VOC generation was calculated as follows:
where denotes the amount of VOCs generated by the flare, kg; denotes the total amount of exhaust gas discharged into the flare, kg; and denotes the combustion efficiency of the flare, %.
Combustion flue gas emissions and process organized emissions of volatile organic pollutants (VOCs), Cl2, HCl, and other organized exhaust emissions of NOx, particulate matter, etc., are accounted for by the actual measurement method. The specific formula is as expressed in Equation (A5):
where indicates the actual emissions of the jth pollutant of the main organized emission outlet of the exhaust gas during the accounting period, t; indicates the measured average emission concentration of the jth pollutant in the ith hour, mg/m3; indicates the dry flue gas volume of the jth pollutant in the ith hour under the standard state, Nm3/h; and n indicates the emission time, h.
For the accounting of VOC emissions in the unorganized exhaust, we mainly use the equation method and TANKS model method. The main accounting formulas are as follows.
Emissions from cooling towers and recirculating cooling systems are quantified according to Equation (A6), while leakage from both the dynamic and static sealing points of equipment is determined through the formulaic approach outlined in Equation (A7).
where denotes the amount of VOCs generated by the cooling tower and the recirculating cooling system, kg; Q denotes the flow rate of the recirculating water in the cooling tower, m3; signifies the concentration of evaporative volatile organic compounds (EVOCs) in the cooling water before exposure to air, mg/L; indicates the concentration of evaporative volatile organic compounds (EVOCs) in the cooling water after exposure to air, mg/L; and t denotes the operational hours of the combustion flue gas emission equipment, h.
where denotes the amount of VOCs generated from equipment leakage during the statistical period, kg; denotes the operating time of sealing point i during the statistical period, h; represents the leakage rate of total organic compounds (TOCs) at sealing point i, kg/h; indicates the average mass fraction of VOCs in the material flowing through sealing point i during the operating period; and denotes the average mass fraction of TOCs in the material flowing through sealing point i during the operating period.
The volatilization loss from organic liquid storage and blending is calculated using the TANKS model method, as recommended in the U.S. Environmental Protection Agency’s “Air Emissions Factors and Quantification (AP-42)” [41,42].
In GHG accounting, the CO2 emissions implied by net purchased electricity and heat are calculated using the coefficient method, while other emission sources are accounted for using the carbon balance method. The specific methods are as follows:
where denotes CO2 emissions from fuel combustion, t; denotes CO2 emissions from flare combustion, t; denotes CO2 emissions during industrial production, t; and denote the CO2 emissions embedded in the net purchased electricity and heat, respectively, t; and denotes the amount of CO2 recovered and utilized, t.
where the subscript i denotes different types of fuel; denotes the amount of fuel combustion, indicating the physical consumption of various fossil fuels, t or m3; denotes the carbon content of fossil fuels, t-C/t or t-C/m3; and denotes the carbon oxidation rate of the fuel, %.
where the subscript i denotes the serial number of the flare; denotes the CO2 emissions from flare gas combustion under normal operating conditions, t; Q denotes the flow rate of flare gas, m3; denotes the total carbon content of other carbon-containing compounds in the flare gas, in addition to CO2, t-C/m3; denotes the carbon oxidation rate of the flare system, %; and denotes the volumetric concentration of CO2 in the flare gas, %.
where the subscript j denotes the number of accidents; denotes the CO2 emissions from flare gas combustion due to the accident, t; denotes the average flare gas flow rate at the jth accident state, m3/h; denotes the duration of the accident, h; denotes the average number of carbon atoms in the molar fraction of the accidental flare gas; and 44 is the molar mass of CO2, g/mol.
where “activity level data” refers to the use of raw materials or the production of products and semi-finished products [43], t; the emission factor should correspond to the activity level data, tCO2/t.
where and denote the amount of CO2 gas recovered for external supply and the amount recovered for feedstock, respectively; PUR denotes the CO2 purity of the gas.
where AD denotes the net purchased electricity and heat consumption of the enterprise, i.e., the net difference between the purchased and external supplies; EF denotes the CO2 emission factor of the electricity and heat supply (average CO2 emission factor of electricity supply and average CO2 emission factor of heat supply in regional grid).
Appendix B

Table A1.
Names of environmental impact midpoint indicators and standardization factors.
Table A1.
Names of environmental impact midpoint indicators and standardization factors.
| Environmental Impact Indicator | Unit | Abbreviation | Standardized Factor |
|---|---|---|---|
| Abiotic depletion | kg Sb eq | ADP element | 1.80 × 10−8 |
| Abiotic depletion (fossil fuels) | MJ | ADP fossil | 3.18 × 10−14 |
| Global warming (GWP100a) | kg CO2 eq | GWP | 1.99 × 10−13 |
| Ozone depletion | kg CFC-11 eq | ODP | 1.12 × 10−8 |
| Human toxicity | kg 1,4-DB eq | HTP | 1.29 × 10−13 |
| Fresh water aquatic ecotoxicity | kg 1,4-DB eq | FAETP | 1.93 × 10−12 |
| Marine aquatic ecotoxicity | kg 1,4-DB eq | MAETP | 8.57 × 10−15 |
| Terrestrial ecotoxicity | kg 1,4-DB eq | TETP | 2.06 × 10−11 |
| Photochemical oxidation | kg C2H4 eq | POCP | 1.18 × 10−10 |
| Acidification | kg SO2 eq | AP | 3.55 × 10−11 |
| Eutrophication | kg PO4-eq | EP | 7.58 × 10−11 |

Table A2.
Chemical industry future (2035) scenario setting and related parameters.
Table A2.
Chemical industry future (2035) scenario setting and related parameters.
| Type | Name | Unit | Refinery Project | Ethylene Project | Chlor-Alkali Project | |||
|---|---|---|---|---|---|---|---|---|
| Baseline Scenario | Low-Carbon Development Scenario | Baseline Scenario | Low-Carbon Development Scenario | Baseline Scenario | Low-Carbon Development Scenario | |||
| Upstream power structure | Thermal power | % | 66.5 | 42.39 | 66.5 | 42.39 | 66.5 | 42.39 |
| Hydroelectricity | % | 15.28 | 18.7 | 15.28 | 18.7 | 15.28 | 18.7 | |
| Nuclear energy | % | 4.72 | 5.65 | 4.72 | 5.65 | 4.72 | 5.65 | |
| Wind power | % | 8.61 | 18.7 | 8.62 | 18.7 | 8.62 | 18.7 | |
| Solar power | % | 4.83 | 14.55 | 4.83 | 14.55 | 4.83 | 14.55 | |
| Process improvement | Substitution of green hydrogen for grey hydrogen | Total energy consumption of electricity and steam decreased by 1.5% | Total energy consumption of electricity and steam decreased by 1.5% | |||||
Note: In the baseline scenario, the power generation structure of externally purchased electricity by enterprises is based on the installed capacity data of various power sources for the benchmark year as published by the National Bureau of Statistics. The installed capacity proportions for thermal, hydropower, nuclear, wind, and photovoltaic power are 66.5%, 15.28%, 4.72%, 8.61%, and 4.83%, respectively.
Appendix C

Table A3.
SH Petrochemical’s process devices, organized air pollutants, control measures, and emission accounting.
Table A3.
SH Petrochemical’s process devices, organized air pollutants, control measures, and emission accounting.
| Device | Serial Number | Source of Pollution | Governance Measures | Exhaust Volume/ (Nm3/h) | Generation (t/a) | Emissions (t/a) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SO2 | NOx | Particulate Matter | NMHC | SO2 | NOx | Particulate Matter | NMHC | |||||
| Boiling Bed Residue Hydrogenation Plant | G1-2-1 | Heating furnace flue gas | Low-sulfur fuel gas, ultra-low-NOx burners | 60,000 | 2.02 | 20.16 | 1.01 | 1.01 | 2.02 | 20.16 | 1.01 | 1.01 |
| Hydrocracker | G1-3-1 | Heating furnace flue gas | Low-sulfur fuel gas, ultra-low-NOx burners | 64,500 | 2.17 | 21.67 | 1.08 | 1.08 | 2.17 | 21.67 | 1.08 | 1.08 |
| Sulfur Recovery Combined Unit | G1-6-1 | Incinerator tail gas | RASOC | 62,590 | 600.94 | / | / | / | 13.15 | / | / | / |
| POX Hydrogen Plant | G1-7-1 | Petroleum rubber silo exhaust | Bag filter | 1000 | / | / | 8.40 | 0.17 | / | / | 0.04 | 0.17 |
| G1-7-2 | Slag pond air release | / | 162 | / | / | / | 0.03 | / | / | / | 0.03 | |
| G1-7-3 | Acid gas Stripping tail gas | Washing tower | 13,056 | / | / | / | 54.67 | / | / | / | 11.68 | |
| Total | 605.12 | 41.83 | 10.49 | 56.95 | 17.33 | 41.83 | 2.13 | 13.96 | ||||
Note: POX: petroleum coke to hydrogen; RASOC: regenerable absorption process for SOx cleanup, i.e., renewable wet flue gas desulfurization technology.

Table A4.
SH Petrochemical’s combustion processes, organic air pollutant control measures, and emission accounting.
Table A4.
SH Petrochemical’s combustion processes, organic air pollutant control measures, and emission accounting.
| Type | Serial Number | End-of-Pipe Process | SO2 Generation (t/a) | SO2 Emissions (t/a) | NOx Generation (t/a) | NOx Emissions (t/a) | Particulate Matter Generation (t/a) | Particulate Matter Emissions (t/a) | VOC Generation (kg/a) | VOC Emissions (kg/a) |
|---|---|---|---|---|---|---|---|---|---|---|
| Oil boiler | MF0342 | Desulfurization—sodium hydroxide; Denitrification—selective catalytic reduction; Dedusting—Venturi | 129.9 | 0.73 | 369.24 | 71.41 | * | 11.77 | 0 | 0 |
| MF0343 | 135.57 | 0.7 | 313.86 | 72.53 | * | 11.42 | 0 | 0 | ||
| Subtotal | 265.47 | 1.43 | 683.1 | 143.94 | * | 23.19 | 0 | 0 | ||
| Heating crude oil | MF0922 | / | 4.13 | 4.13 | 34.84 | 34.84 | 3.4 | 3.4 | 12,619.98 | 12,619.98 |
| MF0923 | 4.13 | 4.13 | 34.84 | 34.84 | 3.4 | 3.4 | 12,619.98 | 12,619.98 | ||
| MF0924 | 4.13 | 4.13 | 34.84 | 34.84 | 3.4 | 3.4 | 12,619.98 | 12,619.98 | ||
| MF0938 | 6.33 | 6.33 | 53.49 | 53.49 | 5.22 | 5.22 | 19,418.95 | 19,418.95 | ||
| MF0939 | 6.33 | 6.33 | 53.49 | 53.49 | 5.22 | 5.22 | 19,418.95 | 19,418.95 | ||
| Subtotal | 25.05 | 25.05 | 211.5 | 211.5 | 20.64 | 20.64 | 76,697.84 | 76,697.84 | ||
| Total | 290.52 | 26.48 | 894.6 | 355.44 | * | 43.83 | 76,697.84 | 76,697.84 |
Note: Data for each sector are from the EIA report, and data for oil boilers are from the 2017 EMS data. “*” indicates missing data.

Table A5.
SK Ethylene’s air pollutant emission accounting.
Table A5.
SK Ethylene’s air pollutant emission accounting.
| Device | NOx | TSP | SO2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Governance Measures | Generation (t/a) | Emission Reduction (t/a) | Emissions (t/a) | Governance Measures | Generation (t/a) | Emission Reduction (t/a) | Emissions (t/a) | Disposal Measures | Generation (t/a) | Emission Reduction (t/a) | Emissions (t/a) | |
| Ethylene plant | PCC’s patented technology for nitrogen removal | 2386.95 | 834.87 | 1552.08 | Not present | 90.19 | 0 | 90.19 | Not present | 2.26 | 0 | 2.26 |
| Ethylbenzene/styrene (EB/SM) plant | Low-nitrogen burners | 384.66 | 134.63 | 250.03 | Not present | 0 | 0 | 0 | Not present | 5.68 | 0 | 5.68 |
| Polystyrene (PS) units | Low-nitrogen burners | 11.79 | 4.13 | 7.66 | Not present | 0 | 0 | 0 | Not present | 0 | 0 | 0 |
| Acrylonitrile (I and II) plant | AOGI PCC denitrification | 861.66 | 424.34 | 437.32 | Bag filter | 1911.82 | 1902.26 | 9.56 | Not present | 8.82 | 0 | 8.82 |
| Sulfuric acid recovery (SAR) unit | Organic catalytic flue gas desulfurization and denitrification | 139.20 | 37.85 | 101.35 | Not present | 0 | 0 | 0 | Organic catalytic flue gas desulfurization and denitrification | 658.54 | 492.83 | 165.70 |
| Butadiene (BEU1 and 2) plant | Incorporation into acrylonitrile (I and II) unit | Incorporation into acrylonitrile (I and II) unit | Incorporation into acrylonitrile (I and II) unit | |||||||||
| Power center | SCR denitrification | 1125.42 | 776.54 | 348.88 | Ammonia desulfurization and dedusting | 19.13 | 6.31 | 12.82 | Ammonia desulfurization and dedusting | 115.20 | 62.21 | 52.99 |
| Combined heat and power supply | Low-nitrogen burners | 1681.07 | 588.37 | 1092.69 | Not present | 0 | 0 | 0 | Not present | 0 | 0 | 0 |
| Total | / | 4203.80 | 1965.86 | 2237.94 | / | 2021.13 | 1908.57 | 112.56 | / | 790.49 | 555.04 | 235.44 |

Table A6.
SK Ethylene’s VOC emission accounting.
Table A6.
SK Ethylene’s VOC emission accounting.
| Type | Segment | Disposal Measures | VOCs | ||
|---|---|---|---|---|---|
| Generation (t/a) | Emission Reduction (t/a) | Emissions (t/a) | |||
| Organized emissions | Combustion flue gas emissions | Absorption tower + AOGI thermal incineration | 9187.33 | 9028.53 | 158.80 |
| Organized emissions from processes | Not present | 0.3 | 0 | 0.3 | |
| Flare emissions | Flare thermal incineration | 23,409.68 | 22,941.04 | 468.64 | |
| Subtotal | 32,597.31 | 31,969.57 | 627.74 | ||
| Unorganized emissions | Organic liquid storage and reconciliation of volatilization losses | AOGI thermal incineration | 162.14 | 153 | 9.13 |
| Leakage at static and dynamic sealing points of equipment | Not present | 259.72 | 0.00 | 259.72 | |
| Cooling tower, circulating water, cooling system release | Not present | 358.98 | 0.00 | 358.98 | |
| Subtotal | 780.84 | 153 | 627.83 | ||
| Total | 33,378.15 | 32,122.57 | 1255.57 | ||

Table A7.
HS Chlor-Alkali’s VOC emission accounting.
Table A7.
HS Chlor-Alkali’s VOC emission accounting.
| Type | Segment | Device | VOCs | Note | |||
|---|---|---|---|---|---|---|---|
| Disposal Measures | Generation (t/a) | Emission Reduction (t/a) | Emissions (t/a) | ||||
| Unorganized emissions | Organic liquid storage and reconciliation of volatilization losses | Storage tank area | Not present | 156.88 | 0.00 | 156.88 | |
| Leakage at static and dynamic sealing points of equipment | / | Not have | 9.58 | 0.00 | 9.58 | ||
| Organized emissions | Organized emissions from processes | Caustic soda plant | Not present | / | / | / | |
| EDC device | High-temperature incineration | 9.20 | 9.11 | 0.09 | |||
| Hydrogen boiler | Not present | / | / | / | |||
| Combustion flue gas emissions | / | / | / | / | / | Use of hydrogen | |
| Flare emissions | / | / | / | / | / | No flare | |
| Total | 175.66 | 9.11 | 166.55 | ||||

Table A8.
SH Petrochemical’s fuel combustion CO2 emissions.
Table A8.
SH Petrochemical’s fuel combustion CO2 emissions.
| Type of Fuel | Annual Use | Unit | Calorific Value (GJ/t, GJ/million m3) | Carbon Content Per Unit Calorific Value (tC/GJ) | Carbon Oxidation Rate | CO2 Emissions (tCO2/a) |
|---|---|---|---|---|---|---|
| Fuel oil | 46,386 | t/a | 41.86 | 0.015 | 0.99 | 317,723.08 |
| Petroleum | 183,026.15 | million m3/a | 390 | 0.015 | 0.99 | 3,964,384.78 |
| Total | 4,282,107.86 | |||||
Note: Energy consumption data are from 2017 environmental statistics.

Table A9.
SK Ethylene project’s fuel combustion CO2 emissions.
Table A9.
SK Ethylene project’s fuel combustion CO2 emissions.
| Type of Fuel | Annual Use | Unit | Calorific Value (GJ/t, GJ/million m(3)) | Carbon Content per Unit Calorific Value (tC/GJ) | Carbon Oxidation Rate | CO2 Emissions (tCO2/a) | Other Information |
|---|---|---|---|---|---|---|---|
| Fuel oil | 95,700 | t/a | 42 | 0.0211 | 0.98 | 304,748.23 | Self-production |
| Fuel gas | 7.9 | Million m3/a | 380 | 0.0153 | 0.99 | 166.73 | Blending of plant-produced hydrogen methane with purchased natural gas, with hydrogen methane components similar to natural gas |
| Total | 304,914.96 | ||||||

Table A10.
SK Ethylene project’s flare CO2 emissions.
Table A10.
SK Ethylene project’s flare CO2 emissions.
| Flare Model | Flare Gas Flow Rate (Million Nm3/a) | Total Carbon Content of Carbon-Containing Compounds (Tons of Carbon/10,000 Nm3) | Carbon Oxidation Rate | CO2 Emissions (tCO2/a) | |
|---|---|---|---|---|---|
| HP-1 | 2639.34 | 6.52 | 0.98 | 61,812.06 | |
| HP-2 | 1376.69 | 9.71 | 0.98 | 48,009.87 | |
| LP-A | 484.04 | 12.50 | 0.98 | 21,736.93 | |
| LP-B | 673.34 | 4.70 | 0.98 | 11,377.80 | |
| LP-C | 2330.20 | 7.85 | 0.98 | 65,768.37 | |
| Low-temperature flare | Inlet pipeline 1 | 0.00 | 13.82 | 0.98 | 0.48 |
| Inlet pipeline 2 | 0.13 | ||||
| Acrylonitrile flare | Craft torch | 7.38 | 10.53 | 0.8 | 318.80 |
| Hydrocyanic acid Norming furnace | 131.40 | ||||
| Total | 209,024.31 | ||||

Table A11.
SK Ethylene project’s process CO2 emissions (ethylene cracker).
Table A11.
SK Ethylene project’s process CO2 emissions (ethylene cracker).
| Serial Number | Average Tail Gas Flow (Nm3/h) | Annual Cumulative Coking Time (h/a) | Volume Concentration of CO2 in Exhaust Gas (%) | CO2 Emissions (tCO2/a) |
|---|---|---|---|---|
| 1 | 90,100 | 216 | 0.20% | 76.68 |
| 2 | 272,000 | 216 | 0.20% | 231.48 |
| 3 | 272,000 | 216 | 0.20% | 231.48 |
| 4 | 252,000 | 216 | 0.20% | 214.46 |
| 5 | 258,000 | 216 | 0.20% | 219.57 |
| 6 | 276,000 | 216 | 0.20% | 234.89 |
| 7 | 220,000 | 216 | 0.20% | 187.23 |
| 8 | 249,000 | 216 | 0.20% | 211.91 |
| 9 | 240,000 | 216 | 0.20% | 204.25 |
| 10 | 120,000 | 216 | 0.20% | 102.12 |
| 11 | 114,000 | 216 | 0.20% | 97.02 |
| Total | 2011.09 | |||

Table A12.
Direct CO2 emissions related to end-of-pipe treatment in SK Ethylene project.
Table A12.
Direct CO2 emissions related to end-of-pipe treatment in SK Ethylene project.
| Pollutant | Department | End-of-Pipe Management Equipment | Direct CO2 Emissions from End-of-Pipe Treatment (t/a) |
|---|---|---|---|
| VOCs | Vertical fixed roof tanks | AOGI thermal incineration | 480.22 |
| Acrylonitrile I | AOGI thermal incineration | 28,337.55 | |
| Total | 28,817.76 |

Table A13.
HS Chlor-Alkali project’s fuel combustion CO2 emissions.
Table A13.
HS Chlor-Alkali project’s fuel combustion CO2 emissions.
| Type of Fuel | Annual Use | Unit | Source | Low-Level Heat Generation (GJ/million Nm3) | Carbon Content per Unit Calorific Value (tC/GJ) | Carbon Oxidation Rate | CO2 Emissions (tCO2/a) |
|---|---|---|---|---|---|---|---|
| Petroleum | 145.2 | million Nm3/a | Natural gas pipeline in chemical zone | 389.31 | 0.0153 | 0.99 | 3139.50 |
| Total | 3139.50 | ||||||

Table A14.
HS Chlor-Alkali’s process CO2 emissions.
Table A14.
HS Chlor-Alkali’s process CO2 emissions.
| Device | CO2 Emissions from Raw Material Consumption | CO2 Emissions from Carbonate Use Processes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carbon-Containing Raw Materials | Dosage (104t/a) | Carbon-Containing Product | Production (10,000 t/a) | CO2 Emissions (tCO2/a) | Carbonate Type | Consumption (t/a) | CO2 Emission Factor (Tons CO2/Tons Carbonate) | Purity of Carbonate (%) | CO2 Emissions (tCO2/a) | |
| Caustic soda plant | / | 0 | Not present | 0 | 0 | Na2CO3 | 5000 | 0.4149 | 100 | 2074.5 |
| EDC device | Ethylene | 12 | Refined EDC | 41 | 12,698.41 | / | 0 | / | / | 0 |
| Total | 14,772.91 | |||||||||

Table A15.
Direct CO2 emissions associated with end-of-pipe management in HS Chlor-Alkali project.
Table A15.
Direct CO2 emissions associated with end-of-pipe management in HS Chlor-Alkali project.
| Pollutant | Device | Exhaust Port | End-of-Pipe Management Equipment | Direct CO2 Emissions from End-of-Pipe Treatment (t/a) |
|---|---|---|---|---|
| VOCs | EDC device | 1# Incinerator | High-temperature incineration | 138.99 |
| 2# Incinerator | High-temperature incineration | |||
| Total | 138.99 | |||
Appendix D

Table A16.
SH Petrochemical’s synergy factors.
Table A16.
SH Petrochemical’s synergy factors.
| Pollutant | Department | Emission Reduction Technologies/ End-of-Pipe Equipment | Equipment Power/ Electricity Consumption Factor | Emission Reduction (t/a) | Electricity Consumption (kWh/a) | Indirect CO2 Emissions (t/a) | Synergistic Coefficient: Amount of Additional CO2 Emitted per Ton of Air Pollutant Reduced (t, both Direct and Indirect) |
|---|---|---|---|---|---|---|---|
| SO2 | Boiling bed residue hydrotreating unit | Low-sulfur fuel gas | / | / | / | / | / |
| Hydrocracker | / | / | / | / | / | ||
| Sulfur recovery combined unit | RASOC renewable wet flue gas | 1280 kW | 587.79 | 10,752,000.00 | 4351.97 | 7.40 | |
| Oil boiler | NaOH | / | 264.04 | / | / | / | |
| NOx | Boiling bed residue hydrotreating unit | Ultra-low-nitrogen burners | / | / | / | / | / |
| Hydrocracker | / | / | / | / | / | ||
| Oil boiler | SCR | 295.5 kW | 539.16 | 2,511,750.00 | 1016.65 | 1.89 | |
| Particulate matter | POX hydrogen plant | Bag filter | 10 kWh/tTSP | 8.36 | 83.58 | 0.03 | 0.0041 |
| Total | 1399.35 | 13,263,833.58 | 5368.66 | 3.84 | |||

Table A17.
SK Ethylene project’s synergistic coefficients.
Table A17.
SK Ethylene project’s synergistic coefficients.
| Pollutant | Department | Emission Reduction Technologies/ End-of-Pipe Equipment | Equipment Power/Electricity Consumption Factor | Emission Reduction (t/a) | Electricity Consumption (kWh/a) | Direct CO2 Emissions (t/a) | Indirect CO2 Emissions (t/a) | Synergistic Coefficient: Amount of Additional CO2 Emitted per Ton of Air Pollutant Reduced (t, both Direct and Indirect) |
|---|---|---|---|---|---|---|---|---|
| NOx | Acrylonitrile I | AOGI PCC denitrification | / | 236.38 | / | 0 | / | / |
| Acrylonitrile II | / | 187.97 | / | 0 | / | / | ||
| Power center | SCR denitrification | 295.5 kW | 776.54 | 2,364,000 | 0 | 956.85 | 1.23 | |
| Subtotal | / | / | 776.54 | 2,364,000 | 0 | 956.85 | 1.23 | |
| Particulate matter | Acrylonitrile I | Bag filter | 10 kWh/tTSP | 1628.59 | 16,285.9 | 0 | 6.59 | 0.00405 |
| Acrylonitrile II | 10 kWh/tTSP | 273.66 | 2736.6 | 0 | 1.11 | 0.00405 | ||
| Subtotal | / | / | 1902.25 | 19,022.5 | 0 | 7.70 | 0.00405 | |
| VOCs | Vertical fixed roof tanks | AOGI thermal incineration | 1538.32 kWh/t (VOC) | 100.27 | 154,247.35 | 480.22 | 62.43 | 3.76 |
| Floating roof tanks | 52.73 | 81,115.61 | 32.83 | |||||
| Acrylonitrile I | 4876.14 | 7,501,058.64 | 28,337.55 | 3036.12 | 3.76 | |||
| Acrylonitrile II | 4114.13 | 6,328,841.37 | 2561.66 | |||||
| Power center | 36.03 | 55,425.92 | 22.43 | |||||
| Ground flare | Flare thermal incineration | 611.41 kW | 22,938.15 | 4,891,307.57 | 209,024.31 | 1979.80 | 9.20 | |
| Low-temperature flare | 0.91 | 1395.99 | 0.57 | |||||
| Acrylonitrile flare | 1.98 | 3027.78 | 1.23 | |||||
| Subtotal | / | / | 32,120.33 | 19,016,420.23 | 237,842.08 | 7697.07 | 7.64 | |
| SO2, particulate matter | Power center | Ammonia desulfurization and dust removal | 1124.3 kW | 62.21 t SO2; 6.31 t TSP | 8,994,400 | 0 | 3640.56 | 53.13 |
| SO2, NOx | Sulfuric acid recovery | Organocatalytic flue gas desulfurization | 697.32 kW | 492.83 t SO2; 37.85 t NOx | 5,578,560 | 0 | 2257.97 | 4.25 |
| Total | 35,398.32 | 35,972,402.73 | 237,842.08 | 14,560.15 | 7.13 | |||

Table A18.
HS Chlor-alkali project’s synergies.
Table A18.
HS Chlor-alkali project’s synergies.
| Pollutant | Device | Exhaust Port | End-of-pipe Management Equipment | Equipment Power/ Electricity Consumption Factor | Emission Reduction (t/a) | Electricity Consumption (kWh/a) | Direct CO2 Emissions (t/a) | Indirect CO2 Emissions (t/a) | Synergistic Coefficient: Amount of Additional CO2 Emitted per Ton of Air Pollutant Reduced (t, both Direct and Indirect) |
|---|---|---|---|---|---|---|---|---|---|
| Cl2 | Caustic soda plant | 1# Waste chlorine absorption tower | Alkali absorption | 66,458.98 kWh/(ton Cl2) | 8.73 | 711,111.11 | 0 | 287.83 | 26.90 |
| 2# Waste chlorine absorption tower | 0.67 | ||||||||
| 3# Waste chlorine absorption tower | 1.30 | ||||||||
| Subtotal | 10.70 | 711,111.11 | 0.00 | 287.83 | |||||
| HCl | Caustic soda plant | 1# Hydrochloric acid tail gas treatment (A furnace) | Falling film absorption + water jet | 1,052,380.95 kWh/(ton HCl) | 0.20 | 2,103,634.59 | 0 | 851.47 | 425.95 |
| 1# Hydrochloric acid tail gas treatment (B furnace) | 0.00 | ||||||||
| 1# Hydrochloric acid tail gas treatment (C furnace) | 0.00 | ||||||||
| 1# Hydrochloric acid tail gas treatment (D furnace) | 0.11 | ||||||||
| 1# Hydrochloric acid tail gas treatment (E furnace) | 0.24 | ||||||||
| 1# Hydrochloric acid tail gas treatment (F furnace) | 1.44 | ||||||||
| Subtotal | 2.00 | 2,103,634.59 | 0.00 | 851.47 | |||||
| VOCs | EDC device | 1# Incinerator | High-temperature incineration | 136,915.60 kWh/(tonVOCs) | 4.56 | 1,247,287.57 | 138.99 | 504.85 | 70.67 |
| 2# Incinerator | 4.56 | ||||||||
| Subtotal | 9.11 | 1,247,287.57 | 138.99 | 504.85 | |||||
| Total | 21.81 | 4,062,033.27 | 138.99 | 1644.15 | 81.76 | ||||
Appendix E

Table A19.
SH Petrochemical’s life cycle environmental impact assessment results for the production of one ton of refined diesel fuel.
Table A19.
SH Petrochemical’s life cycle environmental impact assessment results for the production of one ton of refined diesel fuel.
| Environmental Impact Indicator | Unit | Abbreviation | Standardized Factor | Standardized Environmental Load Value | Percentage of Environmental Load |
|---|---|---|---|---|---|
| Abiotic depletion | ADP element | kg Sb eq | 7.28 × 10−5 | 1.31 × 10−12 | 0.02% |
| Abiotic depletion (fossil fuels) | ADP fossil | MJ | 118,867.92 | 3.78 × 10−9 | 69.48% |
| Global warming (GWP100a) | GWP | kg CO2 eq | 1035.18 | 1.71 × 10−10 | 3.79% |
| Ozone depletion | ODP | kg CFC-11 eq | 3.60 × 10−5 | 4.07 × 10−13 | 0.01% |
| Human toxicity | HTP | kg 1,4-DB eq | 182.95 | 2.32 × 10−11 | 0.43% |
| Fresh water aquatic ecotoxicity | FAETP | kg 1,4-DB eq | 1.58 | 2.97 × 10−12 | 0.06% |
| Marine aquatic ecotoxicity | MAETP | kg 1,4-DB eq | 1.31 × 105 | 9.89 × 10−10 | 20.59% |
| Terrestrial ecotoxicity | TETP | kg 1,4-DB eq | 0.16 | 2.97 × 10−12 | 0.06% |
| Photochemical oxidation | POCP | kg C2H4 eq | 1.04 × 10−1 | 1.20 × 10−11 | 0.23% |
| Acidification | AP | kg SO2 eq | 1.20 | 3.89 × 10−11 | 0.78% |
| Eutrophication | EP | kg PO4- eq | 3.27 | 2.47 × 10−10 | 4.56% |
| Abiotic depletion | / | / | / | 5.44 × 10−9 | 100.00% |

Table A20.
SK Ethylene’s life cycle environmental impact assessment results for the production of one ton of ethylene.
Table A20.
SK Ethylene’s life cycle environmental impact assessment results for the production of one ton of ethylene.
| Environmental Impact Indicator | Unit | Abbreviation | Standardized Factor | Standardized Environmental Load Value | Percentage of Environmental Load |
|---|---|---|---|---|---|
| Abiotic depletion | ADP element | kg Sb eq | 3.05 × 10−4 | 3.60 × 10−12 | 0.05% |
| Abiotic depletion (fossil fuels) | ADP fossil | MJ | 1.19 × 105 | 3.80 × 10−9 | 53.94% |
| Global warming (GWP100a) | GWP | kg CO2 eq | 1.83 × 103 | 3.63 × 10−10 | 5.15% |
| Ozone depletion | ODP | kg CFC-11 eq | 1.53 × 10−3 | 1.71 × 10−11 | 0.24% |
| Human toxicity | HTP | kg 1,4-DB eq | 261 | 3.37 × 10−11 | 0.48% |
| Fresh water aquatic ecotoxicity | FAETP | kg 1,4-DB eq | 39.5 | 7.63 × 10−11 | 1.08% |
| Marine aquatic ecotoxicity | MAETP | kg 1,4-DB eq | 2.58 × 105 | 2.21 × 10−9 | 31.37% |
| Terrestrial ecotoxicity | TETP | kg 1,4-DB eq | 0.921 | 1.90 × 10−11 | 0.27% |
| Photochemical oxidation | POCP | kg C2H4 eq | 0.438 | 5.17 × 10−11 | 0.73% |
| Acidification | AP | kg SO2 eq | 10.6 | 3.77 × 10−10 | 5.35% |
| Eutrophication | EP | kg PO4- eq | 1.24 | 9.40 × 10−11 | 1.33% |
| Abiotic depletion | / | / | / | 7.05 × 10−9 | 100.00% |

Table A21.
HS Chlor-Alkali’s life cycle environmental impact assessment results for the production of one ton of chlor-alkali.
Table A21.
HS Chlor-Alkali’s life cycle environmental impact assessment results for the production of one ton of chlor-alkali.
| Environmental Impact Indicator | Unit | Abbreviation | Standardized Factor | Standardized Environmental Load Value | Percentage of Environmental Load |
|---|---|---|---|---|---|
| Abiotic depletion | ADP element | kg Sb eq | 0.00504 | 5.95 × 10−11 | 9.22% |
| Abiotic depletion (fossil fuels) | ADP fossil | MJ | 6.94 × 103 | 2.21 × 10−10 | 34.23% |
| Global warming (GWP100a) | GWP | kg CO2 eq | 805 | 1.60 × 10−10 | 24.78% |
| Ozone depletion | ODP | kg CFC-11 eq | 9.72 × 10−6 | 1.09 × 10−13 | 0.02% |
| Human toxicity | HTP | kg 1,4-DB eq | 41.2 | 5.32 × 10−12 | 0.82% |
| Fresh water aquatic ecotoxicity | FAETP | kg 1,4-DB eq | 1.21 | 2.34 × 10−12 | 0.36% |
| Marine aquatic ecotoxicity | MAETP | kg 1,4-DB eq | 1.68 × 104 | 1.44 × 10−10 | 22.31% |
| Terrestrial ecotoxicity | TETP | kg 1,4-DB eq | 1.47 × 10−1 | 3.03 × 10−12 | 0.47% |
| Photochemical oxidation | POCP | kg C2H4 eq | 4.70 × 10−2 | 5.55 × 10−12 | 0.86% |
| Acidification | AP | kg SO2 eq | 1.08 | 3.83 × 10−11 | 5.93% |
| Eutrophication | EP | kg PO4- eq | 8.47 × 10−2 | 6.42 × 10−12 | 0.99% |
| Abiotic depletion | / | / | / | 6.46 × 10−10 | 100.00% |
Appendix F

Table A22.
Full list of acronyms.
Table A22.
Full list of acronyms.
| Abbreviation | Full Name |
|---|---|
| AOGI | Acrylonitrile Gas Incineration |
| GHG | Greenhouse Gas |
| CGE | Computable General Equilibrium |
| IES | Integrated Environmental Strategies |
| IIASA | International Institute for Applied Systems Analysis |
| LCA | Life Cycle Assessment |
| RASOC | Regenerable Absorption Process for SOx Cleanup |
| NMHC | Non-Methane Total Hydrocarbons |
| FGD | Flue Gas Desulfurization |
| SCR | Selective Catalytic Reduction |
References
- Arrow, K.J. Global Climate Change: A Challenge to Policy. Econ. Voice 2007, 4, 2. [Google Scholar] [CrossRef]
- Zhao, Q. A Review of Pathways to Carbon Neutrality from Renewable Energy and Carbon Capture. E3S Web Conf. 2021, 245, 01018. [Google Scholar] [CrossRef]
- Pang, L.; Wen, H.; Chang, J.; Li, Y.; Cai, B.; Lei, Y.; Yan, G.; Lv, C.; Zhang, L.; Qi, Z.; et al. Pathway of Carbon Emission Peak for China’s Petrochemical and Chemical Industries. Res. Environ. Sci. 2022, 35, 356–367. [Google Scholar] [CrossRef]
- Chen, F. Air Pollution Control Strategy of Petrochemical Enterprises. Chem. Eng. Des. Commun. 2020, 46, 199–212. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhang, Y.; Zhao, Y.; Miao, C.; An, J. Spatiotemporal Characteristics and Influencing Factors of the Synergistic Effect of Pollution Reduction and Carbon Reduction in China. Environ. Sci. 2024, 45, 4993–5002. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, Y.; Zhao, J.; Zhang, X.; Lu, G. Analysis and suggestions of the synergy management and development of air quality pollutant emission inventory and greenhouse gas emission inventory—Helping synergize the reduction of pollution and carbon emissions. Environ. Prot. Sci. 2024, 50, 49–53. [Google Scholar] [CrossRef]
- Bollen, J.; Brink, C. Air pollution policy in Europe: Quantifying the interaction with greenhouse gases and climate change policies. Energy Econ. 2014, 46, 202–215. [Google Scholar] [CrossRef]
- Brendemoen, A.; Vennemo, H. A Climate Treaty and the Norwegian Economy: ACGE Assessment. Energy J. 1994, 15, 77–93. [Google Scholar]
- Mardones, C.; Ortega, J. The individual and combined impact of environmental taxes in Chile− A flexible computable general equilibrium analysis. J. Environ. Manag. 2023, 325, 116508. [Google Scholar] [CrossRef]
- West, J.J.; Osnaya, P.; Laguna, I.; Martínez, J.; Fernández, A. Co-control of urban air pollutants and greenhouse gases in Mexico City. Environ. Sci. Technol. 2004, 38, 3474–3481. [Google Scholar] [CrossRef]
- Pu, Y. Research on the Emission Characteristics and Cost Prediction of Air Pollutants for China’s Thermal Power Generation: A GAINS-China Model-Based Spatial Analysis. Master’s Thesis, Jilin University, Changchun, China, 2020. [Google Scholar]
- Wagner, F.; Amann, M.; Borken-Kleefeld, J.; Cofala, J.; Höglund-Isaksson, L.; Purohit, P.; Rafaj, P.; Schöpp, W.; Winiwarter, W. Sectoral marginal abatement cost curves: Implications for mitigation pledges and air pollution co-benefits for Annex I countries. Sustain. Sci. 2012, 7, 169–184. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.; Wang, D.; Wu, H.; Li, Y.; Li, Y.; Qiao, Q.; Yin, Z. An evaluation method for synergistic effect of air pollutants and CO2 emission reduction in the Chinese petroleum refining technology. J. Environ. Manag. 2024, 371, 123169. [Google Scholar] [CrossRef]
- Chan, Y.; Tang, H.; Li, X.; Ma, W.; Tang, W. Correction: Chan et al. Analysis of the Synergies of Cutting Air Pollutants and Greenhouse Gas Emissions in an Integrated Iron and Steel Enterprise in China. Sustainability 2023, 15, 13231. Sustainability 2025, 17, 2900. [Google Scholar] [CrossRef]
- Cai, J.; Gao, S.; Sun, X.; Jiang, P.; Zhen, J.; Zhang, M. Synthesis of research on synergies between environmental, economic and social development. China Popul. Resour. Environ. 2016, S2, 4. [Google Scholar]
- Gu, A.; Teng, F.; Feng, X. Effects of pollution control measures on carbon emission reduction in China: Evidence from the 11th and 12th Five-Year Plans. Clim. Policy 2018, 18, 198–209. [Google Scholar] [CrossRef]
- Wang, S.; Lv, L.; Zhang, B.; Wang, S.; Wu, J.; Fu, J.; Luo, H. Multi Objective Programming Model of Low-Cost Path for China’s Peaking Carbon Dioxide Emissions and Carbon Neutrality. Res. Environ. Sci. 2021, 34, 2044–2055. [Google Scholar] [CrossRef]
- Li, J.; Li, H. Decomposition of carbon emission factors and analysis of emission reduction potential of petrochemical industry in Beijing-Tianjin-Hebei under the perspective of industrial transfer. Res. Environ. Sci. 2020, 33, 324–332. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, S.; Zhang, Z.-J.; Qu, Y.-Z.; Liu, T.-S. Scenario modeling and effect assessment of pollution reduction and carbon reduction synergistic control in Beijing. Environ. Sci. 2023, 44, 1998–2008. [Google Scholar] [CrossRef]
- Tang, Y. Research on the Accounting of Synergistic Emission Reduction Effect of Air Pollutants and Greenhouse Gases and Load Optimization Control of Thermal Power Plants. Master’s Thesis, North China Electric Power University, Beijing, China, 2014. [Google Scholar]
- Ministry of Environmental Protection. Calculation Methods for Actual Pollutant Emissions in 17 Industries Including Thermal Power under Emission Permit Management (Including Emission Factors and Material Balance Methods) (Provisional); Ministry of Environmental Protection: Beijing, China, 2017. [Google Scholar]
- Chinese Academy of Environmental Sciences. Guidelines for the Compilation of Provincial Greenhouse Gas Inventories; Chinese Academy of Environmental Sciences: Beijing, China, 2011. [Google Scholar]
- Wang, Y.; Ren, Y. Synergistic Strategies for Carbon Emission Reduction and Atmospheric Environment Management. Low Carbon World 2024, 14, 43–45. [Google Scholar] [CrossRef]
- O’Neill, N.F.; Ma, J.M.; Walther, D.C.; Brockway, L.R.; Ding, C.; Lin, J. A modified total equivalent warming impact analysis: Addressing direct and indirect emissions due to corrosion. Sci. Total Environ. 2020, 741, 140312. [Google Scholar] [CrossRef]
- Liu, T.; Bian, X.; Wu, S.; Liang, S.; Xu, Q.; Wei, X. Overview and outlook of carbon emission accounting for power systems. Power Syst. Prot. Control 2024, 52, 176–187. [Google Scholar] [CrossRef]
- Tang, H. Accounting for Greenhouse Gas Emissions in Iron and Steel Enterprises and Analyzing the Synergistic Effect of Air Pollutant Emission Reduction and Greenhouse Gas Emission; Fudan University: Shanghai, China, 2021. [Google Scholar]
- Liu, K.; Li, X.; Liu, T.; Li, B.; Dai, Y.; Meng, C.; Hao, Y. Example analysis of evaluation of synergistic control effect of pollution reduction and carbon reduction in iron and steel enterprises’ technological reform projects. Environ. Eng. 2022, 780–783. [Google Scholar] [CrossRef]
- Mao, X.; Zeng, A.; Liu, S.; Hu, T.; Xing, Y. Evaluation of synergistic control effects of sulfur, nitrogen and carbon in iron and steel industry. J. Environ. Sci. 2012, 32, 1253–1260. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, T.; Shen, X.; Ma, X.; Hong, J. Development of life cycle assessment method. Resour. Sci. 2021, 43, 446–455. [Google Scholar]
- ISO 14040:2006; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006.
- Petrescu, L.; Chisalita, D.-A.; Cormos, C.-C.; Manzolini, G.; Cobden, P.; van Dijk, H.A.J. Life Cycle Assessment of SEWGS Technology Applied to Integrated Steel Plants. Sustainability 2019, 11, 1825. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Cang, D. LCA life cycle evaluation of pelletizing process in iron and steel industry based on GaBi software. Metall. Energy 2017, 36, 3–6+32. [Google Scholar]
- Shi, W. Life Cycle Assessment of Different Flue Gas Desulfurization Processes in Coal-Fired Power Plants. Master’s Thesis, Shandong University, Jinan, China, 2016. [Google Scholar]
- Dreyer, L.C.; Niemann, A.L.; Hauschild, M.Z. Comparison of Three Different LCIA Methods: EDIP97, CML2001 and Eco-indicator 99: Does it matter which one you choose? Int. J. Life Cycle Assess. 2003, 8, 191–200. [Google Scholar] [CrossRef]
- International Iron and Steel Institute. World Steel Life Cycle Inventory Methodology Report. 2000. Available online: https://worldsteel.org/zhhans/publications/bookshop/life-cycle-inventory-report-2018/ (accessed on 16 May 2025).
- Duan, L.; Li, Z.; Pu, X. Scenario modeling of air quality improvement in Chengdu-Chongqing area in the medium and long term: A comprehensive strategy for pollution reduction and carbon reduction. China Environ. Sci. 2024, 44, 1756–1768. [Google Scholar] [CrossRef]
- Zhao, C.; Yuan, P.; Bian, W.; Tong, G.; Niu, Q. Application and Development of Green and Low-Carbon Technologies in Chlor-alkali Chemical Industry. China Chlor-Alkali 2023, 11, 54–57. [Google Scholar]
- Sun, J.; Kong, Y.; Chen, Y.; Xiang, T.; Gao, B.; Luo, S. A Review of Whole Chain Carbon Emission Accounting Methods for Power Systems. Autom. Electr. Power Syst. 2024, accepted. [Google Scholar]
- Liu, S.; Deng, C. Key Points of Strong Accounting of Pollutant Sources in Environmental Impact Assessment. Leather Manuf. Environ. Technol. 2022, 3, 192–194. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, N.; Guo, S. Problems and Suggestions of Carbon Emission Assessment and Accounting in China’s Chemical Industry. Environ. Prot. Oil Gas Fields 2023, 33, 5–11. [Google Scholar] [CrossRef]
- Liang, S. Improvement of VOCs Emission Inventory for Anthropogenic Sources in China and Its Characterization. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2022. [Google Scholar] [CrossRef]
- Shanghai Environmental Protection Bureau. Calculation Method for Volatile Organic Compound Emissions in the Petrochemical Industry in Shanghai (Revised in 2017); Shanghai Environmental Protection Bureau: Shanghai, China, 2017. [Google Scholar]
- Shanghai Ecological and Environmental Bureau. Shanghai Guidelines for Greenhouse Gas Emission Accounting and Reporting (Provisional); Shanghai Ecological and Environmental Bureau: Shanghai, China, 2022. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).