Soil Carbon Sequestration: A Mechanistic Perspective on Limitations and Future Possibilities
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy and Databases
- “Soil carbon sequestration” AND (“biotic limitation” OR “microbial necromass” OR “carbon use efficiency”)
- “Abiotic limitation” AND (“temperature sensitivity” OR “moisture variability”)
- “Mineral-associated organic matter” OR “MAOM formation”
- “Biochar” OR “nanomaterials” OR “MOF” AND “carbon storage”
- “Microbial gene editing” OR “synthetic microbiome” AND “soil C”
- “Root architecture” OR “rhizosphere exudation” AND “carbon stabilization”
- “Carbon sequestration” AND “economic feasibility” OR “scalability”
- “Carbon sequestration” AND “agronomic management”
- “Carbon sequestration” AND “biodiversity”
2.2. Inclusion and Exclusion Criteria
- ○
- Presented empirical or modeling-based evidence on soil C mechanisms (biological, chemical, or physical);
- ○
- Addressed field, mesocosm, or laboratory scales with relevance to agricultural soils;
- ○
- Discussed limitations, unresolved contradictions, or novel interventions related to C sequestration;
- ○
- Were peer-reviewed articles, reviews, or meta-analyses published in English.
- ○
- Lacked mechanistic details or were purely descriptive without quantitative data;
2.3. Data Extraction and Synthesis
- Photosynthetic and root-level constraints;
- Microbial contributions and limitations (necromass, respiration, viral shunt);
- Environmental/abiotic factors (temperature, moisture, pH);
- Soil structural and mineralogical influences (texture, aggregation, MAOM);
- Emerging interventions and scalability (engineered materials, microbial engineering).
3. Limitations
3.1. Biotic Limitations
3.1.1. Photosynthetic Capacity and C Allocation
3.1.2. Root Architecture
3.1.3. Plant-Root Chemical Composition, Rhizodeposition, and Plant–Microbe Interactions
3.1.4. Microorganisms
3.2. Abiotic Limitations
3.2.1. Temperature and Moisture
3.2.2. Nutrients
3.3. Structural and Physical Soil Characteristics
3.4. Chemical Limitations
4. Possibilities and Opportunities
4.1. Photosynthetic Modification
4.2. Modification of Root Systems and Rhizodeposition
4.3. C-Capturing Minerals
4.4. Synthetic Poly-Carboxylic Compounds
4.5. Phytolith Formation
4.6. Responsive Hydrogels
4.7. Nanomaterials
4.8. Microbial Modification
4.9. Deliberate Phage Infection
5. Management and Human Dimension
6. Future Research Directions
- i.
- Engineering the Plant-Microbe-Mineral Interface for Carbon Persistence
- ii.
- Quantifying the Net Carbon Balance of the Soil Viral Shunt and Microbial Predation
- iii.
- Developing Multi-Functional “Smart” Soil Amendments
- iv.
- Elucidating Mechanisms of Deep Soil Carbon Persistence
- v.
- Assessing Ecological and Economic Feasibility of Advanced Interventions
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AMF | Arbuscular Mycorrhizal Fungi |
| C | Carbon |
| Ca | Calcium |
| C:N | Carbon-to-nitrogen ratio |
| C:N:P | Carbon, nitrogen, and phosphorus ratio |
| CO2 | Carbon dioxide |
| CRISPR-Cas9 | Clustered Regularly Interspaced Short Palindromic Repeats and CRISPR-associated protein 9 |
| CSP | Conservation Stewardship Program |
| CUE | Carbon use efficiency |
| DOC | Dissolved organic carbon |
| DRO1 | Deeper Rooting 1 |
| eCO2 | elevated carbon dioxide |
| EcM | Ectomycorrhizal |
| EPS | Extracellular polymeric substance |
| EQIP | Environmental Quality Incentives Program |
| Fe/Al | Iron/Aluminum |
| GHG | Greenhouse gases |
| GMOs | Genetically Modified Organisms |
| GPP | Gross Primary Productivity |
| GRSP | Glomalin-related soil protein |
| K | Potassium |
| LCA | Life cycle assessment |
| MAOC | Mineral-associated organic carbon |
| MAOM | Mineral-associated organic matter |
| Mg | Magnesium |
| Mn | Manganese |
| Mo | Molybdenum |
| MOFs | Metal–organic frameworks |
| MRV | Measurement, reporting, and verification |
| MWD | Mean weight diameter |
| N | Nitrogen |
| N2 | Dinitrogen |
| NanoSIMS | Nanoscale Secondary Ion Mass Spectrometry |
| NPP | Net primary productivity |
| O2 | Oxygen |
| OM | Organic matter |
| P | Phosphorus |
| Pg | Petagrams |
| PhytOC | Phytolith-occluded carbon |
| PMC | Potentially mineralizable carbon |
| POC | Particulate organic carbon |
| POM | Particulate organic matter |
| POX-C | Permanganate oxidizable carbon |
| rbcS | Ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit |
| ROI | Return on investment |
| SAPs | Superabsorbent polymers |
| SCCH | Sustainable carbon-capture hydrogels |
| SOC | Soil organic carbon |
| SOM | Soil organic matter |
| TCA | Tricarboxylic acid |
| TEAs | Techno-economic analyses |
| VOC-C | Volatile organic compound-carbon |
| yr−1 | per year |
References
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Li, H.; Luijkx, I.T.; Olsen, A.; et al. Global Carbon Budget 2024. Earth Syst. Sci. Data Discuss. 2024, 17, 965–1039. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-Smart Soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef]
- Lal, R. Digging Deeper: A Holistic Perspective of Factors Affecting Soil Organic Carbon Sequestration in Agroecosystems. Glob. Change Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef] [PubMed]
- Paustian, K.; Collier, S.; Baldock, J.; Burgess, R.; Creque, J.; DeLonge, M.; Dungait, J.; Ellert, B.; Frank, S.; Goddard, T.; et al. Quantifying Carbon for Agricultural Soil Management: From the Current Status toward a Global Soil Information System. Carbon Manag. 2019, 10, 567–587. [Google Scholar] [CrossRef]
- Lal, R. Soil Carbon Sequestration to Mitigate Climate Change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Martin, M.P.; Dimassi, B.; Román Dobarco, M.; Guenet, B.; Arrouays, D.; Angers, D.A.; Blache, F.; Huard, F.; Soussana, J.-F.; Pellerin, S. Feasibility of the 4 per 1000 Aspirational Target for Soil Carbon: A Case Study for France. Glob. Change Biol. 2021, 27, 2458–2477. [Google Scholar] [CrossRef]
- Minasny, B.; Malone, B.P.; McBratney, A.B.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Das, B.S.; et al. Soil Carbon 4 per Mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- de Vries, W. Soil Carbon 4 per Mille: A Good Initiative but Let’s Manage Not Only the Soil but Also the Expectations: Comment on Minasny et al. (2017) Geoderma 292: 59–86. Geoderma 2018, 309, 111–112. [Google Scholar] [CrossRef]
- Kon Kam King, J.; Granjou, C.; Fournil, J.; Cecillon, L. Soil Sciences and the French 4 per 1000 Initiative—The Promises of Underground Carbon. Energy Res. Soc. Sci. 2018, 45, 144–152. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Amundson, R. Managing for Soil Carbon Sequestration: Let’s Get Realistic. Glob. Change Biol. 2019, 25, 386–389. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil Carbon Debt of 12,000 Years of Human Land Use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef] [PubMed]
- FAO. Emissions Due to Agriculture. Global, Regional and Country Trends 2000–2018|Policy Support and Governance|Food and Agriculture Organization of the United Nations. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/cc09fbbc-eb1d-436b-a88a-bed42a1f12f3/content (accessed on 1 July 2024).
- Jandl, R.; Rodeghiero, M.; Martinez, C.; Cotrufo, M.F.; Bampa, F.; van Wesemael, B.; Harrison, R.B.; Guerrini, I.A.; Richter, D.D., Jr.; Rustad, L.; et al. Current Status, Uncertainty and Future Needs in Soil Organic Carbon Monitoring. Sci. Total Environ. 2014, 468–469, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Soussana, J.-F.; Angers, D.; Schipper, L.; Chenu, C.; Rasse, D.P.; Batjes, N.H.; van Egmond, F.; McNeill, S.; Kuhnert, M.; et al. How to Measure, Report and Verify Soil Carbon Change to Realize the Potential of Soil Carbon Sequestration for Atmospheric Greenhouse Gas Removal. Glob. Change Biol. 2020, 26, 219–241. [Google Scholar] [CrossRef]
- Abbas, F.; Hammad, H.M.; Ishaq, W.; Farooque, A.A.; Bakhat, H.F.; Zia, Z.; Fahad, S.; Farhad, W.; Cerdà, A. A Review of Soil Carbon Dynamics Resulting from Agricultural Practices. J. Environ. Manag. 2020, 268, 110319. [Google Scholar] [CrossRef]
- Beillouin, D.; Cardinael, R.; Berre, D.; Boyer, A.; Corbeels, M.; Fallot, A.; Feder, F.; Demenois, J. A Global Overview of Studies about Land Management, Land-Use Change, and Climate Change Effects on Soil Organic Carbon. Glob. Change Biol. 2022, 28, 1690–1702. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Cover Crops and Soil Ecosystem Engineers. Agron. J. 2022, 114, 3096–3117. [Google Scholar] [CrossRef]
- Das, S.; Liptzin, D.; Maharjan, B. Long-Term Manure Application Improves Soil Health and Stabilizes Carbon in Continuous Maize Production System. Geoderma 2023, 430, 116338. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Wu, J. The Effect of Land Degradation and Restoration on Particulate and Mineral-Associated Organic Carbon. Appl. Soil Ecol. 2024, 196, 105322. [Google Scholar] [CrossRef]
- Luo, L.; Wang, J.; Lv, J.; Liu, Z.; Sun, T.; Yang, Y.; Zhu, Y.-G. Carbon Sequestration Strategies in Soil Using Biochar: Advances, Challenges, and Opportunities. Environ. Sci. Technol. 2023, 57, 11357–11372. [Google Scholar] [CrossRef]
- Janzen, H.H.; van Groenigen, K.J.; Powlson, D.S.; Schwinghamer, T.; van Groenigen, J.W. Photosynthetic Limits on Carbon Sequestration in Croplands. Geoderma 2022, 416, 115810. [Google Scholar] [CrossRef]
- Busch, F.A.; Sage, R.F. The Sensitivity of Photosynthesis to O2 and CO2 Concentration Identifies Strong Rubisco Control above the Thermal Optimum. New Phytol. 2017, 213, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Ruehr, S.; Keenan, T.F.; Williams, C.; Zhou, Y.; Lu, X.; Bastos, A.; Canadell, J.G.; Prentice, I.C.; Sitch, S.; Terrer, C. Evidence and Attribution of the Enhanced Land Carbon Sink. Nat. Rev. Earth Environ. 2023, 4, 518–534. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Zhu, B.; Cheng, W. Root Effects on Soil Organic Carbon: A Double-Edged Sword. New Phytol. 2021, 230, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Terrer, C.; Phillips, R.P.; Hungate, B.A.; Rosende, J.; Pett-Ridge, J.; Craig, M.E.; van Groenigen, K.J.; Keenan, T.F.; Sulman, B.N.; Stocker, B.D.; et al. A Trade-off between Plant and Soil Carbon Storage under Elevated CO2. Nature 2021, 591, 599–603. [Google Scholar] [CrossRef]
- Terrer, C.; Jackson, R.B.; Prentice, I.C.; Keenan, T.F.; Kaiser, C.; Vicca, S.; Fisher, J.B.; Reich, P.B.; Stocker, B.D.; Hungate, B.A.; et al. Nitrogen and Phosphorus Constrain the CO2 Fertilization of Global Plant Biomass. Nat. Clim. Change 2019, 9, 684–689. [Google Scholar] [CrossRef]
- Lal, R.; Negassa, W.; Lorenz, K. Carbon Sequestration in Soil. Curr. Opin. Environ. Sustain. 2015, 15, 79–86. [Google Scholar] [CrossRef]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil Carbon Sequestration Accelerated by Restoration of Grassland Biodiversity. Nat. Commun. 2019, 10, 718. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. What Is the Maximum Efficiency with Which Photosynthesis Can Convert Solar Energy into Biomass? Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving Photosynthesis and Crop Productivity by Accelerating Recovery from Photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- Matthews, M.L. Engineering Photosynthesis, Nature’s Carbon Capture Machine. PLOS Biol. 2023, 21, e3002183. [Google Scholar] [CrossRef]
- Ito, A. Constraining Size-Dependence of Vegetation Respiration Rates. Sci. Rep. 2020, 10, 4304. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global Patterns of Terrestrial Nitrogen and Phosphorus Limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Fatichi, S.; Pappas, C.; Zscheischler, J.; Leuzinger, S. Modelling Carbon Sources and Sinks in Terrestrial Vegetation. New Phytol. 2019, 221, 652–668. [Google Scholar] [CrossRef] [PubMed]
- Poirier, V.; Roumet, C.; Munson, A.D. The Root of the Matter: Linking Root Traits and Soil Organic Matter Stabilization Processes. Soil Biol. Biochem. 2018, 120, 246–259. [Google Scholar] [CrossRef]
- Poirier, V.; Roumet, C.; Angers, D.A.; Munson, A.D. Species and Root Traits Impact Macroaggregation in the Rhizospheric Soil of a Mediterranean Common Garden Experiment. Plant Soil 2018, 424, 289–302. [Google Scholar] [CrossRef]
- Wilpiszeski, R.L.; Aufrecht, J.A.; Retterer, S.T.; Sullivan, M.B.; Graham, D.E.; Pierce, E.M.; Zablocki, O.D.; Palumbo, A.V.; Elias, D.A. Soil Aggregate Microbial Communities: Towards Understanding Microbiome Interactions at Biologically Relevant Scales. Appl. Environ. Microbiol. 2019, 85, e00324-19. [Google Scholar] [CrossRef]
- Tian, S.; Liu, X.; Jin, B.; Zhao, X. Contribution of Fine Roots to Soil Organic Carbon Accumulation in Different Desert Communities in the Sangong River Basin. Int. J. Environ. Res. Public Health 2022, 19, 10936. [Google Scholar] [CrossRef]
- Schäfer, E.D.; Owen, M.R.; Band, L.R.; Farcot, E.; Bennett, M.J.; Lynch, J.P. Modeling Root Loss Reveals Impacts on Nutrient Uptake and Crop Development. Plant Physiol. 2022, 190, 2260–2278. [Google Scholar] [CrossRef]
- Ma, S.; He, F.; Tian, D.; Zou, D.; Yan, Z.; Yang, Y.; Zhou, T.; Huang, K.; Shen, H.; Fang, J. Variations and Determinants of Carbon Content in Plants: A Global Synthesis. Biogeosciences 2018, 15, 693–702. [Google Scholar] [CrossRef]
- Kiem, R.; Kögel-Knabner, I. Refractory Organic Carbon in Particle-Size Fractions of Arable Soils II: Organic Carbon in Relation to Mineral Surface Area and Iron Oxides in Fractions < 6 Μm. Org. Geochem. 2002, 33, 1699–1713. [Google Scholar] [CrossRef]
- Peixoto, L.; Olesen, J.E.; Elsgaard, L.; Enggrob, K.L.; Banfield, C.C.; Dippold, M.A.; Nicolaisen, M.H.; Bak, F.; Zang, H.; Dresbøll, D.B.; et al. Deep-Rooted Perennial Crops Differ in Capacity to Stabilize C Inputs in Deep Soil Layers. Sci. Rep. 2022, 12, 5952. [Google Scholar] [CrossRef] [PubMed]
- Rees, R.M.; Bingham, I.J.; Baddeley, J.A.; Watson, C.A. The Role of Plants and Land Management in Sequestering Soil Carbon in Temperate Arable and Grassland Ecosystems. Geoderma 2005, 128, 130–154. [Google Scholar] [CrossRef]
- Waring, B.G.; Smith, K.R.; Belluau, M.; Khlifa, R.; Messier, C.; Munson, A.; Paquette, A. Soil Carbon Pools Are Affected by Species Identity and Productivity in a Tree Common Garden Experiment. Front. For. Glob. Change 2022, 5, 1032321. [Google Scholar] [CrossRef]
- Averill, C.; Turner, B.L.; Finzi, A.C. Mycorrhiza-Mediated Competition between Plants and Decomposers Drives Soil Carbon Storage. Nature 2014, 505, 543–545. [Google Scholar] [CrossRef]
- Carrillo, Y.; Bell, C.; Koyama, A.; Canarini, A.; Boot, C.M.; Wallenstein, M.; Pendall, E. Plant Traits, Stoichiometry and Microbes as Drivers of Decomposition in the Rhizosphere in a Temperate Grassland. J. Ecol. 2017, 105, 1750–1765. [Google Scholar] [CrossRef]
- Sollins, P.; Homann, P.; Caldwell, B.A. Stabilization and Destabilization of Soil Organic Matter: Mechanisms and Controls. Geoderma 1996, 74, 65–105. [Google Scholar] [CrossRef]
- Finzi, A.C.; Abramoff, R.Z.; Spiller, K.S.; Brzostek, E.R.; Darby, B.A.; Kramer, M.A.; Phillips, R.P. Rhizosphere Processes Are Quantitatively Important Components of Terrestrial Carbon and Nutrient Cycles. Glob. Change Biol. 2015, 21, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Dueñas, F.J.; Martínez, A.T. Microbial Degradation of Lignin: How a Bulky Recalcitrant Polymer Is Efficiently Recycled in Nature and How We Can Take Advantage of This. Microb. Biotechnol. 2009, 2, 164–177. [Google Scholar] [CrossRef]
- Harman-Ware, A.E.; Sparks, S.; Addison, B.; Kalluri, U.C. Importance of Suberin Biopolymer in Plant Function, Contributions to Soil Organic Carbon and in the Production of Bio-Derived Energy and Materials. Biotechnol. Biofuels 2021, 14, 75. [Google Scholar] [CrossRef]
- Winkler, A.; Haumaier, L.; Zech, W. Insoluble Alkyl Carbon Components in Soils Derive Mainly from Cutin and Suberin. Org. Geochem. 2005, 36, 519–529. [Google Scholar] [CrossRef]
- Bugg, T.D.H. The Chemical Logic of Enzymatic Lignin Degradation. Chem. Commun. 2024, 60, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Villarino, S.H.; Pinto, P.; Jackson, R.B.; Piñeiro, G. Plant Rhizodeposition: A Key Factor for Soil Organic Matter Formation in Stable Fractions. Sci. Adv. 2021, 7, eabd3176. [Google Scholar] [CrossRef] [PubMed]
- Pausch, J.; Kuzyakov, Y. Carbon Input by Roots into the Soil: Quantification of Rhizodeposition from Root to Ecosystem Scale. Glob. Change Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Preece, C.; Peñuelas, J.; Giri, J. Soil Carbon Sequestration by Root Exudates. Trends Plant Sci. 2022, 27, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y. Review: Factors Affecting Rhizosphere Priming Effects. J. Plant Nutr. Soil Sci. 2002, 165, 382–396. [Google Scholar] [CrossRef]
- Yan, S.; Yin, L.; Dijkstra, F.A.; Wang, P.; Cheng, W. Priming Effect on Soil Carbon Decomposition by Root Exudate Surrogates: A Meta-Analysis. Soil Biol. Biochem. 2023, 178, 108955. [Google Scholar] [CrossRef]
- Camenzind, T.; Mason-Jones, K.; Mansour, I.; Rillig, M.C.; Lehmann, J. Formation of Necromass-Derived Soil Organic Carbon Determined by Microbial Death Pathways. Nat. Geosci. 2023, 16, 115–122. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Creamer, C.; Whitaker, J. Deconstructing the Microbial Necromass Continuum to Inform Soil Carbon Sequestration. Funct. Ecol. 2022, 36, 1396–1410. [Google Scholar] [CrossRef]
- Adamczyk, S.; Mäkipää, R.; Lehtonen, A.; Adamczyk, B. Precise Method to Measure Fungal and Bacterial Necromass Using High Pressure Liquid Chromatography with Fluorescence Detector Adjusted to Inorganic, Organic and Peat Soils. Pedobiologia 2024, 106, 150977. [Google Scholar] [CrossRef]
- Craig, M.E.; Mayes, M.A.; Sulman, B.N.; Walker, A.P. Biological Mechanisms May Contribute to Soil Carbon Saturation Patterns. Glob. Change Biol. 2021, 27, 2633–2644. [Google Scholar] [CrossRef]
- Miltner, A.; Zheng, T.; Liang, C.; Kästner, M. Microbial Necromass as a Source for Soil Organic Matter Formation—Implications for Soil Processes. In Proceedings of the Copernicus Meetings, Online, 3–8 May 2020. [Google Scholar]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial Necromass as the Source of Soil Organic Carbon in Global Ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Zhang, Y.; Morrissey, E.; Liu, Y.; Sun, L.; Qu, L.; Sang, C.; Zhang, H.; Li, G.; et al. Integrating Microbial Community Properties, Biomass and Necromass to Predict Cropland Soil Organic Carbon. ISME Commun. 2023, 3, 86. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ni, B.; Wang, X.; Deng, Y.; Tao, L.; Zhou, X.; Deng, J. Effect of Forest Soil Viruses on Bacterial Community Succession and the Implication for Soil Carbon Sequestration. Sci. Total Environ. 2023, 892, 164800. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, S.; Zhao, X.; Zhao, X.; Mason-Jones, K.; Zhu, Z.; Redmile-Gordon, M.; Li, Y.; Chen, J.; Kuzyakov, Y.; et al. Experimental Evidence for the Impact of Phages on Mineralization of Soil-Derived Dissolved Organic Matter under Different Temperature Regimes. Sci. Total Environ. 2022, 846, 157517. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.N.; Parra-Saldívar, R. Soil Carbon Sequestration—An Interplay between Soil Microbial Community and Soil Organic Matter Dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- Geyer, K.M.; Kyker-Snowman, E.; Grandy, A.S.; Frey, S.D. Microbial Carbon Use Efficiency: Accounting for Population, Community, and Ecosystem-Scale Controls over the Fate of Metabolized Organic Matter. Biogeochemistry 2016, 127, 173–188. [Google Scholar] [CrossRef]
- Duran, P.; Mora, M.d.l.L.; Matus, F.; Barra, P.J.; Jofré, I.; Kuzyakov, Y.; Merino, C. Biological Crusts to Increase Soil Carbon Sequestration: New Challenges in a New Environment. Biology 2021, 10, 1190. [Google Scholar] [CrossRef]
- Keiluweit, M.; Wanzek, T.; Kleber, M.; Nico, P.; Fendorf, S. Anaerobic Microsites Have an Unaccounted Role in Soil Carbon Stabilization. Nat. Commun. 2017, 8, 1771. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Friedlingstein, P.; Sitch, S.; Anthoni, P.; Arneth, A.; Arora, V.K.; Bastrikov, V.; Delire, C.; Goll, D.S.; Jain, A.; et al. Process-Oriented Analysis of Dominant Sources of Uncertainty in the Land Carbon Sink. Nat. Commun. 2022, 13, 4781. [Google Scholar] [CrossRef]
- Ke, P.; Ciais, P.; Sitch, S.; Li, W.; Bastos, A.; Liu, Z.; Xu, Y.; Gui, X.; Bian, J.; Goll, D.S.; et al. Low Latency Carbon Budget Analysis Reveals a Large Decline of the Land Carbon Sink in 2023. Natl. Sci. Rev. 2024, 11, nwae367. [Google Scholar] [CrossRef]
- Holik, L.; Vranova, V.; Foltynova, L.; Acosta, M. Forest Soil Properties under Elevated CO2: A Five-Year Experiment. Eur. J. Soil Biol. 2021, 106, 103346. [Google Scholar] [CrossRef]
- Schäfer, E.D.; Ajmera, I.; Farcot, E.; Owen, M.R.; Band, L.R.; Lynch, J.P. In Silico Evidence for the Utility of Parsimonious Root Phenotypes for Improved Vegetative Growth and Carbon Sequestration under Drought. Front. Plant Sci. 2022, 13, 1010165. [Google Scholar] [CrossRef]
- Lu, M.; Wang, S.; Malhotra, A.; Tumber-Dávila, S.J.; Weintraub-Leff, S.; McCormack, M.L.; Wang, X.T.; Jackson, R.B. A Continental Scale Analysis Reveals Widespread Root Bimodality. Nat. Commun. 2025, 16, 5281. [Google Scholar] [CrossRef] [PubMed]
- Friggens, N.L.; Hugelius, G.; Kokelj, S.V.; Murton, J.B.; Phoenix, G.K.; Hartley, I.P. Positive Rhizosphere Priming Accelerates Carbon Release from Permafrost Soils. Nat. Commun. 2025, 16, 3576. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, L.A.; Sala, O.E. Global Patterns and Climatic Controls of Belowground Net Carbon Fixation. Proc. Natl. Acad. Sci. USA 2020, 117, 20038–20043. [Google Scholar] [CrossRef] [PubMed]
- Perakis, S.S.; Matkins, J.J.; Hibbs, D.E. Interactions of Tissue and Fertilizer Nitrogen on Decomposition Dynamics of Lignin-Rich Conifer Litter. Ecosphere 2012, 3, art54. [Google Scholar] [CrossRef]
- Kohl, L.; Wanek, W.; Keiblinger, K.; Hämmerle, I.; Fuchslueger, L.; Schneider, T.; Riedel, K.; Eberl, L.; Zechmeister-Boltenstern, S.; Richter, A. Nutrient Controls on Carbohydrate and Lignin Decomposition in Beech Litter. Geoderma 2023, 429, 116276. [Google Scholar] [CrossRef]
- Buckeridge, K.M.; Mason, K.E.; Ostle, N.; McNamara, N.P.; Grant, H.K.; Whitaker, J. Microbial Necromass Carbon and Nitrogen Persistence Are Decoupled in Agricultural Grassland Soils. Commun. Earth Environ. 2022, 3, 114. [Google Scholar] [CrossRef]
- Albright, M.B.N.; Gallegos-Graves, L.V.; Feeser, K.L.; Montoya, K.; Emerson, J.B.; Shakya, M.; Dunbar, J. Experimental Evidence for the Impact of Soil Viruses on Carbon Cycling during Surface Plant Litter Decomposition. ISME Commun. 2022, 2, 24. [Google Scholar] [CrossRef]
- Osburn, E.D.; Baer, S.G.; Evans, S.E.; McBride, S.G.; Strickland, M.S. Effects of Experimentally Elevated Virus Abundance on Soil Carbon Cycling across Varying Ecosystem Types. Soil Biol. Biochem. 2024, 198, 109556. [Google Scholar] [CrossRef]
- Yan, G.; Fan, C.; Zheng, J.; Liu, G.; Yu, J.; Guo, Z.; Cao, W.; Wang, L.; Wang, W.; Meng, Q.; et al. Forest Carbon Stocks Increase with Higher Dominance of Ectomycorrhizal Trees in High Latitude Forests. Nat. Commun. 2024, 15, 5959. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Matthews, B.; Sandén, H.; Katzensteiner, K.; Hagedorn, F.; Gorfer, M.; Berger, H.; Berger, T.W.; Godbold, D.L.; Rewald, B. Soil Fertility Determines Whether Ectomycorrhizal Fungi Accelerate or Decelerate Decomposition in a Temperate Forest. New Phytol. 2023, 239, 325–339. [Google Scholar] [CrossRef]
- Jia, Y.; Kuzyakov, Y.; Wang, G.; Tan, W.; Zhu, B.; Feng, X. Temperature Sensitivity of Decomposition of Soil Organic Matter Fractions Increases with Their Turnover Time. Land Degrad. Dev. 2020, 31, 632–645. [Google Scholar] [CrossRef]
- Georgiou, K.; Koven, C.D.; Wieder, W.R.; Hartman, M.D.; Riley, W.J.; Pett-Ridge, J.; Bouskill, N.J.; Abramoff, R.Z.; Slessarev, E.W.; Ahlström, A.; et al. Emergent Temperature Sensitivity of Soil Organic Carbon Driven by Mineral Associations. Nat. Geosci. 2024, 17, 205–212. [Google Scholar] [CrossRef]
- Hassan, W.; Li, Y.; Saba, T.; Wu, J.; Bashir, S.; Bashir, S.; Gatasheh, M.K.; Diao, Z.-H.; Chen, Z. Temperature Responsiveness of Soil Carbon Fractions, Microbes, Extracellular Enzymes and CO2 Emission: Mitigating Role of Texture. PeerJ 2022, 10, e13151. [Google Scholar] [CrossRef]
- Soong, J.L.; Castanha, C.; Hicks Pries, C.E.; Ofiti, N.; Porras, R.C.; Riley, W.J.; Schmidt, M.W.I.; Torn, M.S. Five Years of Whole-Soil Warming Led to Loss of Subsoil Carbon Stocks and Increased CO2 Efflux. Sci. Adv. 2021, 7, eabd1343. [Google Scholar] [CrossRef]
- Humphrey, V.; Berg, A.; Ciais, P.; Gentine, P.; Jung, M.; Reichstein, M.; Seneviratne, S.I.; Frankenberg, C. Soil Moisture–Atmosphere Feedback Dominates Land Carbon Uptake Variability. Nature 2021, 592, 65–69. [Google Scholar] [CrossRef]
- Heckman, K.A.; Possinger, A.R.; Badgley, B.D.; Bowman, M.M.; Gallo, A.C.; Hatten, J.A.; Nave, L.E.; SanClements, M.D.; Swanston, C.W.; Weiglein, T.L.; et al. Moisture-Driven Divergence in Mineral-Associated Soil Carbon Persistence. Proc. Natl. Acad. Sci. USA 2023, 120, e2210044120. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Gelderman, T.M. Soil Moisture Controls on Temperature Sensitivity of Soil Organic Carbon Decomposition for a Mesic Grassland. Soil Biol. Biochem. 2011, 43, 455–457. [Google Scholar] [CrossRef]
- Huang, X.; Terrer, C.; Dijkstra, F.A.; Hungate, B.A.; Zhang, W.; van Groenigen, K.J. New Soil Carbon Sequestration with Nitrogen Enrichment: A Meta-Analysis. Plant Soil 2020, 454, 299–310. [Google Scholar] [CrossRef]
- Rocci, K.S.; Lavallee, J.M.; Stewart, C.E.; Cotrufo, M.F. Soil Organic Carbon Response to Global Environmental Change Depends on Its Distribution between Mineral-Associated and Particulate Organic Matter: A Meta-Analysis. Sci. Total Environ. 2021, 793, 148569. [Google Scholar] [CrossRef]
- Tang, B.; Rocci, K.S.; Lehmann, A.; Rillig, M.C. Nitrogen Increases Soil Organic Carbon Accrual and Alters Its Functionality. Glob. Change Biol. 2023, 29, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil Extracellular Enzyme Activities, Soil Carbon and Nitrogen Storage under Nitrogen Fertilization: A Meta-Analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- van Groenigen, J.W.; van Kessel, C.; Hungate, B.A.; Oenema, O.; Powlson, D.S.; van Groenigen, K.J. Sequestering Soil Organic Carbon: A Nitrogen Dilemma. Environ. Sci. Technol. 2017, 51, 4738–4739. [Google Scholar] [CrossRef] [PubMed]
- Bijay-Singh; Craswell, E. Fertilizers and Nitrate Pollution of Surface and Ground Water: An Increasingly Pervasive Global Problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar] [CrossRef]
- Bei, S.; Li, X.; Kuyper, T.W.; Chadwick, D.R.; Zhang, J. Nitrogen Availability Mediates the Priming Effect of Soil Organic Matter by Preferentially Altering the Straw Carbon-Assimilating Microbial Community. Sci. Total Environ. 2022, 815, 152882. [Google Scholar] [CrossRef]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef]
- Miao, F.; Wang, S.; Yuan, Y.; Chen, Y.; Guo, E.; Li, Y. The Addition of a High Concentration of Phosphorus Reduces the Diversity of Arbuscular Mycorrhizal Fungi in Temperate Agroecosystems. Diversity 2023, 15, 1045. [Google Scholar] [CrossRef]
- Li, L.; Luo, Z.; Li, L.; Niu, Y.; Zhang, Y.; He, R.; Liu, J.; Nian, L. Long-Term Phosphorus Fertilization Reveals the Phosphorus Limitation Shaping the Soil Micro-Food Web Stability in the Loess Plateau. Front. Microbiol. 2024, 14, 1256269. [Google Scholar] [CrossRef]
- Mattila, T.J.; Vihanto, N. Agricultural Limitations to Soil Carbon Sequestration: Plant Growth, Microbial Activity, and Carbon Stabilization. Agric. Ecosyst. Environ. 2024, 367, 108986. [Google Scholar] [CrossRef]
- Trierweiler, A.M.; Winter, K.; Hedin, L.O. Rising CO2 Accelerates Phosphorus and Molybdenum Limitation of N2-Fixation in Young Tropical Trees. Plant Soil 2018, 429, 363–373. [Google Scholar] [CrossRef]
- Wichard, T.; Mishra, B.; Myneni, S.C.B.; Bellenger, J.-P.; Kraepiel, A.M.L. Storage and Bioavailability of Molybdenum in Soils Increased by Organic Matter Complexation. Nature Geosci. 2009, 2, 625–629. [Google Scholar] [CrossRef]
- Barron, A.R.; Wurzburger, N.; Bellenger, J.P.; Wright, S.J.; Kraepiel, A.M.L.; Hedin, L.O. Molybdenum Limitation of Asymbiotic Nitrogen Fixation in Tropical Forest Soils. Nature Geosci. 2009, 2, 42–45. [Google Scholar] [CrossRef]
- Churchman, G.J.; Singh, M.; Schapel, A.; Sarkar, B.; Bolan, N. Clay Minerals as the Key to the Sequestration of Carbon in Soils. Clays Clay Miner. 2020, 68, 135–143. [Google Scholar] [CrossRef]
- Rodríguez-Albarracín, H.S.; Demattê, J.A.M.; Rosin, N.A.; Contreras, A.E.D.; Silvero, N.E.Q.; Cerri, C.E.P.; de Sousa Mendes, W.; Tayebi, M. Potential of Soil Minerals to Sequester Soil Organic Carbon. Geoderma 2023, 436, 116549. [Google Scholar] [CrossRef]
- Gulde, S.; Chung, H.; Amelung, W.; Chang, C.; Six, J. Soil Carbon Saturation Controls Labile and Stable Carbon Pool Dynamics. Soil Sci. Soc. Am. J. 2008, 72, 605–612. [Google Scholar] [CrossRef]
- Stewart, C.; Paustian, K.; Conant, R.; Plante, A.; Six, J. Soil Carbon Saturation: Concept, Evidence and Evaluation. Biogeochemistry 2007, 86, 19–31. [Google Scholar] [CrossRef]
- Guillaume, T.; Makowski, D.; Libohova, Z.; Bragazza, L.; Sallaku, F.; Sinaj, S. Soil Organic Carbon Saturation in Cropland-Grassland Systems: Storage Potential and Soil Quality. Geoderma 2022, 406, 115529. [Google Scholar] [CrossRef]
- Vogel, C.; Mueller, C.W.; Höschen, C.; Buegger, F.; Heister, K.; Schulz, S.; Schloter, M.; Kögel-Knabner, I. Submicron Structures Provide Preferential Spots for Carbon and Nitrogen Sequestration in Soils. Nat. Commun. 2014, 5, 2947. [Google Scholar] [CrossRef]
- Six, J.; Doetterl, S.; Laub, M.; Müller, C.R.; Van de Broek, M. The Six Rights of How and When to Test for Soil C Saturation. SOIL 2024, 10, 275–279. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Hübner, R.; Spörlein, P.; Geuß, U.; Hangen, E.; Reischl, A.; Schilling, B.; von Lützow, M.; Kögel-Knabner, I. Carbon Sequestration Potential of Soils in Southeast Germany Derived from Stable Soil Organic Carbon Saturation. Glob. Change Biol. 2014, 20, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Arrouays, D.; Angers, D.A.; Martin, M.P.; Walter, C. Soil Carbon Stocks under Different Land Uses and the Applicability of the Soil Carbon Saturation Concept. Soil Tillage Res. 2019, 188, 53–58. [Google Scholar] [CrossRef]
- Georgiou, K.; Jackson, R.B.; Vindušková, O.; Abramoff, R.Z.; Ahlström, A.; Feng, W.; Harden, J.W.; Pellegrini, A.F.A.; Polley, H.W.; Soong, J.L.; et al. Global Stocks and Capacity of Mineral-Associated Soil Organic Carbon. Nat. Commun. 2022, 13, 3797. [Google Scholar] [CrossRef]
- Karstens, K.; Bodirsky, B.L.; Dietrich, J.P.; Dondini, M.; Heinke, J.; Kuhnert, M.; Müller, C.; Rolinski, S.; Smith, P.; Weindl, I.; et al. Management-Induced Changes in Soil Organic Carbon on Global Croplands. Biogeosciences 2022, 19, 5125–5149. [Google Scholar] [CrossRef]
- Chevallier, T.; Blanchart, E.; Albrecht, A.; Feller, C. The Physical Protection of Soil Organic Carbon in Aggregates: A Mechanism of Carbon Storage in a Vertisol under Pasture and Market Gardening (Martinique, West Indies). Agric. Ecosyst. Environ. 2004, 103, 375–387. [Google Scholar] [CrossRef]
- Mustafa, A.; Minggang, X.; Ali Shah, S.A.; Abrar, M.M.; Nan, S.; Baoren, W.; Zejiang, C.; Saeed, Q.; Naveed, M.; Mehmood, K.; et al. Soil Aggregation and Soil Aggregate Stability Regulate Organic Carbon and Nitrogen Storage in a Red Soil of Southern China. J. Environ. Manag. 2020, 270, 110894. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, C.; Wang, J.; Meng, Q.; Yuan, Y.; Ma, X.; Liu, X.; Zhu, Y.; Ding, G.; Zhang, J.; et al. Soil Aggregates Stability and Storage of Soil Organic Carbon Respond to Cropping Systems on Black Soils of Northeast China. Sci. Rep. 2020, 10, 265. [Google Scholar] [CrossRef]
- Yang, J.Q.; Zhang, X.; Bourg, I.C.; Stone, H.A. 4D Imaging Reveals Mechanisms of Clay-Carbon Protection and Release. Nat. Commun. 2021, 12, 622. [Google Scholar] [CrossRef]
- Witzgall, K.; Vidal, A.; Schubert, D.I.; Höschen, C.; Schweizer, S.A.; Buegger, F.; Pouteau, V.; Chenu, C.; Mueller, C.W. Particulate Organic Matter as a Functional Soil Component for Persistent Soil Organic Carbon. Nat. Commun. 2021, 12, 4115. [Google Scholar] [CrossRef]
- Yu, W.; Huang, W.; Weintraub-Leff, S.R.; Hall, S.J. Where and Why Do Particulate Organic Matter (POM) and Mineral-Associated Organic Matter (MAOM) Differ among Diverse Soils? Soil Biol. Biochem. 2022, 172, 108756. [Google Scholar] [CrossRef]
- Wagai, R.; Kajiura, M.; Asano, M. Iron and Aluminum Association with Microbially Processed Organic Matter via Meso-Density Aggregate Formation across Soils: Organo-Metallic Glue Hypothesis. SOIL 2020, 6, 597–627. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Chapter One—Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 130, pp. 1–140. [Google Scholar]
- Shabtai, I.A.; Wilhelm, R.C.; Schweizer, S.A.; Höschen, C.; Buckley, D.H.; Lehmann, J. Calcium Promotes Persistent Soil Organic Matter by Altering Microbial Transformation of Plant Litter. Nat. Commun. 2023, 14, 6609. [Google Scholar] [CrossRef] [PubMed]
- Buss, W.; Hasemer, H.; Sokol, N.W.; Rohling, E.J.; Borevitz, J. Applying Minerals to Soil to Draw down Atmospheric Carbon Dioxide through Synergistic Organic and Inorganic Pathways. Commun. Earth Environ. 2024, 5, 602. [Google Scholar] [CrossRef]
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium-Mediated Stabilisation of Soil Organic Carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef]
- Chen, J.; Seven, J.; Zilla, T.; Dippold, M.A.; Blagodatskaya, E.; Kuzyakov, Y. Microbial C:N:P Stoichiometry and Turnover Depend on Nutrients Availability in Soil: A 14C, 15N and 33P Triple Labelling Study. Soil Biol. Biochem. 2019, 131, 206–216. [Google Scholar] [CrossRef]
- Adhikari, D.; Sowers, T.; Stuckey, J.W.; Wang, X.; Sparks, D.L.; Yang, Y. Formation and Redox Reactivity of Ferrihydrite-Organic Carbon-Calcium Co-Precipitates. Geochim. Cosmochim. Acta 2018, 244, 86–98. [Google Scholar] [CrossRef]
- Pan, W.; Kan, J.; Inamdar, S.; Chen, C.; Sparks, D. Dissimilatory Microbial Iron Reduction Release DOC (Dissolved Organic Carbon) from Carbon-Ferrihydrite Association. Soil Biol. Biochem. 2016, 103, 232–240. [Google Scholar] [CrossRef]
- Wang, C.; Kuzyakov, Y. Soil Organic Matter Priming: The pH Effects. Glob. Change Biol. 2024, 30, e17349. [Google Scholar] [CrossRef]
- Pan, Y.; Koopmans, G.F.; Bonten, L.T.C.; Song, J.; Luo, Y.; Temminghoff, E.J.M.; Comans, R.N.J. Influence of pH on the Redox Chemistry of Metal (Hydr)Oxides and Organic Matter in Paddy Soils. J. Soils Sediments 2014, 14, 1713–1726. [Google Scholar] [CrossRef]
- Berthelin, J.; Laba, M.; Lemaire, G.; Powlson, D.; Tessier, D.; Wander, M.; Baveye, P.C. Soil Carbon Sequestration for Climate Change Mitigation: Mineralization Kinetics of Organic Inputs as an Overlooked Limitation. Eur. J. Soil Sci. 2022, 73, e13221. [Google Scholar] [CrossRef]
- Woo, D.K.; Seo, Y. Effects of Elevated Temperature and Abnormal Precipitation on Soil Carbon and Nitrogen Dynamics in a Pinus Densiflora Forest. Front. For. Glob. Change 2022, 5, 1051210. [Google Scholar] [CrossRef]
- Siregar, I.H.; Camps-Arbestain, M.; Wang, T.; Kirschbaum, M.U.F.; Kereszturi, G.; Palmer, A. Higher Temperature Accelerates Carbon Cycling in a Temperate Montane Forest without Decreasing Soil Carbon Stocks. Geoderma Reg. 2024, 39, e00889. [Google Scholar] [CrossRef]
- Yang, X.; Ma, S.; Huang, E.; Zhang, D.; Chen, G.; Zhu, J.; Ji, C.; Zhu, B.; Liu, L.; Fang, J. Nitrogen Addition Promotes Soil Carbon Accumulation Globally. Sci. China Life Sci. 2025, 68, 284–293. [Google Scholar] [CrossRef]
- Xu, C.; Xu, X.; Ju, C.; Chen, H.Y.H.; Wilsey, B.J.; Luo, Y.; Fan, W. Long-Term, Amplified Responses of Soil Organic Carbon to Nitrogen Addition Worldwide. Glob. Change Biol. 2021, 27, 1170–1180. [Google Scholar] [CrossRef]
- McGrath, C.R.; Pries, C.E.H.; Nguyen, N.; Glazer, B.; Lio, S. Minerals Limit the Deep Soil Respiration Response to Warming in a Tropical Andisol. Biogeochem. Lett. 2022, 161, 85–99. [Google Scholar] [CrossRef]
- Fernández-Catinot, F.; Pestoni, S.; Gallardo, N.; Vaieretti, M.V.; Pérez Harguindeguy, N. No Detectable Upper Limit When Predicting Soil Mineral-Associated Organic Carbon Stabilization Capacity in Temperate Grassland of Central Argentina Mountains. Geoderma Reg. 2023, 35, e00722. [Google Scholar] [CrossRef]
- Barati, B.; Zeng, K.; Baeyens, J.; Wang, S.; Addy, M.; Gan, S.-Y.; El-Fatah Abomohra, A. Recent Progress in Genetically Modified Microalgae for Enhanced Carbon Dioxide Sequestration. Biomass Bioenergy 2021, 145, 105927. [Google Scholar] [CrossRef]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning Photosynthesis to Sustainably Meet Global Food and Bioenergy Demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef]
- Kaldenhoff, R. Mechanisms Underlying CO2 Diffusion in Leaves. Curr. Opin. Plant Biol. 2012, 15, 276–281. [Google Scholar] [CrossRef]
- Hanson, M.R.; Lin, M.T.; Carmo-Silva, A.E.; Parry, M.A.J. Towards Engineering Carboxysomes into C3 Plants. Plant J. 2016, 87, 38–50. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Quick, W.P.; Furbank, R.T. The Development of C 4 Rice: Current Progress and Future Challenges. Science 2012, 336, 1671–1672. [Google Scholar] [CrossRef]
- Murchie, E.H.; Niyogi, K.K. Manipulation of Photoprotection to Improve Plant Photosynthesis. Plant Physiol. 2011, 155, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.; Amasino, R.M. Inhibition of Leaf Senescence by Autoregulated Production of Cytokinin. Science 1995, 270, 1986–1988. [Google Scholar] [CrossRef] [PubMed]
- John, I.; Drake, R.; Farrell, A.; Cooper, W.; Lee, P.; Horton, P.; Grierson, D. Delayed Leaf Senescence in Ethylene-deficient ACC-oxidase Antisense Tomato Plants: Molecular and Physiological Analysis. Plant J. 1995, 7, 483–490. [Google Scholar] [CrossRef]
- Leister, D. Genetic Engineering, Synthetic Biology and the Light Reactions of Photosynthesis. Plant Physiol. 2019, 179, 778–793. [Google Scholar] [CrossRef] [PubMed]
- MIT Enhanced Photosynthesis in Crops (EPiC)|Abdul Latif Jameel Water and Food Systems Lab (J-WAFS). Available online: https://jwafs.mit.edu/projects/2023/enhanced-photosynthesis-crops (accessed on 7 February 2025).
- Song, Q.; Chu, C.; Parry, M.A.J.; Zhu, X.-G. Genetics-Based Dynamic Systems Model of Canopy Photosynthesis: The Key to Improve Light and Resource Use Efficiencies for Crops. Food Energy Secur. 2016, 5, 18–25. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N.; et al. Control of Root System Architecture by DEEPER ROOTING 1 Increases Rice Yield under Drought Conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar] [CrossRef]
- Arai-Sanoh, Y.; Takai, T.; Yoshinaga, S.; Nakano, H.; Kojima, M.; Sakakibara, H.; Kondo, M.; Uga, Y. Deep Rooting Conferred by DEEPER ROOTING 1 Enhances Rice Yield in Paddy Fields. Sci. Rep. 2014, 4, 5563. [Google Scholar] [CrossRef] [PubMed]
- Joseph Fernando, E.A.; Selvaraj, M.; Uga, Y.; Busch, W.; Bowers, H.; Tohme, J. Going Deep: Roots, Carbon, and Analyzing Subsoil Carbon Dynamics. Mol. Plant 2024, 17, 1–3. [Google Scholar] [CrossRef]
- Schneider, H.M.; Strock, C.F.; Hanlon, M.T.; Vanhees, D.J.; Perkins, A.C.; Ajmera, I.B.; Sidhu, J.S.; Mooney, S.J.; Brown, K.M.; Lynch, J.P. Multiseriate Cortical Sclerenchyma Enhance Root Penetration in Compacted Soils. Proc. Natl. Acad. Sci. USA 2021, 118, e2012087118. [Google Scholar] [CrossRef]
- Silver, W.L.; Miya, R.K. Global Patterns in Root Decomposition: Comparisons of Climate and Litter Quality Effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Mommer, L.; De Vries, F.T. Going Underground: Root Traits as Drivers of Ecosystem Processes. Trends Ecol. Evol. 2014, 29, 692–699. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere Size and Shape: Temporal Dynamics and Spatial Stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]
- Nie, M.; Lu, M.; Bell, J.; Raut, S.; Pendall, E. Altered Root Traits Due to Elevated CO2: A Meta-Analysis. Glob. Ecol. Biogeogr. 2013, 22, 1095–1105. [Google Scholar] [CrossRef]
- Chari, N.R.; Taylor, B.N. Soil Organic Matter Formation and Loss Are Mediated by Root Exudates in a Temperate Forest. Nat. Geosci. 2022, 15, 1011–1016. [Google Scholar] [CrossRef]
- Oburger, E.; Jones, D.L. Sampling Root Exudates—Mission Impossible? Rhizosphere 2018, 6, 116–133. [Google Scholar] [CrossRef]
- Harnessing Plants Initiative. Salk Institute for Biological Studies. Available online: https://www.salk.edu/harnessing-plants-initiative/ (accessed on 30 April 2025).
- Srivastava, R.K.; Yetgin, A. An Overall Review on Influence of Root Architecture on Soil Carbon Sequestration Potential. Theor. Exp. Plant Physiol. 2024, 36, 165–178. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A Review of Mineral Carbonation Technologies to Sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon Capture, Storage and Utilisation Technologies: A Critical Analysis and Comparison of Their Life Cycle Environmental Impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Renforth, P.; Manning, D.A.C.; Lopez-Capel, E. Carbonate Precipitation in Artificial Soils as a Sink for Atmospheric Carbon Dioxide. Appl. Geochem. 2009, 24, 1757–1764. [Google Scholar] [CrossRef]
- Hartmann, J.; West, A.J.; Renforth, P.; Köhler, P.; De La Rocha, C.L.; Wolf-Gladrow, D.A.; Dürr, H.H.; Scheffran, J. Enhanced Chemical Weathering as a Geoengineering Strategy to Reduce Atmospheric Carbon Dioxide, Supply Nutrients, and Mitigate Ocean Acidification. Rev. Geophys. 2013, 51, 113–149. [Google Scholar] [CrossRef]
- Renforth, P.; Manning, D.A. Laboratory Carbonation of Artificial Silicate Gels Enhanced by Citrate: Implications for Engineered Pedogenic Carbonate Formation. Int. J. Greenh. Gas Control 2011, 5, 1578–1586. [Google Scholar] [CrossRef]
- Matter, J.M.; Kelemen, P.B. Permanent Storage of Carbon Dioxide in Geological Reservoirs by Mineral Carbonation. Nat. Geosci. 2009, 2, 837–841. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Matter, J.; Streit, E.E.; Rudge, J.F.; Curry, W.B.; Blusztajn, J. Rates and Mechanisms of Mineral Carbonation in Peridotite: Natural Processes and Recipes for Enhanced, in Situ CO2 Capture and Storage. Annu. Rev. Earth Planet. Sci. 2011, 39, 545–576. [Google Scholar] [CrossRef]
- Neupane, A.; Herndon, E.M.; Whitman, T.; Faiia, A.M.; Jagadamma, S. Manganese Effects on Plant Residue Decomposition and Carbon Distribution in Soil Fractions Depend on Soil Nitrogen Availability. Soil Biol. Biochem. 2023, 178, 108964. [Google Scholar] [CrossRef]
- Al-Sakkari, E.G.; Ragab, A.; Dagdougui, H.; Boffito, D.C.; Amazouz, M. Carbon Capture, Utilization and Sequestration Systems Design and Operation Optimization: Assessment and Perspectives of Artificial Intelligence Opportunities. Sci. Total Environ. 2024, 917, 170085. [Google Scholar] [PubMed]
- Zeni, C.; Pinsler, R.; Zügner, D.; Fowler, A.; Horton, M.; Fu, X.; Wang, Z.; Shysheya, A.; Crabbé, J.; Ueda, S.; et al. A Generative Model for Inorganic Materials Design. Nature 2025, 639, 624–632. [Google Scholar] [CrossRef]
- Park, H.; Yan, X.; Zhu, R.; Huerta, E.A.; Chaudhuri, S.; Cooper, D.; Foster, I.; Tajkhorshid, E. A Generative Artificial Intelligence Framework Based on a Molecular Diffusion Model for the Design of Metal-Organic Frameworks for Carbon Capture. Commun. Chem. 2024, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Curti, L.; Moore, O.W.; Babakhani, P.; Xiao, K.-Q.; Woulds, C.; Bray, A.W.; Fisher, B.J.; Kazemian, M.; Kaulich, B.; Peacock, C.L. Carboxyl-Richness Controls Organic Carbon Preservation during Coprecipitation with Iron (Oxyhydr) Oxides in the Natural Environment. Commun. Earth Environ. 2021, 2, 229. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization Mechanisms of Soil Organic Matter: Implications for C-Saturation of Soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Dziejarski, B.; Serafin, J.; Andersson, K.; Krzyżyńska, R. CO2 Capture Materials: A Review of Current Trends and Future Challenges. Mater. Today Sustain. 2023, 24, 100483. [Google Scholar] [CrossRef]
- Thapa, B.; Mowrer, J. Soil Carbon and Aggregate Stability Are Positively Related and Increased under Combined Soil Amendment, Tillage, and Cover Cropping Practices. Soil Sci. Soc. Am. J. 2024, 88, 730–744. [Google Scholar] [CrossRef]
- Tan, L.; Fan, X.; Yan, G.; Peng, M.; Zhang, N.; Ye, M.; Gao, Z.; Song, A.; Nikolic, M.; Liang, Y. Sequestration Potential of Phytolith Occluded Carbon in China’s Paddy Rice (Oryza Sativa L.) Systems. Sci. Total Environ. 2021, 774, 145696. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Z.; Hao, Q.; Wang, Y.; Ding, F.; Song, A. Phytolith-Occluded Carbon Storages in Forest Litter Layers in Southern China: Implications for Evaluation of Long-Term Forest Carbon Budget. Front. Plant Sci. 2019, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Q.; Tang, T.; Chen, X.; Luo, X. Silicon Fertilizer Application Promotes Phytolith Accumulation in Rice Plants. Front. Plant Sci. 2019, 10, 425. [Google Scholar] [CrossRef]
- Anjum, M.; Prakash, N.B. Production of Phytolith and PhytOC and Distribution of Extractable Si Pools in Aerobic Rice as Influenced by Different Si Sources. Front. Plant Sci. 2023, 14, 1146416. [Google Scholar] [CrossRef] [PubMed]
- Soratto, R.P.; Crusciol, C.A.C.; Castro, G.S.A.; da Costa, C.H.M.; Ferrari Neto, J. Leaf Application of Silicic Acid to White Oat and Wheat. Rev. Bras. Ciênc. Solo 2012, 36, 1538–1544. [Google Scholar] [CrossRef]
- Chen, Y.; He, D.; Wu, H.; Li, Y.; Li, P.; Huang, H.; Liao, X.; Qiu, Q.; Liu, J.; Liu, Y.; et al. Silicon Fertiliser Application Increases the Terrestrial Ecosystem Carbon Pool at the Global Scale. Geoderma 2024, 442, 116806. [Google Scholar] [CrossRef]
- Azeem, M.K.; Islam, A.; Khan, R.U.; Rasool, A.; Qureshi, M.A.U.R.; Rizwan, M.; Sher, F.; Rasheed, T. Eco-friendly Three-dimensional Hydrogels for Sustainable Agricultural Applications: Current and Future Scenarios. Polym. Adv. Technol. 2023, 34, 3046–3062. [Google Scholar] [CrossRef]
- Jansen-van Vuuren, R.D.; Naficy, S.; Ramezani, M.; Cunningham, M.; Jessop, P. CO 2-Responsive Gels. Chem. Soc. Rev. 2023, 52, 3470–3542. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, A.A.; Rehman, O.; Rashid, M.; Alvi, S.; Raza, A.; Waheed, A.; Irfan, M.; Saleem, S.; Iqbal, M.; Mujtaba, G. Potentials of hydrogels in rainfed soil to conserve soil moisture and fertility to maximize the wheat yield. Soil Environ. 2020, 39, 204–210. [Google Scholar]
- Smagin, A.V.; Sadovnikova, N.B.; Belyaeva, E.A.; Krivtsova, V.N.; Shoba, S.A.; Smagina, M.V. Gel-Forming Soil Conditioners of Combined Action: Field Trials in Agriculture and Urban Landscaping. Polymers 2022, 14, 5131. [Google Scholar] [CrossRef]
- Krishnan, M.R.; Alsharaeh, E.H. Polymer Gel Amended Sandy Soil with Enhanced Water Storage and Extended Release Capabilities for Sustainable Desert Agriculture. J. Polym. Sci. Eng. 2023, 6, 2892. [Google Scholar] [CrossRef]
- Zhang, W.; Mu, X.; Xu, Y.; Ma, G.; Lei, Z. Research Progress of Environment-Responsive Hydrogel Applications in Agriculture. Express Polym. Lett. 2024, 18, 193–213. [Google Scholar] [CrossRef]
- Guo, Y.; Bolongaro, V.; Hatton, T.A. Scalable Biomass-Derived Hydrogels for Sustainable Carbon Dioxide Capture. Nano Lett. 2023, 23, 9697–9703. [Google Scholar] [CrossRef]
- Youns, Y.T.; Manshad, A.K.; Ali, J.A. Sustainable Aspects behind the Application of Nanotechnology in CO2 Sequestration. Fuel 2023, 349, 128680. [Google Scholar] [CrossRef]
- Pramanik, P.; Ray, P.; Maity, A.; Das, S.; Ramakrishnan, S.; Dixit, P. Nanotechnology for Improved Carbon Management in Soil. In Carbon Management in Tropical and Sub-Tropical Terrestrial Systems; Ghosh, P.K., Mahanta, S.K., Mandal, D., Mandal, B., Ramakrishnan, S., Eds.; Springer: Singapore, 2020; pp. 403–415. ISBN 978-981-13-9628-1. [Google Scholar]
- Wang, S.; Liu, Y.; Wang, X.; Xiang, H.; Kong, D.; Wei, N.; Guo, W.; Sun, H. Effects of Concentration-Dependent Graphene on Maize Seedling Development and Soil Nutrients. Sci. Rep. 2023, 13, 2650. [Google Scholar] [CrossRef]
- Bayat, H.; Kolahchi, Z.; Valaey, S.; Rastgou, M.; Mahdavi, S. Iron and Magnesium Nano-Oxide Effects on Some Physical and Mechanical Properties of a Loamy Hypocalcic Cambisol. Geoderma 2019, 335, 57–68. [Google Scholar] [CrossRef]
- Kannan, G.; Sujatha, E.R. Geotechnical Behaviour of Nano-Silica Stabilized Organic Soil. Geomech. Eng. 2022, 28, 239–253. [Google Scholar]
- Sinha, T.; Singh, B.P.; Nandi, K.; Das, K. Nanomaterials in Soil Health Management and Crop Production. In Modern Nanotechnology: Volume 1: Environmental Sustainability and Remediation; Malik, J.A., Sadiq Mohamed, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 77–99. ISBN 978-3-031-31111-6. [Google Scholar]
- Fierer, N.; Walsh, C.M. Can We Manipulate the Soil Microbiome to Promote Carbon Sequestration in Croplands? PLoS Biol. 2023, 21, e3002207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, X.; Zhong, S.; Yin, G.; Gao, Y.; He, X. Recalcitrant Carbon Components in Glomalin-Related Soil Protein Facilitate Soil Organic Carbon Preservation in Tropical Forests. Sci. Rep. 2017, 7, 2391. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.M.F.; Tabita, F.R. Microbial Ribulose 1,5-Bisphosphate Carboxylase/Oxygenase: A Molecule for Phylogenetic and Enzymological Investigation. FEMS Microbiol. Lett. 1997, 146, 13–22. [Google Scholar] [CrossRef]
- Mao, Y.; Catherall, E.; Díaz-Ramos, A.; Greiff, G.R.L.; Azinas, S.; Gunn, L.; McCormick, A.J. The Small Subunit of Rubisco and Its Potential as an Engineering Target. J. Exp. Bot. 2022, 74, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, M.; Pandey, A.; Thakur, I.S. Genomic Analysis of Carbon Dioxide Sequestering Bacterium for Exopolysaccharides Production. Sci. Rep. 2019, 9, 4270. [Google Scholar] [CrossRef]
- Kanao, T.; Fukui, T.; Atomi, H.; Imanaka, T. ATP-Citrate Lyase from the Green Sulfur Bacterium Chlorobium Limicola Is a Heteromeric Enzyme Composed of Two Distinct Gene Products. Eur. J. Biochem. 2001, 268, 1670–1678. [Google Scholar] [CrossRef]
- Garritano, A.N.; Song, W.; Thomas, T. Carbon Fixation Pathways across the Bacterial and Archaeal Tree of Life. PNAS Nexus 2022, 1, pgac226. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, Y.; Yue, X.; Wang, S.; Yang, K.; Xu, Y.; Shen, Q.; Friman, V.-P.; Wei, Z. The Role of Rhizosphere Phages in Soil Health. FEMS Microbiol. Ecol. 2024, 100, fiae052. [Google Scholar] [CrossRef]
- Trubl, G.; Jang, H.B.; Roux, S.; Emerson, J.B.; Solonenko, N.; Vik, D.R.; Solden, L.; Ellenbogen, J.; Runyon, A.T.; Bolduc, B. Soil Viruses Are Underexplored Players in Ecosystem Carbon Processing. mSystems 2018, 3, E00076-18. [Google Scholar] [CrossRef]
- Braga, L.P.P.; Spor, A.; Kot, W.; Breuil, M.-C.; Hansen, L.H.; Setubal, J.C.; Philippot, L. Impact of Phages on Soil Bacterial Communities and Nitrogen Availability under Different Assembly Scenarios. Microbiome 2020, 8, 52. [Google Scholar] [CrossRef]
- Breitbart, M.; Bonnain, C.; Malki, K.; Sawaya, N.A. Phage Puppet Masters of the Marine Microbial Realm. Nat. Microbiol. 2018, 3, 754–766. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.P.; Burgess, S.J.; Doran, L.; Hansen, J.; Manukyan, L.; Maryn, N.; Gotarkar, D.; Leonelli, L.; Niyogi, K.K.; Long, S.P. Soybean Photosynthesis and Crop Yield Are Improved by Accelerating Recovery from Photoprotection. Science 2022, 377, 851–854. [Google Scholar] [CrossRef]
- Salesse-Smith, C.E.; Lochocki, E.B.; Doran, L.; Haas, B.E.; Stutz, S.S.; Long, S.P. Greater Mesophyll Conductance and Leaf Photosynthesis in the Field through Modified Cell Wall Porosity and Thickness via AtCGR3 Expression in Tobacco. Plant Biotechnol. J. 2024, 22, 2504–2517. [Google Scholar] [CrossRef]
- Dhungana, I.; Kantar, M.B.; Nguyen, N.H. Root Exudate Composition from Different Plant Species Influences the Growth of Rhizosphere Bacteria. Rhizosphere 2022, 25, 100645. [Google Scholar] [CrossRef]
- Roy, D.; Sinha, A.K.; Rakesh, S.; Rao, K.K.; Sahoo, S.; Bhattacharya, P.M.; Mitra, B.; Mukhopadhyay, P.; Padbhushan, R. Addition of Biofertilizers with Crop Residue in Conservation Agriculture Improves Soil Carbon Sequestration: A Long-Term Field Study. Trop. Ecol. 2025, 66, 119–131. [Google Scholar] [CrossRef]
- Kommunikation PhD Defense by Anastasios Marantos. Available online: https://nbi.ku.dk/english/calendar/activities_23/phd-defense-by-anastasios-marantos/ (accessed on 23 June 2025).
- Kuzyakov, Y.; Mason-Jones, K. Viruses in Soil: Nano-Scale Undead Drivers of Microbial Life, Biogeochemical Turnover and Ecosystem Functions. Soil Biol. Biochem. 2018, 127, 305–317. [Google Scholar] [CrossRef]
- Acharya, U.; Lal, R.; Chandra, R. Data Driven Approach on In-Situ Soil Carbon Measurement. Carbon Manag. 2022, 13, 401–419. [Google Scholar] [CrossRef]
- Yuan, Q.; Sheng, W.; Zhang, Z.; Li, H.; Zhang, M. Recent Advances in Soil Nutrient Monitoring: A Review. In Sensing Technologies for Field and In-House Crop Production: Technology Review and Case Studies; Zhang, M., Li, H., Sheng, W., Qiu, R., Zhang, Z., Eds.; Springer Nature: Singapore, 2023; pp. 19–38. ISBN 978-981-99-7927-1. [Google Scholar]
- Abdalqadir, M.; Hughes, D.; Rezaei Gomari, S.; Rafiq, U. A State of the Art of Review on Factors Affecting the Enhanced Weathering in Agricultural Soil: Strategies for Carbon Sequestration and Climate Mitigation. Environ. Sci. Pollut. Res. 2024, 31, 19047–19070. [Google Scholar] [CrossRef]
- Di Rauso Simeone, G.; Maennicke, H.; Bromm, T.; Glaser, B. Artificial Formation of Benzene Polycarboxylic Acids during Sample Processing of Black Carbon Analysis: The Role of Organic Carbon Amount. Chem. Biol. Technol. Agric. 2024, 11, 8. [Google Scholar] [CrossRef]
- Suji, M.; Davamani, V.; Periyasamy, D.; Thambiyannan, S.; Subramanian, S.; Parameswari, E.; Ramasamy, S.P.; Murugan, P. Silicon Fertilization for Carbon Sequestration Through PhytOC Production in Plants. Commun. Soil Sci. Plant Anal. 2025, 56, 292–318. [Google Scholar] [CrossRef]
- Parr, J.F.; Sullivan, L.A. Soil Carbon Sequestration in Phytoliths. Soil Biol. Biochem. 2005, 37, 117–124. [Google Scholar] [CrossRef]
- Acharya, B.S.; Dodla, S.; Wang, J.J.; Pavuluri, K.; Darapuneni, M.; Dattamudi, S.; Maharjan, B.; Kharel, G. Biochar Impacts on Soil Water Dynamics: Knowns, Unknowns, and Research Directions. Biochar 2024, 6, 34. [Google Scholar] [CrossRef]
- Toushif, P.K.; Santosh, S.; Wagmare, B.; Rakesh, S.; Madegowda, J.; Dey, A.N.; Dinesha, S. Nanomaterials for Soil Carbon Sequestration: Implications in Agroforestry. In Nanomaterials in Agroforestry Systems; Jabborova, D., Sarkar, D., Rakesh, S., Datta, R., Singh, S., Eds.; Springer Nature: Singapore, 2025; pp. 129–141. ISBN 978-981-96-1337-3. [Google Scholar]
- Enebe, M.C.; Ray, R.L.; Griffin, R.W. Carbon Sequestration and Soil Responses to Soil Amendments—A Review. J. Hazard. Mater. Adv. 2025, 18, 100714. [Google Scholar] [CrossRef]
- Nilahyane, A.; Ghimire, R.; Sharma Acharya, B.; Schipanski, M.E.; West, C.P.; Obour, A.K. Overcoming Agricultural Sustainability Challenges in Water-Limited Environments through Soil Health and Water Conservation: Insights from the Ogallala Aquifer Region, USA. Int. J. Agric. Sustain. 2023, 21, 2211484. [Google Scholar] [CrossRef]
- Brewer, K.M.; Gaudin, A.C. Potential of Crop-Livestock Integration to Enhance Carbon Sequestration and Agroecosystem Functioning in Semi-Arid Croplands. Soil Biol. Biochem. 2020, 149, 107936. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Laird, D.A.; Heaton, E.A.; Rathke, S.; Acharya, B.S. Soil Carbon Increased by Twice the Amount of Biochar Carbon Applied after 6 Years: Field Evidence of Negative Priming. GCB Bioenergy 2020, 12, 240–251. [Google Scholar] [CrossRef]
- Gross, A.; Bromm, T.; Glaser, B. Soil Organic Carbon Sequestration after Biochar Application: A Global Meta-Analysis. Agronomy 2021, 11, 2474. [Google Scholar] [CrossRef]
- Ascenzi, I.; Hilbers, J.P.; van Katwijk, M.M.; Huijbregts, M.A.J.; Hanssen, S.V. Increased but Not Pristine Soil Organic Carbon Stocks in Restored Ecosystems. Nat. Commun. 2025, 16, 637. [Google Scholar] [CrossRef]
- Tian, D.; Xiang, Y.; Seabloom, E.; Wang, J.; Jia, X.; Li, T.; Li, Z.; Yang, J.; Guo, H.; Niu, S. Soil Carbon Sequestration Benefits of Active versus Natural Restoration Vary with Initial Carbon Content and Soil Layer. Commun. Earth Environ. 2023, 4, 83. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, P.; Li, P.; Li, Z.; Min, Z.; Sun, J.; Cui, L.; Niu, H.; Zu, P.; Cao, M. Effects of Vegetation Restoration on Soil Organic Carbon in the Loess Plateau: A Meta-Analysis. Land Degrad. Dev. 2023, 34, 2088–2097. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Isbell, F.; Arce-Plata, M.I.; Di Marco, M.; Harfoot, M.; Johnson, J.; Lerman, S.B.; Miller, B.W.; Morelli, T.L.; Mori, A.S.; et al. Biodiversity Loss Reduces Global Terrestrial Carbon Storage. Nat. Commun. 2024, 15, 4354. [Google Scholar] [CrossRef] [PubMed]
- Spohn, M.; Bagchi, S.; Biederman, L.A.; Borer, E.T.; Bråthen, K.A.; Bugalho, M.N.; Caldeira, M.C.; Catford, J.A.; Collins, S.L.; Eisenhauer, N.; et al. The Positive Effect of Plant Diversity on Soil Carbon Depends on Climate. Nat. Commun. 2023, 14, 6624. [Google Scholar] [CrossRef] [PubMed]
- Colunga, S. Soil Carbon Sequestration Efforts in Arid and Semi-Arid Climates Under Conservation Agriculture and Reforestation. Master’s Thesis, University of Texas Rio Grande Valley, Edinburg, TX, USA, 2024. [Google Scholar]
- Basak, B.B.; Sarkar, B.; Saha, A.; Sarkar, A.; Mandal, S.; Biswas, J.K.; Wang, H.; Bolan, N.S. Revamping Highly Weathered Soils in the Tropics with Biochar Application: What We Know and What Is Needed. Sci. Total Environ. 2022, 822, 153461. [Google Scholar] [CrossRef] [PubMed]
- Lessmann, M.; Ros, G.H.; Young, M.D.; de Vries, W. Global Variation in Soil Carbon Sequestration Potential through Improved Cropland Management. Glob. Change Biol. 2022, 28, 1162–1177. [Google Scholar] [CrossRef]
- Mayer, S.; Wiesmeier, M.; Sakamoto, E.; Hübner, R.; Cardinael, R.; Kühnel, A.; Kögel-Knabner, I. Soil Organic Carbon Sequestration in Temperate Agroforestry Systems—A Meta-Analysis. Agric. Ecosyst. Environ. 2022, 323, 107689. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Alori, E.T.; Ogunbode, T.O.; Sangoyomi, T.; Oriade, O.A. Enhancing Organic Carbon Content in Tropical Soils: Strategies for Sustainable Agriculture and Climate Change Mitigation. Open Agric. J. 2023, 17, e18743315282476. [Google Scholar] [CrossRef]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Lyamlouli, K. Olive Mill Waste Sludge: From Permanent Pollution to a Highly Beneficial Organic Biofertilizer: A Critical Review and Future Perspectives. Ecotoxicol. Environ. Saf. 2023, 259, 114997. [Google Scholar] [CrossRef]
- Surdoval, A.; Jain, M.; Blair, E.; Wang, H.; Blesh, J. Financial Incentive Programs and Farm Diversification with Cover Crops: Assessing Opportunities and Challenges. Environ. Res. Lett. 2024, 19, 044063. [Google Scholar] [CrossRef]
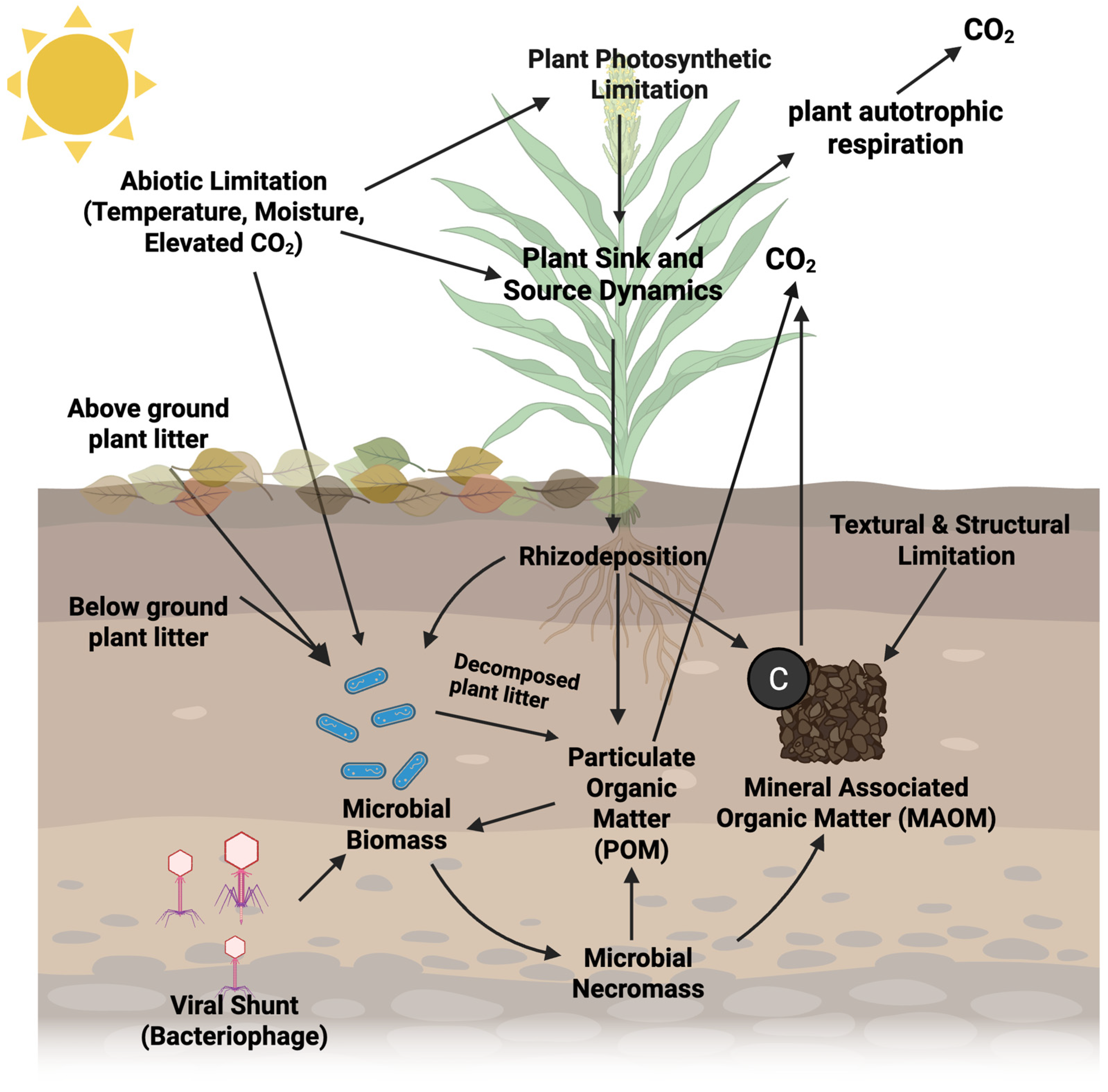

| Processes | Current Understanding | Contradictions | SOC Implications | Knowledge Gaps | References |
|---|---|---|---|---|---|
| Photosynthetic C supply | Net land sink driven mainly by rising CO2: 1.2 ± 0.2 Pg C yr−1 (1960–1969) → 3.5 ± 0.8 Pg C yr−1 (2011–2020). | Extreme heat/drought in 2023 drove the land sink near zero, indicating climatic overrides on CO2-fertilization. | Confirms that additional plant C input is available for sequestration but is increasingly counter balanced by warming feedback | Understanding partitioning of above- and belowground C allocation at global scale in changing plant productivity under elevated CO2. Lack of photo–respiration tradeoffs and nutrient feedback to MRV models and tools. | [71,72] |
| eCO2 effects on plant–soil allocation | Meta-analysis of 108 eCO2 experiments: SOC rose 8 ± 2% in grasslands, remained 0 ± 2% in forests; greatest plant biomass gains frequently reduced SOC via nutrient mining. | Five-year mountain beech–spruce FACE found no detectable SOC change despite eCO2. | Demonstrates allocation trade-off; nutrient constraints limit conversion of extra NPP to stable SOC | Evaluation of threshold points where nutrient addition flips the trade-off is necessary. | [25,73] |
| Root architecture (“Steep, Cheap & Deep” maize) | Roots contain ~45.6% carbon and contribute 30–40% of SOC, often with multi-millennial turnover in deep soil. Root-derived C inputs range from 0.1 to 2.8 t C ha−1 yr−1. | 13CO2 tracing shows root activity after thaw increased SOC loss by 31% via priming of deep pools. | Deep inputs target sub-saturated mineral surfaces → higher MAOC potential | Mechanistic drivers of bimodal rooting (species traits vs. soil constraints) remain unresolved. Lack of in situ measurements of how deep-root inputs translate into MAOC vs. primed losses. Standardized protocols for sampling > 1 m depths are still scarce, impeding trait-based breeding and model calibration. | [74,75,76] |
| Belowground C allocation fraction | Belowground NPP 24.7 Pg C yr−1 = 46% of terrestrial C fixation. | Exudate additions raised mineralization and DOC, converting “resistant” SOC into CO2 (meta-analysis). | Large flux highlights leverage of root/rhizodeposition pathways | Chemical fate of this flux (POC vs. MAOC) remains poorly constrained. | [57,77] |
| Root chemistry and rhizodeposition | Rhizodeposits supply up to ~5% of GPP; contribute 10–40% of MAOC depending on soil mineralogy. | High-N litter trials increase mineralization of lignin, reducing its assumed recalcitrance. | Confirms chemical gateway to stable pools | Need compound-specific turnover rates under drought, high temperature, and high CO2. | [78,79] |
| Microbial biomass vs. necromass | Amino-sugar proxies show 50–80% of SOC is necromass; cropland 51%, grassland 47%, forest 35% (0–20 cm). | Isotopically labelled necromass became mineral-associated in 3 days, but 50% respired within 8 months under intensive management. | Validates necromass as dominant stable pool | Quantitative understanding of microbial turnover, including virus-mediated C balances across depth and soil types, predator-driven shifts in CUE and necromass, especially in deep soil and under shifting aerobic/anaerobic conditions. | [64,80] |
| Soil viral shunt | Viral addition raised cumulative respiration by 30% over 41 d within one soil and altered DOC/N coupling. | Mesocosms with elevated virus abundance reduced respiration by 3–6% via a kill-the-host strategy. | Viral-induced lysis can both liberate DOC (priming) and add necromass | Quantify net C balance across soil types, moisture regimes and agronomic managements. | [81,82] |
| Mycorrhizal type | Forest plots with high ectomycorrhizal (EcM) dominance stored significantly more soil C (model and inventory of >4000 plots). | Fertility-gradient study shows EcM fungi either accelerate or slow decomposition depending on N/P status. | Points to mycorrhizal trait filtering as a management lever | Determine absolute % increase and mechanisms (enzyme repression vs. litter quality) in non-boreal biomes. | [83,84] |
| Processes | Current Understanding | Contradictory Findings | SOC Implication | Knowledge Gaps | References |
|---|---|---|---|---|---|
| Temperature and moisture | SOC responds negatively to increasing temperature, with a coefficient of 0.24. Earth system model analysis shows that frequent droughts can reduce the current land carbon sink by 2–3 Gt C per year. Unprotected POC is more sensitive than protected carbon; >28% higher loss with 10 °C increase in temperature. | Field warming in temperate forests often yields Q10 < 1.5 with no net SOC loss, likely due to substrate depletion. | Temperature sensitivity is ecosystem dependent, lab-predictions can overestimate field losses. | Precise moisture thresholds at which MAOC flips from sink to source and representation of VOC-C pathways released during drought–rewet pulses. | [134,135] |
| Nitrogen fertilization | Meta-analysis of long-term N fertilization found it increased SOC stocks by a mean of 4–7%. | Nitrogen addition can increase SOC loss, and there is no significant impact on SOC content. | Nutrient additions can redistribute carbon between soil pools (from POC to MAOC) rather than uniformly increasing the total SOC stock. The net outcome depends on the balance of production and decomposition. | Defining multi-element thresholds (N, P, and micronutrients) that maximize net SOC sequestration without causing nutrient saturation, priming of old carbon loss, or eutrophication. | [136,137] |
| Textural limitation (soil saturation) | Globally, subsoils (30–100 cm) in croplands are estimated to be, on average, at only 46% of their C saturation capacity, indicating a large potential sink. | In soils rich in reactive minerals such as Andisols, SOC can reach an apparent saturation point where additional carbon inputs do not lead to further SOC accrual, implying mineralogy-specific ceilings on storage. | The potential for SOC sequestration varies strongly with soil mineralogy. Universal “carbon deficit” models fail to capture these mineral-specific limitations, especially in volcanic and oxide-rich soils. | The specific chemical or physical mechanisms that drive the early onset of carbon saturation in soils rich in iron and aluminum oxides. | [138] |
| Chemical pools of carbon (MAOC) | Radiocarbon (14C) dating of bulk MAOC often reveals mean residence times of hundreds to thousands of years, suggesting long-term stability. | Despite its old average age, a significant fraction of MAOC can be rapidly mineralized. Studies using enzyme additions have shown that newer, more labile MAOC can be decomposed within years, especially under nutrient enrichment. | The persistence of MAOC is controlled more by its physical and chemical accessibility to microbes and enzymes rather than its intrinsic chemical structure or age. This stability can be modulated by nutrient status. | Partitioning the loss of MAOC into distinct pathways (enzyme-driven decomposition vs. physical desorption from minerals) under the combined pressures of global warming and fertilization. | [139] |
| Limitation | Solution | Applicability and Target Systems | Scalability and Economic Feasibility | Current Status | Select References |
|---|---|---|---|---|---|
| Photosynthetic Capacity | Photosynthetic Modification | Primarily targets major C3 crops (wheat, rice, soybeans) that have lower photosynthetic efficiency than C4 crops. Most effective in high-input agricultural systems where light and nutrients are not limiting factors. | Low–medium. High R&D costs. Regulatory hurdles for genetically modified organisms (GMOs) are significant. Once developed, scaling through seed distribution is feasible, but requires widespread farmer adoption and adapted agronomic practices. | Laboratory/Early Field Trials. Researchers have successfully engineered modifications in model plants (tobacco) and some crops, demonstrating yield increases. Not yet commercially available. | [31,209,210] |
| Root Architecture and C Allocation | Modification of Root Systems and Genetic Engineering | Broadly applicable to all annual and perennial cropping systems, especially in regions with deep soils or those prone to drought. Essential for moving carbon into more stable, deeper soil layers. | Medium. Breeding is a proven, scalable pathway. Genetic engineering faces similar regulatory and public acceptance hurdles as photosynthetic modification. The perennial grain Kernza® is an early commercial example. | Research for early commercialization. Specific genes (e.g., DRO1) identified and tested. Perennial grain varieties such as Kernza® are available on a limited commercial scale. Widespread availability of deep-rooting staple crops is still years away. | [42,151] |
| Priming Effect and Root Chemistry | Modification of Rhizodeposition and Root Chemistry | Applicable to agricultural systems where maximizing the efficiency of carbon inputs is critical, particularly in soils with low carbon saturation potential or where organic amendments are used. | Low. Requires advanced genetic selection and engineering, making R&D costs high. The link between specific exudates and long-term carbon stabilization needs more field validation before commercial breeding efforts can be scaled. | Conceptual/Laboratory Research. Specific compounds have been identified that reduce priming in lab settings. Breeding programs are beginning to consider root chemistry as a selection trait, but it is not yet a primary focus. | [211] |
| Microbial CUE and Necromass Formation | Microbial Modification and Management (Bio-fertilizers) | Best suited for degraded soils or intensive agricultural systems where native microbial communities may be suboptimal. Efficacy is highly dependent on soil type, climate, and management practices. | High. Production of microbial inoculants is well established and relatively low cost. The main challenge is ensuring product stability (shelf life) and consistent performance across diverse field conditions. | Commercially available. Bio-fertilizers and microbial soil amendments are widely available, but their specific formulation for carbon sequestration is an emerging market. Product consistency and efficacy remain variable. | [212] |
| Viral and Predator–Prey Dynamics | Deliberate Phage Infection | Highly targeted approach, potentially useful for controlling specific microbial groups known to accelerate carbon loss in certain high-value agricultural systems (e.g., horticulture) or in bioremediation contexts. | Very low. Phage therapy for soil applications is highly complex. Identifying, isolating, and deploying effective phages at a field scale is a massive logistical and ecological challenge. Cost-prohibitive for broadacre agriculture currently. | Conceptual/Early Laboratory Research. Phage biology is well understood, but its application for manipulating broad soil ecosystem functions such as carbon cycling is in its infancy. No field-scale applications for this purpose exist. | [213,214] |
| Temperature and Moisture Stress | Responsive Hydrogels | Most applicable to arid and semi-arid regions, sandy soil with low water-holding capacity, and high-value horticulture to mitigate drought stress and improve water use efficiency. | Medium–low. The cost of hydrogels can be prohibitive for large acres of row crops. Concerns about the long-term fate and potential microplastic pollution from non-biodegradable polymers limit scalability. Biodegradable options are emerging but are more expensive. | Commercially Available/Niche Application. Used in horticulture, landscaping, and forestry. Research into biodegradable hydrogels and their large-scale agricultural use is ongoing. | [187,188] |
| Nutrient Limitation | Precision Nutrient Management and Bio-fertilizers | Universally applicable to all managed agricultural systems. Essential for optimizing plant growth and carbon inputs while minimizing environmental impacts such as nitrous oxide emissions and nutrient runoff. | High. Technology for precision application (e.g., variable rate technology) is commercially available and becoming more affordable. Bio-fertilizers are scalable. The main barrier is upfront investment and farmer training. | Commercially available and increasingly adopted. Widely adopted in developed agricultural economies. Continued innovation focuses on integrating more data layers (soil sensors, satellite imagery) for higher precision. | [215,216] |
| Carbon Saturation | C-Capturing Minerals (Enhanced Weathering) | Best suited for acidic agricultural soils where crushed silicate rocks (such as basalt) can provide a liming co-benefit. Requires accessible sources of suitable rock and infrastructure for grinding and transport. | Medium. Scalability is limited by the proximity of farms to rock quarries, transportation costs, and the energy required for grinding. Supply chains need to be developed. Potentially very large scale if logistical and economic hurdles are overcome. | Field Trials and early commercial projects. Several startups and research projects are conducting large-scale field trials. Carbon credits from enhanced weathering are being sold on the voluntary market, but the science of measurement, reporting, and verification (MRV) is still developing. | [217] |
| Chemical Instability of SOC | Synthetic Poly-carboxylic Compounds | Potential application in degraded soils or as an additive to other organic amendments (e.g., compost) to increase the formation of stable organo–mineral complexes. | Low. Currently, these are specialty chemicals, not produced at a scale or cost suitable for agriculture. The environmental impact and long-term stability of synthetic polymers in soil require extensive study. | Conceptual/Laboratory Research. The principles of organo–mineral stabilization are well known. Some commercial products use carboxylic acids as fertilizer additives, but their design for long-term carbon sequestration is not established. | [218] |
| Limited Formation of Stable C Pools | Phytolith Formation Enhancement | Applicable to crops that are high in silica accumulators, such as rice, sugarcane, wheat, and bamboo. Most effective in soils with available silicon or where silicon-based fertilizers are applied. | Medium. Application of silicate amendments (e.g., slag, diatomaceous earth, rice husk biochar) is logistically feasible. Breeding for higher silica uptake is a viable long-term strategy. | Research/Field Trials. The role of PhytOC in carbon sequestration is well documented. Field trials applying silicon sources have shown increased phytolith production. It is not yet a mainstream, managed sequestration practice. | [219,220] |
| Physical Protection and Aggregation | Nanomaterials and Biochar | Biochar: widely applicable across most soil types and systems. Nanomaterials: highly experimental, targeted for specific soil conditioning challenges such as improving aggregation or water retention. | Biochar: High. Scalable, with production systems ranging from small on-farm units to large industrial plants. Cost and quality control are key variables. Nanomaterials: very low. Production costs are extremely high, and major concerns about ecotoxicity and environmental fate prevent any consideration for large-scale agricultural use at present. | Biochar: commercially Available and increasingly adopted. A well-established soil amendment with a growing market. Nanomaterials: laboratory research. Exclusively in the research phase for soil applications; no commercial use in agriculture for sequestration. | [221,222,223] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Beegum, S.; Acharya, B.S.; Panday, D. Soil Carbon Sequestration: A Mechanistic Perspective on Limitations and Future Possibilities. Sustainability 2025, 17, 6015. https://doi.org/10.3390/su17136015
Das S, Beegum S, Acharya BS, Panday D. Soil Carbon Sequestration: A Mechanistic Perspective on Limitations and Future Possibilities. Sustainability. 2025; 17(13):6015. https://doi.org/10.3390/su17136015
Chicago/Turabian StyleDas, Saurav, Sahila Beegum, Bharat Sharma Acharya, and Dinesh Panday. 2025. "Soil Carbon Sequestration: A Mechanistic Perspective on Limitations and Future Possibilities" Sustainability 17, no. 13: 6015. https://doi.org/10.3390/su17136015
APA StyleDas, S., Beegum, S., Acharya, B. S., & Panday, D. (2025). Soil Carbon Sequestration: A Mechanistic Perspective on Limitations and Future Possibilities. Sustainability, 17(13), 6015. https://doi.org/10.3390/su17136015








