Influence of Biochar Foliar Application on Malvazija Istarska Grapevine Physiology
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Photosynthetic Activity of Grapevine Leaves
3.2. Leaf Water Potential of Grapevine Leaves
3.3. Elemental Composition of Grapevine Leaves
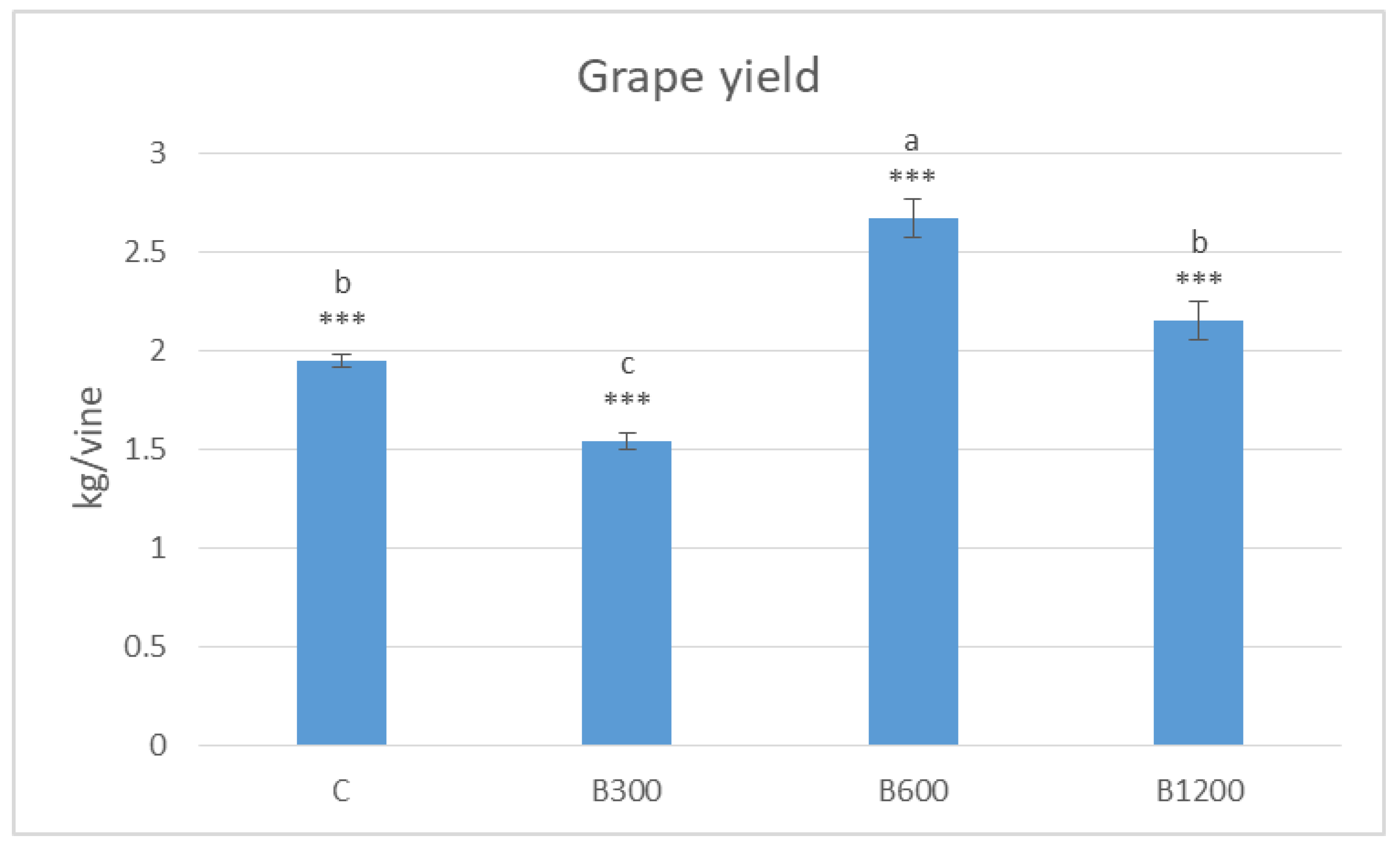
3.4. Grape Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marín, D.; Armengol, J.; Carbonell-Bejerano, P.; Escalona, J.M.; Gramaje, D.; Hernández-Montes, E.; Intrigliolo, D.S.; Martínez-Zapater, J.M.; Medrano, H.; Mirás-Avalos, J.M.; et al. Challenges of Viticulture Adaptation to Global Change: Tackling the Issue from the Roots. Aust. J. Grape Wine Res. 2021, 27, 8–25. [Google Scholar] [CrossRef]
- Schultz, H.R.; Jones, G.V. Climate Induced Historic and Future Changes in Viticulture. J. Wine Res. 2010, 21, 137–145. [Google Scholar] [CrossRef]
- Alsafadi, K.; Bi, S.; Bashir, B.; Alsalman, A.; Srivastava, A.K. Future Scenarios of Bioclimatic Viticulture Indices in the Eastern Mediterranean: Insights into Sustainable Vineyard Management in a Changing Climate. Sustainability 2023, 15, 11740. [Google Scholar] [CrossRef]
- Xyrafis, E.G.; Fraga, H.; Nakas, C.T.; Koundouras, S. A Study on the Effects of Climate Change on Viticulture on Santorini Island. OENO One 2022, 56, 259–273. [Google Scholar] [CrossRef]
- Ponti, L.; Gutierrez, A.P.; Boggia, A.; Neteler, M. Analysis of Grape Production in the Face of Climate Change. Climate 2018, 6, 20. [Google Scholar] [CrossRef]
- Prelac, M.; Palčić, I.; Cvitan, D.; Anđelini, D.; Repajić, M.; Ćurko, J.; Kovačević, T.K.; Goreta Ban, S.; Užila, Z.; Ban, D.; et al. Biochar from Grapevine Pruning Residues as an Efficient Adsorbent of Polyphenolic Compounds. Materials 2023, 16, 4716. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Cayuela, M.L.; Rasse, D.P.; Sánchez-Monedero, M.A. Biochars from Mediterranean Agroindustry Residues: Physicochemical Properties Relevant for C Sequestration and Soil Water Retention. ACS Sustain. Chem. Eng. 2019, 7, 4724–4733. [Google Scholar] [CrossRef]
- Anđelini, D.; Cvitan, D.; Prelac, M.; Pasković, I.; Černe, M.; Nemet, I.; Major, N.; Ban, S.G.; Užila, Z.; Ferri, T.Z.; et al. Biochar from Grapevine-Pruning Residues Is Affected by Grapevine Rootstock and Pyrolysis Temperature. Sustainability 2023, 15, 4851. [Google Scholar] [CrossRef]
- Beatrice, P.; Dalle Fratte, M.; Baronti, S.; Miali, A.; Genesio, L.; Vaccari, F.P.; Cerabolini, B.E.L.; Montagnoli, A.; Leuzinger, S.; Santorufo, L.; et al. The Long-Term Effect of Biochar Application to Vitis vinifera L. Reduces Fibrous and Pioneer Root Production and Increases Their Turnover Rate in the Upper Soil Layers. Front. Plant Sci. 2024, 15, 1384065. [Google Scholar] [CrossRef]
- Lucchetta, V.; Loesch, M.; Chitarrini, G.; Dordevic, N.; Matteazzi, A.; Sanoll, C.; Robatscher, P.; Raifer, B. The Use of Biochar as a Soil Amendment Did Not Affect Wine Quality in a Müller Thurgau Vineyard in South Tyrol (Italy). Laimbg. J. 2024, 6. [Google Scholar] [CrossRef]
- Rafique, R.; Ahmad, T.; Ahmed, M.; Azam Khan, M. Exploring Key Physiological Attributes of Grapevine Cultivars under the Influence of Seasonal Environmental Variability. OENO One 2023, 57, 381–397. [Google Scholar] [CrossRef]
- Kobus Hunter, J.J.; Tarricone, L.; Volschenk, C.; Giacalone, C.; Melo, M.S.; Zorer, R. Grapevine Physiological Response to Row Orientation-Induced Spatial Radiation and Microclimate Changes. OENO One 2020, 54, 411–433. [Google Scholar] [CrossRef]
- Genesio, L.; Miglietta, F.; Baronti, S.; Vaccari, F.P. Biochar Increases Vineyard Productivity without Affecting Grape Quality: Results from a Four Years Field Experiment in Tuscany. Agric. Ecosyst. Environ. 2015, 201, 20–25. [Google Scholar] [CrossRef]
- Premalatha, R.P.; Poorna Bindu, J.; Nivetha, E.; Malarvizhi, P.; Manorama, K.; Parameswari, E.; Davamani, V. A Review on Biochar’s Effect on Soil Properties and Crop Growth. Front. Energy Res. 2023, 11, 1092637. [Google Scholar] [CrossRef]
- Abd Elwahed, M.S.; Abd El-Aziz, M.E.; Shaaban, E.A.; Salama, D.M. New Trend to Use Biochar as Foliar Application for Wheat Plants (Triticum aestivum). J. Plant Nutr. 2019, 42, 1180–1191. [Google Scholar] [CrossRef]
- Williams, L.E. Physiological Tools to Assess Vine Water Status for Use in Vineyard Irrigation Management: Review and Update. Proc. Acta Hortic. 2017, 1157, 151–166. [Google Scholar] [CrossRef]
- Kumar, A.; Joseph, S.; Graber, E.R.; Taherysoosavi, S.; Mitchell, D.R.G.; Munroe, P.; Tsechansky, L.; Lerdahl, O.; Aker, W.; Sæbø, M. Fertilizing Behavior of Extract of Organomineral-Activated Biochar: Low-Dose Foliar Application for Promoting Lettuce Growth. Chem. Biol. Technol. Agric. 2021, 8, 38. [Google Scholar] [CrossRef]
- Shahzadi, J.; Zaib-Un-Nisa; Ali, N.; Iftikhar, M.; Shah, A.A.; Ashraf, M.Y.; Chao, C.; Shaffique, S.; Gatasheh, M.K. Foliar Application of Nano Biochar Solution Elevates Tomato Productivity by Counteracting the Effect of Salt Stress Insights into Morphological Physiological and Biochemical Indices. Sci. Rep. 2025, 15, 3205. [Google Scholar] [CrossRef]
- Palčić, I.; Jagatić Korenika, A.-M.; Jakobović, S.; Pasković, I.; Major, N.; Ban, D.; Ban, S.G.; Karoglan, M.; Petek, M.; Ćustić Herak, M.; et al. Soil Type Affects Grape Juice Free Amino Acids Profile during Ripening of Cv. Malvasia Istriana (Vitis vinifera L.). New Zeal. J. Crop Hortic. Sci. 2020, 48, 22–33. [Google Scholar] [CrossRef]
- Torres, N.; Yu, R.; Martínez-Lüscher, J.; Kostaki, E.; Kurtural, S.K. Application of Fractions of Crop Evapotranspiration Affects Carbon Partitioning of Grapevine Differentially in a Hot Climate. Front. Plant Sci. 2021, 12, 633600. [Google Scholar] [CrossRef]
- DIN ISO 10390:2021; Soil quality—Determination of pH. Beuth Verlag: Berlin, Germany, 2021.
- The European Biochar Certificate (EBC). Available online: https://www.european-biochar.org/en (accessed on 4 June 2025).
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a System for Identifying Grapevine Growth Stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Egri, D.; Pârvulescu, O.C.; Ion, V.A.; Răducanu, C.E.; Calcan, S.I.; Bădulescu, L.; Madjar, R.; Orbeci, C.; Dobre, T.; Moț, A.; et al. Vine Pruning-Derived Biochar for Agronomic Benefits. Agronomy 2022, 12, 2730. [Google Scholar] [CrossRef]
- de Assunção, H.H.T.; Campos, S.F.B.; Sousa, L.A.; Lemes, E.M.; Zandonadi, C.H.S.; da Cunha, J.P.A.R. Adjuvants plus Phytosanitary Products and the Effects on the Physical-Chemical Properties of the Spray Liquids. Biosci. J. 2019, 35, 1878–1885. [Google Scholar] [CrossRef]
- Mataffo, A.; Scognamiglio, P.; Dente, A.; Strollo, D.; Colla, G.; Rouphael, Y.; Basile, B. Foliar Application of an Amino Acid-Enriched Urea Fertilizer on ‘Greco’ Grapevines at Full Veraison Increases Berry Yeast-Assimilable Nitrogen Content. Plants 2020, 9, 619. [Google Scholar] [CrossRef]
- Daler, S.; Özkol, Y. Effects of 5-Aminolevulinic Acid (5-ALA) on Morphological and Physiological Characteristics of Grapevine against Salt Stress. Turk. J. Agric. Food Sci. Technol. 2024, 12, 575–585. [Google Scholar] [CrossRef]
- Mubashir, A.; Nisa, Z.U.; Shah, A.A.; Kiran, M.; Hussain, I.; Ali, N.; Zhang, L.; Madnay, M.M.Y.; Alsiary, W.A.; Korany, S.M.; et al. Effect of Foliar Application of Nano-Nutrients Solution on Growth and Biochemical Attributes of Tomato (Solanum lycopersicum) under Drought Stress. Front. Plant Sci. 2023, 13, 1066790. [Google Scholar] [CrossRef]
- Tourajzadeh, O.; Piri, H.; Naserin, A.; Cahri, M.; Tourajzadeh, O.; Piri, H.; Naserin, A.; mahdi Cahri, M. Effect of Nano Biochar Addition and Deficit Irrigation on Growth, Physiology and Water Productivity of Quinoa Plants under Salinity Conditions. Environ. Exp. Bot. 2024, 217, 105564. [Google Scholar] [CrossRef]
- Deloire, A.; Pellegrino, A.; Rogiers, S. A Few Words on Grapevine Leaf Water Potential. IVES Tech. Rev. Vine Wine 2020. [Google Scholar] [CrossRef]
- Prieto, J.A.; Louarn, G.; Perez Peña, J.; Ojeda, H.; Simonneau, T.; Lebon, E. A Leaf Gas Exchange Model That Accounts for Intra-Canopy Variability by Considering Leaf Nitrogen Content and Local Acclimation to Radiation in Grapevine (Vitis vinifera L.). Plant Cell Environ. 2012, 35, 1313–1328. [Google Scholar] [CrossRef]
- Gallo, A.E.; Christophe, A.; Poupard, M.; Boulord, R.; Rolland, G.; Prieto, J.A.; Simonneau, T.; Pallas, B. Effects of Acclimation to Long-Term Shading on Photosynthesis in Grapevines: Roles of Non-Structural Carbohydrates and Stomatal Conductance. Physiol. Plant. 2024, 176, e14636. [Google Scholar] [CrossRef]
- Matthews, M.A.; Ishii, R.; Anderson, M.M.; O’Mahony, M. Dependence of Wine Sensory Attributes on Vine Water Status. J. Sci. Food Agric. 1990, 51, 321–335. [Google Scholar] [CrossRef]
- Shani, M.Y.; Ahmad, S.; Ashraf, M.Y.; Nawaz, M.; Arshad, I.; Anjum, A.; De Mastro, F.; Cocozza, C.; Khan, Z.; Gul, N.; et al. Nano-Biochar Suspension Mediated Alterations in Growth, Physio-Biochemical Activities and Nutrient Content in Wheat (Triticum aestivum L.) at the Vegetative Stage. Plants 2024, 13, 2347. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Noureen, S.; Ali, S.; Anwar, S.; Rehman, M.Z.U.; Qayyum, M.F.; Hussain, A. Influence of Biochar Amendment and Foliar Application of Iron Oxide Nanoparticles on Growth, Photosynthesis, and Cadmium Accumulation in Rice Biomass. J. Soils Sediments 2019, 19, 3749–3759. [Google Scholar] [CrossRef]
- Muhammad Mehmood, H.; Yasin Ashraf, M.; Iqra Almas, H.; Zaib-un-Nisa; Ali, N.; Khaliq, B.; Ahmad Ansari, M.; Singh, R.; Gul, S. Synergistic Effects of Soil and Foliar Nano-Biochar on Growth, Nitrogen Metabolism and Mineral Uptake in Wheat Varieties. J. King Saud Univ. Sci. 2024, 36, 103392. [Google Scholar] [CrossRef]
- Verdenal, T.; Spangenberg, J.E.; Zufferey, V.; Lorenzini, F.; Spring, J.L.; Viret, O. Effect of Fertilisation Timing on the Partitioning of Foliar-Applied Nitrogen in Vitis vinifera Cv. Chasselas: A 15N Labelling Approach. Aust. J. Grape Wine Res. 2015, 21, 110–117. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Leng, X.; Mu, Q.; Wang, X.; Li, X.; Zhu, X.; Shangguan, L.; Fang, J. Transporters, Chaperones, and P-Type ATPases Controlling Grapevine Copper Homeostasis. Funct. Integr. Genom. 2015, 15, 673–684. [Google Scholar] [CrossRef]
- Palčić, A.P.; Jeromel, A.; Pecina, M.; Palčić, I.; Gluhić, D.; Petek, M.; Ćustić, M.H. Decreased Leaf Potassium Content Affects the Chemical Composition of Must for Sparkling Wine Production. Horticulturae 2022, 8, 512. [Google Scholar] [CrossRef]
- Likar, M.; Stres, B.; Rusjan, D.; Vogel-Mikuš, K.; Regvar, M. Grapevine Leaf Ionome Is Shaped by Soil Factors and Plant Age. Plant Soil Environ. 2022, 68, 415–423. [Google Scholar] [CrossRef]
- Zhao, Z.; Chu, C.; Zhou, D.; Sha, Z.; Wu, S. Soil Nutrient Status and the Relation with Planting Area, Planting Age and Grape Varieties in Urban Vineyards in Shanghai. Heliyon 2019, 5, e02362. [Google Scholar] [CrossRef]
- Conradie, W.J.; Raath, P.J.; Mulidzi, A.R.; Howell, C.L. Dry Matter Accumulation, Seasonal Uptake and Partitioning of Mineral Nutrients by Vitis vinifera L. Cv. Sultanina Grapevines in the Lower Orange River Region of South Africa–A Preliminary Investigation. S. Afr. J. Enol. Vitic. 2022, 43, 46–57. [Google Scholar] [CrossRef]
- Pradubsuk, S.; Davenport, J.R. Seasonal Distribution of Micronutrients in Mature “Concord” Grape: Boron, Iron, Manganese, Copper, and Zinc. J. Am. Soc. Hortic. Sci. 2011, 136, 69–77. [Google Scholar] [CrossRef]
- Mahaut, L.; Violle, C.; Renard, D. Complementary Mechanisms Stabilize National Food Production. Sci. Rep. 2021, 11, 4922. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.W.M.; Samy, M.M.; Sany, H.; Eid, R.R.; Rashad, H.M.; Abdeldaym, E.A. Nanopotassium, Nanosilicon, and Biochar Applications Improve Potato Salt Tolerance by Modulating Photosynthesis, Water Status, and Biochemical Constituents. Sustainability 2022, 14, 723. [Google Scholar] [CrossRef]
- Flexas, J.; Galmés, J.; Gallé, A.; Gulías, J.; Pou, A.; Ribas-Carbo, M.; Tomàs, M.; Medrano, H. Improving Water Use Efficiency in Grapevines: Potential Physiological Targets for Biotechnological Improvement. Aust. J. Grape Wine Res. 2010, 16, 106–121. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; De Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]

| Parameter | Unit | Value |
|---|---|---|
| pH | / | 9.79 ± 0.05 |
| EC | µS/cm | 792 ± 65.9 |
| ash | % | 8.36 ± 0.01 |
| total carbon (TC) | % | 73.1 ± 0.43 |
| specific surface area (SSA) | m2g−1 | 2.07 ± 0.14 |
| N | % | 1.06 ± 0.01 |
| P | g/kg | 27.2 ± 0.21 |
| K | g/kg | 22.8 ± 0.78 |
| Mg | g/kg | 27.5 ± 1.67 |
| S | g/kg | 12.4 ± 0.30 |
| Ca | g/kg | 187 ± 9.61 |
| Cu | mg/kg | 4.65 ± 0.25 |
| Mn | mg/kg | 6.56 ± 1.22 |
| Mo | mg/kg | 0.11 ± 0.00 |
| Zn | mg/kg | 2.69 ± 0.01 |
| Foliar Suspension | pH | EC (µS/cm) |
|---|---|---|
| C | 6.15 ± 0.45 | 1.80 ± 0.30 |
| B300 | 9.21 ± 0.26 | 36.0 ± 1.04 |
| B600 | 9.42 ± 0.32 | 57.5 ± 1.82 |
| B1200 | 9.34 ± 0.04 | 92.0 ± 2.27 |
| Net Photosynthetic Assimilation Rate (A) and Intercellular Carbon Dioxide Concentration (Ci) in Grapevine Leaves | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Phenological Stage | |||||||
| Flowering (S19) | Setting (S27) | Veraison (S35) | Harvest (S38) | |||||
| A | Ci | A | Ci | A | Ci | A | Ci | |
| µmol m−2 s−1 | µmol mol−1 | µmol m−2 s−1 | µmol mol−1 | µmol m−2 s−1 | µmol mol−1 | µmol m−2 s−1 | µmol mol−1 | |
| C | 2.72 ± 0.13 | 377 ± 2.14 | 3.00 ± 0.27 | 376 ± 0.88 | 3.02 ± 0.34 | 350 ± 5.25 | 2.23 ± 0.41 | 500 ± 8.63 |
| B300 | 2.73 ± 0.20 | 378 ± 0.80 | 4.10 ± 0.08 | 375 ± 4.95 | 2.93 ± 0.19 | 333 ± 8.51 | 1.97 ± 0.50 | 538 ± 32.3 |
| B600 | 2.92 ± 0.44 | 371 ± 5.64 | 3.37 ± 0.39 | 372 ± 0.41 | 3.99 ± 0.33 | 356 ± 3.23 | 3.45 ± 0.49 | 315 ± 13.9 |
| B1200 | 2.01 ± 0.41 | 376 ± 5.97 | 3.35 ± 0.09 | 374 ± 0.28 | 3.66 ± 0.36 | 348 ± 8.07 | 2.81 ± 0.71 | 308 ± 4.07 |
| p value | 0.283 | 0.609 | 0.925 | 0.783 | 0.122 | 0.168 | 0.294 | 0.051 |
| significance | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Leaf Water Potential (MPa) | ||||
|---|---|---|---|---|
| Treatment | Phenological Stage | |||
| Flowering (S19) | Setting (S27) | Veraison (S35) | Harvest (S38) | |
| C | −0.47 ± 0.08 | −0.70 ± 0.03 | −0.77 ± 0.04 | −0.55 ± 0.00 b |
| B300 | −0.47 ± 0.03 | −0.73 ± 0.06 | −0.80 ± 0.10 | −0.68 ± 0.03 a |
| B600 | −0.53 ± 0.09 | −0.68 ± 0.06 | −0.85 ± 0.08 | −0.58 ± 0.02 ab |
| B1200 | −0.77 ± 0.09 | −0.80 ± 0.08 | −0.98 ± 0.07 | −0.68 ± 0.04 a |
| p value | 0.116 | 0.544 | 0.282 | 0.022 |
| significance | n.s. | n.s. | n.s. | * |
| Macroelement Content of Grapevine Leaves | ||||||
|---|---|---|---|---|---|---|
| Element | N | P | K | Mg | Ca | S |
| Unit | % | g/kg | g/kg | g/kg | g/kg | g/kg |
| Flowering (S19) | ||||||
| C | 3.53 ± 0.02 c | 15.3 ± 0.97 b | 11.8 ± 0.58 b | 2.38 ± 0.11 ab | 15.3 ± 0.97 b | 2.24 ± 0.11 |
| B300 | 3.45 ± 0.02 b | 18.3 ± 0.41 a | 12.6 ± 0.3 b | 2.62 ± 0.07 a | 18.3 ± 0.41 a | 2.4 ± 0.09 |
| B600 | 3.8 ± 0.02 a | 16.3 ± 0.07 ab | 17.7 ± 0.18 a | 2.3 ± 0.02 b | 16.3 ± 0.07 ab | 2.52 ± 0.02 |
| B1200 | 3.81 ± 0.01 a | 17.2 ± 0.2 ab | 17.3 ± 0.08 a | 2.11 ± 0.01 b | 17.2 ± 0.2 ab | 2.55 ± 0.01 |
| p value | <0.001 | <0.001 | <0.001 | 0.004 | 0.025 | 0.058 |
| significance | *** | *** | *** | ** | * | n.s. |
| Setting (S27) | ||||||
| C | 3.01 ± 0.01 a | 2.38 ± 0.01 a | 13.2 ± 0.07 c | 3.01 ± 0.01 a | 22.8 ± 0.13 c | 2.39 ± 0.01 a |
| B300 | 2.84 ± 0 b | 2.34 ± 0.02 a | 11.1 ± 0.15 d | 2.73 ± 0.05 b | 20.8 ± 0.35 d | 2.4 ± 0.01 b |
| B600 | 2.86 ± 0.04 b | 2.24 ± 0 b | 18.4 ± 0.1 a | 2.22 ± 0.02 c | 25 ± 0.2 a | 2.34 ± 0.01 b |
| B1200 | 2.91 ± 0.03 ab | 2.34 ± 0.02 a | 16.1 ± 0.32 b | 2.31 ± 0.04 c | 24 ± 0.1 b | 2.46 ± 0.02 a |
| p value | 0.004 | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 |
| significance | ** | *** | *** | *** | *** | ** |
| Veraison (S35) | ||||||
| C | 2.14 ± 0.01 b | 2.00 ± 0.04 | 9.82 ± 0.27 c | 2.29 ± 0.03 c | 27.5 ± 0.67 b | 1.87 ± 0.06 b |
| B300 | 2.00 ± 0.00 c | 1.91 ± 0.08 | 8.44 ± 0.38 d | 3.41 ± 0.15 a | 32.4 ± 1.74 a | 1.94 ± 0.13 ab |
| B600 | 2.17 ± 0.02 ab | 2.00 ± 0.01 | 14.1 ± 0.3 a | 2.38 ± 0.00 bc | 32.9 ± 0.01 a | 2.08 ± 0.02 ab |
| B1200 | 2.22 ± 0.02 a | 1.89 ± 0.03 | 12.1 ± 0.07 b | 2.74 ± 0.03 b | 34.2 ± 0.27 a | 2.21 ± 0.03 a |
| p value | <0.001 | 0.306 | <0.001 | <0.001 | 0.005 | 0.051 |
| significance | *** | n.s. | *** | *** | ** | * |
| Harvest (S38) | ||||||
| C | 2.02 ± 0.01 bc | 2.99 ± 0.03 | 7.25 ± 0.03 b | 2.46 ± 0.02 b | 29.63 ± 0.67 | 1.78 ± 0.02 |
| B300 | 2.01 ± 0.02 c | 3.32 ± 0.01 | 6.95 ± 0.02 b | 3 ± 0.03 a | 35.83 ± 0.41 | 1.83 ± 0.01 |
| B600 | 2.11 ± 0.01 a | 2.95 ± 0.03 | 10.23 ± 0.04 a | 2.36 ± 0.12 b | 36.04 ± 1.32 | 2.05 ± 0.03 |
| B1200 | 2.07 ± 0.01 ab | 3.25 ± 0.41 | 10.13 ± 0.48 a | 2.45 ± 0.08 b | 30.33 ± 8.04 | 2.22 ± 0.20 |
| p value | 0.002 | 0.536 | <0.001 | 0.001 | 0.573 | 0,050 |
| significance | ** | n.s. | *** | ** | n.s. | n.s. |
| Microelement Content of Grapevine Leaves | ||||||||
|---|---|---|---|---|---|---|---|---|
| Element | B | Cu | Fe | Mn | Mo | Na | Si | Zn |
| Unit | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg | mg/kg |
| Flowering (S19) | ||||||||
| C | 46.5 ± 0.94 d | 7.10 ± 0.29 b | 76.1 ± 2.72 | 33.8 ± 0.69 b | 0.84 ± 0.03 | 15.3 ± 0.97 b | 379 ± 12.5 b | 12.0 ± 0.29 b |
| B300 | 60.9 ± 1.41 b | 8.16 ± 0.19 a | 80.5 ± 2.01 | 44.4 ± 1.02 a | 0.92 ± 0.04 | 18.3 ± 0.41 a | 672 ± 22.5 a | 14.6 ± 0.35 a |
| B600 | 54.0 ± 0.43 c | 8.17 ± 0.04 a | 75.9 ± 0.17 | 36.0 ± 0.20 b | 0.93 ± 0.02 | 16.3 ± 0.07 ab | 402 ± 2.62 b | 13.0 ± 0.14 b |
| B1200 | 65.1 ± 0.2 a | 8.52 ± 0.02 a | 78.6 ± 0.74 | 42.4 ± 0.17 a | 0.91 ± 0.04 | 17.2 ± 0.2 ab | 407 ± 21.3 b | 14.7 ± 0.05 a |
| p value | <0.001 | 0.002 | 0.268 | <0.001 | 0.226 | 0.027 | <0.001 | <0.001 |
| significance | *** | ** | n.s. | *** | n.s. | * | *** | *** |
| Setting (S27) | ||||||||
| C | 44.3 ± 0.13 c | 6.90 ± 0.02 ab | 82.6 ± 1.21 ab | 48.3 ± 0.22 c | 0.89 ± 0.03 | 26.3 ± 0.23 b | 403 ± 18.3 b | 12 ± 0.06 a |
| B300 | 45.8 ± 0.4 c | 6.41 ± 0.03 b | 83.2 ± 1.41 ab | 41.5 ± 0.51 b | 0.84 ± 0.02 | 22 ± 0.26 d | 412 ± 12.9 b | 9.02 ± 0.12 b |
| B600 | 56.7 ± 0.13 b | 6.18 ± 0.06 b | 79.7 ± 0.28 ab | 53.4 ± 0.19 a | 0.86 ± 0.01 | 23.6 ± 0.28 c | 680 ± 16.1 a | 10.7 ± 0.12 a |
| B1200 | 67.5 ± 0.74 a | 7.48 ± 0.37 a | 77.5 ± 1.5 b | 52.2 ± 0.5 a | 0.92 ± 0.02 | 28.0 ± 0.35 a | 695 ± 34.9 a | 12.0 ± 0.61 a |
| p value | <0.001 | 0.006 | 0.033 | <0.001 | 0.089 | <0.001 | <0.001 | <0.001 |
| significance | *** | ** | * | *** | n.s. | *** | *** | *** |
| Veraison (S35) | ||||||||
| C | 69.6 ± 1.42 b | 233 ± 4.67 b | 107 ± 2.57 a | 52.4 ± 0.82 c | 69.6 ± 1.42 b | 77.1 ± 1.88 b | 592.7 ± 9.02 | 9.99 ± 0.3 c |
| B300 | 72.4 ± 3.94 b | 292 ± 14.43 c | 97.5 ± 6.07 ab | 62.3 ± 3.04 b | 72.4 ± 3.94 b | 93.2 ± 4.68 a | 637.9 ± 38.5 | 10.4 ± 0.49 c |
| B600 | 74.7 ± 1.07 ab | 465 ± 2.82 a | 95.8 ± 1.01 ab | 64.6 ± 0.36 ab | 74.7 ± 1.07 ab | 96.7 ± 2.76 a | 643.5 ± 47.2 | 14.9 ± 0.25 a |
| B1200 | 83.6 ± 1.61 a | 486 ± 9.70 a | 88.3 ± 0.75 b | 69.8 ± 0.73 a | 83.6 ± 1.61 a | 87.4 ± 3.70 ab | 718.1 ± 33.8 | 13.0 ± 0.24 b |
| p value | 0.012 | <0.001 | 0.031 | <0.001 | 0.041 | 0.016 | 0.167 | <0.001 |
| significance | * | *** | * | *** | * | * | n.s. | *** |
| Harvest (S38) | ||||||||
| C | 67.3 ± 0.67 | 99.5 ± 5.31 | 86.3 ± 0.51 a | 52.2 ± 0.12 b | 0.87 ± 0.02 ab | 73.4 ± 2.16 | 852 ± 3.63 a | 11.2 ± 0.19 b |
| B300 | 79.2 ± 0.15 | 133 ± 2.86 | 93.8 ± 4.72 a | 61.9 ± 0.17 ab | 0.91 ± 0.01 a | 65.6 ± 0.72 | 893 ± 16.2 a | 12.4 ± 0.06 b |
| B600 | 73.3 ± 4.23 | 257 ± 25.68 | 63.3 ± 8.57 b | 65.3 ± 0.64 a | 0.78 ± 0.01 c | 69.8 ± 2.54 | 407 ± 16.9 b | 14.2 ± 1.43 ab |
| B1200 | 82.7 ± 5.49 | 206 ± 98.73 | 79.9 ± 8.57 ab | 59.6 ± 5.12 ab | 0.8 ± 0.02 bc | 63.7 ± 8.56 | 407 ± 46.7 b | 17.7 ± 1.82 a |
| p value | 0.058 | 0.202 | 0.014 | 0.037 | 0.002 | 0.495 | <0.001 | 0.018 |
| significance | n.s. | n.s. | * | * | ** | n.s. | *** | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palčić, I.; Anđelini, D.; Prelac, M.; Pasković, I.; Černe, M.; Major, N.; Goreta Ban, S.; Užila, Z.; Bubola, M.; Ban, D.; et al. Influence of Biochar Foliar Application on Malvazija Istarska Grapevine Physiology. Sustainability 2025, 17, 5947. https://doi.org/10.3390/su17135947
Palčić I, Anđelini D, Prelac M, Pasković I, Černe M, Major N, Goreta Ban S, Užila Z, Bubola M, Ban D, et al. Influence of Biochar Foliar Application on Malvazija Istarska Grapevine Physiology. Sustainability. 2025; 17(13):5947. https://doi.org/10.3390/su17135947
Chicago/Turabian StylePalčić, Igor, Dominik Anđelini, Melissa Prelac, Igor Pasković, Marko Černe, Nikola Major, Smiljana Goreta Ban, Zoran Užila, Marijan Bubola, Dean Ban, and et al. 2025. "Influence of Biochar Foliar Application on Malvazija Istarska Grapevine Physiology" Sustainability 17, no. 13: 5947. https://doi.org/10.3390/su17135947
APA StylePalčić, I., Anđelini, D., Prelac, M., Pasković, I., Černe, M., Major, N., Goreta Ban, S., Užila, Z., Bubola, M., Ban, D., Nemet, I., Karažija, T., Petek, M., Jagatić Korenika, A.-M., & Cvitan, D. (2025). Influence of Biochar Foliar Application on Malvazija Istarska Grapevine Physiology. Sustainability, 17(13), 5947. https://doi.org/10.3390/su17135947















