Reactive Filtration Water Treatment: A Retrospective Review of Sustainable Sand Filtration Re-Engineered for Advanced Nutrient Removal and Recovery, Micropollutant Destructive Removal, and Net-Negative CO2e Emissions with Biochar
Abstract
1. Introduction
2. Processes and Mechanisms of Reactive Filtration
2.1. Reactive Filtration (RF)
2.2. RF with Catalytic Oxidation (Fe-CatOx-RF)
2.3. RF with Biochar (Fe-BC-RF)
2.4. RF for Mercury and Arsenic Removal
2.5. RF for Denitrification
2.6. Life Cycle Assessment (LCA) and Techno-Economic Analysis (TEA)
2.7. RF Configurations
3. Results
3.1. P Removal from Laboratory Studies
3.2. P Removal from Field Pilot-Scale Studies
3.3. Micropollutant Removal Performance from Field Pilot and Large-Scale Studies
3.4. Nutrient Recovery Performance from Field Pilot Studies
3.5. P, Hg, Cu, and N Removal from Large-Scale Studies
3.6. TEA Case Studies on RF at Different Flow Rates
4. Conclusions
5. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Biochar |
| CatOx | Catalytic Oxidation |
| CAPEX | Capital Expenditure |
| ECHO | Enforcement Compliance History Online |
| EBPR | Enhanced Biological Phosphorus Removal |
| GAC | Granulated Activated Carbon |
| GWP | Global Warming Potential |
| HAB | Harmful Algal Blooms |
| HFO-RF | Hydrous Ferric Oxide Reactive Filtration |
| LCA | Life Cycle Assessment |
| LCOW | Levelized Cost of Water |
| LoQ | Limits of Quantification |
| MGD | Million Gallons per Day |
| OP | Ortho-Phosphorus |
| OPEX | Operating Expenditure |
| RF | Reactive Filtration |
| SGD | Sustainable Development Goals |
| SLW | Synthetic Lake Water |
| TEA | Techno-Economic Analysis |
| TP | Total Phosphorus |
| UI | University of Idaho |
| USEPA | United States Environmental Protection Agency |
| WRRF | Water Resource and Recovery Facilities |
References
- The 17 Goals-Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 6 November 2023).
- Śniatała, B.; Kurniawan, T.A.; Sobotka, D.; Mąkinia, J.; Othman, M.H.D. Macro-nutrients recovery from liquid waste as a sustainable resource for production of recovered mineral fertilizer: Uncovering alternative options to sustain global food security cost-effectively. Sci. Total Environ. 2023, 856, 159283. [Google Scholar] [CrossRef] [PubMed]
- Head, B.W. The Rise of ‘Wicked Problems’—Uncertainty, Complexity and Divergence. In Wicked Problems in Public Policy: Understanding and Responding to Complex Challenges; Springer: Berlin/Heidelberg, Germany, 2022; pp. 21–36. [Google Scholar] [CrossRef]
- Davis, J.; Horwitz, P. PLOS Water: A bridge and a voice for the water sectors. PLOS Water 2022, 1, e0000018. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Anderson, D.M.; Cembella, A.D.; Enevoldsen, H.O. Manual on Harmful Marine Microalgae; UNESCO: Paris, France, 2004. [Google Scholar]
- Almanassra, W.; McKay, G.; Kochkodan, V.; Atieh, M.A.; Al-Ansari, T. A state of the art review on phosphate removal from water by biochars. Chem. Eng. J. 2021, 409, 128211. [Google Scholar] [CrossRef]
- Rock, P. United States Geological Survey. 2023. Available online: https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-phosphate.pdf (accessed on 5 December 2023).
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and regional potential of wastewater as a water, nutrient and energy source. In Natural Resources Forum; Wiley Online Library: Hoboken, NJ, USA, 2020; Volume 44, pp. 40–51. [Google Scholar] [CrossRef]
- Daramola, D.A.; Hatzell, M.C. Energy Demand of Nitrogen and Phosphorus Based Fertilizers and Approaches to Circularity. ACS Energy Lett. 2023, 8, 1493–1501. [Google Scholar] [CrossRef]
- Cordell, D.; Rosemarin, A.; Schröder, J.J.; Smit, A. Towards global phosphorus security: A systems framework for phosphorus recovery and reuse options. Chemosphere 2011, 84, 747–758. [Google Scholar] [CrossRef]
- European Sustainable Phosphorus Platform. Available online: https://phosphorusplatform.eu/ (accessed on 13 November 2023).
- Sustainable Phosphorus Alliance. Available online: https://phosphorusalliance.org/ (accessed on 13 November 2023).
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and recovery of phosphate from water using sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Di Capua, F.; de Sario, S.; Ferraro, A.; Petrella, A.; Race, M.; Pirozzi, F.; Fratino, U.; Spasiano, D. Phosphorous removal and recovery from urban wastewater: Current practices and new directions. Sci. Total Environ. 2022, 823, 153750. [Google Scholar] [CrossRef]
- Kumar, P.S.; Korving, L.; van Loosdrecht, M.C.; Witkamp, G.-J. Adsorption as a technology to achieve ultra-low concentrations of phosphate: Research gaps and economic analysis. Water Res. X 2019, 4, 100029. [Google Scholar] [CrossRef]
- Zheng, Y.; Wan, Y.; Zhang, Y.; Huang, J.; Yang, Y.; Tsang, D.C.; Wang, H.; Chen, H.; Gao, B. Recovery of phosphorus from wastewater: A review based on current phosphorous removal technologies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1148–1172. [Google Scholar] [CrossRef]
- Sedlak, R. Phosphorus and Nitrogen Removal from Municipal Wastewater: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Wathugala, G.; Suzuki, T.; Kurihara, Y. Removal of nitrogen, phosphorus and COD from waste water using sand filtration system with Phragmites australis. Water Res. 1987, 21, 1217–1224. [Google Scholar] [CrossRef]
- Yildiz, E. Phosphate removal from water by fly ash using crossflow microfiltration. Sep. Purif. Technol. 2004, 35, 241–252. [Google Scholar] [CrossRef]
- Erickson, J.; Gulliver, J.S.; Weiss, P.T. Enhanced sand filtration for storm water phosphorus removal. J. Environ. Eng. 2007, 133, 485–497. [Google Scholar] [CrossRef]
- Leo, P.; Chai, W.; Mohammad, A.; Qi, Y.; Hoedley, A.; Chai, S.-P. Phosphorus removal using nanofiltration membranes. Water Sci. Technol. 2011, 64, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Stephenson, T.; Pearce, P. Phosphorus removal by chemical precipitation in a biological aerated filter. Water Res. 1997, 31, 2557–2563. [Google Scholar] [CrossRef]
- Ngo, H.-H.; Guo, W. Membrane fouling control and enhanced phosphorus removal in an aerated submerged membrane bioreactor using modified green bioflocculant. Bioresour. Technol. 2009, 100, 4289–4291. [Google Scholar] [CrossRef]
- Awual, M.R.; Shenashen, M.; Jyo, A.; Shiwaku, H.; Yaita, T. Preparing of novel fibrous ligand exchange adsorbent for rapid column-mode trace phosphate removal from water. J. Ind. Eng. Chem. 2014, 20, 2840–2847. [Google Scholar] [CrossRef]
- Luo, X.; Wang, X.; Bao, S.; Liu, X.; Zhang, W.; Fang, T. Adsorption of phosphate in water using one-step synthesized zirconium-loaded reduced graphene oxide. Sci. Rep. 2016, 6, 39108. [Google Scholar] [CrossRef]
- Genz, A.; Kornmüller, A.; Jekel, M. Advanced phosphorus removal from membrane filtrates by adsorption on activated aluminium oxide and granulated ferric hydroxide. Water Res. 2004, 38, 3523–3530. [Google Scholar] [CrossRef]
- Barnard, J.L. Biological nutrient removal without the addition of chemicals. Water Res. 1975, 9, 485–490. [Google Scholar] [CrossRef]
- Boelee, N.; Temmink, H.; Janssen, M.; Buisman, C.; Wijffels, R. Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms. Water Res. 2011, 45, 5925–5933. [Google Scholar] [CrossRef]
- Buhmann, A.; Papenbrock, J. Biofiltering of aquaculture effluents by halophytic plants: Basic principles, current uses and future perspectives. Environ. Exp. Bot. 2013, 92, 122–133. [Google Scholar] [CrossRef]
- Oehmen, A.; Lemos, P.C.; Carvalho, G.; Yuan, Z.; Keller, J.; Blackall, L.L.; Reis, M.A. Advances in enhanced biological phosphorus removal: From micro to macro scale. Water Res. 2007, 41, 2271–2300. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.H.; McIlroy, S.J.; Albertsen, M.; Nierychlo, M. Re-evaluating the microbiology of the enhanced biological phosphorus removal process. Curr. Opin. Biotechnol. 2019, 57, 111–118. [Google Scholar] [CrossRef]
- Korchef, A.; Saidou, H.; Amor, M.B. Phosphate recovery through struvite precipitation by CO2 removal: Effect of magnesium, phosphate and ammonium concentrations. J. Hazard. Mater. 2011, 186, 602–613. [Google Scholar] [CrossRef]
- Saidou, H.; Moussa, S.B.; Amor, M.B. Influence of airflow rate and substrate nature on heterogeneous struvite precipitation. Environ. Technol. 2009, 30, 75–83. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Mehariya, S.; Molino, A.; Calabrò, V. Biofuel production and phosphorus recovery through an integrated treatment of agro-industrial waste. Sustainability 2018, 11, 52. [Google Scholar] [CrossRef]
- Fattah, K.P.; Mavinic, D.S.; Koch, F.A.; Jacob, C. Determining the feasibility of phosphorus recovery as struvite from filter press centrate in a secondary wastewater treatment plant. J. Environ. Sci. Health Part A 2008, 43, 756–764. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Abarca, R.R.M.; de Luna, M.D.G.; Huang, Y.-H.; Lu, M.-C. Recovery of phosphorus from synthetic wastewaters by struvite crystallization in a fluidized-bed reactor: Effects of pH, phosphate concentration and coexisting ions. Chemosphere 2017, 173, 466–473. [Google Scholar] [CrossRef]
- Suzuki, K.; Tanaka, Y.; Kuroda, K.; Hanajima, D.; Fukumoto, Y.; Yasuda, T.; Waki, M. Removal and recovery of phosphorous from swine wastewater by demonstration crystallization reactor and struvite accumulation device. Bioresour. Technol. 2007, 98, 1573–1578. [Google Scholar] [CrossRef]
- Fattah, K.; Sabrina, N.; Mavinic, D.; Koch, F. Reducing operating costs for struvite formation with a carbon dioxide stripper. Water Sci. Technol. 2008, 58, 957–962. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Molinari, R. Advances in struvite precipitation technologies for nutrients removal and recovery from aqueous waste and wastewater. Sustainability 2020, 12, 7538. [Google Scholar] [CrossRef]
- Wu, H.; Vaneeckhaute, C. Nutrient recovery from wastewater: A review on the integrated Physicochemical technologies of ammonia stripping, adsorption and struvite precipitation. Chem. Eng. J. 2022, 433, 133664. [Google Scholar] [CrossRef]
- Sena, M.; Seib, M.; Noguera, D.R.; Hicks, A. Environmental impacts of phosphorus recovery through struvite precipitation in wastewater treatment. J. Clean. Prod. 2021, 280, 124222. [Google Scholar] [CrossRef]
- Kim, J.; Deng, Q.; Benjamin, M.M. Simultaneous removal of phosphorus and foulants in a hybrid coagulation/membrane filtration system. Water Res. 2008, 42, 2017–2024. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Ullman, J.L. Removal of phosphorus; BOD, and pharmaceuticals by rapid rate sand filtration and ultrafiltration systems. J. Environ. Eng. 2016, 142, 06016006. [Google Scholar] [CrossRef]
- Newcombe, R.L.; Rule, R.A.; Hart, B.K.; Möller, G. Phosphorus removal from municipal wastewater by hydrous ferric oxide reactive filtration and coupled chemically enhanced secondary treatment: Part I-performance. Water Environ. Res. A Res. Publ. Water Environ. Fed. 2008, 80, 238–247. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef]
- Shepherd, J.G.; Sohi, S.P.; Heal, K.V. Optimising the recovery and re-use of phosphorus from wastewater effluent for sustainable fertiliser development. Water Res. 2016, 94, 155–165. [Google Scholar] [CrossRef]
- Yu, P.; Baker, M.C.; Crump, A.R.; Vogler, M.; Strawn, D.G.; Möller, G. Biochar integrated reactive filtration of wastewater for P removal and recovery, micropollutant catalytic oxidation, and negative CO2e: Process operation and mechanism. Water Environ. Res. 2023, 95, e10926. [Google Scholar] [CrossRef]
- Nobaharan, K.; Novair, S.B.; Lajayer, B.A.; van Hullebusch, E.D. Phosphorus removal from wastewater: The potential use of biochar and the key controlling factors. Water 2021, 13, 517. [Google Scholar] [CrossRef]
- Bakshi, S.; Laird, D.A.; Smith, R.G.; Brown, R.C. Capture and release of orthophosphate by Fe-modified biochars: Mechanisms and environmental applications. ACS Sustain. Chem. Eng. 2021, 9, 658–668. [Google Scholar] [CrossRef]
- Bashar, R.; Gungor, K.; Karthikeyan, K.; Barak, P. Cost effectiveness of phosphorus removal processes in municipal wastewater treatment. Chemosphere 2018, 197, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, R.L.; Strawn, D.G.; Grant, T.M.; Childers, S.E.; Möller, G. Phosphorus removal from municipal wastewater by hydrous ferric oxide reactive filtration and coupled chemically enhanced secondary treatment: Part II—Mechanism. Water Environ. Res. 2008, 80, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, R.L.; Hart, B.K.; Möller, G. Arsenic removal from water by moving bed active filtration. J. Environ. Eng. 2006, 132, 5–12. [Google Scholar] [CrossRef]
- Baker, M.C.; McCarthy, D.; Taslakya, L.; Henchion, G.; Mannion, R.; Strawn, D.G.; Gregory, M. Iron-Ozone Catalytic Oxidation Reactive Filtration of Municipal Wastewater at Field Pilot and Full-Scale with High-Efficiency Pollutant Removal and Potential Negative CO2e with Biochar. Water Environ. Res. 2023, 95, e10876. [Google Scholar] [CrossRef]
- Möller, G.; Baker, M.; Dunkel, C.; Shrestha, D.S.; Strawn, D.G. New Tech™: Resource Recovery in a Novel Functionalized Biochar, Catalytic Oxidation, Reactive Filtration Water Treatment for N/P Removal/Recovery and Pathogen/Priority Substance Destruction. In Proceedings of the 10th European Waste Water Management Conference, Manchester, UK, 11–12 October 2016; Available online: https://www.aquaenviro.co.uk/wp-content/uploads/2017/03/Gregory-M%C3%B6ller-paper.pdf (accessed on 8 January 2024).
- Möller, G.; Brackney, K.M.; Korus, R.A.; Keller, M.K., Jr.; Hart, B.K.; Newcombe, R.L. Reactive Filtration. U.S. Patent 7,399,416; B2 Patent, Appl. 10/727,963, 13 November 2008. [Google Scholar]
- Möller, G.; Strawn, D. Biochar Water Treatment. U.S. Patent 10,351,455; B2 Patent, Appl. 14/549,342, 28 May 2015. [Google Scholar]
- Strawn, D.G.; Crump, A.R.; Peak, D.; Garcia-Perez, M.; Möller, G. Reactivity of Fe-amended biochar for phosphorus removal and recycling from wastewater. PLoS Water 2023, 2, e0000092. [Google Scholar] [CrossRef]
- Beutel, M.W.; Dent, S.R.; Newcombe, R.L.; Möller, G. Mercury removal from municipal secondary effluent with hydrous ferric oxide reactive filtration. Water Environ. Res. 2018, 91, 132–143. [Google Scholar] [CrossRef]
- Möller, G.; Newcombe, R.L. Water Treatment Method. U.S. Patent 8,080,163 B2, 20 December 2011. [Google Scholar]
- Taslakyan, L.; Baker, M.C.; Shrestha, D.S.; Strawn, D.G.; Möller, G. CO2e footprint and eco-impact of ultralow phosphorus removal by hydrous ferric oxide reactive filtration: A municipal wastewater LCA case study. Water Environ. Res. 2022, 94, e10777. [Google Scholar] [CrossRef]
- Taslakyan, L.; Baker, M.; Strawn, D.G.; Möller, G. Biochar-integrated reactive filtration of tertiary wastewater for P removal and recovery, micropollutant catalytic oxidation, and negative CO2e: Life cycle assessment and techno-economic Analysis. Water Environ. Res. 2023, 95, e10926. [Google Scholar] [CrossRef]
- Garca-Montao, J.; Prez-Estrada, L.; Oller, I.; Maldonado, M.I.; Torrades, F.; Peral, J. Pilot plant scale reactive dyes degradation by solar photo-Fenton and biological processes. J. Photochem. Photobiol. A Chem. 2008, 195, 205–214. [Google Scholar] [CrossRef]
- Pera-Titus, M.; Garc, V.; Baos, M.A.; Gimnez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Shin, S.; Yoon, H.; Jang, J. Polymer-encapsulated iron oxide nanoparticles as highly efficient Fenton catalysts. Catal. Commun. 2008, 10, 178–182. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Yan, L.; Bing, J.; Wu, H. The behavior of ozone on different iron oxides surface sites in water. Sci. Rep. 2019, 9, 14752. [Google Scholar] [CrossRef]
- Pestovsky, O.; Stoian, S.; Bominaar, E.L.; Shan, X.; Münck, E.; Que, L., Jr.; Bakac, A. Aqueous FeIV=O: Spectroscopic Identification and Oxo-Group Exchange. Angew. Chem. Int. Ed. 2005, 44, 6871–6874. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, H.; Jiang, Z.; Xing, B. Combined effects of biochar properties and soil conditions on plant growth: A meta-analysis. Sci. Total Environ. 2020, 713, 136635. [Google Scholar] [CrossRef]
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 9338. [Google Scholar] [CrossRef]
- Miroshnichenko, D.; Zhylina, M.; Shmeltser, K. Modern uses of biochar in various technologies and industries. A review. Chem. Chem. Technol. 2024, 18, 232–243. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K.; Bhattacharyya, D. Structure—Mechanics property relationship of waste derived biochars. Sci. Total Environ. 2015, 538, 611–620. [Google Scholar] [CrossRef]
- Dunkel, C. GHG Emissions and Energy Usage Assessment of Phosphorus Recovery from Municipal Wastewater Systems Utilizing Biochar-Catalytic Oxidation-Reactive Filtration; University of Idaho: Moscow, ID, USA, 2016. [Google Scholar]
- Perez-Mercado, L.F.; Lalander, C.; Berger, C.; Dalahmeh, S.S. Potential of biochar filters for onsite wastewater treatment: Effects of biochar type, physical properties and operating conditions. Water 2018, 10, 1835. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H.; Li, H.; Li, J.; Zhou, W. Biochar stability assessment methods: A review. Sci. Total Environ. 2019, 647, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Khanzada, N.K.; Farid, M.U.; Kharraz, J.A.; Choi, J.; Tang, C.Y.; Nghiem, L.D.; Jang, A.; An, A.K. Removal of organic micropollutants using advanced membrane-based water and wastewater treatment: A review. J. Membr. Sci. 2020, 598, 117672. [Google Scholar] [CrossRef]
- Arsenic. World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 14 December 2023).
- USEPA. Drinking Water Arsenic Rule History. Available online: https://www.epa.gov/dwreginfo/drinking-water-arsenic-rule-history (accessed on 10 June 2024).
- Hall, B.; Bodaly, R.; Fudge, R.; Rudd, J.; Rosenberg, D. Food as the dominant pathway of methylmercury uptake by fish. Water Air Soil Pollut. 1997, 100, 13–24. [Google Scholar] [CrossRef]
- USEPA. National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 10 June 2024).
- USEPA. Guidelines for Eating Fish That Contain Mercury. Available online: https://www.epa.gov/mercury/guidelines-eating-fish-contain-mercury (accessed on 10 June 2024).
- Diz, H.R.; Novak, J.T. Fluidized bed for removing iron and acidity from acid mine drainage. J. Environ. Eng. 1998, 124, 701–708. [Google Scholar] [CrossRef]
- Thirunavukkarasu, O.S.; Viraraghavan, T.; Subramanian, K. Removal of arsenic in drinking water by iron oxide-coated sand and ferrihydrite—Batch studies. Water Qual. Res. J. 2001, 36, 55–70. [Google Scholar] [CrossRef]
- Lo, S.-L.; Jeng, H.-T.; Lai, C.-H. Characteristics and adsorption properties of iron-coated sand. Water Sci. Technol. 1997, 35, 63–70. [Google Scholar] [CrossRef]
- Henneberry, Y.K.; Kraus, T.E.; Fleck, J.A.; Krabbenhoft, D.P.; Bachand, P.M.; Horwath, W.R. Removal of inorganic mercury and methylmercury from surface waters following coagulation of dissolved organic matter with metal-based salts. Sci. Total Environ. 2011, 409, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Urgun-Demirtas, M.; Gillenwater, P.; Negri, M.C.; Lin, Y.; Snyder, S.; Doctor, R.; Pierce, L.; Alvarado, J. Achieving the Great Lakes Initiative mercury limits in oil refinery effluent. Water Environ. Res. 2013, 85, 77–86. [Google Scholar] [CrossRef]
- Dzombak, D.A.; Morel, F.M. Surface Complexation Modeling: Hydrous Ferric Oxide; John Wiley & Sons: Hoboken, NJ, USA, 1991. [Google Scholar]
- USEPA. Wastewater Technology Fact Sheet Denitrification Filters. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100IL79.txt (accessed on 26 March 2024).
- Metcalf, L.; Eddy, H.P.; Tchobanoglous, G. Wastewater Engineering: Treatment, Disposal, and Reuse; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Chan, T.; Koodie, T.; Sloper, M.; Wiggam, R. Harnessing the power in nitrifying sand filters. In Proceedings of the 11th European Waste Water Management Conference, Leeds, UK, 3–4 October 2017; Available online: https://www.aquaenviro.co.uk/wp-content/uploads/2018/03/201711thEWWM-Chan_TF_et_al-NSF.pdf (accessed on 10 April 2024).
- Lee, C.O.; Boe-Hansen, R.; Musovic, S.; Smets, B.; Albrechtsen, H.-J.; Binning, P. Effects of dynamic operating conditions on nitrification in biological rapid sand filters for drinking water treatment. Water Res. 2014, 64, 226–236. [Google Scholar] [CrossRef]
- Moore, K. Optimizing Recirculating Media Filters for Ammonia Treatment. Available online: https://dnr.mo.gov/document-search/optimizing-recirculating-media-filters-ammonia-treatment-pub2737/pub2737 (accessed on 2 February 2024).
- Koopman, B.; Stevens, C.M.; Wonderlick, C.A. Denitrification in a moving bed upflow sand filter. Res. J. Water Pollut. Control. Fed. 1990, 62, 239–245. Available online: https://www.jstor.org/stable/25043827 (accessed on 2 February 2024).
- BlueNite Biological Denitrification. Available online: https://nexom.com/bluenite/ (accessed on 6 June 2024).
- Reactive Filtration for Ultra-Low Phosphorus & Metals. Available online: https://nexom.com/bluepro-3/ (accessed on 20 November 2023).
- Chang, C.-C.; DiGiovanni, K.; Mei, Y.; Research, W.E. Sustainability. Water 2019, 91, 1129–1149. [Google Scholar] [CrossRef]
- Osman, A.I.; Fawzy, S.; Farghali, M.; El-Azazy, M.; Elgarahy, A.M.; Fahim, R.A.; Maksoud, M.A.; Ajlan, A.A.; Yousry, M.; Saleem, Y.; et al. Biochar for agronomy, animal farming, anaerobic digestion, composting, water treatment, soil remediation, construction, energy storage, and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2385–2485. [Google Scholar] [CrossRef] [PubMed]
- Choubert, J.-M.; Ruel, S.M.; Miege, C.; Coquery, M. Rethinking micropollutant removal assessment methods for wastewater treatment plants–how to get more robust data? Water Sci. Technol. 2017, 75, 2964–2972. [Google Scholar] [CrossRef] [PubMed]
- Little, T. Method validation essentials, limit of blank, limit of detection, and limit of quantitation. BioPharm Int. 2015, 28. Available online: https://www.biopharminternational.com/view/method-validation-essentials-limit-blank-limit-detection-and-limit-quantitation (accessed on 13 March 2024).
- Helsel, R. Less than obvious-statistical treatment of data below the detection limit. Environ. Sci. Technol. 1990, 24, 1766–1774. [Google Scholar] [CrossRef]
- Altmann, J.; Rehfeld, D.; Trder, K.; Sperlich, A.; Jekel, M. Combination of granular activated carbon adsorption and deep-bed filtration as a single advanced wastewater treatment step for organic micropollutant and phosphorus removal. Water Res. 2016, 92, 131–139. [Google Scholar] [CrossRef]
- Marciczyk, M.; Ok, Y.S.; Oleszczuk, P. From waste to fertilizer: Nutrient recovery from wastewater by pristine and engineered biochars. Chemosphere 2022, 306, 135310. [Google Scholar] [CrossRef]
- Laxmisagara Sagar, K.; Strawn, D.G.; Crump, A.R.; Baker, M.; Möller, G. Availability of Recycled Phosphorus on Biochar Reacted with Wastewater to Support Growth of Lactuca sativa. Soil Syst. 2024, 8, 93. [Google Scholar] [CrossRef]
- USEPA. Water Quality Guidances for the Great Lakes System: Supplementary Information Document (SID). Available online: https://www.epa.gov/sites/default/files/2018-10/documents/water-quality-great-lakes-sid.pdf (accessed on 2 February 2024).
- USEPA. Echo Data Base—Effluent Charts. Available online: https://echo.epa.gov/effluent-charts#AL0060887 (accessed on 10 June 2024).
- Ragsdale, D. Advanced Wastewater Treatment to Achieve Low Concentration of Phosphorus; EPA Region 10; United States Environmental Protection Agency (EPA), Office of Water and Watersheds: Seattle, WA, USA, 2007. Available online: https://www.epa.gov/sites/default/files/2019-02/documents/advanced-wastewater-treatment-low-concentration-phosphorus.pdf (accessed on 14 December 2023).
- Seiple, T.E.; Skaggs, R.L.; Fillmore, L.; Coleman, A.M. Municipal wastewater sludge as a renewable, cost-effective feedstock for transportation biofuels using hydrothermal liquefaction. J. Environ. Manag. 2020, 270, 110852. [Google Scholar] [CrossRef]






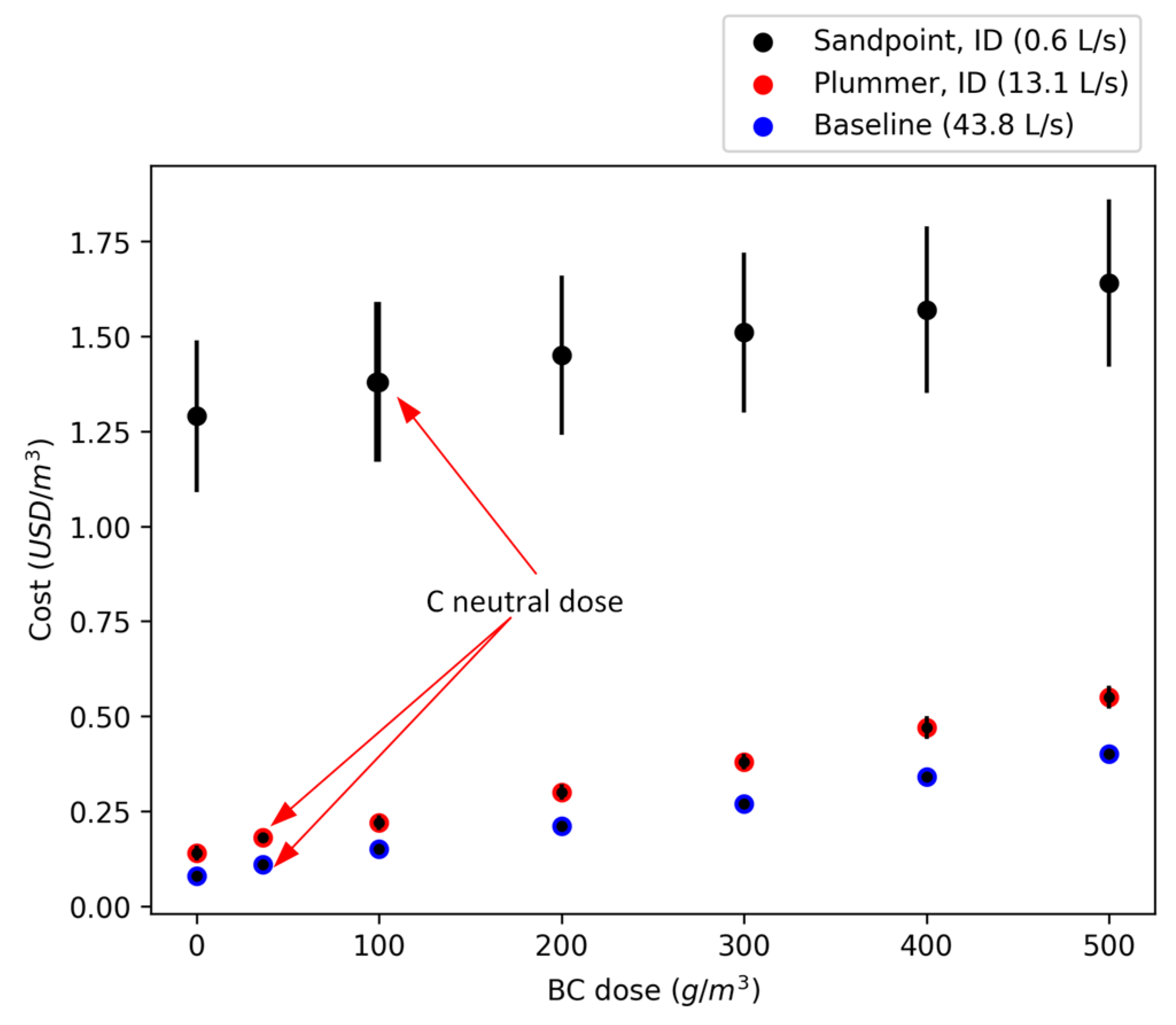
| WRRF | Flow Rate (L/s) | Biochar Dose (g/m3) |
|---|---|---|
| Sandpoint, ID, USA | 0.6 | 0, 100, 200, 300, 400, 500 |
| Plummer, ID, USA | 13.1 | 0, 100, 200, 300, 400, 500 |
| Baseline model | 43.8 | 0, 100, 200, 300, 400, 500 |
| Configuration | Site | Application | Flow Rate (L/s) | Type of Study | Year |
|---|---|---|---|---|---|
| RF | Fruitland, ID, USA | Municipal | 0.63 | Pilot [52] | 2006 |
| RF | Hayden, ID, USA | Municipal | 11 | Full-scale installation [44,51,58] | 2008 |
| RF | Marlborough, MA, USA | Municipal | 127.3 | Full-scale installation [94] | 2012 |
| RF | Hibbing, MN, USA | Municipal | 1.57 | Pilot [58] | 2018 |
| RF | Virginia, MN, USA | Municipal | 1.57 | Pilot [58] | 2018 |
| RF | Int. Falls, MN, USA | Municipal | 132 | Full-scale installation [58] | 2018 |
| Serial-RF | Plummer, ID, USA | Municipal | 13.89 | Full-scale installation, LCA, TEA [60,61] | 2022 |
| RF | Sandpoint, ID, USA | Municipal | 0.55–0.59 | Pilot [47] | 2023 |
| RF | Moscow, ID, USA | Synthetic | 0.032–0.063 | Laboratory [47] | 2023 |
| Serial-RF | Citronelle, AL, USA | Municipal | 17.53 | Full-scale installation [94] | 2016 |
| RF | Bloomer, WI, USA | Municipal | 16.17 | Full-scale installation [94] | 2021 |
| RF | Burrillville, RI, USA | Municipal | 65.72 | Full-scale installation [94] | 2017 |
| RF | Indian Head, MD, USA | Municipal | 26.29 | Full-scale installation [94] | 2011 |
| Fe-BC-RF | Moscow, ID, USA | Synthetic | 0.032–0.063 | Laboratory [47] | 2023 |
| Fe-CatOx-RF | Moscow, ID, USA | Municipal | 0.41–0.45 | Pilot [53] | 2023 |
| Fe-CatOx-RF | Horwich, UK | Municipal | 10–16 | Full-scale installation [53] | 2023 |
| Fe-CatOx-RF | Sandpoint, ID, USA | Municipal | 0.55–0.59 | Pilot [47] | 2023 |
| Fe-CatOx-BC-RF | Moscow, ID, USA | Municipal | 0.6–1.0 | Pilot [47,54] | 2016, 2023 |
| Fe-CatOx-BC-RF | Troy, ID, USA | Municipal | 0.6–1.0 | Pilot [47,54] | 2016, 2023 |
| Fe-CatOx-BC-RF | Sandpoint, ID, USA | Municipal | 0.55–0.59 | Pilot, LCA, TEA [47,54,61] | 2023 |
| Configuration | Site | Application | Flow Rate (L/s) | Target | Effluent (mg/L) | Removal (%) |
|---|---|---|---|---|---|---|
| RF | Fruitland, ID, USA | Municipal | 0.63 | As | 0.0033 | 91.8 |
| RF | Hibbing, MN, USA | Municipal | 1.57 | Hg | 1.9 × 10−6 | 95.6 |
| RF | Hibbing, MN, USA | Municipal | 1.57 | P | 0.08 | 96.4 |
| RF | Virginia, MN, USA | Municipal | 1.57 | Hg | 8 × 10−7 | 81.0 |
| RF | Virginia, MN, USA | Municipal | 1.57 | P | 0.03 | 94.3 |
| RF | Sandpoint, ID, USA | Municipal | 0.55–0.59 | P | 0.10 | 96.4 |
| Fe-CatOx-RF | Moscow, ID, USA | Municipal | 0.41–0.45 | P | 0.03 | 86.9 |
| Fe-CatOx-RF | Sandpoint, ID, USA | Municipal | 0.55–0.59 | P | 0.44 | 88.3 |
| Fe-CatOx-BC-RF | Moscow, ID, USA | Municipal | 0.6–1.0 | P | 0.02 | 83.9 |
| Fe-CatOx-BC-RF | Troy, ID, USA | Municipal | 0.6–1.0 | P | 0.03 | 99.5 |
| Fe-CatOx-BC-RF | Sandpoint, ID, USA | Municipal | 0.55–0.59 | P | 0.18–0.51 | 88.8–95.2 |
| Excellent (>90%) | Good (70–90%) | Fair (40–70%) | Low (20–40%) | Poor (<20%) | |
|---|---|---|---|---|---|
| Analytes | |||||
| Acetaminophen | ❺❼ | ❷ | |||
| Androstenedione | ❹ | ❺ | |||
| Bisphenol A | ❶❷❸❹❺❻❼ | ||||
| Caffeine | ❶❷❸❹❺❻❼ | ❺ | |||
| Carbamazepine | ❶❷❸❹❺❻ | ||||
| DEET | ❶❸❹❺❻ | ❷ | |||
| Diclofenac | ❶❷❸❹❺❻ | ||||
| Dilantin | ❶❷❸❹❻ | ❺ | |||
| Estrone | ❷❸❹❺❻ | ❼ | |||
| Fluoxetine | ❶❷❸❹❺❻ | ❼ | |||
| Gemfibrozil | ❶❷❸❹❺❻❼ | ||||
| Hydrocodone | ❶❷❸❹❺❻ | ||||
| Ibuprofen | ❶❷❸❹❻❼ | ❺ | |||
| Meprobamate | ❶❸❻ | ❷❹ | ❺ | ||
| Methadone | ❶❷❸❹❻ | ❺ | |||
| Naproxen | ❶❷❸❹❺❻❼ | ||||
| Oxybenzone | ❷❸❹ | ||||
| Salicylic Acid | ❺ | ❹ | ❷ | ||
| Sulfamethoxazole | ❶❷❸❹❻❼ | ❺ | |||
| Trimethoprim | ❶❷❸❹❺❻ | ❼ |
| P (mg/kg BC) | N (mg/kg BC) | ||||
|---|---|---|---|---|---|
| Total | Formic Acid Extractable P | Total | KCI Extractable NO3 | ||
| Fe-BCP | Native | 757 | 18 | 810 | 14 |
| Recovered | 1600 (2.1×) | 206 (11.4×) | 3040 (3.8×) | 510 (36.4×) | |
| Reject | 7510 (9.9×) | 199 (11×) | 4030 (5.0×) | 413 (29.5×) | |
| BCP | Native | 806 | 124 | 790 | 16 |
| Recovered | 931 (1.2×) | 434 (3.5×) | 680 (0.86×) | 111 (6.9×) | |
| Reject | 14,200 (17.6×) | 300 (2.4×) | 4910 (6.2×) | 146 (9.1×) | |
| BCB | Native | 46,400 | 6360 | 29,000 | - |
| Recovered | 45,900 (0.99×) | 4480 (0.70×) | 27,600 (0.95×) | - | |
| Configuration | Site | Application | Flow Rate (L/s) | Target | Effluent (mg/L) | Removal (%) |
|---|---|---|---|---|---|---|
| RF | Hayden, ID, USA | Municipal | 11 | Hg | 4.6 × 10−7 | 53 |
| RF | Hayden, ID, USA | Municipal | 11 | P | 0.03 | 98.7 |
| RF | Marlborough, MA, USA | Municipal | 127.3 | P | 0.7–1.0 | 77.8–93 |
| RF | Int. Falls, MN, USA | Municipal | 132 | Hg | 2.7 × 10−6 | 96.9 |
| RF | Int. Falls, MN, USA | Municipal | 132 | P | 0.59 | 83.2 |
| Serial-RF | Plummer, ID, USA | Municipal | 13.89 | P | 0.05 | 97.5 |
| Serial-RF | Citronelle, AL, USA | Municipal | 17.53 | P | 0.022 | 99.0–99.9 |
| RF | Bloomer, WI, USA | Municipal | 16.17 | P | 0.075 | 90.6 |
| RF | Burrilleville, RI, USA | Municipal | 65.72 | Cu | 0.0075 | 50 |
| RF | Burrilleville, RI, USA | Municipal | 65.72 | P | 0.08 | 84–94.7 |
| RF | Indian Head, MD, USA | Municipal | 26.29 | N | 1.5 | - |
| RF | Indian Head, MD, USA | Municipal | 26.29 | P | 0.30 | - |
| Fe-CatOx-RF | Horwich, UK | Municipal | 10–16 | P | 0.19 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Baker, M.C.; Taslakyan, L.; Strawn, D.G.; Möller, G. Reactive Filtration Water Treatment: A Retrospective Review of Sustainable Sand Filtration Re-Engineered for Advanced Nutrient Removal and Recovery, Micropollutant Destructive Removal, and Net-Negative CO2e Emissions with Biochar. Sustainability 2025, 17, 5799. https://doi.org/10.3390/su17135799
Yu P, Baker MC, Taslakyan L, Strawn DG, Möller G. Reactive Filtration Water Treatment: A Retrospective Review of Sustainable Sand Filtration Re-Engineered for Advanced Nutrient Removal and Recovery, Micropollutant Destructive Removal, and Net-Negative CO2e Emissions with Biochar. Sustainability. 2025; 17(13):5799. https://doi.org/10.3390/su17135799
Chicago/Turabian StyleYu, Paulo, Martin C. Baker, Lusine Taslakyan, Daniel G. Strawn, and Gregory Möller. 2025. "Reactive Filtration Water Treatment: A Retrospective Review of Sustainable Sand Filtration Re-Engineered for Advanced Nutrient Removal and Recovery, Micropollutant Destructive Removal, and Net-Negative CO2e Emissions with Biochar" Sustainability 17, no. 13: 5799. https://doi.org/10.3390/su17135799
APA StyleYu, P., Baker, M. C., Taslakyan, L., Strawn, D. G., & Möller, G. (2025). Reactive Filtration Water Treatment: A Retrospective Review of Sustainable Sand Filtration Re-Engineered for Advanced Nutrient Removal and Recovery, Micropollutant Destructive Removal, and Net-Negative CO2e Emissions with Biochar. Sustainability, 17(13), 5799. https://doi.org/10.3390/su17135799










