Abstract
In arid and semi-arid areas, soil is blown up by the wind because of its loose structure. Wind erosion causes soil quality and fertility loss, land degradation, air pollution, disruption of ecological balance, and agricultural and livestock losses. Consequently, there is an immediate imperative for methods to mitigate the impacts of wind erosion. SICP (soybean urease-induced carbonate precipitation) has emerged as a promising biogeotechnical technology in mitigating wind erosion in arid and semi-arid regions. To enhance bio-cementation efficacy and treatment efficiency of SICP, aluminum chloride (AlCl3) was employed as an additive to strengthen the SICP process. Multiple SICP treatment cycles with AlCl3 additive were conducted on Tengger Desert sand specimens, with the specimens treated without AlCl3 as the control group. The potential mechanisms by which AlCl3 enhances SICP may have two aspects: (1) its flocculation effect accelerates the salting-out of proteinaceous organic matter in the SICP solution, retaining these materials as nucleation sites within soil pores; (2) the highly charged Al3+ cations adsorb onto negatively charged sand particle surfaces, acting as cores to attract and coalesce free CaCO3 in solution, thereby promoting preferential precipitation at particle surfaces and interparticle contacts. This mechanism enhances CaCO3 cementation efficiency, as evidenced by 2.69–3.89-fold increases in penetration resistance at the optimal 0.01 M AlCl3 concentration, without reducing CaCO3 production. Wind erosion tests showed an 88% reduction in maximum erosion rate (from 1142.6 to 135.3 g·m−2·min−1), directly correlated with improved microstructural density observed via SEM (spherical CaCO3 aggregates at particle interfaces). Economic analysis revealed a 50% cost reduction due to fewer treatment cycles, validating the method’s sustainability. These findings highlight AlCl3-modified SICP as a robust, cost-effective strategy for wind erosion control in arid zones, with broad implications for biogeotechnical applications.
1. Introduction
Land desertification represents a significant ecological and environmental challenge on a global scale. As a key driver of land degradation, wind erosion exacerbates this challenge. Wind erosion in arid and semi-arid regions leads to a multitude of adverse effects, including soil quality and fertility loss, land degradation, air pollution, disruption of ecological balance, and agricultural and livestock losses [1,2]. Therefore, effective control and prevention measures against wind erosion are crucial for sustainable land management in these regions.
Numerous strategies have been employed to mitigate wind erosion. First, vegetation cover is used to stabilize the soil and reduce wind erosion [3]. However, establishing and maintaining vegetation requires significant time and extensive resource investment, posing critical challenges in arid or resource-poor regions. Additionally, soil and water conservation structures (e.g., terraces and windbreak walls) alter wind flow and intensity, thereby decreasing erosion [4]. Despite their effectiveness, these structures require substantial financial investment and engineering expertise, potentially impacting land use. Furthermore, soil stabilizers enhance soil cohesion and stability [5], mitigating wind erosion risks. However, certain stabilizers may cause adverse environmental impacts or entail prohibitively high costs.
In recent years, bio-induced CaCO3 precipitation has received considerable attention from researchers as a new type of soil treatment technique. Specifically, bio-induced CaCO3 precipitation technologies are mainly divided into two categories: microbial-induced carbonate precipitation (MICP) and enzyme-induced carbonate precipitation (EICP). The mechanisms of the two are similar, and the reaction is shown as follows:
Hydrolysis of urea by urease enzymes produces CaCO3 between soil particles. The bonding effect of CaCO3 leads to an increased soil strength [6,7,8,9,10,11,12]. Furthermore, bio-induced CaCO3 precipitation techniques have demonstrated significant promise in suppressing dust pollution and mitigating wind erosion [13,14,15,16,17].
The MICP method relies on bacteria, such as Bacillus pasteurii, as a source of urease. Unlike MICP, the urease of the EICP method is extracted directly from plants. In addition, the size of the urease molecule is 12 nm [18]. This is much smaller than the diameters of bacteria, which are generally larger than 300 nm [19]. For example, Bacillus pasteurii has a diameter of approximately 2800 nm [20]. This smaller size of the urease molecule allows it to penetrate into smaller pores, making EICP more effective in treating fine-grained soils [21].
Numerous methods have been investigated to enhance the effectiveness of bio-induced CaCO3 precipitation techniques in soil treatment. For example, Refei et al. [22] demonstrated that the addition of sodium alginate (SA) to cement increased the unconfined compressive strength (UCS) of soil, with 2 M cementation solution and 1% SA resulting in the highest UCS of 1711.4 kPa, which was 3.4 times higher than without the addition of SA. Additionally, Wu et al. [23] added xanthan gum to the cement to improve the penetration resistance of sand specimens. The highest resistance was achieved when the xanthan gum concentration was 1 g·L−1 and the cement concentration was 0.3 M, 1.9 times higher than without xanthan gum, reaching 7 MPa. Wang and Tao [24] used a new polyvinyl alcohol (PVA)-modified MICP, which was optimal when the cement concentration was 1 M, resulting in a UCS of 418.9 kPa, 2.2 times higher than that of the conventional method. Yuan et al. [25] used Na-Mt to modify the EICP, and an 8% Na-Mt addition increased the UCS to 2.4 times that of the conventional method. Sun et al. [26] used polyvinyl acetate (PVAc) and polyethylene glycol (PEG) to optimize EICP; the EICP-PVAc-PEG treatment with 50 g/L PVAc and 30 g/L PEG was most suitable for dust control. Yao et al. [27] added wool fibers to sand to increase the UCS of soil, with a 0.1% wool fiber content resulting in a 3.4-fold increase. Miyake et al. [28] used casein to enhance the UCS of biocemented sand. In the case of 0.893 M urea, 0.581 M CaCl2, 2.6 g/L urease, and 38.87 g/L casein, the UCS reached 2 MPa. Despite the successes of these methods, further development is needed to address remaining challenges, such as the need for large quantities of materials and labor and the potential for xanthan gum and some polymers to degrade over time [17].
Previous studies have confirmed that AlCl3 flocculants significantly enhance the strength of sand reinforced by MICP. After five treatment cycles, the UCS of MICP-treated sand columns with 0.02 M AlCl3 reached 2 MPa, 27.7 times higher than that of the conventional MICP method [29]. Compared with the aforementioned additives, AlCl3 not only exhibits more pronounced strengthening effects on MICP-reinforced sand but also requires a lower dosage. Its strengthening mechanism differs from those of other additives: hydrogel-based additives such as SA [22], xanthan gum [23], PVA [24], and PEG [26] form network structures in the sand to create a composite bonding effect with MICP; casein [28] and Na-Mt [25] enhance the reinforcement of sand by providing additional nucleation sites for calcium carbonate precipitation in MICP. In contrast, the mechanism by which AlCl3 enhances MICP involves the generation of Al3+ through the introduction of AlCl3 during the MICP reaction, leading to the precipitation of aluminum hydroxide flocculates. These flocculants exhibit remarkable adsorption properties, efficiently capturing unbound precipitated calcium carbonate in the pore network and altering the spatial distribution of calcium carbonate precipitation. As a result, more calcium carbonate is transformed into an “effective” form as a binder rather than solely serving as a filler [30]. Therefore, given the similar reaction principles between MICP and SICP (with the only difference being the source of urease), it is hypothesized that AlCl3 may also exert a strengthening effect on SICP-reinforced sand. To improve the wind erosion control efficiency of SICP and reduce costs, we investigated the reinforcement effects of SICP with a small amount of AlCl3 added to desert sand from the Tengger Desert. By testing the penetration resistance, wind erosion rate, calcium carbonate content, and calcium carbonate morphology, the wind erosion resistance of desert sand treated with SICP-AlCl3 was evaluated, and the strengthening mechanism of AlCl3 flocculants on SICP was deeply explored.
2. Materials and Methods
2.1. Sand
The sand was sourced from the Tengger Desert (104°17′ E, 36°59′ N) in Ningxia, China. Using the sieving method, the particle size distribution of the sand was obtained, as illustrated in Figure 1. The D10, D30, and D60 of the sand values are 0.11 mm, 0.146 mm, and 0.19 mm, respectively. Calculated from D10, D30 and D60, Cu = 1.73 and Cc = 1.02. Based on these values, the sand is classified as poorly graded sand (SP) according to the Unified Soil Classification System (ASTM D2487-06) [31]. The moisture content, specific gravity (Gs), and dry density of the sand are 0.31, 2.66, and 1.46 g·cm−3. X-ray diffraction (XRD) analysis was conducted on the sand, indicating that its main composition is quartz [32].
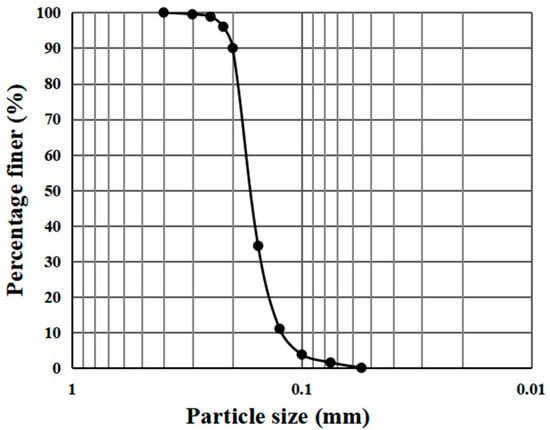
Figure 1.
Particle size distribution of the raw sand grains.
2.2. Soybean Crude Urease (SCU) and Cementation Solution (CS)
- (1)
- Crude urease was extracted from soybeans, a legume rich in urease, following the method of Yan et al. [33]. The procedure of the method was as follows:
- (2)
- First, 60 g of soy flour was mixed with 1000 mL of deionized water and stirred for 1 h. The bean powder solution was filtered using a 200-mesh filter, and 0.67 g of CaCl2 was added to the solution and stirred for 1 min.
- (3)
- The solution was centrifuged at 4000 rpm and 4 °C for 10 min, and the SCU solution was obtained by filtering the solution using a 200-mesh filter.
- (4)
- The urease activity of SCU, representing the rate of enzymatic hydrolysis of urea, was evaluated using the electrical conductivity change of 1 M urea at 25 °C, following the method proposed by Whiffin et al. [34]. The urease activity in this paper is 8.9 mM·min−1. The CS was composed of urea and calcium chloride, and the concentration of CS used in this study (after mixing with SCU 1:1 by volume) was 0.5 M.
2.3. Aluminum Chloride (AlCl3)
In this study, AlCl3·6H2O was used. After the CS was configured, AlCl3·6H2O was added to the solution. In the preliminary experiments, the predefined concentration range for AlCl3 addition was 0–0.5 M. Analysis of the experimental results revealed that the effective concentration range for AlCl3 to strengthen SICP is between 0.004 and 0.015 M. When the concentration of AlCl3 exceeds this range, it exhibits no significant strengthening effect on SICP. Therefore, the concentration of AlCl3 selected was 0, 0.004, 0.008, 0.01, and 0.015 M.
2.4. Specimen Preparation and Treatment
According to Hamdan et al. [14], a shallow pan was used as a mold in this study. The test mold was a rectangular metal pan with dimensions of 24 cm × 17 cm × 4 cm. The molds were filled with pre-weighed desert sand to achieve a bulk density of 1.51 g·cm−3. As reported by Meng et al. [17], the treatment solution was uniformly sprayed onto the sand surface at a dose of 4 L·m−2, with a 24 h interval between each spray. After treatment, the specimens were rinsed with deionized water at a rate of 12 L·m−2. Then, the specimens were dried in an oven at 60 °C and then cooled to room temperature. The penetration resistance, wind erosion, and CaCO3 content were then determined.
Table 1 shows all of the treatment schemes. Only schemes with no more than three treatments were considered because preliminary experiments were conducted before the main test. In the preliminary experiments, there were schemes involving more than three treatments. However, we observed that when the fourth treatment was performed, the treatment solution became congested on the surface of the sample, which undoubtedly led to a waste of the treatment solution. Therefore, only schemes with no more than three treatments were carried out.

Table 1.
Different treatments of sandy soil.
Five specimens were prepared for each treatment scheme.
2.5. Viscosity of Treatment Solution (SCU + CS+ AlCl3) and Thickness of Crust Layer
The addition of AlCl3 to the treatment solution may increase its viscosity and reduce its permeability in sandy soil, thus reducing the thickness of the crust layer formed on the surface. To test the viscosity of the treatment solution after adding different concentrations of AlCl3, an NDJ-5 (8)B rotary digital display viscometer (rotor 0, speed 60 rpm, range 1–10 mPa·S, accuracy ±3%) was used. After drying the specimens, the crust layer was removed, and its thickness was measured using a vernier caliper.
2.6. Surface Penetration Test
A digital micro-penetrometer (HP-1k, Aidebao, Beijing, China) was utilized to evaluate the penetration resistance of the specimens. The cross-section of the digital micro-penetrometer was round with a diameter of 5 mm. During the measurement, the digital display push–pull meter was fixed on an HLD hand-crank test bench (Aidebao, China). The specimen was placed on the horizontal test bench, and the maximum force was recorded when the probe penetrated the crust layer of the specimen. The penetration resistance was calculated as the force divided by the cross-sectional area of the probe. To reduce the error, six measurement points were taken for each specimen.
2.7. CaCO3 Content Test
The formation of a crust layer on the surface of the specimen was attributed to the filling and cementing effect of CaCO3. To evaluate the chemical conversion efficiency of SICP, the CaCO3 content of the crust layer was measured. The calcium carbonate content in the surface crust layer was measured using the Ethylenediaminetetraacetic Acid (EDTA) Titration Method [35,36,37]. After completion of the penetration test, a 5 g sample was taken for testing.
2.8. Wind and Sand Erosion Test
The wind and sand erosion equipment used in this study was as referenced by Liu et al. [38]. Based on the in situ meteorological monitoring in the Tengger Desert [39], the local wind speeds ranged from 5 to 15 m·s−1. The pre-test results showed that the wind erosion rates of the specimens were too small under the wind and sand erosion at 5 and 10 m·s−1. To obtain more conservative results, the most severe conditions were selected for the indoor tests, specifically a wind speed of 15 m·s−1. If good results can still be achieved at this wind speed, the wind erosion resistance of sand treated with SICP-AlCl3 can also exhibit good performance at lower wind speeds.
When the wind speed exceeded the critical wind speed, the sand particles were separated from the ground surface, and they impacted and abraded the ground surface. As the wind speed increased, the number of sand particles blown up by the wind increased, resulting in a significant increase in the abrasion of the surface [40]. The sand transfer rate of 512 g/min was as referenced by Liu et al. [38]. The wind and sand erosion test was conducted until the crust layer of the specimen was broken. The specimen was weighed every 5 min to monitor the breakage of the crust layer.
2.9. SEM Analysis
The dried crust layer was prepared for SEM analysis. Scanning electron microscopy (SEM) was used to investigate the microstructures and morphology of the precipitates.
2.10. pH of the Treated Sand
After treating the specimens, the soil pH was assessed using a soil pH analyzer. The pH measurements were taken at hourly intervals over the first six hours and then at four-hour intervals from 6 to 26 h.
3. Results
3.1. Viscosity of the Treatment Solution and Thickness of the Crust Layer
As shown in Figure 2, the AlCl3 additive had little effect on the viscosity of the treatment solution. This suggested that the AlCl3 additive did not reduce the permeability of the solution in the sand. As shown in Figure 3, after one, two, and three treatments, the thickness of the crust layer was approximately 1.3, 2.0, and 3.0 cm, respectively, with or without the AlCl3 additive. This suggested that the AlCl3 additive did not change the viscosity of the treatment solution or reduce the thickness of the crust layer.

Figure 2.
Viscosity of the treatment solution.
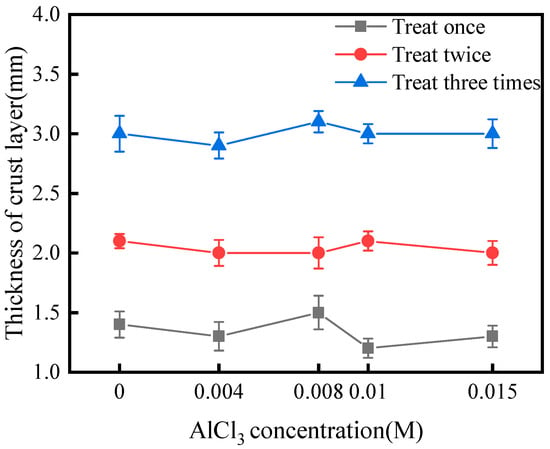
Figure 3.
Thickness of the crust layer.
3.2. CaCO3 Content
Figure 4 shows that the CaCO3 content of the crust layer was almost independent of the concentration of AlCl3 additive after treatment with SICP and AlCl3 additive. After one, two, and three treatments, the CaCO3 content of the crust layer was approximately 1.0%, 1.5%, and 2.5%, respectively. The results are in agreement with Meng et al. [37]. Thus, it can be concluded that the AlCl3 additive did not inhibit urease-induced CaCO3 precipitation within the concentration range studied.
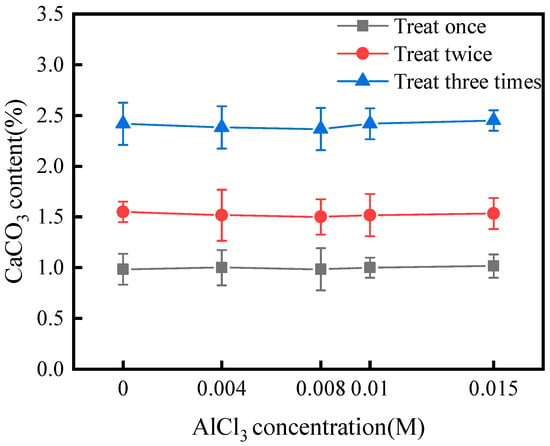
Figure 4.
CaCO3 content of specimens treated with different treatment schemes.
3.3. Penetration Resistance
Figure 5 shows that the penetration resistance of the SICP-treated and SICP with AlCl3 additive-treated desert sand increased with increasing number of spraying cycles. For example, the penetration resistance increased from 3.45 to 12.52 MPa when the number of spraying cycles increased from one to two. The increase in cementation hardness of the soil can be attributed to the enhanced production of CaCO3 in the soil.
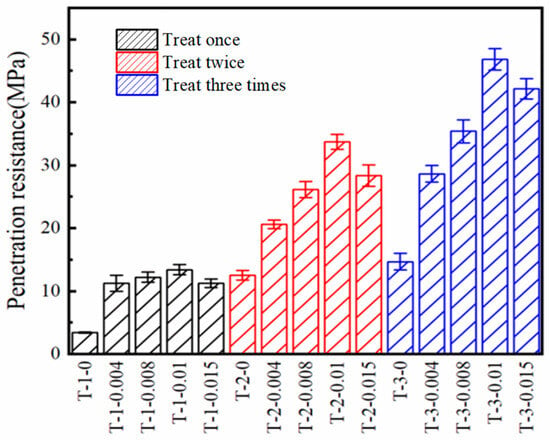
Figure 5.
Penetration resistance of desert sand treated with different treatment schemes.
Comparing the penetration resistance of the SICP with AlCl3 additive-treated and SICP-treated specimens, the AlCl3 additive resulted in a significant increase in the penetration resistance of the specimens. For instance, after one treatment, the penetration resistance of the T-1-0 specimen was 3.45 MPa, and that of the T-1-0.01 specimen was 13.42 MPa, an increase of 289%. In previous studies, a penetration resistance of 13.42 MPa was not achieved at the 1% CaCO3 content (Figure 6) [17,38,41]. After two treatments, the penetration resistance of the T-2-0 was 12.52 MPa, and that of the T-2-0.01 specimen was 33.68 MPa, an increase of 169%. After three treatments, the penetration resistance of the T-3-0 specimen was 14.69 MPa, and that of the T-3-0.01 specimen was 46.84 MPa, an increase of 219%. It can be concluded that the maximum number of treatments for this desert sand was three, and the ultimate penetration resistances of the SICP-treated and SICP with AlCl3 additive-treated specimens were 14.69 and 46.84 MPa, respectively. This indicated that the AlCl3 additive resulted in a 219% increase in the ultimate penetration resistance of the SICP-treated specimen.

Figure 6.
Relationship between CaCO3 content and penetration resistance [17,38,41].
In Figure 6, the relationship between the CaCO3 content and the penetration resistance is illustrated. The SICP-treated specimens demonstrated an increase in penetration resistance from 3.45 to 14.69 MPa as the CaCO3 content was raised from 1% to 2.5%. However, the CaCO3 content did not correlate with the penetration resistance when the treatment scheme was different. For example, although the CaCO3 content of the T-2-0.01 specimen was only 1.7%, the penetration resistance reached 33.68 MPa. The findings indicated that the strength of the bio-cementation is influenced not only by the CaCO3 content but also by the location, morphology, and size of the CaCO3 production [42,43,44].
According to Cui et al. [45], the presence of CaCO3 crystals at the particle-particle contacts contributes more significantly to the improvement of soil strength compared to those located at the surfaces of individual particles. AlCl3 is a flocculant. The soybean urease solution contains numerous organic substances with protein-like characteristics. The introduction of the flocculant can enhance the salting-out process and lead to the retention of organic matter in the soil pores during solution percolation. These precipitated organic materials were retained within soil pores during solution percolation, serving as nucleation sites to augment the overall strength of the soil [46]. AlCl3 produced a large number of highly charged cations in solution, which were adsorbed on the surfaces of the negatively charged sand particles as cores to adsorb and coalesce the free CaCO3 in solution [47,48]. During the reaction of SICP, the amount of CaCO3 that only served to fill the pores was reduced, and more CaCO3 was precipitated on the surfaces of the sand particles and at the particle–particle contacts.
3.4. Wind Erosion Resistance
Figure 7 shows the damage to the specimens after wind and sand erosion. The development of damage to the crust layers of the specimens during wind and sand erosion was observed. At the wind speed of 15 m·s−1, the SICP-treated specimen was penetrated by abrasion after 25 min of wind and sand erosion (Figure 7a,b). By contrast, the SICP with AlCl3 additive-treated specimen was better retained, with only a few shallow grooves after 60 min of wind and sand erosion (Figure 7c,d). The maximum abrasion depth was about 3 mm, which was much less than the thickness of the crust layer, indicating that the AlCl3 additive improved the resistance of the specimen to wind and sand erosion.

Figure 7.
Damage to the crust layer of specimens under wind and sand erosion: (a,b) T-1-0, (a) 5 min, (b) 25 min, (c,d) T-1-0.01, (c) 25 min, (d) 60 min.
Figure 8a–c shows the variation of the wind erosion rate of the SICP-treated and SICP with AlCl3 additive-treated specimens with time. The wind erosion rate gradually increased with the extension of the wind and sand erosion time. This indicated that the wind erosion resistance of the specimens gradually decreased during the long period of wind and sand erosion.

Figure 8.
Wind erosion rates of specimens under wind and sand erosion: (a) treated once, (b) treated twice, (c) treated three times, (d) relationship between wind erosion rate and penetration resistance.
By comparing the wind erosion rates of the SICP-treated and SICP with AlCl3 additive-treated specimens, it was concluded that the AlCl3 additive could significantly reduce the wind erosion rates of the SICP-treated specimens, demonstrating that the AlCl3 additive could enhance the wind erosion resistance of the specimens. The wind erosion rates of the specimens after one treatment (Figure 8a) decreased in a certain range with increasing concentration of AlCl3. As the concentration of AlCl3 increased from 0 to 0.01 M, the maximum wind erosion rate of the specimens decreased from 1142.6 to 135.3 g·m−2·min−1. However, when the concentration of AlCl3 increased to 0.015 M, the maximum wind erosion rate of the specimens increased to 355.9 g·m−2·min−1. As a micro-efficient additive, AlCl3 has an optimal dosage. When the dosage exceeds this optimal value, its effect will decline. The possible mechanistic reasons may include: (1) charge reversal and double-layer compression caused by excessive Al3+, where saturation of Al3+ adsorption may induce local positive polarization of particle surface charges, leading to re-enhanced electrostatic repulsion between particles, while high ionic strength compresses the electric double layer to disrupt the flocculation structure and hinder calcium carbonate formation on sand particle surfaces; and (2) colloidal encapsulation and decreased cementation efficiency, as excessive Al3+ hydrolyzes to form Al (OH)3 colloids that coat sand particle surfaces, impeding effective contact between CaCO3 and particles, reducing cementation strength, and thereby increasing the wind erosion rate.
The wind erosion rates of the specimens after two treatments (Figure 8b) decreased with increasing concentration of AlCl3. The maximum wind erosion rate of the specimens was the smallest at 47.5 g·m−2·min−1 when the concentration of AlCl3 was 0.01 M. After three treatments, the maximum wind erosion rate of the specimen was the smallest at 35.6 g·m−2·min−1 when the concentration of AlCl3 was 0.01 M. Thus, the optimal concentration for AlCl3 to improve the treatment effect of SICP was 0.01 M. Figure 8c shows the wind erosion rate of the specimen after three treatments. It can be seen from the figure that when the AlCl3 addition amount is 0.01 M, the wind erosion rate is the lowest, at 19.7 g·m−2·min−1.
Figure 8d shows the relationship between the wind erosion rates and the penetration resistances of the specimens. The strength of sand plays a crucial role in determining the rate of wind erosion, as it directly affects its capacity to withstand wind shear and sand particle impingement. As the penetration resistance increased, the wind erosion rate gradually decreased. When the penetration resistance increased from 3.45 to 46.84 MPa, the corresponding maximum wind erosion rate decreased from 1142.6 to 19.7 g·m−2·min−1. This indicated that the wind erosion resistance of the specimens increased as the penetration resistance increased.
3.5. SEM Imaging
The XRD results of the sand sample produced by SICP and SICP-AlCl3 are demonstrated in Figure 9. Clear and sharp diffraction peaks showed good crystallinity. Based on the distribution of diffraction peaks, a mineral crystalline phase of CaCO3-calcite was detected. This confirmed that CaCO3 is the primary cementing substance in both SICP and SICP-AlCl3 methods.

Figure 9.
SEM images and XRD patterns of the treated crust layers: (a) microscale identification of the SICP-treated sand (T-1-0), and (b) microscale identification of the SICP with AlCl3-treated sand (T-1-0.01).
Figure 9 shows the cementation morphology of the SICP-treated and SICP with AlCl3 additive-treated specimens. The treated specimen exhibited a crystalline morphology with a large amount of CaCO3 precipitated on the surfaces of the sand particles with interstices, thus providing a strong binding force to withstand a high shear strength [49,50]. The divergence in crystal morphology between the CaCO3 produced through SICP treatment and the SICP with AlCl3 treatment is evident. Specifically, following SICP treatment, the CaCO3 crystals present on the surface of specimens exhibit a rhombic structure, whereas the combination of SICP with AlCl3 results in the formation of spherical CaCO3 crystals. Figure 9b reveals that the interface between the sand particles exhibits not only calcium carbonate crystals but also a colloid-like layer. This suggests that the SICP with AlCl3 has a greater potential for enhancing sand solidification. The incorporation of colloid-like material into the sand promotes a more cohesive and integrated structure, resulting in an enhanced cementation effect.
3.6. Economic Benefit Analysis
The costs associated with the SICP method and the SICP method with AlCl3 were compared and analyzed. To facilitate a fair comparison, it was assumed that the controlled wind erosion area covered 10,000 m2 and that both solutions achieved a surface strength of 13 MPa. The results of the cost comparison are presented in Table 2. The cost of the conventional SICP solution amounted to CNY ¥24,320, while the cost of the SICP solution with aluminum chloride totaled CNY ¥12,340.78. The addition of AlCl3 resulted in a 50% reduction in cost.

Table 2.
Cost analysis of a 10,000 m2 area with soil surface strength up to 13 MPa under different wind erosion control schemes.
4. Discussion
4.1. Mechanism Analysis
The results of this study demonstrate that the addition of AlCl3 to the SICP treatment significantly enhances the wind erosion resistance of desert sand. This enhancement can be attributed to several factors. First, the AlCl3 additive did not inhibit the CaCO3 precipitation process, as evidenced by the similar CaCO3 content in specimens with and without AlCl3. This is an important finding, as it indicates that the additive does not interfere with the fundamental mechanism of SICP. Second, the significant increase in penetration resistance with AlCl3 addition (up to 289% after one treatment) suggests that the additive alters the quality rather than the quantity of CaCO3 precipitation. This is further supported by the SEM imaging, which revealed differences in crystal morphology between SICP-treated and SICP with AlCl3-treated specimens. The formation of spherical CaCO3 crystals in the presence of AlCl3, compared to rhombic structures in conventional SICP, may contribute to the enhanced mechanical properties. Additionally, the presence of a colloid-like layer at particle interfaces in AlCl3-treated specimens suggests that the flocculant promotes a more cohesive structure.
4.2. Limitations
High levels of aluminum ions in the soil can lead to soil acidification and affect plant growth. Given that the treatment solution volume was 4 L/m2, approximately equivalent to the void volume of the 1 cm untreated soil, the influence depth of the aluminum-containing treatment solution was estimated to be around 1.2 cm, with negligible effects on the underlying soil layers. There is no universally applicable threshold for aluminum ion content in soil that encompasses all scenarios, as it varies based on factors such as geographical location, soil type, and ecosystem characteristics. Additionally, the tolerance to aluminum ions differs among plant species and organisms. Elevated aluminum ion levels can have detrimental effects on plant growth, particularly in acidic soils or in the presence of anthropogenic pollutants such as aluminum mine waste. Notably, some plant species may experience heightened aluminum ion toxicity when soil pH drops below 5.5 due to increased dissolution and release of aluminum ions under lower pH conditions [52]. Soils typically contain aluminum in inert forms, such as aluminosilicates [53], which are nontoxic under high soil pH (>5.5).
Figure 10 presents the temporal changes in soil pH. The pH of the SICP and SICP with AlCl3-treated soils exhibited a peak around 4 h of reaction, followed by a gradual decrease. The final pH of the SICP-treated soil was weakly basic, approximately 7.8. In contrast, the pH of the SICP with AlCl3-treated soil eventually reached a neutral level of around 7. Therefore, it can be concluded that the soil does not experience acidification at this aluminum ion content. Despite observing a higher concentration of residual aluminum ions in the surface crust layer compared to the subsoil, this method remains highly promising. This is because it enhances soil strength by 3.89 times and reduces the cost of wind and sand control by half, in comparison to conventional approaches. Consequently, the aforementioned benefits render this method economically and environmentally appealing.
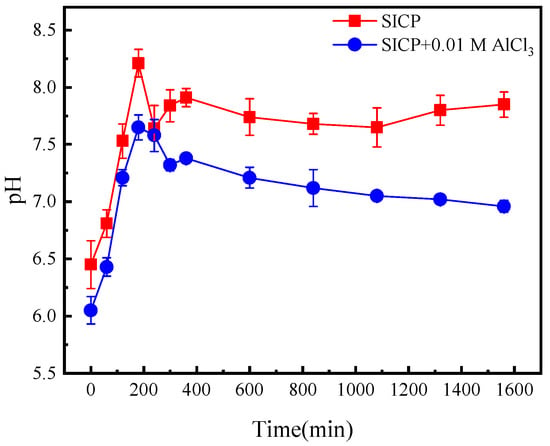
Figure 10.
Changes in pH of the treated sand at different times.
5. Conclusions
Based on the experimental results investigating the AlCl3-additive-optimized SICP method for wind erosion control, the following conclusions can be drawn:
- AlCl3 could improve the penetration resistance of the SICP-treated specimens. The penetration resistance of the SICP with 0.01 M AlCl3 additive-treated specimens reached 13.42 MPa after one treatment, which was 289% higher than that of the SICP-treated specimen. The AlCl3 additive could significantly improve the wind erosion resistance of the SICP-treated specimens, with optimal concentrations of 0.01 M. In addition, the wind erosion resistances of the specimens were positively correlated with the penetration resistance.
- The flocculation effect of AlCl3 intensified the salting-out process of organic matter in the SICP solution, causing more organic matter to be retained in the pores as nucleation sites. AlCl3 produced some highly charged cations in solution, which were adsorbed on the surface of negatively charged sand particles as a core to adsorb and coalesce free CaCO3 in solution. These resulted in more CaCO3 precipitation on the surface of the sand particles and at the contact of the sand particles, thus enhancing the cementation effect of the CaCO3. This may be the reason why AlCl3 enhances the penetration resistance and wind erosion resistance of SICP-treated specimens.
- Economic analysis indicates that incorporating AlCl3 into the SICP method can reduce the overall cost of sand consolidation by approximately 50%, making it a more cost-effective approach.
While this study demonstrates the promising potential of AlCl3-modified SICP for wind erosion control, several aspects warrant further investigation. The experiments were conducted under controlled laboratory conditions; future research should focus on field-scale trials to validate these findings under real-world environmental conditions, including variations in temperature, humidity, and rainfall. The long-term durability and weathering resistance of the treated sand also require a comprehensive assessment. The applicability of this method to different types of sandy soils with varying mineralogy and particle size distributions should also be explored. Finally, further optimization of treatment parameters, such as AlCl3 concentration, application frequency, and curing conditions, could potentially lead to even greater improvements in performance and cost-effectiveness.
Author Contributions
Conceptualization, L.L. (Liangliang Li) and J.Z.; methodology, L.L. (Liangliang Li) and J.Z.; software, R.W. and D.D.; validation, J.P., L.L. (Lingxiao Liu) and J.Z.; formal analysis, L.L. (Liangliang Li) and L.L.; investigation, L.L. (Liangliang Li) and J.Z.; resources, J.H. and Y.G.; data curation, R.W., D.D. and L.L. (Lingxiao Liu); writing—original draft preparation, L.L. (Liangliang Li); writing—review and editing, J.Z. and J.P.; visualization, J.P.; supervision, J.H.; project administration, Y.G.; funding acquisition, J.Z. and Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Major Scientific Research Projects (Natural Science Category) of Higher Education Institutions in Anhui Province for 2024: 2024AH040233 and National Natural Science Foundation of China [Grant No. 51978244 and 51578214].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ravi, S.; D’Odorico, P.; Over, T.M.; Zobeck, T.M. On the effect of air humidity on soil susceptibility to wind erosion: The case of air-dry soils. Geophys. Res. Lett. 2004, 31, L09501. [Google Scholar] [CrossRef]
- Sepehr, A.; Hassanzadeh, M.; Rodriguez-Caballero, E. The protective role of cyanobacteria on soil stability in two Aridisols in northeastern Iran. Geoderma Reg. 2019, 16, e00201. [Google Scholar] [CrossRef]
- Shumack, S.; Fisher, A.; Hesse, P.P. Refining medium resolution fractional cover for arid Australia to detect vegetation dynamics and wind erosion susceptibility on longitudinal dunes. Remote Sens. Environ. 2021, 265, 112647. [Google Scholar] [CrossRef]
- Tian, M.; Fu, X.; Yang, W.; Feng, C.; Gao, J. Design of wind-break walls and their application in Hulunbeier sandy land control. J. Environ. Eng. Technol. 2021, 11, 970–975. [Google Scholar]
- Erci, V.; Seker, C.; Basaran, M.; Erpul, G. Determining the effectiveness of some soil stabilizers in wind erosion prevention using wind tunnel experiments. Land Degrad. Dev. 2021, 32, 2962–2977. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Ivanov, V.; Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Bio/Technol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Van Paassen, L.A.; Daza, C.M.; Staal, M.; Sorokin, D.Y.; Der, Z.W.V.; Van Loosdrecht, M.C. Potential soil reinforcement by biological denitrification. Ecol. Eng. 2010, 36, 168–175. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K.; Santamarina, C. Factors affecting efficiency of microbially induced calcite precipitation. J. Geotech. Geoenviron. 2012, 138, 992–1001. [Google Scholar] [CrossRef]
- Cheng, L.; Cord Ruwisch, R. In situ soil cementation with ureolytic bacteria by surface percolation. Ecol. Eng. 2012, 42, 64–72. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, C.; Tang, C.S.; Xie, Y.H.; Yin, L.Y.; Cheng, Q.; Shi, B. Bio-remediation of desiccation cracking in clayey soils through microbially induced calcite precipitation (MICP). Eng. Geol. 2020, 264, 105389. [Google Scholar] [CrossRef]
- Liu, H.; Chu, J.; Kavazanjian, E. Biogeotechnics: A new frontier in geotechnical engineering for sustainability. Biogeotechnics 2023, 1, 100001. [Google Scholar] [CrossRef]
- Meyer, F.D.; Bang, S.; Min, S.; Stetler, L.D.; Bang, S.S. Microbiologically-induced soil stabilization: Application of Sporosarcina pasteurii for fugitive dust control. In Geo-Frontiers 2011: Advances in Geotechnical Engineering; Han, J., Alzamora, D.E., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2011; pp. 4002–4011. [Google Scholar]
- Hamdan, N.; Kavazanjian, E.J. Enzyme-induced carbonate mineral precipitation for fugitive dust control. Géotechnique 2016, 66, 546–555. [Google Scholar] [CrossRef]
- Maleki, M.; Ebrahimi, S.; Asadzadeh, F.; Tabrizi, M.E. Performance of microbial-induced carbonate precipitation on wind erosion control of sandy soil. Int. J. Environ. Sci. Technol. 2016, 13, 937–944. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wang, H.; Yuan, J.; Fan, G. Enhanced rainfall erosion durability of enzymatically induced carbonate precipitation for dust control. Sci. Total Environ. 2021, 791, 148369. [Google Scholar] [CrossRef]
- Meng, H.; Gao, Y.; He, J.; Qi, Y.; Hang, L. Microbially induced carbonate precipitation for wind erosion control of desert soil: Field-scale tests. Geoderma 2021, 383, 114723. [Google Scholar] [CrossRef]
- Blakely, R.L.; Zerner, B. Jack Bean Urease: The First Nickel Enzyme. J. Mol. Catal. 1984, 23, 263–292. [Google Scholar] [CrossRef]
- Hamdan, N.; Kavazanjian, E. Carbonate Cementation via Plant Derived Urease. In Proceedings of the 18th International Conference on Soil Mechanics and Geotechnical Engineering, Paris, France, 2–6 September 2013; pp. 2489–2492. [Google Scholar]
- Dominique, J.T.; Mark, O.C.; Vernon, R.P. Transport of Sporosarcina pasteurii in sandstone and its significance for subsurface engineering technologies. Appl. Geochem. 2014, 42, 38–44. [Google Scholar]
- Almajed, A.; Tirkolaei, H.K.; Kavazanjian, E. Baseline investigation on enzyme induced calcium carbonate precipitation. J. Geotech. Geoenviron. Eng. 2018, 144, 04018081. [Google Scholar] [CrossRef]
- Refaei, M.; Arab, M.G.; Omar, M.; Omar, M. Sandy soil improvement through biopolymer-assisted EICP. In Geo-Congress 2020: Geotechnical Earthquake Engineering and Special Topics; Arduino, P., Boulanger, R.W., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2020; pp. 625–634. [Google Scholar]
- Wu, M.; Gao, Y.; He, J. Laboratory study on use of soybean urease-induced calcium carbonate precipitation with xanthan gum for stabilization of desert sand against wind erosion. Chin. J. Geotech. Eng. 2020, 42, 1914–1921. [Google Scholar]
- Wang, X.; Tao, J. Polymer-modified microbially induced carbonate precipitation for one-shot targeted and localized soil improvement. Acta Geotech. 2018, 14, 657–671. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, K.; Zhang, C.; Zhao, Z. Mechanical properties of Na-montmorillonite-modified EICP-treated silty sand. Environ. Sci. Pollut. Res. 2022, 29, 10332–10344. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wang, H.; Yuan, J.; Fan, G.; Xia, J. Suppression of dust pollution by double-network material based on enzymatic calcification. Constr. Build. Mater. 2021, 312, 125432. [Google Scholar] [CrossRef]
- Yao, D.; Wu, J.; Wang, G.; Wang, P.; Zheng, J.; Yan, J.; Yan, Y. Effect of wool fiber addition on the reinforcement of loose sands by microbially induced carbonate precipitation (MICP): Mechanical property and underlying mechanism. Acta Geotech. 2021, 16, 1401–1416. [Google Scholar] [CrossRef]
- Miyake, M.; Kim, D.; Hata, T. Casein-assisted enhancement of the compressive strength of biocemented sand. Sci. Rep. 2022, 12, 12754. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Peng, J.; Li, L. Accelerated reinforcement of calcareous sand via biomineralization with aluminum ion flocculant. Appl. Biochem. Biotechnol. 2023, 195, 7197–7213. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.J.; Peng, J.; He, J.; Li, L.L.; Jiang, Z.; Tang, J.H. Effects of Adding Aluminum Ion Flocculant on MICP Reinforcement of Sand. Acta Geotech. 2024, 19, 3505–3517. [Google Scholar] [CrossRef]
- ASTM D2487–06; Standard Classification of Soils for Engineering Purposes. ASTM: West Conshohocken, PA, USA, 2006.
- Liu, L.; Chen, Y.; Gao, Y.; Liu, B.i.; Zhou, Y.; Li, C. Effect of urease enrichment degree of multiple sources of urease on bio-cementation efficacy via enzyme-induced carbonate precipitation. Can. Geotec. J. 2023, 60, 1923–1937. [Google Scholar] [CrossRef]
- Yan, B.; Zhou, Y.; Li, C.; Shu, S.; Gao, Y. Modified SICP method to mitigate the effect of bio-clogging by excess protein from soybean crude urease extracts for biocementation process. Acta Geotech. 2023, 18, 5047–5062. [Google Scholar] [CrossRef]
- Whiffin, V.S.; Van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- ISO 6058:1984; ISO Water Quality: Determination of Calcium Content: EDTA Titrimetric Method. ISO: Geneva, Switzerland, 1984.
- He, J.; Gao, Y.; Gu, Z.; Chu, J.; Wang, L. Characterization of crude bacterial urease for CaCO3 precipitation and cementation of silty sand. J. Mater. Civ. Eng. 2020, 32, 04020071. [Google Scholar] [CrossRef]
- Meng, H.; Shu, S.; Gao, Y.; Yan, B.; He, J. Multiple-phase enzyme-induced carbonate precipitation (EICP) method for soil improvement. Eng. Geol. 2021, 294, 106374. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Y.; Geng, W.; Song, J.; Zhou, Y.; Li, C. Comparison of jack bean and soybean crude ureases on surface stabilization of desert sand via enzyme-induced carbonate precipitation. Geoderma 2023, 435, 116504. [Google Scholar] [CrossRef]
- China Meteorological Data Service Centre. Available online: http://data.cma.cn/dataService/cdcindex/datacode/A.0012.0001/show_value/normal.html (accessed on 17 February 2022).
- Cheng, X. Experimental Research on Sand Incipience Law in Wind-Blown-Sand Two Phase Flow; Tsinghua University: Beijing, China, 2003. [Google Scholar]
- Gao, Y.; Meng, H.; He, J.; Qi, Y.; Hang, L. Field trial on use of soybean crude extract for carbonate precipitation and wind erosion control of sandy soil. J. Cent. South. Univ. 2020, 27, 3320–3333. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Mujah, D. Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation. Can. Geotech. J. 2013, 50, 81–90. [Google Scholar] [CrossRef]
- Simatupang, M.; Okamura, M. Liquefaction resistance of sand remediated with carbonate precipitation at different degrees of saturation during curing. Soils Found. 2017, 57, 619–631. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S. Enzyme induced calcium carbonate precipitation and its engineering application: A systematic review and meta-analysis. Constr. Build. Mater. 2021, 308, 125000. [Google Scholar] [CrossRef]
- Cui, M.J.; Zheng, J.J.; Zhang, R.J.; Lai, H.J.; Zhang, J. Influence of cementation level on the strength behaviour of bio-cemented sand. Acta Geotech. 2017, 12, 971–986. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; He, J.; Zhou, Y.; Geng, W. An experimental investigation of wind-erosion resistance of desert sand cemented by soybean-urease induced carbonate precipitation. Geoderma 2023, 429, 116231. [Google Scholar] [CrossRef]
- Thiloththama, H.K.; Nawarathna, K.N.; Masahiro, F.; Momoko, T.; Satoru, K. Effects of cationic polypeptide on CaCO3 crystallization and sand solidification by microbial-induced carbonate precipitation. ACS Sustain. Chem. Eng. 2018, 6, 10315–10322. [Google Scholar]
- Jada, A.; Ait Akbour, R.; Douch, J. Surface charge and adsorption from water onto quartz sand of humic acid. Chemosphere 2006, 64, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Shahin, M.A.; Mujah, D. Influence of key environmental conditions on microbially induced cementation for soil stabilization. J. Geotech. Geoenviron. 2017, 143, 04016083. [Google Scholar] [CrossRef]
- Hoang, T.; Alleman, J.; Cetin, B.; Choi, S.G. Engineering Properties of Biocementation coarse- and fine-grained sand catalyzed by bacterial cells and bacterial enzyme. J. Mater. Civ. Eng. 2020, 32, 04020030. [Google Scholar] [CrossRef]
- Wu, M.; Hu, X.; Zhang, Q. Preparation and performance evaluation of environment-friendly biological dust suppressant. J. Clean. Prod. 2020, 273, 123162. [Google Scholar] [CrossRef]
- Munyaneza, V.; Zhang, W.; Haider, S.; Xu, F.; Wang, C.; Ding, G. Strategies for Alleviating Aluminum Toxicity in Soils and Plants. Plant Soil 2024, 504, 167–190. [Google Scholar] [CrossRef]
- Huang, C.F.; Yamaji, N.; Mitani, N. A Bacterial-Type ABC Transporter Is Involved in Aluminum Tolerance in Rice. Plant Cell 2009, 21, 655–667. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).