Abstract
The aim of this scoping review is to investigate the potential development of an alternative material derived from renewable biological resources such as goldenberry calyx and modified cassava starch as the matrix. Moreover, this paper reviews the impact of combining starch and lignocellulosic fiber on improving the properties of bioplastic materials. The goldenberry calyx is a type of lignocellulosic waste with a low moisture content, which offers logistical advantages, as a high moisture content can accelerate waste deterioration. However, studies on the utilization of goldenberry calyx are scarce. In addition, due to its low cost and availability, starch is the main polysaccharide for biofilm development as a matrix. Combining these two materials can result in a composite material with suitable and adequate properties for packaging applications, although no studies have been published on this specific combination. Starch and lignocellulosic fiber are complementary as the properties of starch biopolymers improve when a hydrophobic material (lignocellulosic fibers) is incorporated. Moreover, starch strengthens fibers by enhancing their biodegradability through its water absorption capacity. In this study, modified cassava starch, with its higher amylose content, is suggested for use, as the proportion of amylose correlates with enhanced bioplastic properties.
1. Introduction
There is evidence of a significant increase in organic waste, leading to CO2 emissions that contribute to the greenhouse effect. The widespread use of plastic derived from fossil fuels for packaging represents a great challenge. However, numerous studies have used organic waste to design biodegradable packaging [1], for instance, Henao-Díaz et al. [2] focused on creating a bioplastic from fruit peel. In this context, considering goldenberry calyx (Physalis peruviana) as a resource for packaging is relevant. Sarango Ortega et al. [3] suggest that this type of waste can be used as a raw material to replace packaging made from conventional plastic.
This review considers it important to clarify the definitions of the terms “biopolymer,” “bioplastic,” “biocomposite,” and “biomaterial.” These concepts are often used interchangeably, although they are closely interrelated. Biopolymers are polymers composed of biomolecules, and bioplastics are manufactured from bio-based polymers [4]. Biocomposites are composite materials that combine natural fibers with biodegradable matrices [5], for example, a material consisting of a cassava starch matrix reinforced with lignocellulosic fiber. Finally, Quiceno et al. [6] acknowledge that biomaterials are biodegradable entities derived from organic sources—such as certain agro-industrial residues—and that they should be utilized in the packaging and plastics markets.
Based on the above, a literature review is conducted on the properties of the goldenberry calyx, including its hydrophobic, antioxidant, and antimicrobial characteristics, to reaffirm its potential as a reinforcement material for cassava starch. Studies on the goldenberry calyx are scarce [7], and, to date, no research has directly addressed the use of Physalis peruviana calyx as a reinforcement material for cassava starch-based biopolymers. In this context, this review aims to explore materials that may complement the properties of goldenberry calyx, such as cassava starch and plasticizers like glycerol. According to Vieira et al. [8], the association of starch and fiber enhances the mechanical, antimicrobial, and morphological properties, improving the structural stability of the biocomposite.
Currently, only a limited number of research articles have explored the use of goldenberry calyx to make biofilms. First, a biocomposite based on calyx and corn starch was created, supporting the potential of goldenberry calyx in producing cellulosic materials suitable for packaging in the food industry [3]. Second, the goldenberry calyx was used to produce a biopolymer to cover a metal upper limb prosthesis [9]. Finally, the calyx was used to produce nanocomposites for food coating [10] and the biomedical and pharmaceutical industries [11]. These applications are made possible by the antibacterial properties of the calyx extract from Physalis peruviana [12]. According to Nocetti et al. [13], the extract of goldenberry calyx contains bioactive compounds such as withanolides, flavonoids, phenols, physaperuvin, saponins, and peruvioses. These bioactive compounds offer significant benefits, including antioxidant, antiproliferative, and antibacterial properties. The phenolic compounds in goldenberry calyx are responsible for its antioxidant activity.
Nanoparticles derived from goldenberry calyx exhibit enhanced antioxidant and antimicrobial properties, making them effective functional agents in biopolymers for active packaging. When used as reinforcement in cassava starch matrices, they improve material performance and expand their potential for food preservation [7]. The encapsulation of bioactive compounds through nanotechnology positions this agricultural by-product as a strategic alternative for the development of sustainable and smart packaging solutions. Moreover, the preservation of antimicrobial properties is an ideal quality in food packaging.
Recent interest in combining starch and fiber has increased significantly, and fibers have been extensively studied as reinforcement agents for their industrial potential [14]. Vásquez Velásquez et al. [15] suggest that the mechanical properties of starch biopolymers improve with the addition of hydrophobic materials, with lignocellulosic waste fiber being a suitable example. Starch not only enhances fiber interaction but also promotes the bonds between particles. Various researchers have suggested combining starch with additional compounds such as waste fibers to improve the performance of biopolymers [16]. Thus, starch from a tuber source such as cassava is considered a recommended option for biopackaging due to its favorable properties. Amaraweera et al. [17] recognize that biofilms made from tuber starch show lower density compared to those derived from cereals, and a low density reduces transportation and raw material costs.
It is important to clarify that this document is a scoping review and does not include experimental procedures for the development of biopolymers. The main objective of this review is to synthesize existing findings to reaffirm the potential of goldenberry calyx as a reinforcement material for cassava starch, particularly in food packaging applications. First, the goldenberry calyx is characterized, followed by a discussion on the relevance of its use. Subsequent sections highlight examples of goldenberry calyx use in the production of biomaterials and the need to reinforce starch with lignocellulosic fiber. Cassava starch was selected in response to the suggestion made in [17]. Accordingly, the next section presents a literature review focused on the use of cassava starch in the production of bioplastics and the benefits of employing modified cassava starch. The final sections discuss the role of plasticizers in cassava starch-based films and present the conclusions.
2. Methodology
This review begins with an initial phase, which recognizes the goldenberry calyx as a valuable resource to produce eco-friendly materials. As observed, research on the use of the goldenberry calyx for biomaterial production is highly limited. Therefore, a basic literature search was conducted using the keywords “physalis peruviana,” “calyx,” “biofilm,” and “cellulose.” From this preliminary phase, findings were identified regarding the characterization and relevance of using the goldenberry calyx, as well as a few pertinent applications aligned with this approach. Applications involving the use of the calyx in biopolymers and nanomaterials were found. In connection with its application in biopolymer fabrication, the importance of lignocellulosic fibers and their potential as starch reinforcement were highlighted. Consequently, additional articles related to the combination of lignocellulosic fiber with starch were reviewed (see Figure 1).
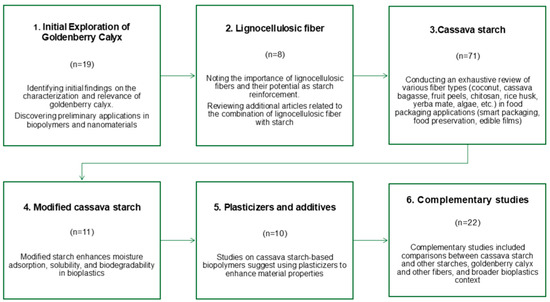
Figure 1.
Phases of review processes (number of articles by phase).
Subsequently, the review focused on starch. Cassava starch was selected based on recommendations favoring the use of tuber-derived starch due to its favorable properties. The search related to starch involved a more detailed process to filter the information, given the abundance of studies on starch-based biofilm production. This search required the inclusion of selection criteria. Accordingly, the method employed for article selection was based on the PRISMA-ScR framework (see Figure 2). This is a scoping review that involves mapping and organizing the available literature, and according to Fernández-Lambert et al. [18], this framework provides guidance for conducting scoping reviews.
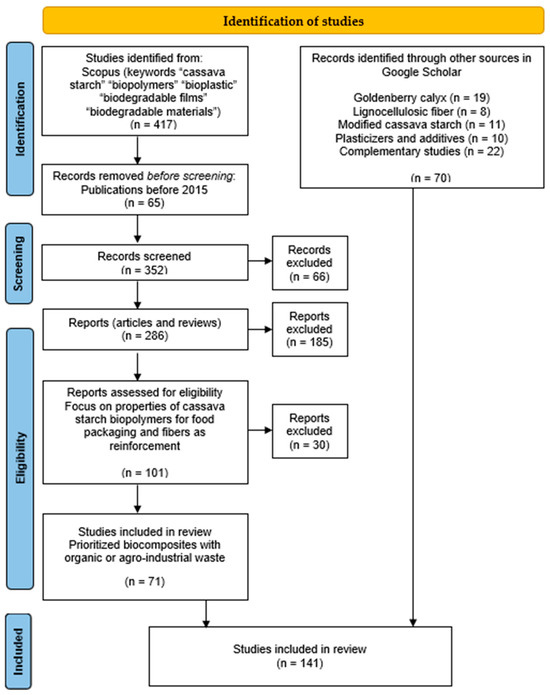
Figure 2.
PRISMA-ScR for the process of searching and filtering reports.
As selection criteria, only records from the year 2015 onward were considered. Additionally, only research and review articles were included, with a particular emphasis on cassava starch-based biopolymers reinforced with fibers, prioritizing fibers derived from agro-industrial residues. An exhaustive review was conducted on various types of fibers, including coconut, cassava bagasse, fruit peels, chitosan, rice husk, yerba mate, algae, and others, with applications in the food packaging industry—such as smart packaging, food preservation, and edible films. The review also focused on the evaluation of biopolymer properties, including mechanical, physicochemical, antioxidant, and antimicrobial characteristics.
For the literature review on cassava starch usage, the process began with the selection of the keywords “cassava starch,” “biopolymer,” “bioplastic,” and “biodegradable films” to conduct searches in databases. This preliminary search was refined to include only documents categorized as articles, reviews, and conference papers. Once the selection criteria were established, the next step was to implement a strategy for organizing the information. VOSviewer® software (version 1.6.18) was used for keyword analysis. The processed information was presented in narrative format, as well as in tables and figures.
Based on the search, studies related to cassava starch-based biopolymers showed two significant peaks in 2022 and 2024, with new research continuing to emerge in 2025. Moreover, the countries with the highest number of publications on this topic are Brazil, Thailand, and Colombia, indicating a strong connection to the potential use of agro-industrial residues from tropical regions for reinforcing cassava starch biopolymers.
As shown in Figure 3, the most impactful keywords include, for example, “food packaging,” “mechanical properties,” “antioxidants,” “essential oils,” “cellulose,” “reinforcement,” “biodegradation,” “films,” “fillers,” and others. Based on these findings, these aspects are explored in greater detail in Section 3.5. Additionally, results from the cassava starch biopolymer literature suggest a preference for using modified starch and incorporating supplementary reinforcements such as plasticizers and essential oils. Therefore, the final sections of this review address these recommendations.
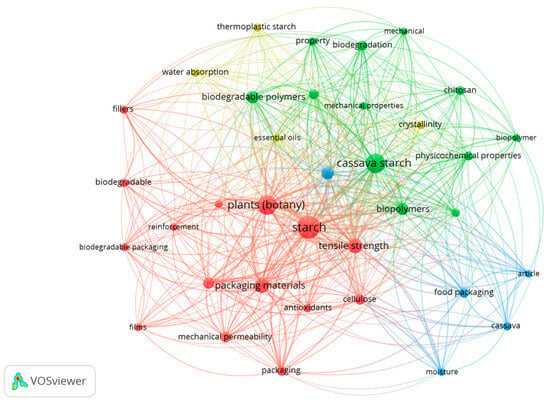
Figure 3.
Keywords used in studies related to cassava starch-based biopolymers: generated by VOSviewer®.
3. Results
3.1. Characterization of Goldenberry Calyx
The goldenberry (Physalis peruviana) belongs to the Solanaceae family, prevalent in tropical America [13], primarily Colombia, Perú, and Ecuador [19]. It is worth noting that the goldenberry was introduced to South Africa by the Spanish and has since spread to various tropical and subtropical regions [20].
This berry has a diameter of 3 cm, and its epicarp is orange, smooth, and waxy. The mesocarp and endocarp make up the yellow “pulp,” which provides an acidic and aromatic juice [21]. The berries contain juicy pulp with 100–300 very small yellowish seeds and are encased in an inflated papery calyx, which accounts for about 5% of the fresh fruit’s weight [20]. However, Physalis peruviana calyx has been studied for its ability to modulate glucose metabolism in in vitro and in vivo settings [20].
Despite the increasing commercial value of this fruit, goldenberries are often sold without the calyx due to consumer preferences, leading to significant waste generation. In this context, utilizing the goldenberry calyx for biocomposite design becomes especially relevant. The calyx, approximately 5 cm in length, possesses a round or elongated morphology [10]. Its function is to protect the goldenberry from external factors such as sunlight, cold, pests, mechanical damage, among others, facilitated by its chemical composition. According to Dwivedi et al. [11], the goldenberry calyx contains bioactive substances like flavonols, phenolics, hydroxycinnamic acid, and quercetin, all of which are known for their medical potential.
It is important to highlight that the goldenberry calyx is generally regarded as a waste product, and its production is abundant. In this context, the valorization of the goldenberry calyx represents a relevant strategy for mitigating the environmental impact associated with organic waste. Colombia is the world’s leading producer of goldenberry. However, in this country, 40% of the harvested fruit is processed into dried berries, jams, syrups, or fruit pulps, while the calyx—representing between 5% and 10% of the fruit’s weight—is discarded. Goldenberry calyx can reach approximately 33 metric tons per hectare of cultivated crop. Moreover, in Colombia, around 18,414 metric tons of calyces were generated in a single year. The goldenberry calyx is considered a problematic residue due to its low bulk density as it tends to occupy a large volume [22]. Moreover, calyx is one of the postharvest by-products and represents an expansive amount of waste, since 1 to 2 tons of biomass is generated per ha of goldenberry crop [23]. Different research works have described the goldenberry calyx as a source of bioactive compounds [23,24,25].
There are some characteristics for calyx which include the use of extracts in some applications and products. Firstly, three phenolic acids and four flavonoids are present in the calyx extracts, demonstrating high antioxidant activity through physiologically relevant cell-based assays, the ability to inhibit advanced glycation end product (AGE) formation and nitric oxide production, and antiproliferative properties [20]. Secondly, the calyx represents an essential source of carbohydrates during the first 20 days of fruit growth and development, and it is a residue rich in bioactive compounds, such as flavonols and hydroxycinnamic acid derivatives [26].
Thirdly, some results show that calyces of Physalis peruviana are rich in carbohydrates (approximately 42.0%). Carbohydrates are the most abundant nutrient in several fruit peels. Additionally, calyces contain fiber (close to 32.0%), fat (2.1%), and protein (6.1%). The protein content of Physalis peruviana calyces is higher compared with the protein content (1.02%) found in physalis fruit juice [27]. Moreover, the ash and moisture contents of calyces are 9.7% and 7.2%, respectively. Additionally, they contain minerals such as calcium (7.50 mg/100 g calyces), iron (1.28 mg/100 g calyces), copper (<0.03 mg/100 g calyces), and zinc (<0.02 mg/100 g calyces) [27]. The goldenberry calyx is a natural fiber and a viable material for alternative products like paper and biopolymer due to its composition, including hemicellulose (40 to 49%), cellulose (26 to 29%), lignin (22–26%), and humidity (27.1%) [28]. In another study, the cellulose content in goldenberry calyx was found to be 29.11%, with the contents of ashes (6.87% to 8.83%) and proteins (6.54% to 10.33%) being lower [29].
Finally, the anti-proliferative bioactivity against HT-29 colon cancer cells of a withanolides-rich extract from a goldenberry (Physalis peruviana L.) calyx was investigated by Foodomics [23], and it was found that the calyx that envelops the goldenberry fruit has a cupuliform structure, similar to a Chinese lantern, formed by sepals or modified leaves [13].
3.2. Relevance of Using Goldenberry Calyx
Goldenberry calyx is a wood compound with a significant proportion of lignin. To assess the relevance of producing biocomposites with the goldenberry calyx, it is essential to evaluate materials with properties similar to goldenberry that are commonly employed in papermaking. González Velandia et al. [28] evaluated the physical and chemical properties of various organic solid wastes intended for paper production. Recognizing cellulose as a crucial element in papermaking, the exploration of alternative materials aimed to contribute to forest conservation. The analysis considered properties such as humidity, solubility in hot and cold water, density, volume, and the contents of holocellulose, cellulose, and lignin for the following residues: goldenberry calyx; the stems of rose, carnation, and sunflower; radish waste; pineapple crown and shell; corn cob leaf and corn bagasse; chrysanthemum and rose petals; orange; passion fruit; lulo; mango; tree tomato; lemon peels; long onion leaf; cassava skin; banana bagasse; and dry grass.
Physical characterization revealed a challenge potentially hindering the use of these materials: a high moisture content, a factor that facilitates the rapid deterioration of plant residues. However, goldenberry calyx is one of the materials with lower moisture (27.1%) compared to other materials, most of which exceed 50%. The calyx also stood out for its 22–26% lignin content in this evaluation. It is noteworthy that lignin, a natural substance in the cell wall, contributes to hardness and resistance. However, in paper production, efforts are made to eliminate it to enhance fiber adhesion. In addition to the goldenberry calyx, other materials noted for their lignin content include cassava peel, pineapple peel, mango peel, passion fruit peel, and corn stem, among others [28]. Several of these high-lignin materials have been used as raw materials [2] and reinforcing fibers to produce starch-based bioplastics, such as pineapple peel [30]. Similarly, pineapple peel was highlighted due to its low density (0.7–0.71 g/cm3), as was the goldenberry calyx (0.59–0.68 g/cm3), which exhibits an even lower density than pineapple peel [28]. Low density is a desirable property in the packaging industry as it helps reduce transportation and raw material costs.
Within the framework of the bioeconomy, lignin stands out due to its aromatic ring structure. This structure is essential for forming strong bonds during lignin polymerization, which confer resistance and stability. However, lignin remains undervalued despite its abundance and potential, and a large portion of it is incinerated for energy production [31]. It is estimated that the pulp and paper industry generates approximately 70 million metric tons of lignin annually. Nonetheless, only 2% of this lignin is processed, while the remainder is used as fuel [32].
Lignin should be valorized because of its multiple desirable properties, including high thermal stability, antioxidant capacity, hydrophobicity, biodegradability, and both antimicrobial and antioxidant activities. Based on these characteristics, lignin-based polymers are ideally suited to produce packaging materials [31]. Moreover, the incorporation of lignin as a reinforcing agent in biopolymers helps reduce both cost and water absorption. Its antioxidant properties arise from phenolic hydroxyl groups capable of scavenging free radicals. Additionally, the thermal stability and water resistance of the resulting composites can also be enhanced with lignin addition [32].
Lignin valorization makes a substantial contribution to the circular economy by reducing waste generation and dependence on non-renewable resources—potentially lowering greenhouse gas emissions by 30–40%. This valorization also yields economic benefits, as lignin-based bioplastics can reduce production costs by up to 20% compared to conventional plastics. Furthermore, the development of lignin-derived products opens new markets and revenue streams [31]. An additional economic advantage lies in the exploration of alternatives beyond cellulose modification, which is a highly costly process [32].
3.3. Use of Goldenberry Calyx in Biocomposites
Fewer studies have investigated the use of goldenberry calyx in making biodegradable products due to goldenberry calyx features shown in Figure 4. However, its use is relevant to produce food packaging due to its beneficial properties. According to Castellanos et al. [22], the cuticular wax layer extracted from the goldenberry calyx provides mechanical strength and viscoelastic properties, and it also helps preserve fruits by forming a hydrophobic interface that regulates excessive transpiration and gas exchange. In addition, goldenberry calyx extracts contain three phenolic acids and four flavonoids, which result in high antioxidant, antibacterial, and antifungal activities [20].
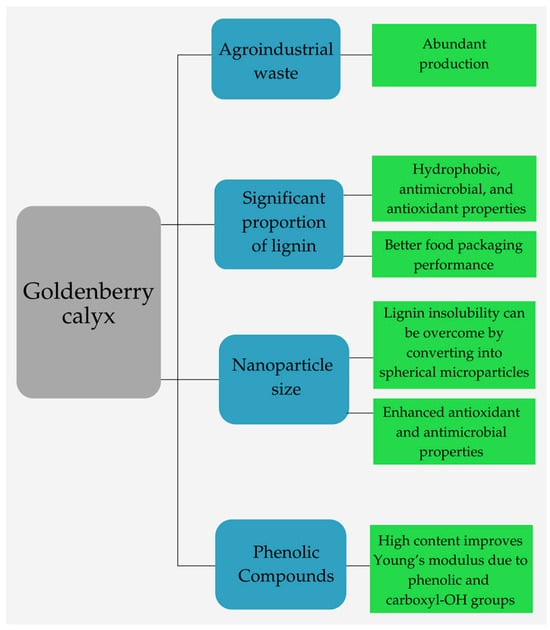
Figure 4.
Features of goldenberry calyx.
A few academic studies have explored the potential of goldenberry calyx for paper and bioplastics. For example, in Ecuador, a study explored the potential of goldenberry calyx to produce bioplastic by mixing it with corn starch and glycerin. Based on the tests performed on the biopolymer, it was concluded that transparency is a property associated with its potential applications, such as coatings and packaging. Regarding resistance, a solubility study was conducted using various solvents, including hexane, ethyl acetate, ethanol, water, and hydrochloric acid. In all cases, the biopolymer retained its initial shape, although it experienced mass loss. This property is also relevant when defining the biopolymer’s intended applications [3].
In Colombia, goldenberry calyx was used to produce a biopolymer to cover a metal upper limb prosthesis. Bioplastics used in prostheses must have esthetic characteristics that imitate the skin of the person who will use the prosthesis (as if it were a glove), such as softness, resistance, and elasticity. This research contributes to reducing plastic use in the health sector. It is worth mentioning that the flexibility of the biopolymer depends on the water content [9].
The final category focuses on the production of nanocomposites; a study explored the biofabrication of CeO2-TiO2 nanocomposites synthesized with phytochemicals derived from Physalis peruviana calyx outer leaves, revealing promising antibacterial and antioxidant properties. These nanocomposites were synthesized through an aqueous extract of the calyx under ultrasonic irradiation. Ultrasound-assisted synthesis offers a fast, energy-efficient, and scalable approach for nanocomposite fabrication, ensuring excellent biocompatibility, uniformity, and stability. The biosynthesized nanocomposites showed clear crystalline structures, a spherical shape, and an average size of 11 nm [11].
The phytochemicals present in the calyx, including polyphenols, flavonoids, and other active biomolecules, contributed to the reduction of metal precursors into oxides and stabilized the nanoparticles by coating them to prevent agglomeration. Additionally, nanoparticle synthesis is advantageous as it enhances antibacterial and antioxidant properties. The role of goldenberry calyx phytochemicals as a bio-template is also noteworthy. Utilizing the calyx provides an alternative to the use of hazardous chemicals in nanoparticle synthesis and contributes to waste management through the valorization of agricultural by-products [11]. These antibacterial properties are an attractive feature for the development of bio-based packaging as they help to better preserve food.
In the context of nanocomposite research, another study investigated the use of edible pectin coatings reinforced with nanocellulose—extracted from the Physalis peruviana calyx—to assess their impact on the physicochemical and physiological quality of goldenberries during refrigeration storage. Edible coatings are essential for preserving fruit quality as they contribute to extending shelf life. Given that the market demands goldenberries without their calyx, its removal accelerates the ripening and senescence processes. Under storage conditions of 12 °C and 80% relative humidity, goldenberries with their calyx remain fresh for up to 24 days, whereas those without calyx retain their post-harvest quality for only 11 days. Edible coatings help prolong shelf life by providing a protective layer made from renewable polymers, which offer desirable properties such as biodegradability and effective barriers against oxygen and moisture [10].
Nanocellulose was extracted from Physalis calyces, and it is recognized that nanocellulose offers superior crystallinity, specific surface area, mechanical strength, surface chemical reactivity, thermal stability, and lower density compared to cellulose. Given these advantages, formulations based on cellulose should be prioritized when valorizing goldenberry calyces. The use of food by-products for packaging development can yield economic benefits for the packaging industry while reducing the environmental impact associated with poor waste management. The application of pectin and nanocellulose-based polymers may delay fruit ripening processes, a property attributed to hydrogen bond formation and the high crystalline content of nanocellulose [10].
These documents lay the foundations for developing a new bioplastic from goldenberry calyx due to the hydrophobic nature of lignin in calyx fibers. Sarango Ortega et al. [3] introduced glycerin as a plasticizing agent with corn starch, and Fauzi et al. [33] suggested that materials with a high glycerol content overcome biofilm fragility by altering hydrogen bonds and increasing intermolecular distances. Finally, the goldenberry calyx contains significant amounts of phenolic compounds [34]. Additionally, lignins with higher concentrations of phenolic and carboxyl -OH groups enhance Young’s modulus (an indicator of a material’s resistance to deformation under longitudinal tension or compression) by promoting stronger hydrogen bond formation between lignin and starch [35].
3.4. Lignocellulosic Material as Reinforcement for Starch-Based Biopolymers
As observed in the previous section, it is possible to produce packages using goldenberry and starch. Subsequent discussions will explore several studies utilizing starch as the primary component in bioplastics. Due to its low cost, wide availability, biodegradability, and low toxicity, starch is the polysaccharide most used as raw material in the production of biofilms. However, prototypes made from starch are known to be water-sensitive [36]. Similarly, starch-based biofilms are still preferred for food packaging because they are homogeneous, odorless, tasteless, and colorless [37]. Numerous studies have recommended combining starch with other compounds such as fibers to enhance its functional properties [16].
Materials with hydrophobic character can improve the mechanical properties of starch biopolymers. A clear example of this type of material is fiber from lignocellulosic waste [15]. This becomes essential as starch bioplastics are known for their weakness and water sensitivity [38]. The main interactions influencing the bonds of lignin include hydrophobic and electrostatic interactions and hydrogen bonding, all of which are influenced by the total content of phenolic –OH and –COOH groups in its chemical structure. Lignin particles form strong hydrogen bonds and physical interactions with starch polymer chains. Many studies have demonstrated that starch and lignin are polymers that can be combined to create a reinforced bioplastic, with the mechanical and barrier properties varying depending on the content of synthetic additives [35].
Due to their biodegradability, affordability, low density, and safety, lignocellulosic fibers are widely researched; their chemical makeup aligns with starch, supporting strong matrix–filler adhesion. In this context the authors base their work on scientific evidence supporting the combination of starch and lignocellulosic material to obtain bioplastics [39]. Adding lignocellulosic fibers enhances many properties, such as tensile strength, elasticity, hardness, and viscoelasticity, in bioplastics. Additionally, starch contributes to the polymer’s properties because it increases its biodegradability due to its water absorption capacity [40]. The water sensitivity of starch enhances the biodegradability of packaging in proportion to its starch content. In other words, the higher the starch content, the faster the biodegradation rate [35].
Lignin serves as an adhesive within the cell wall of wood, binding cellulose and hemicellulose together. This is why it is primarily used as a binder in the chemical industry. The hemicellulose/lignin matrix contributes flexibility, controls the moisture content, and provides protection against pathogens. Moreover, due to its thermoplastic properties (typically ranging from 90 to 170 °C), aromaticity, highly cross-linked structure, and functional groups, lignin is highly reactive. It can interact with various polymers, altering their wettability and enhancing properties such as mechanical strength and antioxidant activity, among others [35].
There was a growing interest in producing bioplastics using a combination of starch and lignocellulosic fiber. This is attributed to the challenges associated with the widespread production of conventional plastics and the issue of organic waste generation [14]. These same authors advocate for further studies that integrate lignocellulosic agricultural residues and starch to develop biopolymers for industrial use as their mechanical properties are comparable to those of synthetic fiber-reinforced materials. Additionally, such combinations involve two economically viable and environmentally friendly sources. The characteristics of biodegradable polymer matrices, such as starch, can be enhanced by incorporating natural fibers, thereby increasing their large-scale applicability. Among future research directions, improving the thermal stability of biopolymers is another key area as this property is known to increase with the addition of reinforcing fibers [41]. Researchers join this call and emphasize the need to explore diverse sources of reinforcement in bioplastic production in the future. Furthermore, numerous articles supported the importance of reinforcing starch in biopolymers with lignocellulosic materials. Sierra Montes et al. [39] and Bergel et al. [42] recommend adding hydrophobic materials to starch-based biofilms to impede water contact with starch, thereby making the material more traction-resistant.
Several materials have been used as reinforcements for starch bioplastics (Table 1). Topinambur, for instance, has been utilized to strengthen a starch-based biofilm, acknowledging the inherent affinity of starch bioplastics for water, which can limit their functionality. However, this issue can be addressed by adding hydrophobic materials, such as topinambur, which increases the tensile strength and elastic modulus [39]. Additionally, rice residues have been applied for reinforcement, and Machado et al. [43] highlight that enhanced broken rice improves properties such as tensile strain and maximum bending stress. Furthermore, incorporating rice husk improves mechanical properties while reducing the water absorption capacity [44].
Moreover, pineapple peel can enhance tensile strength due to an interfacial interaction between the fiber and the starch matrix. However, it is important to carefully control the amount of fiber as high proportions can interfere with the expandability, induce discontinuity, and reduce the breaking strength in the starch matrix [30]. Cellulose nanofibrils derived from a chontaduro agro-industrial residue can also enhance the tensile strength. According to a spectroscopic finding, there is a possible formation of cross-linking between starch and cellulose nanofibrils. This cross-linking can positively influence the strength of starch biofilms [45].
Versino and García [46] studied the influence of the bagasse particle size derived from cassava roots on the properties of cassava starch biocomposites. The authors demonstrated that the impact on the properties of different materials depends on both size and composition. Consequently, it is crucial to evaluate the particle size and distribution of materials intended to reinforce starch-based biofilms. Moreover, Donati et al. [47] combined biofilms based on cassava starch with rice husk ash, resulting in significant improvements to the physical and morphological properties of the biopolymer. For instance, the addition of 20 to 40% ash led to an increase in tensile strength.
In some cases, chemical modifications of lignocellulosic fibers are necessary to improve their interaction with polymer matrices. For example, mercerization involves treating lignocellulosic fibers with an alkaline solution to reduce the contents of lignin, pectin, and other substances present in the fibers. This modification reduces the hydrophilicity of the fibers and/or enhances their adhesion with the polymer matrix, ultimately improving the mechanical properties of the composites [1].

Table 1.
Research focused on obtaining bioplastics from starch and reinforcement materials.
Table 1.
Research focused on obtaining bioplastics from starch and reinforcement materials.
| Research | Year | Source |
|---|---|---|
| The influence of chitosan coating on thermoplastic foams made from potato, cassava, and corn starches was studied. | 2017 | [42] |
| Nanocomposites based on cassava starch reinforced with cellulose nanofibers were developed and analyzed. | 2017 | [48] |
| The incorporation of broken rice flour was studied for its effect on cassava starch-based foams. | 2018 | [43] |
| Cassava starch and bagasse biocomposites were examined considering variations in particle size distribution. | 2019 | [46] |
| Cassava starch foam trays were reinforced using fibers extracted from pineapple shells. | 2019 | [30] |
| Cassava starch films enhanced with cellulose and starch nanoparticles were analyzed for their morphology, barrier function, and mechanical behavior. The results support the application of these additives in developing reinforced materials. | 2020 | [49] |
| Cassava starch films were developed using cellulose nanofibrils derived from peach palm agro-industrial waste. | 2020 | [45] |
| Foams made from cassava starch were rendered biodegradable by integrating rice husk residue as a macro filler. | 2020 | [44] |
| Peanut skin-reinforced native starch foams modified with acetyl groups were assessed for their environmental implications. | 2022 | [41] |
| Rice husk ash was reused as a filler material in biodegradable foams composed of cassava starch. | 2023 | [47] |
| Strawberry packaging was developed using cellulose nanofibrils extracted from wheat and oat residues. | 2023 | [50] |
| Cassava starch biofilms were reinforced with fibers from the aerial parts of topinambur (Helianthus tuberosus). | 2024 | [39] |
3.5. Biopolymers Made from Cassava Starch
Given the context outlined above, there is a significant need for research focused on the biopolymers derived from starch and various lignocellulosic fiber materials. However, to advance this work, it is crucial to select a starch source (in this case, the lignocellulosic material is the goldenberry calyx). Unlike previous works on biopolymers using starch, there is limited research utilizing goldenberry calyx as raw material.
One earlier study explored the potential of goldenberry calyx to produce bioplastic by mixing it with corn starch and glycerin [3]. However, the performance properties of biopolymers depend on the starch source. For example, films made from tuber starch show lower density compared to those derived from cereals [17], and low density is desirable in packaging as it reduces transportation and raw material costs. Thus, starch from a tuber source such as cassava becomes a pertinent option for biopackaging due to its favorable properties. Consequently, exploring alternative approaches to utilize goldenberry calyx, such as using a new starch source in conjunction with the calyx fiber that will be part of the composition of the biocomposite, is relevant. In this case, the selected source is cassava starch, a tuber starch. Figure 5 presents a summary of the main findings regarding the application of cassava starch in biopolymers.
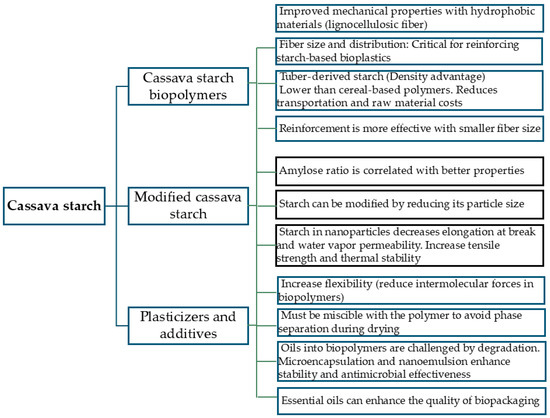
Figure 5.
Cassava starch biopolymers: functional properties and enhancement strategies.
Regarding availability, cassava is accessible year-round. In this context, the utilization of cassava starch represents a feasible process for bioplastic production. The selection of cassava starch as a raw material constitutes a strategy that yields sustainability-related benefits [51]. Firstly, the production of cassava starch-based biopolymers enhances the profitability of cassava processors, may help reduce unemployment, and encourages workers to develop agricultural technology skills and increase their knowledge of bioplastics. Moreover, cassava cultivation has led to significant environmental impacts due to waste generated during its production. Therefore, the development of cassava-based biopolymers can reduce greenhouse gas emissions by managing waste volumes and promoting the production of biodegradable materials [52].
Cassava starch has an amylopectin content ranging from 80% to 90% and an amylose content between 10% and 20% [53]. Numerous studies support its use as a raw material to design bioplastics. The amylose content influences the properties of biofilms [54]. Starch consists of two distinct polymeric structures: amylopectin and amylose. Amylopectin has a branched structure in contrast to the straight-chain formation of amylose. The bond between amylopectin and amylose plays a crucial role in film production as it significantly influences the physical and mechanical properties of the resulting materials [55]. Moreover, a study examined corn, quinoa, and tapioca starches combined with glycerol to produce biofilms. This research determined the oil–water binding capabilities of starches and found that tapioca starch had favorable film-forming properties [56].
The comparison with other types of starch supports the selection of cassava starch. A study evaluated the performance of cassava starch and potato starch in terms of the gelatinization temperature. Cassava starch exhibited a gelatinization temperature between 66 and 68 °C, whereas that of potato starch was 62 °C. A higher gelatinization temperature is associated with a greater degree of crystallinity, which in turn is linked to thermal stability. Furthermore, in bioplastic production, the gelatinization temperature of starch is a critical factor. If the temperature is too low, the starch suspension may gelatinize during the drying process, which is undesirable. In this regard, cassava starch is suitable for exploring alternatives for bioplastic manufacturing [57]. Cassava starch films are recommended for food packaging applications, particularly for fruits and vegetables, due to their mechanical properties, which are comparable to those of low-density polyethylene films commonly used in food packaging [58].
Gelatinization is also related to adhesive properties. However, in this case, the degree of gelatinization plays a crucial role. One study compared the adhesive properties of cassava starch and corn starch, concluding that cassava starch exhibited a higher degree of gelatinization than corn starch. Consequently, the viscosity of cassava starch adhesive was higher than that of corn starch adhesive, indicating its potential for producing higher-viscosity adhesives [59].
Starch-based bioplastics are a viable alternative, although they require reinforcing materials to improve their mechanical strength and thermal stability. For instance, one study developed a bioplastic using cassava root and mango seed reinforced with clay. This demonstrates that cassava by-products can also contribute to reinforcing fibers [18,60]. Moreover, the cassava starch industry is known to generate negative externalities due to cassava bagasse, an agro-industrial residue. Hundreds of tons of this waste are produced annually, leading to significant environmental impacts [18].
In addition, the manufacture of cassava starch generates agro-industrial waste, including cassava peel and bagasse. The production of 1 ton of cassava starch translates into 0.38 tons of husk and 1.4 tons of bagasse. Both wastes are rich in starch, and given the importance of cassava starch in the food industry, utilizing these wastes for biopackaging aligns with sustainability objectives since they do not affect food security. Further research should explore using these wastes to make food biopackaging [61]. A subsequent study a year later sought to create biopolymeric films from starch taken from cassava periderm and bark, carob galactomannan, and cellulose nanofibers—all types of cassava waste [62]. Currently, there are still relatively few studies focusing on the use of starch derived from cassava waste or by-products. These wastes include cassava peel, the primary by-product of cassava tuber peeling, which accounts for more than 5% of the cassava root mass and is typically discarded during the production of cassava flour or starch [63]. In addition, when cassava starch originates from agro-industrial waste, the carbon footprint of biopolymeric films can be further reduced [64].
It is important to mention that cassava peel starch is a viable alternative for food packaging. However, it is important to control the temperature and humidity carefully during food storage [65]. Furthermore, in South America, numerous articles have used cassava starch to produce biofilms, providing a strong foundation for further research. Building on this idea, Vargas-García et al. [66] recognize that South America has enormous potential to take advantage of biomass for developing industries centered around bioplastics as an alternative to counteract environmental pollution generated by synthetic plastics. Cassava starch is widely researched for plastic packaging materials. Recent studies have focused on (i) blending starch with other polymeric materials, (ii) incorporating reinforcing agents, and (iii) adding polyphenols and essential oils to enhance antioxidant and antimicrobial properties [63].
As shown in Table 2, the selected articles can be categorized into four main groups. The first group involves articles that explore the feasibility of preparing food packaging. The second involves studies promoting the use of fiber to improve the properties of cassava starch. The third involves studies focusing on the production of bioplastics and their properties. Finally, certain works evaluate the safety aspect of biopolymers. The search reviewed studies from 2017 onwards.

Table 2.
Studies on the use of cassava starch for bioplastics.
Based on the results presented in the table about using cassava starch for bioplastics in Latin America (Table 2), the most recent advancements in the field can be highlighted. Firstly, emphasis is placed on the use of cellulose nanofibrils derived from wheat and oats for packaging delicate foods such as strawberries [50]. Additionally, Colivet et al. [91] developed biofilms based on cassava starch and watermelon seed oil emulsion. They concluded that adding this emulsion increased the elongation capacity and reduced deformation at the break. This study underscores the potential of reinforcing materials to increase the mechanical resistance and hydrophobicity of cassava starch, rendering them suitable for packaging [82]. Another study found that bioplastic films incorporating chitosan, particularly when combined with cassava starch, show great potential for applications in the food industry. The research determined that using commercial starch for biodegradable plastic film production enhances moisture adsorption, solubility, and biodegradability compared to manually extracted cassava starch [55]. Furthermore, the combination of cassava starch with reinforcing materials also promotes other properties such as antioxidant, antibacterial, and antifungal attributes [63,83].
A study was conducted to evaluate the antibacterial activity of a goldenberry calyx extract. The results confirmed its potential antibacterial properties, paving the way for further research on its application as a natural preservative in the food industry [12]. A study also emerged that evaluated the use of edible pectin coatings reinforced with nanocellulose, which was obtained from the calyx of Physalis peruviana [10].
The proportion of reinforcing material significantly influences the performance of bioplastics; for instance, in the formulation of a biopolymer based on quinoa starch nanocrystals used as reinforcement for cassava starch, the percentage of nanocrystals must be 5% to guarantee the best properties [37]. Similarly, the addition of citric acid as a cross-linker should be 10% to achieve a favorable balance of tensile strength, elongation, and fracture stress [92]. Thus, cassava starch emerges as a promising alternative for producing bioplastics that can serve as packaging for fruits and vegetables, offering good mechanical properties and food safety standards. According to Rada Mendoza et al. [88], food biopackaging must be free of toxic metals to guarantee their quality and safety.
In addition, exploring lignocellulosic fibers to reinforce starch properties is vital because a successful interaction with another source of biopolymers facilitates the production of food packaging [78]. Corralo Spada et al. [44] show that rice husk improves mechanical properties and reduces water absorption compared to starch-only biofilms, making starch and rice husk the ideal combination to produce packaging for cherry tomatoes. Four years later, a study evaluated films made from cassava, potato, corn, yam, and wheat starches to assess their role in cherry tomato preservation [85].
In addition to the results regarding the applications of cassava starch in biopolymers production, it is worth mentioning some advances beyond Latin America. In terms of using fibers as reinforcing materials, Cogon grass is a weed that decreases the hydrophilicity of biodegradable polymers [95]. Jumaidin et al. [96] investigated the effect of durian skin fiber, which also decreases hydrophilicity. Coconut waste was also used as a reinforcing material for biopolymers made from cassava starch [97]. Moreover, cassava starch biopolymers can be reinforced with metakaolin with coconut fiber [98]. Another study investigated the development of food packaging films with two different thicknesses using cassava starch, glycerol, and coconut oil as a plasticizer. The study evaluated their suitability for packaging dry solid foods [99].
Some studies have examined the mechanical and thermal characteristics of thermoplastic cassava starch reinforced with sugarcane bagasse fiber. The authors underscore the importance of the lignin content in reducing hydrophilicity since lignin is hydrophobic [100]. It is necessary to consider the characterization of the fibers that will be used as starch reinforcement [101]. Other determining factors are the fiber’s morphological structure, thermal stability, and chemical composition [102].
Diyana et al. [103] evaluated the effect of pandanus amaryllifolius fiber on the properties of thermoplastic cassava starch/beeswax composites. The addition of pandanus amaryllifolius fiber can improve thermal stability and crystallinity. Another study created biodegradable plastic using rubberized cassava starch and tofu waste, incorporating sorbitol as a plasticizer. The most favorable sample presented a tensile strength of 4291.9 kPa, 35% elongation, 41.94% water uptake, and nearly 80% degradation in soil in around two weeks [104]. This idea provides an opportunity to discuss research findings regarding the properties of biopolymers made with cassava starch.
According to Fan et al. [105], chitosan improves the antibacterial and physicochemical properties of starch by strengthening intermolecular forces and forming new hydrogen bonds, which drive the disappearance of the porous structure on the surface. In a subsequent study, chitosan was added to cassava starch to produce biodegradable films, utilizing its multiple hydroxyl groups. Chitosan enhanced the film’s physicochemical traits, improving compressive strength and water resistance. The bioplastics became stiffer and showed a higher tensile strength as the chitosan levels increased. Conversely, a higher glycerol concentration further reduced the stiffness of the mixture, likely due to the high water vapor content in cassava starch. Chitosan improves the polymer’s mechanical properties, biodegradability, bioadhesiveness, bioactivity, and biocompatibility [106]. Moreover, mussel and shrimp shell wastes were converted into chitosan, offering a natural substitute for toxic chemicals [107].
Chitosan has shown promising properties as a reinforcement for cassava starch, and this potential could also apply to the incorporation of goldenberry calyx fibers. One study compared the properties of chitosan with other components used to preserve fruits. Edible coatings are commonly prepared from biopolymers such as starches, dextrin, cellulose, alginate, chitosan, and pectin, all of which exhibit desirable properties like biodegradability, good oxygen and vapor barrier capabilities, and the ability to reduce moisture loss in fruits. Nanofibers extracted from goldenberry calyx have demonstrated favorable properties for reinforcing pectin in enhancing fruit preservation performance [10]. In addition to fruit preservation, certain substances contribute to extending the shelf life of other types of food products. For instance, maltol has proven to be effective in prolonging the shelf life of bakery product packaging due to its antifungal properties [108].
Another application is the development of edible films. Gao et al. [109] developed an edible film incorporating black rice anthocyanin extract (AEBR) into an acetylated cassava starch (ACS)/carboxymethylcellulose (CMC) matrix to enhance the shelf life of pumpkin seeds and slow their oxidation. The rheological analysis of the solutions showed that AEBR was uniformly dispersed within the polymer matrix and stabilized by hydrogen bonds. A suitable concentration of AEBR was compatible with the polymer matrix, forming a compact film structure that improved the mechanical properties, barrier performance, and opacity. Moreover, the ACS/CMC/AEBR film has the potential to serve not only as an edible coating to extend food shelf life but also as a pH indicator for monitoring food freshness, particularly in meat products. Finally, another example of edible films based on cassava starch involves the incorporation of red cabbage extract. The results indicate a promising packaging material that could serve as a sustainable alternative to plastic packaging [110].
Furthermore, nanocrystals have also been shown to improve water barrier properties and tensile strength when incorporated into starch-based biopolymers [111]. The fiber size has an effect on the mechanical properties of bioplastics [112]. A smaller fiber improves the mechanical properties more than other fiber sizes and shows a better reinforcement effect than larger fiber sizes. Based on the above, a cassava starch-based biopolymer benefits more from reinforcing materials when these have a smaller fiber size.
According to Zúniga Linan et al. [35], when lignin reaches the nanoscale, its surface and other properties are enhanced, expanding its potential applications. For instance, it has been demonstrated that the insolubility and indigestibility of lignin can be overcome by transforming it into spherical microparticles with diameters ranging from 0.1 to 3 µm. This nanoparticle configuration also improves the biodegradability of the material. The spherical shape, with a hydrophobic core made up of methoxy groups and a hydrophilic surface composed of phenolic and aliphatic hydroxyl groups, facilitates the formation of hydrogen bonds. These bonds create heterogeneously distributed microclusters that add surface roughness to the biopolymer, thereby increasing its contact angle with water.
Lignin-containing cellulose nanofibrils can enhance the properties of bioplastic materials when incorporated into their structure [32]. In this context, nature-inspired nanoparticles are at the forefront of current scientific research in advanced nanobiotechnology. Additionally, the use of abundant plant waste resources for nanoparticle production is seen as a more sustainable and eco-friendly approach. The biofabrication of TiO2 nanoparticles from plant waste has been reported using various fruit peels, such as banana, lemon, plum, peach, and kiwi peel extracts. The biogenic synthesis of CeO2-TiO2 nanocomposites using goldenberry calyx is achievable by employing an aqueous extract of the calyx leaves of Physalis peruviana fruits under ultrasonic irradiation. The calyx leaf extract exhibits bioactive properties, making it suitable as a coating during the nanoparticle bio-manufacturing process [11].
Cassava starch is internationally recognized as a topic of research in the food packaging sector. Boonphayak et al. [113] investigated the effects of plasticization using glycerol, sorbitol, and a mixture of glycerol and sorbitol plasticizers while evaluating the effects of bio-SiO2 particles extracted from sugarcane leaves on the mechanical properties and biodegradability of cassava starch-based biofilms. Films containing bio-SiO2 exhibit significantly lower water solubility and moisture content than films without bio-SiO2. Moreover, the inclusion of bio-SiO2 particles enhances the water resistance properties of the biofilms. Overall, the results suggest that cassava starch films, prepared with a mixture of plasticizers and reinforced with bio-SiO2 particles, can be considered a viable and safe material for biodegradable food packaging applications.
Promsorn et al. [114] used gallic acid to reinforce cassava starch biopackaging, while Joshi et al. [115] and Kavas et al. [116] employed polyvinyl alcohol, with the latter authors integrating betanin to enhance antioxidant properties, crucial for the development of food packaging. Dilkushi et al. [117] characterized food biofilms using banana pseudostem and cassava starch. Also, Krümmel et al. [118] formulated cassava starch films with antimicrobial properties by adding either free carvacrol or chia mucilage nanocapsules containing carvacrol to evaluate their effectiveness against foodborne microbes.
It has been demonstrated that the properties of cassava starch biopolymers for food packaging applications improve when the reinforcing fibers are encapsulated nanoparticles. Although there are no studies addressing both goldenberry calyx and cassava starch simultaneously, the use of calyx-derived nanoparticles suggests a promising future for this biocomposite. According to Bazana et al. [119], nanotechnology is one of the most promising technologies in the food industry due to its ability to encapsulate various bioactive compounds extracted from plants. This technology holds potential for preserving phenolic compounds and the antioxidant capacity of goldenberry calyx extracts.
The nanoparticles derived from goldenberry calyx exhibited higher antioxidant activity than those found in the seeds and juice. Moreover, antioxidant efficiency was greater in calyx nanoparticles than in their unprocessed counterpart. This study assessed the occurrence of various mold species (Mucor sp., Aspergillus niger, Penicillium sp., and Alternaria sp.) and yeasts (Saccharomyces cerevisiae and Rhodotorula glutinis). These species showed a more significant reduction in response to goldenberry calyx nanoparticles compared to the untreated calyx. In this regard, nanoparticles also show promising potential for controlling the growth and distribution of microorganisms [7].
3.6. Improving Cassava Starch Properties Through Modification
Throughout this study, it has been evident that integrating reinforcing materials such as lignocellulosic fibers can significantly improve the properties of biofilms made from cassava starch. However, its properties can be more optimal if the starch is previously modified before using it in the development of biopolymers. According to Lin et al. [120], native or unmodified starch is highly hydrophilic and has poor properties. Modification improves its properties, but this depends on factors such as the starch source, reaction conditions, and distribution of functional groups within the starch molecule. Furthermore, as discussed in Section 3.5, the use of commercially available modified starch for bioplastic production improves moisture adsorption, solubility, and biodegradability [55].
Starch consists of amylose and amylopectin, with their proportions varying depending on the source. A higher amylose content is correlated with enhanced mechanical properties, particularly an increase in tensile strength [17]. In starches, amylopectin is the dominant component, and the proportions of amylose and amylopectin present in the starch are crucial to form films [121]. Additionally, the ability to form films depends mainly on the presence of amylose [122]. High-amylose starches form better films than those rich in amylopectin since amylopectin’s branched chains can cause agglomeration and an uneven film structure [123]. Also, amylose reduces the ability of starch to associate with water.
There are several methods to modify starch, with chemical modification employing acetic acid being one approach. This method entails replacing hydroxyl groups of the starch molecule with some ester or ether groups, thereby increasing the proportion of amylose and facilitating molding and gelification [51]. Similarly, cassava starch should be modified with a 5% acetic acid concentration [124]. The final product is a biopolymer with 61.76% amylose and 38.28% amylopectin.
Apart from acetic acid, acetylation is another chemical modification technique with esterification that uses reagents such as acetic anhydride, vinyl acetate, and alkalis. Catalysts are essential to initiate the reaction, wherein starch is first conditioned into an alkaline state to form a starch base before the acetate reagent is added to form starch acetate [125]. Jayarathna et al. [122] emphasizes esterification as an ideal modification method to manufacture biopolymers with hydrophobic properties. Additionally, substituted starches resulting from esterification reactions receive industrial interest due to the enhancement of thermal properties that results from introducing an ester group in a polysaccharide [126].
Modifying starch by replacing free hydroxyl groups with acetyl groups weakens intermolecular bonds, resulting in the formation of an amphiphilic (hydrophilic and hydrophobic) starch. It is important to mention that the degree of substitution significantly impacts the physicochemical and functional properties of acetylated starch. Higher degrees of substitution render the starch more hydrophobic (starches with a low degree of substitution are hydrophilic). However, opting for modified starch with a low degree of substitution is most advisable because it minimizes changes in granule morphology, with acetylation reactions with a low degree of substitution primarily occurring in the amorphous region of the surface of the starch granule. Conversely, high degrees of substitution affect the internal structure of the granule and cause greater damage [125].
Dry heat treatment has emerged as a viable modification process to obtain starch, particularly in producing biopolymers via casting and extrusion techniques. In both cases, dry heat treatment enhances the performance of bioplastics by reducing water permeability and the moisture content. Casting produces thinner bioplastics, while extrusion results in thicker ones [81]. Marta et al. [127] indicate that starch can be modified by reducing its size. Using starch nanoparticles decreases the elongation at break and water vapor permeability and increases bioplastics’ tensile strength and thermal stability.
Starch modification improves mechanical properties, with several studies indicating that biopolymers derived from modified starch are more resistant to bacteria and fungi. For instance, biofilms based on acetylated cassava starch can inhibit fungal growth by up to six times, attributed to the reduction in material hydrophilicity resulting from this modification [125,128]. It is noteworthy that starches undergo physical, chemical, and enzymatic modifications to prepare biocomposites with desired properties. In this context, adding essential oils serves as an additional method to modify starch properties [129].
Essential oils possess antibacterial and antioxidant functions. For example, rosemary oil shows potent antimicrobial effects against Gram-positive (S. epidermidis, S. aureus, and B. subtilis), Gram-negative (P. vulgaris, P. aeruginosa, and E. coli), and fungal strains (C. albicans and A. niger). This oil is one of the most used in starch matrices since it can also act as a plasticizer. Cinnamon essential oil, containing cinnamaldehyde, also demonstrates antibacterial and antioxidant effects. Moreover, adding cinnamon essential oil reduces the moisture content and water solubility of biofilms while providing good thermal stability. Essential oils can also work with other plasticizers, such as glycerol [130].
Finally, starch modification is also useful in adhesive formulations. A study showed the adhesive ability of starch to bond kraft paper substrates. Modifications to achieve this goal include alkaline treatments with NaOH, cold hydrolysis, and starch gelatinization. These techniques exhibit oxidative properties, resulting in adhesive products with improved characteristics. Applying NaOH–urea mixtures to cassava starch at ambient temperature disrupted its structure, triggering cold gelatinization. Changes in adhesive properties vary with the starch concentration and NaOH/urea ratios [131]. Moreover, using cassava starch modified with 1,2,3,4-butane tetracarboxylic acid is a good option to obtain bioadhesives that can bind fibers [132]. Adhesive components play a crucial role in the packaging industry.
3.7. Use of Plasticizers in Starch Biofilms
Based on the findings from studies on cassava starch-based biopolymers presented in Section 3.5, some authors recommend the incorporation of plasticizers to enhance material properties. Maitha et al. [99] used cassava starch, glycerol, and coconut oil as plasticizers for food bioplastics and recommends their use, as plasticizers increase tensile strength by preventing brittleness in the film. Additionally, plasticizers help to mitigate the intermolecular forces between starch polymer chains and soften the matrix. The authors of [113], in turn, recommend glycerol and sorbitol to improve the mechanical properties and biodegradability of cassava starch-based films. These improvements enable the development of a viable and safe material for food packaging applications.
Plasticizers increase the flexibility of biopolymers by reducing intermolecular forces, thereby resulting in a smooth and uniform morphological appearance [133]. Glycerol establishes new hydrogen bonds with starch, disrupting the existing hydrogen bonds between starch chains and loosening chain interactions, ultimately enhancing the elasticity of the matrix [134]. However, it is noteworthy that an increased concentration of glycerol results in a thicker bioplastic. A thicker starch-based plant film layer increases gas permeability, water resistance, and protection [65].
The use of glycerol is recommended because it improves resistance to deterioration [135]. However, the choice of plasticizers should be carefully considered, ensuring compatibility with the biopolymer and miscibility with the polymer to prevent potential separation during the drying process, which could negatively affect the biofilms [136]. Plasticizers are most effective when their structure closely resembles that of the polymer they plasticize. This occurs as polyol polar groups engage with starch hydroxyl groups during plasticization, weakening the internal hydrogen bonds [130]. Also, plasticizers can influence optical and physicochemical properties, and higher concentrations of starch and plasticizers can result in biofilms with low opacity [137].
In addition to biopolymer compatibility, the concentration of the plasticizer is a critical factor to consider. For instance, an increase in glycerol concentration leads to a decrease in tensile strength. This change in properties occurs because plasticizers weaken the intermolecular forces between adjacent macromolecule chains, increase free volume, and consequently result in reduced mechanical resistance [56].
According to Hernández et al. [93], other plasticizing agents, such as citric acid, exhibit promising properties. Beyond plasticizers, various additives can improve the antimicrobial and antioxidant properties of biopackaging. Essential oils can improve the quality of food packaging. Thymus vulgaris essential oil has an excellent antibacterial effect on many bacteria due to its phenolic content, especially thymol and carvacrum [138]. Additionally, oregano essential oil, being a natural food additive with a hydrophobic nature, has important antioxidant and antimicrobial qualities, enhancing the mechanical, barrier, and solubility properties of films [93].
However, incorporating essential oil or other bioactive agents into biofilms is challenging due to oxidation and degradation during extrusion. One promising solution is the microencapsulation of additives [139], which can be achieved in polymeric particles, liposomes, and lipid nanoparticles [130]. Another challenge is the hydrophobic nature of essential oils and the hydrophilic nature of starch, which prevents direct addition to biofilms and results in non-homogeneous microstructures that reduce mechanical properties. To address this, encapsulation in emulsions, particularly Pickering emulsions prepared with cellulose nanocrystals, proves effective in improving compatibility with starch and enhancing the functionality of the biofilm [138].
The formation of nanoemulsions can enhance the stability, bioavailability, and antimicrobial efficacy of essential oils. For instance, lavender essential oil is noted for its antimicrobial and antioxidant properties [140]. This essential oil contains antioxidant and bioactive compounds (linalool, p-cymene, camphor, α-pinene, β-pinene, 1,8-cineole, limonene, terpinen-4-ol, and borneol), which can help reduce oxidative stress. Additionally, its mild aroma compared to other essential oils can better preserve the sensory characteristics of packaged foods [141].
However, its volatility and susceptibility to environmental degradation limit its practical application in food packaging [140]. These issues can be mitigated by preparing nanoemulsions, which enhance the antimicrobial effects of essential oils in film matrices. Essential oil nanoemulsions retain the antimicrobial activity of the oils by adhering to and disrupting microbial cell membranes [141].
4. Conclusions
Starch-based bioplastics exhibit improved mechanical properties when combined with lignocellulosic materials due to their hydrophobic nature. Moreover, starch enhances fiber properties by increasing biodegradability through its water absorption capacity.
When lignin reaches the nanoscale, its surface and other properties are enhanced, expanding its potential applications. For instance, there is a possibility of developing nanocomposites for food coatings, leveraging the antibacterial and antioxidant properties of goldenberry calyx extract. The nanoscale also significantly influences starch and additives such as essential oils.
The use of fibers with a high lignin content, such as those found in goldenberry calyx, could lead to the utilization of the waste produced from this fruit as a reinforcing material in composites with modified and unmodified cassava starch matrices, combining them in such a way as to replace some single-use plastics and solve environmental problems associated with this issue. It should be noted that considerable research is still needed on the influence of important variables such as particle size and the percentage of goldenberry calyx as reinforcement material. For instance, more research is needed on the chemical cohesion between the matrix and the reinforcement and their influence on the mechanical properties of the biomaterial obtained, including the final plasticity and elasticity after combining the fibers and matrix, the final moisture content, and the drying temperature.
Excessive fiber content can interfere with expandability, create discontinuities in the starch matrix, and compromise crack resistance. Another key factor to consider is the morphology of the fiber, along with its thermal stability and chemical composition. The calyx of goldenberry contains significant amounts of phenolic compounds, and lignins with higher concentrations of phenolic and carboxyl -OH groups enhance the Young’s modulus by facilitating the formation of stronger hydrogen bonds between lignin and starch. Cassava starch also exhibits adhesive properties, and likewise, lignin acts as an adhesive within the wood cell wall, binding cellulose and hemicellulose.
Cassava starch is an excellent alternative to use with goldenberry calyx as no significant advancements have been reported in biocomposites combining goldenberry calyx and cassava starch. Moreover, biopolymers made with tuber starch demonstrate lower density compared to those made with cereal starch—an attribute that is especially desirable for packaging applications. Goldenberry calyx has a moisture content of 27.1%, which is relatively low compared to other organic waste materials. This characteristic offers a logistical advantage as high a moisture content accelerates waste degradation.
It is preferable to use modified cassava starch as it contains a higher amylose content, which positively influences the mechanical properties of the biopolymer while reducing its hydrophilic nature. Another recommendation is to use starch derived from cassava residues or by-products as this promotes waste valorization, avoids compromising food security, and addresses a gap in the literature given the limited number of studies focusing on cassava waste-derived starch for biocomposite applications.
In summary, this review article examines the potential of combining goldenberry calyx with modified cassava starch. Future studies are encouraged to develop and assess biocomposites and explore their applications in real-world settings, such as within companies.
Author Contributions
Conceptualization, V.E.T.B. and J.M.G.B.; methodology, V.E.T.B.; software, V.E.T.B.; validation, V.E.T.B. and J.M.G.B.; formal analysis, V.E.T.B. and J.M.G.B.; investigation, V.E.T.B. and J.M.G.B.; resources, V.E.T.B. and J.M.G.B.; data curation, V.E.T.B.; writing—original draft preparation, V.E.T.B.; writing—review and editing, V.E.T.B.; visualization, V.E.T.B.; supervision, V.E.T.B. and J.M.G.B.; project administration, V.E.T.B. and J.M.G.B.; funding acquisition, V.E.T.B. and J.M.G.B. All authors have read and agreed to the published version of the manuscript.
Funding
The research received external support from the National Association of Industrialists (ANDI) under the IP-36 Andi initiative. The APC was handled by Fundación Universitaria Los Libertadores, identified by code ING-15-25.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors are grateful to the Universidad Nacional de Colombia Arts Faculty and National Association Industrialist (ANDI) for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ramos, G.W.; Klosowski, A.B.; Lopes, A.C.; Carvalho, G.A.; Olivato, J.B. How Are the Properties of Starch-Based Composites Influenced by the Chemical Treatment of BSG? Macromol. Symp. 2024, 413, 2400081. [Google Scholar] [CrossRef]
- Henao-Díaz, L.S.; Cadena-Casanova, C.L.; López Bolio, G.I.; Veleva, L.; Azamar-Barrios, J.A.; Hernández-Villegas, M.M.; Córdova-Sánchez, S. Obtaining and characterization films of a bioplastic obtained from passion fruit waste (Passiflora edulis). Agro Productividad. 2021. [Google Scholar] [CrossRef]
- Ortega, Y.S.; Juórez, A.S.; Benítez, L.B.C. Fabricación y análisis general de costo de un Biopolímero naturalo partir del cáliz de Physalis peruviana L (uvilla). Rev. Univ. Guayaquil 2016, 122, 12–18. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Bălțatu, M.S.; Vizureanu, P.; Sandu, A.V.; Achitei, D.C.; Nergis, D.D.B.; Perju, M.C.; Nabiałek, M. Biocomposites: Materials, Properties, and Applications. In Composite Materials Science and Engineering; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- Ciro, S.Q.; Yepes, W.U.; Posada, C.J.C.; Valencia-Arias, A. Revisión de modelos de gestión tecnológica e innovación orientados a la construcción de una metodología para el desarrollo de biomateriales biodegradables. Ingeniare. Rev. Chil. Ingeniería 2024, 31. [Google Scholar] [CrossRef]
- Yehia, H.M.; Elkhadragy, M.F.; Shebl, R.I.; Al-Masoud, A.H.; El-Din, M.F.S. Control some foodborne pathogens, contaminated bacteria and fungi by fabrication calyx cape gooseberry (Physalis peruviana L.) nanoparticles. Food Sci. Technol. 2022, 42, e116021. [Google Scholar] [CrossRef]
- Vieira, A.F.; Pereira, T.R.R.; da Silva, L.P.F.R.; Rodrigues, T.J.A.; de Almeida, R.D.; Santos, N.C.; da Costa, M.E.M.D.; de Araújo, G.T.; Rocha, A.P.T. Mechanical, Antimicrobial and Morphological Properties of Biodegradable Trays Based on Cassava Starch and Agroindustrial Processing Residues. Packag. Technol. Sci. 2024, 38, 195–210. [Google Scholar] [CrossRef]
- Chicaiza, D.S.; Forigua, M.C.; Valásquez, J.E.C. Elaboración de un modelo de biopolímero a partir de residuos orgánicos para el recubrimiento de prótesis de miembros superiores. In Proceedings of the Mujeres en Ingeniería: Empoderamiento, Liderazgo y Compromise, Bogotá, Colombia, 7 September 2021. [Google Scholar] [CrossRef]
- Cárdenas-Barboza, L.C.; Paredes-Córdoba, A.C.; Serna-Cock, L.; Guancha-Chalapud, M.; Torres-León, C. Quality of Physalis peruviana fruits coated with pectin and pectin reinforced with nanocellulose from P. peruviana calyces. Heliyon 2021, 7, e07988. [Google Scholar] [CrossRef]
- Dwivedi, B.; Bhardwaj, D.; Atal, P.K.; Choudhary, D. Bio-inspired facile synthesis of CeO2-TiO2 nanocomposites using calyx leaves extract of Physalis peruviana fruits and their biological assessments: Antibacterial and antioxidant activity. Plant Nano Biol. 2024, 11, 100130. [Google Scholar] [CrossRef]
- Bazana, M.T.; Missel, M.V.; de Deus, C.; Pinto, V.S.; Codevilla, C.F.; Santos, R.C.V.; Silva, C.D.B.D.; de Menezes, C.R. Antibacterial and antibiofilm activity of Physalis peruviana Calyx Extract/Atividade Antibacteriana e antibiofilme do extrato do cálice de Physalis peruviana. Braz. J. Dev. 2020, 6, 77422–77432. [Google Scholar] [CrossRef]
- Nocetti, D.; Núñez, H.; Puente, L.; Espinosa, A.; Romero, F. Composition and biological effects of goldenberry byproducts: An overview. J. Sci. Food Agric. 2020, 100, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Schutz, G.F.; Gonçalves, S.D.Á.; Alves, R.M.V.; Vieira, R.P. A review of starch-based biocomposites reinforced with plant fibers. Int. J. Biol. Macromol. 2024, 261, 129916. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, A.V.V.; Quiroz, H.O.; Sandoval, C.A.S. Evaluación de parámetros óptimos para mejorar la resistencia de biopolímero producido a partir de almidón: Revisión bibliográfica. Rev. Científica Pakamuros 2020, 8, 22–33. [Google Scholar] [CrossRef]
- Ragoubi, M.; Terrié, C.; Leblanc, N. Physico-Chemical, Rheological, and Viscoelastic Properties of Starch Bio-Based Materials. J. Compos. Sci. 2022, 6, 375. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Dassanayake, R.S.; Fernando, N.M.; Wanninayaka, D.B.; Rajapaksha, S.M.; Manamperi, A.; Gangoda, M.; Manchanda, A.; et al. Preparation and Characterization of Dual-Modified Cassava Starch-Based Biodegradable Foams for Sustainable Packaging Applications. ACS Omega 2022, 7, 19579–19590. [Google Scholar] [CrossRef]
- Serpa-Fajardo, J.G.; Hernández-Ramos, E.J.; Fernández-Lambert, G.; Sandoval-Herazo, L.C.; Andrade-Pizarro, R.D. Post-industrial context of cassava bagasse and trend of studies towards a sustainable industry: A scoping review—Part, I. F1000Research 2022, 11, 562. [Google Scholar] [CrossRef]
- Coronado, A.C.M.; Castillo, J.A.G.; Coronado, Y.M. Caracterización de la diversidad genética de uchuva (Physalis peruviana L.) en Boyacá. Biotecnol. Sect. Agropecu. Agroindustrial 2018, 16, 26–33. [Google Scholar]
- Añibarro-Ortega, M.; Dias, M.i.; Petrović, J.; Mandim, F.; Núñez, S.; Soković, M.; López, V.; Barros, L.; Pinela, J. Nutrients, Phytochemicals, and In Vitro Biological Activities of Goldenberry (Physalis peruviana L.) Fruit and Calyx. Plants 2025, 14, 327. [Google Scholar] [CrossRef]
- Gimenez, L.A.S.; Rivas, M.A.; Vignale, N.D.; Gurni, A.A. Caracterización micrográfica de tres frutas tropicales, Musa paradisii L., Physalis peruviana L. y Persea americana Mill. Importancia en el control de calidad botánico de alimentos derivados. Polibotánica 2021, 51, 155–170. [Google Scholar] [CrossRef]
- Castellanos, I.C.; Rojas-Pérez, L.C. Extraction and characterisation of cuticular waxes from Physalis peru-viana calyx. Int. J. Food Sci. Technol. 2024, 59, 1829–1839. [Google Scholar] [CrossRef]
- Ballesteros-Vivas, D.; Alvarez-Rivera, G.; Leon, C.; Morantes, S.J.; Ibánez, E.; Parada-Alfonso, F.; Cifuentes, A.; Valdés, A. Anti-proliferative bioactivity against HT-29 colon cancer cells of a withanolides-rich extract from golden berry (Physalis peruviana L.) calyx investigated by Foodomics. J. Funct. Foods 2019, 63, 103567. [Google Scholar] [CrossRef]
- Lan, Y.-H.; Chang, F.-R.; Pan, M.-J.; Wu, C.-C.; Wu, S.-J.; Chen, S.-L.; Wang, S.-S.; Wu, M.-J.; Wu, Y.-C. New cytotoxic withanolides from Physalis peruviana. Food Chem. 2009, 116, 462–469. [Google Scholar] [CrossRef]
- Calderón, J.; Ruiz, N.; Castellanos, L. Within and between plant variation of 4β-hydroxiwithanolide E in cape gooseberry (Physalis peruviana; Solanaceae). Biochem. Syst. Ecol. 2012, 41, 21–25. [Google Scholar] [CrossRef]
- Puente, L.A.; Pinto-Muñoz, C.A.; Castro, E.S.; Cortés, M. Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: A review. Food Res. Int. 2011, 44, 1733–1740. [Google Scholar] [CrossRef]
- Wahdan, O.A.; Badr, S.E.-S.A.; Abdelfattah, M.S. Phytochemical Analysis, Antibacterial and Anti-cancer Activities of the Physalis peruviana Calyces Growing in Egypt. Food Nutr. J. 2019, 4, 197. [Google Scholar]
- González-Velandia, K.-D.; Daza-Rey, D.; Caballero-Amado, P.-A.; Martínez-González, C. Evaluación de las propiedades físicas y químicas de residuos sólidos orgánicos a emplearse en la elaboración de papel. Luna Azul 2016, 43, 499–517. [Google Scholar] [CrossRef]
- Popova, V.; Stoyanova, M.; Ivanova, T.; Mazova, N.; Stoyanova, A. Comparison of some chemical indices of the fruit calyces of Physalis peruviana L. and Physalis alkekengi L. from different genotypes. AIP Conf. Proc. 2023, 2889, 080007. [Google Scholar] [CrossRef]
- Cabanillas, A.; Nuñez, J.; Cruz-Tirado, J.; Vejarano, R.; Tapia-Blácido, D.R.; Arteaga, H.; Siche, R. Pineapple shell fiber as reinforcement in cassava starch foam trays. Polym. Polym. Compos. 2019, 27, 496–506. [Google Scholar] [CrossRef]
- Ali, S.; Rani, A.; Dar, M.A.; Qaisrani, M.M.; Noman, M.; Yoganathan, K.; Asad, M.; Berhanu, A.; Barwant, M.; Zhu, D. Recent Advances in Characterization and Valorization of Lignin and Its Value-Added Products: Challenges and Future Perspectives. Biomass 2024, 4, 947–977. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef]
- Fauzi, A.R.; Sarofa, U.; Rosida, D.F. Characteristics of Biodegradable Foam with Proportional Treatment of Tapioca Flour and Soybean Peel Flour with Added Glycerol. AJARCDE Asian J. Appl. Res. Community Dev. Empower. 2024, 8, 246–253. [Google Scholar] [CrossRef]
- Aguilar, M.A.C.; Quintana, S.G.C. Fenoles y capacidad antioxidante de psidium guajava, vaccinium myrtillus, selenicereus megalanthus y physalis peruviana de diferentes procedencias. Bioagro 2020, 32, 225–230. [Google Scholar]
- Linan, L.Z.; Fakhouri, F.M.; Nogueira, G.F.; Zoppe, J.; Velasco, J.I. Benefits of Incorporating Lignin into Starch-Based Films: A Brief Review. Polymers 2024, 16, 2285. [Google Scholar] [CrossRef]
- Adeyeye, S.A.O.; Babu, A.S.; Ganesh, P.S. Starch Nanocrystal and its Food Packaging Applications. Curr. Res. Nutr. Food Sci. J. 2023, 11, 1–21. [Google Scholar] [CrossRef]
- Velásquez-Castillo, L.E.; Leite, M.A.; Tisnado, V.J.A.; Ditchfield, C.; Sobral, P.J.D.A.; Moraes, I.C.F. Cassava Starch Films Containing Quinoa Starch Nanocrystals: Physical and Surface Properties. Foods 2023, 12, 576. [Google Scholar] [CrossRef]
- Méité, N.; Konan, L.K.; Tognonvi, M.T.; Oyetola, S. Effect of metakaolin content on mechanical and water barrier properties of cassava starch films. S. Afr. J. Chem. Eng. 2022, 40, 186–194. [Google Scholar] [CrossRef]
- Montes, L.F.S.; Melaj, M.A.; Lorenzo, M.C.; Ribba, L.; Garcia, M.A. Biodegradable Composite Materials Based on Cassava Starch and Reinforced with Topinambur (Helianthus tuberosus) Aerial Part Fiber. Sustain. Polym. Energy 2024, 2, 10004. [Google Scholar] [CrossRef]
- Zarski, A.; Bajer, K.; Kapuśniak, J. Review of the Most Important Methods of Improving the Processing Properties of Starch toward Non-Food Applications. Polymers 2021, 13, 832. [Google Scholar] [CrossRef]
- García-Rengifo, A.R.; Rojas-Bringas, P.M.; De-La-Torre, G.E.; Torres, F.G. Environmental impact of peanut skin-reinforced native starch foams modified by acetylation. Environ. Qual. Manag. 2021, 31, 89–99. [Google Scholar] [CrossRef]
- Bergel, B.F.; da Luz, L.M.; Santana, R.M.C. Comparative study of the influence of chitosan as coating of thermoplastic starch foam from potato, cassava and corn starch. Prog. Org. Coat. 2017, 106, 27–32. [Google Scholar] [CrossRef]
- Machado, C.M.; Longhi, E.M.; Spada, J.C.; Tessaro, I.C. Effect of Broken Rice Flour Addition on Cassava Starch-Based Foams. Starch-Starke 2018, 70, 1700191. [Google Scholar] [CrossRef]
- Spada, J.C.; Jasper, A.; Tessaro, I.C. Biodegradable Cassava Starch Based Foams Using Rice Husk Waste as Macro Filler. Waste Biomass-Valoriza. 2019, 11, 4315–4325. [Google Scholar] [CrossRef]
- Martins, M.P.; Dagostin, J.L.A.; Franco, T.S.; de Muñiz, G.I.B.; Masson, M.L. Application of Cellulose Nanofibrils Isolated from an Agroindustrial Residue of Peach Palm in Cassava Starch Films. Food Biophys. 2020, 15, 323–334. [Google Scholar] [CrossRef]
- Versino, F.; Garcia, M.A. Particle Size Distribution Effect on Cassava Starch and Cassava Bagasse Biocomposites. ACS Sustain. Chem. Eng. 2018, 7, 1052–1060. [Google Scholar] [CrossRef]
- Donati, N.; Spada, J.C.; Tessaro, I.C. Recycling rice husk ash as a filler on biodegradable cassava starch-based foams. Polym. Bull. 2022, 80, 10231–10248. [Google Scholar] [CrossRef]
- Santana, J.S.; Rosário, J.M.D.; Pola, C.C.; Otoni, C.G.; FerreiraSoares, N.D.F.; Camilloto, G.P.; Cruz, R.S. Cassava starch-based nanocomposites reinforced with cellulose nanofibers extracted from sisal. J. Appl. Polym. Sci. 2016, 134, 44637. [Google Scholar] [CrossRef]
- Santana, J.S.; Costa, É.K.D.C.; Rodrigues, P.R.; Correia, P.R.C.; Cruz, R.S.; Druzian, J.I. Morphological, barrier, and mechanical properties of cassava starch films reinforced with cellulose and starch nanoparticles. J. Appl. Polym. Sci. 2018, 136, 47001. [Google Scholar] [CrossRef]
- Lago, R.C.D.; Zitha, E.Z.M.; de Oliveira, A.L.M.; de Abreu, D.J.M.; Carvalho, E.E.N.; Piccoli, R.H.; Tonoli, G.H.D.; Boas, E.V.d.B.V. Effect of coating with co-product-based bionanocomposites on the quality of strawberries under refrigerated storage. Sci. Hortic. 2022, 309, 111668. [Google Scholar] [CrossRef]
- Martinez, C.V.V.; Murillo, X.S.Z.; Demera, M.H.D.; Briones, G.A.B.; Palacios, C.A.C. Almidones de Cáscara de Yuca (Manihot Esculenta) y Papa (Solanum Tuberosum) para Producción de Bioplásticos: Propiedades Mecánicas y Efecto Gelatinizante. Rev. Bases De La Cienc. 2021, 6, 137–152. [Google Scholar] [CrossRef]
- Lilavanichakul, A.; Yoksan, R. Development of Bioplastics from Cassava toward the Sustainability of Cassava Value Chain in Thailand. Sustainability 2023, 15, 14713. [Google Scholar] [CrossRef]
- Fernando, N.M.L.; Amaraweera, A.P.S.M.; Gunawardane, O.H.P.; Wanninayaka, W.M.D.B.; Manipura, A.; Manamperi, W.A.R.; Gunathilake, C.A.; Kulathunga, K.M.A.K. Sustainable Biorefinery Approach for Cassava: A Review. Eng. J. Inst. Eng. Sri Lanka 2022, 55, 71–88. [Google Scholar] [CrossRef]
- Figueiró, C.D.S.; Trojaner, M.R.; Calcagno, C.I.W.; Santana, R.M.C. Rheological and structural characterization of cassava starches foam with low and high amylose contents. J. Polym. Res. 2022, 29, 1–8. [Google Scholar] [CrossRef]
- Dodino-Duarte, I.; Quiroz-Ortega, L.A.; Arias-Benítez, J.C.; García-León, R.A. Development of biodegradable plastic films from cassava starch. DYNA 2024, 91, 75–85. [Google Scholar] [CrossRef]
- Bishnoi, A.; Chandla, N.K.; Talwar, G.; Khatkar, S.K.; Sharma, A.; Anupam, R. Mechanical Strength, Solubility, and Functional Studies of Developed Composite Biopolymeric Film. J. Food Process. Preserv. 2023, 2023, 5108490. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. In Proceedings of the 2nd International Symposium on Green Technology for Value Chains, Jakarta, Indonesia, 23–24 October 2017. [Google Scholar]
- Luchese, C.L.; Benelli, P.; Spada, J.C.; Tessaro, I.C. Impact of the starch source on the physicochemical properties and biodegradability of different starch-based films. J. Appl. Polym. Sci. 2018, 135, 46564. [Google Scholar] [CrossRef]
- Chen, X.; Yao, W.; Gao, F.; Zheng, D.; Wang, Q.; Cao, J.; Tan, H.; Zhang, Y. Physicochemical Properties Comparative Analysis of Corn Starch and Cassava Starch, and Comparative Analysis as Adhesive. J. Renew. Mater. 2021, 9, 979–992. [Google Scholar] [CrossRef]
- Tezera, A.; Abera, D.; Bantie, Z.; Bisenebt, B.; Beyene, A.; Deslaegn, E.; Nega, T.; Demeke, M.; Aregahegn, S.; Abrham, T.; et al. Evaluation and Optimization of Sustainable Ecofriendly Bio-based Micro-composites for Smart Packaging Application Using Micro-Clay Particles as Reinforcement. In Green Energy and Technology; Springer Nature: Berlin, Germany, 2025. [Google Scholar] [CrossRef]
- Thuppahige, V.T.W.; Moghaddam, L.; Welsh, Z.G.; Wang, T.; Karim, A. Investigation of critical properties of Cassava (Manihot esculenta) peel and bagasse as starch-rich fibrous agro-industrial wastes for biodegradable food packaging. Food Chem. 2023, 422, 136200. [Google Scholar] [CrossRef]
- Fronza, P.; Batista, M.J.P.A.; Franca, A.S.; Oliveira, L.S. Bionanocomposite Based on Cassava Waste Starch, Locust Bean Galactomannan, and Cassava Waste Cellulose Nanofibers. Foods 2024, 13, 202. [Google Scholar] [CrossRef]
- Silveira, Y.D.O.; Franca, A.S.; Oliveira, L.S. Cassava Waste Starch as a Source of Bioplastics: Development of a Polymeric Film with Antimicrobial Properties. Foods 2025, 14, 113. [Google Scholar] [CrossRef]
- Santana, I.; Felix, M.; Bengoechea, C. Sustainable Biocomposites Based on Invasive Rugulopteryx okamurae Seaweed and Cassava Starch. Sustainability 2023, 16, 76. [Google Scholar] [CrossRef]
- Supardi, Y.A.; Karmini, M. Effect of using cassava and glycerol as food storage on the quality of bioplastic packaged food. Heal. Low-Resour. Settings 2023, 11, 1–13. [Google Scholar] [CrossRef]
- Vargas-García, Y.; Pazmiño-Sánchez, J.; Dávila-Rincón, J. Potencial de Biomasa en América del Sur para la Producción de Bioplásticos. Rev. Politec. 2021, 48, 7–20. [Google Scholar] [CrossRef]
- Muñoz, S.B.; Riera, M.A. Residuos de la cáscara de yuca y cera de abejas como potenciales materiales de partida para la producción de bioplásticos. Av. Química 2020, 15, 3–11. [Google Scholar]
- Florencia, V.; López, O.V.; García, M.A. Exploitation of by-products from cassava and ahipa starch extraction as filler of thermoplastic corn starch. Compos. Part B: Eng. 2020, 182, 107653. [Google Scholar] [CrossRef]
- Mantovan, J.; Bersaneti, G.T.; Faria-Tischer, P.C.; Celligoi, M.A.P.C.; Mali, S. Use of microbial levan in edible films based on cassava starch. Food Packag. Shelf Life 2018, 18, 31–36. [Google Scholar] [CrossRef]
- Andretta, R.; Luchese, C.L.; Tessaro, I.C.; Spada, J.C. Development and characterization of pH-indicator films based on cassava starch and blueberry residue by thermocompression. Food Hydrocoll. 2019, 93, 317–324. [Google Scholar] [CrossRef]
- Engel, J.B.; Ambrosi, A.; Tessaro, I.C. Development of biodegradable starch-based foams incorporated with grape stalks for food packaging. Carbohydr. Polym. 2019, 225, 115234. [Google Scholar] [CrossRef]
- Knapp, M.A.; dos Santos, D.F.; Pilatti-Riccio, D.; Deon, V.G.; dos Santos, G.H.F.; Pinto, V.Z. Yerba mate extract in active starch films: Mechanical and antioxidant properties. J. Food Process. Preserv. 2019, 43, 13897. [Google Scholar] [CrossRef]
- Xavier, T.D.N.; de Oliveira, V.R.L.; Leite, R.H.d.L.; Aroucha, E.M.M.; dos Santos, F.K.G. Filmes biopoliméricos baseados em fécula, quitosana e cera de carnaúba e suas propriedades. Mater. Jan. 2020, 25, 1517–707620200004. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Molina, G.; Pelissari, F.M. Biodegradable trays based on cassava starch blended with agroindustrial residues. Compos. Part B: Eng. 2020, 183, 107682. [Google Scholar] [CrossRef]
- Ferreira, L.F.; de Oliveira, A.C.S.; de Oliveira Begali, D.; de Sena Neto, A.R.; Martins, M.A.; de Oliveira, J.E.; Borges, S.V.; Yoshida, M.I.; Tonoli, G.H.D.; Dias, M.V. Characterization of cassava starch/soy protein isolate blends obtained by extrusion and thermocompression. Ind. Crops Prod. 2021, 160, 113092. [Google Scholar] [CrossRef]
- Versino, F.; López, O.V.; García, M.A. Sunflower Oil Industry By-product as Natural Filler of Biocomposite Foams for Packaging Applications. J. Polym. Environ. 2021, 29, 1869–1879. [Google Scholar] [CrossRef]
- Carpes, S.T.; Bertotto, C.; Bilck, A.P.; Yamashita, F.; Anjos, O.; Siddique, A.B.; Harrison, S.M.; Brunton, N.P. Bio-based films prepared with apple pomace: Volatiles compound composition and mechanical, antioxidant and antibacterial properties. LWT 2021, 144, 111241. [Google Scholar] [CrossRef]
- Pereira, G.V.D.S.; Neves, E.M.P.X.; Albuquerque, G.A.; Rêgo, J.D.A.R.D.; Cardoso, D.N.P.; Brasil, D.D.S.B.; Joele, M.R.S.P. Effect of the Mixture of Polymers on the Rheological and Technological Properties of Composite Films of Acoupa Weakfish (Cynoscion acoupa) and Cassava Starch (Manihot esculenta C.). Food Bioprocess Technol. 2021, 14, 1199–1215. [Google Scholar] [CrossRef]
- Laureanti, E.J.G.; Paiva, T.S.; Tasso, I.D.S.; Dallabona, I.D.; Helm, C.V.; Jorge, L.M.D.M.; Jorge, R.M.M. Development of active cassava starch films reinforced with waste from industrial wine production and enriched with pink pepper extract. J. Appl. Polym. Sci. 2021, 138, 50922. [Google Scholar] [CrossRef]
- Araya, J.; Esquivel, M.; Jimenez, G.; Navia, D.; Poveda, L. Antimicrobial activity and physicochemical characterization of thermoplastic films based on bitter cassava starch, nanocellulose and rosemary essential oil. J. Plast. Film. Sheeting 2021, 38, 46–71. [Google Scholar] [CrossRef]
- La Fuente, C.I.; Siqueira, L.D.V.; Augusto, P.E.D.; Tadini, C.C. Casting and extrusion processes to produce bio-based plastics using cassava starch modified by the dry heat treatment (DHT). Innov. Food Sci. Emerg. Technol. 2022, 75, 102906. [Google Scholar] [CrossRef]
- González, C.M.O.; Schelegueda, L.I.; Ruiz-Henestrosa, V.M.P.; Campos, C.A.; Basanta, M.F.; Gerschenson, L.N. Cassava Starch Films with Anthocyanins and Betalains from Agroindustrial by-Products: Their Use for Intelligent Label Development. Foods 2022, 11, 3361. [Google Scholar] [CrossRef]
- Bertotto, C.; Bilck, A.P.; Yamashita, F.; Anjos, O.; Siddique, A.B.; Harrison, S.M.; Brunton, N.P.; Carpes, S.T. Development of a biodegradable plastic film extruded with the addition of a Brazilian propolis by-product. LWT 2022, 157, 113124. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Quintero-Borregales, L.M.; Goyanes, S.; Bernal, C.; Famá, L. A Biodegradable and Active Bilayer Nanocomposite Obtained from Starch-Yerba Mate Extract and Starch-TiO2 Nanoparticles Films for Packaging Applications. Starch-Starke 2023, 76, 2300048. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Villabona-Ortíz, Á.; Ortega-Toro, R. Sustainable Starch-Based Films from Cereals and Tubers: A Comparative Study on Cherry Tomato Preservation. Polymers 2024, 16, 2913. [Google Scholar] [CrossRef]
- Rada-Mendoza, M.D.P.; Villamiel-Guerra, M.D.M.; Hoyos-Saavedra, O.L.; Alvira, L.F. Quantification of lead using atomic absorption spectrometry in thermoformed and biodegradable flexible films made from cassava (Manihot esculenta crantz). DYNA 2018, 85, 236–242. [Google Scholar] [CrossRef]
- Rada-Mendoza, M.; Arciniegas-Herrera, J.L.; Hoyos-Saavedra, O.L.; Del Castillo, R.; Chito-Trujillo, D. Atomic absorption spectrometry for the quantification of cadmium in thermoformed and biodegradable flexible films made from cassava (Manihot esculenta crantz). J. Thermoplast. Compos. Mater. 2019, 34, 657–670. [Google Scholar] [CrossRef]
- Maite, R.-M.; Diana, C.-T.; Lucía, H.-S.O.; Luis, A.-H.J.; Janneth, M.-T.N. Determination of zinc in cassava based polymeric materials. J. Thermoplast. Compos. Mater. 2021, 36, 615–625. [Google Scholar] [CrossRef]
- Luchese, C.L.; Pavoni, J.M.F.; dos Santos, N.Z.; Quines, L.K.; Pollo, L.D.; Spada, J.C.; Tessaro, I.C. Effect of chitosan addition on the properties of films prepared with corn and cassava starches. J. Food Sci. Technol. 2018, 55, 2963–2973. [Google Scholar] [CrossRef]
- Alarcón-Hernández, C.d.J.; González-García, E.A.; Medina-Torres, L.; Morales-Pacheco, P. Rheological effect of the concentration of nanoparticles in cassava starch. MRS Adv. 2019, 4, 2889–2896. [Google Scholar] [CrossRef]
- Colivet, J.; Garcia, V.A.d.S.; Lourenço, R.V.; Yoshida, C.M.P.; de Oliveira, A.L.; Vanin, F.M.; de Carvalho, R.A. Characterization of Films Produced with Cross-Linked Cassava Starch and Emulsions of Watermelon Seed Oils. Foods 2022, 11, 3803. [Google Scholar] [CrossRef]
- Ramos, A.R.; Manalo, R.D.; Ong, H.L.; Herrera, M.U.; Balela, M.D.L. The Effects of Citric Acid as Crosslinking Agent on Selected Properties of Cassava Starch Films. Mater. Sci. Forum 2023, 1087, 29–34. [Google Scholar] [CrossRef]
- Hernández, M.S.; Ludueña, L.N.; Flores, S.K. Citric acid, chitosan and oregano essential oil impact on physical and antimicrobial properties of cassava starch films. Carbohydr. Polym. Technol. Appl. 2023, 1–13. [Google Scholar] [CrossRef]
- Lacovone, C.; Guz, L.; Famá, L. Sustainable innovations in food packaging: Antioxidant basil-enriched cassava starch films with UV protection and enhanced water and mechanical resistance. Food Packag. Shelf Life 2024, 45, 101324. [Google Scholar] [CrossRef]
- Ridhwan, J.; Mohamad, R.; Fareeq, R.; Faudzi, M.; Aziz; Ruzanna, A.; Keshavarz; Meysam; Salleh; Suhaimi, T.; et al. Water Transport Properties of Thermoplastic Cassava Starch/Beeswax Reinforced with Cogon Grass Fiber. J. Adv. Res. Fluid Mech. Therm. Sci. 2022, 95, 55–61. [Google Scholar] [CrossRef]
- Jumaidin, R.; Wong, J.; Aziz, A.R.; Keshavarz, M.; Salleh, T.S.; Ilyas, R.A.; Munir, F.A.; Taha, M.M.; Razali, N.; Saleman, A.R.; et al. Water Absorption and Environmental Properties of Thermoplastic Cassava Starch Reinforced with Durian Skin Fiber. J. Adv. Res. Fluid Mech. Therm. Sci. 2022, 96, 115–121. [Google Scholar] [CrossRef]
- Pongsa, U.; Sangrayub, P.; Saengkhiao, P.; Lumsakul, P.; Kaweegitbundit, P.; Kasemsiri, P.; Hiziroglu, S. Properties of biodegradable foam composites made from coconut residue as a function of the re-inforcing phase of cassava starch. Eng. Appl. Sci. Res. 2023, 50, 270–277. [Google Scholar] [CrossRef]
- Méité, N.; Kouakou, L.P.M.-S.; Kouamé, A.N.; Kouassi, S.S.; e Silva, C.S.; Sandé, S.L.S.; Pereira, S.D.F.P.; Konan, L.K. Physical and chemical properties of cassava starch biopolymer reinforced with coconut fiber and/or metakaolin. J. Indian Chem. Soc. 2024, 101, 101185. [Google Scholar] [CrossRef]
- Maitha, I.M.; Okoth, M.W.; Njue, L.G.; Mbuge, D.O. Development of eco-friendly food packaging bio-film from cassava starch plasticized with coconut oil. East Afr. J. Sci. Technol. Innov. 2024, 5, 1–14. [Google Scholar]
- Jumaidin, R.; Rahman, A.H.A.; Sapuan, S.M.; Rushdan, A.I. Effect of sugarcane bagasse on thermal and mechanical properties of thermoplastic cassava starch/beeswax composites. Phys. Sci. Rev. 2022, 9, 1–15. [Google Scholar] [CrossRef]
- Kamaruddin, Z.H.; Jumaidin, R.; Ilyas, R.A.; Selamat, M.Z.; Alamjuri, R.H.; Yusof, F.A.M. Biocomposite of Cassava Starch-Cymbopogan Citratus Fibre: Mechanical, Thermal and Biodegradation Properties. Polymers 2022, 14, 514. [Google Scholar] [CrossRef] [PubMed]
- Tarique, J.; Sapuan, S.; Zainudin, E.; Khalina, A.; Ilyas, R.; Hazrati, K.; Aliyu, I. A comparative review of the effects of different fibre concentrations on arrowroot fibre and other fibre-reinforced composite films. Mater. Today Proc. 2022, 74, 411–414. [Google Scholar] [CrossRef]
- Diyana, Z.N.; Jumaidin, R.; Selamat, M.Z.; Suan, M.S.M.; Hazrati, K.Z.; Yusof, F.A.M.; Ilyas, R.A.; Eldin, S.M. Effect of Pandanus Amaryllifolius Fibre on Physio-Mechanical, Thermal and Biodegradability of Thermoplastic Cassava Starch/Beeswax Composites. J. Polym. Environ. 2023, 32, 1406–1422. [Google Scholar] [CrossRef]
- Marhaini, M.; Fernianti, D.; Harbi, J.; Sintya, G. Sorbitol-Based Biodegradable Plastics from Rubberized Cassava Starch and Tofu Dregs Starch. J. Ecol. Eng. 2024, 25, 380–385. [Google Scholar] [CrossRef]
- Fan, W.; Zhao, J.; Zong, H.; Li, Q. Effect of chitosan concentration on physicochemical properties of starch-based straws. Green Mater. 2023, 11, 50–59. [Google Scholar] [CrossRef]
- Albar, N.; Anuar, S.T.; Azmi, A.A.; Soh, S.K.C.; Bhubalan, K.; Ibrahim, Y.S.; Khalik, W.M.A.W.M.; Abdullah, N.S.; Yahya, N.K.E. Chemical, Mechanical, and Wettability Properties of Bioplastic Material from Manihot esculenta Cassava–Chitosan Blends as Plastic Alternative. Starch-Starke 2024, 77, 2300278. [Google Scholar] [CrossRef]
- Worawut, K.; Phungpis, B.; Kaewkumsan, P.; Noppawan, P. Film Development of Chitosan from Mussel Shells and Pacific White Shrimp Shells. ASEAN J. Sci. Technol. Rep. 2024, 27, 254066. [Google Scholar] [CrossRef]
- Promhuad, K.; Bumbudsanpharoke, N.; Wadaugsorn, K.; Sonchaeng, U.; Harnkarnsujarit, N. Maltol-Incorporated Acetylated Cassava Starch Films for Shelf-Life-Extension Packaging of Bakery Products. Polymers 2022, 14, 5342. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Sun, H.; Nagassa, M.; Li, X.; Pei, H.; Liu, S.; Gu, Y.; He, S. Edible film preparation by anthocyanin extract addition into acetylated cassava starch/sodium carboxymethyl cellulose matrix for oxidation inhibition of pumpkin seeds. Int. J. Biol. Macromol. 2024, 267, 131439. [Google Scholar] [CrossRef]
- Jati, I.R.A.; Kamaluddin, M.A.; Utomo, A.R.; Setijawaty, E.; Edward, E.; Nugraha, D.T. Red cabbage and eggshell powder as active agent on cassava starch-based edible films: It’s physicochemical properties and application. Nutr. Food Sci. 2024, 55, 423–437. [Google Scholar] [CrossRef]
- Thipchai, P.; Punyodom, W.; Jantanasakulwong, K.; Thanakkasaranee, S.; Hinmo, S.; Pratinthong, K.; Kasi, G.; Rachtanapun, P. Preparation and Characterization of Cellulose Nanocrystals from Bamboos and Their Application in Cassava Starch-Based Film. Polymers 2023, 15, 2622. [Google Scholar] [CrossRef] [PubMed]
- Jumaidin, R.; Gazari, A.S.; Kamaruddin, Z.H.; Zakaria, N.H.; Kudus, S.I.A.; Yob, M.S.; Munir, F.A.; Keshavarz, M. Mechanical Properties of Thermoplastic Cassava Starch/Coconut Fibre Composites: Effect of Fibre Size. Pertanika J. Sci. Technol. 2024, 32, 91–113. [Google Scholar] [CrossRef]
- Boonphayak, P.; Muenyong, N.; Chinchao, R.; Khansumled, S. Development of a Biodegradable Cassava Starch Biofilm based on a Combination of Plasticizers with Bio-SiO2 Extracted from Sugarcane Leaves. Starch-Starke 2024, 77, 2300164. [Google Scholar] [CrossRef]
- Promsorn, J.; Harnkarnsujarit, N. Oxygen absorbing food packaging made by extrusion compounding of thermoplastic cassava starch with gallic acid. Food Control. 2022, 142, 109273. [Google Scholar] [CrossRef]
- Joshi, P.; Gupta, K.; Uniyal, P.; Jana, A.; Banerjee, A.; Kumar, N.; Ghosh, D.; Srivastava, M.; Ray, A.; Khatri, O.P. Cassava starch-derived aerogels as biodegradable packaging materials. Mater. Chem. Phys. 2022, 296, 127282. [Google Scholar] [CrossRef]
- Kavas, E.; Terzioğlu, P.; Sıcak, Y. Betanin and Nano-Calcium Carbonate Incorporated Polyvinyl alcohol/Starch Films for Active and Intelligent Packaging Applications. J. Polym. Environ. 2023, 31, 4919–4929. [Google Scholar] [CrossRef]
- Dilkushi, H.; Jayarathna, S.; Manipura, A.; Chamara, H.; Edirisinghe, D.; Vidanarachchi, J.; Priyashantha, H. Development and characterization of biocomposite films using banana pseudostem, cassava starch and poly(vinyl alcohol): A sustainable packaging alternative. Carbohydr. Polym. Technol. Appl. 2024, 7, 100472. [Google Scholar] [CrossRef]
- Krümmel, A.; Pagno, C.H.; Malheiros, P.D.S. Active Films of Cassava Starch Incorporated with Carvacrol Nanocapsules. Foods 2024, 13, 1141. [Google Scholar] [CrossRef]
- Bazana, M.T.; da Silveira Cáceres de Menezes, M.F.; da Silva, S.S.; Deus, C.; Lucas, B.N.; Codevilla, C.F.; Mazutti, M.; Barin, J.S.; de Bona da Silva, C.; de Menezes, C.R. Study of stability and antioxidant capacity of nanoemulsions containing the extract of the calyx of Physalis peruviana. In Proceedings of the 1st International Congress on Bioactive Compounds and 2nd International Workshop on Bioactive Compounds, Galoá, Fiji, 22–23 November 2018; Available online: https://proceedings.science/icbc/icbc-2018/papers/study-of-stability-and-antioxidant-capacity-of-nanoemulsions-containing-the-extr?lang=en (accessed on 22 April 2025).
- Lin, Z.; Cheng, H.; He, K.; McClements, D.J.; Jin, Z.; Xu, Z.; Meng, M.; Peng, X.; Chen, L. Recent progress in the hydrophobic modification of starch-based films. Food Hydrocoll. 2024, 151, 109860. [Google Scholar] [CrossRef]
- Kumari, S.; Kaur, B.P.; Thiruvalluvan, M. Ultrasound modified millet starch: Changes in functional, pasting, thermal, structural, in vitro digestibility properties, and potential food applications. Food Hydrocoll. 2024, 153, 110008. [Google Scholar] [CrossRef]
- Jayarathna, S.; Andersson, M.; Andersson, R. Recent Advances in Starch-Based Blends and Composites for Bioplastics Applications. Polymers 2022, 14, 4557. [Google Scholar] [CrossRef] [PubMed]
- Sivapragasam, N.; Maqsood, S.; Rupasinghe, H.V. Berry bioactive compounds immobilized in starch matrix for active and intelligent packaging: A review. Futur. Foods 2024, 10, 100397. [Google Scholar] [CrossRef]
- Cuaces, A.F.V.; Moreira, W.L.C.; Anchundia, B.J.C. Obtención de polímeros biodegradables a partir del almidón de yuca. MQRInvestigar 2023, 7, 2680–2700. [Google Scholar] [CrossRef]
- Subroto, E.; Cahyana, Y.; Indiarto, R.; Rahmah, T.A. Modification of Starches and Flours by Acetylation and Its Dual Modifications: A Review of Impact on Physicochemical Properties and Their Applications. Polymers 2023, 15, 2990. [Google Scholar] [CrossRef]
- León-Méndez, G.; León-Méndez, D.; Monroy-Arellano, M.R.; Espriella-Angarita, D.L.; Herrera-Barros, A. Modificación química de almidones Chemical modification of starches through esterification reactions and their potential use in the cosmetic industry. AVFT Arch. Venez. De Farmacol. Y Terapéutica 2020, 5, 620–629. [Google Scholar] [CrossRef]
- Marta, H.; Wijaya, C.; Sukri, N.; Cahyana, Y.; Mohammad, M. A Comprehensive Study on Starch Nanoparticle Potential as a Reinforcing Material in Bioplastic. Polymers 2022, 14, 4875. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.S.; Ludueña, L.N.; Flores, S.K. Combined effect of oregano essential oil and glycerol on physicochemical properties of antimicrobial films based on chitosan and acetylated cassava starch. Food Hydrocoll. 2024, 156, 110259. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Suri, S.; Barua, S.; Phimolsiripol, Y. Native and modified biodegradable starch-based packaging for shelf-life extension and safety of fruits/vegetables. Int. J. Food Sci. Technol. 2022, 58, 862–870. [Google Scholar] [CrossRef]
- Muñoz-Gimena, P.F.; Oliver-Cuenca, V.; Peponi, L.; López, D. A Review on Reinforcements and Additives in Starch-Based Composites for Food Packaging. Polymers 2023, 15, 2972. [Google Scholar] [CrossRef] [PubMed]
- Monroy, Y.; Cabezas, D.M.; Rivero, S.; García, M.A. Structural analysis and adhesive capacity of cassava starch modified with NaOH:urea mixtures. Int. J. Adhes. Adhes. 2023, 126, 103470. [Google Scholar] [CrossRef]
- Monroy, Y.; García, M.; Deladino, L.; Rivero, S. Valorization of a by-product of the yerba mate industry by assembling with cassava starch adhesive for packaging material production. Int. J. Biol. Macromol. 2024, 266, 131271. [Google Scholar] [CrossRef]
- Reyes, G.V.C.; Marinero-Orantes, E.A.; Funes-Guadrón, C.R.; Toruño, P.J. Biopolímeros para uso agro industrial: Alternativa sostenible para la elaboración de una película de almidón termo plástico biodegradable. Rev. Iberoam. Bioecon. Cambio Clim. 2020, 6, 1359–1382. [Google Scholar] [CrossRef]
- Tafa, T.G.; Engida, A.M. Preparation of green film with improved physicochemical properties and enhanced antimicrobial activity using ingredients from cassava peel, bamboo leaf and rosemary leaf. Heliyon 2022, 8, 10130. [Google Scholar] [CrossRef]
- Nogueira, M.R.; Paiva, P.D.D.O.; Neto, A.R.D.C.; dos Reis, M.V.; Nascimento, Â.M.P.; Timoteo, C.D.O. Starch-based films for Red Torch ginger inflorescences postharvest conservation. Cienc. E Agrotecnologia 2023, 47, 017822. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A. Compatibilization of biopolymer blends: A review. Adv. Ind. Eng. Polym. Res. 2024, 7, 373–404. [Google Scholar] [CrossRef]
- Mueller, E.; Hoffmann, T.G.; Schmitz, F.R.W.; Helm, C.V.; Roy, S.; Bertoli, S.L.; de Souza, C.K. Development of ternary polymeric films based on cassava starch, pea flour and green banana flour for food packaging. Int. J. Biol. Macromol. 2023, 256, 128436. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.; Wei, Y.; Liu, P.; Deng, X.; Lei, Y.; Zhang, J. Preparation and properties of films loaded with cellulose nanocrystals stabilized Thymus vulgaris essential oil Pickering emulsion based on modified tapioca starch/polyvinyl alcohol. Food Chem. 2023, 435, 137597. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.D.D.F.; Siqueira, L.D.V.; Tadini, C.C.; Favaro-Trindade, C.S. Characterization of Cassava Starch Extruded Sheets Incorporated with Tucumã Oil Microparticles. Processes 2023, 11, 876. [Google Scholar] [CrossRef]
- Khan, A.A.; Ullah, M.W.; Qayum, A.; Khalifa, I.; Ul-Islam, M.; Bacha, S.A.S.; Zeb, U.; Yao, F.-J.; Alharbi, S.A.; Shrahili, M.; et al. Structure-property relationship of ultrasound-assisted nanoemulsion-impregnated bioactive polysaccharide films for enhanced shelf life of mushrooms. Food Packag. Shelf Life 2024, 46, 101372. [Google Scholar] [CrossRef]
- Khan, A.A.; Cui, F.-J.; Ullah, M.W.; Qayum, A.; Khalifa, I.; Bacha, S.A.S.; Ying, Z.-Z.; Khan, I.; Zeb, U.; Alarfaj, A.A.; et al. Fabrication and characterization of bioactive curdlan and sodium alginate films for enhancing the shelf life of Volvariella volvacea. Food Biosci. 2024, 62, 105137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).