The Possibilities of Using Non-Traditional Raw Materials for Fertilizing Products
Abstract
1. Introduction
1.1. The Development and Trends of Sustainability Awareness
1.2. The Expansion of the Insect Market
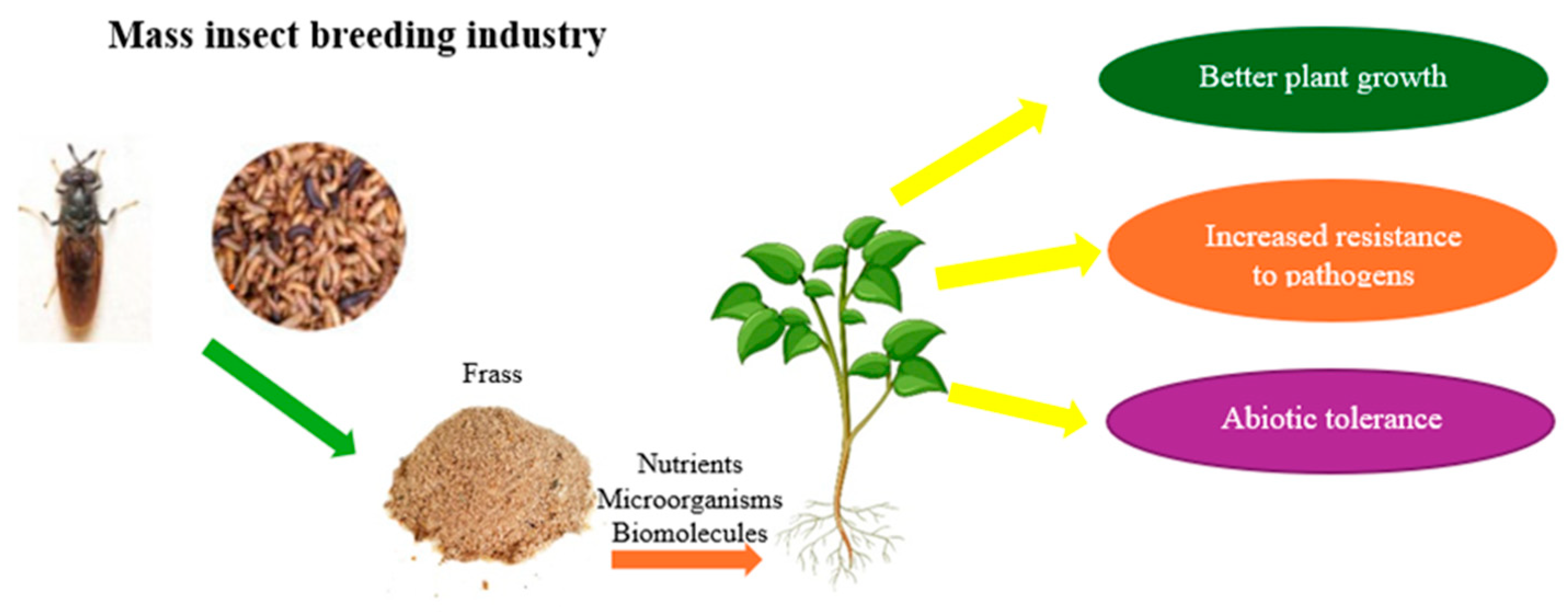
1.3. Opportunities for the Utilization of Generated Waste
2. Materials and Methods
2.1. Materials and Sample Preparation for Analysis
2.2. Physical Analysis
2.3. Chemical Analysis
2.4. Instrumental Analysis
2.5. Statistical Data Analysis
3. Results and Discussion
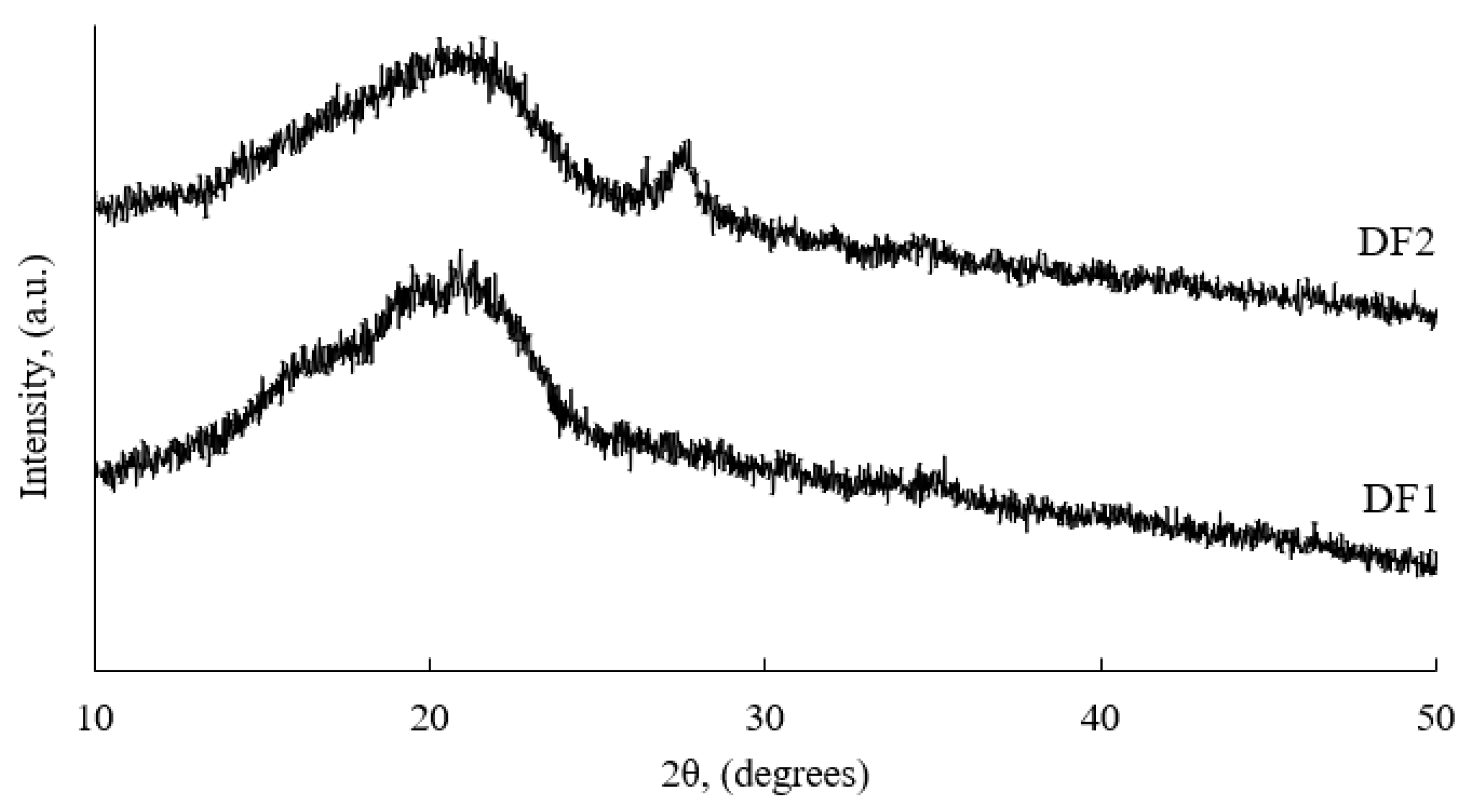
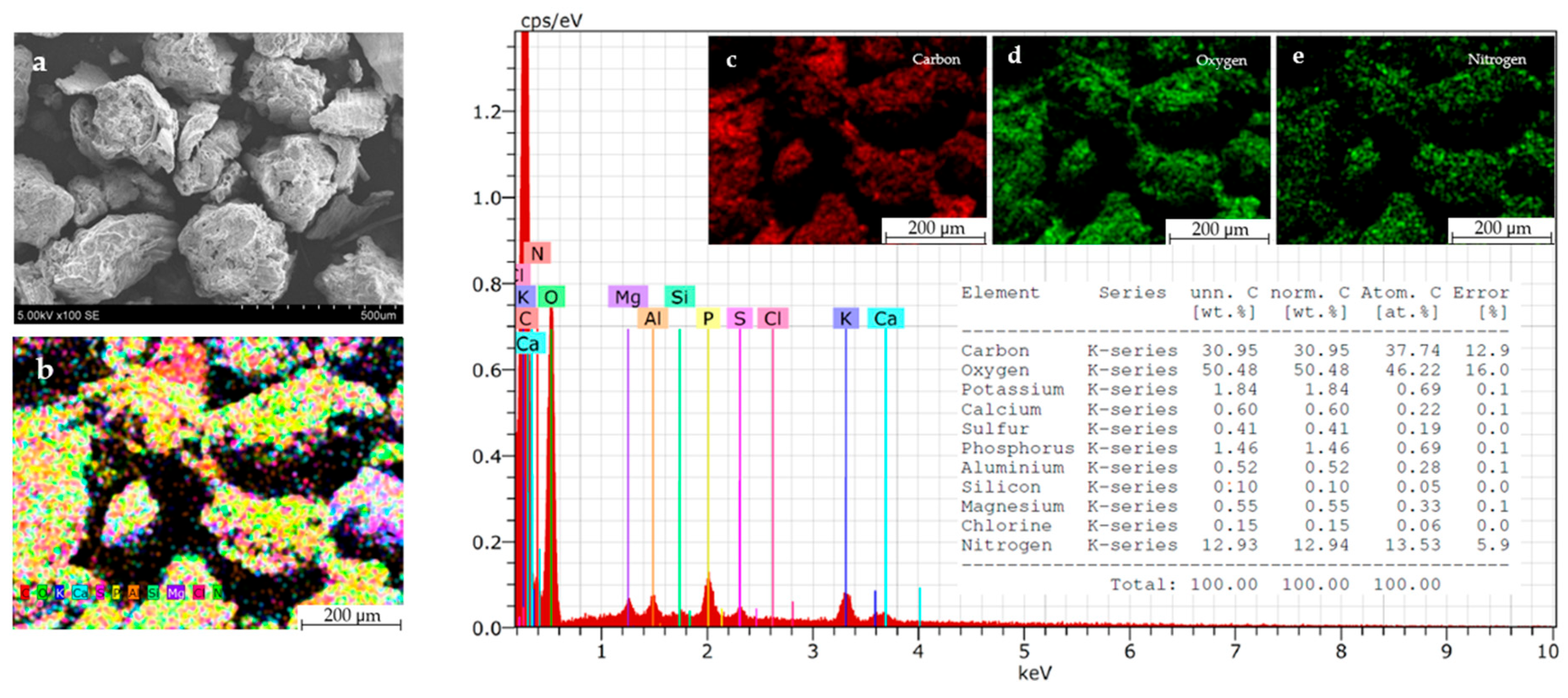
3.1. The Physical Properties and Chemical Composition of Frass Samples
3.2. The Chemical Composition of Frass Ash
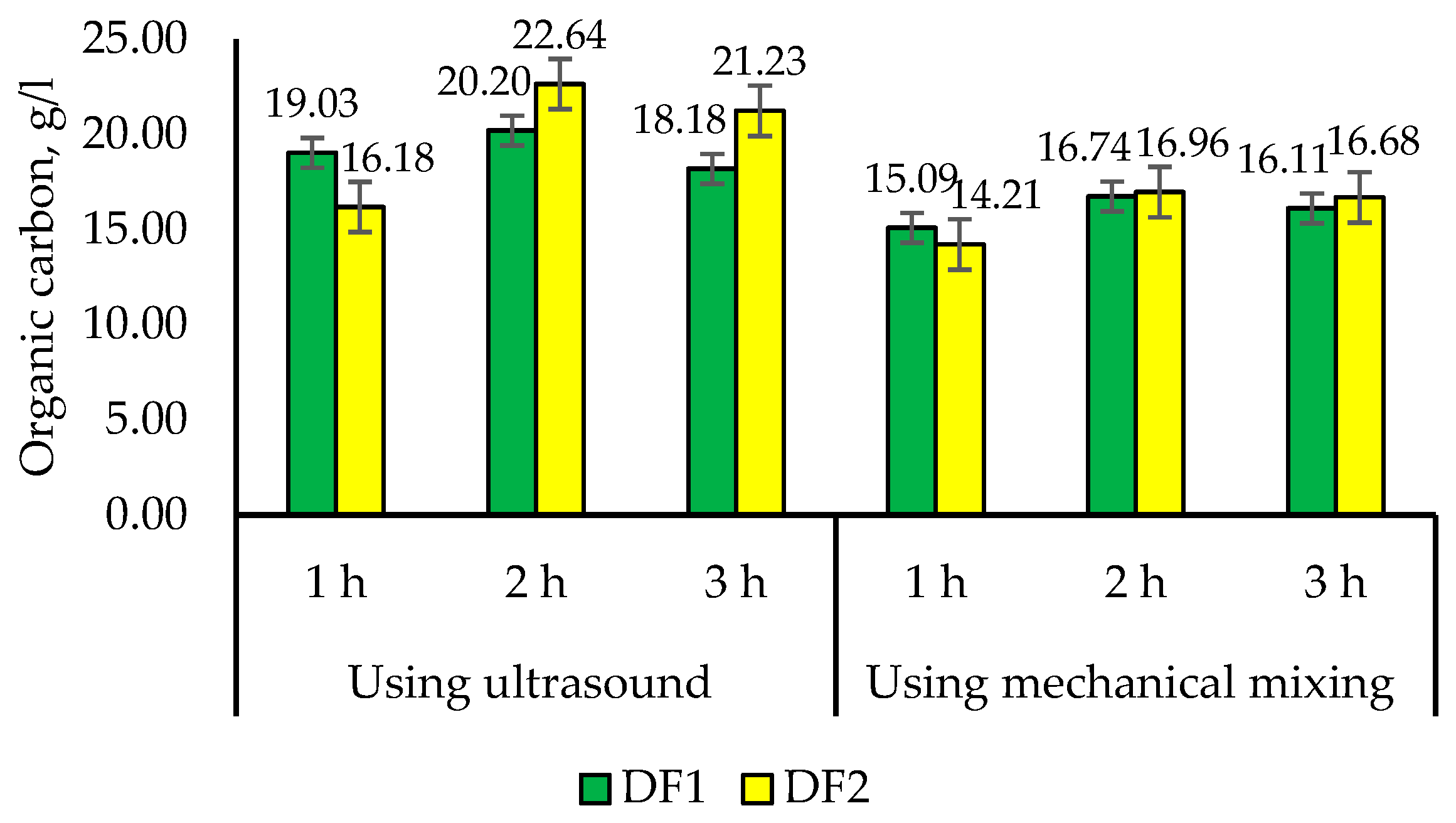
3.3. Preparation of Frass Extracts and Their Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission: Brussels, Belgium, 2020.
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Malik, K.; Moore, J.M.; Kamboj, B.R.; Malik, S.; Malik, V.K.; Arya, S.; Singh, K.; Mahanta, S.; Bishnoi, D.K. Valorisation of agri-food waste for bioactive compounds: Recent trends and future sustainable challenges. Molecules 2024, 29, 2055. [Google Scholar] [CrossRef]
- Leni, G.; Caligiani, A.; Sforza, S. Bioconversion of agri-food waste and by-products through insects: A new valorization opportunity. In Valorization of Agri-Food Wastes and By-Products; Academic Press: Cambridge, MA, USA, 2021; pp. 809–828. [Google Scholar]
- Errico, S.; Spagnoletta, A.; Verardi, A.; Moliterni, S.; Dimatteo, S.; Sangiorgio, P. Tenebrio molitor as a source of interesting natural compounds, their recovery processes, biological effects, and safety aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 148–197. [Google Scholar] [CrossRef]
- Liceaga, A.M. Edible insects, a valuable protein source from ancient to modern times. Adv. Food Nutr. Res. 2022, 101, 129–152. [Google Scholar] [PubMed]
- Tang, C.; Yang, D.; Liao, H.; Sun, H.; Liu, C.; Wei, L.; Li, F. Edible insects as a food source: A review. Food Prod. Process. Nutr. 2019, 1, 8. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Jung, C. Insects used as food and feed: Isn’t that what we all need? Foods 2020, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed 2020, 6, 27–44. [Google Scholar] [CrossRef]
- Grand View Research. Edible Insects Market Size, Share & Trends Analysis Report by Product (Beetles, Caterpillar, Cricket), by Application (Powder, Protein Bars), by Region (North America, Europe, Asia Pacific, Latin America, MEA), and Segment Forecasts, 2025–2030; Grand View Research: San Francisco, CA, USA, 2024. [Google Scholar]
- Research and Markets. Insect Protein Market by Application (Food & Beverages, Animal Nutrition, Pharmaceutical & Cosmetics), Insect Type (Cricket, Grasshoppers, Ants, Mealworms, Black Soldier Flies, and Others), Distribution Channel and Region-Global Forecast to 2027. 2022. Available online: https://www.researchandmarkets.com/reports/5651252/insect-protein-market-by-insect-type-cricket?srsltid=AfmBOoqVWhSur86voGS0yu_7DSAoBhb1AYTmXaFzrT2hXxiJB6btkA0j (accessed on 6 May 2025).
- European Commission. Commission Regulation (EU) 2021/1372 of 17 August 2021 Amending Annex IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council as Regards the Prohibition to Feed Non-Ruminant Farmed Animals, Other than Fur Animals, with Protein Derived from Animals (Text with EEA Relevance). Off. J. Eur. Union 2021, 1–10. [Google Scholar]
- European Food Safety Authority (EFSA). Nutrition, Novel Foods and Food Allergens (NDA) Panel. Available online: https://www.efsa.europa.eu/en/science/scientific-committee-and-panels/nda (accessed on 6 May 2025).
- Adamaki-Sotiraki, C.; Rumbos, C.I.; Athanassiou, C.G. From a stored-product pest to a promising protein source: A U-turn of human perspective for the yellow mealworm Tenebrio molitor. J. Pest Sci. 2025, 98, 113–129. [Google Scholar] [CrossRef]
- Moruzzo, R.; Riccioli, F.; Espinosa Diaz, S.; Secci, C.; Poli, G.; Mancini, S. Mealworm (Tenebrio molitor): Potential and challenges to promote circular economy. Animals 2021, 11, 2568. [Google Scholar] [CrossRef]
- Nava, A.L.; Higareda, T.E.; Barreto, C.; Rodríguez, R.; Márquez, I.; Palacios, M.L. Circular economy approach for mealworm industrial production for human consumption. IOP Conf. Ser. Earth Environ. Sci. 2020, 463, 012087. [Google Scholar] [CrossRef]
- Vrontaki, M.; Adamaki-Sotiraki, C.; Rumbos, C.I.; Anastasiadis, A.; Athanassiou, C.G. Valorization of local agricultural by-products as nutritional substrates for Tenebrio molitor larvae: A sustainable approach to alternative protein production. Environ. Sci. Pollut. Res. 2024, 31, 35760–35768. [Google Scholar] [CrossRef] [PubMed]
- Kee, P.E.; Cheng, Y.S.; Chang, J.S.; Yim, H.S.; Tan, J.C.Y.; Lam, S.S.; Lan, J.C.W.; Ng, H.S.; Khoo, K.S. Insect biorefinery: A circular economy concept for biowaste conversion to value-added products. Environ. Res. 2023, 221, 115284. [Google Scholar] [CrossRef] [PubMed]
- Lopes, I.G.; Yong, J.W.; Lalander, C. Frass derived from black soldier fly larvae treatment of biodegradable wastes. A critical review and future perspectives. Waste Manag. 2022, 142, 65–76. [Google Scholar] [CrossRef]
- Yu, Z.; Xie, C.; Zhang, Z.; Huang, Z.; Zhou, J.; Wang, C. Microbial fermentation and black soldier fly feeding to enhance maize straw degradation. Chemosphere 2024, 353, 141498. [Google Scholar] [CrossRef]
- He, L.; Zhang, Y.; Ding, M.Q.; Li, M.X.; Ding, J.; Bai, S.W.; Wu, Q.L.; Zhao, L.; Cao, G.L.; Ren, N.Q.; et al. Sustainable strategy for lignocellulosic crop wastes reduction by Tenebrio molitor Linnaeus (mealworm) and potential use of mealworm frass as a fertilizer. J. Clean. Prod. 2021, 325, 129301. [Google Scholar] [CrossRef]
- Katchali, M.; Senagi, K.; Richard, E.; Beesigamukama, D.; Tanga, C.M.; Athanasiou, G.; Zahariadis, T.; Casciano, D.; Lazarou, A.; Tonnang, H.E.Z. Unveiling Environmental Influences on Sustainable Fertilizer Production through Insect Farming. Sustainability 2024, 16, 3746. [Google Scholar] [CrossRef]
- Smitt, E.; de Vries, W. Potential benefits of using Hermetia illucens frass as a soil amendment on food production and for environmental impact reduction. Curr. Opin. Green Sustain. Chem. 2020, 25, 100335. [Google Scholar] [CrossRef]
- Kotsou, K.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Lalas, S.I. Exploiting Agri-Food Waste as Feed for Tenebrio molitor Larvae Rearing: A Review. Foods 2024, 13, 1027. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Harahap, I.A.; Osei-Owusu, J.; Saikia, T.; Wu, Y.S.; Fernando, I.; Perestrelo, R.; Câmara, J.S. Bioconversion of organic waste by insects–A comprehensive review. Process Saf. Environ. Prot. 2024, 187, 1–25. [Google Scholar] [CrossRef]
- Kim, S.Y.; Chung, T.H.; Kim, S.H.; Song, S.; Kim, N. Recycling agricultural wastes as feed for mealworm (Tenebrio molitor). Korean J. Appl. Entomol. 2014, 53, 365–371. [Google Scholar] [CrossRef]
- Wu, Q.; Tao, H.; Wong, M.H. Feeding and metabolism effects of three common microplastics on Tenebrio molitor L. Environ. Geochem. Health 2019, 41, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.Y.; Chen, Z.; Chen, J.; Yu, H.; Zhou, X.; Criddle, C.S.; Wu, W.M.; Zhang, Y. Biodegradation of polyvinyl chloride (PVC) in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae. Environ. Int. 2020, 145, 106106. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Du, R.; Henriquez, F.N.; Liu, H.; Chan, S.Y.; Leong, C.M.; Lui, M.Y. Enhancing Plastic Decomposition in Mealworms (Tenebrio molitor): The Role of Nutritional Amino Acids and Water. Adv. Energy Sustain. Res. 2025, 6, 2400378. [Google Scholar] [CrossRef]
- Koyunoğlu, C. Biofuel production utilizing Tenebrio molitor: A sustainable approach for organic waste management. Int. J. Thermofluids 2024, 22, 100603. [Google Scholar] [CrossRef]
- Hénault-Ethier, L.; Quinche, M.; Reid, B.; Hotte, N.; Fortin, A.; Normandin, É.; Renaud, G.L.R.; Zadeh, A.R.; Deschamps, M.H.; Vandenberg, G. Opportunities and challenges in upcycling agri-food byproducts to generate insect manure (frass): A literature review. Waste Manag. 2024, 176, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Amorim, H.C.; Ashworth, A.J.; Arsi, K.; Rojas, M.G.; Morales-Ramos, J.A.; Donoghue, A.; Robinson, K. Insect frass composition and potential use as an organic fertilizer in circular economies. J. Econ. Entomol. 2024, 117, 1261–1268. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Faucon, M.P.; Dulaurent, A.M. Potential use of mealworm frass as a fertilizer: Impact on crop growth and soil properties. Sci. Rep. 2020, 10, 4659. [Google Scholar] [CrossRef]
- Beesigamukama, D.; Subramanian, S.; Tanga, C.M. Nutrient quality and maturity status of frass fertilizer from nine edible insects. Sci. Rep. 2022, 12, 7182. [Google Scholar] [CrossRef]
- Watson, C.; Schlösser, C.; Vögerl, J.; Wichern, F. Excellent excrement? Frass impacts on a soil’s microbial community, processes and metal bioavailability. Appl. Soil Ecol. 2021, 168, 104110. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.Y.; Nurfikari, A.; Van De Zande, E.M.; Wantulla, M.; Van Loon, J.J.; De Boer, W.; Dicke, M. Insect frass and exuviae to promote plant growth and health. Trends Plant Sci. 2022, 27, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Nyanzira, A.; Machona, O.; Matongorere, M.; Chidzwondo, F.; Mangoyi, R. Analysis of frass excreted by Tenebrio molitor for use as fertilizer. Entomol. Appl. Sci. Lett. 2023, 10, 29–37. [Google Scholar] [CrossRef]
- Foscari, A.; Dalla Costa, L.; Tulli, F.; Uboni, C.; Fellet, G. Frass from Tenebrio molitor as alternative to NPK-mineral fertilization: Results from a germination test and pot experiment on sunflower. Ital. J. Agron. 2024, 19, 100010. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Dulaurent, A.M. Assessment of the short-term fertilizer potential of mealworm frass using a pot experiment. Front. Sustain. Food Syst. 2021, 5, 714596. [Google Scholar] [CrossRef]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm frass as a potential biofertilizer and abiotic stress tolerance-inductor in plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Khatami, N.; Guerrero, P.; Martín, P.; Quintela, E.; Ramos, V.; Saa, L.; Cortajarena, A.L.; Caba, K.D.L.; Camarero-Espinosa, S.; Abarrategi, A. Valorization of biological waste from insect-based food industry: Assessment of chitin and chitosan potential. Carbohydr. Polym. 2024, 324, 121529. [Google Scholar] [CrossRef]
- Weaver, D.K.; McFarlane, J.E.; Alli, I. Repellency of volatile fatty acids present in frass of larval yellow mealworms, Tenebrio molitor L. (Coleoptera: Tenebrionidae), to larval conspecifics. J. Chem. Ecol. 1990, 16, 585–593. [Google Scholar] [CrossRef]
- Urrutia, R.I.; Jesser, E.N.; Gutierrez, V.S.; Rodriguez, S.; Gumilar, F.; Murray, A.P.; Volpe, M.A.; Werdin-González, J.O. From waste to food and bioinsecticides: An innovative system integrating Tenebrio molitor bioconversion and pyrolysis bio-oil production. Chemosphere 2023, 340, 139847. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef]
- Gong, B.Q.; Wang, F.Z.; Li, J.F. Hide-and-seek: Chitin-triggered plant immunity and fungal counterstrategies. Trends Plant Sci. 2020, 25, 805–816. [Google Scholar] [CrossRef]
- Kim, H.B.; Lee, J.H.; Lee, Y.J.; Rho, J.S.; Lee, J.M.; Kim, S.H.; Park, J.H.; Seo, D.C. Adsorption characteristics and mechanism of Cd by mealworm frass. Appl. Biol. Chem. 2025, 68, 5. [Google Scholar] [CrossRef]
- Wedwitschka, H.; Gallegos Ibanez, D.; Jáquez, D.R. Biogas Production from Residues of Industrial Insect Protein Production from Black Soldier Fly Larvae Hermetia illucens (L.): An Evaluation of Different Insect Frass Samples. Processes 2023, 11, 362. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Muda, K.; Steven, R.; Mustapha, M.; Ibrahim, H.U.; Ouahbi, T. Insect frass as a substrate to stimulate native ureolytic bacteria for microbial-induced carbonate precipitation in soil biocementation. Biomass Convers. Biorefinery 2024, 14, 25849–25872. [Google Scholar] [CrossRef]
- Hodge, S. Beetles Sale: Could Insect Farming Feed Us All Help Save Planet? Wētā 2022, 56, 1–12. [Google Scholar]
- Poveda, J. Insect frass in the development of sustainable agriculture. A review. Agron. Sustain. Dev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Chavez, M.; Uchanski, M. Insect left-over substrate as plant fertiliser. J. Insects Food Feed 2021, 7, 683–694. [Google Scholar] [CrossRef]
- Van Huis, A.; Rumpold, B.A.; van der Fels-Klerx, H.J.; Tomberlin, J.K. Advancing edible insects as food and feed in a circular economy. J. Insects Food Feed 2021, 7, 935–948. [Google Scholar] [CrossRef]
- Hodge, S.; Conway, J. The effects of insect frass fertilizer and biochar on the shoot growth of chicory and plantain, two forage herbs commonly used in multispecies swards. Agronomy 2022, 12, 2459. [Google Scholar] [CrossRef]
- EN 1482-1:2007; Fertilizers and Liming Materials—Sampling and Sample Preparation—Part 1: Sampling. European Committee for Standardization (CEN): Bruxelles, Belgium, 2007. Available online: https://standards.iteh.ai/catalog/standards/cen/0b07d50f-496f-4e38-9765-b7687501d382/en-1482-1-2007 (accessed on 12 May 2025).
- EN 1482-2:2007; Fertilizers and Liming Materials—Sampling and Sample Preparation—Part 2: Sample Preparation. European Committee for Standardization (CEN): Bruxelles, Belgium, 2007. Available online: https://standards.iteh.ai/catalog/standards/cen/7721782c-cacf-4781-8b78-3b9c2df3d2cd/en-1482-2-2007 (accessed on 12 May 2025).
- Retsch GmbH. Sieving Methods of Sieve Analysis. Available online: https://www.retsch.com/applications/knowledge-base/sieve-analysis/ (accessed on 6 May 2025).
- LST EN 13040:2008; Soil Improvers and Growing Media—Sample Preparation for Chemical and Physical Tests, Determination of Dry Matter Content, Moisture Content and Laboratory Compacted Bulk Density. Lithuanian Standard: Vilnius, Lithuania, 2008.
- LST EN 13037:2012; Soil Improvers and Growing Media—Determination of pH. Lithuanian Standard: Vilnius, Lithuania, 2012.
- LST EN 13654-1:2002; Soil Improvers and Growing Media—Determination of Nitrogen. Part 1: Modified Kjeldahl Method. Lithuanian Standard: Vilnius, Lithuania, 2002.
- UR-Lex. Regulation (EC) No 2003/2003 of the European Parliament and of the Council of 13 October 2003 Relating to Fertilisers. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32003R2003 (accessed on 6 May 2025).
- LST EN 13039:2012; Soil Improvers and Growing Media—Determination of Organic Matter Content and Ash. Lithuanian Standard: Vilnius, Lithuania, 2012.
- EN 1484:1997; Water Analysis—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). European Committee for Standardization (CEN): Brussels, Belgium, 1997.
- Lucideon. Scanning Electron Microscopy (SEM) with Energy Dispersive X-Ray Analysis (EDX) Testing Technique. Available online: https://www.lucideon.com/testing-characterisation/techniques/sem-edx (accessed on 6 May 2025).
- SAS Institute Inc. SAS/STAT® 9.3 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- National University. Statistics Resources. Available online: https://resources.nu.edu/statsresources/assumptions (accessed on 12 May 2025).
- Carpi, A. Introduction to Descriptive Statistics: Using Mean, Median, and Standard Deviation. Visionlearning. Available online: https://www.visionlearning.com/en/library/Chemistry/1/Introduction-to-Descriptive-Statistics/218 (accessed on 12 May 2025).
- Divaks. About Us. Available online: https://divaks.com/about-us/ (accessed on 6 May 2025).
- Luengnaruemitchai, A.; Anupapwisetkul, C. Surface morphology and cellulose structure of Napier grass pretreated with the ionic liquid 1-ethyl-3-methylimidazolium acetate combined with either water or dimethyl sulfoxide as a co-solvent under microwave irradiation. Biomass Convers. Biorefinery 2020, 10, 435–446. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J. Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Jena, K.; Ananta, S.; Akthar, J.; Patnaik, A.; Das, S.; Singh, J.; Sathyanarayana, K.; Kar, P.K.; Das, B.K.; Hassan, M.A.; et al. Physical, biochemical and antimicrobial characterization of chitosan prepared from tasar silkworm pupae waste. Environ. Technol. Innov. 2023, 31, 103200. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vazvani, M.G.; Vatankhah, M.; Kennedy, J.F. Chitin-induced disease resistance in plants: A review. Int. J. Biol. Macromol. 2024, 266, 131105. [Google Scholar]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Akcay, C.; Yalcin, M. Morphological and chemical analysis of Hylotrupes bajulus (old house borer) larvae-damaged wood and its FTIR characterization. Cellulose 2021, 28, 1295–1310. [Google Scholar] [CrossRef]
- Zhu, P.; Gong, S.; Deng, M.; Xia, B.; Yang, Y.; Tang, J.; Qian, G.; Yu, F.; Goonetilleke, A.; Li, X. Biodegradation of waste refrigerator polyurethane by mealworms. Front. Environ. Sci. Eng. 2023, 17, 38. [Google Scholar] [CrossRef]
- ISO 10694:1995; Soil Quality—Determination of Organic and Total Carbon After Dry Combustion (Elementary Analysis). International Organization for Standardization (ISO): Geneva, Switzerland, 1995. Available online: https://www.iso.org/standard/18782.html (accessed on 6 May 2025).
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Reduction of ammonia emissions from laying hen manure in a closed composting process using gas-permeable membrane technology. Agronomy 2021, 11, 2384. [Google Scholar] [CrossRef]
- Li, X.; Guo, Z.; Ma, Y.; Leite, P.A.; Li, Z.; Wu, G.L. Incorporating Cattle Manure Improves Hydraulic Properties and Enhances Infiltration Rates of Low-Infiltrability Saline-Sodic Soils. Land Degrad. Dev. 2025, 36, 945–953. [Google Scholar] [CrossRef]
- Guo, X.X.; Liu, H.T.; Wu, S.B. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef]
- EN 13039:2011; Soil Improvers and Growing Media—Determination of Organic Matter Content and Ash. European Committee for Standardization (CEN): Bruxelles, Belgium, 2011. Available online: https://standards.iteh.ai/catalog/standards/cen/825ecadd-2c72-4dc9-a38c-1e4bdeac857f/en-13039-2011 (accessed on 6 May 2025).
- Demeyer, A.; Nkana, J.V.; Verloo, M.G. Characteristics of wood ash and in-fluence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Moragues-Saitua, L.; Arias-González, A.; Blanco, F.; Benito-Carnero, G.; Gartzia-Bengoetxea, N. Effects of biochar and wood ash amendments in the soil-water-plant environment of two temperate forest plantations. Front. For. Glob. Change 2023, 5, 878217. [Google Scholar] [CrossRef]
- Kim, N.; Watmough, S.A.; Yan, N.D. Wood ash amendments as a potential solution to widespread calcium decline in eastern Canadian forests. Environ. Rev. 2022, 30, 485–500. [Google Scholar] [CrossRef]
- Pettit, R.E. Organic Matter, Humus, Humate, Humic Acid, Fulvic Acid and Humin: Their Importance in Soil Fertility and Plant Health. Available online: https://humates.com/wp-content/uploads/2020/04/ORGANICMATTERPettit.pdf (accessed on 6 May 2025).
- Nieweś, D.; Huculak-Mączka, M.; Braun-Giwerska, M.; Marecka, K.; Tyc, A.; Biegun, M.; Hoffmann, K.; Hoffmann, J. Ultrasound-Assisted Extraction of Humic Substances from Peat: Assessment of Process Efficiency and Products’ Quality. Molecules 2022, 27, 3413. [Google Scholar] [CrossRef] [PubMed]




| Sample * | 10% Solution pH | Moisture, % | Bulk Density, kg/m3 | Fractional Composition, % | |||
|---|---|---|---|---|---|---|---|
| >1 mm | 0.5–1 mm | 0.2–0.5 mm | <0.2 mm | ||||
| DF1 | 5.85 ± 0.05 | 7.41 ± 0.51 | 524.89 ± 0.22 | 0.05 ± 0.83 | 1.98 ± 0.43 | 93.36 ± 1.01 | 4.61 ± 0.69 |
| DF2 | 5.45 ± 0.07 | 8.76 ± 0.32 | 393.81 ± 0.35 | 0.01 ± 0.51 | 1.95 ± 0.93 | 95.75 ± 0.86 | 2.29 ± 0.72 |
| Sample * | Primary and Secondary Macronutrients, % | ||||||
| N | P2O5 | K2O | CaO | MgO | Na | S | |
| DF1 | 3.25 ± 0.32 | 2.06 ± 0.21 | 2.33 ± 0.19 | 0.69 ± 0.22 | 1.5 ± 0.10 | 0.38 ± 0.13 | 9.25 ± 0.31 |
| DF2 | 3.87 ± 0.21 | 2.74 ± 0.45 | 2.60 ± 0.09 | 0.91 ± 0.20 | 1.24 ± 0.19 | – | 8.99 ± 0.04 |
| Sample | Microelements, mg/kg | ||||||
| Zn | Mn | Cu | Fe | Co | Mo | ||
| DF1 | – | – | – | 615.63 ± 0.55 | 7.23 ± 0.25 | – | |
| DF2 | – | – | – | 284.05 ± 0.17 | 2.47 ± 0.12 | – | |
| Sample | Heavy metals, mg/kg | ||||||
| Cd | Pb | Hg | Ni | Cr | As | ||
| DF1 | – | 11.23 ± 0.11 | <0.02 | 2.50 ± 0.31 | 30.69 ± 0.08 | <0.09 | |
| DF2 | – | 18.03 ± 0.09 | <0.02 | 4.20 ± 0.22 | 32.46 ± 0.19 | <0.09 | |
| Sample * | N, % | P2O5, % | K2O, % | |||
|---|---|---|---|---|---|---|
| H2O 1 | HCl 2 | H2O | HCl | H2O | HCl | |
| DF1 | 0.15 ± 0.09 | 0.42 ± 0.51 | 5.52 ± 0.51 | 30.50 ± 0.62 | 21.30 ± 0.12 | 31.52 ± 0.22 |
| DF2 | 0.21 ± 0.51 | 0.62 ± 0.51 | 2.28 ± 0.51 | 21.29 ± 0.43 | 10.56 ± 0.20 | 18.98 ± 0.47 |
| Sample * | Conditions | pH | Density, g/cm3 | Kinematic Viscosity, mm2/s | |
|---|---|---|---|---|---|
| DF1 | Using ultrasound | 1 h | 13.20 ± 0.09 | 1.145 ± 0.05 | 3.301 ± 0.11 |
| 2 h | 13.15 ± 0.07 | 1.148 ± 0.07 | 3.320 ± 0.08 | ||
| 3 h | 13.20 ± 0.10 | 1.147 ± 0.10 | 3.318 ± 0.12 | ||
| Using mechanical mixing | 1 h | 13.15 ± 0.11 | 1.143 ± 0.05 | 3.311 ± 0.15 | |
| 2 h | 13.18 ± 0.05 | 1.147 ± 0.08 | 3.290 ± 0.10 | ||
| 3 h | 13.20 ± 0.04 | 1.144 ± 0.04 | 3.305 ± 0.09 | ||
| DF2 | Using ultrasound | 1 h | 13.10 ± 0.08 | 1.142 ± 0.10 | 2.621 ± 0.31 |
| 2 h | 13.20 ± 0.04 | 1.141 ± 0.09 | 2.599 ± 0.25 | ||
| 3 h | 13.22 ± 0.09 | 1.145 ± 0.05 | 2.579 ± 0.20 | ||
| Using mechanical mixing | 1 h | 13.10 ± 0.12 | 1.140 ± 0.02 | 2.512 ± 0.21 | |
| 2 h | 13.40 ± 0.08 | 1.142 ± 0.05 | 2.522 ± 0.11 | ||
| 3 h | 13.30 ± 0.05 | 1.144 ± 0.02 | 2.519 ± 0.19 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudinskaitė, G.; Paleckienė, R. The Possibilities of Using Non-Traditional Raw Materials for Fertilizing Products. Sustainability 2025, 17, 5710. https://doi.org/10.3390/su17135710
Gudinskaitė G, Paleckienė R. The Possibilities of Using Non-Traditional Raw Materials for Fertilizing Products. Sustainability. 2025; 17(13):5710. https://doi.org/10.3390/su17135710
Chicago/Turabian StyleGudinskaitė, Goda, and Rasa Paleckienė. 2025. "The Possibilities of Using Non-Traditional Raw Materials for Fertilizing Products" Sustainability 17, no. 13: 5710. https://doi.org/10.3390/su17135710
APA StyleGudinskaitė, G., & Paleckienė, R. (2025). The Possibilities of Using Non-Traditional Raw Materials for Fertilizing Products. Sustainability, 17(13), 5710. https://doi.org/10.3390/su17135710






