Advancing Phytoremediation: A Review of Soil Amendments for Heavy Metal Contamination Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Methodology
2.2. Research Trends over Time
2.3. Leading Countries in Research Output
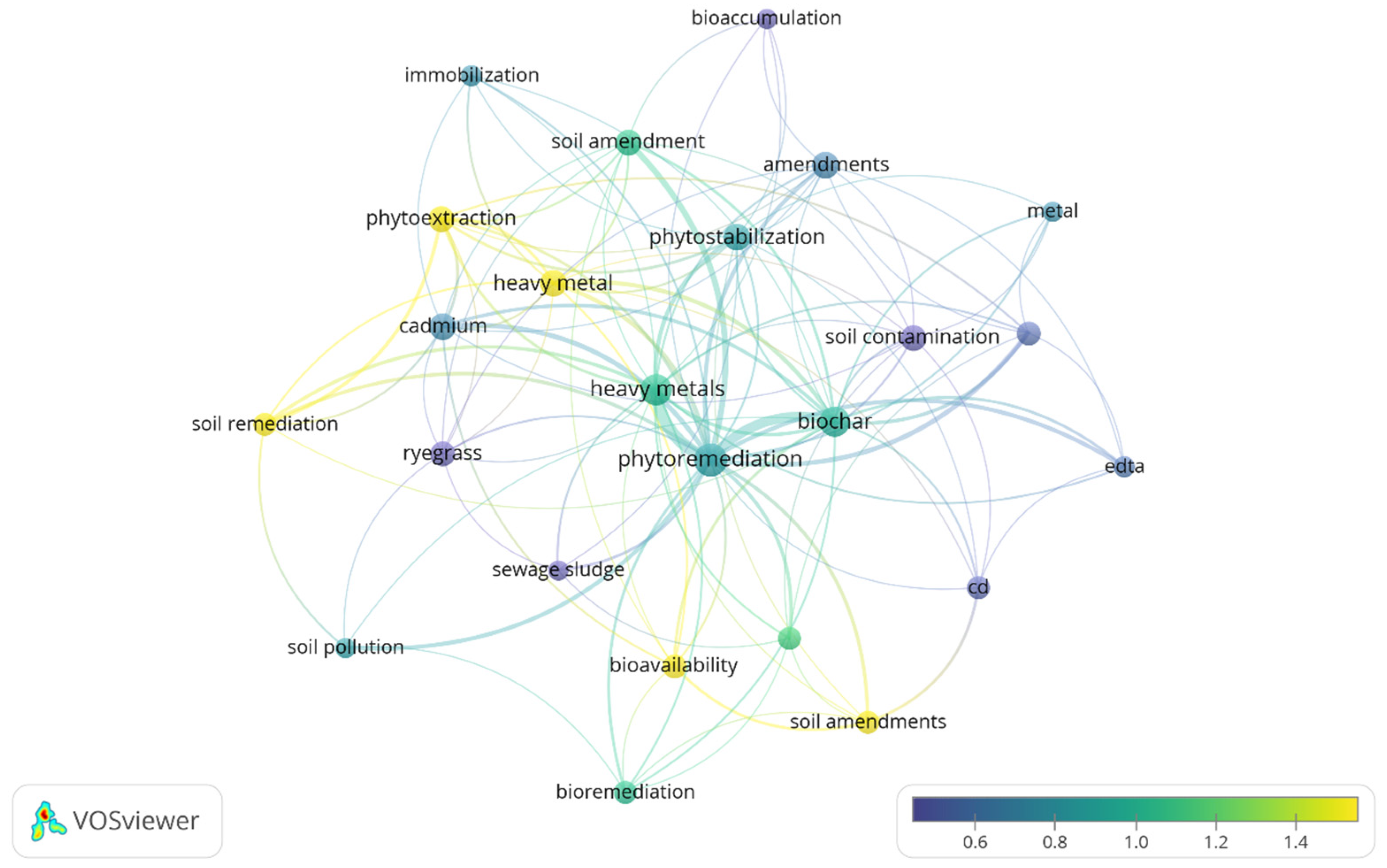
2.4. Keyword Co-Occurrence Analysis
- Phytoremediation mechanisms such as phytoextraction, phytostabilisation and bioaccumulation.
- HM contaminants such as Cd, Pb, As and Cr.
- Soil amendment strategies using biochar, immobilisation agents and organic amendments as commonly studied solutions.
- Bioremediation approaches focusing on microbial interactions, mycorrhizal fungi and bioavailability reduction.
- Environmental impact considerations, including soil pollution, remediation effectiveness and sustainability.
2.5. Document Co-Citation Analysis
3. Amendments Used in Phytoremediation
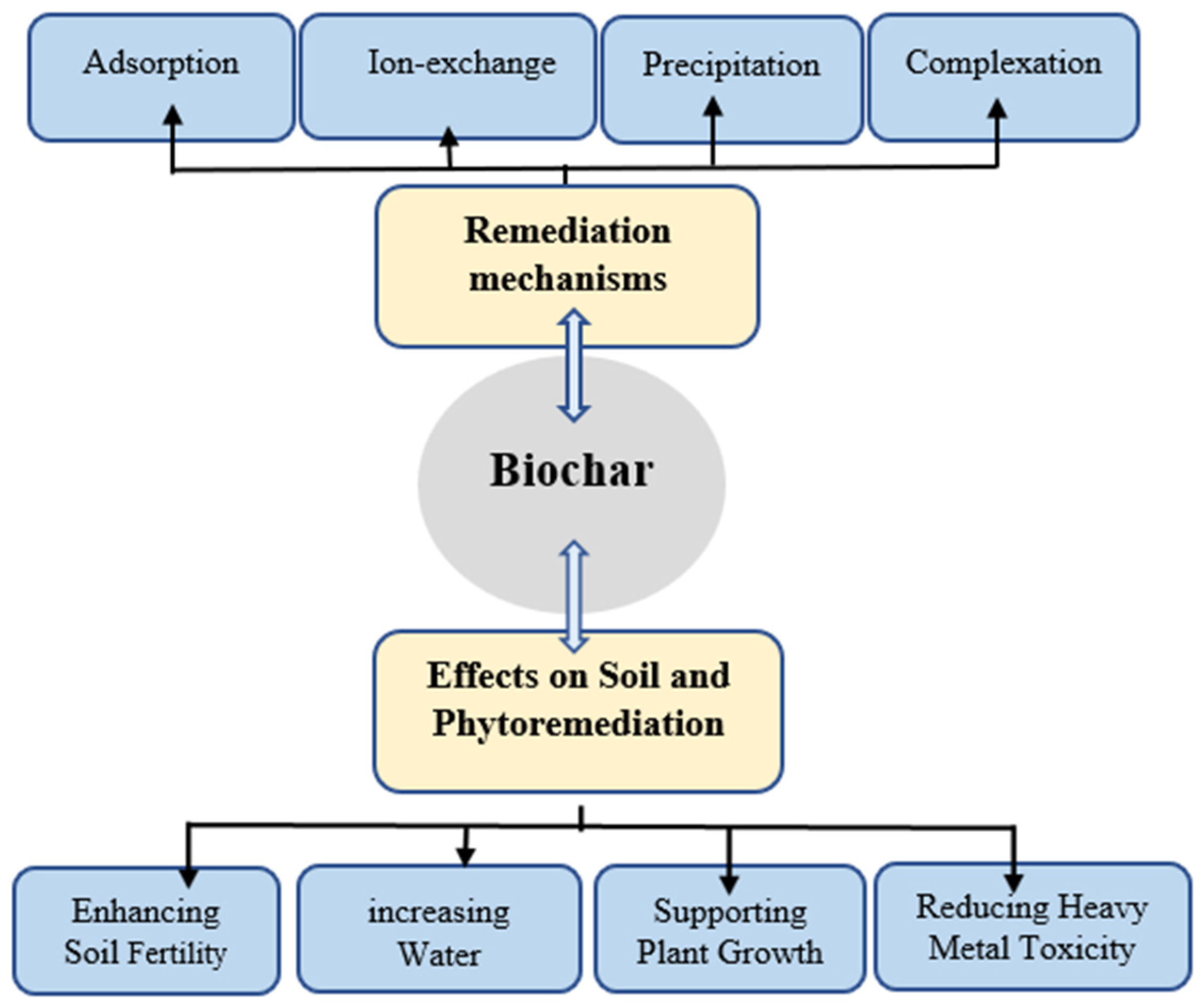
3.1. Biochar
3.1.1. Properties and Mechanisms of Biochar in Phytoremediation
3.1.2. Biochar in Combination with Other Amendments
3.1.3. Limitations, Potential Risks and Critical Assessment of Biochar Applications in Phytoremediation Studies
3.2. Compost and Vermicompost
3.2.1. Challenges and Limitations
3.2.2. Future Directions and Research Gaps
3.3. Plant Exudates and Extracts
3.3.1. Root Exudates in Phytoremediation
3.3.2. Plant Extracts as Phytoremediation Enhancers
3.3.3. Challenges and Future Direction in Using Exudates and Extracts
3.4. Microbial Agents
3.4.1. Plant Growth-Promoting Bacteria (PGPR)
- Limited effectiveness at high metal concentrations: The effectiveness of PGPR may decrease in soils with high HM levels probably owing to toxic effects, restricting their application to moderately contaminated environments.
- Extended remediation time: PGPR-based remediation is slower than physical and chemical remediation and often requires years to achieve significant results.
- Complex interaction dependencies: PGPR activity is influenced by environmental conditions, the availability of supplementary nutrients and pollutants and interactions with existing microbial populations. These dependencies introduce challenges in terms of precision and practical management.
| Host Plant | PGPR Strain | Origin | Key Beneficial Properties | Observed Effects on Plant Growth and Soil Quality | Reference |
|---|---|---|---|---|---|
| Helianthus annuus | Trichoderma harzianum, Azotobacter chroococcum and Bacillus subtilis | Cd-contaminated soil | Reduces Cd bioavailability, enhances antioxidant activity and improves nutrient uptake (indole-3-acetic acid (IAA) and siderophores). | Increased biomass, reduced oxidative damage, enhanced metabolite and enzyme activity and reduced Cd levels. | Abeed et al. [113] |
| Sorghum bicolour | Bacillus thuringiensis SE1C2 + biochar (5%) | Cd- and Zn-contaminated soil | Enhance PGPB colonisation and improve stress tolerance (siderophores, IAA). | Increased shoot/root growth, chlorophyll content and antioxidant activity and reduced Cd and Zn uptake. | Anbuganesan et al. [114] |
| Brassica juncea | Consortium-BC8 (Klebsiella variicola and Pseudomonas otitidis) | Ni- and Pb-contaminated soil from mines and dumpsites. | Forms a biofilm on roots and enhances metal solubility and uptake (IAA, siderophores and phytoextraction enhancement). | Increased vegetative growth, Ni phytoextraction (TF 1.58) and Pb phytostabilisation. | Sharma and Saraf [115] |
| Brassica juncea | Bacillus sp. Kz5 and Enterobacter sp. Kz15 | Isolated from the rhizospheres of plants grown in copper mine soils | Enhances Cd uptake and improves root morphology and soil health (IAA, siderophores and phytoextraction enhancement). | Increased biomass, Cd concentration, root morphology, photosynthetic activity and rhizosphere soil properties. | Zhang et al. [116] |
| Lolium multiflorum | Pseudomonas aeruginosa | Isolated from Cu–Cd co-contaminated soil | IAA and siderophores | Increased growth, Cu and Cd uptake and shoot translocation. Biomass increased by 43.1% (T7 vs. T1) and approximately 89% or Cu and Cd were removed. | Shi et al. [117] |
| Triticum aestivum | Bacillus cereus | - | Enhance antioxidant enzymes (superoxide dismutase (SOD), glutathione S-transferase (GST) and ascorbate peroxidase (APX)) and reduce reactive oxygen species (ROS) and HM bioavailability (IAA and siderophores). | Improved growth rate, photosynthetic efficiency and stress tolerance. | Direk et al. [118] |
| Helianthus annuus | Brucella intermedium (E1) and Bacillus velezensis (EW8) | Ni–Cd battery waste-contaminated soil | Enhanced antioxidant enzyme activity (IAA and siderophores). | Increased metal accumulation, improved soil quality index and enhanced plant growth. | Kriti et al. [119] |
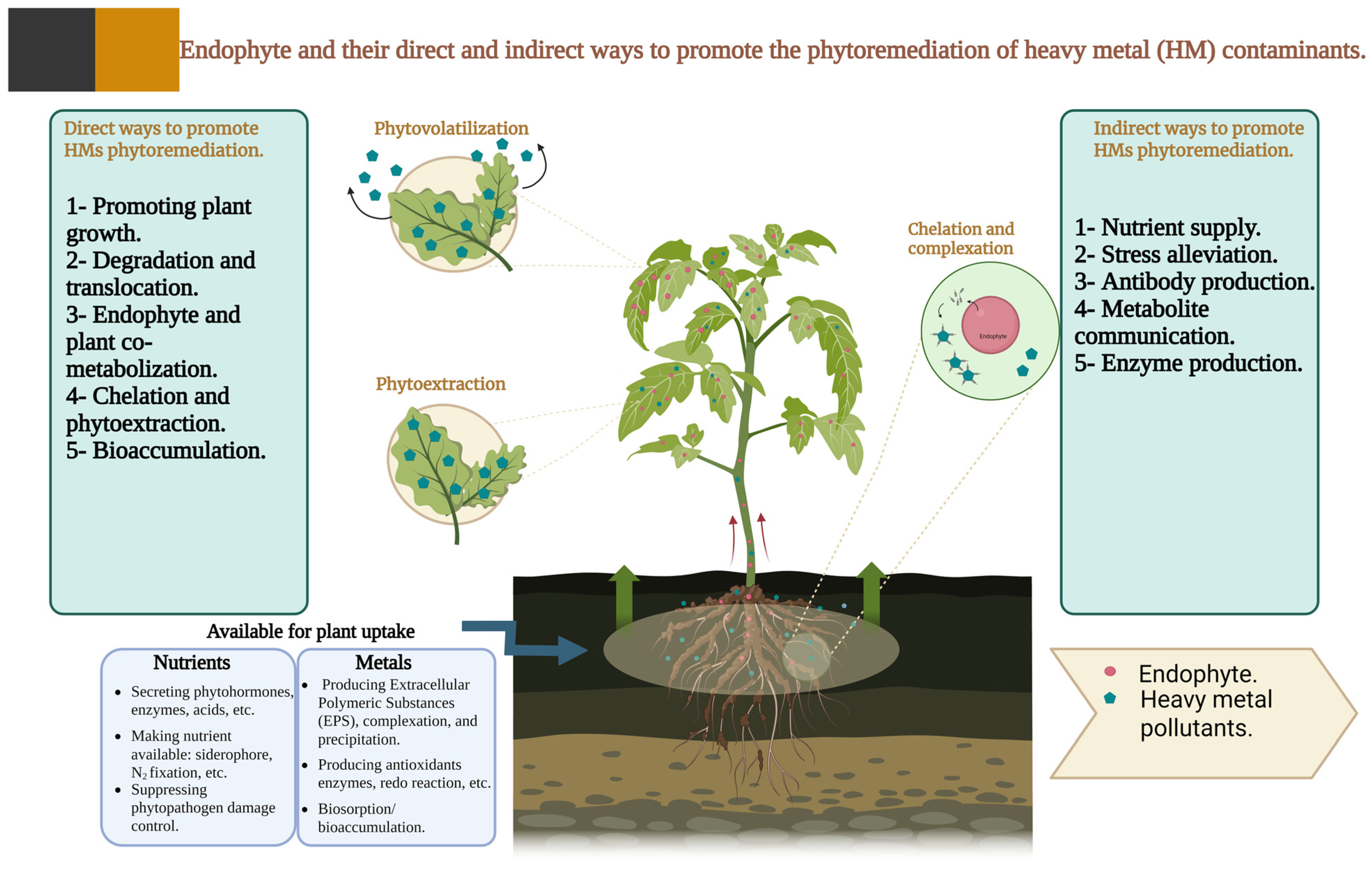
3.4.2. Endophytic Bacteria
- Survival in contaminated soils: Endophytes may struggle to survive or establish in heavily contaminated soils, particularly in the absence of suitable host plants.
- Host compatibility: Not all bacterial endophytes that colonise one plant species or cultivar are able to colonise others, leading to host specificity and limiting their broad applicability in phytoremediation.
3.4.3. Arbuscular Mycorrhizal Fungi (AMF)
| Bacterial Strains | Plant Species | Heavy Metals | Type of Soil | Significance and Impact | References |
|---|---|---|---|---|---|
| Kocuria sp. (LC2, LC3 and LC5), Enterobacter sp. (LC1, LC4 and LC6) and Kosakonia sp. (LC7) | Solanum nigrum | As | Soil with high As concentrations | Enhanced plant growth in Solanum nigrum. Increased bioaccumulation and root-to-shoot transport of As. | Mukherjee et al. [130] |
| Micrococcus yunnanensis SMJ12, Vibrio sagamiensis SMJ18 and Salinicola peritrichatus SMJ30 | Spartina maritima | As, Cu and Zn | HM-contaminated soil | Endophytic bacteria exhibited resistance to multiple HMs and metalloids. Displayed plant growth-promoting properties. | Mesa et al. [131] |
| Bacillus sp. SLS18 | Solanum nigrum L. | Cd | Mine tailing soil | Isolation of 30 Cd-tolerant bacterial endophytes from the roots, stems and leaves of Solanum nigrum L. | Luo et al. [132] |
| Paenibacillus sp. RM | Tridax procumbens | Cu, Zn, Pb and As | - | Highly resistant to Cu, Zn, Pb and As. Produced growth-promoting substances that enhanced metal tolerance and bioremediation potential. | Govarthanan et al. [133] |
| Enterobacter sp. (strain SVUB4) | Eichhornia crassipes | Cd and Zn | - | Exhibited several plant growth-promoting traits. Demonstrated the ability to grow in the presence of Cd and Zn. | El-Deeb et al. [134] |
| Pseudomonas sp. Lk9 | Solanum nigrum L. | Cd, Zn and Cu | - | Inoculation with Pseudomonas sp. Lk9 led to improved Fe and P mineral availability in soil, enhanced soil HM availability, increased Solanum. nigrum shoot dry biomass and greater total accumulation of HMs. | Chen et al. [135] |
- Need for field testing: Most AMF studies are conducted under laboratory conditions, and research on their effectiveness in real-world contaminated soils is limited.
- Limited understanding of nutrient pathways: The mechanisms through which AMF acquire nutrients under HM stress, particularly during long-distance metal transport in host plants, remain poorly understood.
- Synergies with other microbes: AMF frequently interact with bacteria in the rhizosphere; however, the full potential of these interactions, such as AMF–PGPR combinations, for enhancing phytoremediation requires further investigation.
- Pathogen proliferation: Some microbial amendments, particularly non-native strains, may exhibit pathogenic traits that pose risks to plant, animal or human health. Ensuring microbial safety is essential.
- Nutrient runoff: The addition of nutrients to enhance microbial activity can lead to nutrient leaching, potentially causing eutrophication in nearby water bodies.
- Horizontal gene transfer: The exchange of genetic material between introduced microbes and native soil organisms can lead to unintended ecological consequences, particularly when genetically engineered microbes are involved.
- Microbe–microbe interactions: Microbial agents such as PGPR and AMF can interact synergistically to enhance phytoremediation. For example, PGPR may facilitate AMF colonisation, improve metal uptake and increase plant tolerance to HMs.
- Organic and microbial amendment: Combining organic and microbial amendments can enhance phytoremediation. For example, humic substances improve soil conditions, creating a favourable environment for microbial activity that mobilises metals for plant uptake.
- Plant–microbe synergies: Different types of microbes (PGPR, endophytes and AMF) provide complementary benefits at different plant interaction sites, from root-associated processes to internal plant functions, thereby strengthening plant health, increasing plant stress tolerance and enhancing HM accumulation.
3.5. Chelating Agents
3.5.1. Synthetic Chelators: Ethylenediaminetetraacetic Acid (EDTA) and, Diethylenetriaminepentaacetic Acid (DTPA)
3.5.2. Biodegradable Chelators: Nitrilotriacetic Acid (NTA) and Ethylenediamine-N,N′-Disuccinic Acid (EDDS)
3.5.3. Natural Chelators: Organic Acids
3.5.4. Combination Approaches, Synergies and Future Directions
4. Economic Feasibility of Implementing Soil Amendments in Phytoremediation Projects
Critical Evaluation and Feasibility of Large-Scale Use
5. Molecular and Genetic Basis of Phytoremediation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| AMF | Arbuscular mycorrhizal fungi |
| APG | Alkyl polyglucoside |
| APX | Ascorbate peroxidase |
| AtHMA4 | Arabidopsis thaliana heavy metal ATPase 4 |
| BM | Beneficial microorganisms |
| bZIP | Basic leucine zipper |
| CEC | Cation-exchange capacity |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| DOC | Dissolved organic carbon |
| DPTA | Diethylenetriamine pentaacetate |
| EDDS | Ethylenediamine-N,N′-disuccinic |
| EDTA | Ethylenediaminetetraacetic acid |
| EPS | Exopolysaccharide |
| GSH | Glutathione synthetase |
| GST | Glutathione S-transferase |
| HMA | Heavy metal ATPase |
| IAA | Indole-3-acetic acid |
| JA | Jasmonic acid |
| merA | Mercuric reductase |
| merB | Organomercurial lyase |
| MYB | Myeloblastosis |
| NRAMP | Natural resistance-associated macrophage protein |
| NTA | Nitrilotriacetic acid |
| PCS | Phytochelatin synthase |
| PGPB | Plant growth-promoting bacteria |
| PGPR | Plant growth-promoting rhizobacteria |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SSA | Specific surface area |
| TFs | Transcription factors |
| WOS | Web of Science |
References
- Priya, A.K.; Muruganandam, M.; Ali, S.S.; Kornaros, M. Clean-Up of Heavy Metals from Contaminated Soil by Phytoremediation: A Multidisciplinary and Eco-Friendly Approach. Toxics 2023, 11, 422. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Arthur, E.L.; Rice, P.J.; Rice, P.J.; Anderson, T.A.; Baladi, S.M.; Henderson, K.L.D.; Coats, J.R. Phytoremediation—An Overview. CRC Crit. Rev. Plant Sci. 2005, 24, 109–122. [Google Scholar] [CrossRef]
- Wang, L.; Rinklebe, J.; Tack, F.M.G.; Hou, D. A Review of Green Remediation Strategies for Heavy Metal Contaminated Soil. Soil. Use Manag. 2021, 37, 936–963. [Google Scholar] [CrossRef]
- Gerhardt, K.E.; Huang, X.D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and Rhizoremediation of Organic Soil Contaminants: Potential and Challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
- Bolan, N.S.; Park, J.H.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilization: A Green Approach to Contaminant Containment. Adv. Agron. 2011, 112, 145–204. [Google Scholar] [CrossRef]
- Olguín, E.J.; Sánchez-Galván, G. Heavy Metal Removal in Phytofiltration and Phycoremediation: The Need to Differentiate between Bioadsorption and Bioaccumulation. New Biotechnol. 2012, 30, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Enhancing Phytoremediation of Soils Polluted with Heavy Metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Hasan, M.M.; Uddin, M.N.; Ara-Sharmeen, I.; Alharby, H.F.; Alzahrani, Y.; Hakeem, K.R.; Zhang, L. Assisting Phytoremediation of Heavy Metals Using Chemical Amendments. Plants 2019, 8, 295. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and Opportunities in the Phytoremediation of Heavy Metals Contaminated Soils: A Review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lv, J.; Xie, J.; Yu, J.; Li, J.; Zhang, J.; Tang, C.; Niu, T.; Patience, B.E. Effect of Slow-Release Fertilizer on Soil Fertility and Growth and Quality of Wintering Chinese Chives (Allium Tuberm Rottler Ex Spreng.) in Greenhouses. Sci. Rep. 2021, 11, 8070. [Google Scholar] [CrossRef]
- Hou, D.; Bolan, N.S.; Tsang, D.C.W.; Kirkham, M.B.; O’Connor, D. Sustainable Soil Use and Management: An Interdisciplinary and Systematic Approach. Sci. Total Environ. 2020, 729, 138961. [Google Scholar] [CrossRef]
- Lenoir, I.; Lounes-Hadj Sahraoui, A.; Fontaine, J. Arbuscular Mycorrhizal Fungal-Assisted Phytoremediation of Soil Contaminated with Persistent Organic Pollutants: A Review. Eur. J. Soil. Sci. 2016, 67, 624–640. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of Heavy Metal(Loid)s Contaminated Soils—To Mobilize or to Immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation Strategies for Soils Contaminated with Heavy Metals: Modifications and Future Perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Koptsik, G.N. Problems and Prospects Concerning the Phytoremediation of Heavy Metal Polluted Soils: A Review. Eurasian Soil. Sci. 2014, 47, 923–939. [Google Scholar] [CrossRef]
- Chirakkara, R.A.; Cameselle, C.; Reddy, K.R. Assessing the Applicability of Phytoremediation of Soils with Mixed Organic and Heavy Metal Contaminants. Rev. Environ. Sci. Bio/Technol. 2016, 15, 299–326. [Google Scholar] [CrossRef]
- Gascó, G.; Álvarez, M.L.; Paz-Ferreiro, J.; Méndez, A. Combining Phytoextraction by Brassica Napus and Biochar Amendment for the Remediation of a Mining Soil in Riotinto (Spain). Chemosphere 2019, 231, 562–570. [Google Scholar] [CrossRef]
- Mosa, A.; El-Ghamry, A.; Tolba, M. Functionalized Biochar Derived from Heavy Metal Rich Feedstock: Phosphate Recovery and Reusing the Exhausted Biochar as an Enriched Soil Amendment. Chemosphere 2018, 198, 351–363. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of Phytoremediation and Biochar to Remediate Heavy Metal Polluted Soils: A Review. Solid. Earth 2014, 5, 65–75. [Google Scholar] [CrossRef]
- Laird, D.A. The Charcoal Vision: A Win–Win–Win Scenario for Simultaneously Producing Bioenergy, Permanently Sequestering Carbon, While Improving Soil and Water Quality. Agron. J. 2008, 100, 178–181. [Google Scholar] [CrossRef]
- Kabir, E.; Kim, K.H.; Kwon, E.E. Biochar as a Tool for the Improvement of Soil and Environment. Front. Environ. Sci. 2023, 11, 1324533. [Google Scholar] [CrossRef]
- Ok, Y.S.; Bhatnagar, A.; Hou, D.; Bhaskar, T.; Mašek, O. Advances in Algal Biochar: Production, Characterization and Applications. Bioresour. Technol. 2020, 317. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar Application for the Remediation of Heavy Metal Polluted Land: A Review of in Situ Field Trials. Sci. Total Environ. 2018, 619–620, 815–826. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An Overview on Engineering the Surface Area and Porosity of Biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative Distribution of Pb2+ Sorption Mechanisms by Sludge-Derived Biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef]
- Rees, F.; Simonnot, M.O.; Morel, J.L. Short-Term Effects of Biochar on Soil Heavy Metal Mobility Are Controlled by Intra-Particle Diffusion and Soil PH Increase. Eur. J. Soil. Sci. 2014, 65, 149–161. [Google Scholar] [CrossRef]
- Geng, N.; Kang, X.; Yan, X.; Yin, N.; Wang, H.; Pan, H.; Yang, Q.; Lou, Y.; Zhuge, Y. Biochar Mitigation of Soil Acidification and Carbon Sequestration Is Influenced by Materials and Temperature. Ecotoxicol. Environ. Saf. 2022, 232, 113241. [Google Scholar] [CrossRef]
- Gezahegn, S.; Sain, M.; Thomas, S.C. Variation in Feedstock Wood Chemistry Strongly Influences Biochar Liming Potential. Soil Syst. 2019, 3, 26. [Google Scholar] [CrossRef]
- Jafri, N.; Wong, W.Y.; Doshi, V.; Yoon, L.W.; Cheah, K.H. A Review on Production and Characterization of Biochars for Application in Direct Carbon Fuel Cells. Process Saf. Environ. Prot. 2018, 118, 152–166. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock Choice, Pyrolysis Temperature and Type Influence Biochar Characteristics: A Comprehensive Meta-Data Analysis Review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Narayanan, M.; Ma, Y. Influences of Biochar on Bioremediation/Phytoremediation Potential of Metal-Contaminated Soils. Front. Microbiol. 2022, 13, 929730. [Google Scholar] [CrossRef]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. Mutual Relationships of Biochar and Soil PH, CEC, and Exchangeable Base Cations in a Model Laboratory Experiment. J. Soils Sediments 2019, 19, 2405–2416. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of Biochar on Soil Available Inorganic Nitrogen: A Review and Meta-Analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar Elemental Composition and Factors Influencing Nutrient Retention. Biochar Environ. Manag. 2015, 139–163. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of Biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, J.; Tang, G.; Bao, D.; Wang, T.; Kong, D. Impacts and Mechanisms of Biochar on Soil Microorganisms. Plant Soil Environ. 2023, 69, 45–54. [Google Scholar] [CrossRef]
- Adirianto, B.; Bachtiar, T. Effect of Biochar in Soil on Microbial Diversity: A Meta-Analysis. IOP Conf. Ser. Earth Environ. Sci. 2023, 1263, 012047. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A Quantitative Review of the Effects of Biochar Application to Soils on Crop Productivity Using Meta-Analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil. Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Lu, K.; Yang, X.; Shen, J.; Robinson, B.; Huang, H.; Liu, D.; Bolan, N.; Pei, J.; Wang, H. Effect of Bamboo and Rice Straw Biochars on the Bioavailability of Cd, Cu, Pb and Zn to Sedum Plumbizincicola. Agric. Ecosyst. Environ. 2014, 191, 124–132. [Google Scholar] [CrossRef]
- Su, Y.; Peng, S.; Xu, G.; Gao, Q.; Chen, J.; Lu, X.; Duan, B. Effect of Cornstalk Biochar on Phytoremediation of Pb-Contaminated Soil by Females and Males of Populus Deltoides (Salicaceae). Physiol. Plant 2023, 175, e13986. [Google Scholar] [CrossRef]
- Pračke, K.; Száková, J.; Tlustoš, P. Biochar Applications Enhance the Phytoextraction Potential of Salix Smithiana [Willd. (Willow) in Heavily Contaminated Soil: Potential for a Sustainable Remediation Method? J. Soils Sediments 2022, 22, 905–915. [Google Scholar] [CrossRef]
- Manori, S.; Shah, V.; Soni, V.; Dutta, K.; Daverey, A. Phytoremediation of Cadmium-Contaminated Soil by Bidens Pilosa L.: Impact of Pine Needle Biochar Amendment. Environ. Sci. Pollut. Res. 2021, 28, 58872–58884. [Google Scholar] [CrossRef]
- Li, X.; Xiao, J.; Salam, M.M.A.; Ma, C.; Chen, G. Impacts of Bamboo Biochar on the Phytoremediation Potential of Salix Psammophila Grown in Multi-Metals Contaminated Soil. Int. J. Phytoremediat. 2021, 23, 387–399. [Google Scholar] [CrossRef]
- Gu, P.; Zhang, Y.; Xie, H.; Wei, J.; Zhang, X.; Huang, X.; Wang, J.; Lou, X. Effect of Cornstalk Biochar on Phytoremediation of Cd-Contaminated Soil by Beta Vulgaris Var. Cicla L. Ecotoxicol. Environ. Saf. 2020, 205, 111144. [Google Scholar] [CrossRef]
- Rees, F.; Sterckeman, T.; Morel, J.L. Biochar-Assisted Phytoextraction of Cd and Zn by Noccaea Caerulescens on a Contaminated Soil: A Four-Year Lysimeter Study. Sci. Total Environ. 2020, 707. [Google Scholar] [CrossRef]
- Břendová, K.; Tlustoš, P.; Száková, J. Biochar Immobilizes Cadmium and Zinc and Improves Phytoextraction Potential of Willow Plants on Extremely Contaminated Soil. Plant Soil Environ. 2015, 61, 303–308. [Google Scholar] [CrossRef]
- Ahmad, M.; Soo Lee, S.; Yang, J.E.; Ro, H.M.; Han Lee, Y.; Sik Ok, Y. Effects of Soil Dilution and Amendments (Mussel Shell, Cow Bone, and Biochar) on Pb Availability and Phytotoxicity in Military Shooting Range Soil. Ecotoxicol. Environ. Saf. 2012, 79, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Fellet, G.; Marmiroli, M.; Marchiol, L. Elements Uptake by Metal Accumulator Species Grown on Mine Tailings Amended with Three Types of Biochar. Sci. Total Environ. 2014, 468–469, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, G.; Tian, Q.; Liu, C.; Chen, J. Biochar Remediates Cadmium and Lead Contaminated Soil by Stimulating Beneficial Fungus aspergillus Spp. Environ. Pollut. 2024, 359, 124601. [Google Scholar] [CrossRef]
- Peiris, C.; Alahakoon, Y.A.; Malaweera Arachchi, U.; Mlsna, T.E.; Gunatilake, S.R.; Zhang, X. Phosphorus-Enriched Biochar for the Remediation of Heavy Metal Contaminated Soil. J. Agric. Food Res. 2023, 12, 100546. [Google Scholar] [CrossRef]
- Wu, J.; Fu, X.; Zhao, L.; Lv, J.; Lv, S.; Shang, J.; Lv, J.; Du, S.; Guo, H.; Ma, F. Biochar as a Partner of Plants and Beneficial Microorganisms to Assist In-Situ Bioremediation of Heavy Metal Contaminated Soil. Sci. Total Environ. 2024, 923, 171442. [Google Scholar] [CrossRef]
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.P.; Gerber, H. Biochar Organic Fertilizers from Natural Resources as Substitute for Mineral Fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, L.; Li, Y.; Brookes, P.C.; Xu, J.; Luo, Y. The Effects of Combinations of Biochar, Lime, and Organic Fertilizer on Nitrification and Nitrifiers. Biol. Fertil. Soils 2017, 53, 77–87. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zou, D.; Hu, X.; Zhou, L.; Wu, Z.; Yang, Y.; Xiao, Z. Nanoscale Zerovalent Iron, Carbon Nanotubes and Biochar Facilitated the Phytoremediation of Cadmium Contaminated Sediments by Changing Cadmium Fractions, Sediments Properties and Bacterial Community Structure. Ecotoxicol. Environ. Saf. 2021, 208, 111510. [Google Scholar] [CrossRef]
- Rathika, R.; Srinivasan, P.; Alkahtani, J.; Al-Humaid, L.A.; Alwahibi, M.S.; Mythili, R.; Selvankumar, T. Influence of Biochar and EDTA on Enhanced Phytoremediation of Lead Contaminated Soil by Brassica juncea. Chemosphere 2021, 271, 129513. [Google Scholar] [CrossRef]
- Hartley, W.; Dickinson, N.M.; Riby, P.; Lepp, N.W. Arsenic Mobility in Brownfield Soils Amended with Green Waste Compost or Biochar and Planted with Miscanthus. Environ. Pollut. 2009, 157, 2654–2662. [Google Scholar] [CrossRef]
- Lebrun, M.; De Zio, E.; Miard, F.; Scippa, G.S.; Renzone, G.; Scaloni, A.; Bourgerie, S.; Morabito, D.; Trupiano, D. Amending an As/Pb Contaminated Soil with Biochar, Compost and Iron Grit: Effect on Salix Viminalis Growth, Root Proteome Profiles and Metal(Loid) Accumulation Indexes. Chemosphere 2020, 244, 125397. [Google Scholar] [CrossRef]
- Simiele, M.; Lebrun, M.; Miard, F.; Trupiano, D.; Poupart, P.; Forestier, O.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Assisted Phytoremediation of a Former Mine Soil Using Biochar and Iron Sulphate: Effects on As Soil Immobilization and Accumulation in Three Salicaceae Species. Sci. Total Environ. 2020, 710, 136203. [Google Scholar] [CrossRef]
- Deebika, P.; Sheela, A.M.; Ilamathi, R. Biochar and Compost-Based Phytoremediation of Crude Oil Contaminated Soil. Indian. J. Sci. Technol. 2021, 14, 220–228. [Google Scholar] [CrossRef]
- Liu, W.; Huo, R.; Xu, J.; Liang, S.; Li, J.; Zhao, T.; Wang, S. Effects of Biochar on Nitrogen Transformation and Heavy Metals in Sludge Composting. Bioresour. Technol. 2017, 235, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Jahantab, E.; Jafari, M.; Roohi, R.; Aman, M.S.; Moameri, M.; Zare, S. Application of Artificial Neural Network Model for the Identification the Effect of Municipal Waste Compost and Biochar on Phytoremediation of Contaminated Soils. J. Geochem. Explor. 2020, 208, 106399. [Google Scholar] [CrossRef]
- Forján, R.; Rodríguez-Vila, A.; Cerqueira, B.; Covelo, E.F. Comparison of Compost with Biochar versus Technosol with Biochar in the Reduction of Metal Pore Water Concentrations in a Mine Soil. J. Geochem. Explor. 2018, 192, 103–111. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of Biochar and Greenwaste Compost Amendments on Mobility, Bioavailability and Toxicity of Inorganic and Organic Contaminants in a Multi-Element Polluted Soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The Forms of Alkalis in the Biochar Produced from Crop Residues at Different Temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil. Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-Chemical Properties and Microbial Responses in Biochar-Amended Soils: Mechanisms and Future Directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Tisserant, A.; Cherubini, F. Potentials, Limitations, Co-Benefits, and Trade-Offs of Biochar Applications to Soils for Climate Change Mitigation. Land 2019, 8, 179. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential Hazards of Biochar: The Negative Environmental Impacts of Biochar Applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd_Allah, E.F.; et al. Biochar Production and Characteristics, Its Impacts on Soil Health, Crop Production, and Yield Enhancement: A Review. Plants 2024, 13, 166. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; Van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An Investigation into the Reactions of Biochar in Soil. Soil. Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Kookana, R.S. The Role of Biochar in Modifying the Environmental Fate, Bioavailability, and Efficacy of Pesticides in Soils: A Review. Soil. Res. 2010, 48, 627–637. [Google Scholar] [CrossRef]
- Vijay, V.; Shreedhar, S.; Adlak, K.; Payyanad, S.; Sreedharan, V.; Gopi, G.; Sophia van der Voort, T.; Malarvizhi, P.; Yi, S.; Gebert, J.; et al. Review of Large-Scale Biochar Field-Trials for Soil Amendment and the Observed Influences on Crop Yield Variations. Front. Energy Res. 2021, 9, 710766. [Google Scholar] [CrossRef]
- Pandey, J.; Sarkar, S.; Pandey, V.C. Compost-Assisted Phytoremediation. Assist. Phytoremediat. 2022, 243–264. [Google Scholar] [CrossRef]
- Alvarenga, P.; Gonçalves, A.P.; Fernandes, R.M.; de Varennes, A.; Vallini, G.; Duarte, E.; Cunha-Queda, A.C. Organic Residues as Immobilizing Agents in Aided Phytostabilization: (I) Effects on Soil Chemical Characteristics. Chemosphere 2009, 74, 1292–1300. [Google Scholar] [CrossRef]
- Vamerali, T.; Bandiera, M.; Mosca, G. Field Crops for Phytoremediation of Metal-Contaminated Land. A Review. Environ. Chem. Lett. 2009, 8, 1–17. [Google Scholar] [CrossRef]
- Ratnasari, A.; Syafiuddin, A.; Mehmood, M.A.; Boopathy, R. A Review of the Vermicomposting Process of Organic and Inorganic Waste in Soils: Additives Effects, Bioconversion Process, and Recommendations. Bioresour. Technol. Rep. 2023, 21, 101332. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, J.; Tang, Z.; Zhao, Y.; Wang, C. Vermiremediation of Organically Contaminated Soils: Concepts, Current Status, and Future Perspectives. Appl. Soil. Ecol. 2020, 147, 103377. [Google Scholar] [CrossRef]
- Al-Tawarah, B.; Alasasfa, M.A.; Mahadeen, A.Y. Efficacy of Compost and Vermicompost on Growth, Yield and Nutrients Content of Common Beans Crop (Phaseolus Vulgaris L.). J. Ecol. Eng. 2024, 25, 215–226. [Google Scholar] [CrossRef]
- Rehman, S.U.; De Castro, F.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermicompost: Enhancing Plant Growth and Combating Abiotic and Biotic Stress. Agronomy 2023, 13, 1134. [Google Scholar] [CrossRef]
- Brunetti, G.; Farrag, K.; Soler-Rovira, P.; Ferrara, M.; Nigro, F.; Senesi, N. The Effect of Compost and Bacillus Licheniformis on the Phytoextraction of Cr, Cu, Pb and Zn by Three Brassicaceae Species from Contaminated Soils in the Apulia Region, Southern Italy. Geoderma 2012, 170, 322–330. [Google Scholar] [CrossRef]
- Liu, B.; Wu, C.; Pan, P.; Fu, Y.; He, Z.; Wu, L.; Li, Q. Remediation Effectiveness of Vermicompost for a Potentially Toxic Metal-Contaminated Tropical Acidic Soil in China. Ecotoxicol. Environ. Saf. 2019, 182, 109394. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Pandey, A.K.; Bundela, P.S.; Wong, J.W.C.; Li, R.; Zhang, Z. Co-Composting of Gelatin Industry Sludge Combined with Organic Fraction of Municipal Solid Waste and Poultry Waste Employing Zeolite Mixed with Enriched Nitrifying Bacterial Consortium. Bioresour. Technol. 2016, 213, 181–189. [Google Scholar] [CrossRef]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble Salts in Compost and Their Effects on Soil and Plants: A Review. Compost. Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Shrestha, P.; Bellitürk, K.; Görres, J.H. Phytoremediation of Heavy Metal-Contaminated Soil by Switchgrass: A Comparative Study Utilizing Different Composts and Coir Fiber on Pollution Remediation, Plant Productivity, and Nutrient Leaching. Int. J. Environ. Res. Public Health 2019, 16, 1261. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, X.; Luo, L.; Zhou, Y.; Wei, J.; Chen, A.; Tang, L.; Wu, H.; Deng, Y.; Zhang, F.; et al. Remediation of Cu, Pb, Zn and Cd-Contaminated Agricultural Soil Using a Combined Red Mud and Compost Amendment. Int. Biodeterior. Biodegrad. 2017, 118, 73–81. [Google Scholar] [CrossRef]
- Huang, X.; Luo, D.; Chen, X.; Wei, L.; Liu, Y.; Wu, Q.; Xiao, T.; Mai, X.; Liu, G.; Liu, L. Insights into Heavy Metals Leakage in Chelator-Induced Phytoextraction of Pb- and Tl-Contaminated Soil. Int. J. Environ. Res. Public Health 2019, 16, 1328. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Cho, K.S.; Yun, J. Phytoremediaton Strategies for Co-Contaminated Soils: Overcoming Challenges, Enhancing Efficiency, and Exploring Future Advancements and Innovations. Processes 2025, 13, 132. [Google Scholar] [CrossRef]
- Bender, L.A.; Demarco, C.F.; Pieniz, S.; Carlos, F.S.; Quadro, M.S.; Andreazza, R. An Integrated Approach to Pb Bioremediation: Role of Bacteria in Enhancing Phytoremediation. Sustainability 2025, 17, 1386. [Google Scholar] [CrossRef]
- De Sena, A.; Mosdossy, K.; Whalen, J.K.; Madramootoo, C.A. Root Exudates and Microorganisms. Encycl. Soils Environ. Second Ed. 2023, 343–356. [Google Scholar] [CrossRef]
- Xing, Q.; Cao, X.; Tan, C.; Sun, L.; Deng, Y.; Yang, J.; Tu, C. Effects of Single and Combined Applications of Three Root Exudates of Sedum Plumbizincicola on the Phytoremediation Efficiency of Paddy Soil Contaminated with Cd. Front. Environ. Sci. 2023, 10, 1086753. [Google Scholar] [CrossRef]
- Williams, A.; Langridge, H.; Straathof, A.L.; Fox, G.; Muhammadali, H.; Hollywood, K.A.; Xu, Y.; Goodacre, R.; de Vries, F.T. Comparing Root Exudate Collection Techniques: An Improved Hybrid Method. Soil. Biol. Biochem. 2021, 161, 108391. [Google Scholar] [CrossRef]
- Agarwal, P.; Vibhandik, R.; Agrahari, R.; Daverey, A.; Rani, R. Role of Root Exudates on the Soil Microbial Diversity and Biogeochemistry of Heavy Metals. Appl. Biochem. Biotechnol. 2024, 196, 2673–2693. [Google Scholar] [CrossRef]
- Phillips, L.A.; Greer, C.W.; Farrell, R.E.; Germida, J.J. Plant Root Exudates Impact the Hydrocarbon Degradation Potential of a Weathered-Hydrocarbon Contaminated Soil. Appl. Soil. Ecol. 2012, 52, 56–64. [Google Scholar] [CrossRef]
- Han, R.; Dai, H.; Guo, B.; Noori, A.; Sun, W.; Wei, S. The Potential of Medicinal Plant Extracts in Improving the Phytoremediation Capacity of Solanum Nigrum L. for Heavy Metal Contaminated Soil. Ecotoxicol. Environ. Saf. 2021, 220, 112411. [Google Scholar] [CrossRef]
- Pandey, V.V.; Bhattacharya, A.; Pandey, A. Plant Growth-Promoting Microbiomes: History and Their Role in Agricultural Crop Improvement. In Plant-Microbe Interactions—Recent Advances in Molecular and Biochemical Approaches: Volume 1: Overview of Biochemical and Physiological Alterations During Plant-Microbe Interactions; Academic Press: Cambridge, MA, USA, 2023; pp. 1–44. [Google Scholar] [CrossRef]
- Godlewska, K.; Ronga, D.; Michalak, I. Plant Extracts—Importance in Sustainable Agriculture. Ital. J. Agron. 2021, 16. [Google Scholar] [CrossRef]
- Stingu, A.; Volf, I.; Popa, V.I.; Gostin, I. New Approaches Concerning the Utilization of Natural Amendments in Cadmium Phytoremediation. Ind. Crops Prod. 2012, 35, 53–60. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Chandra, R.; Kumar, V.; Tripathi, S.; Sharma, P. Heavy Metal Phytoextraction Potential of Native Weeds and Grasses from Endocrine-Disrupting Chemicals Rich Complex Distillery Sludge and Their Histological Observations during in-Situ Phytoremediation. Ecol. Eng. 2018, 111, 143–156. [Google Scholar] [CrossRef]
- Alves, A.R.A.; Yin, Q.; Oliveira, R.S.; Silva, E.F.; Novo, L.A.B. Plant Growth-Promoting Bacteria in Phytoremediation of Metal-Polluted Soils: Current Knowledge and Future Directions. Sci. Total Environ. 2022, 838, 156435. [Google Scholar] [CrossRef] [PubMed]
- Rabani, M.S.; Hameed, I.; Mir, T.A.; Wani, B.A.; Gupta, M.K.; Habib, A.; Jan, M.; Hussain, H.; Tripathi, S.; Pathak, A.; et al. Microbial-Assisted Phytoremediation. In Phytoremediation: Biotechnological Strategies for Promoting Invigorating Environment; Kumar, A., Singh, V.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 91–114. [Google Scholar] [CrossRef]
- Tiodar, E.D.; Văcar, C.L.; Podar, D. Phytoremediation and Microorganisms-Assisted Phytoremediation of Mercury-Contaminated Soils: Challenges and Perspectives. Int. J. Environ. Res. Public Health 2021, 18, 2435. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Freitas, H. Inoculation of Plant Growth Promoting Bacterium Achromobacter Xylosoxidans Strain Ax10 for the Improvement of Copper Phytoextraction by Brassica juncea. J. Environ. Manag. 2009, 90, 831–837. [Google Scholar] [CrossRef]
- Tribedi, P.; Goswami, M.; Chakraborty, P.; Mukherjee, K.; Mitra, G.; Bhattacharyya, P.; Dey, S. Bioaugmentation and Biostimulation: A Potential Strategy for Environmental Remediation. J. Microbiol. Exp. 2018, 6, 223–231. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R.; Gamalero, E.; Glick, B.R. Use of Plant Growth-Promoting Bacteria to Facilitate Phytoremediation. AIMS Microbiol. 2024, 10, 415–448. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 871, 402666. [Google Scholar] [CrossRef]
- Tak, H.I.; Ahmad, F.; Babalola, O.O. Advances in the Application of Plant Growth-Promoting Rhizobacteria in Phytoremediation of Heavy Metals. Rev. Environ. Contam. Toxicol. 2013, 223, 33–52. [Google Scholar] [CrossRef]
- Abeed, A.H.A.; Mahdy, R.E.; Alshehri, D.; Hammami, I.; Eissa, M.A.; Abdel Latef, A.A.H.; Mahmoud, G.A.E. Induction of Resilience Strategies against Biochemical Deteriorations Prompted by Severe Cadmium Stress in Sunflower Plant When Trichoderma and Bacterial Inoculation Were Used as Biofertilizers. Front. Plant Sci. 2022, 13, 1004173. [Google Scholar] [CrossRef]
- Anbuganesan, V.; Vishnupradeep, R.; Mehnaz, N.; Kumar, A.; Freitas, H.; Rajkumar, M. Synergistic Effect of Biochar and Plant Growth Promoting Bacteria Improve the Growth and Phytostabilization Potential of Sorghum Bicolor in Cd and Zn Contaminated Soils. Rhizosphere 2024, 29, 100844. [Google Scholar] [CrossRef]
- Sharma, S.; Saraf, M. Biofilm-Forming Plant Growth-Promoting Rhizobacterial Consortia Isolated from Mines and Dumpsites Assist Green Remediation of Toxic Metal (Ni and Pb) Using Brassica Juncea. Biol. Futur. 2023, 74, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, X.; Tao, Y.; Ke, T.; Wu, W.; Liao, K.; Li, X.; Zeng, Y.; Chen, C.; Chen, L. Effect of Plant Growth–Promoting Rhizobacteria on Oilseed Rape Brassica Juncea and Phytoextraction of Cadmium. J. Soils Sediments 2023, 23, 3472–3484. [Google Scholar] [CrossRef]
- Shi, G.; Hu, J.; Ding, F.; Li, S.; Shi, W.; Chen, Y. Exogenous Pseudomonas Aeruginosa Application Improved the Phytoremediation Efficiency of Lolium Multiflorum Lam on Cu–Cd Co-Contaminated Soil. Environ. Technol. Innov. 2022, 27, 102489. [Google Scholar] [CrossRef]
- Direk, A.; Arikan-Abdulveli, B.; Ozfidan-Konakci, C.; Yildiztugay, E.; Uysal, A. Effects of Bacillus Cereus on Physiological and Biochemical Characteristics of Wheat under Arsenic and Cadmium Stress: A Biological Agent to Reduce Heavy Metal Stress. Plant Stress 2024, 12, 100458. [Google Scholar] [CrossRef]
- Kriti; Kumari, B.; Singh, G.; Gautam, A.; Sinam, G.; Pal, S.; Anshu; Mishra, K.; Mallick, S. Enhancement in Ni–Cd Phytoremediation Efficiency of Helianthus annuus L. from Battery Waste Contaminated Soil by Bacterial Augmentation, Isolated from E-Waste Contaminated Sites. Int. J. Environ. Res. 2023, 17, 18. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.L.; Zhang, Q.; Verma, R.; Obi, N.; Dvinskikh, S.; Elmore, M.T.; Verma, S.K.; Gond, S.K.; Kowalski, K.P. Review: Endophytic Microbes and Their Potential Applications in Crop Management. Pest. Manag. Sci. 2019, 75, 2558–2565. [Google Scholar] [CrossRef]
- Pinski, A.; Betekhtin, A.; Hupert-Kocurek, K.; Mur, L.A.J.; Hasterok, R. Defining the Genetic Basis of Plant–Endophytic Bacteria Interactions. Int. J. Mol. Sci. 2019, 20, 1947. [Google Scholar] [CrossRef]
- Ali, B.; Hafeez, A.; Javed, M.A.; Afridi, M.S.; Abbasi, H.A.; Qayyum, A.; Batool, T.; Ullah, A.; Marc, R.A.; Jaouni, S.K.A.; et al. Role of Endophytic Bacteria in Salinity Stress Amelioration by Physiological and Molecular Mechanisms of Defense: A Comprehensive Review. S. Afr. J. Bot. 2022, 151, 33–46. [Google Scholar] [CrossRef]
- Barzanti, R.; Ozino, F.; Bazzicalupo, M.; Gabbrielli, R.; Galardi, F.; Gonnelli, C.; Mengoni, A. Isolation and Characterization of Endophytic Bacteria from the Nickel Hyperaccumulator Plant Alyssum bertolonii. Microb. Ecol. 2007, 53, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Ae, N.; Freitas, H. Endophytic Bacteria and Their Potential to Enhance Heavy Metal Phytoextraction. Chemosphere 2009, 77, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A.; Reynolds, C.M. Bacteria and Phytoremediation: New Uses for Endophytic Bacteria in Plants. Trends Biotechnol. 2005, 23, 6–8. [Google Scholar] [CrossRef]
- French, K.E. Engineering Mycorrhizal Symbioses to Alter Plant Metabolism and Improve Crop Health. Front. Microbiol. 2017, 8, 279045. [Google Scholar] [CrossRef]
- Vilela, L.A.F.; Barbosa, M.V. Contribution of Arbuscular Mycorrhizal Fungi in Promoting Cadmium Tolerance in Plants. In Cadmium Tolerance in Plants; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 553–586. [Google Scholar] [CrossRef]
- Millar, N.S.; Bennett, A.E. Stressed out Symbiotes: Hypotheses for the Influence of Abiotic Stress on Arbuscular Mycorrhizal Fungi. Oecologia 2016, 182, 625–641. [Google Scholar] [CrossRef]
- Wu, J.T.; Wang, L.; Zhao, L.; Huang, X.C.; Ma, F. Arbuscular Mycorrhizal Fungi Effect Growth and Photosynthesis of Phragmites Australis (Cav.) Trin Ex. Steudel under Copper Stress. Plant Biol. 2020, 22, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, G.; Saha, C.; Naskar, N.; Mukherjee, A.; Mukherjee, A.; Lahiri, S.; Majumder, A.L.; Seal, A. An Endophytic Bacterial Consortium Modulates Multiple Strategies to Improve Arsenic Phytoremediation Efficacy in Solanum Nigrum. Sci. Rep. 2018, 8, 6979. [Google Scholar] [CrossRef]
- Mesa, J.; Mateos-Naranjo, E.; Caviedes, M.A.; Redondo-Gómez, S.; Pajuelo, E.; Rodríguez-Llorente, I.D. Endophytic Cultivable Bacteria of the Metal Bioaccumulator Spartina Maritima Improve Plant Growth but Not Metal Uptake in Polluted Marshes Soils. Front. Microbiol. 2015, 6, 1450. [Google Scholar] [CrossRef]
- Luo, S.; Chen, L.; Chen, J.; Xiao, X.; Xu, T.; Wan, Y.; Rao, C.; Liu, C.; Liu, Y.; Lai, C.; et al. Analysis and Characterization of Cultivable Heavy Metal-Resistant Bacterial Endophytes Isolated from Cd-Hyperaccumulator Solanum Nigrum L. and Their Potential Use for Phytoremediation. Chemosphere 2011, 85, 1130–1138. [Google Scholar] [CrossRef]
- Govarthanan, M.; Mythili, R.; Selvankumar, T.; Kamala-Kannan, S.; Rajasekar, A.; Chang, Y.C. Bioremediation of Heavy Metals Using an Endophytic Bacterium paenibacillus sp. RM Isolated from the Roots of Tridax Procumbens. 3 Biotech 2016, 6, 242. [Google Scholar] [CrossRef]
- El-Deeb, B.; Gherbawy, Y.; Hassan, S. Molecular Characterization of Endophytic Bacteria from Metal Hyperaccumulator Aquatic Plant (Eichhornia crassipes) and Its Role in Heavy Metal Removal. Geomicrobiol. J. 2012, 29, 906–915. [Google Scholar] [CrossRef]
- Chen, L.; Luo, S.; Li, X.; Wan, Y.; Chen, J.; Liu, C. Interaction of Cd-Hyperaccumulator Solanum nigrum L. and Functional Endophyte pseudomonas sp. Lk9 on Soil Heavy Metals Uptake. Soil. Biol. Biochem. 2014, 68, 300–308. [Google Scholar] [CrossRef]
- Boorboori, M.R.; Zhang, H.Y. Arbuscular Mycorrhizal Fungi Are an Influential Factor in Improving the Phytoremediation of Arsenic, Cadmium, Lead, and Chromium. J. Fungi 2022, 8, 176. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Saxena, S. Arbuscular Mycorrhizal Fungi (AMF) from Heavy Metal-Contaminated Soils: Molecular Approach and Application in Phytoremediation. In Biofertilizers for Sustainable Agriculture and Environment; Yadav, A.N., Saxena, S., Giri, R.P., Prasad, R., Wu, Q.S., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 489–500. [Google Scholar] [CrossRef]
- Abdelhameed, R.E.; Metwally, R.A. Alleviation of Cadmium Stress by Arbuscular Mycorrhizal Symbiosis. Int. J. Phytoremediat. 2019, 21, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Li, B.; Jiang, M.; Li, T.; He, Y.; Li, Y.; Wang, Y. Effects of Arbuscular Mycorrhizal Fungi on the Growth and Heavy Metal Accumulation of Bermudagrass [Cynodon dactylon (L.) Pers.] Grown in a Lead–Zinc Mine Wasteland. Int. J. Phytoremediat. 2019, 21, 849–856. [Google Scholar] [CrossRef]
- Abbaslou, H.; Bakhtiari, S.; Hashemi, S.S. Rehabilitation of Iron Ore Mine Soil Contaminated with Heavy Metals Using Rosemary Phytoremediation-Assisted Mycorrhizal Arbuscular Fungi Bioaugmentation and Fibrous Clay Mineral Immobilization. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 431–441. [Google Scholar] [CrossRef]
- Abu-Elsaoud, A.M.; Nafady, N.A.; Abdel-Azeem, A.M. Arbuscular Mycorrhizal Strategy for Zinc Mycoremediation and Diminished Translocation to Shoots and Grains in Wheat. PLoS ONE 2017, 12, e0188220. [Google Scholar] [CrossRef]
- Jiang, Q.Y.; Zhuo, F.; Long, S.H.; Zhao, H.D.; Yang, D.J.; Ye, Z.H.; Li, S.S.; Jing, Y.X. Can Arbuscular Mycorrhizal Fungi Reduce Cd Uptake and Alleviate Cd Toxicity of Lonicera Japonica Grown in Cd-Added Soils? Sci. Rep. 2016, 6, 21805. [Google Scholar] [CrossRef]
- Hosseinniaee, S.; Jafari, M.; Tavili, A.; Zare, S.; Cappai, G. Chelate Facilitated Phytoextraction of Pb, Cd, and Zn from a Lead–Zinc Mine Contaminated Soil by Three Accumulator Plants. Sci. Rep. 2023, 13, 21185. [Google Scholar] [CrossRef]
- Guo, D.; Ali, A.; Ren, C.; Du, J.; Li, R.; Lahori, A.H.; Xiao, R.; Zhang, Z.; Zhang, Z. EDTA and Organic Acids Assisted Phytoextraction of Cd and Zn from a Smelter Contaminated Soil by Potherb Mustard (Brassica juncea, Coss) and Evaluation of Its Bioindicators. Ecotoxicol. Environ. Saf. 2019, 167, 396–403. [Google Scholar] [CrossRef]
- Hegazy, R.; El- Swaify, Z.A.; Abd Elkawy, A.M.; Radwan, A.M. Phyto-Extraction Potential of Duranta Erecta’s Against Heavy Metal Assisting by EDTA and Citric Acid for Mitigating Industrial Polluted Soil. Egypt. J. Agron. 2024, 46, 157–170. [Google Scholar] [CrossRef]
- Gul, I.; Manzoor, M.; Silvestre, J.; Rizwan, M.; Hina, K.; Kallerhoff, J.; Arshad, M. EDTA-Assisted Phytoextraction of Lead and Cadmium by Pelargonium Cultivars Grown on Spiked Soil. Int. J. Phytoremediat. 2019, 21, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Perveen, K.; Khan, F.; Sayyed, R.Z.; Hock, O.G.; Bhatt, S.C.; Singh, J.; Qamar, M.O. Effect of Different Levels of EDTA on Phytoextraction of Heavy Metal and Growth of Brassica juncea L. Front. Microbiol. 2023, 14, 1228117. [Google Scholar] [CrossRef]
- Hsiao, K.H.; Kao, P.H.; Hseu, Z.Y. Effects of Chelators on Chromium and Nickel Uptake by Brassica Juncea on Serpentine-Mine Tailings for Phytoextraction. J. Hazard. Mater. 2007, 148, 366–376. [Google Scholar] [CrossRef]
- Hart, G.; Gilly, A.; Koether, M.; McElroy, T.; Greipsson, S. Phytoextraction of Lead (Pb) Contaminated Soil by Switchgrass (Panicum virgatum L.): Impact of BAP and NTA Applications. Front. Energy Res. 2022, 10, 1032404. [Google Scholar] [CrossRef]
- de Souza Freitas, E.V.; do Nascimento, C.W.A. The Use of NTA for Lead Phytoextraction from Soil from a Battery Recycling Site. J. Hazard. Mater. 2009, 171, 833–837. [Google Scholar] [CrossRef]
- Zhang, Z.; Sarkar, D.; Sidhu, V.; Warke, M.; Datta, R. Impact of EDDS Dosage on Lead Phytoextraction in Contaminated Urban Residential Soils. Front. Sustain. Cities 2022, 3, 773467. [Google Scholar] [CrossRef]
- Wang, X.; Fernandes de Souza, M.; Li, H.; Qiu, J.; Ok, Y.S.; Meers, E. Biodegradation and Effects of EDDS and NTA on Zn in Soil Solutions during Phytoextraction by Alfalfa in Soils with Three Zn Levels. Chemosphere 2022, 292, 133519. [Google Scholar] [CrossRef]
- Meers, E.; Ruttens, A.; Hopgood, M.J.; Samson, D.; Tack, F.M.G. Comparison of EDTA and EDDS as Potential Soil Amendments for Enhanced Phytoextraction of Heavy Metals. Chemosphere 2005, 58, 1011–1022. [Google Scholar] [CrossRef]
- De Araújo, J.D.C.T.; Do Nascimento, C.W.A. Phytoextraction of Lead from Soil from a Battery Recycling Site: The Use of Citric Acid and NTA. Water Air Soil. Pollut. 2010, 211, 113–120. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Effect of Citric Acid on Phytoextraction Potential of Cucurbita Pepo, Lagenaria Siceraria, and Raphanus Sativus Plants Exposed to Multi-Metal Stress. Sci. Rep. 2023, 13, 13070. [Google Scholar] [CrossRef] [PubMed]
- Fomina, M.; Hillier, S.; Charnock, J.M.; Melville, K.; Alexander, I.J.; Gadd, G.M. Role of Oxalic Acid Overexcretion in Transformations of Toxic Metal Minerals by Beauveria Caledonica. Appl. Environ. Microbiol. 2005, 71, 371–381. [Google Scholar] [CrossRef]
- Qiu, L.; Gao, W.; Wang, Z.; Li, B.; Sun, W.; Gao, P.; Sun, X.; Song, B.; Zhang, Y.; Kong, T.; et al. Citric Acid and AMF Inoculation Combination–Assisted Phytoextraction of Vanadium (V) by Medicago Sativa in V Mining Contaminated Soil. Environ. Sci. Pollut. Res. 2021, 28, 67472–67486. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rizwan, M.; Kamran, M.; Mohamed, I.A.A.; khan, Z.; Bamagoos, A.A.; Alharby, H.F.; Hakeem, K.R.; et al. Individual and Combined Application of EDTA and Citric Acid Assisted Phytoextraction of Copper Using Jute (Corchorus capsularis L.) Seedlings. Environ. Technol. Innov. 2020, 19, 100895. [Google Scholar] [CrossRef]
- Cay, S. Assessment of Tea Saponin and Citric Acid–Assisted Phytoextraction of Pb-Contaminated Soil by Salvia Virgata Jacq. Environ. Sci. Pollut. Res. 2023, 30, 49771–49778. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Cost–Benefit Calculation of Phytoremediation Technology for Heavy-Metal-Contaminated Soil. Sci. Total Environ. 2016, 563–564, 796–802. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Rodgers, H.; Norton, J.; Norton, U.; van Diepen, L.T.A. Sustaining Vulnerable Agroecosystems with Compost: Lasting Benefits to Soil Health and Carbon Storage in Semiarid Winter Wheat (Triticum aestivum, L.). Appl. Soil. Ecol. 2024, 204, 105716. [Google Scholar] [CrossRef]
- Albtoosh, A.F.; Alnsour, M.A.; Hajar, H.A.; Lagum, A.A. Techno-Economic and Environmental Sustainability Assessment of a Sewage Sludge Composting Plant: A Case Study. Waste Biomass Valorization 2024, 15, 5275–5292. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial Inoculants: Reviewing the Past, Discussing the Present and Previewing an Outstanding Future for the Use of Beneficial Bacteria in Agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef]
- You, Y.; Dou, J.; Xue, Y.; Jin, N.; Yang, K. Chelating Agents in Assisting Phytoremediation of Uranium-Contaminated Soils: A Review. Sustainability 2022, 14, 6379. [Google Scholar] [CrossRef]
- Clemens, S.; Ma, J.F. Toxic Heavy Metal and Metalloid Accumulation in Crop Plants and Foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Ali, A.; Wan, Y.; Zhou, G. Genome-Wide Identification of Heavy-Metal ATPases Genes in Areca Catechu: Investigating Their Functionality under Heavy Metal Exposure. BMC Plant Biol. 2024, 24, 484. [Google Scholar] [CrossRef]
- Mendoza-Cózatl, D.G.; Butko, E.; Springer, F.; Torpey, J.W.; Komives, E.A.; Kehr, J.; Schroeder, J.I. Identification of High Levels of Phytochelatins, Glutathione and Cadmium in the Phloem Sap of Brassica Napus. A Role for Thiol-Peptides in the Long-Distance Transport of Cadmium and the Effect of Cadmium on Iron Translocation. Plant J. 2008, 54, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Juraniec, M.; Baliardini, C.; Meyer, C.L. Tolerance to Cadmium in Plants: The Special Case of Hyperaccumulators. BioMetals 2013, 26, 633–638. [Google Scholar] [CrossRef]
- Faizan, M.; Alam, P.; Hussain, A.; Karabulut, F.; Tonny, S.H.; Cheng, S.H.; Yusuf, M.; Adil, M.F.; Sehar, S.; Alomrani, S.O.; et al. Phytochelatins: Key Regulator against Heavy Metal Toxicity in Plants. Plant Stress. 2024, 11, 100355. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Archana, G. Cytosolic Expression of Synthetic Phytochelatin and Bacterial Metallothionein Genes in Deinococcus radiodurans R1 for Enhanced Tolerance and Bioaccumulation of Cadmium. BioMetals 2014, 27, 471–482. [Google Scholar] [CrossRef]
- Blindauer, C.A. Metallothioneins. In Binding, Transport and Storage of Metal Ions in Biological Cells; Maret, W., Ed.; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 606–665. [Google Scholar] [CrossRef]
- Gupta, D.K.; Vandenhove, H.; Inouhe, M. Role of Phytochelatins in Heavy Metal Stress and Detoxification Mechanisms in Plants. Heavy Metal. Stress. Plants 2013, 73–94. [Google Scholar] [CrossRef]
- Li, S.; Han, X.; Lu, Z.; Qiu, W.; Yu, M.; Li, H.; He, Z.; Zhuo, R. MAPK Cascades and Transcriptional Factors: Regulation of Heavy Metal Tolerance in Plants. Int. J. Mol. Sci. 2022, 23, 4463. [Google Scholar] [CrossRef] [PubMed]
- Kosakivska, I.V.; Babenko, L.M.; Romanenko, K.O.; Korotka, I.Y.; Potters, G. Molecular Mechanisms of Plant Adaptive Responses to Heavy Metals Stress. Cell Biol. Int. 2021, 45, 258–272. [Google Scholar] [CrossRef]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone Priming: Regulator for Heavy Metal Stress in Plants. J. Plant Growth Regul. 2018, 38, 739–752. [Google Scholar] [CrossRef]
- Kumar, K.; Shinde, A.; Aeron, V.; Verma, A.; Arif, N.S. Genetic Engineering of Plants for Phytoremediation: Advances and Challenges. J. Plant Biochem. Biotechnol. 2022, 32, 12–30. [Google Scholar] [CrossRef]
- Li, R.; Wu, H.; Ding, J.; Li, N.; Fu, W.; Gan, L.; Li, Y. Transgenic MerA and MerB Expression Reduces Mercury Contamination in Vegetables and Grains Grown in Mercury-Contaminated Soil. Plant Cell Rep. 2020, 39, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A. Phytoremediation of Heavy Metal-Contaminated Soils: Recent Advances, Challenges, and Future Prospects. In Bioremediation for Environmental Sustainability: Toxicity, Mechanisms of Contaminants Degradation, Detoxification and Challenges; Elsevier: Amsterdam, The Netherlands, 2021; pp. 29–51. [Google Scholar] [CrossRef]
- Rahman, S.U.; Nawaz, M.F.; Gul, S.; Yasin, G.; Hussain, B.; Li, Y.; Cheng, H. State-of-the-Art OMICS Strategies Against Toxic Effects of Heavy Metals in Plants: A Review. Ecotoxicol. Environ. Saf. 2022, 242, 113952. [Google Scholar] [CrossRef] [PubMed]


| Country | Documents | Citations | Total Link Strength |
|---|---|---|---|
| China | 73 | 5585 | 28 |
| Pakistan | 14 | 2536 | 16 |
| United States | 23 | 2680 | 15 |
| Australia | 11 | 2435 | 11 |
| Poland | 14 | 464 | 9 |
| Spain | 16 | 822 | 9 |
| France | 12 | 522 | 7 |
| India | 25 | 1877 | 4 |
| Italy | 9 | 406 | 4 |
| Iran | 8 | 156 | 3 |
| Keyword | Frequency |
|---|---|
| Heavy metals | 120 |
| Phytoremediation | 98 |
| Soil amendments | 85 |
| Biochar | 72 |
| Immobilisation | 65 |
| Cadmium | 60 |
| Soil remediation | 58 |
| Bioremediation | 54 |
| Document | Citations |
|---|---|
| Ali et al. [14] | 3137 |
| Bolan et al. [15] | 1748 |
| Sarwar et al. [16] | 1131 |
| Mahar et al. [10] | 996 |
| Koptsik [17] | 165 |
| Chirakkara et al. [18] | 129 |
| Gascó et al. [19] | 101 |
| Mosa et al. [20] | 96 |
| Feedstock and Pyrolysis Degree | HMs | Plant Species | Key Observations and Effects | References |
|---|---|---|---|---|
| Bamboo, rice straw (>500 °C) | Cd, Cu, Pb and Zn | Sedum plumbizincicola | Increased the aboveground biomass of Sedum plumbizincicola while reducing the solubility and accumulation of Cd, Cu, Pb and Zn. | Lu et al. [44] |
| Corn straw (≥500 °C) | Pb | Populus deltoides (male and female) | Biochar enhanced total biomass by 29% in females and 26% in males under Pb stress. Without biochar, biomass was reduced by 11% in females and 3% in males. Enhanced antioxidative response in males. Improved soil microbial diversity and stability. | Su et al. [45] |
| Coconut shells (800 °C) | Cd, Pb and Zn | Salix smithiana Willd. | Increased phytoextraction efficiency. High biochar application (5, 10 and 15% w/w) reduced metal phytotoxicity in soil solution, improved biomass growth in Salix smithiana and enhanced HM uptake by plants in the amendment treatments. | Pračke et al. [46] |
| Pine needle (500 °C) | Cd | Bidens pilosa L. | Biochar amendment enhanced Cd accumulation in roots and shoots, promoting dry weight and root elongation. | Manori et al. [47] |
| Bamboo biochar (600 °C) | Cd and Zn | Salix psammophila | Stimulated Cu, Cd and Zn accumulation in plant tissues. The 3% bamboo biochar (BBC-3%) treatment significantly improved Cd and Zn uptake. Bamboo biochar amendment improved the transfer and bioconcentration factor values of Cd, Zn and Cu compared with the control. | Li et al. [48] |
| Cornstalk biochar (500 °C) | Cd | Beta vulgaris var. cicla L. | Increased Cd concentrations in leaves (36%) and roots (52%). Root dry weight increased by 267% and Cd accumulation increased by 206%. | Gu et al. [49] |
| Wood-derived biochar | Cd and Zn | Noccaea caerulescens | Improved seed germination and plant survival. Increased root surface density. The hyperaccumulating plants removed approximately 40% of the initial Cd contamination from the soil. | Rees et al. [50] |
| Coconut shell biochar | Cd and Zn | Salix × smithiana (willow) | Reduced Cd and Zn leachate concentrations by 99% in all biochar treatments. Biochar significantly increased biomass production. | Břendová et al. [51] |
| Oak wood (400 °C) | Pb | Lactuca sativa | Biochar reduced Pb bioavailability by 75.8% and bioaccessibility by 12.5% in soil. Increased seed germination by 360% and root length by 189% compared to unamended soil. Improved soil quality and supported plant growth. | Ahmad et al. [52] |
| Pruning residues in orchards (550 °C), fir tree pellets (350–400 °C) and manure pellets mixed with fir tree pellets | Cd and Pb | Anthyllis vulneraria subsp. polyphylla, Noccaea rotundifolium subsp. cepaeifolium and Poa alpina subsp. alpina | Different biochars influenced pH, EC, CEC and metal bioavailability. Manure pellets and pruning residue biochar reduced Cd and Pb in plant shoots. Manure pellet biochar at a 1.5% dose increased biomass production. Biochar effects vary with feedstock and soil characteristics. | Fellet et al. [53] |
| Aspect | Compost | Vermicompost |
|---|---|---|
| Nutrient Content | Low levels of macro- and micro-nutrients and primarily enhance soil fertility through organic matter. | High concentrations of macro- and micro-nutrients (N, P, K, Ca, Mg and Zn) are due to earthworm processing [81,82]. |
| Soil Structure Improvement | Enhances soil aeration, moisture retention and overall structure [83]. | Improves porosity, aeration and water-holding capacity and reduces bulk density [82]. |
| Microbial Activity | Increases microbial diversity and activity in the soil [83]. | Significantly enhances microbial populations and improves nutrient cycling [84]. |
| Heavy Metal Stabilisation | Reduces the bioavailability of HMs through immobilisation [83]. | Enhances the phytostabilisation potential and effectively adsorbs HMs. |
| Promotion of Plant Growth | Supports plant growth by improving nutrient availability and soil conditions [83]. | Promotes root development and overall plant vigour owing to enriched nutrients and growth hormones. |
| Contaminant Degradation | Facilitates organic pollutant degradation through enhanced microbial activity [83]. | Stimulates microbial bioremediation for effective pollutant breakdown [84]. |
| Application Rate | Typically requires higher application rates for effectiveness. | More effective at lower application rates owing to higher nutrient availability [84]. |
| Production Time | Longer production time (several months). | Shorter production time (few weeks to months). |
| Mycorrhizal Species | Host Plant | Heavy Metals | Significance and Impact | References |
|---|---|---|---|---|
| Glomus monosporum, Glomus clarum and Gigaspora nigra | Trigonella sp. | Cd | AMF improved Trigonella plant growth, chlorophyll content and protein levels in Trigonella under Cd stress. Reduced Cd translocation and oxidative damage while enhancing antioxidant activity. | Abdelhameed and Metwally [138] |
| Funneliformis mosseae and Diversispora spurcum (AMF) | Bermudagrass (Cynodon dactylon (L.) Pers.) | Pb, Zn and Cd | Diversispora spurcum significantly enhanced bermudagrass growth and HM uptake. AMF increased soil pH and nutrient levels (P and S) while reducing the availability of Pb and Zn in soil. Decreased Pb translocation in shoots improves bermudagrass suitability for mine wasteland restoration. | Zhan et al. [139] |
| Rhizophagus irregularis (AMF) | Common Reed (Phragmites australis) | Cu | AMF inoculation promoted plant growth and improved physiological activity in Phragmites australis under Cu stress. | Wu et al. [129] |
| Glomus mosseae and Glomus intraradices (AMF) | Rosemary (Rosmarinus officinalis) | Cu, Zn, Mn, Cd, Pb and Fe | AMF facilitated plant survival in metal-contaminated soil by enhancing nutrient uptake, reducing metal toxicity and facilitating metal absorption. | Abbaslou et al. [140] |
| Funneliformis geosporum (AMF) | Wheat (Triticum aestivum L. cv. Gemmeza-10) | Zn | Inoculation with F. geosporum significantly reduced Zn accumulation and inhibited its translocation to wheat shoots and grains. | Abu-Elsaoud et al. [141] |
| Glomus versiforme and Rhizophagus intraradices (AMF) | Lonicera japonica | Cd | AMF reduced Cd levels in the shoots and roots of Lonicera japonica, increased P acquisition and enhanced antioxidant activity (catalase (CAT), ascorbate peroxidase (APX) and glutathione reductase (GR)), leading to improved plant growth and Cd tolerance and making the plant safer for use in Cd-contaminated soils. | Jiang et al. [142] |
| Amendment | Cost Considerations/Hectare | Effectiveness | Scalability | Environmental Impact |
|---|---|---|---|---|
| Biochar | Varies between USD 4000 and USD 8000 depending on feedstock and processing | High | Medium | Positive (carbon sequestration) |
| Compost | USD 250–USD 500 when locally sourced | Moderate | High | Positive (waste reduction) |
| Microbial Agents | Costs vary with production and monitoring activities | High | Low–Medium | Positive (biodegradable) |
| Chelating Agents | USD 2190–USD 10,950 with environmental risks | Very High | Low | Negative (metal leaching risk) |
| Plant Extracts | Included in project costs | Moderate | Low | Positive (biodegradable) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamma, A.A.; Lejcuś, K.; Fiałkiewicz, W.; Marczak, D. Advancing Phytoremediation: A Review of Soil Amendments for Heavy Metal Contamination Management. Sustainability 2025, 17, 5688. https://doi.org/10.3390/su17135688
Tamma AA, Lejcuś K, Fiałkiewicz W, Marczak D. Advancing Phytoremediation: A Review of Soil Amendments for Heavy Metal Contamination Management. Sustainability. 2025; 17(13):5688. https://doi.org/10.3390/su17135688
Chicago/Turabian StyleTamma, Ahmed Abderrafaa, Krzysztof Lejcuś, Wiesław Fiałkiewicz, and Daria Marczak. 2025. "Advancing Phytoremediation: A Review of Soil Amendments for Heavy Metal Contamination Management" Sustainability 17, no. 13: 5688. https://doi.org/10.3390/su17135688
APA StyleTamma, A. A., Lejcuś, K., Fiałkiewicz, W., & Marczak, D. (2025). Advancing Phytoremediation: A Review of Soil Amendments for Heavy Metal Contamination Management. Sustainability, 17(13), 5688. https://doi.org/10.3390/su17135688






