Environmental Sustainability of Nile Tilapia Reared in Biofloc Technology (BFT) System: Evaluation of Carbon, Nitrogen, and Phosphorus Dynamics and Indicators of Sustainability
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Fish, Experimental Conditions, and Feeding
2.3. Growth Performance Variables
- Survival rate (SR; %) = (final number of fish/starting number of fish) × 100;
- Weight gain (WG; g) = final weight (g) − initial weight (g);
- Apparent feed conversion (AFC) = feed consumption (g)/weight gain (g);
- Specific growth rate (SGR; %/day) = 100 − [ln final weigh (g) − ln initial weight (g)/trial period].
2.4. Water Quality Monitoring
2.5. Nutrient Quantification in the System
2.6. Nutrient Mass Balance
- Initial water (IW) = TC, TN, or TP concentration analyzed in the initial water (mg L−1) × tank water volume (L);
- Water replacement (WR) = TC, TN, or TP concentration analyzed in the water supply (mg L−1) × volume of water used (L);
- Initial biomass of fish (IB) = TC, TN, or TP concentration analyzed in the initial carcass (g kg−1) × initial biomass of fish (kg);
- Feed (F) = TC, TN, or TP concentration analyzed in the feed (g kg−1 based on dry matter) × total amount of feed provided (kg);
- Total nutrient input (TNI) = IW + WR + IB + F.
- Final water (FW) = TC, TN, or TP concentration analyzed in final water (mg L−1) × tank water volume (L);
- Solids removed (SOR) = TC, TN, or TP concentration analyzed in the solids removed during the test (g kg−1) × volume of solids discarded (L);
- Final biomass of fish (FB) = TC, TN, or TP concentration analyzed in the final carcass (g kg−1) × final biomass of fish (kg);
- Total nutrient output (TNO) = FW + SOR + FB.
2.7. Indicators of Sustainability
2.8. Statistical Analysis
3. Results
3.1. Water Quality
3.2. Nutrient Quantification
3.3. Mass Balance
3.4. Sustainability Indicators
3.5. Productive Performance
4. Discussion
4.1. Water Quality
4.2. Nutrient Balance
4.3. Sustainability Metrics
4.4. Growth Efficiency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFC | Apparent Feed Conversion |
| BFT | Biofloc Technology System |
| FB | Final Biomass of Fish |
| FW | Final Water |
| IB | Initial Biomass of Fish |
| IW | Initial Water |
| NH3 | Ammonia |
| NO2-N | Nitrogen-Nitrite |
| NO3-N | Nitrogen-Nitrate |
| Phosphate | |
| SGR | Specific Growth Rate |
| SOR | Solids Removed |
| SR | Survival Rate |
| SS | Sedimentable Solids |
| TC | Total Carbon |
| TN | Total Nitrogen |
| TNI | Total Nutrient Input; |
| TNO | Total Nutrient Output |
| TP | Total Phosphorus |
| TSS | Total Suspended Solids |
| UNF | Unaccounted Nutrient Fraction |
| WG | Weight Gain |
| WR | Water Replacement |
References
- FAO (Food and Agricultural Organization). The State of World Fisheries and Aquaculture—Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Coldebella, A.; Godoy, A.C.; Gentelini, A.L.; Piana, P.A.; Coldebella, P.F.; Boscolo, W.R.; Feiden, A. Nitrogen and phosphorus dynamics in Nile tilapia farming in excavated rearing ponds. Res. Soc. Dev. 2020, 9, e1319119699. [Google Scholar] [CrossRef]
- Valenti, W.C.; Barros, H.P.; Moraes-Valenti, P.; Bueno, G.W.; Cavalli, R.O. Aquaculture in Brazil: Past, present and future. Aquac. Rep. 2021, 19, 100611. [Google Scholar] [CrossRef]
- Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Guidebook, 3rd ed.; World Aquaculture Society: Sorrento, FL, USA, 2014. [Google Scholar]
- Vijayan, K.K.; Panigrahi, A. Training Manual on Biofloc Technology for Nursery and Growout Aquaculture; Central Institute of Brackishwater Aquaculture: Tamil Nadu, India, 2019. [Google Scholar]
- Avnimelech, Y. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture 2007, 264, 140–147. [Google Scholar] [CrossRef]
- Gallardo-Collí, A.; Pérez-Fuentes, M.; Pérez-Rostro, C.I.; Hernández-Vergara, M.P. Compensatory growth of Nile tilapia Oreochromis niloticus, L. subjected to cyclic periods of feed restriction and feeding in a biofloc system. Aquac. Res. 2020, 51, 1813–1823. [Google Scholar] [CrossRef]
- Hisano, H.; Parisi, J.; Cardoso, I.L.; Ferri, G.H.; Ferreira, P.M.F. Dietary protein reduction for Nile tilapia fingerlings reared in biofloc technology. J. World Aquac. Soc. 2020, 51, 452–462. [Google Scholar] [CrossRef]
- Ghamkhar, R.; Boxman, S.E.; Main, K.L.; Zhang, Q.; Trotz, M.A.; Hicks, A. Life cycle assessment of aquaculture systems: Does burden shifting occur with an increase in production intensity? Aquac. Eng. 2021, 92, 102130. [Google Scholar] [CrossRef]
- David, L.H.; Pinho, S.M.; Keesman, K.J.; Garcia, F. Assessing the sustainability of tilapia farming in biofloc-based culture using emergy synthesis. Ecol. Indic. 2021, 131, 108186. [Google Scholar] [CrossRef]
- Pinho, S.M.; David, L.H.; Garcia, F.; Portella, M.C.; Keesman, K.J. Sustainability assessment of FLOCponics compared to stand-alone hydroponic and biofloc systems using emergy synthesis. Ecol. Indic. 2022, 141, 109092. [Google Scholar] [CrossRef]
- Chang, C.C.; Chang, K.C.; Lin, W.C.; Wu, M.H. Carbon footprint analysis in the aquaculture industry: Assessment of an ecological shrimp farm. J. Clean. Prod. 2017, 168, 1101–1107. [Google Scholar] [CrossRef]
- Valenti, W.; Kimpara, J.; Preto, B.; Moraes-Valenti, P. Indicators of sustainability to assess aquaculture systems. Ecol. Indic. 2018, 88, 402–413. [Google Scholar] [CrossRef]
- Samuel-Fitwi, B.; Wuertz, S.; Schroeder, J.P.; Schulz, C. Sustainability assessment tools to support aquaculture development. J. Clean. Prod. 2012, 32, 183–192. [Google Scholar] [CrossRef]
- Abakari, G.; Luo, G.; Kombat, E.O. Dynamics of nitrogenous compounds and their control in biofloc technology (BFT) systems: A review. Aquac. Fish. 2021, 6, 441–447. [Google Scholar] [CrossRef]
- Legarda, E.C.; Poli, M.A.; Martins, M.A.; Pereira, S.A.; Martins, M.L.; Machado, C.; de Lorenzo, M.A.; Vieira, F.N. Integrated recirculating aquaculture system for mullet and shrimp using biofloc technology. Aquaculture 2019, 512, 734308. [Google Scholar] [CrossRef]
- Jørgensen, S.E.; Johnsen, I. Chapter 2—Mass Conservation. In Principles of Environmental Science and Technology; Jørgensen, S.E., Johnsen, I., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 33, pp. 29–142. [Google Scholar]
- Golterman, H.L.; Clymo, R.S.; Ohnstad, M.A.M. Methods for Physical and Chemical Analysis of Fresh Waters; Hand-book: London, UK, 1978. [Google Scholar]
- Lorenzen, C.J. Determination of chlorophyll and pheo-pigments: Spectrophotometric equations. Limnol. Oceanogr. 1967, 12, 343–346. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Sea-Water Analysis, 2nd ed.; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972. [Google Scholar]
- Baird, R.; Bridgewater, L. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- AOAC. Official Methods of Analysis, 21st ed.; The Association of Official Analytical Chemists: Washington, DC, USA, 2019. [Google Scholar]
- Silva, K.R.; Wasielesky, W.; Abreu, P.C. Nitrogen and Phosphorus Dynamics in the Biofloc Production of the Pacific White Shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2013, 44, 30–41. [Google Scholar] [CrossRef]
- Sahu, B.C.; Adhikari, S.; Mahapatra, A.S.; Dey, L. Nitrogen, Phosphorus, and Carbon Budgets in Polyculture Ponds of Indian Major Carps and Giant Freshwater Prawn in Orissa State, India. J. Appl. Aquac. 2015, 27, 365–376. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Fish and Shrimp; The National Academies Press: Washington, DC, USA, 2011.
- Boyd, C.E.; Tucker, C.; McNevin, A.; Bostick, K.; Clay, J. Indicators of resource use efficiency and environmental performance in fish and crustacean aquaculture. Rev. Fish. Sci. 2007, 15, 327–360. [Google Scholar] [CrossRef]
- Lewis-Beck, C.; Lewis-Beck, M.S. Applied Regression: An Introduction, 2nd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Fialho, N.S.; Valenti, W.C.; David, F.S.; Godoy, E.M.; Proença, D.C.; Roubach, R.; Wolff Bueno, G. Environmental sustainability of Nile tilapia net-cage culture in a neotropical region. Ecol. Indic. 2021, 129, 108008. [Google Scholar] [CrossRef]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Klower Academic Publisher: Boston, MA, USA, 1998. [Google Scholar]
- Colt, J. Water quality requirements for reuse systems. Aquac. Eng. 2006, 34, 143–156. [Google Scholar] [CrossRef]
- Avnimelech, Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia-nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Robles-Porchas, G.R.; Gollas-Galván, T.; Martínez-Porchas, M.; Martínez-Cordova, L.R.; Miranda-Baeza, A.; Vargas-Albores, F. The nitrification process for nitrogen removal in biofloc system aquaculture. Rev. Aquac. 2020, 12, 2228–2249. [Google Scholar] [CrossRef]
- Ogello, E.O.; Outa, N.O.; Obiero, K.O.; Kyule, D.N.; Munguti, J.M. The prospects of biofloc technology (BFT) for sustainable aquaculture development. Sci. Afr. 2021, 14, e01053. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Tilapia Culture; CABI Publishing: Wallingford, UK, 2006. [Google Scholar]
- Turker, H.; Eversole, A.G.; Brune, D.E. Effect of temperature and phytoplankton concentration on Nile tilapia Oreochromis niloticus (L.) filtration rate. Aquac. Res. 2003, 34, 453–460. [Google Scholar] [CrossRef]
- Semyalo, R.; Rohrlack, T.; Kayiira, D.; Kizito, Y.S.; Byarujali, S.; Nyakairu, G.; Larsson, P. On the diet of Nile tilapia in two eutrophic tropical lakes containing toxin producing cyanobacteria. Limnologica 2011, 41, 30–36. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Biofloc Production Systems for Aquaculture Southern Regional Aquaculture Center; SRAC Publ: Stoneville, MS, USA, 2013. [Google Scholar]
- Azim, M.E.; Little, D.C. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Stockhausen, L.; Vilvert, M.P.; Silva, M.; Dartora, A.; Krainz, R.; Ferreira, G.B.; Silva, L.R.; Jatobá, A. Practical diet with total replacement of fishmeal by soybean meal for Nile tilapia: Growth performance and health effects. Ciência Anim. Bras. 2022, 23, e-71567E. [Google Scholar] [CrossRef]
- Cao, B.; Abakari, G.; Luo, G.; Tan, H.; Xia, W. Comparative analysis of nitrogen and phosphorus budgets in a bioflocs aquaculture system and recirculation aquaculture system during over-wintering of tilapia (GIFT, Oreochromis niloticus). Aquac. Eng. 2020, 89, 102026. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef] [PubMed]
- Sklan, D.; Prag, T.; Lupatsch, I. Apparent digestibility coefficients of feed ingredients and their prediction in diets for tilapia Oreochromis niloticus x Oreochromis aureus (Teleostei, Cichlidae). Aquac. Res. 2004, 35, 358–364. [Google Scholar] [CrossRef]
- Flickinger, D.L.; Costa, G.A.; Dantas, D.P.; Proença, D.C.; David, F.S.; Durborow, R.M.; Moraes-Valenti, P.; Valenti, W.C. The budget of carbon in the farming of the Amazon river prawn and tambaqui fish in earthen pond monoculture and integrated multitrophic systems. Aquac. Rep. 2020, 17, 100340. [Google Scholar] [CrossRef]
- Oliveira, L.K.; Pilz, L.; Furtado, P.S.; Ballester, E.L.C.; Bicudo, Á.J.d.A. Growth, nutritional efficiency, and profitability of juvenile GIFT strain of Nile tilapia (Oreochromis niloticus) reared in biofloc system on graded feeding rates. Aquaculture 2021, 541, 736830. [Google Scholar] [CrossRef]
- Gallardo-Collí, A.; Pérez-Rostro, C.I.; Hernández-Vergara, M.P.; Pérez-Legaspi, I.A. Microeukaryote community and the nutritional composition of the biofloc during Nile tilapia culture in water-reusing biofloc systems. Aquac. Int. 2019, 27, 381–398. [Google Scholar] [CrossRef]
- Neto, H.S.; Santaella, S.T.; Nunes, A.J.P. Bioavailability of crude protein and lipid from biofloc meals produced in an activated sludge system for white shrimp, Litopenaeus vannamei. Rev. Bras. Zootec. 2015, 44, 269–275. [Google Scholar] [CrossRef]
- Wurts, W.A.; Durborow, R.M. Interactions of pH, Carbon Dioxide, Alkalinity and Hardness in Fish Ponds. SRAC Publ. 1992, 464, 1–4. [Google Scholar]
- Vasanth, M.; Muralidhar, M.; Saraswathy, R.; Nagavel, A.; Dayal, J.S.; Jayanthi, M.; Lalitha, N.; Kumararaja, P.; Vijayan, K.K. Methodological approach for the collection and simultaneous estimation of greenhouse gases emission from aquaculture ponds. Environ. Monit. Assess. 2016, 188, 1–10. [Google Scholar] [CrossRef]
- Moura, R.S.T.; Valenti, W.C.; Henry-Silva, G.G. Sustainability of Nile tilapia net-cage culture in a reservoir in a semi-arid region. Ecol. Indic. 2016, 66, 574–582. [Google Scholar] [CrossRef]
- Haridas, H.; Verma, A.K.; Rathore, G.; Prakash, C.; Sawant, P.B.; Babitha Rani, A.M. Enhanced growth and immuno-physiological response of Genetically Improved Farmed Tilapia in indoor biofloc units at different stocking densities. Aquac. Res. 2017, 48, 4346–4355. [Google Scholar] [CrossRef]
- Choueri, R.B.; Azevedo, J.A.R. Biodiversidade e impacto de grandes empreendimentos hidrelétricos na Bacia Tocantins-Araguaia: Uma análise sistêmica. Soc. Nat. 2017, 29, 443–457. [Google Scholar] [CrossRef]
- Ekasari, J.; Rivandi, D.R.; Firdausi, A.P.; Surawidjaja, E.H.; Zairin, M.; Bossier, P.; De Schryver, P. Biofloc technology positively affects Nile tilapia (Oreochromis niloticus) larvae performance. Aquaculture 2015, 441, 72–77. [Google Scholar] [CrossRef]
- Silva, M.A.; de Alvarenga, É.R.; Alves, G.F.O.; Manduca, L.G.; Turra, E.M.; de Brito, T.S.; de Sales, S.C.M.; da Silva Junior, A.F.; Borges, W.J.M.; Teixeira, E.A. Crude protein levels in diets for two growth stages of Nile tilapia (Oreochromis niloticus) in a biofloc system. Aquac. Res. 2018, 49, 2693–2703. [Google Scholar] [CrossRef]
- Hisano, H.; Pinheiro, V.R.; Losekann, M.E.; Moura e Silva, M.S.G. Effect of feeding frequency on water quality, growth, and hematological parameters of Nile tilapia Oreochromis niloticus reared using biofloc technology. J. Appl. Aquac. 2021, 33, 96–110. [Google Scholar] [CrossRef]
- Luo, G.; Gao, Q.; Wang, C.; Liu, W.; Sun, D.; Li, L.; Tan, H. Growth, digestive activity, welfare, and partial cost-effectiveness of genetically improved farmed tilapia (Oreochromis niloticus) cultured in a recirculating aquaculture system and an indoor biofloc system. Aquaculture 2014, 422–423, 1–7. [Google Scholar] [CrossRef]
- Long, L.; Yang, J.; Li, Y.; Guan, C.; Wu, F. Effect of biofloc technology on growth, digestive enzyme activity, hematology, and immune response of genetically improved farmed tilapia (Oreochromis niloticus). Aquaculture 2015, 448, 135–141. [Google Scholar] [CrossRef]
- Mirzakhani, N.; Ebrahimi, E.; Jalali, S.A.H.; Ekasari, J. Growth performance, intestinal morphology and nonspecific immunity response of Nile tilapia (Oreochromis niloticus) fry cultured in biofloc systems with different carbon sources and input C:N ratios. Aquaculture 2019, 512, 734235. [Google Scholar] [CrossRef]
- Gullian-Klanian, M.; Arámburu-Adame, C. Performance of Nile tilapia Oreochromis niloticus fingerlings in a hyper-intensive recirculating aquaculture system with low water exchange. Lat. Am. J. Aquat. Res. 2013, 41, 150–162. [Google Scholar] [CrossRef]
- Rodde, C.; Chatain, B.; Vandeputte, M.; Trinh, T.Q.; Benzie, J.A.H.; de Verdal, H. Can individual feed conversion ratio at commercial size be predicted from juvenile performance in individually reared Nile tilapia Oreochromis niloticus? Aquac. Reports 2020, 17, 100349. [Google Scholar] [CrossRef]
- Montanhini Neto, R.; Ostrensky, A. Nutrient load estimation in the waste of Nile tilapia Oreochromis niloticus (L.) reared in cages in tropical climate conditions. Aquac. Res. 2015, 46, 1309–1322. [Google Scholar] [CrossRef]
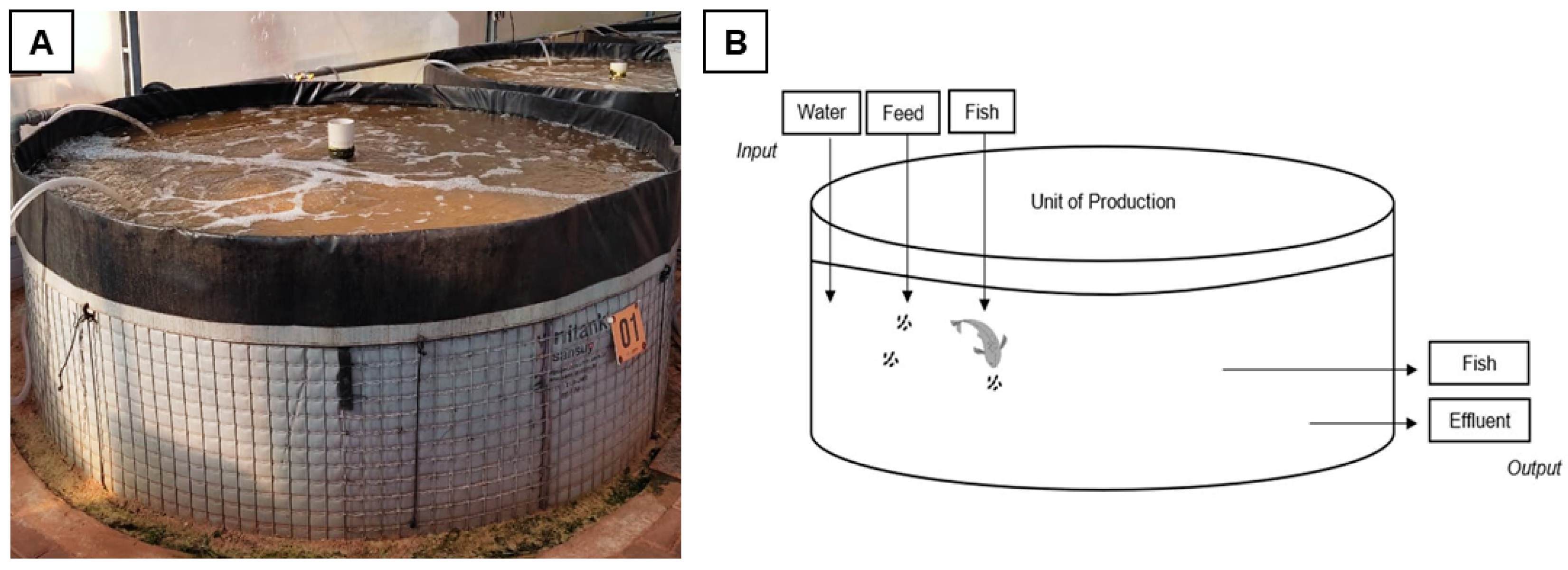
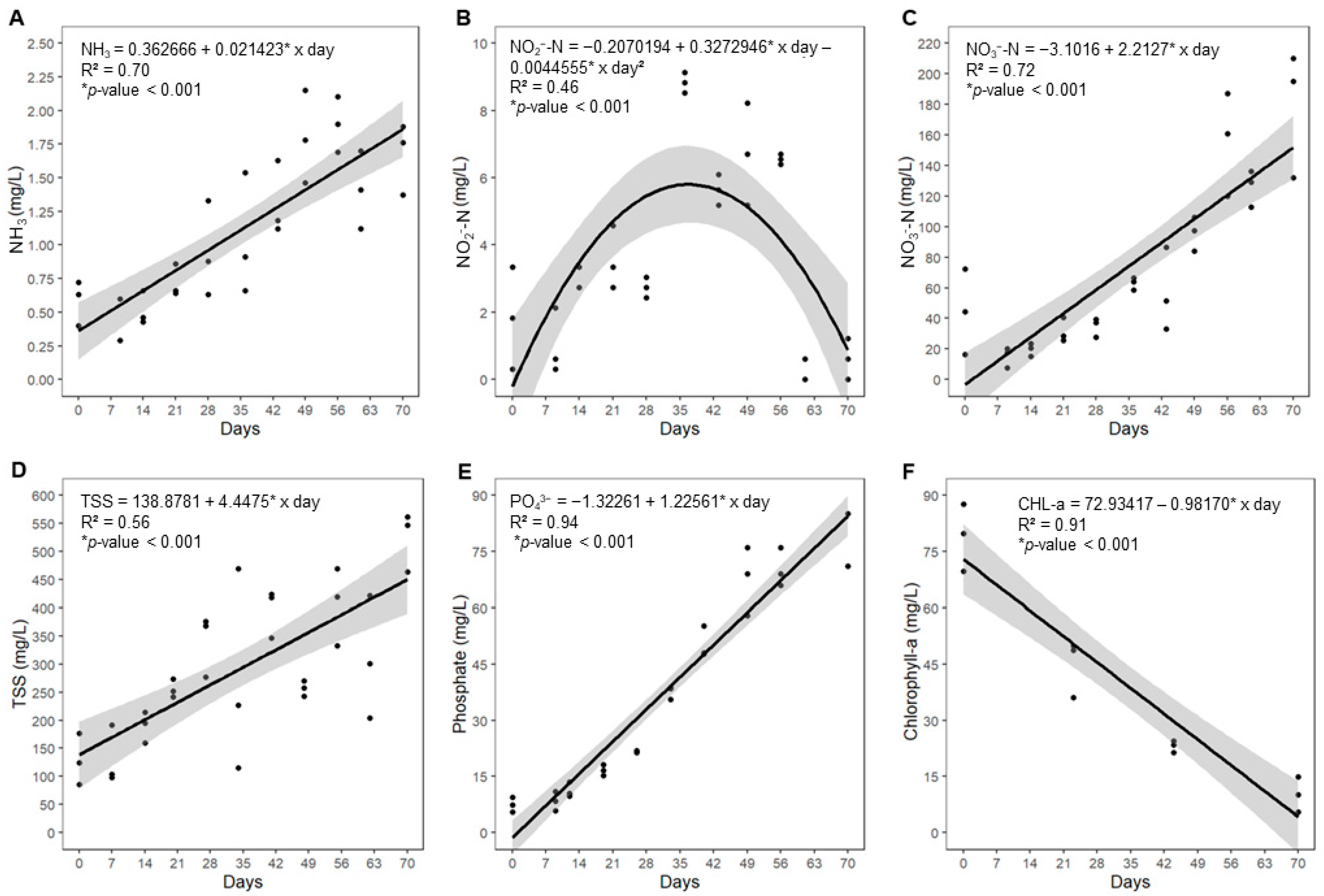
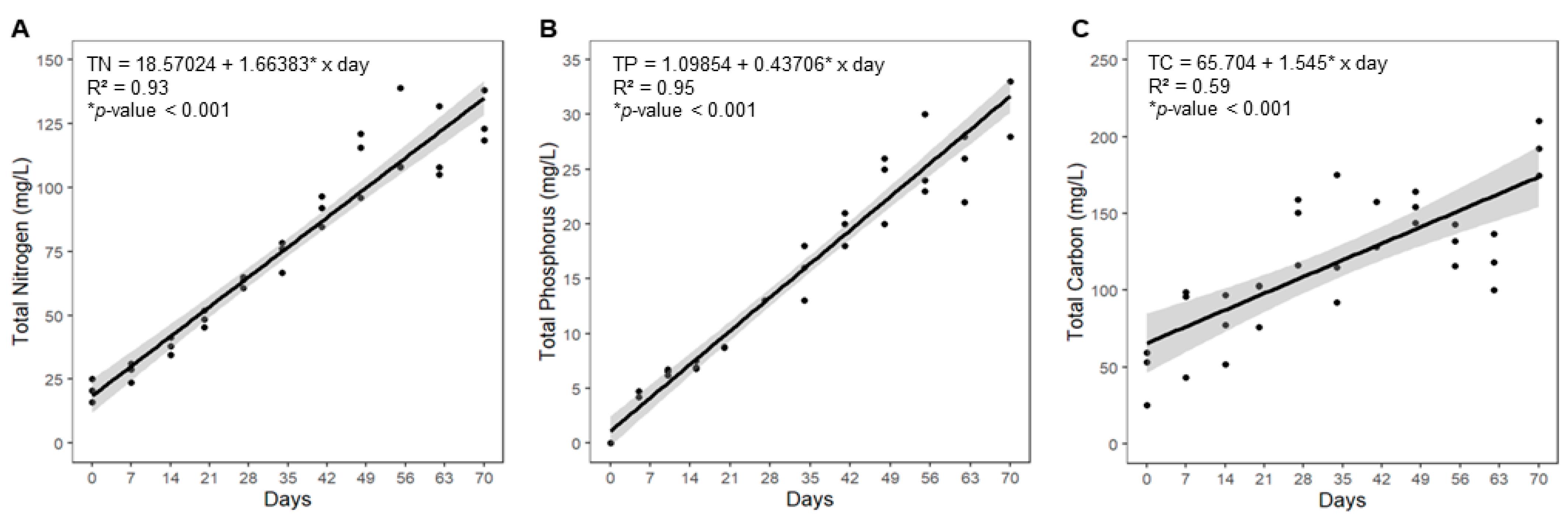
| Category | Indicator | Formula |
|---|---|---|
| Use of the resources | Use of space 1 | E = area used (hectare)/production (tons) |
| Use of water 2 | A = volume used (m3)/production (tons) | |
| Use of energy 2 | EN = pump power (hp) × aeration time (h.) × 0.745 kW/hp/production (kg) × 0.9 (MJ kg −1) | |
| Use of nutrients 1 | U = nutrients applied (kg)/production (tons) | |
| Efficiency in using resources | Production actually used 1 | PEU (%) = (production − not used) × 100 |
| Efficiency in the use of nutrients 1 | EU (%) = (nutrient mass in fish/nutrient mass applied) × 100 | |
| Release of pollutants | Eutrophication potential—carbon, nitrogen, and phosphorus released into water 1 | PE = mass of nutrient released in the effluent (kg)/total production (tons) |
| Risk of production for the conservation of genetics and biodiversity | Produced species risk—increasing levels of impact according to the organism produced 1 | REC = 1, 2, 3, 4, 5, 6 or 8 |
| Variables 1 | Feed | Biofloc 2 |
|---|---|---|
| Carbon (%) | 42.9 | 33.97 ± 1.16 |
| Nitrogen (%) | 4.55 | 4.64 ± 0.23 |
| Phosphorus (%) | 1.01 | 2.62 ± 0.17 |
| Crude Protein (%) | 28.44 | 29.98 ± 1.40 |
| Crude Fiber (%) | 4.18 | 6.17 ± 0.68 |
| Ether extract (%) | 5.54 | 0.81 ± 0.13 |
| Mineral Matter (%) | 6.94 | 27.67 ± 1.66 |
| Total Carbon (TC) | Total Nitrogen (TN) | Total Phosphorus (TP) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD (kg) 1 | % 2 | Mean ± SD (kg) 1 | % 2 | Mean ± SD (g) 1 | % 2 | |
| Initial water + replacement | 0.36 ± 0.06 | 2.30 ± 0.23 | 0.09 ± 0.02 | 4.93 ± 0.76 | 0.12 ± 0.03 | 0.03 ± 0.01 |
| Feed | 14.75 ± 0.85 | 94.35 ± 0.18 | 1.56 ± 0.09 | 88.36 ± 0.54 | 399.84 ± 23.06 | 89.26 ± 0.27 |
| Fish initial biomass | 0.52 ± 0.02 | 3.35 ± 0.09 | 0.12 ± 0.00 | 6.71 ± 0.23 | 47.96 ± 1.79 | 10.72 ± 0.27 |
| Total input | 15.63 ± 0.92 | 100.00 | 1.77 ± 0.11 | 100.00 | 447.92 ± 24.68 | 100.00 |
| Effluent (final water + solids) | 2.58 ± 0.38 | 34.32 ± 3.07 | 0.67 ± 0.07 | 44.84 ± 3.00 | 194.92 ± 11.35 | 45.53 ± 1.33 |
| Fish final biomass | 4.91 ± 0.35 | 65.68 ± 3.07 | 0.83 ± 0.02 | 55.16 ± 3.00 | 233.49 ± 18.95 | 54.47 ± 1.33 |
| Total output | 7.49 ± 0.62 | 100.00 | 1.50 ± 0.06 | 100.00 | 428.42 ± 30.30 | 100.00 |
| Unaccounted portion | 8.15 ± 0.31 | 0.27 ± 0.17 | 19.50 ± 9.85 | |||
| Retention rate (%) | 29.74 ± 1.42 | 45.38 ± 2.76 | 46.34 ± 1.72 | |||
| Category | Indicators | Cages 1 | BFT 2 |
|---|---|---|---|
| Use of resources | Use of space (ha ton−1) | 0.0014 | 0.0150 ± 0.0008 |
| Use of water (m3 ton−1) | 0.7540 | 135.30 ± 8.20 | |
| Carbon use (kg of TC ton−1) | 700.00 | 442.47 ± 12.77 | |
| Nitrogen use (kg of TN ton−1) | 77.50 | 46.63 ± 1.36 | |
| Phosphorus use (kg of TP ton−1) | 18.25 | 10.24 ± 0.05 | |
| Use of energy (MJ kg−1) | 0.028 | 114.59 ± 6.95 | |
| Efficiency in using resources | Efficiency in the use of TC (%) | - | 29.74 ± 1.41 |
| Efficiency in the use of TN (%) | 25.82 | 45.56 ± 2.86 | |
| Efficiency in the use of TP (%) | 16.87 | 46.56 ± 1.62 | |
| Production actually used (%) | 100.00 | 100.00 ± 0.00 | |
| Release of pollutants | TC eutrophication potential (kg ton−1) | - | 57.39 ± 7.64 |
| TN eutrophication potential (kg ton−1) | 59.50 | 20.02 ± 2.74 | |
| TP eutrophication potential (kg ton−1) | 22.00 | 5.70 ± 0.50 | |
| Risk of production for the conservation of genetics and biodiversity | Produced species risk | 5 | 4 |
| Variables | Unit of Production | Mean ± SD 1 | ||
|---|---|---|---|---|
| BFT 1 | BFT 2 | BFT 3 | ||
| Survival rate (%) | 98.19 | 95.96 | 100.00 | 98.05 ± 2.02 |
| Initial weight (g) | 3.46 | 3.34 | 3.45 | 3.42 ± 0.07 |
| Final weight (g) | 21.80 | 19.77 | 19.73 | 20.43 ± 1.19 |
| Weight gain (g/fish) | 18.34 | 16.43 | 16.27 | 17.01 ± 1.15 |
| Apparent feed conversion | 1.03 | 1.03 | 1.08 | 1.05 ± 0.03 |
| Specific growth rate (%/day) | 3.06 | 2.74 | 2.71 | 2.84 ± 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blatt, T.L.d.S.; Cardoso, A.J.d.S.; Watanabe, A.L.; Neto, C.C.B.; Hisano, H. Environmental Sustainability of Nile Tilapia Reared in Biofloc Technology (BFT) System: Evaluation of Carbon, Nitrogen, and Phosphorus Dynamics and Indicators of Sustainability. Sustainability 2025, 17, 5670. https://doi.org/10.3390/su17135670
Blatt TLdS, Cardoso AJdS, Watanabe AL, Neto CCB, Hisano H. Environmental Sustainability of Nile Tilapia Reared in Biofloc Technology (BFT) System: Evaluation of Carbon, Nitrogen, and Phosphorus Dynamics and Indicators of Sustainability. Sustainability. 2025; 17(13):5670. https://doi.org/10.3390/su17135670
Chicago/Turabian StyleBlatt, Tainara Laise da Silva, Alex Júnio da Silva Cardoso, André Luiz Watanabe, Celso Carlos Buglione Neto, and Hamilton Hisano. 2025. "Environmental Sustainability of Nile Tilapia Reared in Biofloc Technology (BFT) System: Evaluation of Carbon, Nitrogen, and Phosphorus Dynamics and Indicators of Sustainability" Sustainability 17, no. 13: 5670. https://doi.org/10.3390/su17135670
APA StyleBlatt, T. L. d. S., Cardoso, A. J. d. S., Watanabe, A. L., Neto, C. C. B., & Hisano, H. (2025). Environmental Sustainability of Nile Tilapia Reared in Biofloc Technology (BFT) System: Evaluation of Carbon, Nitrogen, and Phosphorus Dynamics and Indicators of Sustainability. Sustainability, 17(13), 5670. https://doi.org/10.3390/su17135670







