A Review on Phytoremediation of Decommissioned Mines and Quarries in Ontario: A Sustainable Approach
Abstract
1. Introduction
1.1. Current State of Decommissioned Quarries and Mines
1.2. Toxins and Their Impacts on Environmental Health
1.3. Current Research on Phytoremediation in Quarries
1.4. Phytoremediation
2. Methods
3. Results
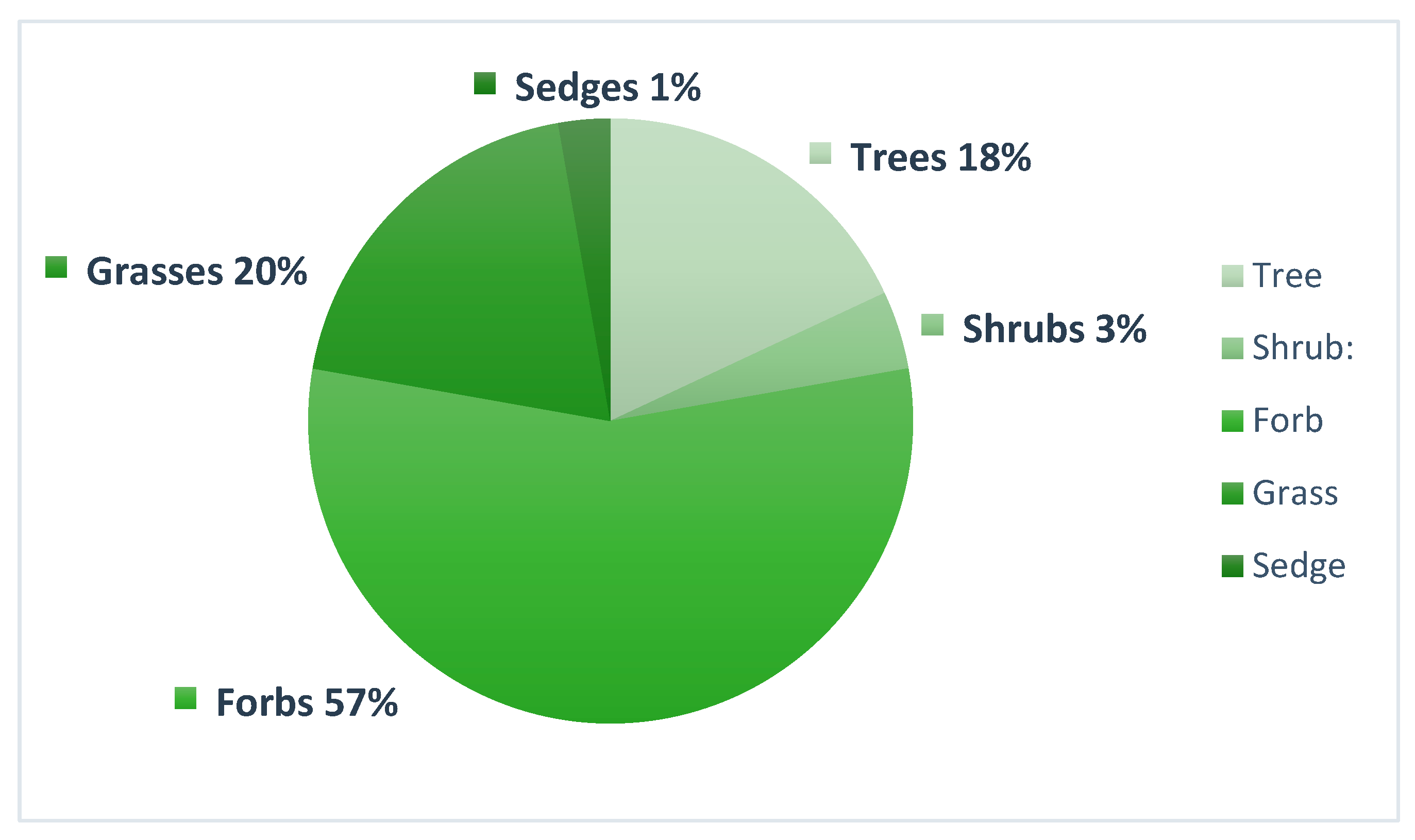
3.1. Plant Species Capable of Phytoremediation
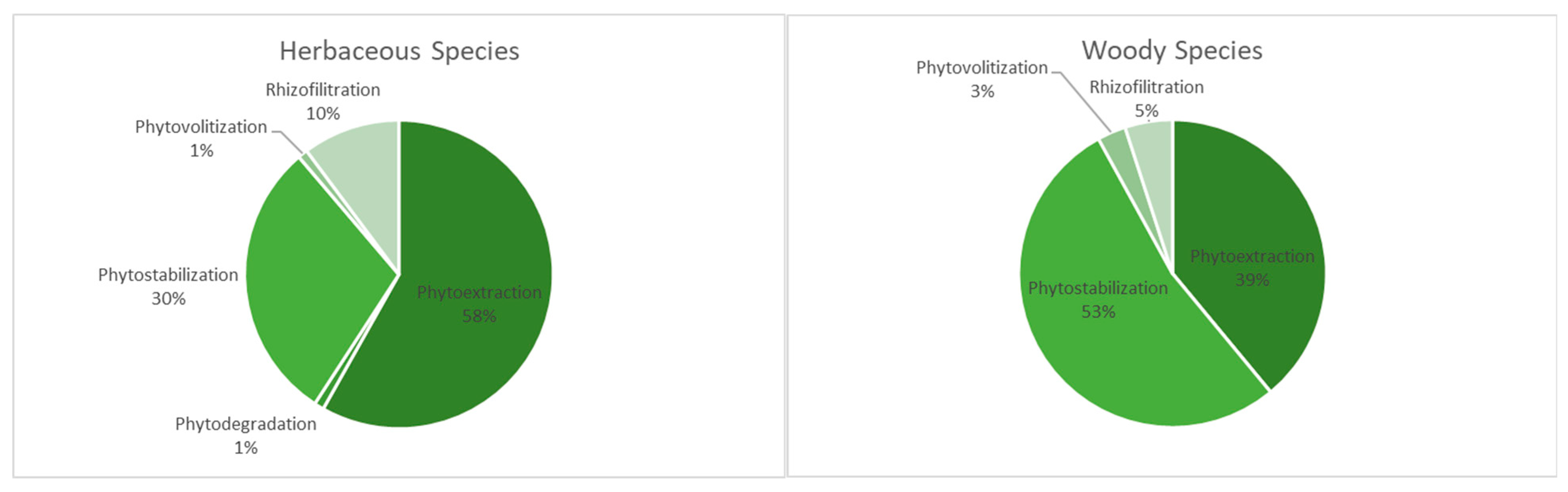
3.2. Remediation Abilities of Plant Species
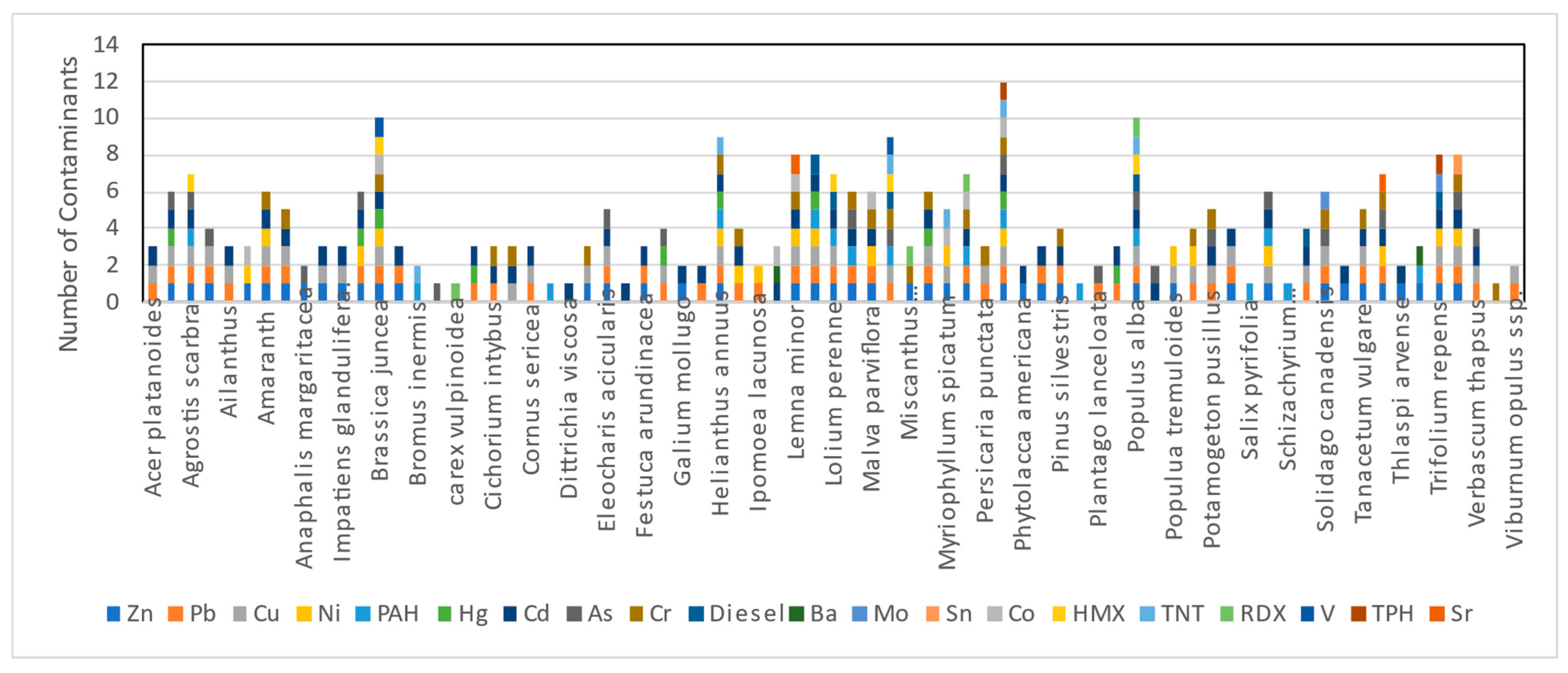
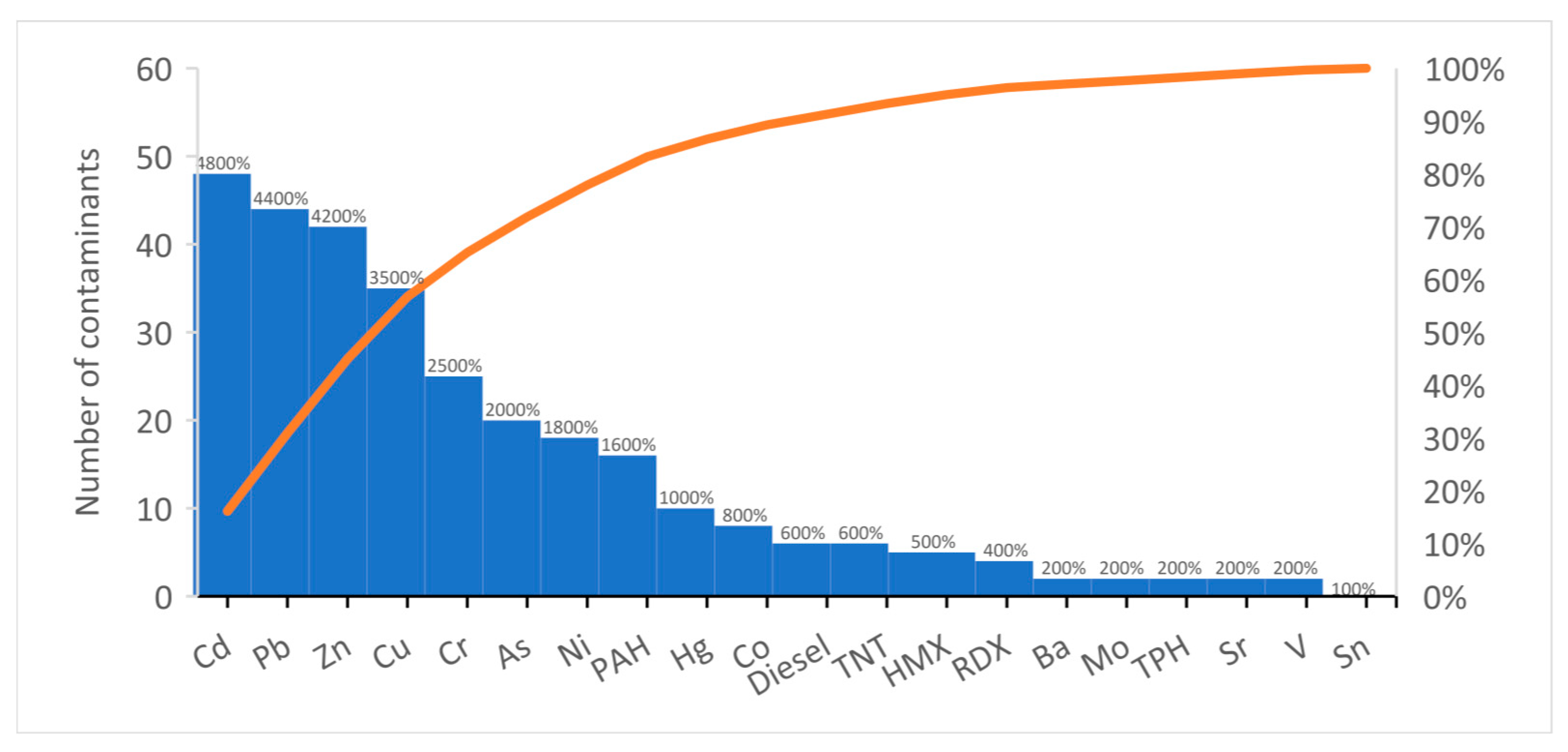
3.3. Contaminate vs. Plant Species
4. Discussion
4.1. Scarring the Landscape Through Excavation
4.2. Damage to Biodiversity
4.3. Importance of New Habitat Development
4.4. Spontaneous Succession of Native Plants in Abandoned Quarries
4.5. Phytoremediation of Contaminated Quarry Sites
4.6. Native vs. Technical Plantings
4.7. Plant Morphology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Common Name | Scientific Name | Family | Contaminates | Mechanism of Phytoremediation | Metal Accumulation mg/kg | pH Preference | Suggested for Rehabilitation | References |
|---|---|---|---|---|---|---|---|---|
| Alfalfa | Medicago sativa | Fabaceae | Zn, Diesel, V, TNT, PAH, Hydrocarbon, HMX, Pb, Cu, Cr, As | V, TNT, PAH–Phytostabilization; Diesel, Cu, Cd, | Pb-43,300 | 6.8 | no | [98,99,100,101,102,103,104,105,106] |
| Amur Silvergrass | Miscanthus sacchariferous | Poaceae | RDX, Zn, Cr | Phytoextraction and Phytostabilization | yes | [107] | ||
| Bent grass | Agrostis spp. | Poaceae | Zn, Pb, Cu, As, Cd, PAH, TNT | Phytoextraction | yes | [108,109] | ||
| Bermuda grass | Cynodon dactylon | Poaceae | TPH | Rhizodegradation | [110] | |||
| Birch | Betula papyrifera | Betulaceae | Hg, Cd, Pb, Zn Ni, As | Phytostabilization | 1000 | yes | [111,112,113] | |
| Bushgrass | Calamagrostis epigejos | Poeacea | As | Phytostabilization | [114] | |||
| Birds Trefoil | Lotus corniculatus | Fabaceae | Zn, PAH, Cd, Cr, Pb, As | Phytostabilization | yes | [114,115,116,117] | ||
| Milk thistle | Silybum marianum | Asteraceae | Pb, Cu, Cd, Diesel | Phytoextraction | Zn 270.9, Cd 72.3, Pb 401.8 | 7.4 | no | [118,119] |
| Fleabane | Dittrichia viscosa | Asteraceae | Cd | Phytoextraction | no | [108] | ||
| Canadian Goldenrod | Solidago canadensis | Asteraceae | Pb, Zn, As, Mo, Sb, Pb, Cu, Cr | Phyto stabilize, Phytoextraction | Zn 520, Pb 520 | no | [78,120,121] | |
| Cattail | Typha latifolia | Typhaceae | As, Pb, Sn, Cd, Cr, Pb, Zn, Ni, Cu | Phytoextraction, Pb-Phytostabilization | 6 | yes | [122,123,124,125] | |
| Chicory | Cichorium intybus | Asteraceae | Cr, Cd, Pb | Cd, Pb-Phytoextraction; Cr-Phytostabilization | 0 | Cd 2.43, Pb 12.25, Cr 1730 | no | [126,127] |
| Chinese mustard | Brassica juncea | Brassicaceae | Pb, Cu, Ni, Hg, Cd, V, Zn, Cr, HMX, Au, Co | Ni-Phytostabilization; Pb, Cu, Hg, Cd, V, Zn-Phytoextraction | Ni-3916, Cr | 5.7 | yes | [82,128,129,130,131,132,133,134,135] |
| Common reed | Phragmites australis | Poaceae | Pb, Zn, Cu, Ni, Hg, Cd, Cr, As, HMX, RDX, TNT, V, PAH, Co, Hydrocarbons (with bacteria) | Cu, Ni, Zn, Hg-Phytoextraction; Pb, Cd, As, Co, Ba-Phytostabilization | yes | [123,136,137,138,139,140,141,142,143,144] | ||
| Common Chickweed | Stellaria media | Caryophyllaceae | Cd, Zn | Phytoextraction | no | [145,146] | ||
| Common Mallow | Malva parviflora | Malvaceae | Zn, Pb, Ni, Cd, Cr, Co | Phytoextraction | no | [147] | ||
| Common Pokeweed | Phytolacca americana | Phytolaccaceae | Cd, Zn | Phytoextraction | no | [148,149] | ||
| Common ragweed | Ambrosia artemisiifolia | Asteraceae | Pb, Zn, Cd, Cu, Cr | Phytostabilization, Phytoextration | Zn 2332, Pb 305 | 5.5–7.5 | no | [150] |
| Common Sunflower | Helianthus annuus | Asteraceae | TNT, Hg, Pb, Zn, Cd, Cu, Cr, Ni, PAH | Rhizofiltration; Hg, Zn, Pb-Phtyoextraction | 5.3 | yes | [131,135,151,152,153,154] | |
| Common water hyacinth | Muscari neglectum | Pontederiaceae | Zn, Pb, Cu, Hg, Cd, Cr, | Rhizofilitration | Hg 5600 | all pH | no | [155,156] |
| Common wormwood | Artemisia vulgaris | Asteraceae | Zn, Cd, Cu | Phytoextraction | no | [145,157] | ||
| Common Yarrow | Achillea millefolium | Asteraceae | Pb, Cu, Cd, As, Zn, Hg | Phytoextraction | no | [145,157,158,159] | ||
| Creeping Bent grass | Agrostis scarbra | Poaceae | Cd | Phytoextraction | yes | [109] | ||
| Pondweed | Potamogeton spp. | Potamogetonaceae | Pb, Cu, Ni, Cr, | Phytoextraction | no | [160,161] | ||
| Dandelion | Taraxacum officinale | Asteraceae | Cd, As, Pb, Zn, Ni, Cr, Sr | Phytoextraction | no | [80,118,162,163] | ||
| Red-Osier Dogwood | Cornus sericea | Cd, Cu, Pb | Phyto | [164] | ||||
| Scots Pine | Pinus silvestris | Pinaceae | Diesel, Cd, Pb, Zn | Phytostabilization | yes | [165,166] | ||
| Eurasian watermilfoil | Myriophyllum spicatum | Haloragaceae | Co, TNT, Ni, Cu, Zn | Phytoextraction Transformation | no | [167,168] | ||
| Fescue | Festuca arundinacea | Poaceae | Cr, Cd, Pb, Zn, | Phytoextraction | yes | [169] | ||
| Creeping red fescue | Festuca rubra | Poaceae | Cu, Pb, As | Phytoextraction | [114,170] | |||
| Field bindweed | Convolvulus arvensis | Polygonaceae | Cu, Cd, Cr, | Phytoextraction | no | [171] | ||
| Fox sedge | Carex vulpinoidea | Cyperaceae | RDX | Phytoextraction | no | [161] | ||
| Golden Rod | Solidago canadensis | Asteraceae | Zn, Pb, Mo, Cu, | Zn-Phytostabilization; Pb, As, Mo-Phytoextraction | Zn-450 | no | [120,172,173] | |
| Bedstraw | Galium mollugo | Rubiaceae | Zn, Cd, Cu | Phytostabilization | Zn-28.4 | 5.8 | no | [145,174] |
| Himalayan Balsam | Impatiens glandulifera. | Balsaminaceae | Cd, Cu, Zn, B | [175] | ||||
| Honey Locust | Gleditid Tricanthos | Fabaceae | Pb, Cd | Phytoextraction | yes | [176] | ||
| Kentucky Blue grass | Poa pratensis ssp. pratensis | Poaceae | Pb, Cd, Hg | RDX-Phytostabilization; Zn-Phytoextraction | 5.5 | yes | [177,178,179,180] | |
| Lambs quarter | Chenopodium album | Chenopodiaceae | Cd, Pb Hg | Phytoextraction | no | [181] | ||
| Lesser duckweed | Lemna minor | Lemnaceae | Pb, Cu, Ni, Cd, Cr, Zn, Co, Sr, BPA | Phytoextraction | no | [182,183] | ||
| Little Blue stem | Schizachyrium scoparium | Poeceae | PAH | [184,185] | ||||
| Morning glory | Ipomoea spp. | Convolvulaceae | Pb, Ni, | V-Phytostabilization; Pb, Ni-Phytoextraction | no | [186] | ||
| Mullein | Verbascum thapsus | Scrophulariaceae | Pb, As, Cd, Cu, Cr | Phytoextraction | Pb 1840, Zn 7807, Cd 141, As 37.8 | 6.5–7.8 | [187,188] | |
| Narrow-Leaved plantain | Plantago lanceloata | Plantaginaceae | Pb, As | Phytoextraction | yes | [106] | ||
| Norway maple | Acer platanoides | Aceraceae | Pb, Cd, Cu, | Phytoextraction | 5.28 | yes | [189] | |
| Old field Cinquefoil | Potentilla simplex | Rosaceae | Zn, Pb, Cu, Cd | Pb-Phytoextraction, Zn-Phytostabilization | no | [164,174] | ||
| Orchard grass | Dactylis glomerata | Poaceae | PAH, | Phytostabilization | yes | [78,190] | ||
| Pearly everlast | Anaphalis margaritacea | Asteraceae | As, Al, Zn | Phytostabilization | 95 | no | [191] | |
| Penny cress | Thlaspi arvense | Brassicaceae | Zn, Cd, Ni | Phytostabilization | no | [192] | ||
| Perennial ryegrass | Lolium perenne | Poaceae | Zn, Pb, Cu, PAH, Cd, HMX, Diesel | Zn, Cu, As-Phytodegradation; Pb, Cu, PAH-Phytoextraction | 4.5–8 | yes | [100,100,118,152,165,193,194,195,196] | |
| Pondweed | Potamogeton pusillus | Potamogetonaceae | Cd, Cr, Cu, As, Pb | Rhizofiltration | no | [144] | ||
| Poplar | Populus alba | Salicaceae | Zn, Pb, Cu, As, Diesel, HMX, RDX, TNT, Cd, Sa, | yes | [134,197,198,199,200] | |||
| Poplar | Populus balamifera | Salicaceae | As, Cd | [102,134,201] | ||||
| Rapeseed | Brassica napus | Brassicaceae | Zn, Pb, Cd | Phytoextraction | no | [202,203] | ||
| Red clover | Trifolium pratense | Fabaceae | Zn, PAH, | Phytoextraction | 80 | yes | [195,204] | |
| Red fescue | Festuca rubra | Poaceae | Hg, Diesel, Cu, Cd, Ni, Pb, Zn | Phytoextraction | 84 | yes | [205,206] | |
| Norway Spruce | Picea abies | Pinaceae | Pb, Zn, Cd | Phytoextraction | yes | [100,166,207] | ||
| Small Alyssum | Alyssum | Brassicaceae | Ni, Zn, Co | Phytostabilization | no | [134,208,209] | ||
| Scots Pine | Pinus Silvestris | Pinaceae | Cd, Cr Pb, Zn | [166,210] | ||||
| Smartweed | Persicaria punctata | Polygonaceae | Pb, Cu, Cr | Phytoextraction | no | [211] | ||
| Smooth Brome | Bromus inermis | Poaceae | PAH, TNT | Phytoextraction | no | [212] | ||
| Spikegrass | Eleocharis acicularis | Cyperacea | Cu, Cd, Zn, As, Pb | phytoextraction | [213,214] | |||
| St John’s Wort | Hypericum perforatum | Clusiaceae | Cr, Pb, Cd Ni | Phytoextraction | Cu 6.34, Zn 68.31, Pb 47.51 | no | [215] | |
| Switchgrass | Panicum vigratum | Poaceae | Pb, Cr, Cd, Zn, PAH, RDX, Cu | Pb, Cd, Hg, As-Phytoextraction; Cr-Phytostabilization | 1543 | 3.4–6.1 | no | [183,184,216,217,218] |
| Tall Fescue | Lolium arundinaceum | Poaceae | Zn, Pb, Cu, Cd, Diesel, Hg, Ni, PAH | As-Photoextraction; Cu-Phytodegradtion; Zn-Phytostabilization; Pb-Rhizofilitration | no | [205,219,220] | ||
| Tamarack | Larix laricina | Pinaceae | Ba, Cd, Co | Phytostabilization | 280 | yes | [221,222] | |
| Tansy | Tanacetum vulgare | Asteraceae | Zn, Pb, Cu, Cd, Cr | Phytoextraction | no | [150,223] | ||
| Tree of heaven | Ailanthus | Simaroubaceae | Pb, Cu, Cd | Phytoextraction | 2037 | yes | [224] | |
| Trembling Aspen | Populus tremuloides | Salicaceae | Cu, Ni, Zn | Phytostabilization and Phytoextraction | [225] | |||
| Tuffed hairgrass | Deschampsia cespitosa | Poaceae | Cu, Cr, Zn | Phytostabilization | yes | [225] | ||
| Verbena | Verbena officinalis | Verbenaceae | Cr, | Phytoextraction | no | [194] | ||
| Viburnum (awabuki) | Viburnum opulus ssp. | Caprifoliaceae | Pb, Cu | Phytoextraction | no | [226] | ||
| White Amaranth | Amaranth | Amaranthaceae | Cu, Pb, Cd, Ni, Cr, Zn | Phytoextraction | 6.9–5.5 pH | yes | [227] | |
| White clover | Trifolium repens | Fabaceae | diesel, As, TPHs, Cd, Zn | Rhizodegradation | Diesel 80 | 7.5 | yes | [165,228,229,230,231] |
| White spruce | Picea glauca | Pinaceae | As | Phytostabilization | 3642 | yes | [222] | |
| Willow | Salix spp. | Salicaceae | Cu, Ni, PAH, As, Cd, Zn | Phytostabilization | 5.0–6.0 | yes | [232,233,234] | |
| Scientific Name | Family | Acronym | Native? | C | W | Physiognomy |
|---|---|---|---|---|---|---|
| Acer platanoides | n/a | ACEPLAT | non-native | 0 | 5 | tree |
| Achillea millefolium ssp. lanulosa | n/a | ACHMILLL | native | 0 | 3 | forb |
| Agrostis perennans | n/a | AGRPERE | native | 5 | 1 | grass |
| Agrostis scabra | n/a | AGRSCAB | native | 6 | 0 | grass |
| Ailanthus altissima | n/a | AILALTI | non-native | 0 | 5 | tree |
| Amaranthus albus | n/a | AMAALBU | non-native | 0 | 3 | forb |
| Ambrosia artemisiifolia | n/a | AMBARTE | native | 0 | 3 | forb |
| Anaphalis margaritacea | n/a | ANAMARG | native | 3 | 5 | forb |
| Artemisia vulgaris | n/a | ARTVULG | non-native | 0 | 5 | forb |
| Betula papyrifera | n/a | BETPAPY | native | 2 | 2 | tree |
| Brassica juncea | n/a | BRAJUNC | non-native | 0 | 5 | forb |
| Brassica napus | n/a | BRANAPU | non-native | 0 | 5 | forb |
| Bromus inermis ssp. inermis | n/a | BROINERI | non-native | 0 | 5 | grass |
| Calamagrostis epigejos | n/a | CALEPIG | non-native | 0 | 0 | grass |
| Camassia scilloides | n/a | CAMSCIL | native | 10 | −1 | forb |
| Carex vulpinoidea | n/a | CARVULP | native | 3 | −5 | sedge |
| Chenopodium album | n/a | CHEALBU | non-native | 0 | 1 | forb |
| Cichorium intybus | n/a | CICINTY | non-native | 0 | 5 | forb |
| Convolvulus arvensis | n/a | CONARVE | non-native | 0 | 5 | forb |
| Dactylis glomerata | n/a | DACGLOM | non-native | 0 | 3 | grass |
| Deschampsia cespitosa ssp. cespitosa | n/a | DESCESPC | native | 9 | −4 | grass |
| Festuca arundinacea | n/a | FESARUN | non-native | 0 | 2 | grass |
| Festuca rubra | n/a | FESRUBR | non-native | 0 | 1 | grass |
| Galium mollugo | n/a | GALMOLL | non-native | 0 | 5 | forb |
| Gleditsia triacanthos | n/a | GLETRIA | native | 3 | 0 | tree |
| Helianthus annuus | n/a | HELANNU | non-native | 0 | 1 | forb |
| Hypericum perforatum | n/a | HYPPERF | non-native | 0 | 5 | forb |
| Impatiens glandulifera | n/a | IMPGLAN | non-native | 0 | −3 | forb |
| Ipomoea pandurata | n/a | IPOPAND | native | 9 | 3 | forb |
| Larix laricina | n/a | LARLARI | native | 7 | −3 | tree |
| Lemna minor | n/a | LEMMINO | native | 2 | −5 | forb |
| Lobularia maritima | n/a | LOBMARI | non-native | 0 | 5 | forb |
| Lolium perenne | n/a | LOLPERE | non-native | 0 | 3 | grass |
| Lotus corniculata | n/a | LOTCORN | non-native | 0 | 1 | forb |
| Malva neglecta | n/a | MALNEGL | non-native | 0 | 5 | forb |
| Medicago sativa | n/a | MEDSATI | non-native | 0 | 5 | forb |
| Miscanthus sacchariflorus | n/a | MISSACC | non-native | 0 | 5 | grass |
| Muscari botryoides | n/a | MUSBOTR | non-native | 0 | 5 | forb |
| Myriophyllum spicatum | n/a | MYRSPIC | non-native | 0 | −5 | forb |
| Panicum virgatum | n/a | PANVIRG | native | 6 | −1 | grass |
| Phragmites australis (P. communis) | n/a | PHRAUST | native | 0 | −4 | grass |
| Phytolacca americana | n/a | PHYAMER | native | 3 | 1 | forb |
| Picea abies | n/a | PICABIE | non-native | 0 | 5 | tree |
| Picea glauca | n/a | PICGLAU | native | 6 | 3 | tree |
| Pinus strobus | n/a | PINSTRO | native | 4 | 3 | tree |
| Pinus sylvestris | n/a | PINSYLV | non-native | 0 | 5 | tree |
| Plantago lanceolata | n/a | PLALANC | non-native | 0 | 0 | forb |
| Poa pratensis | n/a | POAPRAT | native | 0 | 1 | grass |
| Polygonum achoreum | n/a | POLACHO | native | 0 | 5 | forb |
| Populus alba | n/a | POPALBA | non-native | 0 | 5 | tree |
| Populus balsamifera | n/a | POPBALS | native | 4 | −3 | tree |
| Populus tremuloides | n/a | POPTREM | native | 2 | 0 | tree |
| Potamogeton alpinus | n/a | POTALPI | native | 10 | −5 | forb |
| Potamogeton crispus | n/a | POTCRIS | non-native | 0 | −5 | forb |
| Potamogeton pusillus | n/a | POTPUSI | native | 5 | −5 | forb |
| Potentilla simplex | n/a | POTSIMP | native | 3 | 4 | forb |
| Salix alba | n/a | SALALBA | non-native | 0 | −3 | tree |
| Salix pyrifolia | n/a | SALPYRI | native | 10 | −4 | shrub |
| Schizachyrium scoparium (andropogon s.) | n/a | SCHSCOP | native | 7 | 3 | grass |
| Solidago canadensis | n/a | SOLCANA | native | 1 | 3 | forb |
| Stellaria media | n/a | STEMEDI | non-native | 0 | 3 | forb |
| Tanacetum vulgare | n/a | TANVULG | non-native | 0 | 5 | forb |
| Taraxacum officinale | n/a | TAROFFI | non-native | 0 | 3 | forb |
| Thlaspi arvense | n/a | THLARVE | non-native | 0 | 5 | forb |
| Trifolium pratense | n/a | TRIPRAT | non-native | 0 | 2 | forb |
| Trifolium repens | n/a | TRIREPE | non-native | 0 | 2 | forb |
| Typha latifolia | n/a | TYPLATI | native | 3 | −5 | forb |
| Verbascum thapsus | n/a | VERTHAP | non-native | 0 | 5 | forb |
| Verbena simplex | n/a | VERSIMP | native | 9 | 5 | forb |
| Viburnum opulus | n/a | VIBOPUL | non-native | 0 | 0 | shrub |
References
- Trimble, K.D.; Seibert, M. An Evolution of Reclamation Approaches Through the Life of a Southern Ontario Gravel Pit. J. Am. Soc. Min. Reclam. 2002, 2002, 344–361. [Google Scholar] [CrossRef]
- Home. Available online: https://toarc.com/ (accessed on 11 March 2025).
- TOARC—About Us & the Aggregate Resources Trust. TOARC—Ont. Aggreg. Resour. Corp. Available online: https://toarc.com/about-us/ (accessed on 11 March 2025).
- Corry, R.; Lafortezza, R.; Brown, R. Ecological Functionality of Landscapes with Alternative Rehabilitations of Depleted Aggregate Sites. Int. J. Min. Reclam. Environ. 2010, 24, 216–232. [Google Scholar] [CrossRef]
- Ontario Stone, Sand, Gravel Association (OSSGA). Available online: https://www.ossga.com/home/ (accessed on 11 March 2025).
- Piryonesi, S.M.; Carnegie, D.; Weissling, L. Best Management Practices for Aggregate Pit and Quarry Rehabilitation in Ontario; Ontario Society of Professional Engineers: Toronto, ON, Canada, 2021. [Google Scholar]
- Lowe, S.B. Trees & Shrubs for the Improvement and Rehabilitation of Pits and Quarries in Ontario; Ontario Ministry of Natural Resources, Mineral Resources Branch: Toronto, ON, Canada, 1979; ISBN 978-0-7743-4784-6. [Google Scholar]
- Pitz, C.; Piqueray, J.; Monty, A.; Mahy, G. Naturally Recruited Herbaceous Vegetation in Abandoned Belgian Limestone Quarries: Towards Habitats of Conservation Interest Analogues? Folia Geobot. 2018, 53, 147–158. [Google Scholar] [CrossRef]
- Richardson, P.; Murphy, S. Rapid Ecological Restoration for Aggregate Sites (RERAS) 2021; The Ontario Aggregate Resources Corporation: Burlington, ON, Canada, 2021. [Google Scholar]
- Řehounková, K.; Prach, K. Spontaneous Vegetation Succession in Gravel–Sand Pits: A Potential for Restoration. Restor. Ecol. 2008, 16, 305–312. [Google Scholar] [CrossRef]
- Bouzekri, S.; El Fadili, H.; El Hachimi, M.L.; El Mahi, M.; Lotfi, E.M. Assessment of Trace Metals Contamination in Sediment and Surface Water of Quarry Lakes from the Abandoned Pb Mine Zaida, High Moulouya-Morocco. Environ. Dev. Sustain. 2020, 22, 7013–7031. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of Heavy Metals—Concepts and Applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Lago-Vila, M.; Rodríguez-Seijo, A.; Arenas-Lago, D.; Andrade, L.; Vega, M.F.A. Heavy Metal Content and Toxicity of Mine and Quarry Soils. J. Soils Sediments 2017, 17, 1331–1348. [Google Scholar] [CrossRef]
- Ma, N. Critical Issues of Sustainability Associated with Quarry Activities. Asp. Min. Miner. Sci. 2018, 1, 58–65. [Google Scholar] [CrossRef]
- Hatva, T. Effect of Gravel Extraction on Groundwater. Future Groundwater Resources at Risk. IAHS Publ.-Ser. Proc. Rep.-Intern Assoc Hydrol. Sci. 1994, 222, 427–434. [Google Scholar]
- Ochelebe, I.; Nkebem, G.E.; Kudamnya, E.A. Assessment of Heavy Metals Concentration and Enrichment Levels in Soils around Quarries and Barite Mine Sites in Part of Akamkpa and Biase Area, Southeastern Nigeria. J. Geosci. Environ. Prot. 2020, 8, 107–128. [Google Scholar] [CrossRef]
- Vucic, J.M.; Cohen, R.S.; Gray, D.K.; Murdoch, A.D.; Shuvo, A.; Sharma, S. Young Gravel-Pit Lakes along Canada’s Dempster Highway: How Do They Compare with Natural Lakes? Arct. Antarct. Alp. Res. 2019, 51, 25–39. [Google Scholar] [CrossRef]
- Kalubi, K.N.; Mehes-Smith, M.; Narendrula, R.; Michael, P.; Omri, A. Molecular Analysis of Red Maple (Acer rubrum) Populations from a Reclaimed Mining Region in Northern Ontario (Canada): Soil Metal Accumulation and Translocation in Plants. Ecotoxicology 2015, 24, 636–647. [Google Scholar] [CrossRef]
- Krawczyk, R.; Lis, Ł.; Urbaniak, J. Water Parameters and Species Composition of Macrophytes in Reclamation Lakes in the Area of a Former Sulphur Borehole Mine (SE Poland). Ann. Univ. Mariae Curie-Sklodowska Sect. C Biol. 2017, 71, 27. [Google Scholar] [CrossRef]
- Münzel, T.; Hahad, O.; Daiber, A.; Landrigan, P.J. Soil and Water Pollution and Human Health: What Should Cardiologists Worry About? Cardiovasc. Res. 2023, 119, 440–449. [Google Scholar] [CrossRef]
- Stephenson, C.; Black, C.R. One Step Forward, Two Steps Back: The Evolution of Phytoremediation into Commercial Technologies. Biosci. Horiz. Int. J. Stud. Res. 2014, 7, hzu009. [Google Scholar] [CrossRef]
- Ifon, B.E.; Togbé, A.C.F.; Tometin, L.A.S.; Suanon, F.; Yessoufou, A.; Ifon, B.E.; Togbé, A.C.F.; Tometin, L.A.S.; Suanon, F.; Yessoufou, A. Metal-Contaminated Soil Remediation: Phytoremediation, Chemical Leaching and Electrochemical Remediation. In Metals in Soil—Contamination and Remediation; IntechOpen: London, UK, 2019; ISBN 978-1-78985-776-4. [Google Scholar]
- Munford, K.E.; Asemaninejad, A.; Basiliko, N.; Mykytczuk, N.C.S.; Glasauer, S.; McGarry, S.; Watmough, S.A. Native Plants Facilitate Vegetation Succession on Amended and Unamended Mine Tailings. Int. J. Phytoremediation 2022, 24, 963–974. [Google Scholar] [CrossRef]
- Saier, M.H.; Trevors, J.T. Phytoremediation. Water Air. Soil Pollut. 2010, 205, 61–63. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Elements with More Toxic Effects. In Principles of Plant Nutrition; Mengel, K., Kirkby, E.A., Kosegarten, H., Appel, T., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 657–673. ISBN 978-1-4020-0008-9. [Google Scholar]
- Cataldo, D.A.; Wildung, R.E. Soil and Plant Factors Influencing the Accumulation of Heavy Metals by Plants. Environ. Health Perspect. 1978, 27, 149–159. [Google Scholar] [CrossRef]
- Nedjimi, B. Phytoremediation: A Sustainable Environmental Technology for Heavy Metals Decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Bothe, H.; Słomka, A. Divergent Biology of Facultative Heavy Metal Plants. J. Plant Physiol. 2017, 219, 45–61. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, Plant Selection and Enhancement by Natural and Synthetic Agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Bes, C.M.; Mench, M.; Aulen, M.; Gaste, H.; Taberly, J. Spatial Variation of Plant Communities and Shoot Cu Concentrations of Plant Species at a Timber Treatment Site. Plant Soil 2009, 330, 267–280. [Google Scholar] [CrossRef]
- Barrutia, O.; Artetxe, U.; Hernández, A.; Olano, J.M.; García-Plazaola, J.I.; Garbisu, C.; Becerril, J.M. Native Plant Communities in an Abandoned Pb-Zn Mining Area of Northern Spain: Implications for Phytoremediation and Germplasm Preservation. Int. J. Phytoremediation 2011, 13, 256–270. [Google Scholar] [CrossRef]
- Banásová, V.; Ďurišová, E.; Nadubinská, M.; Gurinová, E.; Čiamporová, M. Natural Vegetation, Metal Accumulation and Tolerance in Plants Growing on Heavy Metal Rich Soils. In Bio-Geo Interactions in Metal-Contaminated Soils; Springer: Berlin/Heidelberg, Germany, 2012; pp. 233–250. ISBN 978-3-642-23327-2. [Google Scholar]
- Rascio, N.; Navari-Izzo, F. Heavy Metal Hyperaccumulating Plants: How and Why Do They Do It? And What Makes Them so Interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Cronk, J.K.; Fennessy, M.S. Wetland Plants: Biology and Ecology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4200-3292-5. [Google Scholar]
- El-Liethy, M.A.; Dakhil, M.A.; El-Keblawy, A.; Abdelaal, M.; Halmy, M.W.A.; Elgarhy, A.H.; Kamika, I.; El-Sherbeny, G.A.; Mwaheb, M.A. Temporal Phytoremediation Potential for Heavy Metals and Bacterial Abundance in Drainage Water. Sci. Rep. 2022, 12, 8223. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation Technologies and Their Mechanism for Removal of Heavy Metal from Contaminated Soil: An Approach for a Sustainable Environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef]
- Kumar, G.; Bhatt, P.; Lal, S.; Kumar, G.; Bhatt, P.; Lal, S. Phytoremediation: A Synergistic Interaction between Plants and Microbes for Removal of Petroleum Hydrocarbons. In Soil Contamination—Threats and Sustainable Solutions; IntechOpen: London, UK, 2021; ISBN 978-1-83880-754-2. [Google Scholar]
- Jayapal, A.; Chaterjee, T.; Sahariah, B.P. Bioremediation Techniques for the Treatment of Mine Tailings: A Review. Soil Ecol. Lett. 2023, 5, 220149. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Fang, Z.; Shi, G.; Lou, L.; Ren, K.; Cai, Q. Italian Ryegrass–Rice Rotation System for Biomass Production and Cadmium Removal from Contaminated Paddy Fields. J. Soils Sediments 2020, 20, 874–882. [Google Scholar] [CrossRef]
- Phang, L.-Y.; Mingyuan, L.; Mohammadi, M.; Tee, C.-S.; Yuswan, M.H.; Cheng, W.-H.; Lai, K.-S. Phytoremediation as a Viable Ecological and Socioeconomic Management Strategy. Environ. Sci. Pollut. Res. 2024, 31, 50126–50141. [Google Scholar] [CrossRef]
- Spyreas, G. Floristic Quality Assessment: A Critique, a Defense, and a Primer. Ecosphere 2019, 10, e02825. [Google Scholar] [CrossRef]
- Swink, F.; Wilhelm, G. Plants of the Chicago Region; The Morton Arboretum: Lyle, IL, USA, 1979. [Google Scholar]
- Abdullah, D.M.H. Factor-Cluster Analysis and Enrichment Study of Mangrove Sediments—An Example from Mengkabong Sabah. Malays. J. Anal. Sci. 2007, 11, 421–430. [Google Scholar]
- Valdez-Hernández, M.; Gil-Medina, R.; López-Martínez, J.O.; Torrescano-Valle, N.; Cabanillas-Terán, N.; Islebe, G.A. Succession and the Relationship between Vegetation and Soil in the Marl Quarries of the Yucatan Peninsula, Mexico. Forests 2019, 10, 116. [Google Scholar] [CrossRef]
- Bradshaw, A. Restoration of Mined Lands—Using Natural Processes. Ecol. Eng. 1997, 8, 255–269. [Google Scholar] [CrossRef]
- Gathuru Gladys The Effect of Tree Species on the Chemical Properties of Soil in the Rehabilitation of a Limestone Quarry at Athi River, Kenya. J. Environ. Sci. Eng. A 2017, 6, 178. [CrossRef]
- Tomlinson, S.; Matthes, U.; Richardson, P.J.; Larson, D.W. The Ecological Equivalence of Quarry Floors to Alvars. Appl. Veg. Sci. 2008, 11, 73–82. [Google Scholar] [CrossRef]
- Rani, B. Impact of Quarrying and Crushing on Soil Quality: A Case Study in Tumkur District, Karnataka. Int. J. Res. Granthaalayah 2017, 5. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; Andrade, M.L. Characterization of Soil Physico-Chemical Parameters and Limitations for Revegetation in Serpentine Quarry Soils (NW Spain). J. Soils Sediments 2017, 17, 1321–1330. [Google Scholar] [CrossRef]
- Key, R. Bare Ground and the Conservation of Invertebrates. Br. Wildl. 2000, 11, 183–191. [Google Scholar]
- Bodsworth, E.; Shepherd, P.; Plant, C. Exotic Plant Species on Brownfield Land: Their Value to Invertebrates of Nature Conservation Importance; English Nature: Ashford, UK, 2005. [Google Scholar]
- Kirby, P. Habitat Management for Invertebrates: A Practical Handbook, 2nd ed.; Pelagic Publishing: Exeter, UK, 2013; ISBN 978-1-907807-51-0. [Google Scholar]
- Perzley, J. A Comparison of Brownfield and Old-Field Plant Communities; Rutgers University: New Brunswick, NJ, USA, 2018. [Google Scholar]
- Prach, K.; Lencová, K.; Řehounková, K.; Dvořáková, H.; Jírová, A.; Konvalinková, P.; Mudrák, O.; Novák, J.; Trnková, R. Spontaneous Vegetation Succession at Different Central European Mining Sites: A Comparison across Seres. Environ. Sci. Pollut. Res. 2013, 20, 7680–7685. [Google Scholar] [CrossRef]
- Prach, K.; Řehounková, K.; Lencová, K.; Jírová, A.; Konvalinková, P.; Mudrák, O.; Študent, V.; Vaněček, Z.; Tichý, L.; Petřík, P.; et al. Vegetation Succession in Restoration of Disturbed Sites in Central Europe: The Direction of Succession and Species Richness across 19 Seres. Appl. Veg. Sci. 2014, 17, 193–200. [Google Scholar] [CrossRef]
- Novák, J.; Konvička, M. Proximity of Valuable Habitats Affects Succession Patterns in Abandoned Quarries. Ecol. Eng. 2006, 26, 113–122. [Google Scholar] [CrossRef]
- Reinhardt, E.G.; Dalby, A.P.; Kumar, A.; Patterson, R.T. Arcellaceans as Pollution Indicators in Mine Tailing Contaminated Lakes near Cobalt, Ontario, Canada. Micropaleontology 1998, 44, 131. [Google Scholar] [CrossRef]
- Janečková, P.; Řehounková, K.; Vítovcová, K.; Šebelíková, L.; Prach, K. Spontaneous Succession on Road Verges—An Effective Approach with Minimum Effort. Land Degrad. Dev. 2021, 32, 2726–2734. [Google Scholar] [CrossRef]
- Prach, K.; Řehounková, K.; Řehounek, J.; Konvalinková, P. Ecological Restoration of Central European Mining Sites: A Summary of a Multi-Site Analysis. Landsc. Res. 2011, 36, 263–268. [Google Scholar] [CrossRef]
- Wiegleb, G.; Felinks, B. Primary Succession in Post-Mining Landscapes of Lower Lusatia—Chance or Necessity. Ecol. Eng. 2001, 17, 199–217. [Google Scholar] [CrossRef]
- Šálek, M. Spontaneous Succession on Opencast Mining Sites: Implications for Bird Biodiversity. J. Appl. Ecol. 2012, 49, 1417–1425. [Google Scholar] [CrossRef]
- Son, D.; Alday, J.G.; Chu, Y.; Lee, E.J.; Park, S.; Lee, H. Plant Species Colonization in Newly Created Road Habitats of South Korea: Insights for More Effective Restoration. Sci. Total Environ. 2020, 719, 137476. [Google Scholar] [CrossRef] [PubMed]
- About|Gravel Watch Ontario. Available online: https://gravelwatch.org/about-2/ (accessed on 13 March 2025).
- Alday, J.G.; Marrs, R.H.; Martínez-Ruiz, C. Soil and Vegetation Development during Early Succession on Restored Coal Wastes: A Six-Year Permanent Plot Study. Plant Soil 2012, 353, 305–320. [Google Scholar] [CrossRef]
- Alifragki, M.G.; Pavlatou-Ve, A.K.; Orfanoudakis, M.Z. Phytoremediation Affects Microbial Development on a Limestone Quarry. Int. J. Phytoremediation 2018, 20, 957–963. [Google Scholar] [CrossRef]
- Panz, K.; Miksch, K. Phytoremediation of Soil Contaminated with Explosive Compounds. In Biological Remediation of Explosive Residues; Singh, S.N., Ed.; Environmental Science and Engineering; Springer International Publishing: Cham, Switzerland, 2014; pp. 235–257. ISBN 978-3-319-01082-3. [Google Scholar]
- Awa, S.H.; Hadibarata, T. Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: A Review. Water Air. Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Greipsson, S. Phytoremediation. Nat. Educ. 2011, 3, 7. [Google Scholar]
- Mang, K.C.; Ntushelo, K. Phytoextraction and Phytostabilisation Approaches of Heavy Metal Remediation in Acid Mine Drainage with Case Studies: A Review. Appl. Ecol. Environ. Res. 2019, 17, 6129–6149. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Shann, J.R.; Crowley, D.E.; Anderson, T.A. Phytoremediation of Contaminated Water and Soil. In Phytoremediation of Soil and Water Contaminants; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1997; Volume 664, pp. 2–17. ISBN 978-0-8412-3503-8. [Google Scholar]
- Wang, C.; Wu, B.; Jiang, K.; Wei, M.; Wang, S. Effects of Different Concentrations and Types of Cu and Pb on Soil N-Fixing Bacterial Communities in the Wheat Rhizosphere. Appl. Soil Ecol. 2019, 144, 51–59. [Google Scholar] [CrossRef]
- Raklami, A.; Meddich, A.; Oufdou, K.; Baslam, M. Plants—Microorganisms-Based Bioremediation for Heavy Metal Cleanup: Recent Developments, Phytoremediation Techniques, Regulation Mechanisms, and Molecular Responses. Int. J. Mol. Sci. 2022, 23, 5031. [Google Scholar] [CrossRef] [PubMed]
- Shikha, D.; Singh, P.K. In Situ Phytoremediation of Heavy Metal–Contaminated Soil and Groundwater: A Green Inventive Approach. Environ. Sci. Pollut. Res. 2021, 28, 4104–4124. [Google Scholar] [CrossRef] [PubMed]
- Cristaldi, A.; Conti, G.O.; Jho, E.H.; Zuccarello, P.; Grasso, A.; Copat, C.; Ferrante, M. Phytoremediation of Contaminated Soils by Heavy Metals and PAHs. A Brief Review. Environ. Technol. Innov. 2017, 8, 309–326. [Google Scholar] [CrossRef]
- Vamerali, T.; Guarise, M.; Ganis, A.; Mosca, G. Effects of Water and Nitrogen Management on Fibrous Root Distribution and Turnover in Sugar Beet. Eur. J. Agron. 2009, 31, 69–76. [Google Scholar] [CrossRef]
- Martin, T.A.; Ruby, M.V. Review of in Situ Remediation Technologies for Lead, Zinc, and Cadmium in Soil. Remediat. J. 2004, 14, 35–53. [Google Scholar] [CrossRef]
- Keever, C. Causes of Succession on Old Fields of the Piedmont, North Carolina. Ecol. Monogr. 1950, 20, 229–250. [Google Scholar] [CrossRef]
- Mol, C. Bioremediation of Contaminated Soils from Mine Sites Using Native Plants in Northwestern Ontario; Lakehead University: Raybay, ON, Canada, 2016. [Google Scholar]
- Adams, P.; Lamoureux, S. The Use of Native Northern Plants for the Re-Vegetation of Arctic Mine Tailings and Mine Waste. Environ. Nat. Resour. 2005, 67. Available online: https://www.gov.nt.ca/sites/ecc/files/wkss_northern_plants_re-vegetation-2005.pdf (accessed on 11 March 2025).
- Cheng, H.; Liu, Q.; Ma, M.; Liu, Y.; Wang, W.; Ning, W. Cadmium Tolerance, Distribution, and Accumulation in Taraxacum ohwianum Kitam. as a Potential Cd-Hyperaccumulator. Int. J. Phytoremediation 2019, 21, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.V.L.; Brown, K.S. Phylogeny of the Nymphalidae (Lepidoptera). Syst. Biol. 2004, 53, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Salt, D.E.; Blaylock, M.; Kumar, N.P.B.A.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. Phytoremediation: A Novel Strategy for the Removal of Toxic Metals from the Environment Using Plants. Nat. Biotechnol. 1995, 13, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Whiting, S.N.; Reeves, R.D.; Richards, D.; Johnson, M.S.; Cooke, J.A.; Malaisse, F.; Paton, A.; Smith, J.A.C.; Angle, J.S.; Chaney, R.L.; et al. Research Priorities for Conservation of Metallophyte Biodiversity and Their Potential for Restoration and Site Remediation. Restor. Ecol. 2004, 12, 106–116. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Z.M. Mercury Toxicity, Molecular Response and Tolerance in Higher Plants. BioMetals 2012, 25, 847–857. [Google Scholar] [CrossRef]
- Majumder, A.; Jha, S. Hairy Roots: A Promising Tool for Phytoremediation. In Microorganisms in Environmental Management; Springer: Dordrecht, The Netherlands, 2012; pp. 607–629. ISBN 978-94-007-2229-3. [Google Scholar]
- Courtin, G.M. Birch Coppice Woodlands near the Sudbury Smelters: Dynamics of a Forest Monoculture. In Restoration and Recovery of an Industrial Region; Springer: New York, NY, USA, 1995; pp. 233–245. ISBN 978-1-4612-2520-1. [Google Scholar]
- Nyenda, T.; Gwenzi, W.; Gwata, C.; Jacobs, S.M. Leguminous Tree Species Create Islands of Fertility and Influence the Understory Vegetation on Nickel-Mine Tailings of Different Ages. Ecol. Eng. 2020, 155, 105902. [Google Scholar] [CrossRef]
- Grime, J.P. Vegetation Classification by Reference to Strategies. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
- Wielgolaski, F.E. Primary Productivity of Alpine Meadow Communities. In Fennoscandian Tundra Ecosystems; Springer: Berlin/Heidelberg, Germany, 1975; pp. 121–128. ISBN 978-3-642-80937-8. [Google Scholar]
- Peura, S.; Saetre, P.; Ehnvall, B.; Nilsson, M.B.; Öquist, M.G. Plant Functional Type and Peat Properties Determine Elemental Transfer in Boreal Mire Vegetation. Heliyon 2024, 10, e38925. [Google Scholar] [CrossRef]
- Schück, M.; Greger, M. Plant Traits Related to the Heavy Metal Removal Capacities of Wetland Plants. Int. J. Phytoremediation 2020, 22, 427–435. [Google Scholar] [CrossRef]
- Cronk, J.; Fennessy, S. Wetland Plants. In Encyclopedia of Inland Waters; Academic Press: Cambridge, MA, USA, 2009; pp. 590–598. ISBN 978-0-12-370626-3. [Google Scholar]
- Tripathi, R.D.; Tripathi, P.; Dwivedi, S.; Kumar, A.; Mishra, A.; Chauhan, P.S.; Norton, G.J.; Nautiyal, C.S. Roles for Root Iron Plaque in Sequestration and Uptake of Heavy Metals and Metalloids in Aquatic and Wetland Plants. Metallomics 2014, 6, 1789–1800. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy Metals Toxicity in Plants: An Overview on the Role of Glutathione and Phytochelatins in Heavy Metal Stress Tolerance of Plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and Molecular Mechanism Involved in Cold Stress Tolerance in Plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, N.; Yu, L.; Han, Z.; Guo, Y.; Ndombi, S.N.; Zhang, H.; Jiang, J.; Duan, Y.; Zou, Z.; et al. Plant Resistance Inducer AMHA Enhances Antioxidant Capacities to Promote Cold Tolerance by Regulating the Upgrade of Glutathione S-Transferase in Tea Plant. Hortic. Res. 2025, 12, uhaf073. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular Mechanisms for Heavy Metal Detoxification and Tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Gan, C.; Chen, T.; Yang, J. Growth Responses and Accumulation of Vanadium in Alfalfa, Milkvetch Root, and Swamp Morning Glory and Their Potential in Phytoremediation. Bull. Environ. Contam. Toxicol. 2021, 107, 559–564. [Google Scholar] [CrossRef]
- Futughe, A.; Purchase, D.; Jones, H. Phytoremediation; Shmaefsky, B., Ed.; Using Native Plants; Springer: Cham, Switzerland, 2020; pp. 285–327. ISBN 978-3-030-00098-1. [Google Scholar]
- Groom, C.A.; Halasz, A.; Paquet, L.; Morris, N.; Olivier, L.; Dubois, C.; Hawari, J. Accumulation of HMX (Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine) in Indigenous and Agricultural Plants Grown in HMX-Contaminated Anti-Tank Firing-Range Soil. Environ. Sci. Technol. 2002, 36, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Muratova, A.; Hübner, T.; Tischer, S.; Turkovskaya, O.; Möder, M.; Kuschk, P. Plant–Rhizosphere-Microflora Association During Phytoremediation of PAH-Contaminated Soil. Int. J. Phytoremediation 2003, 5, 137–151. [Google Scholar] [CrossRef]
- Hannink, N.K.; Rosser, S.J.; Bruce, N.C. Phytoremediation of Explosives. Crit. Rev. Plant Sci. 2002, 21, 511–538. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Song, Z.; Wang, D.; Qiu, W. Chelator Complexes Enhanced Amaranthus hypochondriacus L. Phytoremediation Efficiency in Cd-Contaminated Soils. Chemosphere 2019, 237, 124480. [Google Scholar] [CrossRef]
- Song, X.Y.; Li, X.X.; Wang, Y.; Hu, X.J. Long-Term Phytoremediation Process of Diesel Oil-Contaminated Soil. Adv. Mater. Res. 2012, 414, 280–283. [Google Scholar] [CrossRef]
- Qu, J.; Wang, L.; Yuan, X.; Cong, Q.; Guan, S.S. Effects of Ammonium Molybdate on Phytoremediation by Alfalfa Plants and (Im)Mobilization of Toxic Metals in Soils. Environ. Earth Sci. 2011, 64, 2175–2182. [Google Scholar] [CrossRef]
- Matanzas, N.; Afif, E.; Díaz, T.E.; Gallego, J.R. Phytoremediation Potential of Native Herbaceous Plant Species Growing on a Paradigmatic Brownfield Site. Water Air. Soil Pollut. 2021, 232, 290. [Google Scholar] [CrossRef]
- Park, J.; Bae, B. Uptake and Transformation of RDX by Perennial Plants in Poaceae Family (Amur Silver Grass and Reed Canary Grass) under Hydroponic Culture Conditions. J. Korean Soc. Environ. Eng. 2014, 36, 237–245. [Google Scholar] [CrossRef]
- Fernández, R.; Bertrand, A.; Casares, A.; García, R.; González, A.; Tamés, R.S. Cadmium Accumulation and Its Effect on the in Vitro Growth of Woody Fleabane and Mycorrhized White Birch. Environ. Pollut. 2008, 152, 522–529. [Google Scholar] [CrossRef]
- Yuan, J.; Bai, Y.; Chao, Y.; Sun, X.; He, C.; Liang, X.; Xie, L.; Han, L. Genome-Wide Analysis Reveals Four Key Transcription Factors Associated with Cadmium Stress in Creeping Bentgrass (Agrostis stolonifera L.). PeerJ 2018, 6, e5191. [Google Scholar] [CrossRef] [PubMed]
- Nguemté, P.M.; Djumyom Wafo, G.V.; Djocgoue, P.F.; Kengne Noumsi, I.M.; Wanko, N.A. Potentialities of Six Plant Species on Phytoremediation Attempts of Fuel Oil-Contaminated Soils—PAHs Impacts on Bioconcentration and Translocation Factors. In Proceedings of the 4th World Congress on New Technologies, Madrid, Spain, 19–21 August 2018. [Google Scholar]
- Rezek, J.; der Wiesche, C.; Mackova, M.; Zadrazil, F.; Macek, T. Biodegradation of Pahs in Long-Term Contaminated Soil Cultivated with European White Birch (Betula pendula) and Red Mulberry (Morus rubra) Tree. Int. J. Phytoremediation 2009, 11, 65–80. [Google Scholar] [CrossRef]
- Dmuchowski, W.; Gozdowski, D.; Brągoszewska, P.; Baczewska, A.H.; Suwara, I. Phytoremediation of Zinc Contaminated Soils Using Silver Birch (Betula pendula Roth). Ecol. Eng. 2014, 71, 32–35. [Google Scholar] [CrossRef]
- Fernández-Fuego, D.; Bertrand, A.; González, A. Metal Accumulation and Detoxification Mechanisms in Mycorrhizal Betula pubescens. Environ. Pollut. 2017, 231, 1153–1162. [Google Scholar] [CrossRef]
- Dradrach, A.; Karczewska, A.; Szopka, K.; Lewińska, K. Accumulation of Arsenic by Plants Growing in the Sites Strongly Contaminated by Historical Mining in the Sudetes Region of Poland. Int. J. Environ. Res. Public Health 2020, 17, 3342. [Google Scholar] [CrossRef]
- Yousaf, S.; Ripka, K.; Reichenauer, T.G.; Andria, V.; Afzal, M.; Sessitsch, A. Hydrocarbon Degradation and Plant Colonization by Selected Bacterial Strains Isolated from Italian Ryegrass and Birdsfoot Trefoil. J. Appl. Microbiol. 2010, 109, 1389–1401. [Google Scholar] [CrossRef]
- Masu, S.; Grecu, E.; Ionica, H.; Petrescu, M. For a Sustainable Development: Phytoremediation of Oil-Polluted Soils by Using Birdsfoot Trefoil Crops. Romanian Biotechnol. Lett. 2016, 22, 12010–12017. [Google Scholar]
- Moussa, H.; Serag, M.; Elgendy, M.; Mohesien, M. Heavy Metals Biosorption Using Dry Biomass of Lotus corniculatus L. and Amaranthus viridis L. Egypt. J. Chem. 2022, 65, 1275–1282. [Google Scholar] [CrossRef]
- Hammami, H.; Alaie, E.; Dastgheib, S.M.M. The Ability of Silybum marianum to Phytoremediate Cadmium and/or Diesel Oil from the Soil. Int. J. Phytoremediation 2018, 20, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Papadimou, S.G.; Golia, E.E.; Barbayiannis, N.; Tsiropoulos, N.G. Dual Role of the Hyperaccumulator Silybum marianum (L.) Gaertn. in Circular Economy: Production of Silymarin, a Valuable Secondary Metabolite, and Remediation of Heavy Metal Contaminated Soils. Sustain. Chem. Pharm. 2024, 38, 101454. [Google Scholar] [CrossRef]
- Bielecka, A.; Królak, E. Solidago Canadensis as a Bioaccumulator and Phytoremediator of Pb and Zn. Environ. Sci. Pollut. Res. 2019, 26, 36942–36951. [Google Scholar] [CrossRef]
- Demková, L.; Jezný, T.; Bobulska, L. Assessment of Soil Heavy Metal Pollution in a Former Mining Area—Before and After the End of Mining Activities. Soil Water Res. 2017, 12, 229–236. [Google Scholar] [CrossRef]
- Kumari, A.; Lal, B.; Rai, U.N. Assessment of Native Plant Species for Phytoremediation of Heavy Metals Growing in the Vicinity of NTPC Sites, Kahalgaon, India. Int. J. Phytoremediation 2016, 18, 592–597. [Google Scholar] [CrossRef]
- Githuku, C.R.; Ndambuki, J.M.; Salim, R.W.; Badejo, A.A. Treatment Potential of Typha Latifolia in Removal of Heavy Metals from Wastewater Using Constructed Wetlands. In Proceedings of the 41st WEDC International Conference, Nakuru, Kenya, 9–13 July 2018. [Google Scholar]
- Jomjun, N.; Siripen, T.; Maliwan, S.; Jintapat, N.; Prasak, T.; Somporn, C.; Petch, P. Phytoremediation of Arsenic in Submerged Soil by Wetland Plants. Int. J. Phytoremediation 2010, 13, 35–46. [Google Scholar] [CrossRef]
- Chandra, R.; Yadav, S. Phytoremediation of CD, CR, CU, MN, FE, NI, PB and ZN from Aqueous Solution Using Phragmites cummunis, Typha angustifolia and Cyperus esculentus. Int. J. Phytoremediation 2011, 13, 580–591. [Google Scholar] [CrossRef]
- Sekara, A.; Poniedziałek, M.; Ciura, J.; Jedrszczyk, E. Zinc and Copper Accumulation and Distribution in the Tissues of Nine Crops: Implications for Phytoreme. Pol. J. Environ. Stud. 2005, 14, 829–883. [Google Scholar]
- Bursztyn Fuentes, A.L.; José, C.; de los Ríos, A.; do Carmo, L.I.; de Iorio, A.F.; Rendina, A.E. Phytoextraction of Heavy Metals from a Multiply Contaminated Dredged Sediment by Chicory (Cichorium intybus L.) and Castor Bean (Ricinus communis L.) Enhanced with EDTA, NTA, and Citric Acid Application. Int. J. Phytoremediation 2018, 20, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.T.; Naidoo, K.; Nokes, J.; Walker, T.; Orton, F. Indicative Assessment of the Feasibility of Ni and Au Phytomining in Australia. J. Clean. Prod. 2009, 17, 194–200. [Google Scholar] [CrossRef]
- Dushenkov, V.; Kumar, P.B.A.N.; Motto, H.; Raskin, I. Rhizofiltration: The Use of Plants to Remove Heavy Metals from Aqueous Streams. Environ. Sci. Technol. 1995, 29, 1239–1245. [Google Scholar] [CrossRef]
- Moreno, F.N.; Anderson, C.W.N.; Stewart, R.B.; Robinson, B.H. Phytoremediation of Mercury-Contaminated Mine Tailings by Induced Plant-Mercury Accumulation. Environ. Pract. 2004, 6, 165–175. [Google Scholar] [CrossRef]
- Cassina, L.; Tassi, E.; Pedron, F.; Petruzzelli, G.; Ambrosini, P.; Barbafieri, M. Using a Plant Hormone and a Thioligand to Improve Phytoremediation of Hg-Contaminated Soil from a Petrochemical Plant. J. Hazard. Mater. 2012, 231–232, 36–42. [Google Scholar] [CrossRef]
- Mbangi, A.; Muchaonyerwa, P.; Zengeni, R. Accumulation of Multiple Heavy Metals in Plants Grown on Soil Treated with Sewage Sludge for More than 50 Years Presents Health Risks and an Opportunity for Phyto-Remediation. Water SA 2018, 44, 569–576. [Google Scholar] [CrossRef]
- Mahey, S.; Kumar, R.; Sharma, M.; Kumar, V.; Bhardwaj, R. A Critical Review on Toxicity of Cobalt and Its Bioremediation Strategies. SN Appl. Sci. 2020, 2, 1279. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and Opportunities in the Phytoremediation of Heavy Metals Contaminated Soils: A Review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Azirun, S.; Boyce, A. Enhanced Accumulation of Copper and Lead in Amaranth (Amaranthus paniculatus), Indian Mustard (Brassica juncea) and Sunflower (Helianthus annuus). PLoS ONE 2013, 8, e62941. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0062941 (accessed on 13 March 2025). [CrossRef]
- Bello, A.O.; Tawabini, B.S.; Khalil, A.B.; Boland, C.R.; Saleh, T.A. Phytoremediation of Cadmium-, Lead- and Nickel-Contaminated Water by Phragmites australis in Hydroponic Systems. Ecol. Eng. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Morari, F.; Ferro, N.D.; Cocco, E. Municipal Wastewater Treatment with Phragmites australis L. and Typha latifolia L. for Irrigation Reuse. Boron and Heavy Metals. Water Air Soil Pollut. 2015, 226, 56. [Google Scholar] [CrossRef]
- Gomes, H.I.; Mayes, W.M.; Whitby, P.; Rogerson, M. Constructed Wetlands for Steel Slag Leachate Management: Partitioning of Arsenic, Chromium, and Vanadium in Waters, Sediments, and Plants. J. Environ. Manag. 2019, 243, 30–38. [Google Scholar] [CrossRef]
- Nepovim, A.; Hebner, A.; Soudek, P.; Gerth, A.; Thomas, H.; Smrcek, S.; Vanek, T. Degradation of 2,4,6-Trinitrotoluene by Selected Helophytes. Chemosphere 2005, 60, 1454–1461. [Google Scholar] [CrossRef]
- Panz, K.; Miksch, K. Phytoremediation of Explosives (TNT, RDX, HMX) by Wild-Type and Transgenic Plants. J. Environ. Manag. 2012, 113, 85–92. [Google Scholar] [CrossRef]
- Kovačević, M.; Andrejic, G.; Šinžar-Sekulić, J.; Rakić, T.; Dželetović, Ž. Bioaccumulation of Heavy Metals in Common Reed (Phragmites australis) Growing Spontaneously on Highly Contaminated Mine Tailing Ponds in Serbia and Potential Use of This Species in Phytoremediation. Bot. Serbica 2019, 43, 85–95. [Google Scholar] [CrossRef]
- Fahid, M.; Arslan, M.; Shabir, G.; Younus, S.; Yasmeen, T.; Rizwan, M.; Siddique, K.; Ahmad, S.R.; Tahseen, R.; Iqbal, S.; et al. Phragmites australis in Combination with Hydrocarbons Degrading Bacteria Is a Suitable Option for Remediation of Diesel-Contaminated Water in Floating Wetlands. Chemosphere 2020, 240, 124890. [Google Scholar] [CrossRef] [PubMed]
- Lizama-Allende, K.; Ayala, J.; Jaque, I.; Echeverría, P. The Removal of Arsenic and Metals from Highly Acidic Water in Horizontal Subsurface Flow Constructed Wetlands with Alternative Supporting Media. J. Hazard. Mater. 2021, 408, 124832. [Google Scholar] [CrossRef]
- Hamidian, A.H.; Atashgahi, M.; Khorasani, N. Phytoremediation of Heavy Metals (Cd, Pb and V) in Gas Refinery Wastewater Using Common Reed (Phragmitis australis). Int. J. Aquat. Biol. 2014, 2, 29–35. [Google Scholar] [CrossRef]
- Antoniadis, V.; Shaheen, S.M.; Stärk, H.-J.; Wennrich, R.; Levizou, E.; Merbach, I.; Rinklebe, J. Phytoremediation Potential of Twelve Wild Plant Species for Toxic Elements in a Contaminated Soil. Environ. Int. 2021, 146, 106233. [Google Scholar] [CrossRef]
- Naz, R.; Khan, M.S.; Hafeez, A.; Fazil, M.; Khan, M.N.; Ali, B.; Javed, M.A.; Imran, M.; Shati, A.A.; Alfaifi, M.Y.; et al. Assessment of Phytoremediation Potential of Native Plant Species Naturally Growing in a Heavy Metal-Polluted Industrial Soils. Braz. J. Biol. 2024, 84, e264473. [Google Scholar] [CrossRef]
- Galal, T.M.; Shedeed, Z.A.; Hassan, L.M. Hazards Assessment of the Intake of Trace Metals by Common Mallow (Malva parviflora K.) Growing in Polluted Soils. Int. J. Phytoremediation 2019, 21, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.B.; Zhou, Y. Cadmium and Zinc Bioaccumulation by Phytolacca americana from Hydroponic Media and Contaminated Soils. Int. J. Phytoremediation 2019, 21, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Liu, X.; Zhang, C.; Dong, S.; Liu, Q.; Deng, M. Cd Uptake by Phytolacca americana L. Promoted by Cornstalk Biochar Amendments in Cd-Contaminated Soil. Int. J. Phytoremediation 2020, 22, 251–258. [Google Scholar] [CrossRef]
- Minkina, T.M.; Mandzhieva, S.S.; Chaplygin, V.A.; Motuzova, G.V.; Burachevskaya, M.V.; Bauer, T.V.; Sushkova, S.N.; Nevidomskaya, D.G. Effect of Aerotechnogenic Emissions on the Content of Heavy Metals in Herbaceous Plants of the Lower Don Region. Eurasian Soil Sci. 2017, 50, 746–756. [Google Scholar] [CrossRef]
- Pedron, F.; Petruzzelli, G.; Barbafieri, M.; Tassi, E.; Ambrosini, P.; Patata, L. Mercury Mobilization in a Contaminated Industrial Soil for Phytoremediation. Commun. Soil Sci. Plant Anal. 2011, 42, 2767–2777. [Google Scholar] [CrossRef]
- Padmavathiamma, P.K.; Li, L.Y. Phytoremediation of Metal-Contaminated Soil in Temperate Humid Regions of British Columbia, Canada. Int. J. Phytoremediation 2009, 11, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Ndubueze, E. Potential of Five Plant Species for Phytoremediation of Metal-PAH-Pesticide Contaminated Soil. Master’s Thesis, The University of Western Ontario, London, ON, Canada, 2018. [Google Scholar]
- Lee, I.; Baek, K.; Kim, H.; Kim, S.; Kim, J.; Kwon, Y.; Chang, Y.; Bae, B. Phytoremediation of Soil Co-Contaminated with Heavy Metals and TNT Using Four Plant Species. J. Environ. Sci. Health Part A 2007, 42, 2039–2045. [Google Scholar] [CrossRef]
- Churko, E.E.; Nhamo, L.; Chitakira, M. Phytoremediation Capacity of Water Hyacinth (Eichhornia crassipes) as a Nature-Based Solution for Contaminants and Physicochemical Characterization of Lake Water. Water 2023, 15, 2540. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and Toxicity of Hexavalent Chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Murtić, S.; Zahirović, Ć.; Čivić, H.; Sijahović, E.; Jurković, J.; Avdić, J.; Šahinović, E.; Podrug, A. Phytoaccumulation of Heavy Metals in Native Plants Growing on Soils in the Spreča River Valley, Bosnia and Herzegovina. Plant Soil Environ. 2021, 67, 533–540. [Google Scholar] [CrossRef]
- Nujkić, M.; Milić, S.; Spalović, B.; Dardas, A.; Alagić, S.; Ljubić, D.; Papludis, A. Saponaria officinalis L. and Achillea millefolium L. as Possible Indicators of Trace Elements Pollution Caused by Mining and Metallurgical Activities in Bor, Serbia. Environ. Sci. Pollut. Res. 2020, 27, 44969–44982. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, X.; Anderson, C.W.N.; Xing, Y.; Shang, L. Remediation of Mercury Contaminated Sites—A Review. J. Hazard. Mater. 2012, 221–222, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Norouznia, H.; Hamidian, A.H. Phytoremediation Efficiency of Pondweed (Potamogeton crispus) in Removing Heavy Metals (Cu, Cr, Pb, As and Cd) from Water of Anzali Wetland. Int. J. Aquat. Biol. 2014, 2, 206–214. [Google Scholar] [CrossRef]
- Best, E.P.; Zappi, M.E.; Fredrickson, H.L.; Sprecher, S.L.; Larson, S.L.; Ochman, M. Screening of Aquatic and Wetland Plant Species for Phytoremediation of Explosives-Contaminated Groundwater from the Iowa Army Ammunition Plant. Ann. N. Y. Acad. Sci. 1997, 829, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Collier, M.H.; Boughter, S.A.; Dameron, M.P.; Gribbins, K.M.; Keane, B.; Shann, J.R.; Rogstad, S.H. Uptake and Distribution of Copper, Lead, and Zinc in Dandelions (Taraxacum officinale; Asteraceae) Sampled from Polluted and Nonpolluted Soils. J. Torrey Bot. Soc. 2017, 144, 47–57. [Google Scholar] [CrossRef]
- Vural, A. Trace Element Accumulation Behavior, Ability, and Propensity of Taraxacum officinale F.H. Wigg (Dandelion). Environ. Sci. Pollut. Res. 2024, 31, 16667–16684. [Google Scholar] [CrossRef]
- Meiman, P.J.; Davis, N.R.; Brummer, J.E.; Ippolito, J.A. Riparian Shrub Metal Concentrations and Growth in Amended Fluvial Mine Tailings. Water Air Soil Pollut. 2012, 223, 1815–1829. [Google Scholar] [CrossRef]
- Palmroth, M.R.T.; Pichtel, J.; Puhakka, J.A. Phytoremediation of Subarctic Soil Contaminated with Diesel Fuel. Bioresour. Technol. 2002, 84, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Placek, A.; Grobelak, A.; Kacprzak, M. Improving the Phytoremediation of Heavy Metals Contaminated Soil by Use of Sewage Sludge. Int. J. Phytoremediation 2016, 18, 605–618. [Google Scholar] [CrossRef]
- Saleh, H.M.; Moussa, H.R.; Mahmoud, H.H.; El-Saied, F.A.; Dawoud, M.; Abdel Wahed, R.S. Potential of the Submerged Plant Myriophyllum spicatum for Treatment of Aquatic Environments Contaminated with Stable or Radioactive Cobalt and Cesium. Prog. Nucl. Energy 2020, 118, 103147. [Google Scholar] [CrossRef]
- Pavlostathis, S.G.; Comstock, K.K.; Jacobson, M.E.; Saunders, F.M. Transformaton of 2,4,6-Trinitrotoluene by the Aquatic Plant Myriophyllum spicatum. Environ. Toxicol. Chem. 1998, 17, 2266–2273. [Google Scholar] [CrossRef]
- Khashij, S.; Karimi, B.; Makhdoumi, P. Phytoremediation with Festuca Arundinacea: A Mini Review. Int. J. Health Life Sci. 2018, 4, e86625. [Google Scholar] [CrossRef]
- Cuske, M.; Karczewska, A.; Gałka, B.; Dradrach, A. Some Adverse Effects of Soil Amendment with Organic Materials—The Case of Soils Polluted by Copper Industry Phytostabilized with Red Fescue. Int. J. Phytoremediation 2016, 18, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Gardea-Torresdey, J.L.; Peralta-Videa, J.R.; Montes, M.; de la Rosa, G.; Corral-Diaz, B. Bioaccumulation of Cadmium, Chromium and Copper by Convolvulus arvensis L.: Impact on Plant Growth and Uptake of Nutritional Elements. Bioresour. Technol. 2004, 92, 229–235. [Google Scholar] [CrossRef]
- Bielecka, A.; Królak, E. The Accumulation of Mn and Cu in the Morphological Parts of Solidago Canadensis under Different Soil Conditions. PeerJ 2019, 7, e8175. [Google Scholar] [CrossRef]
- Ficko, S.A.; Rutter, A.; Zeeb, B.A. Potential for Phytoextraction of PCBs from Contaminated Soils Using Weeds. Sci. Total Environ. 2010, 408, 3469–3476. [Google Scholar] [CrossRef]
- Zykova, I.V.; Isakov, V.A. Study of the Adsorption of Heavy Metal Ions from Binary and Multicomponent Solutions by Ceramic Chips. IOP Conf. Ser. Earth Environ. Sci. 2020, 613, 12169. [Google Scholar] [CrossRef]
- Miletić, Z.; Jarić, S.; Jonjev, M.; Mitrović, M.; Pavlović, D.; Matić, M.; Pavlović, P. Phytoremediation Potential of Invasive Plant Species for Potentially Toxic Elements along the Sava River Upstream. Heliyon 2024, 10, e33798. [Google Scholar] [CrossRef]
- Dizaji, E.; Kafi, M.; Khalighi, A.; Jari, S. Phytoremediation of Lead and Cadmium by Thornless Honey Loccust Trees’ (Gleditsia triacanthos L. Var. Inermis) in Contaminatedsoil near the Tehran-Karaj Highway. Indian J. Agric. Res. 2016, 50, 579–583. [Google Scholar] [CrossRef][Green Version]
- Putra, R.S.; Ohkawa, Y.; Tanaka, S. Application of EAPR System on the Removal of Lead from Sandy Soil and Uptake by Kentucky Bluegrass (Poa pratensis L.). Sep. Purif. Technol. 2013, 102, 34–42. [Google Scholar] [CrossRef]
- Xian, J.; Wang, Y.; Niu, K.; Ma, H.; Ma, X. Transcriptional Regulation and Expression Network Responding to Cadmium Stress in a Cd-Tolerant Perennial Grass Poa pratensis. Chemosphere 2020, 250, 126158. [Google Scholar] [CrossRef] [PubMed]
- Sas-Nowosielska, A.; Galimska-Stypa, R.; Kucharski, R.; Zielonka, U.; Małkowski, E.; Gray, L. Remediation Aspect of Microbial Changes of Plant Rhizosphere in Mercury Contaminated Soil. Environ. Monit. Assess. 2008, 137, 101–109. [Google Scholar] [CrossRef]
- Gołda, S.; Korzeniowska, J. Comparison of Phytoremediation Potential of Three Grass Species in Soil Contaminated with Cadmium. Ochr. Srodowiska Zasobów Nat. 2016, 27, 8–14. [Google Scholar] [CrossRef]
- Flores-Calla, S.S.; Villanueva-Salas, J.A.; Diaz-Rodriguez, K.; Gonzales-Condori, E.G. Removal of Lead, Cadmium, and Mercury in Monometallic and Trimetallic Aqueous Systems Using Chenopodium album L. Scientifica 2024, 2024, 6842159. [Google Scholar] [CrossRef]
- Ekperusi, A.O.; Sikoki, F.D.; Nwachukwu, E.O. Application of Common Duckweed (Lemna minor) in Phytoremediation of Chemicals in the Environment: State and Future Perspective. Chemosphere 2019, 223, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Phouthavong-Murphy, J.C.; Merrill, A.K.; Zamule, S.; Giacherio, D.; Brown, B.; Roote, C.; Das, P. Phytoremediation Potential of Switchgrass (Panicum virgatum), Two United States Native Varieties, to Remove Bisphenol-A (BPA) from Aqueous Media. Sci. Rep. 2020, 10, 835. [Google Scholar] [CrossRef]
- Pradhan, S.; Conrad, J.; Paterek, J.; Srivastava, V. Potential of Phytoremediation for Treatment of PAHs in Soil at MGP Sites. J. Soil Contam. 1998, 7, 467–480. [Google Scholar] [CrossRef]
- Wiltse, C.; Rooney, W.; Chen, Z.; Schwab, A.; Banks, M. Greenhouse Evaluation of Agronomic and Crude Oilphytoremediation Potential among Alfalfa Genotypes. J. Environ. Qual. 1998, 27, 169–173. [Google Scholar] [CrossRef]
- Valizadeh, R.; Mahdavian, L. Phytoremediation and Absorption Isotherms of Heavy Metal Ions by Convolvulus tricolor (CTC). Int. J. Phytoremediation 2016, 18, 329–336. [Google Scholar] [CrossRef]
- Kavousi, H.R.; Karimi, M.R.; Neghab, M.G. Assessment the Copper-Induced Changes in Antioxidant Defense Mechanisms and Copper Phytoremediation Potential of Common Mullein (Verbascum thapsus L.). Environ. Sci. Pollut. Res. Int. 2021, 28, 18070–18080. [Google Scholar] [CrossRef]
- Čudić, V.; Stojiljković, D.; Jovović, A. Phytoremediation Potential of Wild Plants Growing on Soil Contaminated with Heavy Metals. Arch. Ind. Hyg. Toxicol. 2016, 67, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Mleczek, M.; Goliński, P.; Krzesłowska, M.; Gąsecka, M.; Magdziak, Z.; Rutkowski, P.; Budzyńska, S.; Waliszewska, B.; Kozubik, T.; Karolewski, Z.; et al. Phytoextraction of Potentially Toxic Elements by Six Tree Species Growing on Hazardous Mining Sludge. Environ. Sci. Pollut. Res. Int. 2017, 24, 22183–22195. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, G.; Olsen, R.A. Sorption and Biological Removal of Creosote-Contaminants from Groundwater in Soil/Sand Vegetated with Orchard Grass (Dactylis glomerata). Adv. Environ. Res. 2004, 8, 313–327. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytoremediation of Mine Tailings in Temperate and Arid Environments. Rev. Environ. Sci. Biotechnol. 2008, 7, 47–59. [Google Scholar] [CrossRef]
- Ghosh, P.; Konar, A.; Roy, A.; Chatterjee, S.; Dalal, D. Phytoremediation Technology: A Review. Int. J. Agric. Plant Sci. 2023, 5, 44–49. [Google Scholar]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of Green Waste Compost and Biochar Soil Amendments for Reducing Lead and Copper Mobility and Uptake to Ryegrass. J. Hazard. Mater. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Rehman, G.; Muhammad, J.; Ilyas, M.; Subhanullah, M.; Ullah, K.; Massimzhan, M.; Toktar, M.; Bektay, Y.; Kalybekov, M.; Ydyrys, A.; et al. Phytoremediation of Heavy Metals from Soil and Their Effects on Plant Physiology—A Review. ES Mater. Manuf. 2024, 26, 1298. [Google Scholar] [CrossRef]
- Cook, R.L.; Hesterberg, D. Comparison of Trees and Grasses for Rhizoremediation of Petroleum Hydrocarbons. Int. J. Phytoremediation 2013, 15, 844–860. [Google Scholar] [CrossRef]
- Hou, F.S.L.; Milke, M.W.; Leung, D.W.M.; MacPherson, D.J. Variations in Phytoremediation Performance with Diesel-Contaminated Soil. Environ. Technol. 2001, 22, 215–222. [Google Scholar] [CrossRef]
- Yoon, J.M.; Aken, B.V.; Schnoor, J.L. Leaching of Contaminated Leaves Following Uptake and Phytoremediation of RDX, HMX, and TNT by Poplar. Int. J. Phytoremediation 2006, 8, 81–94. [Google Scholar] [CrossRef]
- El-Gendy, A.S.; Svingos, S.; Brice, D.; Garretson, J.H.; Schnoor, J. Assessments of the Efficacy of a Long-Term Application of a Phytoremediation System Using Hybrid Poplar Trees at Former Oil Tank Farm Sites. Water Environ. Res. Res. Publ. Water Environ. Fed. 2009, 81, 486–498. [Google Scholar] [CrossRef]
- Singh, S.N.; Mishra, S. Phytoremediation of TNT and RDX. In Biological Remediation of Explosive Residues; Singh, S.N., Ed.; Environmental Science and Engineering; Springer International Publishing: Cham, Switzerland, 2014; pp. 371–392. ISBN 978-3-319-01082-3. [Google Scholar]
- Li, C.; Jiang, Y.; Shao, Y.; Gao, G.; Fan, M.; Zhang, L.; Zhang, S.; Xiang, J.; Hu, S.; Wang, Y.; et al. Quantification of Degree of Interactions during Co-Pyrolysis of Nine Typical Carbonaceous Wastes. Renew. Energy 2024, 227, 120569. [Google Scholar] [CrossRef]
- Koptsik, G.N. Problems and Prospects Concerning the Phytoremediation of Heavy Metal Polluted Soils: A Review. Eurasian Soil Sci. 2014, 47, 923–940. [Google Scholar] [CrossRef]
- Pan, F.; Meng, Q.; Luo, S.; Shen, J.; Chen, B.; Khan, K.Y.; Japenga, J.; Ma, X.; Yang, X.; Feng, Y. Enhanced Cd Extraction of Oilseed Rape (Brassica napus) by Plant Growth-Promoting Bacteria Isolated from Cd Hyperaccumulator Sedum alfredii Hance. Int. J. Phytoremediation 2017, 19, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Angelova, V. Potential of Rapeseed (Brassica napus L.) for Phytoremediation of Soils Contaminated with Heavy Metals. J. Environ. Prot. Ecol. 2017, 18, 468–478. [Google Scholar]
- Mikalajūnė, A.; Jasulaitytė, G. Cleaning the Soil from Zinc Using Red Clovers “Arimaičiai”. Moksl.–Liet. Ateitis Sci.–Future Lith. 2010, 2, 60–65. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Gałązka, A.; Kucharski, J. Role of Festuca rubra and Festuca Arundinacea in Determinig the Functional and Genetic Diversity of Microorganisms and of the Enzymatic Activity in the Soil Polluted with Diesel Oil. Environ. Sci. Pollut. Res. Int. 2019, 26, 27738–27751. [Google Scholar] [CrossRef] [PubMed]
- Dradrach, A.; Karczewska, A.; Szopka, K. Arsenic Accumulation by Red Fescue (Festuca rubra) Growing in Mine Affected Soils—Findings from the Field and Greenhouse Studies—ScienceDirect. Chemosphere 2020, 248, 126045. [Google Scholar] [CrossRef] [PubMed]
- Grobelak, A.; Placek, A.; Grosser, A.; Singh, B.R.; Almås, Å.R.; Napora, A.; Kacprzak, M. Effects of Single Sewage Sludge Application on Soil Phytoremediation. J. Clean. Prod. 2017, 155, 189–197. [Google Scholar] [CrossRef]
- Kukier, U.; Peters, C.A.; Chaney, R.L.; Angle, J.S.; Roseberg, R.J. The Effect of pH on Metal Accumulation in Two Alyssum Species. J. Environ. Qual. 2004, 33, 2090–2102. [Google Scholar] [CrossRef]
- Krämer, U.; Grime, G.W.; Smith, J.A.C.; Hawes, C.R.; Baker, A.J.M. Micro-PIXE as a Technique for Studying Nickel Localization in Leaves of the Hyperaccumulator Plant Alyssum lesbiacum. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1997, 130, 346–350. [Google Scholar] [CrossRef]
- Wegiel, A.; Bielinis, E. Polowy Heavy Metals Accumulation in Scots Pine Stands of Different Densities Growing on Not Contaminated Forest Area (Northwestern Poland). Cent. Fuer Gesamte Forstwes. Austrian J. For. Sci. 2018, 135, 259–281. [Google Scholar]
- Qian, J.; Zayed, A.; Zhu, Y.; Yu, M.; Terry, N. Phytoaccumulation of Trace Elements by Wetland Plants: III. Uptake and Accumulation of Ten Trace Elements by Twelve Plant Species. J. Environ. Qual. 1999, 28, 1448–1455. [Google Scholar] [CrossRef]
- Yan, L.; Penttinen, P.; Simojoki, A.; Stoddard, F.L.; Lindström, K. Perennial Crop Growth in Oil-Contaminated Soil in a Boreal Climate. Sci. Total Environ. 2015, 532, 752–761. [Google Scholar] [CrossRef][Green Version]
- Gafur, N.; Sakakibara, M.; Sera, K. Phytoremediation of Heavy Metal-Polluted Mine Drainage by Eleocharis Acicularis. Environ. Sci. Indian J. 2017, 13, 131. [Google Scholar]
- Ernst, W.H.O. Phytoextraction of Mine Wastes—Options and Impossibilities. Geochemistry 2005, 65, 29–42. [Google Scholar] [CrossRef]
- Murch, S.J.; Haq, K.; Rupasinghe, H.P.V.; Saxena, P.K. Nickel Contamination Affects Growth and Secondary Metabolite Composition of St. John’s Wort (Hypericum perforatum L.). Environ. Exp. Bot. 2003, 49, 251–257. [Google Scholar] [CrossRef]
- Cary, T.J.; Rylott, L.; Zhang, L.; Routsong, R.; Palazzo, A.J.; Strand, S.E.; Bruce, N.C. Field Trial Demonstrating Phytoremediation of the Military Explosive RDX Using XplAB-Expressing Switchgrass in-the-Field. Nat. Biotechnol. 2021, 39, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Suljić, A.; Bečić, E.; Husejnović, M.Š.; Ademović, Z. St John’s Wort as a Possible Tool for Remediation of the Soil Contaminated with Heavy Metals. In Interdisciplinary Advances in Sustainable Development II; Springer: Cham, Switzerland, 2024; pp. 61–69. [Google Scholar]
- Pavlova, D.; Karadjova, I.; Krasteva, I. Essential and Toxic Element Concentrations in Hypericum Perforatum. Aust. J. Bot. 2015, 63, 152–158. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, D.; Xu, Z.; Yuan, S.; Li, Y.; Wang, L. Effect of Overlying Water pH, Dissolved Oxygen and Temperature on Heavy Metal Release from River Sediments under Laboratory Conditions. Arch. Environ. Prot. 2017, 43, 28–36. [Google Scholar] [CrossRef]
- Taylor, R.W.; Ibeabuchi, I.O.; Sistani, K.R.; Shuford, J.W. Heavy Metal Concentration in Forage Grasses and Extractability from Some Acid Mine Spoils. Water Air. Soil Pollut. 1993, 68, 363–372. [Google Scholar] [CrossRef]
- Gagnon, V.; Rodrigue-Morin, M.; Migneault, M.; Tardif, A.; Garneau, L.; Lalonde, S.; Shipley, B.; Greer, C.W.; Bellenger, J.-P.; Roy, S. Survival, Growth and Element Translocation by 4 Plant Species Growing on Acidogenic Gold Mine Tailings in Québec. Ecol. Eng. 2020, 151, 105855. [Google Scholar] [CrossRef]
- Renault, S. Phytoremediation and Revegetation of Mine Tailings and Bio-Ore Production: Progress Report on Plant Growth in Amended Tailings and Metal Accumulation in Seedlings Planted at Central Manitoba (Au) Minesite (NTS 52L13). In Report of Activities 2004, Manitoba Industry, Economic Development and Mines, Manitoba Geological Survey; Province of Manitoba: Winnipeg, MB, Canada, 2004. [Google Scholar]
- Jasion, M.; Samecka-Cymerman, A.; Kolon, K.; Kempers, A.J. Tanacetum Vulgare as a Bioindicator of Trace-Metal Contamination: A Study of a Naturally Colonized Open-Pit Lignite Mine. Arch. Environ. Contam. Toxicol. 2013, 65, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Abbaslou, H.; Bakhtiari, S. Phytoremediation Potential of Heavy Metals by Two Native Pasture Plants (Eucalyptus grandis and Ailanthus altissima) Assisted with AMF and Fibrous Minerals in Contaminated Mining Regions. Pollution 2017, 3, 471–486. [Google Scholar]
- Mehes-Smith, M.; Nkongolo, K.K. Physiological and Cytological Responses of Deschampsia cespitosa and Populus tremuloides to Soil Metal Contamination. Water Air. Soil Pollut. 2015, 226, 125. [Google Scholar] [CrossRef]
- Kang, M.; Tian, Y.; Peng, S.; Wang, M. Effect of Dissolved Oxygen and Nutrient Levels on Heavy Metal Contents and Fractions in River Surface Sediments. Sci. Total Environ. 2019, 648, 861–870. [Google Scholar] [CrossRef]
- Rahim, A.; Soeprobowati, T.R. Bioaccumulation of Lead (Pb) by the Common Water Hyacinth Eichhornia crassipes (Mart.) Solms in Batujai Reservoir, Central Lombok Regency, Indonesia. Aquac. Aquar. Conserv. Legis. 2018, 11, 1435–1444. [Google Scholar]
- Shen, Y.; Wang, W.; Li, H.; Zhao, M.; Yue, B.; Chang, G.; Li, Z.; Gao, T. A Pilot Experiment for Phytoremediation of Petroleum Contaminated Soil by Trifolium repens L. with Weathering in an Arid and Semi-Arid Region in China. In Environmental Pollution Governance and Ecological Remediation Technology; Springer: Cham, Switzerland, 2023; pp. 679–690. [Google Scholar]
- Sotiriou, V.; Michas, G.; Xiong, L.; Drosos, M.; Vlachostergios, D.; Papadaki, M.; Mihalakakou, G.; Kargiotidou, A.; Tziouvalekas, M.; Salachas, G.; et al. Effects of Heavy Metal Ions on White Clover (Trifolium repens L.) Growth in Cd, Pb and Zn Contaminated Soils Using Zeolite. Soil Sci. Environ. 2023, 2, 4. [Google Scholar] [CrossRef]
- Simiele, M.; Lebrun, M.; Cioppo, G.; Scippa, G.; Trupiano, D.; Bourgerie, S.; Morabito, D. Evaluation of Different Amendment Combinations Associated with Trifolium repens to Stabilize Pb and As in a Mine-Contaminated Soil. Water Air. Soil Pollut. 2020, 231, 539. [Google Scholar] [CrossRef]
- Memić, S.; Bektić, S.; Huseinović, S. Effect of Soil Composition on Heavy Metal Uptake and Distribution in White Clover (Trifolium repens L.). J. Appl. Life Sci. Int. 2023, 26, 87–95. [Google Scholar] [CrossRef]
- Courchesne, F.; Turmel, M.-C.; Cloutier-Hurteau, B.; Constantineau, S.; Munro, L.; Labrecque, M. Phytoextraction of Soil Trace Elements by Willow during a Phytoremediation Trial in Southern Québec, Canada. Int. J. Phytoremediation 2017, 19, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Gervais-Bergeron, B.; Chagnon, P.-L.; Labrecque, M. Willow Aboveground and Belowground Traits Can Predict Phytoremediation Services. Plants 2021, 10, 1824. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.A.; Wang, X.; Muiznieks, I.A.; Ekuan, G.; Ruszaj, M.; Cortellucci, R.; Domroes, D.; Karscig, G.; Newman, T.; Crampton, R.S.; et al. Remediation of Trichloroethylene in an Artificial Aquifer with Trees: A Controlled Field Study. Environ. Sci. Technol. 1999, 33, 2257–2265. [Google Scholar] [CrossRef]
| Gladys [46] n = 1 | Lago-Vila [13] n = 3 | Tomlinso et al. [47] n = 65 | Rani et al. [48] n = 32 | Rodriguez-Seijo et al. [49] n = 1 | Recommended Values | |
|---|---|---|---|---|---|---|
| pH | 8.98 | 5.99–7.87 | 7.68 | 5.1–8.8 | 7.87–8.05 | 6.3–7.5 |
| TKN | 0.005 | 0.03–2.75 | 4.96 | 8.1–113 | 0.042–UL * | 0.2–0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koornneef, K.; Kurissery, S.; Kanavillil, N. A Review on Phytoremediation of Decommissioned Mines and Quarries in Ontario: A Sustainable Approach. Sustainability 2025, 17, 5475. https://doi.org/10.3390/su17125475
Koornneef K, Kurissery S, Kanavillil N. A Review on Phytoremediation of Decommissioned Mines and Quarries in Ontario: A Sustainable Approach. Sustainability. 2025; 17(12):5475. https://doi.org/10.3390/su17125475
Chicago/Turabian StyleKoornneef, Karen, Sreekumari Kurissery, and Nandakumar Kanavillil. 2025. "A Review on Phytoremediation of Decommissioned Mines and Quarries in Ontario: A Sustainable Approach" Sustainability 17, no. 12: 5475. https://doi.org/10.3390/su17125475
APA StyleKoornneef, K., Kurissery, S., & Kanavillil, N. (2025). A Review on Phytoremediation of Decommissioned Mines and Quarries in Ontario: A Sustainable Approach. Sustainability, 17(12), 5475. https://doi.org/10.3390/su17125475







