Valorisation of Inorganic Fractions of Waste Generated by Hydrothermal Treatment of Sewage Sludge in Alkaline Cement
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
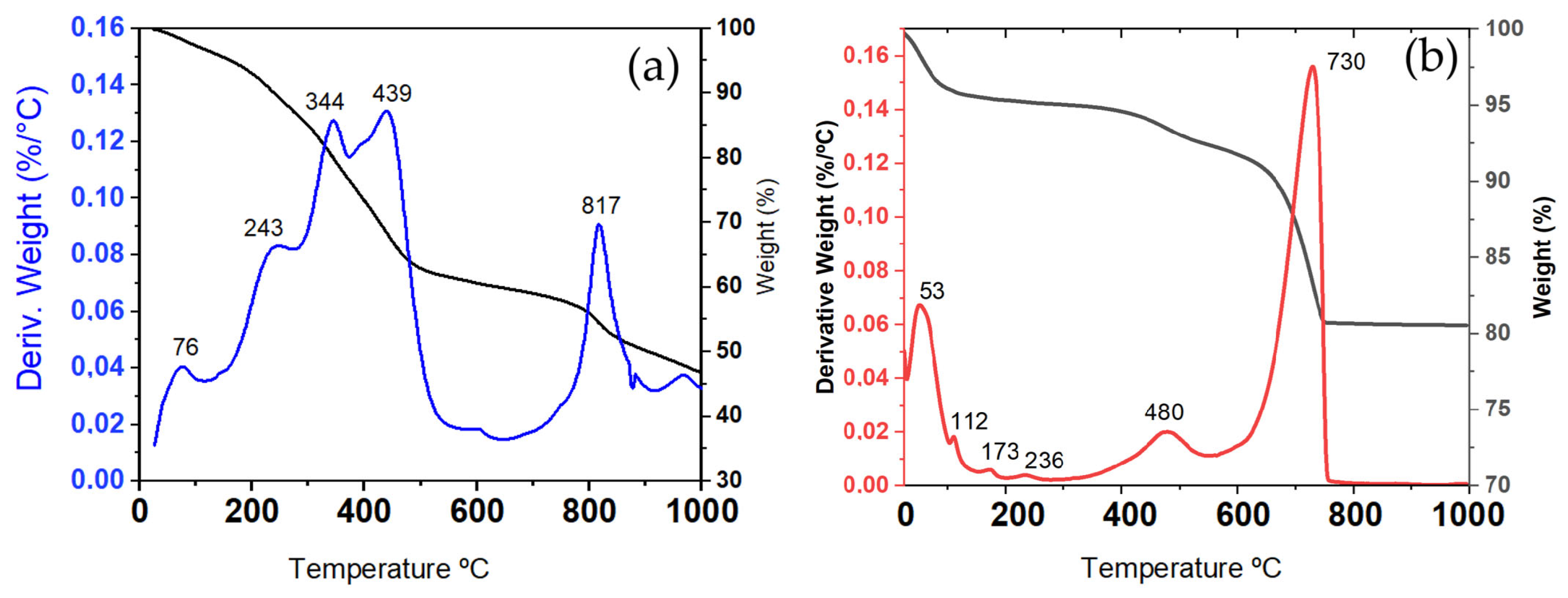
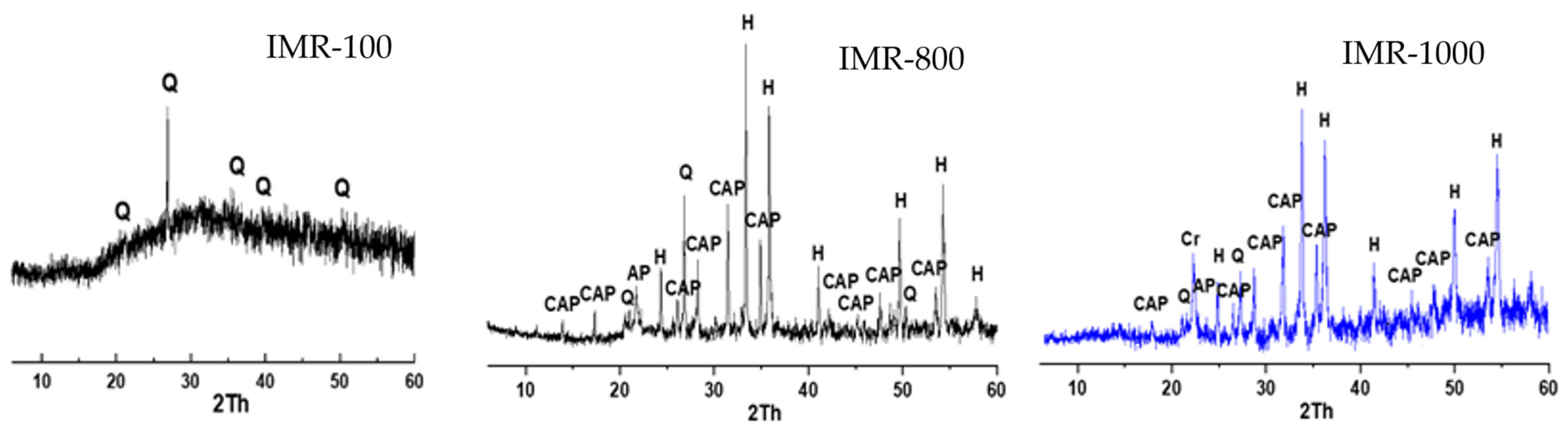
3.1. Material Characterisation
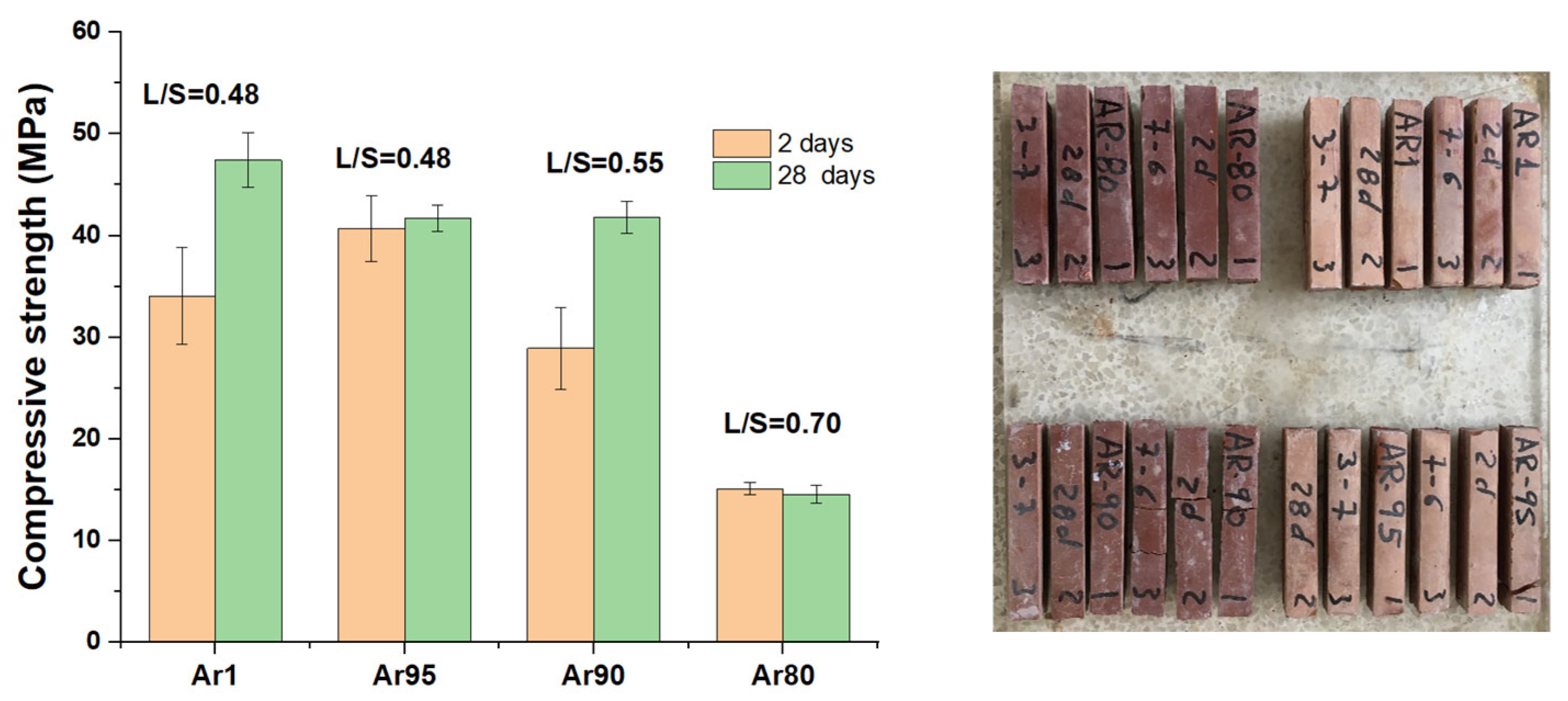
3.2. Alkaline Activation
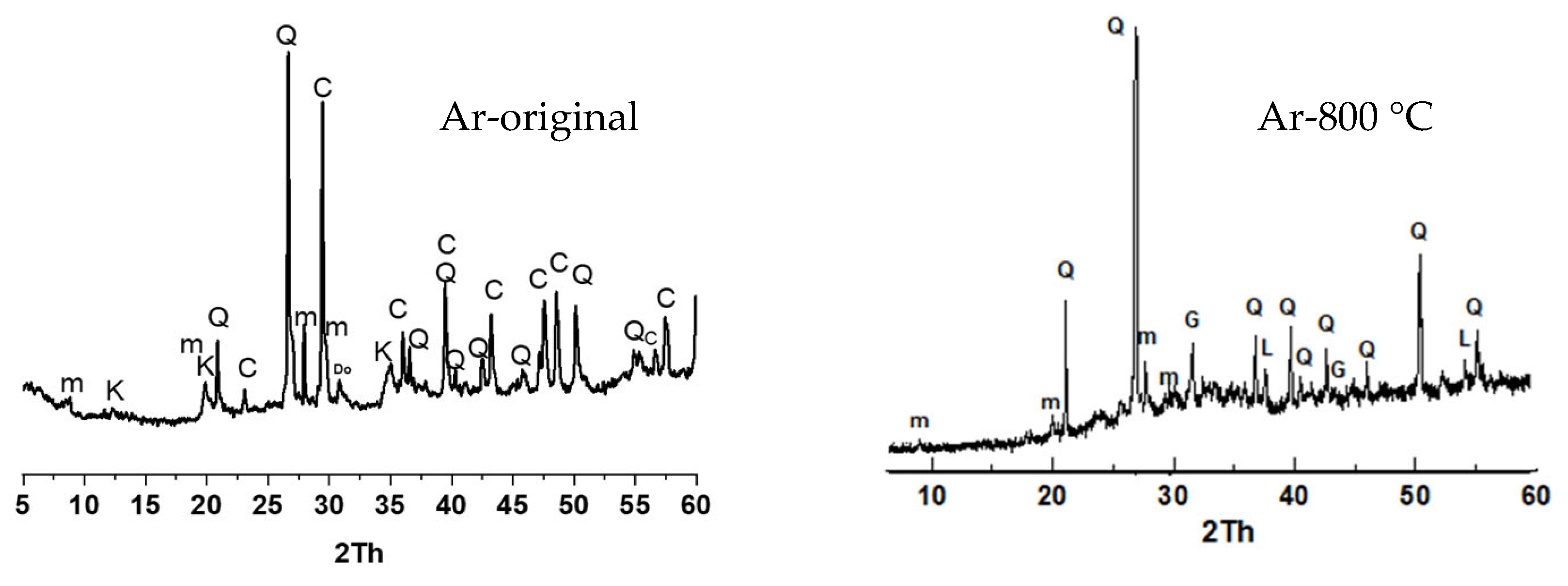
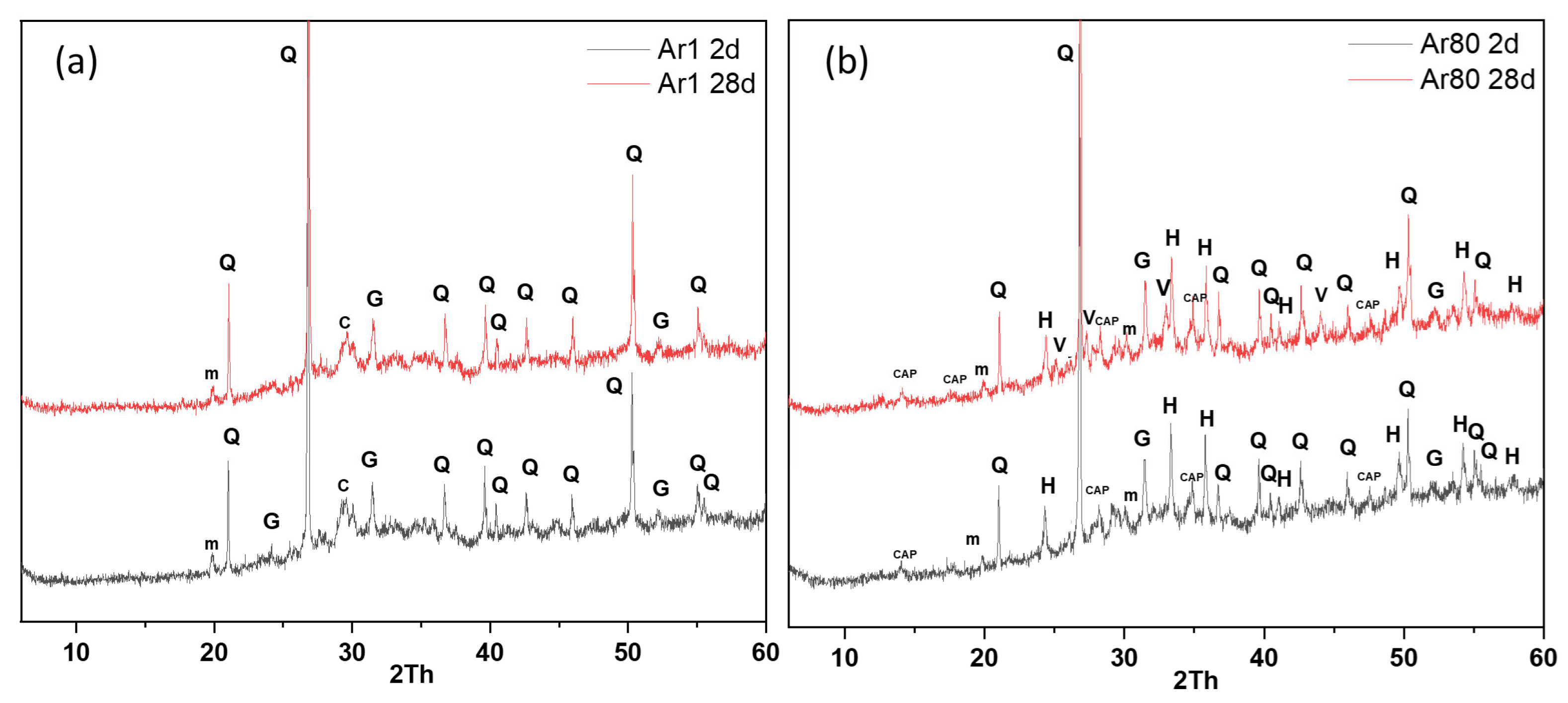
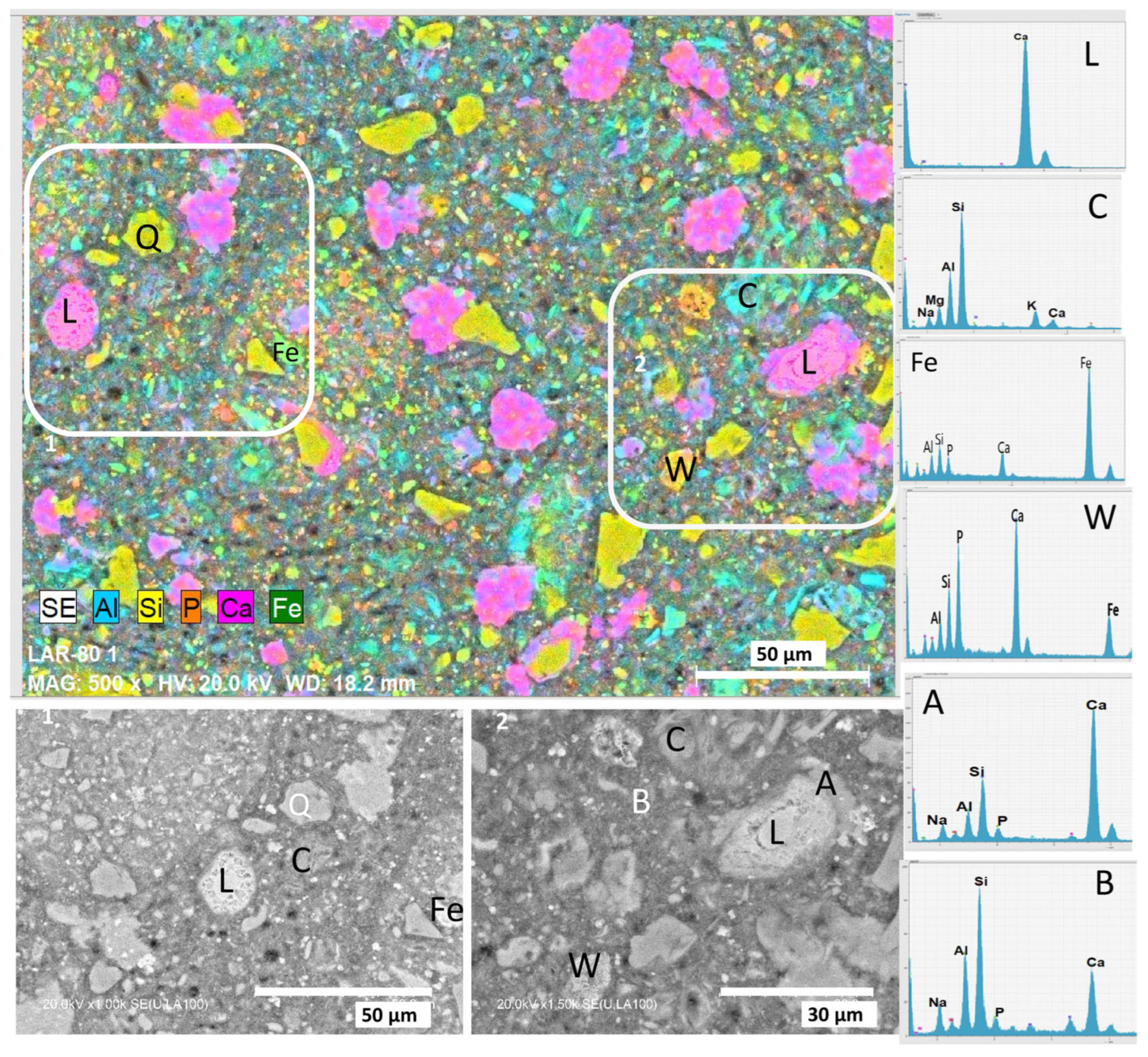
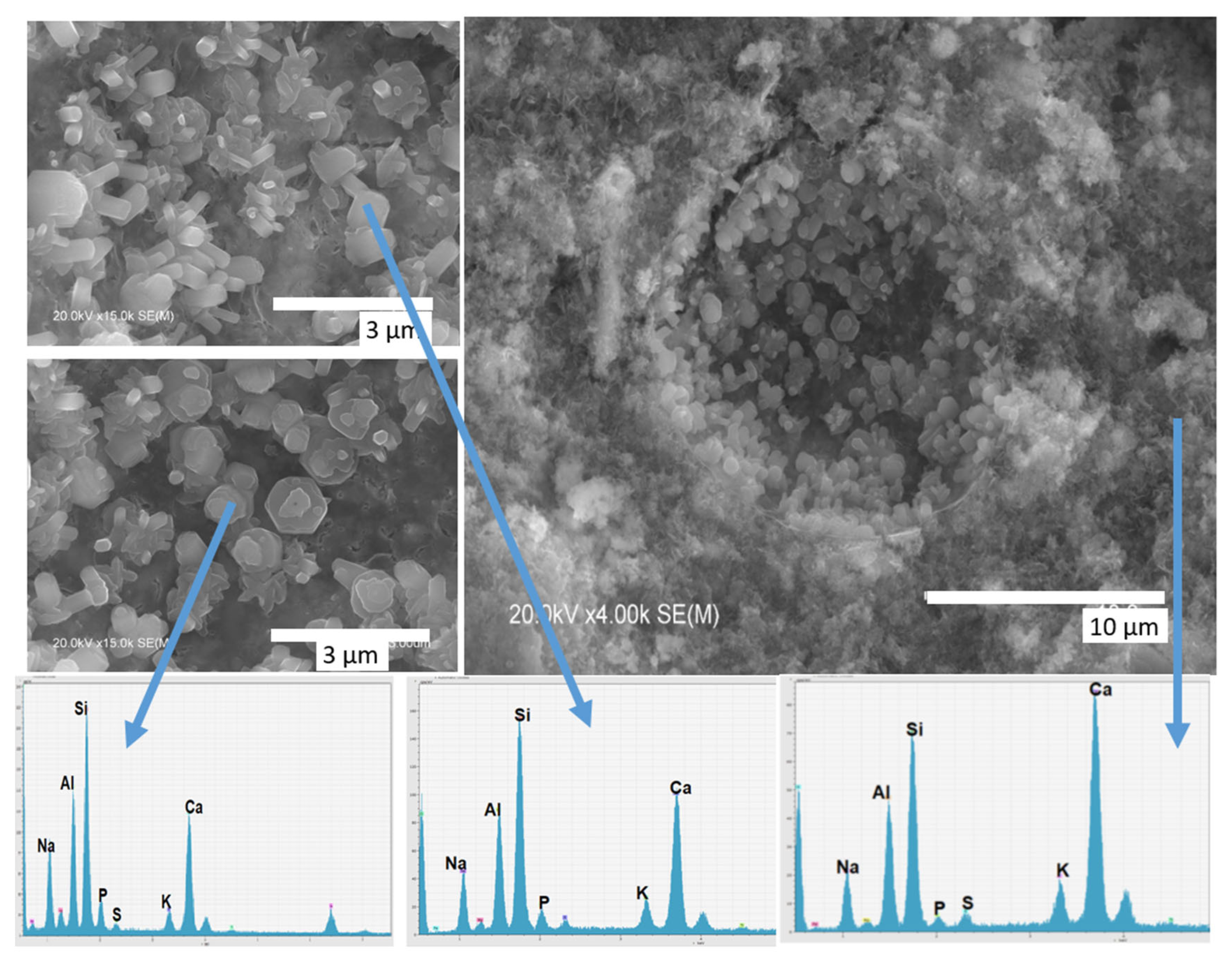
3.2.1. Characterisation of the Reaction Products
3.2.2. Leaching Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HTL | Hydrothermal liquefaction process |
| IMR | Inorganic mineral residue |
| XRD | X-ray diffraction |
| Power Diffraction File | |
| TG/DTG | Thermogravimetric analysis/derivative thermogravimetric Analyses |
| EDX | Energy-dispersive X-ray spectroscopy |
References
- Christopher Selvam, D.; Yuvarajan Devarajan Raja, T. Sewage sludge as a sustainable feedstock for biodiesel: Advances in conversion technologies and catalytic applications. Results Eng. 2025, 25, 104000. [Google Scholar] [CrossRef]
- Zhang, O.; Gao, L.; Li, W.; Xiao, L. Predicting sludge generation patterns carbon reduction potential under shared socioeconomic pathways. J. Environ. Manag. 2022, 322, 116088. [Google Scholar] [CrossRef]
- Aryanfar, Y.; Keçebas, A.; Nourbakhsh, A.S.; García, J.L.A.; Fernández, B.; Wu, W. Production of biodiesel from industrial sludge: Recent progress, challenges, perspective. Processes 2024, 12, 2517. [Google Scholar] [CrossRef]
- Agencia Europea de Medio Ambiente (AEMA). Urban Waste Water Treatment for 21st Century Challenges. Available online: https://www.eea.europa.eu/en/analysis/publications/urban-waste-water-treatment-for (accessed on 5 May 2025).
- Krause, M.J.; Bronstein, K. Estimating national sludge generation disposal from US drinking water wastewater treatment plants. J. Clean. Prod. 2024, 453, 142121. [Google Scholar] [CrossRef]
- Fan, Y.; Hornung, U.; Dahmen, N. Hydrothermal liquefaction of sewage sludge for biofuel application: A review on fundamentals, current challenges and strategies. Biomass Bioenergy 2022, 165, 106570. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Li, R.; Wu, Y. Hydrothermal Carbonization and Liquefaction of Sludge for Harmless and Resource Purposes: A Review. Energy Fuels 2020, 34, 13268–13290. [Google Scholar] [CrossRef]
- Guo, S.; Wu, Y.; Jia, Z.; Qi, X.; Wang, W. Sodium-based activators in alkali-activated materials: Classification and comparison. J. Build. Eng. 2023, 70, 106397. [Google Scholar] [CrossRef]
- Matsimbe, J.; Dinka, M.; Olukanni, D.; Musonda, I. Geopolypmer: A Systematic Review of Methodologies. Mater 2022, 15, 6852. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Fernández-Jiménez, A.; Chen, B.; Bing, L.; Leng, Z.; Dai, J.G. Low-carbon cementitious materials: Scale-up potential, environmental impact and barriers. Constr. Build. Mater. 2024, 455, 139087. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, O.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T.M. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Kryvenko, P.; Rudenko, I.; Sikora, P.; Sanytsky, M.; Konstantynovskyi, O.; Kropyvnytska, T. Alkali-activated cements as sustainable materials for repairing building construction: A review. J. Build. Engin. 2024, 90, 109399. [Google Scholar] [CrossRef]
- Rodríguez, E.; Bernal, S.; Mejía de Gutiérrez, R.; Puertas, F. Alternative concrete based on alkali-activated slag. Mater. Construcción 2008, 58, 53–67. [Google Scholar] [CrossRef]
- Haha, M.B.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast furnace slags. Cem. Concr. Res. 2012, 42, 74–83. [Google Scholar] [CrossRef]
- Fu, Q.; Bu, M.; Zhang, Z.; Xu, W.; Yuan, Q.; Niu, D. Hydration Characteristics and Microstructure of Alkali-Activated Slag Concrete: A Review. Res. Civ. Eng. Mater.―Rev. Eng. 2023, 20, 162–179. [Google Scholar] [CrossRef]
- Boakye, K.; Khorami, M. Impact of Low-Reactivity Calcined Clay on the Performance of Fly Ash-Based Geopolymer Mortar. Sustainability 2023, 15, 13556. [Google Scholar] [CrossRef]
- Hoyos-Montilla, A.; Tobón, J.I.; Puertas, F. Role of calcium hydroxide in the alkaline activation of coal fly ash. Cem. Concr. Compos. 2023, 137, 104925. [Google Scholar] [CrossRef]
- Ma, Y.; Gong, J.; Ye, G.; Fu, J. Effect of Activator and Mineral Admixtures on the Autogenous Shrinkage of Alkali-Activated Slag/Fly Ash. Sustainability 2023, 15, 16101. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Pontikes, Y.; Elsen, J.; Cizer, Ö. Comparing the reactivity of different natural clays under thermal and alkali activation. RILEM Tech. Lett. 2019, 4, 74–80. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhang, Z.; Ma, Y.; Wang, H. Recent progress of utilization of activated kaolinitic clay in cementitious construction materials. Compos. Part B Eng. 2021, 211, 108636. [Google Scholar] [CrossRef]
- Rivera, J.; Castro, F.; Fernández-Jiménez, A.; Cristelo, N. Alkali-activated cements from urban, mining and agro-industrial waste: State-of-the- art and opportunities. Waste Biomass Valorization 2021, 12, 2665–2683. [Google Scholar] [CrossRef]
- Occhipinti, R.; Fernández-Jiménez, A.; Palomo, A.; Tarantino, S.C.; Zema, M. Sulfate-bearing clay and Pietra Serena sludge: Raw materials for the development of alkali activated binders. Constr. Build. Mater. 2021, 301, 124030. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Neupane, K. Evaluation of environmental sustainability of one-part geopolymer binder concrete. Clean. Mater. 2022, 6, 100138. [Google Scholar] [CrossRef]
- Liu, M.; Dai, W.; Jin, W.; Li, M.; Yang, X.; Han, Y.; Huang, M. Mix proportion design and carbon emission assessment of high strength geopolymer concrete based on ternary solid waste. Sci. Rep. 2024, 14, 24989. [Google Scholar] [CrossRef] [PubMed]
- Cong, P.; Du, R.; Gao, H.; Chen, Z. Comparison and assessment of carbon dioxide emissions between alkali-activated materials and OPC cement concrete. J. Traffic Transp. Eng. 2024, 11, 918–938. [Google Scholar] [CrossRef]
- Amran, M.; Al-Fakih, A.; Chu, S.H.; Fediuk, R.; Haruna, S.; Azevedo, A.; Vatin, N. Long-term durability properties of geopolymer concrete: An in-depth review. Case Stud. Constr. Mater. 2021, 15, e00661. [Google Scholar] [CrossRef]
- Wong, L.S. Durability Performance of Geopolymer Concrete: A Review. Polymers 2022, 14, 868. [Google Scholar] [CrossRef]
- Paruthi, S.; Rahman, I.; Khan, A.H.; Sharma, N.; Alyaseen, A. Strength, durability, and economic analysis of GGBS-based geopolymer concrete with silica fume under harsh conditions. Sci. Rep. 2024, 14, 31572. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Understanding the Filler Effect on the Nucleation and Growth of C-S-H. J. Am. Ceram. Soc. 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- Poppe, A.M.; De Schutter, G. Cement hydration in the presence of high filler contents. Cem. Concr. Res. 2005, 35, 2290–2299. [Google Scholar] [CrossRef]
- Fernandez, R.; Martirena, F.; Scrivener, K.L. The origin of the pozzolanic activity of calcined clay minerals: A comparison between kaolinite, illite and montmorillonite. Cem. Concr. Res. 2011, 41, 113–122. [Google Scholar] [CrossRef]
- Danner, T.; Justnes, H. The Influence of Production Parameters on Pozzolanic Reactivity of Calcined Clays. Nord. Concr. Res. 2018, 59, 1–12. [Google Scholar] [CrossRef]
- Dannera, T.; Nordenb, G.; Justnes, H. Characterisation of calcined raw clays suitable as supplementary cementitious materials. Appl. Clay Sci. 2018, 162, 391–402. [Google Scholar] [CrossRef]
- D’Elia, A.; Pinto, D.; Eramo, G.; Laviano, R.; Palomo, A.; Fernández-Jiménez, A. Effect of Alkali Concentration on the Activation of Carbonate-High Illite Clay. Appl. Sci. 2020, 10, 2203. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Puertas, F.; Palacios, M.; Manzano, H.; Dolado, J.S.; Rico, A.; Rodríguez, J. A model for the C–A–S–H gel formed in alkali-activated slag cements. J. Eur. Ceram. Soc. 2011, 31, 2043–2056. [Google Scholar] [CrossRef]
- Liu, C.; Tao, Y.; Nie, S.; Chen, Y.; Li, Z.; Poon, C.S.; Ye, G. Dissolution of cations in C-(N,K-)A-S-H gels at the nanoscale. Compos. Part B Eng. 2025, 297, 112337. [Google Scholar] [CrossRef]
- Martín-Rodríguez, P.; García-Lodeiro, I.; Fernández-Carrasco, L.; Blanco-Varela, M.T.; Palomo, A.; Fernández-Jiménez, A. Artificial precursor for Alkaline Cements. Compos. Part B Eng. 2025, 296, 112216. [Google Scholar] [CrossRef]
- Su, Y.; Luo, B.; Luo, Z.; Xu, F.; Huang, H.; Long, Z.; Shen, C. Mechanical characteristics and solidification mechanism of slag/ fly ash-based geopolymer and cement solidified organic clay: A comparative study. J. Build. Eng. 2023, 71, 106459. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Escalante-García, J.I. Structural transition to well-ordered phases of NaOH-activated slag-metakaolin cements aged by 6 years. Cem. Concr. Res. 2022, 156, 106791. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Donatello, S.; Fernández-Jiménez, A.; Palomo, Á. Hydration of hybrid alkaline cement containing a very large proportion of fly ash: A descriptive model. Materials 2016, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patiño, C.L.; Zhang, Y. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int/teams/environment-climate-change-and-health/water-sanitation-and-health/chemical-hazards-in-drinking-water (accessed on 12 May 2025).
- United States Environmental Protection Agency. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 12 May 2025).
- Palomo, A.; Palacios, M. Alkali-activated cementitious materials: Alternative matrices for the immobilisation of hazardous wastes—Part, I.I. Stabilisation of chromium and lead. Cem. Concr. Res. 2003, 33, 289–295. [Google Scholar] [CrossRef]
- Zhang, J.; Provis, J.L.; Feng, D.; van Deventer, J.S.J. Geopolymers for immobilization of Cr6+, Cd2+, and Pb2+. J. Hazard. Mater. 2008, 157, 587–598. [Google Scholar] [CrossRef]
- Genua, F.; Lancellotti, I.; Leonelli, C. Geopolymer-Based Stabilization of Heavy Metals, the Role of Chemical Agents in Encapsulation and Adsorption: Review. Polymers 2025, 17, 670. [Google Scholar] [CrossRef]
- Sun, Z.; Li, X.; Gan, M.; Ji, Z.; Fan, X.; Xing, J. Converting Municipal Solid Waste Incineration Fly Ash and Municipal Sludge into Environmentally Compatible Alkali-Activated Material. Sustainability 2024, 16, 7912. [Google Scholar] [CrossRef]
- Sun, T.; Chen, J.; Lei, X.; Zhou, C. Detoxification and Immobilization of Chromite Ore Processing Residue with Metakaolin-Based Geopolymer. J. Environ. Chem. Eng. 2014, 2, 304–309. [Google Scholar] [CrossRef]
- Zhang, J.; Provis, J.L.; Feng, D.; Van Deventer, J.S.J. The Role of Sulfide in the Immobilization of Cr(VI) in Fly Ash Geopolymers. Cem. Concr. Res. 2008, 38, 681–688. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Zhou, S.; Lei, X. Reduction/Immobilization Processes of Hexavalent Chromium Using Metakaolin-Based Geopolymer. J. Environ. Chem. Eng. 2017, 5, 373–380. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Wang, H.; Zhou, S.; Wu, H.; Lei, X. Detoxification/Immobilization of Hexavalent Chromium Using Metakaolin-Based Geopolymer Coupled with Ferrous Chloride. J. Environ. Chem. Eng. 2016, 4, 2084–2089. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.; Huang, J.; Shi, H. Detoxification and Solidification of Heavy Metal of Chromium Using Fly Ash-Based Geopolymer with Chemical Agents. Constr. Build. Mater. 2017, 151, 394–404. [Google Scholar] [CrossRef]
- Ding, X.H.; Luo, B.; Zhou, H.T.; Chen, Y.H. Generalized solutions for advection–dispersion transport equations subject to time- and space-dependent internal and boundary sources. Comput. Geotech. 2025, 178, 106944. [Google Scholar] [CrossRef]




| Name | Binder (B) | Liquid Activator | L/B Ratio | Curing Conditions | |
|---|---|---|---|---|---|
| IMR (2 h, 800 °C) | % ArC (2 h, 800 °C) | ||||
| Ar1 | 0% | 100% | NaOH, 6 M | 0.48 | 20 h, 85 °C |
| Ar95 | 5% | 95% | NaOH, 6 M | 0.48 | 20 h, 85 °C |
| Ar90 | 10% | 90% | NaOH, 6 M | 0.55 | 20 h, 85 °C |
| Ar80 | 20% | 80% | NaOH, 6 M | 0.70 | 20 h, 85 °C |
| % Oxides | IMR-100 | IMR-800 | IMR-1000 | Ar-Original | Ar-800 |
|---|---|---|---|---|---|
| SiO2 | 5.30 | 14.77 | 15.78 | 37.83 | 48.99 |
| Al2O3 | 3.99 | 9.69 | 10.72 | 12.03 | 14.56 |
| CaO | 7.99 | 14.42 | 16.49 | 21.22 | 21.83 |
| MgO | 0.47 | 1.53 | 1.75 | 2.16 | 2.97 |
| Na2O | 0.06 | 0.44 | 0.56 | 0.33 | 0.55 |
| K2O | 0.38 | 0.72 | 0.82 | 2.64 | 3.03 |
| P2O5 | 10.65 | 22.24 | 24.03 | 0.07 | 0.09 |
| Fe2O3 | 14.99 | 25.08 | 28.04 | 5.79 | 5.87 |
| TiO2 | 0.31 | 0.54 | 0.59 | 0.86 | 0.77 |
| SO3 | 1.58 | 0.65 | 0.04 | 0.58 | 1.01 |
| Cl | 1.13 | 0.01 | — | 0.16 | 0.12 |
| MnO | 0.03 | 0.06 | 0.07 | 0.04 | 0.04 |
| ZnO | 0.28 | 0.41 | 0.46 | 0.01 | 0.011 |
| Others | 0.31 | 0.26 | 0.65 | 0.04 | 0.16 |
| * LoI | 52.53 | 9.18 | 0.00 | 18.19 | 0.00 |
| % Oxides | IMR-800 | AR1-800 | Ar95 | Ar90 | Ar80 |
|---|---|---|---|---|---|
| SiO2 + CaO + Al2O3 | 38.87 | 85.38 | 83.06 | 80.73 | 76.08 |
| CaO/SiO2 | 0.97 | 0.44 | 0.45 | 0.46 | 0.48 |
| Al2O3/SiO2 | 0.66 | 0.29 | 0.30 | 0.31 | 0.32 |
| Heavy Metal | Ar80 | 1 Acceptance Limits for Inert Materials | |
|---|---|---|---|
| 28 Days | Limit for Disposal in Landfill According to WHO | Limit for Drinking Water According to WHO | |
| As | (mg/L) < 0.005 | 0.05–0.25 mg/L | 0.01 mg/L (10 µg/L) |
| Pb | (mg/L) < 0.020 | 0.5–5.0 mg/L | 0.01 mg/L (10 µg/L) |
| Ni | 0.051 (mg/L) | 0.1–0.5 mg/L | 0.02 mg/L (20 µg/L) |
| Cu | 0.020 (mg/L) | 2 mg/L–5 mg/L | 2.00 mg/L |
| Cr | 0.485 (mg/L) | 0.1–1.0 mg/L | 0.05 mg/L (50 µg/L) |
| Zn | 0.067 (mg/L) | 3 mg/L–10 mg/L | 3.00 mg/L |
| Cd | (mg/L) < 0.010 | 0.01–0.2 mg/L | 0.003 mg/L (3 µg/L) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Jiménez, A.; Maltseva, O.; Palomo, A.; Marian, N.M.; Sturini, M.; Riccardi, M.P.; Zema, M.; Tarantino, S.C. Valorisation of Inorganic Fractions of Waste Generated by Hydrothermal Treatment of Sewage Sludge in Alkaline Cement. Sustainability 2025, 17, 5413. https://doi.org/10.3390/su17125413
Fernández-Jiménez A, Maltseva O, Palomo A, Marian NM, Sturini M, Riccardi MP, Zema M, Tarantino SC. Valorisation of Inorganic Fractions of Waste Generated by Hydrothermal Treatment of Sewage Sludge in Alkaline Cement. Sustainability. 2025; 17(12):5413. https://doi.org/10.3390/su17125413
Chicago/Turabian StyleFernández-Jiménez, Ana, Olga Maltseva, Angel Palomo, Narcisa Mihaela Marian, Michela Sturini, Maria Pia Riccardi, Michele Zema, and Serena C. Tarantino. 2025. "Valorisation of Inorganic Fractions of Waste Generated by Hydrothermal Treatment of Sewage Sludge in Alkaline Cement" Sustainability 17, no. 12: 5413. https://doi.org/10.3390/su17125413
APA StyleFernández-Jiménez, A., Maltseva, O., Palomo, A., Marian, N. M., Sturini, M., Riccardi, M. P., Zema, M., & Tarantino, S. C. (2025). Valorisation of Inorganic Fractions of Waste Generated by Hydrothermal Treatment of Sewage Sludge in Alkaline Cement. Sustainability, 17(12), 5413. https://doi.org/10.3390/su17125413









