Abstract
The sustainability of the natural gas-to-methanol (NGTM) and methanol-to-gasoline (MTG) processes are assessed in this systematic review as a potential substitute in the global energy transition. Methanol offers itself as a versatile and less carbon-intensive substitute for conventional gasoline in light of growing environmental concerns and the demand for cleaner fuels. This review’s rationale is to assess MTG’s ability to lessen environmental impact while preserving compatibility with current fuel infrastructure. The goal is to examine methanol and gasoline’s effects on the environment, society, and economy throughout their life cycles. This review used a two-phase systematic literature review methodology, filtering and evaluating studies that were indexed by Scopus using bibliometric and thematic analysis. A total of 25 documents were reviewed, in which 22 documents analyzed part of this study, and 68% employed LCA or techno-economic analysis, with the U.S. contributing 35% of the overall publications. A comparative analysis of the reviewed literature indicates that methanol-based fuels offer significantly lower greenhouse gas (GHG) emissions and life cycle environmental impacts than gasoline, particularly when combined with carbon capture and renewable feedstocks. This review also highlights benefits, such as improved safety and energy security, while acknowledging challenges, including high production costs, infrastructure adaptation, and toxicity concerns. Several drawbacks are high manufacturing costs, the necessity to adjust infrastructure, and toxicity issues. The report suggests investing in renewable methanol production, AI-driven process optimization, and robust legislative frameworks for integrating green fuels. The life cycle sustainability assessment (LCSA) of NGTM and MTG systems should be investigated in future studies, particularly in light of different feedstock and regional circumstances. The findings emphasize NGTM and MTG’s strategic role in aligning with several UN Sustainable Development Goals (SDGs) and add to the worldwide conversation on sustainable fuels. A strong transition necessitates multi-stakeholder cooperation, innovation, and supporting policies to fully realize the sustainability promise of cleaner fuels like methanol.
1. Introduction
Methanol and gasoline are two prominent fuels used in transportation and industry, each with unique characteristics, production processes, and environmental implications. Methanol has garnered attention as a sustainable alternative to gasoline due to its ability to reduce GHG emissions and its versatility in production methods, including pathways from natural gas, biomass, and even carbon dioxide. This review aims to explore the sustainability of methanol and gasoline from environmental, social, and economic perspectives, leading to a critical analysis of MTG process-based natural gas as a viable energy transition pathway.
1.1. Environmental Impact
Methanol demonstrates significant environmental advantages over gasoline. As a cleaner fuel, when produced via renewable pathways such as biomass gasification or CO2 hydrogenation using green hydrogen, it can reduce CO2 emissions by up to 95% compared to conventional gasoline. This substantial reduction is possible because the carbon used in these processes is biogenic or recycled, meaning it does not contribute additional fossil carbon to the atmosphere. Methanol also significantly reduces NOx and SOx emissions, resulting in improved air quality and a mitigated impact on climate change. Methanol’s production process generates fewer particulates, making it less damaging to both the environment and human health. Moreover, advancements in carbon capture technologies and renewable energy integration can further enhance methanol’s sustainability profile [1].
However, certain challenges persist. Methanol’s high solubility in water raises concerns about its impact on aquatic ecosystems, where accidental spills could lead to water contamination. Additionally, methanol’s indirect contribution to global warming through hydroxyl radical depletion underscores the need for careful management and further research into minimizing these effects [2].
In contrast, gasoline’s environmental footprint remains a significant challenge. The combustion of gasoline releases approximately 19 pounds of CO2 per gallon, making it a major contributor to global GHG emissions. Gasoline production is also linked to widespread air, water, and soil pollution. Refining processes often generate harmful byproducts, while oil spills and leaks cause long-term damage to ecosystems. The lower heating value (LHV) of methanol is around 12–16 MJ/kg [3], whereas that of gasoline is approximately 42–44 MJ/kg [4]. This means that more methanol is needed to generate the same amount of energy. However, the amount of CO2 released during the burning of methanol derived from renewable sources is far lower, even when adjusted per unit of energy. Despite efforts to improve the sustainability of gasoline through advanced refining techniques, its inherent environmental drawbacks make the case for exploring alternative fuels such as methanol [5].
1.2. Social Effects
The social implications of fuel production and use are critical in evaluating sustainability. Methanol offers several social benefits compared to gasoline. Its lower toxicity reduces risks associated with storage and transport, making it safer for workers and communities. Methanol’s ability to be produced from diverse feedstocks, including biomass and waste carbon dioxide, can help reduce dependence on imported petroleum, thereby enhancing national energy resilience. This adaptability is particularly valuable for nations seeking to diversify their energy portfolios and strengthen their resilience against geopolitical risks [6].
However, there are significant health and safety risks associated with methanol due to its high toxicity, water solubility, and corrosive nature [7]. Accidental exposure can have serious health consequences, such as blindness or death, particularly if it occurs through ingestion or inhalation. Due to its corrosive nature, storage tanks, pipes, and transportation systems must adhere to stringent material compatibility standards. As a result, although methanol offers flexibility in production, it must be handled with strict safety procedures and containment measures to minimize risks to employees and the community.
Furthermore, the global methanol industry supports a range of employment opportunities, from production to downstream applications. Methanol plants create jobs in manufacturing, transportation, and chemical industries, contributing to economic stability in both developed and developing regions. This positive social impact contrasts sharply with the challenges posed by gasoline production.
Gasoline’s reliance on crude oil fosters geopolitical tensions, as supply chains are often vulnerable to conflicts and price volatility. The production and refining of gasoline expose workers and local communities to hazardous pollutants, increasing risks of respiratory illnesses and other health problems. High-profile incidents, such as oil spills and refinery explosions, underscore the dangers associated with gasoline production and highlight the need for safer alternatives [8].
1.3. Economic Growth
Methanol’s expanding market reflects its potential as a key player in the transition to sustainable energy. Valued at USD 38 billion in 2023, the methanol market is projected to grow to USD 56.2 billion by 2033, driven by increasing demand for cleaner fuels and industrial applications. The flexibility of methanol production, utilizing domestic feedstocks such as natural gas and biomass, enhances energy independence while creating economic opportunities at the local level. Governments and industries investing in methanol production also benefit from job creation and supporting infrastructure development [9].
Nevertheless, the economic feasibility of methanol is influenced by production costs, particularly for renewable pathways. Advances in technologies like carbon capture and green hydrogen integration are expected to reduce costs, making renewable methanol more competitive in the global market. In contrast, gasoline remains deeply entrenched in the global economy. With a market size of USD 128.8 billion in 2023, gasoline continues to dominate transportation fuel demand. However, its price volatility and environmental costs underscore the need for alternatives like methanol. MTG process-based natural gas offers a promising solution by leveraging methanol to produce gasoline-compatible fuels, providing a pathway to reduce dependency on crude oil while meeting existing infrastructure demands [10].
1.4. Objective and Rationale of the Review
The transition to sustainable energy sources necessitates a thorough evaluation of NGTM and the MTG processes. Methanol’s environmental benefits, including lower GHG emissions and reduced air pollution, position it as a strong alternative to traditional fuels. Socially, its safer handling, reduced toxicity, and contribution to energy security make it an appealing option for governments and industries aiming to mitigate the negative impacts of fossil fuels. Economically, methanol’s growing market potential and flexibility in production pathways highlight its viability as a key player in energy transitions [11].
Despite these advantages, the adoption of methanol is not without challenges. Production costs, particularly for renewable methanol, remain higher than those of conventional fuels. Infrastructure for large-scale methanol use and distribution requires significant investment and adaptation. Public perception and regulatory frameworks also play crucial roles in determining methanol’s future [12]. Similarly, while promising, MTG process-based natural gas must address issues such as energy efficiency, scalability, and lifecycle emissions to align with global sustainability goals [13].
This review explores these dimensions better to understand methanol’s potential in decarbonizing energy systems. By examining environmental, social, and economic factors, this study aims to identify the opportunities and barriers to integrating methanol into existing energy frameworks. The findings will contribute to the broader discourse on sustainable energy solutions and inform strategies for achieving global decarbonization targets.
2. Literature Review
2.1. Global Assessment of the Methanol and Gasoline Industries’ Sustainability
This review offers a thorough literature evaluation with the goal of pointing out methodological and application-oriented flaws in the subject. This systematic review follows the PRISMA 2020 guidelines and has been registered with the Open Science Framework (OSF) under the registration ID: 24vtq. The registration details are publicly available at https://osf.io/24vtq/ (accessed on 9 May 2025). Figure 1 illustrates the research framework of the global systematic review process. Targeted keywords and filtering strategies were used to find pertinent publications in the Scopus database. This review was carried out using a two-phase, systematic methodology. The first phase comprised a thorough review of the body of existing research to ascertain the total number of studies addressing sustainability concerns pertaining to the methanol and gasoline industries. The advanced query search was (TITLE-ABS-KEY (methanol) AND TITLE-ABS-KEY (gasoline) AND TITLE-ABS-KEY (sustainability) OR TITLE-ABS-KEY (sustainable) AND TITLE-ABS-KEY (life AND cycle), and was employed in the initial stage to look for publications formed since the original work was published. In the first stage, 25 pertinent publications were gathered, including journal articles, conference proceedings, and review papers. The second phase carried out a thorough analysis of 22 chosen sources. Numerous criteria, such as author names, publication year, affiliated nations, research methodology, systems studied, research scope, and publication formats, were used to systematically classify these documents. Due to methodological heterogeneity—particularly differences in system boundaries, functional units, and data assumptions—a comparative synthesis was conducted through qualitative rather than quantitative alignment. Key insights were derived by grouping studies based on their analytical focus, modeling scope (e.g., cradle-to-gate, well-to-wheel), and integration of sustainability indicators. This approach enabled the identification of cross-cutting themes and prevailing best practices across the reviewed literature. Table 1 presents the bibliometric analysis of methanol and gasoline products and their relevant sustainability studies from the Scopus database.
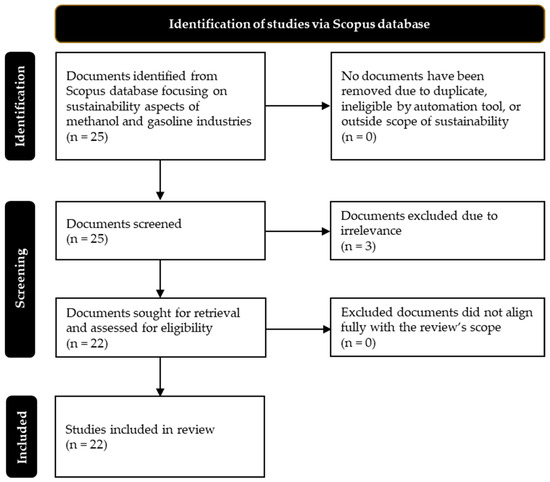
Figure 1.
Framework of the global systematic review process following PRISMA 2020 guidelines.

Table 1.
Bibliometric analysis of methanol and gasoline products with sustainability studies from the Scopus database.
To ensure that the literature included in this study was relevant to the broader MTG sector, an automated filtering method was initially applied to identify thematically aligned publications. However, most of the retrieved papers focused on specific application domains—such as automotive and vehicles, carbon utilization, alternative fuels, energy systems, biorefineries, biofuels, and hydrogen technologies—rather than addressing broader sustainable development metrics. To refine the dataset and maintain focus, a second manual screening phase was implemented to exclude studies falling outside the scope of this work.
This research concentrates on assessing technological developments within MTG process-based natural gas. As such, studies limited to technical materials, safety or risk assessments, and life cycle assessment (LCA)-based evaluations of biofuels were excluded. The aim was to preserve a clear focus on MTG’s potential contribution to sustainable energy transitions.
Figure 2 displays the yearly distribution of publications related to NGTM and MTG sustainability from 2002 to 2024, as indexed in SCOPUS. Early outputs were sporadic, with minor peaks in 2002, 2007, and 2009. After a quiet period, research began to gain traction around 2017. From 2019 onward, publication activity increased significantly, stabilizing at a higher level, with 2–3 publications annually. This trend indicates a recent and growing academic focus on the sustainability of NGTM and MTG pathways in cleaner energy transitions.
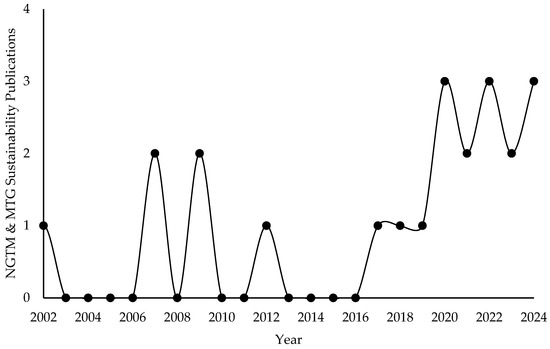
Figure 2.
Annual trends in methanol and gasoline sustainability publications indexed in SCOPUS (2002–2024).
The bibliometric analysis results presented in Figure 3a–d offer a multidimensional view of the academic landscape. The geographical breakdown shows the United States as the leading contributor to MTG-related research, accounting for 35% of total publications. Switzerland follows with 10%, while Canada, China, Germany, and the United Kingdom each contribute 6%. Other countries collectively represent 31%, reflecting widespread yet regionally concentrated research efforts.
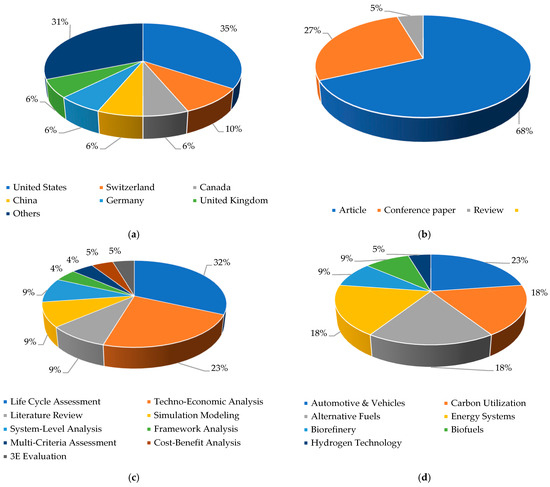
Figure 3.
Bibliometric analysis of methanol and gasoline sustainability studies: Insights by (a) country, (b) type of document, (c) assessment methodology, and (d) thematic focus.
In terms of document type, journal articles dominate the field, comprising 68% of the reviewed publications. Conference papers account for 27%, illustrating the presence of ongoing professional dialogue, while review articles make up the remaining 5%. This distribution indicates strong academic engagement supported by a solid foundation of original research.
Methodologically, the literature heavily favors quantitative and system-level approaches. LCA and techno-economic analysis are the most prevalent, representing 32% and 23%, respectively. Additional contributions include simulation modeling, system-level analysis, and literature reviews (each 9%), with smaller shares dedicated to framework analysis, cost–benefit analysis, and 3E evaluation. This highlights a clear preference for holistic and evaluative tools in MTG-based natural gas research.
Regarding the thematic scope of application, the most prominent areas include automotive and vehicles (23%), followed closely by carbon utilization, alternative fuels, and energy systems (each at 18%). Less frequent attention is given to sectors such as biofuels, biorefineries, and hydrogen technologies. This distribution demonstrates a broad yet uneven focus, emphasizing the versatility of MTG technology across sectors while also identifying areas with untapped research potential.
2.2. NGTM and MTG Processes and Supply Chain
2.2.1. Natural Gas to Methanol
The conversion of natural gas to methanol involves three main stages: feedstock preparation, syngas production, and methanol synthesis. Natural gas, primarily composed of methane (CH4), undergoes purification to remove impurities such as sulfur compounds, carbon dioxide, and nitrogen. These impurities are harmful to downstream catalysts and are removed using chemical absorption techniques, typically amine-based solutions [36]. The purified gas, with methane content exceeding 95%, is then processed to produce syngas via steam reforming or autothermal reforming. Steam reforming involves the reaction of methane with steam over nickel catalysts at high temperatures (700–1000 °C) and pressures, generating a blend of hydrogen (H2) and carbon monoxide (CO). Autothermal reforming combines partial oxidation and steam reforming, offering enhanced energy efficiency and operational flexibility [37]. Finally, syngas is compressed and catalytically converted into methanol using copper-based catalysts under moderate conditions (200–300 °C, 50–100 bar), with water as a byproduct [38].
The supply chain of methanol begins with natural gas extraction and transportation to processing facilities. Purified natural gas is converted into methanol at large-scale production plants. Methanol integrates seamlessly into existing chemical and fuel supply chains as a versatile feedstock. It is stored and transported using specialized tanks and infrastructure, ensuring safety and efficiency. With global production exceeding 70 million metric tons annually, methanol plays a vital role in various applications, including fuels, petrochemicals, and renewable energy initiatives [39].
2.2.2. Methanol to Gasoline
The MTG process transforms methanol into gasoline-range hydrocarbons through catalytic reactions. Methanol is vaporized, superheated, and fed into reactors, where it is converted into dimethyl ether (DME) and light olefins. To produce gasoline, these olefins further oligomerize into higher hydrocarbons, including paraffins, naphthenes, and aromatics. ExxonMobil’s fluid-bed MTG technology optimizes the process, reducing capital and operational costs while enhancing scalability. The resulting gasoline meets modern fuel standards, featuring low sulfur and benzene content, making it suitable for direct use or blending [10].
The supply chain for MTG-produced gasoline integrates with the existing fuel distribution infrastructure. Methanol from natural gas or renewable sources is transported to MTG plants for conversion. The gasoline product is then distributed through traditional channels, including pipelines, tankers, and retail outlets. The MTG process reduces reliance on crude oil, diversifies feedstock sources, and contributes to a sustainable energy future. Its adaptability to renewable methanol sources further enhances its environmental and economic benefits [39].
Figure 4 briefly illustrates the NGTM and MTG process chain.
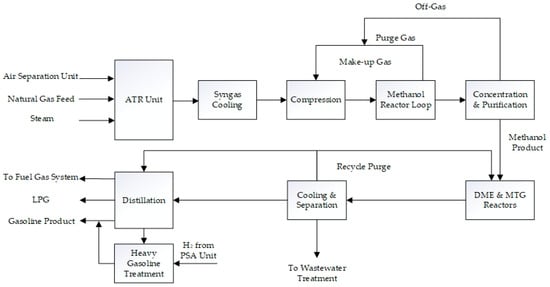
Figure 4.
Block flow diagram of the NGTM and MTG process chain.
2.3. Comparative Fuel Characteristics of Methane, Methanol, and Gasoline
Assessing the suitability of prospective fuels for energy transition techniques requires an understanding of their physicochemical characteristics. Methanol, gasoline, and natural gas (mostly methane) are essential components in continuous fuel diversification initiatives. A comparison of their main characteristics is shown in Table 2, with a focus on performance indicators that affect storage, combustion behavior, compatibility with infrastructure, and environmental impact.

Table 2.
Key physical and chemical properties of natural gas (as methane), methanol, and gasoline.
Due to its high-octane rating and natural oxygen content, methanol burns cleaner and more completely than other fuels. However, due to its poor energy density, more fuel is needed to produce the same amount of energy. Although methane has a high calorific value and remarkable knock resistance, its gaseous form and cryogenic boiling point make storage and transportation extremely difficult. On an energy-equivalent basis, gasoline remains the most carbon-intensive fuel, despite having the highest energy content of any liquid fuel and being fully compatible with the existing distribution system.
These trade-offs illustrate the need for transitional fuel technologies such as NGTM and MTG. NGTM enables the conversion of methane into a liquid fuel with enhanced usability, while MTG further processes methanol into gasoline-compatible hydrocarbons. This conversion pathway retains environmental benefits while leveraging existing gasoline infrastructure, making it a technically and economically viable option in the global shift toward cleaner transportation fuels.
2.4. Research Gap Identified
A thorough review of the literature found that the majority of international research on energy sources focuses on industries including power generation, transportation, and MTG production. However, research explicitly examining the LCA of MTG generated from natural gas as a practical, sustainable, and environmentally responsible substitute for traditional fuels is conspicuously lacking. A thorough LCSA of the MTG value chain, from methanol manufacturing to gasoline conversion, final distribution, and consumption, has also not been carried out in any research.
Furthermore, compared to conventional fuel sources, the MTG pathway has shown better social, economic, and environmental performance, resulting in less pollution and better outcomes. Despite this, no cohesive literature study currently incorporates the best practices for MTG supply chain development. Transportation logistics, supply route selection, refueling station infrastructure, energy resiliency, MTG safety and sustainability measures, policy-driven methods for sustainable development, and general process optimization are all included in this review.
3. Methanol and Gasoline Uses in the World
Methanol ranks among the top five most traded chemicals at the worldwide level due to its primary application as a chemical feedstock for the production of compounds such as dimethyl ether, acetic acid, formaldehyde, and methyl tertiary butyl ether (MTBE) [49], which are critical for the production of a variety of products, such as antifreeze, paints, plastics, silicones, adhesives, and resins [50]. Methanol’s versatility stretches to energy applications since it is categorized as the most attractive alternative fuel because of its plentiful availability, low expense, and eco-friendly combustion properties. Therefore, methanol currently functions as a competitive fuel for engines in multiple marine and vehicle organizations [51], as well as for internal combustion engines due to its higher oxygen concentration and lower carbon proportion, leading to more complete combustion [52]. This enables decreased environmental emissions of nitrogen oxides (NOx), carbon dioxide (CO2), and soot. On the other hand, internal combustion engines powered by traditional fossil fuels constitute a considerable environmental hazard by releasing substantial amounts of pollutants that play a role in global warming and the overall depletion of natural resources [53].
Moreover, methanol presents a channel for cleaner energy solutions as it contributes to the production of hydrogen for fuel cells, which is regarded as a clean fuel because it restrictively produces heat, electricity, and water. This is achieved via methanol reforming, particularly methanol steam reforming, because it achieves the optimum quantity of hydrogen [54]. Furthermore, methanol’s role in the methanol-to-olefins (MTO) process accentuates its relevance in producing light olefins. Methanol can be synthesized from syngas (CO/CO2/H2), which can be extracted from various feedstocks or alternative carbon sources, such as coal, natural gas, biomass, CO2, and municipal waste, and the MTO process is acknowledged as one of the most promising non-petroleum pathways for producing propylene and ethylene. These are two fundamental components of the petrochemical industry that contribute significantly to generating fine chemicals and polymers, including polyethylene and polypropylene [55].
A total of 124 nations revealed their intentions to become carbon neutral and reach net-zero carbon emissions by 2050 or 2060 as of February 2021 [56], and GHGs that are key contributors to climate change, such as carbon dioxide and methane, are minimized when methanol undergoes combustion. Additionally, reduced amounts of the harmful compounds and particulates that contribute to smog are produced. Methanol is an elastic fuel that can be adopted for a variety of applications, such as heating, cooking, generating electricity, and automobile operation. As a consequence, the use of methanol as a source of energy could greatly reduce carbon emissions while at the same time encouraging the future transition to cleaner, more environmentally friendly energy sources [57].
Adopting methanol as a feedstock plays a role in the production of high-octane, gasoline-range hydrocarbons at reduced costs through the MTG mechanism [58]. MTG represents a technically feasible pathway for generating renewable gasoline [59], as approximately 40% of the energy consumed in transportation around the world is allocated to lead passenger vehicles, which are mainly driven by gasoline-powered spark ignition (SI) engines, and the daily universal requirement for gasoline exceeds 4.8 billion liters [60], which emphasizes its widespread application and essential function in transportation.
One of the main energy supplies for the international transportation sector is fossil fuels, specifically gasoline [61]. Furthermore, gasoline is crucial for operation in piston-type aircraft engines, with nearly 230,000 piston-powered aircraft depending on 100 low lead (100LL) aviation gasoline (AVGAS) for effective operation [62]. This is the most prevalent and well-known type of aviation gasoline on a global scale in regards to production and consumption [63].
Moreover, additives like ethanol are often blended with gasoline to enhance performance and decrease pollutants. According to studies, the most efficient additives for gasoline fuel at the present time are alcohols, mainly ethanol. Better octane ratings and significant decreases in GHG emissions are only two of the many benefits of these additions. Crucially, relying on them does not seem to result in any potential hazards to the surroundings [64]. For instance, E10, a renowned ethanol-to-gasoline blend that is made up of 10% ethanol and 90% gasoline, continues to rise in popularity and now makes up over 98% of gasoline in the US [65]. In conclusion, gasoline is necessary for almost every type of transportation, including cars and light aircraft, to enable them to keep operating universally.
4. Challenges, Opportunities, and Future Directions for Sustainable Fuel Transition
As global energy demands increase and environmental concerns grow, the shift from traditional fossil fuels to cleaner alternatives has become imperative. Methanol and gasoline, despite their challenges, remain key players in the transition to sustainable transportation fuels. This chapter explores their challenges, opportunities, and strategic pathways, integrating diverse perspectives such as innovation, artificial intelligence (AI), and alignment with SDGs.
4.1. Challenges in Using Methanol and Gasoline as Fuels
Methanol and gasoline face a variety of challenges as transportation fuels. Methanol, while promising due to its clean-burning properties, has a lower energy density than gasoline, leading to reduced mileage and requiring frequent refueling [66]. Its corrosive nature necessitates modifications to storage, distribution, and vehicle systems, increasing costs. Moreover, methanol’s toxicity raises concerns about handling and accidental exposure.
On the other hand, gasoline is deeply entrenched in the global energy infrastructure, making it difficult to displace despite its environmental drawbacks. The production and consumption of gasoline contribute significantly to GHG emissions, air pollution, and reliance on finite crude oil reserves. These challenges necessitate innovative approaches to optimize the use of both fuels while mitigating their adverse impacts [67].
4.2. Selecting Sustainable Supply Options
The supply options for methanol and gasoline have profound implications for sustainability. Currently, most methanol is produced from natural gas, a carbon-intensive process. However, emerging technologies offer greener alternatives, such as CO2 hydrogenation and biomass gasification. These pathways enable the integration of renewable energy, reducing the carbon footprint of methanol production [68].
For gasoline, blending biofuels such as ethanol or biodiesel in the supply chain can help reduce its environmental impact. Policymakers must prioritize LCA to determine the most sustainable supply options, balancing production costs, environmental impact, and feedstock availability. Investments in carbon-neutral pathways, such as recycling captured CO2 in fuel production, can redefine supply chains for both methanol and gasoline [69].
4.3. Technological Innovation and Design Limitations
Innovation is crucial to overcoming the design limitations associated with methanol and gasoline. Methanol engines require corrosion-resistant materials and system adaptations to accommodate its chemical properties. Advanced fuel injection systems, dual-fuel engines, and hybrid configurations are potential solutions to enhance performance and efficiency [70].
Gasoline engines, although mature, can benefit from innovations such as turbocharging, variable compression ratios, and hybridization to reduce emissions and improve fuel economy. To maximize these advancements in practical contexts, AI is being increasingly utilized. For example, real-time combustion analysis and adaptive regulation are now possible with AI-powered engine control units, which enhance engine response while reducing pollutants and fuel consumption. Companies like Siemens and General Motors have demonstrated increased engine dependability and decreased downtime in large fleet operations by applying machine learning algorithms to predictive maintenance systems [71]. AI-driven predictive maintenance systems and real-time performance optimization can further enhance engine longevity and reduce operational costs [72].
Along with current modifications, new research suggests that methanol-compatible fuel cells and microturbine technologies could be developed to circumvent the constraints of internal combustion completely. These systems have the potential to reduce emissions and increase energy efficiency, especially in stationary or industrial applications. Additionally, AI has been utilized in digital twin models for methanol pilot plants, such as those developed in Europe as part of the Horizon 2020 program [73]. In these models, neural networks are utilized to simulate and optimize process parameters under dynamic conditions, including catalyst activity, reaction temperatures, and energy input. Furthermore, pilot methanol plants are increasingly integrating digital twin models and machine learning algorithms to optimize energy consumption, feedstock utilization, and emissions management in real time. These developments mark a paradigm change toward smarter, more flexible, and cleaner fuel systems rather than merely minor enhancements. To accelerate this shift, research into reliable AI platforms that integrate engine dynamics with emissions performance is crucial [74].
4.4. Energy Security and Resilience
Energy security remains a critical concern for nations seeking to reduce dependency on foreign oil. Methanol’s flexibility as a feedstock-derived fuel, capable of being produced from natural gas, coal, or biomass, enhances its role in diversifying energy sources [75]. Countries rich in renewable resources can harness solar, wind, and hydro energy to produce green methanol, ensuring a reliable domestic supply.
While currently dominant, gasoline poses energy security risks due to geopolitical volatility in oil-producing regions. Integrating renewable components and reducing reliance on imports can mitigate these risks [76]. A dual approach leveraging methanol’s flexibility and gasoline’s established infrastructure can strengthen national energy resilience.
4.5. Sustainability and Safety Considerations
The environmental sustainability of methanol and gasoline hinges on reducing their lifecycle emissions. Methanol offers a lower carbon footprint during combustion and can align with circular economy principles through CO2 recycling. However, methanol’s toxicity presents a significant safety challenge that surpasses many other fuels, including gasoline and natural gas. Stringent handling and operational standards are essential to ensure its safe use [77]. Methanol is considerably more toxic than ethanol and biodiesel, both of which are generally regarded as safer alternatives in terms of human exposure and environmental impact. Ethanol’s lower toxicity allows its widespread use in blends like E10 and E85, while biodiesel—produced from vegetable oils or animal fats—is biodegradable and non-toxic [78]. Dimethyl ether (DME), a derivative of methanol, also offers reduced toxicity and favorable combustion behavior [79].
In comparison to gasoline, methanol burns cleaner with fewer emissions, but poses a greater toxicological risk upon ingestion or prolonged exposure. Gasoline, although flammable and carcinogenic due to compounds like benzene, is less acutely toxic than methanol in small exposures and has an extensively developed handling framework. Natural gas (primarily methane) is less toxic overall but introduces asphyxiation and explosion hazards, particularly in confined spaces [80].
These comparisons highlight the necessity for methanol-specific safety interventions, including robust containment systems, real-time leak detection, and comprehensive emergency response planning. Its adoption as a sustainable fuel must, therefore, be balanced against its higher toxicity risks relative to other conventional and alternative fuels.
Gasoline’s sustainability is tied to incorporating renewable additives and improving fuel efficiency. Its widespread infrastructure provides an advantage, but emissions from production and use continue to harm the environment. Developing advanced safety protocols and adopting cleaner technologies are essential to address these concerns [81].
4.6. Aligning with Sustainable Development Goals (SDGs)
The combined use of NGTM and MTG technologies plays a vital role in supporting the global transition toward more sustainable and responsible energy systems. These fuel pathways not only offer technical and economic advantages but also contribute meaningfully to several of the UN SDGs. SDG mapping serves as a strategic tool for aligning industrial practices with sustainability’s environmental, social, and economic pillars. For NGTM and MTG systems, SDG alignment provides a framework to evaluate lifecycle impacts, inform policy and industry decisions, and drive innovation in low-emission fuel production.
This alignment is explored through three core dimensions: environmental impact, human impact, and economic impact, and is supported by multi-stakeholder cooperation among industry, academia, and government.
4.6.1. Environmental Impact: Eco-Friendly Practices and Climate Action
NGTM and MTG processes, when optimized with sustainable technologies, offer significant potential for environmental enhancement. NGTM enables the production of low-carbon methanol by integrating carbon capture, utilization, and storage (CCUS) systems, reducing CO2 emissions at the source. Methanol is further converted into gasoline via the MTG process, which creates a synthetic fuel alternative with clean combustion and renewable energy inputs, and with a lower lifecycle carbon footprint [82].
Water conservation is prioritized through zero-liquid discharge, rainwater harvesting, and advanced wastewater treatment. Biodiversity and land preservation are achieved by minimizing land-use change, enforcing recycling policies, and implementing emissions control measures. Furthermore, AI-driven predictive maintenance and energy-saving protocols support overall operational efficiency and waste reduction. These comprehensive sustainability strategies align with
- SDG 6: Ensuring universal access to clean water sources and sufficient sanitation;
- SDG 7: Increasing access to affordable, sustainable, and dependable energy;
- SDG 9: Encouraging technical innovation, industrial growth, and robust infrastructure;
- SDG 13: Acting quickly to mitigate the effects of climate change;
- SDG 14: Preserving maritime biodiversity and marine habitats;
- SDG 15: Promoting sustainable land use and protecting terrestrial ecosystems.
4.6.2. Human Impact: Health, Safety, and Community Development
The successful deployment of NGTM and MTG technologies must also prioritize human well-being. Advanced emission control devices reduce harmful pollutants such as NOx, SOx, and VOCs, improving air quality and public health outcomes [83]. Worker safety is ensured through comprehensive occupational health programs and adherence to international labor standards.
The facilities are expected to contribute positively to surrounding communities by supporting environmental awareness campaigns, local infrastructure development, and educational outreach. Investments in green technology training help build a skilled workforce capable of operating and maintaining sustainable fuel systems. These efforts reflect alignment with
- SDG 3: Promoting general well-being and ensuring healthy lives for people of all ages;
- SDG 4: Promoting high-quality, inclusive, and equitable education for all;
- SDG 8: Facilitating access to productive employment and inclusive economic growth;
- SDG 11: Encouraging inclusive, resilient, and ecologically sustainable urban growth.
4.6.3. Economic Impact: Growth, Innovation, and Responsible Business Practices
Economically, NGTM and MTG plants are drivers of industrial diversification, job creation, and regional development. They offer new professional opportunities in engineering, operations, and environmental compliance, while promoting safe, fair, and well-compensated employment. The facilities adopt circular economy principles, such as waste reuse, energy recovery, and lean supply chains, leading to cost savings and resource efficiency [84].
Moreover, collaboration with academic institutions, certification under standards like ISO 14001, and active participation in global sustainability programs ensure long-term responsible growth. The MTG segment, in particular, contributes to fuel security by converting domestic methanol into gasoline, thus enhancing energy resilience and economic independence. These practices support
- SDG 8: Encouraging sustainable economic growth and equitable job opportunities;
- SDG 12: Promoting sustainable patterns of production and consumption as well as effective resource use;
- SDG 17: Increasing international cooperation and partnerships to accomplish sustainable goals.
4.6.4. Strategic SDG Mapping and Integration
The selection of SDGs for NGTM–MTG integration was guided by three sustainability lenses [85]:
- Environmental Dimension: Technologies that support emissions reduction, clean energy generation, and natural resource conservation (SDGs 6, 7, 13, 14, 15).
- Social Dimension: Initiatives aimed at health, education, workforce safety, and community development (SDGs 3, 4, 8, 11).
- Economic Dimension: Emphasis on innovation, productivity, circular economy adoption, and global cooperation (SDGs 8, 9, 12, 17).
Both NGTM and MTG technologies demonstrate a robust alignment with the United Nations SDG framework. These fuel pathways not only enhance clean energy availability (SDG 7) and support sustainable industrial infrastructure (SDG 9), but also play a pivotal role in climate change mitigation (SDG 13). Their successful integration into national and global energy strategies will require collaborative efforts across policy, research, and private sectors.
4.7. Sustainable Development Strategies
A comprehensive strategy for sustainable fuel development should focus on the following:
- Research and Development: Investments in renewable methanol production technologies, such as solar-driven CO2 hydrogenation, can significantly lower carbon intensity.
- Policy Support: Governments should establish incentives for renewable methanol production and mandate blending ratios for biofuels in gasoline.
- Public Awareness: Educating stakeholders on the benefits of alternative fuels and addressing safety concerns are critical for consumer acceptance.
- Global Collaboration: Sharing best practices and fostering international partnerships can accelerate the adoption of sustainable fuel technologies.
4.8. Policymakers’ Role and Opportunities
Policymakers are uniquely positioned to drive the transition to sustainable fuels. By implementing carbon pricing, supporting renewable fuel infrastructure, and setting stringent emission standards, they can create a conducive environment for methanol and gasoline alternatives [86]. Programs to subsidize green methanol production and incentivize private-sector investment in clean technologies are particularly impactful.
Additionally, aligning fuel policies with international climate agreements, such as the Paris Accord, ensures accountability and progress toward long-term sustainability goals.
4.9. Future Research Directions
Research efforts should prioritize addressing key challenges and exploring untapped opportunities. Areas for future investigation include the following:
- AI in Fuel Systems: Leveraging AI for predictive analytics in engine design, fuel optimization, and supply chain management. To enable smart engines that maximize both fuel economy and emission profiles, future research should investigate AI models that integrate real-time combustion metrics with environmental outcomes. It is also possible to investigate applications in autonomous fuel management systems.
- Carbon-Neutral Pathways: Advancing CO2 capture technologies and integrating renewable hydrogen production for green methanol synthesis. Decentralized, small-scale methanol production facilities utilizing modular electrolyzers and carbon capture systems should be considered in future research. In isolated or off-grid areas, these could offer local green fuel production.
- Circular Economy Models: Exploring the feasibility of fully recycling CO2 into fuel production. Sustainability standards may be redefined by closed-loop methanol production, in which CO2 released during combustion is absorbed and recycled. Pilot demonstration projects and complete techno-economic validation are needed for this concept.
- Energy-Efficient Infrastructure: Developing materials and systems to reduce energy losses in fuel storage, transport, and use. Infrastructure innovation should prioritize the use of phase-change thermal buffers, nanocomposites, and enhanced insulation materials to reduce energy loss during transportation.
- Safety Innovations: Designing next-generation handling and containment systems to mitigate methanol’s toxicity and flammability risks. Emerging safety technologies, including predictive hazard analytics, autonomous valve shutoff systems, and AI-based leak detection, can significantly improve safety in storage and distribution. It is necessary to validate these by modeling high-risk scenarios.
These research priorities can significantly enhance the viability and sustainability of methanol and gasoline as transportation fuels.
4.10. Benchmarking and Continuous Improvement
Benchmarking best practices across industries and regions is essential for identifying opportunities for continuous improvement. For instance, comparing the lifecycle impacts of methanol produced from natural gas versus biomass can inform optimal production pathways. Similarly, assessing the success of policy incentives in countries adopting green methanol can provide valuable lessons for other regions. It is recommended that a consistent cross-industry approach be adopted to further enhance the efficacy of benchmarking. Economic efficiency (e.g., cost per ton of CO2 averted), socio-economic advantages (e.g., job-years created per fuel plant), and lifetime data (e.g., CO2-equivalent per MJ) should all be incorporated into this framework. These measures enable the making of insightful comparisons and aid in identifying high performers.
Establishing an open-access global benchmarking archive, possibly under the auspices of organizations such as the IEA, UNEP, or UNIDO, may also promote global cooperation, expedite technology transfer, and increase capacity in developing nations.
Methodologies for continuous improvement, such as ISO 50001 energy management systems, Lean Manufacturing, or Six Sigma, can also be applied methodically to supply chain and fuel production processes. These technologies can help standardize sustainability gains, improve process efficiency, and reduce waste. Ultimately, benchmarking should be used to drive proactive innovation and help stakeholders anticipate and fix performance gaps throughout the fuel lifecycle, in addition to tracking historical performance [87].
Methanol and gasoline remain pivotal in the global energy transition. While they face challenges such as high production costs, safety concerns, and infrastructure adaptation, innovation, sustainability, and energy security opportunities are immense. Stakeholders can unlock their full potential by aligning these fuels with SDGs, leveraging technological advancements, and fostering policy support [88]. The path forward requires collaborative action from governments, industry leaders, researchers, and consumers. Through innovation, strategic policymaking, and continuous improvement, methanol and gasoline can play a transformative role in achieving a sustainable, energy-secure future.
5. Conclusions and Future Work
With emphasis on the NGTM and MTG processes, this paper offers a thorough summary of the body of research on the sustainability of methanol and gasoline. According to this review, methanol has a clear environmental advantage over regular gasoline, especially when it comes from renewable resources or is produced by CO2 hydrogenation. Key findings show that burning methanol produces a cleaner-burning option that is consistent with climate mitigation methods, as well as significantly lower GHG emissions and NOx and SOx pollutants. The market for methanol is expanding quickly at an economic level due to its adaptability and rising demand in the chemical and fuel industries. Methanol’s decentralized production capacity, reduced toxicity, and safer handling profile all contribute to its social acceptance and energy security.
According to the bibliometric analysis, China, Switzerland, and the United States are the main contributors to the growing global research efforts focused on sustainable methanol production. A common topic across the studied publications is technological innovation, namely in the areas of carbon capture and green hydrogen integration. Additionally, by enabling methanol-derived fuels to be compatible with current fuel infrastructure, the MTG approach lowers transition costs and provides a strategic advantage.
Notwithstanding these advantages, a number of drawbacks were noted. Because methanol has a lower energy density than gasoline, engine and infrastructure adjustments are required. Furthermore, due to its toxicity and corrosive nature, better safety procedures and material compatibility are required. Higher production costs when using renewable methods also make MTG less economically viable, though these are predicted to decrease as technology advances.
To improve economic viability, future research should prioritize reducing the levelized cost of methanol (LCM) from renewable sources. Enhancing the energy return on investment (EROI) of MTG plants and increasing carbon conversion efficiency in NGTM systems are also critical to process sustainability.
From an environmental standpoint, a comprehensive LCSA across diverse geographical and feedstock scenarios is essential for achieving net-zero or even negative GHG emissions, particularly when integrated with CCUS and green hydrogen technologies. Social research should focus on quantifying the health and safety advantages of renewable fuels compared to conventional fuels, thereby supporting broader public and policy acceptance. Future directions also include implementing circular economy strategies, such as CO2-to-fuel recycling and zero-liquid discharge systems, investing in AI-driven process optimization, and benchmarking global best practices. Overall, research should aim to develop methanol-based fuels that are energy-efficient, cost-competitive, and environmentally sustainable, in line with the UN SDGs.
MTG process-based natural gas can be viably used to diversify energy portfolios and decarbonize the transportation industry. Its strategic significance is highlighted by its alignment with the Sustainable Development Goals of the UN, especially SDGs 7 and 13. To overcome current obstacles and fully fulfill MTG’s promise as a sustainable fuel pathway, industry, government, and academia must work together to make the shift effective.
Author Contributions
Conceptualization, H.A.-Y.; methodology, H.A.-Y.; software, H.A.-Y.; validation, H.A.-Y. and A.A.K.; formal analysis, H.A.-Y. and A.A.K.; investigation, H.A.-Y. and S.A.; resources, H.A.-Y.; data curation, H.A.-Y.; writing—original draft preparation, H.A.-Y.; writing—review and editing, H.A.-Y., S.A. and A.A.K.; visualization, H.A.-Y.; supervision, H.A.-Y. and S.A.; project administration, H.A.-Y.; funding acquisition, H.A.-Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded and supported by the Qatar National Research Fund [UREP31-141-2-044]. The findings achieved herein are solely the responsibility of the authors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The authors would like to acknowledge the Qatar Research, Development and Innovation (QRDI) Council for funding the research and the Qatar National Library for supporting open-access publishing.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial intelligence |
| AVGAS | Aviation gasoline |
| CCUS | Carbon capture, utilization, and storage |
| DME | Dimethyl ether |
| EROI | Energy return on investment |
| GHG | Greenhouse gas |
| LCA | Life cycle assessment |
| LCM | Levelized cost of methanol |
| LCSA | Life cycle sustainability assessment |
| MTBE | Methyl tertiary butyl ether |
| MTG | Methanol-to-gasoline |
| MTO | Methanol-to-olefins |
| NGTM | Natural gas-to-methanol |
| SDGs | Sustainable development goals |
| SI | Spark ignition |
References
- Methanol Institute. Carbon Footprint of Methanol; Methanol Institute: Alexandria, VA, USA, 2022. [Google Scholar]
- Bræstrup, F.; Kristensen, T.B. Emissions from Ship Engines Using Methanol as a New, Green Fuel. Available online: https://forcetechnology.com/en/articles/emissions-ship-engines-methanol-new-green-fuel (accessed on 11 December 2024).
- Iaquaniello, G.; Centi, G.; Salladini, A.; Palo, E.; Perathoner, S.; Spadaccini, L. Waste-to-methanol: Process and economics assessment. Bioresour. Technol. 2017, 243, 611–619. [Google Scholar] [CrossRef]
- Pfleger, G.S.; Teubler, R.; Schober, S. A novel gas chromatographic method for high-resolution analysis of gasoline fuels that enables the calculation of CHO ratio, higher and lower heating value, density and energy density. Fuel 2024, 376, 132704. [Google Scholar] [CrossRef]
- US EPA. Overview of Greenhouse Gases. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 11 December 2024).
- Klein, T. Methanol: A Future-Proof Fuel a Primer Prepared for the Methanol Institute; Methanol Institute: Alexandria, VA, USA, 2020. [Google Scholar]
- Abubakar, M.; Arif, M.M.; Kausar, H.; Khan, S.H.; Nisar, W.; Shahzad, K. Methanol Formation, Toxicity and its Impact on the Human Nervous System and Liver: Methanol Formation, Toxicity and its Impact. Pak. J. Health Sci. 2023, 4, 12–20. [Google Scholar] [CrossRef]
- Ekpenyong, C.E.; Asuquo, A.E. Recent advances in occupational and environmental health hazards of workers exposed to gasoline compounds. Int. J. Occup. Med. Environ. Health 2017, 30, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, T.; Morosuk, T.; Tsatsaronis, G. Exergy-based evaluation of methanol production from natural gas with CO2 utilization. Energy 2017, 141, 2528–2539. [Google Scholar] [CrossRef]
- ExxonMobil Product Solutions. Methanol to Gasoline Technology (MTG). Available online: www.exxonmobilchemical.com/en/catalysts-and-technology-licensing/methanol-to-gasoline-technology (accessed on 9 January 2025).
- Yang, S.; Chen, Q.; Liu, Z.; Wang, Y.; Tang, Z.; Sun, Y. Performance analysis of the wind energy integrated with a natural-gas-to-methanol process. Energy Convers. Manag. 2018, 173, 735–742. [Google Scholar] [CrossRef]
- Tabibian, S.S.; Sharifzadeh, M. Statistical and analytical investigation of methanol applications, production technologies, value-chain and economy with a special focus on renewable methanol. Renew. Sustain. Energy Rev. 2023, 179, 113281. [Google Scholar] [CrossRef]
- Nesterenko, N.; Medeiros-Costa, I.C.; Clatworthy, E.B.; Cruchade, H.; Konnov, S.V.; Dath, J.-P.; Gilson, J.-P.; Mintova, S. Methane-to-chemicals: A pathway to decarbonization. Natl. Sci. Rev. 2023, 10, nwad116. [Google Scholar] [CrossRef]
- Biswal, S.; Das, S.R.; Saha, N.; Mishra, P.C. Environmental sustainability assessment of gasoline and methanol blended smart fuel for reduced emission formation. Environ. Dev. Sustain. 2024, 26, 26753–26784. [Google Scholar] [CrossRef]
- Kexel, J.; Pischinger, S.; Balazs, A.; Schroeder, B.; Wegner, H. Sustainable Propulsion in a Post-Fossil Energy World: Life-Cycle Assessment of Renewable Fuel and Electrified Propulsion Concepts. SAE Tech. Pap. 2024, 1–18. [Google Scholar] [CrossRef]
- Boretti, A. Carbon dioxide hydrogenation for sustainable energy storage. Int. J. Hydrogen Energy 2024, 58, 1386–1395. [Google Scholar] [CrossRef]
- Moretti, C.; Patil, V.; Falter, C.; Geissbühler, L.; Patt, A.; Steinfeld, A. Technical, economic and environmental analysis of solar thermochemical production of drop-in fuels. Sci. Total Environ. 2023, 901, 166005. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.C.; Scheidemantle, B.; Hanes, R.J.; Bartling, A.W.; Grundl, N.J.; Clark, R.J.; Biddy, M.J.; Tao, L.; Trinh, C.T.; Guss, A.M. Economics and global warming potential of a commercial-scale delignifying biorefinery based on co-solvent enhanced lignocellulosic fractionation to produce alcohols, sustainable aviation fuels, and co-products from biomass. Energy Environ. Sci. 2024, 17, 1202–1215. [Google Scholar] [CrossRef]
- Luo, L.; Wang, H.; Li, C.; Hu, Y. Life cycle assessment of methanol vehicles from energy, environmental and economic perspectives. Energy Rep. 2022, 8, 5487–5500. [Google Scholar] [CrossRef]
- Ryu, K.H.; Kim, B.; Heo, S. Sustainability analysis framework based on global market dynamics: A carbon capture and utilization industry case. Renew. Sustain. Energy Rev. 2022, 166, 112639. [Google Scholar] [CrossRef]
- Benavides, P.T.; Bartling, A.W.; Phillips, S.D.; Hawkins, T.R.; Singh, A.; Zaimes, G.G.; Wiatrowski, M.; Harris, K.; Burli, P.H.; Hartley, D.; et al. Identification of Key Drivers of Cost and Environmental Impact for Biomass-Derived Fuel for Advanced Multimode Engines Based on Techno-Economic and Life Cycle Analysis. ACS Sustain. Chem. Eng. 2022, 10, 10465–10475. [Google Scholar] [CrossRef]
- Bartling, A.W.; Stone, M.L.; Hanes, R.J.; Bhatt, A.; Zhang, Y.; Biddy, M.J.; Davis, R.; Kruger, J.S.; Thornburg, N.E.; Luterbacher, J.S. Techno-economic analysis and life cycle assessment of a biorefinery utilizing reductive catalytic fractionation. Energy Environ. Sci. 2021, 14, 4147–4168. [Google Scholar] [CrossRef]
- Sarp, S.; Gonzalez Hernandez, S.; Chen, C.; Sheehan, S.W. Alcohol Production from Carbon Dioxide: Methanol as a Fuel and Chemical Feedstock. Joule 2021, 5, 59–76. [Google Scholar] [CrossRef]
- Al-Qahtani, A.; González-Garay, A.; Bernardi, A.; Galán-Martín, Á.; Pozo, C.; Dowell, N.M.; Chachuat, B.; Guillén-Gosálbez, G. Electricity grid decarbonisation or green methanol fuel? A life-cycle modelling and analysis of today’s transportation-power nexus. Appl. Energy 2020, 265, 114718. [Google Scholar] [CrossRef]
- Torres, H.; Camacho, K.; Macken, N. A life cycle assessment of biodiesel fuel produced from waste cooking oil. In Proceedings of the ASME Power Conference, Online, 4–5 August 2020; p. V001T008A006. [Google Scholar]
- Lopez, N.S.; Soliman, J.; Biona, J.B.M.; Fulton, L. Cost-benefit analysis of alternative vehicles in the Philippines using immediate and distant future scenarios. Transp. Res. Part D Transp. Environ. 2020, 82, 102308. [Google Scholar] [CrossRef]
- Hoseinzade, L.; Adams, T.A. Techno-economic and environmental analyses of a novel, sustainable process for production of liquid fuels using helium heat transfer. Appl. Energy 2019, 236, 850–866. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. Life cycle environmental impact assessments and comparisons of alternative fuels for clean vehicles. Resour. Conserv. Recycl. 2018, 132, 141–157. [Google Scholar] [CrossRef]
- Sundaram, S.; Kolb, G.; Hessel, V.; Wang, Q. Energy-Efficient Routes for the Production of Gasoline from Biogas and Pyrolysis Oil—Process Design and Life-Cycle Assessment. Ind. Eng. Chem. Res. 2017, 56, 3373–3387. [Google Scholar] [CrossRef]
- Kim, J.; Johnson, T.A.; Miller, J.E.; Stechel, E.B.; Maravelias, C.T. Fuel production from CO2 using solar-thermal energy: System level analysis. Energy Environ. Sci. 2012, 5, 8417–8429. [Google Scholar] [CrossRef]
- Tarka, T.J.; Chen, C. Gasoline from coal: The methanol-to-gasoline process. In Proceedings of the 26th Annual International Pittsburgh Coal Conference 2009, PCC 2009, Pittsburgh, PA, USA, 20–23 September 2009; pp. 1504–1559. [Google Scholar]
- Renó, M.L.G.; Lora, E.E.S.; Palacio, J.C.E.; Venturini, O.J.; Buchgeister, J.; Almazan, O. A LCA (life cycle assessment) of the methanol production from sugarcane bagasse. Energy 2011, 36, 3716–3726. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P. Carbohydrate Is a Promising Hydrogen Carrier for Transportation. In Proceedings of the AIChE Annual Meeting, Salt Lake City, UT, USA, 4–9 November 2007; p. 649c. [Google Scholar]
- Zhou, Z.; Jiang, H.; Qin, L. Life cycle sustainability assessment of fuels. Fuel 2007, 86, 256–263. [Google Scholar] [CrossRef]
- Joshi, S.; Lave, L.; Maclean, H.; Lankey, R. A Life Cycle Comparison of Alternative Transportation Fuels. SAE Tech. Pap. 2000, 1–11. [Google Scholar] [CrossRef]
- Kikkawa, Y.; Nozawa, N.; Inoue, H.; Sato, E. Method of Removing Sulfur Compounds from Natural Gas. U.S. Patent 7780933B2, 24 August 2010. [Google Scholar]
- Authayanun, S.; Arpornwichanop, A.; Paengjuntuek, W.; Assabumrungrat, S. Thermodynamic study of hydrogen production from crude glycerol autothermal reforming for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 6617–6623. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef]
- Gierse, M.; Ali, R.E.; Ouda, M.; Schaadt, A.; Sauer, J.; Hebling, C. 6-Power-to-DME: A cornerstone towards a sustainable energy system. In Power to Fuel; Spazzafumo, G., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 123–151. [Google Scholar]
- Perry, C.E. Handbook. In Chemical Engineers “Handbook”, 3rd ed.; McGraw-Hill Book Company: New York, NY, USA, 1963. [Google Scholar]
- Mehra, R.K.; Duan, H.; Juknelevičius, R.; Ma, F.; Li, J. Progress in hydrogen enriched compressed natural gas (HCNG) internal combustion engines—A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 1458–1498. [Google Scholar] [CrossRef]
- Gong, C.; Si, X.; Liu, F. Combined effects of excess air ratio and EGR rate on combustion and emissions behaviors of a GDI engine with CO2 as simulated EGR (CO2) at low load. Fuel 2021, 293, 120442. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kaviti, A.K.; Mehra, R.; Mer, K.K.S. Progress in performance analysis of ethanol-gasoline blends on SI engine. Renew. Sustain. Energy Rev. 2017, 69, 324–340. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Alternative Fuels Data Center (AFDC). Available online: https://afdc.energy.gov (accessed on 9 May 2025).
- U.S. Department for Energy. Alternative Fuels Data Center: Methanol. Available online: https://afdc.energy.gov/fuels/emerging-methanol (accessed on 9 January 2025).
- Spencer, A.B.; Colonna, G.R. NFPA Pocket Guide to Hazardous Materials; Jones & Bartlett Learning: Burlington, MA, USA, 2003. [Google Scholar]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Gupta, H.N. Fundamentals of Internal Combustion Engines; PHI Learning Pvt. Ltd.: Delhi, India, 2012. [Google Scholar]
- Verhelst, S.; Turner, J.W.G.; Sileghem, L.; Vancoillie, J. Methanol as a fuel for internal combustion engines. Prog. Energy Combust. Sci. 2019, 70, 43–88. [Google Scholar] [CrossRef]
- Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105. [Google Scholar] [CrossRef]
- Lv, J.; Sun, Y.; Zhang, Z.; Fang, Y. Optimization of operational parameters of marine methanol dual-fuel engine based on RSM-MOPSO. Process Saf. Environ. Prot. 2024, 191, 2634–2652. [Google Scholar] [CrossRef]
- Zhen, X. Chapter 11-Methanol As An Internal Combustion on Engine Fuel. In Methanol; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 313–337. [Google Scholar]
- Dong, P.; Zhang, Y.; Wang, Y.; Long, W.; Tian, J.; Tian, H.; Nishida, K. Application and extension of diesel spray theory in analysis of methanol spray characteristics under high-pressure injection conditions. Green Energy Resour. 2024, 2, 100103. [Google Scholar] [CrossRef]
- Mohammed Abbas, A.H.; Cheralathan, K.K.; Porpatham, E.; Arumugam, S.K. Hydrogen generation using methanol steam reforming–catalysts, reactors, and thermo-chemical recuperation. Renew. Sustain. Energy Rev. 2024, 191, 114147. [Google Scholar] [CrossRef]
- Alshafei, F.H.; Zones, S.I.; Davis, M.E. Improving the propylene selectivity in the methanol-to-olefins reaction over CIT-17, a SAT-type molecular sieve. Chem. Eng. J. 2024, 482, 149045. [Google Scholar] [CrossRef]
- Chen, L.; Msigwa, G.; Yang, M.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Strategies to achieve a carbon neutral society: A review. Environ. Chem. Lett. 2022, 20, 2277–2310. [Google Scholar] [CrossRef]
- Wolday, A.K.; Ramteke, M. Surrogate model-based optimization of methanol synthesis process for multiple objectives: A pathway towards achieving sustainable development goals. Chem. Eng. Res. Des. 2024, 204, 172–182. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Chen, G.; Yao, J.; Yan, B.; Yi, W.; Tian, C.; Zao, H. Enhanced methanol to gasoline performance using nanosheet-like SAPO-11 catalyst. J. Energy Inst. 2023, 111, 101424. [Google Scholar] [CrossRef]
- MacDonald, J.; Lopez-Pintor, D.; Matsubara, N.; Kitano, K.; Yamada, R. A chemical kinetic analysis of knock propensity of methanol-to-gasoline fuel. Fuel 2025, 382, 133787. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Farooq, A.; Kalghatgi, G.T. Recent progress in gasoline surrogate fuels. Prog. Energy Combust. Sci. 2018, 65, 67–108. [Google Scholar] [CrossRef]
- Rosdi, S.M.; Erdiwansyah; Ghazali, M.F.; Mamat, R. Evaluation of engine performance and emissions using blends of gasoline, ethanol, and fusel oil. Case Stud. Chem. Environ. Eng. 2025, 11, 101065. [Google Scholar] [CrossRef]
- Kumar, T.; Mohsin, R.; Majid, Z.A.; Ghafir, M.F.A.; Yusuf, N.K.; Kim, J.; Wash, A.M.; Sahri, D.M. Response surface methodology application in optimization of performance and exhaust emissions of RON 98, aviation gasoline 100LL and the blends in Lycoming O-320 engine. Fuel 2019, 256, 115909. [Google Scholar] [CrossRef]
- Ershov, M.A.; Klimov, N.A.; Savelenko, V.D.; Makhova, U.A.; Burov, N.O.; Karpunin-Ozherovskiy, E.V.; Aleksanyan, D.R.; Donskaya, E.S.; Mukhina, D.Y.; Kapustin, V.M.; et al. An Overview of the Global Market, Fleet, and Components in the Field of Aviation Gasoline. Aerospace 2023, 10, 863. [Google Scholar] [CrossRef]
- Sarlak, M.; Pirouzfar, V.; Sakhaeinia, H.; Alihosseini, A. Combustion of gasoline with oxygen-containing and nano-additives: An experimental study, modeling, optimization, and analysis survey. J. Taiwan Inst. Chem. Eng. 2024, 159, 105452. [Google Scholar] [CrossRef]
- Awad, O.I.; Zhou, B.; Chen, Z.; Kadirgama, K.; Mohammed, M.N.; Ramasamy, D. Influence of PODE1 additive into ethanol-gasoline blends (E10) on fuel properties and phase stability. Heliyon 2023, 9, e22364. [Google Scholar] [CrossRef]
- Gonzalez-Garay, A.; Heuberger-Austin, C.; Fu, X.; Klokkenburg, M.; Zhang, D.; van der Made, A.; Shah, N. Unravelling the potential of sustainable aviation fuels to decarbonise the aviation sector. Energy Environ. Sci. 2022, 15, 3291–3309. [Google Scholar] [CrossRef]
- Bazaluk, O.; Havrysh, V.; Nitsenko, V.; Baležentis, T.; Streimikiene, D.; Tarkhanova, E.A. Assessment of Green Methanol Production Potential and Related Economic and Environmental Benefits: The Case of China. Energies 2020, 13, 3113. [Google Scholar] [CrossRef]
- Deka, T.J.; Osman, A.I.; Baruah, D.C.; Rooney, D.W. Methanol fuel production, utilization, and techno-economy: A review. Environ. Chem. Lett. 2022, 20, 3525–3554. [Google Scholar] [CrossRef]
- Chang, W.-R.; Hwang, J.-J.; Wu, W. Environmental impact and sustainability study on biofuels for transportation applications. Renew. Sustain. Energy Rev. 2017, 67, 277–288. [Google Scholar] [CrossRef]
- Khedr, A.M.; El-Adawy, M.; Ismael, M.A.; Qador, A.; Abdelhafez, A.; Ben-Mansour, R.; Habib, M.A.; Nemitallah, M.A. Recent Fuel-Based Advancements of Internal Combustion Engines: Status and Perspectives. Energy Fuels 2025, 39, 5099–5132. [Google Scholar] [CrossRef]
- Shah, J. Integration of AI-based predictive maintenance for energy-efficient mechanical systems. World J. Adv. Eng. Technol. Sci. 2024, 11, 664–673. [Google Scholar] [CrossRef]
- García, A.; Monsalve-Serrano, J.; Martínez-Boggio, S.; Wittek, K. Potential of hybrid powertrains in a variable compression ratio downsized turbocharged VVA Spark Ignition engine. Energy 2020, 195, 117039. [Google Scholar] [CrossRef]
- Rataj, M.; Lodi, C.; Zawieska, J.; Stepniak, M.; Cheimariotis, I.; Grosso, M.; Piazza, F.; Marotta, A. New and Emerging Transport Technologies and Trends in European Research and Innovation Projects 2024; Publications Office of the European Union: Luxembourg City, Luxembourg, 2025. [Google Scholar]
- Nweje, U.; Taiwo, M. Leveraging Artificial Intelligence for predictive supply chain management, focus on how AI-driven tools are revolutionizing demand forecasting and inventory optimization. Int. J. Sci. Res. Arch. 2025, 14, 230–250. [Google Scholar] [CrossRef]
- Amanpreet Kaur Jassal, U. Advanced Solid Catalysis for Biomass Conversion into High Value-Added Chemicals. In Solid Base Catalysts; Springer: Berlin/Heidelberg, Germany, 2024; pp. 97–127. [Google Scholar]
- Ibekwe, E.; Etukudoh, E.; Nwokediegwu, Z.; Umoh, A.; Adefemi, A.; Ilojianya, V.; Magash, T. Energy Security in the Global Context: A Comprehensive Review of Geopolitical Dynamics and Policies. Eng. Sci. Technol. J. 2024, 5, 152–168. [Google Scholar] [CrossRef]
- Khalifa, R.; Alherbawi, M.; Bicer, Y.; Al-Ansari, T. Fueling circularity: A thorough review of circular practices in the aviation sector with sustainable fuel solutions. Resour. Conserv. Recycl. Adv. 2024, 23, 200223. [Google Scholar] [CrossRef]
- Marchuk, А.; Likhanov, V.; Lopatin, O. Alternative energy: Methanol, ethanol and alcohol esters of rapeseed oil as eco-friendly biofuel. Теoретическая и Прикладная Экoлoгия 2019, 3, 80–86. [Google Scholar] [CrossRef]
- Awad, O.I.; Ma, X.; Kamil, M.; Ali, O.M.; Ma, Y.; Shuai, S. Overview of polyoxymethylene dimethyl ether additive as an eco-friendly fuel for an internal combustion engine: Current application and environmental impacts. Sci. Total Environ. 2020, 715, 136849. [Google Scholar] [CrossRef] [PubMed]
- Reese, E.; Kimbrough, R.D. Acute toxicity of gasoline and some additives. Environ. Health Perspect. 1993, 101, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, G. Development of Fuel/Engine Systems—The Way Forward to Sustainable Transport. Engineering 2019, 5, 510–518. [Google Scholar] [CrossRef]
- Patil, T.; Naji, A.; Mondal, U.; Pandey, I.; Unnarkat, A.; Dharaskar, S. Sustainable methanol production from carbon dioxide: Advances, challenges, and future prospects. Environ. Sci. Pollut. Res. 2024, 31, 44608–44648. [Google Scholar] [CrossRef]
- United Nations. From Vision to Action: Explaining UNDP’s Digital Transformation Framework; United Nations Development Programme: New York, NY, USA, 2023; pp. 1–21. [Google Scholar]
- Waly, M.M.; Mickovski, S.B.; Thomson, C. Application of Circular Economy in Oil and Gas Produced Water Treatment. Sustainability 2023, 15, 2132. [Google Scholar] [CrossRef]
- Solis-Jacome, A.; Ramírez-Márquez, C.; Morales-Cabrera, M.A.; Ponce-Ortega, J.M. Methanol production from residual streams of natural gas sweetening for achieving the sustainable development goals. Chem. Eng. Process.-Process Intensif. 2024, 199, 109746. [Google Scholar] [CrossRef]
- Reddy, V.J.; Hariram, N.P.; Maity, R.; Ghazali, M.F.; Kumarasamy, S. Sustainable E-Fuels: Green Hydrogen, Methanol and Ammonia for Carbon-Neutral Transportation. World Electr. Veh. J. 2023, 14, 349. [Google Scholar] [CrossRef]
- Ghelani, H. Six Sigma and Continuous Improvement Strategies: A Comparative Analysis in Global Manufacturing Industries. Int. J. Sci. Res. Manag. IJSRM 2024, 11, 954–972. [Google Scholar] [CrossRef]
- Usman, M.; Jamil, M.K.; Ashraf, W.M.; Saqib, S.; Ahmad, T.; Fouad, Y.; Raza, H.; Ashfaq, U.; Pervaiz, A. AI-driven optimization of ethanol-powered internal combustion engines in alignment with multiple SDGs: A sustainable energy transition. Energy Convers. Manag. X 2023, 20, 100438. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).