Ranking Bacteria for Carbon Capture and Self-Healing in Concrete: Performance, Encapsulation, and Sustainability
Abstract
1. Introduction
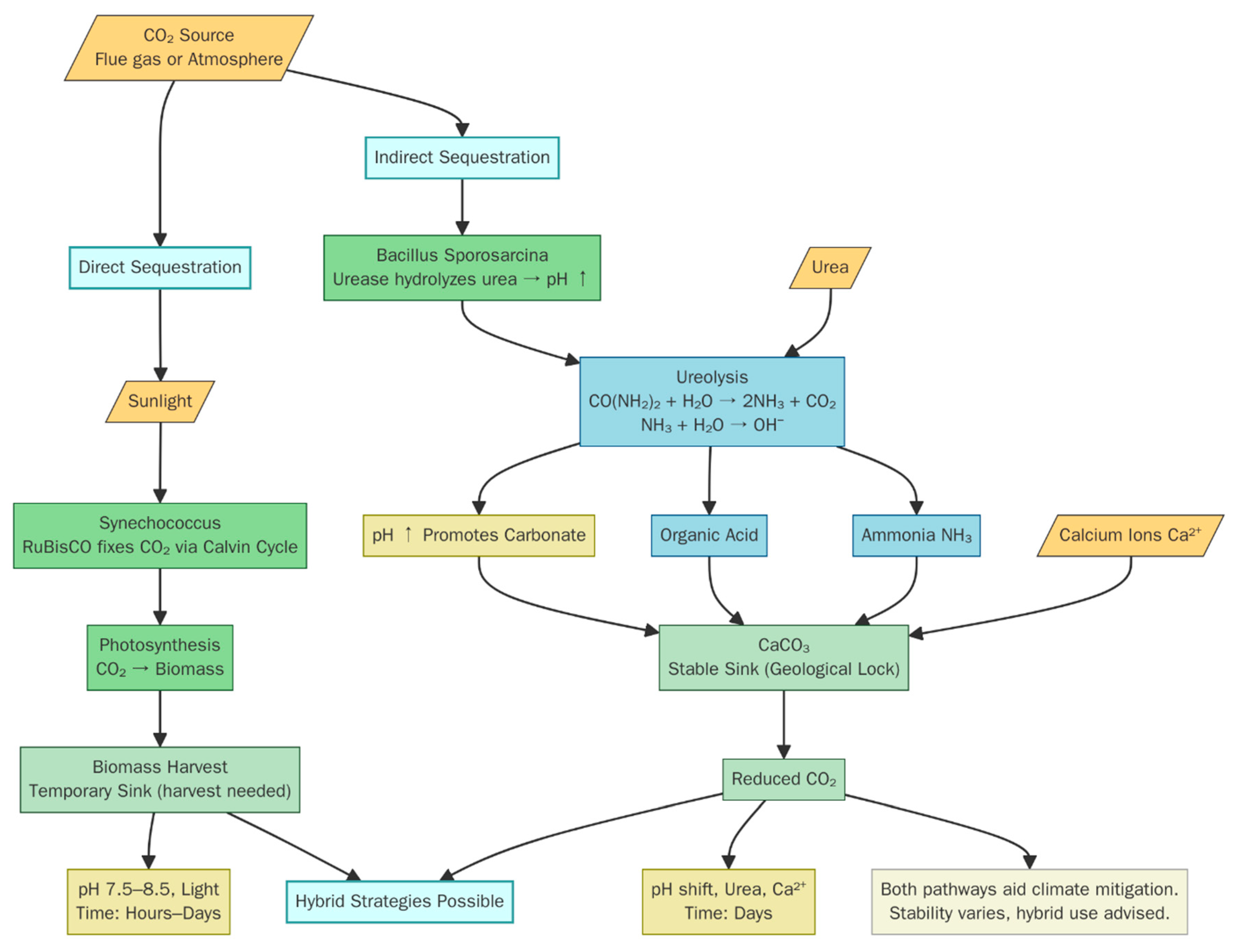
2. Direct and Indirect CO2 Sequestration
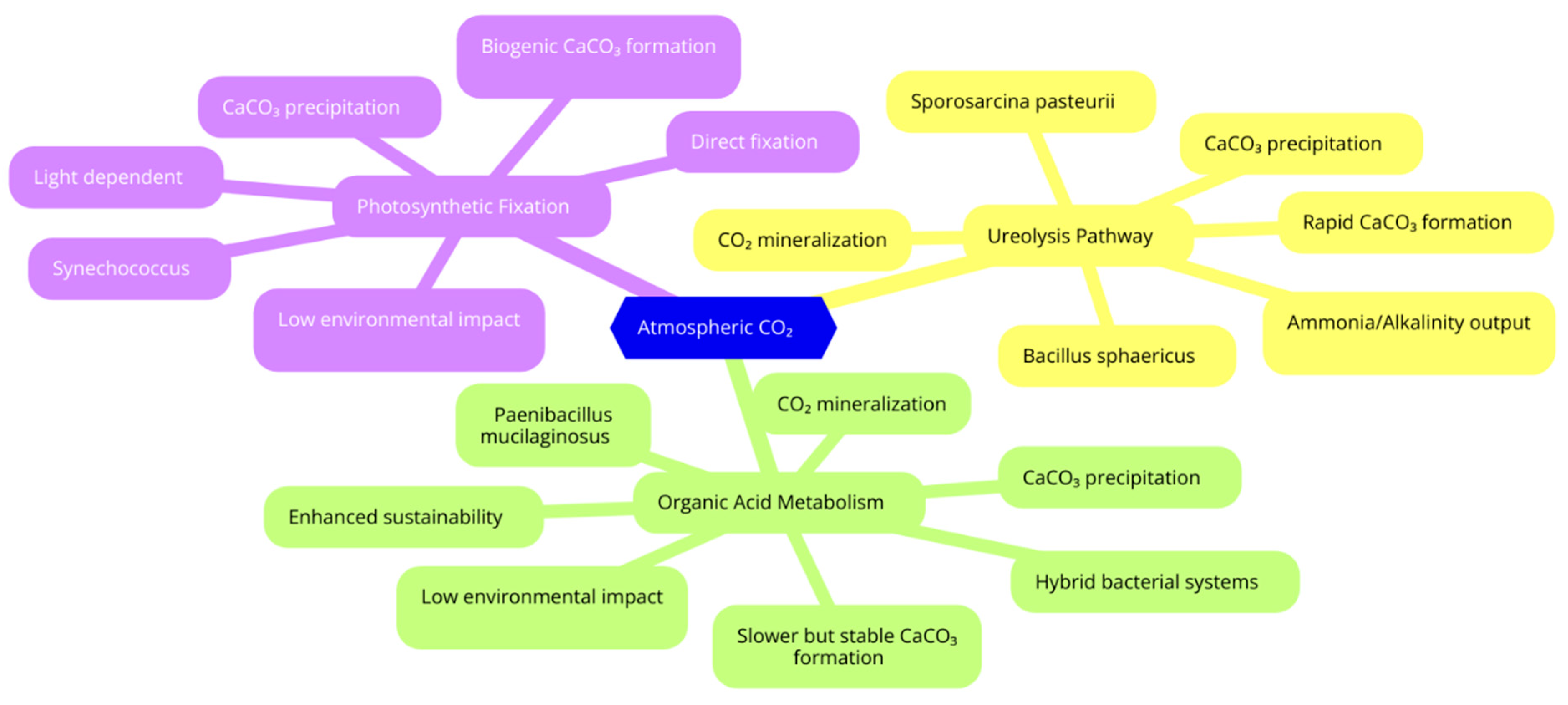
2.1. Efficiency of CO2 Sequestration Among Key Bacteria
2.2. Comparative Analysis of Carbon Mineralization Rates per Unit Biomass
2.3. Influence of Bacterial Metabolism on the Extent and Permanence of CO2 Storage
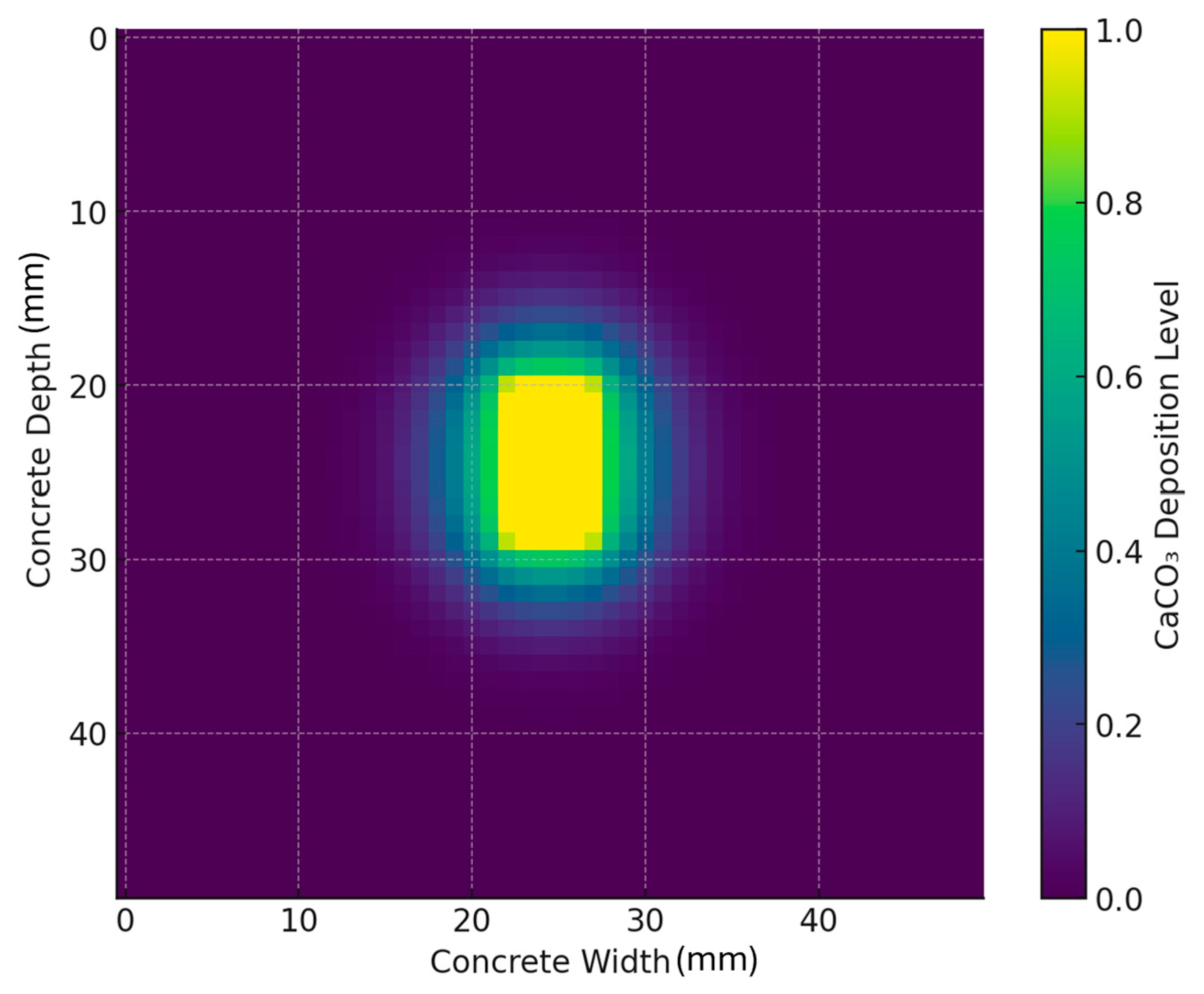
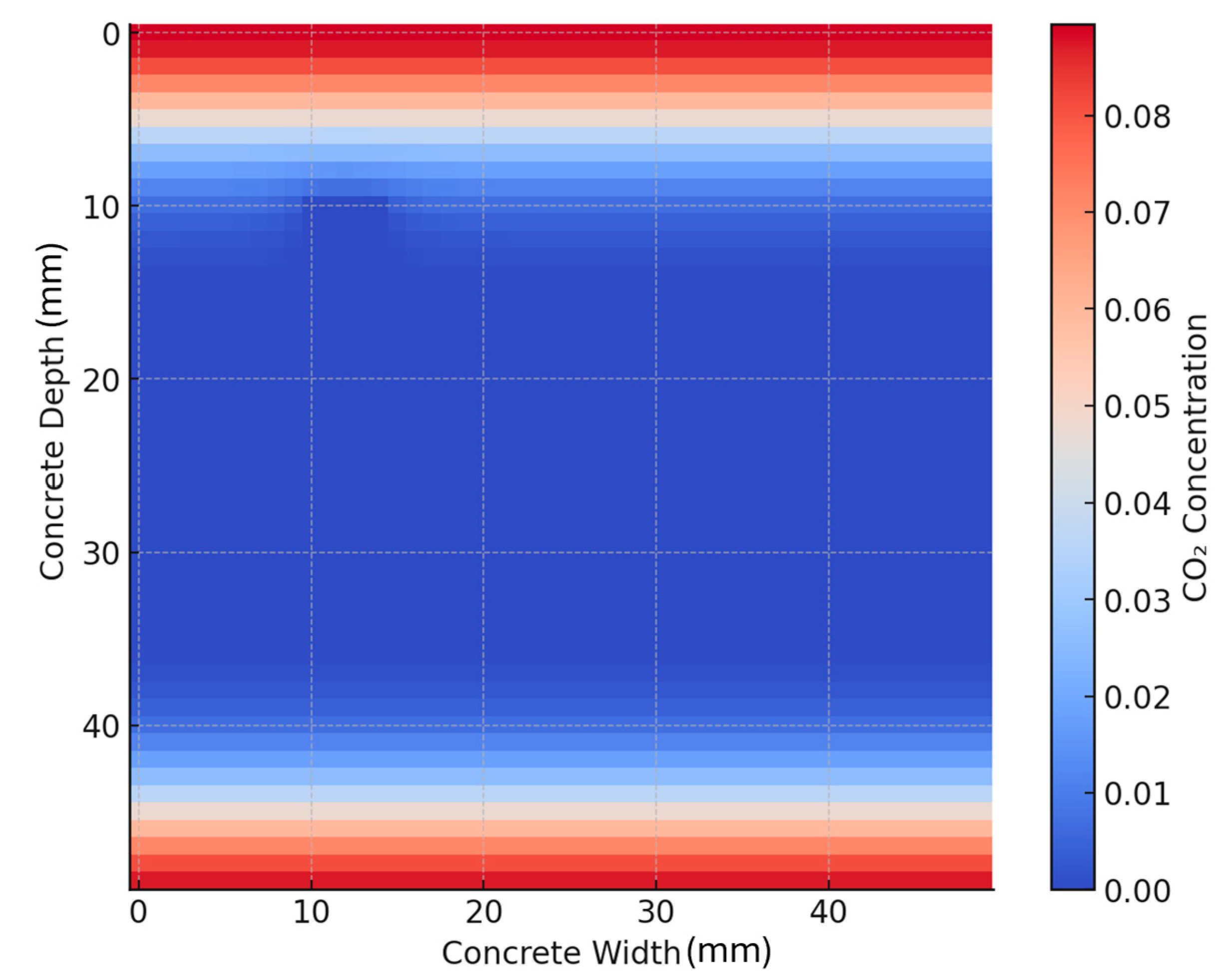
2.4. Finite Difference Method (FDM)
- The CaCO3 deposition rate governed by bacterial ureolysis;
- The diffusion of calcium ions (Ca2+) and carbonate ions (CO32−) within the crack;
- The precipitation kinetics, where mineralization occurs in regions with high ion concentrations.
3. Ranking Bacteria for Self-Healing Carbon-Capturing Concrete
4. Bacterial Encapsulation in Enhancing Self-Healing and CO2 Sequestration in Concrete
4.1. Types of Encapsulation Techniques
4.2. Impact of Encapsulation on Mechanical Properties of Bacterial Concrete
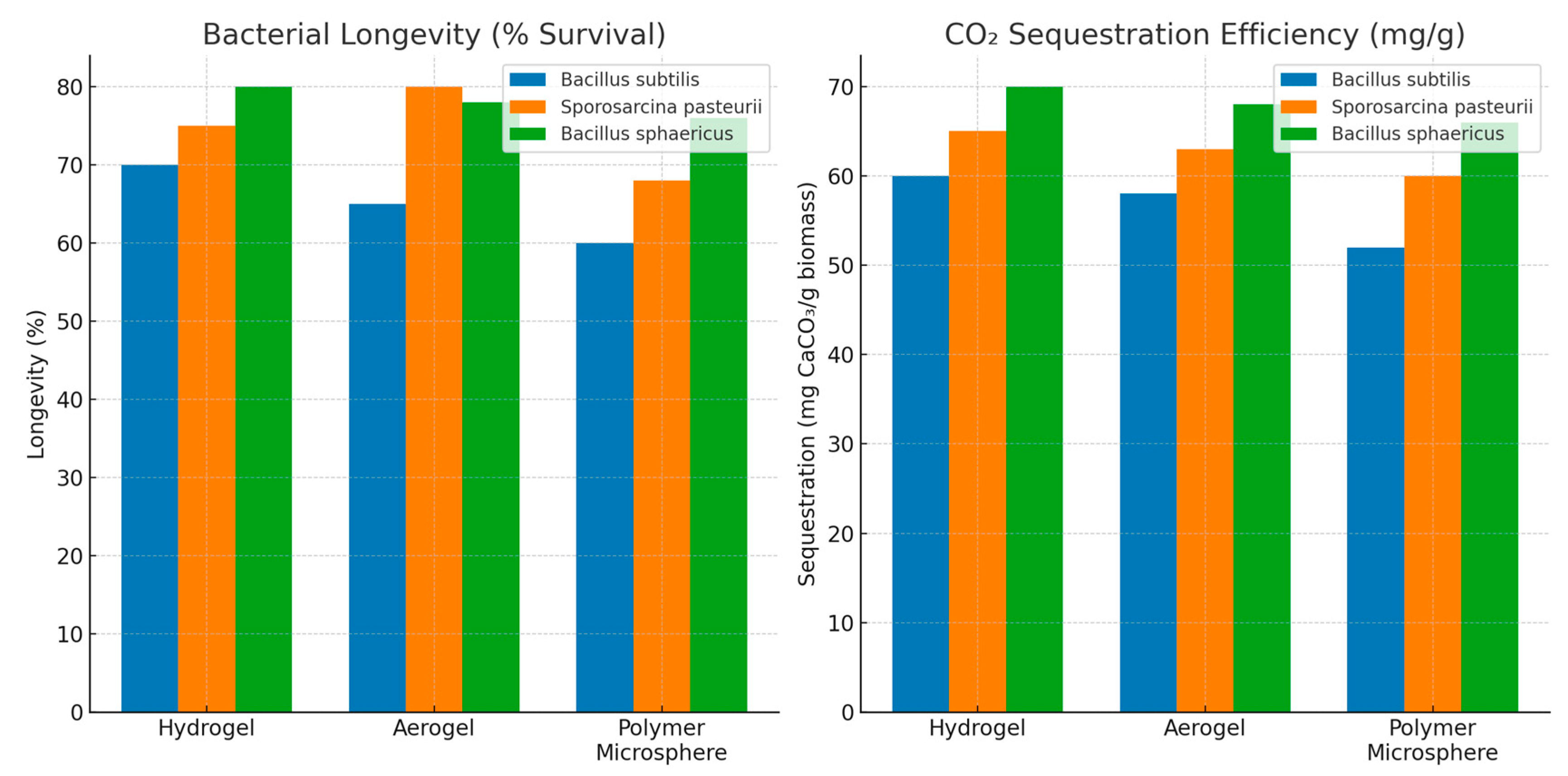
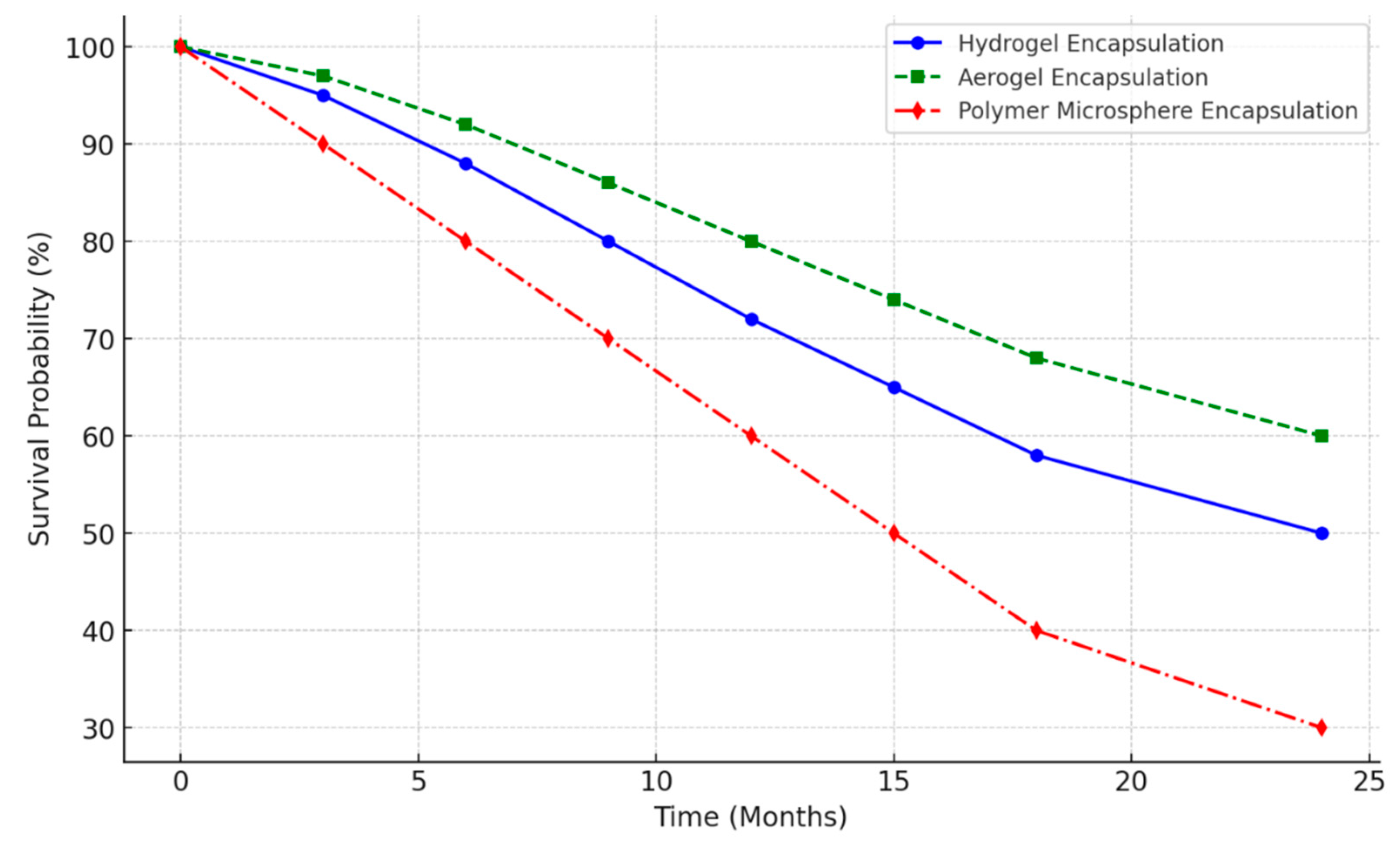
4.3. Impact of Encapsulation on Bacterial Efficiency
5. Microstructure Analysis of CaCO3 Crystals Formed via Microbially Induced Calcite Precipitation
6. Sustainability and Environmental Impact
6.1. Carbon Footprint Reduction and Long-Term Performance
6.2. Life Cycle Assessment (LCA) of Bacterial Self-Healing Concrete
6.2.1. Goal and Scope Definition
6.2.2. LCA Phases Considered
6.2.3. Sustainability Implications and Future Considerations
6.2.4. Process Flow for Bacterial-Based Self-Healing Concrete (BBSHC) Production and Life Cycle
6.3. Life Cycle Cost and Industrial Feasibility
6.3.1. Cost Comparison
6.3.2. Practical Challenges and Feasibility of Large-Scale Adoption
6.3.3. Future Prospects: Making Bacterial Concrete Industrially Viable
6.4. Regulatory and Safety Considerations
6.4.1. Environmental Regulations for Biogenic Carbonate Precipitation and NH3 Release
6.4.2. Structural and Material Safety Standards
6.4.3. Potential for Genetically Modified Bacteria in Future Applications
6.4.4. Future Regulatory Framework Development
7. Conclusions
- Five carbonate-precipitating bacterial strains were screened under high-pH concrete conditions to identify the most efficient survivors and CaCO3 producers.
- A novel encapsulation method was validated, maintaining bacterial viability for over 6 months and enabling >80% crack healing efficiency.
- A cradle-to-gate life cycle assessment (LCA) quantified the net CO2 sequestration benefits, confirming the environmental advantages of microbial concrete.
- An integrated performance framework was developed, linking bacterial activity, the crack healing rates, and the environmental impacts to guide practical applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbol | Definition/Description |
| Ci,j | CO2 concentration (CFD-inspired simulation) or CaCO3 concentration (FDM crack healing model) at grid cell (i,j) |
| D | Diffusion coefficient for ions (Ca2+, CO32−) or CO2 in the reaction–diffusion equations (normalized to 0.1) |
| Δ(C)i,j | Discrete Laplacian (5-point stencil) applied to concentration C at cell (i,j) |
| R | Precipitation (sequestration) reaction rate per time step per active cell (e.g., 0.02 units/timestep in CO2 model) |
| Bi,j | Binary mask indicating bacterial presence at cell (i,j) (1 if bacteria present/active, 0 otherwise) |
| i,j | Integer indices for rows and columns in the 2D simulation grid (both FDM- and CFD-inspired use a 50 × 50 grid) |
| t | Time variable in discrete time steps for both FDM- and CFD-inspired simulations |
| Ca2+ | Calcium ion (divalent cation) used in reaction–diffusion descriptions of MICP |
| CO32− | Carbonate ion (divalent anion) produced during ureolysis or organic acid metabolism; participates in CaCO3 precipitation |
| CO2 | Carbon dioxide molecule (gaseous form for direct fixation or produced during bacterial metabolism) |
| CaCO3 | Calcium carbonate (precipitated mineral); primary biomineral formed during MICP |
| OD600 | Optical density at 600 nm, used to quantify cell concentration (e.g., OD600 = 5.0 for Sporosarcina pasteurii) |
| 105 CFU/mL | Colony-forming units per milliliter; optimal bacterial dose for Bacillus sphaericus in biocementation experiments |
| σ2 | Variance in performance scores in Monte Carlo sensitivity analysis (e.g., σ2 = 0.021 for Bacillus sphaericus) |
| mg CaCO3/g biomass | Units for carbonate precipitation efficiency (e.g., 50–100 mg CaCO3 per gram of bacterial biomass) |
| mm2 | Area unit for each grid cell in the 50 × 50 FDM/CFD model (1 mm2 per cell) |
| Abbreviation | Definition |
| CO2 | Carbon Dioxide |
| CaCO3 | Calcium Carbonate |
| MICP | Microbially Induced Calcium Carbonate Precipitation |
| LCA | Life Cycle Assessment |
| pH | Power of Hydrogen (measure of acidity/alkalinity) |
| NH3 | Ammonia |
| CO32− | Carbonate Ion |
| HCO3− | Bicarbonate Ion |
| WCF | Waste Concrete Fines |
| OD600 | Optical Density at 600 nm |
| CFU | Colony-Forming Unit(s) |
| SEM | Scanning Electron Microscopy |
| EDS | Energy-Dispersive X-Ray Spectroscopy |
| XRD | X-Ray Diffraction |
| FDM | Finite Difference Method |
| CFD | Computational Fluid Dynamics |
| MCP | Mineral Carbonation Process |
| AAC | Alkali-Activated Concrete |
| BC | Bacterial Concrete |
| DE | Diatomaceous Earth |
| LWA | Lightweight Aggregate(s) |
| CaAlg | Calcium–Alginate |
| SAP | Superabsorbent Polymer |
| PU | Polyurethane |
| CSA | Calcium Sulfoaluminate |
| AFt | Ettringite (and/or Thaumasite) Phases |
| EV | Expanded Vermiculite |
| CFBB | Carbon Fiber Bacteria Balls |
| RA | Recycled Brick Aggregate |
| EPS | Extracellular Polymeric Substance (exopolysaccharide) |
| GWP | Global Warming Potential (kg CO2-eq/m3) |
| MJ | Megajoule (unit for embodied energy, MJ/m3) |
| kg CO2-eq/m3 | Kilograms of CO2 Equivalent per Cubic Meter (unit for GWP) |
References
- Shivaprasad, K.N.; Yang, H.-M.; Singh, J.K. A path to carbon neutrality in construction: An overview of recent progress in recycled cement usage. J. CO2 Util. 2024, 83, 102816. [Google Scholar] [CrossRef]
- Khaiyum, M.Z.; Sarker, S.; Kabir, G. Evaluation of Carbon Emission Factors in the Cement Industry: An Emerging Economy Context. Sustainability 2023, 15, 15407. [Google Scholar] [CrossRef]
- Liu, C.; Xing, L.; Liu, H.; Huang, W.; Nong, X.; Xu, X. Experimental on repair performance and complete stress-strain curve of self-healing recycled concrete under uniaxial loading. Constr. Build. Mater. 2021, 285, 122900. [Google Scholar] [CrossRef]
- Kaushal, V.; Saeed, E. Sustainable and Innovative Self-Healing Concrete Technologies to Mitigate Environmental Impacts in Construction. CivilEng 2024, 5, 549–558. [Google Scholar] [CrossRef]
- Wong, P.Y.; Mal, J.; Sandak, A.; Luo, L.; Jian, J.; Pradhan, N. Advances in microbial self-healing concrete: A critical review of mechanisms, developments, and future directions. Sci. Total Environ. 2024, 947, 174553. [Google Scholar] [CrossRef]
- Adhikary, S.K.; Rathod, N.; Adhikary, S.D.; Kumar, A.; Perumal, P. Chemical-based self-healing concrete: A review. Discov. Civ. Eng. 2024, 1, 119. [Google Scholar] [CrossRef]
- Cappellesso, V.G.; Van Mullem, T.; Gruyaert, E.; Van Tittelboom, K.; De Belie, N. Self-healing concrete with a bacteria-based or crystalline admixture as healing agent to prevent chloride ingress and corrosion in a marine environment. Dev. Built Environ. 2024, 19, 100486. [Google Scholar] [CrossRef]
- Javeed, Y.; Goh, Y.; Mo, K.H.; Yap, S.P.; Leo, B.F. Microbial self-healing in concrete: A comprehensive exploration of bacterial viability, implementation techniques, and mechanical properties. J. Mater. Res. Technol. 2024, 29, 2376–2395. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Wong, C.S.; Rajasekar, A.; Ling, J.H.; Laiche, A.B.; Basri, H.F.; Sivakumar, G.; Ouahbi, T. Bio-Based Solutions for Concrete Infrastructure: A Review of Microbial-Induced Carbonate Precipitation in Crack Healing. Buildings 2025, 15, 1052. [Google Scholar] [CrossRef]
- Ahmad, I.; Shokouhian, M.; Owolabi, D.; Jenkins, M.; McLemore, G.L. Assessment of Biogenic Healing Capability, Mechanical Properties, and Freeze–Thaw Durability of Bacterial-Based Concrete Using Bacillus subtilis, Bacillus sphaericus, and Bacillus megaterium. Buildings 2025, 15, 943. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, D.; Koner, S.; Hseu, Z.-Y.; Hsu, B.-M. Microbial induce carbonate precipitation derive bio-concrete formation: A sustainable solution for carbon sequestration and eco-friendly construction. Environ. Res. 2025, 270, 121006. [Google Scholar] [CrossRef] [PubMed]
- van der Bergh, J.M.; Miljević, B.; Vučetić, S.; Šovljanski, O.; Markov, S.; Riley, M.; Ranogajec, J.; Bras, A. Comparison of Microbially Induced Healing Solutions for Crack Repairs of Cement-Based Infrastructure. Sustainability 2021, 13, 4287. [Google Scholar] [CrossRef]
- Jiang, L.; Xia, H.; Wang, W.; Zhang, Y.; Li, Z. Applications of microbially induced calcium carbonate precipitation in civil engineering practice: A state-of-the-art review. Constr. Build. Mater. 2023, 404, 133227. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, C.-S.; Jiang, N.-J.; Pan, X.-H.; Liu, B.; Wang, Y.-J.; Shi, B. Microbial-induced carbonate precipitation (MICP) technology: A review on the fundamentals and engineering applications. Environ. Earth Sci. 2023, 82, 229. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Ngu, L.H.; Ong, D.E.L.; Nissom, P.M. Low-cost cultivation of Sporosarcina pasteurii strain in food-grade yeast extract medium for microbially induced carbonate precipitation (MICP) application. Biocatal. Agric. Biotechnol. 2019, 17, 247–255. [Google Scholar] [CrossRef]
- Carter, M.S.; Tuttle, M.J.; Mancini, J.A.; Martineau, R.; Hung, C.-S.; Gupta, M.K. Microbially Induced Calcium Carbonate Precipitation by Sporosarcina pasteurii: A Case Study in Optimizing Biological CaCO3 Precipitation. Appl. Environ. Microbiol. 2023, 89, e01794-22. [Google Scholar] [CrossRef]
- Yamasamit, N.; Sangkeaw, P.; Jitchaijaroen, W.; Thongchom, C.; Keawsawasvong, S.; Kamchoom, V. Effect of Bacillus subtilis on mechanical and self-healing properties in mortar with different crack widths and curing conditions. Sci. Rep. 2023, 13, 7844. [Google Scholar] [CrossRef]
- Islam, M.M.; Hoque, N.; Islam, M.; Ibney Gias, I. An Experimental Study on the Strength and Crack Healing Performance of E. coli Bacteria-Induced Microbial Concrete. Adv. Civ. Eng. 2022, 2022, 3060230. [Google Scholar] [CrossRef]
- Mangan, N.M.; Brenner, M.P. Systems analysis of the CO2 concentrating mechanism in cyanobacteria. eLife 2014, 3, e02043. [Google Scholar] [CrossRef]
- Durall, C.; Lindblad, P. Mechanisms of carbon fixation and engineering for increased carbon fixation in cyanobacteria. Algal Res. 2015, 11, 263–270. [Google Scholar] [CrossRef]
- Hoffmann, T.D.; Reeksting, B.J.; Gebhard, S. Bacteria-induced mineral precipitation: A mechanistic review. Microbiology 2021, 167, 001049. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, H.; Li, Y. Biomineralization Induced by Cells of Sporosarcina pasteurii: Mechanisms, Applications and Challenges. Microorganisms 2021, 9, 2396. [Google Scholar] [CrossRef] [PubMed]
- Chuo, S.C.; Mohamed, S.F.; Mohd Setapar, S.H.; Ahmad, A.; Jawaid, M.; Wani, W.A.; Yaqoob, A.A.; Mohamad Ibrahim, M.N. Insights into the Current Trends in the Utilization of Bacteria for Microbially Induced Calcium Carbonate Precipitation. Materials 2020, 13, 4993. [Google Scholar] [CrossRef]
- Tan, K.; Wu, S.; Ding, S. Carriers of Healing Agents in Biological Self-Healing Concrete. Adv. Mater. Sci. Eng. 2023, 2023, 7179162. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Yao, W.; Kulminskaya, A.A.; Shah, S.P. Microbial-inspired self-healing of concrete cracks by sodium silicate-coated recycled concrete aggregates served as bacterial carrier. Front. Struct. Civ. Eng. 2024, 18, 14–29. [Google Scholar] [CrossRef]
- Kim, H.; Son, H. Utilization of Bio-Mineral Carbonation for Enhancing CO2 Sequestration and Mechanical Properties in Cementitious Materials. Buildings 2022, 12, 744. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Q.; Zhang, X.; Luo, M. Microbial self-healing of cracks in cement-based materials and its influencing factors. Front. Struct. Civ. Eng. 2023, 17, 1630–1642. [Google Scholar] [CrossRef]
- Demo, P.; Přeučil, F.; Prošek, Z.; Tichá, P.; Domonkos, M. Self-Healing of Cementitious Materials via Bacteria: A Theoretical Study. Crystals 2022, 12, 920. [Google Scholar] [CrossRef]
- Bandlamudi, R.K.; Kar, A.; Ray Dutta, J. A review of durability improvement in concrete due to bacterial inclusions. Front. Built Environ. 2023, 9, 1095949. [Google Scholar] [CrossRef]
- Ramagiri, K.K.; Chintha, R.; Bandlamudi, R.K.; Kara De Maeijer, P.; Kar, A. Cradle-to-Gate Life Cycle and Economic Assessment of Sustainable Concrete Mixes—Alkali-Activated Concrete (AAC) and Bacterial Concrete (BC). Infrastructures 2021, 6, 104. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Memon, Z.A.; Balasbaneh, A.T.; Al-Kutti, W.A.; Mokhtar, N.; Othman, N.; Juki, M.I.; Noman, E.A.; Algaifi, H.A. Critical Analysis for Life Cycle Assessment of Bio-Cementitious Materials Production and Sustainable Solutions. Sustainability 2022, 14, 1920. [Google Scholar] [CrossRef]
- Garces, J.I.T.; Dollente, I.J.; Beltran, A.B.; Tan, R.R.; Promentilla, M.A.B. Life cycle assessment of self-healing geopolymer concrete. Clean. Eng. Technol. 2021, 4, 100147. [Google Scholar] [CrossRef]
- Jansson, C.; Northen, T. Calcifying cyanobacteria—The potential of biomineralization for carbon capture and storage. Curr. Opin. Biotechnol. 2010, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Lamérand, C.; Shirokova, L.S.; Bénézeth, P.; Rols, J.-L.; Pokrovsky, O.S. Carbon sequestration potential of Mg carbonate and silicate biomineralization in the presence of cyanobacterium Synechococcus. Chem. Geol. 2022, 599, 120854. [Google Scholar] [CrossRef]
- Kamennaya, N.A.; Ajo-Franklin, C.M.; Northen, T.; Jansson, C. Cyanobacteria as Biocatalysts for Carbonate Mineralization. Minerals 2012, 2, 338–364. [Google Scholar] [CrossRef]
- Wilcox, S.M.; Mulligan, C.N.; Neculita, C.M. Mineral Carbonation for Carbon Sequestration: A Case for MCP and MICP. Int. J. Mol. Sci. 2025, 26, 2230. [Google Scholar] [CrossRef]
- Goodchild-Michelman, I.M.; Church, G.M.; Schubert, M.G.; Tang, T.-C. Light and carbon: Synthetic biology toward new cyanobacteria-based living biomaterials. Mater. Today Bio 2023, 19, 100583. [Google Scholar] [CrossRef]
- Kazemian, M.; Shafei, B. Carbon sequestration and storage in concrete: A state-of-the-art review of compositions, methods, and developments. J. CO2 Util. 2023, 70, 102443. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Pang, S.D. Healing cement mortar by immobilization of bacteria in biochar: An integrated approach of self-healing and carbon sequestration. Cem. Concr. Compos. 2018, 86, 238–254. [Google Scholar] [CrossRef]
- Taharia, M.; Dey, D.; Das, K.; Sukul, U.; Chen, J.-S.; Banerjee, P.; Dey, G.; Sharma, R.K.; Lin, P.-Y.; Chen, C.-Y. Microbial induced carbonate precipitation for remediation of heavy metals, ions and radioactive elements: A comprehensive exploration of prospective applications in water and soil treatment. Ecotoxicol. Environ. Saf. 2024, 271, 115990. [Google Scholar] [CrossRef]
- Fahimizadeh, M.; Pasbakhsh, P.; Mae, L.S.; Tan, J.B.L.; Raman, R.K.S. Multifunctional, Sustainable, and Biological Non-Ureolytic Self-Healing Systems for Cement-Based Materials. Engineering 2022, 13, 217–237. [Google Scholar] [CrossRef]
- Nežerka, V.; Holeček, P.; Somr, M.; Tichá, P.; Domonkos, M.; Stiborová, H. On the Possibility of Using Bacteria for Recycling Finest Fractions of Concrete Waste: A Critical Review. Rev. Environ. Sci. Biotechnol. 2023, 22, 1–24. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Saha, A.; Ghosh, D.; Dam, B.; Samanta, A.K.; Dutta, S. Microbial Repairing of Concrete & Its Role in CO2 Sequestration: A Critical Review. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 7. [Google Scholar] [CrossRef]
- Saxena, A.; Abraham, A.; Sang, B.-I. Bio-Concrete for the Modern Era: Paving the Way for Future Construction. J. Ceram. Process. Res. 2024, 25, 1122–1141. [Google Scholar] [CrossRef]
- Elgendy, I.M.; Elkaliny, N.E.; Saleh, H.M.; Darwish, G.O.; Almostafa, M.M.; Metwally, K.; Yahya, G.; Mahmoud, Y.A.-G. Bacteria-Powered Self-Healing Concrete: Breakthroughs, Challenges, and Future Prospects. J. Ind. Microbiol. Biotechnol. 2025, 52, kuae051. [Google Scholar] [CrossRef]
- Wang, J.; Ersan, Y.C.; Boon, N.; De Belie, N. Application of Microorganisms in Concrete: A Promising Sustainable Strategy to Improve Concrete Durability. Appl. Microbiol. Biotechnol. 2016, 100, 2993–3007. [Google Scholar] [CrossRef]
- Andalib, R.; Abd Majid, M.Z.; Hussin, M.W.; Ponraj, M.; Keyvanfar, A.; Mirza, J.; Lee, H.-S. Optimum Concentration of Bacillus megaterium for Strengthening Structural Concrete. Constr. Build. Mater. 2016, 118, 180–193. [Google Scholar] [CrossRef]
- D’Costa, S.S. Mechanism of Biotransformation of Phosphogypsum by Urease and Carbonic Anhydrase Produced by Lysinibacillus sphaericus. Master’s Thesis, Goa University, Panaji, India, 2023. [Google Scholar]
- Okyay, T.O. Carbon Dioxide Sequestration Through Microbially-Induced Calcium Carbonate Precipitation Using Ureolytic Environmental Microorganisms. Available online: https://uh-ir.tdl.org/server/api/core/bitstreams/9dfd05bb-9ff7-4c25-a785-1cd8e175c540/content (accessed on 4 June 2025).
- Zhu, X.; Mignon, A.; Nielsen, S.D.; Zieger, S.E.; Koren, K.; Boon, N.; De Belie, N. Viability determination of Bacillus sphaericus after encapsulation in hydrogel for self-healing concrete via microcalorimetry and in situ oxygen concentration measurements. Cem. Concr. Compos. 2021, 119, 104006. [Google Scholar] [CrossRef]
- Chuenchom, C.; Intarasoontron, J.; Sorasitthiyanukarn, F.N.; Chindasiriphan, P.; Jongvivatsakul, P.; Thaiboonrod, S.; Likitlersuang, S.; Pungrasmi, W.; Rojsitthisak, P. Enhancing concrete self-healing capabilities of Bacillus sphaericus spores through the encapsulation in biopolymeric microcapsules. J. Sustain. Cem.-Based Mater. 2024, 13, 1582–1595. [Google Scholar] [CrossRef]
- Luhar, S.; Luhar, I.; Shaikh, F.U.A. A Review on the Performance Evaluation of Autonomous Self-Healing Bacterial Concrete: Mechanisms, Strength, Durability, and Microstructural Properties. J. Compos. Sci. 2022, 6, 23. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X. Self-healing of concrete cracks by use of bacteria-containing low alkali cementitious material. Constr. Build. Mater. 2018, 167, 1–14. [Google Scholar] [CrossRef]
- Khaliq, W.; Ehsan, M.B. Crack healing in concrete using various bio influenced self-healing techniques. Constr. Build. Mater. 2016, 102, 349–357. [Google Scholar] [CrossRef]
- Ivaškė, A.; Gribniak, V.; Jakubovskis, R.; Urbonavičius, J. Bacterial Viability in Self-Healing Concrete: A Case Study of Non-Ureolytic Bacillus Species. Microorganisms 2023, 11, 2402. [Google Scholar] [CrossRef] [PubMed]
- Fahimizadeh, M.; Abeyratne, A.; Mae, L.; Singh, R.; Pasbakhsh, P. Biological Self-Healing of Cement Paste and Mortar by Non-Ureolytic Bacteria Encapsulated in Alginate Hydrogel Capsules. Materials 2020, 13, 3711. [Google Scholar] [CrossRef]
- González, L.M.; Mukhitov, N.; Voigt, C.A. Resilient living materials built by printing bacterial spores. Nat. Chem. Biol. 2020, 16, 126–133. [Google Scholar] [CrossRef]
- Thongchom, C.; Laemthong, T.; Sangkeaw, P.; Yamasamit, N.; Keawsawasvong, S. Evaluation of encapsulated Bacillus subtilis bio-mortars for use under acidic conditions. Sci. Rep. 2024, 14, 25947. [Google Scholar] [CrossRef]
- Adilah, R.N.; Chiu, S.-T.; Hu, S.-Y.; Ballantyne, R.; Happy, N.; Cheng, A.-C.; Liu, C.-H. Improvement in the probiotic efficacy of Bacillus subtilis E20-stimulates growth and health status of white shrimp, Litopenaeus vannamei via encapsulation in alginate and coated with chitosan. Fish Shellfish Immunol. 2022, 125, 74–83. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef]
- Aytekin, B.; Mardani, A.; Yazıcı, Ş. State-of-art review of bacteria-based self-healing concrete: Biomineralization process, crack healing, and mechanical properties. Constr. Build. Mater. 2023, 378, 131198. [Google Scholar] [CrossRef]
- Mohammed, H.; Ortoneda-Pedrola, M.; Nakouti, I.; Bras, A. Experimental characterisation of non-encapsulated bio-based concrete with self-healing capacity. Constr. Build. Mater. 2020, 256, 119411. [Google Scholar] [CrossRef]
- Maqbool, S.; Singh, K. Effect of bacterial encapsulation in concrete: A review on applications and effects. Mater. Today Proc. 2020, 33, 1713–1719. [Google Scholar] [CrossRef]
- Yaqoob Wani, I.; Singh, K. Effect of encapsulated bacteria on concrete properties: A review. Mater. Today Proc. 2020, 33, 1706–1712. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Palou, M.T.; Varela, H.; Gunwant, D.; Kalauni, K.; Barluenga, G. Experimental and numerical study on post-fire self-healing concrete for enhanced durability. Sci. Rep. 2025, 15, 9731. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Gunwant, D.; Kalauni, K.; Palou, M.T. Experimental and numerical study on sustainable post-fire repair of concrete structures using bacterial self-healing mechanisms. Constr. Build. Mater. 2025, 474, 141175. [Google Scholar] [CrossRef]
- Zadeike, D.; Gaizauskaite, Z.; Basinskiene, L.; Zvirdauskiene, R.; Cizeikiene, D. Exploring Calcium Alginate-Based Gels for Encapsulation of Lacticaseibacillus paracasei to Enhance Stability in Functional Breadmaking. Gels 2024, 10, 641. [Google Scholar] [CrossRef]
- Klikova, K.; Holecek, P.; Nezerka, V.; Prosek, Z.; Konakova, D.; Demnerova, K.; Stiborova, H. Application of Sporosarcina pasteurii for the biomineralization of calcite in the treatment of waste concrete fines. Environ. Sci. Pollut. Res. 2025. [Google Scholar] [CrossRef]
- Wani, S.; Jan Gęca, M.; Selvaraj, T.; Shanmuga Priya, T. Assessing the influence of Bacillus megaterium and Bacillus sphaericus in cementitious materials: Promoting sustainability towards strength, durability and crack repair. Ain Shams Eng. J. 2024, 15, 102748. [Google Scholar] [CrossRef]
- Kliková, K.; Holeček, P.; Koňáková, D.; Stiborová, H.; Nežerka, V. Exploiting Bacillus pseudofirmus and Bacillus cohnii to promote CaCO3 and AFt phase formation for stabilizing waste concrete fines. Cem. Concr. Compos. 2025, 155, 105839. [Google Scholar] [CrossRef]
- Zhan, Q.; Zhou, J.; Wang, S.; Su, Y.; Liu, B.; Yu, X.; Pan, Z.; Qian, C. Crack self-healing of cement-based materials by microorganisms immobilized in expanded vermiculite. Constr. Build. Mater. 2021, 272, 121610. [Google Scholar] [CrossRef]
- Srinivas M, K.; Alengaram, U.J.; Ibrahim, S.; Phang, S.M.; Vello, V.; Jun, H.K.; Alnahhal, A.M. Evaluation of crack healing potential of cement mortar incorporated with blue-green microalgae. J. Build. Eng. 2021, 44, 102958. [Google Scholar] [CrossRef]
- ISO 14040; Life Cycle Assessment. ISO: Geneva, Switzerland, 2006.
- ISO 14044; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. ISO: Geneva, Switzerland, 2006.
- Ecoinvent Version 3.8. Available online: https://support.ecoinvent.org/ecoinvent-version-3.8 (accessed on 30 May 2025).
- OpenLCA.org|OpenLCA is a Free, Professional Life Cycle Assessment (LCA) and Footprint Software with a Broad Range of Features and Many Available Databases, Created by GreenDelta Since 2006. Available online: https://www.openlca.org/ (accessed on 4 May 2025).
- SimaPro-Sustainability Insights for Informed Changemakers. Available online: https://simapro.com/ (accessed on 30 May 2025).
- Justo-Reinoso, I.; Arena, N.; Reeksting, B.J.; Gebhard, S.; Paine, K. Bacteria-based self-healing concrete—A life cycle assessment perspective. Dev. Built Environ. 2023, 16, 100244. [Google Scholar] [CrossRef]
- ACI 318; Building Code Requirements for Structural Concrete and Commentary. American Concrete Institute: Farmington Hills, MI, USA, 2022.
- EN 1992-1-1; Eurocode 2: Design of Concrete Structures—Part 1-1: General Rules and Rules for Buildings. The European Union Per Regulation: Brussels, Belgium, 2004.
- ASTM C150; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2012.
- ISO 50500; Innovation Management. ISO: Geneva, Switzerland, 2017.

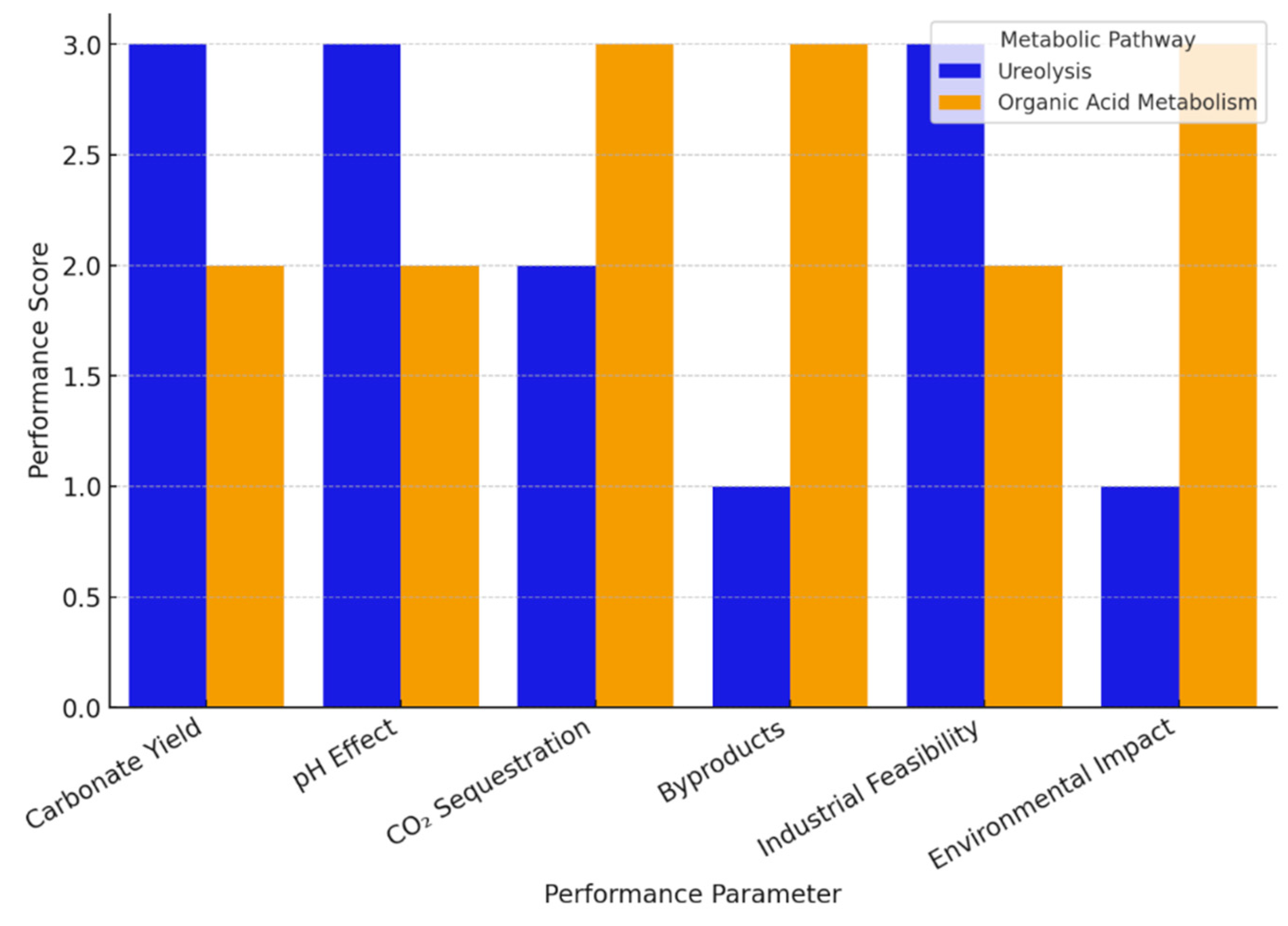

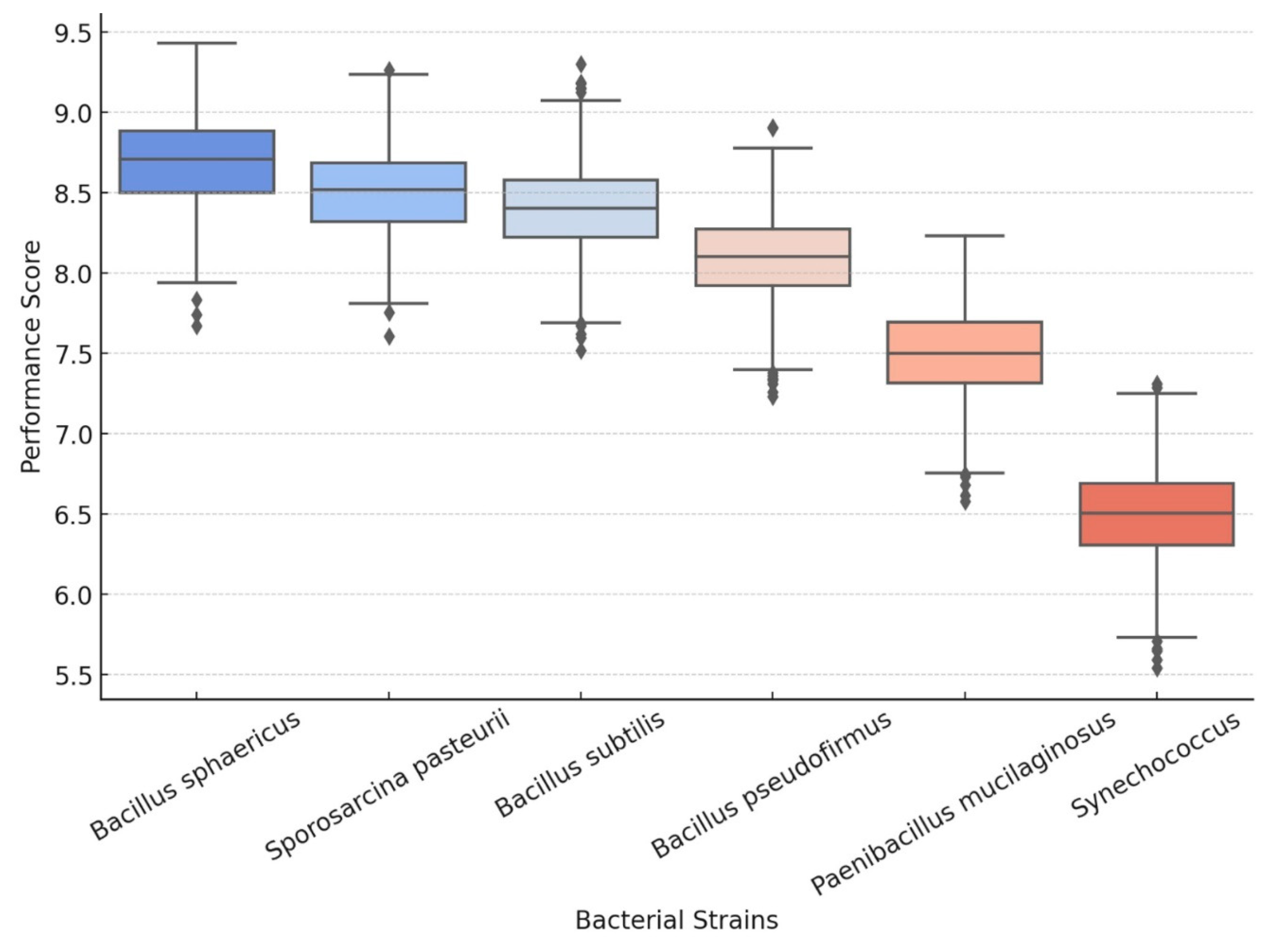
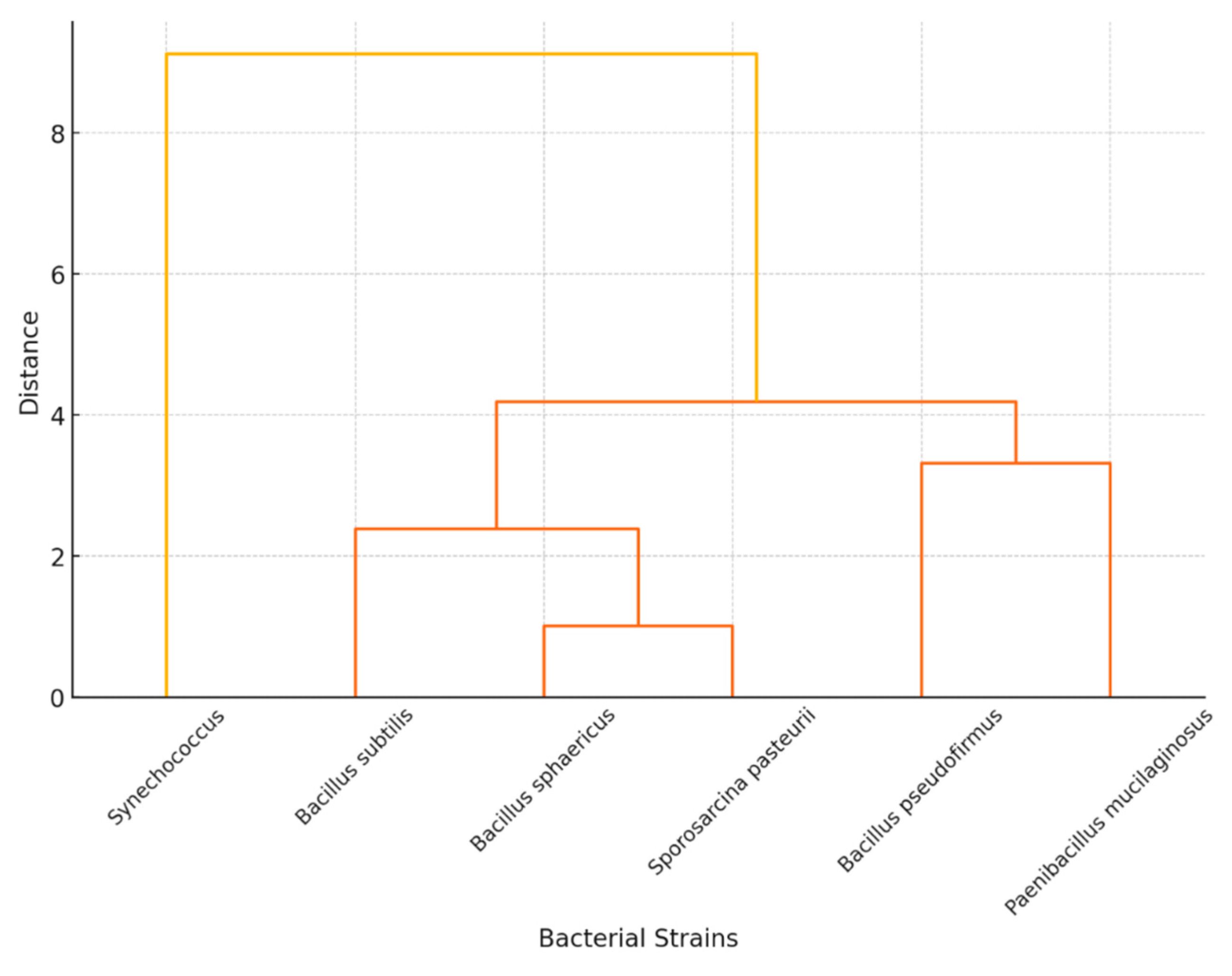
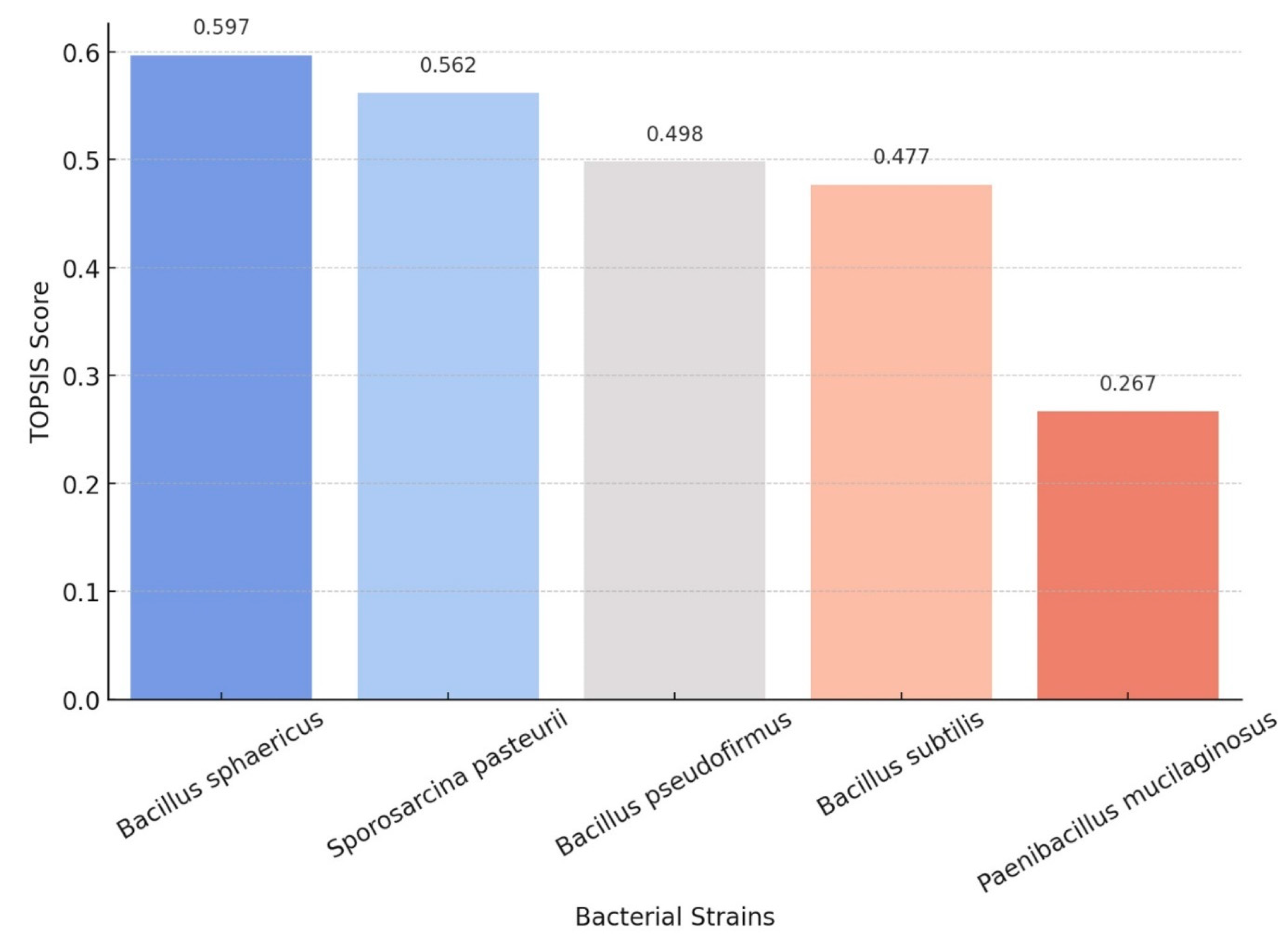


| Parameter | Ureolysis (Urease-Driven) | Organic Acid Metabolism (Fermentation-Driven) |
|---|---|---|
| Primary Bacteria | Sporosarcina pasteurii, Bacillus sphaericus | Bacillus pseudofirmus, Paenibacillus mucilaginosus |
| Carbonate Yield | High (fast CaCO3 precipitation) | Moderate (slower deposition) |
| pH Effect | Strong pH increase (>9) | Mild pH increase |
| CO2 Sequestration | Indirect (via ureolysis byproducts) | Indirect (via bicarbonate formation) |
| Byproducts | NH3 emissions | CO2, organic acids (sustainable) |
| Industrial Feasibility | High (fast reaction, proven application) | Moderate (requires carbon source) |
| Environmental Impact | NH3 pollution concerns | Low environmental impact |
| Bacterium | Precipitation Rate (mg CaCO3/g Biomass) | Primary Mechanism | Byproducts | Long-Term Stability | Refs. |
|---|---|---|---|---|---|
| Sporosarcina pasteurii | 80–100 | Ureolysis | NH3 | Moderate | [42] |
| Bacillus sphaericus | 75–90 | Ureolysis | NH3 | Moderate | [23] |
| Bacillus subtilis | 65–75 | Ureolysis + Organic Acid | NH3 + CO2 | Moderate–High | [43] |
| Bacillus pseudofirmus | 45–55 | Organic Acid Metabolism | CO2, Organic Acids | High | [44] |
| Paenibacillus mucilaginosus | 30–45 | Organic Acid Metabolism | CO2, Organic Acids | Very High | [45] |
| Synechococcus spp. | 100–120 | Photosynthesis | Oxygen, Carbonates | Low | [46] |
| Bacillus megaterium | 55–65 | Ureolysis + Organic Acid | NH3 + CO2 | Moderate–High | [47] |
| Lysinibacillus sphaericus | 85–95 | Ureolysis | NH3 | Moderate | [48] |
| Pseudomonas fluorescens | 40–50 | Organic Acid Metabolism | CO2, Organic Acids | High | [49] |
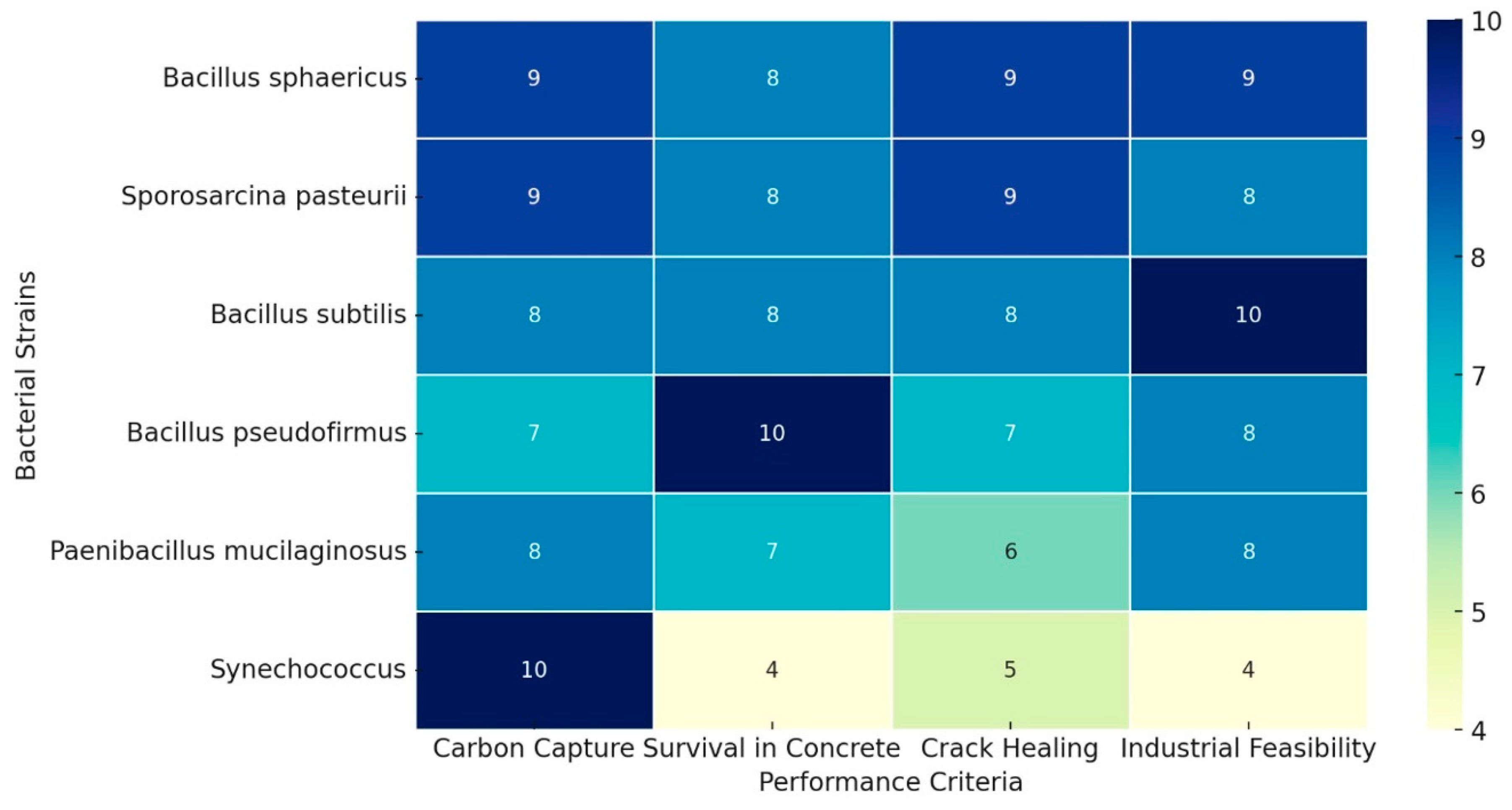
| Bacterium | Carbon Capture (40%) | Survival in Concrete (30%) | Crack Healing (10%) | Industrial Feasibility (20%) | Weighted Score (100) |
|---|---|---|---|---|---|
| Bacillus sphaericus | 9—High | 8—High | 9—High | 9—High | 87 |
| Sporosarcina pasteurii | 9—High | 8—High | 9—High | 8—Med–High | 85 |
| Bacillus subtilis | 8—High | 8—High | 8—High | 10—High | 84 |
| Bacillus pseudofirmus | 7—Mod | 10—Very High | 7—Mod | 8—Med–High | 81 |
| Paenibacillus mucilaginosus | 8—High | 7—Mod | 6—Mod | 8—Med–High | 75 |
| Synechococcus (cyanobacterium) | 10—Very High | 4—Low | 5—Low–Mod | 4—Low | 65 |
| Carrier | Dosage | Healing Performance | Compressive Strength Influence |
|---|---|---|---|
| Pluronic Hydrogel (non-ionic SAP) [52,61] | ~1% by cement mass | Heals 0.3–0.5 mm cracks with 40–90% healing ratio; 68% permeability reduction | ≈50% reduction vs. control (large voids upon drying) |
| Ca–Alginate Hydrogel (methacrylate-modified) [52,61] | 1% by cement mass | Heals ≈ 0.5 mm cracks; supplies internal moisture even at 90% RH | ≈30% compressive/≈23% tensile strength loss at 1% dosage (macrovoids on water release) |
| Chitosan-Enhanced CaAlg [56,63] | 1% total (CaAlg + chitosan, by cement mass) | Heals 1-mm-wide cracks with up to 40 mm depth; improved binder deposition | +10.28% (compressive)/+13.79% (flexural) vs. plain CaAlg—still below unmodified control |
| Diatomaceous Earth [61,62] | 5% by cement weight | 68% permeability reduction; 50% less water absorption | ≈10–15% reduction at 14 days (voids remain after nutrient leaching) |
| Expanded-Clay LWA [52,61] | 3% by volume | Heals ≈ 0.46 mm cracks in 2 weeks; maintains spore viability ≥ 6 months | ≈10% reduction at 14 days (increased porosity/air voids) |
| Melamine Microcapsules [52] | 3–5% by cement weight | Heals ≈ 0.97 mm cracks with 48–80% healing ratio | ≈34% drop at 5% dosage (greater loss at 5% vs. 3%) |
| Polyurethane (PU) Foam [52,62] | ≈5% by cement weight (typical) | Limited CaCO3 precipitation; modest permeability reduction | ≈10–15% compressive strength loss due to void formation (less severe than hydrogels) |
| Silica Gel [56,63] | ≈5% by cement weight (typical) | Improves permeability (≤68% reduction) | ≈10–15% early-age compressive strength loss |
| Expanded Perlite [63,64] | 5% by volume | Good CaCO3 precipitation; effective crack sealing | ≈10–20% compressive strength loss at 5% (porosity from leaching) |
| CSA-Based Encapsulation [64] | 100% CSA (carrier cement) for spores | Fully heals ≈ 0.394 mm cracks | ≈15% early-age compressive strength drop; faster recovery during healing cycles |
| Zeolite (diatomite-immobilized) [64] | ≈5% by cement weight (typical) | Reduces water absorption by one-third; good recovery after healing | ≈10% compressive strength penalty at 14 days |
| Fly Ash + SAP (superabsorbent polymer) [63,64] | 0.5% SAP by cement mass + varying fly ash ratios | SAP swells to seal cracks; healing improves as fly ash content increases | ≈15% compressive strength loss at 0.5% SAP by cement mass |
| Expanded Clay (cement paste-coated) [65] | 10–20% by volume | Effective post-fire self-healing; crack closure via Bacillus subtilis after exposure to 800 °C | 6.11–13.36% reduction vs. control (M40 grade) after 28 days |
| Carbon Fiber Bacteria Balls (CFBB) [65] | 10–20% by volume | Superior thermal protection and post-fire crack healing | 3.26–11.12% reduction after 28 days (better retention vs. expanded clay) |
| Gelatin Capsules Coated with Cement Paste [66] | 10–20% by volume | Maintains bacterial viability post-fire and enables self-healing activation | 9.25–17.43% reduction vs. control |
| Recycled Brick Aggregate (RA) with Bacteria [66] | 10–20% by volume | Effective healing and strength gain post-fire | 14.22–21.16% reduction after 28 days |
| Bacterium | Without Encapsulation (Original Score) | With Encapsulation (Revised Score) | Key Impact of Encapsulation |
|---|---|---|---|
| Bacillus sphaericus | 87 | 92 | Encapsulation increases crack healing and extends viability. |
| Sporosarcina pasteurii | 85 | 90 | Remains highly effective, with encapsulation improving survival. |
| Bacillus subtilis | 84 | 89 | Encapsulation in hydrogels not only increases bacterial survival but also accelerates carbonate formation, improving healing speed. |
| Bacillus pseudofirmus | 81 | 85 | Extreme alkaliphile, encapsulation boosts retention but healing is still slower. |
| Paenibacillus mucilaginosus | 75 | 82 | Encapsulation overcomes viability issues, increasing CO2 capture potential. |
| Synechococcus (cyanobacteria) | 65 | 75 | Only viable with engineered light exposure or surface application. |
| Bacterial Strain | Observations | Microstructure |
|---|---|---|
| Bacillus subtilis [17] |
| 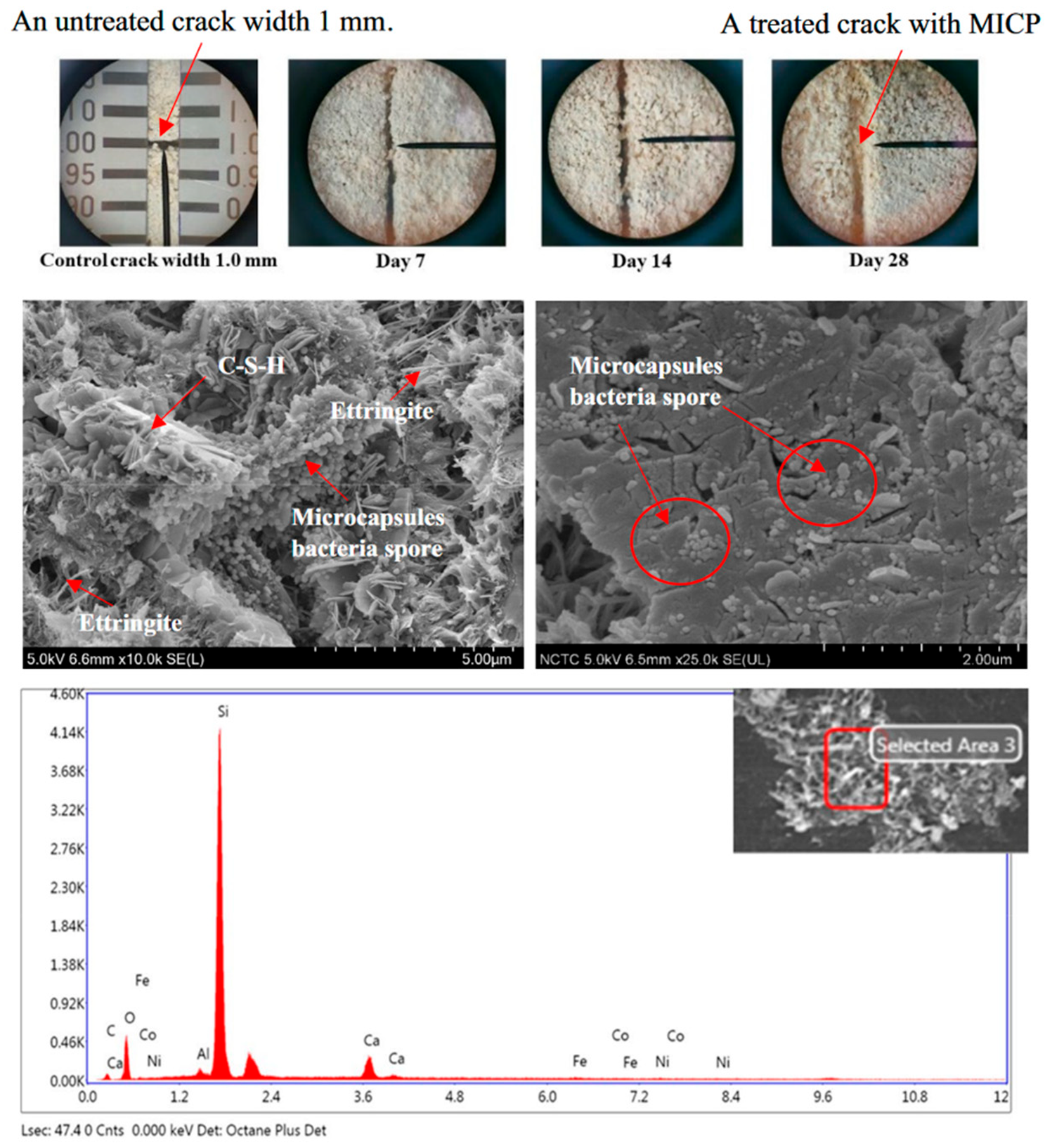 |
| Sporosarcina pasteurii [68] |
| 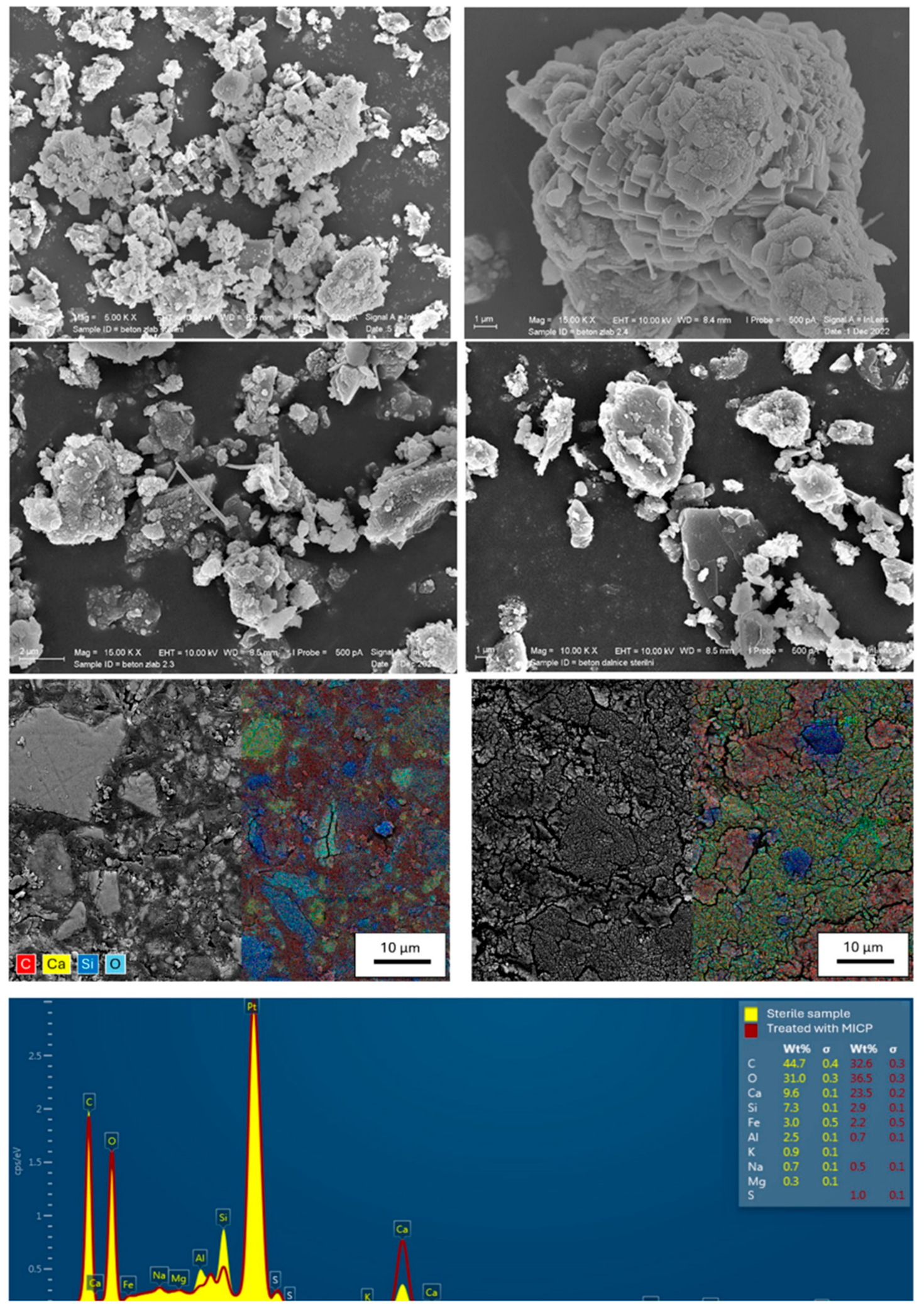 |
| Bacillus sphaericus (BS) [69] |
| 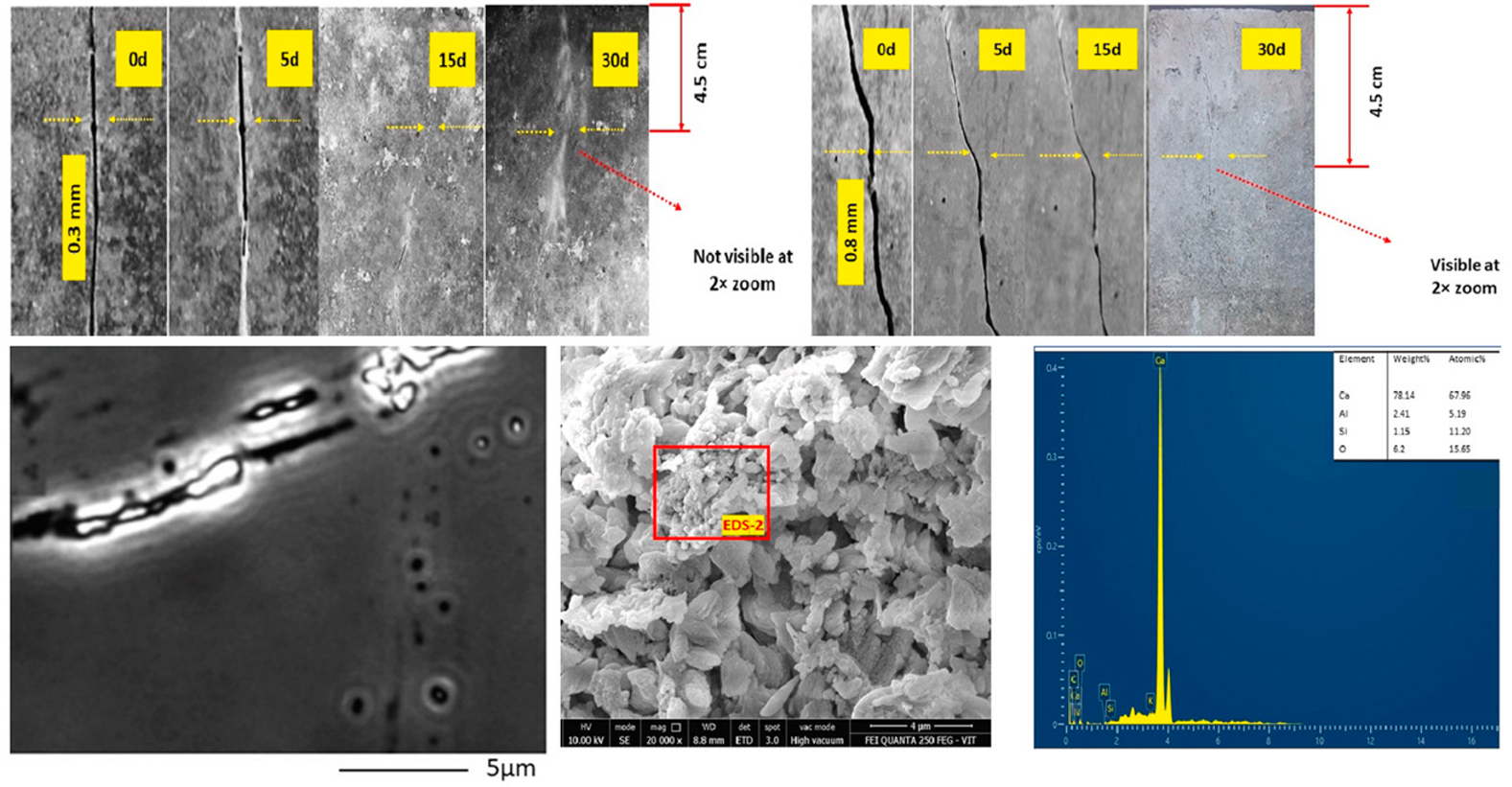 |
| Bacillus pseudofirmus [70] |
| 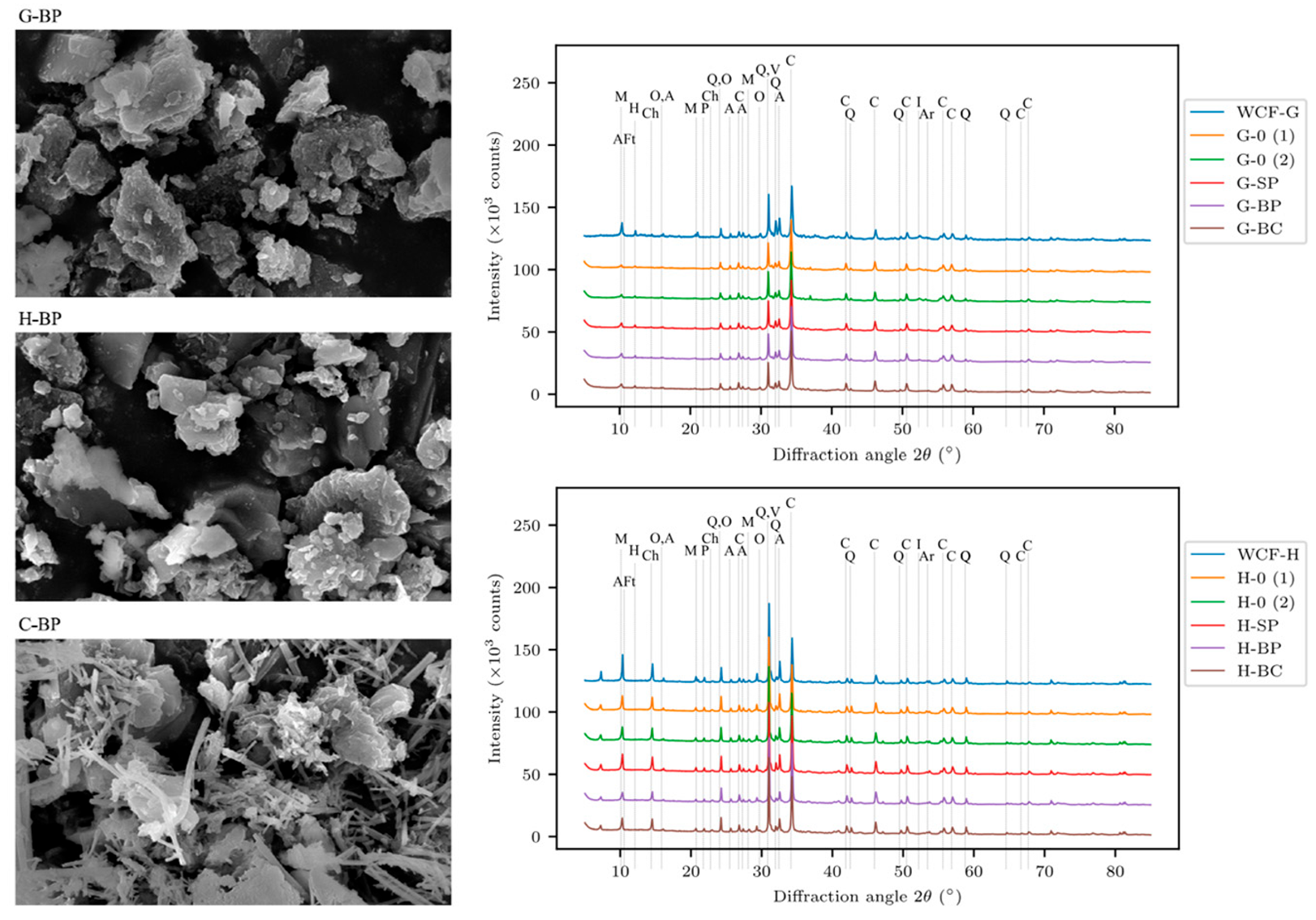 |
| Paenibacillus mucilaginosus [71] |
|  |
| Synechococcus (cyanobacterium) [72] |
|  |
| LCA Element (per ISO 14040/14044) | Description for Bacteria-Based Concrete | Data Source/Tool Used |
|---|---|---|
| 1. Goal and Scope Definition | Assess environmental performance of bacterial self-healing concrete vs. conventional concrete | Not Applicable |
| 2. Functional Unit | 1 m3 of bacterial concrete panel with self-healing capability | Not Applicable |
| 3. System Boundaries | Cradle-to-gate: includes bacteria cultivation, encapsulation, concrete production, and CO2 uptake | Ecoinvent v3.8, OpenLCA, Justo-Reinoso et al. (2023) [78] |
| 4. Life Cycle Inventory (LCI) | Incorporates estimates for CaCO3 precipitation (mg/g biomass), CO2 uptake (kg/m3), and cost inputs | Partially from literature; Ecoinvent |
| 5. Impact Categories | Impact assessment conducted using midpoint method—CML-IA baseline indicators, including global warming potential, cost efficiency (LCC), and material efficiency, in SimaPro 9.5 | Inferred from CO2 savings and costs |
| 6. Allocation Rules | System expansion applied to credit CO2 sequestration; cut-off approach used for nutrient and byproduct emissions unless otherwise noted | Not Applicable |
| 7. Data Quality and Uncertainty | Literature-derived estimates; few empirical measurements | Not Applicable |
| 8. Interpretation | Bacterial concrete enables ~35% cost savings and sequesters up to 22.5 kg CO2/m3 | Justo-Reinoso et al. (2023)/calculations [78] |
| 9. Uncertainty/Sensitivity | Uncertainty addressed via scenario range (15–30 kg CO2/m3); a Monte Carlo simulation on CO2 capture variability is proposed for future work | Not Applicable |
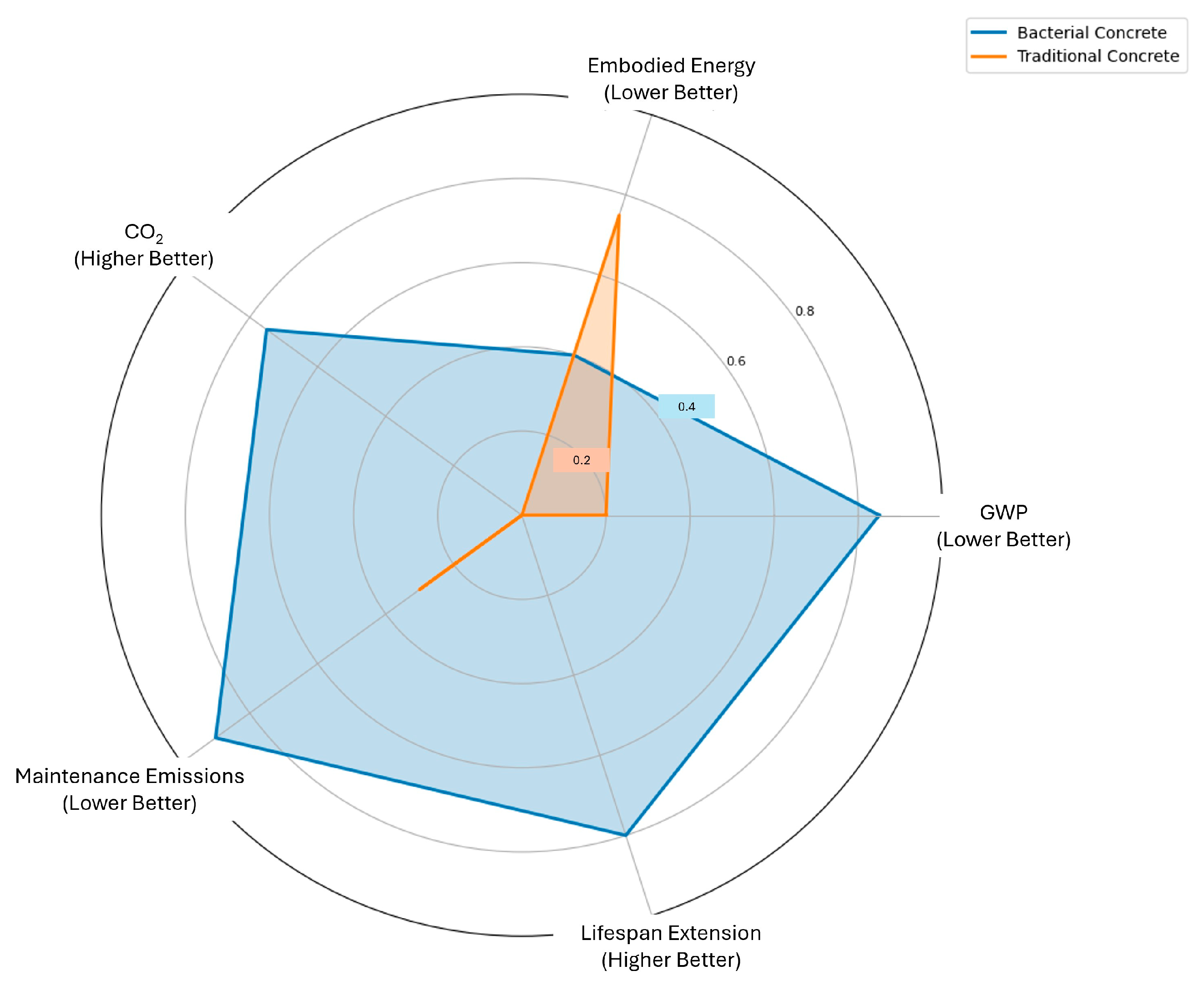
| LCA Indicator | Bacterial Self-Healing Concrete | Traditional Concrete(With Periodic Repairs) |
|---|---|---|
| Global Warming Potential (GWP)—kg CO2-eq/m3 | 200–250 | 350–450 |
| Embodied Energy (MJ/m3) | 2800–3500 | 2600–3000 |
| CO2 Sequestered over Lifetime (kg/m3) | 15–30 | Negligible |
| Maintenance-Related Emissions (kg CO2/m3 over 50 years) | 50–100 | 200–350 |
| Structural Lifespan Extension (%) | 30–50% | Baseline (no extension) |
| Cost Factor | Bacterial Self-Healing Concrete (per m3) | Conventional Concrete + Repair (per m3 over 50 Years) |
|---|---|---|
| Initial Material Cost | USD 150–250 (including encapsulation) | USD 80–120 |
| Bacterial Cultivation and Encapsulation | USD 30–50 | Not applicable |
| Installation and Construction Cost | USD 30–40 | USD 30–40 |
| Repair and Maintenance Costs (50 years) | USD 50–100 (minimal intervention required) | USD 250–500 (multiple repairs over time) |
| Total Life Cycle Cost (50 years) | USD 260–440 | USD 360–660 |
| Overall Cost Savings | 20–35% savings over the life cycle | Higher cumulative costs due to frequent repairs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vedrtnam, A.; Kalauni, K.; Palou, M.T. Ranking Bacteria for Carbon Capture and Self-Healing in Concrete: Performance, Encapsulation, and Sustainability. Sustainability 2025, 17, 5353. https://doi.org/10.3390/su17125353
Vedrtnam A, Kalauni K, Palou MT. Ranking Bacteria for Carbon Capture and Self-Healing in Concrete: Performance, Encapsulation, and Sustainability. Sustainability. 2025; 17(12):5353. https://doi.org/10.3390/su17125353
Chicago/Turabian StyleVedrtnam, Ajitanshu, Kishor Kalauni, and Martin T. Palou. 2025. "Ranking Bacteria for Carbon Capture and Self-Healing in Concrete: Performance, Encapsulation, and Sustainability" Sustainability 17, no. 12: 5353. https://doi.org/10.3390/su17125353
APA StyleVedrtnam, A., Kalauni, K., & Palou, M. T. (2025). Ranking Bacteria for Carbon Capture and Self-Healing in Concrete: Performance, Encapsulation, and Sustainability. Sustainability, 17(12), 5353. https://doi.org/10.3390/su17125353







