Flowering Patterns of Cornus mas L. in the Landscape Phenology of Roadside Green Infrastructure Under Climate Change Conditions in Serbia
Abstract
1. Introduction
2. Materials and Methods
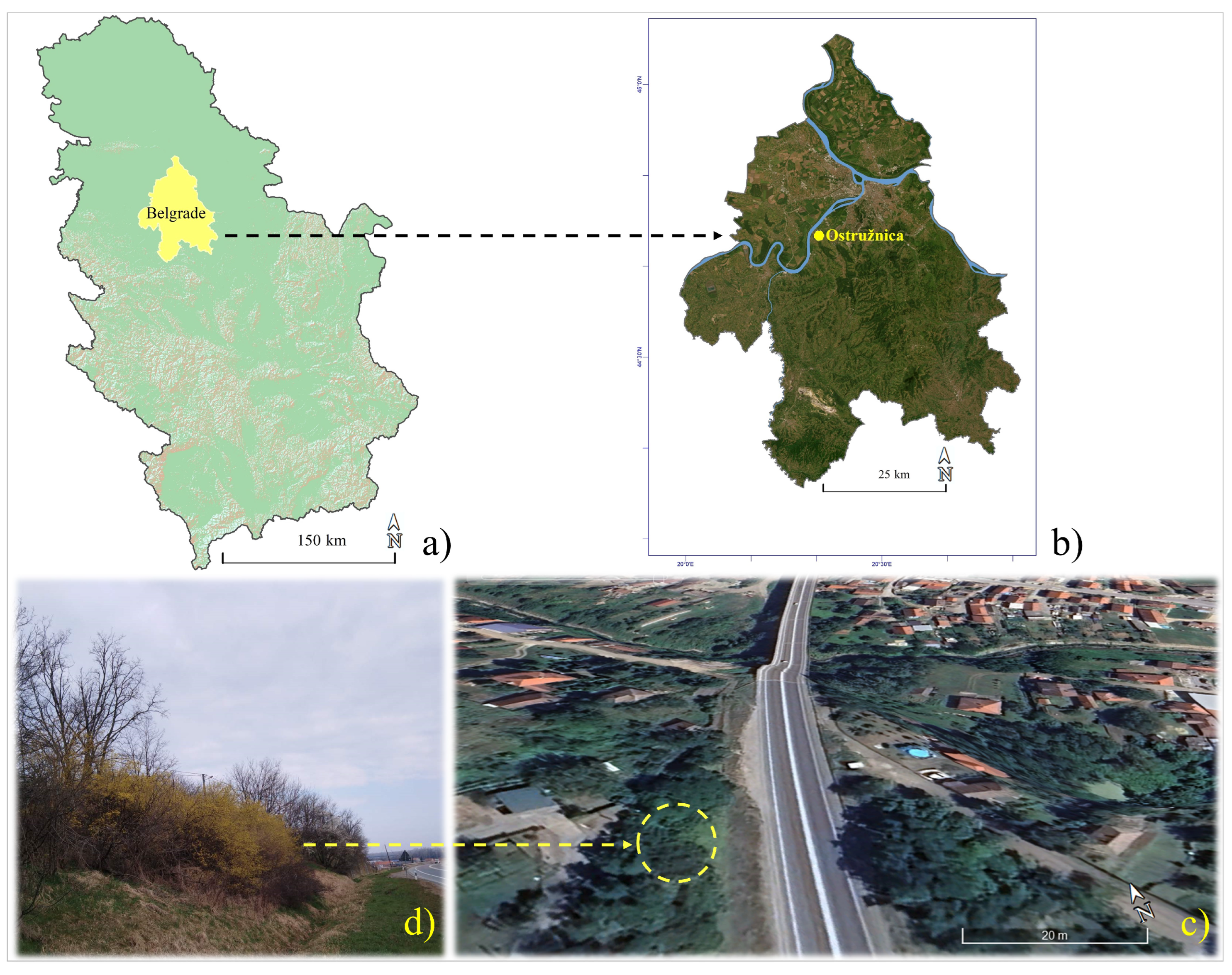
2.1. Study Site
2.2. Data Analysis
Phenology, Seasonality and Climatic Variables
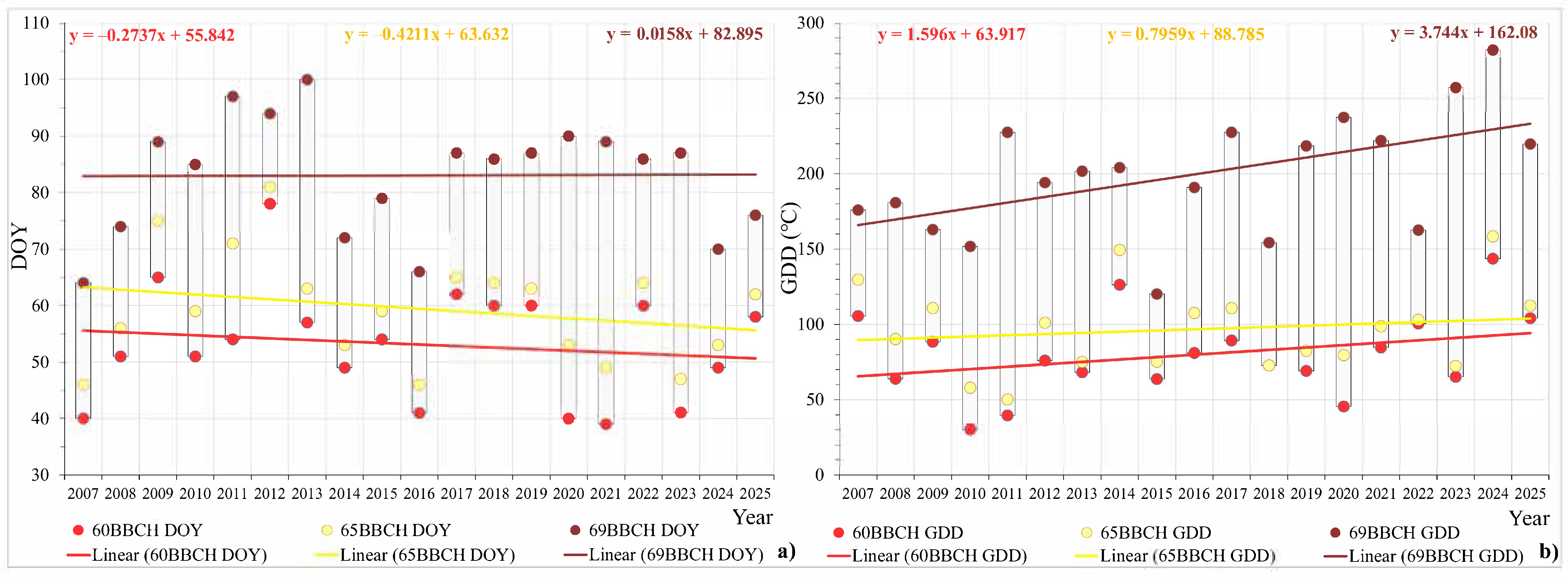
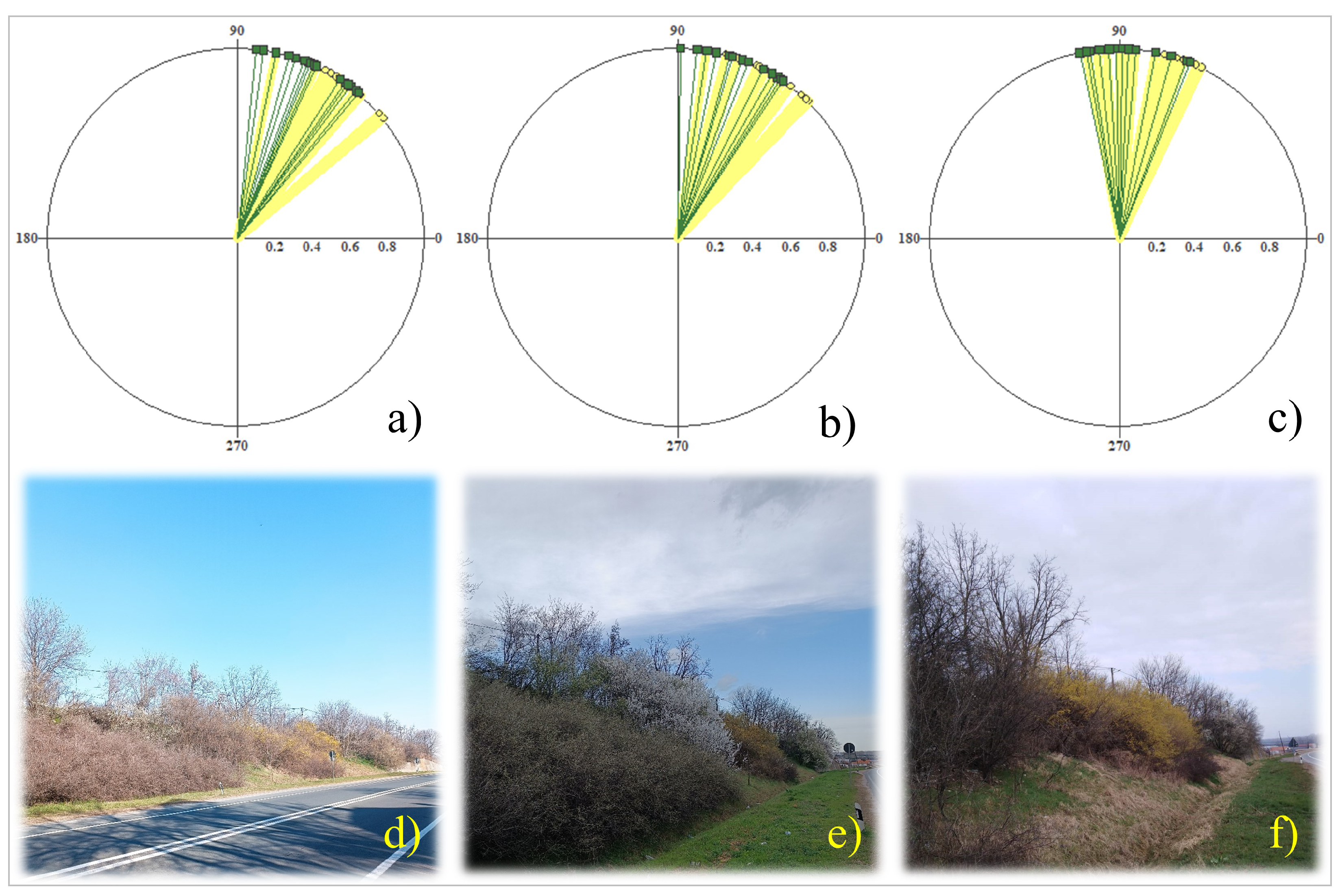
3. Results
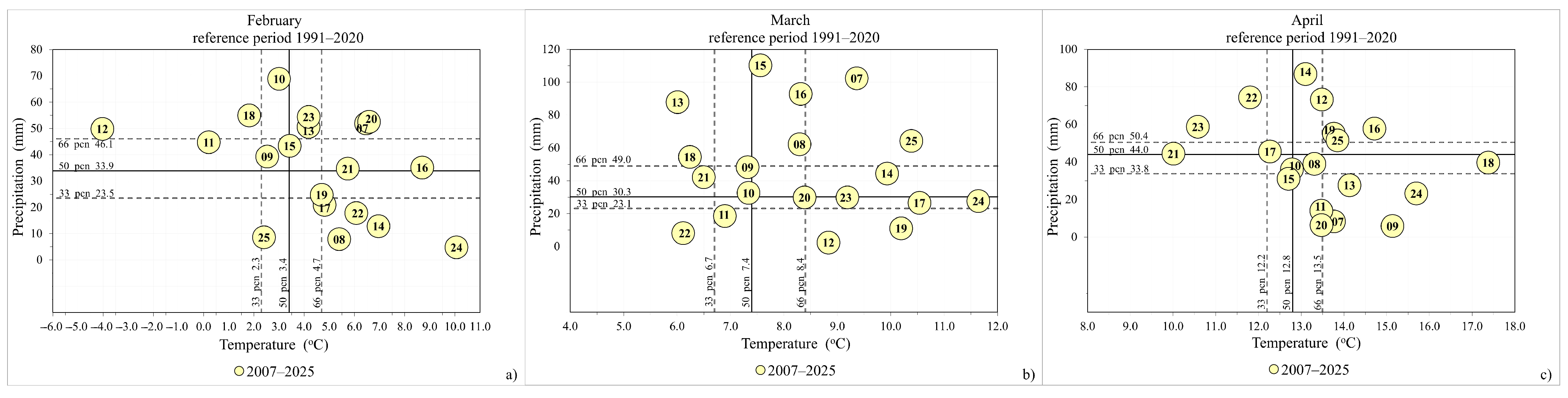
3.1. The Influence of Air Temperature and Precipitation on the Flowering Phenological Patterns of Cornelian Cherry
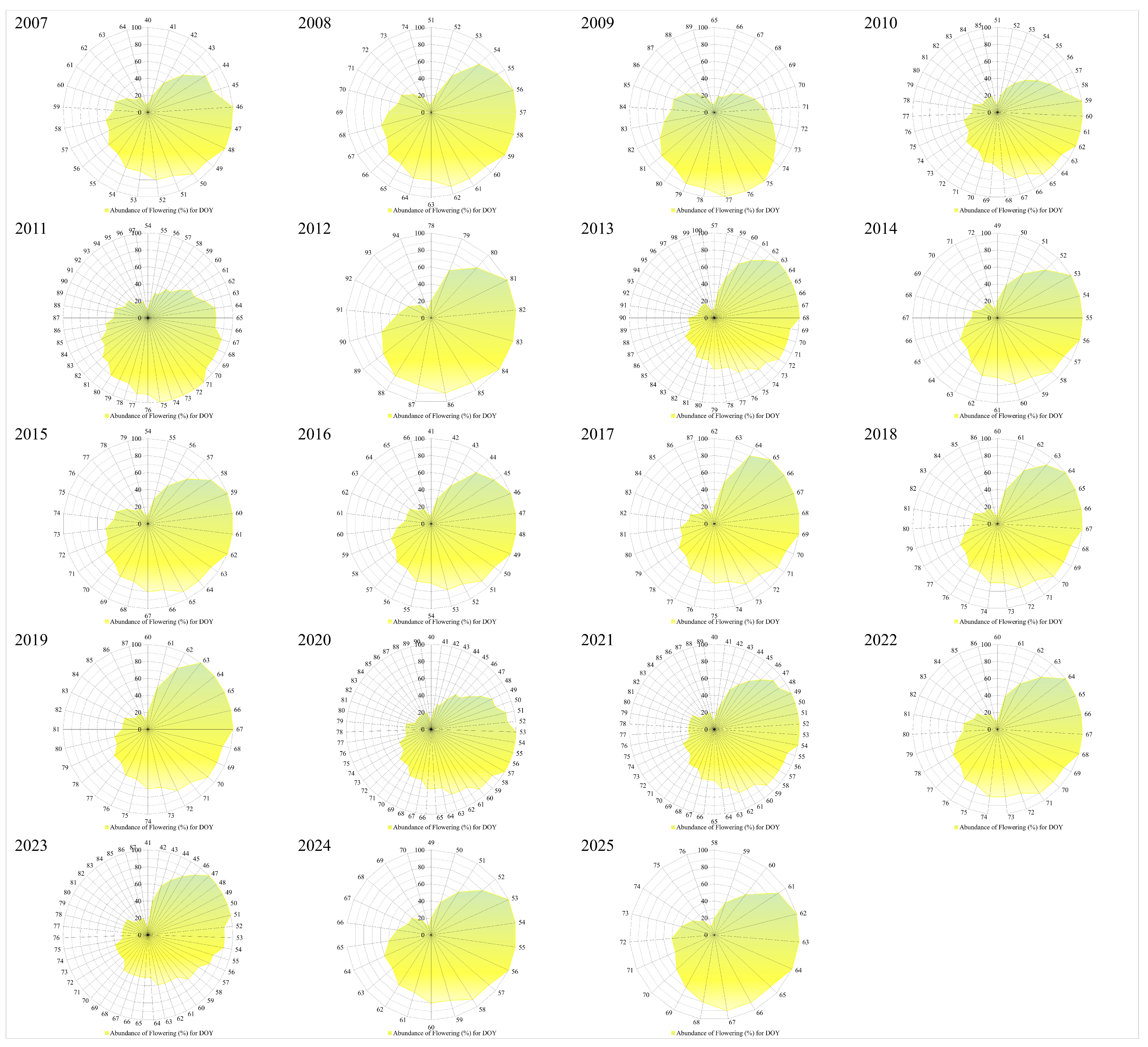
3.2. Phenology of Flowering, Seasonality, and Climatic Variables
3.3. Guidelines for Modelling the Landscape Phenology of Roadside Green Infrastructure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, M.D.; Betancourt, J.L.; Weltzin, J.F. From Caprio’s lilacs to the USA National Phenology Network. Front. Ecol. Environ. 2012, 10, 324–327. [Google Scholar] [CrossRef]
- Celton, J.M.; Martinez, S.; Jammes, M.J.; Bechtim, A.; Salvi, S.; Legave, J.M.; Costes, E. Deciphering the genetic determinism of bud phenology in apple progenies: A new insight into chilling and heat requirement effects on flowering dates and positional candidate genes. New Phytol. 2011, 192, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.M.; Smith, R.L. Elements of Ecology, 8th ed.; Benjamin Cummings: San Francisco, CA, USA, 2012. [Google Scholar]
- Hopkins, A.D. The bioclimatic law. Mon. Weather. Rev. 1920, 48, 355. [Google Scholar] [CrossRef]
- Coeterier, J.F. Hoe Beleven wij Onze Omgeving? Resultaten van 25 Jaar Omgevingspsychologisch Onderzoek in Stad en Landschap; Alterra, Research Instituut voor de Groene Ruimte: Wageningen, The Netherlands, 2000. [Google Scholar]
- Stolze, M.; Piorr, A.; Haring, A.; Dabbert, S. The Environmental Impacts of Organic Farming in Europe. Organic Farming in Europe: Economics and Policy; University of Hohenheim: Stuttgart, Germany, 2000; Volume 6. [Google Scholar]
- Olliff-Yang, R.L.; Ackerly, D.D. Topographic heterogeneity lengthens the duration of pollinator resources. Ecol. Evol. 2020, 10, 9301–9312. [Google Scholar] [CrossRef]
- Koebsch, F.; Sonnentag, O.; Järveoja, J.; Peltoniemi, M.; Alekseychik, P.; Aurela, M.; Arslan, A.; Dinsmore, K.; Gianelle, D.; Helfter, C.; et al. Refining the role of phenology in regulating gross ecosystem productivity across European peatlands. Glob. Change Biol. 2020, 26, 876–887. [Google Scholar] [CrossRef]
- Ruml, M.; Vulić, T. Spatial-temporal distribution of the general flowering of plum požegača in Serbia. Prostorno-vremenska distribucija opšteg cvetanja šljive požegače u Srbiji; Arhiv za poljoprivredne nauke. J. Sci. Agric. Res. 2004, 65, 61–69. (In Serbian) [Google Scholar]
- Piao, S.; Friedlingstein, P.; Ciais, P.; Viovy, N.; Demarty, J. Growing Season Extension and its Impact on Terrestrial Carbon Cycle in the Northern Hemisphere over the Past 2 Decades. Glob. Biogeochem. Cycles 2007, 21, GB3018. [Google Scholar] [CrossRef]
- Ocokoljić, M.; Petrov, D. Decorative Dendrology; Univerzitet u Beogradu-Šumarski Fakultet: Belgrade, Serbia, 2022; p. 409. (In Serbian) [Google Scholar]
- Foster, S. Forest Pharmacy: Medicinal Plants in American Forests; Forest History Society: Durham, NC, USA, 1955; p. 57. [Google Scholar]
- Milberg, P.; Bergman, K.O.; Cronvall, E.; Eriksson, Ĺ.I.; Glimskär, A.; Islamovic, A. Flower abundance and vegetation height as predictors for nectarfeeding insect occurrence in Swedish semi-natural grasslands. Agric. Ecosyst. Environ. 2016, 230, 47–54. [Google Scholar] [CrossRef]
- Bernes, C.; Bullock, J.M.; Jakobsson, S.; Rundlöf, M.; Verheyen, K.; Lindborg, R. How are biodiversity and dispersal of species affected by the management of roadsides? A systematic map protocol. Environ. Evid. 2017, 5, 4. [Google Scholar] [CrossRef][Green Version]
- Akyol, A.; Orucu, O. Investigation of Cornelian Cherry (Cornus mas L.) in the Scope of Non-Wood Forest Products According to Climate Change Scenarios and Species Distribution Mode. Eur. J. Sci. Technol. 2019, 17, 224–233. [Google Scholar] [CrossRef]
- Zhang, K.; Chui, T.F.M. Linking hydrological and bioecological benefits of green infrastructures across spatial scales—A literature review. Sci. Total Environ. 2019, 646, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- Boira, J.V.; Berzi, M. Liberalization, Trans-European Corridors and EU Funds: A New Scenario in the Relationship between Rail Networks and Mediterranean Cities. Land 2023, 12, 1986. [Google Scholar] [CrossRef]
- Hapriyanto, A.R.; Azmi, H. Implementation of Green Infrastructure in Sustainable Transportation in Supporting Urban Mobility: A Literature Review. Eng. Proc. 2025, 84, 25. [Google Scholar] [CrossRef]
- Anjos, L.; Gaistardo, C.; Deckers, J.; Dondeyne, S.; Eberhardt, E.; Gerasimova, M.; Harms, B.; Jones, A.; Krasilnikov, P.; Reinsch, T.; et al. World Reference Base for Soil Resources 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; Schad, P., Van Huyssteen, C., Micheli, E., Eds.; FAO: Rome, Italy, 2015; JRC91947. [Google Scholar]
- Meier, U. BBCH-Monograph. Growth Stages of Plants; Blackwell Wissenschafts-Verla: Berlin, Germany; Vienna, Austria, 1997; p. 622. [Google Scholar]
- Koch, E.; Bruns, E.; Chmielewski, F.M.; Defila, C.; Lipa, W.; Menzel, A. Guidelines for plant phenological observations. World Clim. Data Monit. Programme 2007, 1484, 1–41. [Google Scholar]
- Lalić, B.; Ejcinger, J.; Dalamarta, A.; Orlandini, S.; Firanj Sremac, A.; Paher, B. Meteorology and Climatology for Agronomists; Meteorologija i klimatologija za agronome; Univerzitet u Novom Sadu-Poljoprivredni Fakultet: Novi Sad, Serbia, 2021; p. 219. (In Serbian) [Google Scholar]
- Stilinović, S. Seed Production of Forest and Ornamental Trees and Shrubs; Semenarstvo Šumskog i Ukrasnog Drveća i Žbunja; University of Belgrade—Faculty of Forestry: Belgrade, Serbia, 1985; p. 399. (In Serbian) [Google Scholar]
- Morellato, L.P.C.; Alberti, L.F.; Hudson, I.L. Applications of Circular Statistics in Plant Phenology: A Case Studies Approach. In Phenological Research: Methods for Environmental and Climate Change Analysis; Hudson, I.L., Keatley, M.R., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 339–359. [Google Scholar] [CrossRef]
- Migliavacca, M.; Sonnentag, O.; Keenan, T.F.; Cescatti, A.; O’Keefe, J.; Richardson, A.D. On the uncertainty of phenological responses to climate change, and implications for a terrestrial biosphere model. Biogeosciences 2012, 9, 2063–2083. [Google Scholar] [CrossRef]
- Ibanez-Rueda, N.; Guillen-Royo, M.; Guardiola, J. Pro-Environmental Behavior, Connectedness to Nature, and Wellbeing Dimensions among Granada Students. Sustainability 2020, 12, 9171. [Google Scholar] [CrossRef]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Vujičić, D.; Vasiljević, N.; Radić, B.; Tutundžić, A.; Galečić, N.; Skočajić, D.; Ocokoljić, M. Conceptualisation of the Regulatory Framework of Green Infrastructure for Urban Development: Identifying Barriers and Drivers. Land 2024, 13, 692. [Google Scholar] [CrossRef]
- Gebru, G.H. Creating Interconnected Outdoor Green Infrastructure in The Case of Aksum Historical Sites, Ethiopia. CSID J. Infrastruct. Dev. 2023, 6, 2. [Google Scholar]
- Di Turo, F.; Medeghini, L. How Green Possibilities Can Help in a Future Sustainable Conservation of Cultural Heritage in Europe. Sustainability 2021, 13, 3609. [Google Scholar] [CrossRef]
- Grabowski, Z.J.; McPhearson, T.; Matsler, A.M.; Groffman, P.; Pickett, S.T.A. What is green infrastructure? A study ofdefinitions in US city planning. Front. Ecol. Environ. 2022, 20, 152–160. [Google Scholar] [CrossRef]
- Chatzimentor, A.; Apostolopoulou, E.; Mazaris, A.D. A review of green infrastructure research in Europe: Challenges and opportunities. Landsc. Urban Plan. 2020, 198, 103775. [Google Scholar] [CrossRef]
- Milton, S.J.; Dean, W.R.J. Plant invasions in arid areas: Special problems and solutions—A South African perspective. Biol. Invasions 2010, 12, 3935–3948. [Google Scholar] [CrossRef]
- Stojanović, V.; Jovanović, I. Survey of invasive and potentially invasive plant species in the Republic of Serbia and the environment with the aim of determining their status at the national level. Nat. Conserv. 2018, 68, 41–59. (In Serbian) [Google Scholar]
- Kalansuriya, C.M.; Pannila, A.S.; Sonnadara, D.U.J. Effect of Roadside Vegetation on the Reduction of Traffic Noise Levels. In Proceedings of the Technical Sessions Conference, Colombo, Sri Lanka, 14 March 2009; Institute of Physics Sri Lanka (IPSL): Colombo, Sri Lanka, 2009; Volume 25, pp. 1–6. [Google Scholar]
- Batrićević, A.; Batanjski, V. Zaštita Životinja u Srbiji—Kaznenopravni i Ekološki Aspekti; Institut za Kriminološka i Sociološka Istraživanja: Beograd, Srbija, 2014; p. 231. [Google Scholar]
- Ocokoljić, M.; Petrov, D.; Vujičić, D.; Galečić, N. Effects of Climate Parameters on Blackthorn in Agroforestry Ecotones as Green road Infrastructure Elements. In Proceedings of the XIV International Scientific Agriculture Symposium AGROSYM, Jahorina, Bosnia and Hercegovina, 5–8 October 2023; Kovacevic, D., Ed.; Faculty of Agriculture: East Sarajevo, Bosnia and Hercegovina, 2023; pp. 853–858. [Google Scholar]
- Csontos, P.; Tamás, J.; Kalapos, T. Correlation between age basal diameter of Fraxinus ornus L. in three ecologically contrasting habitats. Acta Bot. Hung. 2001, 43, 127–136. [Google Scholar] [CrossRef]
- Bijelić, S.; Gološin, B.; Cerović, S.; Bogdanović, B. Pomological Characteristics of Cornelian Cherry (Cornus mas L.) Selections in Serbia and the Possibility of Growing in Intensive Organic Orchards. Acta Univ. Agric. Silvic. Mendel. Brun. 2015, 63, 1101–1104. [Google Scholar] [CrossRef]
- Jaćimović, V.; Božović, D.J.; Veličković, N.; Šebek, G. Biological-pomological characteristics of dogwood genotypes (Cornus mas L.) from the area of Gornji Polimlje. Jugosl. Voćarstvo 2000, 34, 99–105. (In Serbian) [Google Scholar]
- Dörken, V. Pflanzenporträt: Cornus mas—Kornelkirsche (Cornaceae). Jahrb. Boch. Bot. Vereins 2010, 1, 213–215. [Google Scholar]
- Cevriye, M. Studies on the Structure of Flowers and Inflorescences of Cornelian Cherry (Cornus mas L.). Not. Bot. Horti Agrobot. 2012, 40, 53–57. [Google Scholar]
- Klimenko, S. The Cornelian cherry (Cornus mas L.)—Collection, preservation and utilization of genetic resources. J. Fruit. Ornam. Plant Res. 2004, 12, 93–98. [Google Scholar]
- Vujanić-Varga, D. Pomology; Univerzitet u Novom Sadu—Poljoprivredni fakultet: Novi Sad, Serbia, 1987. (In Serbian) [Google Scholar]
- Ocokoljić, M.; Petrov, D.; Galečić, N.; Skočajić, D.; Košanin, O.; Simović, I. Phenological Flowering Patterns of Woody Plants in the Function of Landscape Design: Case Study Belgrade. Land 2023, 12, 706. [Google Scholar] [CrossRef]
- Čukanović, J.; Ljubojević, M.; Djordjević, S.; Narandžić, T.; Petrov, D.; Ocokoljić, M. The Impact of Climate Variability on the Blooming of Fraxinus ornus ‘Globosa’ as a Component of Novi Sad’s (Serbia) Green Infrastructure. Sustainability 2024, 16, 8404. [Google Scholar] [CrossRef]
- Blonsky, M.; Nagarajan, A.; Ghosh, S.; McKenna, K.; Veda, S.; Kroposki, B. Potential Impacts of Transportation and Building Electrification on the Grid: A Review of Electrification Projections and Their Effects on Grid Infrastructure, Operation, and Planning. Curr. Sustain. Energy Rep. 2019, 6, 169–176. [Google Scholar] [CrossRef]
- Chrzova, B.; Grabovac, A.; Hala, M.; Lalic, J.; Bashota, V.; Bjeloš, M.; Latal, S.; Naunov, M.; Semani’c, H.; Wilson, A.; et al. Western Balkans at the Crossroads: Assessing Influences of Non-Western External Actors; Prague Security Studies Institute: Prague, Czech Republic, 2019. [Google Scholar]
- Karaduman, H.A.; Karaman-Akgul, A.; Çağlar, M.; Akbaş, H.E. The relationship between logistics performance and carbon emissions: An empirical investigation on Balkan countries. Int. J. Clim. Change Strateg. Manag. 2020, 12, 449–461. [Google Scholar] [CrossRef]
- European Commission. Sustainable and Smart Mobility Strategy-Putting European Transport on Track for the Future; European Commission: Brussels, Belgium, 2020.
- Petrov, D.; Ocokoljić, M.; Galečić, N.; Skočajić, D.; Simović, I. Adaptability of Prunus cerasifera Ehrh. to Climate Changes in Multifunctional Landscape. Atmosphere 2024, 15, 335. [Google Scholar] [CrossRef]
- John, C.; Kerby, J.T.; Stephenson, T.R.; Post, E. Fine-scale landscape phenology revealed through time-lapse imagery: Implications for conservation and management of an endangered migratory herbivore. Remote. Sens. Ecol. Conserv. 2023, 9, 628–640. [Google Scholar] [CrossRef]
- Wilczek, A.M.; Burghardt, L.T.; Cobb, A.R.; Cooper, M.D.; Welch, S.M.; Schmitt, J. Genetic and physiological bases for phenological responses to current and predicted climates. Philos. Trans. R. Soc. Lond. B Biol. Sc. 2010, 365, 3129–3147. [Google Scholar] [CrossRef]
- Saxena, K.G.; Rao, K.S. Climate Change and Vegetation Phenology. In Reproductive Ecology of Flowering Plants: Patterns and Processes; Tandon, R., Shivanna, K., Koul, M., Eds.; Springer: Singapore, 2020; pp. 25–39. [Google Scholar] [CrossRef]
- Miller-Rushing, A.J.; Katsuki, T.; Primack, R.B.; Ishii, Y.; Lee, S.D.; Higuchi, H. Impact of global warming on a group of related species and their hybrids: Cherry tree (Rosaceae) flowering at Mt. Takao, Japan. Am. J. Bot. 2007, 94, 1470–1478. [Google Scholar] [CrossRef]
- Inouye, D.W. Effects of climate change on phenology, frost damage, and floral abundance of montane wild flowers. Ecology 2008, 89, 353–362. [Google Scholar] [CrossRef]
- Cushman, S.A. Generalizing Boltzmann configurational entropy to surfaces, point patterns and landscape mosaics. Entropy 2021, 23, 1616. [Google Scholar] [CrossRef]
- Burroughs, W.J. Gardening and climate change. Weather 2002, 5, 151–157. [Google Scholar] [CrossRef][Green Version]
- Forrest, J.; Thomson, J.D. Pollinator experience, neophobia and the evolution of flowering time. Proc. R. Soc. B 2009, 276, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Tarun, K.; Bhanu, V. GDD (Growing Degree Days): Phenology Forecasting for Crops. Just Agric. 2022, 2, 2582–8223. [Google Scholar]
- Feng, C.M.; Xiang, Q.Y.; Franks, R.G. Phylogeny-based developmental analyses illuminate evolution of inflorescence architectures in dogwoods (Cornus sl., Cornaceae). New Phytol. 2011, 191, 850–869. [Google Scholar] [CrossRef]
- Silva, I.A.; Da Silva, D.M.; De Carvalho, G.H.; Batalha, M.A. Reproductive phenology of Brazilian savannas and riparian forests: Environmental and phylogenetic issues. Ann. For. Sci. 2011, 68, 1207–1215. [Google Scholar] [CrossRef]
- CaraDonna, P.J.; Inouye, D.W. Phenological responses to climate change do not exhibit phylogenetic signal in a subalpine plant community. Ecology 2015, 96, 355–361. [Google Scholar] [CrossRef]


| Parameter | Kendall’s Tau | p-Value | Sen’s Slope |
|---|---|---|---|
| 60BBCH DOY | −0.066 | 0.725 | −0.125 |
| 65BBCH DOY | −0.143 | 0.419 | −0.500 |
| 69BBCH DOY | 0.000 | 1.000 | 0.000 |
| 60BBCH GDD | 0.205 | 0.234 | 1.600 |
| 65BBCH GDD | 0.099 | 0.576 | 0.862 |
| 69BBCH GDD | 0.392 | 0.021 * | 4.029 |
| Phenological Observations | DOY | GDD | |||||
|---|---|---|---|---|---|---|---|
| Parameters | 60BBCH | 65BBCH | 69BBCH | 60BBCH | 65BBCH | 69BBCH | |
| N | 19 | 19 | 19 | 19 | 19 | 19 | |
| Min | 39 | 46 | 64 | 30.1 | 50.2 | 120.1 | |
| Max | 78 | 81 | 100 | 143.6 | 158.4 | 282.1 | |
| Sum | 1009 | 1129 | 1578 | 1517.6 | 1838.1 | 3791 | |
| Mean | 53.10526 | 59.42105 | 83.05263 | 79.87368 | 96.74211 | 199.5263 | |
| Std. error | 2.358132 | 2.233865 | 2.346422 | 6.490402 | 6.570621 | 9.174924 | |
| Variance | 105.655 | 94.81287 | 104.6082 | 800.3809 | 820.2881 | 1599.405 | |
| Stand. dev | 10.27886 | 9.73719 | 10.22781 | 28.291 | 28.64067 | 39.99257 | |
| Median | 54 | 59 | 86 | 76 | 98.7 | 201.7 | |
| 25 prcntil | 41 | 53 | 74 | 63.9 | 75.1 | 162.9 | |
| 75 prcntil | 60 | 64 | 89 | 100.5 | 110.8 | 227.4 | |
| Skewness | 0.4552988 | 0.480130 | −0.38977 | 0.4267544 | 0.5603838 | 0.0544626 | |
| Kurtosis | 0.3206357 | −0.10568 | −0.66438 | 0.3621626 | 0.0771781 | −0.096638 | |
| Geom. mean | 52.17779 | 58.68262 | 82.43102 | 74.84929 | 92.78957 | 195.5902 | |
| Coeff. var | 19.35563 | 16.38677 | 12.31486 | 35.41968 | 29.60518 | 20.04376 | |
| Variable | Min | Max | Mean | Std. Dev. |
|---|---|---|---|---|
| No. of days 60BBCH-65BBCH | 3.0 | 17.0 | 6.3 | 3.8 |
| Average T in 60BBCH-65BBCH DOY | −2.0 | 15.3 | 6.5 | 4.3 |
| No. of days 65BBCH-69BBCH | 13.0 | 40.0 | 23.6 | 8.7 |
| Average T in 65BBCH-69BBCH DOY | 5.3 | 11.9 | 8.4 | 2.2 |
| No. of days 60BBCH-69BBCH | 16.0 | 50.0 | 29.9 | 10.8 |
| Average T in 60BBCH-69BBCH DOY | 4.9 | 12.5 | 7.9 | 2.1 |
| Parameter | Kendall’s Tau | p-Value | Sen’s Slope |
|---|---|---|---|
| No. of days 60BBCH-65BBCH | −0.218 | 0.226 | −0.111 |
| Average T in 60BBCH-65BBCH DOY | −0.228 | 0.184 | −0.159 |
| No. of days 65BBCH-69BBCH | 0.228 | 0.193 | 0.364 |
| Average T in 65BBCH-69BBCH DOY | 0.099 | 0.576 | 0.083 |
| No. of days 60BBCH-69BBCH | 0.174 | 0.325 | 0.167 |
| Average T in 60BBCH-69BBCH DOY | 0.029 | 0.889 | 0.033 |
| Mean Air Temperatures (°C) | |||||||||||||
| Months Period | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Tmean 1991/2020 | 1.0 | 3.0 | 7.5 | 12.9 | 17.6 | 21.4 | 23.3 | 23.2 | 18.0 | 12.8 | 7.4 | 2.2 | 12.5 |
| Tmean 2007/2024 | 2.0 | 4.5 | 8.3 | 13.4 | 17.8 | 22.3 | 24.3 | 24.2 | 18.9 | 13.3 | 8.1 | 3.4 | 13.4 |
| Tmean 2025 | 4.0 | 2.4 | 10.4 | 13.8 | - | - | - | - | - | - | - | - | - |
| Mean Maximum Air Temperatures (°C) | |||||||||||||
| Tmax 1991/2020 | 4.5 | 7.4 | 12.9 | 18.4 | 23.2 | 26.9 | 29.0 | 29.3 | 24.1 | 18.5 | 11.9 | 5.5 | 17.6 |
| Tmax 2007/2024 | 5.6 | 9.0 | 13.5 | 19.0 | 23.2 | 27.6 | 30.0 | 30.3 | 24.8 | 18.9 | 12.6 | 6.8 | 18.4 |
| Tmax 2025 | 7.9 | 7.4 | 16.2 | 19.3 | - | - | - | - | - | - | - | - | - |
| Mean Minimum Air Temperatures (°C) | |||||||||||||
| Tmin 1991/2020 | −2.3 | −1.0 | 2.6 | 7.1 | 11.8 | 15.4 | 16.8 | 16.9 | 12.6 | 7.9 | 3.6 | −0.9 | 7.5 |
| Tmin 2007/2024 | −1.4 | 0.4 | 3.3 | 7.4 | 12.2 | 16.3 | 17.8 | 17.6 | 13.3 | 8.4 | 4.3 | 0.3 | 8.3 |
| Tmin 2025 | 0.1 | −1.5 | 5.1 | 8.2 | - | - | - | - | - | - | - | - | - |
| Mean Monthly and Annual Relative Humidity (%) | |||||||||||||
| Mean 1991/2020 | 85.0 | 78.0 | 68.8 | 64.9 | 66.2 | 66.4 | 63.1 | 62.5 | 68.8 | 74.6 | 79.9 | 85.3 | 72.0 |
| Mean 2007/2024 | 82.6 | 74.4 | 65.9 | 61.3 | 65.4 | 64.3 | 58.4 | 58.2 | 64.9 | 72.0 | 78.4 | 83.0 | 69.1 |
| Mean 2025 | 81.3 | 66.8 | 65.3 | 65.8 | - | - | - | - | - | - | - | - | - |
| Total and Mean Precipitation Amounts (mm) | |||||||||||||
| Period | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | ∑ |
| ∑1991/2020 | 42.4 | 34.0 | 41.7 | 47.4 | 68.1 | 80.1 | 58.2 | 54.0 | 56.0 | 50.7 | 45.5 | 48.3 | 626.4 |
| ∑2007/2024 | 46.0 | 37.2 | 46.2 | 40.5 | 83.1 | 76.4 | 51.6 | 44.0 | 53.9 | 47.9 | 50.8 | 49.7 | 627.3 |
| ∑2025 | 16.4 | 8.6 | 64.3 | 51.5 | - | - | - | - | - | - | - | - | - |
| Tmean (°C) | Perc. Cat. * 1991–2020 | Tmean (°C) 1991–2020 | 1991–2020 33.Perc. | 1991–2020 50.Perc. | 1991–2020 66.Perc. | Terciles ** Cat. |
|---|---|---|---|---|---|---|
| February | ||||||
| 2.4 | N | 3.0 | 2.3 | 3.4 | 4.7 | 0 |
| March | ||||||
| 10.4 | VW | 7.5 | 6.7 | 7.4 | 8.4 | 1 |
| April | ||||||
| 13.8 | W | 12.9 | 12.2 | 12.8 | 13.5 | 1 |
| Sum (mm) | Perc. Cat. * | Sum (mm) 1991–2020 | 1991–2020 | 1991–2020 | 1991–2020 | Terciles ** |
|---|---|---|---|---|---|---|
| 1991–2020 | 33.Perc. | 50.Perc. | 66.Perc. | Cat. | ||
| February | ||||||
| 8.6 | VD | 34.0 | 23.5 | 33.9 | 46.1 | −1 |
| March | ||||||
| 64.3 | W | 41.7 | 23.1 | 30.3 | 49.0 | 1 |
| April | ||||||
| 51.5 | N | 47.4 | 33.8 | 44.0 | 50.4 | 1 |
| Parameter | 60BBCH DOY | 65BBCH DOY | 69BBCH DOY |
|---|---|---|---|
| Sample size | 19 | 19 | 19 |
| Vector r | 0.9849 | 0.9864 | 0.985 |
| Rayleigh R | 18.7125 | 18.7416 | 18.7148 |
| Rayleigh Z | 18.4293 | 18.4867 | 18.4339 |
| u | 4.443 | 4.443 | 4.443 |
| p value | <0.01 | <0.01 | <0.01 |
| Mean date | Late February | Early March | Mid-March |
| Mean angle | 53.084 | 59.4019 | 83.0708 |
| Species | (1) | (2) | (1) | (2) | (1) | (2) |
|---|---|---|---|---|---|---|
| Parameter | 60BBCH DOY | 65BBCH DOY | 69BBCH DOY | |||
| Sample size | 19 | 19 | 19 | 19 | 19 | 19 |
| Mean angle | 53.084 | 64.881 | 59.4019 | 69.0879 | 83.0708 | 88.2384 |
| Vector r | 0.9849 | 0.9824 | 0.9864 | 0.9828 | 0.985 | 0.984 |
| Variance of the mean angle | 0.0303 | 0.0352 | 0.0272 | 0.0345 | 0.03 | 0.0321 |
| Standard error of mean angle | 9.9674 | 10.7471 | 9.4492 | 10.6354 | 9.9275 | 10.2578 |
| Parameter | 60BBCH DOY | 65BBCH DOY | 69BBCH DOY |
| Sample size | 19 | 19 | 19 |
| F | 11.595 | 8.3019 | 2.3495 |
| p value | 0.002 | 0.0067 | 0.1304 |
| Circular correlation | |||
| Raa (correlation coefficient) | 0.7087 | 0.8464 | 0.6677 |
| p-value | 0.0007 | 0 | 0.0018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocokoljić, M.; Galečić, N.; Skočajić, D.; Čukanović, J.; Đorđević, S.; Kolarov, R.; Petrov, D. Flowering Patterns of Cornus mas L. in the Landscape Phenology of Roadside Green Infrastructure Under Climate Change Conditions in Serbia. Sustainability 2025, 17, 5334. https://doi.org/10.3390/su17125334
Ocokoljić M, Galečić N, Skočajić D, Čukanović J, Đorđević S, Kolarov R, Petrov D. Flowering Patterns of Cornus mas L. in the Landscape Phenology of Roadside Green Infrastructure Under Climate Change Conditions in Serbia. Sustainability. 2025; 17(12):5334. https://doi.org/10.3390/su17125334
Chicago/Turabian StyleOcokoljić, Mirjana, Nevenka Galečić, Dejan Skočajić, Jelena Čukanović, Sara Đorđević, Radenka Kolarov, and Djurdja Petrov. 2025. "Flowering Patterns of Cornus mas L. in the Landscape Phenology of Roadside Green Infrastructure Under Climate Change Conditions in Serbia" Sustainability 17, no. 12: 5334. https://doi.org/10.3390/su17125334
APA StyleOcokoljić, M., Galečić, N., Skočajić, D., Čukanović, J., Đorđević, S., Kolarov, R., & Petrov, D. (2025). Flowering Patterns of Cornus mas L. in the Landscape Phenology of Roadside Green Infrastructure Under Climate Change Conditions in Serbia. Sustainability, 17(12), 5334. https://doi.org/10.3390/su17125334









