Habitat Suitability Dynamics of Yellow River Delta Nature Reserves for Rare Waterbirds
Abstract
1. Introduction
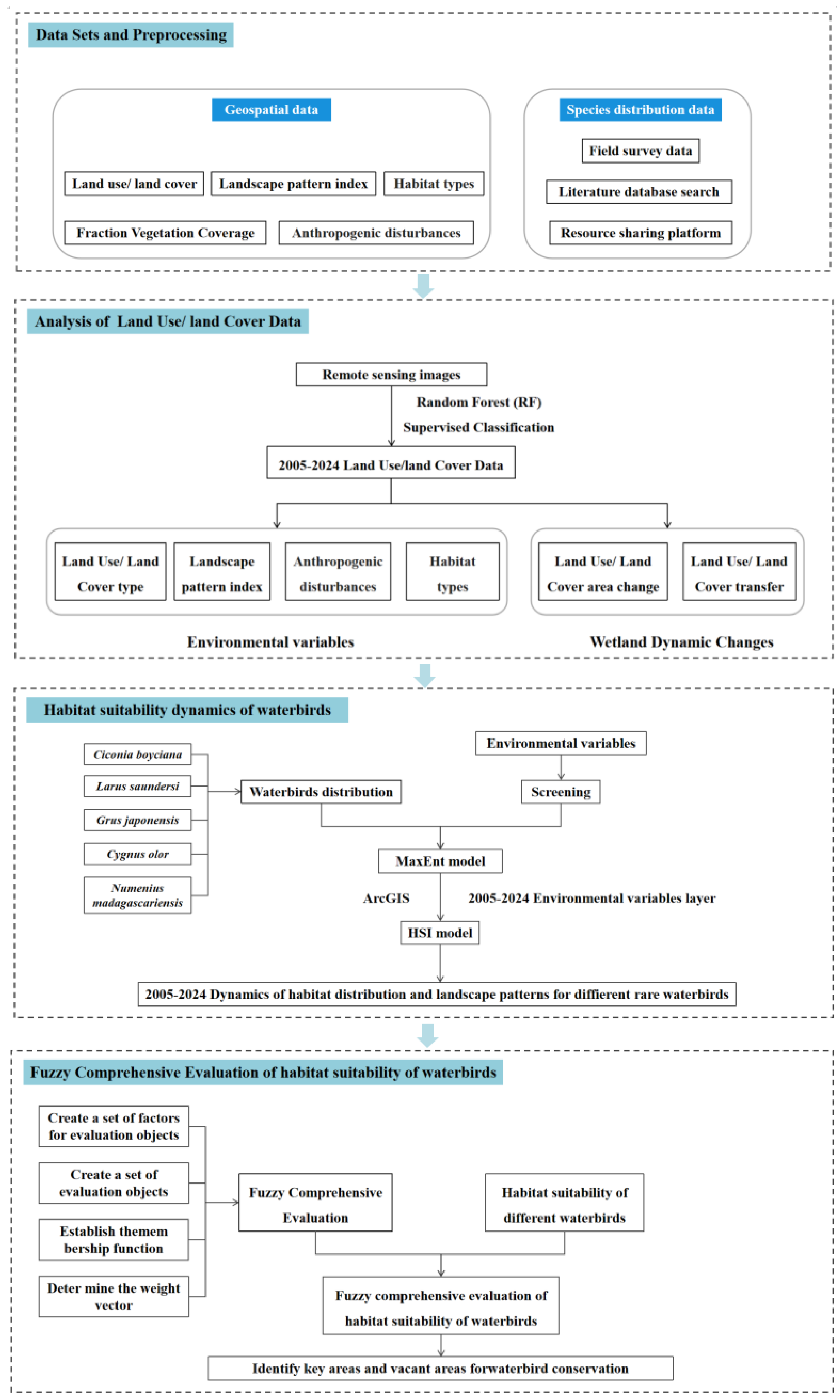
2. Materials and Methods
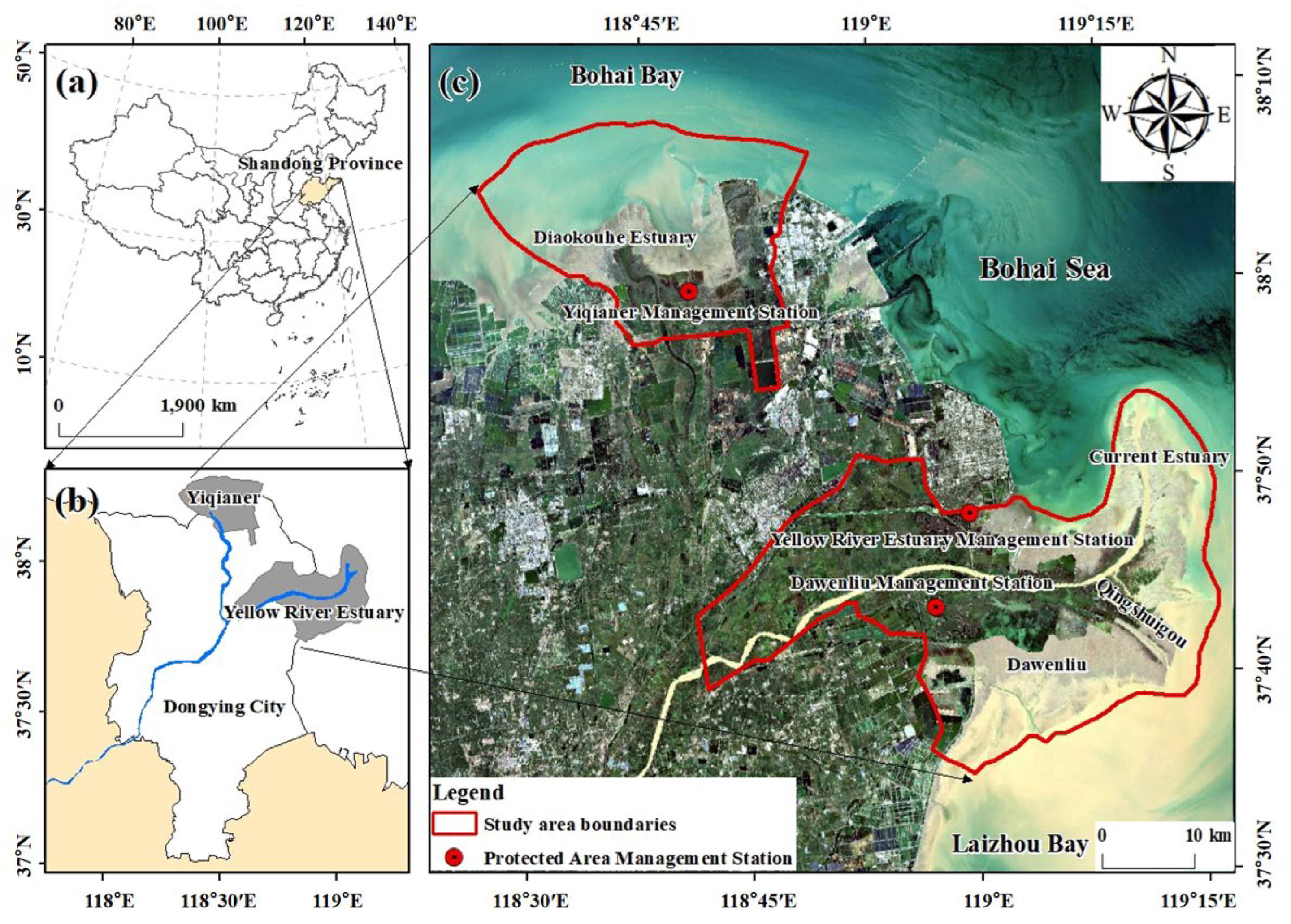
2.1. Study Area
2.2. Data and Methods
2.2.1. Target Species Selection
Conservation Status and International Concern
Regional Distribution and Occurrence Frequency
Ecological Function and Indicator Potential
Ecological Niche Complementarity and Representativeness
2.2.2. Data Sources and Processing
Species Distribution Data
Environmental Variable Data
2.2.3. Research Methods
Habitat Suitability Analysis
Habitat Landscape Pattern Analysis
Fuzzy Comprehensive Evaluation Method
3. Results
3.1. Overview of Waterbirds in the Study Area
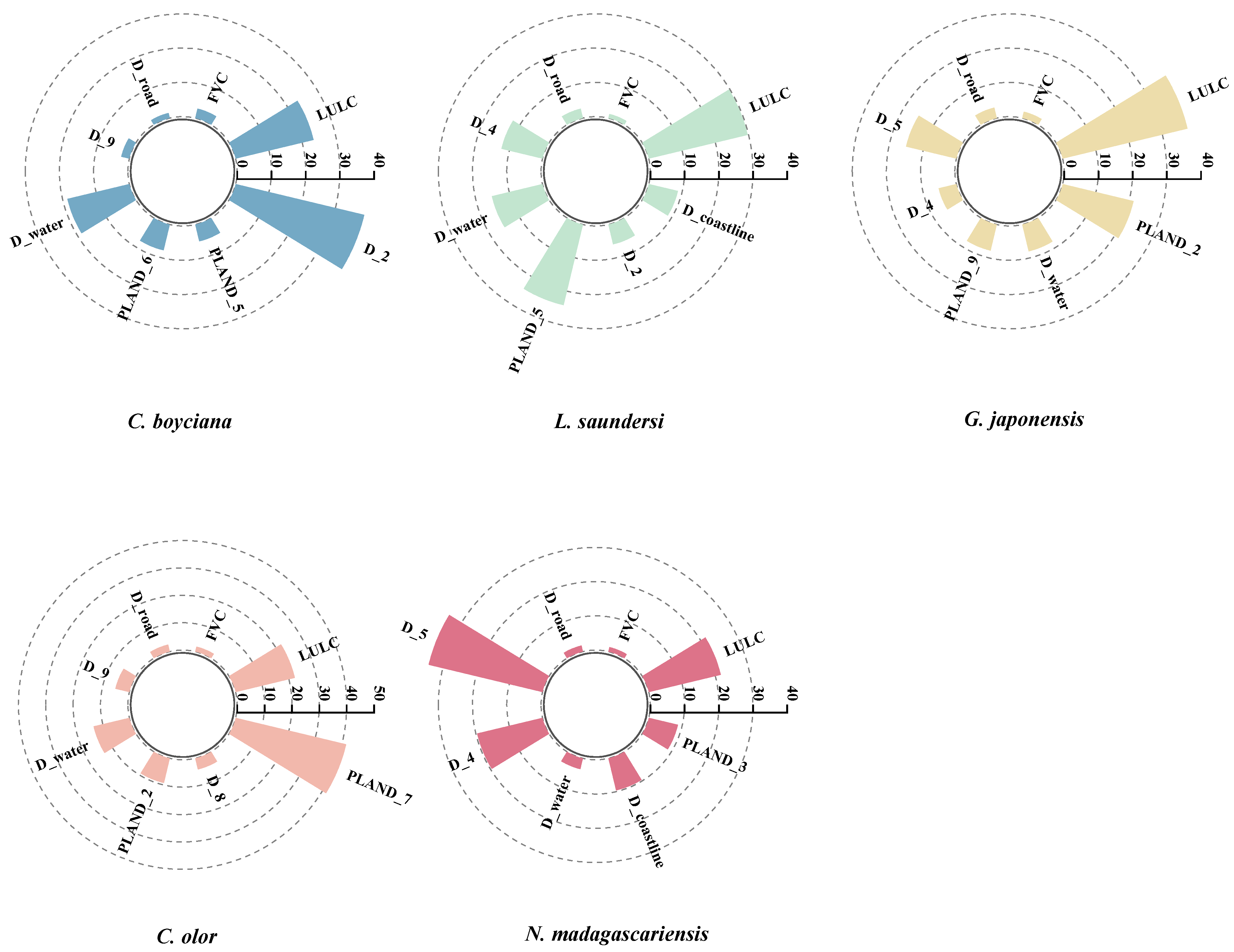
3.2. Environmental Variable Analysis
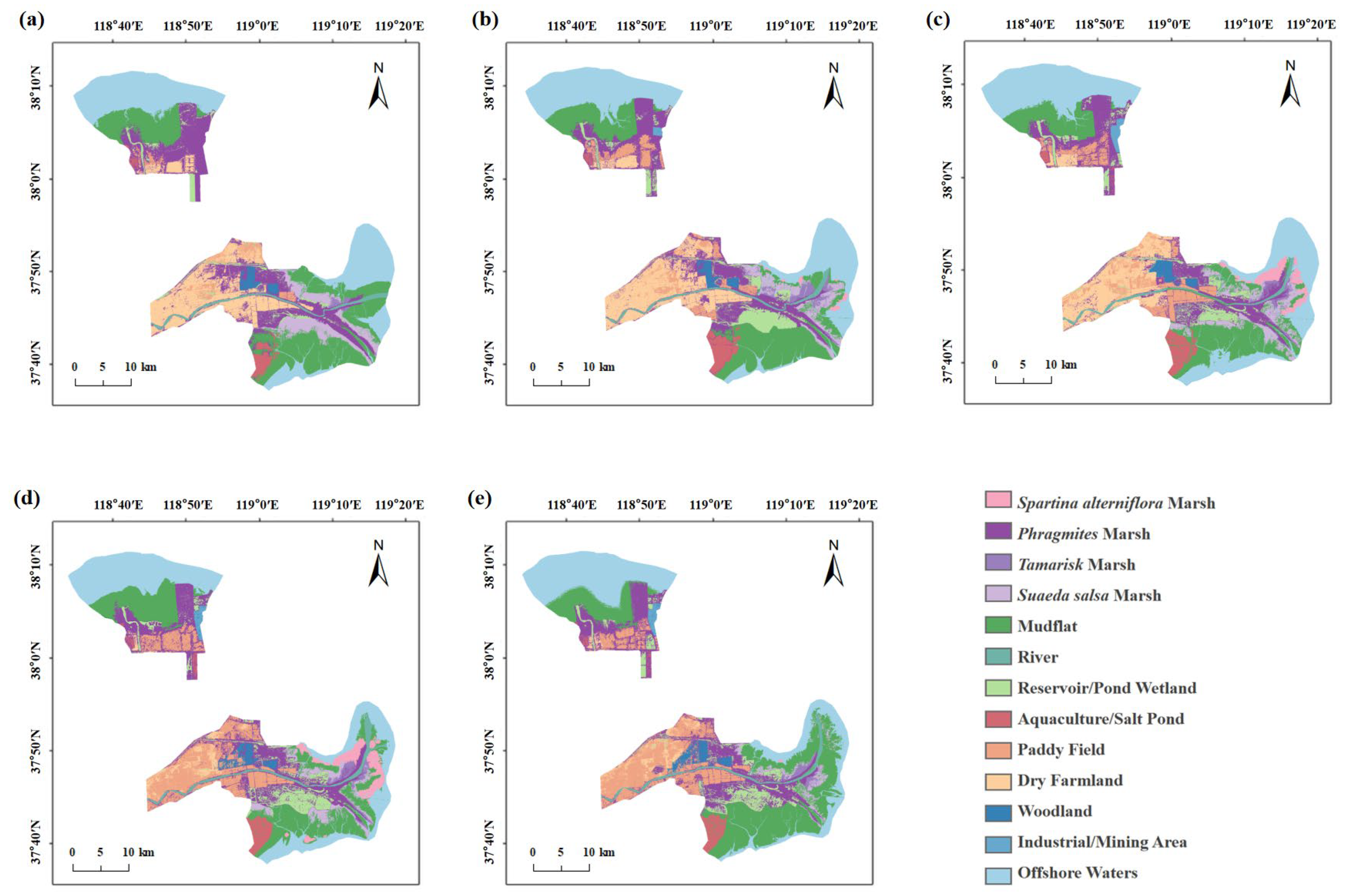
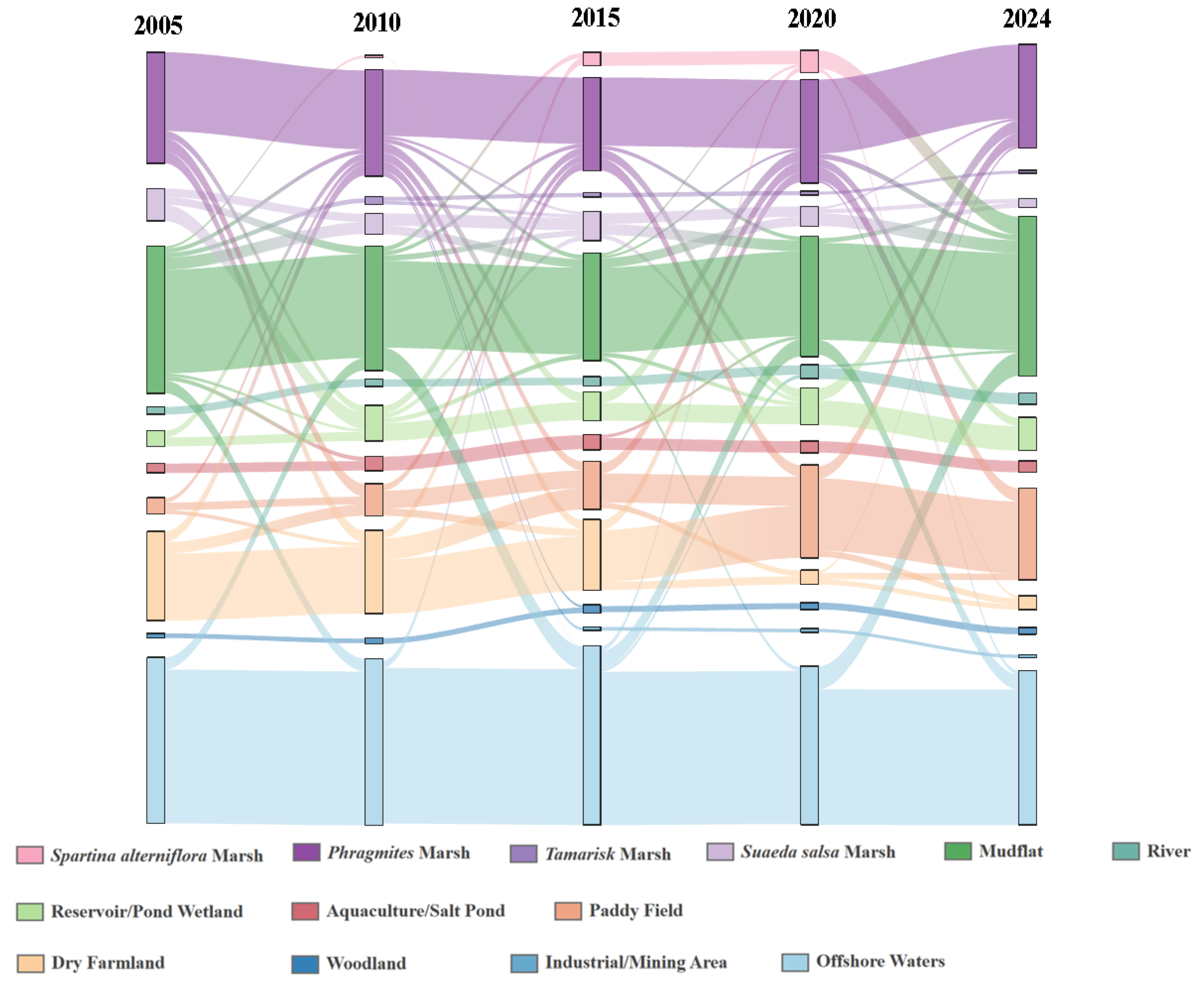
3.3. Wetland Dynamics in the YRDNR
3.4. Dynamics of Habitat Suitability for Rare Waterbirds
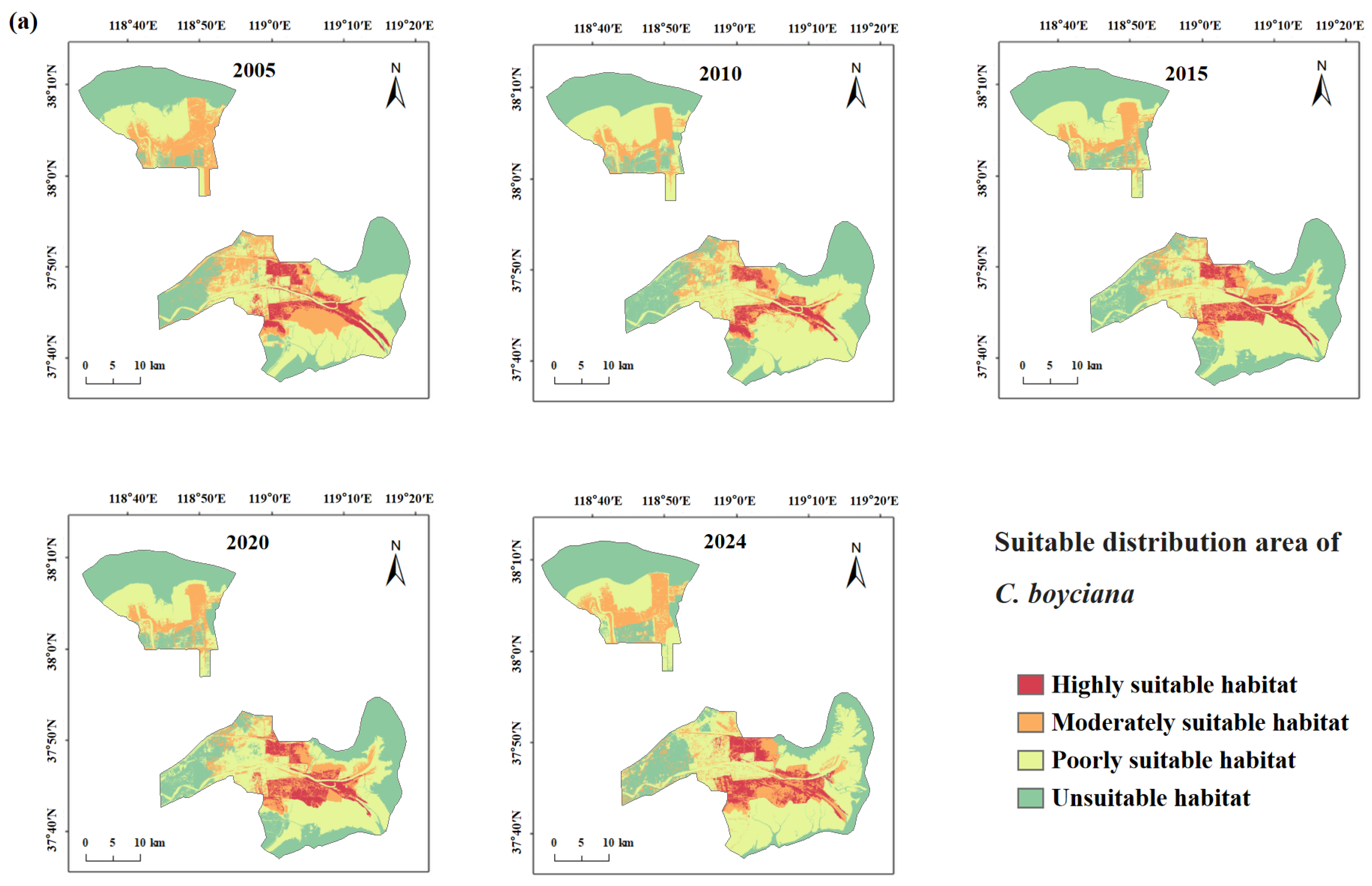
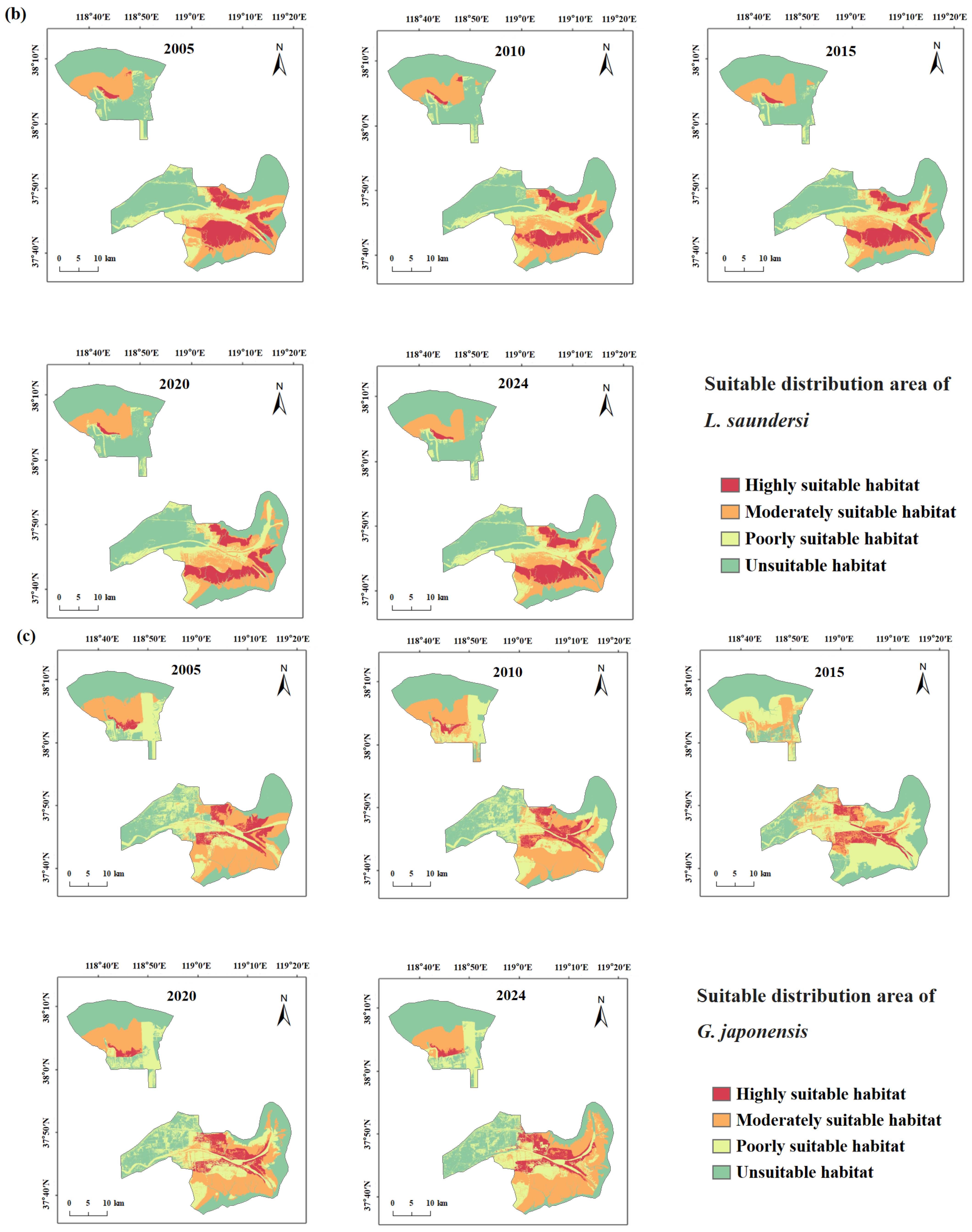
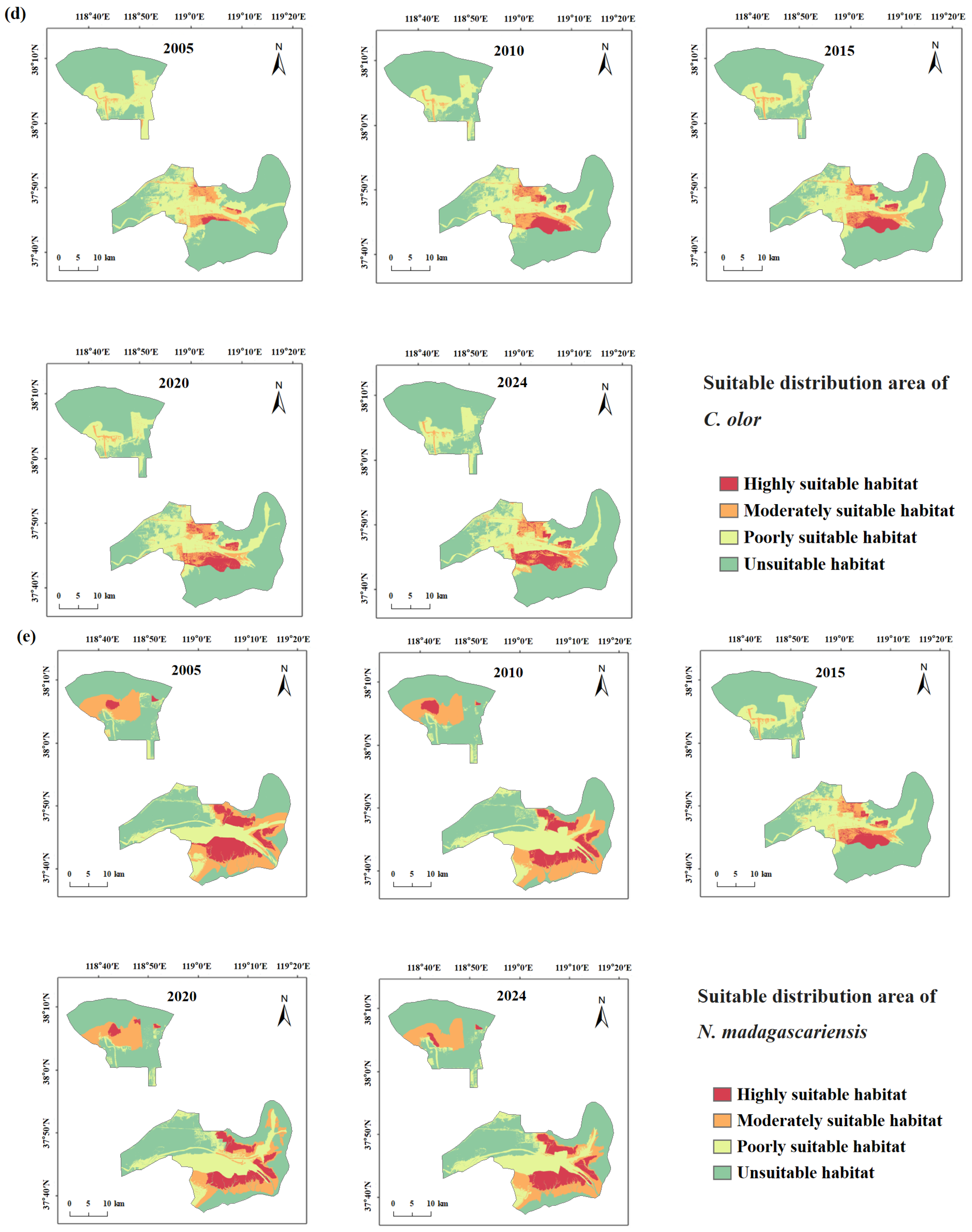
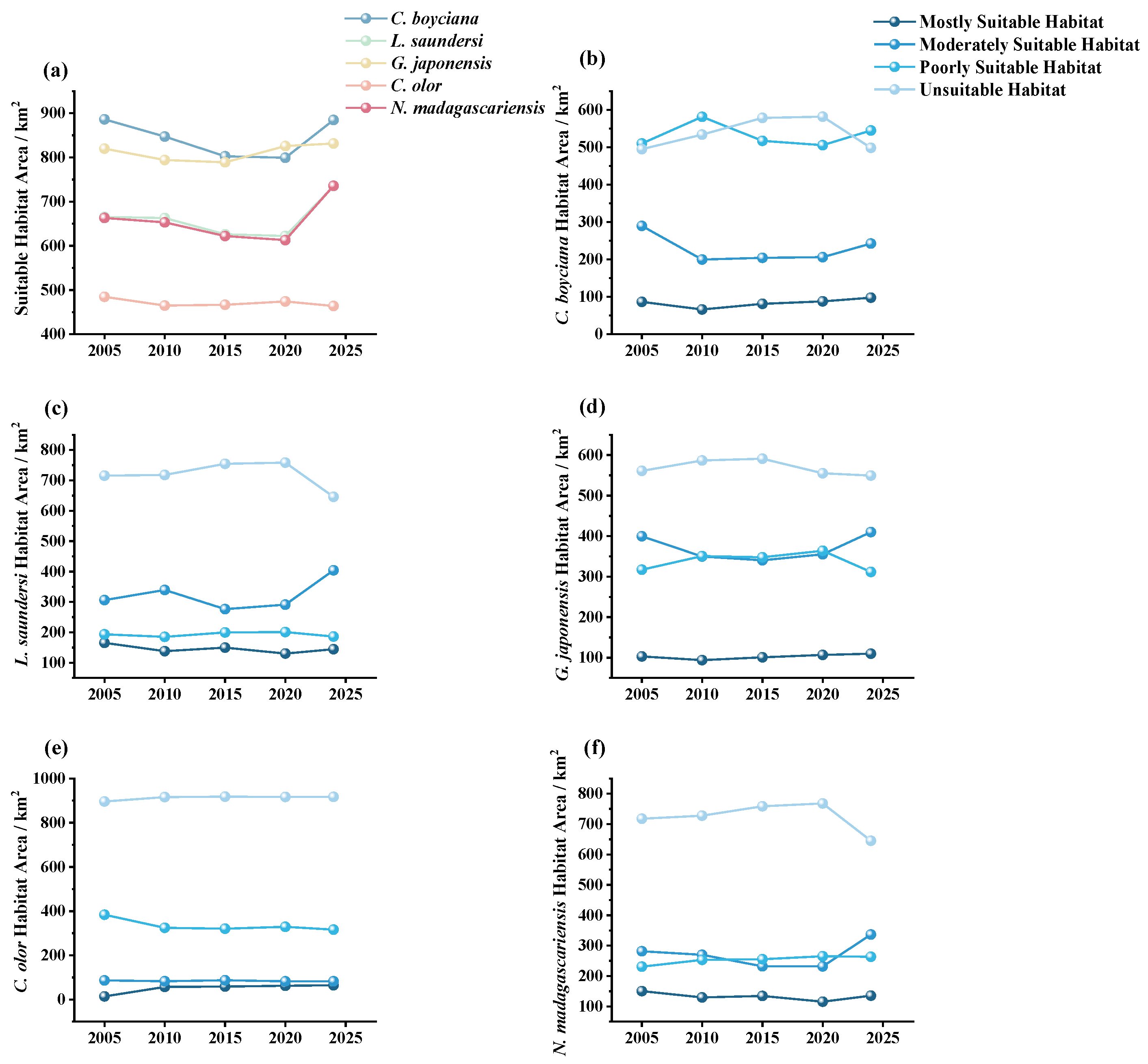
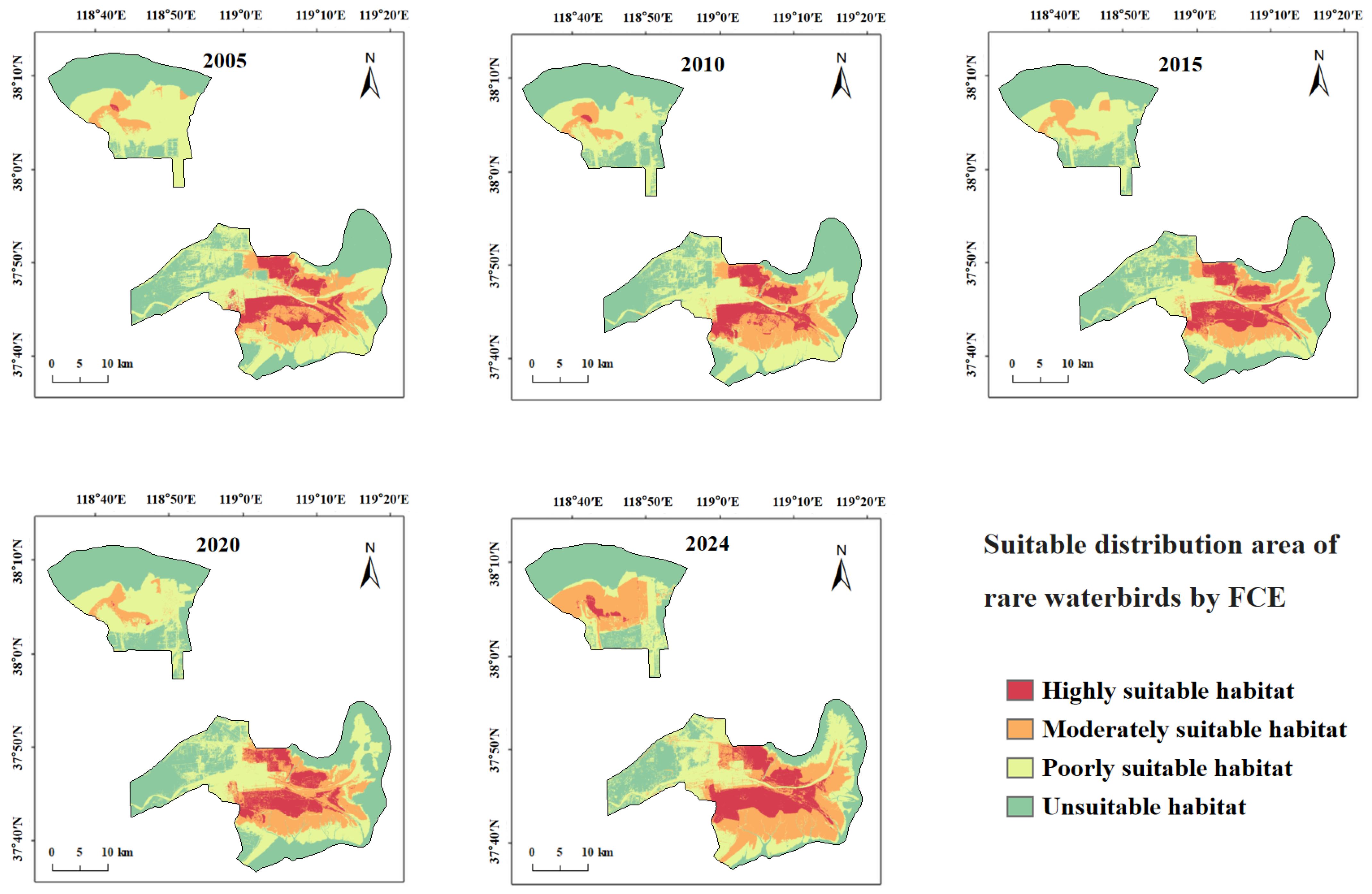
3.4.1. Spatiotemporal Changes in Suitable Habitat for Target Species
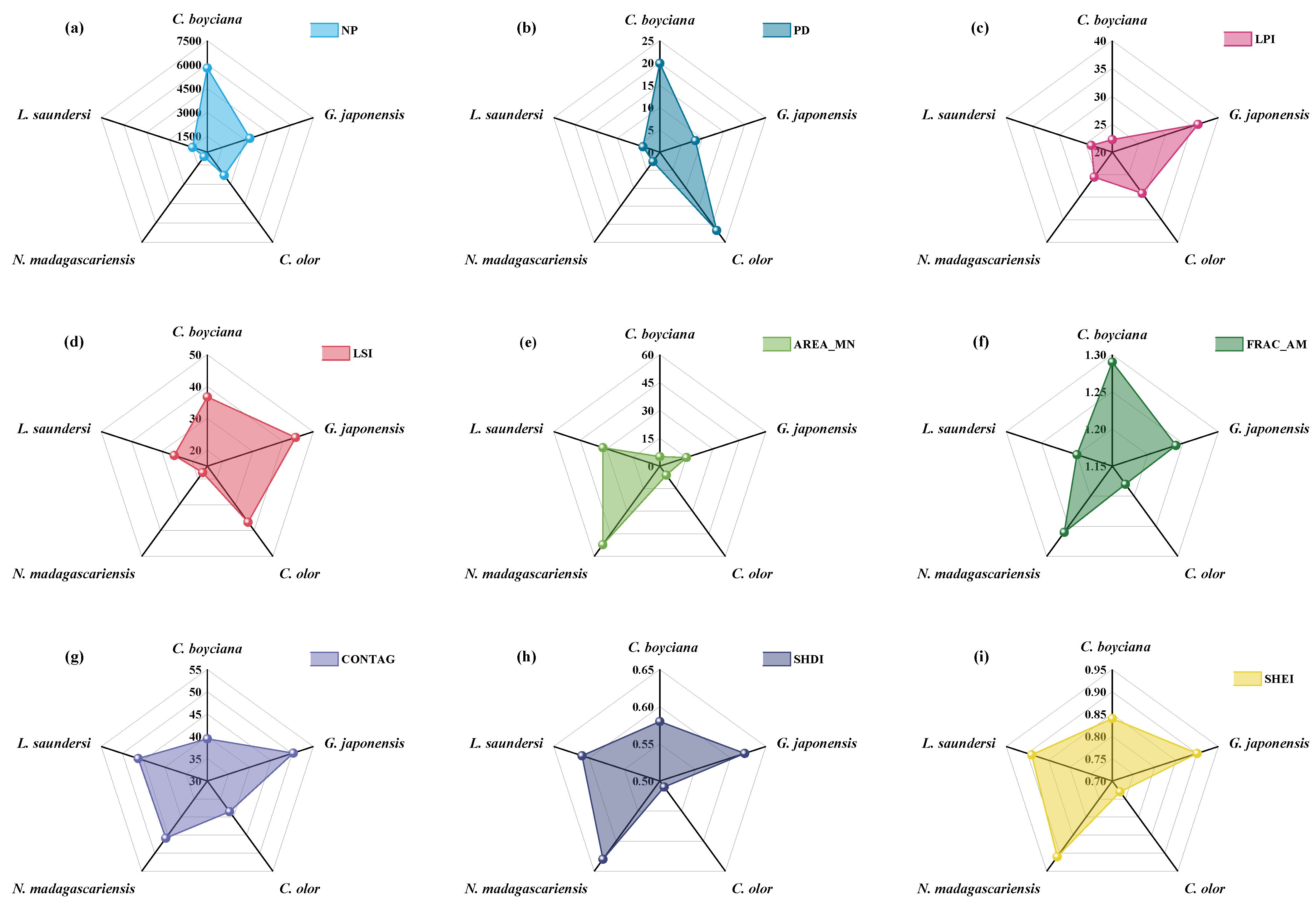
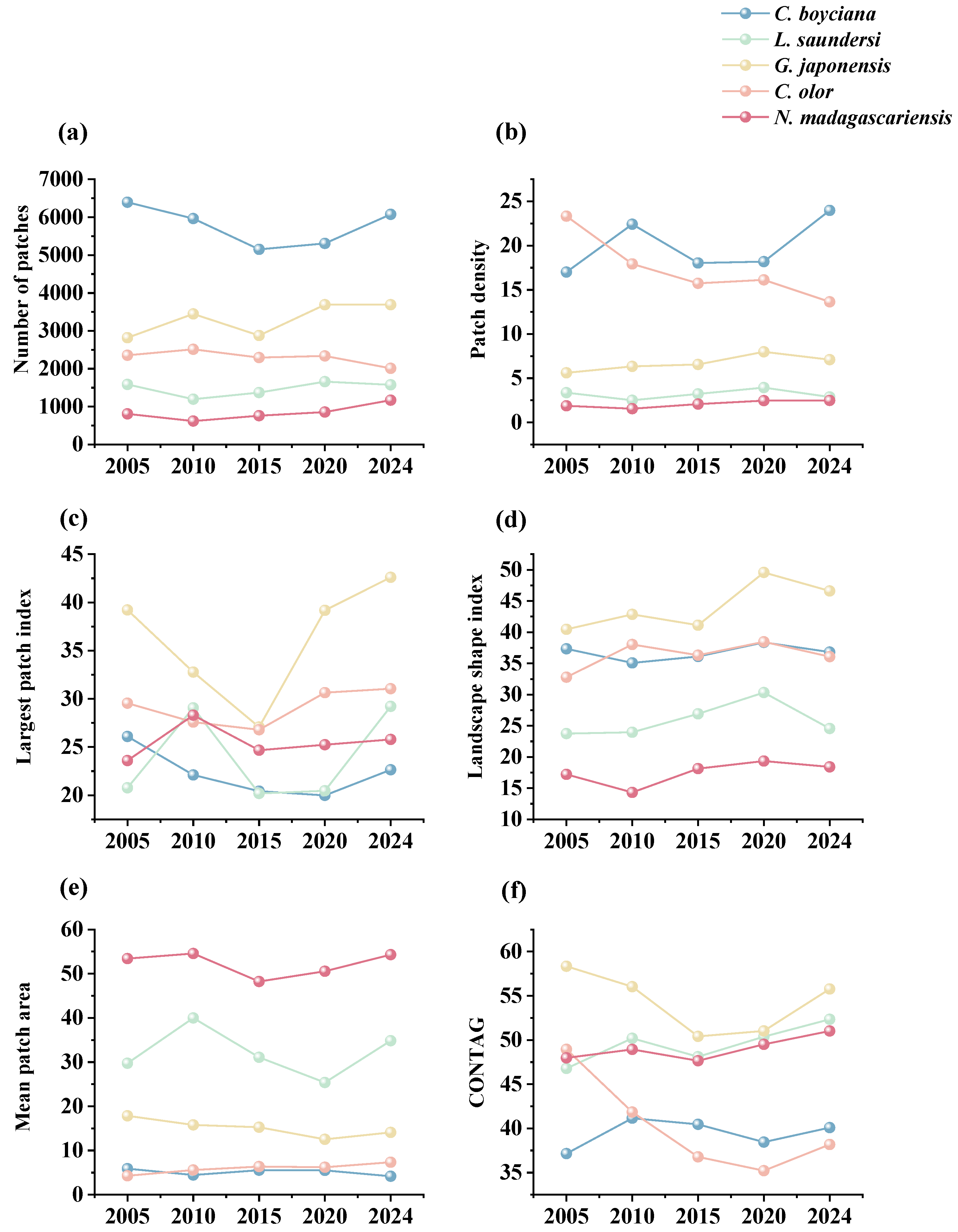
3.4.2. Changes in Landscape Pattern of Suitable Habitat
3.4.3. Fuzzy Comprehensive Evaluation Results of Rare Waterbird Habitat Suitability
4. Discussion
4.1. Discussion of Findings
4.1.1. Differential Influence of Environmental Variables on Waterbird Species
4.1.2. Response of Rare Waterbird Habitats to Wetland Dynamics
- (1)
- Spartina alterniflora Invasion and Control: Introduced in 1989 for shoreline stabilization, Spartina alterniflora began expanding rapidly across the intertidal zones after 2008, displacing native species such as Suaeda, Phragmites, and seagrasses [66]. This study found that after 2010, the suitable habitat area and mean patch size (AREA_MN) for L. saundersi and N. madagascariensis declined, while the patch density (PD) and landscape shape index (LSI) increased, patterns that correspond with habitat fragmentation due to Spartina alterniflora’s encroachment. These shorebirds rely on saltmarshes and tidal flats for breeding and foraging, feeding primarily on small fish and benthic invertebrates [61]. The invasion of Spartina alterniflora degrades the benthic habitat quality, reduces biodiversity, and limits access to feeding grounds, negatively impacting reproductive success [67]. After 2021, management efforts began restoring mudflat patches in the reserve [68]. As a result, the proportion of unsuitable habitat declined for L. saundersi, and the number of highly suitable patches (NP) stabilized. However, the Largest Patch Index (LPI) recovered more slowly, suggesting that while the habitat structure improved, core area recovery lagged behind.
- (2)
- Human Development Activities: Following the establishment of the Yellow River Delta Efficient Ecological Economic Zone in 2010, coastal development intensified. Natural wetlands and unused land were increasingly converted into aquaculture ponds and saltpans, leading to a steady increase in artificial wetland coverage [69]. This transformation directly threatened species dependent on natural habitats (e.g., L. saundersi and N. madagascariensis), while benefiting those reliant on freshwater availability (e.g., C. boyciana and C. olor) [70]. In this study, C. boyciana’s habitat showed high NP and PD values, indicating fragmented and dispersed habitat patches lacking dominant core areas, consistent with prior assessments of wetland fragmentation in the delta [71]. For C. olor, the expansion in open water bodies (pond wetlands and saltpans) resulted in a 71.04% increase in AREA_MN. Patch shapes remained regular and structurally stable, as reflected by low FRAC_AM and LSI values. Although landscape diversity (SHDI < 0.6) remained low, a high connectivity and spatial continuity met the species’ need for safe, open aquatic habitats [72]. It is important to note that while the conversion from natural to functionally homogeneous artificial wetlands may support short-term increases in habitat area and population size, it may also reduce overall ecosystem resilience and heterogeneity, potentially undermining community diversity and reproductive stability [73,74].
- (3)
- Ecological Restoration Projects: To improve wetland health, the Yellow River Conservancy Commission initiated ecological water replenishment projects in 2010, targeting the restoration of degraded freshwater wetlands. These efforts effectively improved the water–salt balance and vegetation recovery within the reserve, curbing fragmentation in the northern mudflats and facilitating land accretion in the modern delta region [75]. The resulting mudflat expansion benefited habitat availability for L. saundersi, G. japonensis, and N. madagascariensis. Simultaneously, Phragmites became the dominant vegetation in restored areas [75], providing an optimal habitat for species like C. boyciana and G. japonensis that favor Phragmites marshes. This study observed that the highly suitable habitats for G. japonensis and C. boyciana expanded significantly following ecological water supplementation. Metrics such as CONTAG and LPI also improved, indicating not only an increase in habitat area but also an enhanced spatial cohesion and structural integration of habitat patches.
4.1.3. Habitat Suitability Dynamics Based on FCE Evaluation
4.2. Conservation Recommendations
4.3. Limitations and Future Directions
5. Conclusions
- (1)
- The integrated modeling approach effectively supports long-term habitat suitability assessment for waterbirds. The combination of MaxEnt and HSI models enabled precise, multi-species, multi-factor, long-term habitat suitability evaluations. The results confirm the reliability and applicability of this approach for monitoring species habitats and analyzing spatiotemporal changes.
- (2)
- Suitable Habitat distribution patterns are highly heterogeneous. Clear interspecific differences in habitat preferences were observed: C. boyciana was primarily associated with Phragmites–pond composite wetlands; L. saundersi and N. madagascariensis depended on continuous mudflats; G. japonensis favored Phragmites marshes; and C. olor preferred large open water bodies. Suitable Habitats were mainly distributed along the Yellow River mainstem and in the southern intertidal zone.
- (3)
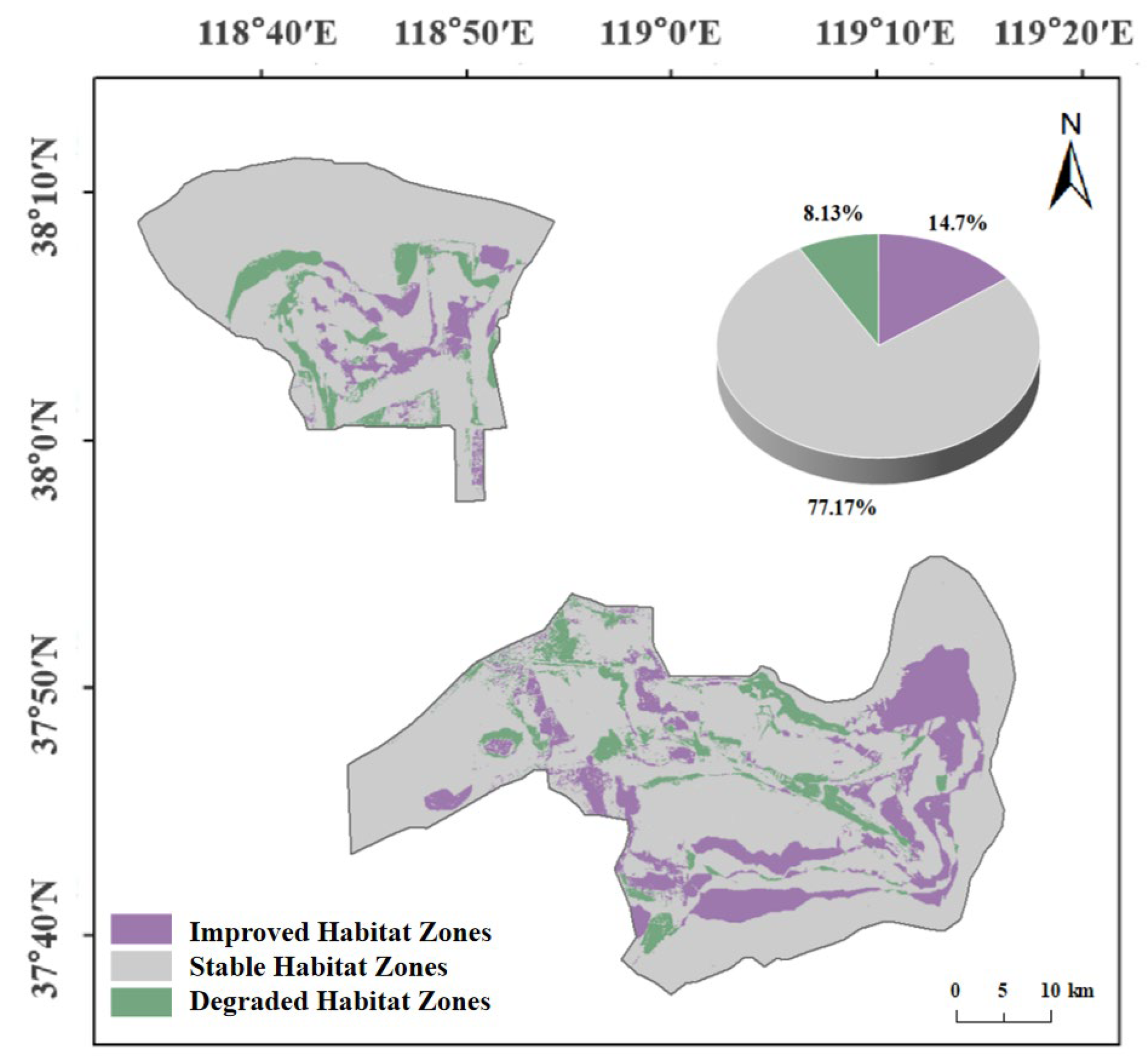
- Habitat suitability declined and then recovered during the study period, reflecting the early effectiveness of ecological restoration. From 2010 to 2020, the area of Mostly Suitable Habitat for several species declined. However, following the implementation of Spartina alterniflora’s removal and wetland water replenishment projects after 2020, habitat quality improved significantly. By 2024, the total area of Mostly Suitable Habitat exceeded that of 2005.
- (4)
- Landscape pattern changes reflect trends in habitat fragmentation and connectivity. The Highly Suitable Habitats of L. saundersi and N. madagascariensis were characterized by a low fragmentation and high spatial aggregation. For C. boyciana and G. japonensis, although patch numbers were high, habitat connectivity improved. C. olor occupied large, structurally simple patches. Overall, the landscape structure became increasingly complex.
- (5)
- Habitat changes were strongly influenced by wetland succession and human activities. The reduction in mudflats and Phragmites marshes, along with the expansion of artificial wetlands such as paddy fields and pond systems, altered the habitat structure for some species. In contrast, ecological restoration projects enhanced the connectivity and diversity of Mostly Suitable Habitat, playing a positive role in overall habitat quality.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, N.; Ma, Z. Ecological Restoration of Coastal Wetlands in China: Current Status and Suggestions. Biol. Conserv. 2024, 291, 110513. [Google Scholar] [CrossRef]
- Mokany, K.; Ware, C.; Harwood, T.D.; Schmidt, R.K.; Ferrier, S. Habitat-based Biodiversity Assessment for Ecosystem Accounting in the Murray-Darling Basin. Conserv. Biol. 2022, 36, e13915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ouyang, Z. Focusing on Rapid Urbanization Areas Can Control the Rapid Loss of Migratory Water Bird Habitats in China. Glob. Ecol. Conserv. 2019, 20, e00801. [Google Scholar] [CrossRef]
- Blankespoor, B.; Dasgupta, S.; Laplante, B. Sea-Level Rise and Coastal Wetlands. Ambio 2014, 43, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Schuerch, M.; Spencer, T.; Temmerman, S.; Kirwan, M.L.; Wolff, C.; Lincke, D.; McOwen, C.J.; Pickering, M.D.; Reef, R.; Vafeidis, A.T. Future Response of Global Coastal Wetlands to Sea-Level Rise. Nature 2018, 561, 231–234. [Google Scholar] [CrossRef]
- Streck, C. Synergies between the Kunming-Montreal Global Biodiversity Framework and the Paris Agreement: The Role of Policy Milestones, Monitoring Frameworks and Safeguards. Clim. Policy 2023, 23, 800–811. [Google Scholar] [CrossRef]
- Wang, W.; Feng, C.; Liu, F.; Li, J. Biodiversity Conservation in China: A Review of Recent Studies and Practices. Environ. Sci. Ecotechnology 2020, 2, 100025. [Google Scholar] [CrossRef]
- Duan, H.; Yu, X.; Xia, S.; Zhang, G. Waterbird Habitat Loss: Fringes of the Yellow and Bohai Seas along the East Asian-Australasian Flyway. Land Degrad. Dev. 2021, 32, 4174–4182. [Google Scholar] [CrossRef]
- Ma, T.; Li, X.; Bai, J.; Cui, B. Habitat Modification in Relation to Coastal Reclamation and Its Impacts on Waterbirds along China’s Coast. Glob. Ecol. Conserv. 2019, 17, e00585. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Li, Y.; Dong, B.; Qiu, C.; Yang, J.; Zong, Y.; Chen, H.; Zhao, Y.; Zhang, Y. Study on Habitat Suitability and Environmental Variable Thresholds of Rare Waterbirds. Sci. Total Environ. 2021, 785, 147316. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wang, W.; Fang, W.-T.; Chiang, Y.-T.; Liu, X.; Ju, H. Vegetation Successions of Coastal Wetlands in Southern Laizhou Bay, Bohai Sea, Northern China, Influenced by the Changes in Relative Surface Elevation and Soil Salinity. J. Environ. Manag. 2021, 293, 112964. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.-L.; Chih, W.-C.; Lai, Y.-C.; Lee, P.-F.; Lien, Y.-Y. Aquaculture Ponds as Important High-Tide Habitats for Waterbirds along the West Coast of Taiwan. Ornithol. Sci. 2018, 17, 55–67. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Dai, L.; Liu, H.; Li, Y.; Qiu, C.; Zhou, Y.; Chen, H.; Dong, B.; Zhao, Y. Study on the Effect of Habitat Function Change on Waterbird Diversity and Guilds in Yancheng Coastal Wetlands Based on Structure-Function Coupling. Ecol. Indic. 2021, 122, 107223. [Google Scholar] [CrossRef]
- Xie, Y.; Zou, J.; Chen, Y.; Li, F.; Jiang, Q. Are Wading Birds the Ideal Focal Species for Broader Bird Conservation? A Cost-Effective Approach to Ecological Network Planning. Ecol. Indic. 2024, 160, 111785. [Google Scholar] [CrossRef]
- Yang, X.; Liu, W.; Li, S.; Ma, Z.; Chen, C.; Gu, W.; Qu, M.; Zhang, C.; Ding, Z.; Xu, Y. Restoration of Urban Waterbird Diversity: A Case Study of the Construction of a Waterbird Ecological Corridor in the Guangdong-Hong Kong-Macao Greater Bay Area, Southern China. Glob. Ecol. Conserv. 2022, 39, e02277. [Google Scholar] [CrossRef]
- Simberloff, D. Flagships, Umbrellas, and Keystones: Is Single-Species Management Passé in the Landscape Era? Biol. Conserv. 1998, 83, 247–257. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.; Kim, S.H.; Lee, E.J. Identifying High-Priority Conservation Areas for Endangered Waterbirds Using a Flagship Species in the Korean DMZ. Ecol. Eng. 2021, 159, 106080. [Google Scholar] [CrossRef]
- Duan, H.; Xia, S.; Jackson, M.V.; Zhao, N.; Liu, Y.U.; Teng, J.; Meng, Z.; Yu, X.; Shi, J. Identifying New Sites of Significance to Waterbirds Conservation and Their Habitat Modification in the Yellow and Bohai Seas in China. Glob. Ecol. Conserv. 2020, 22, e01031. [Google Scholar] [CrossRef]
- Yang, W.; Pei, J.; Li, X.X.; Sun, T.; Wang, W.Y. Systematic Assessment and Countermeasures for Ecological Restoration of Degraded Wetlands in the Yellow River Delta. J. Beijing Norm. Univ. Nat. Sci. 2018, 54, 98–103. [Google Scholar] [CrossRef]
- Kaky, E.; Nolan, V.; Alatawi, A.; Gilbert, F. A Comparison between Ensemble and MaxEnt Species Distribution Modelling Approaches for Conservation: A Case Study with Egyptian Medicinal Plants. Ecol. Inform. 2020, 60, 101150. [Google Scholar] [CrossRef]
- Yan, Z.S.; Shao, M.Q.; Wang, J.Y. Prediction of Wintering Habitat Suitability and Key Influencing Factors for the Common Crane in China. Chin. J. Appl. Ecol. 2025, 36, 578–586. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, X.Y.; Zhou, J.M. Habitat Suitability Evaluation Model and Validation for the Giant Panda Based on Geographical Detector. J. Geo-Inf. Sci. 2016, 18, 767–778. [Google Scholar]
- Chen, F.; Zhang, J.J.; Wang, L.F.; Xiong, C.Y.; Fan, H. Spatiotemporal Changes and Conservation Effectiveness of Asian Elephant Habitats in Transboundary Areas of China and Laos. Acta Ecol. Sin. 2024, 44, 10222–10233. [Google Scholar] [CrossRef]
- Zhang, J.J.; Chen, F.; Xie, F.; Zhang, X.; Yin, W.P.; Fan, H. Long-Term Habitat Dynamics of Asian Elephants and Their Influence on Human-Elephant Conflicts: A Habitat Suitability Assessment Based on Integrated MaxEnt and HSI Models. Acta Ecol. Sin. 2023, 43, 3807–3818. [Google Scholar]
- Cong, P.; Chen, K.; Qu, L.; Han, J. Dynamic Changes in the Wetland Landscape Pattern of the Yellow River Delta from 1976 to 2016 Based on Satellite Data. Chin. Geogr. Sci. 2019, 29, 372–381. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Hou, X.; Wang, X.; Li, X.; Zhang, Y. Habitat Suitability Assessment for Saunders’s Gull (Saundersilarus saundersi) in the Yellow River Delta, China. Ecol. Inform. 2024, 79, 102393. [Google Scholar] [CrossRef]
- Ryu, J.; Liu, K.; McCloskey, T.A.; Yun, S.-L. An Integrated Methodology for Assessing Ecosystem Response to Environmental Stressors under Changing Climatic Conditions in Coastal Wetlands. Heliyon 2023, 9, e21263. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hou, X.; Song, Y.; Shan, K.; Zhu, S.; Yu, X.; Mo, X. Assessing Changes of Habitat Quality for Shorebirds in Stopover Sites: A Case Study in Yellow River Delta, China. Wetlands 2019, 39, 67–77. [Google Scholar] [CrossRef]
- Ren, G.; Zhao, Y.; Wang, J.; Wu, P.; Ma, Y. Ecological Effects Analysis of Spartina alterniflora Invasion within Yellow River Delta Using Long Time Series Remote Sensing Imagery. Estuar. Coast. Shelf Sci. 2021, 249, 107111. [Google Scholar] [CrossRef]
- Liu, L.; Han, M.; Liu, Y.B.; Pan, B. Spatial Distribution of Wetland Vegetation Biomass and Its Influencing Factors in the Yellow River Delta Nature Reserve. Acta Ecol. Sin. 2017, 37, 4346–4355. [Google Scholar]
- Liu, C.; Wang, Y.; Wang, C.; Sheng, H.; Xu, M.M.; Liu, S.W. Wetland Information Extraction in the Yellow River Delta Using Combined Sentinel-1 and Sentinel-2 Imagery. Mar. Sci. 2023, 47, 2–14. [Google Scholar]
- Ottinger, M.; Kuenzer, C.; Liu, G.; Wang, S.; Dech, S. Monitoring Land Cover Dynamics in the Yellow River Delta from 1995 to 2010 Based on Landsat 5 TM. Appl. Geogr. 2013, 44, 53–68. [Google Scholar] [CrossRef]
- Li, F.; Xie, Y.H.; Chen, X.S.; Deng, Z.M.; Li, X. Composition and Ecological Niches of Aquatic Plants in the Yellow River Delta Wetlands. Acta Ecol. Sin. 2009, 29, 6257–6265. [Google Scholar]
- Wang, Y.; Wu, Y.N.; Yan, G.X.; Zhang, Z.M. Spatial Point Pattern Analysis of Mixed Phragmites and Suaeda Salsa Communities in Coastal Wetlands of the Yellow River Delta. Ecol. Sci. 2020, 39, 51–59. [Google Scholar] [CrossRef]
- Xing, S.J.; Xi, J.B.; Zhang, J.F.; Song, Y.M.; Ma, B.Y. Basic Characteristics and Major Types of Vegetation in the Yellow River Delta. J. Northeast. For. Univ. 2003, 6, 85–86. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2025-1. 2025. Available online: https://www.iucnredlist.org (accessed on 10 May 2025).
- Jiang, Z.G. List, Standards and Management of National Protected Species in China. Biodiversity 2019, 27, 698. [Google Scholar]
- Jiang, Z.G.; Xie, F.; Li, C.; Zhang, Y.; Wang, Z.; Zheng, G. China’s Red List of Biodiversity: Vertebrates. In Amphibians; Science Press: Beijing, China, 2021; Volume 4. [Google Scholar]
- Sun, X.P.; Zhang, Y.L.; Cao, M.C.; Zhu, S.Y.; Shan, K. Analysis of Autumn and Winter Waterbird Communities and Their Habitat Relationships in the Yellow River Delta Nature Reserve. J. Ecol. Rural Environ. 2015, 31, 514–521. [Google Scholar]
- Huang, Z.Q.; Che, C.G.; Tan, H.T.; Bi, Z.G.; Liu, W.H.; Zhang, X.T.; Zhang, S.Y.; Li, D.L. Survey and Population Monitoring of Waterbird Diversity in the Yellow River Delta. Shandong For. Sci. Technol. 2018, 48, 41–45. [Google Scholar]
- Zhu, Z.Q.; Liu, Z.X.; Xie, Y.C.; He, J.L.; Li, J.Q.; Xu, J.L.; Zhang, Z.W. Impact of Oil Extraction on Waterbird Diversity and Distribution in the Yellow River Delta Nature Reserve. Acta Ecol. Sin. 2023, 43, 7157–7169. [Google Scholar] [CrossRef]
- Lambeck, R.J. Focal Species: A Multi-species Umbrella for Nature Conservation: Especies Focales: Una Sombrilla Multiespecífica Para Conservar La Naturaleza. Conserv. Biol. 1997, 11, 849–856. [Google Scholar] [CrossRef]
- Roberge, J.-M.; Angelstam, P.E.R. Usefulness of the Umbrella Species Concept as a Conservation Tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Liu, Y.L. Birds of the Yellow River Delta; China Forestry Publishing House: Beijing, China, 2013; ISBN 978-7-5038-6740-8. [Google Scholar]
- MacKinnon, J.; Phillipps, K. A Field Guide to the Birds of China; Hunan Education Press: Changsha, China, 2000; ISBN 978-7-5355-3224-4. [Google Scholar]
- Chai, Z.W.; Lei, W.P.; Mo, X.Q.; Que, P.J.; Shang, C.H.; Yang, J.W.; Zhang, Z.W. Avian Diversity in Beidagang Wetland Nature Reserve, Tianjin. Wetl. Sci. 2020, 18, 667–678. [Google Scholar] [CrossRef]
- Duan, Y.B.; Tian, X.H.; Ma, J.Z.; Zhu, S.Y.; Shan, K. Foraging Habitat Use by Oriental Storks during the Breeding Season in the Yellow River Delta. Acta Ecol. Sin. 2015, 35, 2628–2634. [Google Scholar]
- Cao, M.C.; Liu, G.H.; Xu, H.G. Multi-Scale Habitat Selection Mechanisms of Red-Crowned Cranes: A Case Study of the Yellow River Delta Nature Reserve. Acta Ecol. Sin. 2011, 31, 6344–6352. [Google Scholar] [CrossRef]
- Zhu, M.C.; Cao, M.C.; Wang, Z.X.; Xu, H.G.; Lei, J.C.; Wu, Y.; Zhao, J. Fuzzy Comprehensive Evaluation of Waterbird Habitat Suitability in the Yellow River Delta Nature Reserve. J. Cent. China Norm. Univ. Nat. Sci. 2015, 49, 287–294. [Google Scholar] [CrossRef]
- Panthi, S.; Pariyar, S.; Low, M. Factors Influencing the Global Distribution of the Endangered Egyptian Vulture. Sci. Rep. 2021, 11, 21901. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting Species Distributions from Small Numbers of Occurrence Records: A Test Case Using Cryptic Geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Zadeh, L.A. Fuzzy Sets, Information and Control. Inf. Control 1965, 8, 338–353. [Google Scholar] [CrossRef]
- Sun, X.; Shen, J.; Xiao, Y.; Li, S.; Cao, M. Habitat Suitability and Potential Biological Corridors for Waterbirds in Yancheng Coastal Wetland of China. Ecol. Indic. 2023, 148, 110090. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, H.F.; Li, L.H.; Yan, Y.J. Evaluation of Agricultural Water and Land Resource Carrying Capacity and Identification of Its Influencing Factors in Kazakhstan. Arid Zone Res. 2020, 37, 254–263. [Google Scholar] [CrossRef]
- Xu, M.D.; Cao, L.; He, J.; Li, J.; Wang, H.R. Fuzzy Comprehensive Evaluation of Ecological Environmental Vulnerability Based on GIS. Soil Water Conserv. China 2011, 6, 19–21. [Google Scholar] [CrossRef]
- Chang, Y.; Chang, C.; Li, Y.; Liu, M.; Lv, J.; Hu, Y. Predicting Dynamics of the Potential Breeding Habitat of Larus saundersi by MaxEnt Model under Changing Land-Use Conditions in Wetland Nature Reserve of Liaohe Estuary, China. Remote Sens. 2022, 14, 552. [Google Scholar] [CrossRef]
- Jiang, H.X.; Hou, Y.Q.; Chu, G.Z.; Qian, F.W.; Wang, H.; Zhang, G.G.; Zheng, G.M. Breeding Population Dynamics and Habitat Transition of Saunders’s Gull Larus Saundersi in Yancheng National Nature Reserve, China. Bird Conserv. Int. 2010, 20, 13–24. [Google Scholar] [CrossRef]
- Li, D.; Liu, K.; Gao, Y.; Wu, Y.; Hou, X. Stopover Habitat Use of Coastal Pied Avocet Revealed by Satellite Tracking and Remote Sensing Technology. Glob. Ecol. Conserv. 2024, 56, e03290. [Google Scholar] [CrossRef]
- Cao, M.C.; Sun, X.P.; Le, Z.F.; Cui, P.; Gong, X.; Xu, H.G. Analysis of Wintering Habitat Changes of Red-Crowned Cranes Based on the MaxEnt Model: A Case Study of Yancheng Nature Reserve. J. Ecol. Rural Environ. 2016, 32, 964–970. [Google Scholar]
- Li, Y.; Lu, L.Y.; Tian, X.H.; Duan, Y.B. Predicted Distribution of Suitable Habitats for Oriental Storks in Shandong Province. Acta Ecol. Sin. 2023, 43, 2194–2201. [Google Scholar]
- Wang, C.; Wang, G.; Dai, L.; Liu, H.; Li, Y.; Zhou, Y.; Chen, H.; Dong, B.; Lv, S.; Zhao, Y. Diverse Usage of Waterbird Habitats and Spatial Management in Yancheng Coastal Wetlands. Ecol. Indic. 2020, 117, 106583. [Google Scholar] [CrossRef]
- Min, Y.K.; Ke, Y.H.; Han, Y.; Yin, X.L.; Zhou, D.M. Dynamic Monitoring of Spartina alterniflora Removal Using Time-Series Sentinel-2 and GF-1 Imagery. J. Remote Sens. 2023, 27, 1467–1479. [Google Scholar]
- Chen, P.; Zhang, Y.; Zhu, X.J.; Lu, C.H. Ecological Impact of Spartina alterniflora Invasion on Birds. Acta Ecol. Sin. 2019, 39, 2282–2290. [Google Scholar]
- Li, Z.J.; Wang, Z.Y.; Liu, X.T.; Sun, S.C.; Ma, X.K.; Zuo, Y.N. Effects of Spartina alterniflora Control on Wetland Use and Landscape Pattern in the Yellow River Delta: Using Random Forest and Improved CA-Markov Model. Acta Ecol. Sin. 2024, 44, 8366–8382. [Google Scholar] [CrossRef]
- Gao, H.L.; Li, D.W.; Li, P.P.; Li, X.H.; Di, G.S. Spatiotemporal Analysis of Coastline Changes in the Yellow River Delta from 2020 to 2024. Surv. Mapp. Eng. 2025, 34, 22–30. [Google Scholar] [CrossRef]
- Rajpar, M.N.; Ahmad, S.; Zakaria, M.; Ahmad, A.; Guo, X.; Nabi, G.; Wanghe, K. Artificial Wetlands as Alternative Habitat for a Wide Range of Waterbird Species. Ecol. Indic. 2022, 138, 108855. [Google Scholar] [CrossRef]
- Kong, X.L.; Li, Y.L.; Han, M.; Tian, L.X.; Niu, X.R.; Zhu, J.Q.; Wang, M.; Huang, S.P. Distribution and Driving Factors of Natural Wetlands in the Yellow River Delta across Three Periods Since 1990. Wetl. Sci. 2020, 18, 603–612. [Google Scholar] [CrossRef]
- Gayet, G.; Matthieu, G.; François, M.; Hervé, F.; Laurence, C.; Joël, B. Annual Use of Man-Made Wetlands by the Mute Swan (Cygnus Olor). J. Environ. Manag. 2013, 120, 120–126. [Google Scholar] [CrossRef]
- Cheng, C.; Ma, Z. Conservation Interventions Are Required to Improve Bird Breeding Performance in Artificial Wetlands. Biol. Conserv. 2023, 278, 109872. [Google Scholar] [CrossRef]
- Fan, J.; Wang, X.; Wu, W.; Chen, W.; Ma, Q.; Ma, Z. Function of Restored Wetlands for Waterbird Conservation in the Yellow Sea Coast. Sci. Total Environ. 2021, 756, 144061. [Google Scholar] [CrossRef]
- Pang, B.; Yang, W.X.; Cui, B.S.; Zhang, S.Y.; Xie, T.; Ning, Z.H.; Gao, F.; Zhang, H.S. Evaluation of Vegetation Restoration Effects in the Biodiversity Conservation Project of the Yellow River Delta Wetlands. Environ. Eng. 2023, 41, 213–221. [Google Scholar] [CrossRef]
- Li, D.; Chen, S.; Lloyd, H.; Zhu, S.; Shan, K.; Zhang, Z. The Importance of Artificial Habitats to Migratory Waterbirds within a Natural/Artificial Wetland Mosaic, Yellow River Delta, China. Bird Conserv. Int. 2013, 23, 184–198. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, W.; Wei, J.; Jia, S.; Wang, A.; Huang, Y.; Zhao, Y. Estimation of Ecological Water Supplement for Typical Bird Protection in the Yellow River Delta Wetland. Ecol. Indic. 2021, 127, 107783. [Google Scholar] [CrossRef]
- Casazza, M.L.; McDuie, F.; Jones, S.; Lorenz, A.A.; Overton, C.T.; Yee, J.; Feldheim, C.L.; Ackerman, J.T.; Thorne, K.M. Waterfowl Use of Wetland Habitats Informs Wetland Restoration Designs for Multi-species Benefits. J. Appl. Ecol. 2021, 58, 1910–1920. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, P.; Pan, B.; Zou, Y.; Xie, Y.; Zhu, F.; Chen, X.; Li, F.; Deng, Z.; Zhang, H.; et al. Wetland Restoration in the East Dongting Lake Effectively Increased Waterbird Diversity by Improving Habitat Quality. Glob. Ecol. Conserv. 2021, 27, e01535. [Google Scholar] [CrossRef]
- Kan, Z.; Chen, B.; Yu, W.; Chen, G.; Ma, Z.; Hu, W.; Liao, J.; Du, H. Forecasting Land-Cover Change Effects on Waterbirds in Xiamen Bay, China: Determining Prospective Species Winners and Losers. Mar. Environ. Res. 2023, 188, 106003. [Google Scholar] [CrossRef]
| Species | Pictures | Conservation/Endangered Status | Habitat and History Distribution | Ecological Function/Indicator Role | Migratory Status and Representativeness |
|---|---|---|---|---|---|
| C. boyciana |  | IUCN: Endangered (EN); National Class I Protected Species (China); CBRL: Endangered (EN) | Shallow wetlands, swamps, and Phragmites marshes; choose tall transmission towers or artificial nest-building frames for nesting; mainly distributed in the Yellow River estuary and reservoir and pond wetlands in the YRDNR | Highly sensitive to water quality and connectivity; reflects health of river–pond systems | Long-distance migrant; indicates long-term habitat changes |
| L. saundersi |  | IUCN: Vulnerable (VU); National Class I Protected Species (China); CBRL: Vulnerable (VU) | Coastal saline–alkaline mudflats and intertidal zones; mainly distributed in coastal mudflats and salt marshes in the YRDNR | Depends on continuous mudflats and benthic resources; sensitive to intertidal structure and disturbance | Migratory species; indicates tidal flat integrity and fragmentation |
| G. japonensis |  | IUCN: Endangered (EN); National Class I Protected Species (China); CBRL: Endangered (EN) | Shallow wetlands and marshes with abundant riparian vegetation; mainly distributed in northern Phragmites marshes and shallow wetlands in the YRDNR | Strongly responds to vegetation composition and habitat connectivity; flagship species for wetland health | Long-distance migrant; reflects wetland continuity and quality |
| C. olor |  | IUCN: Least Concern (LC); National Class II Protected Species (China); CBRL: Near Threatened (NT) | Large freshwater lakes, reservoirs, and rivers; mainly distributed in pond wetlands and Phragmites marshes characterized by large expanses of open water in the YRDNR | Depends on extensive open water; useful indicator for artificial wetland restoration outcomes | Resident species; represents restored aquatic ecosystems |
| N. madagascariensis |  | IUCN: Endangered (EN); National Class II Protected Species (China); CBRL: Vulnerable (VU) | Intertidal mudflats and estuarine sandbars; mainly distributed in southern tidal flats and salt marshes in the YRDNR | Benthic forager; highly sensitive to sediment structure and mudflat degradation | Asian–Australasian Flyway; reflects mudflat quality dynamics |
| Variable | Abbreviation (Unit) | Description | Impact on the Habitat | |
|---|---|---|---|---|
| Land Use/Land Cover Type | LULC (−) | Natural or anthropogenic land cover and usage | Provides the material basis for habitat environments | |
| Landscape Metrics | Percentage of Landscape | PLAND(%) | Percentage of each patch type in the landscape | Reflect landscape characteristics and habitat quality |
| Shannon’s Diversity Index | SHDI (−) | Diversity of landscape composition | ||
| Contagion Index | CONTAG (%) | Degree of aggregation or dispersion of patch types across the landscape | ||
| Vegetation Coverage | FVC (%) | Level of vegetation growth and coverage | Provides cover, nesting, and foraging resources for waterbirds | |
| Human Disturbance | Distance to Roads | D_road (km) | Euclidean distance to nearest road | Negatively affects waterbird roosting and breeding |
| Distance to Industrial/Mining Facilities | D_industrial/mining facilities (km) | Euclidean distance to industrial/mining facilities | ||
| Habitat Selection Variables | Distance to Different Land Use/Land Cover Types | D_different land use/land cover types (km) | Euclidean distance to different Land Use/Land Cover types | Reflects the accessibility of food and water sources for waterbirds |
| Distance to Coastline | D_coastline (km) | Euclidean distance to the coastline | ||
| Distance to Water | D_water (km) | Euclidean distance to all water | ||
| Level 1 Classification | Level 2 Classification | Type Description | Code |
|---|---|---|---|
| Natural Wetlands | Spartina alterniflora Marsh | Intertidal marsh dominated by Spartina alterniflora | 1 |
| Phragmites Marsh | Intertidal and freshwater marsh dominated by Phragmites spp. | 2 | |
| Tamarisk Marsh | Intertidal marsh dominated by Tamarix spp. | 3 | |
| Suaeda Marsh | Intertidal marsh dominated by Suaeda spp. | 4 | |
| Mudflat | Vegetation cover <30%, composed of muddy/sandy substrate | 5 | |
| River | Perennial and seasonal/intermittent rivers | 6 | |
| Artificial Wetlands | Reservoir/Pond Wetland | Permanent water bodies including reservoirs and artificial freshwater restoration areas | 7 |
| Aquaculture/Salt Pond | Man-made wetlands for aquaculture or salt production | 8 | |
| Paddy Field | Fields suitable for rice cultivation or seasonally flooded/waterlogged | 9 | |
| Non-wetlands | Dry Farmland | Arable land for crops such as wheat, cotton, and maize without irrigation infrastructure | 10 |
| Woodland | Land dominated by herbaceous plants, trees, or shrubs (excluding marshy grassland); high vegetation cover | 11 | |
| Industrial/Mining Area | Built-up areas including settlements, transportation, industrial, and other impervious surfaces | 12 | |
| Offshore Waters | Permanently submerged coastal, estuarine, and bay waters, typically less than 6 m deep at low tide | 13 |
| Species | Key Variable (Abbreviation) |
|---|---|
| C. boyciana | Land Use/Land Cover (LULC), Fractional Vegetation Cover (FVC), Distance to roads (D_road), Distance to paddy fields (D_9), Distance to water (D_water), Distance to Phragmites Marsh(D_2), Proportion of river landscape (PLAND_6), Proportion of mudflat landscape (PLAND_5) |
| L. saundersi | Land Use/Land Cover (LULC), Fractional Vegetation Cover (FVC), Distance to roads (D_road), Distance to water (D_water), Distance to coastline (D_coastline), Proportion of mudflat landscape (PLAND_5), Distance to Suaeda Marsh (D_4), Distance to Phragmites Marsh (D_2) |
| G. japonensis | Land Use/Land Cover (LULC), Fractional Vegetation Cover (FVC), Distance to roads (D_road), Proportion of paddy field landscape (PLAND_9), Distance to water (D_water), Distance to mudflats (D_5), Distance to Suaeda Marsh (D_4), Proportion of Phragmites landscape (PLAND_2) |
| C. olor | Land Use/Land Cover (LULC), Fractional Vegetation Cover (FVC), Distance to roads (D_road), Distance to water (D_water), Distance to aquaculture areas (D_8), Proportion of Phragmites landscape (PLAND_2), Distance to paddy fields (D_9), Proportion of reservoir/pond landscape (PLAND_7) |
| N. madagascariensis | Land Use/Land Cover (LULC), Fractional Vegetation Cover (FVC), Distance to roads (D_road), Distance to water (D_water), Distance to coastline (D_coastline), Distance to mudflats (D_5), Distance to Suaeda Marsh(D_4), Proportion of Tamarix landscape (PLAND_3) |
| Evaluation Variable | Δ | ||||
|---|---|---|---|---|---|
| C. boyciana | 0.045 | 0.165 | 0.315 | 0.540 | 0.045 |
| L. saundersi | 0.085 | 0.220 | 0.395 | 0.680 | 0.055 |
| G. japonensis | 0.120 | 0.275 | 0.485 | 0.750 | 0.065 |
| C. olor | 0.155 | 0.335 | 0.58 | 0.820 | 0.075 |
| N. madagascariensis | 0.195 | 0.400 | 0.660 | 0.890 | 0.085 |
| Species | Conservation Priority Weight | Habitat Type Weight | Total Weight |
|---|---|---|---|
| C. boyciana | 0.230 | 0.286 | 0.263 |
| L. saundersi | 0.197 | 0.214 | 0.207 |
| G. japonensis | 0.180 | 0.071 | 0.115 |
| C. olor | 0.213 | 0.286 | 0.257 |
| N. madagascariensis | 0.180 | 0.143 | 0.158 |
| Land Cover Type | 2005 | 2010 | 2015 | 2020 | 2024 |
|---|---|---|---|---|---|
| Natural wetlands | 695.44 | 599.45 | 598.93 | 595.21 | 586.97 |
| Artificial wetlands | 118.65 | 211.55 | 231.45 | 278.97 | 288.07 |
| Total Wetlands | 814.09 | 811.00 | 830.38 | 874.18 | 875.03 |
| Non-wetlands | 556.45 | 559.54 | 540.13 | 496.35 | 495.55 |
| Land Use/Land Cover Type | 2005 | 2024 | ||||||
|---|---|---|---|---|---|---|---|---|
| The Proportion in the Mostly Suitable Habitat (%) | The Proportion in the Moderately Suitable Habitat (%) | The Proportion in the Poorly Suitable Habitat (%) | The Proportion in the Unsuitable Habitat (%) | The Proportion in the Mostly Suitable Habitat (%) | The Proportion in the Moderately Suitable Habitat (%) | The Proportion in the Poorly Suitable Habitat (%) | The Proportion in the Unsuitable Habitat (%) | |
| Spartina alterniflora Marsh | - | - | - | - | - | - | - | 0.08 |
| Phragmites Marsh | 38.63 | 30.90 | 30.05 | 3.74 | 41.25 | 31.62 | 18.49 | 2.23 |
| Tamarisk Marsh | 2.30 | 0.67 | - | - | 2.14 | 3.07 | 0.40 | - |
| Suaeda Marsh | 31.69 | 7.40 | 1.20 | 0.78 | 5.91 | 4.85 | 0.85 | 0.34 |
| Mudflat | 14.32 | 34.96 | 29.44 | 17.82 | 19.29 | 34.53 | 35.86 | 17.40 |
| River | 0.53 | 3.69 | 1.73 | 1.50 | - | 3.54 | 2.95 | 1.47 |
| Reservoir/Pond Wetland | 7.19 | 5.36 | 3.56 | 0.25 | 29.83 | 5.10 | 3.95 | 0.27 |
| Aquaculture/Salt Pond | 0.33 | 0.88 | 4.24 | 0.85 | 0.00 | 2.84 | 3.43 | 1.31 |
| Paddy Field | 1.16 | 3.03 | 5.27 | 1.26 | 1.04 | 11.03 | 20.00 | 17.36 |
| Dry Farmland | 3.38 | 9.32 | 19.76 | 16.00 | - | 0.23 | 4.03 | 2.61 |
| Woodland | - | 3.04 | 2.58 | - | 0.32 | 2.41 | 4.37 | 0.34 |
| Industrial/Mining Area | - | - | - | - | - | - | 1.44 | 0.49 |
| Offshore Waters | 0.46 | 0.76 | 2.17 | 57.79 | 0.20 | 0.77 | 4.23 | 56.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Chi, Y.; Zhong, Y.; Wang, Q. Habitat Suitability Dynamics of Yellow River Delta Nature Reserves for Rare Waterbirds. Sustainability 2025, 17, 5326. https://doi.org/10.3390/su17125326
Wang H, Chi Y, Zhong Y, Wang Q. Habitat Suitability Dynamics of Yellow River Delta Nature Reserves for Rare Waterbirds. Sustainability. 2025; 17(12):5326. https://doi.org/10.3390/su17125326
Chicago/Turabian StyleWang, Hongli, Yunyi Chi, Yujie Zhong, and Qiang Wang. 2025. "Habitat Suitability Dynamics of Yellow River Delta Nature Reserves for Rare Waterbirds" Sustainability 17, no. 12: 5326. https://doi.org/10.3390/su17125326
APA StyleWang, H., Chi, Y., Zhong, Y., & Wang, Q. (2025). Habitat Suitability Dynamics of Yellow River Delta Nature Reserves for Rare Waterbirds. Sustainability, 17(12), 5326. https://doi.org/10.3390/su17125326




