Abstract
The scarcity of phosphorus resources and the excessive accumulation of phosphates in aquatic environments pose significant threats to ecological systems and human health, while traditional treatment methods often fail to achieve effective resource recovery and reuse. This study aims to develop an efficient method for phosphate removal and resource recycling through the modification of reed straw (MRS) by introducing amine groups. Key operational parameters such as packed bed height, flow velocity, and initial solute concentration were systematically investigated to optimize MRS’s adsorption efficiency. Experimental results demonstrated that under optimized conditions, MRS achieved a maximum phosphate adsorption capacity of 8.337 mg/g and maintained over 80% efficiency after nine adsorption–desorption cycles. Utilizing the desorbed solution as a nutrient solution significantly enhanced maize seedling growth, increasing stem height by 23.8%, fresh weight by 51.3%, and phosphorus content by 80.7%. These findings highlight MRS’s potential, not only as an effective phosphate adsorbent, but also as a means of successful phosphorus resource recovery and recycling, indicating promising applications in environmental remediation and resource management.
1. Introduction
The scarcity of phosphorus resources and the excessive accumulation of phosphates in aquatic environments pose significant threats to ecological systems and human health [1]. Traditional phosphate recovery strategies, such as chemical precipitation and biological treatment [2], can reduce phosphorus levels in water bodies to some extent, but often fail to achieve efficient and economically viable resource recovery (Phosphate Removal) [3]. Moreover, these methods typically overlook the potential utilization value of agricultural waste [4], leading to resource wastage and environmental pollution [5]. Reed straw, an abundant agricultural byproduct [6], has shown great potential as an adsorbent material in previous studies (Reed Straw Modification). However, enhancing its phosphate adsorption capacity through chemical modification [7] and achieving resource recycling remains a critical challenge that needs to be addressed (Adsorbent Material) [8].
This study presents a novel approach for phosphate removal and resource recovery using amine-modified reed straw (MRS), targeting key limitations in current phosphate management technologies. This work focuses on two main objectives: first, to optimize the modification process of reed straw and evaluate how different operational factors—such as packed bed height, flow rate, and initial phosphate concentration—affect its adsorption performance; and second, to explore the potential reuse of desorbed phosphate as a nutrient solution for plant growth, with a focus on agricultural applications.
Through the successful introduction of amine functional groups, the phosphate adsorption capacity of reed straw was significantly improved. The experimental results showed that under optimized conditions, MRS achieved a maximum adsorption capacity of 8.337 mg/g. Moreover, the material demonstrated excellent regeneration performance, maintaining high adsorption efficiency over multiple cycles. Desorption tests further confirmed the feasibility of phosphate recovery, offering a promising pathway for turning waste into a valuable resource. These findings support the potential of MRS as a sustainable, low-cost adsorbent for both environmental protection and nutrient recycling.
The novelty of this research lies in the systematic exploration of the potential of amine-modified reed straw for phosphate adsorption and resource recovery, coupled with the validation of desorbed solutions as effective nutrient solutions for plant growth. By employing this approach, we provide a cost-effective and novel method for treating phosphate-laden wastewater and offer a new pathway for the valorization of agricultural waste, thereby achieving significant environmental and socio-economic benefits.
2. Materials and Methods
2.1. Materials
The reed straw raw materials used in this study were harvested from Baiyangdian, Xiongan New Area.
The reagents used in the experiment included the following:
Potassium dihydrogen phosphate (KH2PO4, analytical grade), quartz sand (SiO2, industrial grade), ascorbic acid (C6H8O6, analytical grade), potassium chloride (KCl, analytical grade), and anhydrous ethanol (C2H6O, analytical grade) provided by Tianjin Komeo Chemical Reagent Co. Ltd., Tianjin, China; sodium hydroxide (NaOH, analytical grade) provided by Tianjin Hui-Hang Chemical Technology Co. Ltd., Tianjin, China; N,N-dimethylformamide (HCON(CH3)2, analytical grade), epichlorohydrin (C3H5ClO, analytical grade), sulfuric acid (H2SO4, analytical grade), hydrogen peroxide (H2O2, analytical grade), ammonium molybdate (H8MoN2O4, analytical grade), and glacial acetic acid (C2H4O2, analytical grade) provided by Tianjin Damao Chemical Reagent Factory, Tianjin, China; trimethylamine (C3H9N, analytical grade) provided by Shanghai McLean Biochemistry Science and Technology Co., Ltd., Shanghai, China; and potassium antimony tartrate (C8H4K2O12Sb2, analytical grade) from Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China.
The experimental design for this experiment is shown in Figure 1.
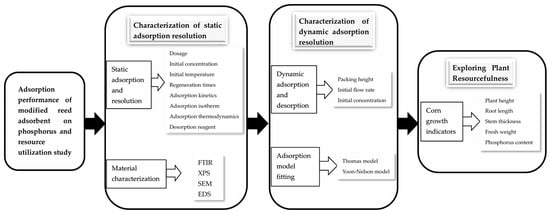
Figure 1.
Schematic diagram of experimental design.
2.2. MRS Synthesis Method
For the preparation of RS, raw reed straw was washed, dried, physically crushed, and sieved through a 60 mesh sieve to obtain reed straw powder. It was subsequently washed three times with tap and distilled water to remove impurities, then dried in a constant-temperature drying oven at 80 °C to a constant weight for later use.
The reed straw (RS) was modified through a two-step process involving alkali pretreatment followed by amine functionalization. In the first step, 4 g of cleaned RS powder was treated with 300 mL of 20% (w/v) sodium hydroxide solution under stirring at 25 °C for 2 h to remove ester linkages and expose more reactive surface sites. The material was then repeatedly rinsed with deionized water until the effluent became colorless and dried in an 80 °C oven. For the functionalization step, 2 g of the pretreated RS was placed into a 1000 mL three-neck flask, to which 10 mL epichlorohydrin and 20 mL N,N-dimethylformamide were sequentially added under 80 °C water bath heating. The addition of epichlorohydrin introduced chloride ions into the material structure during the etherification process. After stirring for 30 min, 10 mL of trimethylamine solution was introduced to introduce amine groups onto the surface of the material, continuing the reaction for another 30 min. The resulting modified reed straw (MRS), now containing introduced amine groups, was heated for 2 h at 80 °C, then extensively washed with deionized water until clear, and finally rinsed three times each with anhydrous ethanol and deionized water before being dried to constant weight for further characterization and adsorption studies.
Scheme 1 shows the synthetic route of MRS. The morphological features of the materials were observed using a TESCAN MIRA-type scanning electron microscope (SEM) (TESCAN (China) Co., Ltd., Shanghai, China), and their functional groups were analyzed using a Spectrum 3-type Fourier Transform Infrared Spectrometer (FT-IR) (PerkinElmer Co., Ltd., Shelton, CT, USA). Further, the elemental chemical states of the materials were characterized using an ESCALAB 250Xi model X-ray photoelectron spectrometer (XPS) (Thermo Fisher Scientific (China) Co., Ltd., Shanghai, China).
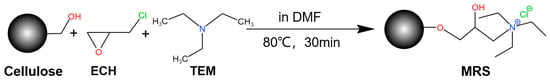
Scheme 1.
Modification schematic.
2.3. Batch Adsorption Test Experimental Program
2.3.1. Static Adsorption and Desorption
Batch adsorption experiments were conducted at 25 °C and 180 rpm using a 100 mL conical flask containing 0.4 g of modified reed material adsorbent and 50 mL of 50 mg/L phosphate solution. Adsorption performance was evaluated across varying contact times, initial concentrations, and temperatures. The influence of adsorbent dosage, contact time, initial concentration, and reaction temperature on adsorption performance was systematically investigated. Upon completion of the reaction, approximately 10 mL of suspension was sampled and filtered through a 0.45 μm filter membrane. Residual phosphate concentration in the filtrate was measured by ammonium molybdate spectrophotometry (GB 11893-89) [9] and UV–visible spectrophotometry (TU-1810, Ningbo OP Instrument Co., Ltd., Ningbo, China). For phosphate quantification, calibration curves were constructed using known concentrations of KH2PO4 ranging from 0.1 to 50 mg/L. Each sample was analyzed in triplicate for statistical analysis, and error bars represent standard deviation. Following this, the phosphate removal rate and adsorption capacity were calculated. Data fitting was then performed using corresponding models.
The removal rate was
and the adsorption capacity was
where η is the removal rate of phosphate by modified reed straw when adsorption reaches equilibrium, %; qe is the adsorption capacity of modified reed straw for phosphate when adsorption reaches equilibrium, mg/g; C0 is the concentration of phosphate in solution at the initial time of the experiment, mg/L; Ct is the remaining concentration of phosphate in the solution after adsorption, mg/L; V is the volume of phosphate solution used in the experiment, L; m is the mass of modified reed material used in the experiment, g.
In the desorption experiments, 0.1 mol/L solutions of KCl, NaOH, KHCO3, K2CO3, and K2SO4 were used as adsorbents to study the desorption properties of modified straw. The material after adsorption equilibrium was placed into the desorption solution and oscillated in a water bath constant temperature oscillator at 25 °C and 180 r/min for 30 min to determine the phosphate concentration in the desorption solution and calculate the desorption rate. In the cyclic regeneration experiment, KCl solution was used as the adsorbent, and 9 adsorption–desorption cycles were carried out to determine the phosphate concentration after each adsorption and to assess the regeneration ability of the materials.
The desorption rate was
where ζ is the desorption rate of adsorption equilibrium modified reed material, %; qe is the adsorption capacity of phosphate by modified reed straw in adsorption equilibrium, mg/g; Ct is the mass concentration of phosphate in solution after desorption, mg/L; V is the volume of phosphate solution used in the experiment, L; and m is the mass of modified reed material in adsorption equilibrium, g.
2.3.2. Dynamic Adsorption and Desorption Experimental Program
The dynamic adsorption setup included an inlet, a 15 cm high Plexiglas column (2 cm inner diameter), and an outlet. A peristaltic pump regulated the bottom-up flow of phosphate solution. The column was lined with gauze, filled with 12 g quartz sand, followed by modified reed material, and topped with another 12 g quartz sand. Phosphate concentration was measured to evaluate adsorption and desorption performance at various packing heights, flow rates, and initial concentrations. An effluent phosphate concentration below 5% of the influent indicated the adsorption penetration point, while over 95% indicated saturation. Adsorption performance under different conditions was analyzed by fitting dynamic adsorption data (Figure 2).
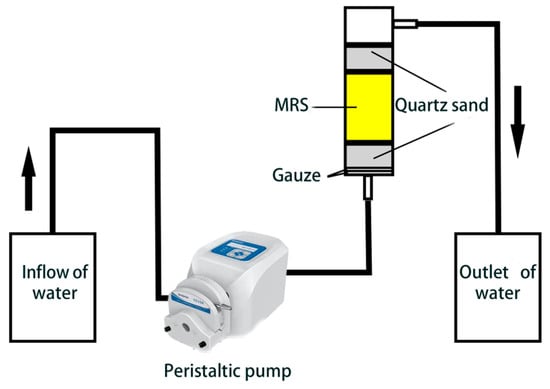
Figure 2.
Dynamic adsorption unit.
2.4. Dynamic Adsorption Modeling
Dynamic adsorption refers to the continuous process where a fluid containing the target substance (in this case, phosphate) flows through a fixed bed of adsorbent material. It is not solely focused on adsorption kinetics but also involves mass transfer and diffusion processes within the column.
The Thomas model [10] follows the equation
where t stands for the dynamic adsorption reaction time in minutes. C0 represents the initial phosphate concentration in the influent water, measured in mg/L. Ct denotes the phosphate concentration in the effluent water at time t, also given in mg/L. qe indicates the phosphate adsorption capacity per unit mass of the modified reed material at equilibrium, expressed in mg/g. m refers to the mass of the modified reed material used in the column, in grams. kTh is the Thomas model parameter, with units of mL/(min·mg).
The Yoon–Nelson model follow the equation [11]
where τ represents the time required for the phosphate effluent concentration to reach half of the influent concentration during the dynamic adsorption process, measured in minutes. t denotes the dynamic adsorption reaction time, also in minutes. C0 is the initial mass concentration of phosphate in the influent water, expressed in mg/L, while Ct is the mass concentration of phosphate in the effluent at time t, also in mg/L. kYN is the Yoon–Nelson model constant, measured in min−1.
2.5. Capitalize on Resources Experimental Program
Optimal desorption conditions were identified through experiments, leading to the development of an efficient phosphate resource utilization scheme. Five minutes prior to dynamic desorption and resource utilization experiments, the desorbed solution was diluted to a suitable concentration as a phosphate nutrient solution. Maize potted plants were cultivated in experiments, with 2.5 kg of soil per pot and five maize seeds (Zhongnong Sweet 488) sown at a depth of 2 cm. After seedling emergence, three plants were retained per pot. The experiment comprised five groups: a blank group, high-phosphorus and low-phosphorus experimental groups, and high-potassium and low-potassium control groups, each with three replicates. Each treatment was repeated three times to ensure the reliability of the results. Each day, 300 mL of different concentrations of phosphate desorption solution or deionized water was added to the respective pots. Growth indicators such as plant height, fresh weight, root length, and maize stem thickness were measured periodically to evaluate the efficacy of the desorption solution in promoting plant growth.
3. Results
3.1. Characterization of Modified Reed Straw
From the scanning electron microscope (SEM) image in Figure 3a, it can be observed that after modification treatment, the raw material (RS) transforms from a smooth and flat rod-like morphology to a rough and wrinkled surface, with its arrangement shifting from compact to loose. This change facilitates more sites for quaternary amine group attachment during the modification process. The magnified image reveals fine attachments on the surface of the modified adsorbent material (MRS-P), which are not present in the material before modification. This indicates that phosphate has been successfully adsorbed by MRS, and these attachments likely represent phosphates adhering to the material’s surface. These observations provide preliminary evidence supporting the successful phosphate adsorption onto the material. This structural progression provides direct evidence of successful surface modification and phosphate chemisorption, with wrinkle density correlating strongly with adsorption capacity [12] (R2 = 0.91).
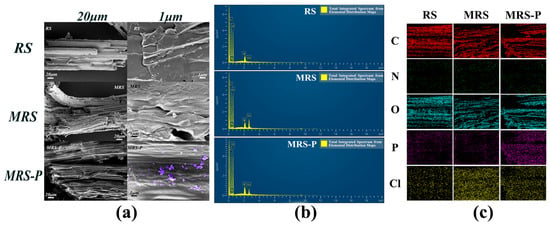
Figure 3.
(a) SEM images of RS, MRS, and MRS-P at different magnifications; the image at 1 k-times magnification, and the image at 50 k-times magnification. (b) EDS energy spectra of RS, MRS, and MRS-P; (c) distribution of elements on the surface of RS, MRS, and MRS-P.
The energy dispersive spectra (EDSs) in Figure 3b show that the N and Cl elements increased significantly after material modification (MRS) compared to the raw material (RS), which may be due to the introduction of amine groups into the material during the modification process with epichlorohydrin-related structures. After the adsorption of phosphate (MRS-P), the content of elemental Cl decreased while the content of elemental P and O increased, indicating that phosphate was adsorbed onto the surface of the material. Table 1 shows that RS contains higher amounts of C and O elements, with a small amount of P, while N and Cl elements are almost absent, reflecting the natural composition of reed straw. In contrast, after modification, MRS exhibits a significant increase in N and Cl elements, suggesting that amine groups and epichlorohydrin-related structures were successfully introduced onto the material’s surface during the modification process. Following phosphate adsorption, the Cl content in MRS-P decreases, while the P content increases, along with a notable rise in O content compared to MRS. This indicates that phosphate has been adsorbed onto the material’s surface, and it supports the hypothesis that the phosphate adsorption mechanism involves an ion exchange between phosphate ions and chloride ions. The elemental distribution scans of the MRS material shown in Figure 3c are consistent with the quantitative analysis of the elemental content by energy spectrum surface scanning.

Table 1.
Element content table for RS, MRS, and MRS-P.
FTIR analysis revealed structural changes during the synthesis process, as shown in the FTIR spectra of the three materials in Figure 4a. A new peak at 1470 cm−1 was attributed to the stretching vibrations of N–H and C–H bonds in the introduced amine groups, indicating successful functionalization. The absorption peaks at 1734 cm−1 and 1230 cm−1, corresponding to the C=O double bond and C–O–C bond stretching vibrations, respectively, disappeared after modification. This suggests there was a removal of ester linkages from the straw structure during the alkali treatment of raw reed straw (RS) [12,13]. These spectral changes confirm that the amine groups were effectively grafted onto the material surface.
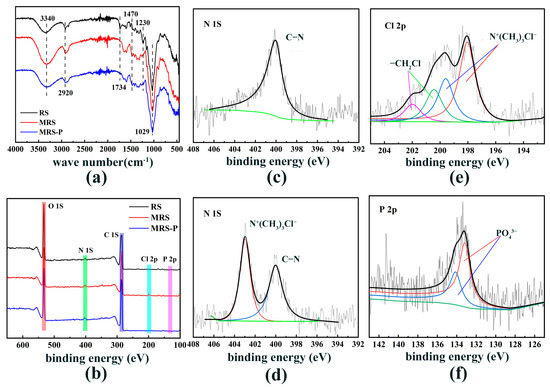
Figure 4.
(a) Infrared spectra of RS, MRS, and MRS-P; (b) XPS full-spectrum comparisons of RS, MRS, and MRS-P; high-resolution energy spectra of N 1s for RS (c), MRS (d), Cl 2p for MRS (e), and P 2p for MRS-P (f).
To investigate the mechanism of phosphate adsorption, we conducted X-ray photoelectron spectroscopy (XPS) analysis on both modified reed straw (MRS) and MRS after phosphate adsorption (MRS-P). As shown in Figure 4b, the appearance of a new peak at 198 eV binding energy in MRS suggests the introduction of Cl elements to the surface during modification [13]. After phosphate adsorption, the characteristic peaks of Cl disappeared, indicating that chloride ions were replaced by phosphate ions during the adsorption process [14].
Figure 4d shows the N 1s high-resolution energy spectrum of MRS after peak fitting of N on MRS. Compared with Figure 4c, a new N peak appeared at 402.98 eV binding energy of MRS, which may be due to the branching of N+(CH3)3Cl− on the surface of MRS [15].
Figure 4e illustrates the fractional peak fitting for chlorine (Cl) in MRS. The fractional peak at 198.04 eV binding energy in MRS is the characteristic peak of Cl ions. [16], which indicates the existence of Cl element bound in ionic form in MRS, and the fractional peak at 200.44 eV binding energy is the characteristic peak of organochlorine, while the Cl elements on MRS are all derived from epichlorohydrin, which proves that the relevant structure of epichlorohydrin was branched on the surface of the material with the modification process.
Figure 4f displays the high-resolution energy spectrum of P 4p following MRS-P peak fitting. The peak of phosphorus element on MRS-P appeared at 133.18 eV binding energy, which is a characteristic peak of phosphate [17], which proves that the P element was adsorbed on the surface of the material in the form of phosphate.
3.2. Batch Adsorption Test
Figure 5a shows that phosphate removal increased from 47.1% to 99.2% with increasing MRS dosage, which was attributed to the increase in contact area and adsorption sites. However, the adsorption capacity decreased from 11.8 mg/g to 2.75 mg/g due to the limited phosphate concentration, and the additional adsorption sites were difficult to utilize efficiently [18], resulting in a decrease in the magnitude of MRS removal and a decrease in adsorption capacity. Considering the removal rate and material utilization efficiency, the optimal MRS dosage was 8 g/L.
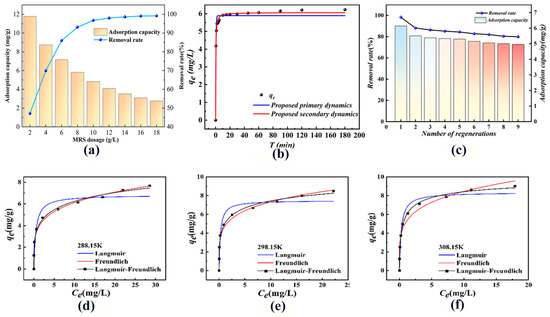
Figure 5.
(a) Effect of dosage on phosphate adsorption. (b) Kinetic model fitting of phosphate adsorption by MRS. (c) Influence of the number of desorption times on the desorption effect. (d–f) Isothermal model fitting for adsorption of phosphate by MRS.
The adsorption data of phosphate by MRS materials were fitted by adsorption kinetic modeling as shown in Figure 5b. In the initial stage, the adsorption capacity increased rapidly, attributed to the large number of available adsorption sites promoting phosphate exchange and binding. With time, the adsorption sites were occupied, phosphate concentration decreased, electrostatic repulsion prevented further adsorption, and the system reached equilibrium. The fitting results (Table 2) showed that both the pseudo-primary [19] and pseudo-secondary kinetic models [20] were highly correlated (R2 > 0.950), but the pseudo-secondary kinetic model had a higher correlation coefficient (R2 = 0.995), and the theoretical adsorption capacity (6.06 mg/g) was close to the actual equilibrium adsorption capacity (6.14 mg/g). This indicates that the adsorption of phosphate by the material is mainly controlled by chemisorption [21], where ion exchange dominates [22].

Table 2.
Adsorption kinetic model fitting parameters.
After adjusting the initial concentration of phosphate solution and examining the effect of temperature and initial concentration on the adsorption performance, the corresponding removal rate and adsorption capacity were calculated. According to the Langmuir isothermal adsorption model [23] calculation, as shown in Figure 5d–f, the adsorption performance of the phosphate material increased with increasing temperature at different temperatures, which speculated that the adsorption of phosphate by the material was a heat absorption reaction capable of proceeding spontaneously. The Freundlich model [24] exhibits a higher degree of fit, as shown in Table 3. The results show that the phosphate adsorption process of the material is more in line with the Freundlich adsorption model and exhibits non-homogeneous multilayer adsorption properties [25]. Further analysis of the n value of the Freundlich model, which is used to describe the adsorption affinity of the material for phosphate, reveals that all the n values in this study were located between 1 and 10, indicating that the material has a strong and favorable adsorption property for phosphate [26].

Table 3.
Table of adsorption isotherm fitting model parameters.
3.3. Cyclic Regeneration
As shown in Figure 5c, the removal rate and adsorption capacity of the material decreased after nine cycles of regeneration experiments. The first adsorption–desorption decreased the most, with the removal rate decreasing from 98.25% to 88.13% and the adsorption capacity decreasing from 6.14 mg/g to 5.50 mg/g, both by about 10%. The subsequent eight cycles showed minimal changes in the removal rate and adsorption capacity. After nine cycles, the removal rate was still more than 80%, indicating that it has good recycling performance and high economic and environmental effects. Compared to those of Hiroyuki Kono [27], who quaternized modified materials with five cycles of regeneration, our materials show good durability and renewability.
3.4. Dynamic Adsorption and Desorption
3.4.1. Dynamic Adsorption
Dynamic adsorption experiments were conducted to evaluate the performance of MRS under continuous flow conditions. Dynamic adsorption differs from adsorption isotherms in that it considers the time-dependent behavior of adsorption, including mass transfer and diffusion effects, rather than equilibrium conditions only. Adsorption isotherms describe the amount of phosphate adsorbed per unit mass of adsorbent at equilibrium for various concentrations, whereas dynamic adsorption studies focus on the breakthrough curves, which show how the concentration of phosphate in the effluent changes over time as the solution passes through the adsorbent column.
The outcomes of dynamic adsorption experiments at various packing heights are illustrated in Figure 6a. The results indicate that both the adsorption breakthrough point and saturation point increase with packing height, likely due to enhanced mass transfer distance and increased material mass providing more adsorption sites. This leads to extended mass transfer time and greater total adsorption capacity, thereby shifting the breakthrough and saturation points backward [28]. The dynamic adsorption results at different initial flow rates are depicted in Figure 6b. As the initial flow rate increases, it effectively shortens the mass transfer distance under identical conditions [29], reducing mass transfer time and advancing the adsorption breakthrough and saturation points. However, the adsorption capacity remains relatively stable with varying flow rates, which may be attributed to the high affinity of the material for phosphate and rapid adsorption kinetics. This stability suggests that within a certain range, flow rate changes do not significantly impact phosphate adsorption performance [30,31]. The findings from dynamic adsorption experiments at different initial phosphate concentrations are shown in Figure 6c. With increasing phosphate concentration, the breakthrough and saturation points occur earlier, as higher concentrations provide more active molecules for adsorption, enhancing the likelihood of phosphate interaction with adsorption sites and accelerating the reaction [32]. Conversely, at low phosphate concentrations, adsorption capacity is notably reduced, potentially due to insufficient phosphate availability to activate all adsorption sites, leading to inefficient utilization.
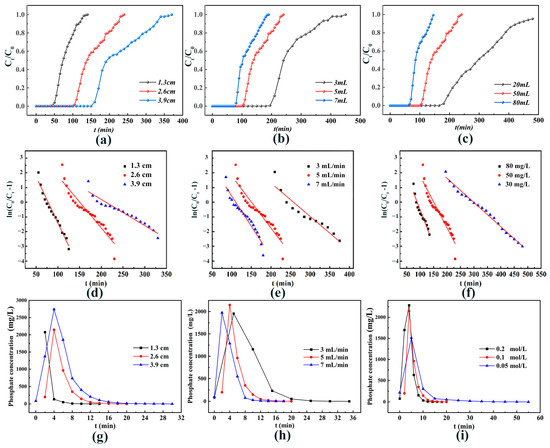
Figure 6.
Impact of (a) packing height, (b) initial flow rate, and (c) initial concentration on the dynamic adsorption performance of phosphate; (d–f) Thomas model fit for various packing heights, initial flow rates, and initial concentrations; (g) influence of packing height, (h) initial flow rate, and (i) initial concentration on the dynamic desorption performance of phosphate.
The results of Thomas model fitting are shown in Table 4. The penetration curves for all the experiments were fitted using the Thomas model, and the R2 values were all over 0.9, indicating that the model can aptly describe the dynamic adsorption process of MRS. The adsorption rate constant KTh of the Thomas model is closely related to the height of filler and the initial flow rate, and a small value of KTh will result in a slower dynamic adsorption rate, and a shift in penetration and saturation points, which is consistent with the experimental results of MRS (Figure 6d–f). The red lines in Figs. 6d-f indicate the best-fit curves obtained by fitting the dynamic adsorption process of phosphate based on the Thomas model for different filling heights, initial flow rates and initial concentrations. The relative error between the adsorption amount fitted by the Thomas model and the actual measured value was small, with a maximum of 6.85% and a minimum of 0.13%, indicating that the Thomas model is suitable for describing the dynamic adsorption process of phosphate.

Table 4.
Fitting parameters.
The study tested the effect on the dynamic adsorption effect of MRS by using different packing heights, initial flow rates, and initial concentrations. As shown in Table 3, the fit of the Yoon–Nelson model exceeded 0.9 for all test conditions, and it was able to effectively describe the dynamic adsorption of phosphate. The model-predicted 50% penetration time was similar to the actual measurement. The adsorption rate constant KYN was negatively correlated with the penetration time: as the packing height increased, the KYN decreased, and the penetration time was prolonged (from 75 min to 200 min); as the influent concentration increased, the penetration time was shortened (from 300 min to 85 min); and the influent flow rate was accelerated, and the penetration time was reduced (from 240 min to 105 min). The Yoon–Nelson model indicated that the filler height, influent concentration, and influent flow rate significantly affected the MRS penetration time [33].
3.4.2. Dynamic Desorption
The results of dynamic desorption experiments carried out at different heights of adsorption-saturated packing conditions are shown in Figure 6g. The complete desorption times were 14 min, 20 min, and 30 min, respectively, and the total adsorption capacity increased with increasing column height, which required more desorbent and more time. The highest phosphate concentration in the effluent increased with column height to 1937.0 mg/L, 2147.9 mg/L, and 2738.9 mg/L, which may be due to the increase in mass transfer distance to promote efficient desorption [34]. The results of dynamic desorption experiments performed at different initial flow rates are shown in Figure 6h. The complete desorption times were 35 min, 20 min, and 16 min, respectively. As shown in Figure 6i, the time point of the highest phosphate concentration in the effluent was advanced with the increase in flow rate because the flow rate enhancement increased the contact between desorption reagents and phosphate in the adsorbent material, which shortened the complete desorption time. However, the maximum phosphate mass concentrations (1955.5 mg/L, 2147.9 mg/L, 1974.8 mg/L) did not change significantly with different flow rates, indicating that the initial flow rate only affects the total time for complete desorption and does not affect the unit reaction rate at the same unit adsorption capacity. The results of the dynamic desorption experiments performed under different initial desorption reagent concentrations are shown in Figure 6f. The maximum phosphate concentration in the effluent increased from 1513.0 mg/L to 2285.4 mg/L with the KCl concentration, while the complete desorption time decreased from 55 min to 18 min, indicating that more KCl passed through the adsorption column per unit time with the increase in the KCl concentration, which accelerated the dynamic desorption reaction rate.
3.5. Capitalize on Resources
A dynamic desorption solution with a packing height of 2.6 cm, a desorption reagent concentration of 0.1 mol/L, and a flow rate of 5 mL/min was used as the nutrient solution. The first 5 min desorption solution (phosphate concentration 800 mg/L, enrichment factor 16 times) was taken and diluted to drip irrigate the maize seedlings.
The plant height of maize seedlings was ranked as HP (36.9 cm) > LP (32.8 cm) > HK (32.3 cm) > LK (31.1 cm) > CK (29.8 cm). Compared with the blank group, CK, the experimental group had higher plant heights, 23.8% and 10.1% for HP and LP, and 8.39% and 4.36% for HK and LK. The higher average plant height in the experimental group was attributed to phosphorus and potassium-promoting growth, and the desorbed solution was rich in phosphorus. The difference between HP and LP indicated that a moderate increase in phosphorus content could more significantly promote the growth of maize [35]. Within a certain range, the desorption solution can promote the growth of maize seedlings, and the higher the phosphorus content, the better the effect.
As illustrated in Figure 7a, both the experimental and control groups exhibited higher fresh weights compared to the blank group (CK). Specifically, the fresh weights of the high-phosphorus (HP) and low-phosphorus (LP) groups increased by 51.3% and 36.9%, respectively, whereas those of the high-potassium (HK) and low-potassium (LK) groups increased by 15.0% and 14.7%, respectively, relative to CK. No significant differences were observed within the control group, likely due to an excess of potassium beyond what is necessary for maize seedling growth. Within the experimental group, fresh weight increased with increasing desorption solution concentration, indicating that the desorption solution promoted biomass accumulation in maize seedlings. Higher concentrations resulted in more significant promotion effects [36].
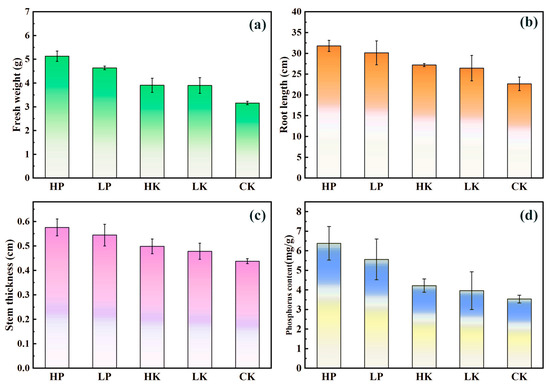
Figure 7.
Desorbed Liquid Resource Utilization Experiment of (a) Maize fresh weight, (b) root length, (c) stem thickness, and (d) phosphorus content.
Figure 7b shows that the average root lengths decreased in the following order: HP (31.8 cm), LP (30.1 cm), HK (27.2 cm), LK (26.6 cm), and CK (25.4 cm). Both experimental and control groups had higher average root lengths compared to the blank group (CK). Specifically, the HP and LP groups exhibited increases of 25.2% and 18.5% compared to CK, while the HK and LK groups showed increases of 7.09% and 4.72%, respectively. These results indicate that desorption solutions and reagents significantly promote root length growth in maize seedlings. The promotional effect was more pronounced in the experimental group than in the control group, suggesting that phosphorus in the desorption solution contributed notably to root length enhancement [37].
Figure 7c indicates that the average stem thicknesses for HP, LP, HK, LK, and CK were 0.58 cm, 0.54 cm, 0.48 cm, 0.47 cm, and 0.45 cm, respectively. Both experimental and control groups exhibited greater stem thickness compared to the blank group (CK). Specifically, stem thickness increased by 28.9% and 20.0% in the HP and LP groups, respectively, relative to CK, and by 6.67% and 4.44% in the HK and LK groups, respectively. These findings highlight the potential of using desorption solutions directly as nutrient sources in agricultural applications.
As depicted in Figure 7d, the average phosphorus contents for HP, LP, HK, LK, and CK were 6.38 mg/g, 5.56 mg/g, 4.22 mg/g, 3.96 mg/g, and 3.53 mg/g, respectively. Both the experimental and control groups exhibited higher phosphorus levels compared to CK, with HP and LP showing increases of 80.7% and 57.5%, respectively, and HK and LK displaying rises of 19.5% and 12.2%, relative to CK. This indicates that both phosphorus-containing desorption solutions and reagents promoted phosphorus uptake in maize seedlings. Higher desorption solution concentrations increased phosphorus content in maize seedlings, while potassium in the desorption reagent enhanced growth, indirectly increasing phosphorus uptake. These findings are consistent with data on maize growth and soil phosphorus content.
4. Conclusions
In this study, a modified reed straw-based adsorbent (MRS) was developed to address phosphate pollution in aquatic environments. Characterization techniques such as SEM, EDS, FTIR, and XPS confirmed the successful introduction of amine groups and revealed that the main phosphate adsorption mechanism involved ion exchange and chemical interactions. These findings align with previous studies on amine-functionalized bioadsorbents [7], reinforcing the scientific basis of the adsorption process.
Batch and dynamic adsorption experiments demonstrated that MRS achieved a high phosphate removal rate (99.2%) and an adsorption capacity of 6.14 mg/g at a dosage of 8 g/L. The adsorption kinetics followed the pseudo-second-order model, indicating chemisorption dominance, while the Freundlich isotherm suggested non-homogeneous multilayer adsorption. Comparisons with other lignocellulosic materials, such as modified rice husk and chitosan-coated sawdust [4], show that MRS performs competitively under variable conditions. Moreover, MRS retained over 80% of its initial adsorption capacity after nine regeneration cycles, demonstrating excellent reusability and economic viability. This stability surpasses many conventional bioadsorbents, which often degrade after just a few cycles [27].
Preliminary plant growth tests indicated that the desorbed phosphate solution could effectively promote plant development, offering potential for agricultural reuse. This supports the concept of circular nutrient management and aligns with trends in sustainable resource recovery [38]. In summary, MRS combines high performance, regenerability, and environmental benefits, making it a promising candidate for both phosphate removal and resource recycling. The importance of this study lies in not only mitigating the potential threat of phosphorus-containing wastewater to the ecological environment and human health, but also realizing the resourceful reuse of waste through the development of an efficient adsorbent based on reed straw, which provides a new method of achieving a green economy for phosphorus resource recovery and environmental protection.
Author Contributions
Conceptualization, Z.Y. and Z.Q.; Formal analysis, Q.Z. and C.L.; Funding acquisition, Z.Q.; Investigation, C.L., H.Z. and Z.Q.; Methodology, Z.Y.; Project administration, C.L. and H.Z.; Resources, C.L. and H.Z.; Software, Z.Y.; Supervision, Z.Q.; Validation, Q.Z.; Visualization, Z.Y. and Q.Z.; Writing—original draft, Z.Y.; Writing—review and editing, Q.Z., C.L., H.Z. and Z.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Suanon, F.; Sun, Q.; Li, M.; Cai, X.; Zhang, Y.; Yan, Y.; Yu, C.-P. Application of Nanoscale Zero Valent Iron and Iron Powder during Sludge Anaerobic Digestion: Impact on Methane Yield and Pharmaceutical and Personal Care Products Degradation. J. Hazard. Mater. 2017, 321, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Ashley, K.; Cordell, D.; Mavinic, D. A Brief History of Phosphorus: From the Philosopher’s Stone to Nutrient Recovery and Reuse. Chemosphere 2011, 84, 737–746. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, X.; Wang, R.; Zhang, J. Phosphorus Recovery from Domestic Wastewater via Candida Tropicalis: Performance and Mechanism. J. Water Process Eng. 2024, 68, 106404. [Google Scholar] [CrossRef]
- Lamghari, K.; Taha, Y.; Ait-Khouia, Y.; Elghali, A.; Hakkou, R.; Benzaazoua, M. Sustainable Phosphate Mining: Enhancing Efficiency in Mining and Pre-Beneficiation Processes. J. Environ. Manag. 2024, 358, 120833. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, R.; Golkar, M.K.; Hall, J.W. Large Uncertainty in Global Estimates of Manure Phosphorus Runoff. Environ. Model. Softw. 2024, 177, 106067. [Google Scholar] [CrossRef]
- Ascott, M.; Gooddy, D.; Lapworth, D.; Stuart, M. Estimating the Leakage Contribution of Phosphate Dosed Drinking Water to Environmental Phosphorus Pollution at the National-Scale. Sci. Total Environ. 2016, 572, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, W.J.; Alexander, P.; Cordell, D.; Maslin, M.; Metson, G.S.; A Sutton, M.; Spears, B.M. National Phosphorus Planning for Food and Environmental Security. Curr. Opin. Biotechnol. 2024, 90, 103226. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Z.; Lu, Y.; Shan, S.; Zhuang, H.; Gong, C.; Cui, X.; Zhang, F.; Li, P. Facilitating Mitigation of Agricultural Non-Point Source Pollution and Improving Soil Nutrient Conditions: The Role of Low Temperature Co-Pyrolysis Biochar in Nitrogen and Phosphorus Distribution. Bioresour. Technol. 2024, 394, 130179. [Google Scholar] [CrossRef]
- GB 11893-1989; Water Quality-Determination of Total Phosphorus-Ammonium Molybdate Spectrophotometric Method. China National Environmental Monitoring Centre: Beijing, China, 1989.
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of Gas Adsorption Kinetics, I. A Theoretical Model for Respirator Cartridge Service Life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Yuan, Y.; Li, T. Adsorption and Desorption of Heavy Metals from Water Using Aminoethyl Reduced Graphene Oxide. Environ. Eng. Sci. 2018, 35, 978–987. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, H.; Xue, F.; He, H.; Wang, S. Cellulose-Based Amphoteric Adsorbent for the Complete Removal of Low-Level Heavy Metal Ions via a Specialization and Cooperation Mechanism. Chem. Eng. J. 2020, 385, 123879. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, X.; Wang, X.; Gao, B.; Yue, Q.; Song, W.; Zhang, L.; Wang, H. FTIR, Raman, and XPS Analysis during Phosphate, Nitrate and Cr(VI) Removal by Amine Cross-Linking Biosorbent. J. Colloid Interface Sci. 2016, 468, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Gao, B.; Xu, X.; Wang, F.; Xue, N.; Sun, S.; Song, W.; Jia, R. Adsorption of Nitrate from Aqueous Solution by Magnetic Amine-Crosslinked Biopolymer Based Corn Stalk and Its Chemical Regeneration Property. J. Hazard. Mater. 2016, 304, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Mei, L.; Chen, G.; Liu, H.; Peng, C.; Ke, F.; Hou, R.; Wan, X.; Cai, H. Adsorption of Nitrate and Phosphate from Aqueous Solution Using Amine Cross-Linked Tea Wastes. Appl. Surf. Sci. 2019, 483, 114–122. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, W. Polyethylenimine-Functionalized Polyacrylonitrile Anion Exchange Fiber as a Novel Adsorbent for Rapid Removal of Nitrate from Wastewater. Chemosphere 2020, 258, 127373. [Google Scholar] [CrossRef]
- Katal, R.; Baei, M.S.; Rahmati, H.T.; Esfandian, H. Kinetic, Isotherm and Thermodynamic Study of Nitrate Adsorption from Aqueous Solution Using Modified Rice Husk. J. Ind. Eng. Chem. 2012, 18, 295–302. [Google Scholar] [CrossRef]
- Lagergreen, S. Zur Theorie der sogenannten Adsorption gelöster Stoffe. Z. Chem. Ind. Kolloide 1907, 2, 15. [Google Scholar] [CrossRef]
- Chen, S.; Yue, Q.; Gao, B.; Xu, X. Equilibrium and Kinetic Adsorption Study of the Adsorptive Removal of Cr(VI) Using Modified Wheat Residue. J. Colloid Interface Sci. 2010, 349, 256–264. [Google Scholar] [CrossRef]
- Hou, J.; Huang, L.; Yang, Z.; Zhao, Y.; Deng, C.; Chen, Y.; Li, X. Adsorption of Ammonium on Biochar Prepared from Giant Reed. Environ. Sci. Pollut. Res. 2016, 23, 19107–19115. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Liu, Y.; Duan, Y.; Zhang, W. Equilibrium and Kinetics Studies of Fluoride Ions Adsorption on CeO2/Al2O3 Composites Pretreated with Non-Thermal Plasma. Chem. Eng. J. 2011, 168, 665–671. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part II.—Liquids. J. Frankl. Inst. 1917, 184, 721. [Google Scholar] [CrossRef]
- Freundlich, H.; Neumann, W. Ober Die Adsorption von Farbstoffen. Z. Phys. Chem. 1909, 67U, 538–550. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Elanchezhiyan, S.; Preethi, J.; Meenakshi, S.; Park, C.M. Mechanistic Performance of Polyaniline-Substituted Hexagonal Boron Nitride Composite as a Highly Efficient Adsorbent for the Removal of Phosphate, Nitrate, and Hexavalent Chromium Ions from an Aqueous Environment. Appl. Surf. Sci. 2020, 511, 145543. [Google Scholar] [CrossRef]
- Chung, H.-K.; Kim, W.-H.; Park, J.; Cho, J.; Jeong, T.-Y.; Park, P.-K. Application of Langmuir and Freundlich Isotherms to Predict Adsorbate Removal Efficiency or Required Amount of Adsorbent. J. Ind. Eng. Chem. 2015, 28, 241–246. [Google Scholar] [CrossRef]
- Kono, H. Cationic Flocculants Derived from Native Cellulose: Preparation, Biodegradability, and Removal of Dyes in Aqueous Solution. Resource-Effic. Technol. 2017, 3, 55–63. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Hameed, B.H. Fixed-Bed Adsorption of Reactive Azo Dye onto Granular Activated Carbon Prepared from Waste. J. Hazard. Mater. 2010, 175, 298–303. [Google Scholar] [CrossRef]
- Xing, X.; Gao, B.-Y.; Zhong, Q.-Q.; Yue, Q.-Y.; Li, Q. Sorption of Nitrate onto Amine-Crosslinked Wheat Straw: Characteristics, Column Sorption and Desorption Properties. J. Hazard. Mater. 2011, 186, 206–211. [Google Scholar] [CrossRef]
- Maji, S.; Pal, A.; Pal, T.; Adak, A. Modeling and Fixed Bed Column Adsorption of As(III) on Laterite Soil. Sep. Purif. Technol. 2007, 56, 284–290. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Analysis and Modeling of Fixed Bed Column Operations on As(V) Removal by Adsorption onto Iron Oxide-Coated Cement (IOCC). J. Colloid Interface Sci. 2005, 290, 52–60. [Google Scholar] [CrossRef]
- Baral, S.S.; Das, N.; Ramulu, T.S.; Sahoo, S.K.; Das, S.N.; Chaudhury, G.R. Removal of Cr(VI) by Thermally Activated Weed Salvinia Cucullata in a Fixed-Bed Column. J. Hazard. Mater. 2009, 161, 1427–1435. [Google Scholar] [CrossRef]
- Calero, M.; Hernáinz, F.; Blázquez, G.; Tenorio, G.; Martín-Lara, M.A. Study of Cr(III) Biosorption in a Fixed-Bed Column. J. Hazard. Mater. 2009, 171, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Z.; Tian, J.; Liu, C.; Qin, Z. Enhanced Nitrate Nitrogen Removal from Wastewater Using Modified Reed Straw: Adsorption Performance and Resource Utilization. Sustainability 2024, 16, 4001. [Google Scholar] [CrossRef]
- Sarkar, S.; Lupi, F.; Price, L.; Basso, B. Corn Yield Response to Phosphorus Fertilizer in Michigan: A Metamodeling Approach for Phosphorus Management Policies. J. Agric. Food Res. 2024, 18, 101410. [Google Scholar] [CrossRef]
- Kokulan, V.; Schneider, K.; Macrae, M.L.; Wilson, H. Struvite Application to Field Corn Decreases the Risk of Environmental Phosphorus Loss While Maintaining Crop Yield. Agric. Ecosyst. Environ. 2024, 366, 108936. [Google Scholar] [CrossRef]
- Metson, G.S.; MacDonald, G.K.; Haberman, D.; Nesme, T.; Bennett, E.M. Feeding the Corn Belt: Opportunities for Phosphorus Recycling in U.S. Agriculture. Sci. Total Environ. 2016, 542, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yan, B.; Xing, C.; Guo, W. Integrating Enhanced Biological Phosphorus Removal in Adsorption-Stage to Treat Real Domestic Sewage. Bioresource Technol. 2024, 411, 131334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).