Environmental Impacts of Shale Gas Development on Groundwater, and Flowback and Produced Water Treatment Management: A Review
Abstract
1. Introduction
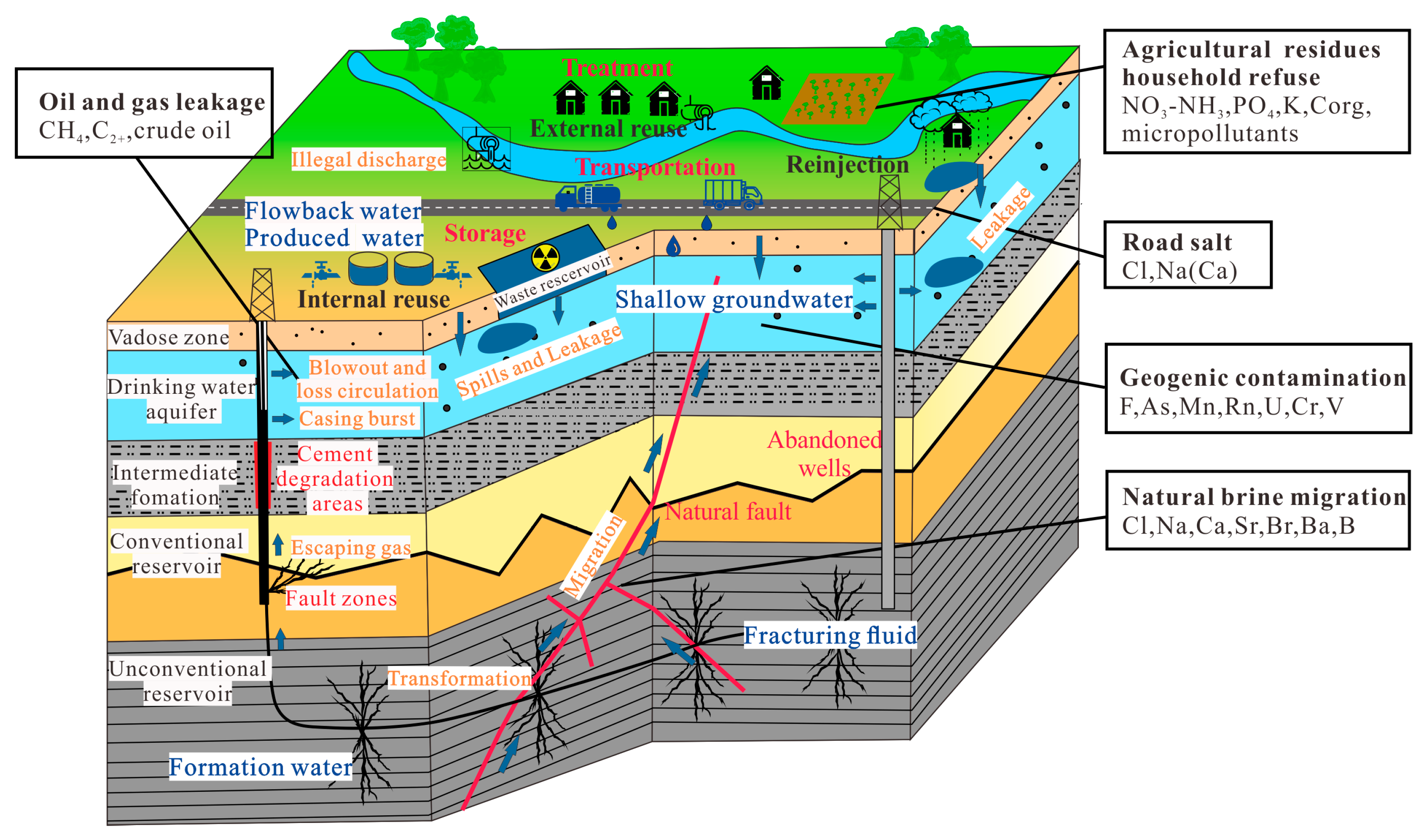
2. Effects of the Whole Progress of Shale Gas Development on Groundwater Water
2.1. Exploration and Preparation
2.2. Drilling
2.3. Hydraulic Fracking
2.4. Flowback and Gas Production
2.5. Freshwater Resource Consumption
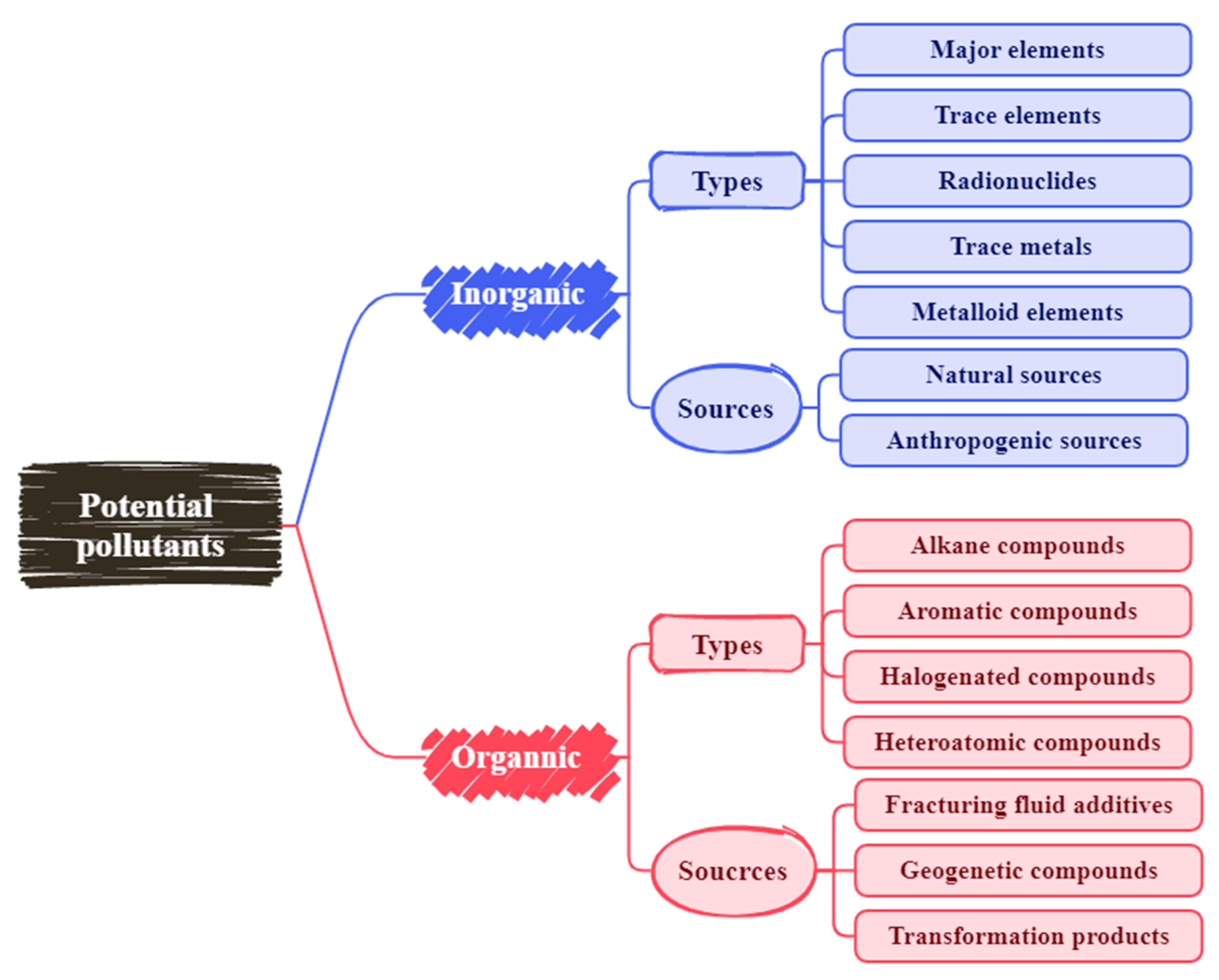
3. Types and Sources of Potential Pollutants
3.1. Inorganic Pollutants
3.1.1. Types of Inorganic Pollutants
3.1.2. Sources of Inorganic Pollutants
3.2. Organic Pollutants
3.2.1. Types of Organic Pollutants
3.2.2. Sources of Organic Pollutants
3.2.3. Hazards of Organic Pollutants
4. Shale Gas Wastewater Treatment
4.1. Strategies of Shale Gas Wastewater Treatment
4.1.1. Wastewater Reinjection

4.1.2. Wastewater Internal Reuse
4.1.3. Wastewater External Reuse
4.2. Technology of Shale Gas Wastewater Treatment
4.2.1. Primary Treatment
4.2.2. Secondary Treatment
4.2.3. Tertiary Treatment
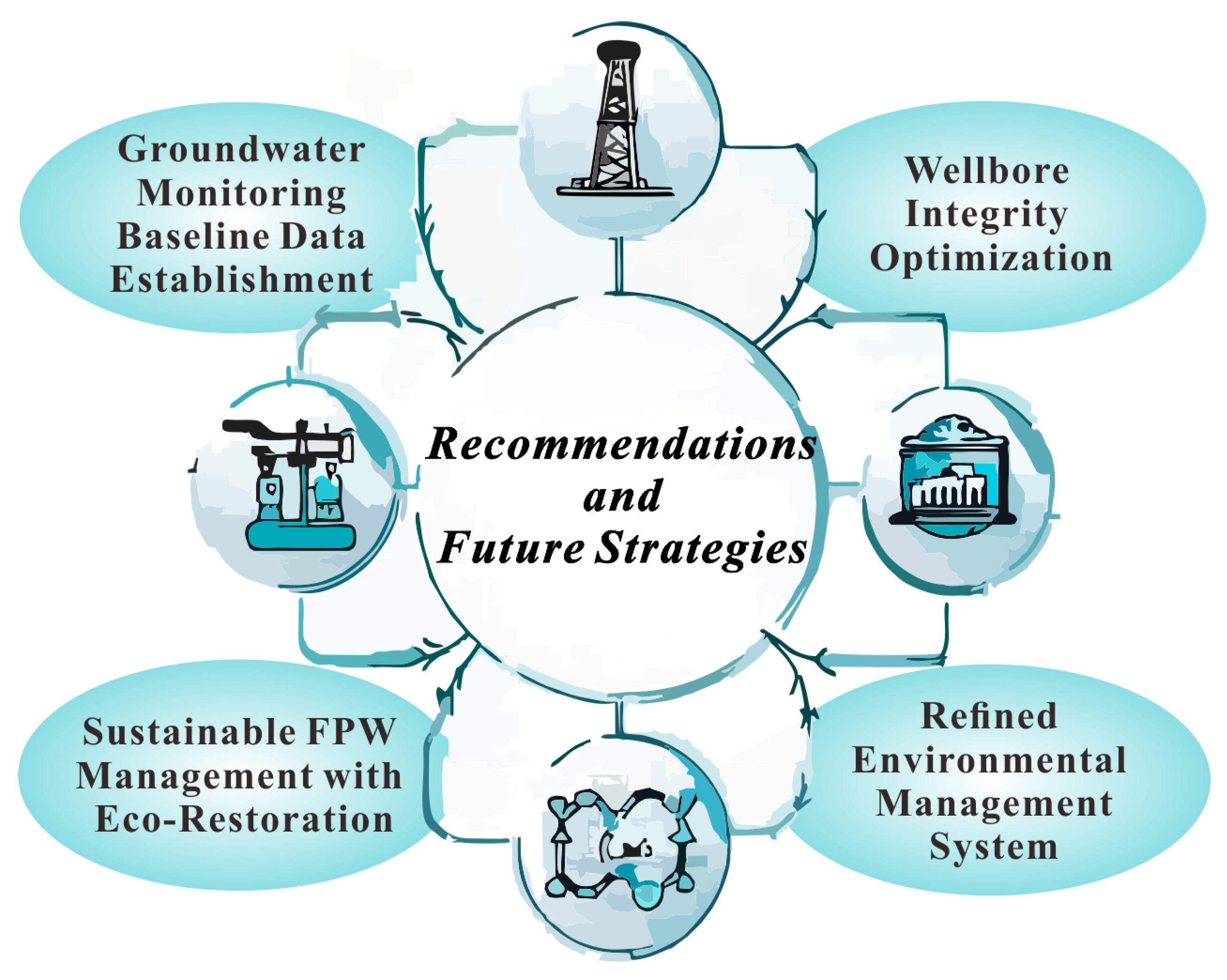
5. Recommendations and Future Strategies
5.1. Optimization of Wellbore Integrity Design and Construction
5.2. Sustainable FPW Management with Eco-Restoration
5.3. Strengthening Groundwater Pollution Monitoring and Baseline Data Establishment
5.4. Establishment of a Refined Environmental Management System
6. Conclusions
Funding
Conflicts of Interest
References
- Howarth, R.W.; Ingraffea, A.; Engelder, T. Should Fracking Stop? Nature 2011, 477, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, L.; Wang, G.; Wang, W.; Zhao, R.; Sun, X.; Wang, D. A Review of the Development in Shale Oil and Gas Wastewater Desalination. Sci. Total Environ. 2023, 873, 162376. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Lu, H.; Xia, J. Tradeoffs in Water and Carbon Footprints of Shale Gas, Natural Gas, and Coal in China. Fuel 2020, 263, 116778. [Google Scholar] [CrossRef]
- Zhang, K.; Yuan, X.; Han, F. Editorial: Reservoir Formation Conditions and Enrichment Mechanisms of Shale Oil and Gas. Front. Earth Sci. 2023, 10, 1047581. [Google Scholar] [CrossRef]
- Zhu, B.; Meng, J.; Pan, R.; Hu, H.; Song, C.; Zhu, Z.; Jin, J. New Insights into the Evaluation Criteria for High-Quality Deep Marine Shale Gas Reservoirs in the Longmaxi Formation: Evidence from Organic Matter Pore Development Characteristics. Front. Ecol. Evol. 2023, 11, 1138991. [Google Scholar] [CrossRef]
- Mukuhira, Y.; Fehler, M.C.; Ito, T.; Asanuma, H.; Häring, M.O. Injection-Induced Seismicity Size Distribution Dependent on Shear Stress. Geophys. Res. Lett. 2021, 48, e2020GL090934. [Google Scholar] [CrossRef]
- Hagos, F.Y.; Abd Aziz, A.R.; Zainal, E.Z.; Mofijur, M.; Ahmed, S.F. Recovery of Gas Waste from the Petroleum Industry: A Review. Environ. Chem. Lett. 2022, 20, 263–281. [Google Scholar] [CrossRef]
- Harkness, J.S.; Darrah, T.H.; Warner, N.R.; Whyte, C.J.; Moore, M.T.; Millot, R.; Kloppmann, W.; Jackson, R.B.; Vengosh, A. The Geochemistry of Naturally Occurring Methane and Saline Groundwater in an Area of Unconventional Shale Gas Development. Geochim. Cosmochim. Acta 2017, 208, 302–334. [Google Scholar] [CrossRef]
- Huang, T.; Pang, Z.; Li, Z.; Li, Y.; Hao, Y. A Framework to Determine Sensitive Inorganic Monitoring Indicators for Tracing Groundwater Contamination by Produced Formation Water from Shale Gas Development in the Fuling Gasfield, SW China. J. Hydrol. 2020, 581, 124403. [Google Scholar] [CrossRef]
- Vengosh, A.; Jackson, R.B.; Warner, N.; Darrah, T.H.; Kondash, A. A Critical Review of the Risks to Water Resources from Unconventional Shale Gas Development and Hydraulic Fracturing in the United States. Environ. Sci. Technol. 2014, 48, 8334–8348. [Google Scholar] [CrossRef]
- Danforth, C.; Chiu, W.A.; Rusyn, I.; Schultz, K.; Bolden, A.; Kwiatkowski, C.; Craft, E. An Integrative Method for Identification and Prioritization of Constituents of Concern in Produced Water from Onshore Oil and Gas Extraction. Environ. Int. 2020, 134, 105280. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Shu, J.; Xie, W.; Su, Y.; He, Q.; Liu, B. Characterizing Hazardous Substances of Shale Gas Wastewater from the Upper Yangtze River: A Focus on Heavy Metals and Organic Compounds. J. Hazard. Mater. 2024, 469, 133873. [Google Scholar] [CrossRef] [PubMed]
- Kondash, A.J.; Albright, E.; Vengosh, A. Quantity of Flowback and Produced Waters from Unconventional Oil and Gas Exploration. Sci. Total Environ. 2017, 574, 314–321. [Google Scholar] [CrossRef]
- Zou, C.; Ni, Y.; Li, J.; Kondash, A.; Coyte, R.; Lauer, N.; Cui, H.; Liao, F.; Vengosh, A. The Water Footprint of Hydraulic Fracturing in Sichuan Basin, China. Sci. Total Environ. 2018, 630, 349–356. [Google Scholar] [CrossRef]
- He, X.; Li, P.; Shi, H.; Xiao, Y.; Guo, Y.; Zhao, H. Identifying Strontium Sources of Flowback Fluid and Groundwater Pollution Using 87Sr/86Sr and Geochemical Model in Sulige Gasfield, China. Chemosphere 2022, 306, 135594. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Tian, L.; Tang, P.; Cui, J.; Wang, T.; Zhu, Y.; Bai, Y.; Tiraferri, A.; Crittenden, J.C.; Liu, B. Shale Gas Wastewater Characterization: Comprehensive Detection, Evaluation of Valuable Metals, and Environmental Risks of Heavy Metals and Radionuclides. Water Res. 2022, 220, 118703. [Google Scholar] [CrossRef]
- Burden, S.; Cluff, M.A.; DeHaven, L.E.; Roberts, C.; Sharkley, S.L.; Singer, A. Review of State and Industry Spill Data: Characterization of Hydraulic Fracturing-Related Spills; U.S. Environmental Protection Agency Office of Research and Development: Washington, DC, USA, 2015. [Google Scholar]
- Hill, C.B.; Yadav, O.P.; Khan, E. Examining Hydraulic Fracturing Chemicals: A Temporal and Comparative Analysis. Water Res. 2022, 208, 117878. [Google Scholar] [CrossRef]
- Bondu, R.; Kloppmann, W.; Naumenko-Dèzes, M.O.; Humez, P.; Mayer, B. Potential Impacts of Shale Gas Development on Inorganic Groundwater Chemistry: Implications for Environmental Baseline Assessment in Shallow Aquifers. Environ. Sci. Technol. 2021, 55, 9657–9671. [Google Scholar] [CrossRef]
- Rashidi Gooya, H.; Katibeh, H.; Maleki, A. Forecasting Groundwater Fluctuations Caused by Earthquakes Using Fuzzy Logic and AHP Method: A Case Study from Iran. Earth Sci. Inf. 2024, 17, 2143–2158. [Google Scholar] [CrossRef]
- Kim, J.; Kaown, D.; Park, I.-W.; Lee, K.-K. Hydrogeochemical and Microbial Signatures of Deep and Shallow Groundwater near the Enhanced Geothermal System in Response to the Earthquake. Environ. Earth Sci. 2023, 82, 87. [Google Scholar] [CrossRef]
- Tasker, T.L.; Burgos, W.D.; Piotrowski, P.; Castillo-Meza, L.; Blewett, T.A.; Ganow, K.B.; Stallworth, A.; Delompré, P.L.M.; Goss, G.G.; Fowler, L.B.; et al. Environmental and Human Health Impacts of Spreading Oil and Gas Wastewater on Roads. Environ. Sci. Technol. 2018, 52, 7081–7091. [Google Scholar] [CrossRef]
- Uliasz-Misiak, B.; Winid, B.; Lewandowska-Śmierzchalska, J.; Matuła, R. Impact of Road Transport on Groundwater Quality. Sci. Total Environ. 2022, 824, 153804. [Google Scholar] [CrossRef] [PubMed]
- Forman, R.T.; Sperling, D.; Bissonette, J.A.; Clevenger, A.P.; Cutshall, C.D.; Dale, V.H.; Fahrig, L.; France, R.; Goldman, C.R.; Heanue, K.; et al. Road Ecology: Science and Solutions; Island Press: Washington, DC, USA, 2003; p. 482. [Google Scholar]
- Piwnicki, P. Road Projects–Waters in the Reports on the Environmental Impact Assessment. Bud. Archit. 2016, 15, 31–39. [Google Scholar] [CrossRef]
- Hossain, M.E.; Al-Majed, A.A. Fundamentals of Sustainable Drilling Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Alkalbani, A.M.; Chala, G.T. A Comprehensive Review of Nanotechnology Applications in Oil and Gas Well Drilling Operations. Energies 2024, 17, 798. [Google Scholar] [CrossRef]
- Berger, B.; Anderson, K. Modern Petroleum: A Basic Primer of the Industry; Oil & Gas Journal: Tulsa, OK, USA, 1981. [Google Scholar]
- Pereira, L.B.; Sad, C.M.S.; Castro, E.V.R.; Filgueiras, P.R.; Lacerda, V. Environmental Impacts Related to Drilling Fluid Waste and Treatment Methods: A Critical Review. Fuel 2022, 310, 122301. [Google Scholar] [CrossRef]
- Davies, R.J.; Almond, S.; Ward, R.S.; Jackson, R.B.; Adams, C.; Worrall, F.; Herringshaw, L.G.; Gluyas, J.G.; Whitehead, M.A. Oil and Gas Wells and Their Integrity: Implications for Shale and Unconventional Resource Exploitation. Mar. Pet. Geol. 2014, 56, 239–254. [Google Scholar] [CrossRef]
- Lefebvre, R. Mechanisms Leading to Potential Impacts of Shale Gas Development on Groundwater Quality. WIREs Water 2017, 4, e1188. [Google Scholar] [CrossRef]
- Bachu, S.; Valencia, R.L. Well Integrity Challenges and Risk Mitigation Measures. Bridge 2014, 44, 28–34. [Google Scholar]
- Kiran, R.; Teodoriu, C.; Dadmohammadi, Y.; Nygaard, R.; Wood, D.; Mokhtari, M.; Salehi, S. Identification and Evaluation of Well Integrity and Causes of Failure of Well Integrity Barriers (A Review). J. Nat. Gas Sci. Eng. 2017, 45, 511–526. [Google Scholar] [CrossRef]
- Dusseault, M.B.; Gray, M.N.; Nawrocki, P.A. Why Oilwells Leak: Cement Behavior and Long-Term Consequences. In Proceedings of the SPE International Oil & Gas Conference and Exhibition in China (IOGCEC), Beijing, China, 7–10 November 2000. [Google Scholar]
- Qi, P.; Tang, Y.; Zhang, C. Review and Prospects of Groundwater Pollution Control in Shale Gas Exploitation. Water Supply 2021, 21, 4382–4390. [Google Scholar] [CrossRef]
- Ibukun, M.; Elyan, E.; Amish, M.; Njuguna, J.; Oluyemi, G.F. A Review of Well Life Cycle Integrity Challenges in the Oil and Gas Industry and Its Implications for Sustained Casing Pressure (SCP). Energies 2024, 17, 5562. [Google Scholar] [CrossRef]
- Nowamooz, A.; Lemieux, J.-M.; Molson, J.; Therrien, R. Numerical Investigation of Methane and Formation Fluid Leakage along the Casing of a Decommissioned Shale Gas Well. Water Resour. Res. 2015, 51, 4592–4622. [Google Scholar] [CrossRef]
- Chen, W.; Wan, W.; Zhao, Y.; He, H.; Wu, Q.; Zhou, Y.; Xie, S. Mechanical Damage Evolution and Mechanism of Sandstone with Prefabricated Parallel Double Fissures under High-Humidity Condition. Bull. Eng. Geol. Environ. 2022, 81, 245. [Google Scholar] [CrossRef]
- Chen, W.; Liu, J.; Liu, W.; Peng, W.; Zhao, Y.; Wu, Q.; Wang, Y.; Wan, W.; Li, S.; Peng, H.; et al. Lateral Deformation and Acoustic Emission Characteristics of Dam Bedrock under Various River Flow Scouring Rates. J. Mater. Res. Technol. 2023, 26, 3245–3271. [Google Scholar] [CrossRef]
- Chen, W.; Liu, J.; Peng, W.; Zhao, Y.; Luo, S.; Wan, W.; Wu, Q.; Wang, Y.; Li, S.; Tang, X.; et al. Aging Deterioration of Mechanical Properties on Coal-Rock Combinations Considering Hydro-Chemical Corrosion. Energy 2023, 282, 128770. [Google Scholar] [CrossRef]
- Flewelling, S.A.; Sharma, M. Constraints on Upward Migration of Hydraulic Fracturing Fluid and Brine. Ground Water 2014, 52, 9–19. [Google Scholar] [CrossRef]
- Birdsell, D.T.; Rajaram, H.; Dempsey, D.; Viswanathan, H.S. Hydraulic Fracturing Fluid Migration in the Subsurface: A Review and Expanded Modeling Results. Water Resour. Res. 2015, 51, 7159–7188. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J. A Review of Fracturing Fluid Systems Used for Hydraulic Fracturing of Oil and Gas Wells. J. Appl. Polym. Sci. 2014, 131, 40735. [Google Scholar] [CrossRef]
- Ferrer, I.; Thurman, E.M. Chemical Constituents and Analytical Approaches for Hydraulic Fracturing Waters. Trends Environ. Anal. Chem. 2015, 5, 18–25. [Google Scholar] [CrossRef]
- Brannon, H.D.; Pulsinelli, R.J. Breaker Concentrations Required To Improve the Permeability of Proppant Packs Damaged by Concentrated Linear and Borate-Crosslinked Fracturing Fluids. SPE Prod. Eng. 1992, 7, 338–342. [Google Scholar] [CrossRef]
- Gregory, K.B.; Vidic, R.D.; Dzombak, D.A. Water Management Challenges Associated with the Production of Shale Gas by Hydraulic Fracturing. Elements 2011, 7, 181–186. [Google Scholar] [CrossRef]
- Stringfellow, W.T.; Domen, J.K.; Camarillo, M.K.; Sandelin, W.L.; Borglin, S. Physical, Chemical, and Biological Characteristics of Compounds Used in Hydraulic Fracturing. J. Hazard. Mater. 2014, 275, 37–54. [Google Scholar] [CrossRef]
- Zoback, M.D.; Arent, D.J. Shale Gas: Development Opportunities and Challenges. Bridge 2014, 44, NREL/JA-6A50-61466. [Google Scholar]
- Davies, R.J.; Mathias, S.A.; Moss, J.; Hustoft, S.; Newport, L. Hydraulic Fractures: How Far Can They Go? Mar. Pet. Geol. 2012, 37, 1–6. [Google Scholar] [CrossRef]
- Lange, T.; Sauter, M.; Heitfeld, M.; Schetelig, K.; Brosig, K.; Jahnke, W.; Kissinger, A.; Helmig, R.; Ebigbo, A.; Class, H. Hydraulic Fracturing in Unconventional Gas Reservoirs: Risks in the Geological System Part 1. Environ. Earth Sci. 2013, 70, 3839–3853. [Google Scholar] [CrossRef]
- Kissinger, A.; Helmig, R.; Ebigbo, A.; Class, H.; Lange, T.; Sauter, M.; Heitfeld, M.; Klünker, J.; Jahnke, W. Hydraulic Fracturing in Unconventional Gas Reservoirs: Risks in the Geological System, Part 2: Modelling the Transport of Fracturing Fluids, Brine and Methane. Environ. Earth Sci. 2013, 70, 3855–3873. [Google Scholar] [CrossRef]
- Reagan, M.T.; Moridis, G.J.; Keen, N.D.; Johnson, J.N. Numerical Simulation of the Environmental Impact of Hydraulic Fracturing of Tight/Shale Gas Reservoirs on Near-surface Groundwater: Background, Base Cases, Shallow Reservoirs, Short-term Gas, and Water Transport. Water Resour. Res. 2015, 51, 2543–2573. [Google Scholar] [CrossRef]
- Missimer, T.M.; Maliva, R.G. Hydraulic Fracturing in Southern Florida: A Critical Analysis of Potential Environmental Impacts. Nat. Resour. Res. 2020, 29, 3385–3411. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, D.; Bian, H. Quantifying Aquifer Contamination Risk from Casing Rupture Using Support Vector Machine: A Comprehensive Assessment. Stoch. Environ. Res. Risk Assess. 2024, 38, 923–936. [Google Scholar] [CrossRef]
- Dusseault, M.; Jackson, R. Seepage Pathway Assessment for Natural Gas to Shallow Groundwater during Well Stimulation, in Production, and after Abandonment. Environ. Geosci. 2014, 21, 107–126. [Google Scholar] [CrossRef]
- Gassiat, C.; Gleeson, T.; Lefebvre, R.; McKenzie, J. Hydraulic Fracturing in Faulted Sedimentary Basins: Numerical Simulation of Potential Contamination of Shallow Aquifers over Long Time Scales. Water Resour. Res. 2013, 49, 8310–8327. [Google Scholar] [CrossRef]
- Flewelling, S.A.; Tymchak, M.P.; Warpinski, N. Hydraulic Fracture Height Limits and Fault Interactions in Tight Oil and Gas Formations. Geophys. Res. Lett. 2013, 40, 3602–3606. [Google Scholar] [CrossRef]
- Rahm, B.G.; Vedachalam, S.; Bertoia, L.R.; Mehta, D.; Vanka, V.S.; Riha, S.J. Shale Gas Operator Violations in the Marcellus and What They Tell Us about Water Resource Risks. Energy Policy 2015, 82, 1–11. [Google Scholar] [CrossRef]
- Hammond, P.A.; Wen, T.; Woda, J.; Oakley, D. Pathways and Environmental Impacts of Methane Migration: Case Studies in the Marcellus Shale, USA. Geofluids 2024, 2024, 9290873. [Google Scholar] [CrossRef]
- Barbot, E.; Vidic, N.S.; Gregory, K.B.; Vidic, R.D. Spatial and Temporal Correlation of Water Quality Parameters of Produced Waters from Devonian-Age Shale Following Hydraulic Fracturing. Environ. Sci. Technol. 2013, 47, 2562–2569. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, S.; Li, L.; Zhang, B.; Yao, L.; Sui, J.; Chen, J. Geochemical Characteristics of Shale Gas Hydraulic Fracturing Flowback/Produced Water in Zheng’an Block, Northern Guizhou Province, China. J. Nat. Gas Geosci. 2024, 9, 27–37. [Google Scholar] [CrossRef]
- Warner, N.R.; Darrah, T.H.; Jackson, R.B.; Millot, R.; Kloppmann, W.; Vengosh, A. New Tracers Identify Hydraulic Fracturing Fluids and Accidental Releases from Oil and Gas Operations. Environ. Sci. Technol. 2014, 48, 12552–12560. [Google Scholar] [CrossRef]
- Ni, Y.; Zou, C.; Cui, H.; Li, J.; Lauer, N.E.; Harkness, J.S.; Kondash, A.J.; Coyte, R.M.; Dwyer, G.S.; Liu, D.; et al. Origin of Flowback and Produced Waters from Sichuan Basin, China. Environ. Sci. Technol. 2018, 52, 14519–14527. [Google Scholar] [CrossRef]
- Warner, N.R.; Jackson, R.B.; Darrah, T.H.; Osborn, S.G.; Down, A.; Zhao, K.; White, A.; Vengosh, A. Geochemical Evidence for Possible Natural Migration of Marcellus Formation Brine to Shallow Aquifers in Pennsylvania. Proc. Natl. Acad. Sci. USA 2012, 109, 11961–11966. [Google Scholar] [CrossRef]
- Bordeleau, G.; Rivard, C.; Lavoie, D.; Lefebvre, R.; Malet, X.; Ladevèze, P. Geochemistry of Groundwater in the Saint-Édouard Area, Quebec, Canada, and Its Influence on the Distribution of Methane in Shallow Aquifers. Appl. Geochem. 2018, 89, 92–108. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, J.; Liu, Y.; Tao, L.; Wu, J.; Zhu, P.; Huang, H.; Zheng, H.; Huang, T. Salinization of Groundwater in Shale Gas Extraction Area in the Sichuan Basin, China: Implications for Water Protection in Shale Regions with Well-Developed Faults. Sci. Total Environ. 2024, 915, 170065. [Google Scholar] [CrossRef]
- U.S. EPA. Hydraulic Fracturing for Oil and Gas: Impacts from the Hydraulic Fracturing Water Cycle on Drinking Water Resources in the United States (Final Report); U.S. Environmental Protection Agency: Washington, DC, USA, 2016. [Google Scholar]
- Clancy, S.A.; Worrall, F.; Davies, R.J.; Gluyas, J.G. The Potential for Spills and Leaks of Contaminated Liquids from Shale Gas Developments. Sci. Total Environ. 2018, 626, 1463–1473. [Google Scholar] [CrossRef]
- Jon, W.S. US Produced Water Volumes & Management Practices in 2021; Ground Water Protection Council: Oklahoma City, OK, USA, 2022. [Google Scholar]
- Parker, K.M.; Zeng, T.; Harkness, J.; Vengosh, A.; Mitch, W.A. Enhanced Formation of Disinfection Byproducts in Shale Gas Wastewater-Impacted Drinking Water Supplies. Environ. Sci. Technol. 2014, 48, 11161–11169. [Google Scholar] [CrossRef]
- Ni, Y.; Yao, L.; Sui, J.; Chen, J.; Liu, F.; Wang, F.; Zhu, G.; Vengosh, A. Shale Gas Wastewater Geochemistry and Impact on the Quality of Surface Water in Sichuan Basin. Sci. Total Environ. 2022, 851, 158371. [Google Scholar] [CrossRef]
- Holzman, D.C. Methane Found in Well Water near Fracking Sites. Environ. Health Perspect. 2011, 119, a289. [Google Scholar] [CrossRef]
- Jackson, R.B.; Vengosh, A.; Darrah, T.H.; Warner, N.R.; Down, A.; Poreda, R.J.; Osborn, S.G.; Zhao, K.; Karr, J.D. Increased Stray Gas Abundance in a Subset of Drinking Water Wells near Marcellus Shale Gas Extraction. Proc. Natl. Acad. Sci. USA 2013, 110, 11250–11255. [Google Scholar] [CrossRef]
- Vidic, R.D.; Brantley, S.L.; Vandenbossche, J.M.; Yoxtheimer, D.; Abad, J.D. Impact of Shale Gas Development on Regional Water Quality. Science 2013, 340, 1235009. [Google Scholar] [CrossRef]
- Woda, J.; Wen, T.; Oakley, D.; Yoxtheimer, D.; Engelder, T.; Castro, M.C.; Brantley, S.L. Detecting and Explaining Why Aquifers Occasionally Become Degraded near Hydraulically Fractured Shale Gas Wells. Proc. Natl. Acad. Sci. USA 2018, 115, 12349–12358. [Google Scholar] [CrossRef]
- Chang, Y.; Huang, R.; Masanet, E. The Energy, Water, and Air Pollution Implications of Tapping China’s Shale Gas Reserves. Resour. Conserv. Recycl. 2014, 91, 100–108. [Google Scholar] [CrossRef]
- Vandecasteele, I.; Marí Rivero, I.; Sala, S.; Baranzelli, C.; Barranco, R.; Batelaan, O.; Lavalle, C. Impact of Shale Gas Development on Water Resources: A Case Study in Northern Poland. Environ. Manag. 2015, 55, 1285–1299. [Google Scholar] [CrossRef]
- Guo, M.; Lu, X.; Nielsen, C.P.; McElroy, M.B.; Shi, W.; Chen, Y.; Xu, Y. Prospects for Shale Gas Production in China: Implications for Water Demand. Renew. Sustain. Energy Rev. 2016, 66, 742–750. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Reedy, R.C.; Male, F.; Walsh, M. Water Issues Related to Transitioning from Conventional to Unconventional Oil Production in the Permian Basin. Environ. Sci. Technol. 2017, 51, 10903–10912. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, M.; Bentley, Y.; Feng, L.; Zhang, C. Water Use for Shale Gas Extraction in the Sichuan Basin, China. J. Environ. Manag. 2018, 226, 13–21. [Google Scholar] [CrossRef]
- Nicot, J.-P.; Scanlon, B.R. Water Use for Shale-Gas Production in Texas, U.S. Environ. Sci. Technol. 2012, 46, 3580–3586. [Google Scholar] [CrossRef]
- Schmid, K.; Yoxtheimer, D. Wastewater Recycling and Reuse Trends in Pennsylvania Shale Gas Wells. Environ. Geosci. 2015, 22, 115–125. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Reedy, R.C.; Nicot, J.P. Will Water Scarcity in Semiarid Regions Limit Hydraulic Fracturing of Shale Plays? Environ. Res. Lett. 2014, 9, 124011. [Google Scholar] [CrossRef]
- Shih, J.-S.; Saiers, J.E.; Anisfeld, S.C.; Chu, Z.; Muehlenbachs, L.A.; Olmstead, S.M. Characterization and Analysis of Liquid Waste from Marcellus Shale Gas Development. Environ. Sci. Technol. 2015, 49, 9557–9565. [Google Scholar] [CrossRef]
- Chang, H.; Li, T.; Liu, B.; Vidic, R.D.; Elimelech, M.; Crittenden, J.C. Potential and Implemented Membrane-Based Technologies for the Treatment and Reuse of Flowback and Produced Water from Shale Gas and Oil Plays: A Review. Desalination 2019, 455, 34–57. [Google Scholar] [CrossRef]
- Wang, H.; Lu, L.; Chen, X.; Bian, Y.; Ren, Z.J. Geochemical and Microbial Characterizations of Flowback and Produced Water in Three Shale Oil and Gas Plays in the Central and Western United States. Water Res. 2019, 164, 114942. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhuang, Y.; Li, J.; Zhou, Z.; Chen, S. Feasibility Evaluation of the Treatment and Recycling of Shale Gas Produced Water: A Case Study of the First Shale Gas Field in the Eastern Sichuan Basin, China. Environ. Sci. Water Res. Technol. 2019, 5, 358–369. [Google Scholar] [CrossRef]
- Abass, O.K.; Zhuo, M.; Zhang, K. Concomitant Degradation of Complex Organics and Metals Recovery from Fracking Wastewater: Roles of Nano Zerovalent Iron Initiated Oxidation and Adsorption. Chem. Eng. J. 2017, 328, 159–171. [Google Scholar] [CrossRef]
- Xu, T.; Wang, L.; Wang, X.; Li, T.; Zhan, X. Heavy Metal Pollution of Oil-Based Drill Cuttings at a Shale Gas Drilling Field in Chongqing, China: A Human Health Risk Assessment for the Workers. Ecotoxicol. Environ. Saf. 2018, 165, 160–163. [Google Scholar] [CrossRef]
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, and State Environmental Protection Administration. GB 5085.3-2007; Identification Standard for Hazardous Wastes: Leaching Toxicity Identification. China Environmental Science Press: Beijing, China, 2007.
- Zhang, H.; Zhou, X.; Wang, L.; Wang, W.; Xu, J. Concentrations and Potential Health Risks of Strontium in Drinking Water from Xi’an, Northwest China. Ecotoxicol. Environ. Saf. 2018, 164, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.L.; Jew, A.D.; Dustin, M.K.; Thomas, D.L.; Joe-Wong, C.M.; Bargar, J.R.; Johnson, N.; Brown, G.E.; Maher, K. Element Release and Reaction-Induced Porosity Alteration during Shale-Hydraulic Fracturing Fluid Interactions. Appl. Geochem. 2017, 82, 47–62. [Google Scholar] [CrossRef]
- Dustin, M.K.; Bargar, J.R.; Jew, A.D.; Harrison, A.L.; Joe-Wong, C.; Thomas, D.L.; Brown, G.E.; Maher, K. Shale Kerogen: Hydraulic Fracturing Fluid Interactions and Contaminant Release. Energy Fuels 2018, 32, 8966–8977. [Google Scholar] [CrossRef]
- Huang, T.; Li, Z.; Mayer, B.; Nightingale, M.; Li, X.; Li, G.; Long, Y.; Pang, Z. Identification of Geochemical Processes During Hydraulic Fracturing of a Shale Gas Reservoir: A Controlled Field and Laboratory Water-Rock Interaction Experiment. Geophys. Res. Lett. 2020, 47, e2020GL090420. [Google Scholar] [CrossRef]
- Darrah, T.H.; Vengosh, A.; Jackson, R.B.; Warner, N.R.; Poreda, R.J. Noble Gases Identify the Mechanisms of Fugitive Gas Contamination in Drinking-Water Wells Overlying the Marcellus and Barnett Shales. Proc. Natl. Acad. Sci. USA 2014, 111, 14076–14081. [Google Scholar] [CrossRef] [PubMed]
- Kreisserman, Y.; Emmanuel, S. Release of Particulate Iron Sulfide during Shale-Fluid Interaction. Environ. Sci. Technol. 2018, 52, 638–643. [Google Scholar] [CrossRef]
- Pearce, J.K.; Turner, L.; Pandey, D. Experimental and Predicted Geochemical Shale-Water Reactions: Roseneath and Murteree Shales of the Cooper Basin. Int. J. Coal Geol. 2018, 187, 30–44. [Google Scholar] [CrossRef]
- Mehta, N.; Kocar, B.D. Geochemical Conditions Conducive for Retention of Trace Elements and Radionuclides during Shale–Fluid Interactions. Environ. Sci. Process. Impacts 2019, 21, 1764–1776. [Google Scholar] [CrossRef]
- Phan, T.T.; Hakala, J.A.; Sharma, S. Application of Isotopic and Geochemical Signals in Unconventional Oil and Gas Reservoir Produced Waters toward Characterizing in Situ Geochemical Fluid-Shale Reactions. Sci. Total Environ. 2020, 714, 136867. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.; Capo, R.C.; Stewart, B.W.; Graney, J.R.; Johnson, J.D.; Sharma, S.; Toro, J. Trace Metal Distribution and Mobility in Drill Cuttings and Produced Waters from Marcellus Shale Gas Extraction: Uranium, Arsenic, Barium. Appl. Geochem. 2015, 60, 89–103. [Google Scholar] [CrossRef]
- Eitrheim, E.S.; May, D.; Forbes, T.Z.; Nelson, A.W. Disequilibrium of Naturally Occurring Radioactive Materials (NORM) in Drill Cuttings from a Horizontal Drilling Operation. Environ. Sci. Technol. Lett. 2016, 3, 425–429. [Google Scholar] [CrossRef]
- Tisherman, R.; Bain, D.J. Alkali Earth Ratios Differentiate Conventional and Unconventional Hydrocarbon Brine Contamination. Sci. Total Environ. 2019, 695, 133944. [Google Scholar] [CrossRef]
- Jackson, R.E.; Heagle, D.J. Sampling Domestic/Farm Wells for Baseline Groundwater Quality and Fugitive Gas. Hydrogeol. J. 2016, 24, 269–272. [Google Scholar] [CrossRef]
- Iqbal, J.; Su, C.; Abbas, H.; Jiang, J.; Han, Z.; Baloch, M.Y.J.; Xie, X. Prediction of Nitrate Concentration and the Impact of Land Use Types on Groundwater in the Nansi Lake Basin. J. Hazard. Mater. 2025, 487, 137185. [Google Scholar] [CrossRef]
- Dugan, H.A.; Arnott, S.E. The Ecosystem Implications of Road Salt as a Pollutant of Freshwaters. Wiley Interdiscip. Rev. Water 2023, 10, e1629. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, M.; Wang, P.; Shi, B. Chemical Characterization in Hydraulic Fracturing Flowback and Produced Water (HF-FPW) of Shale Gas in Sichuan of China. Environ. Sci. Pollut. Res. 2020, 27, 26532–26542. [Google Scholar] [CrossRef]
- Piotrowski, P.K.; Weggler, B.A.; Yoxtheimer, D.A.; Kelly, C.N.; Barth-Naftilan, E.; Saiers, J.E.; Dorman, F.L. Elucidating Environmental Fingerprinting Mechanisms of Unconventional Gas Development through Hydrocarbon Analysis. Anal. Chem. 2018, 90, 5466–5473. [Google Scholar] [CrossRef]
- Strong, L.C.; Gould, T.; Kasinkas, L.; Sadowsky, M.J.; Aksan, A.; Wackett, L.P. Biodegradation in Waters from Hydraulic Fracturing: Chemistry, Microbiology, and Engineering. J. Environ. Eng. 2014, 140, B4013001. [Google Scholar] [CrossRef]
- He, Y.; Sun, C.; Zhang, Y.; Folkerts, E.J.; Martin, J.W.; Goss, G.G. Developmental Toxicity of the Organic Fraction from Hydraulic Fracturing Flowback and Produced Waters to Early Life Stages of Zebrafish (Danio Rerio). Environ. Sci. Technol. 2018, 52, 3820–3830. [Google Scholar] [CrossRef]
- Wu, K.; Cui, W.; Ren, G.; An, J.; Zheng, K.; Zeng, X.; Ouyang, M.; Yu, Z. Organoiodines in Effluents of a Shale-Fracturing Wastewater Treatment Plant. Environ. Chem. Lett. 2023, 21, 1943–1949. [Google Scholar] [CrossRef]
- Luek, J.L.; Schmitt-Kopplin, P.; Mouser, P.J.; Petty, W.T.; Richardson, S.D.; Gonsior, M. Halogenated Organic Compounds Identified in Hydraulic Fracturing Wastewaters Using Ultrahigh Resolution Mass Spectrometry. Environ. Sci. Technol. 2017, 51, 5377–5385. [Google Scholar] [CrossRef] [PubMed]
- Wolford, R.A. Characterization of Organics in the Marcellus Shale Flowback and Produced Waters; The Pennsylvania State University: University Park, PA, USA, 2011. [Google Scholar]
- Maguire-Boyle, S.J.; Barron, A.R. Organic Compounds in Produced Waters from Shale Gas Wells. Environ. Sci. Process. Impacts 2014, 16, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, K.; Sumner, A.J.; Karatum, O.; Nelson, R.K.; Drollette, B.D.; O’Connor, M.P.; D’Ambro, E.L.; Getzinger, G.J.; Ferguson, P.L.; Reddy, C.M.; et al. Indications of Transformation Products from Hydraulic Fracturing Additives in Shale-Gas Wastewater. Environ. Sci. Technol. 2016, 50, 8036–8048. [Google Scholar] [CrossRef]
- Lester, Y.; Ferrer, I.; Thurman, E.M.; Sitterley, K.A.; Korak, J.A.; Aiken, G.; Linden, K.G. Characterization of Hydraulic Fracturing Flowback Water in Colorado: Implications for Water Treatment. Sci. Total Environ. 2015, 512–513, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Li, J.; Flynn, S.L.; Nesbø, C.L.; Sun, C.; Von Gunten, K.; Lanoil, B.D.; Goss, G.G.; Martin, J.W.; Alessi, D.S. Temporal Changes in Microbial Community Composition and Geochemistry in Flowback and Produced Water from the Duvernay Formation. ACS Earth Space Chem. 2019, 3, 1047–1057. [Google Scholar] [CrossRef]
- Luek, J.L.; Gonsior, M. Organic Compounds in Hydraulic Fracturing Fluids and Wastewaters: A Review. Water Res. 2017, 123, 536–548. [Google Scholar] [CrossRef]
- Ziemkiewicz, P.F.; Thomas He, Y. Evolution of Water Chemistry during Marcellus Shale Gas Development: A Case Study in West Virginia. Chemosphere 2015, 134, 224–231. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Olutona, G.O.; Olumayede, E.G.; Akintayo, C.O.; Ximba, B.J. Phenols, Flame Retardants and Phthalates in Water and Wastewater—A Global Problem. Water Sci. Technol. 2016, 74, 1025–1038. [Google Scholar] [CrossRef]
- Hale, S.E.; Arp, H.P.H.; Schliebner, I.; Neumann, M. What’s in a Name: Persistent, Mobile, and Toxic (PMT) and Very Persistent and Very Mobile (vPvM) Substances. Environ. Sci. Technol. 2020, 54, 14790–14792. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.E.; Arp, H.P.H.; Schliebner, I.; Neumann, M. Persistent, Mobile and Toxic (PMT) and Very Persistent and Very Mobile (vPvM) Substances Pose an Equivalent Level of Concern to Persistent, Bioaccumulative and Toxic (PBT) and Very Persistent and Very Bioaccumulative (vPvB) Substances under REACH. Environ. Sci. Eur. 2020, 32, 155. [Google Scholar] [CrossRef]
- Jin, B.; Huang, C.; Yu, Y.; Zhang, G.; Arp, H.P.H. The Need to Adopt an International PMT Strategy to Protect Drinking Water Resources. Environ. Sci. Technol. 2020, 54, 11651–11653. [Google Scholar] [CrossRef]
- Jin, B.; Han, M.; Huang, C.; Arp, H.P.H.; Zhang, G. Towards Improved Characterization of the Fate and Impact of Hydraulic Fracturing Chemicals to Better Secure Regional Water Quality. Environ. Sci. Process. Impacts 2022, 24, 497–503. [Google Scholar] [CrossRef]
- Huang, C.; Jin, B.; Han, M.; Zhang, G.; Arp, H.P.H. Identifying Persistent, Mobile and Toxic (PMT) Organic Compounds Detected in Shale Gas Wastewater. Sci. Total Environ. 2023, 858, 159821. [Google Scholar] [CrossRef]
- Wang, Z.; Han, M.; Jin, B. Identifying Candidate Persistent, Mobile, and Toxic (PMT) and Very Persistent and Very Mobile (vPvM) Substances in Shale Gas Drilling Fluids by Combining Nontarget Analysis and Machine Learning Model. Environ. Sci. Technol. Lett. 2024, 11, 114–121. [Google Scholar] [CrossRef]
- Lutz, B.D.; Lewis, A.N.; Doyle, M.W. Generation, Transport, and Disposal of Wastewater Associated with Marcellus Shale Gas Development. Water Resour. Res. 2013, 49, 647–656. [Google Scholar] [CrossRef]
- Zhao, T. Treatment Technology of Shale Gas Fracturing Flowback Fluid: A Mini Review. Front. Energy Res. 2023, 11, 1245552. [Google Scholar] [CrossRef]
- National Development and Reform Commission. SY/T 6596—2004; Recommended Practice for Produced-Water Reinjection in Gas Field. Petroleum Industry Press: Beijing, China, 2004.
- State Energy Administration. SY/T 7640—2021; Environmental Protection Specification for Produced Water Reinjection in Unconventional Gas Fields. Petroleum Industry Press: Beijing, China, 2021.
- Guo, C.; Chang, H.; Liu, B.; He, Q.; Xiong, B.; Kumar, M.; Zydney, A.L. A Combined Ultrafiltration–Reverse Osmosis Process for External Reuse of Weiyuan Shale Gas Flowback and Produced Water. Environ. Sci. Water Res. Technol. 2018, 4, 942–955. [Google Scholar] [CrossRef]
- Ellsworth, W.L. Injection-Induced Earthquakes. Science 2013, 341, 1225942. [Google Scholar] [CrossRef]
- Xiang, L.; Huang, D.; Kang, J.; Xu, R. Study on the Status of Shale Gas Development Fracturing and Returning Liquid Treatment in Southwest China and Its Emission Standard. Adv. Environ. Prot. 2019, 9, 575–583. [Google Scholar] [CrossRef]
- Olsson, O.; Weichgrebe, D.; Rosenwinkel, K.-H. Hydraulic Fracturing Wastewater in Germany: Composition, Treatment, Concerns. Environ. Earth Sci. 2013, 70, 3895–3906. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Arias Chavez, L.H.; Ben-Sasson, M.; Romero-Vargas Castrillón, S.; Yip, N.Y.; Elimelech, M. Desalination and Reuse of High-Salinity Shale Gas Produced Water: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2013, 47, 9569–9583. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.M.; Bhamidimarri, R. A Review of the Issues and Treatment Options for Wastewater from Shale Gas Extraction by Hydraulic Fracturing. Fuel 2016, 182, 292–303. [Google Scholar] [CrossRef]
- Kargbo, D.M.; Wilhelm, R.G.; Campbell, D.J. Natural Gas Plays in the Marcellus Shale: Challenges and Potential Opportunities. Environ. Sci. Technol. 2010, 44, 5679–5684. [Google Scholar] [CrossRef]
- Blauch, M.E.; Myers, R.R.; Moore, T.R.; Lipinski, B.A.; Houston, N.A. Marcellus Shale Post-Frac Flowback Waters—Where Is All the Salt Coming from and What Are the Implications? In Proceedings of the SPE Eastern Regional Meeting, Charleston, WV, USA, 23 September 2009; p. SPE-125740-MS. [Google Scholar]
- Henderson, C.; Acharya, H.; Matis, H.; Kommepalli, H.; Moore, B.; Wang, H. Cost Effective Recovery of Low-TDS Frac Flowback Water for Re-Use; General Electric Co.: Boston, MA, USA, 2011; p. 1030557. [Google Scholar]
- Keister, T. Conversion of Marcellus Production Wastewater into Salable Products. In Proceedings of the Shale Energy Engineering 2014, Pittsburgh, PA, USA, 21–23 July 2014; American Society of Civil Engineers: Pittsburgh, PA, USA, 2014; pp. 85–94. [Google Scholar]
- National Energy Administration. NB/T 14002.3-2022; Shale Gas—Reservoir Stimulation—Part 3: Recycling and Disposal Methods of Fracturing Flowback Water. Petroleum Industry Press: Beijing, China, 2022.
- Jin, Y.X.; Bao, K.; Peng, L.; Sun, J.R.; Chen, J.W.; Fan, X.J.; Xu, H. Sources Analysis and Treatment Measures of Shale Gas Well Wastewater in Southeast Chongqing Area. Guangzhou Chem. Ind. 2021, 49, 79–82. [Google Scholar]
- Mantell, M.E. Produced Water Reuse and Recycling Challenges and Opportunities across Major Shale Plays. In Proceedings of the EPA Hydraulic Fracturing Study Technical Workshop, Oklahoma City, OK, USA, 29–30 March 2011; Volume 4. [Google Scholar]
- Akob, D.M.; Mumford, A.C.; Orem, W.; Engle, M.A.; Klinges, J.G.; Kent, D.B.; Cozzarelli, I.M. Wastewater Disposal from Unconventional Oil and Gas Development Degrades Stream Quality at a West Virginia Injection Facility. Environ. Sci. Technol. 2016, 50, 5517–5525. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Zou, C.; Cui, H.; Ni, Y.; Liu, J.; Wu, W.; Zhang, L.; Coyte, R.; Kondash, A.; et al. Recycling Flowback Water for Hydraulic Fracturing in Sichuan Basin, China: Implications for Gas Production, Water Footprint, and Water Quality of Regenerated Flowback Water. Fuel 2020, 272, 117621. [Google Scholar] [CrossRef]
- Zou, C.; Dong, D.; Wang, Y.; Li, X.; Huang, J.; Wang, S.; Guan, Q.; Zhang, C.; Wang, H.; Liu, H.; et al. Shale Gas in China: Characteristics, Challenges and Prospects (I). Pet. Explor. Dev. 2015, 42, 753–767. [Google Scholar] [CrossRef]
- Ord, J. Strategies for Disposing Waste Water. In Proceedings of the UK Shale Gas Environmental Summit, London, UK, 27–28 October 2014. [Google Scholar]
- Liang, T.; Shao, L.; Yao, E.; Zuo, J.; Liu, X.; Zhang, B.; Zhou, F. Study on Fluid-Rock Interaction and Reuse of Flowback Fluid for Gel Fracturing in Desert Area. Geofluids 2018, 2018, 8948961. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced Water Characteristics, Treatment and Reuse: A Review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, L.; Shu, J.; Wu, Q.; Liu, B. Potential Hazards of Typical Small Molecular Organic Matters in Shale Gas Wastewater for Wheat Irrigation: 2-Butoxyethanol and Dimethylbenzylamine. Environ. Pollut. 2024, 340, 122729. [Google Scholar] [CrossRef] [PubMed]
- Pica, N.E.; Carlson, K.; Steiner, J.J.; Waskom, R. Produced Water Reuse for Irrigation of Non-Food Biofuel Crops: Effects on Switchgrass and Rapeseed Germination, Physiology and Biomass Yield. Ind. Crops Prod. 2017, 100, 65–76. [Google Scholar] [CrossRef]
- Chang, H.; Liu, S.; Tong, T.; He, Q.; Crittenden, J.C.; Vidic, R.D.; Liu, B. On-Site Treatment of Shale Gas Flowback and Produced Water in Sichuan Basin by Fertilizer Drawn Forward Osmosis for Irrigation. Environ. Sci. Technol. 2020, 54, 10926–10935. [Google Scholar] [CrossRef]
- Costa, T.C.; Hendges, L.T.; Temochko, B.; Mazur, L.P.; Marinho, B.A.; Weschenfelder, S.E.; Florido, P.L.; Da Silva, A.; Ulson De Souza, A.A.; Guelli Ulson De Souza, S.M.A. Evaluation of the Technical and Environmental Feasibility of Adsorption Process to Remove Water Soluble Organics from Produced Water: A Review. J. Pet. Sci. Eng. 2022, 208, 109360. [Google Scholar] [CrossRef]
- Mauter, M.S.; Palmer, V.R. Expert Elicitation of Trends in Marcellus Oil and Gas Wastewater Management. J. Environ. Eng. 2014, 140, B4014004. [Google Scholar] [CrossRef]
- Jang, E.; Jang, Y.; Chung, E. Lithium Recovery from Shale Gas Produced Water Using Solvent Extraction. Appl. Geochem. 2017, 78, 343–350. [Google Scholar] [CrossRef]
- Chang, H.; Liu, B.; Crittenden, J.C.; Vidic, R.D. Resource Recovery and Reuse for Hydraulic Fracturing Wastewater in Unconventional Shale Gas and Oil Extraction. Environ. Sci. Technol. 2019, 53, 13547–13548. [Google Scholar] [CrossRef]
- Tian, L.; Chang, H.; Tang, P.; Li, T.; Zhang, X.; Liu, S.; He, Q.; Wang, T.; Yang, J.; Bai, Y.; et al. Rare Earth Elements Occurrence and Economical Recovery Strategy from Shale Gas Wastewater in the Sichuan Basin, China. ACS Sustain. Chem. Eng. 2020, 8, 11914–11920. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, K.; Zhang, D.; Zhang, L.; Huang, Y. Treatment and Nutrient Recovery of Synthetic Flowback Water from Shale Gas Extraction by Air-cathode (PMo/CB) Microbial Desalination Cells. J. Chem. Technol. Biotechnol. 2021, 96, 262–272. [Google Scholar] [CrossRef]
- Karapataki, C. Techno-Economic Analysis of Water Management Options for Unconventional Natural Gas Developments in the Marcellus Shale. Ph.D. Dissertation, Massachusetts Institute of Technology, Cambridge, MA, USA, 2012. [Google Scholar]
- Mohammad-Pajooh, E.; Weichgrebe, D.; Cuff, G.; Tosarkani, B.M.; Rosenwinkel, K.-H. On-Site Treatment of Flowback and Produced Water from Shale Gas Hydraulic Fracturing: A Review and Economic Evaluation. Chemosphere 2018, 212, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.H.; Cai, Q.Q.; Li, R.; Jothinathan, L.; Lee, B.C.Y.; Ng, O.H.; Guo, J.; Ong, S.L.; Hu, J.Y. Reverse Osmosis Concentrate Treatment by Microbubble Ozonation-Biological Activated Carbon Process: Organics Removal Performance and Environmental Impact Assessment. Sci. Total Environ. 2021, 798, 149289. [Google Scholar] [CrossRef]
- Igunnu, E.T.; Chen, G.Z. Produced Water Treatment Technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef]
- Hayes, T.; Arthur, D. Overview of Emerging Produced Water Treatment Technologies. In Proceedings of the 11th Annual International Petroleum Environmental Conference, Albuquerque, NM, USA, 12–15 October 2004; Volume 201512. [Google Scholar]
- Parker, W.; Monteith, H. Stripping of VOC’s from Dissolved Air Flotation. Environ. Prog. 1996, 15, 73–81. [Google Scholar] [CrossRef]
- Sattler, A.; Hightower, M.; Nenoff, T.; Pless, J.; Phillips, M.; Brister, B.; Arnold, R.; Cather, M.; McPherson, B. Managing Coal Bed Methane Produced Water for Beneficial Uses, Initially Using the San Juan and Raton Basins as a Model. In Interim Progress Report Prepared for NETL; Sandia National Laboratory: Albuquerque, NM, USA, 2004. [Google Scholar]
- Lee, Y.; Cui, M.; Choi, J.; Jeong, Y.; Khim, J. Demonstration and Evaluation of Potential Configuration Options for Shale-Wastewater Treatment Plant by Combining Several Unit Processes. J. Clean. Prod. 2019, 232, 867–876. [Google Scholar] [CrossRef]
- Nadella, M.; Sharma, R.; Chellam, S. Fit-for-Purpose Treatment of Produced Water with Iron and Polymeric Coagulant for Reuse in Hydraulic Fracturing: Temperature Effects on Aggregation and High-Rate Sedimentation. Water Res. 2020, 170, 115330. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A Comprehensive Review of Electrocoagulation for Water Treatment: Potentials and Challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Z.; Zhang, X.; Liu, Y.; Chen, Y. Electro-Flocculation Pretreatment Experiments of Shale Gas Drilling Wastewater. Nat. Gas Ind. B 2020, 7, 309–316. [Google Scholar] [CrossRef]
- Esmaeilirad, N.; Carlson, K.; Omur Ozbek, P. Influence of Softening Sequencing on Electrocoagulation Treatment of Produced Water. J. Hazard. Mater. 2015, 283, 721–729. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J. Fe-Based Fenton-like Catalysts for Water Treatment: Catalytic Mechanisms and Applications. J. Mol. Liq. 2021, 332, 115755. [Google Scholar] [CrossRef]
- Wu, S.; Yin, X.; Liu, Z.; Zhang, W.; Huang, Y.; Dong, F.; Zhang, D. Electrolyte Flow-Driven Coupling of Oxygen Evolution Reaction and CO2 Electroreduction for Promoting Selective HCOOH Production under Acidic Conditions. ACS Sustain. Chem. Eng. 2025, 13, 7062–7073. [Google Scholar] [CrossRef]
- Luo, M.; Yang, H.; Wang, K.; Song, F.; He, Y.; Zhang, Y.; Zhong, C. Coupling Iron-Carbon Micro-Electrolysis with Persulfate Advanced Oxidation for Hydraulic Fracturing Return Fluid Treatment. Chemosphere 2023, 313, 137415. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tang, X.; Li, R.; Liu, X.; Zhang, P.; Gong, Y. Tunable Microscopic Aggregation Morphology of α-Ni(OH)2 for Enhanced Photocatalytic Degradation of Fracturing Flowback Fluid with Ozone Synergy. Front. Mater. 2022, 9, 995276. [Google Scholar] [CrossRef]
- Matsushita, S.; Komizo, D.; Cao, L.T.T.; Aoi, Y.; Kindaichi, T.; Ozaki, N.; Imachi, H.; Ohashi, A. Production of Biogenic Manganese Oxides Coupled with Methane Oxidation in a Bioreactor for Removing Metals from Wastewater. Water Res. 2018, 130, 224–233. [Google Scholar] [CrossRef]
- Riley, S.M.; Oliveira, J.M.S.; Regnery, J.; Cath, T.Y. Hybrid Membrane Bio-Systems for Sustainable Treatment of Oil and Gas Produced Water and Fracturing Flowback Water. Sep. Purif. Technol. 2016, 171, 297–311. [Google Scholar] [CrossRef]
- Zilverentant, A.; Van Nieuwkerk, A.; Vance, I.; Watlow, A.; Rees, M. Pilot-Scale Membrane Bioreactor Treats Produced Water. In World Oil; Gulf Publishing Co.: Houston, TX, USA, 2009. [Google Scholar]
- Mozo, I.; Stricot, M.; Lesage, N.; Spérandio, M. Fate of Hazardous Aromatic Substances in Membrane Bioreactors. Water Res. 2011, 45, 4551–4561. [Google Scholar] [CrossRef]
- Freedman, D.E.; Riley, S.M.; Jones, Z.L.; Rosenblum, J.S.; Sharp, J.O.; Spear, J.R.; Cath, T.Y. Biologically Active Filtration for Fracturing Flowback and Produced Water Treatment. J. Water Process Eng. 2017, 18, 29–40. [Google Scholar] [CrossRef]
- Nadav, N. Boron Removal from Seawater Reverse Osmosis Permeate Utilizing Selective Ion Exchange Resin. Desalination 1999, 124, 131–135. [Google Scholar] [CrossRef]
- Cooley, H.; Donnelly, K. Hydraulic Fracturing and Water Resources: What Do We Know and Need to Know? In The World’s Water: The Biennial Report on Freshwater Resources; Island Press: Washington, DC, USA, 2014; pp. 63–81. [Google Scholar]
- Zhang, D.; Che, Z.; Ding, A.; Peng, S.; Lu, P. Reviews and prospects of shale gas flowback and produced water treatment technology selection. J. Saf. Environ. 2021, 21, 2761–2773. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Hancock, N.T.; Nowosielski-Slepowron, M.S.; McGurgan, G.D. Pilot Demonstration of the NH3/CO2 Forward Osmosis Desalination Process on High Salinity Brines. Desalination 2013, 312, 67–74. [Google Scholar] [CrossRef]
- Li, X.-M.; Zhao, B.; Wang, Z.; Xie, M.; Song, J.; Nghiem, L.D.; He, T.; Yang, C.; Li, C.; Chen, G. Water Reclamation from Shale Gas Drilling Flow-Back Fluid Using a Novel Forward Osmosis–Vacuum Membrane Distillation Hybrid System. Water Sci. Technol. 2014, 69, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Huang, X.; Gao, C.; Gao, X. Application of an Integrated System of Coagulation and Electrodialysis for Treatment of Wastewater Produced by Fracturing. Desalin. Water Treat. 2015, 55, 2034–2043. [Google Scholar] [CrossRef]
- Cho, H.; Choi, Y.; Lee, S. Effect of Pretreatment and Operating Conditions on the Performance of Membrane Distillation for the Treatment of Shale Gas Wastewater. Desalination 2018, 437, 195–209. [Google Scholar] [CrossRef]
- Kalfa, A.; Shapira, B.; Shopin, A.; Cohen, I.; Avraham, E.; Aurbach, D. Capacitive Deionization for Wastewater Treatment: Opportunities and Challenges. Chemosphere 2020, 241, 125003. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Chen, M.; Liu, Y.; Liang, Q.; Tu, W.; Chen, Y.; Liang, J. Development of Shale Gas in China and Treatment Options for Wastewater Produced from the Exploitation: Sustainability Lessons from the United States. J. Environ. Eng. 2020, 146, 4020103. [Google Scholar] [CrossRef]
- Al-Karaghouli, A.; Kazmerski, L.L. Energy Consumption and Water Production Cost of Conventional and Renewable-Energy-Powered Desalination Processes. Renew. Sustain. Energy Rev. 2013, 24, 343–356. [Google Scholar] [CrossRef]
- Feria-Dı́az, J.J.; López-Méndez, M.C.; Rodrı́guez-Miranda, J.P.; Sandoval-Herazo, L.C.; Correa-Mahecha, F. Commercial Thermal Technologies for Desalination of Water from Renewable Energies: A State of the Art Review. Processes 2021, 9, 262. [Google Scholar] [CrossRef]
- Nabil, I.; Abdalla, A.M.; Mansour, T.M.; Shehata, A.I.; Dawood, M.M.K. Salinity Impacts on Humidification Dehumidification (HDH) Desalination Systems: Review. Environ. Sci. Pollut. Res. 2024, 31, 1907–1925. [Google Scholar] [CrossRef]
- Shalaby, S.M.; Kabeel, A.E.; Moharram, B.M.; Fleafl, A.H. Experimental Study of Hybrid Solar Humidification Dehumidification System for Extremely Saline Water Desalination. Energy Convers. Manag. 2021, 235, 114021. [Google Scholar] [CrossRef]
- Jamil, M.A.; Zubair, S.M. On Thermoeconomic Analysis of a Single-Effect Mechanical Vapor Compression Desalination System. Desalination 2017, 420, 292–307. [Google Scholar] [CrossRef]
- Farahat, M.A.; Fath, H.E.S.; El-Sharkawy, I.I.; Ookawara, S.; Ahmed, M. Energy/Exergy Analysis of Solar Driven Mechanical Vapor Compression Desalination System with Nano-Filtration Pretreatment. Desalination 2021, 509, 115078. [Google Scholar] [CrossRef]
- Hayes, T.D.; Halldorson, B.; Horner, P.H.; Ewing, J.J.R.; Werline, J.R.; Severin, B.F. Mechanical Vapor Recompression for the Treatment of Shale-Gas Flowback Water. Oil Gas Facil. 2014, 3, 54–62. [Google Scholar] [CrossRef]
| Additive | Purpose | Fraction % |
|---|---|---|
| Gel Agent | Increases the viscosity of the fracturing fluid, enhances sand suspension, aids in proppant transport | 0.05 |
| Crosslinking Agent | Chemically binds individual gel polymer molecules to maintain fluid viscosity, facilitating proppant transport | 0.007 |
| Lubricant | Reduces the interfacial tension between the fluid and pipe surface, maintaining laminar flow during pumping | 0.07 |
| Breaker | Reverses crosslinking, reduces viscosity, improves gas production efficiency, aids in fracturing fluid recovery | 0.06 |
| pH Control Agent | Enhances the effectiveness of crosslinking agents | 0.01 |
| Acid | Cleans and dissolves minerals, facilitating rock fracturing | 0.15 |
| Corrosion Inhibitor | Prevents corrosion of casings due to acids and salts | 0.002 |
| Scale Inhibitor | Prevents the formation of scale (mineral deposits) within pipelines | 0.09 |
| Iron Control Agent | Prevents iron ion precipitation | 0.006 |
| Clay Stabilizer | Prevents clay swelling in shale formations | 0.120 |
| Biocide | Sterilizes and inhibits bacteria in the fracturing fluid | 0.060 |
| Surfactant | Controls the optimal viscosity of the fracturing fluid, reduces interfacial tension between the fracturing fluid and shale | 0.075 |
| Region | pH | TDS | COD | TOC | TSS | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | Br− | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marcellus Basin, USA | 5.1~8.42 | 680~345,000 | 195~36,600 | 1.2~1530 | 4~7600 | 69.2~117,000 | 37.8~41,000 | 17.3~2550 | 64.2~196,000 | 0~763 | 0.2~1990 | [60] |
| Bakken Basin, USA | 5.0~5.4 | 269,380~295,320 | 1700~2260 | 91~315 | 2660~7500 | 12,271~74,600 | 372~15,346 | 118~1299 | 89~136,220 | 102~531.6 | 37.1~601 | [85] |
| Eagle Ford Basin, USA | 4.3~8.9 | 1033~398,024 | - | - | 160~1559 | 148~123,775 | 1~40,992 | 1~17,203 | 490~245,367 | 1~14,100 | 260 | [85] |
| Denver-Julesburg Basin, USA | 7.0~8.2 | 30,545~30,655 | 2960~3140 | 637~653 | 837~923 | - | - | - | - | - | - | [86] |
| Niobrara/DJ Basin, USA | 6.56~7.42 | 14,220~445,020 | 628~8125 | 95~758 | 80~1297 | 5233~14,794 | 57~1204 | 14~130 | 6524~27,103 | 5.4~258 | 57~259 | [85] |
| Ordos Basin, China | - | 8640~9630 | 1577~2318 | 700~1514 | 80~110 | - | 29.5~508.7 | 3.8~86.2 | 2800~24,700 | 0~38.9 | - | [87] |
| Sichuan Basin (Changning), China | 7.1~7.3 | 30,859~31,204 | 1228~1259 | - | 53~93 | 743.0~1049.1 | 532.1~756.5 | 56.4~62.0 | 541.1~3030.2 | 57.5~257.3 | 309~371 | [88] |
| Sichuan Basin (Fuling), China | 6.58~7.52 | 268,000~402,000 | - | - | - | 12,154~14,654 | 344~501 | 32~52 | 17,620~24,250 | - | 89~114 | [71] |
| Sichuan Basin (Weiyuan), China | - | - | - | - | - | 5856~8887 | 118~316 | 26~49 | 7342~15,253 | - | 40~81 | [63] |
| Sichuan Basin (Anyue), China | - | - | - | - | - | 3113~12,578 | 119~444 | 4~85 | 4645~19,695 | 1.6~286 | 15~80 | [61] |
| Contaminant Type | Typical Substance | Water Quality Indicator | Treatment Method |
|---|---|---|---|
| Suspended Substance | Sand, foulant, bacteria | TSS; Turbidity | 1. C-F and deposition/filtration |
| 2. MF/UF | |||
| Suspended Organic Matter | Oil, colloid, bacteria | OG; TOC; COD; BOD5 | 1. Air flotation |
| 2. Adsorption (with activated carbon, zeolite) | |||
| 3. MF/UF | |||
| 4. Biodegradation | |||
| Dissolved Organic Matter | Benzene series, organic acid, phenols | BTEX; VOCs; Special chemical additives | 1. Adsorption(with activated carbon, organic clay, zeolite, resin) |
| 2. C-F | |||
| 3. Chemical oxidation | |||
| 4. Biodegradation | |||
| 5. NF/RO | |||
| 6. Electrochemical treatment | |||
| Dissolved Polyvalent Metals and Anions | Fouling substance, natural radioactive material | Hardness; Specific metal ions (Fe, Sr, Ba); Specific anions (sulfates, nitrates) | 1. Hardness: Ion exchange |
| 2. Metal: Deposition, filtration, ion exchange, RO | |||
| 3. Radioactive material: Ion exchange, RO | |||
| 4. Anions: Electrochemical, thermal treatment | |||
| Dissolved Monovalent Ions | Na+, K+, NH4+, Cl−, I−, NO3− | Specific ions (Na, Cl, Br); Ammonia | 1. Thermal separation |
| 2. Membrane separation | |||
| 3. Electrochemical treatment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, S.; Zhang, Y.; Lu, P.; Yang, D.; Huang, Y.; Wu, X.; He, P.; Guo, D. Environmental Impacts of Shale Gas Development on Groundwater, and Flowback and Produced Water Treatment Management: A Review. Sustainability 2025, 17, 5209. https://doi.org/10.3390/su17115209
Pan S, Zhang Y, Lu P, Yang D, Huang Y, Wu X, He P, Guo D. Environmental Impacts of Shale Gas Development on Groundwater, and Flowback and Produced Water Treatment Management: A Review. Sustainability. 2025; 17(11):5209. https://doi.org/10.3390/su17115209
Chicago/Turabian StylePan, Shubiao, Ye Zhang, Peili Lu, Demin Yang, Yongkui Huang, Xiaochuan Wu, Pei He, and Dongxin Guo. 2025. "Environmental Impacts of Shale Gas Development on Groundwater, and Flowback and Produced Water Treatment Management: A Review" Sustainability 17, no. 11: 5209. https://doi.org/10.3390/su17115209
APA StylePan, S., Zhang, Y., Lu, P., Yang, D., Huang, Y., Wu, X., He, P., & Guo, D. (2025). Environmental Impacts of Shale Gas Development on Groundwater, and Flowback and Produced Water Treatment Management: A Review. Sustainability, 17(11), 5209. https://doi.org/10.3390/su17115209







