Evaluating the Health Risks of Air Quality and Human Thermal Comfort–Discomfort in Relation to Hospital Admissions in the Greater Athens Area, Greece
Abstract
1. Introduction
2. Materials and Methods
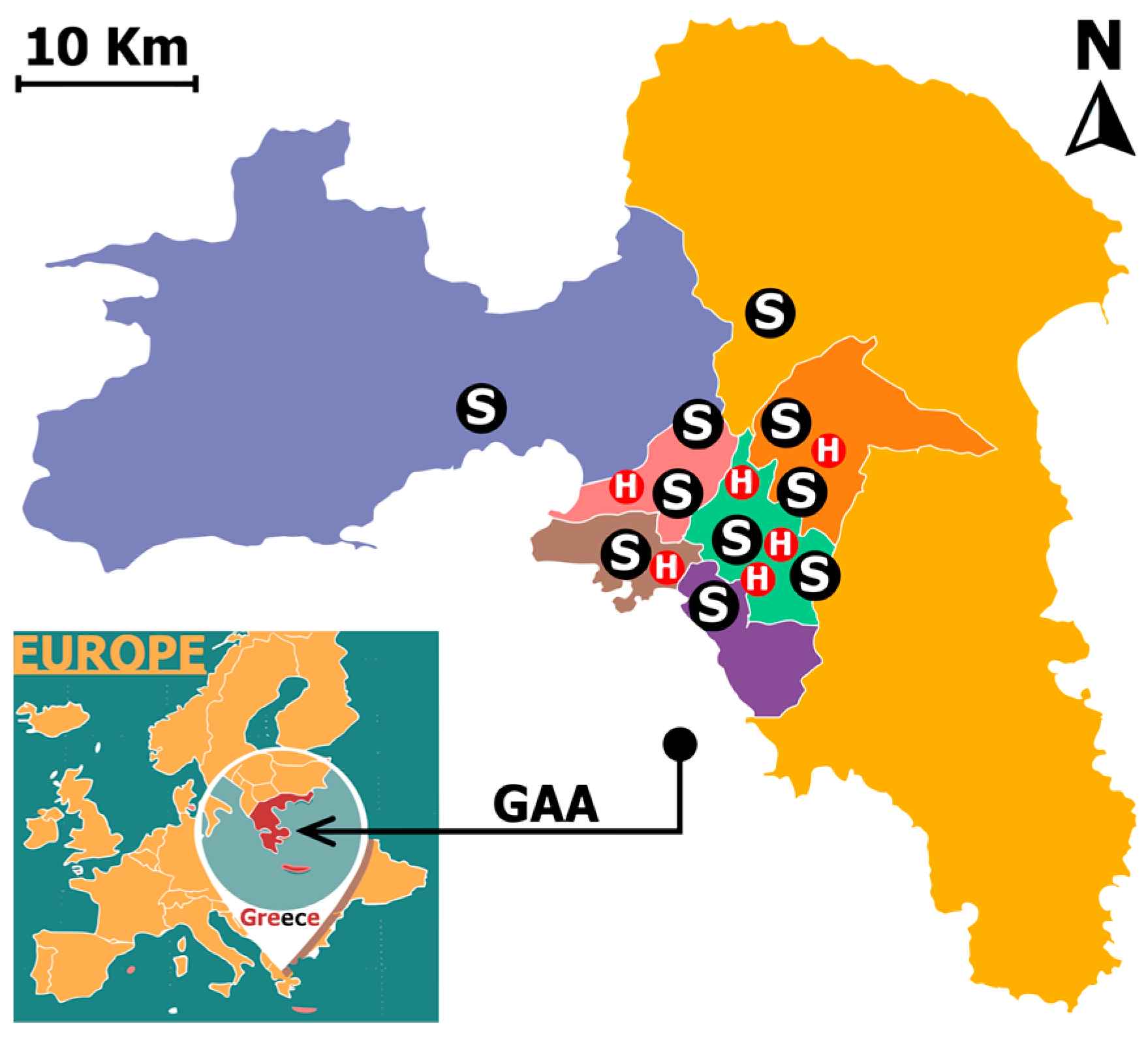
2.1. Study Area Characteristics
2.2. Data Collected
2.2.1. Hospital Admissions
- Evaggelismos (EVA), Konstantopoulio (KON), and Elpis (ELP)—Central GAA Sector;
- Attikon (ATI)—West GAA Sector;
- Tzanio (TZA)—Piraeus (Southwest GAA);
- Sismanoglio (SIS)—North GAA Sector.
- ✓
- Data Availability and Consistency: Hospitals were chosen based on the availability of consistent admissions data covering the study period (2018–2022) to ensure robust analysis;
- ✓
- Relevance to the Study: The selected hospitals include clinics specializing in RD and CVD, as these diseases are strongly linked to air pollution and temperature extremes;
- ✓
- Public Healthcare System Representation: Only public hospitals were included to ensure access to a broad and diverse patient population, avoiding biases related to private healthcare access;
- ✓
- Hospital Capacity and Regional Coverage: The six hospitals in the dataset collectively have a total bed capacity of 2907, which accounts for approximately 34% of the total bed capacity of public hospitals in Attica, standing at 8476 beds as of 2022 [45], ensuring adequate case volume for analysis.
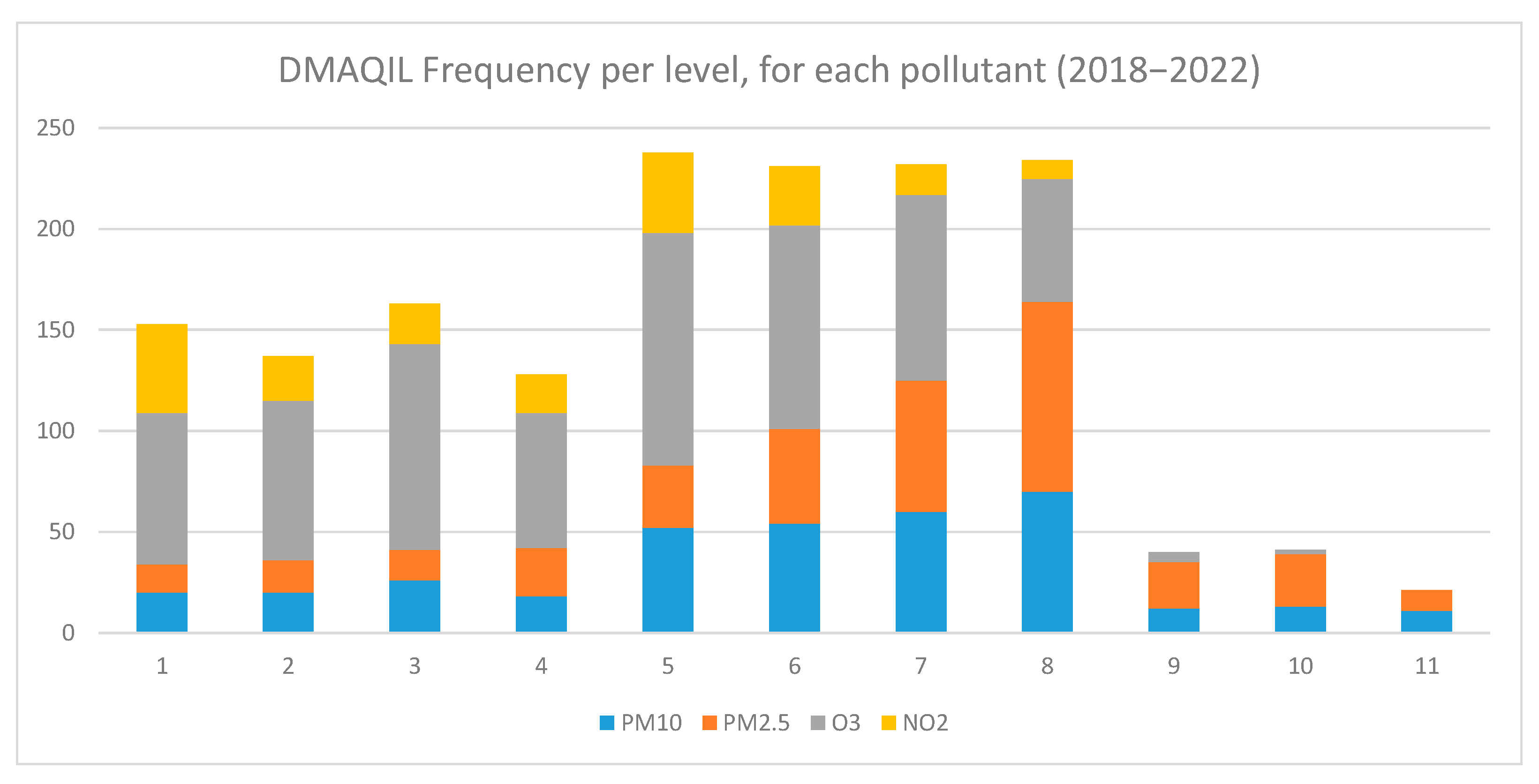
2.2.2. Air Quality Index Levels
2.2.3. Apparent Temperature
2.3. Distributed Lag Non-Linear Model (DLNM)
3. Results
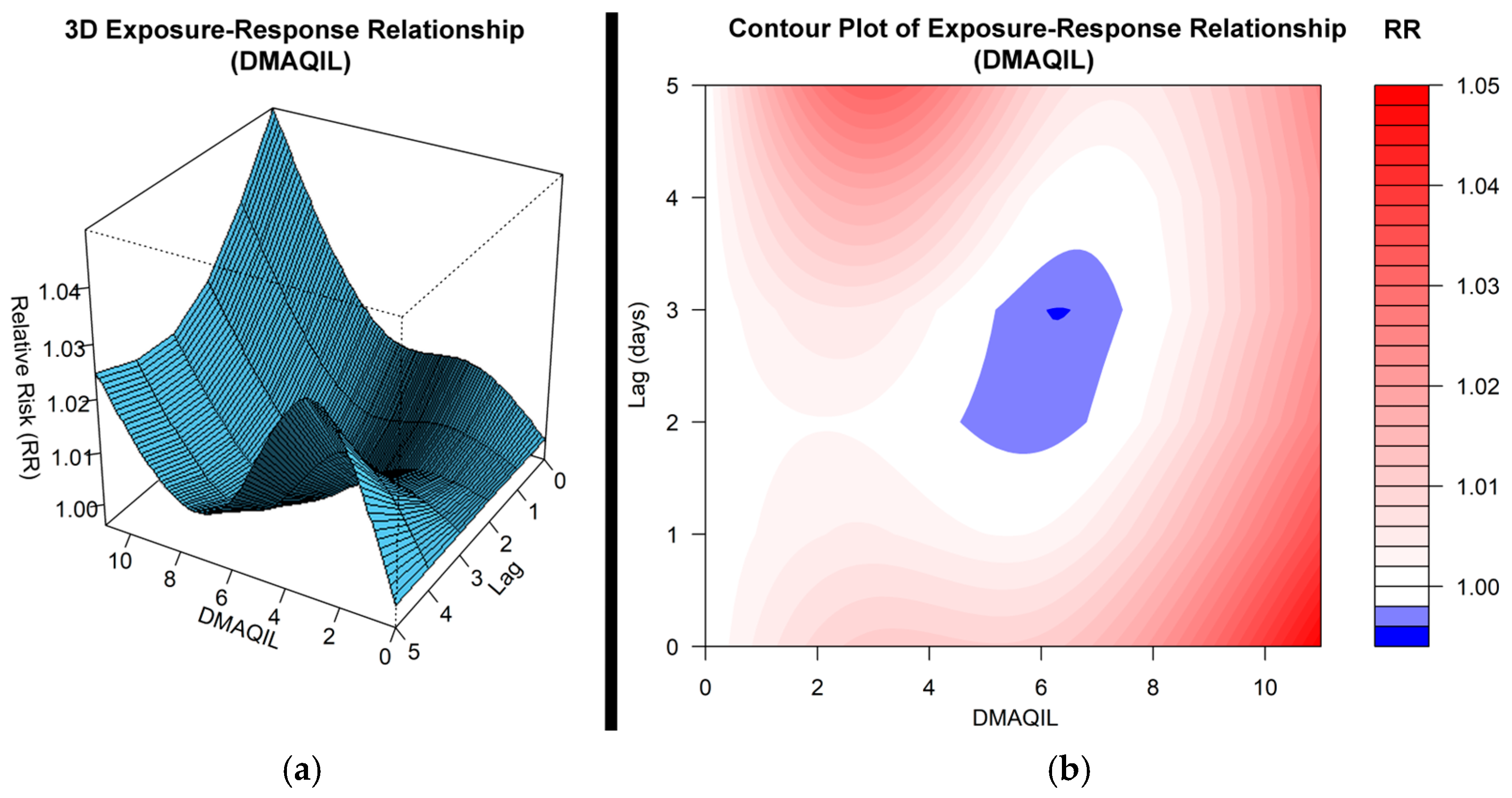
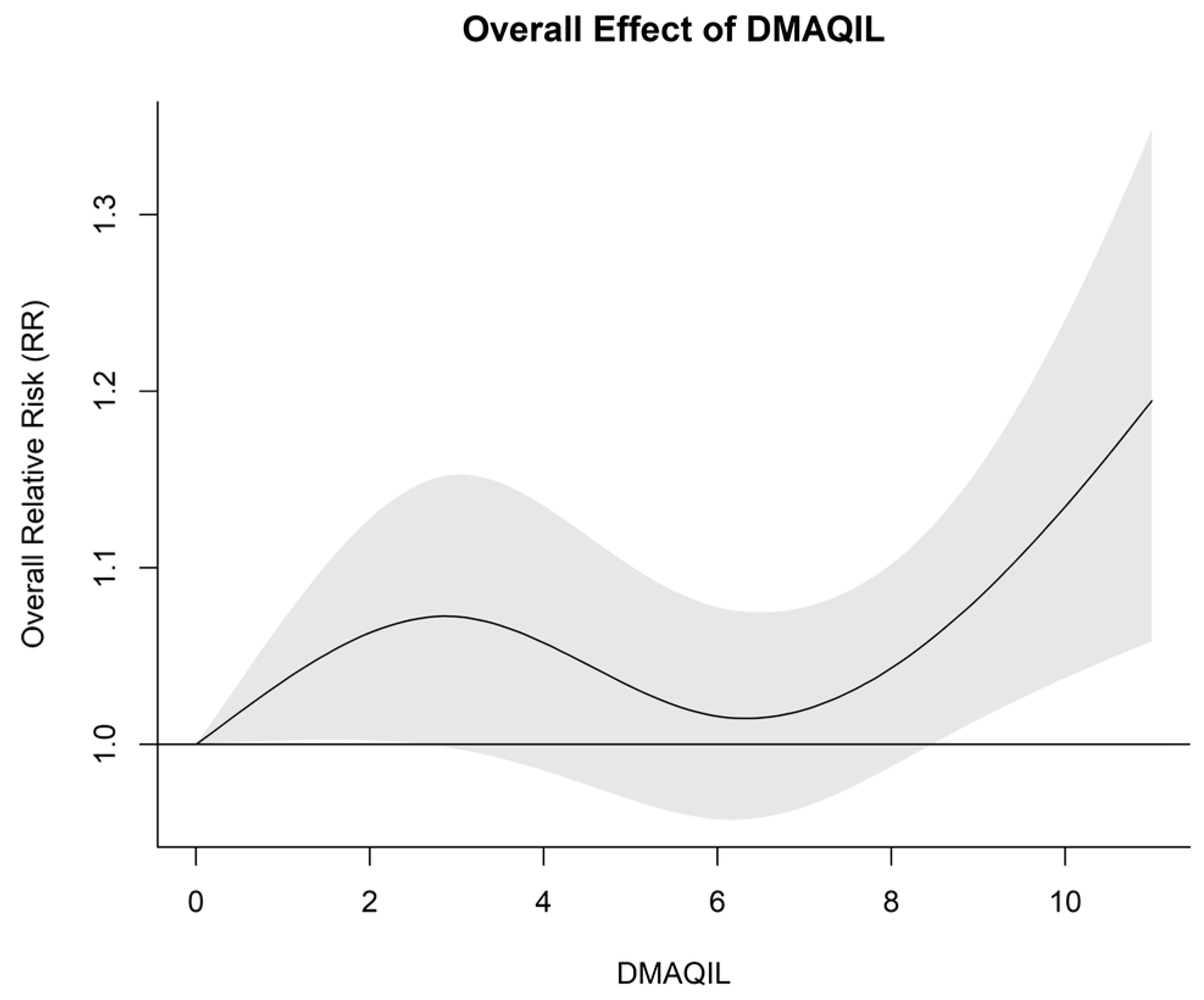
3.1. Impact of the DMAQIL on Hospital Admissions
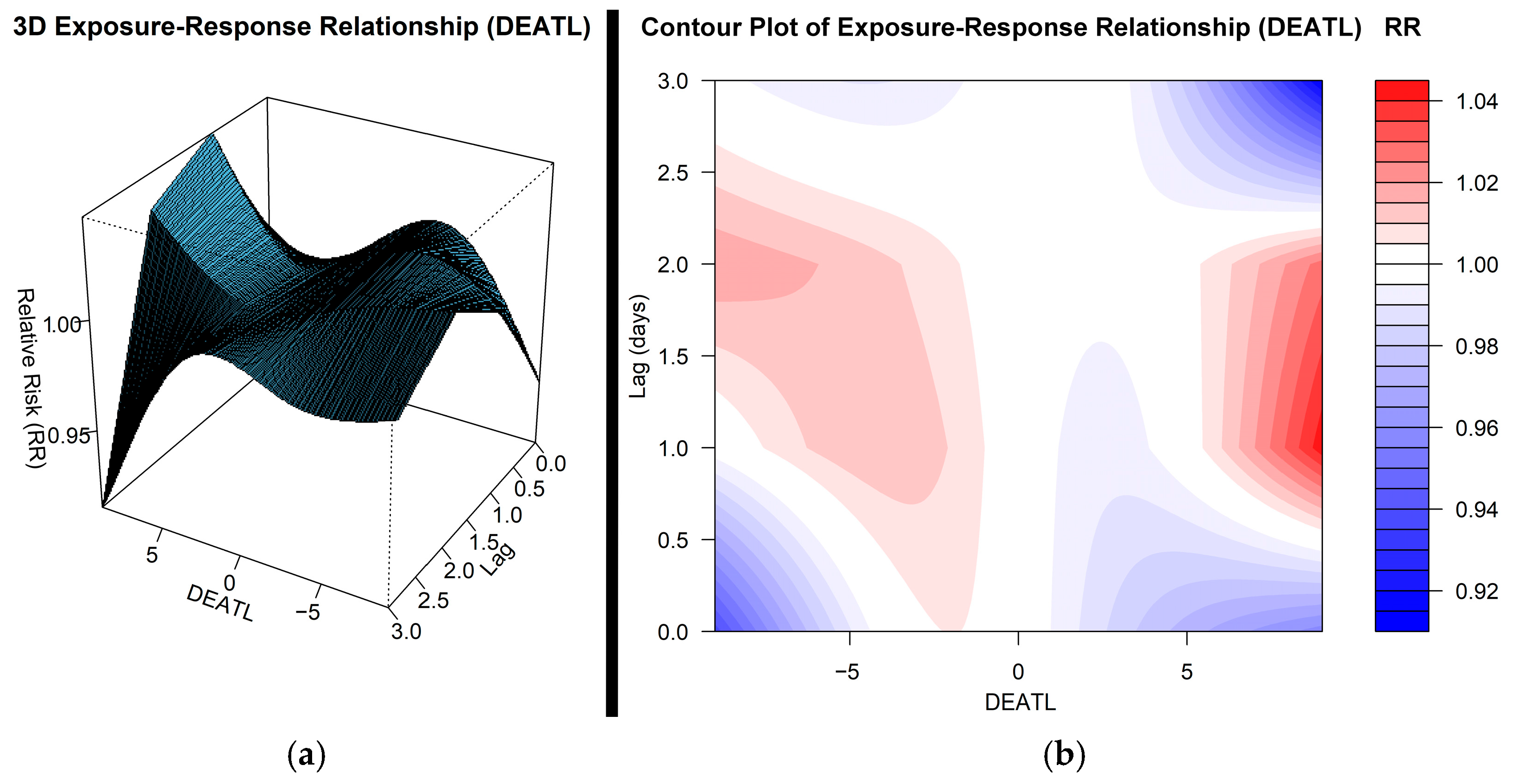
3.2. Impact of DEATL on Hospital Admissions
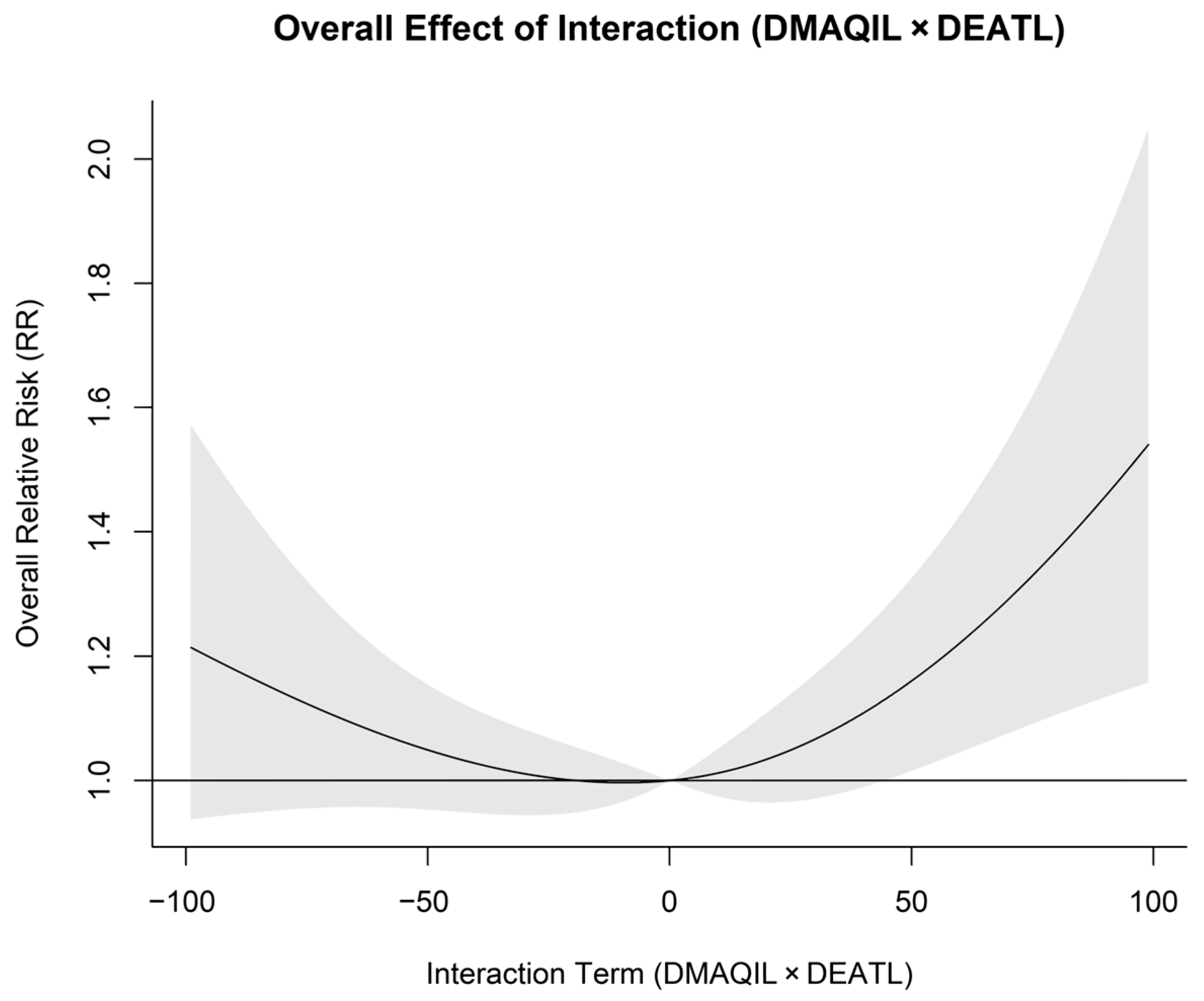
3.3. Impact of Interaction Term on Hospital Admissions
4. Discussion
5. Conclusions
5.1. Policy and Hospital Recommendations
- Hospital Preparedness and Public Health Response
- Hospitals should implement action protocols informed by advanced heat–health–air quality warning systems, allowing for proactive patient management during high-risk periods;
- Emergency departments should be equipped with specialized protocols for handling heatstroke, respiratory distress, and cardiovascular exacerbations linked to environmental exposure;
- Vulnerable populations, such as the elderly, children, and individuals with preexisting conditions, should be prioritized in hospital risk mitigation plans during extreme weather and pollution events.
- Air Quality and Urban Planning Policies
- Policymakers should strengthen air quality regulations by enforcing stricter pollution control measures, particularly for industrial zones and traffic-congested areas, where air pollution is most severe;
- Urban planning should integrate green infrastructure, such as vegetation barriers and urban forests, to reduce Urban Heat Island effects and improve air quality;
- Public awareness campaigns should educate citizens about self-protection measures, such as avoiding outdoor activities during high pollution days and using air filtration systems indoors.
5.2. Implications for Environmental Regulations and Hospital Protocols
5.3. Study Limitations and Potential Biases
- Data quality and measurement bias: Air pollution and meteorological data were obtained from certain monitoring stations, which may not fully capture personal exposure levels or localized environmental variations within the GAA;
- Lack of individual and demographic data: The analysis was based on aggregated hospital admissions rather than individual health records, limiting the ability to control for demographic variations, preexisting medical conditions, socioeconomic factors, or lifestyle behaviors that could influence health risks;
- Potential reporting bias: The dataset excludes cases from private hospitals, which may lead to an incomplete representation of hospital admissions across different socioeconomic groups. Additionally, unreported cases of mild or untreated conditions could result in an underestimation of the true health burden;
- Confounding factors: While the model adjusts for seasonality and long-term trends, additional confounding factors, such as indoor air pollution, occupational exposures, or medication use, were not directly assessed;
- Higher-order Nonlinearities and model assumptions: Although the DLNM with the NB model was selected due to its strong fit and ability to handle overdispersion, GAMs are capable of capturing complex, non-linear relationships in the data. The slightly lower RMSE and MAE values for the GAM suggest that higher-order non-linear effects may exist, which the DLNM does not fully capture.
5.4. Future Research Directions
- Integrate high-resolution patient data to assess individual susceptibility more accurately;
- Expand real-time environmental surveillance by deploying additional monitoring stations for better spatial coverage;
- Develop personalized health risk models that account for socioeconomic disparities in exposure and access to healthcare;
- Investigate potential synergies between pollutants to enhance the understanding of their individual and combined impacts on health outcomes;
- Future research could explore DLNM-GAM hybrid models to capture both the delayed and non-linear effects of environmental exposures while balancing flexibility with interpretability;
- While the current findings provide valuable insights, future studies could benefit from access to larger datasets and more granular hospital or exposure data to further reduce noise and uncertainty in the estimates. Additionally, incorporating machine learning techniques—such as random forests, gradient boosting, or neural networks—may further improve model efficiency by capturing complex, non-linear interactions that traditional approaches may miss. These techniques hold promise for enhancing predictive accuracy and uncovering more nuanced patterns in environmental health relationships.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GAA | Greater Athens Area |
| DMAQIL | Daily Maximum Air Quality Index Level |
| DEATL | Daily Extreme Apparent Temperature Level |
| CVDs | Cardiovascular diseases |
| RDs | Respiratory diseases |
| HAs | Hospital admissions |
| DLNM | Distributed Lag Non-linear Model |
| AQI | Air Quality Index |
| AT | Apparent Temperature |
| RR | Relative Risk |
| ER | Exposure–Response |
| GAMs | Generalized Additive Models |
| NB | Negative Binomial |
| AE | Asymmetric Epanechnikov |
References
- Gao, J.H.; Woodward, A.; Vardoulakis, S.; Kovats, S.; Wilkinson, P.; Li, L.P.; Xu, L.; Li, J.; Yang, J.; Cao, L.; et al. Haze, public health and mitigation measures in China: A review of the current evidence for further policy response. Sci. Total Environ. 2017, 578, 148–157. [Google Scholar] [CrossRef]
- European Environment Agency (EEA). Cutting Air Pollution in Europe Would Prevent Early Deaths, Improve Productivity and Curb Climate Change. Available online: https://www.eea.europa.eu/highlights/cutting-air-pollution-in-europe (accessed on 15 December 2024).
- Duan, R.R.; Hao, K.; Yang, T. Air pollution and chronic obstructive pulmonary disease. Chronic Dis. Transl. Med. 2020, 6, 260–269. [Google Scholar] [CrossRef]
- Jiang, X.Q.; Mei, X.D.; Feng, D. Air pollution and chronic airway diseases: What should people know and do? J. Thorac. Dis. 2016, 8, E31–E40. [Google Scholar] [PubMed]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- De Sario, M.; Katsoujanni, K.; Michelozzi, P. Climate change, extreme weather events, air pollution and respiratory health in Europe. Eur. Respir. J. Off. J. Eur. Soc. Clin. Respir. Physiol. 2013, 42, 826–843. [Google Scholar] [CrossRef]
- USA EPA. Technical Assistance Document for the Reporting of Daily Air Quality—The Air Quality Index (AQI); Environmental Protection Agency: Washington, DC, USA, 2018.
- Jiang, X.; Wei, P.; Luo, Y.; Li, Y.J.A. Air pollutant concentration prediction based on a CEEMDAN-FE-BiLSTM model. Atmosphere 2021, 12, 1452. [Google Scholar] [CrossRef]
- Wang, J.; Du, P.; Hao, Y.; Ma, X.; Niu, T.; Yang, W. An innovative hybrid model based on outlier detection and correction algorithm and heuristic intelligent optimization algorithm for daily air quality index forecasting. J. Environ. Manag. 2020, 255, 109855. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Li, R.; Lu, H. Novel analysis—Forecast system based on multi-objective optimization for air quality index. J. Clean. Prod. 2019, 208, 1365–1383. [Google Scholar] [CrossRef]
- Xie, Y.J.; Zhao, L.J.; Xue, J.; Gao, H.O.; Li, H.Y.; Jiang, R.; Qiu, X.Y.; Zhang, S.H. Methods for defining the scopes and priorities for joint prevention and control of air pollution regions based on data-mining technologies. J. Clean. Prod. 2018, 185, 912–921. [Google Scholar] [CrossRef]
- Thunis, P.; Degraeuwe, B.; Pisoni, E.; Trombetti, M.; Peduzzi, E.; Belis, C.A.; Wilson, J.; Clappier, A.; Vignati, E. PM2.5 source allocation in European cities: A SHERPA modelling study. Atmos. Environ. 2018, 187, 93–106. [Google Scholar] [CrossRef]
- Recast. European Parliament. 2024. Available online: https://eur-lex.europa.eu/eli/dir/2024/2881/oj/eng (accessed on 4 January 2025).
- Poumadère, M.; Mays, C.; Le Mer, S.; Blong, R. The 2003 heat wave in France: Dangerous climate change here and now. Risk Anal. 2005, 25, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ai, S.; Liu, Y.; Qian, Z.; Wang, C.; Sun, J.; Sun, X.; Zhang, S.; Syberg, K.M.; Howard, S.; et al. Hourly Associations Between Ambient Temperature and Emergency Ambulance Calls in One Central Chinese City: Call for an Immediate Emergency Plan. Sci. Total Environ. 2020, 711, 135046. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Armstrong, B.; Hajat, S.; Haines, A.; Wilkinson, P.; Smeeth, L. Heat and Risk of Myocardial Infarction: Hourly Level Case-Crossover Analysis of MINAP Database. BMJ 2012, 345, e8050. [Google Scholar] [CrossRef]
- Liu, Y.; Kan, H.; Xu, J.; Rogers, D.; Peng, L.; Ye, X.; Chen, R.; Zhang, Y.; Wang, W. Temporal Relationship Between Hospital Admissions for Pneumonia and Weather Conditions in Shanghai, China: A Time-Series Analysis. BMJ Open 2014, 4, e004961. [Google Scholar] [CrossRef] [PubMed]
- Kladakis, A.; Fameli, K.-M.; Moustris, K.; Assimakopoulos, V.D.; Nastos, P. Investigation into Atmospheric Pollution Impacts on Hospital Admissions in Attica Using Regression Models. Environ. Sci. Proc. 2023, 26, 25. [Google Scholar]
- Parliari, D.; Economou, T.; Giannaros, C.; Kushta, J.; Melas, D.; Matzarakis, A.; Lelieveld, J. A comprehensive approach for assessing synergistic impact of air quality and thermal conditions on mortality: The case of Thessaloniki, Greece. Urban. Clim. 2024, 56, 102088. [Google Scholar] [CrossRef]
- Schwartz, J. The distributed lag between air pollution and daily deaths. Epidemiology 2000, 11, 320–326. [Google Scholar] [CrossRef]
- Gasparrini, A.; Leone, M. Attributable risk from distributed lag models. BMC Med. Res. Methodol. 2014, 14, 55. [Google Scholar] [CrossRef]
- Singh, N.; Area, A.; Breitner, S.; Zhang, S.; Agewall, S.; Schikowski, T.; Schneider, A. Heat and Cardiovascular Mortality: An Epidemiological Perspective. Circ. Res. 2024, 134, 1098–1112. [Google Scholar] [CrossRef]
- Joshi, M.; Goraya, H.; Joshi, A.; Bartter, T. Climate change and respiratory diseases. Curr. Opin. Pulm. Med. 2020, 26, 119–127. [Google Scholar] [CrossRef]
- Katsouyanni, K.; Analitis, A. Investigating the Synergistic Effects Between Meteorological Variables and Air Pollutants: Results from the European PHEWE, EUROHEAT and CIRCE Projects. Epidemiology 2009, 20, S264. [Google Scholar] [CrossRef]
- Analitis, A.; Donato, F.D.; Scortichini, M.; Lanki, T.; Basagana, X.; Ballester, F.; Astrom, C.; Paldy, A.; Pascal, M.; Gasparrini, A.; et al. Synergistic Effects of Ambient Temperature and Air Pollution on Health in Europe: Results from the PHASE Project. Int. J. Environ. Res. Public. Health 2018, 15, 1856. [Google Scholar] [CrossRef] [PubMed]
- Lepeule, J.; Litonjua, A.A.; Gasparrini, A.; Koutrakis, P.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Lung function association with outdoor temperature and relative humidity and its interaction with air pollution in the elderly. Environ. Res. 2018, 165, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, R.; Kan, H. The Interaction of Ambient Temperature and Air Pollution in China. In Ambient Temperature and Health in China; Springer Nature: Singapore, 2019; pp. 105–116. [Google Scholar]
- Krug, A.; Fenner, D.; Holtmann, A.; Scherer, D. Occurrence and Coupling of Heat and Ozone Events and Their Relation to Mortality Rates in Berlin, Germany, Between 2000 and 2014. Atmosphere 2019, 10, 348. [Google Scholar] [CrossRef]
- Ryti, N.; Guo, Y.; Jaakkola, J. Global Association of Cold Spells and Adverse Health Effects: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2016, 124, 12–22. [Google Scholar] [CrossRef]
- Pozzer, A.; Anenberg, S.C.; Dey, S.; Haines, A.; Lelieveld, J.; Chowdhury, S. Mortality attributable to ambient air pollution: A review of global estimates. GeoHealth 2023, 7, e2022GH000711. [Google Scholar] [CrossRef]
- Fameli, K.-M.; Assimakopoulos, V.D. The New Open Flexible Emission Inventory for Greece and the Greater Athens Area (FEI-GREGAA): Account of Pollutant Sources and Their Importance from 2006 to 2012. Atmos. Environ. 2016, 137, 17–37. [Google Scholar] [CrossRef]
- Chaziris, A.; Yannis, G. A critical assessment of Athens Traffic Restrictions using multiple data sources. Transp. Res. Procedia 2022, 72, 375–382. [Google Scholar] [CrossRef]
- Kallos, G.; Kassomenos, P.; Pielke, R.A. Synoptic and mesoscale weather conditions during air pollution episodes in Athens, Greece. Bound. Layer. Meteorol 1993, 62, 163–184. [Google Scholar] [CrossRef]
- Kuglitsch, F.G.; Toreti, A.; Xoplaki, E.; Della-Marta, P.M.; Zerefos, C.S.; Türke¸s, M.; Luterbacher, J. Heat wave changes in the eastern Mediterranean since 1960. Geophys. Res. Lett. 2010, 37, L04802. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Pal, J.S.; Giorgi, F.; Gao, X. Heat stress intensification in the Mediterranean climate change hotspot. Geophys. Res. Lett. 2007, 34, L11706. [Google Scholar] [CrossRef]
- Ouzeau, G.; Soubeyroux, J.M.; Schneider, M.; Vautard, R.; Planton, S. Heat waves analysis over France in present and future climate: Application of a new method on the EURO-CORDEX ensemble. Clim. Serv. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Founda, D. Evolution of the air temperature in Athens and evidence of climatic change: A review. Adv. Build. Energy Res. 2011, 5, 7–41. [Google Scholar] [CrossRef]
- Founda, D.; Giannakopoulos, C. The exceptionally hot summer of 2007 in Athens, Greece—A typical summer in the future climate? Glob. Planet. Chang. 2009, 67, 227–236. [Google Scholar] [CrossRef]
- Matzarakis, A.; Nastos, P.T. Human-biometeorological assessment of heat waves in Athens. Theor. Appl. Climatol. 2011, 105, 99–106. [Google Scholar] [CrossRef]
- Nastos, P.T.; Matzarakis, A. The effect of air temperature and human thermal indices on mortality in Athens, Greece. Theor. Appl. Climatol. 2012, 108, 591–599. [Google Scholar] [CrossRef]
- Tolika, K. Assessing Heat Waves over Greece Using the Excess Heat Factor (EHF). Climate 2019, 7, 9. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Giannaros, C.; Agathangelidis, I.; Papavasileiou, G.; Galanaki, E.; Kotroni, V.; Lagouvardos, K.; Giannaros, T.M.; Cartalis, C.; Matzarakis, A. The extreme heat wave of July–August 2021 in the Athens urban area (Greece): Atmospheric and human-biometeorological analysis exploiting ultra-high resolution numerical modeling and the local climate zone framework. Sci. Total Environ. 2023, 857, 159300. [Google Scholar] [CrossRef]
- Giannaros, C.; Economou, T.; Parliari, D.; Galanaki, E.; Kotroni, V.; Lagouvardos, K.; Matzarakis, A. A thermo-physiologically consistent approach for studying the heat-health nexus with hierarchical generalized additive modelling: Application in Athens urban area (Greece). Urban Clim. 2024, 857, 159300. [Google Scholar] [CrossRef]
- Ministry of Health. Hospital Admissions and Hospital Beds. Available online: https://www.moh.gov.gr/articles/bihealth/stoixeia-noshleytikhs-kinhshs/10431-klines-noshleythentes-hmeres-noshleias-2021 (accessed on 20 December 2024).
- Lagouvardos, K.; Kotroni, V.; Bezes, A.; Koletsis, I.; Kopania, T.; Mazarakis, N.; Papagiannaki, K.; Vougioukas, S. The automatic weather stations NOANN network of the National Observatory of Athens: Operation and database. Geosci. Data J. 2017, 4, 4–16. [Google Scholar] [CrossRef]
- Steadman, R.G. A Universal Scale of Apparent Temperature. J. Clim. Appl. Meteorol. 1984, 23, 1674–1687. [Google Scholar] [CrossRef]
- Moraes, S.L.; Almendra, R.; Barrozo, L.V. Impact of heat waves and cold spells on cause-specific mortality in the city of São Paulo, Brazil. Int. J. Hyg. Environ. Health 2022, 239, 113861. [Google Scholar] [CrossRef]
- Guo, Y.; Gasparrini, A.; Armstrong, B.G.; Tawatsupa, B.; Tobias, A.; Lavigne, E.; Coelho, M.S.Z.S.; Pan, X.; Kim, H.; Hashizume, M.; et al. Heat Wave and Mortality: A Multicountry, Multicommunity Study. Environ. Health Perspect. 2017, 125, 087006. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Zhou, M.; Yin, P.; Wang, B.; Liu, J.; Chen, Z.; Song, X.; Ou, C.Q.; Liu, Q. Cold spell and mortality in 31 Chinese capital cities: Definitions, vulnerability and implications. Environ. Int. 2019, 128, 271–278. [Google Scholar] [CrossRef]
- Balmain, B.N.; Sabapathy, S.; Louis, M.; Morris, N.R. Aging and Thermoregulatory Control: The Clinical Implications of Exercising under Heat Stress in Older Individuals. Biomed Res. Int. 2018, 8306154. [Google Scholar] [CrossRef]
- Kenny, G.P.; Yardley, J.; Brown, C.; Mph, R.J.S.; Jay, O. Heat stress in older individuals and patients with common chronic diseases. CMAJ 2010, 182, 1053–1060. [Google Scholar] [CrossRef]
- Tansey, E.A.; Johnson, C.D. Recent advances in thermoregulation. Adv. Physiol. Educ. 2014, 39, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.G.; Bell, M.L. Weather-Related Mortality: How Heat, Cold, and Heat Waves Affect Mortality in the United States. Epidemiology 2009, 20, 205–213. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, Z.; Bambrick, H.; Prescott, V.; Wang, N.; Zhang, Y.; Su, H.; Tong, S.; Hu, W. Cardiorespiratory effects of heatwaves: A systematic review and meta-analysis of global epidemiological evidence. Environ. Res. J. 2019, 177, 108610. [Google Scholar] [CrossRef]
- Fouillet, A.; Rey, G.; Laurent, F.; Pavillon, G.; Bellec, S.; Guihenneuc-Jouyaux, C.; Clavel, J.; Jougla, E.; Hémon, D. Excess mortality related to the August 2003 heat wave in France. Int. Arch. Occup. Environ. Health 2006, 80, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Cao, D.; Zheng, D.; Qian, Z.; Huang, C.; Shen, H.; Liu, Y.; Liu, Q.; Sun, J.; Jiao, G.; et al. Cold spells linked with respiratory disease hospitalization, length of hospital stay, and hospital expenses: Exploring cumulative and harvesting effects. Sci. Total Environ. 2023, 863, 160726. [Google Scholar] [CrossRef]
- Ning, Z.; He, S.; Liao, X.; Ma, C.; Wu, J. Health impacts of a cold wave and its economic loss assessment in China’s high-altitude city, Xining. Arch. Public Health 2024, 82, 52. [Google Scholar] [CrossRef]
- Simon, S.D. Understanding the odds ratio and the relative risk. J. Androl. 2001, 22, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat. Med. 2014, 33, 881–899. [Google Scholar] [CrossRef]
- Gasparrini, A.; Armstrong, B.; Kenward, M.G. Distributed lag non-linear models. Stat. Med. 2010, 29, 2224–2234. [Google Scholar] [CrossRef]
- Gasparrini, A. Distributed lag linear and non-linear models in R: The package dlnm. J. Stat. Softw. 2011, 43, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Masselot, P.; Chebana, F.; Bélanger, D.; St-Hilaire, A.; Abdous, B.; Gosselin, P.; Ouarda, T.B.M.J. Aggregating the response in time series regression models, applied to weather-related cardiovascular mortality. Sci. Total Environ. 2018, 628, 217–225. [Google Scholar] [CrossRef]
- Rahman, M.M.; McConnell, R.; Schlaerth, H.; Ko, J.; Silva, S.; Lurmann, F.W.; Palinkas, L.; Johnston, J.; Hurlburt, M.; Yin, H.; et al. The Effects of Coexposure to Extremes of Heat and Particulate Air Pollution on Mortality in California: Implications for Climate Change. Am. J. Respir. Crit. Care Med. 2022, 206, 1117–1127. [Google Scholar] [CrossRef]
- Rai, M.; Stafoggia, M.; de’Donato, F.; Scortichini, M.; Zafeiratou, S.; Vazquez Fernandez, L.; Zhang, S.; Katsouyanni, K.; Samoli, E.; Rao, S.; et al. Heat-related cardiorespiratory mortality: Effect modification by air pollution across 482 cities from 24 countries. Environ. Int. 2023, 174, 107825. [Google Scholar] [CrossRef]
- Xia, Y.; Shi, C.; Li, Y.; Jiang, X.; Ruan, S.; Gao, X.; Chen, Y.; Huang, W.; Li, M.; Xue, R.; et al. Effects of ambient temperature on mortality among elderly residents of Chengdu city in Southwest China, 2016-2020: A distributed-lag non-linear time series analysis. BMC Public. Health. 2023, 23, 149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Breitner, S.; Rai, M.; Nikolaou, N.; Stafoggia, M.; De’ Donato, F.; Samoli, E.; Zafeiratou, S.; Katsouyanni, K.; Rao, S.; et al. Assessment of short-term heat effects on cardiovascular mortality and vulnerability factors using small area data in Europe. Environ. Int. 2023, 179, 108154. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, T.; Yuan, C.; Wu, H.; Gao, L.; Huang, C.; Li, Y.; Li, M.; Xie, M. The underlying mechanisms of PM2.5 and O3 synergistic pollution in East China: Photochemical and heterogeneous interactions. Sci. Total Environ. 2023, 873, 162434. [Google Scholar] [CrossRef]
- Allen, M.J.; Sheridan, S.C. Mortality risks during extreme temperature events (ETEs) using a distributed lag non-linear model. Int. J. Biometeorol. 2015, 62, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Fan, L.; Xie, L.; Ren, Y.; Li, L. Associations between air pollution, climate factors and outpatient visits for eczema in West China Hospital, Chengdu, south-western China: A time series analysis. J Eur Acad. Dermatology Venereol. 2018, 32, 486–494. [Google Scholar] [CrossRef]
- Petkova, E.P.; Gasparrini, A.; Kinney, P.L. Heat and mortality in New York city since the beginning of the 20th century. Epidemiology 2014, 25, 554–560. [Google Scholar] [CrossRef]
- Martins, L.C.; Pereira, L.A.; Lin, C.A.; Santos, U.P.; Prioli, G.; Luiz, O.C.; Saldiva, P.H.; Braga, A.L. The effects of air pollution on cardiovascular diseases: Lag structures. Rev. Saude Publica 2006, 40, 677–683. [Google Scholar] [CrossRef]
- Pothirat, C.; Chaiwong, W.; Liwsrisakun, C.; Bumroongkit, C.; Deesomchok, A.; Theerakittikul, T.; Limsukon, A.; Tajarernmuang, P.; Phetsuk, N. Acute effects of air pollutants on daily mortality and hospitalizations due to cardiovascular and respiratory diseases. J. Thorac. Dis. 2019, 11, 3070–3083. [Google Scholar] [CrossRef]
- Yang, C.; Lei, L.; Li, Y.; Huang, C.; Chen, K.; Bao, J. Bidirectional modification effects on nonlinear associations of summer temperature and air pollution with first-ever stroke morbidity. Ecotoxicol. Environ. Saf. 2024, 272, 116034. [Google Scholar] [CrossRef]
- Yang, L.; Yang, J.; Liu, M.; Sun, X.; Li, T.; Guo, Y.; Hu, K.; Bell, M.L.; Cheng, Q.; Kan, H.; et al. Nonlinear effect of air pollution on adult pneumonia hospital visits in the coastal city of Qingdao, China: A time-series analysis. Environ. Res. 2022, 209, 112754. [Google Scholar] [CrossRef]
- Pan, K.; Lin, F.; Huang, K.; Zeng, S.; Guo, M.; Cao, J.; Dong, H.; Wei, J.; Xi, Q. The effect of short-term exposure to air pollution on the admission of ischemic stroke and its interaction with meteorological factors. Public. Health 2025, 239, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Gosman, R.E.; Sicard, R.M.; Cohen, S.M.; Frank-Ito, D.O. A computational analysis on the impact of multilevel laryngotracheal stenosis on airflow and drug particle dynamics in the upper airway. Exp. Comput. Multiph. Flow. 2023, 5, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Xu, J.; Ning, L.; Feng, T.; Cao, P.; Gao, S.; Shang, P.; Yu, X. Ambient air pollution, temperature and hospital admissions due to respiratory diseases in a cold, industrial city. J. Glob. Health 2022, 12, 04085. [Google Scholar] [CrossRef]
- euPOLIS Project. City of Piraeus. Available online: https://eupolis-project.eu/project/city-of-piraeus/ (accessed on 15 January 2025).
- Wang, Z.; Peng, J.; Liu, P.; Duan, Y.; Huang, S.; Wen, Y.; Liao, Y.; Li, H.; Yan, S.; Cheng, J.; et al. Association between short-term exposure to air pollution and ischemic stroke onset: A time-stratified case-crossover analysis using a distributed lag nonlinear model in Shenzhen, China. Environ. Health A Glob. Access Sci. Source 2020, 19, 1. [Google Scholar] [CrossRef]
- Wu, Z.; Miao, C.; Li, H.; Wu, S.; Gao, H.; Liu, W.; Li, W.; Xu, L.; Liu, G.; Zhu, Y. The lag-effects of meteorological factors and air pollutants on child respiratory diseases in Fuzhou, China. J. Glob. Health 2022, 12, 11010. [Google Scholar] [CrossRef] [PubMed]
- Kassomenos, P.; Papaloukas, C.; Petrakis, M.; Karakitsios, S. Assessment and prediction of short term hospital admissions: The case of Athens, Greece. Atmos. Environ. 2008, 42, 7078–7086. [Google Scholar] [CrossRef]
- Parliari, D.; Cheristanidis, S.; Giannaros, C.; Keppas, S.C.; Papadogiannaki, S.; de’Donato, F.; Sarras, C.; Melas, D. Short-Term Effects of Apparent Temperature on Cause-Specific Mortality in the Urban Area of Thessaloniki, Greece. Atmosphere 2022, 13, 852. [Google Scholar] [CrossRef]
- Bao, J.; Wang, Z.; Yu, C.; Li, X. The influence of temperature on mortality and its Lag effect: A study in four Chinese cities with different latitudes. BMC Public Health 2016, 16, 375. [Google Scholar] [CrossRef]
- Wang, Y.; Achilleos, S.; Salameh, P.; Kouis, P.; Yiallouros, P.; Critselis, E.; Nicolaides, K.; Tymvios, F.; Savvides, C.; Vasiliadou, E.; et al. Temperature and hospital admissions in the Eastern Mediterranean: A case study in Cyprus. Environ. Res. Health 2004, 2, 025004. [Google Scholar] [CrossRef]
- Huang, W.H.; Chen, B.Y.; Kim, H.; Honda, Y.; Guo, Y.L. Significant Effects of Exposure to Relatively Low Level Ozone on Daily Mortality in 17 cities from three Eastern Asian Countries. Environ. Res. 2018, 168, 80–84. [Google Scholar] [CrossRef]
- Bell, M.L.; McDermott, A.; Zeger, S.L.; Samet, J.M.; Dominici, F. Ozone and short-term mortality in 95 USA urban communities, 1987–2000. J. Am. Med. Assoc. 2004, 292, 2372–2378. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.B.; Huang, W.; Huang, X.L.; Zhong, L.J.; Lu, S.E.; Li, Y.; Dai, L.Z.; Zhang, Y.H.; Zhul, T. Estimated acute effects of ambient ozone and nitrogen dioxide on mortality in the Pearl River Delta of Southern China. Environ. Health Perspect. 2012, 120, 393–398. [Google Scholar] [CrossRef]
- Kovats, R.S.; Edwards, S.J.; Charron, D.; Cowden, J.; D’Souza, R.M.; Ebi, K.L.; Gauci, C.; Gerner-Smidt, P.; Hajat, S.; Hales, S.; et al. Climate variability and campylobacter infection: An international study. Int. J. Biometeorol. 2005, 49, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, G.; Hajat, S.; Fadda, E.; Buja, A.; Fedeli, U.; Spolaore, P. Contrasting patterns of hospital admissions and mortality during heat waves: Are deaths from circulatory disease a real excess or an artifact? Med. Hypotheses 2006, 66, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Samet, J.M.; Patz, J.A. Hospital admissions for heart disease: The effects of temperature and humidity. Epidemiology 2004, 15, 755–761. [Google Scholar] [CrossRef]
- Saha, M.V.; Davis, R.E.; Hondula, D. Mortality displacement as a function of heat event strength in 7 USA cities. Am J Epidemiol. 2014, 179, 467–474. [Google Scholar] [CrossRef]
- Iñiguez, C.; Royé, D.; Tobías, A. Contrasting patterns of temperature related mortality and hospitalization by cardiovascular and respiratory diseases in 52 Spanish cities. Environ. Res. 2021, 192, 110191. [Google Scholar] [CrossRef]
- Hanzlíková, H.; Plavcová, E.; Kynčl, J.; Kříž, B.; Kyselý, J. Contrasting patterns of hot spell effects on morbidity and mortality for cardiovascular diseases in the Czech Republic, 1994–2009. Int. J. Biometeorol. 2015, 59, 1673–1684. [Google Scholar] [CrossRef]
- D’Ippoliti, D.; Michelozzi, P.; Marino, C.; de’Donato, F.; Menne, B.; Katsouyanni, K.; Kirchmayer, U.; Analitis, A.; Medina-Ramon, M.; Paldy, A.; et al. The impact of heat waves on mortality in 9 European cities: Results from the EuroHEAT project. Environ. Health 2010, 9, 37. [Google Scholar] [CrossRef]
- Achebak, H.; Devolder, D.; Ballester, J. Trends in temperature-related age-specific and sex-specific mortality from cardiovascular diseases in Spain: A national time-series analysis. Lancet Planet. Health 2019, 3, e297–e306. [Google Scholar] [CrossRef]
- Buya, S.; Lim, A.; Saelim, R.; Musikasuwan, S.; Choosong, T.; Taneepanichskul, N. Impact of air pollution on cardiorespiratory diseases in Southern Thailand. Clin. Epidemiol. Glob. Health 2024, 25, 101501. [Google Scholar] [CrossRef]
- Fong, K.C.; Heo, S.; Lim, C.C.; Kim, H.; Chan, A.; Lee, W.; Stewart, R.; Choi, H.M.; Son, J.Y.; Bell, M.L. The Intersection of Immigrant and Environmental Health: A Scoping Review of Observational Population Exposure and Epidemiologic Studies. Environ. Health Perspect. 2022, 130, 96001. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, E.; Gasparrini, A.; Wang, X.; Wang, X.; Chen, H.; Yagouti, A.; Fleury, D.M.; Cakmak, S. Extreme ambient temperatures and cardiorespiratory emergency room visits: Assessing risk by comorbid health conditions in a time series study. Environ. Health 2014, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J. Is there harvesting in the association of airborne particles with daily deaths and hospital admissions? Epidemiology 2001, 12, 55–61. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, H.; Wu, Y.; Si, Y.; Li, M.; Wu, Y.; Wang, X.; Wang, M.; Chen, L.; Wei, C.; et al. Ambient particulate matter pollution and adult hospital admissions for pneumonia in urban China: A national time series analysis for 2014 through 2017. PLoS Med. 2019, 16, e1003010. [Google Scholar] [CrossRef]
- Bayart, N.E.; Pereira, G.; Reid, C.M.; Nyadanu, S.D.; Badamdorj, O.; Lkhagvasuren, B.; Rumchev, K. Effects of outdoor air pollution on hospital admissions from cardiovascular diseases in the capital city of Mongolia. Atmos. Pollut. Res. 2025, 16, 102338. [Google Scholar] [CrossRef]
- Cao, H.; Xu, R.; Lu, X.; Jiang, W.; Wang, L.; Yu, M.; Wang, W.; Yuan, J. Air pollution, temperature and mumps: A time-series study of independent and interaction effects. Ecotoxicol. Environ. Saf. 2025, 291, 117826. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, L.; Zhang, S.; Ai, L.; Lv, H.; Zhu, C.; Wu, J.; Tan, W. Effects and interaction of air pollution and meteorological factors on pertussis incidence in PR China. Hyg. Environ. Health Adv. 2022, 4, 100036. [Google Scholar] [CrossRef]




| DEATL | DEAT | Percentile | Temperature (°C) | Vapor Pressure (hPa) | Wind Velocity (m/s) |
|---|---|---|---|---|---|
| 9 | Over 37.24 | Over 95th | 31.6–44.4 | 8.1–31.2 | 0.0–4.5 |
| 8 | 35.78 to 37.24 | 90th–95th | 30.1–40.1 | 9.6–30.6 | 0.0–5.4 |
| 7 | 34.54 to 35.78 | 85th–90th | 28.7–39.2 | 7.5–31.9 | 0.0–5.8 |
| 6 | 33.17 to 34.54 | 80th–85th | 27.8–38.2 | 7.2–31.1 | 0.0–6.3 |
| 5 | 31.74 to 33.17 | 75th–80th | 26.6–37.9 | 9.1–29.9 | 0.0–7.6 |
| 4 | 29.62 to 31.74 | 70th–75th | 24.8–36.3 | 6.0–29.7 | 0.0–8.9 |
| 3 | 27.89 to 29.62 | 65th–70th | 23.2–36.0 | 4.8–27.7 | 0.0–9.8 |
| 2 | 24.73 to 27.89 | 60th–65th | 21.1–35.8 | 4.9–26.8 | 0.0–13.0 |
| 1 | 21.31 to 24.73 | 55th–60th | 18.6–31.8 | 5.4–24.1 | 0.0–16.1 |
| 0 | 14.50 to 21.31 | 50th–55th | 13.6–30.9 | 4.5–22.9 | 0.0–17.0 |
| 0 | 11.44 to 14.50 | 45th–50th | 11.2–24.3 | 3.6–17.8 | 0.0–13.4 |
| −1 | 4.51 to 11.44 | 40th–45th | 5.7–18.8 | 3.1–15.9 | 0.0–13.4 |
| −2 | 2.33 to 4.51 | 35th–40th | 3.8–14.6 | 3.1–11.5 | 0.0–13.9 |
| −3 | 1.74 to 2.33 | 30th–35th | 3.3–11.6 | 2.9–9.5 | 0.0–11.6 |
| −4 | 1.51 to 1.74 | 25th–30th | 3.1–10.5 | 3.4–9.1 | 0.0–9.4 |
| −5 | 1.32 to 1.51 | 20th–25th | 3.0–9.7 | 3.2–8.9 | 0.0–9.8 |
| −6 | 1.17 to 1.32 | 15th–20th | 2.9–9.8 | 3.2–9.8 | 0.0–10.3 |
| −7 | −0.27 to 1.17 | 10th–15th | 1.6–12.4 | 2.5–10.4 | 0.0–16.1 |
| −8 | −2.45 to −0.27 | 5th–10th | −0.3–9.4 | 3.0–8.2 | 0.0–12.1 |
| −9 | Up to −2.45 | Up to 5th | −6.5–6.9 | 0.0–7.9 | 0.0–13.9 |
| Parameters | Negative Binomial | Poisson | GAM |
|---|---|---|---|
| AIC | 15,007.05 | 20,535.5 | 20,697.62 |
| BIC | 15,414.5 | 20,937.44 | 20,991.84 |
| Dispersion parameter | 1.123719 | 6.229468 | 6.22823 |
| R | 0.6051255 | 0.6087412 | 0.6078343 |
| RMSE | 14.39894 | 14.3544 | 13.93135 |
| MAE | 11.71858 | 11.69806 | 11.45116 |
| Parameters | Classical DLNM | DLNM + AE Kernel |
|---|---|---|
| AIC | 15,007.05 | 12,152.64 |
| BIC | 15,414.5 | 12,559.68 |
| Dispersion parameter | 1.123719 | 1.094679 |
| R | 0.6051255 | 0.7931329 |
| RMSE | 14.39894 | 6.6336854 |
| MAE | 11.71858 | 5.3533178 |
| MB | −0.00888 | −0.00010 |
| MFB | 10.30241 | 2.086531 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kladakis, A.; Retalis, A.; Giannaros, C.; Vafeiadis, V.; Fameli, K.-M.; Assimakopoulos, V.D.; Moustris, K.; Nastos, P.T. Evaluating the Health Risks of Air Quality and Human Thermal Comfort–Discomfort in Relation to Hospital Admissions in the Greater Athens Area, Greece. Sustainability 2025, 17, 5182. https://doi.org/10.3390/su17115182
Kladakis A, Retalis A, Giannaros C, Vafeiadis V, Fameli K-M, Assimakopoulos VD, Moustris K, Nastos PT. Evaluating the Health Risks of Air Quality and Human Thermal Comfort–Discomfort in Relation to Hospital Admissions in the Greater Athens Area, Greece. Sustainability. 2025; 17(11):5182. https://doi.org/10.3390/su17115182
Chicago/Turabian StyleKladakis, Aggelos, Adrianos Retalis, Christos Giannaros, Vasileios Vafeiadis, Kyriaki-Maria Fameli, Vasiliki D. Assimakopoulos, Konstantinos Moustris, and Panagiotis T. Nastos. 2025. "Evaluating the Health Risks of Air Quality and Human Thermal Comfort–Discomfort in Relation to Hospital Admissions in the Greater Athens Area, Greece" Sustainability 17, no. 11: 5182. https://doi.org/10.3390/su17115182
APA StyleKladakis, A., Retalis, A., Giannaros, C., Vafeiadis, V., Fameli, K.-M., Assimakopoulos, V. D., Moustris, K., & Nastos, P. T. (2025). Evaluating the Health Risks of Air Quality and Human Thermal Comfort–Discomfort in Relation to Hospital Admissions in the Greater Athens Area, Greece. Sustainability, 17(11), 5182. https://doi.org/10.3390/su17115182










