Research on the Optimization of Key Parameters for Heterotrophic Bacteria Assimilation Nitrogen Removal Technology in Aquaculture Tailwater
Abstract
1. Introduction
2. Materials and Methods
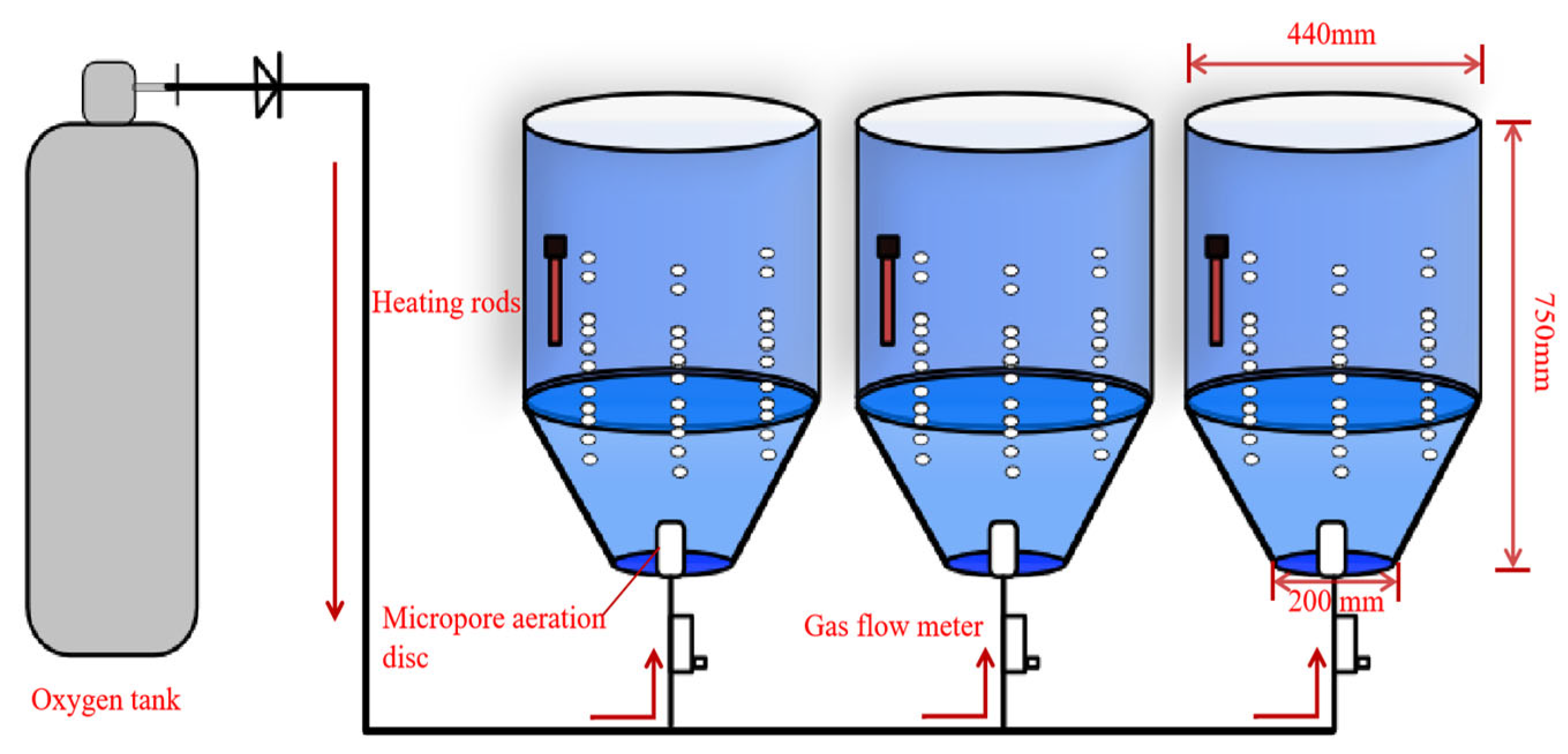
2.1. Experimental Setup
2.2. Experimental Design
2.2.1. Selection of Optimal Carbon Source
2.2.2. Optimization Using Response Surface Methodology
2.3. Analytical Methods
2.3.1. Water Quality Analysis Methods
2.3.2. Microbial Analysis Methods
2.3.3. Data Analysis Methods
3. Results
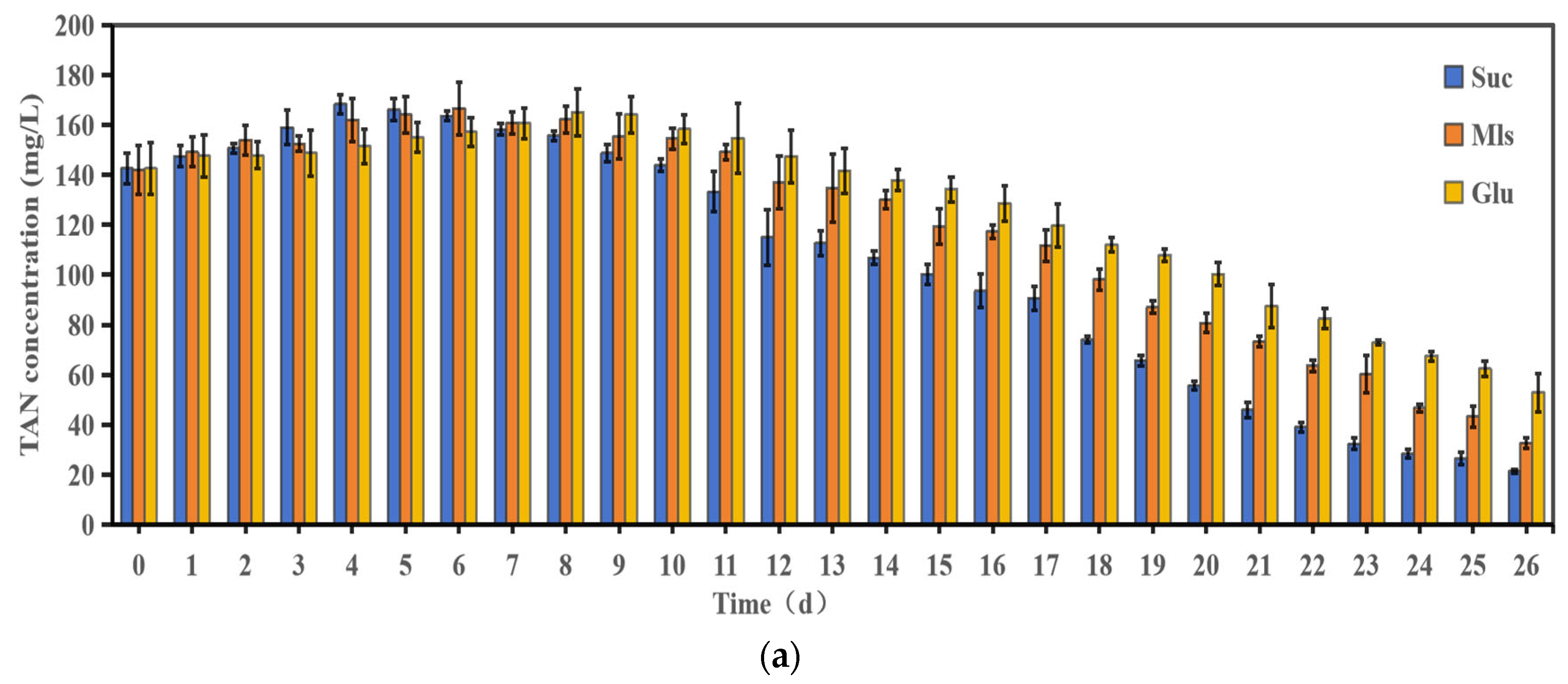
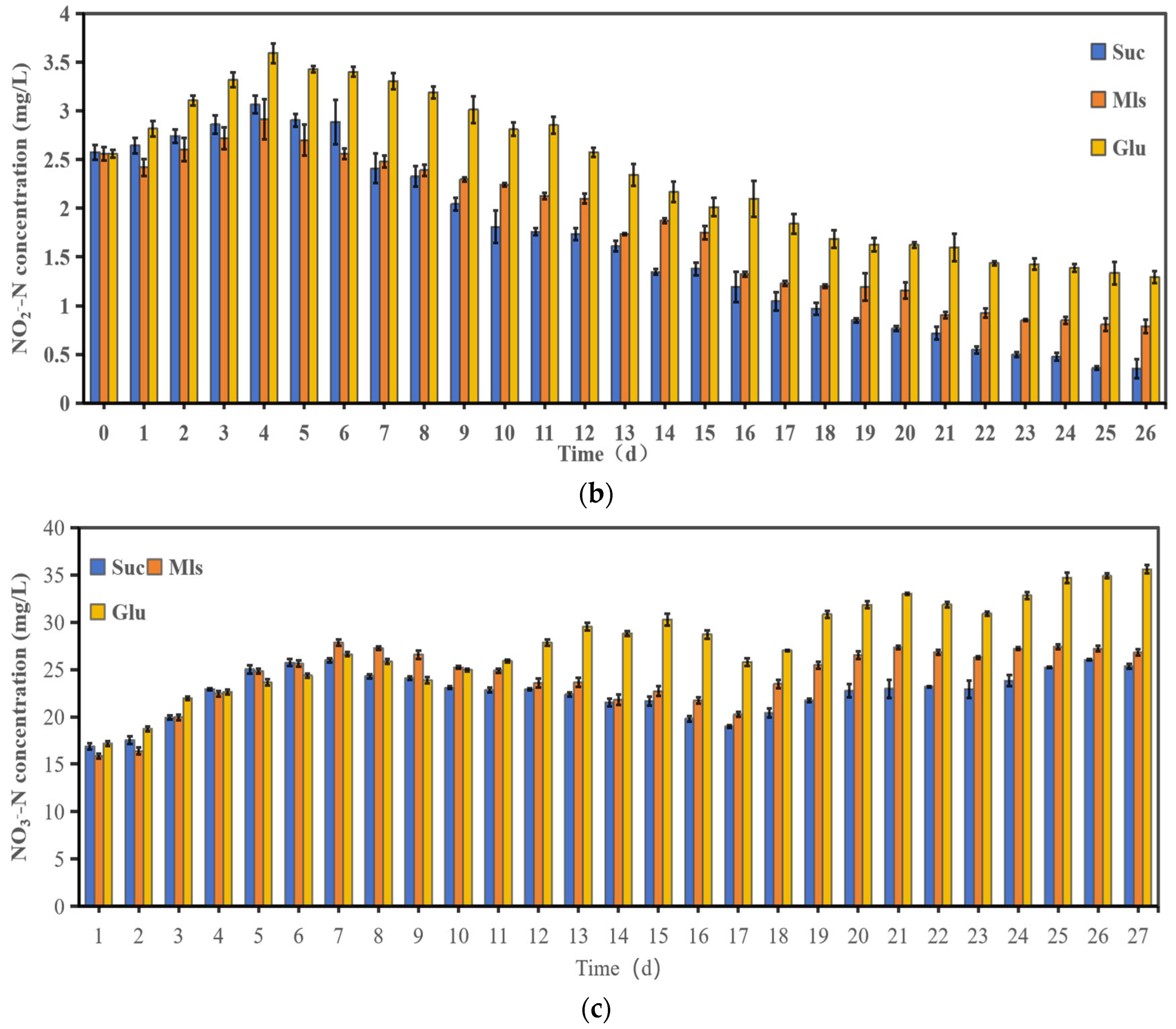
3.1. Water Quality Changes
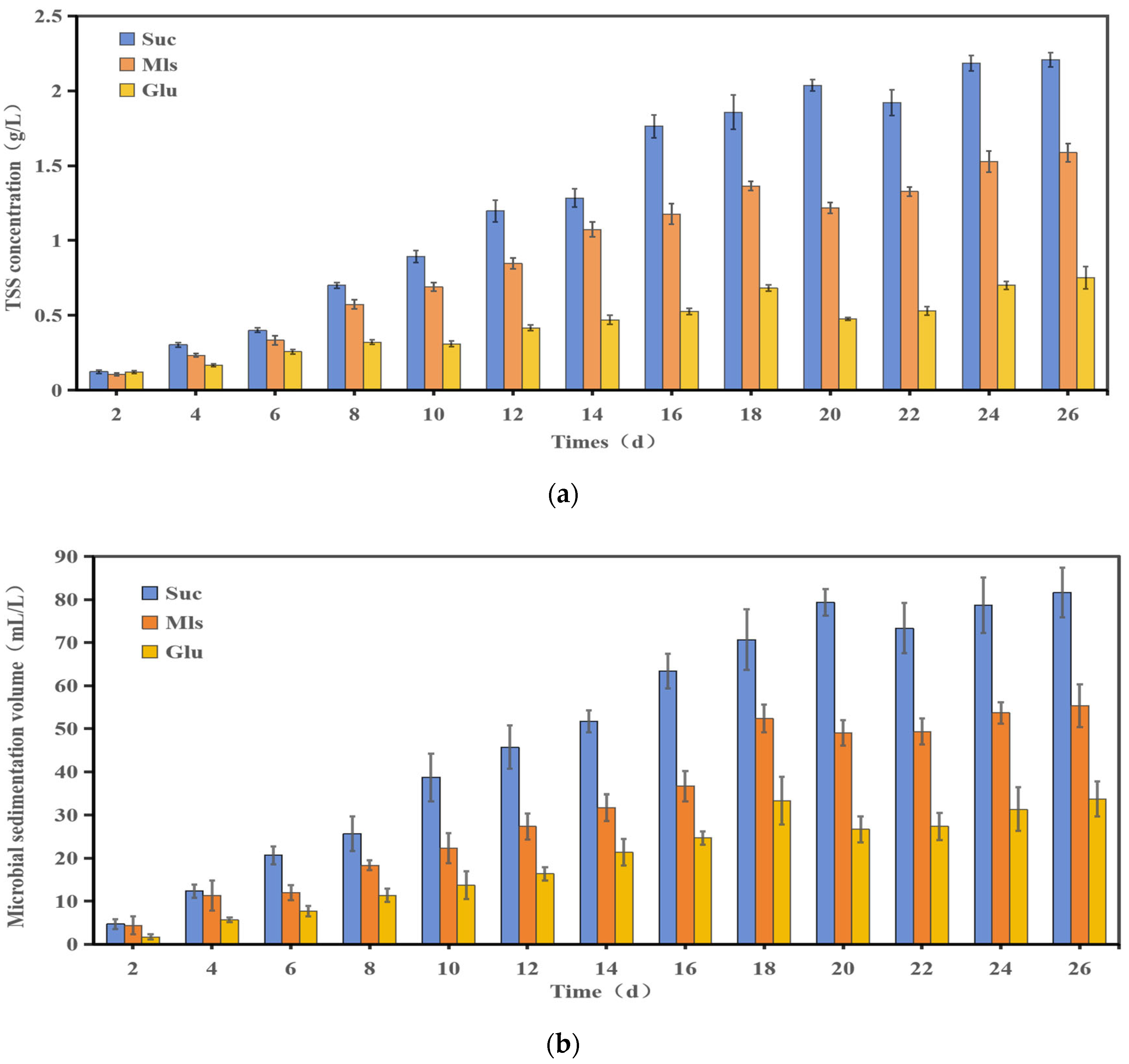
3.2. Microbial Production and Characteristics
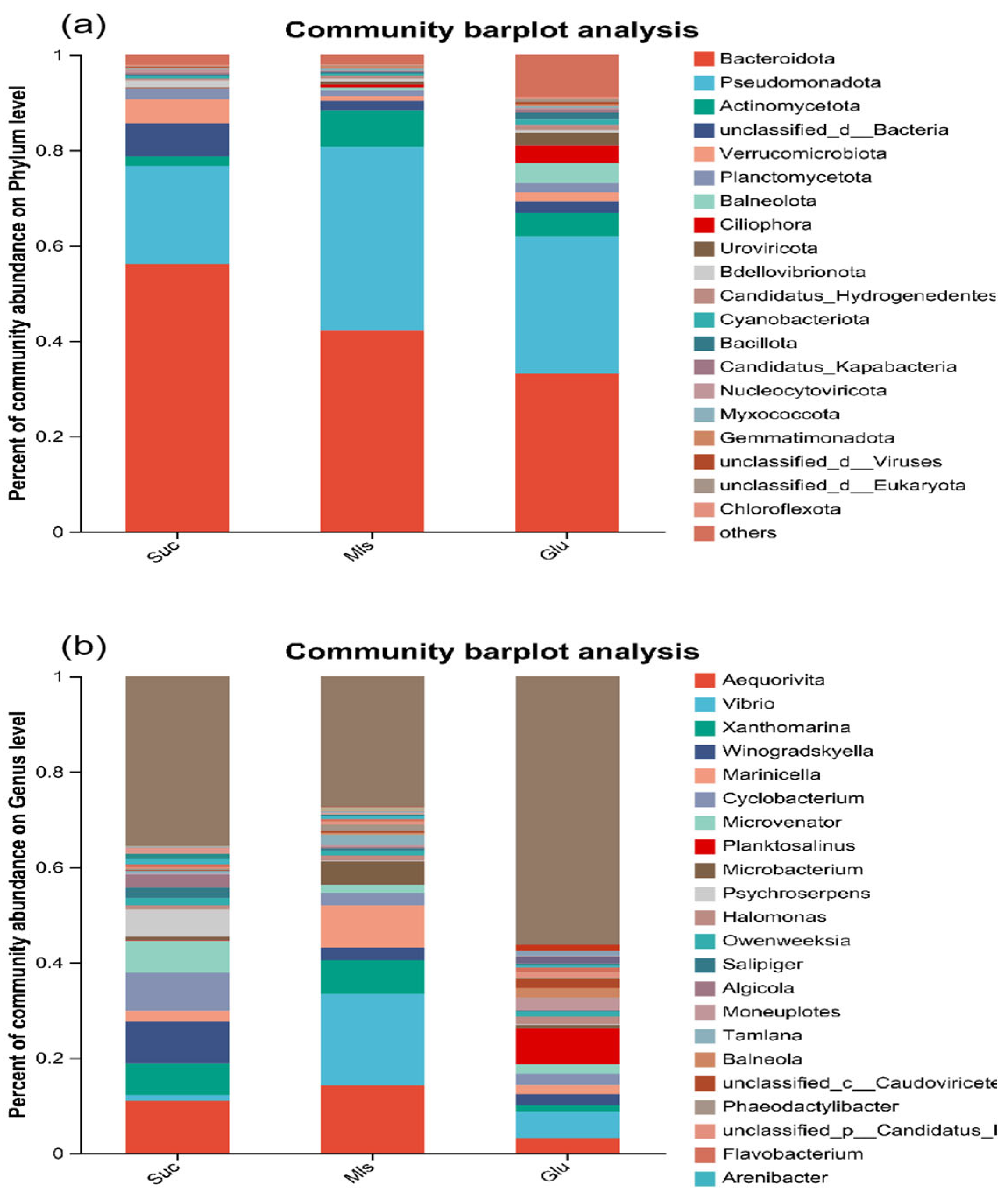
3.3. Microbial Community Structure and Abundance
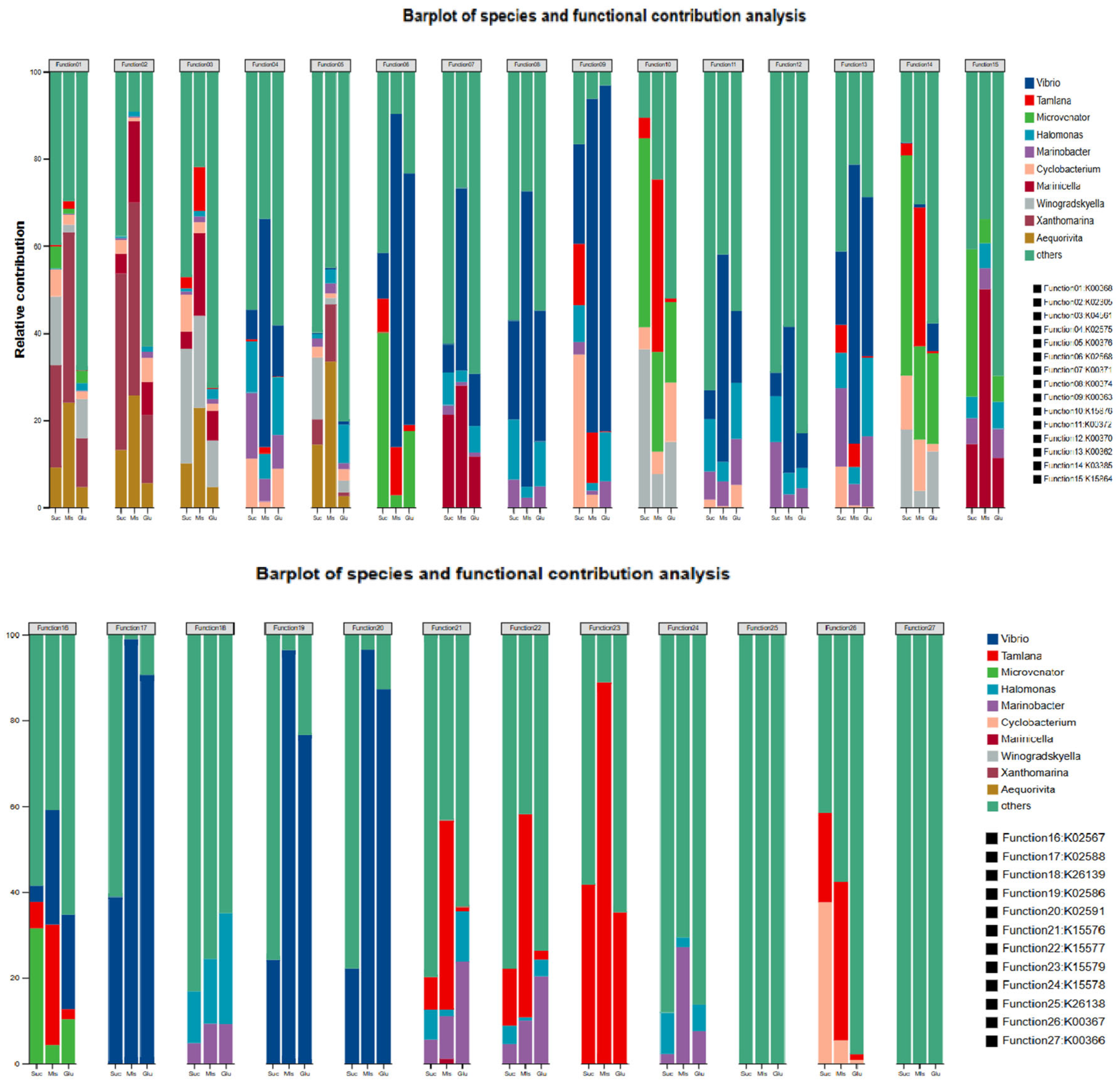
3.4. Metagenomic Metabolic Analysis
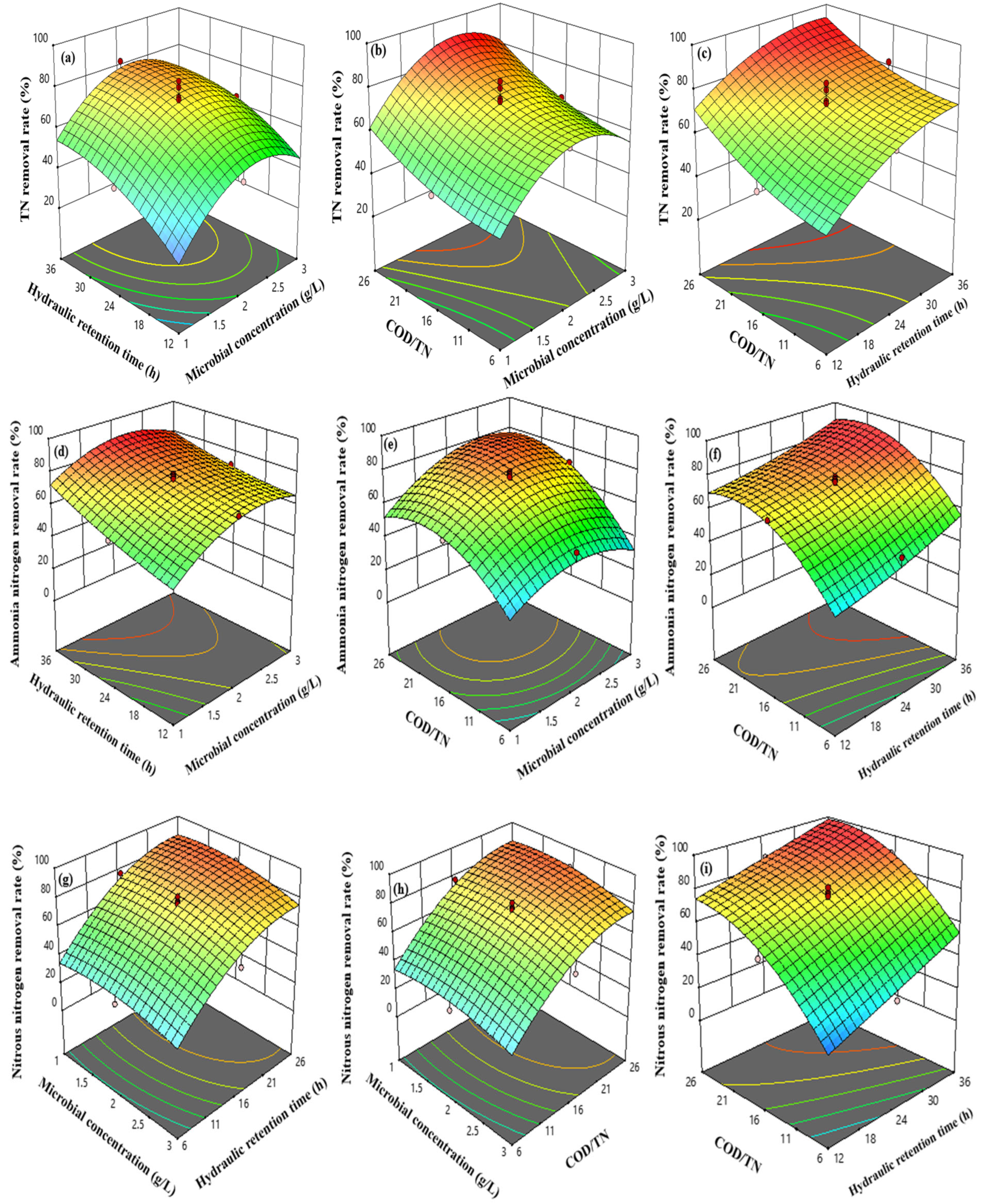
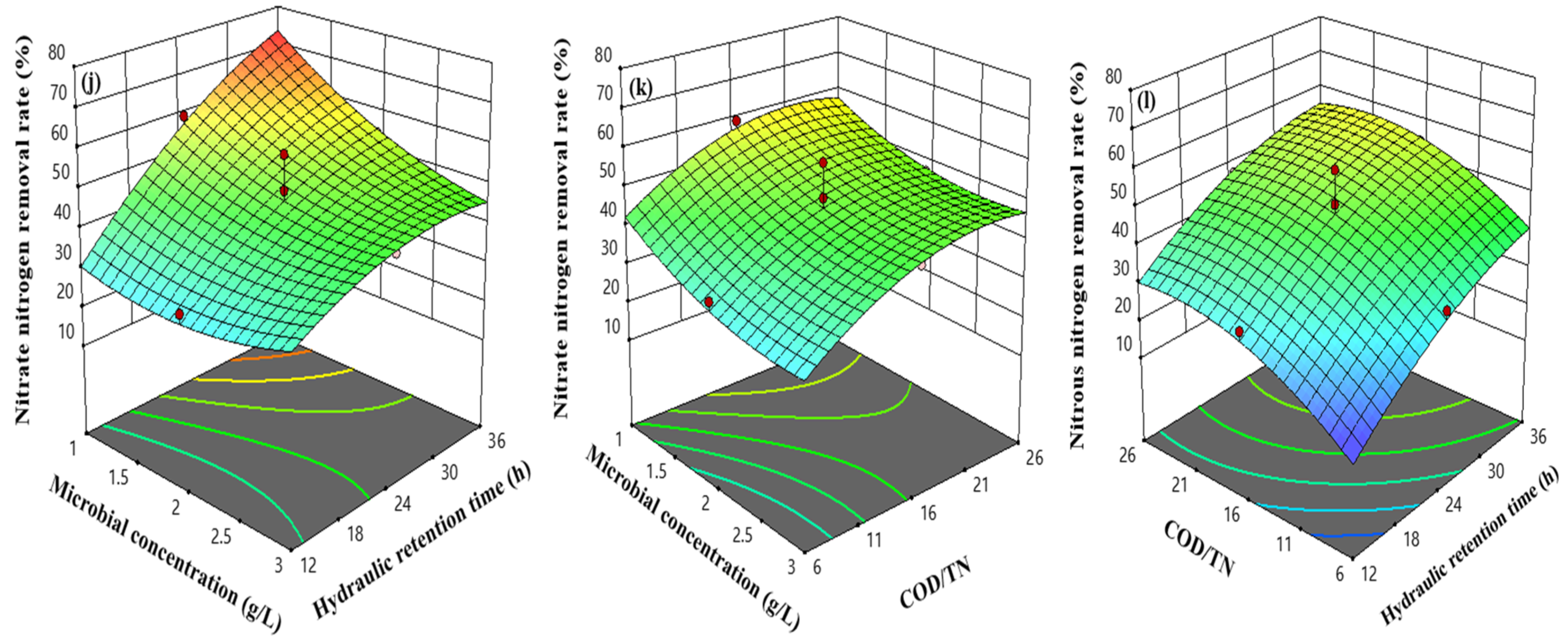
3.5. Response Surface Model Related Data
3.6. Response Surface Interaction Analysis
4. Discussion
4.1. Effects of Different Carbon Sources on Water Quality Indicators of Microbial Culture Systems
4.2. Effect of Carbon Source on the Composition and Distribution of Microbial Communities
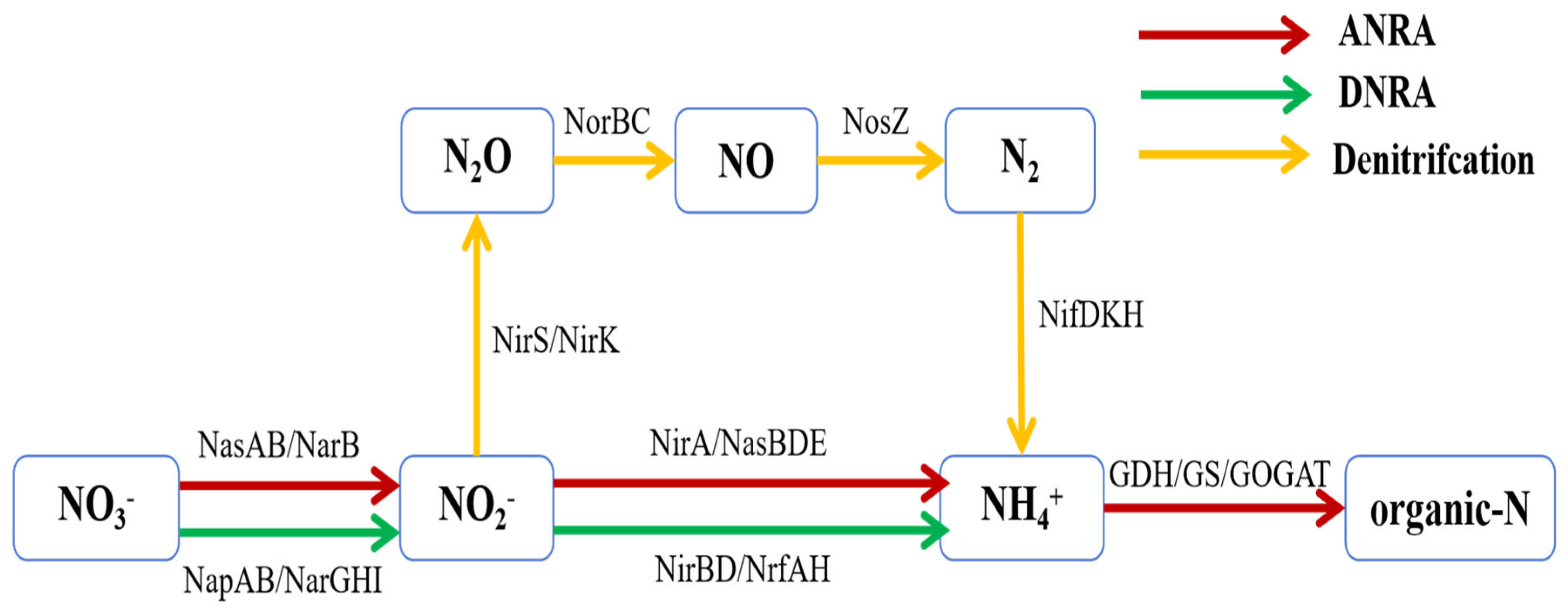
4.3. Effect of Heterotrophic Bacteria Assimilation Reactor Operating Parameters on Nitrogen-Containing Pollutant Removal
4.4. Resource Utilization of Bacterial Clusters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Audelo-Naranjo, J.M.; Martínez-Córdova, L.R.; Voltolina, D. Nitrogen budget in intensive cultures of Litopenaeus vannamei in mesocosms, with zero water exchange and artificial substrates. Rev. Biol. Mar. Oceanogr. 2010, 45, 519–524. [Google Scholar] [CrossRef]
- Chen, Z.; Ge, H.; Chang, Z.; Song, X.; Zhao, F.; Li, J. Nitrogen budget in recirculating aquaculture and water exchange systems for culturing Litopenaeus vannamei. J. Ocean. Univ. China 2018, 17, 905–912. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, T.; Chen, F.; Zhou, L.; Du, Y.; Sun, J. Nitrogen migration law and recycling strategy in an innovative recirculating aquaculture system: Enhancing performance through electrocoagulation. J. Water Process Eng. 2022, 50, 103275. [Google Scholar] [CrossRef]
- Van Rijn, J. Waste treatment in recirculating aquaculture systems. Aquac. Eng. 2013, 53, 49–56. [Google Scholar] [CrossRef]
- Khudyia, O.; Marchenkob, M.; Chebanc, L.; Khudad, L.; Kushniryke, O.; Malishchuk, I. Recirculating aquaculture systems waste water as a medium for increase of phytoplankton and zooplankton biomass. Int. Lett. Nat. Sci. 2016, 54, 1–7. [Google Scholar] [CrossRef]
- Wu, Y.; Song, K. Source, treatment, and disposal of aquaculture solid waste: A review. J. Environ. Eng. 2021, 147, 03120012. [Google Scholar] [CrossRef]
- Smol, M. Circular economy in wastewater treatment plant—Water, energy and raw materials recovery. Energies 2023, 16, 3911. [Google Scholar] [CrossRef]
- Areniello, M.; Matassa, S.; Esposito, G.; Lens, P.N. Biowaste upcycling into second-generation microbial protein through mixed-culture fermentation. Trends Biotechnol. 2023, 41, 197–213. [Google Scholar] [CrossRef]
- Rahimi, S.; Modin, O.; Mijakovic, I. Technologies for biological removal and recovery of nitrogen from wastewater. Biotechnol. Adv. 2020, 43, 107570. [Google Scholar] [CrossRef]
- Gouzy, A.; Poquet, Y.; Neyrolles, O. Nitrogen metabolism in Mycobacterium tuberculosis physiology and virulence. Nat. Rev. Microbiol. 2014, 12, 729–737. [Google Scholar] [CrossRef]
- Kumar, R.; Shimizu, K. Transcriptional regulation of main metabolic pathways of cyoA, cydB, fnr, and fur gene knockout Escherichia coli in C-limited and N-limited aerobic continuous cultures. Microb. Cell Factories 2011, 10, 3. [Google Scholar] [CrossRef]
- Di Capua, F.; Pirozzi, F.; Lens, P.N.; Esposito, G. Electron donors for autotrophic denitrification. Chem. Eng. J. 2019, 362, 922–937. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Wang, H.; Xue, G.; Zhou, M.; Ran, X.; Wang, Y. Sulfur autotrophic denitrification as an efficient nitrogen removals method for wastewater treatment towards lower organic requirement: A review. Water Res. 2023, 245, 120569. [Google Scholar] [CrossRef]
- Francis, C.A.; Beman, J.M.; Kuypers, M.M. New processes and players in the nitrogen cycle: The microbial ecology of anaerobic and archaeal ammonia oxidation. ISME J. 2007, 1, 19–27. [Google Scholar] [CrossRef]
- Winkler, M.K.; Straka, L. New directions in biological nitrogen removal and recovery from wastewater. Curr. Opin. Biotechnol. 2019, 57, 50–55. [Google Scholar] [CrossRef]
- Panigrahi, A.; Sundaram, M.; Chakrapani, S.; Rajasekar, S.; Syama Dayal, J.; Chavali, G. Effect of carbon and nitrogen ratio (C:N) manipulation on the production performance and immunity of Pacific white shrimpLitopenaeus vannamei(Boone, 1931) in a biofloc-based rearing system. Aquac. Res. 2018, 50, 29–41. [Google Scholar] [CrossRef]
- Ray, S.; Scholz, M.; Haritash, A. Kinetics of carbon and nitrogen assimilation by heterotrophic microorganisms during wastewater treatment. Environ. Monit. Assess. 2019, 191, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Jiao, T.; Zhang, W.; Guo, Y.; Han, F.; Lei, J.; Zhou, W. Carbon sources influence on heterotrophic ammonia assimilation: Performance and mechanism. Chem. Eng. J. 2024, 497, 154545. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, W.; Jiang, J.; Li, Y.; Tong, L.; Yang, G. A Continuous Plug-Flow Anaerobic-Multistage Anoxic/Aerobic Process Treating Low-C/N Domestic Sewage: Nutrient Removal, Greenhouse Gas Emissions, and Microbial Community Analysis. Sustainability 2024, 16, 3993. [Google Scholar] [CrossRef]
- Fugimura, M.M.S.; dos Reis Flor, H.; de Melo, E.P.; da Costa, T.V.; Wasielesky, W.; Oshiro, L.M.Y. Brewery residues as a source of organic carbon in Litopenaeus schmitti white shrimp farms with BFT systems. Aquac. Int. 2015, 23, 509–522. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sajjadi, M.M.; Alizadeh, M.; Sourinejad, I. Nursery performance of Pacific white shrimp (Litopenaeus vannamei Boone, 1931) cultivated in a biofloc system: The effect of adding different carbon sources. Aquac. Res. 2017, 48, 1491–1501. [Google Scholar] [CrossRef]
- Verma, A.; Rani, A.B.; Rathore, G.; Saharan, N.; Gora, A.H. Growth, non-specific immunity and disease resistance of Labeo rohita against Aeromonas hydrophila in biofloc systems using different carbon sources. Aquaculture 2016, 457, 61–67. [Google Scholar] [CrossRef]
- Abakari, G.; Luo, G.; Kombat, E.O.; Alhassan, E.H. Supplemental carbon sources applied in biofloc technology aquaculture systems: Types, effects and future research. Rev. Aquac. 2020, 13, 1193–1222. [Google Scholar] [CrossRef]
- Emerenciano, M.; Gaxiola, G.; Cuzon, G. Biofloc Technology (BFT): A Review for Aquaculture Application and Animal Food Industry; IntechOpen: London, UK, 2013. [Google Scholar]
- Zhang, N.; Luo, G.; Tan, H.; Liu, W.; Hou, Z. Growth, digestive enzyme activity and welfare of tilapia (Oreochromis niloticus) reared in a biofloc-based system with poly-β-hydroxybutyric as a carbon source. Aquaculture 2016, 464, 710–717. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 2019. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Bhatt, P.; Huang, J.-Y.; Brown, P.; Shivaram, K.B.; Yakamercan, E.; Simsek, H. Electrochemical treatment of aquaculture wastewater effluent and optimization of the parameters using response surface methodology. Environ. Pollut. 2023, 331, 121864. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, Y.; Su, G.; Wang, H.; Zhang, J.; Tian, H.; Zhou, L.; Qiu, T.; Sun, J. Application of a U-Tube Oxygenator in a Litopenaeus vannamei Recirculating Aquaculture System: Efficiency and Management Models. Water 2023, 15, 4019. [Google Scholar] [CrossRef]
- Mirzoyan, N.; Parnes, S.; Singer, A.; Tal, Y.; Sowers, K.; Gross, A. Quality of brackish aquaculture sludge and its suitability for anaerobic digestion and methane production in an upflow anaerobic sludge blanket (UASB) reactor. Aquaculture 2008, 279, 35–41. [Google Scholar] [CrossRef]
- Lu, J.; Tan, Y.; Tian, S.; Qin, Y.; Zhou, M.; Hu, H.; Zhao, X.; Wang, Z.; Hu, B. Effect of carbon source on carbon and nitrogen metabolism of common heterotrophic nitrification-aerobic denitrification pathway. Chemosphere 2024, 361, 142525. [Google Scholar] [CrossRef]
- Fu, W.; Song, G.; Wang, Y.; Wang, Q.; Duan, P.; Liu, C.; Zhang, X.; Rao, Z. Advances in research into and applications of heterotrophic nitrifying and aerobic denitrifying microorganisms. Front. Environ. Sci. 2022, 10, 887093. [Google Scholar] [CrossRef]
- Ma, S.; Huang, S.; Tian, Y.; Lu, X. Heterotrophic ammonium assimilation: An important driving force for aerobic denitrification of Rhodococcus erythropolis strain Y10. Chemosphere 2022, 291, 132910. [Google Scholar] [CrossRef] [PubMed]
- Martikainen, P.J. Heterotrophic nitrification–An eternal mystery in the nitrogen cycle. Soil Biol. Biochem. 2022, 168, 108611. [Google Scholar] [CrossRef]
- Yao, J.; Li, W.; Ou, D.; Lei, L.; Asif, M.; Liu, Y. Performance and granular characteristics of salt-tolerant aerobic granular reactors response to multiple hypersaline wastewater. Chemosphere 2021, 265, 129170. [Google Scholar] [CrossRef] [PubMed]
- Kurilenko, V.V.; Romanenko, L.A.; Isaeva, M.P.; Svetashev, V.I.; Mikhailov, V.V. Winogradskyella algae sp. nov., a marine bacterium isolated from the brown alga. Antonie Leeuwenhoek 2019, 112, 731–739. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Cai, S.; Lin, L.; Huang, G.; Hu, Z.; Jin, W.; Zheng, Y. Effects of light intensity and salinity on formation and performance of microalgal-bacterial granular sludge. Bioresour. Technol. 2023, 386, 129534. [Google Scholar] [CrossRef]
- Avnimelech, Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- Zhang, K.; Pan, L.; Chen, W.; Wang, C. Effect of using sodium bicarbonate to adjust the pH to different levels on water quality, the growth and the immune response of shrimp Litopenaeus vannamei reared in zero-water exchange biofloc-based culture tanks. Aquac. Res. 2017, 48, 1194–1208. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Forster, I.; Conquest, L.; Dominy, W.; Kuo, W.C.; David Horgen, F. Determination of microbial community structures of shrimp floc cultures by biomarkers and analysis of floc amino acid profiles. Aquac. Res. 2008, 39, 118–133. [Google Scholar] [CrossRef]
- Ahmad, I.; Babitha Rani, A.; Verma, A.; Maqsood, M. Biofloc technology: An emerging avenue in aquatic animal healthcare and nutrition. Aquac. Int. 2017, 25, 1215–1226. [Google Scholar] [CrossRef]
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| X1: Microbial concentration, g/L | 1 | 2 | 3 |
| X2: Hydraulic retention time, h | 12 | 24 | 36 |
| X3: COD/TN | 6 | 16 | 26 |
| Run | Factors | Response | |||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 | Y2 | Y3 | Y4 | |
| 1 | 2 | 24 | 16 | 64 | 79 | 71 | 49 |
| 2 | 3 | 24 | 16 | 64 | 71 | 56 | 44 |
| 3 | 1 | 24 | 16 | 45 | 55 | 77 | 59 |
| 4 | 2 | 24 | 16 | 80 | 77 | 81 | 58 |
| 5 | 2 | 36 | 16 | 82 | 86 | 89 | 54 |
| 6 | 2 | 24 | 16 | 83 | 74 | 77 | 38 |
| 7 | 3 | 36 | 6 | 60 | 43 | 52 | 30 |
| 8 | 2 | 24 | 16 | 65 | 76 | 76 | 42 |
| 9 | 1 | 12 | 6 | 20 | 17 | 22 | 13 |
| 10 | 3 | 12 | 6 | 36 | 23 | 11 | 15 |
| 11 | 2 | 24 | 16 | 75 | 70 | 81 | 40 |
| 12 | 2 | 12 | 16 | 48 | 69 | 55 | 30 |
| 13 | 1 | 36 | 6 | 50 | 44 | 47 | 60 |
| 14 | 2 | 24 | 6 | 66 | 48 | 31 | 33 |
| 15 | 3 | 12 | 26 | 66 | 76 | 62 | 37 |
| 16 | 2 | 24 | 16 | 74 | 78 | 78 | 49 |
| 17 | 1 | 36 | 26 | 67 | 71 | 93 | 73 |
| 18 | 1 | 12 | 26 | 44 | 41 | 77 | 32 |
| 19 | 3 | 36 | 26 | 84 | 86 | 92 | 48 |
| 20 | 2 | 24 | 26 | 88 | 71 | 86 | 47 |
| Indexes | Tailwater Sedimentation Tank | RAS |
|---|---|---|
| TAN (mg/L) | 147 ± 6.35 a | 1.165 ± 0.47 b |
| NO2−-N (mg/L) | 2.1 ± 0.56 a | 0.4607 ± 0.14 b |
| NO3−-N (mg/L) | 16.63 ± 0.64 a | 97.163 ± 14.38 b |
| TN (mg/L) | 217.47 ± 11.52 a | 175.37 ± 9.63 b |
| DO (mg/L) | 0.43 ± 0.16 a | 10.47 ± 1.32 b |
| pH | 7.15 ± 0.2 a | 7.34 ± 0.3 a |
| Indexes | Suc | Mls | Glu |
|---|---|---|---|
| Crude protein (%) | 33.47 ± 0.31 a | 32.71 ± 0.23 b | 31.53 ± 0.45 c |
| Crude fat (%) | 3.95 ± 0.18 a | 3.57 ± 0.13 b | 3.35 ± 0.22 b |
| Crude ash (%) | 23.53 ± 0.14 a | 22.15 ± 0.17 b | 21.37 ± 0.57 c |
| Response Value | R2 | R2Adj | Pred-R2 | CV (%) | Prob > F | AP |
|---|---|---|---|---|---|---|
| Y1 | 0.9424 | 0.8906 | 0.8732 | 9.39 | <0.0001 | 16.9032 |
| Y2 | 0.979 | 0.9601 | 0.8395 | 6.42 | <0.0001 | 25.0467 |
| Y3 | 0.9717 | 0.9463 | 0.8534 | 8.25 | <0.0001 | 22.7513 |
| Y4 | 0.9287 | 0.8645 | 0.8752 | 12.88 | 0.0001 | 15.0398 |
| Parameters | Nitrogen Contaminant Removal Equation |
|---|---|
| (mg/L) | |
| TN | =−80.04364 + 78.91364X1 + 3.88447 X2 − 0.400909 X3 |
| −0.114583X1X2 + 0.162500X1X3 − 0.013542X2X3 | |
| −17.59091X12 − 0.049242X22 + 0.049091X32 | |
| TAN | =−64.16545 + 53.39545X1 + 0.757955X2 + 5.75364X3 |
| −0.28125X1X2 + 0.5625X1X3 − 0.007292X2X3 | |
| −12.13636X12 + 0.016414X22 − 0.156364X32 | |
| NO2−-N | =−38.48000 + 12.20000X1 + 0.92500X2 + 7.54000X3 |
| +0.31250X1X2 − 0.12500X1X3 − 0.020833X2X3 | |
| −5.50000X12 + 7.85369 × 10−17X22 − 0.13500X32 | |
| NO3−-N | =−35.00364 − 10.03636X1 + 4.30530X2 + 3.17909X3 |
| −0.645833X1X2 + 0.10000X1X3 − 0.010417X2X3 | |
| +4.40909X12 − 0.035354X22 − 0.070909X32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, G.; Xu, J.; Du, Y.; Wang, H.; Tian, H.; Zhou, L.; Wang, Y.; Sun, J.; Qiu, T. Research on the Optimization of Key Parameters for Heterotrophic Bacteria Assimilation Nitrogen Removal Technology in Aquaculture Tailwater. Sustainability 2025, 17, 5069. https://doi.org/10.3390/su17115069
Su G, Xu J, Du Y, Wang H, Tian H, Zhou L, Wang Y, Sun J, Qiu T. Research on the Optimization of Key Parameters for Heterotrophic Bacteria Assimilation Nitrogen Removal Technology in Aquaculture Tailwater. Sustainability. 2025; 17(11):5069. https://doi.org/10.3390/su17115069
Chicago/Turabian StyleSu, Guogen, Jianping Xu, Yishuai Du, Hexiang Wang, Huiqin Tian, Li Zhou, Yanfeng Wang, Jianming Sun, and Tianlong Qiu. 2025. "Research on the Optimization of Key Parameters for Heterotrophic Bacteria Assimilation Nitrogen Removal Technology in Aquaculture Tailwater" Sustainability 17, no. 11: 5069. https://doi.org/10.3390/su17115069
APA StyleSu, G., Xu, J., Du, Y., Wang, H., Tian, H., Zhou, L., Wang, Y., Sun, J., & Qiu, T. (2025). Research on the Optimization of Key Parameters for Heterotrophic Bacteria Assimilation Nitrogen Removal Technology in Aquaculture Tailwater. Sustainability, 17(11), 5069. https://doi.org/10.3390/su17115069







