One-Step Synthesis of In Situ Sulfur-Doped Porous Carbons for Efficient CO2 Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Carbon Precursor
2.2. Preparation of Porous Carbons
2.3. Characterization of Porous Carbons
2.4. Adsorption Test of CO2
2.5. Isothermal Adsorption Simulation and Thermodynamic Calculation
3. Results and Discussion
3.1. Physicochemical Characteristics of Porous Carbon
3.2. Pore Structures of Porous Carbon
3.3. CO2 Adsorption of Porous Carbon
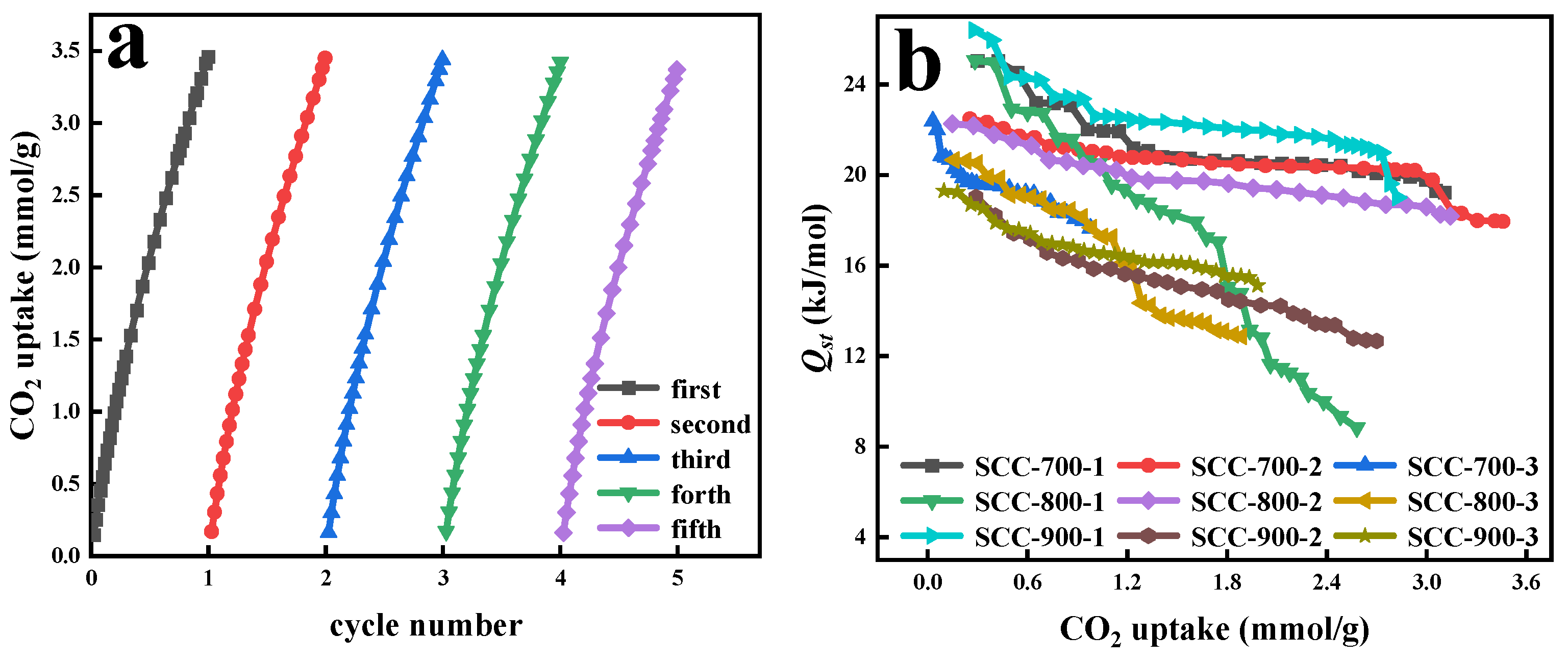
3.4. Analyses of Isothermal Adsorption Simulation and Thermodynamic Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nayak, S.; Goveas, L.C.; Selvaraj, R.; Vinayagam, R.; Manickam, S. Advances in the Utilisation of Carbon-Neutral Technologies for a Sustainable Tomorrow: A Critical Review and the Path Forward. Bioresour. Technol. 2022, 364, 128073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zheng, Y.; Cui, Y. Melamine Assisted Preparation of Nitrogen Doped Activated Carbon from Sustainable Biomass for H2 and CO2 Storage. Int. J. Hydrogen Energy 2023, 48, 17914–17922. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, S.; Li, Y.; Shen, J.; Tian, X.; Ding, M. Highly Dispersed Cu on Hollow Spherical CeO2: An Efficient and Stable Catalyst for the RWGS Reaction. Appl. Catal. B Environ. Energy 2025, 366, 125003. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Othman, R.M.; Ha, W.; Wang, J.; Wang, T.; Zhong, L.; Wang, J.; Pan, W.P. CO2 Adsorption by Coal Fly Ash Zeolite and Modified Zeolite-Templated Carbon. Process Saf. Environ. Prot. 2024, 186, 151–165. [Google Scholar] [CrossRef]
- Gopalan, J.; Buthiyappan, A.; Abdul Raman, A.A. Insight into Metal-Impregnated Biomass Based Activated Carbon for Enhanced Carbon Dioxide Adsorption: A Review. J. Ind. Eng. Chem. 2022, 113, 72–95. [Google Scholar] [CrossRef]
- Ziobrowski, Z.; Rotkegel, A.; Ziobrowski, Z.; Rotkegel, A. Comparison of CO2 Separation Efficiency from Flue Gases Based on Commonly Used Methods and Materials. Materials 2022, 15, 460. [Google Scholar] [CrossRef]
- Hassan, T.N.A.T.; Shariff, A.M.; Pauzi, M.M.M.; Khidzir, M.S.; Surmi, A. Insights on Cryogenic Distillation Technology for Simultaneous CO2 and H2S Removal for Sour Gas Fields. Molecules 2022, 27, 1424. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Deng, S.; Suvarna, M.; Wang, X.; Zhang, W.; Hamilton, S.T.; Alahmed, A.; Jamal, A.; Park, A.H.A.; et al. Recent Advancements in Sustainable Upcycling of Solid Waste into Porous Carbons for Carbon Dioxide Capture. Renew. Sustain. Energy Rev. 2022, 162, 112413. [Google Scholar] [CrossRef]
- Shi, C.; Li, L.; Li, Y. High-Throughput Screening of Hypothetical Aluminosilicate Zeolites for CO2 Capture from Flue Gas. J. CO2 Util. 2020, 42, 101346. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Hussain, S.; Mahmood, Q.; Fteiti, M.; Heo, K.; Ikram, M.; Aizaz Ud Din, M. Towards a Sustainable Conversion of Biomass/Biowaste to Porous Carbons for CO2 Adsorption: Recent Advances, Current Challenges, and Future Directions. Green Chem. 2023, 25, 4941–4980. [Google Scholar] [CrossRef]
- Xu, J.; Xie, Y.; Yao, Q.; Lv, L.; Chu, H. Advances in Sustainable Nano-Biochar: Precursors, Synthesis Methods and Applications. Nanoscale 2024, 16, 15009–15032. [Google Scholar] [CrossRef]
- Zang, P.; Tang, J.; Tao, Y.; Zhang, H.; Wang, X.; Cui, L.; Chen, S.; Zhao, P.; Dong, Y. K2CO3-Doped CaO-Based Sorbent for CO2 Capture: Performance Studies and Promotion Mechanisms. Chem. Eng. J. 2025, 505, 159233. [Google Scholar] [CrossRef]
- Wang, X.; Tarahomi, M.; Sheibani, R.; Xia, C.; Wang, W. Progresses in Lignin, Cellulose, Starch, Chitosan, Chitin, Alginate, and Gum/Carbon Nanotube (Nano)Composites for Environmental Applications: A Review. Int. J. Biol. Macromol. 2023, 241, 124472. [Google Scholar] [CrossRef] [PubMed]
- Soo, X.Y.D.; Lee, J.J.C.; Wu, W.Y.; Tao, L.; Wang, C.; Zhu, Q.; Bu, J. Advancements in CO2 Capture by Absorption and Adsorption: A Comprehensive Review. J. CO2 Util. 2024, 81, 102727. [Google Scholar] [CrossRef]
- Heo, Y.J.; Park, S.J. H2O2/Steam Activation as an Eco-Friendly and Efficient Top-down Approach to Enhancing Porosity on Carbonaceous Materials: The Effect of Inevitable Oxygen Functionalities on CO2 Capture. Green Chem. 2018, 20, 5224–5234. [Google Scholar] [CrossRef]
- Aghel, B.; Behaein, S.; Alobiad, F. CO2 Capture from Biogas by Biomass-Based Adsorbents: A Review. Fuel 2022, 328, 125276. [Google Scholar] [CrossRef]
- Quan, C.; Zhou, Y.; Wang, J.; Wu, C.; Gao, N. Biomass-Based Carbon Materials for CO2 Capture: A Review. J. CO2 Util. 2023, 68, 102373. [Google Scholar] [CrossRef]
- Wang, Y.X.; Ngo, H.H.; Guo, W.S. Preparation of a Specific Bamboo Based Activated Carbon and Its Application for Ciprofloxacin Removal. Sci. Total Environ. 2015, 533, 32–39. [Google Scholar] [CrossRef]
- Kumbhar, D.; Palliyarayil, A.; Reghu, D.; Shrungar, D.; Umapathy, S.; Sil, S. Rapid Discrimination of Porous Bio-Carbon Derived from Nitrogen Rich Biomass Using Raman Spectroscopy and Artificial Intelligence Methods. Carbon 2021, 178, 792–802. [Google Scholar] [CrossRef]
- Khuong, D.A.; Kieu, T.T.; Nakaoka, Y.; Tsubota, T.; Tashima, D.; Nguyen, H.N.; Tanaka, D. The Investigation of Activated Carbon by K2CO3 Activation: Micropores- and Macropores-Dominated Structure. Chemosphere 2022, 299, 134365. [Google Scholar] [CrossRef]
- Zhang, X.; Elsayed, I.; Song, X.; Shmulsky, R.; Hassan, E.B. Microporous Carbon Nanoflakes Derived from Biomass Cork Waste for CO2 Capture. Sci. Total Environ. 2020, 748, 142465. [Google Scholar] [CrossRef]
- Miao, Z.; Wu, J.; Niu, Y.; Guo, Z.; Guo, F.; Zhang, Y. Development of a Novel Type Hierarchical Porous Composite from Coal Gasification Fine Slag for CO2 Capture. Chem. Eng. J. 2022, 435, 134909. [Google Scholar] [CrossRef]
- Serafin, J.; Cruz, O.F. Promising Activated Carbons Derived from Common Oak Leaves and Their Application in CO2 Storage. J. Environ. Chem. Eng. 2022, 10, 107642. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; Alzahrani, A.Y.A.; Ali, H.; Abu-Dief, A.M.; Amin, M.S.; Alenad, A.M.; Al-Mhyawi, S.R.; Al-Hadeethi, Y.; Ajmal, Z.; et al. Recent Advances in Heteroatom-Doped/Hierarchical Porous Carbon Materials: Synthesis, Design and Potential Applications. Prog. Mater. Sci. 2025, 150, 101408. [Google Scholar] [CrossRef]
- Hou, J.; Wen, S.; Chen, J.; Zhao, Q.; Wang, L. Large-Scale Fabrication of Biomass-Derived N, S Co-Doped Porous Carbon with Ultrahigh Surface Area for Oxygen Reduction. Mater. Chem. Phys. 2021, 267, 124601. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Park, S.J. Role of Heteroatoms (Nitrogen and Sulfur)-Dual Doped Corn-Starch Based Porous Carbons for Selective CO2 Adsorption and Separation. J. CO2 Util. 2021, 51, 101641. [Google Scholar] [CrossRef]
- Ma, C.; Lu, T.; Shao, J.; Huang, J.; Hu, X.; Wang, L. Biomass Derived Nitrogen and Sulfur Co-Doped Porous Carbons for Efficient CO2 Adsorption. Sep. Purif. Technol. 2022, 281, 119899. [Google Scholar] [CrossRef]
- GB/T 212-2008; Proximate Analysis of Coal. Standards Press of China: Beijing, China, 2008.
- GB/T 31391-2015; Ultimate Analysis of Coal. Standards Press of China: Beijing, China, 2015.
- Dantas, S.; Struckhoff, K.C.; Thommes, M.; Neimark, A.V. Pore Size Characterization of Micro-Mesoporous Carbons Using CO2 Adsorption. Carbon 2021, 173, 842–848. [Google Scholar] [CrossRef]
- Jin, C.; Sun, J.; Bai, S.; Zhou, Z.; Sun, Y.; Guo, Y.; Wang, R.; Zhao, C. Sawdust Wastes-Derived Porous Carbons for CO2 Adsorption. Part 2. Insight into the CO2 Adsorption Enhancement Mechanism of Low-Doping of Microalgae. J. Environ. Chem. Eng. 2022, 10, 108265. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Bud, J.; Byambajav, E.; Tsubouchi, N. Influence of Ammonia Treatment on the CO2 Adsorption of Activated Carbon. J. Environ. Chem. Eng. 2022, 10, 107273. [Google Scholar] [CrossRef]
- Wang, Q.; He, D.; Mu, J. Hierarchically Porous Carbon from Cork with Tunable Pore Size and N-Doped Structure for Adsorption of Toluene and CO2. Sep. Purif. Technol. 2025, 360, 131256. [Google Scholar] [CrossRef]
- Gautam, S.J.; Vikram, S.; Dziejarski, B.; Sahoo, S. An Environmentally Friendly Synthesis Method of Activated Carbons Based on Subabul (Leucaena leucocephala) Sawdust Waste for CO2 Adsorption. J. Clean. Prod. 2023, 412, 137406. [Google Scholar] [CrossRef]
- Niu, J.; Shen, Y.; Zhang, H.; Li, L.; Guo, S. Preparation of Highly Microporous Activated Carbon by Utilizing Inherent Iron in Coal through CO2 and Steam Co-Activation for Improving CO2 Capture and Methylene Blue Removal. Fuel 2024, 371, 132069. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Tang, M.; Liu, J.; Yuan, J.; Zhao, Y.; Zhang, G. Preparation of N, S Co-Doped Carbon Nanotubes Composites by Coal Pyrolysis for the CO2 Capture. J. Environ. Chem. Eng. 2024, 12, 114452. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Tang, Z.; Zhang, Y. CO2 Capture by Activated and Impregnated Anthracites. Fuel Process. Technol. 2005, 86, 1487–1502. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Tang, M.; Hao, X.; Liu, J.; Zhang, G.; Zhang, Y. Preparation of N, O Co-Doped Carbon Nanotubes and Activated Carbon Composites with Hierarchical Porous Structure for CO2 Adsorption by Coal Pyrolysis. Fuel 2023, 333, 126465. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Liu, N.; Wang, B.; Dong, R. Nitrogen and Sulfur Co-Doped Microporous Carbon Prepared by a Couple of Activating and Functionalized Reagents for Efficient CO2 Capture and Selective CO2/CH4 Separation. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 658, 130732. [Google Scholar] [CrossRef]
- Hai, X.; Ma, B.; Wang, Q.; Bai, Y.; Lv, P.; Song, X.; Wang, X.; Yu, G. Coal Gasification Fine Slag as a Precursor to Prepare Mesoporous Carbon Materials by an Activation-Hydrothermal Two-Step Method for CO2 Adsorption. J. Environ. Manag. 2025, 373, 123590. [Google Scholar] [CrossRef]
- Ismail, I.S.; Singh, G.; Smith, P.; Kim, S.; Yang, J.H.; Joseph, S.; Yusup, S.; Singh, M.; Bansal, V.; Talapaneni, S.N.; et al. Oxygen Functionalized Porous Activated Biocarbons with High Surface Area Derived from Grape Marc for Enhanced Capture of CO2 at Elevated-Pressure. Carbon 2020, 160, 113–124. [Google Scholar] [CrossRef]
- Ma, C.; Bai, J.; Demir, M.; Hu, X.; Liu, S.; Wang, L. Water Chestnut Shell-Derived N/S-Doped Porous Carbons and Their Applications in CO2 Adsorption and Supercapacitor. Fuel 2022, 326, 125119. [Google Scholar] [CrossRef]
- Shi, S.; Liu, Y. Nitrogen-Doped Activated Carbons Derived from Microalgae Pyrolysis by-Products by Microwave/KOH Activation for CO2 Adsorption. Fuel 2021, 306, 121762. [Google Scholar] [CrossRef]
- Li, X.; Hayashi, J.I.; Li, C.Z. FT-Raman Spectroscopic Study of the Evolution of Char Structure during the Pyrolysis of a Victorian Brown Coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]



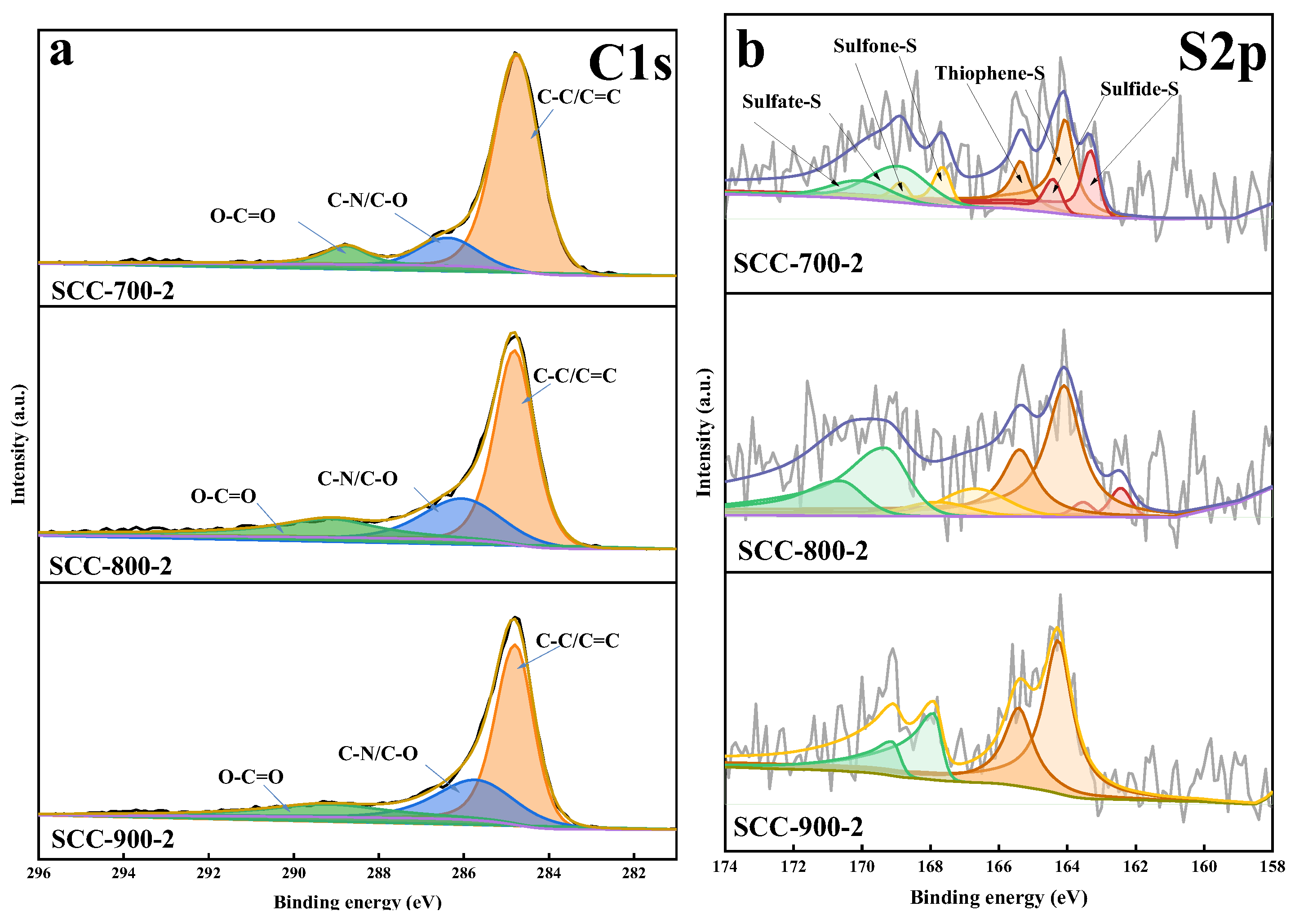




| Sample | Proximate Analysis (wt%) | Ultimate Analysis (wt%, daf) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mad 1 | Ad 2 | Vdaf 3 | C | H | N | S | O 4 | |
| High-sulfur coal | 0.19 | 9.14 | 28.48 | 86.32 | 5.08 | 1.39 | 2.18 | 5.03 |
| Samples | C | O | S |
|---|---|---|---|
| SCC | 83.38 | 16.21 | 0.40 |
| SCC-700-2 | 78.45 | 21.25 | 0.30 |
| SCC-800-2 | 83.10 | 16.65 | 0.25 |
| SCC-900-2 | 88.84 | 11.00 | 0.17 |
| Samples | Sulfur Forms in Total S (%) | |||
|---|---|---|---|---|
| Sulfide-S | Thiophene-S | Sulfone-S | Sulfate-S | |
| SCC | 11.2 | 43.98 | 24.08 | 20.74 |
| SCC-700-2 | 27.21 | 38.15 | 12.16 | 22.49 |
| SCC-800-2 | 7.95 | 47.23 | 11.2 | 33.63 |
| SCC-900-2 | 0 | 62.84 | 0 | 37.16 |
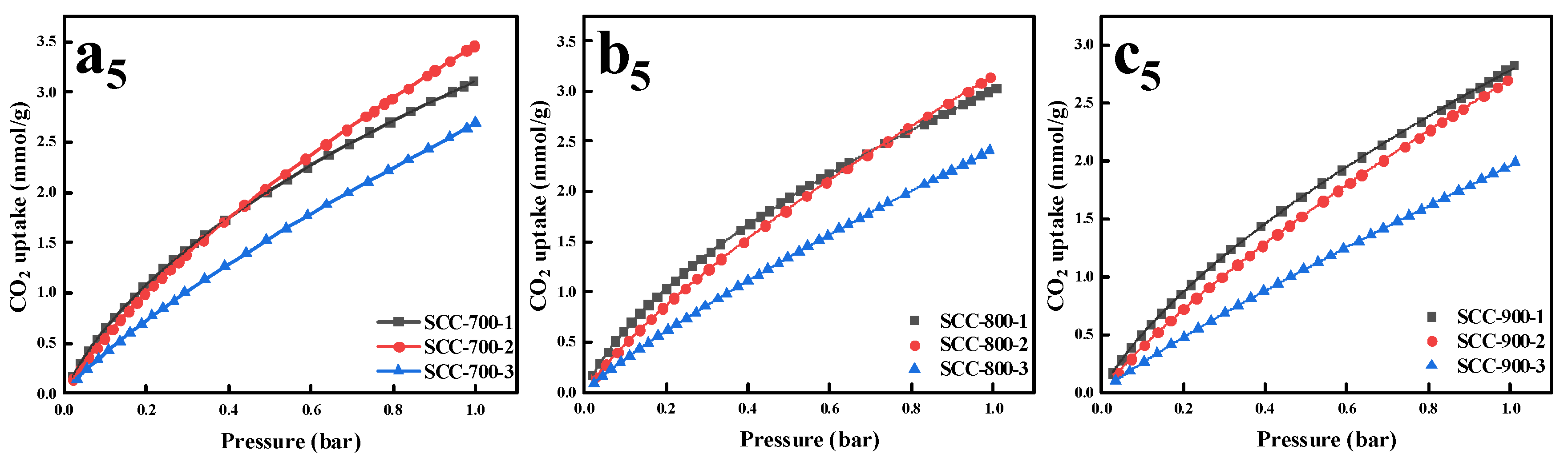
| Samples | SBET (m2/g) | Vt 1 (cm3/g) | Vm 2 (cm3/g) | Vm/Vt (%) | Average Pore Size (nm) | CO2 Capacity (mmol/g) | |||
|---|---|---|---|---|---|---|---|---|---|
| 0 °C | 25 °C | ||||||||
| 0.1 Bar | 1.0 Bar | 0.1 Bar | 1.0 Bar | ||||||
| SCC-700-1 | 804 | 0.47 | 0.39 | 83.0 | 2.34 | 1.25 | 4.59 | 0.65 | 3.11 |
| SCC-700-2 | 1370 | 0.77 | 0.66 | 85.7 | 2.25 | 1.12 | 5.69 | 0.56 | 3.46 |
| SCC-700-3 | 1783 | 1.09 | 0.82 | 75.2 | 2.45 | 0.79 | 4.51 | 0.43 | 2.69 |
| SCC-800-1 | 938 | 0.54 | 0.43 | 79.6 | 2.28 | 1.12 | 4.52 | 0.62 | 3.03 |
| SCC-800-2 | 1626 | 0.89 | 0.78 | 87.6 | 2.19 | 0.95 | 5.08 | 0.51 | 3.14 |
| SCC-800-3 | 2209 | 1.31 | 0.99 | 75.6 | 2.37 | 0.68 | 4.03 | 0.29 | 2.41 |
| SCC-900-1 | 1154 | 0.66 | 0.56 | 84.8 | 2.28 | 1.02 | 4.60 | 0.51 | 2.83 |
| SCC-900-2 | 1817 | 1.07 | 0.78 | 72.9 | 2.36 | 0.67 | 3.97 | 0.40 | 2.70 |
| SCC-900-3 | 1174 | 0.70 | 0.54 | 77.1 | 2.37 | 0.48 | 3.17 | 0.26 | 1.98 |
| Carbon Precursor | Activator and Dosage | SBET (m2/g) | Vt (cm3/g) | CO2 Uptake (mmol/g) | Selectivity | Ref. | |
|---|---|---|---|---|---|---|---|
| 0 °C | 25 °C | ||||||
| Anthracite of Taixi | CO2, steam | 1398 | 0.69 | -- | 1.85 | -- | [35] |
| Shaanxi Shenmu coal | KOH/1 | 1190 | 0.62 | 5.48 | 3.39 | 13.5 | [36] |
| A Pennsylvania anthracite | steam | 928 | 0.44 | -- | 2.76 (30 °C) | -- | [37] |
| Shenmu coal from Shaanxi | KOH/1 | 1245 | 0.61 | 5.66 | 3.46 | 18 | [38] |
| Common oak leaves | KOH/1 | 1842 | 0.88 | 6.17 | 5.44 | 132 | [23] |
| Raw anthracite | NaNH2/2 | 1131 | 0.62 | -- | 3.73 | -- | [39] |
| Industrial solid waste | KOH/3 | 550 | 0.23 | -- | 0.39 | [40] | |
| Grape marc | KOH/3 | 2473 | 1.09 | 6.2 | 3.4 | -- | [41] |
| Water chestnut shell | KOH/2 | 1353 | 0.56 | 6.31 | 4.54 | 23 | [42] |
| Microalgae powders | KOH/2 | 602 | 0.37 | -- | 3.57 | -- | [43] |
| Sulfur-enriched bituminous coal | KOH/2 | 1370 | 0.77 | 5.69 | 3.46 | 17.5 | This work |
| Samples | ΔH (kJ/mol) | ΔS (kJ·K/mol) | ΔG (kJ/mol) | |
|---|---|---|---|---|
| 0 °C | 25 °C | |||
| SCC-700-1 | −10.72 | −26.42 | −7.21 | −7.87 |
| SCC-700-2 | −13.66 | −35.44 | −9.67 | −10.55 |
| SCC-700-3 | −14.11 | −39.12 | −10.67 | −11.65 |
| SCC-800-1 | −11.22 | −28.38 | −7.74 | −8.45 |
| SCC-800-2 | −13.06 | −34.18 | −9.32 | −10.18 |
| SCC-800-3 | −13.77 | −38.79 | −10.58 | −11.55 |
| SCC-900-1 | −13.62 | −37.13 | −10.13 | −11.06 |
| SCC-900-2 | −10.38 | −26.49 | −7.22 | −7.89 |
| SCC-900-3 | −13.19 | −38.65 | −10.54 | −11.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Ma, Y.-P.; Wu, W.-J.; Cao, X.-F.; Fu, Y.-P. One-Step Synthesis of In Situ Sulfur-Doped Porous Carbons for Efficient CO2 Adsorption. Sustainability 2025, 17, 4952. https://doi.org/10.3390/su17114952
Guo J, Ma Y-P, Wu W-J, Cao X-F, Fu Y-P. One-Step Synthesis of In Situ Sulfur-Doped Porous Carbons for Efficient CO2 Adsorption. Sustainability. 2025; 17(11):4952. https://doi.org/10.3390/su17114952
Chicago/Turabian StyleGuo, Jiang, Yun-Peng Ma, Wen-Jun Wu, Xue-Fang Cao, and Yu-Ping Fu. 2025. "One-Step Synthesis of In Situ Sulfur-Doped Porous Carbons for Efficient CO2 Adsorption" Sustainability 17, no. 11: 4952. https://doi.org/10.3390/su17114952
APA StyleGuo, J., Ma, Y.-P., Wu, W.-J., Cao, X.-F., & Fu, Y.-P. (2025). One-Step Synthesis of In Situ Sulfur-Doped Porous Carbons for Efficient CO2 Adsorption. Sustainability, 17(11), 4952. https://doi.org/10.3390/su17114952





