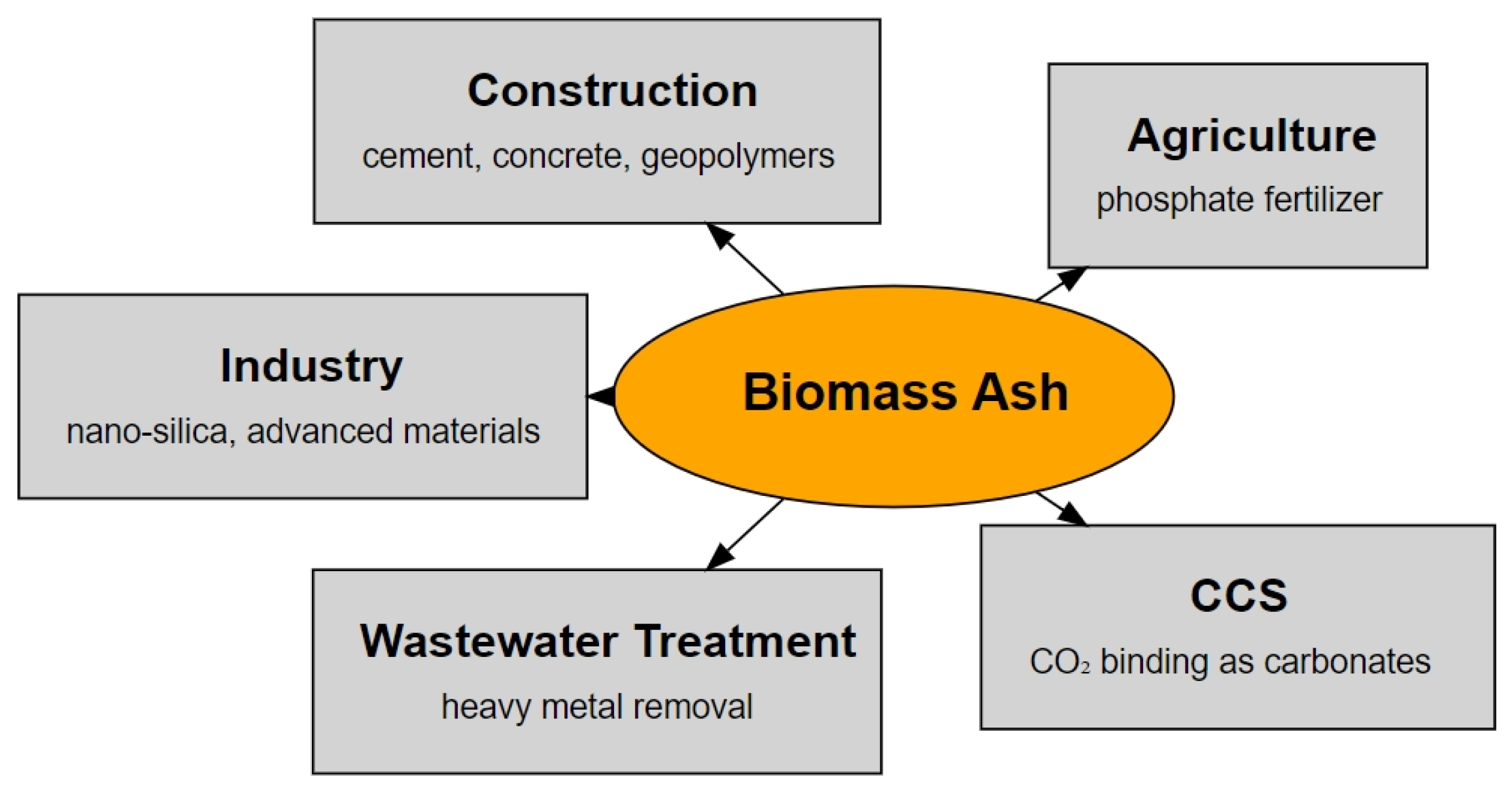
Biomass Ash: A Review of Chemical Compositions and Management Trends
Abstract
1. Introduction
Scientific Originality and Methodology of Review
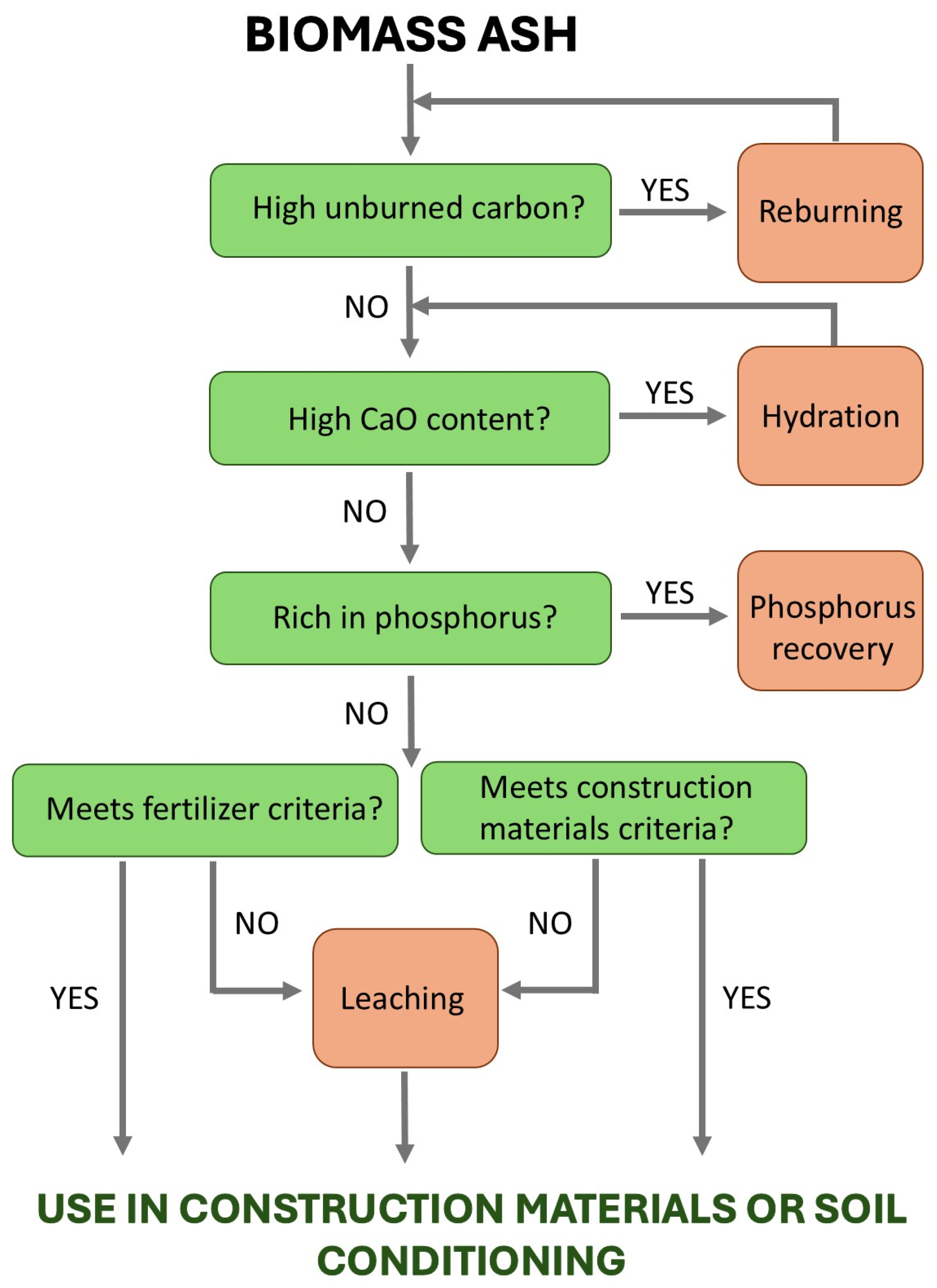
- Identify suitable utilization routes (e.g., soil amendment, construction materials, or advanced materials);
- Predict behavior (e.g., high-temperature corrosion tendency);
- Flag environmental risks (e.g., high heavy metal content).
2. The Quantity of Biomass Ashes
Conclusions
3. Ashes Rich in Silicon (Si)
3.1. Sources of Silicon-Rich Ashes
3.2. Utilization Paths of Silicon-Rich Ashes
3.3. Conclusions
4. Ashes Rich in Phosphorous (P)
4.1. Sources of Phosphorous-Rich Ashes
4.2. Utilization Paths of Phosphorous-Rich Ashes
4.3. Conclusions
5. Ashes Rich in Calcium (Ca)
5.1. Sources of Calcium-Rich Ashes
5.2. Utilization Path of Calcium-Rich Ashes
5.3. Conclusions
6. Ashes with High Chlorine (Cl) Content
6.1. Ashes Rich in Chlorine
6.2. Dangers and Risks
6.3. Remedies
6.4. Conclusions
7. Ashes with High Contents of Metals
7.1. Sources of Metals in Ashes
7.2. Dangers and Risks
7.3. Conclusions
8. Conclusions and Future Directions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Ash content in fuel |
| a.r. | As received |
| CCS | Carbon capture and storage |
| CCU | Carbon capture and utilization |
| d.b. | Dry basis |
| RH | Rice husk |
| LCA | Life cycle assessment |
| LHV | Lower heating value of fuel |
| M | Moisture content in fuel |
| MSW | Municipal solid waste |
| RDF | Refuse-derived fuel |
References
- Mignogna, D.; Szabó, M.; Ceci, P.; Avino, P. Biomass Energy and Biofuels: Perspective, Potentials, and Challenges in the Energy Transition. Sustainability 2024, 16, 7036. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, Y.; Chen, N.; Chen, B. A Review of the Efficient and Thermal Utilization of Biomass Waste. Sustainability 2024, 16, 9506. [Google Scholar] [CrossRef]
- Ufitikirezi, J.d.D.M.; Filip, M.; Ghorbani, M.; Zoubek, T.; Olšan, P.; Bumbálek, R.; Strob, M.; Bartoš, P.; Umurungi, S.N.; Murindangabo, Y.T.; et al. Agricultural Waste Valorization: Exploring Environmentally Friendly Approaches to Bioenergy Conversion. Sustainability 2024, 16, 3617. [Google Scholar] [CrossRef]
- Gładysz, P.; Strojny, M.; Bartela, Ł.; Hacaga, M.; Froehlich, T. Merging Climate Action with Energy Security through CCS—A Multi-Disciplinary Framework for Assessment. Energies 2022, 16, 35. [Google Scholar] [CrossRef]
- Banaś, J.; Utnik-Banaś, K.; Zięba, S. Optimizing Biomass Supply Chains to Power Plants under Ecological and Social Restrictions: Case Study from Poland. Energies 2024, 17, 3136. [Google Scholar] [CrossRef]
- Okunevičiūtė Neverauskienė, L.; Dirma, V.; Tvaronavičienė, M.; Danilevičienė, I. Assessing the Role of Renewable Energy in the Sustainable Economic Growth of the European Union. Energies 2025, 18, 760. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, T.; Niu, Y.; Mukherjee, S.; Abou-Elwafa, S.F.; Nguyen, N.S.H.; Al Aboud, N.M.; Wang, Y.; Pu, M.; Zhang, Y.; et al. A Comprehensive Review on Agricultural Waste Utilization through Sustainable Conversion Techniques, with a Focus on the Additives Effect on the Fate of Phosphorus and Toxic Elements during Composting Process. Sci. Total Environ. 2024, 942, 173567. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Mahamood, R.M.; Jen, T.-C.; Loha, C.; Akinlabi, E.T. An Overview of Biomass Solid Fuels: Biomass Sources, Processing Methods, and Morphological and Microstructural Properties. J. Bioresour. Bioprod. 2023, 8, 333–360. [Google Scholar] [CrossRef]
- Rybak, W.; Moroń, W.; Ferens, W. Dust Ignition Characteristics of Different Coal Ranks, Biomass and Solid Waste. Fuel 2019, 237, 606–618. [Google Scholar] [CrossRef]
- Qu, Z.; Fatehi, H.; Schmidt, F.M. Potassium Release from Biomass Particles during Combustion—Real-Time In Situ TDLAS Detection and Numerical Simulation. Appl. Sci. 2021, 11, 8887. [Google Scholar] [CrossRef]
- Xue, X.; Chen, D.; Song, X.; Dai, X. Hydrothermal and Pyrolysis Treatment for Sewage Sludge: Choice from Product and from Energy Benefit 1. Energy Procedia 2015, 66, 301–304. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Wejkowski, R.; Pronobis, M.; Gołombek, K. High-Temperature Corrosion in a Multifuel Circulating Fluidized Bed (CFB) Boiler Co-Firing Refuse Derived Fuel (RDF) and Hard Coal. Fuel 2022, 324, 124749. [Google Scholar] [CrossRef]
- Chomiak, L. Variation of Lignite Ash in Vertical and Horizontal Sections of Mining Wallsin the Konin Lignite Mine, Central Poland. Geol. Geophys. Environ. 2020, 46, 17. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Abioye, K.J.; Harun, N.Y.; Sufian, S.; Yusuf, M.; Jagaba, A.H.; Ekeoma, B.C.; Kamyab, H.; Sikiru, S.; Waqas, S.; Ibrahim, H. A Review of Biomass Ash Related Problems: Mechanism, Solution, and Outlook. J. Energy Inst. 2024, 112, 101490. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. Biomass Combustion Systems: A Review on the Physical and Chemical Properties of the Ashes. Renew. Sustain. Energy Rev. 2016, 53, 235–242. [Google Scholar] [CrossRef]
- Voshell, S.; Mäkelä, M.; Dahl, O. A Review of Biomass Ash Properties towards Treatment and Recycling. Renew. Sustain. Energy Rev. 2018, 96, 479–486. [Google Scholar] [CrossRef]
- Tan, Z.; Lagerkvist, A. Phosphorus Recovery from the Biomass Ash: A Review. Renew. Sustain. Energy Rev. 2011, 15, 3588–3602. [Google Scholar] [CrossRef]
- Munawar, M.A.; Khoja, A.H.; Naqvi, S.R.; Mehran, M.T.; Hassan, M.; Liaquat, R.; Dawood, U.F. Challenges and Opportunities in Biomass Ash Management and Its Utilization in Novel Applications. Renew. Sustain. Energy Rev. 2021, 150, 111451. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Zhai, J.; Burke, I.T.; Stewart, D.I. Beneficial Management of Biomass Combustion Ashes. Renew. Sustain. Energy Rev. 2021, 151, 111555. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mishra, M.; Suganya, O. The Incorporation of Wood Waste Ash as a Partial Cement Replacement Material for Making Structural Grade Concrete: An Overview. Ain Shams Eng. J. 2015, 6, 429–437. [Google Scholar] [CrossRef]
- Adhikari, S.; Nam, H.; Chakraborty, J.P. Conversion of Solid Wastes to Fuels and Chemicals Through Pyrolysis. In Waste Biorefinery: Potential and Perspectives; Elsevier: Amsterdam, The Netherlands, 2018; pp. 239–263. [Google Scholar] [CrossRef]
- Kuprianov, V.I.; Kaewklum, R.; Sirisomboon, K.; Arromdee, P.; Chakritthakul, S. Combustion and Emission Characteristics of a Swirling Fluidized-Bed Combustor Burning Moisturized Rice Husk. Appl. Energy 2010, 87, 2899–2906. [Google Scholar] [CrossRef]
- Armesto, L.; Bahillo, A.; Veijonen, K.; Cabanillas, A.; Otero, J. Combustion Behaviour of Rice Husk in a Bubbling Fluidised Bed. Biomass Bioenergy 2002, 23, 171–179. [Google Scholar] [CrossRef]
- Fang, M.; Yang, L.; Chen, G.; Shi, Z.; Luo, Z.; Cen, K. Experimental Study on Rice Husk Combustion in a Circulating Fluidized Bed. Fuel Process. Technol. 2004, 85, 1273–1282. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, T.; Wang, J.; Sui, Z.; Wang, L.; Zhang, Y.; Pan, W.-P. Combustion, Emission and Slagging Characteristics for Typical Agricultural Crop Straw Usage in Heating Plants. Thermochim Acta 2021, 702, 178979. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar Nandi, B. Combustion Characteristics of High Ash Indian Coal, Wheat Straw, Wheat Husk and Their Blends. Mater. Sci. Energy Technol. 2021, 4, 274–281. [Google Scholar] [CrossRef]
- Bradna, J.; Malaťák, J.; Hájek, D. The Properties of Wheat Straw Combustion and Use of Fly Ash as a Soil Amendment. Agron. Res. 2016, 14, 1257–1265. [Google Scholar]
- Obernberger, I.; Brunner, T.; Bärnthaler, G. Chemical Properties of Solid Biofuels—Significance and Impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Praspaliauskas, M.; Pedišius, N.; Čepauskienė, D.; Valantinavičius, M. Study of Chemical Composition of Agricultural Residues from Various Agro-Mass Types. Biomass Convers. Biorefin. 2020, 10, 937–948. [Google Scholar] [CrossRef]
- Kowalczyk-juśko, A.; Mazur, A.; Pochwatka, P.; Janczak, D.; Dach, J. Evaluation of the Effects of Using the Giant Miscanthus (Miscanthus × Giganteus) Biomass in Various Energy Conversion Processes. Energies 2022, 15, 3486. [Google Scholar] [CrossRef]
- Lanzerstorfer, C. Combustion of Miscanthus: Composition of the Ash by Particle Size. Energies 2019, 12, 178. [Google Scholar] [CrossRef]
- Baxter, X.C.; Darvell, L.I.; Jones, J.M.; Barraclough, T.; Yates, N.E.; Shield, I. Study of Miscanthus × Giganteus Ash Composition–Variation with Agronomy and Assessment Method. Fuel 2012, 95, 50–62. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.; Darvell, L.I.; Jones, J.M.; Yates, N.; Thain, S.; Donnison, I.S. The Effect of Alkali Metals on Combustion and Pyrolysis of Lolium and Festuca Grasses, Switchgrass and Willow. Fuel 2007, 86, 1560–1569. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, Y.; Darko Matovic, M.; Whalen, J.K. Exploring Switchgrass and Hardwood Combustion on Excess Air and Ash Fouling/Slagging Potential: Laboratory Combustion Test and Thermogravimetric Kinetic Analysis. Energy Convers. Manag. 2015, 97, 409–419. [Google Scholar] [CrossRef]
- Bakker, R.R.; Elbersen, H.W. Managing Ash Content and Quality in Herbaceous Biomass: An Analysis from Plant to Product. In Proceedings of the 14th European Biomass Conference and Exhibition, Paris, France, 17–21 October 2005. [Google Scholar]
- Maj, I.; Kalisz, S.; Ciukaj, S. Properties of Animal-Origin Ash—A Valuable Material for Circular Economy. Energies 2022, 15, 1274. [Google Scholar] [CrossRef]
- Maj, I. Significance and Challenges of Poultry Litter and Cattle Manure as Sustainable Fuels: A Review. Energies 2022, 15, 8981. [Google Scholar] [CrossRef]
- Chen, Y.-C. Effects of Urbanization on Municipal Solid Waste Composition. Waste Manag. 2018, 79, 828–836. [Google Scholar] [CrossRef]
- Shi, T.; Zhou, J.; Ren, J.; Ayub, Y.; Yu, H.; Shen, W.; Li, Q.; Yang, A. Co-Valorisation of Sewage Sludge and Poultry Litter Waste for Hydrogen Production: Gasification Process Design, Sustainability-Oriented Optimization, and Systematic Assessment. Energy 2023, 272, 127131. [Google Scholar] [CrossRef]
- Sakiewicz, P.; Piotrowski, K.; Rajca, M.; Maj, I.; Kalisz, S.; Ober, J.; Karwot, J.; Pagilla, K. Innovative Technological Approach for the Cyclic Nutrients Adsorption by Post-Digestion Sewage Sludge-Based Ash Co-Formed with Some Nanostructural Additives under a Circular Economy Framework. Int. J. Environ. Res. Public Health 2022, 19, 11119. [Google Scholar] [CrossRef]
- Fuller, A.; Carbo, M.; Savat, P.; Kalivodova, J.; Maier, J.; Scheffknecht, G. Results of Fly Ash Quality for Disposal Options from High Thermal Shares up to Pure Biomass Combustion in a Pilot-Scale and Large Scale Pulverized Fuel Power Plants. Renew. Energy 2015, 75, 899–910. [Google Scholar] [CrossRef]
- Sanou, I.; Sawadogo, M.; Seynou, M.; Zerbo, L.; Ouedraogo, R. Study of the Mechanical Behaviour of Mortars Modified with Rice Husk Ash. J. Miner. Mater. Charact. Eng. 2019, 7, 373–384. [Google Scholar] [CrossRef]
- Kang, Q.; Appels, L.; Tan, T.; Dewil, R. Bioethanol from Lignocellulosic Biomass: Current Findings Determine Research Priorities. Sci. World J. 2014, 2014, 298153. [Google Scholar] [CrossRef]
- Coutand, M.; Cyr, M.; Clastres, P. Use of Sewage Sludge Ash as Mineral Admixture in Mortars. Proc. Inst. Civ. Eng. Constr. Mater. 2006, 159, 153–162. [Google Scholar] [CrossRef]
- van Dijen, F.; Pels, J. Classification of Ashes and Identification of Possible Future Utilisations; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Ma, J.F.; Yamaji, N. Silicon Uptake and Accumulation in Higher Plants. Trends Plant Sci 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Silica Extraction from Rice Husk: Comprehensive Review and Applications. Hybrid Adv. 2023, 4, 100111. [Google Scholar] [CrossRef]
- Lv, Y.; Ye, G.; De Schutter, G. Utilization of Miscanthus Combustion Ash as Internal Curing Agent in Cement-Based Materials: Effect on Autogenous Shrinkage. Constr. Build. Mater. 2019, 207, 585–591. [Google Scholar] [CrossRef]
- Kaknics, J.; Michel, R.; Poirier, J. Miscanthus Ash Transformation and Interaction with Bed Materials at High Temperature. Fuel Process. Technol. 2016, 141, 178–184. [Google Scholar] [CrossRef]
- Memon, S.A.; Wahid, I.; Khan, M.K.; Tanoli, M.A.; Bimaganbetova, M. Environmentally Friendly Utilization of Wheat Straw Ash in Cement-Based Composites. Sustainability 2018, 10, 1322. [Google Scholar] [CrossRef]
- Schmitt, V.E.M.; Kaltschmitt, M. Effect of Straw Proportion and Ca- and Al-Containing Additives on Ash Composition and Sintering of Wood–Straw Pellets. Fuel 2013, 109, 551–558. [Google Scholar] [CrossRef]
- Li, F.; Zhao, C.; Li, J.; Li, Y.; Zhao, H.; Fan, H.; Xu, M.; Wang, Z.; Huang, J.; Fang, Y. Investigation on Ash Fusion Behavior Modification of Wheat Straw by Sludge Addition. J. Energy Inst. 2021, 98, 1–10. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Huang, S.; Wei, X.; Li, X.; Wu, S. Comparative Study on the Effects of Wood Dust and Rice Husk on Wheat Straw Gasification Process: Ash Fusion Characteristics and Gasification Reactivity. Fuel 2022, 326, 124942. [Google Scholar] [CrossRef]
- Tsvetkov, M.V.; Podlesnyi, D.N.; Zaichenko, A.Y.; Salganskaya, M.V.; Tsvetkova, Y.Y.; Freiman, V.M.; Salganskii, E.A. Fusibility of Agricultural Plant Waste Ash under the Conditions of High-Temperature Processing. Russ. J. Appl. Chem. 2021, 94, 354–361. [Google Scholar] [CrossRef]
- Buyondo, K.A.; Olupot, P.W.; Kirabira, J.B.; Yusuf, A.A. Optimization of Production Parameters for Rice Husk Ash-Based Geopolymer Cement Using Response Surface Methodology. Case Stud. Constr. Mater. 2020, 13, e00461. [Google Scholar] [CrossRef]
- Singh, N.S.; Thokchom, S.; Debbarma, R. Correlation Study on Microstructure and Mechanical Properties of Rice Husk Ash-Sodium Aluminate Geopolymer Pastes. Adv. Concr. Constr. 2021, 11, 73–80. [Google Scholar]
- Pandey, A.; Kumar, B. Effects of Rice Straw Ash and Micro Silica on Mechanical Properties of Pavement Quality Concrete. J. Build. Eng. 2019, 26, 100889. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R.; Miles, T.R. Combustion Properties of Biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Olatoyan, O.J.; Kareem, M.A.; Adebanjo, A.U.; Olawale, S.O.A.; Alao, K.T. Potential Use of Biomass Ash as a Sustainable Alternative for Fly Ash in Concrete Production: A Review. Hybrid Adv. 2023, 4, 100076. [Google Scholar] [CrossRef]
- Kaminskas, R.; Eisinas, A.; Barauskas, I.; Gaivenis, M. Hydrothermally Treated Biomass Fly Ash as an Additive for Portland Cement. Sustainability 2024, 16, 2754. [Google Scholar] [CrossRef]
- Gomes, C.M.; de Paulo Peruzzi, A. Effective Contribution of Brazilian Rice Husk Silica (RHS) as Eco-Friendly Silica in Concrete Plants. J. Civ. Eng. Archit. 2022, 16, 531–540. [Google Scholar] [CrossRef]
- Malpani, S.K.; Goyal, D. Synthesis, Analysis, and Multi-Faceted Applications of Solid Wastes-Derived Silica Nanoparticles: A Comprehensive Review (2010–2022). Environ. Sci. Pollut. Res. 2022, 30, 28321–28343. [Google Scholar] [CrossRef] [PubMed]
- Andreola, F.; Martín, M.I.; Ferrari, A.M.; Lancellotti, I.; Bondioli, F.; Rincón, J.M.; Romero, M.; Barbieri, L. Technological Properties of Glass-Ceramic Tiles Obtained Using Rice Husk Ash as Silica Precursor. Ceram. Int. 2013, 39, 5427–5435. [Google Scholar] [CrossRef]
- Lee, T.; Othman, R.; Yeoh, F.Y. Development of Photoluminescent Glass Derived from Rice Husk. Biomass Bioenergy 2013, 59, 380–392. [Google Scholar] [CrossRef]
- Lee, C.S.; Matori, K.A.; Ab Aziz, S.H.; Kamari, H.M.; Ismail, I.; Zaid, M.H.M. Fabrication and Characterization of Glass and Glass-Ceramic from Rice Husk Ash as a Potent Material for Opto-Electronic Applications. J. Mater. Sci. Mater. Electron. 2017, 28, 17611–17621. [Google Scholar] [CrossRef]
- Siqueira, E.J.; Yoshida, I.V.P.; Pardini, L.C.; Schiavon, M.A. Preparation and Characterization of Ceramic Composites Derived from Rice Husk Ash and Polysiloxane. Ceram. Int. 2009, 35, 213–220. [Google Scholar] [CrossRef]
- Hossain, S.K.S.; Mathur, L.; Roy, P.K. Rice Husk/Rice Husk Ash as an Alternative Source of Silica in Ceramics: A Review. J. Asian Ceram. Soc. 2018, 6, 299–313. [Google Scholar] [CrossRef]
- Choi, N.W.; Mori, I.; Ohama, Y. Development of Rice Husks–Plastics Composites for Building Materials. Waste Manag. 2006, 26, 189–194. [Google Scholar] [CrossRef]
- Kenechi, N.-O.; Linus, C.; Kayode, A. Utilization of Rice Husk as Reinforcement in Plastic Composites Fabrication-A Review. Am. J. Mater. Synth. Process. 2016, 1, 32–36. [Google Scholar]
- Sae-Oui, P.; Rakdee, C.; Thanmathorn, P. Use of Rice Husk Ash as Filler in Natural Rubber Vulcanizates: In Comparison with Other Commercial Fillers. J. Appl. Polym. Sci. 2002, 83, 2485–2493. [Google Scholar] [CrossRef]
- Arayapranee, W.; Naranong, N.; Rempel, G.L. Application of Rice Husk Ash as Fillers in the Natural Rubber Industry. J. Appl. Polym. Sci. 2005, 98, 34–41. [Google Scholar] [CrossRef]
- Wang, Z.; Smith, A.T.; Wang, W.; Sun, L. Versatile Nanostructures from Rice Husk Biomass for Energy Applications. Angew. Chem. Int. Ed. 2018, 57, 13722–13734. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, S.; Yuvakkumar, R.; Rajendran, V. Nano Silicon from Nano Silica Using Natural Resource (Rha) for Solar Cell Fabrication. Phosphorus Sulfur. Silicon. Relat. Elem. 2013, 188, 1178–1193. [Google Scholar] [CrossRef]
- Bianchini, P.; Merlo, F.; Maraschi, F.; Brescia, R.; Prato, M.; Profumo, A.; Speltini, A. From Rice Husk Ash to Silica-Supported Carbon Nanomaterials: Characterization and Analytical Application for Pre-Concentration of Steroid Hormones from Environmental Waters. Molecules 2023, 28, 745. [Google Scholar] [CrossRef]
- Gariya, D.; Bhamidimarri, R.B.; Satyavathi, B. Functionalized Rice Husk Ash as a Potential Catalytic Monolith: Preparation, Optimization and Application. Biomass. Convers. Biorefin. 2023, 13, 5107–5123. [Google Scholar] [CrossRef]
- Kanchanakul, I.; Srinophakun, T.R.; Kuboon, S.; Kaneko, H.; Kraithong, W.; Miyauchi, M.; Yamaguchi, A. Development of Photothermal Catalyst from Biomass Ash (Bagasse) for Hydrogen Production via Dry Reforming of Methane (DRM): An Experimental Study. Molecules 2023, 28, 4578. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deng, P.; Zhang, Z. Application of Silica-Rich Biomass Ash Solid Waste in Geopolymer Preparation: A Review. Constr. Build. Mater. 2022, 356, 129142. [Google Scholar] [CrossRef]
- Kang, S.H.; Hong, S.G.; Moon, J. The Use of Rice Husk Ash as Reactive Filler in Ultra-High Performance Concrete. Cem. Concr. Res. 2019, 115, 389–400. [Google Scholar] [CrossRef]
- Jittin, V.; Minnu, S.N.; Bahurudeen, A. Potential of Sugarcane Bagasse Ash as Supplementary Cementitious Material and Comparison with Currently Used Rice Husk Ash. Constr. Build. Mater. 2021, 273, 121679. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, C.; Fang, H.; Zhu, W.; Shi, J.; Liu, G. Synthesis of Ordered Mesoporous Silica from Biomass Ash and Its Application in CO2 Adsorption. Environ. Res. 2023, 231, 116070. [Google Scholar] [CrossRef]
- Usas, S.A.; Ricardez-Sandoval, L. Biomass Fly-Ash Derived Li4SiO4 Solid for Pilot-Scale CO2 Capture, Part II: Waste Management and Utilization. Chem. Eng. Res. Des. 2025, 216, 73–89. [Google Scholar] [CrossRef]
- Beck, J.; Brandenstein, J.; Unterberger, S.; Hein, K.R.G. Effects of Sewage Sludge and Meat and Bone Meal Co-Combustion on SCR Catalysts. Appl. Catal. B 2004, 49, 15–25. [Google Scholar] [CrossRef]
- Kowalski, Z.; Makara, A. Sustainable Systems for the Production of District Heating Using Meat-Bone Meal as Biofuel: A Polish Case Study. Energies 2022, 15, 3615. [Google Scholar] [CrossRef]
- Kowalski, Z.; Banach, M.; Makara, A. Optimisation of the Co-Combustion of Meat–Bone Meal and Sewage Sludge in Terms of the Quality Produced Ashes Used as Substitute of Phosphorites. Environ. Sci. Pollut. Res. 2021, 28, 8205–8214. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-H.; Kumar, S.; Ra, C. Solid Waste from Swine Wastewater as a Fuel Source for Heat Production. Asian-Australas. J. Anim. Sci. 2012, 25, 1627–1633. [Google Scholar] [CrossRef]
- Shen, X.; Huang, G.; Yang, Z.; Han, L. Compositional Characteristics and Energy Potential of Chinese Animal Manure by Type and as a Whole. Appl. Energy 2015, 160, 108–119. [Google Scholar] [CrossRef]
- Kasina, M.; Jarosz, K.; Stolarczyk, M.; Göttlicher, J.; Steininger, R.; Michalik, M. Characteristic of Phosphorus Rich Compounds in the Incinerated Sewage Sludge Ashes: A Case for Sustainable Waste Management. Sci. Rep. 2023, 13, 9137. [Google Scholar] [CrossRef]
- Kai, X.; Zhang, Y.; Yang, T.; Wang, J.; Sun, Y.; Zhu, Y. Study on the Effect of Oil Shale Ash on Potassium Retention Characteristics during Pyrolysis of Corn Stalk. J. Anal. Appl. Pyrolysis 2024, 180, 106552. [Google Scholar] [CrossRef]
- Zhao, C.; Bai, Y.; Zhao, W.; Li, Y.; Song, X.; Wang, J.; Su, W.; Lv, P.; Yu, G.; Yao, M. Interaction between Phosphorus and Alkali/Alkaline Earth Metals and Their Effect on Ash Transformation Thermal Kinetics during Phosphorus-Rich Biomass and Coal Co-Gasification. Fuel 2025, 394, 135088. [Google Scholar] [CrossRef]
- He, C.; Du, Y.; Cai, X.; Wang, J.; Qin, Y.; Zhao, Z.; Li, H.; Vassilev, S.V.; Vassileva, C.G. In-Situ Analysis of the Sintering Behavior of Coal Ash and a Phosphorus-Rich Biomass Ash under Gasification Condition. Biomass Bioenergy 2023, 168, 106671. [Google Scholar] [CrossRef]
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The Future Distribution and Production of Global Phosphate Rock Reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Schiemenz, K.; Eichler-Löbermann, B. Biomass Ashes and Their Phosphorus Fertilizing Effect on Different Crops. Nutr. Cycl. Agroecosyst. 2010, 87, 471–482. [Google Scholar] [CrossRef]
- Mozaffari, M.; Russelle, M.P.; Rosen, C.J.; Nater, E.A. Nutrient Supply and Neutralizing Value of Alfalfa Stem Gasification Ash. Soil Sci. Soc. Am. J. 2002, 66, 171–178. [Google Scholar] [CrossRef]
- Faridullah; Irshad, M.; Eneji, A.E.; Mahmood, Q. Plant Nutrient Release from Poultry Litter and Poultry Litter Ash Amended Soils by Various Extraction Methods. J. Plant. Nutr. 2013, 36, 357–371. [Google Scholar] [CrossRef]
- Kan, S.; Yilmaz, F.G.; Yagcioglu, K.D.; Kadioglu, Y.K.; Gezgin, S.; Gunes, A.; Taskin, M.B. Valorization of Poultry Litter Incineration Ash as a Sustainable and Balanced Fertilizer Source. J. Soil Sci. Plant Nutr. 2024, 24, 7570–7580. [Google Scholar] [CrossRef]
- Tominc, S.; Ducman, V.; Wisniewski, W.; Luukkonen, T.; Kirkelund, G.M.; Ottosen, L.M. Recovery of Phosphorus and Metals from the Ash of Sewage Sludge, Municipal Solid Waste, or Wood Biomass: A Review and Proposals for Further Use. Materials 2023, 16, 6948. [Google Scholar] [CrossRef]
- Semerci, N.; Ahadi, S.; Coşgun, S. Comparison of Dried Sludge and Sludge Ash for Phosphorus Recovery with Acidic and Alkaline Leaching. Water Environ. J. 2021, 35, 359–370. [Google Scholar] [CrossRef]
- Guedes, P.; Couto, N.; Ottosen, L.M.; Ribeiro, A.B. Phosphorus Recovery from Sewage Sludge Ash through an Electrodialytic Process. Waste Manag. 2014, 34, 886–892. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Kirkelund, G.M.; Jensen, P.E.; Pedersen, K.B. Extraction of Phosphorus from Sewage Sludge Ash—Influence of Process Variables on the Electrodialytic Process. Sustainability 2023, 15, 13953. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Zhang, Z.Z.; Chen, T.; Tu, S.; Chen, C.; Xu, P.; Hao, T.; Zhou, J.; Yan, B. Improvement of Wet-Chemical Phosphorus Extraction Efficiency in Incinerated Sewage Sludge Ash (ISSA) by Supercritical Hydrothermal Mineral Phase Transformation of ISSA. Sep. Purif. Technol. 2025, 364, 132489. [Google Scholar] [CrossRef]
- Pérez-Piqueres, A.; Ribó, M.; Rodríguez-Carretero, I.; Quiñones, A.; Canet, R. Struvite as a Sustainable Fertilizer in Mediterranean Soils. Agronomy 2023, 13, 1391. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and Messenger. Front. Plant Sci. 2019, 10, 449564. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, H.; Wang, X.; Du, W.; Mikulčić, H.; Duić, N. Study on Extracting Available Salt from Straw/Woody Biomass Ashes and Predicting Its Slagging/Fouling Tendency. J. Clean. Prod. 2017, 155, 164–171. [Google Scholar] [CrossRef]
- Odzijewicz, J.I.; Wołejko, E.; Wydro, U.; Wasil, M.; Jabłońska-Trypuć, A. Utilization of Ashes from Biomass Combustion. Energies 2022, 15, 9653. [Google Scholar] [CrossRef]
- Link, S.; Yrjas, P.; Lindberg, D.; Trikkel, A.; Mikli, V. Ash Melting Behaviour of Reed and Woody Fuels Blends. Fuel 2022, 314, 123051. [Google Scholar] [CrossRef]
- Nik Norizam, N.N.A.; Yang, X.; Ingham, D.; Szuhánszki, J.; Yang, W.; Rezende, J.; Ma, L.; Pourkashanian, M. An Improved Index to Predict the Slagging Propensity of Woody Biomass on High-Temperature Regions in Utility Boilers. J. Energy Inst. 2023, 109, 101272. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Mahmud, S.; Tushar, M.; Leon, M. Ash Analysis of Poultry Litter, Willow and Oats for Combustion in Boilers. J. Biomass Biofuel 2014, 1, 16–26. [Google Scholar] [CrossRef]
- Moura, L.S.d.; Silva, C.C.V.P.d.; Oliveira, S.M.d.; Carneiro, A.M.P.; Lucena, L.C.d.F.L.; Nóbrega, A.C.V.d. Use of Calcium-Rich Wood Biomass Combustion Ashes as Filler in Hot Mix Asphalt. Road Mater. Pavement Des. 2022, 23, 2375–2393. [Google Scholar] [CrossRef]
- Sharko, A.; Louda, P.; Nguyen, V.V.; Buczkowska, K.E.; Stepanchikov, D.; Ercoli, R.; Kascak, P.; Le, V.S. Multicriteria Assessment for Calculating the Optimal Content of Calcium-Rich Fly Ash in Metakaolin-Based Geopolymers. Ceramics 2023, 6, 525–537. [Google Scholar] [CrossRef]
- Cui, J.; Li, J.; Cui, J.; Wang, W.; Wu, Y.; Xu, B.; Chang, Y.; Liu, X.; Li, H.; Yao, D. Removal Effects of a Biomass Bottom Ash Composite on Tailwater Phosphate and Its Application in a Rural Sewage Treatment Plant. Sci. Total Environ. 2022, 812, 152549. [Google Scholar] [CrossRef]
- Uliasz-Bocheńczyk, A. A Comprehensive Review of CO2 Mineral Sequestration Methods Using Coal Fly Ash for Carbon Capture, Utilisation, and Storage (CCUS) Technology. Energies 2024, 17, 5605. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass Waste Utilisation in Low-Carbon Products: Harnessing a Major Potential Resource. Npj Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Koch, R.; Sailer, G.; Paczkowski, S.; Pelz, S.; Poetsch, J.; Müller, J. Lab-Scale Carbonation of Wood Ash for CO2-Sequestration. Energies 2021, 14, 7371. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Petrova, N.L. Mineral Carbonation of Thermally Treated and Weathered Biomass Ashes with Respect to Their CO2 Capture and Storage. Fuel 2022, 321, 124010. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Extra CO2 Capture and Storage by Carbonation of Biomass Ashes. Energy Convers. Manag. 2020, 204, 112331. [Google Scholar] [CrossRef]
- Wang, S. Application of Solid Ash Based Catalysts in Heterogeneous Catalysis. Environ. Sci. Technol. 2008, 42, 7055–7063. [Google Scholar] [CrossRef]
- Yildiz, G.; Ronsse, F.; Venderbosch, R.; van Duren, R.; Kersten, S.R.A.; Prins, W. Effect of Biomass Ash in Catalytic Fast Pyrolysis of Pine Wood. Appl. Catal. B 2015, 168–169, 203–211. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the Catalytic Effects of Alkali and Alkaline Earth Metals (AAEMs) Including Sodium, Potassium, Calcium and Magnesium on the Pyrolysis of Lignocellulosic Biomass and on the Co-Pyrolysis of Coal with Biomass. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Y.; Zeng, Z.; Dai, L.; Peng, Y.; Jiang, L.; Yang, X.; Yue, L.; Liu, Y.; Ruan, R. Study on the Mechanism of Co-Catalyzed Pyrolysis of Biomass by Potassium and Calcium. Bioresour. Technol. 2021, 320, 124415. [Google Scholar] [CrossRef]
- Sun, H.; Sun, K.; Wang, F.; Liu, Y.; Ding, L.; Xu, W.; Sun, Y.; Jiang, J. Catalytic Self-Activation of Ca-Doped Coconut Shell for in-Situ Synthesis of Hierarchical Porous Carbon Supported CaO Transesterification Catalyst. Fuel 2021, 285, 119192. [Google Scholar] [CrossRef]
- Sun, H.; Ma, M.; Fan, M.; Sun, K.; Xu, W.; Wang, K.; Li, B.; Jiang, J. Controllable Preparation of Biomass Derived Mesoporous Activated Carbon Supported Nano-CaO Catalysts for Biodiesel Production. Energy 2022, 261, 125369. [Google Scholar] [CrossRef]
- Nahuelcura, B.; González, M.E.; Gutierrez, N.; Ñanculeo, J.; Romero-García, J.M. Biodiesel Production from Waste Frying Oil (WFO) Using a Biomass Ash-Based Catalyst. Catalysts 2024, 14, 553. [Google Scholar] [CrossRef]
- Saccomani, A.; DE, F.F.; França, X.; Dias, A.; Matos Júnior, J.; Faria, D. Firewood Ash as Calcium Source in the Initial Diet of Broiler Chickens. Rev. Bras. Cienc. Avic. 2016, 18, 645–648. [Google Scholar] [CrossRef]
- Ma, W.; Hoffmann, G.; Schirmer, M.; Chen, G.; Rotter, V.S. Chlorine Characterization and Thermal Behavior in MSW and RDF. J. Hazard. Mater. 2010, 178, 489–498. [Google Scholar] [CrossRef]
- Becidan, M.; Sørum, L.; Frandsen, F.; Pedersen, A.J. Corrosion in Waste-Fired Boilers: A Thermodynamic Study. Fuel 2009, 88, 595–604. [Google Scholar] [CrossRef]
- Lima, A.T.; Ottosen, L.M.; Ribeiro, A.B.; Hansen, H.K. Electrodialytic Removal of Cd from Straw Ash in a Pilot Plant. J. Environ. Sci. Health Part A 2008, 43, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.K.; Pedersen, A.J.; Ottosen, L.M.; Villumsen, A. Speciation and Mobility of Cadmium in Straw and Wood Combustion Fly Ash. Chemosphere 2001, 45, 123–128. [Google Scholar] [CrossRef]
- Maj, I.; Niesporek, K.; Matus, K.; Miccio, F.; Mazzocchi, M.; Łój, P. The Impact of Aluminosilicate Additives upon the Chlorine Distribution and Melting Behavior of Poultry Litter Ash. Energies 2024, 17, 1854. [Google Scholar] [CrossRef]
- Zhovmir, M.M.; Moško, J.; Farták, J.; Jiříček, I.; Pohořelý, M. Complex Study of Straw Suitability for the Production of Nonindustrial Straw Pellets. ACS Omega 2023, 8, 47100–47112. [Google Scholar] [CrossRef]
- Kaniowski, W.; Taler, J.; Wang, X.; Kalemba-Rec, I.; Gajek, M.; Mlonka-Mędrala, A.; Nowak-Woźny, D.; Magdziarz, A. Investigation of Biomass, RDF and Coal Ash-Related Problems: Impact on Metallic Heat Exchanger Surfaces of Boilers. Fuel 2022, 326, 125122. [Google Scholar] [CrossRef]
- Wang, Q.; Han, K.; Wang, P.; Li, S.; Zhang, M. Influence of Additive on Ash and Combustion Characteristics during Biomass Combustion under O2/CO2 Atmosphere. Energy 2020, 195, 116987. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Szymajda, A.; Łaska, G.; Gołombek, K. The Influence of Cow Dung and Mixed Straw Ashes on Steel Corrosion. Renew. Energy 2021, 177, 1198–1211. [Google Scholar] [CrossRef]
- Mlonka-Mędrala, A.; Gołombek, K.; Buk, P.; Cieślik, E.; Nowak, W. The Influence of KCl on Biomass Ash Melting Behaviour and High-Temperature Corrosion of Low-Alloy Steel. Energy 2019, 188, 116062. [Google Scholar] [CrossRef]
- Nielsen, H.P.; Frandsen, F.J.; Dam-Johansen, K.; Baxter, L.L. The Implications of Chlorine-Associated Corrosion on the Operation of Biomass-Fired Boilers. Prog. Energy Combust. Sci. 2000, 26, 283–298. [Google Scholar] [CrossRef]
- Karuana, F.; Prismantoko, A.; Suhendra, N.; Darmawan, A.; Hariana, H.; Darmadi, D.B.; Akhsin Muflikhun, M. Investigation of Austenitic Stainless Steel Corrosion Resistance against Ash Deposits from Co-Combustion Coal and Biomass Waste. Eng. Fail. Anal. 2023, 150, 107368. [Google Scholar] [CrossRef]
- Míguez, J.L.; Porteiro, J.; Behrendt, F.; Blanco, D.; Patiño, D.; Dieguez-Alonso, A. Review of the Use of Additives to Mitigate Operational Problems Associated with the Combustion of Biomass with High Content in Ash-Forming Species. Renew. Sustain. Energy Rev. 2021, 141, 110502. [Google Scholar] [CrossRef]
- Lima, A.T.; Ottosen, L.M.; Pedersen, A.J.; Ribeiro, A.B. Characterization of Fly Ash from Bio and Municipal Waste. Biomass Bioenergy 2008, 32, 277–282. [Google Scholar] [CrossRef]
- Sabour, M.R.; Hatami, A.M.; Nikravan, M.; Zarrabi, H.; Hajbabaie, M. Optimization of Chloride Removal from Fly Ash: A Step towards Sustainable Waste Management. Int. J. Environ. Sci. Technol. 2025, 22, 3149–3162. [Google Scholar] [CrossRef]
- Wang, X.; Gao, M.; Wang, M.; Wu, C.; Wang, Q.; Wang, Y. Chloride Removal from Municipal Solid Waste Incineration Fly Ash Using Lactic Acid Fermentation Broth. Waste Manag. 2021, 130, 23–29. [Google Scholar] [CrossRef]
- Zhao, K.; Hu, Y.; Tian, Y.; Chen, D.; Feng, Y. Chlorine Removal from MSWI Fly Ash by Thermal Treatment: Effects of Iron/Aluminum Additives. J. Environ. Sci. 2020, 88, 112–121. [Google Scholar] [CrossRef]
- Grabke, H.J.; Reese, E.; Spiegel, M. The Effects of Chlorides, Hydrogen Chloride, and Sulfur Dioxide in the Oxidation of Steels below Deposits. Corros. Sci. 1995, 37, 1023–1043. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Hui, S. Ash-Related Issues during Biomass Combustion: Alkali-Induced Slagging, Silicate Melt-Induced Slagging (Ash Fusion), Agglomeration, Corrosion, Ash Utilization, and Related Countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
- Maj, I.; Matus, K. Aluminosilicate Clay Minerals: Kaolin, Bentonite, and Halloysite as Fuel Additives for Thermal Conversion of Biomass and Waste. Energies 2023, 16, 4359. [Google Scholar] [CrossRef]
- Ottosen, L.M.; Sigvardsen, N.M. Heavy Metal Leaching from Wood Ash before and after Hydration and Carbonation. Environ. Sci. Pollut. Res. 2024, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sawidis, T.; Breuste, J.; Mitrovic, M.; Pavlovic, P.; Tsigaridas, K. Trees as Bioindicator of Heavy Metal Pollution in Three European Cities. Environ. Pollut. 2011, 159, 3560–3570. [Google Scholar] [CrossRef]
- Werther, J.; Ogada, T. Sewage Sludge Combustion. Prog. Energy Combust. Sci. 1999, 25, 55–116. [Google Scholar] [CrossRef]
- Benassi, L.; Zanoletti, A.; Depero, L.E.; Bontempi, E. Sewage Sludge Ash Recovery as Valuable Raw Material for Chemical Stabilization of Leachable Heavy Metals. J. Environ. Manag. 2019, 245, 464–470. [Google Scholar] [CrossRef]
- Yoshiie, R.; Nishimura, M.; Moritomi, H. Influence of Ash Composition on Heavy Metal Emissions in Ash Melting Process. Fuel 2002, 81, 1335–1340. [Google Scholar] [CrossRef]
- Huang, B.; Gan, M.; Ji, Z.; Fan, X.; Zhang, D.; Chen, X.; Sun, Z.; Huang, X.; Fan, Y. Recent Progress on the Thermal Treatment and Resource Utilization Technologies of Municipal Waste Incineration Fly Ash: A Review. Process Saf. Environ. Prot. 2022, 159, 547–565. [Google Scholar] [CrossRef]
- Pandey, D.S.; Kwapinska, M.; Leahy, J.J.; Kwapinski, W. Fly Ash from Poultry Litter Gasification–Can It Be Utilised in Agriculture Systems as a Fertiliser? Energy Procedia 2019, 161, 38–46. [Google Scholar] [CrossRef]
- Aljohani, A.S.M. Heavy Metal Toxicity in Poultry: A Comprehensive Review. Front. Vet. Sci. 2023, 10, 1161354. [Google Scholar] [CrossRef]
- Chai, Y.; Chen, A.; Bai, M.; Peng, L.; Shao, J.; Yuan, J.; Shang, C.; Zhang, J.; Huang, H.; Peng, C. Valorization of Heavy Metal Contaminated Biomass: Recycling and Expanding to Functional Materials. J. Clean. Prod. 2022, 366, 132771. [Google Scholar] [CrossRef]
- Pei, Y.; Ike, M.; Shiota, K.; Takaoka, M. The Impacts of Furnace and Fuel Types on the Hazardous Heavy Metal Contents and Leaching Behavior of Woody Biomass Fly Ash. Fuel 2024, 372, 132202. [Google Scholar] [CrossRef]
- Zajac, G.; Szyszlak-Bargłowicz, J.; Szczepanik, M. Influence of Biomass Incineration Temperature on the Content of Selected Heavy Metals in the Ash Used for Fertilizing Purposes. Appl. Sci. 2019, 9, 1790. [Google Scholar] [CrossRef]
- Ajorloo, M.; Ghodrat, M.; Scott, J.; Strezov, V. Heavy Metals Removal/Stabilization from Municipal Solid Waste Incineration Fly Ash: A Review and Recent Trends. J. Mater. Cycles Waste Manag. 2022, 24, 1693–1717. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Cho, D.-W.; Tsang, D.C.W.; Tong, L.; Zhou, Y.; Yang, J.; Hu, Q.; Poon, C.S. Sustainable Stabilization/Solidification of Municipal Solid Waste Incinerator Fly Ash by Incorporation of Green Materials. J. Clean. Prod. 2019, 222, 335–343. [Google Scholar] [CrossRef]
- Huang, T.Y.; Chuieh, P.T. Life Cycle Assessment of Reusing Fly Ash from Municipal Solid Waste Incineration. Procedia. Eng. 2015, 118, 984–991. [Google Scholar] [CrossRef]
- Gong, B.; Deng, Y.; Yang, Y.; Wang, C.; He, Y.; Sun, X.; Liu, Q.; Yang, W. Effects of Microwave-Assisted Thermal Treatment on the Fate of Heavy Metals in Municipal Solid Waste Incineration Fly Ash. Energy Fuels 2017, 31, 12446–12454. [Google Scholar] [CrossRef]
- Li, R.; Zhang, B.; Wang, Y.; Zhao, Y.; Li, F. Leaching Potential of Stabilized Fly Ash from the Incineration of Municipal Solid Waste with a New Polymer. J. Environ. Manag. 2019, 232, 286–294. [Google Scholar] [CrossRef]
- Kurashima, K.; Matsuda, K.; Kumagai, S.; Kameda, T.; Saito, Y.; Yoshioka, T. A Combined Kinetic and Thermodynamic Approach for Interpreting the Complex Interactions during Chloride Volatilization of Heavy Metals in Municipal Solid Waste Fly Ash. Waste Manag. 2019, 87, 204–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Cetin, B.; Likos, W.J.; Edil, T.B. Impacts of PH on Leaching Potential of Elements from MSW Incineration Fly Ash. Fuel 2016, 184, 815–825. [Google Scholar] [CrossRef]
- Xu, D.; Huang, Y.; Jin, X.; Sun, T. Synergistic Treatment of Heavy Metals in Municipal Solid Waste Incineration Fly Ash with Geopolymer and Chemical Stabilizers. Process Saf. Environ. Prot. 2022, 160, 763–774. [Google Scholar] [CrossRef]
- Atanes, E.; Cuesta-García, B.; Nieto-Márquez, A.; Fernández-Martínez, F. A Mixed Separation-Immobilization Method for Soluble Salts Removal and Stabilization of Heavy Metals in Municipal Solid Waste Incineration Fly Ash. J. Environ. Manag. 2019, 240, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, Z.; Li, J.; Bai, T.; Chen, Y. Heavy Metals Behavior of Co-Combustion Ash from Sewage Sludge and Coal Slime: Temperature and Mixing Ratio Dependence, Interactions and Speciation. J. Clean. Prod. 2024, 434, 140435. [Google Scholar] [CrossRef]
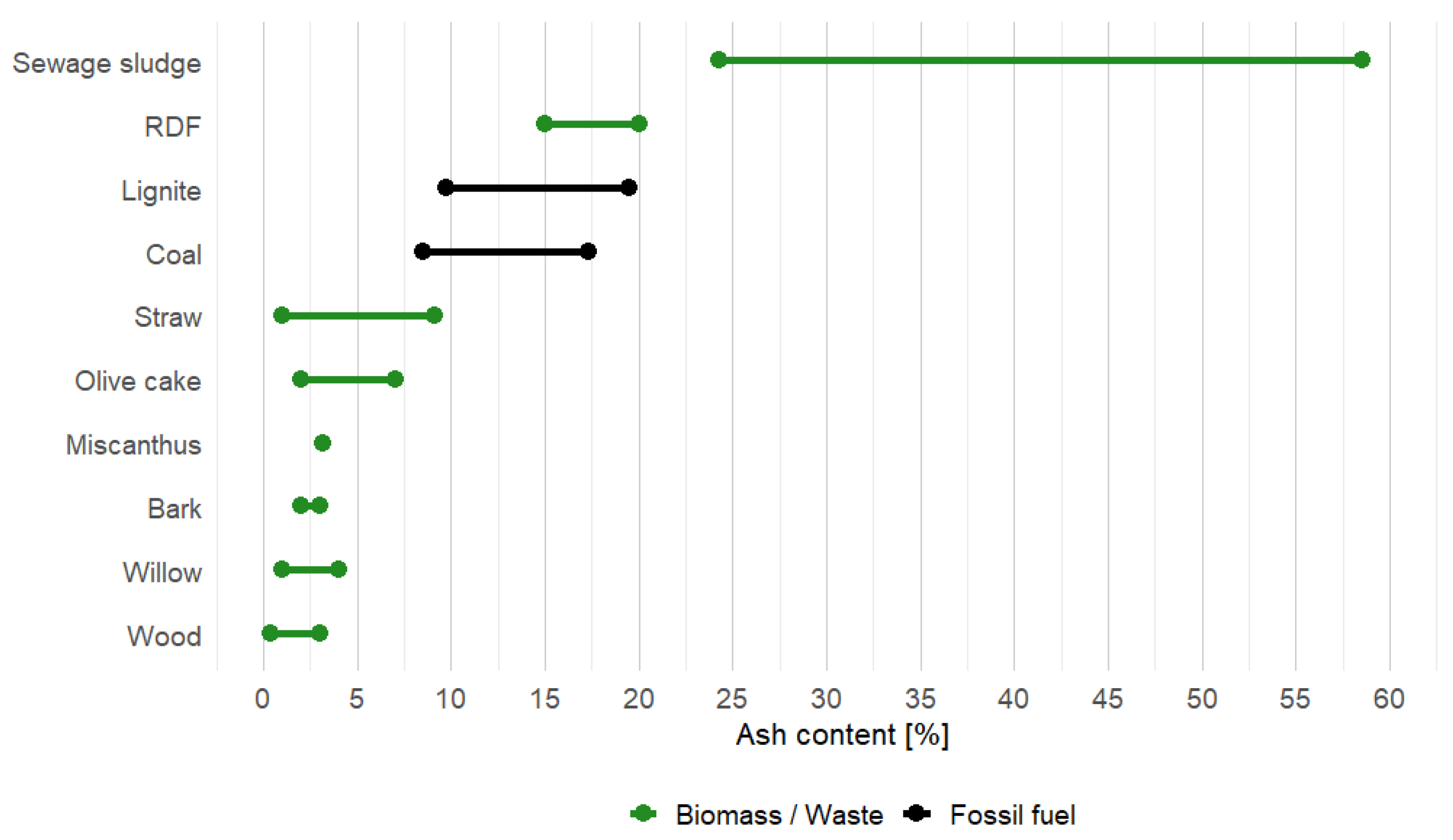
| Fuel | Ash (d.b.) | Moisture (a.r.) | Lower Heating Value (d.b.) | Carbon (d.b.) | Hydrogen (d.b.) | Nitrogen (d.b.) | Sulfur (d.b.) | Chlorine (d.b.) |
|---|---|---|---|---|---|---|---|---|
| A | M | LHV | C | H | N | S | Cl | |
| % | % | MJ/kg | % | % | % | % | % | |
| Coal | 8.5–17.3 | 2–10 | 22–32 | 65.4–87 | 3.5–5 | 0.8–1.5 | 1.2 | <0.6 |
| Lignite | 9.7–19.5 | 10.7 | 16.6 | 43.5 | 4.9 | 0.7 | 2.6 | <0.3 |
| Sewage sludge | 24.3–58.5 | 1.8–60 | 12.5–17.3 | 31.8–36.6 | 4.1–5.9 | 3.7–6.9 | 1.3 | 0.1 |
| RDF | 15–20 | 2.5 | 19.9 | 48.5 | 6.4 | 0.9 | 0.2 | 0.5 |
| Wood | 0.4–3.0 | 50–60 | 18.4–20 | 48–52 | 6.0–6.4 | 0.1–0.5 | <0.05 | <0.06 |
| Bark | 2–3 | 45–65 | 18.5–23 | 48–52 | 5.7–5.8 | 0.3–0.8 | <0.05 | <0.03 |
| Willow | 0.98–4.0 | 8.9–60 | 18.4–19.2 | 47–51 | 5.8–6.7 | 0.2–0.8 | <0.1 | <0.05 |
| Straw | 1.0–9.1 | 10–25 | 16.6–17.4 | 44.3–47 | 5.6–6.0 | 0.4–0.9 | <0.2 | <1.0 |
| Olive cake | 2–7 | 60–70 | 17.5–19 | 48–50 | 5.5–6.5 | 0.5–1.5 | <0.2 | 0.1 |
| Miscanthus | 3.2 | 10.0 | 17.9 | 47.6 | 5.8 | 0.7 | 0.2 | 0.2 |
| Type of Biomass | SiO2 | CaO | K2O | P2O5 | Al2O3 | MgO | Fe2O3 | SO3 | Na2O | TiO2 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
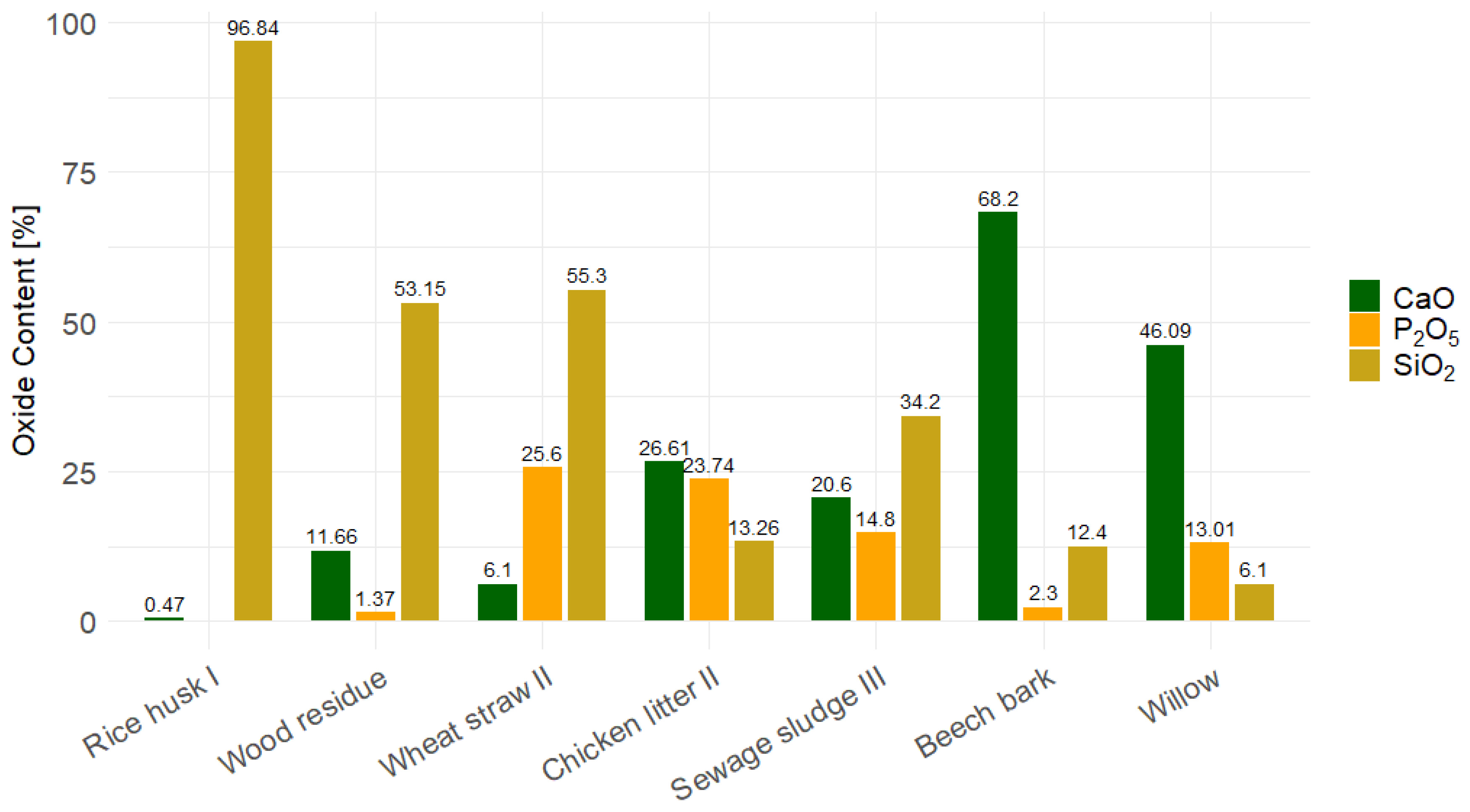
| Rice husk I | 96.84 | 0.47 | 0.81 | n.d. | 1.03 | 0.32 | 0.38 | n.d. | 0.03 | n.d. | [44] |
| Rice husk II | 94.91 | 0.98 | 1.67 | n.d. | 0.37 | 0.26 | 0.79 | 0.09 | 0.02 | n.d. | [57] |
| Rice husk III | 92.19 | 0.09 | 0.05 | n.d. | 0.09 | 0.41 | 0.10 | 0.41 | 1.64 | n.d. | [58] |
| Rice straw I | 79.82 | 0.37 | 1.07 | 3.75 | 1.13 | 7.54 | 0.25 | n.d. | 0.50 | n.d. | [59] |
| Rice straw II | 74.67 | 3.01 | 12.30 | 1.41 | 1.04 | 1.75 | 0.85 | 1.24 | 0.96 | n.d. | [60] |
| Pine chips | 68.18 | 7.89 | 4.51 | 1.56 | 7.04 | 2.43 | 5.45 | 1.19 | 1.2 | 0.55 | [20] |
| Miscanthus I | 56.42 | 10.77 | 19.75 | 5.54 | 0.79 | 3.01 | 0.94 | 2.28 | 0.47 | 0.03 | [45] |
| Miscanthus II | 58.78 | 11.90 | 3.65 | 5.44 | 1.83 | 2.25 | 3.42 | 0.45 | n.d. | n.d. | [50] |
| Miscanthus III | 37.2 | 3.1 | 48.6 | 4.00 | n.d. | 2.3 | n.d. | n.d. | 0.1 | n.d. | [51] |
| Wood residue | 53.15 | 11.66 | 4.85 | 1.37 | 12.64 | 3.06 | 6.24 | 1.99 | 4.47 | 0.57 | [45] |
| Wheat straw I | 40.16 | 8.82 | 34.95 | 1.12 | 0.79 | 2.60 | 0.60 | 3.13 | 2.14 | n.d. | [56] |
| Wheat straw II | 55.3 | 6.1 | 25.6 | 1.3 | 1.9 | 1.1 | 0.7 | 4.4 | 1.7 | 0.1 | [53] |
| Wheat straw III | 64.38 | 4.12 | 8.51 | n.d. | 2.83 | 1.98 | 1.38 | n.d. | n.d. | 1.02 | [52] |
| Type of Biomass | SiO2 | CaO | K2O | P2O5 | Al2O3 | MgO | Fe2O3 | SO3 | Na2O | TiO2 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Swine solid waste | 6.24 | 25.70 | 12.5 | 24.00 | 1.38 | 7.42 | 3.92 | 8.95 | 2.08 | 0.14 | [87] |
| Pig manure | n.d. | 11.46 | 8.99 | 21.49 | n.d. | 9.50 | 2.11 | n.d. | 1.63 | n.d. | [88] |
| Chicken litter I | 7.13 | 28.18 | 20.01 | 22.49 | 1.11 | 6.48 | 4.11 | 1.01 | 6.26 | 0.51 | [38] |
| Chicken litter II | 13.26 | 26.61 | 16.54 | 23.74 | 2.81 | 5.72 | 1.84 | 0.82 | 5.74 | 0.43 | [38] |
| Bone meal I | 2.95 | 41.7 | 2.75 | 36.2 | 0.5 | n.d. | 0.4 | n.d. | 5.9 | n.d. | [84] |
| Bone meal II | n.d. | n.d. | n.d. | 41.0 | 0.0 | 0.33 | 0.014 | n.d. | n.d. | n.d. | [86] |
| Bone meal III | n.d. | n.d. | n.d. | 39.5 | 0.0 | 0.33 | 0.014 | n.d. | n.d. | n.d. | [85] |
| Sewage sludge I | 37.65 | 11.70 | 1.86 | 17.20 | 8.26 | 3.56 | 14.17 | n.d. | 0.69 | 0.94 | [89] |
| Sewage sludge II | 30.20 | 29.49 | 1.87 | 14.72 | 7.36 | 2.83 | 5.80 | 4.20 | 2.07 | 0.58 | [42] |
| Sewage sludge III | 34.2 | 20.6 | 1.7 | 14.8 | 12.6 | 1.9 | 4.7 | 2.8 | 1.0 | 0.9 | [46] |
| Willow | 6.1 | 46.09 | 23.4 | 13.01 | 1.96 | 4.03 | 0.74 | 3.00 | 1.61 | 0.06 | [45] |
| Corn stalk | 38.70 | 11.11 | 24.47 | 7.05 | 0.92 | 1.10 | 0.93 | 2.12 | 0.17 | 0.10 | [90] |
| Blue-green algae | 3.51 | 5.39 | 24.62 | 44.73 | 0.80 | 9.55 | 1.20 | 2.27 | 7.24 | n.d. | [91] |
| Jatropha seed cake | 4.89 | 29.71 | 22.43 | 11.42 | 5.97 | 16.95 | 0.42 | 1.98 | 2.83 | 3.40 | [92] |
| Type of Biomass | SiO2 | CaO | K2O | P2O5 | Al2O3 | MgO | Fe2O3 | SO3 | Na2O | TiO2 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beech bark | 12.40 | 68.20 | 2.60 | 2.30 | 0.12 | 11.50 | 1.10 | 0.80 | 0.90 | 0.10 | [105] |
| Poplar bark | 1.86 | 77.31 | 8.93 | 2.48 | 0.62 | 2.36 | 0.74 | 0.74 | 4.84 | 0.12 | [45] |
| Eucalyptus | n.d. | 57.74 | 9.29 | 2.35 | n.d. | 10.91 | n.d. | n.d. | 1.86 | n.d. | [106] |
| Willow | 6.1 | 46.09 | 23.4 | 13.01 | 1.96 | 4.03 | 0.74 | 3 | 1.61 | 0.06 | [45] |
| Elm bark | 4.48 | 83.46 | 5.47 | 1.62 | 0.12 | 2.49 | 0.37 | 1.00 | 0.87 | 0.12 | [105] |
| Pine wood pellets | 1.5 | 40.9 | 14.3 | 2.9 | 1.0 | 7.1 | 0.6 | 2.4 | 0.3 | n.d. | [107] |
| Pine chips | 5.49 | 45.42 | 11.31 | 7.14 | 4.02 | 10.90 | 0.95 | n.d. | 0.05 | n.d. | [108] |
| Bone meal I | 2.95 | 41.7 | 2.75 | 36.2 | 0.5 | n.d. | 0.4 | n.d. | 5.9 | n.d. | [84] |
| Poultry litter | 2.69 | 65.17 | 6.36 | 17.46 | 0.31 | n.d. | 0.57 | n.d. | 2.48 | 0.02 | [109] |
| Type of Biomass | Cl | Ref. |
|---|---|---|
| Straw I | 21.1 | [128] |
| Straw II | 30–31 | [129] |
| Poultry litter I | 6.36 | [130] |
| Poultry litter II | 5.67 | [38] |
| Cattle manure I | 7.56 | [38] |
| Cattle manure II | 6.55 | [38] |
| Wheat straw | 4.26 | [131] |
| RDF I | 5.07 | [12] |
| RDF II | 6.99 | [132] |
| Rice straw I | 5.63 | [133] |
| Rice straw II | 4.06 | [59] |
| Type of Biomass | Cd | Cr | Pb | Ni | Hg | Cu | Zn | Ref. |
|---|---|---|---|---|---|---|---|---|
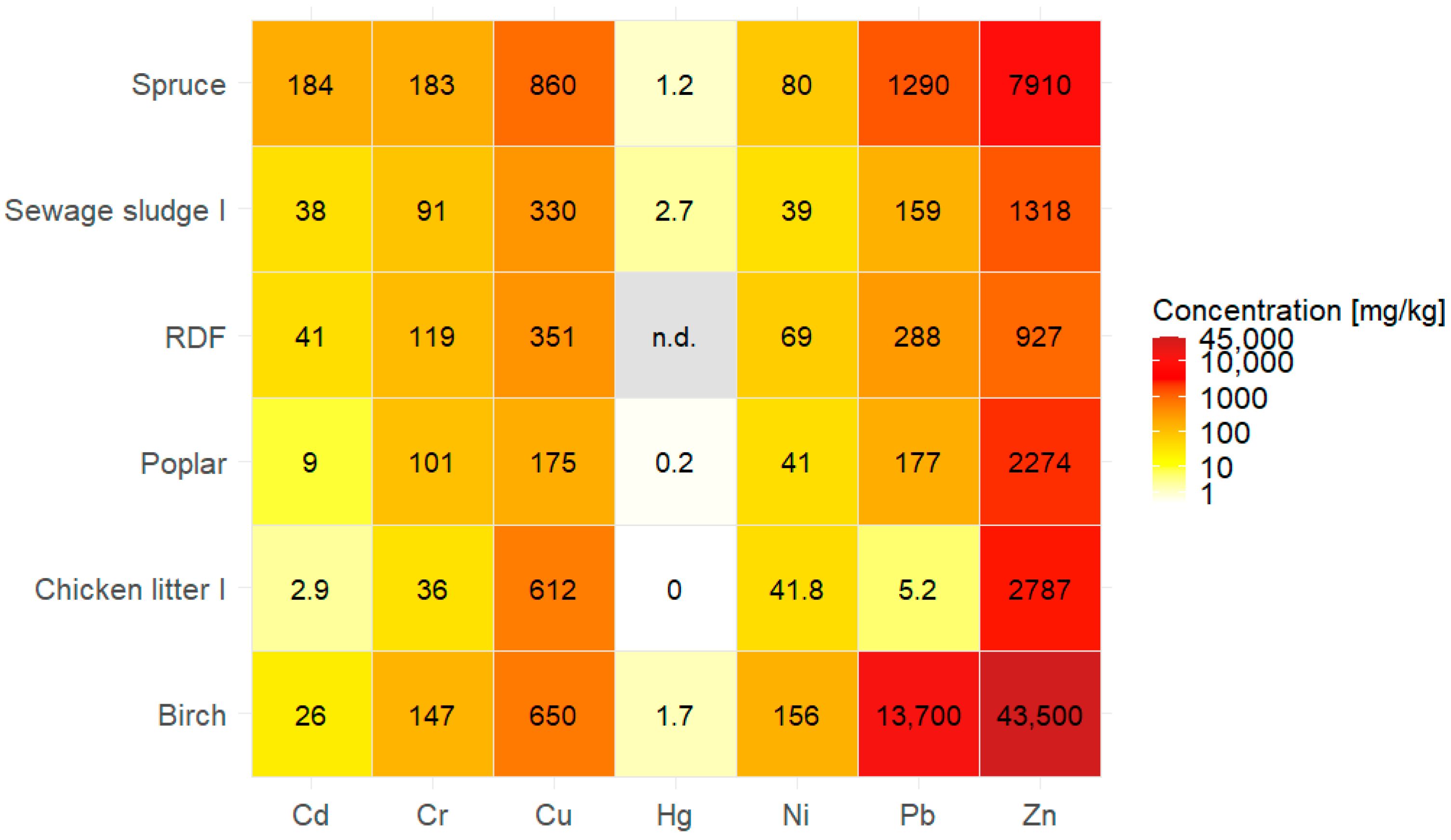
| Birch | 26–203 | 147–508 | 37–13,700 | 18–156 | 0.2–1.7 | 138–650 | 3910–43,500 | [106] |
| Spruce | 4–184 | 183–342 | 8–1290 | 1.4–80 | 0.1–1.2 | 294–860 | 2630–7910 | |
| Poplar | 9 | 101 | 177 | 41 | 0.2 | 175 | 2274 | |
| Sewage sludge I | 38 | 91 | 159 | 39 | 2.7 | 330 | 1318 | [148] |
| Sewage sludge II | 7 | 130 | 285 | 57 | n.d. | 1175 | 2372 | [149] |
| RDF | 41 | 119 | 288 | 69 | n.d. | 351 | 927 | [150] |
| Municipal waste | 36.7 | 157.0 | 1515.0 | n.d. | 35.8 | 563.2 | 3269.0 | [151] |
| Chicken litter I | 2.93 | 36 | 5.24 | 41.8 | <0.05 | 612 | 2787 | [38] |
| Chicken liter II | 0.89 | 20.0 | 3.86 | 74.0 | 7.42 | 600 | 2312 | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maj, I.; Niesporek, K.; Płaza, P.; Maier, J.; Łój, P. Biomass Ash: A Review of Chemical Compositions and Management Trends. Sustainability 2025, 17, 4925. https://doi.org/10.3390/su17114925
Maj I, Niesporek K, Płaza P, Maier J, Łój P. Biomass Ash: A Review of Chemical Compositions and Management Trends. Sustainability. 2025; 17(11):4925. https://doi.org/10.3390/su17114925
Chicago/Turabian StyleMaj, Izabella, Kamil Niesporek, Piotr Płaza, Jörg Maier, and Paweł Łój. 2025. "Biomass Ash: A Review of Chemical Compositions and Management Trends" Sustainability 17, no. 11: 4925. https://doi.org/10.3390/su17114925
APA StyleMaj, I., Niesporek, K., Płaza, P., Maier, J., & Łój, P. (2025). Biomass Ash: A Review of Chemical Compositions and Management Trends. Sustainability, 17(11), 4925. https://doi.org/10.3390/su17114925