Abstract
Recently, the ecological awareness of society and the need to take care of the natural environment have increased significantly. There is also an urgent problem of searching for new, environmentally friendly, and safe for people plant protection techniques using biological preparations, which reduce the intensive and cause significant health problems chemical protection. The study was conducted in a foil tunnel on the ‘Red House’ cultivar roses grown using an adapted method with shoot bending. Maintaining their health under tunnel conditions is often problematic. The study determined the effect of biopreparations on plant health (disease index), photosynthesis parameters, and gas exchange, as well as the species composition of fungi inhabiting roses. The preparations used did not negatively affect the process of photosynthesis and gas exchange. Among the 25 species of fungi obtained from its organs, the polyphagous species Botrytis cinerea dominated; the organs were very often colonized by fungi from the genera Fusarium, Phoma, and Alternaria alternata. The highest concentration of the Biosept 33 SL biopreparation shows a protective effect similar to that of the preparations used in chemical protection, and the degree of leaf blade infection shows a similar level.
Keywords:
biological protection; biopreparations; diseases; fungi; rosa; gas exchange; photosynthesis 1. Introduction
The optimum goal for each producer is to achieve economic benefits at the lowest possible cost, and ecological aspects usually recede into the background. Especially nowadays, it is very important to find a balance between social, economic, and ecological aspects, which is extremely important for the sustainable management of the production of ornamental plants under cover. The production of roses under such conditions is extremely energy and labor-intensive. Rose (Rosa L.) is the most important and popular type of ornamental plant grown for cut flowers. It is not without reason that it is called the queen of flowers, with a huge number of varieties differing in color, shape, and size of flowers that can satisfy the tastes of every consumer. Cut roses are most often bought by customers all year round. In the global market turnover of cut flowers, roses have been in first place for years. The area of rose cultivation is constantly increasing, even in Europe, despite high competitiveness, mainly from South American and African countries [1,2,3,4,5,6].
An alternative to increasing competition and a way of reducing the cost of cut flower production is the introduction of new and innovative cultivation methods. In recent years, the method of growing roses with shoot/stem bending has become increasingly used in greenhouse cultivation [7,8,9,10]. In modern covered cultivation, using the shoot bending method, a specific shape of the rose bush is obtained by pulling out and bending the primary shoot, followed by pulling out the flower shoots and cutting them appropriately, and simultaneously bending the thin and sterile shoots, i.e., low-value shoots [11].
In our climatic conditions, one of the cost-saving methods of producing cut flower roses is cultivation in foil tunnels without heating. Growing roses in unheated foil tunnels, due to the specific conditions (variable thermal conditions between day and night result in condensation of water vapor and high air humidity, location of shoots close to the ground, and high plant density), is not easy and requires highly skilled producers [3]. The large-scale use of pesticides during the production of ornamental plants under covers is a significant problem. In particular, their frequent use against pathogens and pests to obtain roses of a high-quality marketable crop. Their very frequent use has an adverse effect on the health of employees, especially with long-term exposure to the active substances through contact or inhalation, and being in an environment often with limited ventilation, high evaporation, and humidity [12,13,14,15,16,17]. Roses grown in tunnels are characterized by high quality [3].
Furthermore, the low investment in plantation establishment and low maintenance costs, as well as the increase in flowering, and the unwavering popularity of high-quality roses, make it necessary to optimize rose cultivation technologies by introducing new methods of cutting and shaping bushes and changing the method of cutting flower shoots [18]. Methods of forming rose bushes with the bending of some shoots ensure an increase in the number of photosynthetic leaves. Also, flower cutting should be carried out in such a way that the assimilation mass is not depleted [8,9,10,19,20]. The method of growing roses with the bending of shoots is used for year-round greenhouse cultivation. However, it is also possible to use this method in unheated foil tunnels [3,21].
This necessitates systematic chemical protection. An alternative is to use biological preparations that induce plant resistance to pathogenic factors and improve their health. In the cultivation of ornamental plants under covers, especially roses, the use of pesticides on a very large scale and their undesirable effect on the health of people involved in plant cultivation is a very serious problem. This is related not only to their long-term effects in the form of hematological changes in the blood and diseases of the cardiovascular, respiratory, nervous, and digestive systems, but above all to cancer [12,13,14].
Pesticide residues in ornamental plants (although they are often not considered like food products) are problematic in the form of allergies, even among people who are not directly related to flower production, but are involved in their trade, i.e., employees of flower shops and florists, and even their buyers (consumers), are affected by respiratory allergies or skin allergy problems after contact with plants [14,15,17,22,23]. Concerned for human health and the environment, the European Community has acted by introducing directives to reduce chemical protection products in favor of biological preparations [24,25].
In the method of cultivation in a foil tunnel, a major drawback is the maintenance of leaf health on bent shoots that increase the assimilation area of the bush. Therefore, research has been undertaken into the use of health biostimulants to maintain high-quality harvest and to keep the bushes in good condition throughout the growing season, and their effect on gas exchange parameters. Temperature and light are the main factors that determine the rate of growth and development, and thus significantly affect the yield of roses [26,27,28]. The rate of gas exchange is influenced not only by the conditions in the greenhouse, but also to a large extent by the position of shoots on the bush and leaves on them [29,30,31,32].
The physiological processes carried out by the plant are significantly influenced not only by the environmental conditions, but also by the health of the leaves and whole plants. Leaf-inhabiting pathogens lead to a rapid reduction in the process of photosynthesis and gas exchange. They cause morphological, physiological, and biochemical changes, such as a significant decrease in the amount of photosynthetic pigments, thereby limiting the rate of photosynthesis and the resulting reduction in the efficiency of the assimilation apparatus [30,33,34,35,36,37,38,39].
Biopreparations increase yield and plant health by influencing physiological processes such as water, nutrient transport, photosynthesis, and gas exchange, and increase plant resistance to stress conditions, or are immunity activators and, above all, are safe for humans and the environment. In the biopreparation Biosept 33 SL, the biologically active substances are primarily endogenous flavonoids and aliphatic aldehydes, monoterpenes, nootkatone, and glycosides consisting predominantly of naringin. The presence of these substances in the biopreparation is related to their in vitro and in vivo activity. The active compounds contained in this biopreparation act directly on pathogenic factors, inhibiting the development of these microorganisms and at the same time inducing plant resistance, inhibiting the germination of spores, and limiting the growth of germ hyphae by dehydrating the cytoplasm. The preparation causes a decrease in the number of soil pathogens and deformation of their spores, and the disintegration of mycelium hyphae. The treatment of plants with chitosan, which is a biologically active substance in Biochikol 020 PC, is accompanied by phenomena such as increased lignification and the production of phytoalexins and hydrolytic enzymes, which are factors of induced resistance. Chitosan and its derivatives activate a number of immune responses in the plant, such as chitinase synthesis, proteinase inhibitor synthesis, lignification, phytoalexin production, callose deposition, and changes in cell membrane permeability. The biological activity of chitosan is practically manifested by limiting infections of fungi, bacteria, viruses, and viroids [24,40,41,42,43,44,45,46,47,48]. It is also becoming particularly expedient to introduce biopreparations for rose protection in light of the new European Union regulations.
Photosynthetic activity can be determined by parameters such as photosynthetic intensity (Pn), transpiration (E), stomatal conductance (Gs), and intercellular carbon dioxide concentration (Ci) [30,31,49,50,51]. These parameters can also be used to determine the physiological state of the plant as a result of cultivation conditions or care treatments, including the removal or damage to leaves, flowers, fruits or growth cones, or the root system, as well as environmental factors such as wind, hail, and feeding pests that cause mechanical damage to plants. As a result of these stress factors, the hormonal balance is disturbed, which in turn leads to growth and development disorders and consequently to significant changes in the plant’s supply of photosynthetic products. An excess or deficiency of assimilates can affect the intensity of photosynthesis [52,53,54,55].
The aim of this research is to examine the effects of biostimulants on plant health and photosynthesis parameters. The achieved results will allow the development of a method to use health biostimulants to obtain high-quality crops and maintain bushes in good condition throughout the growing season, thereby reducing the use of pesticides.
2. Materials and Methods
2.1. Experimental Design
The research material consisted of roses of the ‘Red House’ variety. It belongs to the tea hybrids—Rosa thea hybrida (TH), the group of large-flowered varieties with medium growth strength. In greenhouse conditions, the shoots reach 50 cm to 60 cm in length and are characterized by weak thorniness, on which large flower buds are placed. Light green leaves, red flowers, without scent (Figure 1).

Figure 1.
(A)—Rose bushes after a strong spring cutting; (B)—shaped rose bush managed with the shoot bending method; (C)—abundantly flowering shrubs of the ‘Red House’ cultivar—first spring bloom; (D)—rose cv. ‘Red House’ at the stage of commercial maturity before harvest (photos by M. Szmagara).
The bushes in the foil tunnel were planted in strips, in two rows 40 cm apart. A distance of 1 m was maintained between the strips of planting. In the rows, the bushes were planted at 25 cm spacing. The experiment was established in completely randomized blocks, in five repetitions, with 4 bushes growing in a plot being one repetition, i.e., 20 bushes for a given combination.
After each growing season in November (3rd decade), and after removing the leaves, the bushes were covered again. In early spring (2nd decade of March), depending on weather conditions, after uncovering the bushes and leveling the soil in the inter-rows, the bushes were pruned. On each bush, depending on the shoot thickness, 3–4 of the strongest shoots were left, pruned above the 3rd dormant bud (Figure 1).
Experiments were conducted each year on bushes prepared this way.
2.2. Weather Parameters
During the conducted research in the foil tunnel, air temperature and humidity measurements were taken, covering the period from the beginning of April to the end of October. The measurements were taken using an automatic air temperature and humidity recorder type AB-171 by Abatronic (Radom, Poland). Monthly average air temperatures and average relative humidity were then calculated.
2.3. Plant Health Assessment
In order to evaluate the effect of biopreparations on plant health, bushes cultivated traditionally and with bent shoots, on which chemical protection was applied, were selected. The second group consisted of shrubs with bent shoots and protected with biopreparations Biosept 33 SL in concentrations of 0.05% and 0.1% and Biochikol 020 PC in concentrations of 0.5% and 2%. The preparations used are considered to be plant resistance stimulants and can be used both prophylactically and as an intervention. The preparation Biosept 33 SL contains 33% of grapefruit (Citrus x paradisi Macfad) seed and pulp extract as a biologically active substance. In Biochikol 020PC, the active substance is chitosan—poly [β-/1,4/2-amino-2-deoxy-D-glucopyranose] (a compound from the group of natural polymers)—20 g per liter of product.
Plant protection was carried out foliar throughout the growing season. A RS- MM 110 03 standard sprayer tip was used to distribute the spray. DMI fungicides were applied at a concentration of 0.1% according to official recommendations. The preparation was applied until the plants were covered with liquid, corresponding to an amount of approximately 30 mL per m2.
This research was carried out during two growing seasons. Plant health was evaluated on the basis of the disease index (DI), which was conducted three times a year, i.e., in spring—in the 2nd decade of May, then a second time—in the 2nd decade of July, and a third time—in the 2nd decade of September based on a 5-grade scale: 0—no symptoms, 1—minor spots on the leaves, 2—necrotic spots on most leaves, 3—wilting of plants and 4—dying of plants. The data were processed using McKinney’s formula [56,57], which generates a numerical disease index (DI) of infestation severity: DI = (Σvn)/(NV) × 100, where v represents the numeric value of the class, n is the number of plants assigned to the class, N is the total number of plants in the repetition, and V is the numerical value of the highest class.
2.4. Mycological Analysis
Fungi were isolated twice during the growing season (in June and September). Then, shoots and leaves with disease symptoms were collected for microscopic and macroscopic laboratory tests. The presence of fungi was determined on the basis of etiological signs found on infected shoots and leaves; these plant organs were subjected to mycological analysis using the artificial culture method [57,58]. The isolates obtained, when brought to the form of pure cultures, were identified to species. The determination of fungi was carried out on the medium used for cultivation or on standard media. Keys and studies on this issue given in Szmagara [59], Marcinkowska [60] and based on Index Fungorum were used to identify fungi.
2.5. The Influence of Biopreparations on Gas Exchange Parameters
To measure the photosynthetic activity of plants, bushes grown in combinations were selected from bushes run via the traditionally controlled approach and by the method of shoot bending. Control I consisted of bushes managed traditionally and chemically protected, while control II consisted of bushes managed by the method with shoot bending and chemically protected. Gas exchange measurements were also taken in plots where bushes were cultivated with the method of shoot bending, and Biosept 33 SL 0.1% and Biochikol 020 PC at a concentration of 2% were applied. On these bushes, measurements were taken on the bent shoots, and the fully developed proper leaves that were located in the middle part of the bent shoots. On the bent shoots, the same leaves remained throughout the growing season.
Measurements were also performed on the erect flowering shoots, which are used to obtain their commercial value, and for fully developed proper leaves, the third leaf counting from the flower bud is always selected. Measurements were carried out five times between the 1st decade of June and the 1st decade of September at intervals of approximately 18–20 days. Measurements were taken on the following dates: 1—the first ten days of June, 2—the first ten days of July, 3—the third ten days of July, 4—the second ten days of August, and 5—the first ten days of September.
During the vegetation period, the following photosynthetic parameters were measured: photosynthetic intensity (Pn) [µmol CO2·m2·s1], transpiration (E) [mmol H2O·m2·s1], stomatal conductance (Gs) [mmol H2O·m2·s1], and intercellular carbon dioxide concentration (Ci) [µmol CO2·m2·s1]. Photosynthetic parameters were measured using a portable infrared gas exchange analyzer—CIRAS-2 Portable Photosynthesis System (Hitchin, Herts, UK). The following conditions were assumed in the analyzer cuvette: external CO2 source, humidity equal to ambient humidity, ambient temperature, and daylight, a measuring cell was placed on the top leaf with a light intensity of 1000 PAR (μmol·m−2·s−1) supplied by a light unit attached to the cuvette [57].
2.6. Statistical Analysis
The obtained results (for the gas exchange and disease index) were statistically analyzed using an ANOVA and Tukey’s confidence intervals at the 5% significance level (α = 0.05). The Pearson correlation coefficients between photosynthesis, transpiration, and the disease index was determined for both types of plots [61,62].
3. Results
3.1. Weather Parameters
During the research period in all years, the highest average air temperature in the foil tunnel occurred in July. In the first year, high temperatures in the foil tunnel were recorded for three summer months (June, July, and August). The average range was from 22.1 °C to 22.5 °C. In the analogous study period the following year, the average monthly temperature did not even exceed 20 °C and ranged between 18.8 °C and 19.6 °C. The lowest average temperature in the spring period, i.e., 13.2 °C, was recorded in April of the first year. That same year, in autumn, the lowest average temperature was recorded in October, at only 11.5 °C, and in September (12.1 °C) the following year of this research (Figure 2).
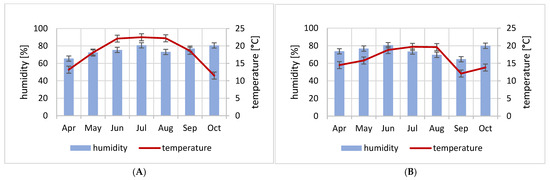
Figure 2.
Average monthly temperature (°C) and relative air humidity (%) in foil tunnel (A)—first year of studies; (B)—second year of studies.
In contrast, the highest monthly average relative humidity above 80% was recorded in July and October of the first year and in June and October of the second year of this research. Throughout the entire study period, the average relative humidity values remained high at around a 70% level, except for April (65.5%) in the first year of the study and September (64.7%) in the second year (Figure 2).
3.2. Plant Health Assessment
Observations of the healthiness of roses in a foil tunnel carried out three times during the growing season showed that disease symptoms occurred on plants both cultivated traditionally, as with shoot bending, and protected chemically or with biopreparations. Characteristic disease symptoms caused by strict pathogens were found on the leaves and the shoots of roses during the growing season, i.e., downy mildew (Peronospora sparsa) and powdery mildew of roses (Sphaeroteca pannosa var. rosae) and black leaf spot (Diplocarpon rosae), as well as other pathogens inhabiting these organs (Figure 3).

Figure 3.
(A)—Disease symptoms caused by strict pathogens on the leaves of the cultivar in late summer. (B)—A diseased leaf of the cv. ‘Red House’ (photos by M. Szmagara).
The average values of the disease index (DI) for both bent and flowering shoots were the lowest on the first test date of the study and increased in the subsequent ones, also, these values differed significantly between the terms. The average value of the disease index of bent shoots increased in the subsequent terms of the study and differed significantly between them. The average disease index of plants showing disease symptoms was the highest on both bent and flowering shoots at the end of the vegetation period, i.e., in the 2nd decade of September, and differed significantly from the other dates (but did not differ from the disease index of flowering shoots in the second date of the study). The average DI of bent shoots at this date was 55.97% in both years of the study, and of flowering shoots, 58.33% (Figure 4).
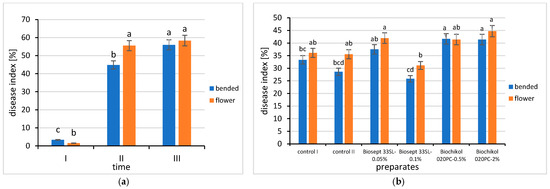
Figure 4.
(a) Average values of the rose shoot disease index during the study period: I—1st decade of May, II—2nd decade of July, III—2nd decade of September; (b) The influence of the bushes forming method and biopreparations on the health of rose shoots based on disease index. Values marked with the same letter are not significantly different (α = 0.05).
The lowest disease index values on bent shoots were recorded for plants treated with a 0.1% concentration of Biosept 33SL (25.83%), and its protective effect was similar to chemical protection, as expressed by the disease index values from both chemically protected control combinations (control II 28.61 and control I—33.33%). The obtained disease index values did not differ significantly from each other. Whereas, values of the DI were recorded for bent shoots protected with both concentrations of Biochikol 020PC (Figure 4).
A similar correlation was observed for flowering shoots, where the lowest average disease index was also the characteristic of plants protected with 0.1% concentration of Biosept 33SL (31.11%), and its value did not differ significantly from the control combinations protected chemically (control II 35.55% and control I—36.11%). In turn, plants sprayed with a 2% concentration of Biochikol 020PC had the highest disease index of leaf blades on flowering shoots, i.e., 44.72% (Figure 4).
3.3. Mycological Analysis
As a result of mycological analysis of rose leaves and shoots during the two years of the study, a total of 2796 isolates of fungi belonging to 25 species were obtained. A total of 1088 isolates, accounting for 38.92%, were collected from the leaves of roses grown in a foil tunnel, and 1708 isolates, accounting for 61.1% of the total fungi isolates, were obtained from shoots (Figure 5).
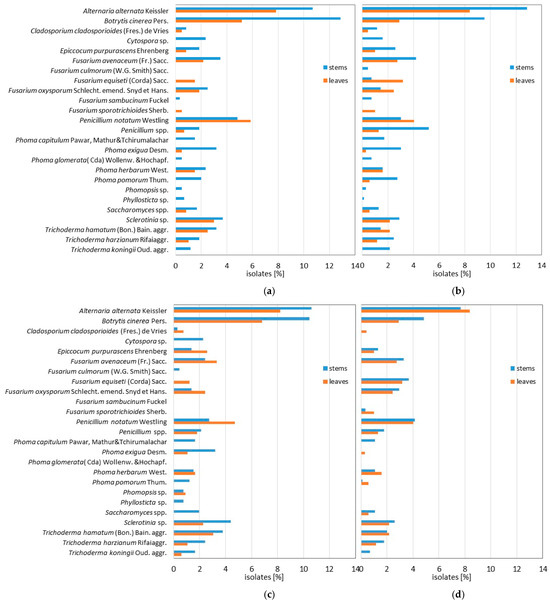
Figure 5.
Fungi colonizing rose shoots and leaves during the study period: (a)—during spring in the first year; (b)—during autumn in the first year; (c)—during spring in the second year; (d)—during autumn in the second year.
Pathogenic fungi of the genus Fusarium were the dominant leaf colonizers during the research period. They accounted for as much as 21.5% of the total number of fungi isolated throughout the entire duration of this study. F. avenaceum and F. oxysporum were isolated most frequently. Very numerous isolates of the polyphagous species Botrytis cinerea were also obtained, accounting for 12.6% of the number of fungi collected from leaves over the entire study period. B. cinerea was obtained in the greatest quantity from these organs during spring periods (Figure 5 and Figure 6). On all study dates, the isolation of A. alternata was very frequent, and its isolates accounted for 20.6% of all fungi obtained from leaves. They were colonized equally frequently by fungi of the genus Phoma, and their isolates represented 6.1% of the isolated fungi. In all study periods, rose leaves were colonized by fungi of the genus Penicillium, including Penicillium notatum and fungi of the genera Cytospora and Sclerotinia (Figure 5 and Figure 6). The polyphagous species Botrytis cinerea was significantly more frequently isolated from shoots, with isolates accounting for 16.6% of all fungi from shoots throughout the study period. The highest number of isolates of this species was obtained in spring, i.e., 12.9% in the first year and 10.5% in the second year of the study. Fungi of the genus Fusarium accounted for 10.5% of the total number of fungi obtained from shoots during the study period (Figure 5 and Figure 6).
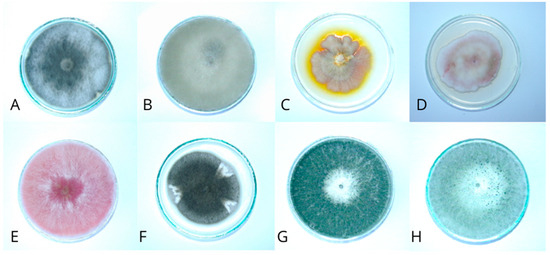
Figure 6.
Eight-day-old colonies on PDA medium: (A)—Alternaria alternata; (B)—Botrytis cinerea; (C)—Epicoccum purpurascens; (D)—Fusarium avenaceum; (E)—Fusarium culmorum; (F)—Phoma glomerata; (G)—Trichoderma harzianum; (H)—Trichoderma koningii.
A. alternata was isolated from shoots very commonly and accounted for 10.7% in the spring of the first year and 12.9% in the autumn, and 10.6% and 7.7% of the fungi obtained from these organs in the second year. Pathogenic species of the genus Phoma were also obtained from shoots in large numbers, and they accounted for 15.2% in the first year and 16.7% in the second year of all fungi obtained from shoots. Fungi of the genus Penicillium were collected from these shoots equally often. Fungi of the genera Phomopsis, Cytospora, and Sclerotinia were also noted on shoots (Figure 5 and Figure 6).
Also, it is worth noting that there was abundant colonization of rose shoots and leaves by saprophytic species of fungi of the genus Trichoderma. T. hamatum was most frequently isolated from shoots in the first and second year of the study in the spring. Numerous isolates of this species were also obtained from leaves in the spring of the second year. Also, numerous isolates of Epicoccum purpurascens were obtained in the first and second years of the study. Most of them were collected in both years of the study from shoots during the autumn period (Figure 5 and Figure 6).
3.4. The Influence of Biopreparations on Gas Exchange Parameters
Measurements of the photosynthetic activity of roses cultivated with the use of biopreparations, regardless of the position of the shoots, the photosynthesis intensity increased until the beginning of July and then gradually decreased until the end of the growing season. Plants sprayed with a 2% concentration of Biochikol 020PC showed a remarkably higher intensity of photosynthesis (Pn), and the obtained numerical values differed significantly from those obtained from bushes grown and protected traditionally, but did not differ from the other average values. The highest photosynthetic intensity was recorded at the second test date, i.e., the turn of the 3rd decade of June and the 1st decade of July, and the numerical values obtained differed significantly from the remaining average values (Figure 7).
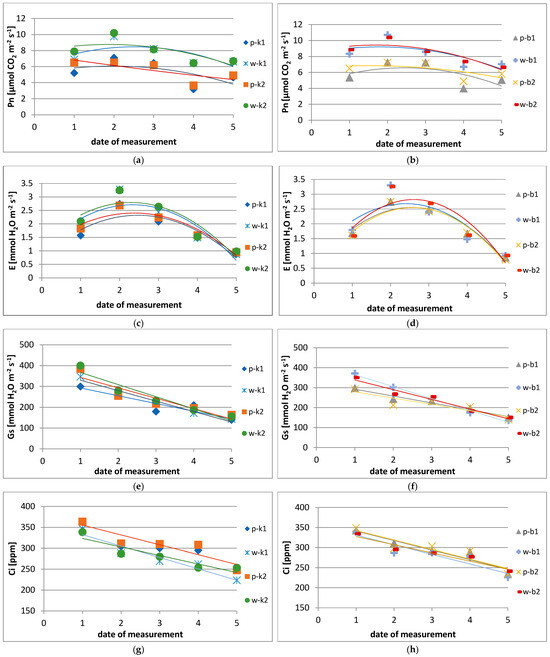
Figure 7.
Photosynthesis and gas exchange parameters depending on the method of protection and shaping of bushes: (a,b)—photosynthesis intensity (Pn); (c,d)—transpiration intensity (E); (e,f)—stomatal conductance intensity (Gs); (g,h)—intercellular carbon dioxide (Ci) concentration. The control combinations (left side) were labeled as follows: pk1-base control 1, wk1-straight control 1, pk2-bent control 2, and wk2-straight control 2. The bioprotected combinations (right side) were labeled as follows: pb1-bent Biosept 0.1%, wb1-straight Biosept 0.1%, pb2-bent Biochikol 2%, and wb2-Biochikol 2%.
However, the highest intensity of photosynthesis in this period was observed in leaves located on erect flowering shoots and sprayed with a 0.1% concentration of Biosept 33SL, slightly lower in the other combinations. On the other hand, leaves located on bent shoots during this period showed the highest photosynthetic intensity in plants sprayed with both biopreparations (Figure 7). A similar relation was also observed in the transpiration intensity (E), and the highest average numerical values differing significantly from the other terms were obtained on the second test date (Figure 7). The highest values of stomatal conductance intensity (Gs) irrespective of the shoot position were recorded on the first measurement terms, i.e., in the 1st decade of June (Figure 7).
In contrast, the leaves of chemically sprayed plants showed the highest intensity of stomatal conductance on erect flowering shoots. Leaves on flowering shoots and those sprayed with Biosept 33SL and Biochikol 020PC also had a similar value. On the other hand, leaves on bent shoots showed the highest mean value of stomatal conductance in plants grown using the adapted method (with shoot bending) and chemically protected (Figure 7). In turn, the highest concentration of intercellular carbon dioxide (Ci) was recorded on the first measurement date and differed from the other dates by decreasing until the end of the vegetation (Figure 7).
4. Discussion
The basis for achieving high harvests in the cultivation of roses in a foil tunnel using the shoot bending method is, first and foremost, the maintenance of high plant health, especially of the leaves and flowers. Pathogens that are not dealt with in time can significantly reduce the yield and deteriorate its quality, or even lead to the complete destruction of the harvest [3,21,63,64]. Maintaining high plant health is hampered by the changing conditions in the foil tunnel, above all, large temperature fluctuations and changes in air humidity. High humidity in autumn is a particularly big concern [3,21].
Nowadays, a very big problem is the use of pesticides to manage pathogens and pests on a very large scale in order to obtain a high-quality yield in the cultivation of ornamental plants under cover, especially roses. This leads to serious health problems for people involved in cultivation, who are particularly exposed to their harmful effects. Employees have, among others, frequently altered blood parameters and problems with cardiovascular, nervous, and digestive diseases, and often suffer from respiratory illnesses and cancer [12,13,14,16,65].
Health problems also affect people who are not directly involved in production but those who are trading flowers, including flower shop employees or florists, and even consumers who buy them in the form of occasional compositions or to decorate their homes and apartments, and come into direct contact with them (pesticide residues in the plants). They are also affected by respiratory allergies as well as contact ones in the form of skin problems [15,17,22,23].
Combining the cultivation of roses in a foil tunnel with the formation of bushes by bending the parts and the use of biological protection can bring tangible benefits in reducing chemicals. This is in line with the new concept of developing horticulture in an integrated and ecological system, and can contribute to protecting the environment and the health of employees.
To date, there has been no research on the effect of the bush forming method combined with the use of biopreparations in the cultivation of roses in foil tunnels. In the present study, the plant disease index used was a reflection of the degree of plant infestation by pathogenic factors and depended on the plant protection method used, the concentration of biopreparations, and the occurrence of weather conditions. Of the biopreparations applied in various concentrations, Biosept 33 SL used at a concentration of 0.1%, demonstrated a favorable effect when the disease indexes of bent shoots were low. The efficiency of the preparation was similar to the chemicals used, as the disease index of plants in control I, chemically protected and traditionally managed, and in control II, where plants were chemically protected and shoot bending was applied, were similar to the disease index of bent shoots protected with Biosept 33SL at a concentration of 0.1%.
The effect of Biosept 33 SL proved to be very beneficial in the conducted studies, and its effectiveness was greater than that of Biochikol 020 PC at both concentrations in the first and second year. A similar relation was also noted for flower shoots. Roses protected with Biosept 33 SL at both concentrations showed similar health to chemically protected bushes. The effective action of Biosept 33 SL in the protection of various plant species is confirmed by many authors [66,67,68,69,70,71,72]. The effect of Biosept 33 SL in the conducted studies was similar to the applied chemical protection. In her studies, Jamiołkowska [71] showed that grapefruit extract (Biosept 33 SL) was more effective than azoxystrobin (Amistar 250 SC) and had a more long-lasting effect, also the substances used delayed fungal spores or inhibited them completely and caused deformation of pathogenic fungal hyphae.
Less effective was the action of Biochikol 020 PC applied at both concentrations, and the value of disease index on the bent shoots was significantly higher than that of the remaining combinations, except for the lowest concentration of Biosept 33 SL. During the period of this study, a high rate of disease on flowering shoots characterized the bushes protected with Biochikol 020 PC at both concentrations, in the second year of the study. Chitosan, which is the active compound in Biochikol 020 PC, is known in the literature as a compound that mobilizes plants to respond rapidly to pathogen attack with so-called elicitors, or inducers of resistance. The activity of the biopreparation may depend significantly on the plant species and variety [73,74]. The fungicidal effectiveness of chitosan also depends on the sensitivity of the fungus species or strain [75,76,77].
The research conducted showed a positive effect of the Biosept 33 SL on the health of roses grown in a foil tunnel. This was expressed by a low infestation of plants, as evidenced by the low disease index. During the term of observations on the influence of the protection method and shaping of bushes on health, it was shown that the average lowest disease index was characteristic of plants protected with Biosept 33 SL at a concentration of 0.1%, and it did not differ from the disease index obtained from control I and II, where the bushes were chemically protected and grown traditionally and with shoot bending. However, the value of the disease index increased on the subsequent observation dates, i.e., the 2nd decade of July and September.
During the vegetation season, plants are exposed to the action of many biotic and abiotic factors. The action and effectiveness of the biological preparation can vary, especially given the multiplicity of influencing factors and the complexity of the processes occurring in the plant. It can be assumed that the plant disease index was influenced not only by the method of protection and the preparations used, but also by the composition of microorganisms inhabiting the organs under study and the physiological properties of the plants, their morphological and anatomical structure, species, and varietal characteristics, weather conditions, or agrotechnical treatments [78,79].
The observations made on the health of roses and the disease index taken into account showed that they were inhabited by strict pathogens causing downy/powdery mildew (Peronospora sparsa), powdery mildew of roses (Sphaeroteca pannosa var. rosae), and black spots on leaves (Diplocarpon rosae). In rose cultivation, they are among the most common and most dangerous, and if not treated in a timely manner, can lead to the total destruction of the harvest [21,63,64,80].
So far, no studies have been carried out on the species composition of fungi colonizing the shoots of roses grown in a foil tunnel, protected with biopreparations and using chemicals. The mycological analyses conducted have shown the influence of the applied protection methods and biopreparations used on the species composition of fungi inhabiting rose shoots and leaves. Particularly important in terms of the decorative value of roses and the photosynthetic process is the abundance of many species of fungi inhabiting the assimilation apparatus. Also essential seems to be fairly frequent isolation of Alternaria alternata, which is considered in many plant species to be the main colonizer of leaves, producing large quantities of toxins, leading to rapid necrosis of the colonized tissues, and consequently, leading to a decrease in the photosynthetic efficiency of plants [81,82,83].
Especially dangerous appears to be the colonization of leaves by pathogenic species, which leads to a rapid reduction in photosynthesis and gas exchange, even during the initial period of disease, when there are no visible symptoms of tissue infection by pathogens. Pathogens also cause morphological, physiological, and biochemical changes, such as a significant decrease in the amount of photosynthetic pigments, and thus reduce the rate of photosynthesis and the resulting decrease in the efficiency of the assimilation apparatus [34,35,36,37,38,39].
The population of fungi inhabiting rose shoots was extensive. The main colonizer of shoots was A. alternata; B. cinerea was also isolated from shoots much more frequently. Most isolates of pathogenic species came from shoots protected with Biochikol 020PC, and the least from chemical protection. Fungi of the genera Fusarium and Phoma were often isolated, significantly more isolates of these pathogens came from shoots of plants protected with Biochikol 020 PC than with Biosept 33 SL or chemically protected, and the number of pathogens isolated indicates the effectiveness of the preparations applied. Rose shoots were also colonized by fungi of the genera Phomopsis and Phylosticta isolated from bio-protected shoots. Numerous saprophytic species of the genera Trichoderma, Epiccocum, and Cladosporium were also isolated from shoots protected with biopreparations, and single isolates of these fungi were also obtained from shoots of chemically protected plants.
In the aspect of biological protection after the application of both biopreparations, an increase in the number of saprophytic fungi of the Trichoderma, Epiccocum, and Cladosporium genera appears to be important, as their numerous occurrence affects the reduction in pathogenic species [84,85,86]. Fungi of the Trichoderma and Epicoccum purpurascens genera are known for their strong competitive abilities against other mycopathogens, their ability to hyperparasite, and produce toxic metabolites. The occurrence of these fungi can be considered very beneficial due to their use as a factor supporting biological control [21,87,88].
In rose cultivation, the abundant occurrence of Botrytis cinerea, which was very frequently isolated in current studies from the above-ground rose organs, is dangerous. The gray mold caused by this polyphagous species is one of the most dangerous diseases of roses. It causes particularly serious problems in maintaining the health of roses in foil tunnels [89,90], where it is known that the prevailing conditions are favorable for its development. Its ability to infect shoots through young parts and flowers of many horticultural plants, as well as rose flowers, has been claimed. Its high harmfulness to many species of horticultural plants has been confirmed so far, both during growth, harvest, and storage. This polyphagous species is also known for its high competitive abilities and can colonize those parts of plants that were originally inhabited by other pathogens [60,90]. In light of the conducted studies, the infection of the above-ground parts of roses should be considered dangerous, which was confirmed by the results of mycological analyses.
The equally frequent isolation of fungi of the genera Fusarium and Phoma from plants entitles the assumption that they may impair the health of roses grown in foil tunnels, as they are known to be highly damaging to many horticultural plant species [21].
A study of the effect of the protection method and the type of biopreparation used on the photosynthetic activity parameters of the plants showed a tendency to improve them. However, in the second year of the study, plants protected with Biosept 33SL were characterized by a significantly lower intensity of stomatal conductance compared to traditionally grown and chemically protected bushes. Ribeiro et al. [91] observed that the intensity of CO2 assimilation and stomatal conductance decreases in plants infected with pathogenic factors, which may also have occurred in the present studies.
In addition, the biopreparations used belong to the so-called biostimulants or bioregulators, which support plant resistance mechanisms and are characterized by a wide range of effects. They affect plant life processes such as photosynthesis, gas exchange, transport of nutrients and water, and increase the resistance of plants to stress conditions, therefore affecting the harvest and plant health [41,42,44,45,48].
With the use of biopreparations, there is also no problem with their negative impact on the health of people working in their cultivation, as in the case of pesticides, which lead to serious health problems for both employees and plant buyers. Pesticide residues in plant tissues also adversely affect the health of people not directly related to cut flower production, but employees involved in their trade, florists, or even buyers [12,13,17,23].
Due to the mode of action and active ingredient, biopreparations are safe for humans and the environment and can significantly affect plant health [24,43,44]. The introduction of biopreparations for the protection of roses in light of the new European Union regulations is also becoming particularly expedient. Concerned for human health and the environment, the European Community has taken action by introducing directives to limit chemical plant protection products in favor of biological preparations [24,25].
5. Conclusions
The biopreparation Biosept 33 SL at its highest concentration shows a similar protective effect to the chemical protection preparations used. The degree of leaf blade infestation in plants protected with this biopreparation, as well as in the control combinations protected chemically, shows a similar level. The low disease index of plants sprayed with this biopreparation indicates its high suitability for protecting roses in foil tunnels.
The disease index of bent and flowering shoots is comparable in the early vegetation season, with the highest values shown by flowering shoots in the autumn. The polyphagous species Botrytis cinerea, which causes gray mold of infected organs, and the fungi of the Penicillium and Alternaria genera, which very often infest the aboveground organs of roses, especially when using bent shoots arranged horizontally close to the ground. Roses were colonized by fungi of the genera Fusarium and Phoma considered pathogenic to many plants.
The course of the intensity of the photosynthetic parameters was shown to vary according to the timing of the measurements. The highest values of these parameters were recorded in the peak part of the vegetation season, then their values systematically decreased towards the end of the vegetation. In general, flowering shoots showed significantly higher values for gas exchange parameters compared to bent shoots, which is also related to their lower infestation.
The conducted research has shown the high effectiveness of biological preparations in the protection of roses, especially Biosept 33SL. Introducing new cultivation methods for rose production and combined with biological protection, is consistent with the new concept of horticulture development in an integrated and ecological system. It can contribute to both reducing the use of chemical protection agents as well as ensuring high yields of roses in foil tunnels, combined with their good health, but this requires further research.
Author Contributions
Conceptualization, M.S.; methodology, M.S., M.K. and B.S.-B.; software, M.S., A.J., W.D. and B.M.; validation, M.S., M.K. and B.S.-B.; formal analysis, M.S., A.S., B.M., W.D. and A.J.; investigation, M.K. and M.S.; resources, M.S.; data curation, M.S.; writing—original draft preparation, M.S. and M.K.; writing—review and editing, M.S. and A.S.; visualization, M.S., M.D.-P., E.P. and A.S.; supervision and project administration, M.S. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DI | Disease Index |
| PAR | Photosynthetically Active Radiation |
| PDA | Potato Dextrose Agar |
References
- Groshkoff, I.; Yakimova, E. Study on the Yield and Cut Flower Quality of Rosa Thea Hybrida. Biotechnol. Biotechnol. Equip. 2000, 14, 75–77. [Google Scholar] [CrossRef][Green Version]
- Jabłońska, L. Ekonomiczne Aspekty Rozwoju Sektora Kwiaciarskiego w Polsce; Wydawnictwo SGGW: Warszawa, Poland, 2007; ISBN 83-7244-856-6. [Google Scholar]
- Hetman, J.; Szmagara, M. Produkcja Róż w Tunelach Foliowych. In Ogrodnictwo Ozdobne Sektorem Gospodarki Narodowej; Wyd SGGW: Warszawa, Poland, 2013; pp. 73–80. [Google Scholar]
- Van ’T Ooster, A.; Bontsema, J.; Van Henten, E.J.; Hemming, S. Model-Based Analysis of Skill Oriented Labour Management in a Multi-Operations and Multi-Worker Static Cut Rose Cultivation System. Biosyst. Eng. 2015, 135, 87–102. [Google Scholar] [CrossRef]
- Monder, M.J. Wybrane Problemy i Kierunki Współczesnej Hodowli Róż. Wiad. Bot. 2017, 61, 1–27. [Google Scholar] [CrossRef]
- Darras, A.I. Implementation of Sustainable Practices to Ornamental Plant Cultivation Worldwide: A Critical Review. Agronomy 2020, 10, 1570. [Google Scholar] [CrossRef]
- Kajihara, S.; Katsutani, N. Effect of Mother-stem Length on Cut Flower Stem Yield and Characteristic Form during the High-rack Training System for Rose Plants. Hortic. Res. Jpn. 2008, 7, 47–50. [Google Scholar] [CrossRef]
- Kajihara, S.; Itou, J.; Katsutani, N.; Goto, T.; Shimaji, H. Partitioning of Photosynthates Originating from Bent Shoots in the Arching and High-Rack Culture Systems of Cut Rose Production. Sci. Hortic. 2009, 121, 485–489. [Google Scholar] [CrossRef]
- Szmagara, M.; Hetman, J.; Pudelska, K.; Kozak, D.; Marcinek, B.; Dudkiewicz-Pietrzyk, M. The Effect of Shoot Bending and Rootstock on Quantity and Quality of Cut Flower of Rose Cv. ‘Red House’ Yield/Wpływ Przeginania Pędów Oraz Podkładki Na Wielkość i Jakość Plonu Ciętych Róż Odmiany ‘Red House. Acta Sci. Pol. Hortorum Cultus 2016, 15, 65–75. [Google Scholar]
- Ohkawa, K. The Past and the Future of Cut Rose Production and Industry in Japan. Acta Hortic. 2010, 870, 21–28. [Google Scholar] [CrossRef]
- Jerzy, M. Kwiaty Cięte Uprawiane Pod Osłonami; Państwowe Wydawnictwo Rolnicze i Leśne: Poznań, Poland, 2006; ISBN 978-83-09-01826-1. [Google Scholar]
- Maharjan, A.; Gautam, R.; Jo, J.; Acharya, M.; Lee, D.; Bahadur, K.C.P.; Gim, J.; Sin, S.; Kim, H.; Kim, C.; et al. Comparison of Overall Immunity Levels among Workers at Grape Orchard, Rose Greenhouse, and Open-Field Onion Farm. Saf. Health Work 2022, 13, 248–254. [Google Scholar] [CrossRef]
- Maharjan, A.; Gautam, R.; Acharya, M.; Jo, J.; Lee, D.; KC, P.B.; Lee, Y.-A.; Kwon, J.-T.; Kim, H.; Kim, K.; et al. Association of Immunotoxicological Indices with Lung Cancer Biomarkers in Poultry, Grape, and Rose Farming Workers. Toxicol. Res. 2023, 39, 739–747. [Google Scholar] [CrossRef]
- Del Prado-Lu, J. Pesticide Exposure, Risk Factors and Health Problems among Cutflower Farmers: A Cross Sectional Study. J. Occup. Med. Toxicol. 2007, 2, 9. [Google Scholar] [CrossRef]
- Toumi, K.; Joly, L.; Vleminckx, C.; Schiffers, B. Risk Assessment of Florists Exposed to Pesticide Residues through Handling of Flowers and Preparing Bouquets. Int. J. Environ. Res. Public Health 2017, 14, 526. [Google Scholar] [CrossRef]
- Nigatu, A.W. Respiratory Health and Acute Pesticide Intoxications Among Workers in the Flower Farm Industry in Ethiopia. Ph.D. Thesis, University of Bergen, Bergen, Norway, 2017. [Google Scholar]
- Toumi, K.; Vleminckx, C.; Van Loco, J.; Schiffers, B. Pesticide Residues on Three Cut Flower Species and Potential Exposure of Florists in Belgium. Int. J. Environ. Res. Public Health 2016, 13, 943. [Google Scholar] [CrossRef]
- Hetman, J. Uprawy Róż w Tunelu Foliowym. Skierniewice 2008, 22, 25–30. [Google Scholar]
- Kool, M.T.N. System Development of Glasshouse Roses. Ph.D. Thesis, Landbouwuniversiteit Wageningen, Wageningen, The Netherlands, 1996. [Google Scholar]
- Särkkä, L. Yield, Quality and Vase Life of Cut Roses in Year-Round Greenhouse Production. Ph.D. Thesis, Helsingin Yliopisto, Helsinki, Finland, 2005. [Google Scholar]
- Szmagara, M. Grzyby Występujące Na Liściach i Pędach Róż Uprawianych w Tunelu Foliowym. Ann. UMCS Sect. EEE 2013, 23, 21–28. [Google Scholar]
- Riu, E.; Monsó, E.; Marin, A.; Magarolas, R.; Radon, K.; Morera, J.; Andreo, F.; Nowak, D. Occupational Risk Factors for Rhinitis in Greenhouse Flower and Ornamental Plant Growers. Am. J. Rhinol. 2008, 22, 361–364. [Google Scholar] [CrossRef]
- Lensen, G.; Coenraads, P.J.; Jungbauer, F.; Schuttelaar, M.-L. Contact Dermatitis Caused by Chlorothalonil on Imported Roses: Irritant or Allergic Reaction? Contact Dermat. 2011, 65, 50–51. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Hetman, B.; Skwaryło-Bednarz, B.; Kopacki, M. Integrowana Ochrona Roślin w Polsce i Unii Europejskiej Oraz Prawne Podstawy Jej Funkcjonowania. Praca Przeglądowa. Ann. Univ. Mariae Curie-Skłodowska Sect. Agric. 2017, 72, 103–111. [Google Scholar] [CrossRef]
- Wojdyła, A.; Łabanowski, G.; Nowak, J.; Boncela, A.; Ptaszek, M.; Czajka, A.; Włodarek, A. Metodyka Integrowanej Ochrony Róży Uprawianej Pod Osłonami; Instytut Ogrodnictwa: Skierniewice, Poland, 2017. [Google Scholar]
- Shin, H.K.; Lieth, J.H.; Kim, S.H. Effects of Temperature on Leaf Area and Flower Size in Rose. Acta Hortic. 2001, 547, 185–191. [Google Scholar] [CrossRef]
- Bredmose, N.; Nielsen, J. Effects of Thermoperiodicity and Plant Population Density on Stem and Flower Elongation, Leaf Development, and Specific Fresh Weight in Single Stemmed Rose (Rosa hybrida L.) Plants. Sci. Hortic. 2004, 100, 169–182. [Google Scholar] [CrossRef]
- Harada, T.; Komagata, T. Effects of Long-Day Treatment Using Fluorescent Lamps and Supplemental Lighting Using White LEDs on the Yield of Cut Rose Flowers. Jpn. Agric. Res. Q. JARQ 2014, 48, 443–448. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lieth, J.H. Effect of Shoot-Bending on Productivity and Economic Value Estimation of Cut-Flower Roses Grown in Coir and UC Mix. Sci. Hortic. 2004, 99, 331–343. [Google Scholar] [CrossRef]
- Paradiso, R.; De Visser, P.H.B.; Arena, C.; Marcelis, L.F.M. Light Response of Photosynthesis and Stomatal Conductance of Rose Leaves in the Canopy Profile: The Effect of Lighting on the Adaxial and the Abaxial Sides. Funct. Plant Biol. 2020, 47, 639–650. [Google Scholar] [CrossRef]
- González-Real, M.M.; Baille, A.; Gutiérrez Colomer, R.P. Leaf Photosynthetic Properties and Radiation Profiles in a Rose Canopy (Rosa hybrida L.) with Bent Shoots. Sci. Hortic. 2007, 114, 177–187. [Google Scholar] [CrossRef]
- Fanourakis, D.; Matkaris, N.; Heuvelink, E.; Carvalho, S.M.P. Effect of Relative Air Humidity on the Stomatal Functionality in Fully Developed Leaves. Acta Hortic. 2010, 870, 83–88. [Google Scholar] [CrossRef]
- Lobato, A.K.S.; Mc, G.-V.; PS Vidigal, F.; Costa, R.C.L.; Cruz, F.J.R.; Santos, D.G.C.; Silva, C.R.; Li, S.; Ll, S. Changes in Photosynthetic Pigment and Carbohydrate Content in Common Bean Cultivars Infected by Colletotrichum lindemuthianum. Plant Soil Environ. 2009, 55, 58–61. [Google Scholar] [CrossRef]
- Lobato, A.; Gonçalves-Vidigal, M.; Vidigal Filho, P.; Andrade, C.; Kvitschal, M.; Bonato, C. Relationships between Leaf Pigments and Photosynthesis in Common Bean Plants Infected by Anthracnose. N. Z. J. Crop Hortic. Sci. 2010, 38, 29–37. [Google Scholar] [CrossRef]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant Physiology Meets Phytopathology: Plant Primary Metabolism and Plant Pathogen Interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef]
- Alves, A.A.; Guimarães, L.M.D.S.; Chaves, A.R.D.M.; DaMatta, F.M.; Alfenas, A.C. Leaf Gas Exchange and Chlorophyll a Fluorescence of Eucalyptus urophylla in Response to Puccinia psidii Infection. Acta Physiol. Plant. 2011, 33, 1831–1839. [Google Scholar] [CrossRef]
- Polanco, L.R.; Rodrigues, F.A.; Nascimento, K.J.T.; Cruz, M.F.A.; Curvelo, C.R.S.; DaMatta, F.M.; Vale, F.X.R. Photosynthetic Gas Exchange and Antioxidative System in Common Bean Plants Infected by Colletotrichum lindemuthianum and Supplied with Silicon. Trop. Plant Pathol. 2014, 39, 35–42. [Google Scholar] [CrossRef]
- Bispo, W.M.d.S.; Araujo, L.; Moreira, W.R.; Silva, L.d.C.; Rodrigues, F.Á. Differential Leaf Gas Exchange Performance of Mango Cultivars Infected by Different Isolates of Ceratocystis fimbriata. Sci. Agric. 2016, 73, 150–158. [Google Scholar] [CrossRef][Green Version]
- Rios, V.S.; Rios, J.A.; Aucique-Pérez, C.E.; Silveira, P.R.; Barros, A.V.; Rodrigues, F.Á. Leaf Gas Exchange and Chlorophyll a Fluorescence in Soybean Leaves Infected by Phakopsora pachyrhizi. J. Phytopathol. 2017, 166, 75–85. [Google Scholar] [CrossRef]
- Orlikowski, L.; Skrzypczak, C.; Wojdyla, A.; Jaworska-Marosz, A. Wyciagi Roslinne i Mikroorganizmy w Ochronie Roslin Przed Chorobami. Zesz. Nauk. Akad. Rol. W Krakowie Ses. Nauk. 2002, 82, 19–32. [Google Scholar]
- Mikiciuk, M.; Dobromilska, R. Assessment of Yield and Physiological Indices of Small-Sized Tomato Cv ‘Bianka F1’ under the Influence of Biostimulators of Marine Algae Origin. Acta Sci. Pol. Hortorum Cultus 2014, 13, 31–41. [Google Scholar]
- Sultana, V.; Baloch, G.; Ara, J.; Ehteshamul-Haque, S.; Athar, M. Seaweeds as Alternative to Chemical Pesticides for the Management of Root Diseases of Sunflower and Tomato. J. Appl. Bot. Food Qual. 2011, 84, 162–168. [Google Scholar]
- Kiełtyka-Dadasiewicz, A.; Król, B. Efekty Dolistnego Stosowania Bio-Algeenu S90 i Biotrissolu T w Uprawie Serdecznika Pospolitego (Leonurus cardiaca L.). Agron. Sci. 2012, 67, 12–20. [Google Scholar] [CrossRef]
- Basak, A. Biostimulators. Definitions, Classification and Legislation. In Monographs Series: Biostimulators in Modern Agriculture, General Aspects; Gawrońska, H., Ed.; Wieś Jutra: Warszawa, Poland, 2008; pp. 7–17. [Google Scholar]
- Dobromilska, R.; Mikiciuk, M.; Gubarewicz, K. Evaluation of Cherry Tomato Yielding and Fruit Mineral Composition after Using-of Bio-Algeen S-90 Preparation. J. Elem. 2008, 13, 491–499. [Google Scholar]
- Truba, M.; Jankowski, K.; Sosnowski, J. Reakcja roślin na stosowanie preparatów biologicznych. Ochr. Śr. Zasobów Nat. 2012, 53, 41–52. [Google Scholar]
- Dudaš, S.; Šola, I.; Sladonja, B.; Erhatić, R.; Ban, D.; Poljuha, D. The Effect of Biostimulant and Fertilizer on “Low Input” Lettuce Production. Acta Bot. Croat. 2016, 75, 253–259. [Google Scholar] [CrossRef]
- Arunkumar, K.; Sivakumar, S.R.; Rengasamy, R. Review on Bioactive Potential in Seaweeds (Marine Macroalgae): A Special Emphasis on Bioactivity of Seaweeds Against Plant Pathogens. Asian J. Plant Sci. 2010, 9, 227–240. [Google Scholar] [CrossRef]
- Kim, S.; Lieth, J.H. A Coupled Model of Photosynthesis, Stomatal Conductance and Transpiration for a Rose Leaf (Rosa hybrida L.). Ann. Bot. 2003, 91, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Chołuj, D.; Kalaji, H.M.; Niemyska, B. Analysis of the Gas Exchange Components in Chilled Tomato Plants. Photosynthetica 1997, 34, 583–589. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Łoboda, T. Fluorescencja Chlorofilu w Badaniach Stanu Fizjologicznego Roślin; Wyd. 2 popr.; Wydawnictwo SGGW: Warszawa, Poland, 2010; ISBN 978-83-7583-119-1. [Google Scholar]
- Muro, J.; Irigoyen, I.; Lamsfus, C. Effect of Defoliation on Onion Crop Yield. Sci. Hortic. 1998, 77, 1–10. [Google Scholar] [CrossRef]
- Egli, D.B.; Bruening, W.P. Source-Sink Relationships, Seed Sucrose Levels and Seed Growth Rates in Soybean. Ann. Bot. 2001, 88, 235–242. [Google Scholar] [CrossRef]
- Bruening, W.P.; Egli, D.B. Relationship between Photosynthesis and Seed Number at Phloem Isolated Nodes in Soybean. Crop Sci. 1999, 39, 1769–1775. [Google Scholar] [CrossRef]
- Blamowski, Z.; Michałek, W.; Rukasz, I. Wpływ Stresu Mechanicznego Na Wymianę Gazową Oraz Na Wzrost Roślin Rzodkiewki Zwyczajnej i Rzepaku Jarego. Acta Sci. Pol. Hortorum Cultus 2003, 2, 3–11. [Google Scholar]
- Łacicowa, B. Metoda Loboratoryjna Szybkiej Oceny Odporności Jęczmienia Jarego Na Helminthosporium Sorokinianum PK et B. Biul. IHAR 1969, 3–4, 61–62. [Google Scholar]
- Szmagara, M.; Kopacki, M.; Skwaryło-Bednarz, B.; Jamiołkowska, A.; Marcinek, B.; Rysiak, K.; Szmagara, A. Assessment of Biometric Parameters and Health of Canna’s Cultivars as Plant Useful in Phytoremediation of Degraded Agrocenoses. Agriculture 2023, 13, 157. [Google Scholar] [CrossRef]
- Machowicz-Stefaniak, Z.; Zalewska, E. Grzyby Występujące Na Nadziemnych Organach Leszczyny [w:] Monitoring Grzybów; Lisiewska, M., Ławrynowicz, M., Eds.; Sekcja Mikologiczna PTB: Lodz, Poland, 2000; pp. 153–166. [Google Scholar]
- Szmagara, M. Biodiversity of Fungi Inhabiting the Highbush Blueberry Stems. Acta Sci. Pol. Hortorum Cultus 2009, 8, 37–50. [Google Scholar]
- Marcinkowska, J. Oznaczanie Rodzajów Grzybów Ważnych w Patologii Roślin; Fundacja “Rozwój SGGW” [Szkoły Głównej Gospodarstwa Wiejskiego]: Warszawa, Poland, 2010; ISBN 978-83-7274-056-4. [Google Scholar]
- Bordens, K.S.; Abbott, B.B. Research Design and Methods. A Process Approach, 7th ed.; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Raudonius, S. Application of Statistics in Plant and Crop Research: Important Issues. Zemdirb.-Agric. 2017, 104, 377–382. [Google Scholar] [CrossRef]
- Wojdyła, A. Wpływ Związków Strobilurynowych Na Rozwój Diplocarpon rosae. Prog. Plant Prot. 2009, 49, 301–304. [Google Scholar]
- Wojdyła, A. Ocena Skuteczności Środka Olejan 85 EC w Ochronie Róży Przed Sphaerotheca pannosa Var. Rosae i Diplocarpon rosae. Zesz. Probl. Postępów Nauk Rol. 2010, 554, 295–302. [Google Scholar]
- Toumi, K.; Vleminckx, C.; Van Loco, J.; Schiffers, B. A Survey of Pesticide Residues in Cut Flowers from Various Countries. Commun. Agric. Appl. Biol. Sci. 2016, 81, 493–502. [Google Scholar]
- Patkowska, E. Effectiveness of Grapefruit Extract and Pythium oligandrum in the Control of Bean and Peas Pathogens. J. Plant Prot. Res. 2006, 46, 15–28. [Google Scholar]
- Sadowski, C.; Lenc, L.; Korpal, W. Investigations on the Possibility of Protection of Organically Grown Red Beet against Fungal Diseases. J. Res. Appl. Agric. Eng. 2007, 52, 38–44. [Google Scholar]
- Sadowski, C.; Lenc, L.; Łukanowski, A. Phytopathological Aspect of Onion Seed Production in Organic Farm. J. Res. Appl. Agric. Eng. 2009, 54, 80–84. [Google Scholar]
- Kaczmarek-Cichosz, R.; Chojnacki, J. Effectivity of Semi-Dry Seeds Dressing Method with Ecological Plant Protection Agent. J. Res. Appl. Agric. Eng. 2010, 55, 152–155. [Google Scholar]
- Jamiołkowska, A. The Influence of Bio-Preparation Biosept 33SL on Fungi Colonizing of Sweet Pepper Plants (Capsicum annum L.) Cultivated in the Field. Electron. J. Pol. Agric. Univ. 2009, 12, 13. [Google Scholar]
- Jamiołkowska, A. Laboratory Effect of Azoxystrobin (Amistar 250 SC) and Grapefruit Extract (Biosept 33 SL) on Growth of Fungi Colonizing Zucchini Plants. Acta Sci. Pol. Hortorum Cultus 2011, 10, 245–257. [Google Scholar]
- Jamiołkowska, A. Effect of Some Biotechnical Preparations on the Growth of Sweet Pepper Plants in the Field Production. Ann. Univ. Mariae Curie-Skłodowska Sect. EEE 2014, 24, 61–70. [Google Scholar]
- Pospieszny, H. Niektore Aspekty Stosowania Chitozanu w Ochronie Roslin. Prog. Plant Prot. 1997, 37, 306–310. [Google Scholar]
- Pastucha, A. Oddzialywanie Chitozanu Na Grzyby Chorobotworcze Dla Soi. Ann. Univ. Mariae Curie-Skłodowska Sect. EEE Hortic. 2001, 9, 61–70. [Google Scholar]
- Wojdyła, A.; Orlikowski, L. Chitozan w Zwalczaniu Grzybów Odglebowych i Nalistnych. Prog. Plant Prot. 1997, 37, 300–305. [Google Scholar]
- Szmagara, M. Biotic and Biotechnical Factors Inhibiting the Growth and Development of Topospora myrtilli (Feltg.) Boerema. Electron. J. Pol. Agric. Univ. 2007, 10, 14. [Google Scholar]
- Szmagara, M. Possibilities of Growth and Development Suppression of Topospora myrtilli (Feltg.) Boerema on Artificial Media and Stems of Highbush Blueberry (Vaccinium corymbosum L.). Acta Sci. Pol. Hortorum Cultus 2008, 7, 103–111. [Google Scholar]
- Whipps, J.M.; Hand, P.; Pink, D.; Bending, G.D. Phyllosphere Microbiology with Special Reference to Diversity and Plant Genotype. J. Appl. Microbiol. 2008, 105, 1744–1755. [Google Scholar] [CrossRef]
- Martyniuk, S.; Oron, J.; Maczka, M. Charakterystyka Mikroorganizmów Występujących Na Kłosach Pszenicy Ozimej Uprawianej w Systemie Konwencjonalnym i Ekologicznym. Prog. Plant Prot. 2009, 49, 1309–1314. [Google Scholar]
- Aegerter, B.J.; Nuñez, J.J.; Davis, R.M. Detection and Management of Downy Mildew in Rose Rootstock. Plant Dis. 2002, 86, 1363–1368. [Google Scholar] [CrossRef]
- Robiglio, A.L.; Lopez, S.E. Mycotoxin Production by Alternaria alternata Strains Isolated from Red Delicious Apples in Argentina. Int. J. Food Microbiol. 1995, 24, 413–417. [Google Scholar] [CrossRef]
- Mesbah, L.; Van der Weerden, G.; Nijkamp, H.; Hille, J. Sensitivity among Species of Solanaceae to AAL Toxins Produced by Alternaria alternata f. Sp. lycopersici. Plant Pathol. 2000, 49, 734–741. [Google Scholar] [CrossRef]
- Jamiołkowska, A. Fungi Isolated from Underground Part of Hot Pepper (Capsicum annum) Plant Cultivated in the Field. Phytopathologia 2009, 51, 37–44. [Google Scholar]
- Patkowska, E. Effect of Bio-Products on Bean Yield and Bacterial and Fungal Communities in the Rhizosphere and Non-Rhizosphere. Pol. J. Environ. Stud. 2009, 18, 255–263. [Google Scholar]
- Nosir, W. New Technique for Rose Production in Soilless Culture System and Disease Reduction. J. Plant Nutr. 2016, 39, 181–188. [Google Scholar] [CrossRef]
- Świerczyńska, I.; Korbas, M.; Horoszkiewicz-Janka, J.; Danilewicz, J. Antagonistic Effect of Trichoderma Viride on Pathogenic Fungi Og the Genus Fusarium in the Presence of Biopreparations. J. Res. Appl. Agric. Eng. 2011, 56, 157–160. [Google Scholar]
- Łacicowa, B. Niektóre Aspekty Wykorzystania Grzybów z Rodzaju Trichoderma i Gliocladium w Biologicznej Ochronie Roślin. Ochr. Roślin 1989, 3, 8–10. [Google Scholar]
- Fokkema, N.J. Strategies for Biocontrol of Foliar Fungus Diseases. In Environmental Biotic Factors in Integrated Plant Disease Control. Polish Phytopathology Society; Mańka, M., Ed.; The Polish Phytopathological Society: Poznań, Poland, 1995; pp. 69–79. [Google Scholar]
- Wojdyła, A.T. Wyciąg z Grejpfruta w Ochronie Chryzantemy i Wierzby Przed Rdzą. Prog. Plant Prot. 2004, 44, 1220–1224. [Google Scholar]
- Wojdyła, A.T. Ochrona Róż Przed Szarą Pleśnią Pod Osłonami i w Przechowalni. In Proceedings of the Ogólnopolska Konferencja “Technika Szklarniowa i Uprawa Róż Pod Osłonami”, Skierniewice, Poland, 25 May 2006; pp. 70–76. [Google Scholar]
- Ribeiro, R.V.; Machado, E.C.; Oliveira, R.F. Growth- and Leaf-Temperature Effects on Photosynthesis of Sweet Orange Seedlings Infected with Xylella Fastidiosa. Plant Pathol. 2004, 53, 334–340. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).