Biological Treatments for VOC-Contaminated Off-Gas: Advances, Challenges, and Energetic Valorization Opportunities
Abstract
1. Introduction
2. Industrial VOC Gaseous Emissions
3. Biological Methods for VOC Emission Treatment
3.1. Biofilter
3.2. Biotrickling Filter
3.3. Bioscrubber
3.4. Bioaugmentation
3.5. Bioreactors for Bioscrubber VOC Treatment Configuration
3.6. Combination of Bioreactors with Other Technologies
3.6.1. Ozone
3.6.2. Photodegradation
3.6.3. Non-Thermal Plasma
3.7. Microbial Fuel Cell
4. Conclusions and Future Perspectives
- Developing multifunctional packing materials that enhance pollutant degradation;
- Exploring synergistic systems that combine conventional biofiltration with other technologies (e.g., MFC, photocatalysis);
- Genetic engineering to create robust microbial consortia capable of degrading a broader range of VOC under diverse conditions;
- Machine learning to predict microbial interactions to optimize bioaugmentation and biofilm stability;
- Conducting comprehensive life cycle assessments and economic analyses to validate sustainability and cost-effectiveness at industrial scales;
- Establishing regulatory frameworks and best practices tailored to the specific VOC profiles of different industrial sectors.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | Active carbon |
| AOP | Advanced Oxidation Processes |
| RO | Alkoxy radical |
| Alkyl radical | |
| ACT | Alternative Control Techniques |
| TAC | Annual cost |
| BTEX | Benzene, toluene, ethylbenzene, and xylene |
| BAT | Best Available Techniques |
| BF | Biofilter |
| BS | Bioscrubber |
| BTF | Biotrickling filter |
| CR | Cancer risk |
| CNF | Carbon nanofibers |
| CNT | Carbon nanotubes |
| CTO | Catalytic thermal oxidizer |
| COD | Chemical oxygen demand |
| CTG | Control Techniques Guidelines |
| DCE | Dichloroethane |
| DCM | Dichloromethane |
| DBD | Dielectric barrier discharge |
| DMDS | Dimethyl disulfide |
| DMS | Dimethyl sulfide |
| DMTS | Dimethyl trisulfide |
| DET | Direct electron transfer |
| EBRT | Empty Bed Resident Time |
| EPA | Environmental Protection Agency |
| ECHA | European Chemicals Agency |
| ESIG | European Solvents Industry Group |
| EU | European Union |
| EPS | Extracellular polymeric substances |
| FFBS | Fixed-film bioscrubber |
| KGL | Gas-liquid partitioning coefficient |
| H | Henry’s Law constant |
| Hydroperoxyl radicals | |
| IED | Industrial Emissions Directive |
| LCR | Lifetime cancer risk |
| MET | Mediated electron transfer |
| MFC | Microbial fuel cells |
| NECD | National Emission reduction Commitments Directive |
| NOx | Nitrogen oxide |
| NMHC | Non-methane hydrocarbons |
| NMVOC | Non-methane volatile organic compound |
| NTP | Non-thermal plasma |
| Log KOW | Octanol–water partition coefficient |
| OVOC | Oxygenated volatile organic compound |
| PPWS | Parallel-plate wet scrubber |
| PA | Peroxyacetyl |
| PAN | Peroxyacetyl nitrate |
| Peroxyl radical | |
| PCO | Photocatalytic oxidation |
| PCR | Photocatalytic reactor |
| PU | Polyurethane |
| PVC | Polyvinyl chloride |
| PT | Propanethiol |
| BREF | Best Available Techniques (BAT) Reference Document |
| RTO | Regenerative thermal oxidizer |
| RE | Removal efficiency |
| SOA | Secondary organic aerosols |
| SCMI | Solid composite microbial inoculant |
| CB | Stumpwood chips and pine bark |
| CBC | Stumpwood chips, pine bark, and compost |
| TMA | Trimethylamine |
| UV | Ultraviolet light |
| US | United States |
| VOC | Volatile organic compounds |
References
- Anand, S.S.; Philip, B.K.; Mehendale, H.M. Volatile Organic Compounds. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 967–970. ISBN 978-0-12-386455-0. [Google Scholar]
- Publications Office of the European Union. Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and Control). Off. J. Eur. Union 2010, 334, 17–119. [Google Scholar]
- Publications Office of the European Union. Directive (EU) 2016/2284 of the European Parliament and of the Council of 14 December 2016 on the Reduction of National Emissions of Certain Atmospheric Pollutants, Amending Directive 2003/35/EC and Repealing Directive 2001/81/EC. Off. J. Eur. Union 2016, 334, 1–31. [Google Scholar]
- Cape, J.N. Effects of Airborne Volatile Organic Compounds on Plants. Environ. Pollut. 2003, 122, 145–157. [Google Scholar] [CrossRef]
- Koppmann, R. (Ed.) Volatile Organic Compounds in the Atmosphere, 1st ed.; Blackwell Pub: Oxford, UK; Ames, IA, USA, 2007; ISBN 978-1-4051-3115-5. [Google Scholar]
- Helmig, D.; Bottenheim, J.; Galbally, I.E.; Lewis, A.; Milton, M.J.T.; Penkett, S.; Plass-Duelmer, C.; Reimann, S.; Tans, P.; Thiel, S. Volatile Organic Compounds in the Global Atmosphere. Eos Trans. Am. Geophys. Union 2009, 90, 513–514. [Google Scholar] [CrossRef]
- Thurston, G.D. Outdoor Air Pollution: Sources, Atmospheric Transport, and Human Health Effects. In International Encyclopedia of Public Health, 2nd ed.; Quah, S.R., Ed.; Academic Press: Oxford, UK, 2017; pp. 367–377. ISBN 978-0-12-803708-9. [Google Scholar]
- Alford, K.L.; Kumar, N. Pulmonary Health Effects of Indoor Volatile Organic Compounds—A Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 1578. [Google Scholar] [CrossRef] [PubMed]
- Sidorova, D.E.; Plyuta, V.A.; Padiy, D.A.; Kupriyanova, E.V.; Roshina, N.V.; Koksharova, O.A.; Khmel, I.A. The Effect of Volatile Organic Compounds on Different Organisms: Agrobacteria, Plants and Insects. Microorganisms 2022, 10, 69. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Wang, C.; Zhou, H. Environmental and Human Health Impacts of Volatile Organic Compounds: A Perspective Review. Chemosphere 2023, 313, 137489. [Google Scholar] [CrossRef]
- Derwent, R.G. Tropospheric Chemistry and Composition|Volatile Organic Compounds Overview: Anthropogenic. In Encyclopedia of Atmospheric Sciences, 2nd ed.; North, G.R., Pyle, J., Zhang, F., Eds.; Academic Press: Oxford, UK, 2015; pp. 265–267. ISBN 978-0-12-382225-3. [Google Scholar]
- Madronich, S.; Shao, M.; Wilson, S.R.; Solomon, K.R.; Longstreth, J.D.; Tang, X.Y. Changes in Air Quality and Tropospheric Composition Due to Depletion of Stratospheric Ozone and Interactions with Changing Climate: Implications for Human and Environmental Health. Photochem. Photobiol. Sci. 2015, 14, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, B.; Wang, S.; Hao, J. Ozone and Secondary Organic Aerosol Formation Potential from Anthropogenic Volatile Organic Compounds Emissions in China. J. Environ. Sci. 2017, 53, 224–237. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, J.; Song, K.; Gong, Y.; Lv, D.; Wan, Z.; Li, T.; Zhang, C.; Lu, S.; Chen, S.; et al. Secondary Organic Aerosol Formation from Semi-Volatile and Intermediate Volatility Organic Compounds in the Fall in Beijing. Atmosphere 2022, 14, 94. [Google Scholar] [CrossRef]
- Castro, L.M.; Pio, C.A.; Harrison, R.M.; Smith, D.J.T. Carbonaceous Aerosol in Urban and Rural European Atmospheres: Estimation of Secondary Organic Carbon Concentrations. Atmos. Environ. 1999, 33, 2771–2781. [Google Scholar] [CrossRef]
- Haagen-Smit, A.J.; Fox, M.M. Photochemical Ozone Formation with Hydrocarbons and Automobile Exhaust. Air Repair 1954, 4, 105–136. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 978-1-119-22116-6. [Google Scholar]
- Kennes, C.; Veiga, M.C. (Eds.) Air Pollution Prevention and Control: Bioreactors and Bioenergy; Wiley, A John Wiley & Sons, Ltd., Publication: Hoboken, NJ, USA, 2013; ISBN 978-1-119-94331-0. [Google Scholar]
- Abas, M.R.B.; Mohamad, S. Hazardous (Organic) Air Pollutants. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Oxford, UK, 2011; pp. 405–416. ISBN 978-0-444-63952-3. [Google Scholar]
- Zhu, J.; Penner, J.E.; Lin, G.; Zhou, C.; Xu, L.; Zhuang, B. Mechanism of SOA Formation Determines Magnitude of Radiative Effects. Proc. Natl. Acad. Sci. USA 2017, 114, 12685–12690. [Google Scholar] [CrossRef]
- Orlando, J.J.; Tyndall, G.S. Laboratory Studies of Organic Peroxy Radical Chemistry: An Overview with Emphasis on Recent Issues of Atmospheric Significance. Chem. Soc. Rev. 2012, 41, 6294. [Google Scholar] [CrossRef]
- Young, C.J.; Washenfelder, R.A.; Edwards, P.M.; Parrish, D.D.; Gilman, J.B.; Kuster, W.C.; Mielke, L.H.; Osthoff, H.D.; Tsai, C.; Pikelnaya, O.; et al. Chlorine as a Primary Radical: Evaluation of Methods to Understand Its Role in Initiation of Oxidative Cycles. Atmos. Chem. Phys. 2014, 14, 3427–3440. [Google Scholar] [CrossRef]
- Edwards, P.M.; Young, C.J. Primary Radical Effectiveness: Do the Different Chemical Reactivities of Hydroxyl and Chlorine Radicals Matter for Tropospheric Oxidation? ACS EST Air 2024, 1, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Le Breton, M.; Hallquist, Å.M.; Pathak, R.K.; Simpson, D.; Wang, Y.; Johansson, J.; Zheng, J.; Yang, Y.; Shang, D.; Wang, H.; et al. Chlorine Oxidation of VOCs at a Semi-Rural Site in Beijing: Significant Chlorine Liberation from ClNO<Sub>2</Sub> and Subsequent Gas- and Particle-Phase Cl–VOC Production. Atmos. Chem. Phys. 2018, 18, 13013–13030. [Google Scholar] [CrossRef]
- Lewis, A.C.; Hopkins, J.R.; Carslaw, D.C.; Hamilton, J.F.; Nelson, B.S.; Stewart, G.; Dernie, J.; Passant, N.; Murrells, T. An Increasing Role for Solvent Emissions and Implications for Future Measurements of Volatile Organic Compounds. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20190328. [Google Scholar] [CrossRef]
- Franzon, L.; Camredon, M.; Valorso, R.; Aumont, B.; Kurtén, T. Ether and Ester Formation from Peroxy Radical Recombination: A Qualitative Reaction Channel Analysis. Atmos. Chem. Phys. 2024, 24, 11679–11699. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Y.; Xu, J.; Lian, M.; Zhang, W.; Wu, W.; Wu, M.; Zhao, J. The Vertical Distribution of VOCs and Their Impact on the Environment: A Review. Atmosphere 2022, 13, 1940. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric Chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Fischer, E.V.; Jacob, D.J.; Yantosca, R.M.; Sulprizio, M.P.; Millet, D.B.; Mao, J.; Paulot, F.; Singh, H.B.; Roiger, A.; Ries, L.; et al. Atmospheric Peroxyacetyl Nitrate (PAN): A Global Budget and Source Attribution. Atmos. Chem. Phys. 2014, 14, 2679–2698. [Google Scholar] [CrossRef] [PubMed]
- LaFranchi, B.W.; Wolfe, G.M.; Thornton, J.A.; Harrold, S.A.; Browne, E.C.; Min, K.E.; Wooldridge, P.J.; Gilman, J.B.; Kuster, W.C.; Goldan, P.D.; et al. Closing the Peroxy Acetyl Nitrate Budget: Observations of Acyl Peroxy Nitrates (PAN, PPN, and MPAN) during BEARPEX 2007. Atmos. Chem. Phys. 2009, 9, 7623–7641. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, L.; Yang, C.; Chen, G.; Ji, X.; Li, L.; Zhang, K.; Hong, Y.; Li, M.; Fan, X.; et al. Trends of Peroxyacetyl Nitrate and Its Impact on Ozone over 2018–2022 in Urban Atmosphere. NPJ Clim. Atmos. Sci. 2024, 7, 192. [Google Scholar] [CrossRef]
- Mellouki, A.; Wallington, T.J.; Chen, J. Atmospheric Chemistry of Oxygenated Volatile Organic Compounds: Impacts on Air Quality and Climate. Chem. Rev. 2015, 115, 3984–4014. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.; Zhao, D.; Liu, R.; Liu, S.; Fu, J.; Zhang, Y.; Ding, H. Review on Catalytic Oxidation of VOCs at Ambient Temperature. Int. J. Mol. Sci. 2022, 23, 13739. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Kumar, A.; Singh, V.; Chakraborty, B.; Kumar, R.; Min, L. Recent Advancement in Organic Aerosol Understanding: A Review of Their Sources, Formation, and Health Impacts. Water Air Soil Pollut. 2023, 234, 750. [Google Scholar] [CrossRef]
- Shuai, J.; Kim, S.; Ryu, H.; Park, J.; Lee, C.K.; Kim, G.-B.; Ultra, V.U.; Yang, W. Health Risk Assessment of Volatile Organic Compounds Exposure near Daegu Dyeing Industrial Complex in South Korea. BMC Public Health 2018, 18, 528. [Google Scholar] [CrossRef]
- Soni, V.; Singh, P.; Shree, V.; Goel, V. Effects of VOCs on Human Health. In Air Pollution and Control; Sharma, N., Agarwal, A.K., Eastwood, P., Gupta, T., Singh, A.P., Eds.; Energy, Environment, and Sustainability; Springer: Singapore, 2018; pp. 119–142. ISBN 978-981-10-7184-3. [Google Scholar]
- Li, A.J.; Pal, V.K.; Kannan, K. A Review of Environmental Occurrence, Toxicity, Biotransformation and Biomonitoring of Volatile Organic Compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Mangotra, A.; Singh, S.K. Volatile Organic Compounds: A Threat to the Environment and Health Hazards to Living Organisms—A Review. J. Biotechnol. 2024, 382, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, R.; Lefebvre-Raine, M.; Gauthier, C.; Bourdin, T.; Bellot, P.; Triffault-Bouchet, G.; Langlois, V.S.; Couture, P. Comparative Toxicity of Conventional and Unconventional Oils during Rainbow Trout (Oncorhynchus mykiss) Embryonic Development: From Molecular to Health Consequences. Chemosphere 2022, 288, 132521. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, T.H.; Shillabeer, N.; Winter, M.J.; Pickford, D.B. Acute and Chronic Effects of Carrier Solvents in Aquatic Organisms: A Critical Review. Aquat. Toxicol. 2006, 76, 69–92. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.C.; Gupta, S. Survival and Reproduction of Some Blue-Green and Green Algae as Affected by Sewage Water, Fertilizer Factory Effluent, Brassica Oil, Phenol, Toluene and Benzene. Folia Microbiol. 2009, 54, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Thaysen, C.; Stevack, K.; Ruffolo, R.; Poirier, D.; De Frond, H.; DeVera, J.; Sheng, G.; Rochman, C.M. Leachate from Expanded Polystyrene Cups Is Toxic to Aquatic Invertebrates (Ceriodaphnia dubia). Front. Mar. Sci. 2018, 5, 71. [Google Scholar] [CrossRef]
- Jakubowska, M.; Białowąs, M.; Stankevičiūtė, M.; Chomiczewska, A.; Jonko-Sobuś, K.; Pažusienė, J.; Hallmann, A.; Bučaitė, A.; Urban-Malinga, B. Effects of Different Types of Primary Microplastics on Early Life Stages of Rainbow Trout (Oncorhynchus mykiss). Sci. Total Environ. 2022, 808, 151909. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J. Trans- and Multigenerational Effects of Isothiazolinone Biocide CMIT/MIT on Genotoxicity and Epigenotoxicity in Daphnia magna. Toxics 2023, 11, 388. [Google Scholar] [CrossRef]
- Zemo, D.A.; Patterson, T.J.; Kristofco, L.; Mohler, R.E.; O’Reilly, K.T.; Ahn, S.; Devine, C.E.; Magaw, R.I.; Sihota, N. Complex Mixture Toxicology: Evaluation of Toxicity to Freshwater Aquatic Receptors from Biodegradation Metabolites in Groundwater at a Crude Oil Release Site, Recent Analogous Results from Other Authors, and Implications for Risk Management. Aquat. Toxicol. 2022, 250, 106247. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Xue, J. Toxic Effects of Disinfection By-Products on Pseudokirchneriella subcapitata and Co-Cultured Algae Community. Sci. Total Environ. 2023, 894, 164760. [Google Scholar] [CrossRef]
- Yen, K.M.; Karl, M.R.; Blatt, L.M.; Simon, M.J.; Winter, R.B.; Fausset, P.R.; Lu, H.S.; Harcourt, A.A.; Chen, K.K. Cloning and Characterization of a Pseudomonas mendocina KR1 Gene Cluster Encoding Toluene-4-Monooxygenase. J. Bacteriol. 1991, 173, 5315–5327. [Google Scholar] [CrossRef]
- Marqués, S.; Ramos, J.L. Transcriptional Control of the Pseudomonas putida TOL Plasmid Catabolic Pathways. Mol. Microbiol. 1993, 9, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Onaca, C.; Kieninger, M.; Engesser, K.-H.; Altenbuchner, J. Degradation of Alkyl Methyl Ketones by Pseudomonas veronii MEK700. J Bacteriol 2007, 189, 3759–3767. [Google Scholar] [CrossRef] [PubMed]
- Benedek, T.; Szentgyörgyi, F.; Gergócs, V.; Menashe, O.; Gonzalez, P.A.F.; Probst, A.J.; Kriszt, B.; Táncsics, A. Potential of Variovorax paradoxus Isolate BFB1_13 for Bioremediation of BTEX Contaminated Sites. AMB Expr. 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Zhang, M.; Toyota, K. Biodegradation of Volatile Organic Compounds and Their Effects on Biodegradability under Co-Existing Conditions. Microbes Environ. 2017, 32, 188–200. [Google Scholar] [CrossRef]
- Wongbunmak, A.; Khiawjan, S.; Suphantharika, M.; Pongtharangkul, T. BTEX Biodegradation by Bacillus amyloliquefaciens Subsp. plantarum W1 and Its Proposed BTEX Biodegradation Pathways. Sci. Rep. 2020, 10, 17408. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, Z.; Dong, Y.; Tao, W.; Wang, B.; Jiang, J.; Guan, X. Biodegradation of Benzene, Toluene, Ethylbenzene, and o-, m-, and p-Xylenes by the Newly Isolated Bacterium comamonas Sp. JB. Appl. Biochem. Biotechnol. 2015, 176, 1700–1708. [Google Scholar] [CrossRef]
- Lee, S.-K.; Lee, S.B. Isolation and Characterization of a Thermotolerant Bacterium Ralstonia Sp. Strain PHS1 That Degrades Benzene, Toluene, Ethylbenzene, and o -Xylene. Appl. Microbiol. Biotechnol. 2001, 56, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Wongbunmak, A.; Khiawjan, S.; Suphantharika, M.; Pongtharangkul, T. BTEX- and Naphthalene-Degrading Bacterium Microbacterium Esteraromaticum Strain SBS1-7 Isolated from Estuarine Sediment. J. Hazard. Mater. 2017, 339, 82–90. [Google Scholar] [CrossRef]
- Surendra, S.V.; Mahalingam, B.L.; Velan, M. Degradation of Monoaromatics by Bacillus pumilus MVSV3. Braz. Arch. Biol. Technol. 2017, 60, e16160319. [Google Scholar] [CrossRef]
- Xue, X.; Wang, H.; Zhai, J.; Nan, X. Biofiltration of Toluene in the Presence of Ethyl Acetate or N-Hexane: Performance and Microbial Community. PLoS ONE 2024, 19, e0302487. [Google Scholar] [CrossRef]
- Ferrando, M.D.; Andreu-Moliner, E. Acute Toxicity of Toluene, Hexane, Xylene, and Benzene to the Rotifers Brachionus calyciflorus and Brachionus plicatilis. Bull. Environ. Contam. Toxicol. 1992, 49, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, J.; Wang, H.; Li, H.; Zhang, H.; Chai, F.; Wang, S. The Characteristics of Ambient Non-Methane Hydrocarbons (NMHCs) in Lanzhou, China. Atmosphere 2019, 10, 745. [Google Scholar] [CrossRef]
- Yang, J. Mass Balance and Unorganized Emission. In From Zero Waste to Material Closed Loop; Springer Nature: Singapore, 2022; pp. 13–18. ISBN 978-981-16-7682-6. [Google Scholar]
- Ren, Y.; Guan, X.; Peng, Y.; Gong, A.; Xie, H.; Chen, S.; Zhang, Q.; Zhang, X.; Wang, W.; Wang, Q. Characterization of VOC Emissions and Health Risk Assessment in the Plastic Manufacturing Industry. J. Environ. Manag. 2024, 357, 120730. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.8: Characterisation of Dose [Concentration]-Response for Human Health; European Chemicals Agency: Helsinki, Finland, 2012. [Google Scholar]
- U.S. Environmental Protection Agency. Risk Assessment Guidance for Superfund, Volume I: Human Health Evaluation Manual (Part A); Office of Emergency and Remedial Response, U.S. Environmental Protection Agency: Washington, DC, USA, 1989.
- U.S. Environmental Protection Agency. ORD Staff Handbook for Developing IRIS Assessments; U.S. Environmental Protection Agency, Office of Research and Development: Washington, DC, USA, 2022.
- LaGrega, M.D.; Buckingham, P.L.; Evans, J.C. Hazardous Waste Management, 2nd ed.; Waveland Press, Inc.: Long Grove, IL, USA, 2010; ISBN 978-1-57766-693-6. [Google Scholar]
- Durmusoglu, E.; Taspinar, F.; Karademir, A. Health Risk Assessment of BTEX Emissions in the Landfill Environment. J. Hazard. Mater. 2010, 176, 870–877. [Google Scholar] [CrossRef]
- Cheng, Z.; Sun, Z.; Zhu, S.; Lou, Z.; Zhu, N.; Feng, L. The Identification and Health Risk Assessment of Odor Emissions from Waste Landfilling and Composting. Sci. Total Environ. 2019, 649, 1038–1044. [Google Scholar] [CrossRef]
- Pinthong, N.; Thepanondh, S.; Kondo, A. Source Identification of VOCs and Their Environmental Health Risk in a Petrochemical Industrial Area. Aerosol. Air Qual. Res. 2022, 22, 210064. [Google Scholar] [CrossRef]
- Li, R.; Yuan, J.; Li, X.; Zhao, S.; Lu, W.; Wang, H.; Zhao, Y. Health Risk Assessment of Volatile Organic Compounds (VOCs) Emitted from Landfill Working Surface via Dispersion Simulation Enhanced by Probability Analysis. Environ. Pollut. 2023, 316, 120535. [Google Scholar] [CrossRef]
- Liu, F.; Tong, L.; Luo, Q.; Ling, Y.; Gu, H.; Lv, Y.; Shi, A.; Liu, H.; Xiao, H.; Huang, C. Emission Characteristics and Health Risk Assessment of Volatile Organic Compounds in Key Industries: A Case Study in the Central Plains of China. Atmosphere 2025, 16, 74. [Google Scholar] [CrossRef]
- Massolo, L.; Rehwagen, M.; Porta, A.; Ronco, A.; Herbarth, O.; Mueller, A. Indoor-Outdoor Distribution and Risk Assessment of Volatile Organic Compounds in the Atmosphere of Industrial and Urban Areas. Environ. Toxicol. 2009, 25, 339–349. [Google Scholar] [CrossRef]
- Hong, S.-H.; Shin, D.-C.; Lee, Y.-J.; Kim, S.-H.; Lim, Y.-W. Health Risk Assessment of Volatile Organic Compounds in Urban Areas. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 1454–1465. [Google Scholar] [CrossRef]
- Tan, T.; Xu, X.; Gu, H.; Cao, L.; Liu, T.; Zhang, Y.; Wang, J.; Chen, M.; Li, H.; Ge, X. The Characteristics, Sources, and Health Risks of Volatile Organic Compounds in an Industrial Area of Nanjing. Toxics 2024, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-H.; Yuan, C.-S.; Lin, C.; Hsu, Z.-P.; Hsu, I.-H.; Wang, L.-C. Comprehensive Analysis of VOCs in an Industrial Harbor City: Spatiotemporal Distribution, Health Risk, and Potential Sources. Aerosol. Air Qual. Res. 2024, 24, 240069. [Google Scholar] [CrossRef]
- Barbeş, S.-B.; Bărbulescu, A.; Barbeș, L. Assessing Benzene and TVOC Pollution and the Carcinogenic and Noncarcinogenic Risks to Workers in an Industrial Plant in Southeastern Romania. Toxics 2024, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Malakan, W.; Thepanondh, S.; Keawboonchu, J.; Kultan, V.; Kondo, A.; Shimadera, H. Integrated Assessment of Inhalation Health Risk and Economic Benefit of Improving Ambient Targeted VOCs in Petrochemical Industrial Area. Air Qual. Atmos. Health 2024, 17, 1885–1903. [Google Scholar] [CrossRef]
- Wypych, G. (Ed.) Handbook of Solvents. Volume 2: Use, Health, and Environment, 3rd ed.; ChemTec Publ: Toronto, ON, Canada, 2019; ISBN 978-1-927885-41-3. [Google Scholar]
- Wu, K.; Yang, X.; Chen, D.; Gu, S.; Lu, Y.; Jiang, Q.; Wang, K.; Ou, Y.; Qian, Y.; Shao, P.; et al. Estimation of Biogenic VOC Emissions and Their Corresponding Impact on Ozone and Secondary Organic Aerosol Formation in China. Atmos. Res. 2020, 231, 104656. [Google Scholar] [CrossRef]
- Loreto, F.; Fares, S. Chapter 4—Biogenic Volatile Organic Compounds and Their Impacts on Biosphere–Atmosphere Interactions. In Developments in Environmental Science; Matyssek, R., Clarke, N., Cudlin, P., Mikkelsen, T.N., Tuovinen, J.-P., Wieser, G., Paoletti, E., Eds.; Climate Change, Air Pollution and Global Challenges; Elsevier: Amsterdam, The Netherlands, 2013; Volume 13, pp. 57–75. [Google Scholar]
- Wang, W.; Yan, Y.; Fang, H.; Li, J.; Zha, S.; Wu, T. Volatile Organic Compound Emissions from Typical Industries: Implications for the Importance of Oxygenated Volatile Organic Compounds. Atmos. Pollut. Res. 2023, 14, 101640. [Google Scholar] [CrossRef]
- Meng, L.; Gao, S.; Zhang, S.; Che, X.; Jiao, Z.; Ren, Y.; Wang, C. Identification of Atmospheric Emerging Contaminants from Industrial Emissions: A Case Study of Halogenated Hydrocarbons Emitted by the Pharmaceutical Industry. Environ. Int. 2024, 192, 109027. [Google Scholar] [CrossRef]
- Tzanakopoulou, V.E.; Narasinghe, K.; Pollitt, M.; Castro-Rodriguez, D.; Gerogiorgis, D.I. Dynamic Simulation and Analysis of Dichloromethane-Acetone, Dichloromethane-Trichloromethane and Dichloromethane-Toluene VOC Mixture Abatement Systems under Transient Feed Conditions. Comput. Chem. Eng. 2024, 187, 108713. [Google Scholar] [CrossRef]
- Imwinkelried, G.; Fermanelli, C.S.; Teruel, M.A.; Saux, C.; Blanco, M.B. Comprehensive Analysis of Soybean Residues Pyrolysis Products. J. Anal. Appl. Pyrolysis 2024, 177, 106367. [Google Scholar] [CrossRef]
- Imwinkelried, G.; Bonetto, L.; Saux, C.; Blanco, M.B. High-Value Products from Chickpea Residues by Thermal Pyrolysis and Its Environmental Impacts. Biomass Bioenergy 2025, 193, 107584. [Google Scholar] [CrossRef]
- Liu, H.; Qin, Y.; Han, X.; Gao, M.; Tong, J.; Li, X.; Niu, M.; Tao, X.; Zhang, P.; Zuo, Y. Characterization of Volatile Organic Compound (VOC) Emission from a Cigarette Production Facility Using Open-Path—Fourier Transform Infrared Spectroscopy (OP-FTIR) and Pollution Rose Diagrams. Instrum. Sci. Technol. 2024, 52, 1–25. [Google Scholar] [CrossRef]
- Lee, H.E.; Kim, J.H.; Seo, D.; Yoon, S.J. Prioritization of Volatile Organic Compound Reduction in the Tire Manufacturing Industry through Speciation of Volatile Organic Compounds Emitted at the Fenceline. Atmosphere 2024, 15, 223. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Yan, Q.; Han, S.; Zhao, Q.; Yang, L.; Liu, Y.; Zhang, R. Typical Industrial Sector-Based Volatile Organic Compounds Source Profiles and Ozone Formation Potentials in Zhengzhou, China. Atmos. Pollut. Res. 2020, 11, 841–850. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Dai, C.; Guo, J.; Zhang, X. Volatile Organic Compounds Emission in the Rubber Products Manufacturing Processes. Environ. Res. 2022, 212, 113485. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Gao, S.; Fu, Q.; Cheng, J.; Chen, X.; Xu, H.; Liang, S.; Zhou, Y.; Ma, Y. Do Volatile Organic Compounds (VOCs) Emitted from Petrochemical Industries Affect Regional PM2.5? Atmos. Res. 2018, 209, 123–130. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, M.; Wang, M.; Chen, C.; Wang, X.; Nie, X.; Wang, W.; Xiong, Q.; Zhang, C.; Li, P.; et al. VOC Emission Characteristics of the Glass Deep-Processing Industry in China. Atmosphere 2023, 14, 179. [Google Scholar] [CrossRef]
- Wang, W.; Shen, X.; Zhang, S.; Lv, R.; Liu, M.; Xu, W.; Chen, Y.; Wang, H. Research on Very Volatile Organic Compounds and Odors from Veneered Medium Density Fiberboard Coated with Water-Based Lacquers. Molecules 2022, 27, 3626. [Google Scholar] [CrossRef]
- Cheng, K.; Hao, W.-W.; Yi, P.; Zhang, Y.; Zhang, J.-Y. Volatile Organic Compounds Emission from Chinese Wood Furniture Coating Industry: Activity-Based Emission Factor, Speciation Profiles, and Provincial Emission Inventory. Aerosol. Air Qual. Res. 2018, 18, 2813–2825. [Google Scholar] [CrossRef]
- Kozicki, M.; Guzik, K. Comparison of VOC Emissions Produced by Different Types of Adhesives Based on Test Chambers. Materials 2021, 14, 1924. [Google Scholar] [CrossRef]
- Ulker, O.C.; Ulker, O.; Hiziroglu, S. Volatile Organic Compounds (VOCs) Emitted from Coated Furniture Units. Coatings 2021, 11, 806. [Google Scholar] [CrossRef]
- Marchesiello, W.M.V.; Spadaccino, G.; Usman, M.; Nardiello, D.; Quinto, M. Determination of Volatile Organic Compounds (VOCs) in Indoor Work Environments by Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry. Environ. Sci. Pollut. Res. 2024, 31, 52804–52814. [Google Scholar] [CrossRef] [PubMed]
- Moura, P.C.; Santos, F.; Fujão, C.; Vassilenko, V. In Situ Indoor Air Volatile Organic Compounds Assessment in a Car Factory Painting Line. Processes 2023, 11, 2259. [Google Scholar] [CrossRef]
- McPherson, K.N.; Jahn, L.G.; Masoud, C.G.; Bhattacharyya, N.; Modi, M.; Patel, K.; Abue, P.; Blomdahl, D.; Misztal, P.K.; Hildebrandt Ruiz, L. Air Pollution from Unconventional Oil and Gas Development in the Eagle Ford Shale. Atmos. Environ. 2024, 338, 120812. [Google Scholar] [CrossRef]
- Das, A.; Giri, B.S.; Manjunatha, R. Systematic Review on Benzene, Toluene, Ethylbenzene, and Xylene (BTEX) Emissions; Health Impact Assessment; and Detection Techniques in Oil and Natural Gas Operations. Environ. Sci. Pollut. Res. 2024, 32, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Jiang, J.; Xia, L.; Xu, H.; Ye, C.; Sun, J.; Gu, R. Investigation and Accounting Research of VOC in Daily and Specialty Ceramic Industry. Coatings 2022, 12, 279. [Google Scholar] [CrossRef]
- Ghobakhloo, S.; Khoshakhlagh, A.H.; Morais, S.; Mazaheri Tehrani, A. Exposure to Volatile Organic Compounds in Paint Production Plants: Levels and Potential Human Health Risks. Toxics 2023, 11, 111. [Google Scholar] [CrossRef]
- Yuan, B.; Shao, M.; Lu, S.; Wang, B. Source Profiles of Volatile Organic Compounds Associated with Solvent Use in Beijing, China. Atmos. Environ. 2010, 44, 1919–1926. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Dai, C.; Wu, H.; Guo, J.; Wang, C.; Zhang, X. Species Profile and Reactivity of Volatile Organic Compounds Emission in Solvent Uses, Industry Activities and from Vehicular Tunnels. J. Environ. Sci. 2024, 135, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Su, G.; Li, C.; Wang, M.; Tan, L.; Gao, L.; Mingge, W.; Wang, Q. Emission Profiles, Ozone Formation Potential and Health-Risk Assessment of Volatile Organic Compounds in Rubber Footwear Industries in China. J. Hazard. Mater. 2019, 375, 52–60. [Google Scholar] [CrossRef]
- Lv, Z.; Liu, X.; Wang, G.; Shao, X.; Li, Z.; Nie, L.; Li, G. Sector-Based Volatile Organic Compounds Emission Characteristics from the Electronics Manufacturing Industry in China. Atmos. Pollut. Res. 2021, 12, 101097. [Google Scholar] [CrossRef]
- European Environment Agency. National Air Pollutant Emissions Data Viewer 2005–2022; European Environment Agency: Copenhagen, Denmark, 2024. [Google Scholar]
- European Solvents Industry Group. Solvent VOC Emission Inventories 2022; European Solvents Industry Group: Brussels, Belgium, 2022. [Google Scholar]
- Pearson, J.K. European Solvent VOC Emission Inventories Based on Industry-Wide Information. Atmos. Environ. 2019, 204, 118–124. [Google Scholar] [CrossRef]
- Simayi, M.; Shi, Y.; Xi, Z.; Ren, J.; Hini, G.; Xie, S. Emission Trends of Industrial VOCs in China since the Clean Air Action and Future Reduction Perspectives. Sci. Total Environ. 2022, 826, 153994. [Google Scholar] [CrossRef] [PubMed]
- European Commission; Joint Research Centre. Best Available Techniques (BAT) Reference Document for Waste Treatment: Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control); Publications Office: Luxembourg, 2018. [Google Scholar]
- European Commission; Joint Research Centre. Best Available Techniques (BAT) Reference Document on Surface Treatment Using Organic Solvents Including Preservation of Wood and Wood Products with Chemicals: Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control); Publications Office: Luxembourg, 2020. [Google Scholar]
- European Commission; Joint Research Centre. Best Available Techniques (BAT) Reference Document for Common Waste Gas Management and Treatment Systems in the Chemical Sector: Industrial Emissions Directive 2010/75/EU (Integrated Pollution Prevention and Control); Publications Office: Luxembourg, 2023. [Google Scholar]
- U.S. Environmental Protection Agency. Control Techniques Guidelines and Alternative Control Techniques Documents for Reducing Ozone-Causing Emissions. Available online: https://www.epa.gov/ground-level-ozone-pollution/control-techniques-guidelines-and-alternative-control-techniques (accessed on 30 April 2025).
- Schiavon, M.; Ragazzi, M.; Rada, E.C.; Torretta, V. Air Pollution Control through Biotrickling Filters: A Review Considering Operational Aspects and Expected Performance. Crit. Rev. Biotechnol. 2016, 36, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Sonigra, P.; Yadav, G. Biological-Based Methods for the Removal of Volatile Organic Compounds (VOCs) and Heavy Metals. Environ. Sci. Pollut. Res. Int. 2021, 28, 2485–2508. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, G.; Ramírez, M.; Cantero, D. 2.22—Biofilters. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, NJ, USA, 2011; pp. 303–318. ISBN 978-0-08-088504-9. [Google Scholar]
- Senatore, V.; Zarra, T.; Galang, M.G.; Oliva, G.; Buonerba, A.; Li, C.-W.; Belgiorno, V.; Naddeo, V. Full-Scale Odor Abatement Technologies in Wastewater Treatment Plants (WWTPs): A Review. Water 2021, 13, 3503. [Google Scholar] [CrossRef]
- Yang, C.; Qian, H.; Li, X.; Cheng, Y.; He, H.; Zeng, G.; Xi, J. Simultaneous Removal of Multicomponent VOCs in Biofilters. Trends Biotechnol. 2018, 36, 673–685. [Google Scholar] [CrossRef]
- Han, M.-F.; Wang, C.; Fu, Y. Treatment of Hydrophobic Volatile Organic Compounds Using Two-Liquid Phase Biofilters. Sci. Total Environ. 2018, 640–641, 1447–1454. [Google Scholar] [CrossRef]
- Lhuissier, M.; Couvert, A.; Dabert, P.; Amrane, A.; Kane, A.; Audic, J.-L.; Dumont, E. Removal of a Mixture of Seven Volatile Organic Compounds (VOCs) Using an Industrial Pilot-Scale Process Combining Absorption in Silicone Oil and Biological Regeneration in a Two-Phase Partitioning Bioreactor (TPPB). Energies 2022, 15, 4576. [Google Scholar] [CrossRef]
- Lv, R.; Kang, J.; Fan, X.; Li, J. Performance of Integral Polypropylene Packing Coated with Polydimethylsiloxane in Biotrickling Filter for Toluene Elimination. Process Saf. Environ. Prot. 2023, 169, 199–211. [Google Scholar] [CrossRef]
- Usevičiūtė, L.; Baltrėnaitė-Gedienė, E.; Baltrėnas, P.; Dutta, S. Acetone, xylene and ammonia removal enhancement in the biofilter packed with steam modified biochar. J. Environ. Eng. Landsc. Manag. 2022, 30, 412–423. [Google Scholar] [CrossRef]
- Re, A.; Schiavon, M.; Torretta, V.; Polvara, E.; Invernizzi, M.; Sironi, S.; Caruson, P. Application of Different Packing Media for the Biofiltration of Gaseous Effluents from Waste Composting. Environ. Technol. 2022, 45, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Orviz, S.; Lebrero, R.; Terrén, L.; Doñate, S.; Esclapez, M.D.; Saúco, L.; Muñoz, R. Evaluation of the Performance of New Plastic Packing Materials from Plastic Waste in Biotrickling Filters for Odour Removal. Process Saf. Environ. Prot. 2024, 191, 2361–2372. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lv, Y.-H.; Wang, C.; Jiang, G.-Y.; Han, M.-F.; Deng, J.-G.; Hsi, H.-C. Microbial Community Evolution and Functional Trade-Offs of Biofilm in Odor Treatment Biofilters. Water Res. 2023, 235, 119917. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yang, B.; Yeung, M.; Xi, J. Quorum Sensing Improved the Low-Temperature Performance of Biofilters Treating Gaseous Toluene. J. Hazard. Mater. 2022, 437, 129277. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, A.A.; Sorial, G.A.; Wendell, D. Performance Evaluation of Fungal Biotrickling Filter for Styrene Destruction: Experimental and Artificial Neural Networks Modeling. Process Saf. Environ. Prot. 2022, 162, 49–60. [Google Scholar] [CrossRef]
- Marycz, M.; Brillowska-Dąbrowska, A.; Muñoz, R.; Gębicki, J. A State of the Art Review on the Use of Fungi in Biofiltration to Remove Volatile Hydrophobic Pollutants. Rev. Environ. Sci. Biotechnol. 2022, 21, 225–246. [Google Scholar] [CrossRef]
- Danila, V.; Zagorskis, A.; Januševičius, T. Effects of Water Content and Irrigation of Packing Materials on the Performance of Biofilters and Biotrickling Filters: A Review. Processes 2022, 10, 1304. [Google Scholar] [CrossRef]
- Lu, L.; Dong, D.; Yeung, M.; Sun, Z.; Xi, J. Sustaining Low Pressure Drop and Homogeneous Flow by Adopting a Fluidized Bed Biofilter Treating Gaseous Toluene. Chemosphere 2022, 291, 132951. [Google Scholar] [CrossRef]
- Boada, E.; Santos-Clotas, E.; Cabrera-Codony, A.; Martín, M.J.; Bañeras, L.; Gich, F. The Core Microbiome Is Responsible for Volatile Silicon and Organic Compounds Degradation during Anoxic Lab Scale Biotrickling Filter Performance. Sci. Total Environ. 2021, 798, 149162. [Google Scholar] [CrossRef]
- Li, Y.; Lv, J.; Xu, Q.; Cai, Y.; Yang, H.; Li, Y.; Yao, Y.; Wang, W.; Liu, N. Study of the Treatment of Organic Waste Gas Containing Benzene by a Low Temperature Plasma-Biological Degradation Method. Atmosphere 2022, 13, 622. [Google Scholar] [CrossRef]
- Merouani, E.F.O.; Ferdowsi, M.; Benyoussef, E.-H.; Malhautier, L.; Buelna, G.; Jones, J.P.; Heitz, M. Biological Mitigation of Methane in Presence of Xylene and Ethylbenzene in Biofilters: Effect of Pollutants Concentrations and Empty Bed Residence Time. Process Saf. Environ. Prot. 2023, 173, 946–960. [Google Scholar] [CrossRef]
- Rybarczyk, P. Removal of Volatile Organic Compounds (VOCs) from Air: Focus on Biotrickling Filtration and Process Modeling. Processes 2022, 10, 2531. [Google Scholar] [CrossRef]
- Wang, C.; Xi, J.-Y.; Hu, H.-Y. Effects of Nitrogen Source, Empty Bed Residence Time and Inlet Concentration on Biofilter Removal of Chlorobenzene. Eng. Life Sci. 2009, 9, 109–115. [Google Scholar] [CrossRef]
- Lv, R.; Kang, J.; Fan, X.; Li, J. Effect of Nitrogen Source and Spray Conditions on the Integral Biotrickling Filter Operation for Hydrophobic Volatile Organic Compounds. J. Environ. Chem. Eng. 2023, 11, 110053. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, L.; Liu, X.; Chen, B.; Zhang, M. Construction and Function of a High-Efficient Synthetic Bacterial Consortium to Degrade Aromatic VOCs. Bioprocess Biosyst. Eng. 2023, 46, 851–865. [Google Scholar] [CrossRef]
- Cheng, Y.; He, H.; Yang, C.; Zeng, G.; Li, X.; Chen, H.; Yu, G. Challenges and Solutions for Biofiltration of Hydrophobic Volatile Organic Compounds. Biotechnol. Adv. 2016, 34, 1091–1102. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, X.; Liu, H.; Yang, C.; Wu, S.; Du, C.; Nie, L.; Zhong, Y. Effect of Presence of Hydrophilic Volatile Organic Compounds on Removal of Hydrophobic N-Hexane in Biotrickling Filters. Chemosphere 2020, 252, 126490. [Google Scholar] [CrossRef]
- Tabernacka, A. Biofiltration of Waste Gas Containing Cyclohexanol, Cyclohexanone and Butanol. Atmosphere 2023, 14, 254. [Google Scholar] [CrossRef]
- Wu, X.; Lin, Y.; Wang, Y.; Dai, M.; Wu, S.; Li, X.; Yang, C. Chemical Structure of Hydrocarbons Significantly Affects Removal Performance and Microbial Responses in Gas Biotrickling Filters. Bioresour. Technol. 2024, 398, 130480. [Google Scholar] [CrossRef]
- Wu, X.; Lin, Y.; Wang, Y.; Wu, S.; Yang, C. Volatile Organic Compound Removal via Biofiltration: Influences, Challenges, and Strategies. Chem. Eng. J. 2023, 471, 144420. [Google Scholar] [CrossRef]
- Nendza, M.; Kosfeld, V.; Schlechtriem, C. Consolidated Octanol/Water Partition Coefficients: Combining Multiple Estimates from Different Methods to Reduce Uncertainties in Log KOW. Environ. Sci. Eur. 2025, 37, 44. [Google Scholar] [CrossRef]
- Wysocka, I.; Gębicki, J.; Namieśnik, J. Technologies for Deodorization of Malodorous Gases. Environ. Sci. Pollut. Res. 2019, 26, 9409–9434. [Google Scholar] [CrossRef] [PubMed]
- Ferdowsi, M.; Avalos Ramirez, A.; Jones, J.P.; Heitz, M. Elimination of Mass Transfer and Kinetic Limited Organic Pollutants in Biofilters: A Review. Int. Biodeterior. Biodegrad. 2017, 119, 336–348. [Google Scholar] [CrossRef]
- Jahan, K.; Balzer, S.; Mosto, P. Toxicity of Nonionic Surfactants; WIT Press: Granada, Spain, 2008; pp. 281–290. [Google Scholar]
- Miller, U.; Sówka, I.; Adamiak, W. The use of surfactant from the Tween group in toluene biofiltration. Arch. Environ. Prot. 2020, 46, 53–57. [Google Scholar] [CrossRef]
- Rezaei, M.; Moussavi, G.; Naddafi, K.; Johnson, M.S. Enhanced Biodegradation of Styrene Vapors in the Biotrickling Filter Inoculated with Biosurfactant-Generating Bacteria under H2O2 Stimulation. Sci. Total Environ. 2020, 704, 135325. [Google Scholar] [CrossRef]
- Fahri, F.; Bacha, K.; Chiki, F.F.; Mbakidi, J.-P.; Panda, S.; Bouquillon, S.; Fourmentin, S. Air Pollution: New Bio-Based Ionic Liquids Absorb Both Hydrophobic and Hydrophilic Volatile Organic Compounds with High Efficiency. Environ. Chem. Lett. 2020, 18, 1403–1411. [Google Scholar] [CrossRef]
- Lamprea Pineda, P.A.; Bruneel, J.; Demeestere, K.; Deraedt, L.; Goetschalckx, T.; Van Langenhove, H.; Walgraeve, C. Absorption of Hydrophobic Volatile Organic Compounds in Renewable Vegetable Oils and Esterified Fatty Acids: Determination of Gas-Liquid Partitioning Coefficients as a Function of Temperature. Chem. Eng. J. 2024, 479, 147531. [Google Scholar] [CrossRef]
- Lamprea Pineda, P.A.; Demeestere, K.; Toledo, M.; Van Langenhove, H.; Walgraeve, C. Enhanced Removal of Hydrophobic Volatile Organic Compounds in Biofilters and Biotrickling Filters: A Review on the Use of Surfactants and the Addition of Hydrophilic Compounds. Chemosphere 2021, 279, 130757. [Google Scholar] [CrossRef]
- Dobslaw, D.; Ortlinghaus, O. Biological Waste Air and Waste Gas Treatment: Overview, Challenges, Operational Efficiency, and Current Trends. Sustainability 2020, 12, 8577. [Google Scholar] [CrossRef]
- Prado, Ó.J.; Gabriel, D.; Lafuente, J. Economical Assessment of the Design, Construction and Operation of Open-Bed Biofilters for Waste Gas Treatment. J. Environ. Manag. 2009, 90, 2515–2523. [Google Scholar] [CrossRef]
- Estrada, J.M.; Kraakman, N.J.R.; Lebrero, R.; Muñoz, R. A Sensitivity Analysis of Process Design Parameters, Commodity Prices and Robustness on the Economics of Odour Abatement Technologies. Biotechnol. Adv. 2012, 30, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Deshusses, M.A.; Cox, H.H.J. A Cost Benefit Approach to Reactor Sizing and Nutrient Supply for Biotrickling Filters for Air Pollution Control. Environ. Prog. 1999, 18, 188–196. [Google Scholar] [CrossRef]
- Deshusses, M.A.; Webster, T.S. Construction and Economics of a Pilot/Full-Scale Biological Trickling Filter Reactor for the Removal of Volatile Organic Compounds from Polluted Air. J. Air Waste Manag. Assoc. 2000, 50, 1947–1956. [Google Scholar] [CrossRef]
- Delhoménie, M.-C.; Heitz, M. Biofiltration of Air: A Review. Crit. Rev. Biotechnol. 2005, 25, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, K.; Kim, K.-H.; Szulejko, J.; Pandey, S.; Singh, R.S.; Giri, B.; Brown, R.; Lee, S. Bio-Filters for the Treatment of VOCs and Odors—A Review. Asian J. Atmos. Environ. 2017, 11, 139. [Google Scholar] [CrossRef]
- Devinny, J.S.; Deshusses, M.A.; Webster, T.S. Biofiltration for Air Pollution Control; Lewis Publishers: Boca Raton, FL, USA, 1999; ISBN 978-1-56670-289-8. [Google Scholar]
- Tomatis, M.; Moreira, M.T.; Xu, H.; Deng, W.; He, J.; Parvez, A.M. Removal of VOCs from Waste Gases Using Various Thermal Oxidizers: A Comparative Study Based on Life Cycle Assessment and Cost Analysis in China. J. Clean. Prod. 2019, 233, 808–818. [Google Scholar] [CrossRef]
- Chou, M.-S.; Cheng, W.-H.; Lee, W.-S. Performance Characteristics of a Regenerative Catalytic Oxidizer for Treating VOC-Contaminated Airstreams. J. Air Waste Manag. Assoc. 2000, 50, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Gospodarek, M.; Rybarczyk, P.; Szulczyński, B.; Gębicki, J. Comparative Evaluation of Selected Biological Methods for the Removal of Hydrophilic and Hydrophobic Odorous VOCs from Air. Processes 2019, 7, 187. [Google Scholar] [CrossRef]
- Liu, J.; Lu, C.; Huang, L.; Sun, J.; Yue, P.; Liu, X.; Kang, X. Performance and Economic Analyses of a Combined Bioreactor for Treating Odors, Volatile Organic Compounds, and Aerosols from a Landfill Site. J. Clean. Prod. 2021, 278, 124161. [Google Scholar] [CrossRef]
- Barbusinski, K.; Kalemba, K.; Kasperczyk, D.; Urbaniec, K.; Kozik, V. Biological Methods for Odor Treatment—A Review. J. Clean. Prod. 2017, 152, 223–241. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.; Zhu, Z.; Jin, B.; Zhu, R.; Li, S. Enhancing Performance Evaluation and Microbial Community Analysis of the Biofilter for Toluene Removal by Adding Polyethylene Glycol-600 into the Nutrient Solution. Bioresour. Technol. 2021, 330, 124954. [Google Scholar] [CrossRef] [PubMed]
- González-Cortés, J.J.; Bruneel, J.; Ramírez, M.; Walgraeve, C. Effect of Hydrophobic Fumed Silica Addition on a Biofilter for Pentane Removal Using SIFT-MS. Chemosphere 2020, 254, 126738. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Xu, M.; Li, J.; Huang, S.; Zhao, G.; Tu, X.; Sun, G.; Guo, J. Structure and Predictive Functional Profiling of Microbial Communities in Two Biotrickling Filters Treated with Continuous/Discontinuous Waste Gases. AMB Expr. 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Malhautier, L.; Khammar, N.; Bayle, S.; Fanlo, J.-L. Biofiltration of Volatile Organic Compounds. Appl. Microbiol. Biotechnol. 2005, 68, 16–22. [Google Scholar] [CrossRef]
- Wu, G.; Conti, B.; Leroux, A.; Brzezinski, R.; Viel, G.; Heitz, M. A High Performance Biofilter for VOC Emission Control. J. Air Waste Manag. Assoc. 1999, 49, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, M.; Chen, R.; Li, Z.; Qian, G.; An, T.; Fu, J.; Sheng, G. Biofiltration Treatment of Odors from Municipal Solid Waste Treatment Plants. Waste Manag. 2009, 29, 2051–2058. [Google Scholar] [CrossRef]
- Chen, X.; Qian, W.; Kong, L.; Xiong, Y.; Tian, S. Performance of a Suspended Biofilter as a New Bioreactor for Removal of Toluene. Biochem. Eng. J. 2015, 98, 56–62. [Google Scholar] [CrossRef]
- Aizpuru, A.; Malhautier, L.; Roux, J.C.; Fanlo, J.L. Biofiltration of a Mixture of Volatile Organic Compounds on Granular Activated Carbon. Biotechnol. Bioeng. 2003, 83, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, Y.; Chai, F.; Ma, J.; Li, L. Biofilters for the Co-Treatment of Volatile Organic Compounds and Odors in a Domestic Waste Landfill Site. J. Clean. Prod. 2020, 277, 124012. [Google Scholar] [CrossRef]
- Muszyński, A.; Tabernacka, A.; Załęska-Radziwiłł, M. How to Reduce the Emission of Microorganisms from a Biofilter Used to Treat Waste Gas from a Food Industry Plant. Atmosphere 2021, 12, 673. [Google Scholar] [CrossRef]
- Guzmán-Beltrán, A.M.; Vela-Aparicio, D.; Montero, S.; Cabeza, I.O.; Brandão, P.F.B. Simultaneous Biofiltration of H2S, NH3, and Toluene Using Compost Made of Chicken Manure and Sugarcane Bagasse as Packing Material. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Huang, Z.; Xie, J.; Ai, X.; Xin, X.; Hong, J. Effects of Filling Methods on the Degradation of Ethyl Acetate and the Microbial Community in Biofilters. Process Saf. Environ. Prot. 2023, 174, 188–199. [Google Scholar] [CrossRef]
- Shang, B.; Zhou, T.; Tao, X.; Chen, Y.; Dong, H. Simultaneous Removal of Ammonia and Volatile Organic Compounds from Composting of Dead Pigs and Manure Using Pilot-Scale Biofilter. J. Air Waste Manag. Assoc. 2021, 71, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, X.; Liu, X.; Yue, P.; Sun, J.; Lu, C. Simultaneous Removal of Bioaerosols, Odors and Volatile Organic Compounds from a Wastewater Treatment Plant by a Full-Scale Integrated Reactor. Process Saf. Environ. Prot. 2020, 144, 2–14. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, J.; Zhang, Y.; Han, C.; Ma, S.; Chen, J.; Li, G.; An, T. Removal of Volatile Organic Compounds (VOCs) Emitted from a Textile Dyeing Wastewater Treatment Plant and the Attenuation of Respiratory Health Risks Using a Pilot-Scale Biofilter. J. Clean. Prod. 2020, 253, 120019. [Google Scholar] [CrossRef]
- Cortés-Castillo, M.; Encinas, A.; Aizpuru, A.; Arriaga, S. Effect of Applying a Magnetic Field on the Biofiltration of Hexane over Long-Term Operation Period. Environ. Sci. Pollut. Res. 2024, 32, 3261–3276. [Google Scholar] [CrossRef]
- Schnelle, K.; Dunn, R.; Ternes, M. Air Pollution Control Technology Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4822-4562-2. [Google Scholar]
- Lebrero, R.; Gondim, A.C.; Pérez, R.; García-Encina, P.A.; Muñoz, R. Comparative Assessment of a Biofilter, a Biotrickling Filter and a Hollow Fiber Membrane Bioreactor for Odor Treatment in Wastewater Treatment Plants. Water Res. 2014, 49, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Mudliar, S.; Giri, B.; Padoley, K.; Satpute, D.; Dixit, R.; Bhatt, P.; Pandey, R.; Juwarkar, A.; Vaidya, A. Bioreactors for Treatment of VOCs and Odours—A Review. J. Environ. Manag. 2010, 91, 1039–1054. [Google Scholar] [CrossRef]
- Cox, H.H.J.; Deshusses, M.A.; Converse, B.M.; Schroeder, E.D.; Iranpour, R. Odor and Volatile Organic Compound Treatment by Biotrickling Filters: Pilot-Scale Studies at Hyperion Treatment Plant. Water Environ. Res. 2002, 74, 557–563. [Google Scholar] [CrossRef]
- Sun, Z.; Ding, C.; Xi, J.; Lu, L.; Yang, B. Enhancing Biofilm Formation in Biofilters for Benzene, Toluene, Ethylbenzene, and Xylene Removal by Modifying the Packing Material Surface. Bioresour. Technol. 2020, 296, 122335. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Lu, B.-H.; Zhou, X.-X.; Li, W. Evaluation of O-Xylene and Other Volatile Organic Compounds Removal Using a Xylene-Acclimated Biotrickling Filter. Environ. Technol. 2013, 34, 2691–2699. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lv, J.-L.; Cai, Y.-L.; Yao, Y.-Y.; Zhang, K.; Ma, C.; Li, J.-X.; Ren, X.-Y.; Hu, J.-J.; Zhao, J.-H. Study on Gaseous Chlorobenzene Treatment by a Bio-Trickling Filter: Degradation Mechanism and Microbial Community. Processes 2022, 10, 1483. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Philip, L.; Murty Bhallamudi, S. Biotrickling Filtration of VOC Emissions from Pharmaceutical Industries. Chem. Eng. J. 2012, 209, 102–112. [Google Scholar] [CrossRef]
- Wu, H.; Guo, C.; Yin, Z.; Quan, Y.; Yin, C. Performance and Bacterial Diversity of Biotrickling Filters Filled with Conductive Packing Material for the Treatment of Toluene. Bioresour. Technol. 2018, 257, 201–209. [Google Scholar] [CrossRef]
- Dobslaw, D.; Schulz, A.; Helbich, S.; Dobslaw, C.; Engesser, K.-H. VOC Removal and Odor Abatement by a Low-Cost Plasma Enhanced Biotrickling Filter Process. J. Environ. Chem. Eng. 2017, 5, 5501–5511. [Google Scholar] [CrossRef]
- Álvarez-Hornos, F.J.; Lafita, C.; Martínez-Soria, V.; Penya-Roja, J.M.; Pérez, M.C.; Gabaldón, C. Evaluation of a Pilot-Scale Biotrickling Filter as a VOC Control Technology for the Plastic Coating Sector. Biochem. Eng. J. 2011, 58–59, 154–161. [Google Scholar] [CrossRef]
- Khoramfar, S.; Jones, K.D.; Boswell, J.; Ghobadi, J.; Paca, J. Evaluation of a Sequential Biotrickling–Biofiltration Unit for Removal of VOCs from the Headspace of Crude Oil Storage Tanks. J. Chem. Technol. Biotechnol. 2018, 93, 1778–1789. [Google Scholar] [CrossRef]
- Bak, A.; Kozik, V.; Dybal, P.; Sulowicz, S.; Kasperczyk, D.; Kus, S.; Barbusinski, K. Abatement Robustness of Volatile Organic Compounds Using Compact Trickle-Bed Bioreactor: Biotreatment of Styrene, Ethanol and Dimethyl Sulfide Mixture in Contaminated Airstream. Int. Biodeterior. Biodegrad. 2017, 119, 316–328. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, G.; Lens, P.N.L.; He, Y.; Qie, L.; Shen, X.; Chen, J.; Cheng, Z.; Chen, D. Enhanced Removal of Mixed VOCs with Different Hydrophobicities by Tween 20 in a Biotrickling Filter: Kinetic Analysis and Biofilm Characteristics. J. Hazard. Mater. 2023, 450, 131063. [Google Scholar] [CrossRef]
- Silva, J.R.; Ascensão, D.S.; Castro, L.M. Removal of Volatile Organic Compounds from Waste Air Stream of a Furniture Factory. Energy Rep. 2020, 6, 250–255. [Google Scholar] [CrossRef]
- Rybarczyk, P.; Cichon, K.; Kucharska, K.; Dobrzyniewski, D.; Szulczyński, B.; Gębicki, J. Packing Incubation and Addition of Rot Fungi Extracts Improve BTEX Elimination from Air in Biotrickling Filters. Molecules 2024, 29, 4431. [Google Scholar] [CrossRef] [PubMed]
- Wongbunmak, A.; Panthongkham, Y.; Suphantharika, M.; Pongtharangkul, T. A Fixed-Film Bioscrubber of Microbacterium Esteraromaticum SBS1-7 for Toluene/Styrene Biodegradation. J. Hazard. Mater. 2021, 418, 126287. [Google Scholar] [CrossRef] [PubMed]
- Malhautier, L.; Lalanne, F.; Fanlo, J.-L. Bioscrubbing as a Treatment for a Complex Mixture of Volatile Organic Compounds: Influence of the Absorption Column Characteristics on performance. Can. J. Civ. Eng. 2009, 36, 1926–1934. [Google Scholar] [CrossRef]
- Bravo, D.; Ferrero, P.; Penya-roja, J.M.; Álvarez-Hornos, F.J.; Gabaldón, C. Control of VOCs from Printing Press Air Emissions by Anaerobic Bioscrubber: Performance and Microbial Community of an on-Site Pilot Unit. J. Environ. Manag. 2017, 197, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.-L.; Tsai, C.-J.; Sheu, S.-R.; Cheng, Y.-H.; Starik, A.M. High-Efficiency Parallel-Plate Wet Scrubber (PPWS) for Soluble Gas Removal. Sep. Purif. Technol. 2015, 142, 189–195. [Google Scholar] [CrossRef]
- Oliveira, F.; Vazquez, L.; França, F. Bioremediation of Contaminated Air Using a Bioscrubber. Environ. Eng. Manag. J. 2009, 8, 381–390. [Google Scholar] [CrossRef]
- Idris, N.F.; Le-Minh, N.; Hayes, J.E.; Stuetz, R.M. Performance of Wet Scrubbers to Remove VOCs from Rubber Emissions. J. Environ. Manag. 2022, 305, 114426. [Google Scholar] [CrossRef]
- Speight, J.G. Chapter 9—Removal of Organic Compounds from the Environment. In Environmental Organic Chemistry for Engineers; Speight, J.G., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 387–432. ISBN 978-0-12-804492-6. [Google Scholar]
- Sun, Y.; Kumar, M.; Wang, L.; Gupta, J.; Tsang, D.C.W. 13—Biotechnology for Soil Decontamination: Opportunity, Challenges, and Prospects for Pesticide Biodegradation. In Bio-Based Materials and Biotechnologies for Eco-Efficient Construction; Pacheco-Torgal, F., Ivanov, V., Tsang, D.C.W., Eds.; Woodhead Publishing Series in Civil and Structural Engineering; Woodhead Publishing: Sawston, UK, 2020; pp. 261–283. ISBN 978-0-12-819481-2. [Google Scholar]
- Darwesh, O.M.; Matter, I.A. Chapter 14—Nanomaterials in the Biological Treatment of Contaminated Soil. In Nanomaterials for Soil Remediation; Amrane, A., Mohan, D., Nguyen, T.A., Assadi, A.A., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 285–300. ISBN 978-0-12-822891-3. [Google Scholar]
- Zhang, S.; Hou, Z.; Du, X.; Li, D.; Lu, X. Assessment of Biostimulation and Bioaugmentation for Removing Chlorinated Volatile Organic Compounds from Groundwater at a Former Manufacture Plant. Biodegradation 2016, 27, 223–236. [Google Scholar] [CrossRef]
- Couto, M.N.P.F.S.; Monteiro, E.; Vasconcelos, M.T.S.D. Mesocosm Trials of Bioremediation of Contaminated Soil of a Petroleum Refinery: Comparison of Natural Attenuation, Biostimulation and Bioaugmentation. Environ. Sci. Pollut. Res. 2010, 17, 1339–1346. [Google Scholar] [CrossRef]
- Gopinath, M.; Pulla, R.H.; Rajmohan, K.S.; Vijay, P.; Muthukumaran, C.; Gurunathan, B. Bioremediation of Volatile Organic Compounds in Biofilters. In Bioremediation: Applications for Environmental Protection and Management; Varjani, S.J., Agarwal, A.K., Gnansounou, E., Gurunathan, B., Eds.; Energy, Environment, and Sustainability; Springer: Singapore, 2018; pp. 301–330. ISBN 978-981-10-7485-1. [Google Scholar]
- Chen, D.-Z.; Zhao, X.-Y.; Miao, X.-P.; Chen, J.; Ye, J.-X.; Cheng, Z.-W.; Zhang, S.-H.; Chen, J.-M. A Solid Composite Microbial Inoculant for the Simultaneous Removal of Volatile Organic Sulfide Compounds: Preparation, Characterization, and Its Bioaugmentation of a Biotrickling Filter. J. Hazard. Mater. 2018, 342, 589–596. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Chen, J.; Luo, Y.; Sun, B.; Chu, G. Performance and Microbial Analysis of a Biotrickling Filter Inoculated by a Specific Bacteria Consortium for Removal of a Simulated Mixture of Pharmaceutical Volatile Organic Compounds. Chem. Eng. J. 2016, 304, 757–765. [Google Scholar] [CrossRef]
- Cheng, Z.; Lu, L.; Kennes, C.; Ye, J.; Yu, J.; Chen, D.; Chen, J. A Composite Microbial Agent Containing Bacterial and Fungal Species: Optimization of the Preparation Process, Analysis of Characteristics, and Use in the Purification for Volatile Organic Compounds. Bioresour. Technol. 2016, 218, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Putmai, N.; Woraruthai, T.; Tirapanampai, C.; Wongnate, T.; Flood, A.E. Biodegradation Characteristics of Mixed Phenol and P-Cresol Contaminants from a Swine Farm Using Bacteria Immobilized in Calcium Alginate Beads. Bioresour. Technol. Rep. 2023, 23, 101528. [Google Scholar] [CrossRef]
- Sun, D.; Li, J.; Xu, M.; An, T.; Sun, G.; Guo, J. Toluene Removal Efficiency, Process Robustness, and Bacterial Diversity of a Biotrickling Filter Inoculated with Burkholderia Sp. Strain T3. Biotechnol. Bioproc. E 2013, 18, 125–134. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, X.; Zhu, S.; Chen, J.; He, Y.; Shi, Y.; Liu, H.; Qin, L. Co-Treatment with Single and Ternary Mixture Gas of Dimethyl Sulfide, Propanethiol, and Toluene by a Macrokinetic Analysis in a Biotrickling Filter Seeded with Alcaligenes Sp. SY1 and Pseudomonas Putida S1. Fermentation 2021, 7, 309. [Google Scholar] [CrossRef]
- Li, Q.; Tang, Z.; Ou Yang, D.; Zhang, J.; Chen, J.; Chen, D. Abatement of Binary Gaseous Chlorinated VOC by Biotrickling Filter: Performance, Interactions, and Microbial Community. Chemosphere 2023, 313, 137542. [Google Scholar] [CrossRef]
- Dezotti, M.; Lippel, G.; Bassin, J.P. Advanced Biological Processes for Wastewater Treatment; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-58834-6. [Google Scholar]
- Ødegaard, H. A Road-Map for Energy-Neutral Wastewater Treatment Plants of the Future Based on Compact Technologies (Including MBBR). Front. Environ. Sci. Eng. 2016, 10, 2. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Stensel, H.D.; Tsuchihashi, R.; Burton, F.L.; Abu-Orf, M.; Bowden, G.; Pfrang, W.; Metcalf, E. (Eds.) Wastewater Engineering: Treatment and Resource Recovery, 5th ed.McGraw-Hill Education: New York, NY, USA, 2014; ISBN 978-0-07-340118-8. [Google Scholar]
- Cheng, H.-H.; Lu, I.-C.; Huang, P.-W.; Wu, Y.-J.; Whang, L.-M. Biological Treatment of Volatile Organic Compounds (VOCs)-Containing Wastewaters from Wet Scrubbers in Semiconductor Industry. Chemosphere 2021, 282, 131137. [Google Scholar] [CrossRef]
- He, C.; Zhuo, M.; Hou, J. Efficient Purification of Toluene Gas by Anoxic Denitrification. Environ. Sci. Pollut. Res. 2021, 28, 11683–11688. [Google Scholar] [CrossRef] [PubMed]
- Mhemid, R.K.S.; Akmirza, I.; Shihab, M.S.; Turker, M.; Alp, K. Ethanethiol Gas Removal in an Anoxic Bio-Scrubber. J. Environ. Manag. 2019, 233, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Mhemid, R.K.S.; Alp, K.; Turker, M.; Akmirza, I.; Shihab, M.S. Removal of Dimethyl Sulphide via a Bio-Scrubber under Anoxic Conditions. Environ. Technol. 2020, 41, 1700–1714. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, G.; Jeyakumar, R.B.; Somanathan, A. Challenges and Emerging Trends in Advanced Oxidation Technologies and Integration of Advanced Oxidation Processes with Biological Processes for Wastewater Treatment. Sustainability 2023, 15, 4235. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Xi, J.; Saingam, P.; Gu, F.; Hu, H.-Y.; Zhao, X. Effect of Continuous Ozone Injection on Performance and Biomass Accumulation of Biofilters Treating Gaseous Toluene. Appl. Microbiol. Biotechnol. 2015, 99, 33–42. [Google Scholar] [CrossRef]
- Senatore, V.; Zarra, T.; Oliva, G.; Belgiorno, V.; Naddeo, V. Volatile Organic Compounds (VOCs) Control by Combining Bio-Scrubber and Ozone Pretreatment. Glob. NEST J. 2020, 22, 143–146. [Google Scholar] [CrossRef]
- Saingam, P.; Baig, Z.; Xu, Y.; Xi, J. Effect of Ozone Injection on the Long-Term Performance and Microbial Community Structure of a VOCs Biofilter. J. Environ. Sci. 2018, 69, 133–140. [Google Scholar] [CrossRef]
- Covarrubias-García, I.; Aizpuru, A.; Arriaga, S. Effect of the Continuous Addition of Ozone on Biomass Clogging Control in a Biofilter Treating Ethyl Acetate Vapors. Sci. Total Environ. 2017, 584–585, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias-García, I.; De Jonge, N.; Arriaga, S.; Nielsen, J.L. Effects of Ozone Treatment on Performance and Microbial Community Composition in Biofiltration Systems Treating Ethyl Acetate Vapours. Chemosphere 2019, 233, 67–75. [Google Scholar] [CrossRef]
- Han, M.-F.; Hu, X.-R.; Wang, Y.-C.; Tong, Z.; Wang, C.; Cheng, Z.-W.; Feng, K.; Qu, M.-M.; Chen, J.-M.; Deng, J.-G.; et al. Comparison of Separated and Combined Photodegradation and Biofiltration Technology for the Treatment of Volatile Organic Compounds: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1325–1355. [Google Scholar] [CrossRef]
- Wang, C.; Xi, J.; Hu, H.; Kang, I. Effects of Design Parameters on Performance and Cost Analysis of Combined Ultraviolet-Biofilter Systems Treating Gaseous Chlorobenzene Based on Mathematical Modeling. Front. Environ. Sci. Eng. 2012, 6, 588–594. [Google Scholar] [CrossRef]
- Rene, E.R.; Veiga, M.C.; Kennes, C. Combined Biological and Physicochemical Waste-Gas Cleaning Techniques. J. Environ. Sci. Health Part A 2012, 47, 920–939. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Mohseni, M. Using UV Pretreatment to Enhance Biofiltration of Mixtures of Aromatic VOCs. J. Hazard. Mater. 2007, 144, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Palau, J.; Penya-Roja, J.M.; Gabaldón, C.; Álvarez-Hornos, F.J.; Martínez-Soria, V. Effect of Pre-treatments Based on UV Photocatalysis and Photo-oxidation on Toluene Biofiltration Performance. J. Chem. Tech. Biotech. 2012, 87, 65–72. [Google Scholar] [CrossRef]
- Mohseni, M.; Zhao, J.L. Coupling Ultraviolet Photolysis and Biofiltration for Enhanced Degradation of Aromatic Air Pollutants. J. Chem. Tech. Biotech 2006, 81, 146–151. [Google Scholar] [CrossRef]
- Wang, C.; Xi, J.-Y.; Hu, H.-Y.; Yao, Y. Advantages of Combined UV Photodegradation and Biofiltration Processes to Treat Gaseous Chlorobenzene. J. Hazard. Mater. 2009, 171, 1120–1125. [Google Scholar] [CrossRef]
- Saucedo-Lucero, J.O.; Arriaga, S. Photocatalytic Oxidation Process Used as a Pretreatment to Improve Hexane Vapors Biofiltration. J. Chem. Tech. Biotech 2015, 90, 907–914. [Google Scholar] [CrossRef]
- Hinojosa-Reyes, M.; Rodríguez-González, V.; Arriaga, S. Enhancing Ethylbenzene Vapors Degradation in a Hybrid System Based on Photocatalytic Oxidation UV/TiO2–In and a Biofiltration Process. J. Hazard. Mater. 2012, 209–210, 365–371. [Google Scholar] [CrossRef]
- Runye, Z.; Christian, K.; Zhuowei, C.; Lichao, L.; Jianming, Y.; Jianmeng, C. Styrene Removal in a Biotrickling Filter and a Combined UV–Biotrickling Filter: Steady- and Transient-State Performance and Microbial Analysis. Chem. Eng. J. 2015, 275, 168–178. [Google Scholar] [CrossRef]
- Mohseni, M.; Prieto, L. Biofiltration of Hydrophobic VOCs Pretreated with UV Photolysis and Photocatalysis. Int. J. Environ. Technol. Manag. 2008, 9, 47. [Google Scholar] [CrossRef]
- Jianming, Y.; Wei, L.; Zhuowei, C.; Yifeng, J.; Wenji, C.; Jianmeng, C. Dichloromethane Removal and Microbial Variations in a Combination of UV Pretreatment and Biotrickling Filtration. J. Hazard. Mater. 2014, 268, 14–22. [Google Scholar] [CrossRef]
- Zeng, P.; Li, J.; Liao, D.; Tu, X.; Xu, M.; Sun, G. Performance of a Combined System of Biotrickling Filter and Photocatalytic Reactor in Treating Waste Gases from a Paint-Manufacturing Plant. Environ. Technol. 2016, 37, 237–244. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, J.; Chen, J.; Chen, Z.; Li, G.; Sun, G.; An, T. Treatment of Organic Waste Gas in a Paint Plant by Combined Technique of Biotrickling Filtration with Photocatalytic Oxidation. Chem. Eng. J. 2012, 200–202, 645–653. [Google Scholar] [CrossRef]
- Amin, N.A.S. Co-Generation of Synthesis Gas and C2+ Hydrocarbons from Methane and Carbon Dioxide in a Hybrid Catalytic-Plasma Reactor: A Review. Fuel 2006, 85, 577–592. [Google Scholar] [CrossRef]
- Palma, D.; Richard, C.; Minella, M. State of the Art and Perspectives about Non-Thermal Plasma Applications for the Removal of PFAS in Water. Chem. Eng. J. Adv. 2022, 10, 100253. [Google Scholar] [CrossRef]
- Bruggeman, P.; Brandenburg, R. Atmospheric Pressure Discharge Filaments and Microplasmas: Physics, Chemistry and Diagnostics. J. Phys. D Appl. Phys. 2013, 46, 464001. [Google Scholar] [CrossRef]
- Fridman, A.; Chirokov, A.; Gutsol, A. Non-Thermal Atmospheric Pressure Discharges. J. Phys. D Appl. Phys. 2005, 38, R1–R24. [Google Scholar] [CrossRef]
- Schiavon, M.; Torretta, V.; Casazza, A.; Ragazzi, M. Non-Thermal Plasma as an Innovative Option for the Abatement of Volatile Organic Compounds: A Review. Water Air Soil Pollut. 2017, 228, 388. [Google Scholar] [CrossRef]
- Feng, X.; Liu, H.; He, C.; Shen, Z.; Wang, T. Synergistic Effects and Mechanism of a Non-Thermal Plasma Catalysis System in Volatile Organic Compound Removal: A Review. Catal. Sci. Technol. 2018, 8, 936–954. [Google Scholar] [CrossRef]
- Belkessa, N.; Assadi, A.A.; Bouzaza, A.; Nguyen-Tri, P.; Amrane, A.; Khezami, L. A Review of Non-Thermal Plasma -Catalysis: The Mutual Influence and Sources of Synergetic Effect for Boosting Volatile Organic Compounds Removal. Environ. Res. 2024, 257, 119333. [Google Scholar] [CrossRef]
- Schiavon, M.; Schiorlin, M.; Torretta, V.; Brandenburg, R.; Ragazzi, M. Non-Thermal Plasma Assisting the Biofiltration of Volatile Organic Compounds. J. Clean. Prod. 2017, 148, 498–508. [Google Scholar] [CrossRef]
- Martini, L.M.; Coller, G.; Schiavon, M.; Cernuto, A.; Ragazzi, M.; Dilecce, G.; Tosi, P. Non-Thermal Plasma in Waste Composting Facilities: From a Laboratory-Scale Experiment to a Scaled-up Economic Model. J. Clean. Prod. 2019, 230, 230–240. [Google Scholar] [CrossRef]
- Huang, H.; He, L.; Wang, Y.; Chen, L.; Hu, H.; Zhang, M. Experimental Study on Toluene Removal by a Two-Stage Plasma-Biofilter System. Plasma Sci. Technol. 2022, 24, 124011. [Google Scholar] [CrossRef]
- Zhang, S.; You, J.; Kennes, C.; Cheng, Z.; Ye, J.; Chen, D.; Chen, J.; Wang, L. Current Advances of VOCs Degradation by Bioelectrochemical Systems: A Review. Chem. Eng. J. 2018, 334, 2625–2637. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial Fuel Cells: An Overview of Current Technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Li, D.; Şahin, S.; Izadi, P.; Ghangrekar, M.; Duteanu, N.M.; Erable, B.; Scott, K.; Yu, E.H. 4.11—Biological and Microbial Fuel Cells. In Comprehensive Renewable Energy, 2nd ed.; Letcher, T.M., Ed.; Elsevier: Oxford, UK, 2022; pp. 290–316. ISBN 978-0-12-819734-9. [Google Scholar]
- Huang, Y.; Zhao, Y.; Tang, C.; Yadav, A.K.; Abbassi, R.; Kang, P.; Cai, Y.; Liu, A.; Yang, A.; Li, M. A Glance of Coupled Water and Wastewater Treatment Systems Based on Microbial Fuel Cells. Sci. Total Environ. 2023, 892, 164599. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, P.; Long, X.; Ma, J.; Li, Y.; Liu, B.; Xu, Z. Research Progress on Using Biological Cathodes in Microbial Fuel Cells for the Treatment of Wastewater Containing Heavy Metals. Front. Microbiol. 2023, 14, 1270431. [Google Scholar] [CrossRef]
- Scott, K.; Yu, E.H.; Ghangrekar, M.M.; Erable, B.; Duteanu, N.M. Biological and Microbial Fuel Cells. In Comprehensive Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2012; pp. 277–300. ISBN 978-0-08-087873-7. [Google Scholar]
- Chen, T.; Zou, C.; Pan, J.; Wang, M.; Qiao, L.; Wang, F.; Zhao, Q.; Cheng, H.; Ding, C.; Yuan, Y. Mapping Research on Microbial Fuel Cells in Wastewater Treatment: A Co-Citation Analysis. Processes 2022, 10, 179. [Google Scholar] [CrossRef]
- Rousseau, D.P.L.; Louage, F.; Wang, Q.; Zhang, R. Constructed Wetlands for Urban Wastewater Treatment: An Overview. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 978-0-12-409548-9. [Google Scholar]
- Ucar, D.; Zhang, Y.; Angelidaki, I. An Overview of Electron Acceptors in Microbial Fuel Cells. Front. Microbiol. 2017, 8, 643. [Google Scholar] [CrossRef]
- Pandit, S.; Das, D. Principles of Microbial Fuel Cell for the Power Generation. In Microbial Fuel Cell: A Bioelectrochemical System that Converts Waste to Watts; Das, D., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 21–41. ISBN 978-3-319-66793-5. [Google Scholar]
- Mahmoodzadeh, F.; Navidjouy, N.; Alizadeh, S.; Rahimnejad, M. Investigation of Microbial Fuel Cell Performance Based on the Nickel Thin Film Modified Electrodes. Sci. Rep. 2023, 13, 20755. [Google Scholar] [CrossRef]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular Electron Transfer Mechanisms between Microorganisms and Minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- Kumar, A.; Hsu, L.H.-H.; Kavanagh, P.; Barrière, F.; Lens, P.N.L.; Lapinsonnière, L.; Lienhard, V.J.H.; Schröder, U.; Jiang, X.; Leech, D. The Ins and Outs of Microorganism–Electrode Electron Transfer Reactions. Nat. Rev. Chem. 2017, 1, 0024. [Google Scholar] [CrossRef]
- Zheng, T.; Li, J.; Ji, Y.; Zhang, W.; Fang, Y.; Xin, F.; Dong, W.; Wei, P.; Ma, J.; Jiang, M. Progress and Prospects of Bioelectrochemical Systems: Electron Transfer and Its Applications in the Microbial Metabolism. Front. Bioeng. Biotechnol. 2020, 8, 10. [Google Scholar] [CrossRef]
- Nawaz, A.; Hafeez, A.; Abbas, S.Z.; Haq, I.U.; Mukhtar, H.; Rafatullah, M. A State of the Art Review on Electron Transfer Mechanisms, Characteristics, Applications and Recent Advancements in Microbial Fuel Cells Technology. Green Chem. Lett. Rev. 2020, 13, 365–381. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E. Electromicrobiology: The Ecophysiology of Phylogenetically Diverse Electroactive Microorganisms. Nat. Rev. Microbiol. 2022, 20, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-L.; Zhu, Y.; Li, J. Electron Transfer Mechanisms, Characteristics and Applications of Biological Cathode Microbial Fuel Cells—A Mini Review. Arab. J. Chem. 2019, 12, 2236–2243. [Google Scholar] [CrossRef]
- Aiyer, K.S. How Does Electron Transfer Occur in Microbial Fuel Cells? World J. Microbiol. Biotechnol. 2020, 36, 19. [Google Scholar] [CrossRef] [PubMed]
- Oveisi, F.; Fallah, N.; Nasernejad, B. Biodegradation of Synthetic Wastewater Containing Styrene in Microbial Fuel Cell: Effect of Adaptation of Microbial Community. Fuel 2021, 305, 121382. [Google Scholar] [CrossRef]
- Mylsamy, P.; Omine, K.; Sivasankar, V. (Eds.) Microbial Fuel Cell Technology for Bioelectricity, 1st ed.; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-92904-0. [Google Scholar]
- Liu, L.; Tsyganova, O.; Lee, D.-J.; Su, A.; Chang, J.-S.; Wang, A.; Ren, N. Anodic Biofilm in Single-Chamber Microbial Fuel Cells Cultivated under Different Temperatures. Int. J. Hydrogen Energy 2012, 37, 15792–15800. [Google Scholar] [CrossRef]
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C.T.; Hassett, D.J.; Gu, T. Microbial Fuel Cell (MFC) Power Performance Improvement through Enhanced Microbial Electrogenicity. Biotechnol. Adv. 2018, 36, 1316–1327. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, S.; Li, P.; Huang, H.; Cen, K. Sensitivity to Oxygen in Microbial Electrochemical Systems Biofilms. iScience 2019, 13, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Akinwumi, O.D.; Dada, E.O.; Agarry, S.E.; Aremu, M.O.; Agbede, O.O.; Alade, A.O.; Aworanti, O.A.; Alao, A.I. Effects of Retention Time, pH, Temperature and Type of Fruit Wastes on the Bioelectricity Generation Performance of Microbial Fuel Cell during the Biotreatment of Pharmaceutical Wastewater: Experimental Study, Optimization and Modelling. Environ. Process. 2024, 11, 51. [Google Scholar] [CrossRef]
- Thakur, S.; Calay, R.K.; Mustafa, M.Y.; Eregno, F.E.; Patil, R.R. Importance of Substrate Type and Its Constituents on Overall Performance of Microbial Fuel Cells. Curr. Res. Biotechnol. 2025, 9, 100272. [Google Scholar] [CrossRef]
- Chen, F.; Liang, B.; Li, Z.-L.; Yang, J.-Q.; Huang, C.; Lyu, M.; Yuan, Y.; Nan, J.; Wang, A.-J. Bioelectrochemical Assisted Dechlorination of Tetrachloroethylene and 1,2-Dichloroethane by Acclimation of Anaerobic Sludge. Chemosphere 2019, 227, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.; Dai, S.; Jin, B. Bioelectrochemical Reaction Kinetics, Mechanisms, and Pathways of Chlorophenol Degradation in MFC Using Different Microbial Consortia. ACS Sustain. Chem. Eng. 2019, 7, 17263–17272. [Google Scholar] [CrossRef]
- Wang, G.; Huang, L.; Zhang, Y. Cathodic Reduction of Hexavalent Chromium [Cr(VI)] Coupled with Electricity Generation in Microbial Fuel Cells. Biotechnol. Lett. 2008, 30, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Huang, L.; Quan, X.; Li Puma, G. Electricity Generation and Bivalent Copper Reduction as a Function of Operation Time and Cathode Electrode Material in Microbial Fuel Cells. J. Power Sources 2016, 307, 705–714. [Google Scholar] [CrossRef]
- Lim, B.S.; Lu, H.; Choi, C.; Liu, Z.X. Recovery of Silver Metal and Electric Power Generation Using a Microbial Fuel Cell. Desalination Water Treat. 2015, 54, 3675–3681. [Google Scholar] [CrossRef]
- Choi, C.; Hu, N.; Lim, B. Cadmium Recovery by Coupling Double Microbial Fuel Cells. Bioresour. Technol. 2014, 170, 361–369. [Google Scholar] [CrossRef]
- Kumbhar, P.; Savla, N.; Banerjee, S.; Mathuriya, A.S.; Sarkar, A.; Khilari, S.; Jadhav, D.A.; Pandit, S. Microbial Electrochemical Heavy Metal Removal: Fundamental to the Recent Development. In Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 521–542. ISBN 978-0-12-821881-5. [Google Scholar]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive Microorganisms in Bioelectrochemical Systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Sydow, A.; Krieg, T.; Mayer, F.; Schrader, J.; Holtmann, D. Electroactive Bacteria—Molecular Mechanisms and Genetic Tools. Appl. Microbiol. Biotechnol. 2014, 98, 8481–8495. [Google Scholar] [CrossRef]
- Luo, J.; Tian, W.; Jin, H.; Yang, J.; Li, J.; Wang, Y.; Shen, W.; Ren, Y.; Zhou, M. Recent Advances in Microbial Fuel Cells: A Review on the Identification Technology, Molecular Tool and Improvement Strategy of Electricigens. Curr. Opin. Electrochem. 2023, 37, 101187. [Google Scholar] [CrossRef]
- Thengumthottathil, V.; Ponnusamy, K.; Naina Mohamed, S. Bioelectrochemical Systems: Exploring Microbial Communities, Interactions, and Electron Transfer. Biochem. Eng. J. 2024, 211, 109442. [Google Scholar] [CrossRef]
- Ren, Z.; Ward, T.E.; Regan, J.M. Electricity Production from Cellulose in a Microbial Fuel Cell Using a Defined Binary Culture. Environ. Sci. Technol. 2007, 41, 4781–4786. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, A.; Rosenbaum, M.A.; Perkins, S.D.; Werner, J.J.; Angenent, L.T. Metabolite-Based Mutualism between Pseudomonas Aeruginosa PA14 and Enterobacter Aerogenes Enhances Current Generation in Bioelectrochemical Systems. Energy Environ. Sci. 2011, 4, 4550. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Dominguez-Benetton, X.; Pant, D. Internal Resistance of Microfluidic Microbial Fuel Cell: Challenges and Potential Opportunities. Bioresour. Technol. 2013, 142, 672–682. [Google Scholar] [CrossRef]
- Wang, W.; Wei, X.; Choi, D.; Lu, X.; Yang, G.; Sun, C. Chapter 1—Electrochemical Cells for Medium- and Large-Scale Energy Storage: Fundamentals. In Advances in Batteries for Medium and Large-Scale Energy Storage; Menictas, C., Skyllas-Kazacos, M., Lim, T.M., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2015; pp. 3–28. ISBN 978-1-78242-013-2. [Google Scholar]
- Barbir, F. Chapter Three—Fuel Cell Electrochemistry. In PEM Fuel Cells, 2nd ed.; Barbir, F., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 33–72. ISBN 978-0-12-387710-9. [Google Scholar]
- Berrueta, A.; San Martín, I.; Sanchis, P.; Ursúa, A. Chapter 6—Lithium-Ion Batteries as Distributed Energy Storage Systems for Microgrids. In Distributed Energy Resources in Microgrids; Chauhan, R.K., Chauhan, K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 143–183. ISBN 978-0-12-817774-7. [Google Scholar]
- Luis, P. Chapter 1—Introduction. In Fundamental Modelling of Membrane Systems; Luis, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–23. ISBN 978-0-12-813483-2. [Google Scholar]
- Luo, H.; Liu, G.; Zhang, R.; Jin, S. Phenol Degradation in Microbial Fuel Cells. Chem. Eng. J. 2009, 147, 259–264. [Google Scholar] [CrossRef]
- Guang, L.; Koomson, D.A.; Jingyu, H.; Ewusi-Mensah, D.; Miwornunyuie, N. Performance of Exoelectrogenic Bacteria Used in Microbial Desalination Cell Technology. Int. J. Environ. Res. Public Health 2020, 17, 1121. [Google Scholar] [CrossRef]
- Wu, C.-H.; Lai, C.-Y.; Lin, C.-W.; Kao, M.-H. Generation of Power by Microbial Fuel Cell with Ferricyanide in Biodegradation of Benzene. CLEAN-Soil Air Water 2013, 41, 390–395. [Google Scholar] [CrossRef]
- Wu, C.-H.; Yet-Pole, I.; Chiu, Y.-H.; Lin, C.-W. Enhancement of Power Generation by Toluene Biodegradation in a Microbial Fuel Cell in the Presence of Pyocyanin. J. Taiwan Inst. Chem. Eng. 2014, 45, 2319–2324. [Google Scholar] [CrossRef]
- Wu, C.-H.; Lin, C.-W. Electricity Generation and Kinetic Aspects of a Biotrickling Filter-Microbial Fuel Cell for the Biofiltration of Ethyl Acetate Vapor from Waste Gas. J. Taiwan Inst. Chem. Eng. 2016, 68, 332–337. [Google Scholar] [CrossRef]
- Wu, C.-H.; Shih, J.-C.; Lin, C.-W. Continuous Production of Power Using Microbial Fuel Cells with Integrated Biotrickling Filter for Ethyl Acetate-Contaminated Air Stream Treatment. Int. J. Hydrogen Energy 2016, 41, 21945–21954. [Google Scholar] [CrossRef]
- Dai, Y.; Guo, Y.; Wang, J.; Li, Y.; Zhang, L.; Liu, X. A Vertically Configured Photocatalytic-Microbial Fuel Cell for Electricity Generation and Gaseous Toluene Degradation. Chemosphere 2021, 285, 131530. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Lin, H.-H.; Wen, S.; Lin, C.-W. Performance of Trickling Bed Microbial Fuel Cell Treating Isopropyl Alcohol Vapor: Effects of Shock-Load and Shut-down Episodes. Chemosphere 2019, 224, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Tsai, S.-L.; Lai, Y.-R.; Lin, C.-W.; Huang, Y.-W. Improving the Performance of Biotrickling Filter Microbial Fuel Cells in Treating Exhaust Gas by Adjusting the Oxygen Content of the Anode Tank. Chemosphere 2021, 278, 130390. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lin, H.-H.; Lin, C.-W. Gaseous Isopropanol Removal in a Microbial Fuel Cell with Deoxidizing Anode: Performance, Anode Characteristics and Microbial Community. J. Hazard. Mater. 2022, 423, 127200. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Y.; Dai, W.; Su, F.; Li, J.; Zhang, C.; Zhang, R.; Liu, P. Simultaneous Removal of Toluene in Gas and Electricity Production by Using a Single-Chamber Microbial Fuel Cell-Biotricking Filter System. ACS Sustain. Chem. Eng. 2022, 10, 8559–8567. [Google Scholar] [CrossRef]
- Yang, Z.; Han, Y.; Yang, R.; Guo, N.; Duan, H.; Zhang, G. Enhancement of Gaseous Ethyl Acetate Removal in a New Bio-Trickling Filter by Integrating with Microbial Fuel Cell. Process Biochem. 2025, 149, 270–276. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial Fuel Cells, a Renewable Energy Technology for Bio-Electricity Generation: A Mini-Review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Abbas, A.A.; Farrag, H.H.; El-Sawy, E.; Allam, N.K. Microbial Fuel Cells with Enhanced Bacterial Catalytic Activity and Stability Using 3D Nanoporous Stainless Steel Anode. J. Clean. Prod. 2021, 285, 124816. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Mungray, A.K.; Arkatkar, A.; Kumar, S.S. Recent Advancement in Scaling-up Applications of Microbial Fuel Cells: From Reality to Practicability. Sustain. Energy Technol. Assess. 2021, 45, 101226. [Google Scholar] [CrossRef]
- Tsekouras, G.J.; Deligianni, P.M.; Kanellos, F.D.; Kontargyri, V.T.; Kontaxis, P.A.; Manousakis, N.M.; Elias, C.N. Microbial Fuel Cell for Wastewater Treatment as Power Plant in Smart Grids: Utopia or Reality? Front. Energy Res. 2022, 10, 843768. [Google Scholar] [CrossRef]
- Saraf, R.; Goyal, A.; Jain, D.; Dutt, K. Microbial Fuel Cell an Alternative for Sustainable Energy Production: Substrate, Challenges and Application. Curr. Green Chem. 2025, 12, 291–308. [Google Scholar] [CrossRef]
- Rossi, R.; Hur, A.Y.; Page, M.A.; Thomas, A.O.; Butkiewicz, J.J.; Jones, D.W.; Baek, G.; Saikaly, P.E.; Cropek, D.M.; Logan, B.E. Pilot Scale Microbial Fuel Cells Using Air Cathodes for Producing Electricity While Treating Wastewater. Water Res. 2022, 215, 118208. [Google Scholar] [CrossRef] [PubMed]
- Blatter, M.; Delabays, L.; Furrer, C.; Huguenin, G.; Cachelin, C.P.; Fischer, F. Stretched 1000-L Microbial Fuel Cell. J. Power Sources 2021, 483, 229130. [Google Scholar] [CrossRef]
- Heinrichmeier, J.; Littfinski, T.; Vasyukova, E.; Steuernagel, L.; Wichern, M. On-Site Performance Evaluation of a 1,000-Litre Microbial Fuel Cell System Using Submergible Multi-Electrode Modules with Air-Cathodes for Sustainable Municipal Wastewater Treatment and Electricity Generation. Water Sci. Technol. 2023, 87, 1969–1981. [Google Scholar] [CrossRef] [PubMed]
- Hiegemann, H.; Herzer, D.; Nettmann, E.; Lübken, M.; Schulte, P.; Schmelz, K.-G.; Gredigk-Hoffmann, S.; Wichern, M. An Integrated 45 L Pilot Microbial Fuel Cell System at a Full-Scale Wastewater Treatment Plant. Bioresour. Technol. 2016, 218, 115–122. [Google Scholar] [CrossRef]
- Jalili, P.; Ala, A.; Nazari, P.; Jalili, B.; Ganji, D.D. A Comprehensive Review of Microbial Fuel Cells Considering Materials, Methods, Structures, and Microorganisms. Heliyon 2024, 10, e25439. [Google Scholar] [CrossRef]
- Bird, H.; Heidrich, E.S.; Leicester, D.D.; Theodosiou, P. Pilot-Scale Microbial Fuel Cells (MFCs): A Meta-Analysis Study to Inform Full-Scale Design Principles for Optimum Wastewater Treatment. J. Clean. Prod. 2022, 346, 131227. [Google Scholar] [CrossRef]
- Ali, H.E.; Hemdan, B.A.; El-Naggar, M.E.; El-Liethy, M.A.; Jadhav, D.A.; El-Hendawy, H.H.; Ali, M.; El-Taweel, G.E. Harnessing the Power of Microbial Fuel Cells as Pioneering Green Technology: Advancing Sustainable Energy and Wastewater Treatment through Innovative Nanotechnology. Bioprocess Biosyst. Eng. 2025, 48, 343–366. [Google Scholar] [CrossRef]
- Li, C.; Liang, D.; Tian, Y.; Liu, S.; He, W.; Li, Z.; Yadav, R.S.; Ma, Y.; Ji, C.; Yi, K.; et al. Sorting Out the Latest Advances in Separators and Pilot-Scale Microbial Electrochemical Systems for Wastewater Treatment: Concomitant Development, Practical Application, and Future Perspective. Environ. Sci. Technol. 2024, 58, 9471–9486. [Google Scholar] [CrossRef]
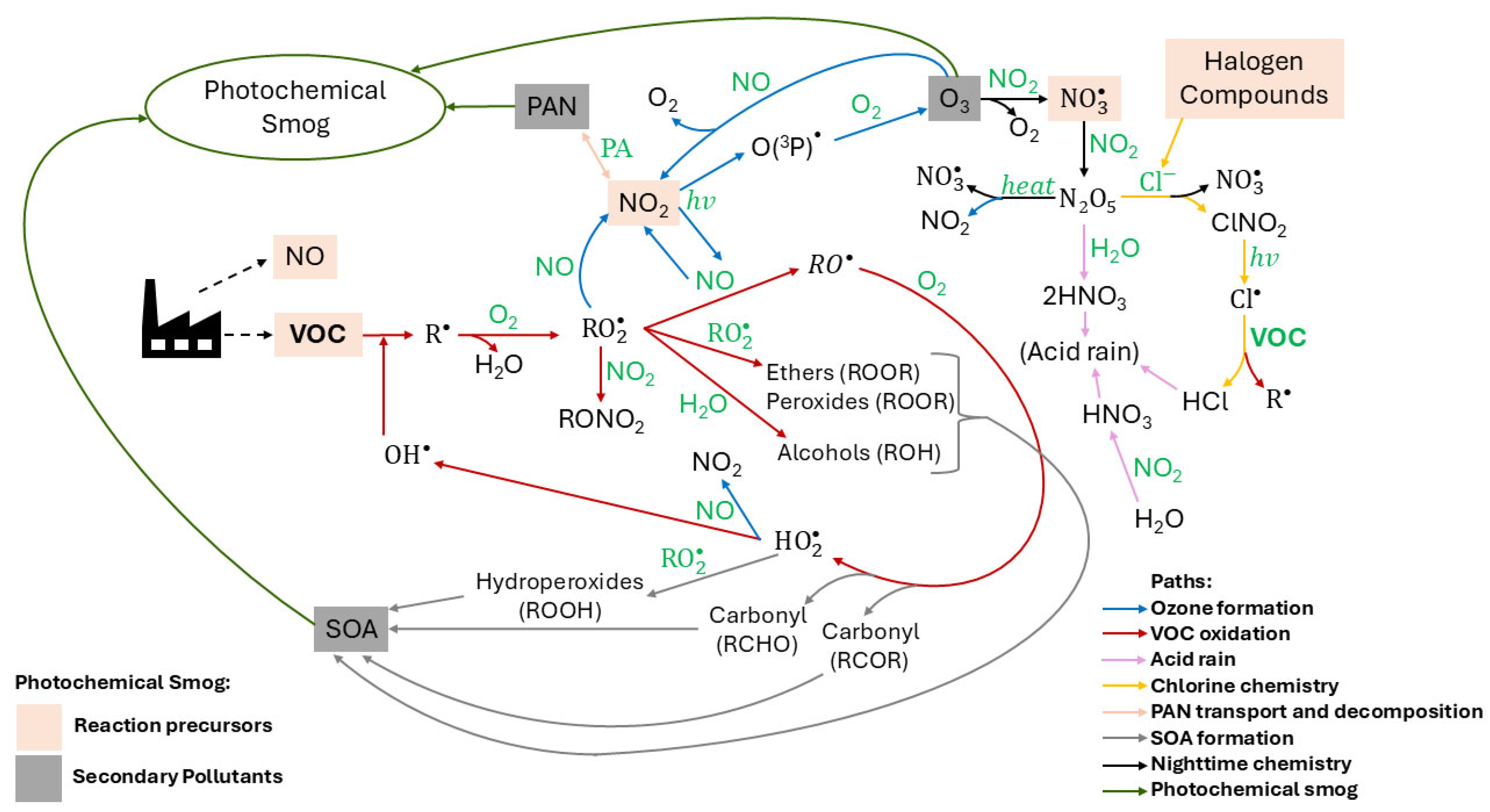
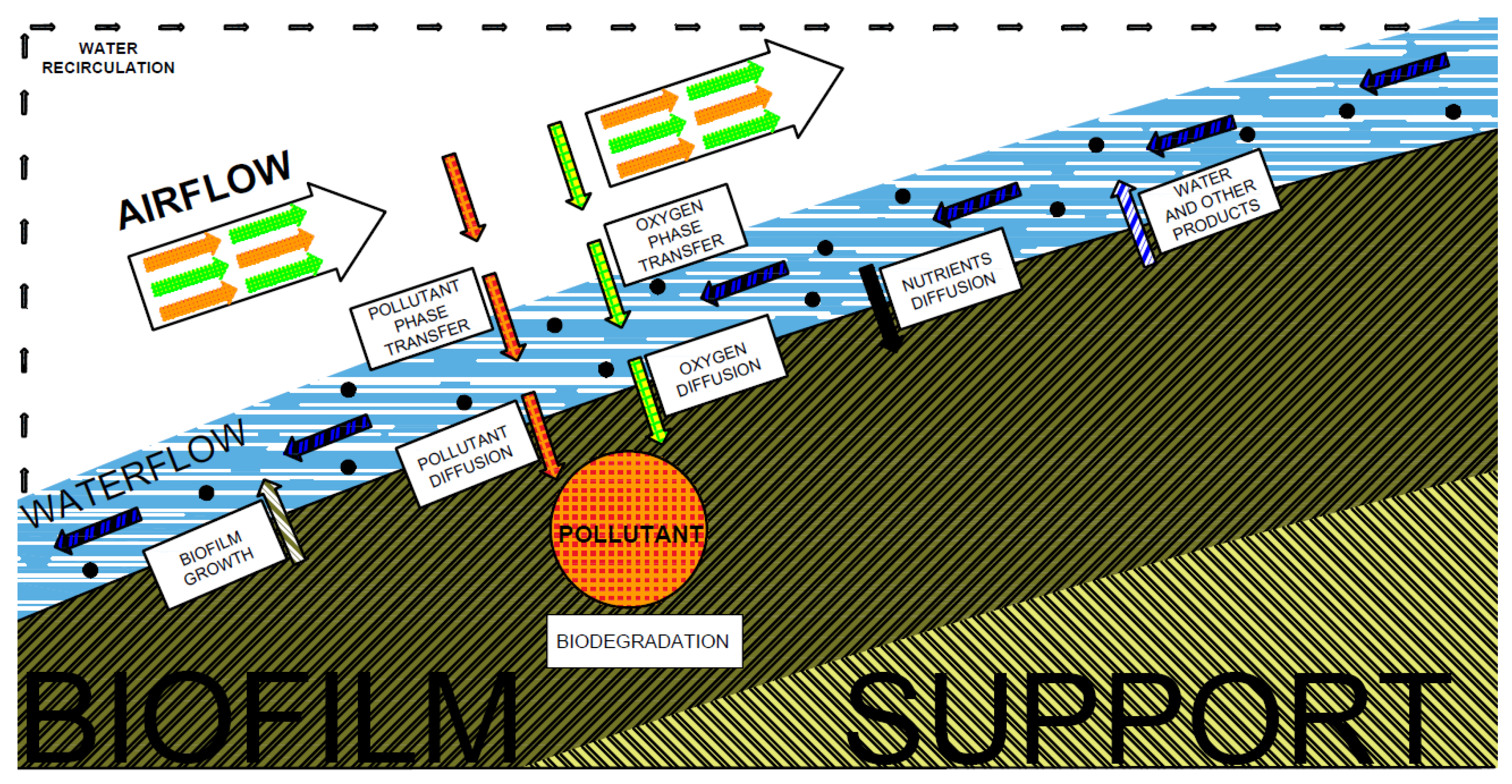
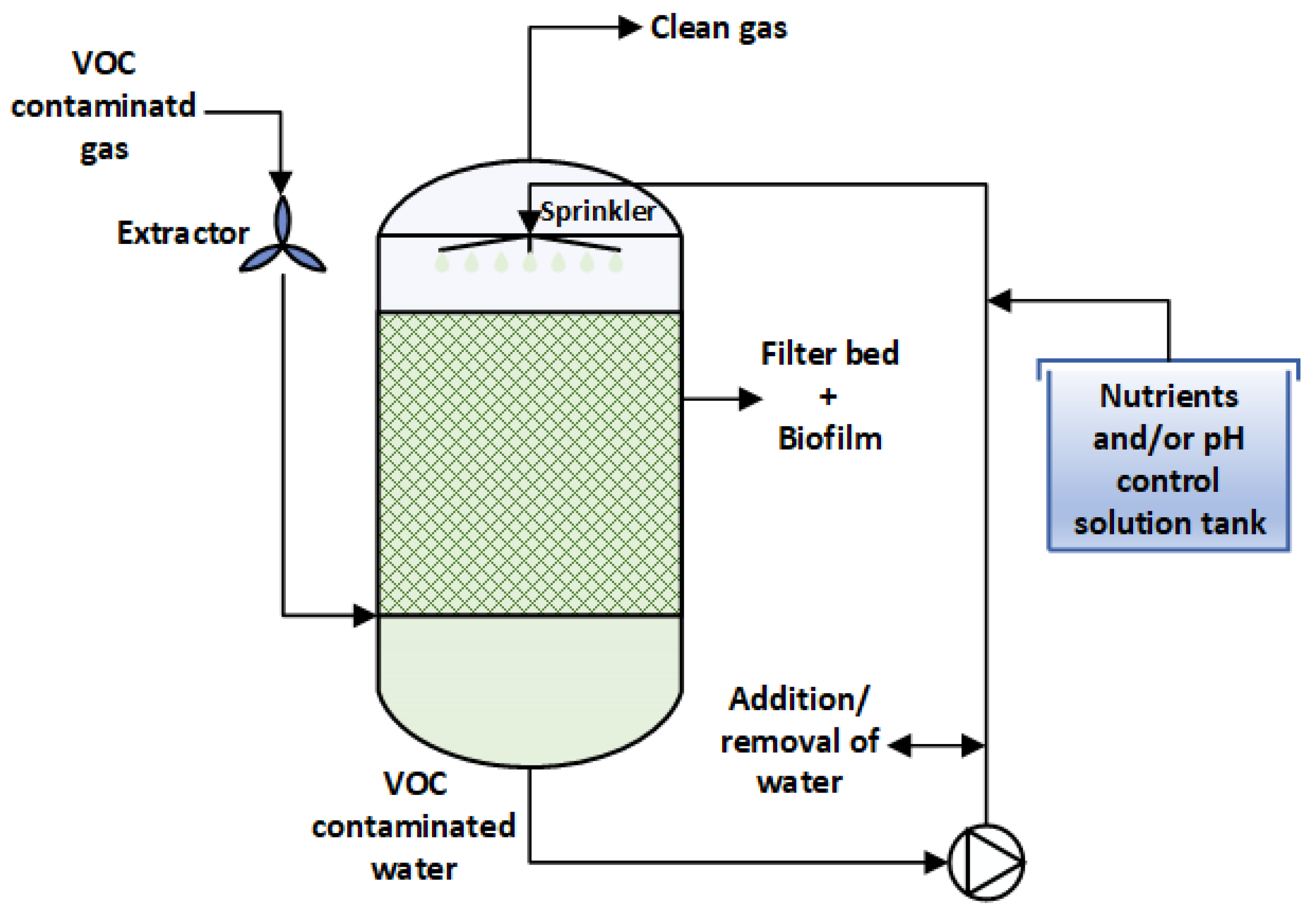

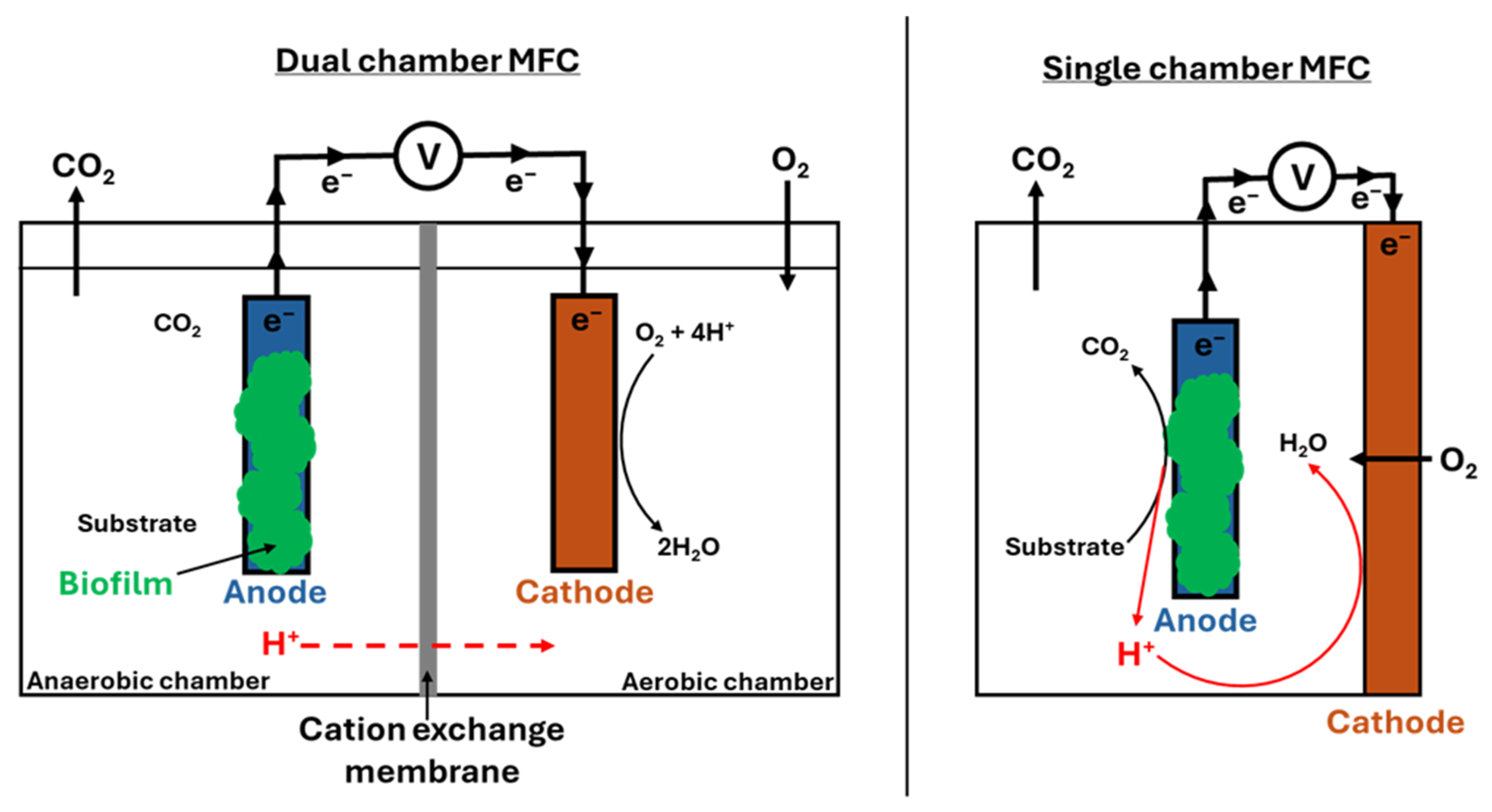
| Industry | Volatile Organic Compound | References |
|---|---|---|
| Pharmaceutical | Halogenated Alkanes: Dichloromethane, Chloroform, 1,2-Dichloropropane, 1,1-Dichloropropanone, Dichloronitromethane, Cyanogen Chloride, Chloroethane; Halogenated Aromatics: Chlorobenzene, Bromobenzene; Halogenated Alkenes: Tetrachloroethylene; Halogenated Methanes: Bromoform; Esters: Ethyl Acetate; Ketones: Acetone; Aromatic Hydrocarbons: Toluene | [82,83] |
| Thermal pyrolysis of high-value products from organic residues | Chickpea Aromatic Hydrocarbons: Methylbenzene; Aldehydes: 2-Methylbutanal, 3-Methylbutanal; Furans: 2-Methylfuran; Alkanes: Octane; Alkenes: 2-Octene; Sulfur Compounds: Dimethyl disulfide; Nitriles: 4-Methylpentanenitrile; Cycloalkenes: 1,3-Dimethyl-1-cyclohexene | [84,85] |
| Soybeans Aromatic Hydrocarbons: Methylbenzene, Ethylbenzene; Nitrogen Compounds: 1-H-Pyrrole, 1-Methyl-1-H-pyrrole; Phenols: Phenol; Aldehydes: 2-Methylbutanal, 3-Methylbutanal; Furans: 2-Methylfuran | ||
| Cigarette | Alcohols: Ethanol, Methanol; Ketones: Acetone; Aromatic Hydrocarbons: Toluene; Phenols: Phenol; Alkanes: Butane; Aldehydes: Formaldehyde | [86] |
| Tire | Alkanes: n-Pentane, 3-Methylhexane, Cyclohexane; Alkenes: Isoprene; Halogenated Hydrocarbons: Methylene Chloride, Carbon Tetrachloride, 1,2-Dichloropropane, Dichlorodifluoromethane; Aromatic Hydrocarbons: m,p-Xylene, Toluene, Ethylbenzene, Styrene | [87] |
| Rubber and plastic products | Halogenated Hydrocarbons: Dichloromethane, 1,2-Dichloroethylene, 1,2-Dichloropropane, Carbon Tetrachloride, Chlorobenzene; Sulfur Compounds: Carbon Disulfide; Aromatic Hydrocarbons: Benzene, Styrene, Naphthalene, Ethylbenzene, Xylene, Toluene, 1,2,4-Trimethylbenzene, 1,3,5-Trimethylbenzene; Ketones: 4-Methyl-2-Pentanone, Acetone, 2-Hexanone; Esters: Methyl methacrylate, Ethyl acetate; Alcohols: Isopropanol, Ethanol; Alkanes and Isomers: Heptane and its isomers, 2-Methylpentane, 3-Methylpentane; Alkenes: 1,3-Butadiene, 1-Decene, 1-Dodecene; Ethers: Tetrahydrofuran, Methyl tert-butyl ether | [62,88,89] |
| Petrochemical | Alkanes and Isomers: Ethane, Propane, 2,3-Dimethylbutane, 2-Methylpentane, 2,2-Dimethylbutane, n-Undecane, Dodecane; Alkenes: Ethylene, Propylene; Aromatic Hydrocarbons: Benzene, Toluene, m,p-Xylene, o-Xylene; Halogenated Hydrocarbons: Chloroform; Alcohols: Ethanol, Isopropanol; Ketones: Acetone, 2-Hexanone | [88,90] |
| Glass | Aromatic Hydrocarbons: Benzene, Toluene, Ethylbenzene, m/p-Xylene, o-Xylene, 4-Ethyltoluene, 1,2,4-Trimethylbenzene, 1,3,5-Trimethylbenzene; Alkanes: n-Hexane, n-Heptane; Alkenes: 1,3-Butadiene, 1-Decene, 1-Dodecene; Halogenated Hydrocarbons: Carbon Tetrachloride, 1,2-Dichloroethane, Tetrachloroethylene, Chlorobenzene; Esters: Ethyl Acetate; Alcohols: Isopropanol; Ketones: Acetone; Ethers: Tetrahydrofuran; Sulfur Compounds: Carbon Disulfide | [91] |
| Fiber board | Halogenated Hydrocarbons: Dichloromethane; Epoxides: 2,3-Epoxy-2-methylbutane; Alkanes: Butane; Alcohols: Ethanol, 1-Butanol, 1,2-Propanediol; Esters: Ethyl acetate, 2-Methylacrylic acid methyl ester; Aldehydes: Acetaldehyde; Furans: 3-Methyl-2(5H)-furanone; Ethers: Tetrahydrofuran, 1,4-Dioxane; Amino Acids: N-Formylglycine; Carboxylic Acids: Acetic acid; Amides: N,N-Dimethylformamide | [92] |
| Furniture | Coating Aromatic Hydrocarbons: Benzene, Toluene, Xylene (m-, p-, and o-xylene), Ethylbenzene, Styrene, p-Dichlorobenzene, 1,2,4-Trimethylbenzene, Chlorobenzene; Aldehydes: Formaldehyde, Acetaldehyde, Nonanal, Decanal; Esters: Butyl acetate, Dimethyl adipate, Dimethyl glutarate, Dimethyl succinate, Ethyl acetate; Ketones: Acetone; Alcohols: Ethanol, Isopropanol, Benzyl alcohol, Methanol; Alkanes: Hexane, Heptane; Alkenes: Propylene, Butylene; Halogenated Hydrocarbons: Dichloromethane, Trichloromethane, Tetrachloroethylene | [93,94,95] |
| Adhesives Alkanes and Isomers: 2-Methylhexane, 3-Methylhexane, n-Hexane, n-Heptane, 3-Methylpentane, 3,4-Dimethylheptane; Cycloalkanes and Isomers: Methylcyclopentane, Ethylcyclopentane, Methylcyclohexane, 1,2,4-Trimethylcyclopentane, 1,2,3-Trimethylcyclopentane, 1,3-Dimethylcyclopentane, 1-Methylcyclohexane; Alcohols: Propylene Glycol, 1-Butanol, 5-Methyl-2-(1-methylethyl)hexan-1-ol, 4,8-Dimethylnonan-1-ol, 6-Methyloctan-1-ol, 1-(2-Butoxy-1-methylethoxy)propan-2-ol, Dihydro-α-Terpineol; Glycols: 2-(2-Butoxyethoxy)ethanol, 2-(2-Butoxyethoxy)ethanol acetate; Esters: 2-(2-Butoxyethoxy)ethyl acetate, Butyl acetate, Ethyl acetate; Ketones: Acetone; Aldehydes: Formaldehyde, 2-Hydroxybenzaldehyde; Halogenated Hydrocarbons: 2-Chlorohexane; Ethers: 1-Methoxypropan-2-ol; Terpenes: Alpha-Pinene; Carboxylic Acids: Acetic Acid | ||
| Painting | Engine manufacturing Aromatic Hydrocarbons: Benzene, (1-Methyldodecyl)-Benzene, 1,3-Dimethyl-p-Xylene, Ethylbenzene, o-Xylene, Toluene; Phenols and Derivatives: Phenol, 4,4′-(1-Methylethylidene)bis-, Phenol derivatives (e.g., 2,4′-Isopropylidenedi-phenol); Alcohols: Benzyl alcohol, Ethanol, 2-(2-Butoxyethoxy)-; Aldehydes: Decanal, Nonanal; Alkanes: Dodecane, Hexadecane, Octadecane, Tetradecane, Undecane, Eicosane; Cycloalkanes: Cyclotetradecane; Carboxylic Acids: n-Hexadecanoic acid | [96,97] |
| Car painting line Alcohols: Ethanol, 2-Propanol, 1-Propanol, Isobutanol, 1-Butanol; Aldehydes: Propanal, Butanal, Pentanal, Hexanal; Ketones: Acetone, 2-Butanone, 2-Hexanone; Carboxylic Acids: Acetic Acid, Propanoic Acid; Aromatic Hydrocarbons: Benzene | ||
| Oil and gas development | Alkanes: Ethane, Propane, n-Butane, Isobutane, n-Pentane, n-Hexane; Cycloalkanes: Cyclohexane; Alkenes: Propylene, Ethylene, Isoprene; Aromatic Hydrocarbons: Benzene, Toluene, Ethylbenzene, Xylenes (o-, m-, p-xylene), Styrene, Naphthalene, Methylnaphthalene, Dimethylnaphthalene; Halogenated Hydrocarbons: Dichloromethane, Chlorinated hydrocarbons; Phenols: Phenol | [98,99] |
| Ceramic | Aromatic Hydrocarbons: Benzene, Toluene, Xylene (o-xylene, m-xylene, p-xylene); Aldehydes: Formaldehyde, Acetaldehyde, Benzaldehyde, Hexanal, Heptanal; Carboxylic Acids: Propionic acid, Acetic acid, Oleic acid, Stearic acid; Alkanes: Hexadecane, Heptadecane, Octadecane; Cycloalkanes: Cyclotetradecane; Phenols: Phenol; Ketones: Acetone, 2-Pentanone | [100] |
| Paint | Aromatic Hydrocarbons: Benzene, Toluene, Ethylbenzene, Xylene (m/p-xylene), Styrene; Alkanes: n-Hexane, n-Heptane, n-Octane, n-Nonane, n-Decane; Halogenated Hydrocarbons: Trichloroethylene, Tetrachloroethylene, Dichlorofluoromethane; Esters: n-Butyl acetate; Ketones: Acetone | [101] |
| Printing | Alkanes: n-Nonane, n-Decane, n-Undecane; Aromatic Hydrocarbons: Toluene, m/p-Xylene; Alcohols: 2-Propanol; Esters: Ethyl acetate | [102,103] |
| Footwear | Alkanes: 3-Methylhexane, 2-Methylhexane, Heptane, n-Hexane, n-Heptane, n-Octane, Ethane; Alkenes: 1-Hexene, Propylene, Ethylene, 4-Methyl-1,3-pentadiene; Alkynes: Acetylene; Aromatic Hydrocarbons: Toluene, Benzene, Chlorobenzene; Ketones: 2-Butanone, 2-Methylethylketone, Acetone, 2-Hexanone; Esters: Ethyl acetate, Methyl Methacrylate; Ethers: Tetrahydrofuran; Halogenated Hydrocarbons: 1,2-Dichloroethylene, Chlorobenzene; Cycloalkanes: Methylcyclopentane, Methylcyclohexane; Sulfur Compounds: Carbon Disulfide; Aldehydes: Acrolein | [88,104] |
| Electronic equipment | Alcohols: Ethanol, Isopropanol; Alkanes: 2,3-Dimethylpentane, n-Undecane, Dodecane, 2-Methylhexane, 2-Methylpentane, 3-Methylpentane, n-Pentane; Cycloalkanes: Cyclohexane; Alkenes: Isoprene; Halogenated Hydrocarbons: Dichlorodifluoromethane, Trichloroethylene; Esters: n-Butyl acetate; Ketones: Acetone | [88,105] |
| Metal product | Alkanes: n-Undecane, Dodecane, n-Decane, Decane, Isopentane, Propane; Halogenated Hydrocarbons: 1,1,2-Tetrachloroethane; Alcohols: Isopropanol; Aromatic Hydrocarbons: Naphthalene; Ketones: Acetone | [88] |
| Technology | Efficiency (% VOC Removal) | Investment Costs (EUR per m3·h−1 Treated Air) | Operating Costs (EUR per 1000 m3 Treated Air) | Scalability/ Industrial Suitability | References |
|---|---|---|---|---|---|
| Biofilter (BF) | High: typically 80–99%, dependent on VOC type | Low: 1.53–12.8 | Low: 0.23–1.0 | Suitable; highly scalable; effectiveness dependent on packing media and moisture control | [152,153,157,158,174,177] |
| Biotrickling filter (BTF) | High: typically 80–99%, excellent for hydrophilic VOC | Moderate: 1.41–18.78 | Low: 0.04–1.27 | Highly scalable; requires effective control of liquid recirculation; optimal for soluble VOC | [117,152,155,157,158,191] |
| Bioscrubber (BS) | Moderate to high: optimal for highly soluble VOC | Moderate to high: 10–32 | Low: 2–6 | Moderate scalability; best suited for VOC with high aqueous solubility | [117,158,162] |
| Thermal Oxidation | Very high: ~95–99%, independent of VOC type | Very high: 12.8–34.83 | High: 1.5–7.67 | Scalable; energy-intensive; economically suitable mainly for concentrated VOC streams | [152,160,161,191] |
| Chemical Scrubbing | Low to high: typically 40–99%, dependent on chemical agent | Moderate to high: 15–30 | Moderate: 5–6 | Moderate scalability; depends heavily on VOC solubility; chemical management and disposal issues limit broader applicability | [117,153,202] |
| Adsorption | High: typically 90–99%, dependent on adsorbent and regeneration | Moderate: 5–12 | High: 10–200 | Scalable but operational costs increase significantly with adsorbent regeneration frequency | [117,157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.R.; Quinta-Ferreira, R.M.; Castro, L.M. Biological Treatments for VOC-Contaminated Off-Gas: Advances, Challenges, and Energetic Valorization Opportunities. Sustainability 2025, 17, 4802. https://doi.org/10.3390/su17114802
Silva JR, Quinta-Ferreira RM, Castro LM. Biological Treatments for VOC-Contaminated Off-Gas: Advances, Challenges, and Energetic Valorization Opportunities. Sustainability. 2025; 17(11):4802. https://doi.org/10.3390/su17114802
Chicago/Turabian StyleSilva, João R., Rosa M. Quinta-Ferreira, and Luís M. Castro. 2025. "Biological Treatments for VOC-Contaminated Off-Gas: Advances, Challenges, and Energetic Valorization Opportunities" Sustainability 17, no. 11: 4802. https://doi.org/10.3390/su17114802
APA StyleSilva, J. R., Quinta-Ferreira, R. M., & Castro, L. M. (2025). Biological Treatments for VOC-Contaminated Off-Gas: Advances, Challenges, and Energetic Valorization Opportunities. Sustainability, 17(11), 4802. https://doi.org/10.3390/su17114802







