Porous Carbon Derived from Pumpkin Tissue as an Efficient Bioanode Toward Wastewater Treatment in Microbial Fuel Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrode Preparation
2.2. Characterization
2.3. MFC Setup and Operation
2.4. Pollutant Removal
2.5. Microbial Community Analysis
3. Results and Discussion
3.1. Characterization of the PBE
3.2. Bioelectricity Generation in MFCs
3.3. Performance of the PBE-MFC
3.4. Biofilm on Anode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newhart, K.B.; Holloway, R.W.; Hering, A.S.; Cath, T.Y. Data-driven performance analyses of wastewater treatment plants: A review. Water Res. 2019, 157, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef]
- Varjani, S. Prospective review on bioelectrochemical systems for wastewater treatment: Achievements, hindrances and role in sustainable environment. Sci. Total Environ. 2022, 841, 156691. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Tang, C.; Yadav, A.K.; Abbassi, R.; Kang, P.; Cai, Y.; Liu, A.; Yang, A.; Li, M. A glance of coupled water and wastewater treatment systems based on microbial fuel cells. Sci. Total Environ. 2023, 892, 164599. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, J.; Fu, X.; Zhao, G. Finite-time Pade-based adaptive FNN controller implementation for microbial fuel cell with delay and multi-disturbance. Int. J. Hydrogen Energy 2025, 98, 1034–1043. [Google Scholar] [CrossRef]
- Huggins, T.; Wang, H.; Kearns, J.; Jenkins, P.; Ren, Z.J. Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour. Technol. 2014, 157, 114–119. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Rodríguez-Couto, S. Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): An overview. Biochem. Eng. J. 2020, 164, 107779. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.; Thomas, A. Carbon-Based Microbial-Fuel-Cell Electrodes: From Conductive Supports to Active Catalysts. Adv. Mater. 2016, 29, 1602547. [Google Scholar] [CrossRef]
- Roberts, A.D.; Li, X.; Zhang, H. Porous carbon spheres and monoliths: Morphology control, pore size tuning and their applications as Li-ion battery anode materials. Chem. Soc. Rev. 2014, 43, 4341–4356. [Google Scholar] [CrossRef]
- Park, S.; Song, J.; Lee, W.C.; Jang, S.; Lee, J.; Kim, J.; Kim, H.-K.; Min, K. Advances in biomass-derived electrode materials for energy storage and circular carbon economy. Chem. Eng. J. 2023, 470, 144234. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Liu, K.; He, S.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, F.; Pu, Y.; Lin, X.; Yin, H.; Tang, X. Nano-Fe3O4 coated on carbon monolith for anode enhancement in microbial fuel cells. J. Environ. Chem. Eng. 2023, 11, 109608. [Google Scholar] [CrossRef]
- Katuri, K.P.; Kamireddy, S.; Kavanagh, P.; Muhammad, A.; Conghaile, P.Ó.; Kumar, A.; Saikaly, P.E.; Leech, D. Electroactive biofilms on surface functionalized anodes: The anode respiring behavior of a novel electroactive bacterium, Desulfuromonas acetexigens. Water Res. 2020, 185, 116284. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, S.; Liu, Y.; Tang, J. Nanostructured Macroporous Bioanode Based on Polyaniline-Modified Natural Loofah Sponge for High-Performance Microbial Fuel Cells. Environ. Sci. Technol. 2013, 47, 14525–14532. [Google Scholar] [CrossRef]
- Zhao, C.; Song, Y.; Chen, H.; Chen, H.; Li, Y.; Lei, A.; Wu, Q.; Zhu, L. Improving the performance of microbial fuel cell stacks via capacitive-hydrogel bioanodes. Int. J. Hydrogen Energy 2025, 97, 708–717. [Google Scholar] [CrossRef]
- Du, J.; Yu, Y.; Zhang, Y.; Liu, L.; Chen, C.; Chen, A. All-Carbon Electrode Directly Derived from Wax Gourd for Supercapacitor. Phys. Status Solidi (a) 2019, 216, 1800798. [Google Scholar] [CrossRef]
- Rethinasabapathy, M.; Lee, J.H.; Roh, K.C.; Kang, S.-M.; Oh, S.Y.; Park, B.; Lee, G.-W.; Cha, Y.L.; Huh, Y.S. Silver grass-derived activated carbon with coexisting micro-, meso- and macropores as excellent bioanodes for microbial colonization and power generation in sustainable microbial fuel cells. Bioresour. Technol. 2020, 300, 122646. [Google Scholar] [CrossRef]
- Bai, S.; Tan, G.; Li, X.; Zhao, Q.; Meng, Y.; Wang, Y.; Zhang, Y.; Xiao, D. Pumpkin-Derived Porous Carbon for Supercapacitors with High Performance. Chem.—Asian J. 2016, 11, 1828–1836. [Google Scholar] [CrossRef]
- HJ 535-2009; Determination of Ammonia Nitrogen in Water Quality—Nessler’s Reagent Spectrophotometric Method. China Standards Press: Beijing, China, 2009.
- GB/T 11893-1989; Determination of Total Phosphorus in Water Quality—Ammonium Molybdate Spectrophotometric Method. China Standards Press: Beijing, China, 1989.
- HJ 924-2017; Technical Requirements and Testing Methods for Rapid Determination Instruments Using COD Photometric Method. China Standards Press: Beijing, China, 2017.
- Qi, X.; Jia, X.; Li, M.; Ye, M.; Wei, Y.; Meng, F.; Fu, S.; Xi, B. Enhancing CO2-reduction methanogenesis in microbial electrosynthesis: Role of oxygen-containing groups on carbon-based cathodes. Bioresour. Technol. 2025, 416, 131830. [Google Scholar] [CrossRef] [PubMed]
- Pajerski, W.; Duch, J.; Ochonska, D.; Golda-Cepa, M.; Brzychczy-Wloch, M.; Kotarba, A. Bacterial attachment to oxygen-functionalized graphenic surfaces. Mater. Sci. Eng. C 2020, 113, 110972. [Google Scholar] [CrossRef]
- Li, H.; Wang, B.; Deng, S.; Dai, J.; Shao, S. Oxygen-containing functional groups on bioelectrode surface enhance expression of c-type cytochromes in biofilm and boost extracellular electron transfer. Bioresour. Technol. 2019, 292, 121995. [Google Scholar] [CrossRef]
- Zakaria, B.S.; Dhar, B.R. Characterization and significance of extracellular polymeric substances, reactive oxygen species, and extracellular electron transfer in methanogenic biocathode. Sci. Rep. 2021, 11, 7933. [Google Scholar] [CrossRef]
- Dong, W.; Xing, J.; Chen, Q.; Huang, Y.; Wu, M.; Yi, P.; Pan, B.; Xing, B. Hydrogen bonds between the oxygen-containing functional groups of biochar and organic contaminants significantly enhance sorption affinity. Chem. Eng. J. 2024, 499, 156654. [Google Scholar] [CrossRef]
- Chen, W.; Wu, D.; Wan, H.; Tang, R.; Li, C.; Wang, G.; Feng, C. Carbon-based cathode as an electron donor driving direct bioelectrochemical denitrification in biofilm-electrode reactors: Role of oxygen functional groups. Carbon 2017, 118, 310–318. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, B.; Yang, J.; Zhou, J.; Xu, Y. Linear discriminant analysis. Nat. Rev. Methods Primers 2024, 4, 1–16. [Google Scholar] [CrossRef]
- Qiu, Y.; Feng, Y.; Yan, Z.; Li, J.; Li, D.; Yan, C.; Liu, G. Improving performance of pilot-scale ecological bed coupled with microbial electrochemical system for urban tail water treatment. Sci. Total Environ. 2023, 865, 161289. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, B.; Kong, Y.; Tao, Y.; Zhao, Z.; Li, Y.; Zhang, Y. Correlation between intracellular electron transfer and gene expression for electrically conductive pili in electroactive bacteria during anaerobic digestion with ethanol. Water Res. 2024, 265, 122307. [Google Scholar] [CrossRef]
- Pu, Y.; Tian, Y.; Hou, S.; Dou, W.; Chen, S. Enhancement of exogenous riboflavin on microbiologically influenced corrosion of nickel by electroactive Desulfovibrio vulgaris biofilm. npj Mater. Degrad. 2023, 7, 7. [Google Scholar] [CrossRef]
- Wang, G.; Chen, L.; Xing, Y.; Sun, C.; Fu, P.; Li, Q.; Chen, R. Biochar establishing syntrophic partnership between exoelectrogens to facilitate extracellular electron transfer. Sci. Total Environ. 2023, 904, 166549. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, W.; Li, L. Dechlorination of dichloromethane by a biofilter enriched with electroactive bacteria: Performance, kinetics, and microbial community. Environ. Res. 2022, 215, 114247. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Zhang, C.; Geng, A.; Sun, Z.; Yang, J.; Xi, J.; Wang, L.; Yang, B. Effect of weak electrical stimulation on m-dichlorobenzene biodegradation in biotrickling filters: Insights from performance and microbial community analysis. Bioresour. Technol. 2023, 390, 129881. [Google Scholar] [CrossRef]
- Li, Z.-R.; Zhang, X.-N.; Wang, H.-C.; Cheng, H.-Y.; Wang, A.-J.; Zhang, Y.-Q.; Cui, C.-W.; Sun, Y.-L. Effects of salinity on sulfur-dominated autotrophic denitrification microorganisms: Microbial community succession, key microorganisms and response mechanisms. Chem. Eng. J. 2023, 478, 147308. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, R.; Jiang, H.; Yang, F. Sulphur-based autotrophic denitrification of wastewater obtained following graphite production: Long-term performance, microbial communities involved, and functional gene analysis. Bioresour. Technol. 2020, 306, 123117. [Google Scholar] [CrossRef]
- Rumora, A.; Hopkins, L.; Yim, K.; Baykus, M.F.; Martinez, L.; Jimenez, L. Detection and Characterization of Electrogenic Bacteria from Soils. BioTech 2023, 12, 65. [Google Scholar] [CrossRef]
- Liang, L.; Yu, Q.; Li, Y.; Zhao, Z.; Fan, S.; Zhang, Y. Effects of magnetite on nitrate-dependent anaerobic oxidation of methane in an anaerobic membrane biofilm reactor: Metatranscriptomic analysis and mechanism prediction. Environ. Technol. Innov. 2023, 32, 103288. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Gu, P.; Yang, K.; Huang, X.; Li, M.; Miao, H. Elucidating applied voltage on the fate of antibiotic resistance genes in microbial electrolysis cell: Focusing on its transmission between anolyte and biofilm microbes. Sci. Total Environ. 2023, 904, 166901. [Google Scholar] [CrossRef]
- Manchon, C.; Muniesa-Merino, F.; Llorente, M.; Esteve-Núñez, A. Microbial photoelectrosynthesis: Feeding purple phototrophic bacteria electricity to produce bacterial biomass. Microb. Biotechnol. 2022, 16, 569–578. [Google Scholar] [CrossRef]
- Xia, Q.; Cheng, J.; Yang, F.; Yi, X.; Huang, W.; Lei, Z.; Wang, D.; Huang, W. Activated carbon and anthraquinone-2,6-disulfonate as electron shuttles for enhancing carbon and nitrogen removal from simultaneous methanogenesis, Feammox and denitrification system. Bioresour. Technol. 2025, 418, 131975. [Google Scholar] [CrossRef]
- Zeng, D.; Miao, J.; Wu, G.; Zhan, X. Nitrogen removal, microbial community and electron transport in an integrated nitrification and denitrification system for ammonium-rich wastewater treatment. Int. Biodeterior. Biodegrad. 2018, 133, 202–209. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; Li, J.; Wang, Y.; Wu, D.; Li, F.; Song, H. Advances in mechanisms and engineering of electroactive biofilms. Biotechnol. Adv. 2023, 66, 108170. [Google Scholar] [CrossRef]
- Thapa, B.S.; Kim, T.; Pandit, S.; Song, Y.E.; Afsharian, Y.P.; Rahimnejad, M.; Kim, J.R.; Oh, S.-E. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef]
- Flemming, H.-C.; van Hullebusch, E.D.; Little, B.J.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. Microbial extracellular polymeric substances in the environment, technology and medicine. Nat. Rev. Microbiol. 2024, 23, 87–105. [Google Scholar] [CrossRef]
- Huang, L.; Puma, G.L. Physiological response of electroactive bacteria via secretion of extracellular polymeric substances in microbial electrochemical processes: A review. Curr. Opin. Electrochem. 2022, 36, 101168. [Google Scholar] [CrossRef]

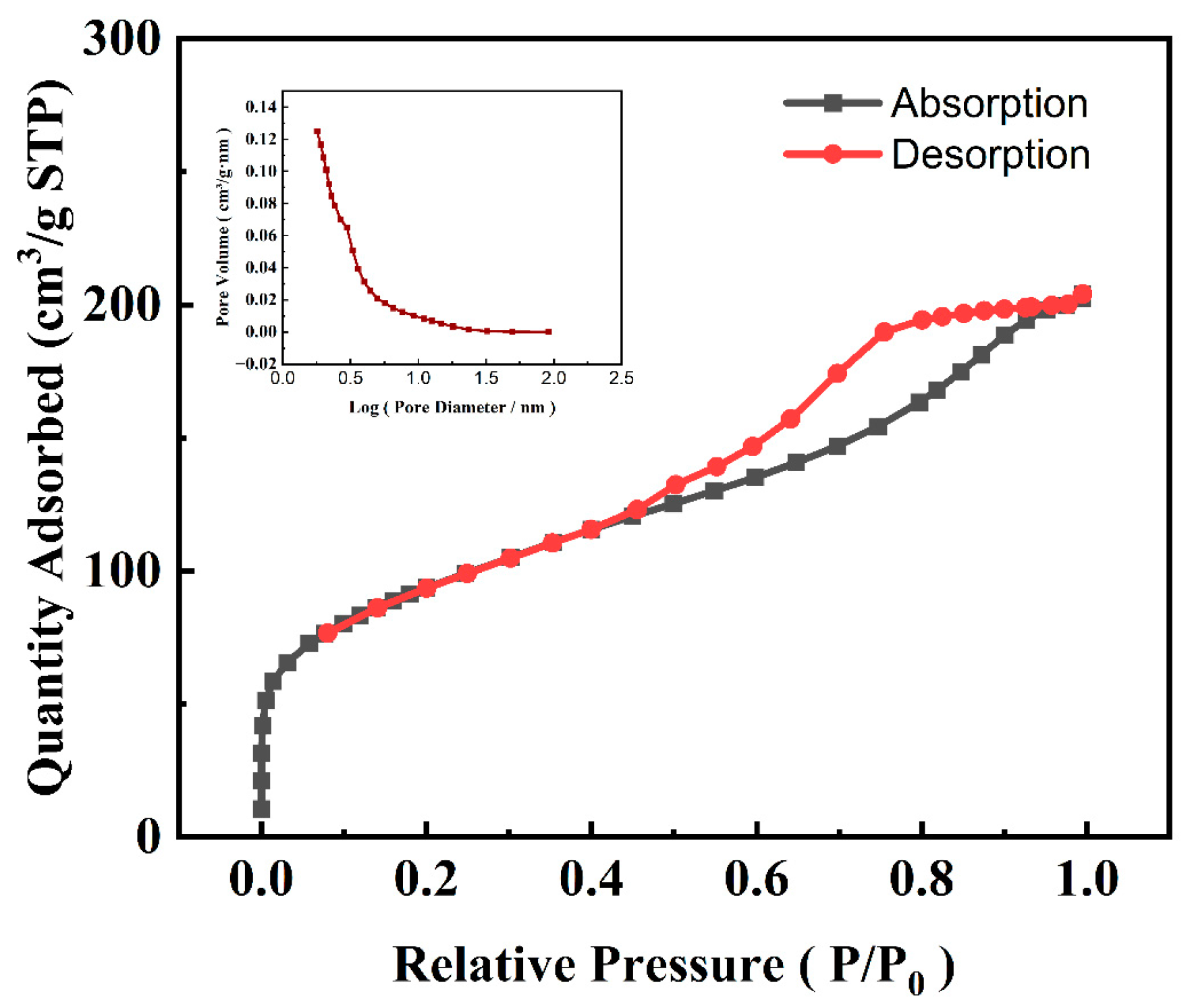



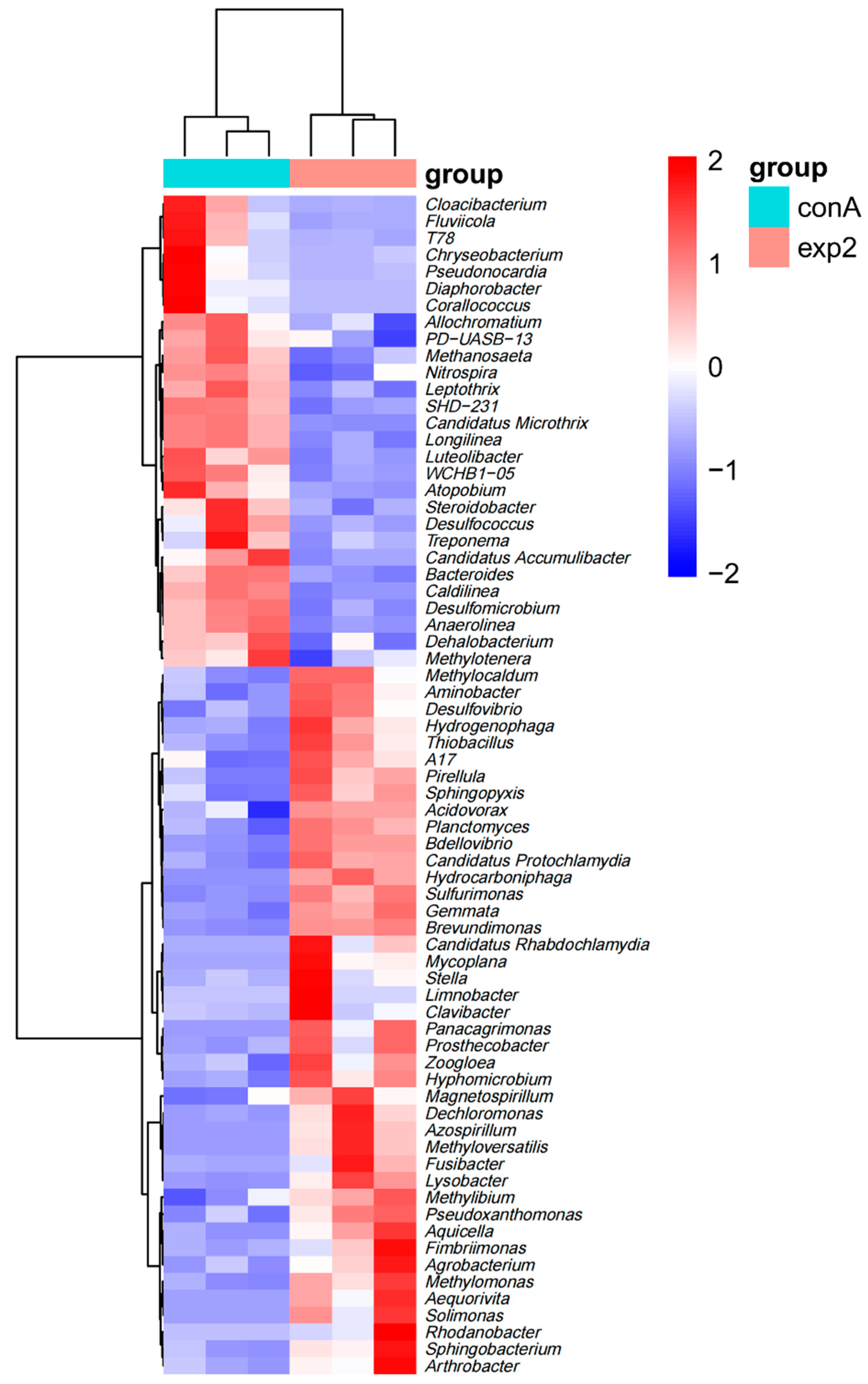
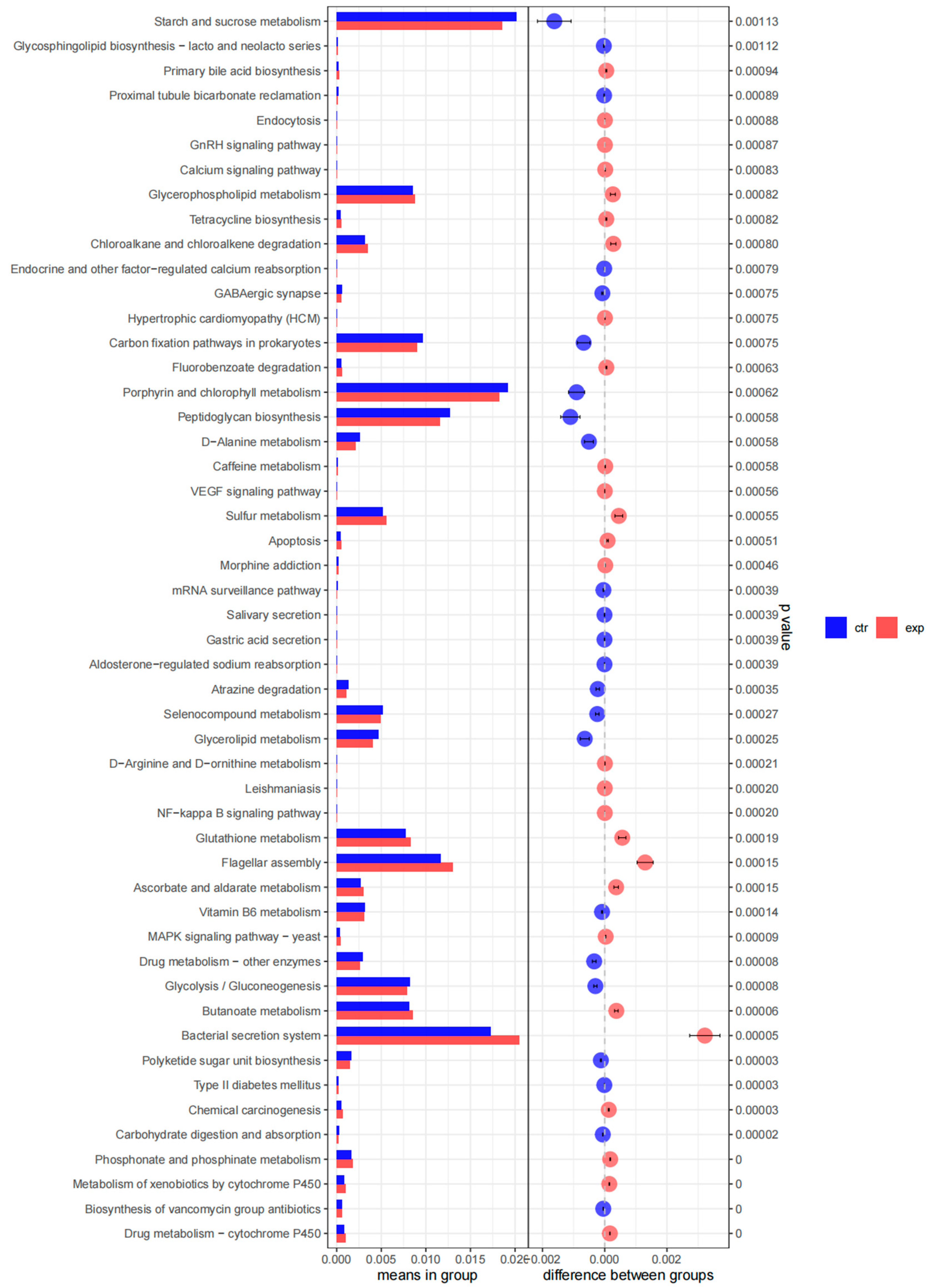
| Pollutant | Time | CB-MFC | PBE-MFC | Significance Between CB-MFC and PBE-MFC |
|---|---|---|---|---|
| COD (mg/L) | 0 h | 182.33 ± 7.51 | 180.00 ± 12.29 | ns (Adjusted p = 0.9808) |
| 48 h | 75.00 ± 3.00 | 52.00 ± 4.00 | * (Adjusted p = 0.0252) | |
| Significance Before/After Treatment | **** (Adjusted p < 0.0001) | **** (Adjusted p < 0.0001) | - | |
| Average Removal Rate | 58.79% | 71.43% | **** (Adjusted p < 0.0001) | |
| NH4+-N (mg/L) | 0 h | 4.84 ± 0.18 | 4.92 ± 0.28 | ns (Adjusted p = 0.9502) |
| 48 h | 2.28 ± 0.13 | 1.43 ± 0.19 | ** (Adjusted p = 0.0040) | |
| Significance Before/After Treatment | **** (Adjusted p < 0.0001) | **** (Adjusted p < 0.0001) | - | |
| Average Removal Rate | 53.75% | 70.18% | **** (Adjusted p < 0.0001) | |
| TP (mg/L) | 0 h | 226.67 ± 8.51 | 227.00 ± 7.00 | ns (Adjusted p = 0.9999) |
| 48 h | 108.67 ± 4.62 | 74.33 ± 3.51 | *** (Adjusted p = 0.0007) | |
| Significance Before/After Treatment | **** (Adjusted p < 0.0001) | **** (Adjusted p < 0.0001) | - | |
| Average Removal Rate | 53.30% | 67.40% | **** (Adjusted p < 0.0001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Yan, X.; Ding, Q.; Xiang, J.; Wei, Z.; Yang, Q.; Xie, K.; Cheng, B.; Xie, X. Porous Carbon Derived from Pumpkin Tissue as an Efficient Bioanode Toward Wastewater Treatment in Microbial Fuel Cells. Sustainability 2025, 17, 4758. https://doi.org/10.3390/su17114758
Liu J, Yan X, Ding Q, Xiang J, Wei Z, Yang Q, Xie K, Cheng B, Xie X. Porous Carbon Derived from Pumpkin Tissue as an Efficient Bioanode Toward Wastewater Treatment in Microbial Fuel Cells. Sustainability. 2025; 17(11):4758. https://doi.org/10.3390/su17114758
Chicago/Turabian StyleLiu, Jiaxin, Xue Yan, Qiang Ding, Jiwu Xiang, Zuna Wei, Qian Yang, Kangwei Xie, Bo Cheng, and Xiaoying Xie. 2025. "Porous Carbon Derived from Pumpkin Tissue as an Efficient Bioanode Toward Wastewater Treatment in Microbial Fuel Cells" Sustainability 17, no. 11: 4758. https://doi.org/10.3390/su17114758
APA StyleLiu, J., Yan, X., Ding, Q., Xiang, J., Wei, Z., Yang, Q., Xie, K., Cheng, B., & Xie, X. (2025). Porous Carbon Derived from Pumpkin Tissue as an Efficient Bioanode Toward Wastewater Treatment in Microbial Fuel Cells. Sustainability, 17(11), 4758. https://doi.org/10.3390/su17114758







